Abstract
Transcranial magnetic resonance (MR)-guided focused ultrasound (FUS) therapy is an emerging and minimally invasive treatment for movement disorders. There are limited reports on its long-term outcomes for tremor-dominant Parkinson’s disease (TDPD). We aimed to investigate the 1-year outcomes of ventralis intermedius (VIM) thalamotomy with FUS in patients with TDPD. Patients with medication-refractory TDPD were enrolled and underwent unilateral VIM-FUS thalamotomy. Neurologists specializing in movement disorders evaluated the tremor symptoms and disability using Parts A, B, and C of the Clinical Rating Scale for Tremor (CRST) at baseline and at 1, 3, and 12 months. In all, 11 patients (mean age: 71.6 years) were included in the analysis. Of these, five were men. The median (interquartile range) improvement from baseline in hand tremor score, the total score, and functional disability score were 87.9% (70.5–100.0), 65.3% (55.7–87.7), and 66.7% (15.5–85.1), respectively, at 12 months postoperatively. This prospective study demonstrated an improvement in the tremor and disability of patients at 12 months after unilateral VIM-FUS thalamotomy for TDPD. In addition, there were no serious persistent adverse events. Our results indicate that VIM-FUS thalamotomy can be safely and effectively used to treat patients with TDPD. A randomized controlled trial with a larger cohort and long blinded period would help investigate the recurrence, adverse effects, placebo effects, and longer efficacy of this technique.
Keywords: magnetic resonance imaging, thalamotomy, transcranial focused ultrasound, tremor-dominant Parkinson’s disease, ventralis intermedius nucleus
Introduction
Tremor-dominant Parkinson’s disease (TDPD) is a subtype of Parkinson’s disease. It is predominantly characterized by tremor with an earlier onset and slower progression than other subtypes.1) However, other Parkinsonian symptoms, such as bradykinesia, rigidity, and postural instability are either absent or mild.2) Resting tremor is usually prominent; however, postural and action tremors may be evident and intolerable. This can be attributed to the associated physical exhaustion and severe restriction in social intercourse.3) Despite a relatively good neurological prognosis, the tremor impairs the quality of life in patients with TDPD.4–7) Furthermore, it may show poor response to medications.8) Therefore, clinicians should consider surgical treatment modalities in medication-refractory cases.
Transcranial magnetic resonance (MR)-guided focused ultrasound (FUS) is a promising and minimally invasive stereotactic treatment.9) There are several reports on the efficacy of ventralis intermedius (VIM) thalamotomy utilizing FUS in improving the quality of life and tremor in patients with TDPD.10–16) Nonetheless, most of them had a follow-up of maximum 6 months, and there is limited literature on its longer efficacy and safety. Thus, we prospectively investigated the 1-year outcomes and adverse events of VIM thalamotomy with FUS for TDPD.
Methods
Patients
We enrolled patients with medication-refractory TDPD, as diagnosed by neurologists specializing in movement disorders (H.I., S.F., and T.K.), between April 2017 and September 2018. TDPD was defined as tremor-dominant with tremor dominant/postural instability and gait difficulty ratio >1.15. This was calculated from the Unified Parkinson’s Disease Rating Scale (UPDRS) in the “on” state according to the criteria postulated by Stebbins et al.17) We enrolled the patients with clinically significant tremor and substantial disability in the performance of daily activities. We defined the former by a score ≥2 on the postural or action item of the Clinical Rating Scale for Tremor (CRST) in the dominant hand, and the latter was defined as a score of ≥2 in any of the disability subsections of the scale. We excluded patients with the following conditions: unstable cardiac conditions, cerebral tumor, intracranial aneurysm or arteriovenous fistula, cognitive impairment (defined by a score of ≤24 on the mini-mental state examination), or history of deep brain stimulation or stereotactic cerebral ablation. This enrollment necessitated a skull density ratio (SDR) of > 0.25 in a screening computed tomography (CT) scan.18) We calculated the SDR as the ratio between the mean values in Hounsfield units for the bone marrow and cortical bone.18) The SDRs were adjusted using an internal formula of InSightec Japan because our CT scanner (Aquilion ONE™ [TSX-301A/2A], Toshiba Medical Systems, Tochigi, Japan) tends to show lower SDRs than the CT scanners of other manufacturers such as GE Healthcare and Philips Healthcare (unpublished internal data).
Ethical approval for standard protocol, clinical registration, and patient consent
We prospectively collected and analyzed the data. We obtained approval from the relevant ethical review board (University hospital Medical Information Network Clinical Trials Registry number: UMIN000033940). We included a patient with a pacemaker from other trial12) after obtaining approval from the ethical review board. We obtained written informed consent from all patients.
FUS procedure
We attached a stereotactic frame onto the skull under local anesthesia. We performed FUS using Exablate Neuro (InSightec, Haifa, Israel) with a 1.5-T MR imaging system (Signa HDx, GE Healthcare, Milwaukee, WI, USA) in all patients with TDPD. The unilateral VIM nucleus was targeted in all cases. The treatment side was decided considering patients’ opinion, dominant hand, and severity of the tremor. We set the target at 6.0 mm anterior to the posterior commissure on the anterior commissure-posterior commissure line, 1.5 mm superior to the anterior commissure-posterior commissure plane, and 12.0 mm lateral to the ipsilateral wall of the third ventricle. The targeted points corresponded to the posterolateral part of the VIM according to the stereotactic atlas of Schaltenbrand and Wahren.19) We then created an additional lesion 0.5 mm anterior to the first one in patients with rigidity and tremor. This facilitated involving the nucleus ventralis oralis posterior.11) We increased the sonication energy in a stepwise fashion during treatment. Moreover, we verified the adequacy of the location, size, shape, and temperature of the heated spot. We confirmed the effects of the technique and the absence of side effects at a temperature that causes a reversible effect. Furthermore, we generated a permanent lesion at a higher temperature. The procedures were terminated when the symptoms improved sufficiently, the temperature did not increase with higher sonication energy, or adverse neurological events were detected taking the patient’s satisfaction and opinion into account. All the procedures were performed, and termination was finally determined by the neurosurgeons and radiologist (K.Y., T.T., and T.Y.). This was followed by assessment of the lesion locations and absence of radiographic complications such as edema, hemorrhage, and extension or elongation of the lesion in the adjacent brain structures of the VIM, including the internal capsule and ventralis caudalis nucleus, using MR imaging on the post-procedural day.
Outcome assessments
The neurologists (H.I., S.F., T.O., and T.K.) used the CRST to evaluate the tremor conditions. Higher scores indicated more severe tremor.20) They examined the CRST scores under medication before the procedure (baseline) and at 1, 3, and 12 months postoperatively. The primary outcome measure was the efficacy, defined as the change in CRST scores of the hand contralateral to the treated VIM (range 0–32) between baseline and 12 months postoperatively. This was calculated using Parts A (three items of the hand component) and B (five items) of the CRST. In contrast, the change from baseline to 12 months in total tremor scores on the CRST (range 0–144) and the functional disability evaluated by Part C of the CRST (range 0–32) were the secondary outcomes. This allowed us to calculate an improvement ratio for the respective time point of assessment as a percent decrease in the CRST score, compared with baseline. Additionally, the levodopa equivalent dose (LED) was recorded at baseline, 1 week, and 1, 3, and 12 months after the procedure.21) We also assessed the complications and tremor recurrences.
Results
Participants
We treated a total of 11 patients with TDPD. Of these, dopamine transporter scan using 123I-ioflupane single-photon emission CT demonstrated reduced radioligand striatal uptake in three patients, and 123I-metaiodobenzylguanidine scintigraphy revealed abnormally high washout rate in two patients. All the 11 patients met the abovementioned criteria for TDPD defined with tremor dominant/postural instability and gait difficulty ratio. One patient was lost to follow-up at 12 months because of an unrelated injury. Table 1 summarizes the patient characteristics. The patients had a mean age (± standard deviation) of 71.6 ± 6.8 years (range: 58–79). There were five men (45.5%) and six women (54.5%). They had a median SDR (interquartile range) of 0.35 (0.30–0.39). The targeted VIM was located in the left hemisphere in seven patients (63.6%). All the 11 patients were right-handed, but four patients desired to treat their left-side tremor because the tremor was more severe on the left side. All the patients were at Hoehn & Yahr stages 1–3, with those at stages 1–2 representing 72.7%. The median scores on the UPDRS Part III were 25 (18–34) at baseline and 9 (5–13) at 12 months postoperatively.
Table 1. Patient characteristics.
| Characteristic | N = 11 |
|---|---|
| Age, yearsa | 71.6 ± 6.8 (58–79) |
| Sex, no. of men (%) | 5 (45.5) |
| MMSEb | 30.0 (29.5–30.0) |
| Skull density ratio | 0.35 (0.29–0.39) |
| Disease duration, years | |
| From onsetb | 6.0 (4.5–9.0) |
| From diagnosisb | 4.0 (3.2–5.5) |
| Baseline scores on CRST | |
| Contralateral hand tremorb | 7 (7–11) |
| Totalb | 20 (17–24) |
| Functional disabilityb | 6 (4–8) |
| UPDRS Part III score at baselineb | 25 (18–34) |
| UPDRS Part III score at 1 yearb | 9 (5–13) |
| Hoehn and Yahr scale at baseline - No. of patients | |
| Stage 1 | 3 |
| Stage 2 | 5 |
| Stage 3 | 3 |
CRST: Clinical Rating Scale for Tremor; MMSE: Mini-Mental State Examination; UPDRS: Unified Parkinson’s Disease Rating Scale
a Mean ± standard deviation (range).
b Median (interquartile range).
FUS
We administered a median number of 9 (9–10) sonications to the patients. They had a median skull surface area of 359.0 cm2 (357.5–375.5) and a skull volume of 243.7 cm3 (229.4–277.4). There were 966 (952–997) active transducer elements in the treatment field. The median maximum delivered energy and maximum temperature in the heated spot were 26087 J (18141–32642) and 57.0°C (56.5–60.0), respectively. Furthermore, the median procedure time from the first to the last sonication was 93.0 min (86.5–97.0), and the median hospital stay was 5.0 days (4.0–16.0). On the T2-weighted MR images on the post-procedural day, the median lesion volume was 152.3 mm3 (125.6–258.3).
Tremor and functional disability
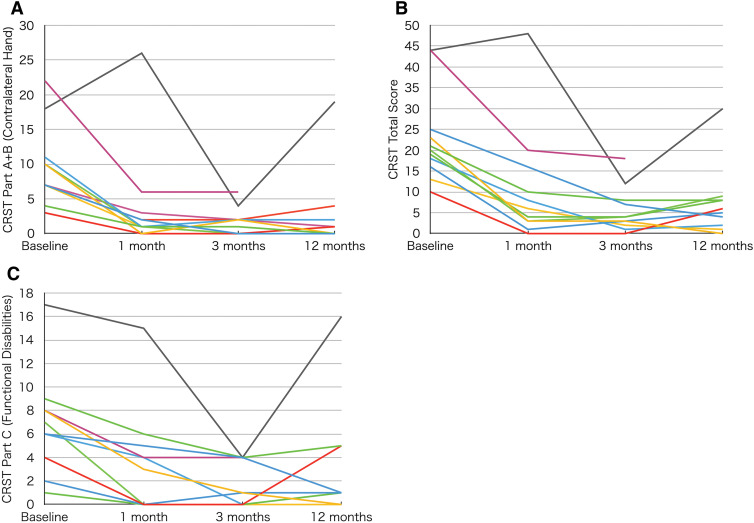
All patients experienced significant improvement in tremor during the procedure. Figure 1 outlines the changes in score on each scale over time. We observed a tremor recurrence in one case at 12 months postoperatively. In this case, the maximum temperature was 49°C, and the lesion volume was 46.2 mm3, which were the lowest and smallest of all the patients.
Fig. 1.
Changes in hand tremor, total, and disability scores over time. Panel A shows the individual hand tremor scores contralateral to the treated hemisphere (Part A + B on the CRST), which revealed tremor improvement, except in one recurrent case. Panel B (total scores on the CRST) and Panel C (Part C on the CRST, disability scores) show the corresponding improvements. CRST: Clinical Rating Scale for Tremor
The median improvement in CRST scores of the hand contralateral to the treated VIM between baseline and 12 months postoperatively was 87.9% (70.5–100.0). The median scores of the treated upper extremity showed the improvement to be 66.7% (50.0–100.0), 100.0% (100.0–100.0), and 100.0% (100.0–100.0) in resting, postural, and action tremors, respectively. Furthermore, the median improvement in total tremor scores on the CRST was 65.3% (55.7–87.7) from baseline to 12 months, and the median improvement of functional disability in Part C on the CRST was 66.7% (15.5–85.1).
Medications
Table 2 shows the time course of LED in the individual cases. The medications were adjusted by the neurologists (H.I., S.F., T.O., and T.K.) according to patients’ opinions based on parkinsonism. The LEDs were reduced in three cases during the follow-up period and remained lower than the baseline in one case at 12 months postoperatively. In contrast, they remained unchanged throughout the follow-up period in five patients, and we observed an increase in four patients at the end of the follow-up.
Table 2. Levodopa equivalent dose in individual cases.
| Case No. | Baseline | 1 week | 1 month | 3 months | 12 months |
|---|---|---|---|---|---|
| 1 | 300 | 300 | 250 | 300 | 300 |
| 2 | 711 | 266 | 399 | 964 | 771 |
| 3 | 0 | 0 | 300 | 300 | 300 |
| 4 | 550 | 350 | 350 | 400 | 500 |
| 5 | 300 | 300 | 300 | 300 | NA |
| 6 | 636 | 636 | 636 | 636 | 732 |
| 7 | 262 | 262 | 262 | 262 | 262 |
| 8* | 0 | 0 | 0 | 0 | 0 |
| 9 | 200 | 200 | 200 | 200 | 200 |
| 10 | 399 | 399 | 399 | 399 | 399 |
| 11 | 300 | 400 | 400 | 400 | 400 |
NA: not applicable
*The patient refused to take medications due to the lack of efficacy.
Adverse events
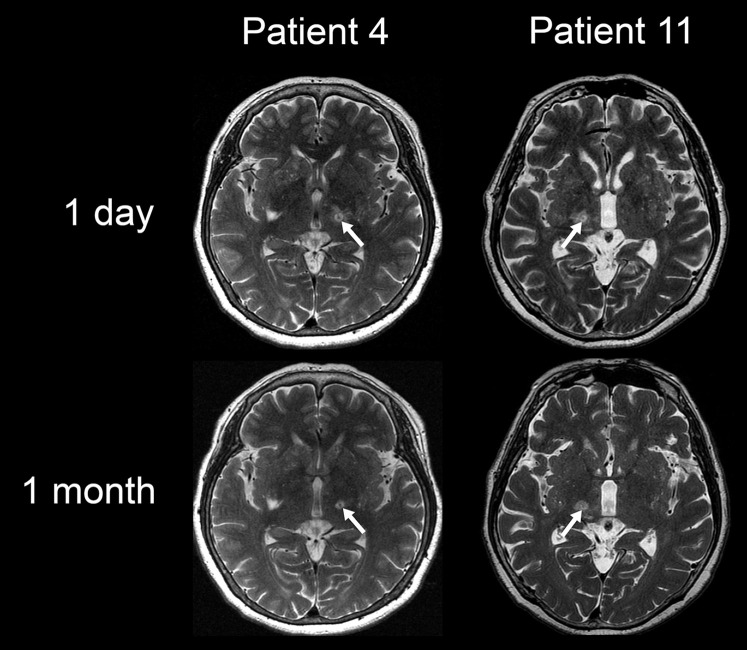
Table 3 summarizes the adverse events recorded during the follow-up period. Most adverse events were mild and transient, and improved within 12 months. There were no records of severe adverse events, although mild dysesthesia was observed in the treated hand in one patient (Patient 11) at 12 months. The lesion showed lateral extension, which was more evident on the MR imaging 1 month after the procedure than the postoperative day (Fig. 2). Headache was the most common adverse event and occurred only during sonication, disappearing immediately after the sonication was terminated. Notably, no delayed adverse events were observed.
Table 3. Adverse events.
| Events | No. of cases | ||||
|---|---|---|---|---|---|
| During procedure | 1 day | 1 month | 3 months | 12 months | |
| Related to thalamotomy | |||||
| Headache | 9 | 0 | 0 | 0 | 0 |
| Floating sensation | 3 | 0 | 0 | 0 | 0 |
| Gait disturbance | 1 | 3 | 1 | 0 | 0 |
| Exacerbation of bradykinesia | 0 | 3 | 0 | 0 | 0 |
| Dysesthesia | 1 | 1 | 1 | 1 | 1 |
| Hemiparesis | 1 | 1 | 0 | 0 | 0 |
| Hypoesthesia | 0 | 1 | 1 | 1 | 0 |
| Ageusia | 0 | 1 | 1 | 0 | 0 |
| Hypotonia | 0 | 1 | 0 | 0 | 0 |
| Bradypragia | 0 | 1 | 0 | 0 | 0 |
| Dysphagia | 0 | 1 | 0 | 0 | 0 |
| Dysarthria | 0 | 1 | 1 | 1 | 0 |
| Related to stereotactic frame | |||||
| Eyelid edema | 0 | 2 | 0 | 0 | 0 |
Fig. 2.
T2-weighted magnetic resonance imaging of demonstrative cases. In the case with dysesthesia remaining at 1 year (Patient 11), the lesion showed lateral extension, which was more evident at 1 month than the postoperative day. In contrast, the demonstrative case without any adverse events (Patient 4) showed a lesion staying in a round shape at 1 month. (White arrows represent lesions created by focused ultrasound thalamotomy.)
Discussion
We found an improvement in the treated-hand tremor after the procedures in all patients. This was concomitant with an improvement in functional disability. Although the improvement persisted in most cases at 12 months, there was recurrence in one case.
Resting tremor is usually prominent in TDPD, whereas postural and action tremors may be evident and disable the patients’ daily activities, lowering their quality of life.3–7) This calls for an improvement in functional disability and tremor. There have been no randomized trials of FUS for TDPD that kept participants blinded for >3 months. Bond et al.16) conducted a randomized crossover clinical trial on VIM-FUS thalamotomy for TDPD. In all, 14 patients treated with unilateral VIM-FUS thalamotomy showed persistent improvement in tremor in the blinded phase at 3 months and in the open-label phase at 12 months. Furthermore, there was an improvement in tremor in the sham cohort, indicating placebo effects, which diminished at 3 months. There was an improvement in the quality of life, as measured by Part C of the CRST and the 39-item Parkinson’s Disease Questionnaire at 3 months. Thereafter, they also reported an improvement in the quality of life in the open-label phase at 12 months.10) They suggested that the placebo effects would not remain long. Thus, the improvement in hand tremor and disability scores in our patients at 12 months could be considered evidence of the effects. However, we cannot completely rule out placebo effects. This warrants a randomized controlled trial with more patients and longer follow-up.
Despite the tremor improvement, the LEDs were unchanged or increased in most cases during the follow-up period. This suggested the lack of association between VIM-FUS thalamotomy for TDPD and the reduction of LEDs. The patients had medication-refractory tremor, confirmed by the facts that high dose of medications did not exert sufficient effects on the tremor or that the doses could not be increased because of their side effects. Therefore, an increase in medication dosage did not affect tremor improvement. The medications were continued or increased for the treatment of other symptoms, such as bradykinesia and rigidity, and to achieve maximum tremor suppression. This also caused the discrepancy between the LEDs and UPDRS Part III scores. The improvement in the UPDRS Part III scores was contributed by tremor reduction. Previous reports suggest that VIM thalamotomy does not significantly improve non-tremor Parkinsonian symptoms, including bradykinesia, rigidity, and gait disturbance; in contrast, researchers have established its effects on Parkinsonian tremor.22–24) Consequently, patients with non-tremor symptoms or progression would need to continue or increase the medications even after VIM-FUS thalamotomy. This is related to the Hoehn & Yahr stages of the patients we treat. As VIM thalamotomy is performed to reduce tremor, we need to consider the merit that the treatment can provide for each patient. Specifically, patients should be treated with the expectation to reduce medications but based on whether VIM thalamotomy will improve their quality of life. From this viewpoint, patients in advanced stages with severe symptoms other than tremors may not be eligible for this treatment. Moreover, because TDPD is a disease with slow progression, with a study showing it takes approximately 20 years on average from the onset to the Hoehn & Yahr stage 4, long-term follow-up is needed after VIM-FUS thalamotomy.
There are limited reports on the recurrence of TDPD symptoms after FUS. Fasano et al. reported that small lesion size may cause recurrence after VIM-FUS thalamotomy for Parkinsonian tremor.25) As mentioned above, the maximum temperature was the lowest, and the lesion volume was the smallest in the recurrent case in our study. Nonetheless, the target was accurate. Therefore, insufficient ablation was the probable cause of this recurrence. However, we cannot rule out other possible factors that contributed to the recurrence as there was only one recurrent case. Further investigations with larger samples and longer follow-up periods are necessary for analyzing the recurrence rates and associated factors.
In the case of the patient whose mild dysesthesia remained at 12 months, the lateral extended part of the lesion possibly involved sensory fibers of the thalamocortical pathway in the medial part of the internal capsule.26) The lesions sometimes suddenly change their shape during treatment. These changes include partial lesion extension and lateral elongation. Exablate Neuro is equipped with filter mask tools to reduce excessive energy and can thereby correct the shape of the lesion after such changes are detected. However, methods to predict shape changes have not yet been established. Therefore, a gradual increase in energy is the only method to avoid adverse events due to changes in shape. However, this will decrease the heating efficiency, and the target might not reach the therapeutic temperatures in cases where the target temperature is difficult to increase.27) Moreover, changes in the lesion shape sometimes occur during sonication aimed to ablate the target. It is difficult to completely exclude the risks for adverse events due to such changes. This necessitates investigations for strict shape control or shape change prediction.
Randomized controlled trials conducted on FUS-VIM thalamotomy showed ataxia in 45.0% of the patients with TDPD and in 19.6% patients with essential tremor after the procedure.16,28) In our study, we observed transient gait disturbance due to ataxia in 27.3% patients. TDPD is a subtype of Parkinson’s disease with relatively mild symptoms and slow progression.2) Nonetheless, its etiology is different from that of essential tremor, and the patients can be vulnerable to adverse events.29–35) Thus, we need to be careful to prevent fall-related events after FUS for TDPD.
Limitations
The small sample size was the primary limitation of our study. Thus, the statistical analysis to detect the factors associated with recurrence or adverse events was not adequate. The open-label, single-arm design and the fact that we could not eliminate the placebo effects were further limitations. Hence, a randomized controlled trial with a larger cohort and sufficiently long blinded phase is warranted for further investigation.
Conclusions
Our results revealed a persistent tremor reduction at 12 months after VIM-FUS thalamotomy for TDPD. In addition, there was a substantial improvement in disability. There were no severe adverse events, and most events were transient. These results indicate that we can safely and effectively treat patients with medication-refractory TDPD by VIM-FUS thalamotomy. Further study is warranted to investigate the recurrence, adverse events, and placebo effects.
Ethical Compliance Statement
Name of the ethics committee or IRB that approved the study: the Tokushukai Ethical Review Board (Tokyo, Japan).
Clinical Trial Registration number: UMIN000033940
Informed Consent
All the patients provided written informed consent.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Conflicts of Interest Disclosure
This study was sponsored by InSightec. The sponsor had no role in the data collection, data analysis, data inter- pretation, writing of the report, or decision to submit for publication. All authors have no conflict of interest. All JNS member authors have registered online Self-reported COI Disclosure Statement Forms through the website.
References
- 1).Marras C: Subtypes of Parkinson’s disease: state of the field and future directions. Curr Opin Neurol 28: 382–386, 2015 [DOI] [PubMed] [Google Scholar]
- 2).Zaidel A, Arkadir D, Israel Z, Bergman H: Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol 22: 387–393, 2009 [DOI] [PubMed] [Google Scholar]
- 3).Deuschl G: New treatment options for tremors. N Engl J Med 342: 505–507, 2000 [DOI] [PubMed] [Google Scholar]
- 4).Marras C, Rochon P, Lang AE: Predicting motor decline and disability in Parkinson disease: a systematic review. Arch Neurol 59: 1724–1728, 2002 [DOI] [PubMed] [Google Scholar]
- 5).Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D: Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord 21: 1123–1130, 2006 [DOI] [PubMed] [Google Scholar]
- 6).Jankovic J, Kapadia AS: Functional decline in Parkinson disease. Arch Neurol 58: 1611–1615, 2001 [DOI] [PubMed] [Google Scholar]
- 7).Marttila RJ, Rinne UK, Marttila RJ: Progression and survival in Parkinson’s disease. Acta Neurol Scand 84: 24–28, 1991 [DOI] [PubMed] [Google Scholar]
- 8).Selikhova M, Kempster PA, Revesz T, Holton JL, Lees AJ: Neuropathological findings in benign tremulous parkinsonism. Mov Disord 28: 145–152, 2013 [DOI] [PubMed] [Google Scholar]
- 9).Bauer R, Martin E, Haegele-Link S, Kaegi G, von Specht M, Werner B: Noninvasive functional neurosurgery using transcranial MR imaging-guided focused ultrasound. Parkinsonism Relat Disord 20: S197–S199, 2014 [DOI] [PubMed] [Google Scholar]
- 10).Sperling SA, Shah BB, Barrett MJ, et al. : Focused ultrasound thalamotomy in Parkinson disease: nonmotor outcomes and quality of life. Neurology 91: e1275–e1284, 2018 [DOI] [PubMed] [Google Scholar]
- 11).Ito H, Fukutake S, Yamamoto K, Yamaguchi T, Taira T, Kamei T: Magnetic resonance imaging-guided focused ultrasound thalamotomy for Parkinson’s disease. Intern Med 57: 1027–1031, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Ito H, Fukutake S, Yamamoto K, et al. : Magnetic resonance imaging-guided focused ultrasound thalamotomy for Parkinson’s disease with cardiac pacemaker: a case report. Mov Disord Clin Pract 5: 339–340, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Yamamoto K, Ito H, Fukutake S, Kamei T, Yamaguchi T, Taira T: Ventralis intermedius thalamotomy with focused ultrasound for patients with low skull density ratio. Mov Disord 34: 1239–1240, 2019 [DOI] [PubMed] [Google Scholar]
- 14).Zaaroor M, Sinai A, Goldsher D, Eran A, Nassar M, Schlesinger I: Magnetic resonance-guided focused ultrasound thalamotomy for tremor: a report of 30 Parkinson’s disease and essential tremor cases. J Neurosurg 128: 202–210, 2018 [DOI] [PubMed] [Google Scholar]
- 15).Moosa S, Martínez-Fernández R, Elias WJ, Del Alamo M, Eisenberg HM, Fishman PS: The role of high-intensity focused ultrasound as a symptomatic treatment for Parkinson’s disease. Mov Disord 34: 1243–1251, 2019 [DOI] [PubMed] [Google Scholar]
- 16).Bond AE, Shah BB, Huss DS, et al. : Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease: a randomized clinical trial. JAMA Neurol 74: 1412–1418, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC: How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord 28: 668–670, 2013 [DOI] [PubMed] [Google Scholar]
- 18).Chang WS, Jung HH, Zadicario E, et al. : Factors associated with successful magnetic resonance-guided focused ultrasound treatment: efficiency of acoustic energy delivery through the skull. J Neurosurg 124: 411–416, 2016 [DOI] [PubMed] [Google Scholar]
- 19).Schaltenbrand G, Wahren W: Atlas for stereotaxy of the human brain. 2nd ed. (Hassler R, ed.). Georg Thieme Publishers; 1977 [Google Scholar]
- 20).Fahn S, Tolosa E, Marín C: Clinical rating scale for tremor. In: Jankovic J, Tolosa E, eds. Parkinson’s disease and movement disorders. 2nd ed. Williams & Wilkins; 1993: 271–280 [Google Scholar]
- 21).Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE: Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25: 2649–2653, 2010 [DOI] [PubMed] [Google Scholar]
- 22).Jankovic J, Cardoso F, Grossman RG, Hamilton WJ: Outcome after stereotactic thalamotomy for parkinsonian, essential, and other types of tremor. Neurosurgery 37: 680–686; discussion 686–687, 1995 [DOI] [PubMed] [Google Scholar]
- 23).Iacono RP, Henderson JM, Lonser RR: Combined stereotactic thalamotomy and posteroventral pallidotomy for Parkinson’s disease. J Image Guid Surg 1: 133–140, 1995 [DOI] [PubMed] [Google Scholar]
- 24).Burchiel KJ: Thalamotomy for movement disorders. Neurosurg Clin N Am 6: 55–71, 1995 [PubMed] [Google Scholar]
- 25).Fasano A, Llinas M, Munhoz RP, Hlasny E, Kucharczyk W, Lozano AM: MRI-guided focused ultrasound thalamotomy in non-ET tremor syndromes. Neurology 89: 771–775, 2017 [DOI] [PubMed] [Google Scholar]
- 26).Groothuis DR, Duncan GW, Fisher CM: The human thalamocortical sensory path in the internal capsule: evidence from a small capsular hemorrhage causing a pure sensory stroke. Ann Neurol 2: 328–331, 1977 [DOI] [PubMed] [Google Scholar]
- 27).Yamamoto K, Ito H, Fukutake S, et al. : Factors associated with heating efficiency in transcranial focused ultrasound therapy. Neurol Med Chir (Tokyo) 60: 594–599, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Elias WJ, Lipsman N, Ondo WG, et al. : A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med 375: 730–739, 2016 [DOI] [PubMed] [Google Scholar]
- 29).Marjama-Lyons J, Koller W: Tremor-predominant Parkinson’s disease. Approaches to treatment. Drugs Aging 16: 273–278, 2000 [DOI] [PubMed] [Google Scholar]
- 30).Rajput AH, Voll A, Rajput ML, Robinson CA, Rajput A: Course in Parkinson disease subtypes: A 39-year clinicopathologic study. Neurology 73: 206–212, 2009 [DOI] [PubMed] [Google Scholar]
- 31).Gallay MN, Jeanmonod D, Liu J, Morel A: Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Struct Funct 212: 443–463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Llinás R, Urbano FJ, Leznik E, Ramírez RR, van Marle HJ: Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci 28: 325–333, 2005 [DOI] [PubMed] [Google Scholar]
- 33).Albin RL, Young AB, Penney JB: The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989 [DOI] [PubMed] [Google Scholar]
- 34).Helmich RC, Toni I, Deuschl G, Bloem BR: The pathophysiology of essential tremor and Parkinson’s tremor. Curr Neurol Neurosci Rep 13: 378, 2013 [DOI] [PubMed] [Google Scholar]
- 35).Nagaseki Y, Shibazaki T, Hirai T, et al. : Long-term follow-up results of selective VIM-thalamotomy. J Neurosurg 65: 296–302, 1986 [DOI] [PubMed] [Google Scholar]