Abstract
Several basic experimental studies have demonstrated that statins have beneficial effects for intracranial aneurysm (IA). Clinical studies on unruptured IAs, however, remain limited to four retrospective studies that have reached different conclusions. This study was the first open-label, multicenter, randomized controlled trial to assess the preventive effects of atorvastatin. Patients with unruptured small saccular IAs were randomly assigned to statin and control groups. The primary endpoint was a composite of aneurysm growth of ≥0.5 mm, new bleb formation confirmed from magnetic resonance (MR) angiography, and rupture. Enrollment was prematurely terminated due to unexpectedly slow enrollment. Of 231 patients (275 target IAs), 110 patients (128 IAs) were randomly assigned to the statin group and 121 patients (147 IAs) to the control group. After excluding 22 dropout patients, 107 IAs in the 93 statin group patients and 140 IAs in the 116 control group patients were finally analyzed. No significant differences of basic characteristics were evident between groups, except for significantly higher systolic pressure in the statin group (P = 0.03). The primary endpoint occurred in 28 IAs (20.0%) in the control group and in 17 IAs (15.9%) in the statin group. No aneurysm rupture was confirmed in either group. Significant beneficial effects of statin for IAs were not demonstrated for the primary endpoint (log-rank P = 0.359). This randomized trial did not establish any preventive effects of atorvastatin for unruptured small IAs. Further studies of larger cohorts are required to clarify the efficacy of statins for patients with unruptured IAs.
Clinical trial registration: UMIN000005135
Keywords: cerebral aneurysm, small unruptured aneurysm, statin, randomized study
Introduction
Subarachnoid hemorrhage (SAH) due to ruptured intracranial aneurysm (IA) is a devastating condition, not only carrying a high risk of permanent neurological deficit or death1–4) but also often affecting relatively young patients in the most productive period of life compared to other stroke subtypes.5) Among the known surrogates for assessing rupture risk of IA, such as location and irregular shape of the IA, hypertension, and smoking habit, IA size is the factor most widely used in therapeutic decision-making for patients with unruptured IAs.6–8) Young to middle-aged patients with large IAs often become candidates for prophylactic surgery involving either endovascular coiling or microsurgical clipping. However, greater difficulty is seen in the decision-making on surgical indications for non-large IAs, due to not only the considerable adverse event rates for both coiling and clipping6,9) but also the favorable natural history of small IAs.10) Primary preventive care for SAH using less-invasive medical treatments would be highly beneficial for some patients with unruptured IAs, considering recent circumstances in which the number of patients with unruptured IAs has been growing due to widespread use of non-invasive diagnostic imaging modalities such as magnetic resonance imaging (MRI).11)
Statins (3-hydroxy-3-methhylglutaryl coenzyme A reductase inhibitors) have been established as potent cholesterol-lowering drugs for the treatment of hyperlipidemia, and have been shown to confer vascular protective effects by inhibiting several inflammatory pathways also known as “pleiotropic” effects. These agents are widely used for both primary and secondary prevention of cardiovascular and cerebrovascular diseases.12–15) Several experimental studies using rodent IA models have demonstrated that statins have beneficial effects for IAs.16–18) Aoki et al. demonstrated suppression of IA progression and regression of degenerative changes in preexisting IA walls following administration of either pitavastatin16) or simvastatin17) using a rat IA model.
However, clinical studies investigating the beneficial effects of statins on unruptured IAs have been limited, with only four retrospective case–control or epidemiological studies published to date.19–22) This study is the first open-label, multicenter, randomized controlled trial to assess the preventive effects of atorvastatin on rupture or growth of unruptured small IAs. The study design was prepared referring to the small unruptured intracranial aneurysm verification (SUAVe) study, the first prospective observational study to clarify the natural history of small IAs in Japan.10)
Methods
Study design
The Small Unruptured Aneurysms Verification-Prevention Effect against Growth of cerebral Aneurysms Study Using Statin (SUAVe-PEGASUS) study was a multicenter, prospective, randomized, open-label, blinded endpoint trial to assess the preventive effects of statin against the growth or rupture of unruptured cerebral aneurysm conducted as an official project of the Japan Neurosurgical Society by a steering committee in the neurosurgical department of Kyoto University (Kyoto, Japan). Patients were recruited from 45 Japanese hospitals (31 university hospitals and 14 local base hospitals). The protocol was created in accordance with the Declaration of Helsinki and approved by the ethics board of Kyoto University Graduate School of Medicine and Kyoto University Hospital (C0505-3), with additional approval obtained from the respective ethics committees of all participating centers. This study is registered with the University hospital Medical Information Network (UMIN000005135). Written informed consent was obtained from all patients prior to enrolment.
Participants
We included patients with unruptured small saccular cerebral aneurysm diagnosed within 3 years of enrollment. Patient age was ≥20 years old and <75 years old. The definition of small aneurysms was those aneurysms with greatest dimension ≥3 mm but ≤5 mm. We excluded those patients who concurrently had aneurysms untreated by clipping or coiling with greatest diameter ≥5 mm. Patients with hyperlipidemia who needed medical therapy were also excluded from this study. Detailed inclusion and exclusion criteria are as follows.
Inclusion criteria: (1) Patients with unruptured small (3mm≤ greatest dimension <5mm) saccular aneurysms diagnosed within 3 years of enrollment. (2) Target aneurysms are located in the circle of Willis or its periphery. (3) Patients with ≥20 and <75 years of age. (4) The management of target aneurysms is follow-up without surgical interventions such as clipping and endovascular coiling. (5) Patients with independence for daily life (modified Rankin scale: 0~2). (6) Patients in whom written informed consent was obtained.
Exclusion criteria: (1) Patients who have a coexisting untreated incidental aneurysm of greatest dimension ≥5mm except for the target aneurysms. (2) Patients within 6 months of surgical intervention for the coexisting aneurysms. (3) Patients with treated coexisting giant aneurysm. (4) Patients with hypercholesterolemia who need medical treatment. (5) Patients with history of hypersensitivity for atorvastatin. (6) Patients on antithrombotic drug. (7) Patients with history of intracerebral hemorrhage. (8) Patients who have a history of ischemic stroke within 6 months of enrollment. (9) Patients with cerebral arteriovenous malformation. (10) Patients with moyamoya disease. (11) Patients with severely impaired hepatic function. (12) Patients with alcohol addiction. (13) Patients during pregnancy or lactation. (14) Patients with uncontrolled high blood pressure. (15) Patients with malignancy. (16) Patients with cardiac failure or a history of ischemic heart disease within 6 months of enrollment. (17) Patients with renal failure. (18) Patients with respiratory failure. (19) Patients who were disqualified as a participant by the investigator for some reason.
Randomization
Patients were randomly assigned in a 1:1 ratio to either the statin-treated group or the non-treated group. As unruptured IA is well known to show a higher rupture rate in patients with a history of SAH than in those patients without such history,8,23) stratified randomization according to the presence or absence of a past history of aneurysmal SAH was performed under a secure, web-based computer-generated procedure by the randomization center (St. Luke's International University Graduate School of Public Health, Tokyo, Japan). Members of the MRI assessment committee were blinded study group assignments.
Statin therapy
In the statin-treated group, 10 mg of atorvastatin was administered at 10 mg/day and statin-related side effects were checked by blood test 1 month after starting follow-up and every 6 months thereafter.
Assessment of aneurysm size and shape
As accurate and consistent MRI assessment of the size and shape of small aneurysms was of paramount importance, a rigorous procedure for radiological diagnosis was adopted in this study. Test images from cerebral MR angiography were submitted to the MRI assessment committee before enrollment from each participating hospital, and both MRI machines and protocol were checked and certified by the committee. The principle that a patient should undergo MRI examination using the same machine and protocol was strictly followed during MRI follow-ups. The protocol for MRI examination is as indicated below.
Image acquisition: (1) Target aneurysms are followed up using 1.5T or 3T MRI machine (3T machine is desirable). (2) Images of MR angiography are taken using three-dimensional time-of-flight (3D-TOF) method. (3) Reference line should be parallel to anterior commissure-posterior commissure (AC-PC) line or orbitomeatal (OM) line. (4) Range of image acquisition should contain coverage from the foramen magnum to the corpus callosum. (5) Spatial resolution is set as high as possible. It is desirable that pixel size is equal to or lower than 0.5×0.5×0.5 mm. (6) In case of 1.5T MRI machine, it is desirable to use imaging techniques such as multiple overlapping thin-slab angiography (MOTSA), tilted optimized non-saturation excitation (TONE), and magnetization transfer contrast (MTC). (6) It is desirable that echo time (TE) is set as water phase is opposed to lipid phase. (7) It is desirable to apply presaturation pulse in the cranial side.
Image processing: (1) Create rotation images of three directions using target maximum intensity projection (MIP) images covering whole cerebral arteries. (2) Create sagittal slab MIP images of bilateral internal carotid arteries. (3) Create MIP images that clearly demonstrate shape and size of the target aneurysm when needed.
Cautions for image acquisition and processing: (1) Participating hospitals must submit test images to the MRI assessment committee before the start of patient enrollment and MRI machine and imaging condition should be certified. (2) The same patient should be followed up using the same MRI machine, the same imaging range, the same imaging parameter, and the same image processing method.
MR angiograms were blindly assessed for enlargement and new bleb formation by two readers (an experienced board-certified radiologist and a neurosurgeon) from among members of the MRI assessment committee. Enlargement was evaluated by the amount of change in either major or minor axis of the aneurysm. Images of the patient obtained every 6 months were evaluated by the same pair of readers and compared with the initial image taken at enrollment in every follow-up. When the pair of readers made a different assessment, inconsistencies were resolved by queries to the committee chairman (M.S.) (Fig. 1).
Fig. 1.
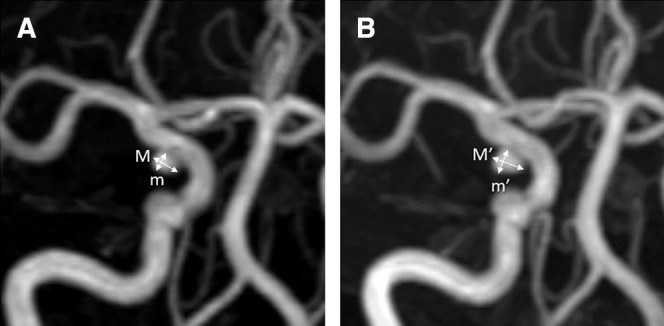
Representative case showing MRI assessment of the size and shape of aneurysm. Every MRI follow-up was obtained in conformity with the initial imaging protocol on the same certified machine and assessed by the same pair of a radiologist and a neurosurgeon. This 59-year-old female patient with right internal carotid artery-posterior communicating artery bifurcation aneurysm reached the primary endpoint at 30 months follow-up due to 0.6 mm enlargement in both major and minor axes by the MRI committee chairman’s final evaluation following a different assessment of the pair readers. Major and minor axes of the aneurysm at enrollment were 3.5 mm and 2.7 mm (A) and those at endpoint were 4.1 mm and 3.3 mm (B), respectively. M: major axis at enrollment, m: minor axis at enrollment, M’: major axis at endpoint, m’: minor axis at endpoint, MRI: magnetic resonance imaging.
Follow-up and outcomes
All study patients were followed up every 6 months at the enrolling hospital until 3 years after randomization. Digital imaging and communications in medicine (DICOM) data from MRI obtained using the same machine and standardized protocol as used at enrollment were submitted to the MRI assessment committee every 6 months until endpoint or close of study. The primary endpoint was a composite of aneurysm growth ≥0.5 mm, new bleb formation, or rupture. The secondary endpoint was the composite of major cardiovascular events. All radiological events comprising the primary endpoint were blindly adjudicated by members of the MRI assessment committee.
Sample size calculation
According to the SUAVe study, as the first prospective observational study of small unruptured IAs in Japan, the rate of enlargement or rupture per 36 months in multiple aneurysms was calculated as 10.1%.10) The event rate in the control group of this trial was therefore estimated as 10%; while data for statin use in the population of the SUAVe study were unavailable, statins were not so widely used in the period of that study. The risk ratio for the statin group compared to the control group was set as 0.5 based on basic studies showing beneficial effects of statins for unruptured IA.16,17) Based on these values, 477 IAs in each group were calculated as necessary to maintain statistical power of 80% with a 5% level of statistical significance using Fisher's exact test. Considering a possible dropout rate of 5%, target enrollment was finally set at 500 IAs in each group for this trial.
Statistical analysis
Categorical variables are expressed as numbers and percentages and were compared using the χ2 test or Fisher’s exact test, as appropriate. Continuous variables are expressed as mean ± standard deviation or median and interquartile range, and compared using Student’s t test or the Wilcoxon rank-sum test based on the normality of their distributions. Endpoints were assessed using Kaplan–Meier methods and compared by log-rank testing. All statistical analyses were performed by a statistician (O.T.) using SPSS software (version 25.0.0.1; IBM, Armonk, NY, USA). All reported P values were two-sided and regarded as significant for values <0.05.
Results
Study population
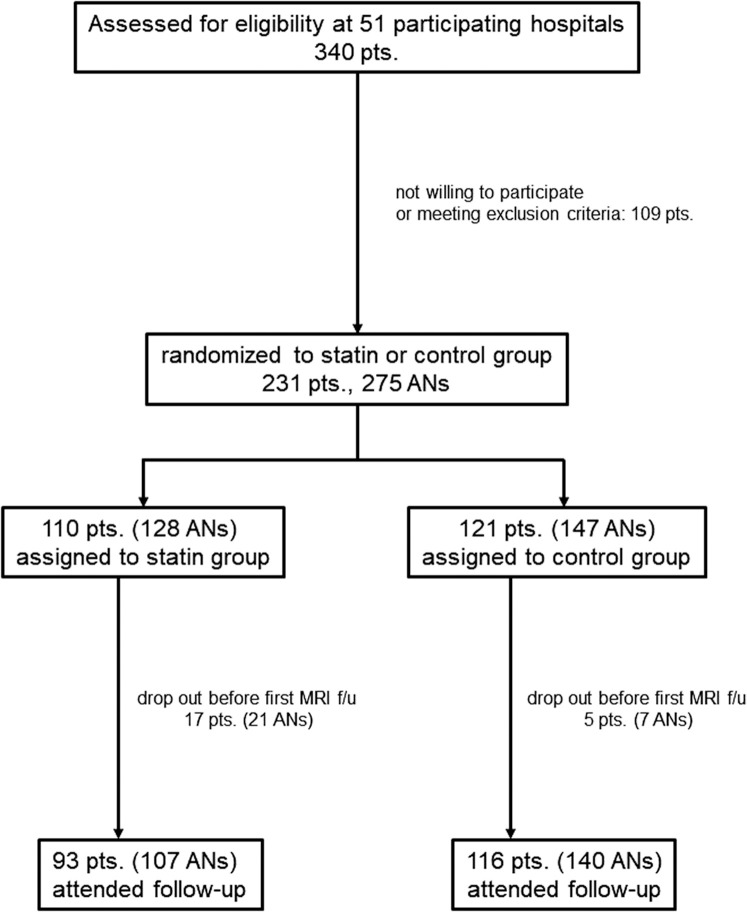
A total of 340 patients were initially enrolled from 45 hospitals between October 25, 2011 and May 30, 2016. After excluding 109 patients (57 patients who did not meet criteria for aneurysm size as judged by the MRI assessment committee and 52 patients who met exclusion criteria confirmed by the enrolling centers), 231 patients were included in the current analysis (Fig. 2). Among the included patients comprising 119 patients with single aneurysm and 112 patients with multiple aneurysms, a total of 387 aneurysms including non-target aneurysms (surgically treated or very small, <3 mm in greatest dimension) and 275 target aneurysms were identified. Of the 231 patients (275 target aneurysms), 110 patients (128 aneurysms) were randomly assigned to the statin group and 121 patients (147 aneurysms) to the control group, and follow-up was started. Seventeen patients (21 aneurysms) in the statin group and 5 patients (7 aneurysms) in the control group dropped out before the first MRI follow-up due to withdrawal of consent or detection of protocol violations. Finally, 107 aneurysms in the 93 statin group patients and 140 aneurysms in the 116control group patients were analyzed in this study.
Fig. 2.
Flow diagram of the study cohort. AN: aneurysm, Pts: patients.
Basic characteristics
Comparing basic characteristics between the statin group and control group, no significant differences were observed in sex, age, number of aneurysms harbored, past histories of SAH or ischemic stroke, smoking, excessive alcohol intake, family history of SAH, or diastolic pressure. Only systolic pressure differed significantly, appearing higher in the statin group (133.2±16.2 mmHg) than in the control group (128.0±11.9 mmHg) (Table 1). No significant differences were also identified in blood examinations for lipid metabolism, liver function, or diabetes mellitus between groups (Table 2).
Table 1. Basic characteristics.
| Variables | Statin (n = 93) | Control (n = 116) | P value |
|---|---|---|---|
| Female sex (%) | 62(66.7) | 71(60.7) | 0.37 |
| Age, y | 61 ± 9 | 60 ± 8 | 0.38 |
| Number of aneurysms (median) | 1 (1–2) | 1 (1–2) | 0.71 |
| Previous SAH (%) | 7 (7.5) | 9 (7.7) | 0.96 |
| Previous ischemic stroke (%) | 2 (2.2) | 2 (1.7) | 1.00 |
| Hypertension (%) | 41 (44.1) | 43 (36.8) | 0.28 |
| Current smoker (%) | 15 (16.1) | 27 (23.1) | 0.21 |
| Excessive alcohol intake (%) | 6 (6.5) | 14 (12.0) | 0.18 |
| Family history of SAH (%) | 5 (5.4) | 13 (11.2) | 0.14 |
| Systolic pressure, mmHg | 133.2 ± 16.2 | 128.0 ± 16.5 | 0.03 |
| Diastolic pressure, mmHg | 78.6 ± 11.8 | 76.8 ± 11.9 | 0.28 |
SAH: subarachnoid hemorrhage.
Table 2. Blood tests at enrollment and 6 months follow-up.
| Variables | Statin (n = 93) | Control (n = 116) | P value |
|---|---|---|---|
| Enrollment | |||
| LDL, mg/dL | 121 ± 28.6 | 118 ± 25.3 | 0.31 |
| HDL, mg/dL | 62.1 ± 15.7 | 62.3 ± 15.8 | 0.85 |
| TG, mg/dL | 138.4 ± 94.1 | 137.2 ± 78.2 | 0.92 |
| CRP, mg/dL | 0.11 ± 0.21 | 0.10 ± 0.17 | 0.86 |
| GOT, U/L | 21.8 ± 6.4 | 23.5 ± 7.9 | 0.29 |
| GPT, U/L | 21.8 ± 12.8 | 20.8 ± 11.7 | 0.54 |
| HbA1C, % | 5.63 ± 0.56 | 5.60 ± 0.46 | 0.75 |
| 6 months | |||
| LDL, mg/dL | 72.8 ± 20.0 | 118.9 ± 26.5 | <0.001 |
| HDL, mg/dL | 64.0 ± 15.1 | 61.6 ± 16.4 | 0.32 |
| TG, mg/dL | 111.3 ± 66.8 | 146.6 ± 99.1 | <0.01 |
| CRP, mg/dL | 0.09 ± 0.18 | 0.11 ± 0.21 | 0.53 |
| GOT, U/L | 23.8 ± 6.4 | 23.4 ± 8.9 | 0.76 |
| GPT, U/L | 24.9 ± 12.8 | 22.5 ± 15.4 | 0.24 |
| HbA1C, % | 5.86 ± 0.64 | 5.62 ± 0.44 | <0.01 |
CRP: C-reactive protein, GOT: glutamic-oxaloacetic transaminase, GPT: glutamic pyruvic transaminase, HbA1C: hemoglobin A1c, HDL: high-density lipoprotein, LDL: low-density lipoprotein, TG: triglyceride.
Locations of target aneurysms
Of the 247 target aneurysms, 105 (42.3%) were in the middle cerebral artery, 87 (35.1%) in the internal carotid artery, 22 (8.9%) in the anterior communicating artery, 19 (7.7%) in the anterior cerebral artery, 7 (2.8%) at the bifurcation of the basilar artery, 6 (2.4%) in the superior cerebellar artery, and 1 (0.4%) in the posterior cerebral artery. The distribution of target aneurysms in this study revealed a very similar pattern to that of the ISAT study after excluding internal carotid artery aneurysms located in the cavernous or paraclinoid portions, as lesions not included in the present analysis.6)
Safety of administering low-dose statin to normolipemic patients
Of the 110 patients in statin group, a total of 20 patients dropped out during follow-up after randomization. Causes of discontinuation were statin-related side effects in six patients, withdrawal of consent in seven patients, non-drug-induced renal failure in one, advanced malignancy in one, pregnancy in one, and moving to a different location in one, respectively. Statin-related side effects comprised asymptomatic mild elevation of liver enzymes in four patients, muscle pain in one, and significant reduction in low-density lipoprotein (LDL) in one patient. No serious adverse effects were identified after low-dose statin administration to normolipemic patients.
In comparing data from blood tests between the statin group and control group after 6 months of enrollment, LDL and triglycerides were significantly lower and hemoglobin (Hb)A1C was significantly higher in the statin group (Table 2).
Outcomes
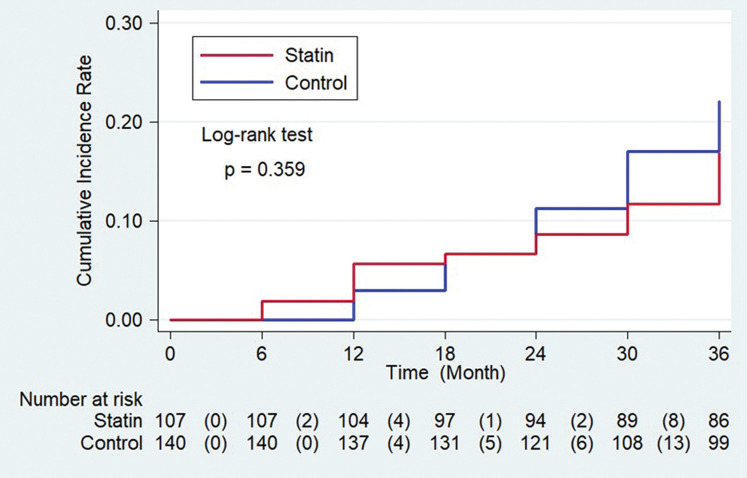
The primary endpoint occurred in 28 aneurysms (20.0%) in the control group and in 17 aneurysms (15.9%) in the statin group (hazard ratio, 0.759; 95% confidence interval, 0.415–1.386). No significant differences were demonstrated for the primary endpoint between the statin group and control group (Fig. 3, log-rank P = 0.359). In the statin group, 17 IAs met the primary endpoint due to enlargement of aneurysm size and 3 IAs met the primary endpoint due to new bleb formation. In the control group, 21 aneurysms met primary endpoint due to enlargement and 7 aneurysms met the primary endpoint due to new bleb formation. No aneurysm ruptures were identified in this study. No instances of secondary endpoint were identified in either group.
Fig. 3.
Cumulative incidence of primary endpoint.
Discussion
In the present study, as the first randomized trial to assess the beneficial effects of statin on unruptured IA, patient enrollment was prematurely terminated because of a much slower than expected enrolments, leading to a severely underpowered sample size. As a result, superiority of the statin group to the control group could not be confirmed for the primary composite endpoint of aneurysm growth ≥0.5 mm, new bleb formation, or rupture while systolic pressure was significantly higher in the statin group among the possible risk factors for SAH.
In the design of this controlled randomized study, we referred to the SUAVe study, which was the first prospective observational study of annual rupture risk associated with incidentally diagnosed small aneurysms in Japan.10) The SUAVe study demonstrated that the average annual rupture rate of unruptured IAs <5 mm in size was as low as 0.54% after following 448 IAs in 374 patients for a mean of 41.0 months, and observed aneurysm growth in 25 patients (6.7%). Although the risk of IA rupture is well accepted as showing considerable ethnic differences and Japanese have a 2.8 times higher risk than Caucasians,8) the general consensus among Japanese neurosurgeons following the publication of the findings from the SUAVe Study has been to adopt a wait-and-scan policy for unruptured small IAs, providing a rationale for randomization and observation without prophylactic surgery and helping to overcome the ethical concerns of our trial. Among several kinds of statins, the efficacy of atorvastatin was assessed in this study, partly because basic experimental studies have demonstrated that different statins showed comparable beneficial efficacy and supported the notion that preventive effects against IAs could be effected through the anti-inflammatory class effects of statins on the NF-κB pathway.16) Another important basis was the fact that atorvastatin was the only statin that had been used in clinical studies for patients with average or lower-than-average cholesterol concentrations and for which safety had been demonstrated at the start of our study.24)
Compared with the results of the SUAVe study,10) the finding that the growth rate was higher and the rupture rate was zero in the present trial is striking (Table 3). Regarding the rupture rate of small IAs, none of the 247 IAs in the SUAVe-PEGASUS trial ruptured, while 7 of 448 (1.9%) IAs developed to SAH in the SUAVe study. This discrepancy could be attributed to the effect of tighter control of lifestyle modifications against SAH with a standardized protocol of MRI follow-up among participants in this trial, which sustained the reasonability of wait and scan policy for incidentally diagnosed small IAs.25) The distribution of IA locations might be another cause of differences in the rupture rate of small IAs. The rupture risk of unruptured IA is widely accepted as depending on the location, with IAs of the anterior communicating artery carrying a higher risk of SAH than those of the middle cerebral artery.7) The proportions of IAs in the middle cerebral artery and anterior communicating artery were 42.3% and 8.9% in our trial, compared to 35.3% and 13.4% in the SUAVe study. With respect to IA growth, 45 of 247 (18.2%) IAs including both the statin group and control group enlarged to reach the primary endpoint during the mean follow-up period of 32.4 months in this study, while 30 of 448 (6.7%) IAs increased in size during the mean follow-up period of 42.5 months in the SUAVe study. Important possible contributors to this discrepancy in IA growth include differences in the definition of enlargement and MRI diagnostic accuracy. The criterion for IA enlargement was as small as 0.5 mm with a meticulous MRI diagnostic procedure as described in this trial, while the definition for IA growth was not described in detail in the SUAVe study. Other possible causes include differences in basic patient characteristics related to risk factors for IA.26) The proportion of patients with multiple IAs was 47.4% in the present trial and 33.2% in the SUAVe study while hypertensive patients comprised 40% and 24.9%, respectively.
Table 3. Comparison between SUAVe study and SUAVe-PEGASUS trial.
| SUAVe study | SUAVe-PEGASUS trial | ||
|---|---|---|---|
| Main results | Target aneurysms, n | 448 | 247 |
| Mean follow-up period, months | 42.5 | 32.4 | |
| Aneurysm growth, % | 6.7 | 18.2 | |
| Aneurysm rupture, % | 1.9 | 0 | |
| Risk factors | Female sex, % | 63.6 | 63.3 |
| Age, year | 61.9 ± 10.3 | 60.8 ± 8.7 | |
| Patients with multiple aneurysms, % | 33.2 | 47.4 | |
| Previous SAH, % | 9.6 | 7.6 | |
| Family history of SAH, % | 8.3 | 8.6 | |
| Hypertension, % | 24.9 | 40 | |
| Aneurysm location | MCA, % | 35.3 | 42.3 |
| ICA, % | 38.6 | 35.1 | |
| Acom, % | 13.4 | 8.9 | |
| ACA, % | 2.7 | 8.1 | |
| BA, % | 7.4 | 5.2 | |
| Others, % | 2.7 | 0.4 |
ACA: anterior carotid artery, Acom: anterior communicating artery, BA: basilar artery, ICA: internal carotid artery, MCA: middle cerebral artery, SAH: subarachnoid hemorrhage, SUAVe: small unruptured intracranial aneurysm verification.
With respect to the safety of administering low-dose statin to non-hyperlipidemic patients with unruptured IAs, 6 of 115 patients (5.2%) in the statin group developed statin-related side effects, none of which were serious, such as asymptomatic elevations in liver enzyme levels. While a lower LDL is one of the accepted risk factors for intracerebral hemorrhage,27,28) studies related to the association between lipid-lowering treatment with statins and hemorrhagic stroke are limited and the results remain somewhat controversial. The SPARCL trial demonstrated that high-dose atorvastatin after stroke or transient ischemic attack was preventive for the overall incidence of stroke and cardiovascular events, but carried a higher risk of hemorrhagic stroke.14) On the other hand, the J-STARS study assessed the benefits of statin for recurrent stroke in Japanese patients and found no significant association between low-dose pravastatin intake and risk of hemorrhagic stroke.29) In addition to reducing cholesterol levels, statins might have antagonistic activity for platelet aggregation and other anti-thrombotic functions that may potentially result in a higher risk of hemorrhagic stroke.30,31) Careful, long-term studies are thus still necessary, although the results of our study with its limited survey period still supported the safety of low-dose statin regarding hemorrhage risk. Another potential side effect of statins under discussion is the development of diabetes mellitus. Sattar et al. conducted a meta-analysis of 13 statin trials including more than 1000 patients and demonstrated statin therapy as associated with a 9% increased risk of incident diabetes, with this deleterious effect tending to increase with age.32) In the present study, HbA1C level was significantly increased at 6 months after starting administration in the statin group, although not severely enough to require further treatment. Diabetes should therefore be kept in mind as an adverse effect of statins, especially when considering long-term use in elderly patients.
Limitations
This trial showed several limitations. In the first place, sample size estimation in the protocol for this trial might have lacked validity because neither clinical case–control nor observational studies assessing the beneficial effects of statin for IA had been published as of the start of the trial, and our calculation was thus based on basic experimental and observational studies regarding the natural course of small IAs in Japan. In addition, the study population was much smaller than initially scheduled because patient recruitment was prematurely terminated due to unexpectedly slow enrollment. The results of this trial must thus be considered inconclusive. Second, this trial might have had problems inherent to the open-label trial design, although the composite primary endpoints consisted of the objective items of IA rupture and morphological changes evaluated on MRI. Patients in the control group might have more intensely adopted abstemious lifestyles against IA growth or rupture. Third, the result that the number of patients who reached the primary endpoint was smaller than expected in this trial clearly demonstrated the need for a larger sample size or longer study period in a similar clinical study. Establishment of much more sensitive biomarkers than morphological ones for high-risk IAs will thus be essential in future clinical studies of medical treatments for small IAs. Fourth, while this trial targeted only small IAs to avoid ethical issues deriving from follow-up without prophylactic surgical treatment of patients with large IAs, it might not be appropriate to subject small unruptured IAs to studies assessing the beneficial effects of medical treatment. Several clinical studies comparing IA walls of various sizes by intraoperative observation or histological examination have shown that small and large IAs tend to display different pathophysiologies.33,34) As discussed in the SUAVe study,10) the discrepancy between the very low risk of rupture during the follow-up of unruptured small IAs and the considerable number of small ruptured IAs in clinical practice could be explained by most ruptured small IAs showing rupture shortly after formation, reducing the window for detection as unruptured IA. Therefore, to see the effects of non-surgical treatment for prevention of rupture or enlargement in already formed IAs, patients with large IAs who do not wish to undergo prophylactic surgery would represent better candidates for these types of clinical study.
Conclusion
The present study is the first clinical and randomized trial to assess the beneficial effects of atorvastatin for unruptured small IAs. Significant benefit as tested by the composite primary endpoint of rupture, enlargement, or new bleb formation was unable to be established because of inadequate statistical power, making this trial inconclusive. Future studies that are larger scale or that adopt more sophisticated surrogate makers for rupture risk are essential.
Funding
This study was supported by the Japan Neurosurgical Society.
Acknowledgments
We wish to express our appreciation to the doctors and clinical research coordinators in the participating hospitals as described below for patient enrollment and collecting data.
Participating hospitals and lead investigators
Kiyohiro Houkin, MD, Department of Neurosurgery, Hokkaido University Graduate School of Medicine; Nobuhiro Mikuni, MD, Department of Neurosurgery, Sapporo Medical University; Toshiaki Osato, MD, Department of Neurosurgery, Nakamura Memorial Hospital; Kuniaki Ogasawara, MD, Department of Neurosurgery, Iwate Medical University; Teiji Tominaga, MD, Department of Neurosurgery, Tohoku University Graduate School of Medicine; Yukihiko Sonoda, MD, Department of Neurosurgery, Yamagata University; Yukihiko Fujii, MD, Department of Neurosurgery, Brain Research Institute, Niigata University; Yoshinori Taniguchi, MD, Department of Neurosurgery, Nagaoka Chuo General Hospital; Toshio Machida, MD, Department of Neurosurgery, Chiba Cerebral and Cardiovascular Center; Nobuhito Saito, MD, Department of Neurosurgery, Faculty of Medicine, The University of Tokyo; Yoshiaki Shiokawa, MD, Department of Neurosurgery, Kyorin University; Takakazu Kawamata, MD, Department of Neurosurgery, Tokyo Women's Medical University; Hajime Arai, MD, Department of Neurosurgery, Juntendo University; Yuichi Murayama, MD, Department of Neurosurgery, The Jikei University School of Medicine; Wataro Tsuruta, MD, Department of Endovascular Neurosurgery, Toranomon Hospital; Toshihiro Kumabe, MD, Department of Neurosurgery, Kitasato University School of Medicine; Hiroyuki Kinouchi, MD, Department of Neurosurgery, Interdisciplinary Graduate School of Medicine and Engineering, University of Yamanashi; Kazuhiro Hongo, MD, Department of Neurosurgery, Shinshu University School of Medicine; Mitsuhito Mase, MD, Department of Neurosurgery, Nagoya City University Graduate School of Medical Sciences; Mitsutoshi Nakada, MD, Department of Neurosurgery, Kanazawa University Hospital; Toshihiko Wakabayashi, MD, Department of Neurosurgery, Nagoya University Graduate School of Medicine; Hidenori Suzuki, MD, Department of Neurosurgery, Mie University Graduate School of Medicine; Toru Iwama, MD, Department of Neurosurgery, Gifu University Graduate School of Medicine; Satoshi Kuroda, MD, Department of Neurosurgery, Graduate School of Medicine and Pharmaceutical Science, University of Toyama; Kazuhiko Nozaki, MD, Department of Neurosurgery, Shiga University of Medical Science; Jun C Takahashi, MD, Department of Neurosurgery, National Cerebral and Cardiovascular Center; Hajime Nakamura, MD, Department of Neurosurgery, Osaka University Graduate School of Medicine; Kenichi Murao, MD, Department of Neuroendovascular Therapy, Shiroyama Hospital; Hiroyuki Nakase, MD, Department of Neurosurgery, Nara Medical University; Nobuyuki Sakai, MD, Department of Neurosurgery, Kobe City Medical Centre General Hospital; Hideyuki Ohnishi, MD, Ohnishi Neurological Centre; Osamu Narumi, MD, Department of Neurosurgery, National Hospital Organization Himeji Medical Center; Isao Date, MD, Department of Neurological Surgery, Graduate School of Medicine, Okayama University; Masaaki Uno, MD, Department of Neurosurgery, Kawasaki Medical School; Kaoru Kurisu, MD, Department of Neurosurgery, Graduate School of Biomedical and Health Sciences, Hiroshima University; Masaki Chin, MD, Department of Neurosurgery, Kurashiki Central Hospital; Michiyasu Suzuki, MD, Department of Neurosurgery, Yamaguchi University School of Medicine; Taketo Hatano, MD, Department of Neurosurgery, Stroke Center, Kokura Memorial Hospital; Koji Iihara, MD, Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University; Tooru Inoue, MD, Department of Neurosurgery, Fukuoka University; Takayuki Matsuo, MD, Department of Neurosurgery, Nagasaki University Graduate School of Biomedical Sciences; Masahiro Yonekura, MD, Department of Neurosurgery, National Nagasaki Medical Center; Koichi Iwasaki, MD, Department of Neurosurgery, Tazuke Kofukai Medical Research Institute and Kitano Hospital; Tatsuya Sasaki, MD, Department of Neurosurgery, Aomori Prefectural Central Hospital.
Conflicts of Interest Disclosure
None.
References
- 1).Biotti D, Jacquin A, Boutarbouch M, et al. : Trends in case-fatality rates in hospitalized nontraumatic subarachnoid hemorrhage: results of a population-based study in Dijon, France, from 1985 to 2006. Neurosurgery 66: 1039–1043; discussion 1043, 2010 [DOI] [PubMed] [Google Scholar]
- 2).Longstreth WT, Nelson LM, Koepsell TD, van Belle G: Clinical course of spontaneous subarachnoid hemorrhage: a population-based study in King County, Washington. Neurology 43: 712–718, 1993 [DOI] [PubMed] [Google Scholar]
- 3).Schievink WI, Wijdicks EF, Parisi JE, Piepgras DG, Whisnant JP: Sudden death from aneurysmal subarachnoid hemorrhage. Neurology 45: 871–874, 1995 [DOI] [PubMed] [Google Scholar]
- 4).van Gijn J, Kerr RS, Rinkel GJ: Subarachnoid haemorrhage. Lancet 369: 306–318, 2007 [DOI] [PubMed] [Google Scholar]
- 5).Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ: Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 8: 635–642, 2009 [DOI] [PubMed] [Google Scholar]
- 6).Wiebers DO, Whisnant JP, Huston J, et al. : Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362: 103–110, 2003 [DOI] [PubMed] [Google Scholar]
- 7).UCAS Japan Investigators. Morita A, Kirino T, et al. : The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366: 2474–2482, 2012 [DOI] [PubMed] [Google Scholar]
- 8).Greving JP, Wermer MJ, Brown RD, et al. : Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 13: 59–66, 2014 [DOI] [PubMed] [Google Scholar]
- 9).Lee T, Baytion M, Sciacca R, Mohr JP, Pile-Spellman J: Aggregate analysis of the literature for unruptured intracranial aneurysm treatment. AJNR Am J Neuroradiol 26: 1902–1908, 2005 [PMC free article] [PubMed] [Google Scholar]
- 10).Sonobe M, Yamazaki T, Yonekura M, Kikuchi H: Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke 41: 1969–1977, 2010 [DOI] [PubMed] [Google Scholar]
- 11).Imaizumi Y, Mizutani T, Shimizu K, Sato Y, Taguchi J: Detection rates and sites of unruptured intracranial aneurysms according to sex and age: an analysis of MR angiography-based brain examinations of 4070 healthy Japanese adults. J Neurosurg 130: 573–578, 2018 [DOI] [PubMed] [Google Scholar]
- 12).Shu HB, Agranoff AB, Nabel EG, et al. : Differential regulation of vascular cell adhesion molecule 1 gene expression by specific NF-kappa B subunits in endothelial and epithelial cells. Mol Cell Biol 13: 6283–6289, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Laufs U, La Fata V, Plutzky J, Liao JK: Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 97: 1129–1135, 1998 [DOI] [PubMed] [Google Scholar]
- 14).Amarenco P, Bogousslavsky J, Callahan A, et al. : High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 355: 549–559, 2006 [DOI] [PubMed] [Google Scholar]
- 15).Kurian KC, Rai P, Sankaran S, Jacob B, Chiong J, Miller AB: The effect of statins in heart failure: beyond its cholesterol-lowering effect. J Card Fail 12: 473–478, 2006 [DOI] [PubMed] [Google Scholar]
- 16).Aoki T, Kataoka H, Ishibashi R, et al. : Pitavastatin suppresses formation and progression of cerebral aneurysms through inhibition of the nuclear factor kappaB pathway. Neurosurgery 64: 357–365; discussion 365–366, 2009 [DOI] [PubMed] [Google Scholar]
- 17).Aoki T, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N: Simvastatin suppresses the progression of experimentally induced cerebral aneurysms in rats. Stroke 39: 1276–1285, 2008 [DOI] [PubMed] [Google Scholar]
- 18).Ledingham JM, Laverty R: Effect of simvastatin given alone and in combination with valsartan or enalapril on blood pressure and the structure of mesenteric resistance arteries and the basilar artery in the genetically hypertensive rat model. Clin Exp Pharmacol Physiol 32: 76–85, 2005 [DOI] [PubMed] [Google Scholar]
- 19).Can A, Castro VM, Dligach D, et al. : Lipid-lowering agents and high HDL (High-density lipoprotein) are inversely associated with intracranial aneurysm rupture. Stroke 49: 1148–1154, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Bekelis K, Smith J, Zhou W, et al. : Statins and subarachnoid hemorrhage in medicare patients with unruptured cerebral aneurysms. Int J Stroke 10: 38–45, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Yoshimura Y, Murakami Y, Saitoh M, et al. : Statin use and risk of cerebral aneurysm rupture: a hospital-based case-control study in Japan. J Stroke Cerebrovasc Dis 23: 343–348, 2014 [DOI] [PubMed] [Google Scholar]
- 22).Marbacher S, Schläppi JA, Fung C, Hüsler J, Beck J, Raabe A: Do statins reduce the risk of aneurysm development? A case-control study. J Neurosurg 116: 638–642, 2012 [DOI] [PubMed] [Google Scholar]
- 23).Murayama Y, Takao H, Ishibashi T, et al. : Risk analysis of unruptured intracranial aneurysms: prospective 10-year cohort study. Stroke 47: 365–371, 2016 [DOI] [PubMed] [Google Scholar]
- 24).Sever PS, Dahlöf B, Poulter NR, et al. : Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-scandinavian cardiac outcomes trial--lipid lowering arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 361: 1149–1158, 2003 [DOI] [PubMed] [Google Scholar]
- 25).Shiue I, Arima H, Hankey GJ, Anderson CS, ACROSS Group : Modifiable lifestyle behaviours account for most cases of subarachnoid haemorrhage: a population-based case-control study in Australasia. J Neurol Sci 313: 92–94, 2012 [DOI] [PubMed] [Google Scholar]
- 26).Suzuki T, Takao H, Rapaka S, et al. : Rupture risk of small unruptured intracranial aneurysms in japanese adults. Stroke 51: 641–643, 2020 [DOI] [PubMed] [Google Scholar]
- 27).Sturgeon JD, Folsom AR, Longstreth WT, Shahar E, Rosamond WD, Cushman M: Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke 38: 2718–2725, 2007 [DOI] [PubMed] [Google Scholar]
- 28).Noda H, Iso H, Irie F, et al. : Low-density lipoprotein cholesterol concentrations and death due to intraparenchymal hemorrhage: the Ibaraki Prefectural Health Study. Circulation 119: 2136–2145, 2009 [DOI] [PubMed] [Google Scholar]
- 29).Hosomi N, Nagai Y, Kohriyama T, et al. : The Japan statin treatment against recurrent stroke (J-STARS): A multicenter, randomized, open-label, parallel-group study. EBioMedicine 2: 1071–1078, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Blum A, Shamburek R: The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis 203: 325–330, 2009 [DOI] [PubMed] [Google Scholar]
- 31).Meier N, Nedeltchev K, Brekenfeld C, et al. : Prior statin use, intracranial hemorrhage, and outcome after intra-arterial thrombolysis for acute ischemic stroke. Stroke 40: 1729–1737, 2009 [DOI] [PubMed] [Google Scholar]
- 32).Sattar N, Preiss D, Murray HM, et al. : Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 375: 735–742, 2010 [DOI] [PubMed] [Google Scholar]
- 33).Kataoka K, Taneda M, Asai T, et al. : Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke 30: 1396–1401, 1999 [DOI] [PubMed] [Google Scholar]
- 34).Kadasi LM, Dent WC, Malek AM: Cerebral aneurysm wall thickness analysis using intraoperative microscopy: effect of size and gender on thin translucent regions. J Neurointerv Surg 5: 201–206, 2013 [DOI] [PubMed] [Google Scholar]