Abstract
Retinal imaging technology is rapidly advancing and can provide ever-increasing amounts of information about the structure, function and molecular composition of retinal tissue in humans in vivo. Most importantly, this information can be obtained rapidly, non-invasively and in many cases using Food and Drug Administration-approved devices that are commercially available. Technologies such as optical coherence tomography have dramatically changed our understanding of retinal disease and in many cases have significantly improved their clinical management. Since the retina is an extension of the brain and shares a common embryological origin with the central nervous system, there has also been intense interest in leveraging the expanding armamentarium of retinal imaging technology to understand, diagnose and monitor neurological diseases. This is particularly appealing because of the high spatial resolution, relatively low-cost and wide availability of retinal imaging modalities such as fundus photography or OCT compared to brain imaging modalities such as magnetic resonance imaging or positron emission tomography. The purpose of this article is to review and synthesize current research about retinal imaging in neurodegenerative disease by providing examples from the literature and elaborating on limitations, challenges and future directions. We begin by providing a general background of the most relevant retinal imaging modalities to ensure that the reader has a foundation on which to understand the clinical studies that are subsequently discussed. We then review the application and results of retinal imaging methodologies to several prevalent neurodegenerative diseases where extensive work has been done including sporadic late onset Alzheimer’s Disease, Parkinson’s Disease and Huntington’s Disease. We also discuss Autosomal Dominant Alzheimer’s Disease and cerebrovascular small vessel disease, where the application of retinal imaging holds promise but data is currently scarce. Although cerebrovascular disease is not generally considered a neurodegenerative process, it is both a confounder and contributor to neurodegenerative disease processes that requires more attention. Finally, we discuss ongoing efforts to overcome the limitations in the field and unmet clinical and scientific needs.
Keywords: Retina, Imaging, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, Cerebral small vessel disease
1. Introduction
The retina is the only portion of the central nervous system (CNS) that is optically accessible for high-resolution imaging. This presents a unique opportunity to study CNS pathology because of the shared embryological origins, structure and physiology with the retina. Over the last several decades, many investigators have attempted to leverage the optical accessibility of the retina to better understand, diagnose and even treat neurodegenerative diseases including sporadic late onset Alzheimer’s Disease (LOAD), Huntington’s Disease (HD), Parkinson’s Disease (PD), Multiple Sclerosis (MS), cerebral small vessel disease (CSVD) and Frontotemporal Dementia (FTD). In many cases these studies have demonstrated significant associations of retinal thickness and function with disease severity and have provided tantalizing possibilities for disease assessment.
The development of imaging techniques such as optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA) have reinvigorated the search for retinal changes that are associated with neurodegenerative disease. Although clear clinical applications are needed, there are several reasons to continue the search for in vivo retinal changes in neurodegenerative disease. First, diagnosis of many neurodegenerative diseases requires significant time, resources and cost. For example, positron emission tomography (PET) and magnetic resonance imaging (MRI) are commonly used in the evaluation of intracranial pathology but both are time-intensive, not universally available and come at significant cost. In addition, neither has the spatial resolution to detect sub-millimeter changes in tissue pathology with clinically useful reproducibility. In contrast, in vivo OCT imaging of the retina provides near histologic level resolution of neurosensory tissue and the recent advent of OCTA provides similar information about retinal blood vessels, including capillaries. Therefore, there exists the possibility that in vivo retinal imaging can provide useful biomarkers of neurodegenerative disease much earlier in the disease process than conventional neuroimaging methods or clinical examination. It is unlikely and impractical to think that retinal imaging will be the sole or even primary diagnostic criteria for any neurodegenerative process, but there are at least three scenarios in which it might prove useful. First, in vivo retinal findings in human subjects with neurodegenerative diseases may provide new insight into the underlying disease pathophysiology. Second, in vivo retinal imaging may be a relatively low-cost and widely available screening tool for assessing risk for certain neurodegenerative disease if appropriate biomarkers are identified. This would be a valuable tool in recruiting appropriate populations for clinical trials and for more resource intensive testing such as brain imaging. Lastly, under the most ideal circumstances, in vivo retinal imaging may be a useful primary or secondary endpoint in clinical trials.
In this article, we discuss the literature on the most prevalent and well-described neurodegenerative diseases with retinal manifestations and examine how rapidly evolving retinal imaging modalities can be leveraged to advance our understanding of these diseases. While there are currently few clinical indications for retinal imaging in neurodegenerative diseases, the opportunity clearly exists and future advances in our understanding of the diseases and imaging methods may enable such indications. By consolidating the knowledge about retinal imaging findings in this comprehensive review we hope to provide a very useful resource for ophthalmologists, neurologists, researchers and students to understand the current state-of-the-art and advance the field in new directions. It is important to mention that many neurodegenerative diseases have extraretinal ophthalmic manifestations (e.g. oculomotor abnormalities) that have been well-described in the neuroophthalmologic literature and which we will not discuss here. We also do not attempt to review the vast body of literature from informative animal models of neurodegenerative disease so that we can focus on the already vast number of applications of retinal imaging in human neurodegenerative disease in this manuscript.
2. Rationale for retinal imaging in neurodegenerative disease
2.1. Embryology
The retina and optic nerve are derived from the neuroectoderm around 23 days of gestation when they invaginate from the diencephalon (Cameron et al., 2017). Therefore the retina shares a common cellular origin with brain tissue and is considered part of the CNS. Retinal neurons have many structural and functional similarities to other neurons in the brain. Retinal ganglion cells (RGC) have axons that extend to the lateral geniculate nucleus (LGN) via a nerve fiber tract complete with an oligodendrocytic myelin sheath. Retinal neurons are generally excitable and have synaptic interconnections mediated by neurotransmitters including acetylcholine, dopamine, glutamate, glycine, and gaba-aminobutyric acid (Gregg et al., 2013). This similarity led to investigation of the pupillary response to dilute tropicamide, a cholinergic antagonist, as a diagnostic test for LOAD, a disease in which CNS cholinergic tone is widely depressed (Scinto et al., 1994; Kardon 1998; Iijima et al., 2003). Similar to other axonal pathways, damage to the optic nerve causes retrograde and anterograde degeneration of the axons, associated cell bodies and target tissues. For example, atrophy of the retina is inversely associated with brain weight in persons with MS (Green et al., 2010). A broad range of biophysical, pathological, clinical and epidemiologic studies demonstrate that retinal blood vessels share many similarities with cerebral vessels and undergo similar pathologic changes implicated in cerebral small vessel disease (CSVD) (Zamir 1976a,b; Sherman 1981; Cogan and Kuwabara 1984; Törnquist and Alm 1986; Frank et al., 1990; Ravalico et al., 1996; Trost et al., 2016). Retinal and cerebral blood vessels share significant similarity in capillary branching angles and organization (Cogan and Kuwabara 1984), carrier-mediated transport functions of blood-retina barrier (BRB) and blood brain barrier (BBB) (Törnquist and Alm 1986), structural features of capillaries and tight junctions (Trost et al., 2016), and pericyte-to-endothelial cell ratio (Frank et al., 1990). In addition to these similarities in health, there are many retinal manifestations of CNS disease as we will discuss below.
2.2. Pathophysiological mechanisms of retinal degeneration in neurodegenerative disease
Given the embryological and physiological similarity between the neurosensory retina, retinal vasculature and brain, there are at least two plausible pathophysiological mechanisms by which neurodegenerative changes may manifest in the retina. One plausible mechanism is that the underlying neurodegenerative process concurrently occurs in retinal tissue as well as in the brain. Abnormal intracellular and extracellular deposition of proteins such as tau, alpha-synuclein (αsyn), and beta-amyloid (Aβ) among others are present in several neurodegenerative disorders and are also found in the aging retina (Löffler et al., 1995; Leger et al., 2011). There is also evidence that Aβ, tau and αsyn accumulate in the retina of subjects with AD (Koronyo-Hamaoui et al., 2011) and PD (Bodis-Wollner et al., 2014), respectively, and may therefore mediate the same neurotoxicity in RGCs as in other CNS tissue. In one study, atrophy of specific classes of RGCs was shown to be associated with disturbance of the sleep-wake cycle in persons with AD (La Morgia, Ross-Cisneros et al., 2016).
A second plausible mechanism is that neurodegenerative changes in the CNS cause some form of retrograde degeneration of ganglion cell axons and associated cell bodies located in the retina (Peng et al., 2020). There is some evidence that diencephalic and brainstem visual centers such as the LGN and superior colliculus manifest senile plaques and neurofibrillary tangles (Leuba and Saini 1995) although the topographic distribution of these changes does not consistently correspond with the patterns of degeneration observed in the retinal nerve fiber layer (RNFL). Analogous evidence exists from case reports of occipital lesions in humans (Meier et al., 2015; Goto et al., 2016). Histopathologic analysis of the visual system in primates with occipital lesions also reveals evidence of transsynaptic degeneration of RGCs (Cowey et al., 2011). Localized defects in the RNFL are also significantly associated with acute and chronic stroke independent of systemic vascular risk factors such as hypertension and diabetes mellitus (Wang et al., 2014). RNFL defects are also significantly associated with infarcts and white matter lesions on MRI in large population-based studies (Kim et al., 2011; Mauschitz et al., 2018). In subjects with PD, the laterality of retinal findings seems to correlate with the more affected hemisphere suggesting a neuroanatomic link (Pilat et al., 2016).
Lastly, it is likely that a combination of concurrent and sequential degeneration is present in variable amounts depending on the specific pathological process and its duration. We should note that these two mechanisms described above are therefore not mutually exclusive.
3. Imaging methodologies
There are numerous methods of imaging the retina ranging from relatively simple color photography to functional imaging of retinal electrical activity. This wealth of imaging technology combined with the optical accessibility of the retina has been one of the main motivations in exploring the role of retinal pathology in neurodegenerative diseases. Broadly speaking these imaging modalities can be divided into those that demonstrate structural features of the retina and those that measure some aspect of retinal function. Structural features include size of retinal vessels, retinal lesions and thickness of retinal layers. Functional features of the retina that are quantified by imaging methods include retinal light sensitivity, blood flow and electrical activity. Below we enumerate and describe the key modalities that have been used to study retinal changes in neurodegenerative diseases. In the subsequent sections we discuss the applications of these imaging modalities in disease-specific contexts. It is important to note that most of these modalities are approved for clinical use in ophthalmology practices by the Food and Drug Administration (FDA). Therefore they provide readily available and clinically feasible tools for assessing retinal pathology in clinical trials and health care settings.
3.1. Structural imaging
The most widely used and well-accepted modalities in retinal imaging provide some measure of retinal structure whether that is the qualitative physical appearance of the retina or a quantitative measure such as thickness. The sections below are not meant to be exhaustive reviews of the imaging methodologies. Rather, these sections are meant to provide an overview of the methods so that the relevance to neurodegenerative diseases discussed in subsequent sections can be more easily understood.
3.1.1. Fundus photography
3.1.1.1. Color fundus imaging.
Color fundus photography (CFP) represents the most common and widely used retinal imaging modality (Yannuzzi et al., 2004; Panwar et al., 2016). CFP was traditionally performed with film but is now available in true color and pseudocolor digital format as well as multi-wavelength scanning laser ophthalmoscopy. These cameras acquire information with relatively low spectral resolution (red, green and blue color channels) but with high spatial resolution approaching dozens of microns. Most fundus cameras require pharmacologic pupillary dilation (mydriasis) which can be a barrier to use in non-ophthalmic settings. Non-mydriatic cameras are becoming more widely available but often have decreased resolution or field-of-view (FOV).
It is important to note that there can be significant artifactual variations in the spectral patterns (color) of the normal retina using pseudocolor digital systems. The color of retinal lesions can be significantly altered by the laser calibration of the camera and media properties of the eye. Therefore, depending on the lesion of interest, some cameras may be more suitable than others. One of the most important variables in CFP is the FOV which can range from 30 degrees of the retinal surface to more than 100° (Panwar et al., 2016). Digital montages of 30–50 degree FOV are also possible to allow for evaluation of peripheral retinal findings (Fig. 1). Common uses of CFP include identifying retinal lesions (e.g. RNFL defects), measuring retinal vascular caliber (e.g. central retinal artery and vein equivalents) and evaluating optic disc appearance (e.g. pallor) in the posterior pole. Modern devices allow for imaging of almost the entire retina and are often referred to as “widefield” (FOV approximately 90°) or “ultra-widefield” (FOV greater than 90°) devices Fig. 2 to contrast with those devices that have more restricted fields (ranging between 30 and 50°; Fig. 1). Widefield imaging has particularly useful applications in identifying peripheral retinal pathology that is difficult to describe and capture with conventional imaging (Kashani et al., 2014a,b). Therefore, one important parameter when considering CFP is that the FOV may exclude relevant pathology especially if it is located in the peripheral retina.
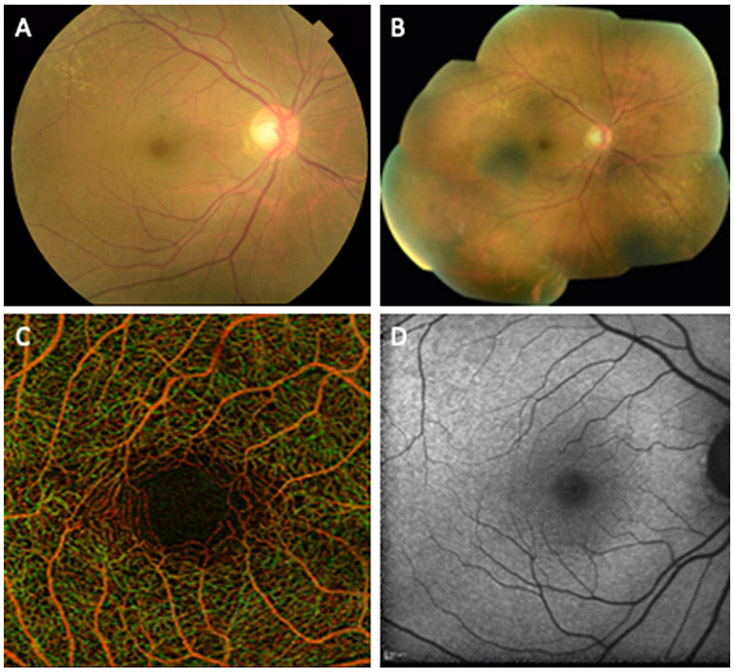
Fig. 1.
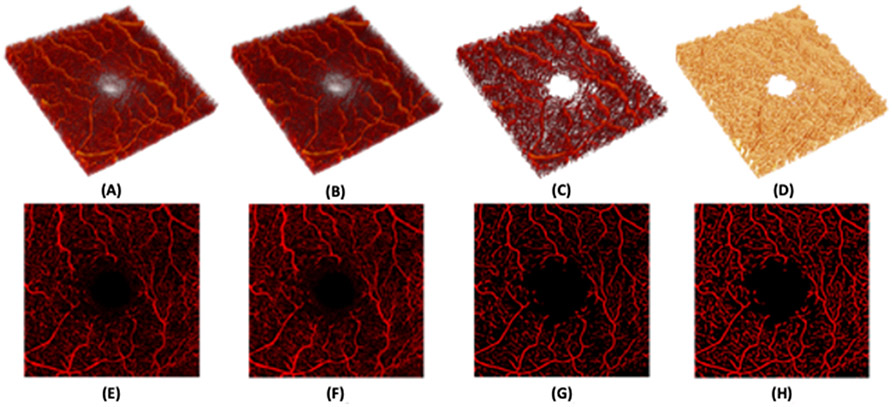
Examples of retinal imaging modalities from a 65 year old female illustrate commonly used methods for evaluation of retinal disease and retinal changes in neurodegenerative diseases. (A) Color fundus photograph illustrating the macula, optic disc and retinal arteries and veins. (B) Digital collage of color fundus images of the same subject demonstrating 60-90° field of view that includes the peripheral retina outside the vascular arcades. (C) Optical coherence tomography angiogram of the parafoveal area illustrating the capillaries in the area and the foveal avascular zone. Red and green pseudocoloring represent the depth of retinal capillaries in the superficial and deep retinal layers, respectively. (D) Short wave fundus autofluorescence image of the macula.
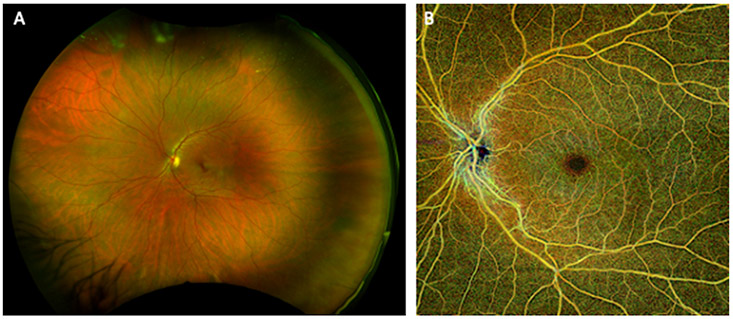
Fig. 2.
Ultra-Widefield fundus photograph and optical coherence tomography angiogram from a human subject. (A) Optos™ pseudocolor ultra-widefield fundus photograph illustrates the peripheral retina where traditional color fundus imaging does not typically reach. (B) Widefield optical coherence tomography angiogram (Zeiss PlexElite™) demonstrates non-invasive imaging of retinal arteries, veins and capillaries beyond the arcades in the same subject.
3.1.1.2. Fundus autofluorescence imaging.
One form of fundus imaging takes advantage of the natural autofluorescent characteristics of the retina and retinal pigment epithelium (RPE). The retina has intrinsic autofluorescence when stimulated by light at several wavelength ranges that is commonly referred to as fundus autofluorescence (FAF) (Sparrow et al., 2020). The FAF properties of the retina are studied at “short wavelength” and “long wavelength” ranges (Kellner et al., 2010). FAF is primarily thought to be a property of the RPE and not the neurosensory retina itself, at least in normal subjects. In addition, FAF of the peripheral retina is relatively poorly studied, only recently becoming possible with the advent of widefield imaging. Quantitative FAF measurements from the retina can vary dramatically, even within the same subject, due to relatively subtle changes in the optical media and light sources (Delori et al., 2011). Therefore, in the absence of carefully calibrated measurements quantitative FAF measurements with current commercially available devices may not be reliable for longitudinal measurements even within the same subject, at least in the absence of any significant calibration efforts.
Stimulation of the retina with short wavelength light (in the range of 400–590 nm) is often referred to as “blue light” FAF and elicits auto-fluorescence between 520 and 800 nm (Fig. 1D). The source of this blue light FAF originates from the bisretinoid family of compounds often referred to as lipofuscin and including A2-glycerophosphoethanolamine, A2E and A2-dihydropyridine-phosphatidylethanolamine (A2-DHP-PE). These compounds represent the product of irreversible chemical reactions of retinaldehyde and the lipid phosphatidylethanolamine that form in the photoreceptors and accumulate in the RPE with age. Normal FAF exhibits a characteristic hypoautofluorescence in the central macula that results from the attenuation of excitation light by macular pigments (xanthophylls). Outside of the central macula, the pattern of blue-light FAF is homogenous except for blood vessels and the disc which lack short-wavelength autofluorescence. In addition to these characteristic spatial variations, short-wavelength autofluorescence shows characteristic age-related changes in normal subjects (Delori et al., 2001). The relatively featureless auto-fluorescence patterns of normal retina provides a useful background for detecting pathological lesions with potential auto-fluorescent properties such as Aβ (Koronyo et al., 2017).
Long-wavelength autofluorescence is commonly generated using near-infrared light between 700 and 800 nm with excitation in the range >800 nm. This form of FAF is primarily from the melanin content of the RPE cells and to a lesser degree from the underlying choroidal melanocytes but it can also be generated by elevated lipofuscin levels in some retinal diseases. In contrast to the short-wavelength auto-fluorescence, long-wavelength autofluorescence has its peak signal intensity in the central macula.
3.1.1.3. Hyperspectral fundus imaging.
Hyperspectral retinal imaging (HRI) is one of the most recently developed forms of fundus photography. Hyperspectral imaging is based on the principal of spectroscopy commonly used for geospatial applications and astronomy. HRI takes advantage of the wide-range of spectral features in the eye as well as advances in spectroscopic imaging over the last several decades (Reshef et al., 2020). HRI requires specialized illumination and detection hardware that allows the high-resolution collection of a broad range of wavelength information across the visible and near-visible electromagnetic spectrum for each pixel in the acquired image. This contrasts with more conventional multispectral CFP (see section 5.1.1.1) which acquires information with lower spectral resolution. The most common application of HRI has been to quantify retinal vascular oxygen content in the form of retinal oximetry (Kashani et al., 2011; Mordant et al., 2011; Jaime et al., 2012). This particular method takes advantage of the well-known spectra of oxy- and deoxyhemoglobin. Several research groups and commercial entities have adopted similar methodology to examine the spectral features of Aβ in eye tissue (More et al., 2019) as well as the human eye in vivo (Hadoux et al., 2019; Sharafi et al., 2019). At least one study has demonstrated a significant correlation between brain PET amyloid burden in humans and retinal spectra associated with Aβ (Hadoux et al., 2019). Further discussion of this imaging modality in disease-specific contexts is provided below and in a recently published review in the context of AD (Gupta et al., 2020; Santangelo et al., 2020).
3.1.2. Fluorescence lifetime imaging ophthalmoscopy
While hyperspectral imaging assesses the breadth of spectral information available from retinal tissue, fluorescence lifetime imaging (FLIO) aims to quantify one specific aspect of the fluorescence of endogenous tissues, namely the duration of fluorescence (Dysli et al., 2017a,b). Of note, FLIO imaging systems are strictly for research and are not FDA approved for clinical use. Excitation of endogenous fluorophores by monochromatic light will cause excitation of natural fluorophores that decay with characteristic lifetimes. The average time between the excitation of the tissue with monochromatic light and the return to the ground state is measured using FLIO. The lifetime of fluorophores is both a characteristic of the fluorophore as well as the surrounding molecular environment. Among the most common retinal fluorophores are phenylalanine, tyrosine, tryptophan, nicotinamide adenine dinucleotide (NADH), flavin adenine dinucleotide (FAD), retinal, hemoglobin, melanin, collagen, lutein, zeazanthin, and lipofuscin. Due to the optical properties of the eye and safety considerations, not all retinal fluorophores are detectable in vivo. FLIO measurements of normal retina have demonstrated discrete patterns for various retinal regions such as the fovea, optic disc, retinal vessels and non-foveal retina (Dysli et al., 2017a,b). Of particular relevance to the current review is the potential use of FLIO to detect molecules implicated in neurodegenerative disease such as Aβ, tau, and huntingtin. We will provide a disease-specific discussion of this methodology in the relevant sections below.
3.1.3. Optical coherence tomography
OCT is a very well-established imaging modality that provides high-resolution images of the retinal structure based on interferometric methods. OCT was first described in 1991 (Huang et al., 1991) and was adopted widely in ophthalmology in the subsequent decades because it allowed quantitative measurements of retinal thickness, choroidal thickness and even sub-layer thickness with micron level precision (Asanad et al., 2019a,b) (Fig. 3). These kinds of measurements were adopted to understand how retinal anatomy can vary in vivo with normal aging, gender and ethnicity (Kashani et al., 2010). OCT measurements also provide the diagnostic basis on which anti-vascular endothelial growth factor agents became widely used in ophthalmology for treatment of macular edema in a wide range of diseases including age-related macular degeneration and diabetic retinopathy (Drexler and Fujimoto 2008). The high-resolution capability of OCT has progressively improved but even the original time-domain system design was rapidly adopted by neurologists to study changes in the RNFL in MS (Petzold et al., 2010) and AD (den Haan et al., 2017). Subsequent studies using more advanced spectral domain systems have replicated and expanded upon the previous studies (den Haan et al., 2017).
Fig. 3.
Example of retinal layer and choroidal identification on enhanced depth imaging optical coherence tomography (EDI-OCT) compared to histology. (A) OCT image depicts retinal layers relative to the corresponding retinal layers on (B) histology in a representative control micrograph stained with hematoxylin and eosin within the macula. (Reproduced under the Creative Commons Attribution-NonCommercial-NoDerivatives (CC BY-NC-ND) 4.0 http://creativecommons.org/licenses/by-nc-nd/4.0/ International License from Asanad S, Ross-Cisneros FN, Nassisi M, Barron E, Karanjia R, Sadun AA. The retina in Alzheimer’s disease: histomorphometric analysis of an ophthalmologic biomarker. Invest Ophthalmol Vis Sci. 2019; 60:1491–1500. https://doi.org/10.1167/iovs.18-25966).
While we will explore the disease specific findings of OCT in subsequent sections of this review, it is important to make a few generalizable points about OCT studies of the retina in neurodegenerative disease. First, most OCT systems image a relatively small portion of the retina ranging from 3 × 3 to 6 × 6mm regions of the central macula or optic disc region. Therefore, the majority of retinal tissue is not sampled with standard OCT imaging protocols. More recently, widefield systems can acquire up to 12 × 12mm FOV (or more) but are costly, not yet commonly available and have lower resolution than smaller scan patterns. Second, there are numerous manufacturers of OCT systems. It is well known that scan patterns, segmentation algorithms and outcome measures vary significantly among manufacturers (Garcia-Martin et al., 2012a,b; Garcia-Martin et al., 2014a,b; Satue et al., 2014; Chan et al., 2019). Therefore, when comparing across studies, it is critical to keep in mind the details of the OCT system and scan parameters used. Third, even within the same commercial system, segmentation algorithms may vary over time and therefore the consistency of measurements made with different generations of the same device may vary in subtle but significant ways (Chan et al., 2019). Fourth, almost all studies that are published to date use two-dimensional representations of retinal features such as thickness and vessel density. These two-dimensional representations of three-dimensional biological structures have inherent limitations which we discuss at the end of this article with respect to future directions.
Lastly, it is important to emphasize that while the resolution of OCT devices is on the micron scale, the reproducibility of measurements depend on the experience and training of users. In experienced hands, the reproducibility of OCT based measurements of retinal thickness can be excellent (Intraclass correlation coefficient = 1.0 (Hu et al., 2015) and the coefficient of variation very low (0.5% in (Hu et al., 2015) and 0.4% in (Obis et al., 2020)). To achieve this level of reproducibility and precision, significant effort has to be expended for training of users and quality control of data.
3.2. Functional imaging
There are several imaging modalities that measure functional aspects of the neurosensory retina and supporting tissues including the RPE and retinal vasculature. These methods are distinctly different from the structural imaging modalities discussed above because they measure an aspect of cellular or tissue function, such as blood flow or electrical activity, rather than provide a static rendering of the retinal structure such as retinal thickness. Below we will review the most relevant of these methodologies so that they can be discussed in disease specific contexts in later sections. The sections below are not meant to be exhaustive reviews of the methodologies. Rather these sections are meant to provide an overview of the method and relevance to neurodegenerative disease in particular.
3.2.1. Optical coherence tomography angiography
OCTA is a recent imaging modality based on OCT that was FDA approved in 2015. OCTA provides information based on the movement of red blood cells within retinal capillaries and OCTA output is often displayed as a static map of retinal capillary density. It is important to keep in mind that while maps of retinal capillary density are often interpreted as anatomic representation of capillaries, they are actually derived from the perfusion state of capillaries. In this sense, OCTA can be considered an assessment of capillary structure and/or perfusion. This technology has also been extensively reviewed elsewhere (Kashani et al., 2017; Borrelli et al., 2018) but is worth discussing here because it is uniquely suited to demonstrate microvascular changes in the retina that may correlate with vascular changes in the CNS. OCTA allows depth-resolved imaging of the retinal vasculature that is far superior to invasive angiography using fluorescein and approaching histologic resolution (Matsunaga et al., 2014; Spaide et al., 2015a,b,c). This allows separation of the retinal capillaries into at least two (or more) capillary plexi commonly referred to as the superficial and deep retinal layers (Figs. 1C and 2B) (Matsunaga et al., 2014; Campbell et al., 2017). In the peripapillary region, there is an additional plexus, the peripapillary radial capillaries, that feeds the RNFL (Matsunaga et al., 2014). OCTA analyses are frequently reported as a density metric such as “vessel density,” “skeleton density” or “perfusion density.” In all cases these values are reporting the detection of red blood cell flow in patent retinal capillaries. By definition, any value reported using OCTA is a “perfusion density” metric because capillaries without flow (or with very slow flow) are not detected. A detailed discussion of OCTA methodology is available elsewhere (Kashani et al., 2017; Spaide et al., 2018). It is notable that most studies performed with OCTA to date only include assessments of the central macula or parafoveal capillaries (Fig. 1C). However, commercially available wide-field OCTA devices that can image the retinal periphery are rapidly becoming available and will likely play an important role in the future (Fig. 2). Similar to OCT based measures of retinal thickness the reproducibility of OCTA measures can be excellent with experienced users, appropriate device settings and quality control steps (Chen C et al., 2016, Conti et al., 2018).
Capillary level changes detected by OCTA correlate with clinical disease severity in diabetic retinopathy (Kim et al., 2016a,b; Kashani et al., 2017), retinal vein occlusion (Kashani et al., 2015; Koulisis et al., 2017) and even inflammatory diseases of the eye (Kim et al., 2016a,b) among many others (Kashani et al., 2017). Several recent studies have demonstrated correlations between capillary features and neurodegenerative disease, most notably in AD (O’Bryhim et al., 2018; Querques et al., 2019). Vascular contributions to cognitive impairment and dementia are common and their diagnosis and monitoring represents a significant unmet medical need. More than 50% of subjects with LOAD pathology have significant comorbid vascular pathology (Schneider et al., 2004; Schneider, Arvanitakis et al. 2007, 2009b; Schneider et al., 2009a). Consensus statements from several leading groups have helped outline the potential relevance of vascular contributions to cognitive impairment and dementia (VCID) (Gorelick et al., 2011; Snyder et al., 2015). These consensus statements called for the development of novel, clinically feasible biomarkers of vascular cognitive impairment and dementia for which OCTA is a promising modality. It is noteworthy that the same limitations that apply to OCT imaging systems apply in large part to OCTA imaging (Spaide et al., 2015a,b,c). Further discussion of this imaging modality in disease-specific contexts is provided in the following sections.
Given the novelty of OCTA it is appropriate to briefly discuss its limitations and the importance of quality control processes in image acquisition and analysis. OCTA images are particularly prone to artifacts, even more so than the underlying OCT images, because they are derived from at least two high-quality and coregistered OCT scans. The most common OCTA artifacts result from the motion of the eye relative to the OCTA device during image acquisition (Spaide et al., 2015a,b,c). Although efforts have been made to circumvent and correct eye motion during image acquisition, this still remains one of the most common artifacts of OCTA images, especially in diseased eyes. Motion correction technologies attempt to correct these movements but also create unique patterns such as stretch artifact, quilting artifact, or duplication artifacts. Another common type of artifact results from loss of signal detection by the device. For example, shadow artifacts results from the blockage of signal by media opacities like floaters. It has been shown that media opacities affect OCTA quantitative measures (Spaide et al., 2015a,b,c). Blink artifacts results from temporary blockage of signal by the eyelid. Advances in eye tracking technology now detect most eye blinks and enable rescanning of the appropriate area. Segmentation errors are another common type of artifact. Although software errors in detection of retinal layer boundaries are seen less frequently in normal eyes, segmentation errors are common in diseased eyes where layer boundaries are irregular or altogether absent (Spaide et al., 2015a,b,c). Image decentration is another problem that appears especially in subjects with difficulty with foveal fixation. Although not technically an artifact, decentration can significantly impact reproducibility of quantitative measurements. Decentration refers to the misalignment between the foveal avascular zone (FAZ) and the center of the OCTA en face image. Decentration severity can be graded based on the distance between the center of FAZ and center of the en face image.
As mentioned before, some of the artifacts seen in OCTA images are intrinsic to the underlying OCT technology, the most common of them being the projection artifact. Projection artifacts occur when the light passing through the superficial vessels is altered by reflection or absorption by the blood cells and surrounding tissue. This light that has passed through the superficial vessels is reflected back by tissues underlying the superficial blood vessels, such as the Retinal Pigment Epithelium (RPE), and is detected by the device. The reflected light from the RPE, creates the false impression of moving red blood cells in vessels within the RPE and, thus, overestimates perfusion density. These projection artifacts are observed in almost all of OCTA images but there are commercially available projection removal algorithms that minimize the impact of these artifacts on images (Spaide et al., 2015a,b,c).
3.2.2. Electroretinography
Electroretinography (ERG) provides a measure of the electrical activity in the neurosensory retina and RPE. ERGs provide information about neuronal and non-neuronal electrical activity in response to a light stimulus (Frishman 2013). The electrical activity of the retina, like other neuronal tissue, is driven by ionic gradients, primarily sodium and potassium, of the cellular components. ERGs provide very useful information across a wide-range of retinal diseases and the pathophysiology of ERG changes, at least for some disease processes, are well understood. While this review will not attempt a comprehensive review of this topic, it is worth noting the key features of ERGs here so that readers can have context when reading disease specific findings in later sections.
ERGs are obtained by surface electrodes placed on the eye and eyelids while simultaneous light stimulation is provided to one or both eyes. ERGs can be tuned to record light information preferentially from rods or cones as well as the downstream retinal circuitry that processes photoreceptor information. For example, the a-wave of the standard or full-field ERG (ffERG) is primarily derived from rods and cones depending on the dark adaptation status of the retina. The b-wave represents responses primarily from Muller and bipolar cells. The c-wave represents electrical activity of the RPE. Because of the summation of electrical activity at the corneal surface, ERGs are not sensitive to disease that affects a relatively small retinal area. There are very useful variations on the ERG methods, such as multifocal ERG (mfERG), which measures predominantly cone function in the central 30 degrees of the macula and pattern reversal ERG (PERG), which measures predominantly ganglion cell activity (Baker et al., 1988; Frishman 2013; Lachowicz and Lubiński 2018).
3.2.3. Visual evoked potentials
Visual evoked potentials (VEP) are not a form of direct retinal imaging but do reveal useful information about retinal function as well as the afferent arm of the visual pathway. A VEP is a measure of the light-induced electrical activity recorded from scalp electrodes placed over the occipital cortex. It is a form of electroencephalography where the signal-to-noise ratio can be significantly enhanced by averaging time-dependent visual stimuli. The most widely used VEP data come from measurements of the amplitude and latency of the initial negative peak (N1), subsequent positive peak (P1 or P100), following negative peak (N2) and ultimate positive peak (P2). Abnormal VEP responses can be due to abnormalities along any portion of the afferent visual pathway from the cornea and lens to the occipital cortex. VEPs are particularly useful in persons in whom an assessment of the visual potential is needed but the subject is unable to cooperate (e.g. children or developmentally delayed adults). Just as with ERG testing, there are useful variations of VEP testing, such a pattern-reversal VEP, which can provide more specific information about particular portions of the visual pathway. VEP abnormalities have been noted in several neurodegenerative diseases including MS and PD, suggesting they are not specific for a particular disease process (Regan and Neima 1984). It is reasonable to assume that any process which causes significant retinal dysfunction (e.g. glaucoma) will also cause abnormalities in the downstream tissues impacting VEP. Therefore, VEPs may have some useful role in assessing visual potential and location of anatomic lesions in neurodegenerative diseases, especially when subjects are unable to cooperate with other forms of testing.
4. Disease specific applications of retinal imaging
4.1. Multiple Sclerosis
Multiple Sclerosis (MS) is an autoimmune disease classically characterized by inflammation and demyelination of the CNS though neuronal and axonal degeneration have more recently come to be appreciated as critical processes in its pathogenesis (Campbell and Mahad 2018). MS impacts about 300,000 people in the United States and more than 2 million people around the world (Schiess and Calabresi 2016). The most characteristic lesions of MS are focal demyelinated plaques in the CNS that are accompanied by inflammation and gliosis. Unlike other neurodegenerative disorders, patients with MS often presents with visual symptoms at the early stages of the disease secondary to retrobulbar optic neuritis. Vision loss is associated with dull retrobulbar pain and often aggravated by eye movement. Common visual symptoms and signs include decreased visual acuity, loss of contrast sensitivity, a worsening red-green color defect, and an enlarging central scotoma, that usually peak at several days to a week (Group 2008). The Optic Neuritis Treatment Trial evaluated 389 subjects with acute unilateral optic neuritis without a diagnosis of MS (Evangelou et al., 2001) and followed them for 15 years (Group 2008). This study showed that the aggregate cumulative probability of developing MS at 15 years was 25% for subjects with ocular presentation without MRI lesions and 72% for patients with one or more intracranial MRI lesions. Therefore, the ocular findings in MS are of well-established clinical significance.
Although MS is the more common cause of optic neuritis (ON), neuromyelitis optica (NMO) and anti-myelin oligodendrocyte glycoprotein (MOG) antibody-positivity can also cause ON. It is clinically important to differentiate MS from NMO because the prognosis and treatment for NMO is different. OCT imaging has played a role in differentiating these disease entities. Although up to 80% of NMO subjects can be diagnosed by having a positive serum NMO antibody, NMO ON typically has more severe peripapillary RNFL (pRNFL) and GCL thinning than in MS (Filippatou et al., 2020a,b). Furthermore, microcystic macular edema is also more common in the inner nuclear layer (INL) of NMO subjects than in MS. Recent OCT based studies demonstrate subclinical RNFL and GCL thinning in eyes of subjects with AQP4-IgG seropositive NMO but without history of ON (Filippatou et al., 2020a,b). Not only do these OCT findings help differentiate NMO from MS, they serve as useful outcome measures in clinical trials (Bennett et al., 2015). Furthermore, it is clinically important to distinguish MS ON and NMO ON from anti-MOG-positive antibody ON by serum antibody testing and not by OCT findings. This antibody is present in one-third of all recurrent ON subjects and its presence predicts a better visual outcome than those with positive-NMO antibodies (Chen et al., 2018).
4.1.1. Optic nerve and peripapillary retina in Multiple Sclerosis
Histopathology of the LGN in subjects with MS has demonstrated significant axonal loss (32–45%) in the optic nerve and optic tract as well as parvocellular neuronal loss (Evangelou et al., 2001). In fact, ON is the initial clinical manifestation of MS in ~25% of patients and optic neuropathy in the form of demyelinating plaques are found in a majority of all postmortem samples, irrespective of any history of ON (Ikuta and Zimmerman 1976; Toussaint et al., 1983; Kerrison et al., 1994; Group 2008). Although not consistently performed, T2 weighted MRI images of the optic nerve can show hyperintense lesions with gadolinium enhancement during acute attacks. In one study, MRI demonstrated hyperintense lesions in 84% of symptomatic and 20% of asymptomatic MS subjects (Miller et al., 1988). Interestingly, cross-sectional and prospective MRI studies demonstrate that ON can be associated with thickening of the optic nerve in the acute phase followed by atrophy later (Hickman et al., 2004). This supports an initial inflammatory insult associated with tissue edema followed by atrophy. This pattern is similar to the pattern of ophthalmoscopic disc changes that are clinically demonstrable if the disease presentation is captured during the acute phase. In many cases ophthalmoscopic examination of the RNFL can demonstrate visible attenuation of the RNFL when a history of ON is present (Elbøl and Work 1990). One limitation of clinical examination is that, in general, at least 50% of the RNFL must be lost for clinically visible findings to be evident (Quigley et al., 1982). Therefore, there is no doubt that involvement of the optic pathway is of major clinical importance in MS but detection of the changes associated with demyelinating lesions by clinical examination, MRI and histopathology each have practical limitations.
The initial demonstration of peripapillary RNFL (pRNFL) attenuation in MS using OCT was of major importance because it provided a much easier, faster and less expensive method of detecting MS activity (Parisi et al., 1999). Numerous studies have replicated and expanded upon the finding using OCT as well as other non-invasive imaging methods (Steel and Waldock 1998; Zaveri et al., 2008). Some degree of pRNFL attenuation occurs in MS subjects regardless of an ON history or changes in visual acuity. The attenuation is greater in magnitude for subjects with ON or measurable visual function deficits. Large meta-analyses of Time-Domain OCT (Petzold et al., 2010) and Spectral Domain OCT (Petzold et al., 2017) have been effectively used to demonstrate that MS activity and duration correlate with thickness of the pRNFL. More recently the availability of higher resolution Spectral Domain OCT devices have allowed detailed analysis of individual retinal layers with remarkable insights into the disease. A meta-analysis of 40 studies and 5776 eyes, revealed that eyes of subjects with MS but without ON have ~7 μm thinner pRNFL than controls and those with history of ON have ~20 μm thinner pRNFL than controls (Petzold et al., 2017). Furthermore, in subjects with clinically isolated syndromes, measurements of GCIPL and RNFL can predict visual function and future disease activity months to years after onset of acute ON (Lambe et al., 2020). Therefore OCT can provide detailed quantitative measurements of the RNFL and macular thickness changes associated with clinical features of MS at a resolution that is not possible with clinical examination or neuroimaging methods. Based on these results, it is very likely that OCT could serve in several roles in the future diagnosis, prognostication and management of MS (Saidha and Naismith 2019).
4.1.2. Degenerative neurosensory retinal changes in Multiple Sclerosis
The RNFL consists of the axonal projections of ganglion cells located in the macula and throughout the retina. Therefore it is not surprising that histopathological evidence of GCL attenuation is also present in MS. A meta-analysis of 40 studies and 5776 eyes demonstrated that the macular RNFL (mRNFL) was ~2 μm thinner in MS subjects without ON and ~6 μm thinner in those with ON compared to controls (Petzold et al., 2017). The GCL and inner plexiform layer (IPL) were ~6 μm thinner in MS subjects without ON and ~16 μm thinner in those with ON. Although these differences are small, they are highly significant and some were even reproducible in a similar study performed much earlier with Time Domain OCT (Petzold et al., 2010; Petzold et al., 2017). Longitudinal data from several studies demonstrated that a 1 μm loss of pRNFL per year may be detectable in large, longitudinal clinical trials of 2–3 years duration aimed at assessing the efficacy of neuroprotective drugs in MS (Petzold et al., 2010; Petzold et al., 2017).
4.1.3. Inflammatory neurosensory retinal changes in Multiple Sclerosis
Retrospective studies demonstrate a correlation between increased INL volume and MRI activity in MS (Gelfand et al., 2012; Saidha et al., 2012). The same meta-analysis of 40 studies and 5776 eyes demonstrated that the INL was about 1 μm thicker in subjects with MS but without ON than control subjects (Knier et al., 2016). A similar small difference in the combined outer nuclear layer (ONL) and outer plexiform layer (OPL) was found. Disease modifying therapy has also been correlated with reduction in INL volume especially in cases where disease activity has essentially become undetectable (Knier et al., 2016). It is notable that an overall decrease in total macular volume is associated with increased disease activity. These apparently conflicting findings highlight the difficulty of identifying consistent, reproducible and relevant retinal changes for particular aspects of the disease process. The opposite changes in RNFL and INL thickness suggest that it is important to evaluate retinal sublayer thickness in addition to overall retinal thickness. For example, it has been hypothesized that inflammation of the INL is associated with increased INL volume while overall loss of RNFL explains the decrease in total macular volume. This hypothesis is supported by histopathology that confirms the presence of inflammatory cells in the inner retina of MS patients (Green et al., 2010). OCT-demonstrated edema in the INL has also been correlated with disease severity (Gelfand et al., 2012). These findings are reminiscent of diffuse tissue infiltration with T-lymphocytes in normal appearing regions of white and gray matter on histopathology (Kutzelnigg et al., 2005). Automated segmentation of retinal layers using deep learning methods have demonstrated impressive predictive ability in MS and may become useful in the clinical setting with appropriate human guidance (He et al., 2019). As we discuss later on (See section 7), novel methods of image registration and analysis may help further refine layer specific analyses that may have layer segmentation bias.
4.1.4. Retinal vascular changes in Multiple Sclerosis
Retinal vascular changes in the form of periphlebitis (inflammation of the veins) are a well-known manifestation of MS (Kerrison et al., 1994). This finding is consistent with subtle perivascular cuffing of mononuclear cells in normal appearing regions of white and gray matter on histopathology (Kutzelnigg et al., 2005). The relationship between phlebitis and perfusion are unclear. MRI-based methods have been able to demonstrate decreased cerebral blood flow within normal appearing areas of white matter and gray matter in MS subjects as compared to controls (Law et al., 2004; Steen, D’haeseleer et al., 2013), but it is unclear if the periphlebitis and blood flow abnormalities are related. Attenuated blood flow in MS also has been demonstrated in the retina independently of obvious periphlebitis. In a study of 16 MS subjects and 17 controls, blood flow velocity and volume in retinal arterioles and venules was significantly lower in MS subjects compared to controls (Jiang et al., 2016). Interestingly, there was no correlation between blood flow and RNFL thickness suggesting that these are independent processes in MS. Similar significant reductions in blood flow have been demonstrated in MS subjects compared to controls in at least one other study (Liu et al., 2019a,b).
In two separate cross-sectional studies of relapsing remitting MS patients compared to healthy controls the density of the retinal capillaries in the SRL of the macula and in the peripapillary region was significantly reduced in those with a history of ON (Murphy et al., 2020; Ulusoy et al., 2020). Capillary density in the DRL was also slightly decreased in the MS group compared to controls. MS-associated ON was also associated with atrophy of the inner retinal layers, mainly the RNFL and ganglion cell-inner plexiform layer (GCIPL). In a study of 68 eyes of 45 MS subjects compared to 55 eyes of 32 healthy controls, optic nerve head blood flow was lowest in MS-associated ON eyes. Although the RNFL and retinal ganglion cell complex (GCC) thicknesses were reduced in the eyes with ON, the OCTA measurements did not correlate with the structural OCT (Spain et al., 2018). Therefore, it appears that optic nerve head blood flow measures an MS effect independent of structural damage. It is worth noting that OCTA based assessment of retinal capillary changes are highly dependent on layer segmentation methods and algorithms which may vary among devices. As we discuss in section 7, novel methods of image analysis that are less dependent on layer segmentation may help further address the relationship of retinal vascular changes to neurosensory changes. In addition, more longitudinal studies will be needed to explain the relationship between perfusion and atrophy over time. In the choriocapillaris layer, higher vessel densities were associated with disease activity (Feucht et al., 2019), while choroidal thinning was correlated with disease duration (Esen et al., 2016). These findings could represent the vasodilatory effects during active inflammation and the resultant atrophy, respectively.
4.1.5. Clinical correlation of retinal changes in Multiple Sclerosis
Retinal thickness changes have been correlated with clinical severity of MS (Gordon-Lipkin et al., 2007; Sepulcre et al., 2007; Toledo et al., 2008; Jiang et al., 2016). Thinning of the GCIPL seems to be the earliest detectable OCT finding in MS and is a more sensitive biomarker for MS than pRNFL (Garcia-Martin et al., 2012a,b; Walter et al., 2012; González-López et al., 2014). GCIPL thinning occurs within weeks of onset of acute ON and can precede the RNFL thinning. GCIPL thinning within the first month of onset of ON is predictive of visual impairment by 6 months (Gabilondo et al., 2015). An inter-eye difference of 4 μm in GCL thickness is predictive of a previous attack of ON in patients with a history of unilateral ON (Nolan-Kenney et al., 2019). The largest and most robust differences between the eyes of MS and control eyes were found in the pRNFL and GCIPL. While these changes are all relatively small, it is very likely that in the correct clinical setting OCT can provide confirmatory or additional evidence of underlying disease activity.
Physiological pRNFL thinning due to age occurs at 0.017% per year starting at 18 years of age, with a 10–20 μm loss over 60 years (Kanamori et al., 2003). Above and beyond this, pRNFL thinning correlates with impaired visual function and pRNFL thickness may predict visual recovery after ON. pRNFL thinning at 75–80 μm has been found to be the threshold level that predicts visual impairment in MS (Costello et al., 2006). A decrease of one line in low-contrast letter acuity correlates with pRNFL thinning of 4 μm. Maximal pRNFL thinning (~10–40 μm) generally occurs after acute ON within 3–6 months and the first detectable difference between the fellow eye occurs between 1 and 2 months, reflecting axonal degeneration immediately after the primary demyelinating event. Stabilization of the pRNFL thickness occurs within 7–12 months from onset of disease (Henderson et al., 2010). Interestingly, an inter-eye difference of 5 μm in pRNFL thickness is predictive of a previous attack of ON in patients with a history of unilateral ON (Nolan-Kenney et al., 2019). pRNFL measurements also correlate with self-reported quality of life measures (Garcia-Martin et al., 2013) and disease progression (Garcia-Martin et al., 2010; Garcia-Martin et al., 2011; Saidha et al., 2013; Martinez-Lapiscina et al., 2016). These stereotypical findings on OCT have led to the use of OCT based endpoints in more than two dozen clinical trials in the past few decades, demonstrating the value of OCT based biomarkers in assessing MS disease activity (Lambe et al., 2020).
4.1.6. Retinal electrophysiology in Multiple Sclerosis
Subjects with MS frequently have delayed VEP even without a clear history of ON. This presumably reflects demyelinating lesions in the visual pathway but not necessarily in the optic nerve (Halliday, McDonald et al. 1972, 1973; Matthews et al., 1977). One prospective study of 29 newly diagnosed MS subjects and 32 controls did not show any association of VEP parameters to intraorbital optic nerve enhancement or atrophy on MRI (Hickman et al., 2004). However, VEP amplitude and latency have been shown to correlate with optic nerve volume in MS patients (Hickman et al., 2002). A significant association exists between PERG latency (and amplitude) and pRNFL thickness in MS subjects with a history of ON (Parisi et al., 1999). Despite abnormal VEP amplitude and latency in subjects with MS with ON compared to controls, the same study demonstrated no association between VEP latency or amplitude and pRNFL thickness (Parisi et al., 1999).
4.2. Late onset Alzheimer’s disease
Alzheimer’s Disease (AD) is a chronic neurodegenerative disorder and the most common form of dementia affecting over 26 million people worldwide (2020). As advancing age is the single strongest risk factor for AD, the term Late Onset AD (LOAD) is used to distinguish its most common form from its more rare autosomal dominant genetic form of younger onset, with a somewhat arbitrary age cut-off of symptom onset of 65 years. LOAD is defined by neuropathologic changes including neuronal loss, gliosis, extracellular accumulation of fibrillar beta-amyloid (Aβ) and intraneuronal cytoskeletal abnormalities consisting in part of hyper-phosphorylated tau (ptau) in the CNS (McKhann et al., 1984; Jack et al., 2011). There are numerous animal models of AD that mimic various aspects of the disease and of significant interest in understanding the pathophysiology of the disease (Lim et al., 2020; Song et al., 2020). Though deposition of diffuse and fibrillar Aβ in the CNS occurs early during the presymptomatic stage of the disease (as early as 20 years prior to the development of overt dementia), ptau accumulation in the form of neurofibrillary tangles is most strongly correlated with neuronal death and clinical symptoms (Nelson et al., 2012). The diagnosis of AD can be made based on clinical parameters alone (“clinically probable AD”) for which specificity and sensitivity can vary between 80 and 90%, or based on post-mortem diagnosis or on AD-specific CSF and PET biomarkers for which the accuracy of detecting AD pathology during life is generally higher (Jack et al., 2011). For in vivo human studies, papers prior to 2000 tended to use clinical diagnoses while AD-specific PET imaging and cerebrospinal fluid biomarkers became available in the early 2000’s (Klunk et al., 2004; Jack et al., 2018). It is important therefore to note what diagnostic criteria are used when interpreting ophthalmologic studies in AD. A detailed review of animal models as well as molecular and cellular findings associated with retinal changes in AD has been published recently (Gupta et al., 2020; Qin et al., 2020).
It is worth noting that ophthalmologic impairments in human contrast sensitivity, color discrimination, motion perception, reading speed and visual fields have been reported in LOAD (Sadun et al., 1987; Katz and Rimmer 1989; Gilmore et al., 2006; Boucart et al., 2015). Some visual dysfunction in LOAD has been associated with degeneration of anterior visual pathways, namely the optic nerve and retina (Hinton et al., 1986). However, it is unclear how early and specifically these ophthalmologic manifestations can be reliably demonstrated in subjects with LOAD (Uhlmann et al., 1991). It would not be surprising if there was objective retinal pathology underlying these changes that was detectable by retinal imaging methods. Below we summarize and synthesize a large body of literature describing retinal imaging findings in LOAD and implications of these findings in future studies. The numerous references in this section are also summarized in Table 1.
Table 1.
Summary of retinal findings in human subjects with late onset Alzheimer’s disease.
Abbreviations: OCT = Optical Coherence Tomography, ERG = Electroretinography, AD = Alzheimer’s Disease, HC = Healthy Controls, pRNFL = peripapillary retinal nerve fiber layer, mRNFL = macular retinal nerve fiber layer, MCI = mild cognitive impairment, RGC = Retinal Ganglion Cells, IPL = Inner Plexiform Layer, FAZ = Foveal Avascular Zone, Blank cells indicate no data or study was available for that parameters.
Complete eye exam includes visual acuity, intraocular pressure measurement, and dilated fundus examination.
4.2.1. Optic nerve changes in AD
In 1986, Hinton et al. provided the first histopathological evidence of optic neuropathy in LOAD after observing diffuse RGC loss and axonal atrophy in postmortem optic nerve tissues derived from severe LOAD patients (Hinton et al., 1986). These findings were independently corroborated on CFP (increased cup-to-disc ratios) and using red free optic disc photographs that demonstrated RNFL defects in LOAD subjects (Tsai et al., 1991). Subsequent histopathology studies further demonstrated degeneration of the RNFL and the RGC, most severe superiorly and inferiorly with respect to the optic nerve (Blanks et al., 1989; Bassi and Sadun 1990; Blanks, Torigoe et al. 1991, 1996a,b; Curcio and Drucker 1993; Blanks et al., 1996a,b; La Morgia, Ross-Cisneros et al., 2016).
OCT has been widely used to quantify retinal and retinal sublayer thinning in living human subjects with LOAD. In a recent meta-analysis comparing 1061 clinically-defined LOAD and 1130 control subjects, there was significant pRNFL thinning in LOAD for all retinal quadrants, most pronounced superiorly and inferiorly (Chan et al., 2019). These OCT findings of superior and inferior retinal atrophy are consistent with retinal histopathological findings in postmortem LOAD eyes described above. The pattern of RNFL loss in LOAD seems to be specific in comparison to some other neurodegenerative diseases (Sadun et al., 1987; La Morgia, Di Vito et al., 2017). Specifically, the superior and inferior RNFL thinning in LOAD is consistent with loss of M-type RGCs that comprise the magnocellular pathway (M-cells), are mainly located in the extramacular retina, and are more involved in low-resolution peripheral vision (Sadun and Bassi 1990). This is in contrast to the P-type RGCs that populate the papillomacular bundle in the temporal region of the optic disc and mediate central vision. This difference may explain the preserved visual acuity that is observed in subjects with advanced LOAD (Sadun et al., 1987) and may be associated with differences in vulnerability to LOAD pathology among RGC subtypes (La Morgia, Di Vito et al., 2017).
4.2.2. Degenerative neurosensory retinal thickness changes in AD
Several lines of evidence demonstrate changes throughout the neurosensory retina in subjects with LOAD. Postmortem histology from subjects with neuropathologically confirmed severe LOAD (Braak Stages V-VI) demonstrate a gradient of retinal thickness reduction whereby thinning was greatest for the RNFL and RGC followed by the INL and ONL in a superotemporal and superonasal pattern with respect to the optic nerve (Asanad et al., 2019a,b) (Fig. 4). These changes have been corroborated by in vivo OCT studies in LOAD. A meta-analysis of 380 clinically-defined LOAD patients and 293 controls from 11 cross-sectional studies showed a weighted mean difference of −15.95 μm (p < 0.0001) in RNFL of LOAD subjects compared to controls (Coppola et al., 2015). Another meta-analysis of 1257 LOAD subjects and 1460 controls from 30 cross-sectional studies showing weighted reductions in the thickness of macular GCIPL (−3.66 μm, p = 0.01), macular volume (−0.23 μm, p = 0.0003), total macular thickness (range −9μm to −14μm; p < 0.0001 for all retinal quadrants) and pRNFL (−5.99 μm, p < 0.0001) in clinically-defined LOAD subjects compared to controls (Chan et al., 2019). Yet another meta-analysis of 887 LOAD subjects and 864 controls showed standardized mean reductions in pRNFL of LOAD subjects (0.98 μm, p < 0.0001) compared to controls. This study also shows a standardized mean reduction in total macular thickness of 0.88 μm (p = 0.0001) (den Haan et al., 2017).
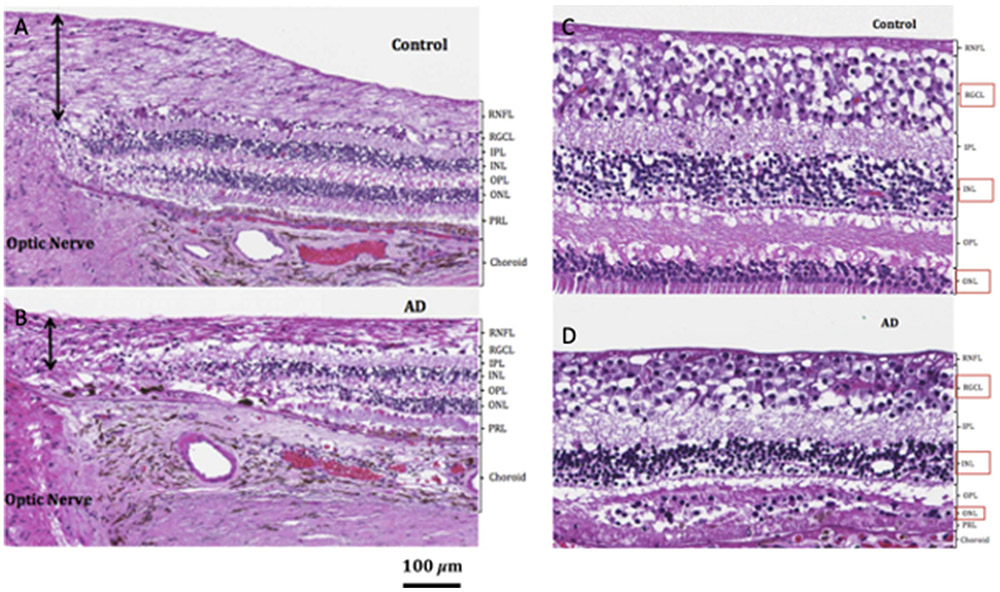
Fig. 4.
Retinal histopathology of AD and control subjects. Light microscopy depicts (A) supero-temporal RNFL (black arrows) in control and (B) AD postmortem tissue. Qualitative assessment of the supero-temporal RGCL, INL, and ONL (marked by red boxes) in (C) representative control and (D) subject with AD depicts supero-temporal RGCL, INL, and ONL thinning most pronounced in the macular region of the subject with AD. All stains are hematoxylin and eosin. (Reproduced under the Creative Commons Attribution-NonCommereial-NoDerivatives (CC BY-NC-ND) 4.0 International License from Asanad S, Ross-Cisneros FN, Nassisi M, Barron E, Karanjia R, Sadun AA. The retina in Alzheimer’s disease: histomorphometric analysis of an ophthalmologic biomarker. Invest Ophthalmol Vis Sci. 2019; 60:1491-1500. https://doi.org/10.1167/iovs.18-25966).
The macula contains more than 50% of the RGCs in the entire retina and RGC bodies are 10–20 times larger than the diameter of their axons. Therefore, macular measurements of GCIPL may be more sensitive than pRNFL measures (Cheung et al., 2015). Specifically, attenuation of the mRNFL, GCL, and IPL collectively known as the retinal ganglion cell complex (GCC) has been reported by several research groups (Kesler et al., 2011; Marziani et al., 2013; Thomson et al., 2015; Cunha et al., 2016; Garcia-Martin et al., 2016; Shao et al., 2018). A large metanalysis comparing OCT measurements of macular thickness in 467 clinically-defined LOAD subjects and 518 controls has further confirmed significant reductions in the GCIPL, GCC, macular full-thickness, and macular volume (Chan et al., 2019).
The findings above, while very encouraging must be considered in the context of at least a few well-conducted studies that have found no significant thinning among subjects that meet clear and objective criteria for probable LOAD (Haan et al., 2019a,b; Sánchez et al., 2020). For example, in subjects with posterior cortical atrophy (PCA), a variant of LOAD disproportionately affecting parietal and occipital cortex, there was no reported association between any measure of retinal thickness and LOAD status (Haan et al., 2019a,b). On the other hand, these investigators found a correlation between atrophy in parietal cortex and RNFL thickness, regardless of the presence of fibrillar Aβ on PET scan (tau-PET was not performed), supporting a transsynaptic mechanism for RNFL thinning independent of amyloid pathology (den Haan et al., 2018a,b). These findings most directly support the idea that transsynaptic retrograde degeneration of neurons is the cause of retinal thinning although further research is needed. They also emphasize the need for pathology or biomarker-based diagnoses to minimize confounding variables such as underlying vascular disease. As we discuss in sections 6.6 and 7, assessment of cerebral vascular pathology in the context of neurodegenerative disease is a major area of unmet medical and research need. Advances in both understanding of the pathophysiology of cerebrovascular disease as well as qualitative and quantitative assessment of capillary level changes may add significant insight into this confounding variable.
4.2.3. Degenerative neurosensory retinal changes in MCI and preclinical AD
One particularly enticing application of retinal imaging in LOAD is to detect incremental progression of disease or subclinical disease changes that are otherwise undetectable with conventional neuroimaging. For example, OCT measured changes in retinal structure and function in subjects with cognitive impairment but without definitive dementia could serve as a useful biomarker in disease prognosis and preventative clinical trials. It is thought that the neurodegenerative process associated with LOAD precedes the onset of symptoms by 20 years or more (Villemagne et al., 2013). In this context, the use of retinal imaging markers may define early changes in neurodegenerative entities including LOAD when interventions may be effective in preserving neuronal tissue. In this section we will review the relevant literature that suggests retinal based biomarkers may be useful in identifying early changes associated with mild cognitive impairment (MCI) and/or preclinical LOAD. Biomarker-based identification of AD pathology is particularly important in this group in whom the diagnosis is not yet clear.
A consistent and significant decrease in pRNFL and macular thickness parameters has been demonstrated in subjects with MCI by several cross-sectional studies and reviewed in at least two meta-analyses (Paquet et al., 2007; Kesler et al., 2011; Ascaso et al., 2014; Coppola et al., 2015; den Haan et al., 2017; Chan et al., 2019). A meta-analysis of 68 MCI patients and 293 controls from 11 cross-sectional studies showed a weighted mean difference of −13.39 μm (p = 0.013) in RNFL of clinically-defined MCI subjects compared to controls(Coppola et al., 2015). Another meta-analysis of 305 clinically-defined MCI subjects and 1460 controls from 30 cross-sectional studies showed weighted reductions in the thickness of macular GCIPL (−10.19 μm, p = 0.05) in MCI subjects compared to controls (Chan et al., 2019). Notably there was no difference in macular volume or pRNFL except for a significant weighted increase in nasal peripapillary thickness. A third meta-analysis of 216 clinically-defined MCI subjects and 864 controls showed standardized mean reductions in pRNFL of 0.71 μm in MCI subjects (p = 0.008) compared to controls. This study also showed a standardized mean reduction in macular thickness of 0.88 μm (p = 0.0001) (den Haan et al., 2017).
These studies support the notion that subclinical retinal changes accompany MCI and may be useful in detecting disease progression in longitudinal studies. For example, a prospective study was reported in 27 cognitively healthy participants with pathologic cerebrospinal fluid Aβ42/tau ratios consistent with the presence of AD pathology, and 16 cognitively healthy controls with normal Aβ42/tau ratios. Mean RNFL was not only significantly thinner in asymptomatic LOAD participants relative to controls, but also demonstrated high sensitivity (87%) but modest specificity (56%) in classifying cognitively healthy individuals with elevated CSF Aβ42/Tau ratios (Asanad et al., 2020) (Fig. 5). These studies have set the stage for larger, multicenter, prospective studies that could formalize these associations.
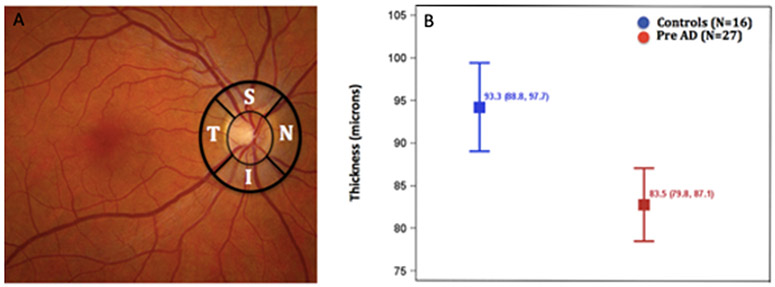
Fig. 5.
Peripapillary retinal nerve fiber layer thickness is reduced in subjects with preclinical AD. (A) The thicknesses of the retinal nerve fiber layer (temporal, superior, nasal, inferior) was measured using OCT in the regions outlined in black. (B) Depicts least-squares mean (95% CI) total retinal nerve fiber layer thickness adjusted for side and region between cognitively healthy controls (blue) and cognitively healthy participants with pathologic CSF Aβ42/Tau levels (red). (Adapted from Asanad S, Fantini M, Sultan W, Nassisi M, Felix CM, Wu J et al. (2020) Retinal nerve fiber layer thickness predicts CSF amyloid/tau before cognitive decline. PLoS ONE 15(5): e0232785. https://doi.org/10.1371/journal.pone.0232785 under the terms of the Creative Commons Attribution License http://creativecommons.org/licenses/by/4.0/).
4.2.4. Inflammatory neurosensory retinal changes in MCI and preclinical AD
In contrast to the above findings, a significant number of well-performed studies report essentially no change or even an increase in retinal thickness in association with early stages of dementia or MCI (Lad et al., 2018; Shao et al., 2018; Alves et al., 2019; Hadoux et al., 2019; Salobrar-García et al., 2019; van de Kreeke et al., 2019; Marquié et al., 2020). For example, one study evaluated retinal thickness in relation to amyloid accumulation in 165 cognitively healthy monozygotic twins among which 18 were Aβ (+). OCT demonstrated no thinning of the pRNFL, mRNFL, GCL, IPL, or total macula. In addition, no significant associations were found between retinal thickness and PET Aβ levels following correction for multiple testing (van de Kreeke et al., 2019). The reason for these negative findings is not clear but may be related to the use of Aβ, which may represent earlier stages of preclinical disease, rather than a neurodegenerative marker.
Shao et al. evaluated macular thickness in 25 clinically-defined LOAD, 24 MCI, and 21 control subjects. Relative to controls, the MCI and LOAD cohort demonstrated macular thinning but also significant ONL and photoreceptor thickening (Shao et al., 2018). Lad et al. analyzed 15 clinically-defined mild-moderate LOAD, 15 MCI, and 18 control subjects. Average pRNFL and GCIPL thicknesses did not significantly differ between groups but areas of thickening were found adjacent to areas of thinning in the pRNFL and GCIPL (Lad et al., 2018). More recently, Marquie et al. conducted a 2-year longitudinal study to investigate the relationships between retinal thickness and PET Aβ levels. OCT showed nasal macular thickening which positively correlated with amyloid uptake (Marquié et al., 2020). Alves et al. evaluated morphological changes in retina and in brain white matter integrity using OCT and diffusion tensor imaging, respectively, among 17 early amyloid-PET defined LOAD and 23 control subjects and noted that INL thickness was positively associated with fractional anisotropy in early LOAD (Alves et al., 2019). Salobrar-Garcia et al. analyzed 39 mild and 21 moderate clinically-defined LOAD patients, and 40 control subjects. Relative to controls, mild LOAD patients showed thinning in the central macula, whereas moderate LOAD patients showed thickening in this region. Both mild and moderate LOAD cohorts showed pronounced thinning of the mRNFL, GCL, and OPL. However, the ONL was significantly thicker in mild and moderate LOAD relative to controls (Salobrar-García et al., 2019). A common interpretation of these results is that there is inflammation early in the neurodegenerative process that could manifest as thickening of specific retinal sublayers. A similar observation has been proposed and demonstrated in MS (Green et al., 2010; Gelfand et al., 2012).
One particularly confounding possibility is that degenerative changes in one retinal layer are offset by thickening from inflammatory changes in other layers. This would result in no detectable change in overall retinal thickness until the degenerative process overtakes the inflammatory stage of the disease. This possibility would explain the absence of any significant change in retinal thickness in a number of studies discussed above. Careful retinal sublayer analysis in larger, prospective studies should help answer this question. In addition, more sophisticated methods of OCT analysis will enable further exploration of layer specific changes and volume changes independently of layer segmentation (see section 7).
4.2.5. Neurosensory retinal amyloid beta deposition in LOAD
A pathologic hallmark of LOAD is deposition of Aβ and ptau in the CNS (McKhann et al., 1984; Montine et al., 2016). Therefore, identification of these proteins in the retina has been an area of intense interest. Retinal Aβ protein deposition in postmortem LOAD retinal tissue and transgenic mice (APPSWE/PS1E9) has been demonstrated following curcumin staining (Koronyo-Hamaoui et al., 2011; Chibhabha et al., 2020; Sidiqi et al., 2020). These findings have been corroborated by recent human studies, which have similarly demonstrated Aβ plaque accumulation highest in the mid-to far-peripheral regions of superior and inferior hemiretinas (Alexandrov et al., 2011; La Morgia, Ross-Cisneros et al., 2016; Koronyo et al., 2017). Immunohistochemistry studies illustrated Aβ distribution localized to the RGC in postmortem LOAD eyes with selective loss of intrinsically photosensitive subtypes of RGCs known as melanopsin-containing RGCs (mRGCs) (La Morgia, Ross-Cisneros et al., 2016). Since mRGCs drive circadian photoentrainment, the authors hypothesized that mRGC loss may also contribute to circadian dysfunction characteristically seen in LOAD (La Morgia, Ross-Cisneros et al., 2016). Koronyo et al. analyzed Aβ plaque deposition in whole mount retinas from 8 LOAD and 7 control postmortem eyes. LOAD retinas showed a 4.7-fold increase in plaque aggregation which correlated with Aβ burden in both the primary visual cortex and entorhinal cortex (Koronyo et al., 2017).
Despite these findings, there is some controversy regarding the detection of these proteinopathies in human retina in LOAD (Blanks et al., 1989; Ho et al., 2014; Williams et al., 2017; den Haan et al., 2018a,b). Ho et al. and Williams et al. were unable to identify Aβ and ptau deposits in retinal cross-sections of autopsy-confirmed LOAD cases relative to age-matched controls (Ho et al., 2014; Williams et al., 2017). Haan et al. reported ptau accumulation in the IPL and OPL in 6 post-mortem LOAD eyes as well as diffuse Aβ deposition throughout the inner retina in both LOAD cases and controls (den Haan et al., 2018a,b). While it is not exactly clear why these studies did not detect pathologic retinal Aβ accumulation, one plausible reason is the relatively limited cross-sectional sampling of the retina in comparison to the retinal whole mounts used in studies with positive Aβ findings in the retina (Alber et al., 2020).
One potentially exciting avenue for detection of retinal Aβ is the use of autofluorescence, hyperspectral imaging and fluorescence lifetime imaging ophthalmoscopy (FLIO). Pilot studies demonstrate significant differences in the fluorescence lifetimes of retinal fluorophores between 7 CSF-defined preclinical LOAD participants and 8 controls using FLIO. Fluorescence lifetimes were not only significantly prolonged in preclinical LOAD retinas, but also correlated with CSF Aβ and tau levels and GCIPL thickness measured by OCT (Sadda et al., 2019). Hadoux and colleagues demonstrated remarkable differences in retinal reflectance patterns using hyperspectral imaging. In addition, retinal hyperspectral scores were notably associated with brain Aβ burden as measured by PET (Hadoux et al., 2019). The pathogenesis of these spectral differences are not clear and much further investigation is needed. However, the general concept of leveraging the spectral properties of Aβ for detection in the retina is promising. A clever variation on this theme is the use of exogenous agents to label amyloid in vivo. For example, Koronyo et al. demonstrate putative amyloid containing lesions in the retina of 10 human subjects with AD compared to 6 healthy controls using a curcumin labelling scheme and fundus autofluorescence imaging (Koronyo et al., 2017). It is not yet clear how quantitative this method can be but prospective clinical studies are in progress.
4.2.6. Subretinal amyloid beta deposition in age-related macular degeneration and LOAD
Aβ is also known to be present in pathologic and non-pathologic age-related changes called drusen (Anderson et al., 2004; Isas et al., 2010). Drusen typically occur in the subretinal space with the greatest prevalence in the posterior pole and show some physiologic age-related accumulation (Hoh Kam et al. 2010). The most common pathologic association of drusen is age-related macular degeneration but numerous studies have examined the potential role of Aβ accumulation in drusen and LOAD (Ohno-Matsui 2011). Unlike intraretinal Aβ deposits, drusen are easily detectable on clinical examination and with a variety of ancillary diagnostic imaging methods. Interestingly, macular drusen area on CFP independently correlates with cerebral Aβ accumulation as measured by florbetapir PET in elderly people without dementia (Fig. 6) (Shoda et al., 2018). Histopathology of specimens from subjects with neuropathologically diagnosed LOAD demonstrates increased numbers of drusen compared with age-matched controls (Ukalovic et al., 2018). These findings are supported by epidemiological findings correlating decreased MMSE and late stages of age-related macular degeneration in the Blue Mountain Eye Study (Pham et al., 2006). However, histopathological assessment of 157 autopsy eyes from subjects greater than 75 years of age did not support an increase in prevalence of age-related macular degeneration among subjects with LOAD (Schwaber et al., 2020). This suggests that the pathophysiology of Aβ accumulation in drusen is likely independent of the severity of age-related macular degeneration.
Fig. 6.
Color fundus photographs and positron emission computed tomograms (PET) demonstrating clinical correlation of retinal and brain imaging findings in (A,B) 74 year old subject without dementia and (C,D) 69 year old subject without dementia. (A) Color fundus photograph of the macula demonstrates numerous drusen (area 7.35 mm2) and (B) F-AV45 (florbetapir) PET yields positive standard uptake values (SUVR) of 1.15 (values greater than or equal to 1.10 are considered positive). (C,D) Similar images for a 69 year old female subject without dementia. (C) This subject had minimal drusen on fundus images and (D) similarly negative SUVR ratio of 1.04. SUVR values indicate the ratio of cortical to cerebellar A-beta accumulation. (Adapted with permission from Shoda C et al., Journal of Alzheimer’s Disease 62 (2018) 239–245 (doi 10.3233/JAD-170956).
4.2.7. Retinal vascular changes in MCI and AD
Several lines of evidence in human LOAD have also provided evidence of retinal vascular pathology. The retina is supplied by two separate vascular structures. The central retinal artery branches from the ophthalmic artery and feeds the retina through additional branches from the optic nerve head directly into the neurosensory retina. This “retinal” blood supply is further subdivided into capillary layers or plexi depending on the depth of the capillary bed within the neurosensory retina and the topographic location within the retina. The retinal circulation is responsible for supplying the inner two-thirds of the neurosensory retina. In contrast, the posterior ciliary arteries also branch from the ophthalmic artery but supply the choroidal circulation which is anatomically distinct from the retina and immediately posterior to it. The choroidal circulation provides the nutrient supply to the outer one-third of the retina. Here we will review relevant findings in both vascular systems in subjects with AD.
Numerous studies have demonstrated changes in LOAD in the large caliber retinal vessels (100–200 μm) emanating from the optic disc including: narrowed vessel caliber (Berisha et al., 2007; Frost et al., 2013; Cheung et al., 2014; Szegedi et al., 2020), increased tortuosity (Frost et al., 2013; Cheung et al., 2014; Williams et al., 2015), reduced blood flow (Berisha et al., 2007; Feke et al., 2015; Szegedi et al., 2020), and altered blood oxygen saturation (Einarsdottir et al., 2016; Stefánsson et al., 2017; Szegedi et al., 2020). Notably increase in arteriolar tortuosity and decrease in caliber have been demonstrated in the cortical microvasculature of transgenic mouse model of AD (Dorr et al., 2012).
In addition to the large caliber vessel changes there are a number of studies demonstrating capillary level changes in the retina of LOAD subjects. Decreased blood flow rates in the precapillary arterioles and post-capillary venules in the macula have been demonstrated (Jiang et al., 2018a,b) corresponding to the decreased flow rates observed in the larger retinal vessels at the disc (Berisha et al., 2007; Feke et al., 2015). Aβ accumulation and subsequent plaque formation in vessel walls may occlude and thus impair blood flow (Berisha et al., 2007; Dorr et al., 2012). Schultz et al. reported significant reduction in capillary pericyte number between retinal sections derived from LOAD and non-demented patient cases (Schultz et al., 2018) reminiscent of the loss of brain pericytes reported in the AD cortex and hippocampus (Sengillo et al., 2013).
Several studies have demonstrated significant attenuation of capillary density or perfusion in the retina of subjects with dementia (Bulut et al., 2018; Jiang et al., 2018a,b) or MCI (Jiang et al., 2018a,b; Yoon et al., 2019). Due to the high cost and limited availability of PET and CSF studies, most of these correlations are often made with only MMSE as measure of disease status and little information is available about comorbid vascular disease. Since the prevalence of comorbid vascular contributions to cognitive impairment and dementia is significant, many of these studies cannot rule-out the confounding role of cardiovascular risk factors versus amyloid angiopathy in the correlation with OCTA based metrics. Notably in a study where MRI and CSF amyloid levels are used to characterize LOAD patients, the correlation between retinal capillary density metrics and LOAD status is less clear (Querques et al., 2019). In this study, a significant impairment of arteriolar reactivity, but not capillary density, was noted in response to flicker stimulation of the retina in subjects with MCI and LOAD compared to normal. This study also accounted for vascular comorbidity. In addition, vascular reactivity impairment was inversely correlated with CSF Aβ levels and directly correlated with GCL thickness (Querques et al., 2019). A reduction in RNFL thickness, retinal arteriolar diameter and blood flow was also recently reported in subjects with clinically-defined LOAD or MCI using a different imaging methodology but flicker induced reactivity was not observed (Doppler OCT and Retinal Vessel Analyzer) (Szegedi et al., 2020).
There is controversy regarding retinal capillary changes in preclinical LOAD. One study of 32 subjects with biomarker positive LOAD (positive CSF Aβ42 or Florbetapir or Pittsburgh compound B PET ligands) but CDR score of 0 showed increasing foveal avascular zone and decreased retinal thickness in biomarker positive subjects compared to biomarker negative controls (O’Bryhim et al., 2018). Notably this study did not report a change in capillary density. In contrast, Haan et al. showed no significant decrease in capillary density or increase in foveal avascular zone in LOAD participants with Aβ PET and CSF positivity, after adjusting for age and sex. There were no associations between any retinal vascular parameters and CSF biomarkers or MMSE scores (Haan et al., 2019a,b). However, there was a positive and significant association between retinal vessel density in the macula and Fazekas score suggesting some form of underlying vascular association (Haan et al., 2019a,b). One limitation of these studies is that assessment of retinal capillary density is limited by layer segmentation methods that vary across commercial devices and analysis methods. As we discuss in Section 7, volume based image analysis methods may help overcome this difficulty.
4.2.8. Choroidal vascular changes in AD
In contrast to the retinal circulation, there are fewer studies examining the choroidal circulation. This may be partly because the choroidal circulation is anatomically separate from the retina, does not share the common embryological origins of the CNS vasculature, and does not have a BRB. Therefore, there is less reason to suspect a direct correlation between changes in the choroidal circulation and CNS pathology. Nevertheless, the choroidal blood supply is an extremely important source of nutrition for the outer retina and may, at least, reflect changes in the metabolic activity of the retina. In the population based Beijing Eye Study on 3009 subjects, there was a significant positive association between OCT measured choroidal thickness and MMSE score even after adjusting for age, axial length, gender, anterior chamber depth, lens thickness, visual acuity and depression score (Jonas et al., 2016).
In a meta-analysis of 203 clinically-defined AD subjects and 307 controls from 5 cross-sectional studies using OCT, subfoveal choroidal thickness was significantly thinner in LOAD subjects than controls (standard mean reduction −1.03 μm, p < 0.001) (Chan et al., 2019). It should be noted that some studies have reported conflicting results for choroidal thickness measurements as well (Gharbiya et al., 2014, Bayhan et al., 2015; Bulut et al., 2016; Trebbastoni et al., 2017; Bulut et al., 2018; Haan et al., 2019a,b; López-de-Eguileta et al., 2020).
A recent histopathological study of choroidal thickness and vascularity in 8 severe LOAD (Braak V-VI) and 11 control postmortem eyes demonstrated global choroidal thinning in the superior hemi-retinas. Intriguingly, regional analysis of the central macular choroid demonstrated significant thickening in CSF-biomarker defined LOAD, which strongly correlated with stromal vessel number (Asanad et al., 2019a,b) (Fig. 7). Quantification of choroidal vasculature using OCT and OCTA is much less developed and more subjective than quantification of retinal circulation (Ferrara et al., 2016) and there is a significant amount of work to be done in assessing choroidal changes in LOAD. Specifically, analysis of choroidal changes on OCT is confounded by the even more obscure borders and anatomy of the choroid and choriocapillaris (in comparison to the less obscure retinal boundaries). As we discuss in Section 7 below, volume based assessments of the choroid and choriocapillaris may help address this technical challenge.
Fig. 7.
Qualitative assessment of the choroid in controls and subjects with Alzheimer’s Disease. Representative light microscopy revealed qualitative (A) superonasal choroidal thinning in patients with AD relative to (B) controls. Representative light microscopy revealed qualitative (C) superotemporal macular choroidal thickening with increased vascularity in patients with AD relative to (D) controls. All stains are hematoxylin and eosin. Abbreviation: AD, Alzheimer’s disease. (Reproduced under the Creative Commons Attribution-NonCommercial-NoDerivatives (CC BY-NC-ND) 4.0 http://creativecommons.org/licenses/by-nc-nd/4.0/ International License from Asanad et al., Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring 11 (2019a) 775–783; doi.org/10.1016/j.dadm.2019.09.005).
4.2.9. Visual pathway electrophysiology of Alzheimer’s disease
Several studies have provided evidence of retinal electrophysiologic dysfunction in LOAD that is complementary to the histopathologic findings of GCL, RNFL and optic nerve atrophy described above. For example, in the retina, multifocal ERG amplitudes were significantly reduced and implicit times were delayed in subjects with AD (Sen et al., 2020a,b). In addition, decreased N95 amplitude and increased implicit time by PERG examination significantly correlated with pRNFL thickness reduction in clinically-defined LOAD (Parisi et al., 2001; Krasodomska et al., 2010; Moschos et al., 2012). PERG measured ganglion cell activity demonstrates significant amplitude reduction while PVEP assessment of retinocortical conduction time and ffERG are normal in LOAD subjects compared to controls. This suggests that signal conduction velocity in the primary visual pathways may be spared from LOAD pathology at least in some stages of the disease (Rimmer et al., 1989). Variations on PERG and VEP stimulation paradigms to further isolate the magnocellular visual pathways in LOAD subjects seem to suggest that they are more affected (Sartucci et al., 2010) in support of histopathologic findings discussed above demonstrating preferential atrophy of larger ganglion cell axonal profiles also indicative of magnocellular neurons (Sadun and Bassi 1990).
4.2.10. Cognitive impairment in AD and retinal thickness
As cognitive decline is the most relevant and salient concomitant of the severity of brain pathology in AD, its correlation with retinal pathology is of interest. Though impairment in episodic memory is the most prominent symptom in early AD, executive function is also affected. Far less commonly, language and visuospatial deficits may be the presenting symptom. Considering this clinical heterogeneity, the large variety of assessment instruments used and the absence of appropriate norms across diverse populations, the use of cognitive impairment and decline as outcome measures in retinal studies poses significant challenges.
One notable trend in the literature assessing retinal findings in subjects with dementia is the widespread use of the MMSE as an outcome measure. The MMSE is a screening instrument. It is easy to administer and is reported in the majority of studies as an assessment of the severity of cognitive impairment in subjects with probable or possible LOAD. In many cases the complete characterization of the AD status of subjects is not presented. The MMSE is a relatively easy screening test (low ceiling) which does not assess all cognitive domains (e.g. does not assess executive function). It exhibits poor sensitivity in relatively healthy participants (Spencer et al., 2013) and in MCI (Arevalo-Rodriguez et al., 2015; Tsoi et al., 2015).
It is therefore not surprising that there are mixed reports on the association of MMSE and retinal thickness parameters. For example, Cipollini et al. illustrated significant thinning in global pRNFL, GCC and total macular volume in 25 clinically-defined LOAD patients in comparison with 17 controls. However, OCT-derived measurements did not correlate with MMSE scores but did correlate with measures of constructional praxis and processing speed (Cipollini et al., 2020). Similarly, in a study of 57 CSF amyloid positive and PET amyloid positive LOAD subjects, MMSE did not correlate with any retinal thickness parameter (Haan et al., 2019a,b). In subjects with PCA, a variant of AD largely affecting the visual cortex, there is no reported association between any measure of retinal thickness and LOAD status including MMSE score (Haan et al., 2019a,b). In contrast, other studies show some association between retinal thickness parameters and MMSE in subjects with LOAD. For example, Cunha et al. show a correlation of 0.33 and 0.43 for average macular thickness and foveal thickness, respectively, with MMSE in a univariate analysis of 24 clinically-defined LOAD patients (mean MMSE = 17) and 24 controls (Cunha et al., 2016). It is likely that more sensitive and comprehensive measures of cognition, perhaps composite measures emphasizing episodic memory and executive function, might be better suited for correlation with retinal changes in AD (Guzmán-Vélez et al., 2018).
4.2.11. Longitudinal studies of retinal imaging in AD and dementia
Longitudinal data seem to provide complementary evidence to support a potential causal association between neurodegenerative changes and retinal atrophy. One longitudinal study of 3289 Dutch adults reports the association of retinal thickness with both incident and prevalent dementia, including LOAD specifically (Mutlu et al., 2018). As expected this study reports that thinner RNFL is associated with an increased risk of dementia overall and more specifically with incident dementia (Hazard Ratio per standard deviation increase in RNFL 1.44 CI 1.19–1.75 for dementia and 1.43 CI 1.15–1.78 for LOAD) but not prevalent dementia. Thinner GCIPL was associated with prevalent dementia (odds ratio per standard deviation decrease in GCIPL 1.37 CI 0.99–1.9) (Mutlu et al., 2018). A second study of 32,038 subjects in the United Kingdom without clinically diagnosed neurodegenerative disease demonstrated that thinner RNFL was associated with worse cognitive performance at baseline and progressively worse cognitive testing 3 years later (Odds Ratio 1.92 CI 1.29–2.85, p < 0.001) (Ko et al., 2018). A 27-month longitudinal study conducted by Santos et al. reported significant mRNFL thinning in preclinical LOAD. Although IPL and ONL thinning were also observed, these thickness changes were reportedly attributed to age-related decline, as expected over the time-span of the study. Notably, only the mRNFL significantly correlated with PET Aβ levels (Santos et al., 2018). One challenge with longitudinal studies is coregistration of images across subjects and across time so that subtle subclinical changes can be detected. As we discuss later on (See section 7.2), novel methods of image registration and analysis may help address this challenge.
4.3. Autosomal Dominant Alzheimer’s disease
As discussed in the previous section, the vast majority of AD cases present late in life with some heritable component (estimated at 58–79%) due to common genetic variants with small effects, and are termed “sporadic” late onset AD (LOAD) (Ringman et al., 2014). However, around 1% of cases are inherited as Autosomal Dominant AD or ADAD (Moulder et al., 2013). Mutations in three genes cause ADAD: presenilin 1 (PSEN1), presenilin 2 (PSEN2), and amyloid precursor protein (APP). All pathogenic mutations increase the relative production of longer-length Aβ species, a finding not consistently observed in LOAD (Szaruga et al., 2015). Mutations in PSEN1 are the most common, accounting for about two-thirds of ADAD cases, APP the second most common, and PSEN2 the least common. For persons carrying pathogenic mutations, the future development of AD can be reliably predicted. Furthermore, the age of disease onset can be estimated as this age tends to be consistent among persons with the same mutation (Ryman et al., 2014). PSEN1 has the youngest age of onset (as early as the 20’s but more commonly in the 40’s or 50’s). Overall, the average age of onset is 45.7 ± 6.8 years, so there is a lower incidence of the comorbidities of aging such as cardiovascular disease and diabetes that frequently confound the diagnosis of LOAD and its underlying pathophysiology. Clinical and pathological phenotypes of ADAD share numerous similarities with LOAD (namely progressive dementia associated with accumulation of β-amyloid plaques and ptau neurofibrillary tangles), but may differ in the mechanisms leading to pathologic accumulation of Aβ (Ringman et al., 2014). Nevertheless, ADAD can serve as a model to study AD pathophysiology, biomarkers, and potential disease modifying therapies in the absence of confounding age-related disease.
Early studies in subjects with ADAD identified elevated plasma Aβ and CSF tau in pre-symptomatic mutation carriers (Ringman et al., 2008). Clinical, biochemical, and radiologic changes in ADAD have well-established temporal associations demonstrating that the disease process is active decades before clinical symptoms present (Bateman et al., 2012) and have been shown to parallel those seen in LOAD (Dubois et al., 2016). For example, while clinical disease activity in ADAD is detectable using the Clinical Dementia Rating sum of boxes testing within 1 year of overt dementia, the earliest change seen is a decrease in Aβ in the CSF ~25 years before presentation. The precuneus shows elevated levels of amyloid deposition on PET using Pittsburgh compound B approximately 25 years before disease onset as well a decline in glucose metabolism as measured by [F18] fluorodeoxyglucose (FDG) PET 17 years before disease onset. Performance on a composite of neuropsychological assessments, including episodic memory, complex attention, processing speed, and a general cognitive screen, are abnormal several years before clinically manifest disease. Hippocampal volume attenuation is detectable a few years before disease onset. This ordering of biomarkers and clinical tests strengthens the hypothesis that clinical diagnosis of AD is made late in the biological cascade and targeting both diagnosis and pharmacologic manipulation of Aβ earlier in the course of the disease may lead to better clinical outcomes. In this context, retinal studies in asymptomatic carriers of ADAD mutations are underway and are of significant potential interest. A recent study showed decreased retinal thickness in 10 presymptomatic carriers of a common PSEN1 mutation relative to 10 matched non-carriers. Differences were most evident in the outer nuclear layer. Interestingly, there was no effect of age on retinal thickness nor were there any differences in retinal vascular parameters assessed on fundus photography (number of branch points, tortuosity, or fractal dimension) (Armstrong et al., 2020). In another recent study, early stage (asymptomatic) carriers of ADAD causing mutations demonstrated increased capillary blood flow and blood flow heterogeneity compared to controls or late stage (symptomatic) carriers of the same mutations (Singer M, Ringman J et al. in press).
4.4. Parkinson’s disease
Parkinson’s Disease (PD) affects more than 10 million people worldwide and is the second most common neurodegenerative disease in the developed world (Archibald et al., 2009; Veys et al., 2019) and numerous animal models have informed out understanding of the disease pathophysiology (Pingale and Gupta 2020). The pathologic hallmark of PD is the abnormal deposition of cytoplasmic inclusions comprising the α-synuclein (αsyn) protein leading to the progressive loss of dopaminergic neurons. In particular, dopamine depletion following neuronal loss in the nigrostriatal pathway leads to characteristic motor symptoms in PD including bradykinesia, resting tremor, rigidity, and postural instability. However, there are numerous non-motor manifestation of PD including alterations in mood and sleep, autonomic nervous system disturbances, visual hallucinations, and dementia (Ortuño-Lizarán et al., 2018). Consistent with this clinical spectrum of extra-motor symptoms in PD, studies have demonstrated αsyn neuropathology in the CNS, peripheral nervous system and various end-organs (Beach et al., 2010). Dopamine and dopaminergic receptors have been demonstrated in the retina via radioligand binding analyses (Borbe et al., 1982) and histopathology (Archibald et al., 2009). Evidence of dopaminergic loss in retina of subjects with PD confirms the presence of neurodegenerative changes in the retina (Archibald et al., 2009). Clinical impairments reported in subjects with PD include reading difficulty, impaired visual acuity, color discrimination, motion perception, and contrast (Archibald et al., 2009; Weil et al., 2016). Notably, the impairment in at least some of these clinical measures, such as contrast sensitivity, is reversible with administration of levodopa (Bulens et al., 1987; Hutton et al., 1993; Giaschi et al., 1997). Though symptoms and signs of extrapyramidal dysfunction and responsiveness to L-dopa make the clinical diagnosis of PD more reliable and not as biomarker-dependent as that of AD, it is more difficult to differentiate dementia with Lewy bodies from AD. In elderly persons with PD or dementia with Lewy bodies, concomitant diseases of aging should be considered as confounders when assessing retinal changes. Below we summarize and synthesize a large body of literature describing retinal imaging findings in PD and implications of these findings in future studies. The numerous references in this section are also summarized in Table 2.
Table 2.
Summary of retinal imaging studies characterizing human subjects with Parkinson’s disease.
Abbreviations: = Optical Coherence Tomography, ERG = Electroretinography, PD = Parkinson’s Disease, pRNFL = peripapillary retinal nerve fiber layer, mRNFL = macular retinal nerve fiber layer, RGC = Retinal Ganglion Cells, IPL = Inner Plexiform Layer, FAZ = Foveal Avascular Zone, Blank cells indicate no data or study was available for that parameters.
Complete eye exam includes visual acuity, intraocular pressure measurement, and dilated fundus examination.
4.4.1. Parkinson’s disease retinal histopathology
The initial evidence for retinal involvement in PD came from histopathologic evidence demonstrating reduced tyrosine hydroxylase immunoreactivity in the retina of 5 subjects with PD (Nguyen-Legros 1988; Witkovsky 2004). This finding has been corroborated and expanded on by several subsequent studies. Histopathology of 4 PD eyes and 12 control eyes demonstrated αsyn immunoreactivity localized within the GCL, IPL and INL corresponding with the anatomic distribution of dopaminergic amacrine cells (Beach et al., 2014; Bodis-Wollner et al., 2014). Prominent accumulation of phosphorylated αsyn deposition has been demonstrated in the RGC of subjects with PD compared to controls and significantly correlates with cortical p-synucleinopathy, disease severity, and functional motor scores (Ortuño-Lizarán et al., 2018). It is notable that at least one study has demonstrated more diffuse αsyn deposition throughout the retina of PD (Ho et al., 2014).
These findings are complemented by high performance liquid chromatography demonstrating modulation of retinal dopamine levels in subjects receiving recent L-DOPA therapy compared to those who did not receive similar therapy (Harnois and Di Paolo 1990). Similar data from rodent and primate models of PD using a neurotoxin for dopaminergic cells, 1-methyl-4-pheny-1,2,3,6-tetrahydropyridine (MPTP), demonstrates a dose-dependent reduction in tyrosine hydroxylase activity in retinal amacrine cells (Ghilardi et al., 1988a,b; Tatton et al., 1990).
4.4.2. Peripapillary RNFL changes in Parkinson’s disease
In a meta-analysis of 1916 subjects with PD and 2006 controls there was a significant association between diagnosis of PD and thinning of the pRNFL (Cohen’s d = −0.42, CI −0.54 to −0.29) and the GCIPL (d = −0.40, CI −0.72 to −0.07) (Chrysou et al., 2019). There was no detectable association with sectoral thinning, duration of disease or severity of symptoms in this study. A number of studies have revealed preferential thinning of the inferior and temporal pRNFL quadrants (Inzelberg et al., 2004; Moschos et al., 2011; Garcia-Martin et al., 2012a,b; Kirbas et al., 2013; La Morgia, Barboni et al., 2013; Sari et al., 2015; Satue et al., 2017; Visser et al., 2018; Sung et al., 2019). However, results have been mixed among other studies (Rohani et al., 2013, Bayhan et al., 2014; Garcia-Martin et al., 2014a,b; Jiménez et al., 2014; Yu et al., 2014; Kaur et al., 2015; Pilat et al., 2016; Pillai et al., 2016; Ucak et al., 2016; Hasanov et al., 2019). Despite the differences in sectoral location, it is clear that retinal thinning, predominantly in the inner retina, is present in subjects with PD. Careful evaluation of device parameters, segmentation algorithms and reliability as well as disease stage may explain why some studies do not report significant retinal thickness changes in the pRNFL (Archibald et al., 2009; Albrecht et al., 2012; Tsironi et al., 2012; Roth et al., 2014; Mailankody et al., 2015). For example, it is possible that changes in macular thickness precede detectable RNFL changes of the ganglion cell body, dendritic field and synaptic connections are the primary site of cell damage. Interestingly, some studies report changes in functional parameters in the absence of structural thinning suggesting that functional changes may precede detectable attenuation in pRNFL thickness (Tsironi et al., 2012).
4.4.3. Macular OCT changes in Parkinson’s disease
In a meta-analysis of 1916 subjects with PD and 2006 controls there was a significant association between diagnosis of PD and thinning of most sectors of the macula (Cohen’s d range −0.37 to −0.57) (Chrysou et al., 2019). The majority of studies investigating the macula in PD have reported significant reduction in one or more layers, especially the mRNFL, GCL, and IPL (Hajee et al., 2009, Cubo et al., 2010; Kaur et al., 2015; Sari et al., 2015; Ucak et al., 2016; Satue et al., 2017; Ahn et al., 2018; Moschos and Chatziralli 2018; Sung et al., 2019). These OCT findings are complementary to the histopathologic findings described in the inner retinal layers (Bodis-Wollner et al., 2014). It has also been proposed that the slope or shape of the foveal pit may have some utility as a marker of PD-specific changes (Shrier et al., 2012; Pilat et al., 2016) although there is significant normal variation in foveal morphology that needs be taken into account when considering such an association.
There is significant disagreement in the literature regarding retinal thickness changes in the outer retina in subjects with PD. Significant thickness changes of the outer retina including the OPL, photoreceptor layer, and RPE layer have been reported in some studies (Müller et al., 2014; Roth et al., 2014; Pilat et al., 2016) and refuted in others (Uchida et al., 2018; Chrysou et al., 2019). Very similar to the layer specific changes reported in subjects with AD, there appear to be layer specific changes in PD which may offset each other (Garcia-Martin et al., 2014a, b). This requires further investigation and may explain the findings from a large number of studies that fail to detect changes in overall retinal thickness (Aaker et al., 2010; Archibald et al., 2011; Tsironi et al., 2012; Schneider et al., 2014; Nowacka et al., 2015; Pillai et al., 2016; Polo et al., 2016). As we discuss later on (See section 7.2), novel methods of image registration and analysis may help circumvent these limitations.
4.4.4. Retinal vascular changes in Parkinson’s disease
Few studies have evaluated the retinal microvasculature in PD. Miri et al. assessed the perifoveal capillary network in 23 PD patients and 13 healthy controls using invasive injection of contrast dye (fluorescein angiography). PD patients exhibited significant reduction in FAZ area and foveal thickness. In addition, foveal thinning was highly associated with disease duration and motor impairment in PD patients (Miri et al., 2015). Choroidal thickness reduction in the subfoveal area as well as in the inner and outer quadrants in PD retina has also been demonstrated (Moschos and Chatziralli 2018). More recently, Kwapong et al. analyzed the SRL and DRL capillary densities in 38 early PD and 28 control subjects using OCTA. SRL density was markedly reduced relative to healthy controls and correlated with GCIPL thickness, suggesting a vascular contribution or consequence of retinal degeneration in PD (Kwapong et al., 2018).
4.4.5. Clinical correlation of retinal changes in PD
Researchers have hypothesized that retinal thinning in PD reflects a primary loss of dopaminergic amacrine cells and a decrease in dopamine neurotransmitter levels in the retina that is presumably reflective of similar changes in the CNS. There are functional implications of this hypothesis including correlation of retinal changes with abnormalities in visual function, motor function and cognitive function. A large meta-analysis of 36 studies (n = 1916 subjects with PD and 2006 controls) found significant thinning of the inner retinal layers in PD but only a few significant correlations were found between retinal thickness and clinical severity, perhaps due to the heterogeneity in the underlying data and methodology among the studies (Chrysou et al., 2019). Nevertheless there are some interesting studies that are worth mentioning. For example, in one study of 14 PD subjects and 14 controls there was a significant association between impaired contrast sensitivity and inner retinal thickness among normal subjects that was absent in subjects with PD (Adam et al., 2013). Direct evidence for a common pathophysiological mechanism in the retina and CNS of PD subjects also exists. Namely, a significant association has been shown between the thickness of the inner retina and dopamine aminotransferase activity in the substantia nigra via PET imaging (Ahn et al., 2018).
Although in most studies the heterogeneity of PD duration, severity and clinical stage precludes rigorous analysis of clinical PD staging and retinal thickness measures (Chrysou et al., 2019) cross-sectional associations between the severity of PD and retinal thinning in the macula (Bayhan et al., 2014; Sari et al., 2015; Pilat et al., 2016; Ahn et al., 2018; Sung et al., 2019) and pRNFL (Jiménez et al., 2014; Mailankody et al., 2015; Pilat et al., 2016) have been reported. In a few studies, the severity and duration of PD has been shown to correlate with the GCIPL layer thickness (Sari et al., 2015). There are very few longitudinal studies to support these cross-sectional associations. At least in one study that evaluated the same 30 PD subjects and 30 controls over a 5 year period, the attenuation in the temporal and supratemporal pRNFL was significantly greater in subjects with PD than controls (Satue et al., 2017). In this same study, PD progression using the Hoehn and Yahr scale also correlated significantly with thinning in the supratemporal pRNFL (Satue et al., 2017). Nevertheless, in some well-performed studies there were no reliable correlations between retinal thickness measures of any kind and severity or duration of motor symptoms using consensus PD assessment tools such as the Unified Parkinson’s Disease Rating Scale (Albrecht et al., 2012).
Cognitive impairment is one of several clinical features of PD that significantly impacts the quality of life of PD subjects. Most PD patients develop dementia and as many as 26% of non-demented subjects have MCI (Litvan et al., 2011). There are generally very few studies that show associations between cognitive impairment in PD and retinal thickness parameters. However, cognitive assessment in PD is subject to the same challenges as those in AD described above. In many of these studies the mean MMSE is above 27 suggesting that a representative spectrum of subjects with cognitive impairment was not available for analysis (Mailankody et al., 2015; Visser et al., 2018). Even in one study of 61 PD subjects and 30 controls with mean MMSE of 24, there was no correlation between cognitive impairment and retinal thickness parameters (Lee et al., 2014). Notably, a significant correlation between MoCA score (which includes measures of executive function and is more sensitive for detecting MCI) and thickness of the macular GCIPL has been demonstrated (Sung et al., 2019). As mentioned earlier, since the GCIPL is the location of the dopaminergic amacrine cells in the retina as well as ganglion cell bodies, this may suggest that detection of early changes in retinal structure require targeted sublayer analyses with careful attention to segmentation methods. As we discuss later on (See section 7.2), novel methods of image registration and analysis may help circumvent these limitations.
4.4.6. Visual pathway electrophysiology in Parkinson’s disease
Some of the earliest work implicating electrophysiological abnormalities in the visual pathway in PD comes from studies using VEP in PD subjects. Specifically, increased VEP latency in response to mid-spatial frequency stimuli have been demonstrated in PD subjects by several studies (Bodis-Wollner and Yahr 1978; Onofrj et al., 1986; Ikeda et al., 1994). These abnormalities are at least partially reversible during L-dopa therapy (Bodis-Wollner and Yahr 1978; Peppe et al., 1995) and are inducible with dopamine antagonists (Onofrj et al., 1986). In at least one study, progression of ERG and PERG changes has been correlated with clinical disease progression (Ikeda et al., 1994). Studies comparing VEP latency and measures of retinal function such as PERG (Nightingale et al., 1986) suggest that at least part of the VEP abnormality is secondary to retinal dysfunction. PERG abnormalities in the retina of subjects with PD are also responsive to administration of L-dopa (Peppe et al., 1995; Tagliati et al., 1996) and can be induced in non-PD subjects and monkey models with selective D2-receptor antagonists I-sulpiride (Tagliati et al., 1994). Primates treated with the MPTP demonstrate decreased retinal dopamine levels, abnormal PVEP latencies and PERG amplitudes as well as systemic signs of PD (Ghilardi et al., 1988a,b). Notably the changes in PVEP and PERG were transiently reversible with administration of levodopa-carbidopa in the primate model (Ghilardi et al., 1988a,b).
Structure-function correlation of the retina support the notion that the electrophysiologic changes described above are part of a common degenerative process of PD. For example, in a group of 46 PD subjects and 33 controls, foveal thickness measurements and PERG N95 amplitude each were remarkably predictive of disease severity and quality of life scores in PD patients (Garcia-Martin et al., 2014a,b). Similarly a combined mfERG and OCT study comprising 53 PD and 41 control subjects found diminished amplitude and prolonged implicit time of the P1 mfERG wave component in addition to macular thinning in PD patients relative to controls. The diagnostic efficacy of ERG and structural parameters when combined greatly outweighed the performances of these biomarkers individually (Huang et al., 2018). However, the association between macular volume, foveal thickness, RNFL thickness and VEP latency appears poor (Altintaş et al., 2008). As we discuss previously, this may be because measurement of specific retinal layers is necessary to detect the relevant pathological process and segmentation errors or variances can easily obscure such subtle changes. As we discuss later on (See section 7.2), novel methods of image registration and analysis may help circumvent these limitations.
4.5. Huntington’s Disease
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder caused by expansion of the cytosine-adenine-guanine (CAG) polyglutamine triplet repeat in the HTT gene on chromosome 4p16.3.(1993) HD has a prevalence and annual incidence of 2.71 and 0.38 in 100,000, respectively (Pringsheim et al., 2012). Numerous animal models have informed our understanding of this disease in humans (Howland et al., 2020). Though somewhat variable, the age of symptom onset among carriers of pathogenic CAG repeat expansions is earlier with longer expansions (Andrew et al., 1993). The histopathological hallmarks of HD include abnormal aggregations of intranuclear and cytoplasmic inclusions of the huntingtin (htt) protein, which lead to progressive cortical and striatal atrophy. Patients classically present with a clinical triad of motor, cognitive, and behavioral abnormalities (Ross et al., 2014). Motor symptoms characteristically include involuntary, jerky, or slow writhing movements (chorea), most obviously involving the upper extremities. Ocular motility deficits such as saccade apraxia, saccade hypometria, and smooth pursuit impairment have also been described in various stages of HD and are reviewed extensively elsewhere (Anderson and MacAskill 2013). Below we summarize and synthesize the available body of literature describing retinal imaging findings in HD and implications of these findings in future studies. The numerous references in this section are also summarized in Table 3.
Table 3.
Summary of retinal imaging studies characterizing human subjects with Huntington’s disease.
| Finding | HD vs Controls | No Difference | Genetically confirmed (CAG) | Complete eye exam* excluding confounding disease |
|---|---|---|---|---|
| OCT | ||||
| pRNFL thinning | Kersten et al., (2015), Gatto et al., (2018), Gulmez Sevim et al., 2019 | Kersten et al., (2015), Gatto et al., (2018), Gulmez Sevim et al., 2019 | Kersten et al., (2015), Gatto et al., (2018), Gulmez Sevim et al., 2019 | |
| mRNFL thinning | Gulmez Sevim et al., 2019, Andrade et al., (2016) | Gulmez Sevim et al., 2019 | Gulmez Sevim et al. 2019, Andrade et al., (2016) | |
| RGC-IPL thinning | Gulmez Sevim et al., 2019, Andrade et al., (2016) | |||
| Total macular thinning | Kersten et al., (2015), Gatto et al., (2018) | Kersten et al., (2015), Gatto et al., (2018) | Kersten et al., (2015), Gatto et al., (2018) | |
| Choroidal thinning | Andrade et al. (2016) | Andrade et al. (2016) | ||
| ERG | ||||
| Inner retinal dysfunction | ||||
| Outer retinal Dysfunction | Pearl et al., (2017); Knapp et al., (2018) | Pearl et al., (2017); Knapp et al., (2018) | Pearl et al., (2017); Knapp et al., (2018) | |
Abbreviations: = Optical Coherence Tomography, ERG = Electroretinography, HD = Huntington’s Disease, pRNFL = peripapillary retinal nerve fiber layer, mRNFL = macular retinal nerve fiber layer, RGC = Retinal Ganglion Cells, IPL = Inner Plexiform Layer, Blank cells indicate no data or study was available for that parameters.
Complete eye exam includes visual acuity, intraocular pressure measurement, and dilated fundus examination.
4.5.1. Huntington’s Disease retinal histopathology
Autopsy-derived brain and retinal tissue samples from 1 HD and 2 control subjects showed the expected findings of significant cortical atrophy associated with htt and ubiquitin deposits in the caudate nucleus and putamen but the retina was devoid of protein aggregations or degenerative changes both macroscopically and histologically (Petrasch-Parwez et al., 2005). This is in contrast to reports in animal models, which have demonstrated progressive retinal degeneration in R6/2 HD mice (Helmlinger et al., 2002). Due to the paucity of human histopathology in HD and the discrepancy between human and animal studies, it is possible that under-sampling in human tissue resulted in false negative finding. It is also possible that retinal manifestations of HD pathology are different than other CNS manifestations.
4.5.2. Huntington’s Disease peripapillary RNFL
Several researchers have shown clinical evidence of pRNFL thinning in HD preferentially of the temporal quadrant (Kersten et al., 2015; Gatto et al., 2018, Gulmez Sevim et al. 2019). Temporal thinning in the pRNFL corresponds with the small axonal fibers comprising the papillomacular bundle which is a pattern of retinal atrophy classically seen in mitochondrial disorders (Carelli et al., 2009). Therefore, despite the absence of histopathologic evidence of retinal involvement there is significant in vivo evidence suggesting pRNFL thinning in HD.
4.5.3. Huntington’s Disease macular RNFL
Although several studies failed to demonstrate any changes or correlations between total macular thickness and HD status (Kersten et al., 2015; Gatto et al., 2018) retinal sublayer analysis has revealed significant thinning for all of the inner retinal layers including the mRNFL, GCL, IPL, and INL. These atrophic changes in the inner retina are coincident with thickening in the outer retinal layers that may explain the absence of significant changes in total retinal thickness measures (Andrade et al., 2016, Gulmez Sevim et al. 2019). Among retinal layers, however, thinning of the GCL correlated the strongest with HD and measures of disease progression which suggest earlier involvement of the ganglion cell bodies in HD as compared to the axonal fibers (Satue et al., 2016, Gulmez Sevim et al. 2019).
4.5.4. Functional impairment and Huntington’s Disease retinal findings
Several studies have reported significant correlations between retinal thickness measures and clinical assessments of HD severity. For example total mean RNFL thickness significantly correlated with total functional capacity scores among 14 genetically confirmed HD subjects and 13 matched controls (Gatto et al., 2018). Both duration of disease (R2 = −0.57, p = 0.006) and Unified Huntington’s Disease Rating Scale (R2 = −0.56, p = 0.01) were significantly and inversely correlated with macular volume in 26 HD patients and 29 controls (Kersten et al., 2015). Notably, these correlations increased in strength following exclusion of presymptomatic patients from the analysis. Other sublayers including the GCL, IPL and mRNFL thicknesses significantly correlate with HD duration, CAG repeat number, and Unified Huntington’s Disease Rating Scale scores (Gulmez Sevim et al. 2019). Similar associations have been reported between clinical measures of HD severity and one or more macular thickness measures in other studies (Andrade et al., 2016).
4.5.5. Visual pathway electrophysiology in Huntington’s Disease
In a recent study comprising 18 HD and 10 control subjects, significantly increased b-wave amplitudes in HD at light- and dark-adapted red flash intensities were reported (Pearl et al., 2017). In addition, greater numbers of CAG triplet repeats correlated with higher b-wave amplitudes. These results expand upon the initial foveal blue light studies by Paulus et al. demonstrating increased photoreceptor thresholds in HD (Paulus et al., 1993), and suggest electrophysiologic dysfunction in retinal cone photoreceptor pathways in HD. More recently, Knapp et al. evaluated retinal structure and function in a 25-year-old man with presymptomatic HD (Knapp et al., 2018). Although retinal structure appeared normal on OCT, ERG amplitudes were decreased in dark- and light-adapted a- and b-waves as well as in light-adapted 30 Hz flicker testing (Knapp et al., 2018). In addition, mfERG showed a decrease in response amplitude suggesting predominant involvement of cone photoreceptors and downstream pathways. These findings in presymptomatic disease may also suggest early involvement of the photoreceptor pathway in HD that precedes retinal morphologic changes measured by OCT. This is also consistent with the retinotopic pattern of degeneration involving the parvocellular neurons of the papillomacular bundle described above (La Morgia, Di Vito et al., 2017). This is at least consistent with the R6/2 mouse model of HD that develops cone-rod degeneration measurable by ERG (Ragauskas et al., 2014). Nevertheless, these results should be interpreted with caution given the small numbers of subjects involved.
4.5.6. Huntington’s Disease visual evoked potentials
The majority of reports have illustrated decreased amplitude with normal peak latency in the VEP responses of HD patients (Ellenberger et al., 1978; Rizzo et al., 1980; Oepen et al., 1981; Josiassen et al., 1984). The magnitude of the amplitude decrease seems to correlate with the severity or duration of the disease (Ellenberger et al., 1978). One potentially confounding variable in these assessments is the effect of medication on observed waveform abnormalities. In particular, VEP amplitudes were lower in HD patients receiving antipsychotic treatment as compared to patients who were not receiving treatment in at least one study (Josiassen et al., 1984). Nevertheless, VEP amplitudes were still significantly lower for both medicated and unmedicated HD patients relative to controls. Importantly, similar VEP irregularities are also seen in psychiatric diseases and are not specific for HD (Josiassen et al., 1984). Notably, at least one study has not been able to demonstrate similar findings using PVEP over a two year period (Ehle et al., 1984).
4.6. Cerebral small vessel disease
Cerebral small vessel disease (CSVD) refers to a group of human cerebrovascular diseases that affect small intraparenchymal arterioles in the CNS (Pantoni 2010; Iadecola 2013; Cannistraro et al., 2019) and many aspects of these diseases have been replicated in animal models (Tuo et al., 2020). Common causes of human CSVD include hypertensive arteriolosclerosis, cerebral amyloidosis, lupus vasculitis, and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). CSVD is responsible for approximately 25% of strokes worldwide (both ischemic and hemorrhagic), and is associated with increased risk of recurrent stroke (Rensma et al., 2018). It is also a primary contributor to cognitive decline, with up to 45% of dementia cases in the general population being associated with CSVD (Gorelick et al., 2011; Gorelick and Pantoni 2013; Sachdev et al., 2014). Current clinical and research practices for identification of CSVD rely on neuroimaging (usually MRI) evidence of parenchymal brain injury caused by CSVD (Wardlaw et al., 2013; Cannistraro et al., 2019; Das et al., 2019). These biomarker findings include, white matter hyperintensity (WMH), lacunar infarcts, cerebral microbleeds (CMB), convexal subarachnoid hemorrhage (cSAH), cortical superficial siderosis (cSS), large hemorrhagic stroke lesions, and enlarged perivascular space (PVS) (Fig. 8) (Wardlaw et al., 2013).
Fig. 8.
Representative MRI findings in cerebral small vessel disease.
Population-based series including imaging and autopsy data identified two primary CVSD subtypes that account for the vast majority of cases (Cannistraro et al., 2019). Hypertension-related Angiopathy (HTNA) is a form of acquired CVSD and represents a small vessel disorder caused by prolonged uncontrolled hypertension. Rather than a single pathological entity, HTNA represents a group of hypertensive cerebral angiopathies that frequently overlap and include hyaline arteriolosclerosis, hyperplastic arteriolosclerosis, segmental arterial disorganization, and microaneurysms (Pantoni 2010). The changes in lumen morphology and biological properties associated with these conditions are thought to lead to a cascade of secondary injury processes including decreased cerebral blood flow (up to and including vascular occlusion and acute ischemic infarction), leakage of blood products from microaneurysm (results in hemorrhagic lesions ranging in severity from CMBs to large hypertensive hemorrhages), and chronic ischemia of oligodendrocytes dysfunction leading to loss of myelin integrity (manifesting as white matter disease) (Wardlaw et al., 2013; Promjunyakul et al., 2018).
Cerebral amyloid angiopathy (CAA) is another form of CVSD that is acquired and caused by deposition of Aβ in the walls of small-to-medium cortical and leptomeningeal arterioles and arteries (Biffi and Greenberg 2011). In turn, the development of amyloid-related angiopathy results in loss of vessel compliance and decreased cerebral vascular reactivity. Ultimately these changes in vessel physiology lead to accumulation of ischemic (WMH) and hemorrhagic (cSAH, CSS, large cortical hemorrhages) lesions, leading to acute stroke and progressive diffuse cortical atrophy (Pantoni 2010). Because of shared links to Aβ deposition CAA is usually associated with AD, with the two conditions representing specific manifestations (vascular vs. parenchymal) of an overarching disorder of amyloid cerebral production, processing and clearance (Greenberg et al., 2020).
4.6.1. Retinal histopathology in cerebral small vessel disease
There have been to date no dedicated studies of retinal histopathology in CVSD, either in the CAA or HTNA form. This in part relates to the ease of acquiring detailed information on vascular structures of interest in vivo by a variety of techniques (fundus photography and OCTA being the most commonly implemented, see below). It is worth mentioning that retinal vascular changes identified in amnestic MCI and AD patients may partially reflect separate contributions from CSVD (either CAA or HTNA), which is comorbid in a large proportion of cases (Matej et al., 2019). Histopathological studies of AD retinas showed pathogenetic Aβ deposits accumulating within and along retinal vasculature in a manner highly reminiscent of CAA (La Morgia, Ross-Cisneros et al., 2016; Koronyo et al., 2017). As for HTNA, histopathological retinal changes associated with hypertension have been consistently associated with risk for cerebrovascular disease (Henderson et al., 2011). Additional studies of retinal histopathology and CSVD are required to identify tissue changes specific to CSVD, and correlate them with characteristics neuroimaging findings and disease progression.
4.6.2. Neurosensory and peripapillary retinal changes in cerebral small vessel disease
Retinal structural changes have so far received limited attention in CSVD investigative efforts. RNFL defects on fundus photography were shown to be associated with WMH in a large study including Korean community-dwelling individuals who participated in health checkups (Kim et al., 2011). RNFL and GCL were shown to be associated with CSVD, primarily in terms of WMH prevalence and severity (Qu et al., 2020). Additional studies, ideally including longitudinal evaluation of both retinal neurosensory changes in relation to the progression of capillary and arteriolar pathology, are warranted at this time. As we discuss in sections 5 and 7, high-resolution capillary imaging of the retina and novel image analysis and registration methods will provide excellent tools for assessing longitudinal and subclinical changes.
4.6.3. Retinal vascular changes in cerebral small vessel disease
For the purposes of our discussion, the retinal vasculature can be divided into macrovascular components that are generally greater than 100 μm in diameter (retinal arteries and venules) and microvascular components (arterioles, venules and capillaries) that are less than 100 μm in diameter. Many studies classify retinal vascular changes as “retinopathy” which is a generic term that applies to almost any vascular changes in the retina. In the context of this manuscript, we will use “retinopathy” to refer specifically to end-stage capillary changes (microaneurysms, cotton wool spots, retinal hemorrhages, hard exudates) that are secondary changes resulting from obliteration or significant structural abnormality of the retinal capillaries. These distinctions are important because color fundus photography is not able to resolve end-arteriolar and capillary changes in the retina but can clearly show the secondary results of chronic capillary pathology such as microaneurysms, cotton wool spots, hemorrhages and hard exudates. In contrast, studies using OCTA are specifically designed to look directly at capillary changes and not secondary effects of capillary damage.
Multiple studies have investigated the association between measurements of neuronal and retinal macrovascular integrity on fundus photography and CSVD (Wu et al., 2017). In addition, retinopathy on fundus imaging is associated with prevalence and progression of cerebral infarcts and WMH in population-based studies (Cooper et al., 2006; Longstreth et al., 2007; Cheung et al., 2010; Hanff et al., 2014). These retinal photography findings were also associated with CSVD neuroimaging markers among patients presenting with acute stroke, and preferentially identified patients presenting with CSVD-related stroke subtypes (primary intracerebral hemorrhage and lacunar infarcts) (Lindley et al., 2009; Ong et al., 2013; Liew et al., 2014; Wei et al., 2016). Among quantitative retinal vasculature metrics arteriolar mean diameters and fractal dimensions (as derived from high-resolution fundus images) were repeatedly found to associate with CSVD MRI markers, especially WMH and lacunar infarcts (Kwa, van der Sande et al., 2002; Doubal et al., 2010; McGrory et al., 2019). In the Rotterdam Study retinal venular diameter did not associate with baseline CSVD severity, but did correlate with progression on follow-up MRI scans (progression of WMH and new lacunar infarcts) (Ikram et al., 2006). A recent study using OCTA has further demonstrated associations between markers of retinal capillary health and function with intracranial vascular health and function (Ashimatey, D’Orazio et al., 2020). In this study, lower retinal capillary perfusion density was significantly associated with worse MRI measures of cerebrovascular reactivity, lower perfusion in the middle cerebral artery perfusion territory as well as impaired cognition (Ashimatey, D’Orazio et al., 2020) (Fig. 9). Collectively these data strongly suggest that retinal capillary changes mirror vascular pathology in the brain at all levels of the vascular tree.
Fig. 9.
Optical coherence tomography angiography (OCTA) images and quantification of retinal capillary changes in a subject with cognitive impairment and control. The figure shows 3 × 3mm2 parafoveal OCTA images of two subjects with CDR-SOB scores = 0 and CDR-SOB = 1, and ages 76 and 73 years, respectively. Neither subject has diabetes but both have hypertension. Images on the second and third columns show the corresponding skeletonized images and pseudo-colored maps of the capillary density. The subject with CDR-SOB = 0 has more localized areas of higher capillary density than the age and medical condition similar subject with CDR-SOB >0. (Reproduced under the Creative Commons Attribution-NonCommercial-NoDerivatives (CC BY-NC-ND) 4.0 http://creativecommons.org/licenses/by-nc-nd/4.0/ International License from Ashimatey et al., Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring in press 2020).
One of the advantages of MRI for CSVD is the ability to distinguish between subtypes, which in turn has direct implications for associated clinical manifestations and prognosis (Cannistraro et al., 2019). To date, few studies distinguished between different forms of CSVD when exploring associations with retinal imaging. Retinopathy lesions on fundus imaging were found to be more common among survivors of CSVD-related stroke compared to other stroke subtypes (Baker et al., 2010a,b; Gobron et al., 2014). Among subtypes of CSVD-related stroke, cases of amyloid-related lobar intracerebral hemorrhage demonstrated the highest prevalence of microvascular wall signs and retinopathy findings on fundus imaging (Baker et al., 2010a,b). A study in rural Ecuador found associations between hypertensive retinopathy and CSVD findings on MRI, but did not distinguish between hypertensive and amyloid subtypes (Del Brutto, Mera et al., 2016). Another study found reduction in arteriolar fractal dimensions to be more common in CSVD-related vascular impairment compared to AD, but did not further distinguish between different small vessel disease etiologies (Jung et al., 2019). While more evidence is needed to definitively assess whether retinal imaging modalities can accurately subtype CSVD, this potential application would directly impact research and clinical care practices.
In addition to OCT/OCTA, other retinal vascular imaging modalities were investigated for association with CSVD. In one study, the diagnosis of Nonarteritic Anterior Ischemic Optic Neuropathy (NAION) was associated with an almost five-fold increase in the prevalence of CSVD (as defined by MRI evaluation) compared to NAION-free controls (Kim et al., 2019a,b). Decreased retinal vasoreactivity determined via Dynamic Vessel Analyzer was associated with impaired cerebral vasoreactivity (assessed via transcranial Doppler), a key marker of CSVD (Bettermann et al., 2017). Central retinal artery blood flow (as determined via Doppler) was notable for lower end-diastolic and mean velocities, and higher pulsatility and resistive indexes among CSVD patients compared to controls. Doppler flow velocities were also associated with WMH severity on MRI (Hiroki et al., 2003). While limited, evidence from these studies suggest that a multimodal approach to retinal imaging may increase the yield of current CSVD research approaches, focused primarily on retinal imaging.
4.6.4. Visual pathway electrophysiology in cerebral small vessel disease
There have been to date no studies leveraging visual pathway electrophysiology to study CSVD. However, multiple studies investigated response in cerebral blood flow to visual pathway stimulation, via either Transcranial Doppler ultrasound or functional MRI. Patients with CAA demonstrate reduced cerebral vascular reactivity to visual pathway stimulation, consistent with imaging and histopathology studies indicating greater disease severity in the occipital lobe (Smith et al., 2008; Peca et al., 2013).
4.6.5. Autosomal dominant cerebral small vessel disease
Thus far there are few studies of retinal changes in genetically-determined CSVD but this is a rapidly growing area of investigation with exciting initial findings. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common genetic form of CSVD (Federico et al., 2012). CADASIL is an autosomal dominant genetic disorder caused by mutations in the NOTCH3 gene, encoding a transmembrane receptor expressed almost exclusively in vascular smooth cells and pericytes (Monet-Leprêtre et al., 2009). Accumulation of the mutated protein product leads to formation of toxic extracellular deposits, leading to alterations in small vessel physiology similar to those observed in CAA. Among genetically-determined forms of CSVD, CADASIL has been the topic of most studies looking into potential application of retinal imaging techniques. In an early study of 10 consecutive subjects with skin biopsy or genetically confirmed CADASIL, color fundus photographs and dye-based retinal angiography demonstrated bilateral arteriolar sheathing in 30% of subjects, arteriolar narrowing in 80% of subjects and arteriovenous nicking in 90% of subjects (Haritoglou et al., 2004). Notably, the prevalence of focal arteriolar narrowing and arteriovenous nicking in population-based studies is much lower that reported in this small cohort (~14%) (Wong et al., 2007). Subsequent studies focused primarily on OCT and OCTA. Genetically confirmed CADASIL cases were shown to have significantly increased OCT-based mean arterial and venous diameters compared to controls (Alten et al., 2014). OCT measurements of arterial and venous mean diameters were also found to correlate with lacunar infarcts and cerebral microbleeds on MRI (Fang et al., 2017). In one study of OCTA in CADASIL, vessel density of the DRL was found to be significantly decreased in affected patients compared to healthy controls (Nelis et al., 2018). Larger and longitudinal studies will be ultimately needed to validated and expand upon these findings.
Additional evidence on the use of retinal imaging has also recently emerged for disorders with mixed small and large vessel CNS manifestations. In Fabry disease, retinal and conjunctival vessel tortuosity represents an important diagnostic finding. Analysis of retinal and conjunctival vasculature demonstrated increased vessels tortuosity in Fabry disease patients (Sodi et al., 2019a,b). Evidence of abnormal RNFL and vessel appearance on OCT have also been reported in smaller studies of Mitochondrial Encephalopathy, Lactic acidosis, and Stroke-like episodes (MELAS) syndrome (Cho and Yu 2015; Sultan et al., 2017) Taken together, these findings suggest that retinal imaging of neuronal and vascular structures may aid in early diagnosis and longitudinal monitoring of rarer genetic forms of CSVD.
4.7. Frontotemporal Dementia
Frontotemporal Dementia (FTD) is a heterogeneous group of neurodegenerative diseases characterized by neuronal and glial proteinaceous inclusions preferentially involving the prefrontal and anterior temporal lobes of the brain. The age of FTD onset is typically younger than in LOAD (peaking in the 60’s) with an incidence ranging from 5 to 22/100,000 person-years with a rough estimate of 20,000–30,000 prevalent cases in the United States (Neary et al., 2005; Knopman and Roberts 2011). FTD is associated with two principal proteinopathies including microtubule-associated protein tau and transactive response DNA-binding protein 43 (TDP-43) and this heterogeneity is captured with the neuropathological term “frontotemporal degenerations” (Irwin et al., 2015). FTD and ALS occur on a spectrum in some families, most commonly in association with C9orf72 expansions.
FTD is predominantly a behavioral disorder featuring significant changes in personality and social conduct. Notably, however, patients may also present with language impairment, cognitive deficits in executive function, and motor symptoms (Neary et al., 2005). Therefore, FTD is more commonly referred to as a group of 3 clinical syndromes: behavioral variant, progressive non-fluent aphasia, and semantic dementia(Neary et al., 2005; Ferrari et al., 2017). These syndromes relate more to the areas of the brain involved rather than the subtype of protein accumulation. The clinical presentation correlates poorly with the underlying proteinopathy and clinical diagnosis remains especially challenging given the phenotypic variability of this disease (Neary et al., 2005). Interestingly, a significant fraction of subjects with presumed FTD are later diagnosed with AD by neuropathology (Kertesz et al., 2005; Knopman et al., 2005; Forman et al., 2006). While the specific pathology of the frontotemporal degenerations has not been histopathologically demonstrated in the human retina, abnormal accumulation of tau has been demonstrated in the aging retina (Leger et al., 2011). Therefore, non-invasive retinal imaging may provide a potentially useful biomarker for this disease though studies of retinal changes in FTD must take into account the considerable genetic and pathological heterogeneity in this group of disorders. Below we summarize and synthesize a large body of literature describing retinal imaging findings in FTD and implications of these findings in future studies. The numerous references in this section are also summarized in Table 4.
Table 4.
Summary of retinal imaging studies characterizing human subjects with frontotemporal dementia.
| Finding | FTD vs Controls | No Difference | Biomarker confirmed (tauopathy, TAR DNA–binding protein 43) |
Complete eye exam* excluding confounding disease |
|---|---|---|---|---|
| OCT | ||||
| pRNFL thinning | Ferrari et al. (2017) | |||
| mRNFL thinning | Kim et al., (2017); Kim et al., 2019a,b | Kim et al., (2017); Kim et al., 2019a,b | ||
| RGC-IPL thinning | Kim et al., (2017); Kim et al., 2019a,b | Kim et al., (2017); Kim et al., 2019a,b | ||
| Total macular thinning | Kim et al., (2017); Kim et al., 2019a,b | Kim et al., (2017); Kim et al., 2019a,b |
Abbreviations: = Optical Coherence Tomography, FTD = Frontotemporal Dementia, pRNFL = peripapillary retinal nerve fiber layer, mRNFL = macular retinal nerve fiber layer, RGC = Retinal Ganglion Cells, IPL = Inner Plexiform Layer, Blank cells indicate no data or study was available for that parameters.
Complete eye exam includes visual acuity, intraocular pressure measurement, and dilated fundus examination.
4.7.1. Neurosensory retinal thickness in Frontotemporal Dementia
Studies of the retina in FTD are very few. Ferrari et al. compared retinal thickness between 17 FTD patients and 49 healthy controls using OCT (Ferrari et al., 2017). This cross-sectional study showed significant pRNFL and GCIPL thinning in FTD relative to controls (Ferrari et al., 2017). However, retinal thickness did not correlate with MMSE scores (Ferrari et al., 2017). Kim et al. measured macular thickness in 38 FTD patients with probable tau proteinopathy and 44 healthy controls using OCT (Kim et al., 2017). After adjusting for age, race and sex, significant thinning of the outer retinal photoreceptor complex, most pronounced in the ONL and in the IS/OS junction (Ellipsoid Zone), was observed in subjects with probable tauopathy but not in those with other subtypes of disease. Specifically, the ONL was about 10% thinner in FTD associated with tauopathy than in controls. In contrast, the inner retinal layers comprising the GCC were relatively unaffected. Outer retinal thickness also correlated significantly with MMSE scores in the whole cohort of FTD subjects (Kim et al., 2017).
A 16-month longitudinal study to further investigate disease progression in the retina showed persistent outer retinal thinning in FTD without significant involvement of the inner retinal layers (Kim et al., 2019a,b). Outer retinal thickness similarly correlated with MMSE scores prospectively, suggesting a possible relationship between the retina and the severity of disease. The investigators hypothesize that since microtubule-associated tau is found in the retina as a photoreceptor ciliary protein, a tau-based mechanism for outer retinal atrophy may be responsible for the observations (Kim et al., 2019a,b). Importantly, Kim et al. also performed objective CSF testing to exclude participants with confounding AD (Kim et al., 2017). Additional morphologic analyses of postmortem eyes correlated with retinal changes in well-characterized, homogeneous FTD cohorts are necessary to improve our understanding of retinal markers for this spectrum of disease.
5. Future directions and conclusions
We have mentioned at several points throughout this article that there is a significant need for longitudinal studies to address the causality and temporal relationship of retinal imaging findings in the diseases we have reviewed. Beyond this there are a number of limitations that are associated with retinal imaging methodologies and studies that are worth further addressing because they significantly impact the conclusions that may be derived from the retina and retinal imaging about neurological processes. In addition, these limitations provide significant opportunity for methodological development in future studies that we will discuss in the next section. For the purposes of this article and discussion we categorize these limitations and future directions in the following groups: field-of-view size, image registration, 2-dimensional versus 3-dimensional analyses, imaging artifacts, and study design. We believe that one or more of these categories underly the often contradictory or inconsistent findings in the literature.
5.1. Wide-field retinal imaging
In general, most studies of retinal findings in neurodegenerative disease are limited by the field-of-view of the imaging modality whether that is fundus photography or OCT based imaging. In the vast majority of cases the field-of-view is limited to the macula or optic disc. Specifically, for OCT imaging the central 6 × 6mm or even 3 × 3mm parafoveal region of the macula is the most commonly imaged region of the retina and for fundus photographs the 30–50° (approximately 10–15 mm; Fig. 1A) immediately adjacent to and temporal to the optic nerve head is the most commonly imaged. It is important to note that by virtue of the study design and imaging device, large areas of the peripheral retina are excluded from analysis in these studies (compare Figs. 1A and 2A). While it is not clear exactly how important the peripheral retina is compared to the central retina, it is worth keeping in mind the sampling bias introduced by this limitation. For example, some histopathological studies suggest that retinal amyloid deposition occurs primarily in the peripheral (extramacular) retina (Koronyo-Hamaoui et al., 2011; Koronyo et al., 2017) and would not be detected by studies that are only assessing central macular images.
The adoption of wide-field imaging methods that we discuss in section 5.1.1 will change this bias in the future and is particularly relevant to studies of neurodegenerative disease. Wide-field color fundus imaging is rapidly becoming the standard-of-care throughout ophthalmology practices and will grow in popularity in research settings as well (Panwar et al., 2016). Wide-field OCT and OCTA are also a subject of intense investigation and technological development (Eastline et al., 2019) (Fig. 2). One important point to keep in mind in evaluating data from different field-of-view OCT and OCTA images is that the image resolution often varies inversely with field-of-view and therefore subtle findings (e.g. capillary level findings) may be difficulty to resolve in larger images. While the wide-field capabilities of these devices are just beginning to be explored it is very likely that technological development and clinical demand will push the development of wide-field OCT and OCTA in the next several years. Implementation of these technologies in studies of retinal changes and neurodegenerative disease will provide data on aspects of retinal anatomy that were not previously available. By imaging more of the peripheral retina, future studies may better explain the differences between histopathologic studies and in vivo imaging studies.
5.2. Image registration
The detection of subtle, subclinical changes in retinal thickness, capillary density and especially sublayer thickness requires precise measurements that are reliable between and among subjects. For example, at least one cross-sectional study that did not find significant differences in average RNFL or GCIPL of subjects with LOAD (or MCI) compared to controls, did report focal regions of RNFL thickening immediately adjacent to areas of thinning (Lad et al., 2018). This suggests that quantifying retinal thickness or sublayer thickness across relatively large regions of retina may obscure subtle changes that cancel each other out. This problem is also encountered in the analysis of retinal images from subjects with retinal disease (e.g. macular edema or retinal atrophy). Therefore, one important consideration in the analysis of retinal imaging data is image registration. Image registration is critical to ensure comparison of analogous retinal regions of the same subject from different time points in longitudinal studies or analogous retinal regions from different subjects in cross-sectional studies. This is particularly relevant in OCT studies of retinal thickness but also applicable to other imaging modalities. Because accurate image registration is difficult, almost all the studies we describe in this review report average retinal thickness values across large regions (often predetermined by commercially available software) of the OCT scan. Image registration between or among subjects is generally not considered at all. This is a significant area of improvement which is particularly applicable to longitudinal studies of the retina in subjects with neurodegenerative disease especially since effect sizes are usually small and may be masked by slight changes in image sampling area.
Image registration is a computational technique for finding one-to-one correspondence between pairs of images and useful for image analysis techniques including quantitative measures of retinal changes (thickness, capillary density), image fusion or stitching. Multiple image registration techniques have been described in the field of medical image processing (Toga and Thompson 2001; Murphy et al., 2008; Teng et al., 2010; Gavaghan et al., 2011; Oliveira and Tavares 2014). These techniques have been used for registration of OCT and OCTA images to evaluate treatment efficiency (Lee et al., 2015) understand retinal diseases (Chen et al., 2014; Lee et al., 2015; Antony et al., 2016), correct motion artifacts (Kraus et al., 2012) and assist with layer segmentation (Duan et al., 2018). The majority of these methods have been performed on 2D images because of the technical challenges and computation cost of 3D image registration. However, the latter is more likely to provide anatomically accurate information for both qualitative and quantitative analyses. More importantly, it is possible and likely that 3D based measurements would capture more subtle retinal changes that are so prevalently (but inconsistently) reported in the neurodegeneration literature. Furthermore, many of the layer specific findings (or lack thereof) among current studies would benefit from a method that allowed qualitative and quantitative evaluation of retinal sections independently of layer segmentation.
Techniques for within and between subject image registration have been proposed previously. Within subject registration of OCTA image volumes has been reported to align vascular pattern of repeated scans (Zhang et al., 2019). However, between-subject OCTA registration is not feasible due to lack of features that can be reliably used for registration. In fact, vascular patterns in OCTA are the dominant features and are not expected to match across subjects. However, since OCTA is generated from more than one OCT image, the same registration for OCT volumes is applicable to OCTA images. In OCT, retinal layer boundaries and foveal pit are considered as distinctive features across subjects (Khansari et al., 2020). These features can be used for reliable registration of between and within subjects OCT image volume. Several methods have been developed for cross-subject OCT registration in 2D (Gibson et al., 2010; Chen et al., 2014; Lee et al., 2015).
Khansari et al. developed an automated 3D registration technique for cross-subject registration of OCT image volumes in subjects with retinal vascular disease (Khansari et al., 2020). This technique consisted of an initial restricted affine transformation to define anatomically consistent volumes of interest. Affine transformation is similar to rigid transformation which also corrects shear and scale between pairs of images. Afterwards, an efficient B-spline transformation using stochastic gradient descent is performed to align layers boundaries and foveal pit within the VOI. B-spline finds local deformation between pair of images and is particularly powerful for 3D registration. The authors showed high accuracy of the registration in terms of aligning eight different retinal layer boundaries in healthy and subjects with diabetic retinopathy complications. This method is particularly unique because the determinant of Jacobian matrix which is partial derivative of the vector-field at each voxel for the non-linear deformation was used to visualize tissue expansion and contraction independently of layer segmentation (Fig. 10). Additionally, tensor-based morphometry was performed for detection of group-wised localized structural changes in different stages of DR. Tensor based morphometry measures significance of local structural differences between groups of images based on voxel level statistics. In Khansari et al., 2020, for each voxel, t-test was performed on Jacobian values between healthy and diabetic subjects to generate a p-value map. Voxels in the p-value map with values less than a threshold (i.e. p < 0.05) were considered to be significantly deformed. Fig. 10 shows example non-linear registration of OCT of subjects with diabetic retinopathy to a normative image which is generated by registering and averaging multiple healthy OCTs. Color-coded Jacobian maps show the magnitude of local retinal expansion and constriction independently of retinal layer segmentation. Once OCT of different subjects are registered, the transformation can be applied to the corresponding OCTA image volume of each subjects. This brings OCTA images into a common space allowing meaningful layer-based analysis of vascular morphology between and among subjects (Sarabi et al., 2019). Future applications of this method can be performed in a layer segmentation independent manner and will likely be more sensitive for detecting the subclinical retinal changes described in various neurodegenerative diseases.
Fig. 10.
Representative 3D registration and Jacobian maps of OCT image volumes in two subjects with diabetic retinopathy using non-linear registration. Images are a cut through the volume. (A) OCT volume of the one subject with visible diabetic macular edema and alterations in the shape of the foveal pit. (B) Cut through the normative atlas image demonstrating retinal structure of a healthy subject. (C) Color-coded Jacobian map demonstrating magnitude of localized contraction and expansion in diseased subject versus healthy subject independent of layer segmentation. (D) OCT volume of another subject with visible tissue loss. (E) Cut through the atlas image which represents the retinal structure of a healthy subject. (F) Color-coded Jacobian map demonstrating magnitude of localized contraction and expansion in diseased subject versus healthy subject independent of layer segmentation. Red indicates areas of expansion (edema). Blue indicates areas of contraction (tissue loss).
5.3. 2D vs 3D OCTA analysis
Another major limitation of retinal imaging that is particularly relevant in studying neurodegenerative disease is that most data (fundus photographs) are available and analyzed only in 2-dimensional (2D) format. However, OCT and OCTA data that are available in 3D volumes are still also largely analyzed in 2D formats. This is partly because of the ease with which 2D analyses can be performed. However, 2D representations (e.g. en face images) of 3D retinal structures are inherently limited because they confound vascular and tissue relationships that are likely to be important in detecting pathological changes, especially subtle ones that are characteristic of neurodegenerative diseases. Unfortunately, there are significant technical challenges associated with rendering and analyzing 3D data. For example, OCTA data are often compromised by high noise levels, vessel discontinuity and low vessel visibility that mainly affects the appearance of small capillaries. Additionally, OCTA imaging artifacts such as motion and projection of large vessels and challenges in image registration negatively impact the true visualization and geometry of the retinal vessel network (Spaide et al., 2015a,b,c; Holmen et al., 2020). To enable true 3D OCTA analyses, multi-scale and multi-orientation curvilinear enhancement techniques need to be developed to separate blood flow from static tissues and obtain a high-quality vessel and tissue maps. Recent studies have demonstrated the potential advantages of 3D OCTA analysis in retinal images. For example, several studies have (Chen et al., 2016; Spaide 2016; Spaide et al., 2017) shown that the correlation of intraretinal fluid and retinal capillaries can be represented effectively by volume rendering analysis of 3D-OCTA data. OCTA 3D vessel density can better quantify foveal ischemia in diabetic retinopathy compared to the 2D vessel density (Wang et al., 2019).
To reduce speckle noise in 2D-OCT images, numerous algorithmic approaches (Adler et al., 2004; Jian et al., 2009; Wong et al., 2010; Fang et al., 2012; Mayer et al., 2012; Xu et al., 2012; Boroomand et al., 2013; Cameron et al., 2013; Luan and Wu 2013) have been developed. Since OCTA is constructed by subtracting several OCT images, some of the proposed techniques for OCT speckle noise reduction that can incorporate the retinal microvascular structures of various size and orientation into the denoising framework are also applicable to OCTA. Among the exiting techniques, the 3D curvelet transform has been applied successfully to both structural OCT (Jian et al., 2010) and OCTA (Shi et al., 2017), but it may be more appropriate for OCTA vessel enhancement. Curvelet is a model-based filtering approach with high directional sensitivity and anisotropy characteristics and it can provide an efficient representation of edges and other singularities along curves. Compared to conventional filtering methods, curvelet can avoid excessive denoising and preserve capillary details in OCTA (Fig. 11). A novel 3D shape modeling framework was recently developed based on curvelet modeling to produce a novel and high-quality 3D OCTA microvascular visualization which had not been previously demonstrated and might provide a unique tool for clinical and scientific analysis (Zhang et al., 2019). This framework includes a surface reconstruction process that is applied to transform 3D discrete OCTA volume representations to 3D continuous triangular surface representations (Fig. 12). The algorithmic advances would make 3D tissue analyses much more feasible and potentially enable more accurate OCT and OCTA based metrics.
Fig. 11.
New Fig 12 Demonstration of the 3D OCTA surface reconstruction and the subsequent shape and Reeb analysis that may be used for future quantitative analyses of retinal vessels. (A) The original 3D OCTA volume is processed to reconstruct (B) the 3D vessels surface representation. Using (B), we can perform intrinsic shape analysis for (C) large and small vessels classification, and (D) Reeb graph analysis to quantify valuable 3D vessel geometry and topology.
Fig. 12.
New Fig 11 Example of OCTA images from a normal subject before and after preprocessing algorithm for image registration and optimization for further analysis. (A) Original 3D-OCTA image volume. (B) Enhanced OCTA volume obtained by applying 3D curvelet denoising on previous panel. (C) OCTA vesselness map generated by computing the optimally oriented flux response of previous panel. (D) OCTA binary vessel mask obtained by applying Otsu’s global thresholding on previous panel. (E–H) Selected enface views of images in above panels.
Hessian-based methods (Frangi et al., 1998; Sato et al., 1998) have been also widely used to identify and enhance tubular structures in both 2D and 3D medical images. The Frangi filter (Frangi et al., 1998) is one of the most common multiscale hessian-based methods that has been applied to OCTA enface images (Yousefi et al., 2015). To construct the vesselness measure, Frangi uses the eigenvalues ratio of the Hessian matrix (second gradient). The tubular vessel shape is usually represented by the two large eigen values and one small eigen value. However, due to the OCTA projection artifact, the 3D deformed geometry of the large vessels won’t be properly detected by the default Frangi vesselness measure. Recently, researchers proposed a projection resolved technique based on the 3D Frangi vesselness measure for suppressing the tail artifact of large vessels in OCTA (Liu et al., 2019a,b). As part of their method, the authors suggested the plateness measure by modifying the 3D Frangi vesselness to measure the probability that a structure belongs to elongated vessels. In addition to the previously mentioned techniques, other filter-based and hybrid methods have been also developed for enhancement of the OCTA vessels. Several groups presented an automatic algorithm by combining a joint Markov-Gibbs model, a Naíve Bayes (NB) classifier, and a 2D connectivity filter for segmentation of 2D superficial and deep retinal maps of normal and diabetic eyes (Chu et al., 2016; Eladawi et al., 2017; Chlebiej et al., 2019). Others have developed an OCT amplitude-decorrelation algorithm to enhance the SNR of flow detection and microvascular network connectivity in cross-sectional images (Jia et al., 2012).
Recently, an effective OCTA 3D vessel enhancement module has also been demonstrated by combining curvelet denoising (Shi et al., 2017) and optimally orientated flux (OOF) (Law and Chung 2008; Sarabi et al., 2019). OOF is a localized multi-scale curvilinear structure detector that computes the vesselness measure based on the projected image gradient at the boundary of a spherical region centered at every image voxel. The main advantage of OOF is providing robust vessel detection response in presence of closely located structures which makes it an ideal tool for vessel enhancement in OCTA dense microvascular networks. Fig. 11 demonstrates pre-processing results of a normal subject using the combined curvelet and OOF approach. The results show that the combination of curvelet and OOF techniques resolved both the vessel discontinuity and noise adjacent to tiny vessels while preserving the microvasculature geometry in 3D-OCTA. In addition, Fig. 11 illustrates the OCTA binary vessel segmentation that is obtained by automatic thresholding on OOF vesselness map using Otsu’s global thresholding (Otsu 1979). The above 3D-OCTA preprocessing framework has been employed to develop 3D-OCTA microvascular shape analysis (Zhang et al., 2019) and study the morphological and topological vessel changes in subjects with diabetic retinopathy (Fig. 12). Future applications of these methods to other data sets from subjects with neurodegeneration may yield novel insights into neurosensory and retinal vascular changes in those diseases without the confounding effects of layer segmentation and 2D analyses (Fig. 11).
5.4. Study design
One of the most significant limitations of studies that attempt to assess any retinal feature in neurodegenerative disease is the study design. It is critical to state clearly from the outset of any study what the purpose of the proposed retinal findings are intended to be as this will guide the essential details of the study design. For example, retinal findings may serve in early detection of subclinical disease, as biomarkers of disease progression/regression, to characterize in vivo disease pathophysiology, or may be only associated clinical findings. Some studies may aim to impact clinical care or enable drug development while other studies may aim to make contributions to our understanding of disease pathophysiology. In any case, given the small effect sizes that are anticipated in most retinal imaging studies it is critical to power studies appropriately from the outset.
Because of the optical accessibility of the retinal tissue and the high resolution imaging methods that are available, it would seem logical to target the use of retinal findings as risk factors for future development of disease. In this context, relatively low cost and rapid retinal screening tests can be used to guide more resource intensive and likely specific screening efforts (such as PET or CSF studies). The relatively high spatial resolution of retinal imaging modalities may also be useful to assess for subclinical disease onset and subclinical disease progression. In this context, a retinal biomarker of early disease or risk must be validated using objective measures of the underlying neurodegenerative disease status (e.g. PET, CSF and blood) because clinical findings can be subjective, subtle or altogether absent. In many cases, these objective measures are not used because of ethical, financial or logistical reasons. For example the availability of PET scanners is relatively limited and lumbar puncture may seem like a relatively risky proposition for otherwise asymptomatic subjects. Nevertheless, studies should strive to use objective diagnostic criteria from blood, brain imaging, CSF, or genetic testing to ensure the most accurate associations with retinal findings. In addition, there are few or no studies that assess retinal findings across a sufficiently large sample and sufficiently broad spectrum of disease severity to rigorously validate the association of the retinal findings with the underlying neurodegenerative disease.
Another major methodological limitation of many studies is the challenge of performing comprehensive ophthalmologic evaluations to establish clear inclusion/exclusion criteria. There are confounding and highly prevalent ophthalmic disorders that can significantly impact retinal measures of thickness, perfusion and neurosensory function. Appropriately anticipating, quantifying and controlling for these confounding variables is possible and requires close collaboration between ophthalmologists and neurologists. While an extensive discussion of each of these diseases is out of the scope of this review article we review the pertinent findings and rationale for carefully considering some of the most prevalent comorbid ocular disease (diabetic retinopathy, glaucoma and myopia) in the exclusion and inclusion criteria of future studies below. There is also the possibility that these disease processes are not confounding. It has been suggested that there are common underlying pathophysiological mechanisms between what are considered primarily retinal diseases and neurodegenerative diseases (Lynch and Abràmoff 2017; Sen et al., 2020a,b).
Myopia was estimated to impact 23% (1.4 billion people) of the world population in 2000 and is expected to increase in prevalence to 50% (4.7 billion people) by 2050 (Holden et al., 2016). Myopia can be progressive in nature with age or relatively stationary. The increased axial length of the eye that occurs in myopia induces magnification error in many types of retinal images that require careful quantification and correction (Bennett et al., 1994; Lee et al., 2018; Llanas et al., 2019). In addition, myopia is associated with pathologic thinning of the choroid which may impact studies assessing choroidal thickness changes in neurodegenerative disease (Ang et al., 2019).
Glaucoma is another ocular disease characterized by subtle, but significant, thinning of the RNFL (Weinreb et al., 2014) and attenuation of the retinal peripapillary capillaries (Zabel et al., 2019). Glaucoma is highly prevalent especially in older demographics and among Asian and African American races (Tham et al., 2014). Glaucoma is also highly underdiagnosed (Quigley 2011; Tham et al., 2014) because it is largely an asymptomatic disease until the disease is very advanced. By some estimates, more than 110 million people worldwide will have some form of glaucoma by 2040. Most importantly, many of these people may not be diagnosed with or know they have the disease for many years. Therefore, some form of glaucoma screening is essential in studies undertaking any evaluation of retinal thickness in subjects with possible or actual neurodegenerative disease.
In 2014 an estimated 422 million people worldwide had diabetes mellitus (WHO Fact Sheet; https://www.who.int/news-room/fact-sheets/detail/diabetes). Diabetic retinopathy is estimated to effect 29% of adults in the United States with diabetes (Zhang et al., 2010). Diabetic retinopathy can cause many retinal manifestations including retinal thickening, retinal thinning and attenuation of retinal capillary density. As with glaucoma, diabetic retinopathy can be essentially asymptomatic for many years or even decades. Therefore, it is critical to assess for the presence of diabetes mellitus in study subjects as well as some assessment of diabetic retinopathy severity. It is equally as important to assess the duration and control of diabetes mellitus.
5.5. Retinal vascular reactivity
Many of the studies of retinal vascular changes in neurodegenerative disease focus on the static aspects of the retinal vasculature (e.g. capillary density, vessel tortuosity, retinal hemorrhages etc). While these are interesting metrics of vascular structure, they are only indirect measures of vascular function, if any measure of function at all. Improvements and advances in retinal imaging now allow measurement of capillary level changes associated with physiologic stimuli For example, OCTA based measurements of retinal capillary blood flow and/or caliber can be reliably made during physiologic fluctuations in inhaled oxygen and carbon dioxide (Ashimatey et al., 2019; Kushner-Lenhoff et al., 2020). This method has been employed to demonstrate preclinical changes in retinal vascular reactivity in subjects with diabetes even before the onset of clinically evidence retinopathy (Ashimatey et al., 2019; Singer et al., 2020). Similar physiologic changes have been measured using non-FDA approved methods such as AO-SLO (Duan, Bedggood et al. 2016, 2017). These methods will allow quantification of retinal vascular function which has not been possible in the past. This may be advantageous for several reasons including having larger and reversible effect sizes that can be more reliably measured. In addition, changes in retinal vascular function likely precede changes in retinal vascular structure. Future application of these methods in cross-sectional and longitudinal studies will provide novel and potentially interesting insights into the pathophysiology of neurodegenerative diseases, especially cerebrovascular disease and Alzheimer’s Disease. When coupled with measures of retinal function, these studies also have the potential to provide new insight into neurovascular coupling.
6. Conclusions
The notion that neurodegenerative changes in the brain are present in the retina is supported by extensive evidence from in vivo retinal imaging studies as well as evidence from histopathology specimens. This evidence is particularly strong and well-established from histopathologic and cross-sectional studies of subjects with Multiple Sclerosis and Alzheimer’s Disease. These studies show significant changes in inner retinal layers and most profoundly in the retinal nerve fiber layer. Interestingly, layer specific findings involving the ganglion cell layer, inner nuclear layer and photoreceptor layers have been demonstrated in different neurodegenerative diseases. This suggests that clinical stages of these chronic diseases may manifest in the retina in differing, and often very subtle ways. For example, the findings that a subtle increase in inner nuclear layer thickness correlates with MRI activity in Multiple Sclerosis suggests that inflammation attributable to the underlying disease process is present in the retina. This hypothesis seems to be supported by histopathologic evidence of inflammatory cells in the inner retina of subjects with Multiple Sclerosis. Advances in OCT analysis algorithms using 3D metrics may provide additional insight into these subtle layer specific changes. More recent findings of amyloid deposition in the neurosensory retina and retinal vasculature suggest that the amyloid pathology that correlates with Alzheimer’s Disease status is also present in the retina. Use of wide-field and hyperspectral imaging methods presents a novel and exciting opportunity in detecting retinal amyloid lesions in vivo. Advances in OCT metrics and hyperspectral imaging are also potentially applicable to Parkinson’s Disease and Huntington’s Disease where cross-sectional and histopathological evidence for retinal involvement also exists.
Unfortunately, the clinical utility of retinal findings in neurodegenerative disease has been limited because, in most cases, the cross-sectional data and associations are from subjects with relatively advanced disease. Multiple Sclerosis is one exception where optic nerve findings and OCT retinal thickness measures have demonstrated clinical utility because the initial manifestations of the disease can be primarily ocular. With the growing evidence that subtle retinal findings are detectable during the presymptomatic stages of Alzheimer’s Disease (as well as other neurodegenerative diseases), reliable and more sensitive retinal imaging methods would present a significant clinical benefit in detection, monitoring and possibly even therapeutic development. At the least, there should be awareness among ophthalmologists, neurologists and clinician-scientists about the growing number of clinical trials that are recruiting subjects at risk for neurodegenerative disease as well as the potential for ocular manifestations of these diseases.
In addition, large scale and prospective studies are also needed to assess the temporal relationship between retinal imaging findings and known biomarkers associated with preclinical neurodegenerative disease (i.e. CSF and PET amyloid etc).
Retinal imaging presents a particularly unique opportunity in at least two largely unexplored fronts, Autosomal Dominant Alzheimer’s Disease and Cerebral Small Vessel Disease. The confounding role of Vascular Cognitive Impairment and Dementia in assessing neurodegenerative changes in the aged population is particularly challenging to address. Ongoing prospective studies (e.g. MarkVCID) using optical coherence tomography angiography have particular promise in detecting and quantifying retinal vascular changes at the capillary level that may be indicative of similar changes in the brain vasculature. Overall, the potential of retinal imaging in understanding of neurodegenerative disease and in its clinical management is very promising but remains to be fully realized. Novel imaging methods (wide-field imaging, optical coherence tomography angiography and hyperspectral imaging) and analysis algorithms (3D versus 2D) will provide more opportunities to apply retinal findings to neurodegenerative disease with very exciting implications scientifically and clinically.
Acknowledgments
Funding sources
This work was supported by the National Eye Institute (USA, K08EY027006 to AHK) and National Institute for Neurological Diseases (USA, UH3NS100614 to AHK), an unrestricted grant from Research to Prevent Blindness (USA) to the USC Department of Ophthalmology, Brightfocus Foundation (USA, CA2020004 to AHK)), National Institutes of Health (USA, R01AG062007 and P30AG066530 to JMR).
List of abbreviations
- CNS
Central Nervous System
- LOAD
Late Onset Alzheimer’s Disease
- ADAD
Autosomal Dominant Alzheimer’s Disease
- HD
Huntington’s Disease
- PD
Parkinson’s Disease
- AD
Alzheimer’s Disease
- FTD
Frontotemporal Dementia
- CSVD
Cerebral Small Vessel Disease
- MS
Multiple Sclerosis
- OCT
Optical Coherence Tomography
- OCTA
Optical Coherence Tomography Angiography
- PET
Positron Emission Tomography
- MRI
Magnetic Resonance Imaging
- RGC
Retinal Ganglion Cells
- LGN
Lateral Geniculate Nucleus
- BRB
Blood Retinal Barrier
- BBB
Blood Brain Barrier
- RNFL
Retinal Nerve Fiber Layer
- pRNFL
Peripapillary RNFL
- mRNFL
Macular RNFL
- αsyn
Alpha-Synuclein
- Aβ
Beta-Amyloid
- ptau
Hyperphosphorylated Tau
- CFP
Color Fundus Photography
- FAF
Fundus Autofluorescence
- FOV
Field-of-View
- RPE
Retinal Pigment Epithelium
- HRI
Hyperspectral Retinal Imaging
- FLIO
Fluorescence Lifetime Imaging Ophthalmoscopy
- ERG
Electroretinography
- mfERG
Multifocal ERG
- PERG
Pattern ERG
- VEP
Visual Evoke Potentials
- ON
Optic Neuritis
- IPL
Inner Plexiform Layer
- INL
Inner Nuclear Layer
- ONL
Outer Nuclear Layer
- OPL
Outer Plexiform Layer
- GCIPL
Ganglion Cell Layer + Inner Plexiform Layer
- GCC
Retinal Ganglion Cell Complex (mRNFL + GCL + IPL)
- PCA
Posterior Cortical Atrophy
- NMO
Neuromyelitis Optica
- MOG
Myelin Oligodendrocyte Glycoprotein
- mRGC
Melanopsin containing RGC
- CSF
Cerebrospinal Fluid
- VCID
Vascular Cognitive Impairment and Dementia
- FAZ
Foveal Avascular Zone
- ffERG
Full Field ERG
- SRL
Superficial Retinal Layer
- DRL
Deep Retinal Layer
- MoCA
Montreal Cognitive Assessment
- MMSE
Mini-Mental Status Exam
- CAG
Cytosine-adenine-guanine
- Htt
huntington protein
- HC
Healthy Control
- VA
Visual Acuity
- DFE
Dilated Fundus Examination
- IOP
Intraocular Pressure
- PSEN1
Presenilin 1
- PSEN2
Presenilin 2
- APP
Amyloid Precursor Protein
- TDP43
Transactive Response DNA Binding Domain Protein 43
- ALS
Amyotrophic Lateral Sclerosis
- CAA
Cerebral Amyloid Angiopathy
- WMH
White Matter Hyperintensity
- CMB
Cerebral Microbleeds
- HTNA
Hypertension Associated Angiopathy
- CADASIL
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
Footnotes
Declaration of competing interest
The authors have no competing interests to declare. AHK received research funding and honoraria from Carl Zeiss Meditec (Dublin, CA, USA). Funding sources did not have any role in the conception, writing or editing of this manuscript.
References
- Aaker GD, Myung JS, Ehrlich JR, Mohammed M, Henchcliffe C, Kiss S, 2010. Detection of retinal changes in Parkinson’s disease with spectral-domain optical coherence tomography. Clin. Ophthalmol 4, 1427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam CR, Shrier E, Ding Y, Glazman S, Bodis-Wollner I, 2013. Correlation of inner retinal thickness evaluated by spectral-domain optical coherence tomography and contrast sensitivity in Parkinson disease. J. Neuro Ophthalmol 33 (2), 137–142. [DOI] [PubMed] [Google Scholar]
- Adler DC, Ko TH, Fujimoto JG, 2004. Speckle reduction in optical coherence tomography images by use of a spatially adaptive wavelet filter. Opt. Lett 29 (24), 2878–2880. [DOI] [PubMed] [Google Scholar]
- Ahn J, Lee JY, Kim TW, Yoon EJ, Oh S, Kim YK, Kim JM, Woo SJ, Kim KW, Jeon B, 2018. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology 91 (11), e1003–e1012. [DOI] [PubMed] [Google Scholar]
- Alber J, Goldfarb D, Thompson LI, Arthur E, Hernandez K, Cheng D, DeBuc DC, Cordeiro F, Provetti-Cunha L, den Haan J, Van Stavern GP, Salloway SP, Sinoff S, Snyder PJ, 2020. Developing retinal biomarkers for the earliest stages of Alzheimer’s disease: What we know, what we don’t, and how to move forward. Alzheimers Dement. 16 (1), 229–243. [DOI] [PubMed] [Google Scholar]
- Albrecht P, Müller AK, Südmeyer M, Ferrea S, Ringelstein M, Cohn E, Aktas O, Dietlein T, Lappas A, Foerster A, Hartung HP, Schnitzler A, Methner A, 2012. Optical coherence tomography in parkinsonian syndromes. PloS One 7 (4), e34891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov PN, Pogue A, Bhattacharjee S, Lukiw WJ, 2011. Retinal amyloid peptides and complement factor H in transgenic models of Alzheimer’s disease. Neuroreport 22 (12), 623–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alten F, Motte J, Ewering C, Osada N, Clemens CR, Kadas EM, Eter N, Paul F, Marziniak M, 2014. Multimodal retinal vessel analysis in CADASIL patients. PloS One 9 (11), e112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altintaş O, Işeri P, Ozkan B, Cağlar Y, 2008. Correlation between retinal morphological and functional findings and clinical severity in Parkinson’s disease. Doc. Ophthalmol 116 (2), 137–146. [DOI] [PubMed] [Google Scholar]
- Alves C, Jorge L, Canário N, Santiago B, Santana I, Castelhano J, Ambrósio AF, Bernardes R, Castelo-Branco M, 2019. Interplay between macular retinal changes and white matter integrity in early Alzheimer’s disease. J. Alzheimers Dis 70 (3), 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV, 2004. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp. Eye Res 78 (2), 243–256. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association, 2020. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf. (Accessed 16 January 2021). [Google Scholar]
- Anderson TJ, MacAskill MR, 2013. Eye movements in patients with neurodegenerative disorders. Nat. Rev. Neurol 9 (2), 74–85. [DOI] [PubMed] [Google Scholar]
- Andrade C, Beato J, Monteiro A, Costa A, Penas S, Guimarães J, Reis FF, Garrett C, 2016. Spectral-domain optical coherence tomography as a potential biomarker in Huntington’s disease. Mov. Disord 31 (3), 377–383. [DOI] [PubMed] [Google Scholar]
- Andrew SE, Goldberg YP, Kremer B, Telenius H, Theilmann J, Adam S, Starr E, Squitieri F, Lin B, Kalchman MA, 1993. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington’s disease. Nat. Genet 4 (4), 398–403. [DOI] [PubMed] [Google Scholar]
- Ang M, Wong CW, Hoang QV, Cheung GCM, Lee SY, Chia A, Saw SM, Ohno-Matsui K, Schmetterer L, 2019. Imaging in myopia: potential biomarkers, current challenges and future developments. Br. J. Ophthalmol 103 (6), 855–862. [DOI] [PubMed] [Google Scholar]
- Antony BJ, Chen M, Carass A, Jedynak BM, Al-Louzi O, Solomon SD, Saidha S, Calabresi PA, Prince JL, 2016. Voxel based morphometry in optical coherence tomography: validation & core findings. Proc. SPIE-Int. Soc. Opt. Eng 9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald NK, Clarke MP, Mosimann UP, Burn DJ, 2009. The retina in Parkinson’s disease. Brain 132 (Pt 5), 1128–1145. [DOI] [PubMed] [Google Scholar]
- Archibald NK, Clarke MP, Mosimann UP, Burn DJ, 2011. Retinal thickness in Parkinson’s disease. Park. Relat. Disord 17 (6), 431–436. [DOI] [PubMed] [Google Scholar]
- Arevalo-Rodriguez I, Smailagic N, Roqué I Figuls M, Ciapponi A, Sanchez-Perez E, Giannakou A, Pedraza OL, Bonfill Cosp X, Cullum S, 2015. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev 3, CD010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong GW, Kim LA, Vingopoulos F, Park JY, Garg I, Kasetty M, Silverman RF, Zeng R, Douglas VP, Lopera F, Baena A, Giraldo M, Norton D, Cronin-Golomb A, Arboleda-Velasquez JF, Quiroz YT, Miller JB, 2020. November. Retinal imaging findings in carriers with PSEN1-associated early-onset familial Alzheimer disease before onset of cognitive symptoms. JAMA Ophthalmol. e204909. 10.1001/jamaophthalmol.2020.4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanad S, Fantini M, Sultan W, Nassisi M, Felix CM, Wu J, Karanjia R, Ross-Cisneros FN, Sagare AP, Zlokovic BV, Chui HC, Pogoda JM, Arakaki X, Fonteh AN, Sadun AA, Harrington MG, 2020. Retinal nerve fiber layer thickness predicts CSF amyloid/tau before cognitive decline. PloS One 15 (5), e0232785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanad S, Ross-Cisneros FN, Barron E, Nassisi M, Sultan W, Karanjia R, Sadun AA, 2019a. The retinal choroid as an oculovascular biomarker for Alzheimer’s dementia: a histopathological study in severe disease. Alzheimers Dement. (Amst.) 11, 775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanad S, Ross-Cisneros FN, Nassisi M, Barron E, Karanjia R, Sadun AA, 2019b. The retina in Alzheimer’s disease: histomorphometric analysis of an ophthalmologic biomarker. Invest. Ophthalmol. Vis. Sci 60 (5), 1491–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascaso FJ, Cruz N, Modrego PJ, Lopez-Anton R, Santabárbara J, Pascual LF, Lobo A, Cristóbal JA, 2014. Retinal alterations in mild cognitive impairment and Alzheimer’s disease: an optical coherence tomography study. J. Neurol 261 (8), 1522–1530. [DOI] [PubMed] [Google Scholar]
- Ashimatey B, D’Orazio L, Ma S, Jann K, Lu H, Jiang X, Wang D, Ringman J, Kashani A, 2020. Lower retinal capillary density in minimal cognitive impairment among older Latinx adults. Alzheimer’s Dementia: Diagn. Assess. Dis. Monit 12, e12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashimatey BS, Green KM, Chu Z, Wang RK, Kashani AH, 2019. Impaired retinal vascular reactivity in diabetic retinopathy as assessed by optical coherence tomography angiography. Invest. Ophthalmol. Vis. Sci 60 (7), 2468–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CL, Hess RR, Olsen BT, Zrenner E, 1988. Current source density analysis of linear and non-linear components of the primate electroretinogram. J. Physiol 407, 155–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ML, Hand PJ, Wong TY, Liew G, Rochtchina E, Mitchell P, Lindley RI, Hankey GJ, Wang JJ, M.-C. R. S. S. Group, 2010a. Retinopathy and lobar intracerebral hemorrhage: insights into pathogenesis. Arch. Neurol 67 (10), 1224–1230. [DOI] [PubMed] [Google Scholar]
- Baker ML, Wang JJ, Liew G, Hand PJ, De Silva DA, Lindley RI, Mitchell P, Wong MC, Rochtchina E, Wong TY, Wardlaw JM, Hankey GJ, M.-C. R. S. S. Group, 2010b. Differential associations of cortical and subcortical cerebral atrophy with retinal vascular signs in patients with acute stroke. Stroke 41 (10), 2143–2150. [DOI] [PubMed] [Google Scholar]
- Bassi CJ, Sadun AA, 1990. Alzheimer’s disease and vision: correlation versus causation, revisited. Ophthalmology 97 (4), 395–397. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC, Network DIA, 2012. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med 367 (9), 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayhan HA, Aslan Bayhan S, Celikbilek A, Tanık N, Gürdal C, 2015. Evaluation of the chorioretinal thickness changes in Alzheimer’s disease using spectral-domain optical coherence tomography. Clin. Exp. Ophthalmol 43 (2), 145–151. [DOI] [PubMed] [Google Scholar]
- Bayhan HA, Aslan Bayhan S, Tanık N, Gürdal C, 2014. The association of spectral-domain optical coherence tomography determined ganglion cell complex parameters and disease severity in Parkinson’s disease. Curr. Eye Res 39 (11), 1117–1122. [DOI] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG, Consortium, A.P.s.D., 2010. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 119 (6), 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Carew J, Serrano G, Adler CH, Shill HA, Sue LI, Sabbagh MN, Akiyama H, Cuenca N, Consortium, A.P.s.D., 2014. Phosphorylated α-synuclein-immunoreactive retinal neuronal elements in Parkinson’s disease subjects. Neurosci. Lett 571, 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AG, Rudnicka AR, Edgar DF, 1994. Improvements on Littmann’s method of determining the size of retinal features by fundus photography. Graefes Arch. Clin. Exp. Ophthalmol 232 (6), 361–367. [DOI] [PubMed] [Google Scholar]
- Bennett JL, de Seze J, Lana-Peixoto M, Palace J, Waldman A, Schippling S, Tenembaum S, Banwell B, Greenberg B, Levy M, Fujihara K, Chan KH, Kim HJ, Asgari N, Sato DK, Saiz A, Wuerfel J, Zimmermann H, Green A, Villoslada P, Paul F, Gjcf-Icc&Br, 2015. Neuromyelitis optica and multiple sclerosis: seeing differences through optical coherence tomography. Mult. Scler 21 (6), 678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL, 2007. Retinal abnormalities in early Alzheimer’s disease. Invest. Ophthalmol. Vis. Sci 48 (5), 2285–2289. [DOI] [PubMed] [Google Scholar]
- Bettermann K, Slocomb J, Shivkumar V, Quillen D, Gardner TW, Lott ME, 2017. Impaired retinal vasoreactivity: an early marker of stroke risk in diabetes. J. Neuroimaging 27 (1), 78–84. [DOI] [PubMed] [Google Scholar]
- Biffi A, Greenberg SM, 2011. Cerebral amyloid angiopathy: a systematic review. J. Clin. Neurol 7 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittersohl D, Stemplewitz B, Keserü M, Buhmann C, Richard G, Hassenstein A, 2015. November. Detection of retinal changes in idiopathic Parkinson’s disease using high-resolution optical coherence tomography and heidelberg retina tomography. Acta Ophthalmol 93 (7), e578–e584. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Hinton DR, Sadun AA, Miller CA, 1989. Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res. 501 (2), 364–372. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Schmidt SY, Torigoe Y, Porrello KV, Hinton DR, Blanks RH, 1996a. Retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol. Aging 17 (3), 385–395. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Torigoe Y, Hinton DR, Blanks RH, 1996b. Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol. Aging 17 (3), 377–384. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Torigoe Y, Hinton DR, Blanks RH, 1991. Retinal degeneration in the macula of patients with Alzheimer’s disease. Ann. N. Y. Acad. Sci 640, 44–46. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Kozlowski PB, Glazman S, Miri S, 2014. α-synuclein in the inner retina in Parkinson disease. Ann. Neurol 75 (6), 964–966. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Yahr MD, 1978. Measurements of visual evoked potentials in Parkinson’s disease. Brain 101 (4), 661–671. [DOI] [PubMed] [Google Scholar]
- Borbe HO, Fehske KJ, Müller WE, Nover A, Woller U, 1982. The demonstration of several neurotransmitter and drug receptors in human retina. Comp. Biochem. Physiol., C 72 (1), 117–119. [DOI] [PubMed] [Google Scholar]
- Boroomand A, Wong A, Li E, Cho DS, Ni B, Bizheva K, 2013. Multi-penalty conditional random field approach to super-resolved reconstruction of optical coherence tomography images. Biomed. Optic Express 4 (10), 2032–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E, Sarraf D, Freund KB, Sadda SR, 2018. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog. Retin. Eye Res 67, 30–55. [DOI] [PubMed] [Google Scholar]
- Boucart M, Bubbico G, Szaffarczyk S, Defoort S, Ponchel A, Waucquier N, Deplanque D, Deguil J, Bordet R, 2015. Donepezil increases contrast sensitivity for the detection of objects in scenes. Behav. Brain Res 292, 443–447. [DOI] [PubMed] [Google Scholar]
- Bulens C, Meerwaldt JD, Van der Wildt GJ, Van Deursen JB, 1987. Effect of levodopa treatment on contrast sensitivity in Parkinson’s disease. Ann. Neurol 22 (3), 365–369. [DOI] [PubMed] [Google Scholar]
- Bulut M, Kurtuluş F, Gözkaya O, Erol MK, Cengiz A, Akidan M, Yaman A, 2018. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br. J. Ophthalmol 102 (2), 233–237. [DOI] [PubMed] [Google Scholar]
- Bulut M, Yaman A, Erol MK, Kurtuluş F, Toslak D, Doğan B, Turgut Çoban D, Kaya Başar E, 2016. Choroidal thickness in patients with mild cognitive impairment and Alzheimer’s type dementia. J. Ophthalmol 2016, 7291257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A, Lui D, Boroomand A, Glaister J, Wong A, Bizheva K, 2013. Stochastic speckle noise compensation in optical coherence tomography using non-stationary spline-based speckle noise modelling. Biomed. Optic Express 4 (9), 1769–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JR, Megaw RD, Tatham AJ, McGrory S, MacGillivray TJ, Doubal FN, Wardlaw JM, Trucco E, Chandran S, Dhillon B, 2017. Lateral thinking - interocular symmetry and asymmetry in neurovascular patterning, in health and disease. Prog. Retin. Eye Res 59, 131–157. [DOI] [PubMed] [Google Scholar]
- Campbell G, Mahad D, 2018. Neurodegeneration in progressive multiple sclerosis. Cold Spring Harb. Perspect. Med 8 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, Huang D, 2017. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci. Rep 7, 42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF, 2019. CNS small vessel disease: a clinical review. Neurology 92 (24), 1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli V, La Morgia C, Valentino ML, Barboni P, Ross-Cisneros FN, Sadun AA, 2009. Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochim. Biophys. Acta 1787 (5), 518–528. [DOI] [PubMed] [Google Scholar]
- Chan VTT, Sun Z, Tang S, Chen LJ, Wong A, Tham CC, Wong TY, Chen C, Ikram MK, Whitson HE, Lad EM, Mok VCT, Cheung CY, 2019. Spectral-domain OCT measurements in Alzheimer’s disease: a systematic review and meta-analysis. Ophthalmology 126 (4), 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C BK, Xin C, Wen J, Gupta D, Zhang Q, Mudumbai RC, Johnstone MA, Chen PP, Wang RK, 2016. Repeatability and reproducibility of optic disc perfusion measurements using optical coherence tomography-based angiography. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Flanagan EP, Jitprapaikulsan J, López-Chiriboga ASS, Fryer JP, Leavitt JA, Weinshenker BG, McKeon A, Tillema JM, Lennon VA, Tobin WO, Keegan BM, Lucchinetti CF, Kantarci OH, McClelland CM, Lee MS, Bennett JL, Pelak VS, Chen Y, VanStavern G, Adesina OO, Eggenberger ER, Acierno MD, Wingerchuk DM, Brazis PW, Sagen J, Pittock SJ, 2018. Myelin oligodendrocyte glycoprotein antibody-positive optic neuritis: clinical characteristics, radiologic clues, and outcome. Am. J. Ophthalmol 195, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Lang A, Ying HS, Calabresi PA, Prince JL, Carass A, 2014. Analysis of macular OCT images using deformable registration. Biomed. Optic Express 5 (7), 2196–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Huang D, Izatt JA, Wang RK, Werner JS, Yasuno Y, 2016. Re: Spaide et al.: volume-rendering optical coherence tomography angiography of macular telangiectasia type 2 (Ophthalmology 2015;122:2261-9). Ophthalmology 123 (3), e24. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Ong YT, Hilal S, Ikram MK, Low S, Ong YL, Venketasubramanian N, Yap P, Seow D, Chen CL, Wong TY, 2015. Retinal ganglion cell analysis using high-definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis 45 (1), 45–56. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, Catindig JA, Venketasubramanian N, Yap P, Seow D, Chen CP, Wong TY, 2014. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimers Dement. 10 (2), 135–142. [DOI] [PubMed] [Google Scholar]
- Cheung N, Mosley T, Islam A, Kawasaki R, Sharrett AR, Klein R, Coker LH, Knopman DS, Shibata DK, Catellier D, Wong TY, 2010. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: a prospective study. Brain 133 (Pt 7), 1987–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibhabha F, Yang Y, Ying K, Jia F, Zhang Q, Ullah S, Liang Z, Xie M, Li F, 2020. Non-invasive optical imaging of retinal Aβ plaques using curcumin loaded polymeric micelles in APP. J. Mater. Chem. B 8 (33), 7438–7452. [DOI] [PubMed] [Google Scholar]
- Chlebiej M, Gorczynska I, Rutkowski A, Kluczewski J, Grzona T, Pijewska E, Sikorski BL, Szkulmowska A, Szkulmowski M, 2019. Quality improvement of OCT angiograms with elliptical directional filtering. Biomed. Optic Express 10 (2), 1013–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KJ, Yu J, 2015. Retinal nerve fibre layer defect associated with MELAS syndrome. Can. J. Ophthalmol 50 (5), e85–88. [DOI] [PubMed] [Google Scholar]
- Chrysou A, Jansonius NM, van Laar T, 2019. Retinal layers in Parkinson’s disease: a meta-analysis of spectral.domain optical coherence tomography studies. Park. Relat. Disord 64, 40–49. [DOI] [PubMed] [Google Scholar]
- Chu Z, Lin J, Gao C, Xin C, Zhang Q, Chen CL, Roisman L, Gregori G, Rosenfeld PJ, Wang RK, 2016. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J. Biomed. Optic 21 (6), 66008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipollini V, Abdolrahimzadeh S, Troili F, De Carolis A, Calafiore S, Scuderi L, Giubilei F, Scuderi G, 2020. September. Neurocognitive assessment and retinal thickness alterations in Alzheimer disease: is there a correlation? J. Neuro Ophthalmol 40 (3), 370–377. [DOI] [PubMed] [Google Scholar]
- Cogan DG, Kuwabara T, 1984. Comparison of retinal and cerebral vasculature in trypsin digest preparations. Br. J. Ophthalmol 68 (1), 10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti FF, Young JM, Silva FQ, Rodrigues EB, Singh RP, 2018. Repeatability of split-spectrum amplitude-decorrelation angiography to assess capillary perfusion density within optical coherence tomography. Ophthalmic Surg. Lasers Imag. Retina 49 (9), e9–e19. [DOI] [PubMed] [Google Scholar]
- Cooper LS, Wong TY, Klein R, Sharrett AR, Bryan RN, Hubbard LD, Couper DJ, Heiss G, Sorlie PD, 2006. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke 37 (1), 82–86. [DOI] [PubMed] [Google Scholar]
- Coppola G, Di Renzo A, Ziccardi L, Martelli F, Fadda A, Manni G, Barboni P, Pierelli F, Sadun AA, Parisi V, 2015. Optical coherence tomography in Alzheimer’s disease: a meta-analysis. PloS One 10 (8), e0134750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, Freedman MS, Zackon DH, Kardon RH, 2006. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann. Neurol 59 (6), 963–969. [DOI] [PubMed] [Google Scholar]
- Cowey A, Alexander I, Stoerig P, 2011. Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemianopic monkeys and humans. Brain 134 (Pt 7), 2149–2157. [DOI] [PubMed] [Google Scholar]
- Cubo E, Tedejo RP, Rodriguez Mendez V, López Peña MJ, Trejo Gabriel Y, Galán JM, 2010. Retina thickness in Parkinson’s disease and essential tremor. Mov. Disord 25 (14), 2461–2462. [DOI] [PubMed] [Google Scholar]
- Cunha LP, Lopes LC, Costa-Cunha LV, Costa CF, Pires LA, Almeida AL, Monteiro ML, 2016. Macular thickness measurements with frequency domain-OCT for quantification of retinal neural loss and its correlation with cognitive impairment in Alzheimer’s disease. PloS One 11 (4), e0153830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Drucker DN, 1993. Retinal ganglion cells in Alzheimer’s disease and aging. Ann. Neurol 33 (3), 248–257. [DOI] [PubMed] [Google Scholar]
- Das AS, Regenhardt RW, Vernooij MW, Blacker D, Charidimou A, Viswanathan A, 2019. Asymptomatic cerebral small vessel disease: insights from population-based studies. J. Stroke 21 (2), 121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Brutto OH, Mera RM, Viteri EM, Pólit J, Ledesma EA, Cano JA, Plaza KJ, Zambrano M, Costa AF, 2016. Hypertensive retinopathy and cerebral small vessel disease in Amerindians living in rural Ecuador: the Atahualpa Project. Int. J. Cardiol 218, 65–68. [DOI] [PubMed] [Google Scholar]
- Delori F, Greenberg JP, Woods RL, Fischer J, Duncker T, Sparrow J, Smith RT, 2011. Quantitative measurements of autofluorescence with the scanning laser ophthalmoscope. Invest. Ophthalmol. Vis. Sci 52 (13), 9379–9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delori FC, Goger DG, Dorey CK, 2001. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest. Ophthalmol. Vis. Sci 42 (8), 1855–1866. [PubMed] [Google Scholar]
- den Haan J, Janssen SF, van de Kreeke JA, Scheltens P, Verbraak FD, Bouwman FH, 2018a. Retinal thickness correlates with parietal cortical atrophy in early-onset Alzheimer’s disease and controls. Alzheimers Dement. (Amst.) 10, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Haan J, Morrema THJ, Verbraak FD, de Boer JF, Scheltens P, Rozemuller AJ, Bergen AAB, Bouwman FH, Hoozemans JJ, 2018b. Amyloid-beta and phosphorylated tau in post-mortem Alzheimer’s disease retinas. Acta Neuropathol. Commun 6 (1), 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Haan J, Verbraak FD, Visser PJ, Bouwman FH, 2017. Retinal thickness in Alzheimer’s disease: a systematic review and meta-analysis. Alzheimers Dement. (Amst.) 6, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr A, Sahota B, Chinta LV, Brown ME, Lai AY, Ma K, Hawkes CA, McLaurin J, Stefanovic B, 2012. Amyloid-β-dependent compromise of microvascular structure and function in a model of Alzheimer’s disease. Brain 135 (Pt 10), 3039–3050. [DOI] [PubMed] [Google Scholar]
- Doubal FN, de Haan R, MacGillivray TJ, Cohn-Hokke PE, Dhillon B, Dennis MS, Wardlaw JM, 2010. Retinal arteriolar geometry is associated with cerebral white matter hyperintensities on magnetic resonance imaging. Int. J. Stroke 5 (6), 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler W, Fujimoto JG, 2008. State-of-the-art retinal optical coherence tomography. Prog. Retin. Eye Res 27 (1), 45–88. [DOI] [PubMed] [Google Scholar]
- Duan A, Bedggood PA, Bui BV, Metha AB, 2016. Evidence of flicker-induced functional Hyperaemia in the smallest vessels of the human retinal blood supply. PloS One 11 (9), e0162621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan A, Bedggood PA, Metha AB, Bui BV, 2017. Reactivity in the human retinal microvasculature measured during acute gas breathing provocations. Sci. Rep 7 (1), 2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Zheng Y, Ding Y, Hou S, Tang Y, Xu Y, Qin M, Wu J, Shen D, Bi H, 2018. A generative model for OCT retinal layer segmentation by groupwise curve Alignment. IEEE Access 6, 25130–25141. [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues JF, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert MO, Holtzman DM, Kivipelto M, Lista S, Molinuevo JL, O’Bryant SE, Rabinovici GD, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J, Jack CR, P. o. t. M. o. t. I. W. G. I. a. t. A. A. s. A. o. T. P. S. o. Ad” July 23 and U. S. A. Washington DC, 2016. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 12 (3), 292–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dysli C, Fink R, Wolf S, Zinkernagel MS, 2017a. Fluorescence lifetimes of drusen in age.related macular degeneration. Invest. Ophthalmol. Vis. Sci 58 (11), 4856–4862. [DOI] [PubMed] [Google Scholar]
- Dysli C, Wolf S, Berezin MY, Sauer L, Hammer M, Zinkernagel MS, 2017b. Fluorescence lifetime imaging ophthalmoscopy. Prog. Retin. Eye Res 60, 120–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastline M, Munk MR, Wolf S, Schaal KB, Ebneter A, Tian M, Giannakaki-Zimmermann H, Zinkernagel MS, 2019. Repeatability of wide-field optical coherence tomography angiography in normal retina. Transl. Vis. Sci. Technol 8 (3), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehle AL, Stewart RM, Lellelid NA, Leventhal NA, 1984. Evoked potentials in Huntington’s disease. A comparative and longitudinal study. Arch. Neurol 41 (4), 379–382. [DOI] [PubMed] [Google Scholar]
- Einarsdottir AB, Hardarson SH, Kristjansdottir JV, Bragason DT, Snaedal J, Stefánsson E, 2016. Retinal oximetry imaging in Alzheimer’s disease. J. Alzheimers Dis 49 (1), 79–83. [DOI] [PubMed] [Google Scholar]
- Eladawi N, Elmogy M, Helmy O, Aboelfetouh A, Riad A, Sandhu H, Schaal S, El-Baz A, 2017. Automatic blood vessels segmentation based on different retinal maps from OCTA scans. Comput. Biol. Med 89, 150–161. [DOI] [PubMed] [Google Scholar]
- Elbøl P, Work K, 1990. Retinal nerve fiber layer in multiple sclerosis. Acta Ophthalmol. 68 (4), 481–486. [DOI] [PubMed] [Google Scholar]
- Ellenberger C, Petro DJ, Ziegler SB, 1978. The visually evoked potential in Huntington disease. Neurology 28 (1), 95–97. [DOI] [PubMed] [Google Scholar]
- Esen E, Sizmaz S, Demir T, Demirkiran M, Unal I, Demircan N, 2016. Evaluation of choroidal vascular changes in patients with multiple sclerosis using enhanced depth imaging optical coherence tomography. Ophthalmologica 235 (2), 65–71. [DOI] [PubMed] [Google Scholar]
- Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM, 2001. Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiple sclerosis. Brain 124 (Pt 9), 1813–1820. [DOI] [PubMed] [Google Scholar]
- Fang L, Li S, Nie Q, Izatt JA, Toth CA, Farsiu S, 2012. Sparsity based denoising of spectral domain optical coherence tomography images. Biomed. Optic Express 3 (5), 927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang XJ, Yu M, Wu Y, Zhang ZH, Wang WW, Wang ZX, Yuan Y, 2017. Study of enhanced depth imaging optical coherence tomography in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Chin. Med. J. (Engl.) 130 (9), 1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico A, Di Donato I, Bianchi S, Di Palma C, Taglia I, Dotti MT, 2012. Hereditary cerebral small vessel diseases: a review. J. Neurol. Sci 322 (1–2), 25–30. [DOI] [PubMed] [Google Scholar]
- Feke GT, Hyman BT, Stern RA, Pasquale LR, 2015. Retinal blood flow in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. (Amst.) 1 (2), 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara D, Waheed NK, Duker JS, 2016. Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog. Retin. Eye Res 52, 130–155. [DOI] [PubMed] [Google Scholar]
- Ferrari L, Huang SC, Magnani G, Ambrosi A, Comi G, Leocani L, 2017. Optical coherence tomography reveals retinal neuroaxonal thinning in frontotemporal dementia as in Alzheimer’s disease. J. Alzheimers Dis 56 (3), 1101–1107. [DOI] [PubMed] [Google Scholar]
- Feucht N, Maier M, Lepennetier G, Pettenkofer M, Wetzlmair C, Daltrozzo T, Scherm P, Zimmer C, Hoshi MM, Hemmer B, Korn T, Knier B, 2019. Optical coherence tomography angiography indicates associations of the retinal vascular network and disease activity in multiple sclerosis. Mult. Scler 25 (2), 224–234. [DOI] [PubMed] [Google Scholar]
- Filippatou AG, Mukharesh L, Saidha S, Calabresi PA, Sotirchos ES, 2020a. AQP4-IgG and MOG-IgG related optic neuritis-prevalence, optical coherence tomography findings, and visual outcomes: a systematic review and meta-analysis. Front. Neurol 11, 540156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippatou AG, Vasileiou ES, He Y, Fitzgerald KC, Kalaitzidis G, Lambe J, Mealy MA, Levy M, Liu Y, Prince JL, Mowry EM, Saidha S, Calabresi PA, Sotirchos ES, 2020b. Evidence of subclinical quantitative retinal layer abnormalities in AQP4-IgG seropositive NMOSD. Mult. Scler 10.1177/1352458520977771, 1352458520977771, Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, Chatterjee A, Hurtig HI, Karlawish JH, Rosen HJ, Van Deerlin V, Lee VM, Miller BL, Trojanowski JQ, Grossman M, 2006. Frontotemporal dementia: clinicopathological correlations. Ann. Neurol 59 (6), 952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangi AF, Niessen WJ, Vincken KL, Viergever MA, 1998. Multiscale Vessel Enhancement Filtering. Springer Berlin Heidelberg, Berlin, Heidelberg. [Google Scholar]
- Frank RN, Turczyn TJ, Das A, 1990. Pericyte coverage of retinal and cerebral capillaries. Invest. Ophthalmol. Vis. Sci 31 (6), 999–1007. [PubMed] [Google Scholar]
- Frishman LJ, 2013. Electrogenesis of the Electroretinogram. Elsevier, New York. [Google Scholar]
- Frost S, Kanagasingam Y, Sohrabi H, Vignarajan J, Bourgeat P, Salvado O, Villemagne V, Rowe CC, Macaulay SL, Szoeke C, Ellis KA, Ames D, Masters CL, Rainey-Smith S, Martins RN, AIBL Research Group, 2013. Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl. Psychiatry 3, e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabilondo I, Martínez-Lapiscina EH, Fraga-Pumar E, Ortiz-Perez S, Torres-Torres R, Andorra M, Lluffiu S, Zubizarreta I, Saiz A, Sanchez-Dalmau B, Villoslada P, 2015. Dynamics of retinal injury after acute optic neuritis. Ann. Neurol 77 (3), 517–528. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin E, Bambo MP, Marques ML, Satue M, Otin S, Larrosa JM, Polo V, Pablo LE, 2016. Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer’s disease. Acta Ophthalmol. 94 (6), e454–459. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin E, Larrosa JM, Polo V, Satue M, Marques ML, Alarcia R, Seral M, Fuertes I, Otin S, Pablo LE, 2014a. Distribution of retinal layer atrophy in patients with Parkinson disease and association with disease severity and duration. Am. J. Ophthalmol 157 (2), 470–478 e472. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin E, Rodriguez-Mena D, Satue M, Almarcegui C, Dolz I, Alarcia R, Seral M, Polo V, Larrosa JM, Pablo LE, 2014b. Electrophysiology and optical coherence tomography to evaluate Parkinson disease severity. Invest. Ophthalmol. Vis. Sci 55 (2), 696–705. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin E, Pablo LE, Herrero R, Satue M, Polo V, Larrosa JM, Martin J, Fernandez J, 2012a. Diagnostic ability of a linear discriminant function for spectral-domain optical coherence tomography in patients with multiple sclerosis. Ophthalmology 119 (8), 1705–1711. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin E, Satue M, Fuertes I, Otin S, Alarcia R, Herrero R, Bambo MP, Fernandez J, Pablo LE, 2012b. Ability and reproducibility of Fourier-domain optical coherence tomography to detect retinal nerve fiber layer atrophy in Parkinson’s disease. Ophthalmology 119 (10), 2161–2167. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin E, Pueyo V, Almarcegui C, Martin J, Ara JR, Sancho E, Pablo LE, Dolz I, Fernandez J, 2011. Risk factors for progressive axonal degeneration of the retinal nerve fibre layer in multiple sclerosis patients. Br. J. Ophthalmol 95 (11), 1577–1582. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin E, Pueyo V, Martin J, Almarcegui C, Ara JR, Dolz I, Honrubia FM, Fernandez FJ, 2010. Progressive changes in the retinal nerve fiber layer in patients with multiple sclerosis. Eur. J. Ophthalmol 20 (1), 167–173. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin E, Rodriguez.Mena D, Herrero R, Almarcegui C, Dolz I, Martin J, Ara JR, Larrosa JM, Polo V, Fernández J, Pablo LE, 2013. Neuro-ophthalmologic evaluation, quality of life, and functional disability in patients with MS. Neurology 81 (1), 76–83. [DOI] [PubMed] [Google Scholar]
- Gatto E, Parisi V, Persi G, Fernandez Rey E, Cesarini M, Luis Etcheverry J, Rivera P, Squitieri F, 2018. Optical coherence tomography (OCT) study in Argentinean Huntington’s disease patients. Int. J. Neurosci 128 (12), 1157–1162. [DOI] [PubMed] [Google Scholar]
- Gavaghan KA, Peterhans M, Oliveira-Santos T, Weber S, 2011. A portable image overlay projection device for computer-aided open liver surgery. IEEE Trans. Biomed. Eng 58 (6), 1855–1864. [DOI] [PubMed] [Google Scholar]
- Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ, 2012. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain 135 (Pt 6), 1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbiya M, Trebbastoni A, Parisi F, Manganiello S, Cruciani F, D’Antonio F, De Vico U, Imbriano L, Campanelli A, De Lena C, 2014. Choroidal thinning as a new finding in Alzheimer’s disease: evidence from enhanced depth imaging spectral domain optical coherence tomography. J. Alzheimers Dis 40 (4), 907–917. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Bodis-Wollner I, Onofrj MC, Marx MS, Glover AA, 1988a. Spatial frequency-dependent abnormalities of the pattern electroretinogram and visual evoked potentials in a parkinsonian monkey model. Brain 111 (Pt 1), 131–149. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Chung E, Bodis-Wollner I, Dvorzniak M, Glover A, Onofrj M, 1988b. Systemic 1-methyl,4-phenyl,1-2-3-6-tetrahydropyridine (MPTP) administration decreases retinal dopamine content in primates. Life Sci. 43 (3), 255–262. [DOI] [PubMed] [Google Scholar]
- Giaschi D, Lang A, Regan D, 1997. Reversible dissociation of sensitivity to dynamic stimuli in Parkinson’s disease: is magnocellular function essential to reading motion-defined letters? Vis. Res 37 (24), 3531–3534. [DOI] [PubMed] [Google Scholar]
- Gibson E, Young M, Sarunic MV, Beg MF, 2010. Optic nerve head registration via hemispherical surface and volume registration. IEEE Trans. Biomed. Eng 57 (10), 2592–2595. [DOI] [PubMed] [Google Scholar]
- Gilmore GC, Spinks RA, Thomas CW, 2006. Age effects in coding tasks: componential analysis and test of the sensory deficit hypothesis. Psychol. Aging 21 (1), 7–18. [DOI] [PubMed] [Google Scholar]
- Gobron C, Erginay A, Massin P, Lutz G, Tessier N, Vicaut E, Chabriat H, 2014. Microvascular retinal abnormalities in acute intracerebral haemorrhage and lacunar infarction. Rev. Neurol. (Paris) 170 (1), 13–18. [DOI] [PubMed] [Google Scholar]
- González-López JJ, Rebolleda G, Leal M, Oblanca N, Muñoz.Negrete FJ, Costa-Frossard L, Alvarez-Cermeño JC, 2014. Comparative diagnostic accuracy of ganglion cell-inner plexiform and retinal nerve fiber layer thickness measures by Cirrus and Spectralis optical coherence tomography in relapsing-remitting multiple sclerosis. BioMed Res. Int 2014, 128517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ, Frohman EM, Cutter G, Calabresi PA, 2007. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 69 (16), 1603–1609. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Pantoni L, 2013. Advances in vascular cognitive impairment. Stroke 44 (2), 307–308. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S, C. o. E. a. P. American Heart Association Stroke Council, Council on Cardiovascular Nursing, C.uncil on Cardiovascular Radiology and Intervention, Council on Cardiovascular Surgery and Anesthesia, 2011. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42 (9), 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Miki A, Yamashita T, Araki S, Takizawa G, Nakagawa M, Ieki Y, Kiryu J, 2016. Sectoral analysis of the retinal nerve fiber layer thinning and its association with visual field loss in homonymous hemianopia caused by post-geniculate lesions using spectral-domain optical coherence tomography. Graefes Arch. Clin. Exp. Ophthalmol 254 (4), 745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R, 2010. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain 133 (Pt 6), 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ, 2020. Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat. Rev. Neurol 16 (1), 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg R, McCall M, Massey S, 2013. Function and Anatomy of the Mammalian Retina. Elsevier, New York. [Google Scholar]
- Group, O.N.S., 2008. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch. Neurol 65 (6), 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulmez Sevim D, Unlu M, Gultekin M, Karaca C, 2019. Retinal single-layer analysis with optical coherence tomography shows inner retinal layer thinning in Huntington’s disease as a potential biomarker. Int. Ophthalmol 39 (3), 611–621. [DOI] [PubMed] [Google Scholar]
- Gupta VB, Chitranshi N, den Haan J, Mirzaei M, You Y, Lim JK, Basavarajappa D, Godinez A, Di Angelantonio S, Sachdev P, Salekdeh GH, Bouwman F, Graham S, Gupta V, 2020. Retinal changes in Alzheimer’s disease-integrated prospects of imaging, functional and molecular advances. Prog. Retin. Eye Res 100899. [DOI] [PubMed] [Google Scholar]
- Guzmán-Vélez E, Jaimes S, Aguirre-Acevedo DC, Norton DJ, Papp KV, Amariglio R, Rentz D, Baena A, Henao E, Tirado V, Muñoz C, Giraldo M, Sperling RA, Lopera F, Quiroz YT, 2018. A three-factor structure of cognitive functioning among unimpaired carriers and non-carriers of autosomal-dominant Alzheimer’s disease. J. Alzheimers Dis 65 (1), 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan JD, van de Kreeke JA, Konijnenberg E, Kate MT, Braber AD, Barkhof F, van Berckel BN, Teunissen CE, Scheltens P, Visser PJ, Verbraak FD, Bouwman FH, 2019a. Retinal thickness as a potential biomarker in patients with amyloid-proven early- and late-onset Alzheimer’s disease. Alzheimers Dement. (Amst.) 11, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan JD, van de Kreeke JA, van Berckel BN, Barkhof F, Teunissen CE, Scheltens P, Verbraak FD, Bouwman FH, 2019b. Is retinal vasculature a biomarker in amyloid proven Alzheimer’s disease? Alzheimers Dement. (Amst.) 11, 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadoux X, Hui F, Lim JKH, Masters CL, Pébay A, Chevalier S, Ha J, Loi S, Fowler CJ, Rowe C, Villemagne VL, Taylor EN, Fluke C, Soucy JP, Lesage F, Sylvestre JP, Rosa-Neto P, Mathotaarachchi S, Gauthier S, Nasreddine ZS, Arbour JD, Rhéaume MA, Beaulieu S, Dirani M, Nguyen CTO, Bui BV, Williamson R, Crowston JG, van Wijngaarden P, 2019. Non-invasive in vivo hyperspectral imaging of the retina for potential biomarker use in Alzheimer’s disease. Nat. Commun 10 (1), 4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, Bodis-Wollner IG, 2009. Inner retinal layer thinning in Parkinson disease. Arch. Ophthalmol 127 (6), 737–741. [DOI] [PubMed] [Google Scholar]
- Halliday AM, McDonald WI, Mushin J, 1972. Delayed visual evoked response in optic neuritis. Lancet 1 (7758), 982–985. [DOI] [PubMed] [Google Scholar]
- Halliday AM, McDonald WI, Mushin J, 1973. Visual evoked response in diagnosis of multiple sclerosis. Br. Med. J 4 (5893), 661–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff TC, Sharrett AR, Mosley TH, Shibata D, Knopman DS, Klein R, Klein BE, Gottesman RF, 2014. Retinal microvascular abnormalities predict progression of brain microvascular disease: an atherosclerosis risk in communities magnetic resonance imaging study. Stroke 45 (4), 1012–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haritoglou C, Rudolph G, Hoops JP, Opherk C, Kampik A, Dichgans M, 2004. Retinal vascular abnormalities in CADASIL. Neurology 62 (7), 1202–1205. [DOI] [PubMed] [Google Scholar]
- Harnois C, Di Paolo T, 1990. Decreased dopamine in the retinas of patients with Parkinson’s disease. Invest. Ophthalmol. Vis. Sci 31 (11), 2473–2475. [PubMed] [Google Scholar]
- Hasanov S, Demirkilinc Biler E, Acarer A, Akkin C, Colakoglu Z, Uretmen O, 2019. Functional and morphological assessment of ocular structures and follow-up of patients with early-stage Parkinson’s disease. Int. Ophthalmol 39 (6), 1255–1262. [DOI] [PubMed] [Google Scholar]
- He Y, Carass A, Liu Y, Jedynak BM, Solomon SD, Saidha S, Calabresi PA, Prince JL, 2019. Deep learning based topology guaranteed surface and MME segmentation of multiple sclerosis subjects from retinal OCT. Biomed. Optic Express 10 (10), 5042–5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmlinger D, Yvert G, Picaud S, Merienne K, Sahel J, Mandel JL, Devys D, 2002. Progressive retinal degeneration and dysfunction in R6 Huntington’s disease mice. Hum. Mol. Genet 11 (26), 3351–3359. [DOI] [PubMed] [Google Scholar]
- Henderson AD, Bruce BB, Newman NJ, Biousse V, 2011. Hypertension-related eye abnormalities and the risk of stroke. Rev. Neurol. Dis 8 (1–2), 1–9. [PMC free article] [PubMed] [Google Scholar]
- Henderson AP, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT, Miller DH, 2010. A preliminary longitudinal study of the retinal nerve fiber layer in progressive multiple sclerosis. J. Neurol 257 (7), 1083–1091. [DOI] [PubMed] [Google Scholar]
- Hickman SJ, Brierley CM, Brex PA, MacManus DG, Scolding NJ, Compston DA, Miller DH, 2002. Continuing optic nerve atrophy following optic neuritis: a serial MRI study. Mult. Scler 8 (4), 339–342. [DOI] [PubMed] [Google Scholar]
- Hickman SJ, Toosy AT, Jones SJ, Altmann DR, Miszkiel KA, MacManus DG, Barker GJ, Plant GT, Thompson AJ, Miller DH, 2004. A serial MRI study following optic nerve mean area in acute optic neuritis. Brain 127 (Pt 11), 2498–2505. [DOI] [PubMed] [Google Scholar]
- Hinton DR, Sadun AA, Blanks JC, Miller CA, 1986. Optic-nerve degeneration in Alzheimer’s disease. N. Engl. J. Med 315 (8), 485–487. [DOI] [PubMed] [Google Scholar]
- Hiroki M, Miyashita K, Yoshida H, Hirai S, Fukuyama H, 2003. Central retinal artery Doppler flow parameters reflect the severity of cerebral small-vessel disease. Stroke 34 (7), e92–94. [DOI] [PubMed] [Google Scholar]
- Ho CY, Troncoso JC, Knox D, Stark W, Eberhart CG, 2014. Beta-amyloid, phospho-tau and alpha-synuclein deposits similar to those in the brain are not identified in the eyes of Alzheimer’s and Parkinson’s disease patients. Brain Pathol. 24 (1), 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh Kam J, Lenassi E, Jeffery G, 2010. Viewing ageing eyes: diverse sites of amyloid Beta accumulation in the ageing mouse retina and the up-regulation of macrophages. PloS One 5 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S, 2016. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 123 (5), 1036–1042. [DOI] [PubMed] [Google Scholar]
- Holmen IC, Konda MS, Pak JW, McDaniel KW, Blodi B, Stepien KE, Domalpally A, 2020. February 1. Prevalence and severity of artifacts in optical coherence tomographic angiograms. JAMA Ophthalmol. 138 (2), 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland D, Ellederova Z, Aronin N, Fernau D, Gallagher J, Taylor A, Hennebold J, Weiss AR, Gray-Edwards H, McBride J, 2020. Large animal models of Huntington’s disease: what we have learned and where we need to go next. J. Huntingtons Dis 9 (3), 201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Gottlieb CB, Barajas DJ, Barnett CJ, Schoenholz T, Sadda SR, 2015. Improved repeatability of retinal thickness measurements using line-scan ophthalmoscope image-based retinal tracking. Ophthalmic Surg. Lasers Imag. Retina 46 (3), 310–314. [DOI] [PubMed] [Google Scholar]
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, 1991. Optical coherence tomography. Science 254 (5035), 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Li Y, Xiao J, Zhang Q, Xu G, Wu G, Liu T, Luo W, 2018. Combination of multifocal electroretinogram and spectral-domain OCT can increase diagnostic efficacy of Parkinson’s disease. Park. Dis 2018, 4163239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton JT, Morris JL, Elias JW, 1993. Levodopa improves spatial contrast sensitivity in Parkinson’s disease. Arch. Neurol 50 (7), 721–724. [DOI] [PubMed] [Google Scholar]
- Iadecola C, 2013. The pathobiology of vascular dementia. Neuron 80 (4), 844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima A, Haida M, Ishikawa N, Ueno A, Minamitani H, Shinohara Y, 2003. Re-evaluation of tropicamide in the pupillary response test for Alzheimer’s disease. Neurobiol. Aging 24 (6), 789–796. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Head GM, Ellis CJ, 1994. Electrophysiological signs of retinal dopamine deficiency in recently diagnosed Parkinson’s disease and a follow up study. Vis. Res 34 (19), 2629–2638. [DOI] [PubMed] [Google Scholar]
- Ikram MK, De Jong FJ, Van Dijk EJ, Prins ND, Hofman A, Breteler MM, De Jong PT, 2006. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain 129 (Pt 1), 182–188. [DOI] [PubMed] [Google Scholar]
- Ikuta F, Zimmerman HM, 1976. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology 26 (6 PT 2), 26–28. [DOI] [PubMed] [Google Scholar]
- Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A, 2004. Retinal nerve fiber layer thinning in Parkinson disease. Vis. Res 44 (24), 2793–2797. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Cairns NJ, Grossman M, McMillan CT, Lee EB, Van Deerlin VM, Lee VM, Trojanowski JQ, 2015. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol. 129 (4), 469–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isas JM, Luibl V, Johnson LV, Kayed R, Wetzel R, Glabe CG, Langen R, Chen J, 2010. Soluble and mature amyloid fibrils in drusen deposits. Invest. Ophthalmol. Vis. Sci 51 (3), 1304–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH, 2011. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7 (3), 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors, 2018. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14 (4), 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaime GR, Kashani AH, Saati S, Martin G, Chader G, Humayun MS, 2012. Acute variations in retinal vascular oxygen content in a rabbit model of retinal venous occlusion. PloS One 7 (11), e50179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D, 2012. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Optic Express 20 (4), 4710–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian Z, Yu L, Rao B, Tromberg BJ, Chen Z, 2010. Three-dimensional speckle suppression in Optical Coherence Tomography based on the curvelet transform. Optic Express 18 (2), 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian Z, Yu Z, Yu L, Rao B, Chen Z, Tromberg BJ, 2009. Speckle attenuation in optical coherence tomography by curvelet shrinkage. Opt. Lett 34 (10), 1516–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Delgado S, Tan J, Liu C, Rammohan KW, DeBuc DC, Lam BL, Feuer WJ, Wang J, 2016. Impaired retinal microcirculation in multiple sclerosis. Mult. Scler 22 (14), 1812–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Liu Y, Wei Y, Shi Y, Wright CB, Sun X, Rundek T, Baumel BS, Landman J, Wang J, 2018a. Impaired retinal microcirculation in patients with Alzheimer’s disease. PloS One 13 (2), e0192154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wei Y, Shi Y, Wright CB, Sun X, Gregori G, Zheng F, Vanner EA, Lam BL, Rundek T, Wang J, 2018b. Altered macular microvasculature in mild cognitive impairment and Alzheimer disease. J. Neuro Ophthalmol 38 (3), 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez B, Ascaso FJ, Cristóbal JA, López del Val J, 2014. Development of a prediction formula of Parkinson disease severity by optical coherence tomography. Mov. Disord 29 (1), 68–74. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Wang YX, Wei WB, Zhu LP, Shao L, Xu L, 2016. Cognitive function and subfoveal choroidal thickness: the Beijing eye study. Ophthalmology 123 (1), 220–222. [DOI] [PubMed] [Google Scholar]
- Josiassen RC, Shagass C, Mancall EL, Roemer RA, 1984. Auditory and visual evoked potentials in Huntington’s disease. Electroencephalogr. Clin. Neurophysiol 57 (2), 113–118. [DOI] [PubMed] [Google Scholar]
- Jung NY, Han JC, Ong YT, Cheung CY, Chen CP, Wong TY, Kim HJ, Kim YJ, Lee J, Lee JS, Jang YK, Kee C, Lee KH, Kim EJ, Seo SW, Na DL, 2019. Retinal microvasculature changes in amyloid-negative subcortical vascular cognitive impairment compared to amyloid-positive Alzheimer’s disease. J. Neurol. Sci 396, 94–101. [DOI] [PubMed] [Google Scholar]
- Kanamori A, Escano MF, Eno A, Nakamura M, Maeda H, Seya R, Ishibashi K, Negi A, 2003. Evaluation of the effect of aging on retinal nerve fiber layer thickness measured by optical coherence tomography. Ophthalmologica 217 (4), 273–278. [DOI] [PubMed] [Google Scholar]
- Kardon RH, 1998. Drop the Alzheimer’s drop test. Neurology 50 (3), 588–591. [DOI] [PubMed] [Google Scholar]
- Kashani AH, Brown KT, Chang E, Drenser KA, Capone A, Trese MT, 2014a. Diversity of retinal vascular anomalies in patients with familial exudative vitreoretinopathy. Ophthalmology 121 (11), 2220–2227. [DOI] [PubMed] [Google Scholar]
- Kashani AH, Learned D, Nudleman E, Drenser KA, Capone A, Trese MT, 2014b. High prevalence of peripheral retinal vascular anomalies in family members of patients with familial exudative vitreoretinopathy. Ophthalmology 121 (1), 262–268. [DOI] [PubMed] [Google Scholar]
- Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, Shi Y, Wang RK, 2017. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog. Retin. Eye Res 60, 66–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani AH, Kirkman E, Martin G, Humayun MS, 2011. Hyperspectral computed tomographic imaging spectroscopy of vascular oxygen gradients in the rabbit retina in vivo. PloS One 6 (9), e24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani AH, Lee SY, Moshfeghi A, Durbin MK, Puliafito CA, 2015. Optical coherence tomography angiography OF retinal venous occlusion. Retina 35 (11), 2323–2331. [DOI] [PubMed] [Google Scholar]
- Kashani AH, Zimmer-Galler IE, Shah SM, Dustin L, Do DV, Eliott D, Haller JA, Nguyen QD, 2010. Retinal thickness analysis by race, gender, and age using Stratus OCT. Am. J. Ophthalmol 149 (3), 496–502 e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Rimmer S, 1989. Ophthalmologic manifestations of Alzheimer’s disease. Surv. Ophthalmol 34 (1), 31–43. [DOI] [PubMed] [Google Scholar]
- Kaur M, Saxena R, Singh D, Behari M, Sharma P, Menon V, 2015. Correlation between structural and functional retinal changes in Parkinson disease. J. Neuro Ophthalmol 35 (3), 254–258. [DOI] [PubMed] [Google Scholar]
- Kellner U, Kellner S, Weinitz S, 2010. Fundus autofluorescence (488 NM) and near-infrared autofluorescence (787 NM) visualize different retinal pigment epithelium alterations in patients with age-related macular degeneration. Retina 30 (1), 6–15. [DOI] [PubMed] [Google Scholar]
- Kerrison JB, Flynn T, Green WR, 1994. Retinal pathologic changes in multiple sclerosis. Retina 14 (5), 445–451. [DOI] [PubMed] [Google Scholar]
- Kersten HM, Danesh-Meyer HV, Kilfoyle DH, Roxburgh RH, 2015. Optical coherence tomography findings in Huntington’s disease: a potential biomarker of disease progression. J. Neurol 262 (11), 2457–2465. [DOI] [PubMed] [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG, 2005. The evolution and pathology of frontotemporal dementia. Brain 128 (Pt 9), 1996–2005. [DOI] [PubMed] [Google Scholar]
- Kesler A, Vakhapova V, Korczyn AD, Naftaliev E, Neudorfer M, 2011. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin. Neurol. Neurosurg 113 (7), 523–526. [DOI] [PubMed] [Google Scholar]
- Khansari MM, Zhang J, Qiao Y, Gahm JK, Sarabi MS, Kashani AH, Shi Y, 2020. Automated deformation-based analysis of 3D optical coherence tomography in diabetic retinopathy. IEEE Trans. Med. Imag 39 (1), 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH, 2016a. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest. Ophthalmol. Vis. Sci 57 (9), OCT362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AY, Rodger DC, Shahidzadeh A, Chu Z, Koulisis N, Burkemper B, Jiang X, Pepple KL, Wang RK, Puliafito CA, Rao NA, Kashani AH, 2016b. Quantifying retinal microvascular changes in uveitis using spectral domain optical coherence tomography angiography (SD-OCTA). Am. J. Ophthalmol 171, 101–112. 10.1016/j.ajo.2016.08.035. Epub 2016 Sep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Grossman M, Song D, Saludades S, Pan W, Dominguez-Perez S, Dunaief JL, Aleman TS, Ying GS, Irwin DJ, 2019a. Persistent and progressive outer retina thinning in frontotemporal degeneration. Front. Neurosci 13, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Jeong HY, Cho KH, Oh SW, Byun SJ, Woo SJ, Yang HK, Hwang JM, Park KH, Kim CK, Park SJ, 2019b. Nonarteritic anterior ischemic optic neuropathy is associated with cerebral small vessel disease. PloS One 14 (11), e0225322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Irwin DJ, Song D, Daniel E, Leveque JD, Raquib AR, Pan W, Ying GS, Aleman TS, Dunaief JL, Grossman M, 2017. Optical coherence tomography identifies outer retina thinning in frontotemporal degeneration. Neurology 89 (15), 1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Park KH, Kwon JW, Jeoung JW, Kim TW, Kim DM, 2011. Retinal nerve fiber layer defect and cerebral small vessel disease. Invest. Ophthalmol. Vis. Sci 52 (9), 6882–6886. [DOI] [PubMed] [Google Scholar]
- Kirbas S, Turkyilmaz K, Tufekci A, Durmus M, 2013. Retinal nerve fiber layer thickness in Parkinson disease. J. Neuro Ophthalmol 33 (1), 62–65. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B, 2004. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol 55 (3), 306–319. [DOI] [PubMed] [Google Scholar]
- Knapp J, VanNasdale DA, Ramsey K, Racine J, 2018. Retinal dysfunction in a presymptomatic patient with Huntington’s disease. Doc. Ophthalmol 136 (3), 213–221. [DOI] [PubMed] [Google Scholar]
- Knier B, Schmidt P, Aly L, Buck D, Berthele A, Mühlau M, Zimmer C, Hemmer B, Korn T, 2016. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain 139 (11), 2855–2863. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Boeve BF, Parisi JE, Dickson DW, Smith GE, Ivnik RJ, Josephs KA, Petersen RC, 2005. Antemortem diagnosis of frontotemporal lobar degeneration. Ann. Neurol 57 (4), 480–488. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Roberts RO, 2011. Estimating the number of persons with frontotemporal lobar degeneration in the US population. J. Mol. Neurosci 45 (3), 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko F, Muthy ZA, Gallacher J, Sudlow C, Rees G, Yang Q, Keane PA, Petzold A, Khaw PT, Reisman C, Strouthidis NG, Foster PJ, Patel PJ, U. B. E. V. Consortium, 2018. Association of retinal nerve fiber layer thinning with current and future cognitive decline: a study using optical coherence tomography. JAMA Neurol. 75 (10), 1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronyo Y, Biggs D, Barron E, Boyer DS, Pearlman JA, Au WJ, Kile SJ, Blanco A, Fuchs DT, Ashfaq A, Frautschy S, Cole GM, Miller CA, Hinton DR, Verdooner SR, Black KL, Koronyo-Hamaoui M, 2017. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2 (16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, Schwartz M, Farkas DL, 2011. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 54 (Suppl. 1), S204–S217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulisis N, Kim AY, Chu Z, Shahidzadeh A, Burkemper B, Olmos de Koo LC, Moshfeghi AA, Ameri H, Puliafito CA, Isozaki VL, Wang RK, Kashani AH, 2017. Quantitative microvascular analysis of retinal venous occlusions by spectral domain optical coherence tomography angiography. PloS One 12 (4), e0176404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasodomska K, Lubiński W, Potemkowski A, Honczarenko K, 2010. Pattern electroretinogram (PERG) and pattern visual evoked potential (PVEP) in the early stages of Alzheimer’s disease. Doc. Ophthalmol 121 (2), 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MF, Potsaid B, Mayer MA, Bock R, Baumann B, Liu JJ, Hornegger J, Fujimoto JG, 2012. Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed. Optic Express 3 (6), 1182–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner-Lenhoff S, Ashimatey BS, Kashani AH, 2020. Retinal vascular reactivity as assessed by optical coherence tomography angiography. JoVE 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H, 2005. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128 (Pt 11), 2705–2712. [DOI] [PubMed] [Google Scholar]
- Kwa VI, van der Sande JJ, Stam J, Tijmes N, Vrooland JL, Group, A.V.M., 2002. Retinal arterial changes correlate with cerebral small-vessel disease. Neurology 59 (10), 1536–1540. [DOI] [PubMed] [Google Scholar]
- Kwapong WR, Ye H, Peng C, Zhuang X, Wang J, Shen M, Lu F, 2018. Retinal microvascular impairment in the early stages of Parkinson’s disease. Invest. Ophthalmol. Vis. Sci 59 (10), 4115–4122. [DOI] [PubMed] [Google Scholar]
- La Morgia C, Barboni P, Rizzo G, Carbonelli M, Savini G, Scaglione C, Capellari S, Bonazza S, Giannoccaro MP, Calandra-Buonaura G, Liguori R, Cortelli P, Martinelli P, Baruzzi A, Carelli V, 2013. Loss of temporal retinal nerve fibers in Parkinson disease: a mitochondrial pattern? Eur. J. Neurol 20 (1), 198–201. [DOI] [PubMed] [Google Scholar]
- La Morgia C, Di Vito L, Carelli V, Carbonelli M, 2017. Patterns of retinal ganglion cell damage in neurodegenerative disorders: parvocellular vs magnocellular degeneration in optical coherence tomography studies. Front. Neurol 8, 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Morgia C, Ross-Cisneros FN, Koronyo Y, Hannibal J, Gallassi R, Cantalupo G, Sambati L, Pan BX, Tozer KR, Barboni P, Provini F, Avanzini P, Carbonelli M, Pelosi A, Chui H, Liguori R, Baruzzi A, Koronyo-Hamaoui M, Sadun AA, Carelli V, 2016. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann. Neurol 79 (1), 90–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowicz E, Lubiński W, 2018. The importance of the electrophysiological tests in the early diagnosis of ganglion cells and/or optic nerve dysfunction coexisting with pituitary adenoma: an overview. Doc. Ophthalmol 137 (3), 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad EM, Mukherjee D, Stinnett SS, Cousins SW, Potter GG, Burke JR, Farsiu S, Whitson HE, 2018. Evaluation of inner retinal layers as biomarkers in mild cognitive impairment to moderate Alzheimer’s disease. PloS One 13 (2), e0192646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe J, Saidha S, Bermel RA, 2020. Optical coherence tomography and multiple sclerosis: update on clinical application and role in clinical trials. Mult. Scler 26 (6), 624–639. [DOI] [PubMed] [Google Scholar]
- Law M, Saindane AM, Ge Y, Babb JS, Johnson G, Mannon LJ, Herbert J, Grossman RI, 2004. Microvascular abnormality in relapsing-remitting multiple sclerosis: perfusion MR imaging findings in normal-appearing white matter. Radiology 231 (3), 645–652. [DOI] [PubMed] [Google Scholar]
- Law MWK, Chung ACS, 2008. Three Dimensional Curvilinear Structure Detection Using Optimally Oriented Flux. Springer Berlin Heidelberg, Berlin, Heidelberg. [Google Scholar]
- Lee JY, Kim JM, Ahn J, Kim HJ, Jeon BS, Kim TW, 2014. Retinal nerve fiber layer thickness and visual hallucinations in Parkinson’s Disease. Mov. Disord 29 (1), 61–67. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Abraham AG, Swenor BK, Sharrett AR, Ramulu PY, 2018. Application of optical coherence tomography in the detection and classification of cognitive decline. J. Curr. Glaucoma Pract 12 (1), 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lebed E, Sarunic MV, Beg MF, 2015. Exact surface registration of retinal surfaces from 3-D optical coherence tomography images. IEEE Trans. Biomed. Eng 62 (2), 609–617. [DOI] [PubMed] [Google Scholar]
- Leger F, Fernagut PO, Canron MH, Léoni S, Vital C, Tison F, Bezard E, Vital A, 2011. Protein aggregation in the aging retina. J. Neuropathol. Exp. Neurol 70 (1), 63–68. [DOI] [PubMed] [Google Scholar]
- Leuba G, Saini K, 1995. Pathology of subcortical visual centres in relation to cortical degeneration in Alzheimer’s disease. Neuropathol. Appl. Neurobiol 21 (5), 410–422. [DOI] [PubMed] [Google Scholar]
- Liew G, Baker ML, Wong TY, Hand PJ, Wang JJ, Mitchell P, De Silva DA, Wong MC, Rochtchina E, Lindley RI, Wardlaw JM, Hankey GJ, M.-C. R. S. S. Group, 2014. Differing associations of white matter lesions and lacunar infarction with retinal microvascular signs. Int. J. Stroke 9 (7), 921–925. [DOI] [PubMed] [Google Scholar]
- Lim JKH, Li QX, He Z, Vingrys AJ, Chinnery HR, Mullen J, Bui BV, Nguyen CTO, 2020. Retinal functional and structural changes in the 5xFAD mouse model of Alzheimer’s disease. Front. Neurosci 14, 862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley RI, Wang JJ, Wong MC, Mitchell P, Liew G, Hand P, Wardlaw J, De Silva DA, Baker M, Rochtchina E, Chen C, Hankey GJ, Chang HM, Fung VS, Gomes L, Wong TY, M.-C. R. a. S. S. M. C. Group, 2009. Retinal microvasculature in acute lacunar stroke: a cross-sectional study. Lancet Neurol. 8 (7), 628–634. [DOI] [PubMed] [Google Scholar]
- Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez-Oroz MC, Tröster AI, Weintraub D, 2011. MDS Task Force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov. Disord 26 (10), 1814–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Carass A, Filippatou A, He Y, Solomon SD, Saidha S, Calabresi PA, Prince JL, F. 2, IEEE, 2019a. Projection artifact suppression for inner retina IN OCT angiography. In: 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019). IEEE, Venice, Italy, Italy, pp. 592–596. [Google Scholar]
- Liu Y, Delgado S, Jiang H, Lin Y, Hernandez J, Deng Y, Gameiro GR, Wang J, 2019b. Retinal tissue perfusion in patients with multiple sclerosis. Curr. Eye Res 44 (10), 1091–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanas S, Linderman RE, Chen FK, Carroll J, 2019. Assessing the use of incorrectly scaled optical coherence tomography angiography images in peer-reviewed studies: a systematic review. JAMA Ophthalmol, 10.1001/jamaophthalmol.2019.4821. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Longstreth W, Larsen EK, Klein R, Wong TY, Sharrett AR, Lefkowitz D, Manolio TA, 2007. Associations between findings on cranial magnetic resonance imaging and retinal photography in the elderly: the Cardiovascular Health Study. Am. J. Epidemiol 165 (1), 78–84. [DOI] [PubMed] [Google Scholar]
- Luan F, Wu Y, 2013. Application of RPCA in optical coherence tomography for speckle noise reduction. Laser Phys. Lett 10 (3), 035603. [Google Scholar]
- Lynch SK, Abràmoff MD, 2017. Diabetic retinopathy is a neurodegenerative disorder. Vis. Res 139, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-de-Eguileta A, Lage C, López-García S, Pozueta A, García-Martínez M, Kazimierczak M, Bravo M, de Arcocha-Torres M, Banzo I, Jimenez-Bonilla J, Cerveró A, Goikoetxea A, Rodríguez-Rodríguez E, Sànchez-Juan P, Casado A, 2020. Evaluation of choroidal thickness in prodromal Alzheimer’s disease defined by amyloid PET. PloS One 15 (9), e0239484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler KU, Edward DP, Tso MO, 1995. Immunoreactivity against tau, amyloid precursor protein, and beta-amyloid in the human retina. Invest. Ophthalmol. Vis. Sci 36 (1), 24–31. [PubMed] [Google Scholar]
- Mailankody P, Battu R, Khanna A, Lenka A, Yadav R, Pal PK, 2015. Optical coherence tomography as a tool to evaluate retinal changes in Parkinson’s disease. Park. Relat. Disord 21 (10), 1164–1169. [DOI] [PubMed] [Google Scholar]
- Marquié M, Valero S, Castilla-Marti M, Martínez J, Rodríguez-Gómez O, Sanabria Á, Tartari JP, Monté-Rubio GC, Sotolongo-Grau O, Alegret M, Pérez-Cordón A, Roberto N, de Rojas I, Moreno-Grau S, Montrreal L, Hernández I, Rosende-Roca M, Mauleón A, Vargas L, Abdelnour C, Gil S, Esteban-De Antonio E, Espinosa A, Ortega G, Lomeña F, Pavia J, Vivas A, Tejero M, Gómez-Chiari M, Simó R, Ciudin A, Hernández C, Orellana A, Benaque A, Ruiz A, Tárraga L, Boada M, group, F.s., 2020. Association between retinal thickness and β-amyloid brain accumulation in individuals with subjective cognitive decline: fundació ACE Healthy Brain Initiative. Alzheimer’s Res. Ther 12 (1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lapiscina EH, Arnow S, Wilson JA, Saidha S, Preiningerova JL, Oberwahrenbrock T, Brandt AU, Pablo LE, Guerrieri S, Gonzalez I, Outteryck O, Mueller AK, Albrecht P, Chan W, Lukas S, Balk LJ, Fraser C, Frederiksen JL, Resto J, Frohman T, Cordano C, Zubizarreta I, Andorra M, Sanchez-Dalmau B, Saiz A, Bermel R, Klistorner A, Petzold A, Schippling S, Costello F, Aktas O, Vermersch P, Oreja-Guevara C, Comi G, Leocani L, Garcia-Martin E, Paul F, Havrdova E, Frohman E, Balcer LJ, Green AJ, Calabresi PA, Villoslada P, consortium, I., 2016. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 15 (6), 574–584. [DOI] [PubMed] [Google Scholar]
- Marziani E, Pomati S, Ramolfo P, Cigada M, Giani A, Mariani C, Staurenghi G, 2013. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci 54 (9), 5953–5958. [DOI] [PubMed] [Google Scholar]
- Matej R, Tesar A, Rusina R, 2019. Alzheimer’s disease and other neurodegenerative dementias in comorbidity: a clinical and neuropathological overview. Clin. Biochem 73, 26–31. [DOI] [PubMed] [Google Scholar]
- Matsunaga D, Yi J, Puliafito CA, Kashani AH, 2014. OCT angiography in healthy human subjects. Ophthalmic Surg. Lasers Imag. Retina 45 (6), 510–515. [DOI] [PubMed] [Google Scholar]
- Matthews WB, Small DG, Small M, Pountney E, 1977. Pattern reversal evoked visual potential in the diagnosis of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 40 (10), 1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauschitz MM, Bonnemaijer PWM, Diers K, Rauscher FG, Elze T, Engel C, Loeffler M, Colijn JM, Ikram MA, Vingerling JR, Williams KM, Hammond CJ, Creuzot.Garcher C, Bron AM, Silva R, Nunes S, Delcourt C, Cougnard-Grégoire A, Holz FG, Klaver CCW, Breteler MMB, Finger RP, Consortium, E.E.E.E., 2018. Systemic and ocular determinants of peripapillary retinal nerve fiber layer thickness measurements in the European eye epidemiology (E3) population. Ophthalmology 125 (10), 1526–1536. [DOI] [PubMed] [Google Scholar]
- Mayer MA, Borsdorf A, Wagner M, Hornegger J, Mardin CY, Tornow RP, 2012. Wavelet denoising of multiframe optical coherence tomography data. Biomed. Optic Express 3 (3), 572–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrory S, Ballerini L, Doubal FN, Staals J, Allerhand M, Valdes-Hernandez MDC, Wang X, MacGillivray T, Doney ASF, Dhillon B, Starr JM, Bastin ME, Trucco E, Deary IJ, Wardlaw JM, 2019. Retinal microvasculature and cerebral small vessel disease in the Lothian nirth cohort 1936 and mild stroke study. Sci. Rep 9 (1), 6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM, 1984. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology 34 (7), 939–944. [DOI] [PubMed] [Google Scholar]
- Meier PG, Maeder P, Kardon RH, Borruat FX, 2015. Homonymous ganglion cell layer thinning after isolated occipital lesion: macular OCT demonstrates transsynaptic retrograde retinal degeneration. J. Neuro Ophthalmol 35 (2), 112–116. [DOI] [PubMed] [Google Scholar]
- Miller DH, Newton MR, van der Poel JC, du Boulay EP, Halliday AM, Kendall BE, Johnson G, MacManus DG, Moseley IF, McDonald WI, 1988. Magnetic resonance imaging of the optic nerve in optic neuritis. Neurology 38 (2), 175–179. [DOI] [PubMed] [Google Scholar]
- Miri S, Shrier EM, Glazman S, Ding Y, Selesnick I, Kozlowski PB, Bodis-Wollner I, 2015. The avascular zone and neuronal remodeling of the fovea in Parkinson disease. Ann. Clin. Transl. Neurol 2 (2), 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monet-Leprêtre M, Bardot B, Lemaire B, Domenga V, Godin O, Dichgans M, Tournier.Lasserve E, Cohen-Tannoudji M, Chabriat H, Joutel A, 2009. Distinct phenotypic and functional features of CADASIL mutations in the Notch3 ligand binding domain. Brain 132 (Pt 6), 1601–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Monsell SE, Beach TG, Bigio EH, Bu Y, Cairns NJ, Frosch M, Henriksen J, Kofler J, Kukull WA, Lee EB, Nelson PT, Schantz AM, Schneider JA, Sonnen JA, Trojanowski JQ, Vinters HV, Zhou XH, Hyman BT, 2016. Multisite assessment of NIA-AA guidelines for the neuropathologic evaluation of Alzheimer’s disease. Alzheimers Dement. 12 (2), 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordant DJ, Al-Abboud I, Muyo G, Gorman A, Sallam A, Ritchie P, Harvey AR, McNaught AI, 2011. Spectral imaging of the retina. Eye 25 (3), 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More SS, Beach JM, McClelland C, Mokhtarzadeh A, Vince R, 2019. In vivo assessment of retinal biomarkers by hyperspectral imaging: early detection of Alzheimer’s disease. ACS Chem. Neurosci 10 (11), 4492–4501. [DOI] [PubMed] [Google Scholar]
- Moschos MM, Chatziralli IP, 2018. Evaluation of choroidal and retinal thickness changes in Parkinson’s disease using spectral domain optical coherence tomography. Semin. Ophthalmol 33 (4), 494–497. [DOI] [PubMed] [Google Scholar]
- Moschos MM, Markopoulos I, Chatziralli I, Rouvas A, Papageorgiou SG, Ladas I, Vassilopoulos D, 2012. Structural and functional impairment of the retina and optic nerve in Alzheimer’s disease. Curr. Alzheimer Res 9 (7), 782–788. [DOI] [PubMed] [Google Scholar]
- Moschos MM, Tagaris G, Markopoulos I, Margetis I, Tsapakis S, Kanakis M, Koutsandrea C, 2011. Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur. J. Ophthalmol 21 (1), 24–29. [DOI] [PubMed] [Google Scholar]
- Moulder KL, Snider BJ, Mills SL, Buckles VD, Santacruz AM, Bateman RJ, Morris JC, 2013. Dominantly Inherited Alzheimer Network: facilitating research and clinical trials. Alzheimer’s Res. Ther 5 (5), 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MJ, Wei Z, Fatyga M, Williamson J, Anscher M, Wallace T, Weiss E, 2008. How does CT image noise affect 3D deformable image registration for image-guided radiotherapy planning? Med. Phys 35 (3), 1145–1153. [DOI] [PubMed] [Google Scholar]
- Murphy OC, Kwakyi O, Iftikhar M, Zafar S, Lambe J, Pellegrini N, Sotirchos ES, Gonzalez-Caldito N, Ogbuokiri E, Filippatou A, Risher H, Cowley N, Feldman S, Fioravante N, Frohman EM, Frohman TC, Balcer LJ, Prince JL, Channa R, Calabresi PA, Saidha S, 2020. Alterations in the retinal vasculature occur in multiple sclerosis and exhibit novel correlations with disability and visual function measures. Mult. Scler 26 (7), 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu U, Colijn JM, Ikram MA, Bonnemaijer PWM, Licher S, Wolters FJ, Tiemeier H, Koudstaal PJ, Klaver CCW, Ikram MK, 2018. Association of retinal neurodegeneration on optical coherence tomography with dementia: a population-based study. JAMA Neurol. 75 (10), 1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller AK, Blasberg C, Südmeyer M, Aktas O, Albrecht P, 2014. Photoreceptor layer thinning in parkinsonian syndromes. Mov. Disord 29 (9), 1222–1223. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden J, Mann D, 2005. Frontotemporal dementia. Lancet Neurol. 4 (11), 771–780. [DOI] [PubMed] [Google Scholar]
- Nelis P, Kleffner I, Burg MC, Clemens CR, Alnawaiseh M, Motte J, Marziniak M, Eter N, Alten F, 2018. OCT-Angiography reveals reduced vessel density in the deep retinal plexus of CADASIL patients. Sci. Rep 8 (1), 8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG, 2012. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol 71 (5), 362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Legros J, 1988. Functional neuroarchitecture of the retina: hypothesis on the dysfunction of retinal dopaminergic circuitry in Parkinson’s disease. Surg. Radiol. Anat 10 (2), 137–144. [DOI] [PubMed] [Google Scholar]
- Nightingale S, Mitchell KW, Howe JW, 1986. Visual evoked cortical potentials and pattern electroretinograms in Parkinson’s disease and control subjects. J. Neurol. Neurosurg. Psychiatry 49 (11), 1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan-Kenney RC, Liu M, Akhand O, Calabresi PA, Paul F, Petzold A, Balk L, Brandt AU, Martínez-Lapiscina EH, Saidha S, Villoslada P, Al-Hassan AA, Behbehani R, Frohman EM, Frohman T, Havla J, Hemmer B, Jiang H, Knier B, Korn T, Leocani L, Papadopoulou A, Pisa M, Zimmermann H, Galetta SL, Balcer LJ, I. M. S. V. S. Consortium, 2019. Optimal intereye difference thresholds by optical coherence tomography in multiple sclerosis: an international study. Ann. Neurol 85 (5), 618–629. [DOI] [PubMed] [Google Scholar]
- Nowacka B, Lubiński W, Honczarenko K, Potemkowski A, Safranow K, 2015. Bioelectrical function and structural assessment of the retina in patients with early stages of Parkinson’s disease (PD). Doc. Ophthalmol 131 (2), 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryhim BE, Apte RS, Kung N, Coble D, Van Stavern GP, 2018. Association of preclinical Alzheimer disease with optical coherence tomographic angiography findings. JAMA Ophthalmol. 136 (11), 1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obis J, Garcia-Martin E, Orduna E, Vilades E, Alarcia R, Rodrigo MJ, Pablo LE, Polo V, Larrosa JM, Satue M, 2020. Reproducibility of retinal and choroidal measurements using swept-source optical coherence tomography in patients with Parkinson’s disease. Arq. Bras. Oftalmol 83 (1), 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oepen G, Doerr M, Thoden U, 1981. Visual (VEP) and somatosensory (SSEP) evoked potentials in Huntington’s chorea. Electroencephalogr. Clin. Neurophysiol 51 (6), 666–670. [DOI] [PubMed] [Google Scholar]
- Ohno-Matsui K, 2011. Parallel findings in age-related macular degeneration and Alzheimer’s disease. Prog. Retin. Eye Res 30 (4), 217–238. [DOI] [PubMed] [Google Scholar]
- Oliveira FP, Tavares JM, 2014. Medical image registration: a review. Comput. Methods Biomech. Biomed. Eng 17 (2), 73–93. [DOI] [PubMed] [Google Scholar]
- Ong YT, De Silva DA, Cheung CY, Chang HM, Chen CP, Wong MC, Wong TY, Ikram MK, 2013. Microvascular structure and network in the retina of patients with ischemic stroke. Stroke 44 (8), 2121–2127. [DOI] [PubMed] [Google Scholar]
- Onofrj M, Ghilardi MF, Basciani M, Gambi D, 1986. Visual evoked potentials in parkinsonism and dopamine blockade reveal a stimulus-dependent dopamine function in humans. J. Neurol. Neurosurg. Psychiatry 49 (10), 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortuño-Lizarán I, Beach TG, Serrano GE, Walker DG, Adler CH, Cuenca N, 2018. Phosphorylated α-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov. Disord 33 (8), 1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu N, 1979. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern 9 (1), 62–66. [Google Scholar]
- Pantoni L, 2010. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9 (7), 689–701. [DOI] [PubMed] [Google Scholar]
- Panwar N, Huang P, Lee J, Keane PA, Chuan TS, Richhariya A, Teoh S, Lim TH, Agrawal R, 2016. Fundus photography in the 21st century–A review of recent technological advances and their implications for worldwide healthcare. Telemed. J. e Health 22 (3), 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet C, Boissonnot M, Roger F, Dighiero P, Gil R, Hugon J, 2007. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett 420 (2), 97–99. [DOI] [PubMed] [Google Scholar]
- Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, Bucci MG, Pierelli F, 1999. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest. Ophthalmol. Vis. Sci 40 (11), 2520–2527. [PubMed] [Google Scholar]
- Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F, 2001. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin. Neurophysiol 112 (10), 1860–1867. [DOI] [PubMed] [Google Scholar]
- Paulus W, Schwarz G, Werner A, Lange H, Bayer A, Hofschuster M, Müller N, Zrenner E, 1993. Impairment of retinal increment thresholds in Huntington’s disease. Ann. Neurol 34 (4), 574–578. [DOI] [PubMed] [Google Scholar]
- Pearl JR, Heath LM, Bergey DE, Kelly JP, Smith C, Laurino MY, Weiss A, Price ND, LaSpada A, Bird TD, Jayadev S, 2017. Enhanced retinal responses in Huntington’s disease patients. J. Huntingtons Dis 6 (3), 237–247. [DOI] [PubMed] [Google Scholar]
- Peca S, McCreary CR, Donaldson E, Kumarpillai G, Shobha N, Sanchez K, Charlton A, Steinback CD, Beaudin AE, Flück D, Pillay N, Fick GH, Poulin MJ, Frayne R, Goodyear BG, Smith EE, 2013. Neurovascular decoupling is associated with severity of cerebral amyloid angiopathy. Neurology 81 (19), 1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Trojanowski JQ, Lee VM, 2020. Protein transmission in neurodegenerative disease. Nat. Rev. Neurol 16 (4), 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppe A, Stanzione P, Pierelli F, De Angelis D, Pierantozzi M, Bernardi G, 1995. Visual alterations in de novo Parkinson’s disease: pattern electroretinogram latencies are more delayed and more reversible by levodopa than are visual evoked potentials. Neurology 45 (6), 1144–1148. [DOI] [PubMed] [Google Scholar]
- Petrasch-Parwez E, Saft C, Schlichting A, Andrich J, Napirei M, Arning L, Wieczorek S, Dermietzel R, Epplen JT, 2005. Is the retina affected in Huntington disease? Acta Neuropathol. 110 (5), 523–525. [DOI] [PubMed] [Google Scholar]
- Petzold A, Balcer LJ, Calabresi PA, Costello F, Frohman TC, Frohman EM, Martinez-Lapiscina EH, Green AJ, Kardon R, Outteryck O, Paul F, Schippling S, Vermersch P, Villoslada P, Balk LJ, Imsvisual E-E, 2017. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 16 (10), 797–812. [DOI] [PubMed] [Google Scholar]
- Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, Calabresi PA, Polman C, 2010. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 9 (9), 921–932. [DOI] [PubMed] [Google Scholar]
- Pham TQ, Kifley A, Mitchell P, Wang JJ, 2006. Relation of age-related macular degeneration and cognitive impairment in an older population. Gerontology 52 (6), 353–358. [DOI] [PubMed] [Google Scholar]
- Pilat A, McLean RJ, Proudlock FA, Maconachie GD, Sheth V, Rajabally YA, Gottlob I, 2016. In vivo morphology of the optic nerve and retina in patients with Parkinson’s disease. Invest. Ophthalmol. Vis. Sci 57 (10), 4420–4427. [DOI] [PubMed] [Google Scholar]
- Pillai JA, Bermel R, Bonner-Jackson A, Rae-Grant A, Fernandez H, Bena J, Jones SE, Ehlers JP, Leverenz JB, 2016. Retinal nerve fiber layer thinning in Alzheimer’s disease: a case-control study in comparison to normal aging, Parkinson’s disease, and non-Alzheimer’s dementia. Am. J. Alzheimers Dis. Other Demen 31 (5), 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingale T, Gupta GL, 2020. Classic and evolving animal models in Parkinson’s disease. Pharmacol. Biochem. Behav 199, 173060. [DOI] [PubMed] [Google Scholar]
- Polo V, Satue M, Rodrigo MJ, Otin S, Alarcia R, Bambo MP, Fuertes MI, Larrosa JM, Pablo LE, Garcia-Martin E, 2016. Visual dysfunction and its correlation with retinal changes in patients with Parkinson’s disease: an observational cross-sectional study. BMJ Open 6 (5), e009658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N, 2012. The incidence and prevalence of Huntington’s disease: a systematic review and meta-analysis. Mov. Disord 27 (9), 1083–1091. [DOI] [PubMed] [Google Scholar]
- Promjunyakul NO, Dodge HH, Lahna D, Boespflug EL, Kaye JA, Rooney WD, Silbert LC, 2018. Baseline NAWM structural integrity and CBF predict periventricular WMH expansion over time. Neurology 90 (24), e2119–e2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin T, Prins S, Groeneveld GJ, Van Westen G, de Vries HE, Wong YC, Bischoff LJM, de Lange ECM, 2020. Utility of animal models to understand human Alzheimer’s disease, using the mastermind research approach to avoid unnecessary further sacrifices of animals. Int. J. Mol. Sci 21 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M, Kwapong WR, Peng C, Cao Y, Lu F, Shen M, Han Z, 2020. Retinal sublayer defect is independently associated with the severity of hypertensive white matter hyperintensity. Brain Behav. 10 (2), e01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querques G, Borrelli E, Sacconi R, De Vitis L, Leocani L, Santangelo R, Magnani G, Comi G, Bandello F, 2019. Functional and morphological changes of the retinal vessels in Alzheimer’s disease and mild cognitive impairment. Sci. Rep 9 (1), 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, 2011. Glaucoma. Lancet 377 (9774), 1367–1377. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM, Green WR, 1982. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch. Ophthalmol 100 (1), 135–146. [DOI] [PubMed] [Google Scholar]
- Ragauskas S, Leinonen H, Puranen J, Rönkkö S, Nymark S, Gurevicius K, Lipponen A, Kontkanen O, Puoliväli J, Tanila H, Kalesnykas G, 2014. Early retinal function deficit without prominent morphological changes in the R6/2 mouse model of Huntington’s disease. PloS One 9 (12), e113317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravalico G, Toffoli G, Pastori G, Crocè M, Calderini S, 1996. Age-related ocular blood flow changes. Invest. Ophthalmol. Vis. Sci 37 (13), 2645–2650. [PubMed] [Google Scholar]
- Regan D, Neima D, 1984. Visual fatigue and visual evoked potentials in multiple sclerosis, glaucoma, ocular hypertension and Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 47 (7), 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA, 2018. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci. Biobehav. Rev 90, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef ER, Miller JB, Vavvas DG, 2020. Hyperspectral imaging of the retina: a review. Int. Ophthalmol. Clin 60 (1), 85–96. [DOI] [PubMed] [Google Scholar]
- Rimmer S, Iragui V, Klauber MR, Katz B, 1989. Retinocortical time exhibits spatial selectivity. Invest. Ophthalmol. Vis. Sci 30 (9), 2045–2049. [PubMed] [Google Scholar]
- Ringman JM, Goate A, Masters CL, Cairns NJ, Danek A, Graff-Radford N, Ghetti B, Morris JC, Network DIA, 2014. Genetic heterogeneity in Alzheimer disease and implications for treatment strategies. Curr. Neurol. Neurosci. Rep 14 (11), 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Younkin SG, Pratico D, Seltzer W, Cole GM, Geschwind DH, Rodriguez-Agudelo Y, Schaffer B, Fein J, Sokolow S, Rosario ER, Gylys KH, Varpetian A, Medina LD, Cummings JL, 2008. Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology 71 (2), 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo PA, Pierelli F, Pozzessere G, Morocutti C, 1980. Pattern visual evoked potentials in Huntington’s disease. Acta Neurol. 2 (2), 128–131. [PubMed] [Google Scholar]
- Rohani M, Langroodi AS, Ghourchian S, Falavarjani KG, SoUdi R, Shahidi G, 2013. Retinal nerve changes in patients with tremor dominant and akinetic rigid Parkinson’s disease. Neurol. Sci 34 (5), 689–693. [DOI] [PubMed] [Google Scholar]
- Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, Scahill RI, Leavitt BR, Stout JC, Paulsen JS, Reilmann R, Unschuld PG, Wexler A, Margolis RL, Tabrizi SJ, 2014. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat. Rev. Neurol 10 (4), 204–216. [DOI] [PubMed] [Google Scholar]
- Roth NM, Saidha S, Zimmermann H, Brandt AU, Isensee J, Benkhellouf-Rutkowska A, Dornauer M, Kühn AA, Müller T, Calabresi PA, Paul F, 2014. Photoreceptor layer thinning in idiopathic Parkinson’s disease. Mov. Disord 29 (9), 1163–1170. [DOI] [PubMed] [Google Scholar]
- Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, Goate A, Frommelt P, Ghetti B, Langbaum JB, Lopera F, Martins R, Masters CL, Mayeux RP, McDade E, Moreno S, Reiman EM, Ringman JM, Salloway S, Schofield PR, Sperling R, Tariot PN, Xiong C, Morris JC, Bateman RJ, Network DIA, 2014. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology 83 (3), 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P, Kalaria R, O’Brien J, Skoog I, Alladi S, Black SE, Blacker D, Blazer DG, Chen C, Chui H, Ganguli M, Jellinger K, Jeste DV, Pasquier F, Paulsen J, Prins N, Rockwood K, Roman G, Scheltens P, I. S. f. V. B. a. C. Disorders, 2014. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis. Assoc. Disord 28 (3), 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadda SR, Borrelli E, Fan W, Ebraheem A, Marion KM, Harrington M, Kwon S, 2019. A pilot study of fluorescence lifetime imaging ophthalmoscopy in preclinical Alzheimer’s disease. Eye 33 (8), 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadun AA, Bassi CJ, 1990. Optic nerve damage in Alzheimer’s disease. Ophthalmology 97 (1), 9–17. [DOI] [PubMed] [Google Scholar]
- Sadun AA, Borchert M, DeVita E, Hinton DR, Bassi CJ, 1987. Assessment of visual impairment in patients with Alzheimer’s disease. Am. J. Ophthalmol 104 (2), 113–120. [DOI] [PubMed] [Google Scholar]
- Saidha S, Naismith RT, 2019. Optical coherence tomography for diagnosing optic neuritis: are we there yet? Neurology 92 (6), 253–254. [DOI] [PubMed] [Google Scholar]
- Saidha S, Sotirchos ES, Ibrahim MA, Crainiceanu CM, Gelfand JM, Sepah YJ, Ratchford JN, Oh J, Seigo MA, Newsome SD, Balcer LJ, Frohman EM, Green AJ, Nguyen QD, Calabresi PA, 2012. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 11 (11), 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidha S, Sotirchos ES, Oh J, Syc SB, Seigo MA, Shiee N, Eckstein C, Durbin MK, Oakley JD, Meyer SA, Frohman TC, Newsome S, Ratchford JN, Balcer LJ, Pham DL, Crainiceanu CM, Frohman EM, Reich DS, Calabresi PA, 2013. Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol. 70 (1), 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salobrar-García E, de Hoz R, Ramírez AI, López-Cuenca I, Rojas P, Vazirani R, Amarante C, Yubero R, Gil P, Pinazo-Durán MD, Salazar JJ, Ramírez JM, 2019. Changes in visual function and retinal structure in the progression of Alzheimer’s disease. PloS One 14 (8), e0220535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo R, Huang SC, Bernasconi MP, Falautano M, Comi G, Magnani G, Leocani L, 2020. Neuro-retina might reflect Alzheimer’s disease stage. J. Alzheimers Dis 77 (4), 1455–1468. [DOI] [PubMed] [Google Scholar]
- Santos CY, Johnson LN, Sinoff SE, Festa EK, Heindel WC, Snyder PJ, 2018. Change in retinal structural anatomy during the preclinical stage of Alzheimer’s disease. Alzheimers Dement. (Amst.) 10, 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabi MS, Gahm JK, Khansari MM, Zhang J, Kashani AH, Shi Y, 2019. An Automated 3D Analysis Framework for Optical Coherence Tomography Angiography. bioRxiv, p. 655175. [Google Scholar]
- Sari ES, Koc R, Yazici A, Sahin G, Ermis SS, 2015. Ganglion cell-inner plexiform layer thickness in patients with Parkinson disease and association with disease severity and duration. J. Neuro Ophthalmol 35 (2), 117–121. [DOI] [PubMed] [Google Scholar]
- Sartucci F, Borghetti D, Bocci T, Murri L, Orsini P, Porciatti V, Origlia N, Domenici L, 2010. Dysfunction of the magnocellular stream in Alzheimer’s disease evaluated by pattern electroretinograms and visual evoked potentials. Brain Res. Bull 82 (3-4), 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Nakajima S, Shiraga N, Atsumi H, Yoshida S, Koller T, Gerig G, Kikinis R, 1998. Three-dimensional multi-scale line filter for segmentation and visualization of curvilinear structures in medical images. Med. Image Anal 2 (2), 143–168. [DOI] [PubMed] [Google Scholar]
- Satue M, Obis J, Rodrigo MJ, Otin S, Fuertes MI, Vilades E, Gracia H, Ara JR, Alarcia R, Polo V, Larrosa JM, Pablo LE, Garcia-Martin E, 2016. Optical coherence tomography as a biomarker for diagnosis, progression, and prognosis of neurodegenerative diseases. J. Ophthalmol 2016, 8503859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satue M, Rodrigo MJ, Obis J, Vilades E, Gracia H, Otin S, Fuertes MI, Alarcia R, Crespo JA, Polo V, Larrosa JM, Pablo LE, Garcia-Martin E, 2017. Evaluation of progressive visual dysfunction and retinal degeneration in patients with Parkinson’s disease. Invest. Ophthalmol. Vis. Sci 58 (2), 1151–1157. [DOI] [PubMed] [Google Scholar]
- Satue M, Seral M, Otin S, Alarcia R, Herrero R, Bambo MP, Fuertes MI, Pablo LE, Garcia-Martin E, 2014. Retinal thinning and correlation with functional disability in patients with Parkinson’s disease. Br. J. Ophthalmol 98 (3), 350–355. [DOI] [PubMed] [Google Scholar]
- Schiess N, Calabresi PA, 2016. Multiple sclerosis. Semin. Neurol 36 (4), 350–356. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA, 2009a. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J. Alzheimers Dis 18 (3), 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA, 2009b. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol 66 (2), 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA, 2007. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69 (24), 2197–2204. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA, 2004. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 62 (7), 1148–1155. [DOI] [PubMed] [Google Scholar]
- Schneider M, Müller HP, Lauda F, Tumani H, Ludolph AC, Kassubek J, Pinkhardt EH, 2014. Retinal single-layer analysis in Parkinsonian syndromes: an optical coherence tomography study. J. Neural. Transm 121 (1), 41–47. [DOI] [PubMed] [Google Scholar]
- Schultz N, Byman E, Wennström M, Bank NB, 2018. Levels of retinal LAPP are altered in Alzheimer’s disease patients and correlate with vascular changes and hippocampal IAPP levels. Neurobiol. Aging 69, 94–101. [DOI] [PubMed] [Google Scholar]
- Schwaber EJ, Thompson AC, Smilnak G, Stinnett SS, Whitson HE, Lad EM, 2020. Co-prevalence of Alzheimer’s disease and age-related macular degeneration established by histopathologic diagnosis. J. Alzheimers Dis 76 (1), 207–215. [DOI] [PubMed] [Google Scholar]
- Scinto LF, Daffner KR, Dressler D, Ransil BI, Rentz D, Weintraub S, Mesulam M, Potter H, 1994. A potential noninvasive neurobiological test for Alzheimer’s disease. Science 266 (5187), 1051–1054. [DOI] [PubMed] [Google Scholar]
- Sen S, Saxena R, Tripathi M, Vibha D, Dhiman R, 2020a. Neurodegeneration in Alzheimer’s disease and glaucoma: overlaps and missing links. Eye 34 (9), 1546–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Saxena R, Vibha D, Tripathi M, Sharma P, Phuljhele S, Tandon R, Kumar P, 2020b. Detection of structural and electrical disturbances in macula and optic nerve in Alzheimer’s patients and their correlation with disease severity. Semin. Ophthalmol 35 (2), 116–125. [DOI] [PubMed] [Google Scholar]
- Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV, 2013. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol. 23 (3), 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, García-Layana A, Bejarano B, Villoslada P, 2007. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology 68 (18), 1488–1494. [DOI] [PubMed] [Google Scholar]
- Shao Y, Jiang H, Wei Y, Shi Y, Shi C, Wright CB, Sun X, Vanner EA, Rodriguez AD, Lam BL, Rundek T, Baumel BS, Gameiro GR, Dong C, Wang J, 2018. Visualization of focal thinning of the ganglion cell-inner plexiform layer in patients with mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis 64 (4), 1261–1273. [DOI] [PubMed] [Google Scholar]
- Sharafi SM, Sylvestre JP, Chevrefils C, Soucy JP, Beaulieu S, Pascoal TA, Arbour JD, Rhéaume MA, Robillard A, Chayer C, Rosa-Neto P, Mathotaarachchi SS, Nasreddine ZS, Gauthier S, Lesage F, 2019. Vascular retinal biomarkers improves the detection of the likely cerebral amyloid status from hyperspectral retinal images. Alzheimers Dement. (N. Y.) 5, 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman TF, 1981. On connecting large vessels to small. The meaning of Murray’s law. J. Gen. Physiol 78 (4), 431–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Gahm J, Kashani AH, 2017. Curvelet-based vessel enhancement for 3D OCT angiography. Invest. Ophthalmol. Vis. Sci 58 (8), 648–648. [Google Scholar]
- Shoda C, Kitagawa Y, Shimada H, Yuzawa M, Tateno A, Okubo Y, 2018. Relationship of area of soft drusen in retina with cerebral amyloid-β accumulation and blood amyloid-β level in the elderly. J. Alzheimers Dis 62 (1), 239–245. [DOI] [PubMed] [Google Scholar]
- Shrier EM, Adam CR, Spund B, Glazman S, Bodis-Wollner I, 2012. Interocular asymmetry of foveal thickness in Parkinson disease. J. Ophthalmol 2012, 728457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiqi A, Wahl D, Lee S, Ma D, To E, Cui J, Beg MF, Sarunic M, Matsubara JA, 2020. Retinal fluorescence imaging with curcumin in an Alzheimer mouse model. Front. Neurosci 14, 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Ashimatey BS, Zhou X, Chu Z, Wang R, Kashani AH, 2020. Impaired layer specific retinal vascular reactivity among diabetic subjects. PloS One 15 (9), e0233871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Vijayappa M, Lima F, Delgado P, Wendell L, Rosand J, Greenberg SM, 2008. Impaired visual evoked flow velocity response in cerebral amyloid angiopathy. Neurology 71 (18), 1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC, 2015. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 11 (6), 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodi A, Lenzetti C, Bacherini D, Finocchio L, Verdina T, Borg I, Cipollini F, Patwary FU, Tanini I, Zoppetti C, Rizzo S, Virgili G, 2019a. Quantitative analysis of conjunctival and retinal vessels in Fabry disease. J. Ophthalmol 2019, 4696429. 10.1155/2019/4696429 eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodi A, Nicolosi C, Vicini G, Lenzetti C, Virgili G, Rizzo S, 2019b. Computer-assisted retinal vessel diameter evaluation in Fabry disease. Eur. J. Ophthalmol 1120672119886985. [DOI] [PubMed] [Google Scholar]
- Song G, Steelman ZA, Finkelstein S, Yang Z, Martin L, Chu KK, Farsiu S, Arshavsky VY, Wax A, 2020. Multimodal coherent imaging of retinal biomarkers of Alzheimer’s disease in a mouse model. Sci. Rep 10 (1), 7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF, 2016. Volume-Rendered optical coherence tomography of retinal vein occlusion pilot study. Am. J. Ophthalmol 165, 133–144. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Fujimoto JG, Waheed NK, 2015a. Image artifacts IN optical coherence tomography angiography. Retina 35 (11), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF, Fujimoto JG, Waheed NK, 2015b. Image artifacts IN optical coherence tomography angiography. Retina 35 (11), 2163–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF, Klancnik JM, Cooney MJ, 2015c. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 133 (1), 45–50. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G, 2018. Optical coherence tomography angiography. Prog. Retin. Eye Res 64, 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF, Suzuki M, Yannuzzi LA, Matet A, Behar-Cohen F, 2017. Volume-rendered angiographic and structural optical coherence tomography angiography of macular telangiectasia type 2. Retina 37 (3), 424–435. [DOI] [PubMed] [Google Scholar]
- Spain RI, Liu L, Zhang X, Jia Y, Tan O, Bourdette D, Huang D, 2018. Optical coherence tomography angiography enhances the detection of optic nerve damage in multiple sclerosis. Br. J. Ophthalmol 102 (4), 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Duncker T, Schuerch K, Paavo M, de Carvalho JRL, 2020. Lessons learned from quantitative fundus autofluorescence. Prog. Retin. Eye Res 74, 100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RJ, Wendell CR, Giggey PP, Katzel LI, Lefkowitz DM, Siegel EL, Waldstein SR, 2013. Psychometric limitations of the mini-mental state examination among nondemented older adults: an evaluation of neurocognitive and magnetic resonance imaging correlates. Exp. Aging Res 39 (4), 382–397. [DOI] [PubMed] [Google Scholar]
- Steel DH, Waldock A, 1998. Measurement of the retinal nerve fibre layer with scanning laser polarimetry in patients with previous demyelinating optic neuritis. J. Neurol. Neurosurg. Psychiatry 64 (4), 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen C, D’haeseleer M, Hoogduin JM, Fierens Y, Cambron M, Mostert JP, Heersema DJ, Koch MW, De Keyser J, 2013. Cerebral white matter blood flow and energy metabolism in multiple sclerosis. Mult. Scler 19 (10), 1282–1289. [DOI] [PubMed] [Google Scholar]
- Stefánsson E, Olafsdottir OB, Einarsdottir AB, Eliasdottir TS, Eysteinsson T, Vehmeijer W, Vandewalle E, Bek T, Hardarson SH, 2017. Retinal oximetry discovers novel biomarkers in retinal and brain diseases. Invest. Ophthalmol. Vis. Sci 58 (6), BIO227–BIO233. [DOI] [PubMed] [Google Scholar]
- Stemplewitz B, Keserü M, Bittersohl D, Buhmann C, Skevas C, Richard G, Hassenstein A, 2015. December. Scanning laser polarimetry and spectral domain optical coherence tomography for the detection of retinal changes in Parkinson’s disease. Acta Ophthalmol. 93 (8), e672–e677. [DOI] [PubMed] [Google Scholar]
- Sultan H, Kellogg C, El-Annan J, 2017. Retinal detachment and microangiopathy in mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes syndrome. Can. J. Ophthalmol 52 (6), e208–e211. [DOI] [PubMed] [Google Scholar]
- Sung MS, Choi SM, Kim J, Ha JY, Kim BC, Heo H, Park SW, 2019. Inner retinal thinning as a biomarker for cognitive impairment in de novo Parkinson’s disease. Sci. Rep 9 (1), 11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaruga M, Veugelen S, Benurwar M, Lismont S, Sepulveda-Falla D, Lleo A, Ryan NS, Lashley T, Fox NC, Murayama S, Gijsen H, De Strooper B, Chávez-Gutiérrez L, 2015. Qualitative changes in human γ-secretase underlie familial Alzheimer’s disease. J. Exp. Med 212 (12), 2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegedi S, Dal-Bianco P, Stögmann E, Traub-Weidinger T, Rainer M, Masching A, Schmidl D, Werkmeister RM, Chua J, Schmetterer L, Garhöfer G, 2020. November. Anatomical and functional changes in the retina in patients with Alzheimer’s disease and mild cognitive impairment. Acta Ophthalmol. 98 (7), e914–e921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez D, Castilla-Marti M, Marquié M, Valero S, Moreno-Grau S, Rodríguez-Gómez O, Piferrer A, Martínez G, Martinez J, Rojas I, Hernández I, Abdelnour C, Rosende-Roca M, Vargas L, Mauleón A, Gil S, Alegret M, Ortega G, Espinosa A, Pérez-Cordón A, Sanabria Á, Roberto N, Ciudin A, Simó R, Hernández C, Tárraga L, Boada M, Ruiz A, 2020. Evaluation of macular thickness and volume tested by optical coherence tomography as biomarkers for Alzheimer’s disease in a memory clinic. Sci. Rep 10 (1), 1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliati M, Bodis-Wollner I, Kovanecz I, Stanzione P, 1994. Spatial frequency tuning of the monkey pattern ERG depends on D2 receptor-linked action of dopamine. Vis. Res 34 (16), 2051–2057. [DOI] [PubMed] [Google Scholar]
- Tagliati M, Bodis-Wollner I, Yahr MD, 1996. The pattern electroretinogram in Parkinson’s disease reveals lack of retinal spatial tuning. Electroencephalogr. Clin. Neurophysiol 100 (1), 1–11. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Kwan MM, Verrier MC, Seniuk NA, Theriault E, 1990. MPTP produces reversible disappearance of tyrosine hydroxylase-containing retinal amacrine cells. Brain Res. 527 (1), 21–31. [DOI] [PubMed] [Google Scholar]
- Teng CC, Shapiro LG, Kalet IJ, 2010. Head and neck lymph node region delineation with image registration. Biomed. Eng. Online 9, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY, 2014. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121 (11), 2081–2090. [DOI] [PubMed] [Google Scholar]
- The Huntington’s Disease Collaborative Research Group, 1993. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72 (6), 971–983. [DOI] [PubMed] [Google Scholar]
- Thomson KL, Yeo JM, Waddell B, Cameron JR, Pal S, 2015. A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement. (Amst.) 1 (2), 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, 2001. The role of image registration in brain mapping. Image Vis Comput. 19 (1–2), 3–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo J, Sepulere J, Salinas-Alaman A, García-Layana A, Murie-Fernandez M, Bejarano B, Villoslada P, 2008. Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult. Scler 14 (7), 906–912. [DOI] [PubMed] [Google Scholar]
- Toussaint D, Périer O, Verstappen A, Bervoets S, 1983. Clinicopathological study of the visual pathways, eyes, and cerebral hemispheres in 32 cases of disseminated sclerosis. J. Clin. Neuro Ophthalmol 3 (3), 211–220. [PubMed] [Google Scholar]
- Trebbastoni A, Marcelli M, Mallone F, D’Antonio F, Imbriano L, Campanelli A, de Lena C, Gharbiya M, 2017. Attenuation of choroidal thickness in patients with Alzheimer disease: evidence from an Italian prospective study. Alzheimer Dis. Assoc. Disord 31 (2), 128–134. [DOI] [PubMed] [Google Scholar]
- Trost A, Lange S, Schroedl F, Bruckner D, Motloch KA, Bogner B, Kaser-Eichberger A, Strohmaier C, Runge C, Aigner L, Rivera FJ, Reitsamer HA, 2016. Brain and retinal pericytes: origin, function and role. Front. Cell. Neurosci 10, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CS, Ritch R, Schwartz B, Lee SS, Miller NR, Chi T, Hsieh FY, 1991. Optic nerve head and nerve fiber layer in Alzheimer’s disease. Arch. Ophthalmol 109 (2), 199–204. [DOI] [PubMed] [Google Scholar]
- Tsironi EE, Dastiridou A, Katsanos A, Dardiotis E, Veliki S, Patramani G, Zacharaki F, Ralli S, Hadjigeorgiou GM, 2012. Perimetric and retinal nerve fiber layer findings in patients with Parkinson’s disease. BMC Ophthalmol. 12, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi KK, Chan JY, Hirai HW, Wong SY, Kwok TC, 2015. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern. Med 175 (9), 1450–1458. [DOI] [PubMed] [Google Scholar]
- Tuo QZ, Zou JJ, Lei P, 2020. October 27. Rodent models of vascular cognitive impairment. J. Mol. Neurosci 10.1007/s12031.020.01733.2. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Törnquist P, Alm A, 1986. Carrier.mediated transport of amino acids through the blood-retinal and the blood-brain barriers. Graefes Arch. Clin. Exp. Ophthalmol 224 (1), 21–25. [DOI] [PubMed] [Google Scholar]
- Ucak T, Alagoz A, Cakir B, Celik E, Bozkurt E, Alagoz G, 2016. Analysis of the retinal nerve fiber and ganglion cell - inner plexiform layer by optical coherence tomography in Parkinson’s patients. Park. Relat. Disord 31, 59–64. [DOI] [PubMed] [Google Scholar]
- Uchida A, Pillai JA, Bermel R, Bonner-Jackson A, Rae-Grant A, Fernandez H, Bena J, Jones SE, Leverenz JB, Srivastava SK, Ehlers JP, 2018. Outer retinal assessment using spectral-domain optical coherence tomography in patients with Alzheimer’s and Parkinson’s disease. Invest. Ophthalmol. Vis. Sci 59 (7), 2768–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann RF, Larson EB, Koepsell TD, Rees TS, Duckert LG, 1991. Visual impairment and cognitive dysfunction in Alzheimer’s disease. J. Gen. Intern. Med 6 (2), 126–132. [DOI] [PubMed] [Google Scholar]
- Ukalovic K, Cao S, Lee S, Tang Q, Beg MF, Sarunic MV, Hsiung GR, Mackenzie IR, Hirsch-Reinshagen V, Cui JZ, Matsubara JA, 2018. Drusen in the peripheral retina of the Alzheimer’s eye. Curr. Alzheimer Res 15 (8), 743–750. [DOI] [PubMed] [Google Scholar]
- Ulusoy MO, Horasanlı B, Işık-Ulusoy S, 2020. Optical coherence tomography angiography findings of multiple sclerosis with or without optic neuritis. Neurol. Res 42 (4), 319–326. [DOI] [PubMed] [Google Scholar]
- van de Kreeke JA, Nguyen HT, den Haan J, Konijnenberg E, Tomassen J, den Braber A, Ten Kate M, Collij L, Yaqub M, van Berckel B, Lammertsma AA, Boomsma DI, Tan HS, Verbraak FD, Visser PJ, 2019. Retinal layer thickness in preclinical Alzheimer’s disease. Acta Ophthalmol. 97 (8), 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veys L, Vandenabeele M, Ortuño-Lizarán I, Baekelandt V, Cuenca N, Moons L, De Groef L, 2019. Retinal α-synuclein deposits in Parkinson’s disease patients and animal models. Acta Neuropathol. 137 (3), 379–395. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA., Salvado O, Szoeke C, Macaulay SL, Martins R, Maruff P, Ames D, Rowe CC, Masters CL, A. I. B. a. L. A. R. Group, 2013. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 12 (4), 357–367. [DOI] [PubMed] [Google Scholar]
- Visser F, Vermeer KA, Ghafaryasl B, Vlaar AMM, Apostolov V, van Hellenberg Hubar J, Weinstein HC, de Boer JF, Berendse HW, 2018. In vivo exploration of retinal nerve fiber layer morphology in Parkinson’s disease patients. J. Neural. Transm 125 (6), 931–936. [DOI] [PubMed] [Google Scholar]
- Walter SD, Ishikawa H, Galetta KM, Sakai RE, Feller DJ, Henderson SB, Wilson JA, Maguire MG, Galetta SL, Frohman E, Calabresi PA, Schuman JS, Balcer LJ, 2012. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology 119 (6), 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Camino A, Pi S, Guo Y, Wang J, Huang D, Hwang TS, Jia Y, 2019. Three-dimensional structural and angiographic evaluation of foveal ischemia in diabetic retinopathy: method and validation. Biomed. Optic Express 10 (7), 3522–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Li Y, Wang C, Xu L, You QS, Wang YX, Zhao L, Wei WB, Zhao X, Jonas JB, 2014. Localized retinal nerve fiber layer defects and stroke. Stroke 45 (6), 1651–1656. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M, 2013. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12 (8), 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Xia Z, Gao H, Gong J, Yan L, Huang Y, Chen F, Zhang W, 2016. Correlation of retinopathy with leukoaraiosis in patients with anterior circulation infarcts. J. Clin. Neurosci 33, 105–110. [DOI] [PubMed] [Google Scholar]
- Weil RS, Schrag AE, Warren JD, Crutch SJ, Lees AJ, Morris HR, 2016. Visual dysfunction in Parkinson’s disease. Brain 139 (11), 2827–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Aung T, Medeiros FA, 2014. The pathophysiology and treatment of glaucoma: a review. J. Am. Med. Assoc 311 (18), 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EA, McGuone D, Frosch MP, Hyman BT, Laver N, Stemmer-Rachamimov A, 2017. Absence of Alzheimer disease neuropathologic changes in eyes of subjects with Alzheimer disease. J. Neuropathol. Exp. Neurol 76 (5), 376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, McGowan AJ, Cardwell CR, Cheung CY, Craig D, Passmore P, Silvestri G, Maxwell AP, McKay GJ, 2015. Retinal microvascular network attenuation in Alzheimer’s disease. Alzheimers Dement. (Amst.) 1 (2), 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, 2004. Dopamine and retinal function. Doc. Ophthalmol 108 (1), 17–40. [DOI] [PubMed] [Google Scholar]
- Wong A, Mishra A, Bizheva K, Clausi DA, 2010. General Bayesian estimation for speckle noise reduction in optical coherence tomography retinal imagery. Optic Express 18 (8), 8338–8352. [DOI] [PubMed] [Google Scholar]
- Wong TY, Wong T, Mitchell P, 2007. The eye in hypertension. Lancet 369 (9559), 425–435. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Wu H, Shi LL, Yu LY, Wang LY, Chen YL, Geng JS, Shi J, Jiang K, Dong JC, 2017. The association between retinal vasculature changes and stroke: a literature review and Meta-analysis. Int. J. Ophthalmol 10 (1), 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Vaswani N, Huang Y, Kang JU, 2012. Modified compressive sensing optical coherence tomography with noise reduction. Opt. Lett 37 (20), 4209–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannuzzi LA, Ober MD, Slakter JS, Spaide RF, Fisher YL, Flower RW, Rosen R, 2004. Ophthalmic fundus imaging: today and beyond. Am. J. Ophthalmol 137 (3), 511–524. [DOI] [PubMed] [Google Scholar]
- Yoon SP, Grewal DS, Thompson AC, Polascik BW, Dunn C, Burke JR, Fekrat S, 2019. Retinal microvascular and neurodegenerative changes in Alzheimer’s disease and mild cognitive impairment compared with control participants. Ophthalmol. Retina 3 (6), 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi S, Liu T, Wang RK, 2015. Segmentation and quantification of blood vessels for OCT-based micro-angiograms using hybrid shape/intensity compounding. Microvasc. Res 97, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JG, Feng YF, Xiang Y, Huang JH, Savini G, Parisi V, Yang WJ, Fu XA, 2014. Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PloS One 9 (1), e85718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P, Kaluzny JJ, Wilkosc-Debczynska M, Gebska-Toloczko M, Suwala K, Zabel K, Zaron A, Kucharski R, Araszkiewicz A, 2019. Comparison of retinal microvasculature in patients with Alzheimer’s disease and primary open-Angle glaucoma by optical coherence tomography angiography. Invest. Ophthalmol. Vis. Sci 60 (10), 3447–3455. [DOI] [PubMed] [Google Scholar]
- Zamir M, 1976a. Optimality principles in arterial branching. J. Theor. Biol 62 (1), 227–251. [DOI] [PubMed] [Google Scholar]
- Zamir M, 1976b. The role of shear forces in arterial branching. J. Gen. Physiol 67 (2), 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri MS, Conger A, Salter A, Frohman TC, Galetta SL, Markowitz CE, Jacobs DA, Cutter GR, Ying GS, Maguire MG, Calabresi PA, Balcer LJ, Frohman EM, 2008. Retinal imaging by laser polarimetry and optical coherence tomography evidence of axonal degeneration in multiple sclerosis. Arch. Neurol 65 (7), 924–928. [DOI] [PubMed] [Google Scholar]
- Zhang J, Qiao Y, Sarabi MS, Khansari MM, Gahm JK, Kashani AH, Shi Y, 2019. May. 3D shape modeling and analysis of retinal micro vasculature in OCT-angiography images. IEEE Trans. Med. Imag 39 (5), 1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, Gregg EW, Albright AL, Klein BE, Klein R, 2010. Prevalence of diabetic retinopathy in the United States, 2005-2008. J. Am. Med. Assoc 304 (6), 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]