Abstract
Breast cancer is one of the most common cancers in women, with the ability to metastasize to secondary organs, which is the main cause of cancer-related deaths. Understanding how breast tumors progress is essential for developing better treatment strategies against breast cancer. Until recently, it has been considered that breast cancer elicits a small immune response. However, it is now clear that breast tumor progression is either prevented by the action of antitumor immunity or exacerbated by proinflammatory cytokines released mainly by the immune cells. In this comprehensive review we first explain antitumor immunity, then continue with how the tumor suppresses and evades the immune response, and next, outline the role of inflammation in breast tumor initiation and progression. We finally review the current immunotherapeutic and immunoengineering strategies against breast cancer as a promising emerging approach for the discovery and design of immune system-based strategies for breast cancer treatment.
Keywords: Antitumor immunity, Immune suppression, Immune evasion, Immunotherapy, Immune cells, immune engineering, Proinflammatory cytokines, Breast cancer progression
Introduction
One in eight women will develop breast cancer in their lifetime. It is estimated that there will be 279,100 new cases of breast cancer and 42,690 deaths in 2020 worldwide.102 Breast tumors have the potential to metastasize to other organs such as bone, lung, and brain,112 which is the main cause of cancer-related deaths.15 Now it is well established that to progress and metastasize successfully, the breast tumor remodels its surrounding tissue and creates its own microenvironment.5 This new microenvironment, known as the tumor microenvironment (TME), includes changes in the extracellular matrix (ECM), the vasculature, and the supporting cells such as stromal cells (fibroblasts and adipocytes), immune cells, and endothelial cells,5,6,13,117 all contributing to tumor progression.
Breast cancer has traditionally been considered one of the cancers with the least immune responses.37,103 Recently, in the last 20 years, there has been an increase in the literature for studies on the immune effects on breast cancer. This has caused a more thorough understanding of the immune system and how it interacts with breast cancer. There are many cells and cytokines involved in the breast cancer immune environment, some with antitumorigenic roles, others with protumorigenic or immunosuppressive functions. These cells and cytokines can also promote the progression of breast cancer through chronic inflammation. Investigating how the immune system functions and affects breast cancer is useful for designing treatments to utilize the immune system and microenvironment to treat breast cancer. It can also enable engineering more predictive models for a better understanding of the breast cancer biology, as well as a more accurate prognosis and better treatment options for breast cancer patients. Scientists have already begun to use the immune response to breast cancer to their advantage and are creating immunotherapies to perform various tasks such as vaccinating against breast cancer, helping enrich the immune system to fight breast cancer, or working in conjunction with chemotherapy to reduce the breast cancer mortality.
This comprehensive review seeks to provide a glance at the whole tumor immune microenvironment in the case of breast cancer by looking at the ways the immune system attacks the tumor, the methods through which the tumor evades the immune response, the role chronic inflammation plays, and strategies used to combat breast cancer. To this end, first, we give a brief introduction on the antitumor immunity and the immune cells involved in antitumor response, then we continue with how the tumors suppress and evade the immune system and give an overview of the cells involved. Next, we explain how inflammation supports cancer progression. Finally, we give brief information on immunotherapy and immune engineering, where state-of-art treatment strategies and engineered models of the immune system are discussed.
Antitumor Immunity
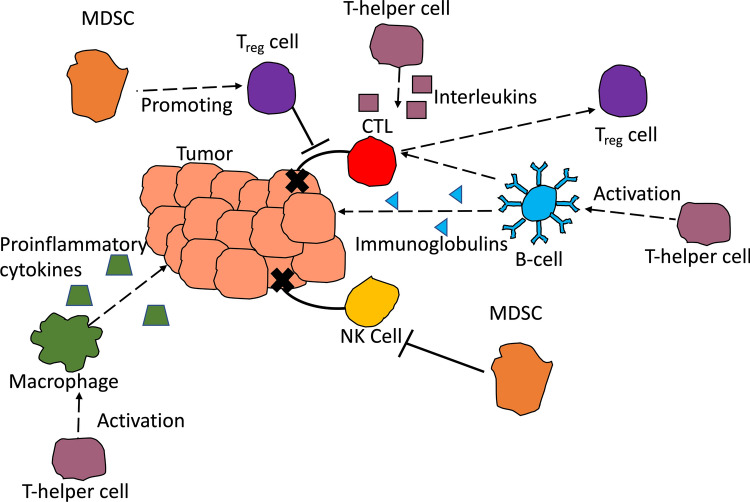
Antitumor immunity is one of the body’s first line of defenses against tumors. In this section, we start by reviewing the immune cells that generate an antitumor response against breast cancer. Innate immune system cells, such as natural killer (NK) cells, attack the primary tumor. These cells have also been thought to prevent neoplasms from developing into fully formed tumors.25 Cytotoxic T-cells are adaptive immune cells that also attack the primary tumor. However, some adaptive immune cells, such as B-cells may have dual roles in the breast tumor immune microenvironment, where they either promote tumor progression or prevent it. A summary of the cell types discussed, and their actions are shown in Fig. 1. The cells referenced in this section are meant to highlight the antitumor immunity function of the specific cells.
Figure 1.
A graphical representation of the cells discussed and their actions on breast tumors. Cytotoxic T-Lymphocytes (CTLs) and Natural Killer (NK) cells directly act on the breast cancer cells and promote antitumor immunity through destroying the cells. B-cells promote antitumor immunity by secreting immunoglobulins that reduce early neoplasms. They promote tumor progression through acting on CTLs and transforming them into Treg cells. Treg cells act by inhibiting CTLs to promote tumor progression. T-helper cells secrete interleukins that promote a proinflammatory environment, activate B-cells and macrophages, and promote CTL anergy. Macrophages secrete proinflammatory cytokines that promote tumor progression. Myeloid Derived Suppressor Cells (MDSCs) promote Treg cell population and suppress NK cells.
Cytotoxic T Lymphocytes (CTLs)
Cytotoxic T lymphocytes are CD8+ cells that play a role in the antitumor immune response. First, an antigen presenting cell (APC), in this case a tumor cell, presents an antigen on its surface that activates the CTLs. The CTLs respond first through clonal expansion, then through lysing the target cell. CTLs act through Fas/FasL binding at the surface of the target cell, which drives the target cell to apoptosis.44
It has been shown previously that elevated levels of CTLs correspond to a better prognosis and better overall survival in triple negative breast cancer23,104 as well as other types of invasive breast ductal carcinomas.84 CTLs, T-cells, and B-cells that have infiltrated into the tumor, tumor infiltrating lymphocytes (TILs), are found more often in higher grade ductal carcinoma in situ and in more invasive carcinomas.113 Overall, an increase in TILs have been proven to have a better prognosis in breast neoplasms.23 Many researchers are currently developing immunotherapeutic strategies based on the use of CTLs as they have more specific targets and are good at killing breast cancer cells and cancer stem cells.23
Natural Killer (NK) Cells
The NK cells interact with a variety of surface receptors on target cells to distinguish between the self and non-self. This interaction allows the NK cells to find and destroy enemies, while avoiding tissue damage. Upon activation, NK cells produce a number of inflammatory cytokines such as CCL5 and XCL1 to crosstalk with T-cells and dendritic cells and initiate an immune response. The presence of these cytokines has been linked to increased cancer patient survival.11 The NK cells, typically, take action through releasing cytotoxic granules to kill the target cells.
The NK cells work to eliminate early tumors and early metastases,76 but the role of NK cells within breast cancer has not been fully elucidated. Various studies87,91 have shown that the NK cell number does not increase in breast cancer as compared to normal breast tissue, but the number of lymphokine-activated killer cells, a population of cells consisting of NK, natural killer T cells, and T cells, activated by lymphokines to kill tumor cells, is increased. On the other hand, in advanced breast cancer, studies showed that the NK cell activity is decreased,8 suggesting that NK cells are active during initial breast tumor development.27 NK cell dysfunction has also been connected to breast cancer progression, indicating that as the disease progresses, the breast cancer cells decrease the activation of NK cells in order to promote immune evasion.74
B-Cells
B-cells are another cell type involved in adaptive immunity. They act by secreting antibodies or immunoglobulins as well as various cytokines. Additionally, the receptors on the surface of the B-cells, surface immunoglobulins, allow the cell to attach to specific antigens and neutralize them. B-cells normally reside in the secondary lymphoid organs, and upon activation they travel to the specific target site.
B-cell’s role in breast cancer is bilateral, as they secrete cytokines that inhibit the antitumorigenic CTLs and NK cells, but have also been found to infiltrate the tumor and promote antitumor immunity.96 The immunoglobulins they secrete may reduce early neoplasms.25 An increase in B-cell count has also been linked to an improved breast cancer prognosis.2,36,116 Despite their role in reducing early neoplasms, B-cells may also lead to breast cancer progression and metastasis. Olkhanud et al. showed that B-cells convert CD4+ or T-helper cells to regulatory T-cells (Tregs), which mediate breast cancer metastasis.81 Inactivation of B-cells has been proven to inhibit metastasis of breast adenocarcinoma cells in a mouse model.60 These findings suggest that B-cells work towards anti-tumor immunity in the beginning stages of the breast tumor, but once the tumor progresses, B-cells begin to be pro-tumorigenic and promote metastasis.
Immune Suppression and Evasion
There are many factors that contribute to breast tumor’s capacity to evade the immune system and eventually suppress the antitumor response. One factor is the immune cells’ inability to identify the tumor as “non-self” or “foreign”, which allows the tumor to escape antitumor activity. As discussed previously, there are multiple different cell types with bilateral roles in breast tumor progression. In this section, we briefly explain the immune cells involved in immune suppression and evasion, and thus breast tumor progression. The cells referenced in this section are meant to highlight the immune suppression and evasion functions of the specific cells.
Regulatory T-Cells (Tregs)
Regulatory T-cells (Tregs), that regulate the immune response by maintaining “self-tolerance”, are one of the most prevalent cell types when it comes to immune suppression and immune evasion, although they account for a low percentage of the overall T-lymphocytes.25 “Self-tolerance” ensures that the immune system is not attacking the body itself. Tregs can inhibit the function of CD8+ CTLs as well as the response of T-helper cells, DCs, NK cells, and B-cells3,16,65,90,108 in order to limit the attack on self. Tregs also promote “self-tolerance” by inhibiting effector cytokines like interleukin (IL)-2 and by producing immunosuppressive cytokines like IL-10.20,24,109 Tregs are CD4+, CD25+ and can be identified by the expression of FOXP3. Treg populations have also been shown to diminish in the case of autoimmune disorders to reduce the immune response against the self.32
It has been proven in breast adenocarcinomas and in spontaneous mouse models of breast cancer that there is an increase of Tregs in the TME.54,71 This corresponds to a poor prognosis with shorter recurrence-free survival and a decrease in overall patient survival.7,77 The ratio of Tregs to CTLs has also been shown to be a predictor of adverse outcomes in patients, with worse outcomes and shorter disease-free survival when Treg to CTL ratio is higher.23 The increase in this ratio is associated with the molecular subtype of breast cancer, with the largest Treg to CTL ratio coming from triple negative breast cancer. These Tregs are members of the T-cell population that infiltrates the breast tumor.38,70 Once they have infiltrated into the breast tumor, they start to expand, which then suppress the function of other effector immune cells so as to help the breast tumor evade the antitumor immune response. The percentage of Tregs was even shown to increase with increasing disease stage in breast cancers from normal tissue to DCIS to invasive carcinoma,7 proving that as the breast cancer becomes more invasive, the TME expands the Treg population. It is thought that an increase in the production of prostaglandin E2 from tumor cells and production of CCL22 from tumor-associated macrophages (TAMs) leads to increased Treg population.21,22,28,40 As the breast tumor proliferates and becomes more invasive, the production of these cytokines increases, which again leads to immune evasion.
T-Helper Cells
T-helper cells are members of the adaptive immune system as they cannot mount an immune response until they have been activated through an APC. T-helper cells activate B-cells, macrophages, cytotoxic T-cells, and other cells involved in the adaptive immune response. T-cells have the potential to differentiate into T-helper 1 (Th1), T-helper 2 (Th2), T-helper 17 (Th17), or T follicular helper (Tfh) cells upon stimulation. The different types of T-helper cells secrete different cytokines in response to an insult. Th1 cells secrete IFN-γ and TNF-α which activate macrophages or CTLs, but can also favor immune evasion.14 Th2 cells secrete interleukins that activate antibody production in B-cells. Th17 cells primarily produce IL-17, while Tfh cells mainly mediate the B-cell response.
Because the T-helper cells can activate various other cells in the adaptive immune system, they are an important mediator in breast cancer antitumor immunity and in immune evasion. Namjoshi et al. showed that the cytokines produced by Th1 cells promoted apoptosis as well as the loss of the HER-2 oncodriver in breast cancer, displaying the role Th1 cells could play in antitumor immunity.79 Conversely, the presence of T-helper cells within the TME correlates with breast tumor progression, metastasis, and an increase in tumor diameter.18,55 The same study14 also proved that the ratio of T-helper cells to CTLs was important, with a greater ratio of T-helper cells to CTLs corresponding to an increase in breast tumor progression, metastasis, and tumor diameter. The interleukins secreted by the Th2 cells decrease T-cell cytotoxicity and mediate T-cell anergy, leading to immune suppression.83 Fu et al. also reported that in postoperative breast cancer patients, the circulating T-helper cells showed immunosuppressive properties.33 These T-helper cells were linked to poor prognosis in these postoperative patients. Gruber et al. showed a correlation between the number of T-helper cells and circulating tumor cells, which both were associated with higher grade breast tumors.42 T-helper cells may also promote both antitumor immunity and immune evasion.
The role of Th17 and Tfh within the immune response to cancer has not been fully elucidated. Th17 cells have been shown to have both a protumorigenic effect and antitumor immunity.45 Il-17, secreted by Th17 cells, decreases with increasing tumor progression, while Th17 cell content was consistent for healthy women, pre-, and post-operative breast cancer patients.4,34 While the Th17 content did not change between patients with or without breast cancer, the ratio of Th17 cells to Tregs in TILs was increased in early breast cancer and decreased in advanced breast cancer, proving that as breast cancer progresses, less Th17 cells and more Tregs infiltrate the breast tumor.110 Th17 cells promote tumorigenesis through secreting angiogenic and anti-apoptotic factors.45 Th17 cells also show antitumor immunity through cytotoxic activity and expressing MHC antigens.45 Tfh cells have been studied even less in the case of breast cancer. Tfh cells were found to activate B-cells in immune therapies.47 In one study, when Tfh cells were detectable in breast tumors, there was an increase in patient survival,43 however, another study has shown no differences in the levels of Tfh cells in breast cancer patients as compared to healthy women.121 The full extent of Th17 and Tfh cells’ response to breast cancer is still under investigation, as conflicting reports of their function and presence within breast cancer leaves their function yet to be discovered.
Macrophages
Macrophages are effector cells within the immune system that use phagocytosis to digest various targets such as cellular debris, cancer cells, and other foreign substances. There are two different subclasses of macrophages entitled M1 and M2 macrophages. M1 macrophages are more proinflammatory while M2 macrophages are more anti-inflammatory. Macrophages change their phenotype based on the immune response needed and the microenvironment. They may promote antitumor immune response, as well as tumor progression.
The first response of macrophages to a neoplasm is from the tissue-resident macrophages that secrete various factors in order to draw other immune cells like NK cells, CTLs, and DCs to the site of action to destroy the neoplasm.25 If the defense is prolonged and becomes chronic, the macrophage response can also become protumorigenic. With chronic infiltration of macrophages, the macrophages secrete cytokines and factors that contribute to tumor cell survival, TME remodeling, and immune suppression.25
TAMs, macrophages that reside in the TME, are altered by the tumor in such a way that they help with tumor development. In a mouse model of breast cancer, increased tumor progression was reported when macrophages infiltrated into the mammary tissue,73 while decreased tumor development as well as decreased metastasis was reported when macrophages did not infiltrate into the tissue.68 Macrophages also regulate vascular endothelial growth factor levels, which controls angiogenesis.67 In an early neoplasm, typically the M1 macrophages invade into the breast tumor and secrete proinflammatory cytokines, which draw other cells to the tumor site. If the macrophages cannot kill the tumor, the proinflammatory cytokines will convert the M1 macrophages into TAMs to promote tumor progression. In the late stages of breast cancer, the TAMs transition to M2 macrophages and secrete other cytokines to drive T-cell differentiation to Th2 cells, as well as immune suppression by Tregs.10 Those breast tumors with high number of M2-TAMs have a higher tumor grade and a lower survival.53
Specifically, within the breast tissue, adipocytes only further aggravate the macrophage response to promote tumorigenesis. The macrophages within the breast tissue form a crown-like structure (CLS) around dead or dying adipocytes. The macrophages phagocytose the dying or dead adipocytes, and thus become lipid-loaded “foam” cells that release a number of proinflammatory cytokines.101 Increased inflammation within the adipose tissue of the breast has been linked with a shorter recurrence-free survival post-mastectomy,49 and with an increase in CLSs caused by macrophages, corresponding to a reduced survival in breast cancer.58 Macrophages have also been shown to help disseminate early breast cancer cells and promote early metastasis.69
Myeloid-Derived Suppressor Cells (MDSCs)
Myeloid-derived suppressor cells (MDSCs) are another immune cell population that is involved in regulating the immune response in a healthy individual as well as in response to various diseases and infections. This population of cells mainly consists of myeloid progenitor cells, and immature dendritic cells, granulocytes, and macrophages. The MDSC population expands in response to various factors, most of which are present at high levels in the TME. MDSCs promote Tregs which suppress the antitumor immune response, while reducing the activity of CTLs. They interact with and affect other cells through cell-to-cell contact, reactive oxygen species production, and multiple other mechanisms. MDSCs have also been shown to suppress DCs and NK cells which have antitumor response.75 As a result, MDSCs also contribute to immune suppression and evasion specifically within the breast tumor environment. An increase in MDSCs in patients with metastatic breast cancer showed worse outcomes and lower overall survival,19 while inhibiting MDSCs improves outcomes and increases survival.75 MDSCs have also been correlated with breast cancer progression and metastasis.9
Inflammation
Inflammation can cause irreparable damage within the body and is typically protumorigenic. Inflammation is caused by various immune and non-immune cells secreting inflammatory cytokines. It also helps with the recruitment of more immune cells to the inflamed tissue. There is both acute and chronic inflammation. Acute inflammation is usually seen in the case of an environmental insult such as injury and infection and lasts a shorter period of time. Chronic inflammation is more persistent and typically does not show symptoms of inflammation. Chronic inflammation can cause a lot of damage through various lymphocytes actually attacking the healthy tissue. This occurs frequently in the breast tissue. In this section, we review the immune cells that secrete proinflammatory cytokines and the resulting consequences these cells have on breast cancer.
Immune Cells Secreting Inflammatory Cytokines
Neutrophils, macrophages, NK cells and T-helper cells all secrete proinflammatory cytokines that contribute to the inflamed breast environment. Macrophages are one of the main mediators of inflammation. They secrete a whole suite of inflammatory cytokines such as tumor necrosis factor (TNF), IL-1, IL-6, IL-8, IL-23, and IL-27.114 IL-6 is an important mediator of breast tumorigenesis through increasing inflammation. T-helper cells recruit the macrophages to the target tissue by producing IFN-γ and TNF-α, as mentioned earlier, which promote an inflammatory environment. Neutrophils also release IFN-γ as well as other proinflammatory interleukins.106 NK cells also secrete proinflammatory interleukins as well as granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, and TNF-α, which, again, lead to recruitment of macrophages to the target site. The inflammatory environment can promote proliferation and expansion of the breast tumor cells and cancer stem cells, as well as angiogenesis.
Proinflammatory Cytokines
There are many different cytokines, mainly produced by macrophages, that contribute to a proinflammatory environment within the breast tissue. Proinflammatory cytokines typically are protumorigenic and can even induce chemoresistance.17 Interleukins are a primary mediator of the inflammatory environment. IL-6 is one of the strongest players involved in chronic inflammation and tumorigenesis. It has been shown that IL-6 creates a feed-forward loop with inflammation that expands the cancer stem cell population.57 IL-6 can also inhibit apoptosis, which benefits breast cancer cell survival.12,62 In addition, IL-6 promotes inflammation, which allows the breast tumor to proliferate and have increased angiogenesis. The advancement of inflammation leads to increases in IL-6 in this feed-forward loop, which is further supports tumor progression. Some interleukins such as IL-12 cause tumor regression through decreasing angiogenesis and remodeling. These cytokines have been used for cytokine therapy to help activate the immune system to promote tumor regression.
TNF-α is another cytokine that contributes to inflammation and, therefore, increases tumorigenesis. TNF-α does this through multiple mechanisms such as interacting with the apoptosis and NF-kB pathways to activate inflammation,118 driving remodeling of blood vessels, and inducing angiogenesis,98 all which contribute to tumor progression. TNF-α has also been shown to increase matrix metalloproteinase (MMP) expression and correlate with disease progression in breast cancer.59 An increase in MMP expression leads to increased breast tumor invasion and progression.80 TNF-α creates an inflammatory phenotype within the cancer associated fibroblasts and mesenchymal stem cells that promote tumor progression through releasing other proinflammatory cytokines.52 In summary, TNF-α promotes breast cancer progression through inducing a proinflammatory environment with other cells and cytokines.
Other interleukins such as IL-1β, IL-23, and IL-27 are also important proinflammatory cytokines. IL-1β promotes breast tumor growth and metastasis through a proinflammatory and proangiogenic environment.46 IL-23 also correlates with a negative prognosis for breast cancer but does this through expanding and maintaining a subset of the Treg cell population.35 IL-27 is similar to IL-1β in that it supports tumor growth and metastasis through a proinflammatory and proangiogenic environment when it is upregulated in the case of breast cancer.72 Important cytokines and their effects on breast cancer are summarized in Table 1. It should be noted that, these are only a summary of a few of the many proinflammatory interleukins and proinflammatory cytokines that play a role in breast cancer proliferation, invasion and metastasis. A thorough review of the role of proinflammatory cytokines in breast cancer can be found elsewhere.39
Table 1.
Proinflammatory cytokines and their effect on breast cancer.
| Cytokine | Effects | Sources |
|---|---|---|
| TNF-α | Activates inflammation, induces angiogenesis, increases MMP production, creates inflammatory environment within stromal cells | 52,59,80,98,118 |
| IFN-γ | Promotes inflammatory environment, recruits macrophages to the tumor site, can inhibit the macrophage from processing the tumor antigen | 61 |
| GM-CSF | Proinflammatory cytokine, activates macrophages to TAMs, associated with EMT and poor prognosis | 105 |
| IL-1β | Creates proinflammatory, proangiogenic environment | 46 |
| IL-6 | Creates a proinflammatory environment, expands cancer stem cell population, inhibits cancer cell apoptosis | 12,57,62 |
| IL-8 | Promotes angiogenesis, promotes cancer cell growth and survival, promotes breast cancer cell invasion | 107 |
| IL-12 | Anti-angiogenic, decreases VEGF and MMP levels, causes breast cancer regression | 26 |
| IL-18 | Increases immunosuppression, promotes migration and invasion | 64,82 |
| IL-23 | Expands and maintains subset of Treg cell population | 35 |
| IL-27 | Creates proinflammatory, proangiogenic environment | 72 |
Immunotherapy
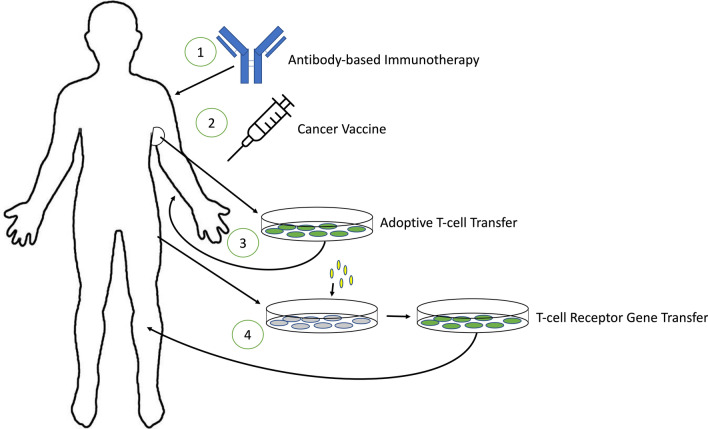
Current treatments for breast cancer include surgical intervention, radiotherapy, chemotherapy, and hormone replacement therapy. These are usually applied to non-invasive or non-metastatic breast cancers. A method that would address invasive and metastatic breast cancers is still missing. Immunotherapy is one promising approach to tackle this need. There are multiple different techniques for immunotherapy. One of the first methods of immunotherapy created was cytokine therapy which uses cytokines to activate certain immune cells to antitumor immunity. Some of these have shown promising results on invasive or metastatic breast cancers, while others have shown an enhanced effect when used in conjunction with chemotherapy.120 Many of these immunotherapies are experimental and are in the process of clinical trials for efficacy and safety but show positive outcomes for the treatment of breast cancer. These methods of immunotherapy are displayed in Fig. 2. This section will cover four of the main treatments being studied for immunotherapy of breast cancer. These immunotherapies have been used as building blocks, with each method building on the base of immunotherapy knowledge and the studies within each method building on each other to better enhance the immunotherapy.
Figure 2.
Graphical representation of four immunotherapy methods discussed in this review. (1) Antibody-based immunotherapy: antibodies are created to target the specific tumor and can be used to target drugs to the site or target specific cytokines. (2) Cancer vaccine: peptides, DNA, or other proteins are injected into the body to activate CTLs to lyse the tumor cells. (3) Adoptive T-cell transfer: a portion of the tumor is resected, and the TILs are cultured from this resection and implanted back into the body to lyse the tumor cells. (4) T-cell receptor gene transfer: T-cells are removed from the body and the TCR gene is transferred to one that can recognize a specific breast cancer antigen. These cells are then implanted back into the body to lyse the tumor cells.
Antibody-Based Immunotherapy
Antibodies can bind to the antigens present on the surface of the tumor cells and mark them, which allows the immune system to recognize and destroy the tumor through a variety of immune responses such as phagocytosis, tumor lysis, inhibiting the invasion and spread of the tumor, or apoptosis that occurs as a result of the immune response. Antibody-based immunotherapy typically focuses on developing antibodies that target antigens specific to a particular cell type, in this case, breast cancer cells. Antibodies have recently been created to target certain cytokines or cytokine receptors to inhibit immune functions or target various points in the immune checkpoint to help inhibit immune evasion by the tumor.120
The main antibodies that have been tested for breast cancer have been monoclonal antibodies for HER-2+ breast cancers. The monoclonal antibody tratsuzumab increased the survival in patients with metastatic breast cancer and had a response rate of 23.1% in patients with HER-2 overexpressing breast cancer.50,63,93,100 This response was only increased when combined with chemotherapy. Another antibody being tested is a monoclonal antibody against the VEGF receptor which showed a 19.1% response rate by itself and a 30.2% response rate in combination with chemotherapy.50 Antibodies are an attractive treatment option as they are more specific than general chemotherapy and can, therefore, mitigate some of the off-target effects. Antibodies could even be used as a targeted drug delivery system to make a drug more specific. There has been some success with targeted antibodies atezolizumab, which targets a transmembrane protein PD-L1 found in breast cancers, and pembrolizumab, which targets another cell surface protein PD-1 found in breast cancers, against triple negative breast cancers.30 The use of these antibodies against triple negative breast cancer shows promise that antibody-based immunotherapies can be developed for more than just HER-2+ tumors.
Cancer Vaccine
Cancer vaccines use CTLs to help kill the tumor cells. This method uses a variety of different tumor specific antigens, such as peptides, DNA, and proteins, to activate the CTLs to lyse the tumor cells expressing that specific antigen. Currently, there are only a few breast cancer specific antigens: HER-2/neu, MUC-1, or NY-ESO-11,85,111 The limited number of available antigens has made it challenging to develop a breast cancer vaccine. However, many more antigens are currently being investigated for the development of a vaccine.
A study showed that 89% of the patients with HER-2 overexpressing breast cancer developed a T-cell immunity in response to the breast cancer vaccine and 82% developed immunoglobulin G antibody immunity, but no clinical response such as tumor lysis or improved survival was observed.89 There have been no promising results from the trials of the NY-ESO-1 breast cancer vaccine. The only breast cancer vaccine that has shown an effect is MUC-1, although not very promising. In a trial containing patients with metastatic breast cancer, only two out of 28 had a partial regression of the tumor in response to the MUC-1 breast cancer vaccine.95 Again, many of these patients had an increase in antigen-specific immune response, but this did not translate to a clinical response.56 There has also been a vaccine developed to stimulate T-cell activation, but the T-cell response has been low with little to no clinical response.29 After more thorough testing and identifying more breast cancer antigens, cancer vaccines could be a viable immunotherapy option in the future.
Adoptive T-Cell Transfer
Adoptive T-cell transfer has been shown to be an effective method for immunotherapy, especially in the case of metastatic cancer. Adoptive T-cell transfer works by resecting a portion of the primary tumor and generating the anti-tumor T lymphocytes. These anti-tumor T lymphocytes are then expanded ex vivo and activated. After expansion and activation, the anti-tumor T lymphocytes are infused back into the patient’s body to elicit an anti-tumor immune response. This immunotherapy has been achieved with great success in the case of melanoma patients who showed a reduction in the metastatic tumors as well as some patients that achieved regression of their cancer.120
It has been shown previously that TILs are present in breast tumors, meaning that adoptive T-cell transfer could be a viable immunotherapy option. TILs were able to be cultured from primary breast tumors in 15 out of 19 patients.97 These cultures were primarily CD4+ T-helper cells with only 21% being CTLs, meaning the cultures had poor lysis capabilities. The TILs lysed the autologous tumor in one out of 12 patients with low lysis and low specificity. Other labs have shown that anti-tumor TILs with tumor reactivity can be generated from primary tumor tissues.29 A clinical trial found that 12 out of 81 patients treated with adoptive T-cell transfer survived over 5 years.51 More research into adoptive T-cell transfer needs to be done before it can be considered as an effective immunotherapy for breast cancer.
T-Cell Receptor Gene Transfer
There are two problems related to the adoptive T-cell transfer: (i) not all patients will have the correct size and accessible tumors, and (ii) only 50% of TIL cultures can generate the specific anti-tumor TILs necessary for this treatment. In order to work around these issues, T-cell receptor (TCR) gene transfer is also being studied. The T-cell is activated through the TCR, which is necessary for specific antigen recognition. Hence, researchers could, theoretically, transfer the specific gene for a TCR to recognize a specific antigen, such as one pertaining to breast cancer. There has been some success with generating specific T-cells that target and kill the melanocytes containing the antigen recognized due to TCR gene transfer94 in the case of melanomas.
In the case of breast cancer, researchers have constructed a chimeric gene of the HER-2/neu monoclonal antibody with the TCR in order to express the TCR with HER-2/neu antigen specificity. This was successful with the cells releasing cytokines upon recognition of HER-2+ breast cancer cells and primary tumors. Cells with the TCR gene transfer also lysed the target cells in vitro with specificity to the HER-2/neu antigen.41 Currently, this is the only work that has been done with HER-2+ cancers, so it will need to be researched if this treatment could be applied to other types of breast cancer. Early results are promising for TCR gene transfer as an immunotherapy, but there are many more clinical trials needed before this will be considered for immunotherapy.
Immune Engineering
Immune engineering, as it relates to breast cancer, is a large field that ranges from machine learning for better breast cancer diagnoses to engineering immune cells for targeting breast cancer. There have been many recent developments in immune engineering. For example, Sahan et al. used machine learning to create a “fuzzy” artificial immune system to better model breast cancer diagnoses.92 They engineered a method to quantitatively capture immune system cells such as B-cells, T-cells, and macrophages through interactions with antigens, cell size, cell shape, and other factors. Polat et al. made the Feature Select Artificial Immune Recognition System to better diagnose breast cancers.86 This is an algorithm that uses an artificial immune system to help make diagnostic decisions based on previous data. Shafiee et al. invented a tissue engineered bone model to investigate the effects of the immune system on breast cancer metastasis.99 Mice models have also become more sophisticated through implanting human immune cells and human cancer cells within the mice in order to study tumor-immune effects.48
There have been recent advances in the field of engineering micro and nanoparticles for immune suppression or stimulation for a therapy to be more effective.78 Recently, researchers have also been using particle-laden cells to target for anti-cancer drugs. Zhao et al. treated lung metastases with erythrocytes attached to chemokine encapsulating nanoparticles.119 This allowed more effector immune cells to infiltrate into the lung metastasis and showed greater animal survival. This could provide a much-needed therapy in the future for breast cancer metastases to the lungs. PEGylated bilirubin nanoparticles were even created to increase the effectiveness of immune-chemotherapy in breast cancer.115
Escobar et al. used genetic engineering to introduce IFN-α to hematopoietic stem cells that will differentiate into macrophages.66 This approach was able to limit tumor progression and metastasis in mouse models of breast cancer. T-cells have long been an attractive cell for immune engineering purposes. Another method for immune engineering has been the development of chimeric antigen receptor (CAR) T-cells. Researchers create chimeric antigen receptors for specific breast tumor antigens and transfer these receptors to the T-cells. CAR-T cells directly interact with these external antigens. Once activated within the body, the CAR-T cells will lyse the target tumor cells presenting these antigens. These CAR-T cells are engineered to target specific breast cancer cells and there have been many new developments with using these cells for breast cancer therapy.31 CAR-NK cells have also been investigated as another potential therapeutic for breast cancer, although most of the trials have been preclinical.88
As the immune system is one of the most complex systems in the body, and some cells may have both antitumor and pro-tumor effects, care must be taken to avoid unwanted outcomes that could lead to tumor progression instead of treating the tumor. For example, B-cells have anti-tumor effects against new neoplasms, they may contribute to tumor progression in the later stages of cancer. Therefore, therapeutics should be applied at the right time, stage, and circumstances.
Concluding Remarks
The immune system plays a dynamic role in the prevention and progression of breast cancer. Cells such as CTLs and NK cells promote antitumor immunity through targeting breast cancer cells. Tregs, macrophages, MDSCs, and T-helper cells aid in the progression of breast cancer through various mechanisms such as inhibiting the function of cytotoxic T-cells, secreting proinflammatory cytokines, promoting metastasis, and others. B-cells may have an antitumorigenic role through releasing tumor-neutralizing antibodies, and a protumorigenic role through suppressing antitumor immunity. Inflammation can cause serious damage to the breast tissue and make it easier for the progression of breast cancer through the increase of proinflammatory cytokines such as interleukins and TNF-α. Immunotherapy and immune engineering are relatively new fields with new discoveries being made constantly. These fields seek to engineer the immune microenvironment or the immune cells to either attack the cancer cells themselves or to release chemotherapeutic drugs to be able to destroy the breast tumor. There has also been work in the immune engineering field to be able to diagnose breast tumors better and give a more accurate prognosis. There is still a long way to go before many of these treatments become valid therapy possibilities, but the studies show promise to be able to engineer the immune system to destroy the breast tumor and promote antitumor immunity.
This paper reviewed the complex tasks that immune cells have in relation to breast cancer. Some cells promote antitumor immunity, while others contribute to tumor progression. Other cells have bilateral roles, as they may either promote cancer or prevent it. Inflammation also plays a key part with proinflammatory cytokines also promoting tumor development. Understanding the immune effects on breast cancer can lead to better therapeutic strategies by improving the abilities of the antitumor cells or inhibiting the cells that promote tumor progression. This knowledge can also be used for creating better models of the immune system. Immune cells should be included in 3D engineered tumors to better study cancer biology and the reaction of immune system against the tumor before they are tested in animal models. Immune cells would allow researchers to create more precise and physiologically relevant breast cancer models. These models would serve as more realistic drug testing platforms, as well as tools for studying the biology of breast cancer. Incorporating immune cells into the model systems will allow for high-throughput evaluation of breast cancer drug candidates. The immune system is very complex, and it may be explored in more detail through engineered models for a better understanding of the immune system’s reactions.
Acknowledgments
This study was funded by NIH Award Number 5R01EB027660-02 and Walther Cancer Foundation, Harper Cancer Research Institute Cancer Cure Ventures Award number 0184.01.
Conflict of interest
Jensen N. Amens, Gökhan Bahçecioglu, and Pinar Zorlutuna declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Apostolopoulos V, Karanikas V, Haurum JS, McKenzie IF. Induction of HLA-A2-restricted CTLs to the mucin 1 human breast cancer antigen. J. Immunol. 1997;159(11):5211–5218. [PubMed] [Google Scholar]
- 2.Arias-Pulido H, Cimino-Mathews A, Chaher N, et al. The combined presence of CD20 + B cells and PD-L1 + tumor-infiltrating lymphocytes in inflammatory breast cancer is prognostic of improved patient outcome. Breast Cancer Res. Treat. 2018;171(2):273–282. doi: 10.1007/s10549-018-4834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003;63(15):4516–4520. [PubMed] [Google Scholar]
- 4.Baharlou R, Atashzar MR, Vasmehjani AA, et al. Reduced levels of T-helper 17-associated cytokines in the serum of patients with breast cancer: indicators for following the course of disease. Cent. Eur. J. Immunol. 2016;41(1):78–85. doi: 10.5114/ceji.2016.58819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahcecioglu G, Basara G, Ellis BW, Ren X, Zorlutuna P. Breast cancer models: engineering the tumor microenvironment. Acta Biomater. 2020;106:1–21. doi: 10.1016/j.actbio.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahcecioglu G, Yue X, Howe E, Guldner I, Stack MS, Nakshatri H, Zhang S, Zorlutuna P. Aged breast extracellular matrix drives mammary epithelial cells to an invasive and cancer-like phenotype. BioRxiv. 2020 doi: 10.1101/2020.09.30.320960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. 2006;24(34):5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int. J. Cancer. 1999;80(6):880–888. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Bergenfelz C, Larsson A-M, von Stedingk K, et al. Systemic monocytic-mdscs are generated from monocytes and correlate with disease progression in breast cancer patients. PLoS ONE. 2015;10(5):e0127028. doi: 10.1371/journal.pone.0127028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 11.Böttcher JP, Bonavita E, Chakravarty P, et al. Nk cells stimulate recruitment of cdc1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172(5):1022–1037.e14. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brocke-Heidrich K, Kretzschmar AK, Pfeifer G, et al. Interleukin-6-dependent gene expression profiles in multiple myeloma INA-6 cells reveal a Bcl-2 family-independent survival pathway closely associated with Stat3 activation. Blood. 2004;103(1):242–251. doi: 10.1182/blood-2003-04-1048. [DOI] [PubMed] [Google Scholar]
- 13.Casey J, Yue X, Nguyen TD, et al. 3D hydrogel-based microwell arrays as a tumor microenvironment model to study breast cancer growth. Biomed. Mater. 2017;12(2):025009. doi: 10.1088/1748-605X/aa5d5c. [DOI] [PubMed] [Google Scholar]
- 14.Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 16.Chen W. Dendritic cells and cd4+cd25+ t regulatory cells: crosstalk between two professionals in immunity versus tolerance. Front. Biosci. 2006;11(1):1360. doi: 10.2741/1889. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Qin Y, Liu S. Cytokines, breast cancer stem cells (BCSCS) and chemoresistance. Clin. Transl. Med. 2018;7(1):6. doi: 10.1186/s40169-018-0205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin Y, Janseens J, Vandepitte J, Vandenbrande J, Opdebeek L, Raus J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992;12(5):1463–1466. [PubMed] [Google Scholar]
- 19.Cole S, Montero A, Garret E, Onicescu MG, Vandenberg T, Hutchens S, Diaz-Montero C. Elevated circulating myeloid derived suppressor cells (MDSC) are associated with inferior overall survival (OS) and correlate with circulating tumor cells (CTC) in patients with metastatic breast cancer. Cancer Res. 2009;69(24 Suppl):4135. doi: 10.1158/0008-5472.sabcs-09-4135. [DOI] [Google Scholar]
- 20.Collison LW, Pillai MR, Chaturvedi V, Vignali DAA. Regulatory t cell suppression is potentiated by target t cells in a cell contact, il-35- and il-10-dependent manner. J. Immunol. 2009;182(10):6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Columba-Cabezas S, Serafini B, Ambrosini E, et al. Induction of macrophage-derived chemokine/CCL22 expression in experimental autoimmune encephalomyelitis and cultured microglia: implications for disease regulation. J. Neuroimmunol. 2002;130(1–2):10–21. doi: 10.1016/S0165-5728(02)00170-4. [DOI] [PubMed] [Google Scholar]
- 22.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 23.de la Cruz-Merino L, Barco-Sánchez A, Henao Carrasco F, et al. New insights into the role of the immune microenvironment in breast carcinoma. Clin. Dev. Immunol. 2013;2013:1–11. doi: 10.1155/2013/785317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de la Cruz-Merino L, Henao-Carrasco F, García-Manrique T, Fernández-Salguero PM, de Villena M. Role of transforming growth factor beta in cancer microenvironment. Clin. Transl. Oncol. 2009;11(11):715–720. doi: 10.1007/s12094-009-0433-8. [DOI] [PubMed] [Google Scholar]
- 25.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9(4):212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dias S, Boyd R, Balkwill F. IL-12 regulates VEGF and MMPs in a murine breast cancer model. Int. J. Cancer. 1998;78(3):361–365. doi: 10.1002/(SICI)1097-0215(19981029)78:3<361::AID-IJC17>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Ellner JJ. Suppressor cells of man. Clin. Immunol. Rev. 1981;1(1):119–214. [PubMed] [Google Scholar]
- 29.Emens LA. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev. Anticancer Ther. 2012;12(12):1597–1611. doi: 10.1586/era.12.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emens LA. Breast cancer immunotherapy: facts and hopes. Clin. Cancer Res. 2018;24(3):511–520. doi: 10.1158/1078-0432.CCR-16-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escobar G, Moi D, Ranghetti A, et al. Genetic engineering of hematopoiesis for targeted ifn- delivery inhibits breast cancer progression. Sci. Transl. Med. 2014;6(217):217ra3. doi: 10.1126/scitranslmed.3006353. [DOI] [PubMed] [Google Scholar]
- 32.Foussat A, Cottrez F, Brun V, Fournier N, Breittmayer J-P, Groux H. A comparative study between t regulatory type 1 and cd4+ cd25+ t cells in the control of inflammation. J. Immunol. 2003;171(10):5018–5026. doi: 10.4049/jimmunol.171.10.5018. [DOI] [PubMed] [Google Scholar]
- 33.Fu G, Miao L, Wang M, et al. The postoperative immunosuppressive phenotypes of peripheral t helper cells are associated with poor prognosis of breast cancer patients. Immunol. Investig. 2017;46(7):647–662. doi: 10.1080/08820139.2017.1360337. [DOI] [PubMed] [Google Scholar]
- 34.Fu G, Miao L, Wang M, et al. The postoperative immunosuppressive phenotypes of peripheral T helper cells are associated with poor prognosis of breast cancer patients. Immunol. Invest. 2017;46(7):647–662. doi: 10.1080/08820139.2017.1360337. [DOI] [PubMed] [Google Scholar]
- 35.Gangemi S, Minciullo P, Adamo B, et al. Clinical significance of circulating interleukin-23 as a prognostic factor in breast cancer patients. J. Cell Biochem. 2012;113(6):2122–2125. doi: 10.1002/jcb.24083. [DOI] [PubMed] [Google Scholar]
- 36.Garaud S, Buisseret L, Solinas C, et al. Tumor-infiltrating B cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019;4(18):e129641. doi: 10.1172/jci.insight.129641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gatti-Mays ME, Balko JM, Gameiro SR, et al. If we build it they will come: targeting the immune response to breast cancer. NPJ Breast Cancer. 2019;5:37. doi: 10.1038/s41523-019-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer. 2008;8(1):57. doi: 10.1186/1471-2407-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr. Drug Targets. 2010;11(9):1133–1146. doi: 10.2174/138945010792006799. [DOI] [PubMed] [Google Scholar]
- 40.Goodwin JS, Ceuppens J. Regulation of the immune response by prostaglandins. J. Clin. Immunol. 1983;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- 41.Gritzapis AD, Mamalaki A, Kretsovali A, et al. Redirecting mouse T hybridoma against human breast and ovarian carcinomas: in vivo activity against HER-2/neu expressing cancer cells. Br. J. Cancer. 2003;88(8):1292–1300. doi: 10.1038/sj.bjc.6600888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gruber I, Landenberger N, Staebler A, et al. Relationship between circulating tumor cells and peripheral T-cells in patients with primary breast cancer. Anticancer Res. 2013;33(5):2233–2238. [PubMed] [Google Scholar]
- 43.Gu-Trantien C, Loi S, Garaud S, et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Invest. 2013;123(7):2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassin D, Garber OG, Meiraz A, Schiffenbauer YS, Berke G. Cytotoxic T lymphocyte perforin and Fas ligand working in concert even when Fas ligand lytic action is still not detectable. Immunology. 2011;133(2):190–196. doi: 10.1111/j.1365-2567.2011.03426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hemdan NY. Anti-cancer versus cancer-promoting effects of the interleukin-17-producing T helper cells. Immunol. Lett. 2013;149(1–2):123–133. doi: 10.1016/j.imlet.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Holen I, Lefley DV, Francis SE, et al. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget. 2016;7(46):75571–75584. doi: 10.18632/oncotarget.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollern DP, Xu N, Thennavan A, et al. B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast cancer. Cell. 2019;179(5):1191–1206.e21. doi: 10.1016/j.cell.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holzapfel BM, Wagner F, Thibaudeau L, Levesque JP, Hutmacher DW. Concise review: humanized models of tumor immunology in the 21st century: convergence of cancer research and tissue engineering. Stem Cells. 2015;33(6):1696–1704. doi: 10.1002/stem.1978. [DOI] [PubMed] [Google Scholar]
- 49.Iyengar NM, Zhou XK, Gucalp A, et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin. Cancer Res. 2016;22(9):2283–2289. doi: 10.1158/1078-0432.CCR-15-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaklamani V, O’Regan RM. New targeted therapies in breast cancer. Semin. Oncol. 2004;31:20–25. doi: 10.1053/j.seminoncol.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 51.Kan N. Analysis of 5-year survival among breast cancer patients with malignant pleural effusion receiving intrapleural OK-432 followed by adoptive transfer with cultured effusion lymphocytes. Gan To Kagaku Ryoho. 2003;30(11):1559–1561. [PubMed] [Google Scholar]
- 52.Katanov C, Lerrer S, Liubomirski Y, et al. Regulation of the inflammatory profile of stromal cells in human breast cancer: prominent roles for TNF-α and the NF-κB pathway. Stem Cell Res. Ther. 2015;6(1):87. doi: 10.1186/s13287-015-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khramtsova, G., C. Liao, and A. Khramtsov et al. The m2/alternatively activated macrophage phenotype correlates with aggressive histopathologic features and poor clinical outcome in early stage breast cancer. In: Poster Discussion Abstracts. American Association for Cancer Research; 2009, pp. 107–107. 10.1158/0008-5472.SABCS-09-107.
- 54.Knutson KL, Dang Y, Lu H, et al. IL-2 immunotoxin therapy modulates tumor-associated regulatory T cells and leads to lasting immune-mediated rejection of breast cancers in neu-transgenic mice. J. Immunol. 2006;177(1):84–91. doi: 10.4049/jimmunol.177.1.84. [DOI] [PubMed] [Google Scholar]
- 55.Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005;2(9):e284. doi: 10.1371/journal.pmed.0020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kontani K, Taguchi O, Ozaki Y, et al. Dendritic cell vaccine immunotherapy of cancer targeting MUC1 mucin. Int. J. Mol. Med. 2003 doi: 10.3892/ijmm.12.4.493. [DOI] [PubMed] [Google Scholar]
- 57.Korkaya H, Kim GI, Davis A, et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol. Cell. 2012;47(4):570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koru-Sengul T, Santander AM, Miao F, et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res. Treat. 2016;158(1):113–126. doi: 10.1007/s10549-016-3847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leber TM, Balkwill FR. Regulation of monocyte MMP-9 production by TNF-alpha and a tumour-derived soluble factor (MMPSF) Br. J. Cancer. 1998;78(6):724–732. doi: 10.1038/bjc.1998.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee-Chang C, Bodogai M, Martin-Montalvo A, et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J Immunol. 2013;191(8):4141–4151. doi: 10.4049/jimmunol.1300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J. Mammary Gland Biol. Neoplasia. 2002;7(2):177–189. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- 62.Leu CM, Wong FH, Chang C, Huang SF, Hu CP. Interleukin-6 acts as an antiapoptotic factor in human esophageal carcinoma cells through the activation of both STAT3 and mitogen-activated protein kinase pathways. Oncogene. 2003;22(49):7809–7818. doi: 10.1038/sj.onc.1207084. [DOI] [PubMed] [Google Scholar]
- 63.Leyland-Jones B. Trastuzumab: hopes and realities. Lancet Oncol. 2002;3(3):137–144. doi: 10.1016/S1470-2045(02)00676-9. [DOI] [PubMed] [Google Scholar]
- 64.Li K, Wei L, Huang Y, et al. Leptin promotes breast cancer cell migration and invasion via IL-18 expression and secretion. Int. J. Oncol. 2016;48(6):2479–2487. doi: 10.3892/ijo.2016.3483. [DOI] [PubMed] [Google Scholar]
- 65.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of b cells by cd4+ cd25+ regulatory t cells. J. Immunol. 2005;175(7):4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 66.Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168(4):724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin EY, Li J-F, Gnatovskiy L, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66(23):11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 68.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 2001;193(6):727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Linde N, Casanova-Acebes M, Sosa MS, et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun. 2018;9(1):21. doi: 10.1038/s41467-017-02481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu F, Lang R, Zhao J, et al. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res. Treat. 2011;130(2):645–655. doi: 10.1007/s10549-011-1647-3. [DOI] [PubMed] [Google Scholar]
- 71.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 2002;169(5):2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 72.Lu D, Zhou X, Yao L, Liu C, Jin F, Wu Y. Clinical implications of the interleukin 27 serum level in breast cancer. J. Investig. Med. 2014;62(3):627–631. doi: 10.2310/JIM.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 73.Maglione JE, Moghanaki D, Young LJ, et al. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61(22):8298–8305. [PubMed] [Google Scholar]
- 74.Mamessier E, Sylvain A, Thibult M-L, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Invest. 2011;121(9):3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Markowitz J, Wesolowski R, Papenfuss T, Brooks TR, Carson WE. Myeloid-derived suppressor cells in breast cancer. Breast Cancer Res. Treat. 2013;140(1):13–21. doi: 10.1007/s10549-013-2618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melief CJ, Kast WM. Lessons from T cell responses to virus induced tumours for cancer eradication in general. Cancer Surv. 1992;13:81–99. [PubMed] [Google Scholar]
- 77.Merlo A, Casalini P, Carcangiu ML, et al. Foxp3 expression and overall survival in breast cancer. J. Clin. Oncol. 2009;27(11):1746–1752. doi: 10.1200/JCO.2008.17.9036. [DOI] [PubMed] [Google Scholar]
- 78.Moon JJ, Huang B, Irvine DJ. Engineering nano- and microparticles to tune immunity. Adv. Mater. 2012;24(28):3724–3746. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Namjoshi P, Showalter L, Czerniecki BJ, Koski GK. T-helper 1-type cytokines induce apoptosis and loss of HER-family oncodriver expression in murine and human breast cancer cells. Oncotarget. 2019;10(57):6006–6020. doi: 10.18632/oncotarget.10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nazir SU, Kumar R, Singh A, et al. Breast cancer invasion and progression by MMP-9 through Ets-1 transcription factor. Gene. 2019;711:143952. doi: 10.1016/j.gene.2019.143952. [DOI] [PubMed] [Google Scholar]
- 81.Olkhanud PB, Damdinsuren B, Bodogai M, et al. Tumor-evoked regulatory b cells promote breast cancer metastasis by converting resting cd4+ t cells to t-regulatory cells. Cancer Res. 2011;71(10):3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park IH, Yang HN, Lee KJ, et al. Tumor-derived IL-18 induces PD-1 expression on immunosuppressive NK cells in triple-negative breast cancer. Oncotarget. 2017;8(20):32722–32730. doi: 10.18632/oncotarget.16281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parker DC. T cell-dependent b cell activation. Annu. Rev. Immunol. 1993;11(1):331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 84.Peng GL, Li L, Guo YW, et al. CD8+ cytotoxic and FoxP3+ regulatory T lymphocytes serve as prognostic factors in breast cancer. Am. J. Transl. Res. 2019;11(8):5039–5053. [PMC free article] [PubMed] [Google Scholar]
- 85.Peoples GE, Goedegebuure PS, Smith R, Linehan DC, Yoshino I, Eberlein TJ. Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc. Natl. Acad. Sci. USA. 1995;92(2):432–436. doi: 10.1073/pnas.92.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Polat K, Sahan S, Kodaz H, Günes S. A new classification method for breast cancer diagnosis: feature selection artificial immune recognition system (FS-AIRS) In: Wang L, Chen K, Ong YS, editors. Advances in Natural Computation, ICNC 2005. Berlin: Springer; 2005. [Google Scholar]
- 87.Pross HF, Sterns E, Macgillis DR. Natural killer cell activity in women at “high risk” for breast cancer, with and without benign breast syndrome. Int. J. Cancer. 1984;34(3):303–308. doi: 10.1002/ijc.2910340303. [DOI] [PubMed] [Google Scholar]
- 88.Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol. Ther. 2017;25(8):1769–1781. doi: 10.1016/j.ymthe.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Investig. 2003;21(6):873–886. doi: 10.1081/CNV-120025091. [DOI] [PubMed] [Google Scholar]
- 90.Romagnani C, Della Chiesa M, Kohler S, et al. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+ CD25hi T regulatory cells. Eur. J. Immunol. 2005;35(8):2452–2458. doi: 10.1002/eji.200526069. [DOI] [PubMed] [Google Scholar]
- 91.Sachs G. Lytic effector cell activity and major depressive disorder in patients with breast cancer: a prospective study. J. Neuroimmunol. 1995;59(1–2):83–89. doi: 10.1016/0165-5728(95)00029-2. [DOI] [PubMed] [Google Scholar]
- 92.Şahan S, Polat K, Kodaz H, Güneş S. A new hybrid method based on fuzzy-artificial immune system and -nn algorithm for breast cancer diagnosis. Comput. Biol. Med. 2007;37(3):415–423. doi: 10.1016/j.compbiomed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 93.Sawaki M, Ito Y, Tada K, et al. Efficacy and safety of trastuzumab as a single agent in heavily pretreated patients with HER-2/neu-overexpressing metastatic breast cancer. Tumori. 2004;90(1):40–43. doi: 10.1177/030089160409000110. [DOI] [PubMed] [Google Scholar]
- 94.Schaft N, Willemsen RA, de Vries J, et al. Peptide fine specificity of anti-glycoprotein 100 CTL is preserved following transfer of engineered TCR alpha beta genes into primary human T lymphocytes. J. Immunol. 2003;170(4):2186–2194. doi: 10.4049/jimmunol.170.4.2186. [DOI] [PubMed] [Google Scholar]
- 95.Scholl S, Squiban P, Bizouarne N, et al. Metastatic breast tumour regression following treatment by a gene-modified vaccinia virus expressing muc1 and il-2. J. Biomed. Biotechnol. 2003;2003(3):194–201. doi: 10.1155/S111072430320704X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti-tumor response and role in carcinogenesis. J. ImmunoTherapy Cancer. 2016 doi: 10.1186/s40425-016-0145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schwartzentruber DJ, Solomon D, Rosenberg SA, Topalian SL. Characterization of lymphocytes infiltrating human breast cancer, specific immune reactivity detected by measuring cytokine secretion. J. Immunother. 1992;12(1):1. doi: 10.1097/00002371-199207000-00001. [DOI] [PubMed] [Google Scholar]
- 98.Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Front. Biosci. 2008;13:5094–5107. doi: 10.2741/3066. [DOI] [PubMed] [Google Scholar]
- 99.Shafiee A, McGovern JA, Lahr CA, et al. Immune system augmentation via humanization using stem/progenitor cells and bioengineering in a breast cancer model study. Int. J. Cancer. 2018;143(6):1470–1482. doi: 10.1002/ijc.31528. [DOI] [PubMed] [Google Scholar]
- 100.Shak S. Overview of the trastuzumab (Herceptin) anti-HER2 monoclonal antibody clinical program in HER2-overexpressing metastatic breast cancer. Herceptin Multinational Investigator Study Group. Semin. Oncol. 1999;26(4 Suppl 12):71–77. [PubMed] [Google Scholar]
- 101.Shapiro H, Pecht T, Shaco-Levy R, et al. Adipose tissue foam cells are present in human obesity. J. Clin. Endocrinol. Metab. 2013;98(3):1173–1181. doi: 10.1210/jc.2012-2745. [DOI] [PubMed] [Google Scholar]
- 102.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 103.Standish LJ, Sweet ES, Novack J, et al. Breast cancer and the immune system. J. Soc. Integr. Oncol. 2008;6(4):158–168. [PMC free article] [PubMed] [Google Scholar]
- 104.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J. Immunother. Cancer. 2016;4(1):59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Su S, Liu Q, Chen J, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25(5):605–620. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 106.Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front. Immunol. 2014;5:508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Todorović-Raković N, Milovanović J. Interleukin-8 in breast cancer progression. J. Interferon Cytokine Res. 2013;33(10):563–570. doi: 10.1089/jir.2013.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trzonkowski P, Szmit E, Myśliwska J, Dobyszuk A, Myśliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin. Immunol. 2004;112(3):258–267. doi: 10.1016/j.clim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 109.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat. Rev. Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang J, Cai D, Ma B, Wu G, Wu J. Skewing the balance of regulatory T-cells and T-helper 17 cells in breast cancer patients. J. Int. Med. Res. 2011;39(3):691–701. doi: 10.1177/147323001103900301. [DOI] [PubMed] [Google Scholar]
- 111.Wang RF, Johnston SL, Zeng G, Topalian SL, Schwartzentruber DJ, Rosenberg SA. A breast and melanoma-shared tumor antigen: T cell responses to antigenic peptides translated from different open reading frames. J. Immunol. 1998;161(7):3598–3606. [PubMed] [Google Scholar]
- 112.Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat. Rev. Cancer. 2005;5(8):591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 113.Wong PY, Staren ED, Tereshkova N, Braun DP. Functional analysis of tumor-infiltrating leukocytes in breast cancer patients. J. Surg. Res. 1998;76(1):95–103. doi: 10.1006/jsre.1998.5301. [DOI] [PubMed] [Google Scholar]
- 114.Yang J, Bahcecioglu G, Zorlutuna P. The extracellular matrix and vesicles modulate the breast tumor microenvironment. Bioengineering. 2020;7(4):124. doi: 10.3390/bioengineering7040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang X, Hu C, Tong F, Liu R, Zhou Y, Qin L, Ouyang L, Gao H. Tumor microenvironment-responsive dual drug dimer-loaded PEGylated bilirubin nanoparticles for improved drug delivery and enhanced immune-chemotherapy of breast cancer. Adv. Funct. Mater. 2019;29:1901896. doi: 10.1002/adfm.201901896. [DOI] [Google Scholar]
- 116.Yeong J, Lim JCT, Lee B, et al. High densities of tumor-associated plasma cells predict improved prognosis in triple negative breast cancer. Front. Immunol. 2018;9:1209. doi: 10.3389/fimmu.2018.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yue X, Nguyen TD, Zellmer V, Zhang S, Zorlutuna P. Stromal cell-laden 3D hydrogel microwell arrays as tumor microenvironment model for studying stiffness dependent stromal cell-cancer interactions. Biomaterials. 2018;170:37–48. doi: 10.1016/j.biomaterials.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 118.Zhang JM, An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao Z, Ukidve A, Krishnan V, et al. Systemic tumour suppression via the preferential accumulation of erythrocyte-anchored chemokine-encapsulating nanoparticles in lung metastases. Nat. Biomed. Eng. 2020 doi: 10.1038/s41551-020-00644-2. [DOI] [PubMed] [Google Scholar]
- 120.Zhou J, Zhong Y. Breast cancer immunotherapy. Cell Mol. Immunol. 2004;1(4):247–255. [PubMed] [Google Scholar]
- 121.Zhu S, Lin J, Qiao G, Wang X, Xu Y. Tim-3 identifies exhausted follicular helper T cells in breast cancer patients. Immunobiology. 2016;221(9):986–993. doi: 10.1016/j.imbio.2016.04.005. [DOI] [PubMed] [Google Scholar]