Abstract
Objectives
The aim of this study was to compare prostate-specific antigen (PSA) kinetics − half-life time (HT), doubling time (DT), and elimination rate PSA (ePSA) in prostate cancer (PCa) monitoring. Implementation of ePSA in clinical practice could help simplify patient monitoring in the remission phase.
Materials and Methods
A total of 49 PCa patients were examined by their PSA tests before prostatectomy and after 30 days, 91 days, and 24 months. Conventional PSA rate of change parameters (HT and DT) were compared to a new clinically understandable ePSA parameter.
Results
We observed that implementation of inverse value (ePSA) rather than HT or DT has distinct advantages: (1) values are valid when PSA is unchanged (ePSA equals zero), (2) the concept of ePSA can be easily understood, as it is a growth fraction, (3) ePSA fluctuates within a narrow range and is thus easy to interpret, and (4) there are no mathematical flaws (no positive skewing).
Conclusion
Exploring ePSA norm as ≤0% could help spot biochemical recurrence in a timely manner. Primary health care providers tend to use an irrelevant PSA threshold, that is, 4.0 ng/mL, in postoperative follow-up. The delayed referrals of patients in remission might be reduced if ePSA testing is adopted.
Keywords: Tumor markers, Cancer relapse, Prostate cancer, Prostate-specific antigen
Highlights of the Study
Cancer recurrence is easier to spot referring to the kinetics of doubling time rather than tumor marker norms.
An inverse value of tumor maker doubling time is a versatile parameter free from mathematical drawbacks.
This study demonstrates that prostate-specific antigen kinetic parameter ePSA can predict biochemical recurrence postoperatively.
The ePSA can be considered for implementation in daily clinical practice.
Introduction
Surgical removal of tumor may result in decline in levels of tumor markers (TMs). TM kinetics in remission are often ignored by primary health care providers (PHCPs) when values oscillate in the range below norm. This approach might delay the recognition of recurrence [1, 2]. A rise in TMs may precede the clinical appearance of recurrence by >6–17 months [1, 3]. The currently explored criteria, namely, doubling time (DT), can be used as an indicator of relapse [4]. However, half-life time (HT)/DT criteria are not convenient in clinical practice for several reasons: (i) HT/DT values are undefined when TM is stable (dividing by 0), (ii) large and highly variable values are hard to interpret, and (iii) mathematical drawbacks, distribution asymmetry is positively skewed [5].
We proposed to study the use of an inverse value of HT/DT in TM monitoring. The concept is identical to the pharmacokinetic elimination rate constant, showing an inverse value of the HT of the amount of drug injected. We refer to this as an elimination rate TM (eTM); this can be defined as a “growth fraction” or “elimination fraction,” and eTM is mathematically valid when there is no change in TMs (eTM equals 0). We hypothesized that monitoring eTM could be helpful in the detection of early recurrence when TM is increasing within the normal range.
Delayed detection of relapse sometimes occurs during primary care follow-up. An example could be a radical prostatectomy (RP) where prostate-specific antigen (PSA) levels should become undetectable following tumor removal [6]. However, PHCPs tend to habitually use a PSA threshold which is too high (4.0 ng/mL) to monitor patients postoperatively [2]. Biochemical recurrence (BCR) of prostate cancer (PCa) must be spotted significantly earlier when PSA = 0.2 ng/mL [7]. It may take years to accomplish a transition of care from PHCPs to oncologists. The risk of delayed referrals and missed opportunities might be linked to the PSA norm embedded in laboratory reports (<4 ng/mL) which is unrelated to postoperative norms (undetectable).
We evaluated the clinical applicability of eTM (ePSA) compared to its inverse equivalent (HT/DT) in PCa patients. The specific ePSA patterns observed postoperatively between days 30 and 91 indicated a BCR of PSA.
Materials and Methods
Calculation of ePSA:
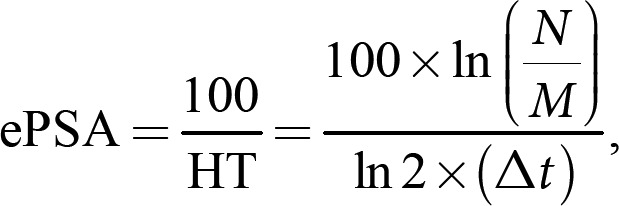
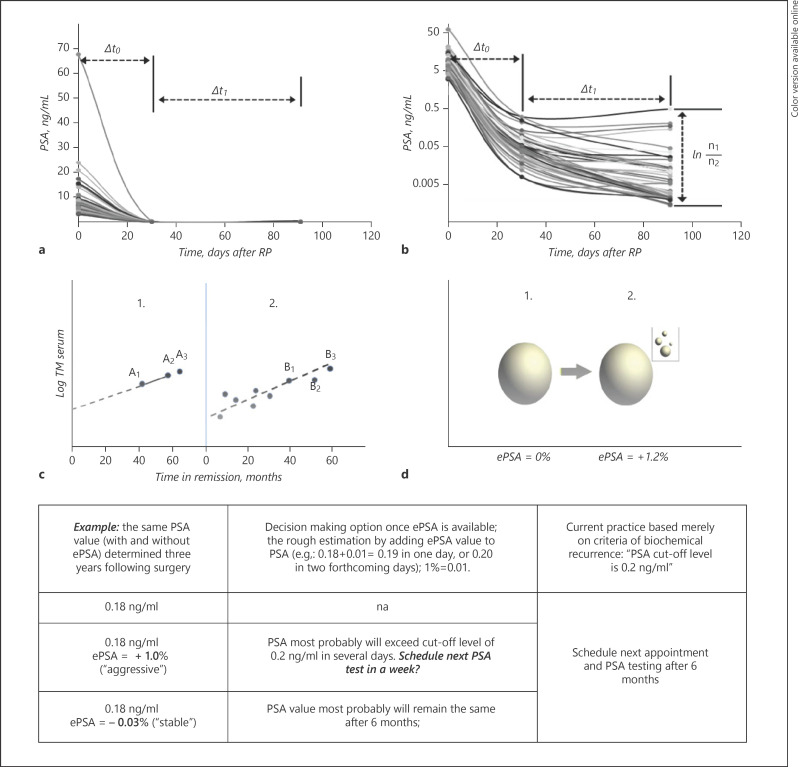
where N = current PSA and M = previous PSA. ePSA values <0 appear if PSA is decreasing and >0 if it is increasing. Δt = time interval, expressed in days between two separate PSA measurements. The ePSA metric is “percent/day” reflecting growth or elimination fraction. In this communication, we use % metric to simplify concept. PSA value adjustment to a specific sampling day (e.g., day 30) was done using the following equation: PSAday30 = PSAM× 2(M − 30)/HT PSAday91 = PSAM× 2(M − 91)/HT, where PSAday30 = PSA value as if it was measured on day 30; PSAM = actual PSA value at M representing a “1-month” time point; M = number of days from RP to PSA measurement; HT = half-life time of PSA from RP to M time point. PSAday91 was used to adjust values to represent a “3-month” time point. ePSA calculations with multiple PSA values (Fig. 1c) can be applied exploring nomograms available for DT calculation [8].
Fig. 1.
The value of logarithm in evaluating TM development. The PSA data (preoperative, day 30 and day 91) of the same cohort are shown using a linear scale (a) and log scale (b). Any claim in colloquial clinical language saying TM “percent increase” (decrease) does not reflect the evolution of TM within a given time interval. Importantly, these claims rarely indicate a precise time interval between two separate TMs. An exact time interval and log TM ratio are two basic components which can describe the “speed” of progress or decay of TM. Trying to squeeze a TM ratio into linear concept of increase [11] results in a discussion on which of the 7 patterns is the most informative. Any new confirmative TM test should be evaluated if it fits into the pattern of the previous two, in a log TM scale only (c). The exact timing of planned A3 point determination is not that important, provided the TM is tested with the same instrument. The availability of multiple TM points is advantageous (c). Calculation of an eTM with multiple points for each patient is available using nomograms with minimal conversion of DT into eTM [8]. A hospital lab can produce automatic reports for eTM if previous data for the same patient are available. A clinician can easily adopt the simplicity of growth fraction concept and can assume that a ∼1% daily increase in TM will result in a roughly 30% increase of TM value within a month (1% per day results roughly in 30% per month) (d). TM, tumor marker; PSA, prostate-specific antigen; eTM, elimination rate TM; DT, doubling time; RP, radical prostatectomy; ePSA, elimination rate PSA.
Patients and Tests
The study was approved by an Institutional Review Board. Informed consent was obtained from all patients. A total of 49 previously untreated patients with a histologically confirmed diagnosis of adenocarcinoma in the prostate received RP at our institute from November 2017 to May 2018. The follow-up PSA data were obtained using an ultrasensitive PSA assay (Cobas e411; Roche Diagnostics, Risch-Rotkreuz, Switzerland). All patients enrolled exhibited normal ranges of liver enzymes, bilirubin, and creatinine during the follow-up. No blood transfusions were done during the period of PSA measurement. Clinicopathological characteristics of the study participants were as follows: age − mean 62.5 ± 6.2 years, BMI − mean 25.1 ± 2.84 kg/m2, and Karnofsky status ≥70%. Adenocarcinoma of the prostate was confirmed based on preoperative biopsy findings. All patients displayed a negative CT scan/MRI and bone scan for metastatic PCa. Conventional open radical retropubic RP was performed extraperitoneally in a retrograde fashion. The laparoscopic extraperitoneal RP was performed using a 5-trocar technique. Total intravenous anesthesia with propofol without sevoflurane or opioid was applied to all study participants. None of the patients received radiotherapy or androgen deprivation therapy within 6 months after the surgery. The post-RP follow-up for each patient included a PSA measurement every 3 months. BCR was defined as a confirmed increase in PSA above 0.2 ng/mL.
Statistical Analysis
IBM-SPSS Statistics 21 (SPSS, Inc., Chicago, IL, USA) was used for data analysis. Binary logistic regression and receiver operating characteristic were performed to determine the effectiveness of ePSA in predicting BCR. p values <0.05 were considered statistically significant.
Results
Preoperative PSA was found as mean 9.0 ± 7.3 ng/mL (median − 8.2 ng/mL); pathological status: 35 patients (pT2) and 14 patients (pT3); Gleason score at surgery was found as follows: 12 patients → (3 + 3), 30 patients → (3 + 4), 6 patients → (4 + 3), and 1 patient → (4 + 4). Positive surgical margins were found in 6 patients. Surgical procedures applied were: open RP (18 patients) and laparoscopic RP (31 patients). The 1-month blood sampling day ranged from day 27 to 38 (mean 31.1; median 30) after RP. The mean PSA-HL was 4.1 days, corresponding to an ePSA = −24.6%. After conforming PSA to an exact 30-day time point, the adjustments ranged from +0.008 to −0.326 ng/ml. A significant slowing in PSA decline was observable during the 1- to 3-month interval (Fig. 1b). Mean PSA-HL at 3 months was +21.4 d, ranging from −238.7 to +374.6 d, and a median of +19.3 d. The calculated mean ePSA value for the same period was −4.67%. A logarithmic scale of postoperative PSA is necessary for PSA pattern visualization (Fig. 1a, b). Four patients (8%) showed a bounce in PSA with values higher at 3 months as compared to their 1-month values. Patients exhibiting a bounce in PSA (positive ePSA) or slowing of PSA decline (ePSA > −2% during the 1- to 3-month interval) were prone to BCR within 24 months after RP: odds ratio (OR) = 1.5, p = 0.006, 99% CI (1.136; 2.139).
Discussion
This study was aimed at reviewing the complexity of methods describing TM kinetics and at proposing a solution to the complexity of implementing the inverse HT/DT parameter. The presence of wide ranging and large numerical HT values was reported in therapeutic docetaxel monitoring [9]. For instance, a very slow response to docetaxel from this study (+12,734 days HT) would instead be a reasonable ePSA of −0.01% (percent PSA decrease per day). The group of patients examined during the postoperative period in our study was ascribed HT/DT or ePSA criteria. The data were conveyed to PHCPs after they were familiarized with the “growth fraction” concept (Fig. 1d) of ePSA. For instance, a patient's PSA before RP was 9.56 ng/mL. PSA levels in this patient were 0.365 ng/mL at 30 days following RP and 0.380 ng/mL at 91 days. While a stabilized PSA in the interval of 30–91 days postoperatively indicates a potential problem, the numerical value of this event and the clinical importance of the PSA oscillations are not available to clinicians in their daily routine. If a clinician is provided with a number as complicated as “minus 1,084.1 days HT,” it is quite difficult to comprehend. Actually, this HT approach is not set as an adopted principle of current medical practice. Instead, we could offer to the clinician the value of 0.1% ePSA derived from the same patient's PSA data. This format would mean that the patient's PSA would increase by 0.1% or roughly 0.0004 ng/mL each day. This information is easy to understand, and it can help establish an approximate BCR time in variable clinical situations (Fig. 1). In fact, up to 67% of PHCPs might not be aware that postoperative PSA norm is categorized as “undetectable” [10]. A norm ePSA of “0 or less” might help PHCPs to spot a pattern of positive ePSA and BCR in a timely fashion. Several methods have been advocated to elucidate the relapse pattern of TM above and below normal range [1, 3, 7, 11]. eTM has the advantage that there is no time restriction for testing (in theory, retesting can be done the next week); furthermore, its value is calculated from the ratio of two separate TM values in natural logarithm. This is overlooked when clinicians evaluate a TM increase or decrease based on a simple, nonlogarithmic ratio of two separate TMs.
The indirect evidence of the TM value pattern was demonstrated in a group of healthy women who were later diagnosed with ovarian cancer [12]. Serum Ca-125 testing revealed that a gradual and persistent increase in normal range was noticeable 3 years preceding the diagnosis. In fact, much of the research on early detection of cancer is focused exclusively on “elevated over the normal range” TM value rather than the “rate of change” of TM. This approach thus consumes an enormous amount of time and resources, only to come to a situation that has little hope of making progress.
For instance, 3 of 4 women with tumors had normal screening tests prior to diagnosis even though they were preselected according to genetic risk criteria, then were tested for Ca-125 and finally were assessed by transvaginal ultrasound [13]. Creating new screening strategies (or strategies to spot a recurrence) built on the rate of change rather than an elevated TM value might open additional opportunities in clinical oncology practice. Importantly, it is not the value itself but rather a steady kinetic pattern in the normal range that could be the earliest indicator of recurrence. The eTM could well serve as a tool to ascribe this in a simple numerical format.
Conclusions
We suggest that the ePSA parameter indicating the percent PSA change per day could be a useful adjunct to regular PSA reports. ePSA is free from drawbacks characteristic of HT and DT. The concept is easily understandable and could be considered for all TMs.
Statement of Ethics
This study was approved by the Regional and Institutional Ethics Committee. It was conducted in accordance with the Declaration of Helsinki (2000) and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent prior to enrollment.
Conflict of Interest Statement
The authors have nothing to declare.
References
- 1.Di Gioia D, Stieber P, Schmidt GP, Nagel D, Heinemann V, Baur-Melnyk A. Early detection of metastatic disease in asymptomatic breast cancer patients with whole-body imaging and defined tumour marker increase. Br J Cancer. 2015 Feb;112((5)):809–18. doi: 10.1038/bjc.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman CH, Caram MEV, Radhakrishnan A, Tsodikov A, Deville C, Burns J, et al. Association between PSA values and surveillance quality after prostate cancer surgery. Cancer Med. 2019, Dec;8((18)):7903–12. doi: 10.1002/cam4.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilder JL, Pavlik E, Straughn JM, Kirby T, Higgins RV, DePriest PD, et al. Clinical implications of a rising serum CA-125 within the normal range in patients with epithelial ovarian cancer: a preliminary investigation. Gynecol Oncol. 2003 May;89((2)):233–5. doi: 10.1016/s0090-8258(03)00051-9. [DOI] [PubMed] [Google Scholar]
- 4.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005 Jul 27;294((4)):433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 5.Mehrara E, Forssell-Aronsson E, Ahlman H, Bernhardt P. Specific growth rate versus doubling time for quantitative characterization of tumor growth rate. Cancer Res. 2007 Apr 15;67((8)):3970–5. doi: 10.1158/0008-5472.CAN-06-3822. [DOI] [PubMed] [Google Scholar]
- 6.Doherty AP, Bower M, Smith GL, Miano R, Mannion EM, Mitchell H, et al. Undetectable ultrasensitive PSA after radical prostatectomy for prostate cancer predicts relapse-free survival. Br J Cancer. 2000;83((11)):1432–6. doi: 10.1054/bjoc.2000.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidelines for radical treatment of locally-advanced disease (section 6.2.4.5.). European Association of Urology. EAU Guidelines Office, Arnhem, The Netherlands. http://uroweb.org/guidelines/compilations-of-all-guidelines/ [Accessed 2020]
- 8.Prediction tools/Prostate cancer nomograms. Memorial Sloan Kettering Cancer Center. PSA doubling time. https://www.mskcc.org/nomograms/prostate/psa_doubling_time [Accessed 2020]
- 9.Hanninen M, Venner P, North S. A rapid PSA half-life following docetaxel chemotherapy is associated with improved survival in hormone refractory prostate cancer. Can Urol Assoc J. 2009 Oct;3((5)):369–74. doi: 10.5489/cuaj.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yassaie O, McLaughlin B, Perera M, Manning T, Lawrentschuk N, Malcolm A. Primary care follow-up of radical prostatectomy patients: a regional New Zealand experience. Prostate Int. 2016 Dec;4((4)):136–9. doi: 10.1016/j.prnil.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu Hassan SO, Nielsen DL, Tuxen MK, Petersen PH, Sölétormos G. Performance of seven criteria to assess CA125 increments among ovarian cancer patients monitored during first-line chemotherapy and the post-therapy follow-up period. Future Sci OA. 2017 Jul 18;3((3)):FSO216. doi: 10.4155/fsoa-2017-0023. PMID: 28884012; PMCID: PMC5583662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson GL, McIntosh M, Wu L, Barnett M, Goodman G, Thorpe JD, et al. Assessing lead time of selected ovarian cancer biomarkers: a nested case-control study. J Natl Cancer Inst. 2010 Jan 6;102((1)):26–38. doi: 10.1093/jnci/djp438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olivier RI, Lubsen-Brandsma MA, Verhoef S, van Beurden M. CA125 and transvaginal ultrasound monitoring in high-risk women cannot prevent the diagnosis of advanced ovarian cancer. Gynecol Oncol. 2006 Jan;100((1)):20–6. doi: 10.1016/j.ygyno.2005.08.038. [DOI] [PubMed] [Google Scholar]