Abstract
Tinea capitis is a common fungal infection in children but is less frequently encountered in adults. Tinea capitis presenting as diffuse hair loss is a rare presentation. When patient presents with a diffuse hair loss, tinea capitis is not commonly thought of as differential diagnosis. Low clinical suspicion can lead to inappropriate empiric treatments that delay diagnosis. Trichoscopy in tinea capitis obviates the need for lengthy and invasive procedure or even KOH examinations in the absence of a side lab for diagnosing this common scalp disorder and also avoids misdiagnosis. Trichoscopy not only differentiates tinea capitis from female patterned hair loss but also aids in differentiating ectothrix from endothrix. Here, we present case reports of tinea capitis masquerading as female pattern hair loss with trichoscopy aiding in diagnosis of endothrix infection.
Keywords: Tinea capitis, Trichoscopy, Diffuse hair loss
Established Facts
Tinea capitis is a common fungal infection in children, but it is less frequently encountered and may have atypical presentation in adults, and therefore, tinea capitis is not commonly thought of as differential diagnosis in adults presenting with hair loss.
Novel Insights
Here, we present a case series of tinea capitis with atypical presentations in adults and children. With this case series, we aim to increase the awareness of the clinician about the importance of trichoscopy in clinical practice to avoid misdiagnosis and delay in treatment.
Introduction
Diffuse hair loss is a common complaint encountered by dermatologists in their daily clinical practice. Most common differential diagnosis in patients presenting with hair loss includes telogen effluvium, female patterned hair loss, or alopecia areata [1]. Tinea capitis is a fungal infection of the scalp. Tinea capitis is a common fungal infection in children but is less frequently encountered in adults. When it occurs in adults, the clinical features may be atypical, and this may lead to misdiagnosis or delay in diagnosis, therefore, improper treatment [2]. Trichoscopy is a useful, handy, noninvasive tool for dermatologists which aids in evaluation and follow-up of patients with hair loss and an immediate, cost-effective way to guide to the right diagnosis and therapy, preventing misdiagnosis.
Case 1
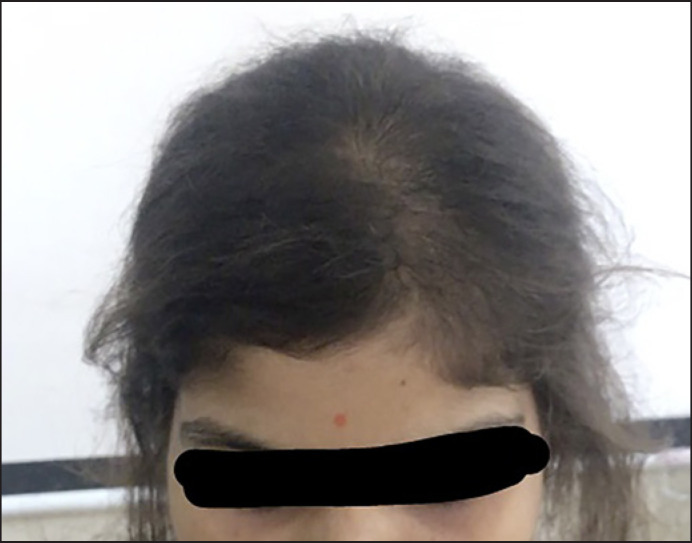
A 20-year-old unmarried female presented with diffuse hair loss since 6 months. There was no history of any menstrual irregularities, any recent major medical or surgical illness, or any major mental stress. On examination, there was a widening of midline partition. Mild scaling was present on scalp (Fig. 1). Hair pull test was positive. Based on these findings, a differential diagnosis of female patterned hair loss with pityriasis sicca (P. Sicca) and telogen effluvium with P. sicca was made. On trichoscopy, hair diameter diversity was <10%. Multiple comma hairs were seen. Broken hair shafts and corkscrew hair were also noted (Fig. 2). KOH mount and fungal culture were negative. Trichoscopy guided 4 mm scalp biopsy was done through corkscrew hair. On histopathology, multiple spores within hair shaft were noted which were positive on periodic acid-stiff stain. Therefore, a final diagnosis of tinea capitis was made. We screened her family members for tinea capitis and other dermatophytic infections. We looked for predisposing factors like HIV, diabetes, contact with animal, and recent history of travel. She was started on tablet Griseofulvin (20 mg/kg/day) in divided doses for 4 weeks and topical application of 2% ketoconazole lotion. After 15 days, she was reassessed. Hair pull test was negative. After 1 month, trichoscopy showed no comma hair or corkscrew hair.
Fig. 1.
Tinea capitis in adult presenting as widening of midline partition of scalp.
Fig. 2.
Trichoscopy shows multiple corkscrew hair and comma shaped hair.
Case 2
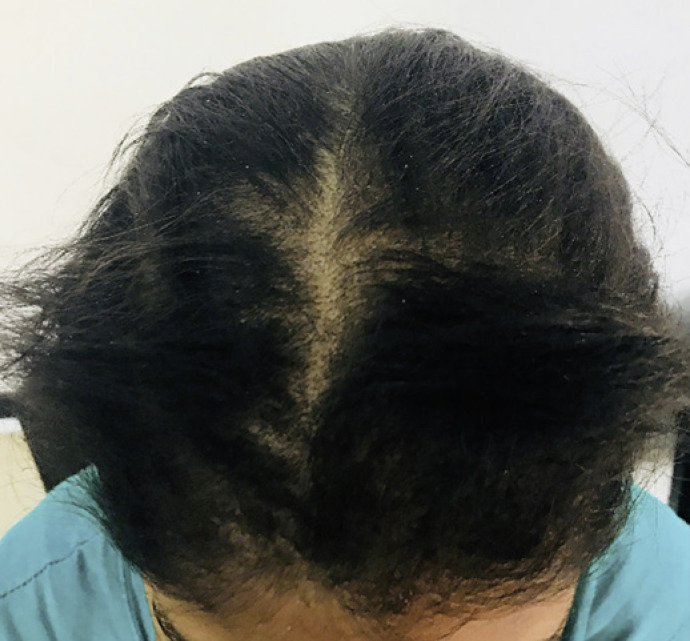
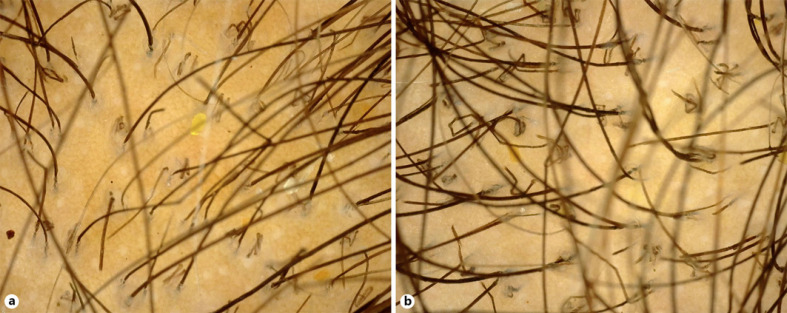
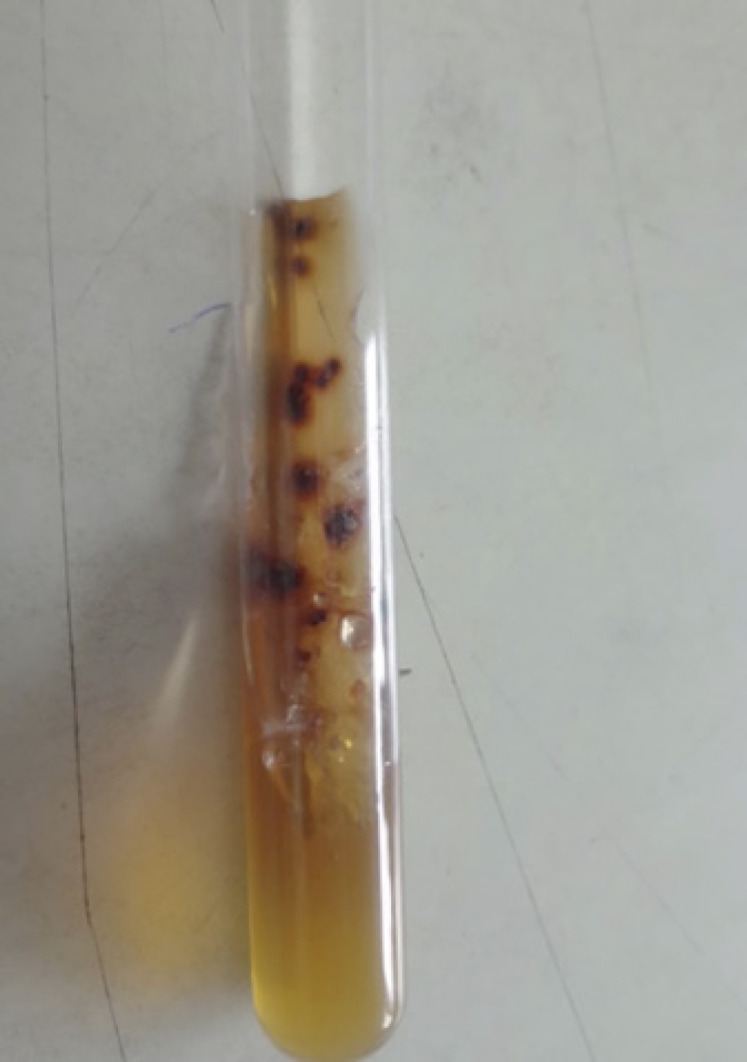
A 23-year-old unmarried female presented with complaint of diffuse hair loss, broken hair, and itching on scalp since 2 years. On examination, there was diffuse sparseness of hair with scaling on scalp (Fig. 3). She visited a private practitioner in past for similar complaint and was treated for female pattern hair loss. Patient did not have any predisposing factor like diabetes or any immunosuppression. We did hair pull test which was negative. We then performed trichoscopy, which revealed few comma, broken, and corkscrew hairs (Fig. 4a, b). Upon these findings, KOH mount and fungal culture were advised. KOH mount with Chicago blue stain revealed numerous spores (Fig. 5). Fungal culture in Sabouraud's dextrose agar revealed growth of violet pigment-producing Trichophyton violaceum (Fig. 6). On the basis of above findings, a diagnosis of tinea capitis was made, and patient was started on T Itraconazole 200 mg daily for 1 month and 2% ketoconazole lotion. Patient did not follow-up further.
Fig. 3.
Tinea capitis in adults: diffuse sparseness of hair with scaling.
Fig. 4.
Trichoscopy of scalp showing comma hair and broken hair (a), corkscrew and zigzag hair (b).
Fig. 5.
Chicago blue staining showing numerous spores.
Fig. 6.
Showing growth of violet pigment-producing Trichophyton violaceum in Sabouraud's dextrose agar.
Case 3
A 19-year-old unmarried female presented with complaint of itching of scalp with hair loss since 2 months (Fig. 7). Clinical examination revealed fragile sparse hair with diffuse scaling on scalp. Hair pull was strongly positive from all areas of scalp. Before presenting to our care centre, patient was treated for telogen effluvium and P. sicca by a private practitioner. We performed trichoscopy of scalp, which revealed few broken and comma hairs. KOH and fungal culture were advised. Fungal culture on Sabouraud's dextrose agar showed colonies of T. rubrum. Diagnosis of tinea capitis was made, and patient was started on griseofulvin (20 mg/kg/day) in 2 divided doses with 2% ketoconazole lotion for 4 weeks. Patient responded well to antifungal treatment in 3 weeks and hair pull became negative.
Fig. 7.
Showing patient with mild scaling of scalp.
Case 4
A 16-year-old female presented with complaint of itching and diffuse hair fall since 1 month. On clinical examination, hair shafts were rough, and scales were present all over the scalp. No localized patches of hair loss were noticed. Patient was treated for telogen effluvium in past. We did hair pull test, which was positive from all areas of the scalp. Patient denied fungal culture. On trichoscopy examination, there were coiled and broken hairs with scaling. Examination on 20% potassium hydroxide scrapping examination showed multiple spores on hair shafts. The biopsy was denied by the patient. A diagnosis of diffuse tinea capitis was made and tablet Griseofulvin (20 mg/kg/day) in 2 divided doses was started for 4 weeks. Patient responded very well, and hair pull became negative after 3 weeks of treatment.
Discussion
Tinea capitis is a common fungal infection in children but is less frequently encountered in adults [2]. Tinea capitis is uncommon in adults due to production of sebum made of fungistatic saturated fatty acids, colonization by Pityrosporum orbicularis which prevents growth of dermatophytes and thicker calibre of adult hair preventing dermatophytic invasion [3]. The predisposing factors for tinea capitis in adults are immunosuppression, diabetes, anaemia, long use of topical or systemic corticosteroids, contact with animal and source of infection elsewhere in body (tinea located elsewhere on the body, contact from infected children or fomites). In postmenopausal women, tinea capitis may occur due to changes in the pH of the scalp and a decrease in quality and quantity in sebum, especially in medium chain fatty acids, as a result of hormonal imbalances that alter these protective effects of sebum [4]. Clinically, children present with pruritus, scaling, “black dots,” hair loss, and posterior cervical lymphadenopathy, whilst tinea capitis in adults is often subtler and may mimic the appearance of seborrheic dermatitis with mild inflammation and scarce scaling, whereas typical kerion is uncommon and favus is rare. Polymorphic and atypical clinical presentations lead to difficulty in diagnosis and delay in treatment [4]. Trichoscopy in tinea capitis obviates the need for lengthy and invasive procedure or even KOH examinations in the absence of a side lab for diagnosing and prevents delay in diagnosis [5]. On trichoscopy, most common trichoscopic features are short broken hairs, followed by black dots, comma shaped hairs, or corkscrew hairs [6]. Slowinska et al. [7] described comma hair as a distinguishing marker for tinea Capitis followed by broken hair and black dots. Comma hairs have an even colour and thickness and marked distal angulation. Comma shape occurs due to hair shafts that have disintegrated, cracked, and bent due to the occurrence of multiple hyphae within the hair shaft [8]. Also, comma hair, corkscrew hair, and pigtail hair are seen only in patients of tinea capitis, thus, are specific features of the same [9]. Other findings are broken hairs, black dots, zigzag hair, and morse code like hair [10]. Trichoscopy features also vary in ectothrix and endothrix infection. Ectothrix infection characterized by accumulation of spores outside the hair shaft which damages the hair shaft partially causing it to bend resulting in comma hair. As the disease progresses, the spores cause transverse perforation of hair shaft leading to transverse fracture of hair which causes horizontal white bands distributed regularly throughout the hair shaft resembling a Morse code. This gives morse code appearance of hair shaft [11]. Horizontal white bands correspond to localized areas of fungal elements responsible for perforation of the hair shaft [12]. At lower magnification, it appears as subtle horizontal white bands whereas empty bands at higher magnification [13]. Such multiple transverse hair fractures lead to zigzag deformation of hair shaft leading to zigzag hair [11]. Complete fracture results in broken hair and black dots. In endothrix infection, there is deformation of the hair shaft associated with loss of intrapilar stiffness is seen [11]. The pigmentation of the hair continues to be normal since the spores are inside the hair shaft. Hence, the signs seen are comma hair, corkscrew hair, broken hair, and black dots. Hughes et al. [14] showed that corkscrew hair, a variant of comma hair is specific in black patients and is marker of endothrix infection. However, Neri et al. [15] reported similar finding in white Italian male. Black dots represent broken hair shaft at the level of scalp. However, our patient presented with asymptomatic widening of midline partition with slight scaling. When a woman presents with scaling, clinician could think of diagnosis of P. sicca and midline parting widening can lead to the misdiagnosis of Female patterned hair loss [16]. Our patient had endothrix invasion. Endothrix dermatophytes are trichophyton tonsurans, trichophyton violaceum and trichophyton schoenleinii, of which, tonsurans and violaceum species are common in India [17]. Microscopy and culture can be negative in few cases of tinea capitis. The rate of false-negative interpretations for KOH microscopy can be >40% even for experienced observers. It can occur due to incorrect or inadequate sample collecting or findings being misinterpreted as artefacts [18]. A study in Nigeria (n = 201) showed 67.60% to be culture negative. Low culture yield could be due to insufficient samples, contaminations, and prior treatment with antifungals [19]. So if trichoscopy is not performed, then such cases of tinea capitis can be missed. Due to epidemic nature of dermatophyte, overcrowding and poor hygiene, in recent years, unusual presentation of tinea capitis is not uncommon [20]. For our patients, poor hygiene, communal sharing of hair care tools [21] and continuous head wrapping (leading to excessive sweating) were contributory. However, routine practice of trichoscopy for patients with hair loss helps in diagnosis of tinea capitis. With such circumstances, it becomes imperative for treating physician to view cases presenting with hair loss or chronic scaling with high index of suspicion. Careful inspection of the scalp for comma hair, corkscrew hair, black dots, morse code hair, zigzag hair and broken hair along with microscopic examination and culturing of plucked black dots or abnormal hairs, rather than just scraping of scales, are both important in confirming the diagnosis [22]. Although Direct KOH observation and mycological culture of skin scrapings are considered gold standard for diagnosis of tinea capitis, they both consume time and money and also could be inconclusive at times. Hence, simple, direct, and quick methods like trichoscopy are required to make a rapid diagnosis and start treatment.
Conclusion
Tinea capitis presenting as female pattern hair loss has not been reported yet to the best of our knowledge. We recommend trichoscopy as a sensitive tool for quick diagnosis and monitoring treatment response in tinea capitis.
Statement of Ethics
Subjects have given their written informed consent to publish their case (including publication of images).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors did not receive any funding.
Author Contributions
All the authors contributed in patient examination and paper writing.
References
- 1.Werner B, Mulinari-Brenner F. Clinical and histological challenge in the diagnosis of diffuse alopecia: female androgenetic alopecia, telogen effluvium and alopecia areata: part II. An Bras Dermatol. 2012;87((6)):884–90. doi: 10.1590/S0365-05962012000600010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181((5–6)):371–8. doi: 10.1007/s11046-016-0004-9. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46((2 Suppl Understanding)):S120–4. doi: 10.1067/mjd.2002.120793. [DOI] [PubMed] [Google Scholar]
- 4.Salazar E, Asz-Sigall D, Vega D, Arenas R. Tinea capitis: unusual chronic presentation in an elderly woman. J Infect Dis Epidemiol. 2018;4:048. [Google Scholar]
- 5.Daroach M, Hanumanthu V, Kumaran MS. Utility of trichoscopy in tinea capitis. Postgrad Med J. 2019;95((1121)):173. doi: 10.1136/postgradmedj-2018-136106. [DOI] [PubMed] [Google Scholar]
- 6.Al-Refu K. Clinical significance of trichoscopy in common causes of hair loss in children: analysis of 134 cases. Int J Trichology. 2018;10((4)):154–61. doi: 10.4103/ijt.ijt_101_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slowinska M, Rudnicka L, Schwartz RA, Kowalska-Oledzka E, Rakowska A, Sicinska J, et al. Comma hairs: a dermatoscopic marker for tinea capitis: a rapid diagnostic method. J Am Acad Dermatol. 2008 Nov;59((5 Suppl)):S77–9. doi: 10.1016/j.jaad.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Elghblawi E. Idiosyncratic findings in trichoscopy of tinea capitis: comma, zigzag hairs, corkscrew, and morse code-like hair. Int J Trichol. 2016;8((4)):180–3. doi: 10.4103/ijt.ijt_92_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Taweel AE, El-Esawy F, Abdel-Salam O. Different trichoscopic features of tinea capitis and alopecia areata in pediatric patients. Dermatol Res Pract. 2014;2014:848763. doi: 10.1155/2014/848763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudnicka L, Olszewska M, Rakowska A, Slowinska M. Trichoscopy update 2011. J Dermatol Case Rep. 2011;5((4)):82–8. doi: 10.3315/jdcr.2011.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourezane Y, Bourezane Y. Analyse des signes trichoscopiques observés chez 24 patients porteurs d'une teigne du cuir chevelu: hypothèses fondées sur la physiopathologie et proposition d'une nouvelle classification. Ann Dermatol Venereol. 2017;144((8–9)):490–6. [Google Scholar]
- 12.Malakar S, Mehta PR, Mukherjee SS. Trichoscopy in pediatric age group. Indian J Paediatr Dermatol [Serial Online] 2018;19:93–101. [Google Scholar]
- 13.Elghblawi E. Idiosyncratic findings in trichoscopy of tinea capitis: comma, zigzag hairs, corkscrew, and morse code-like hair. Int J Trichol. 2016;8((4)):180–3. doi: 10.4103/ijt.ijt_92_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes R, Chiaverini C, Bahadoran P, Lacour JP. Corkscrew hair: a new dermoscopic sign for diagnosis of tinea capitis in black children. Arch Dermatol. 2011;147((3)):355. doi: 10.1001/archdermatol.2011.31. [DOI] [PubMed] [Google Scholar]
- 15.Neri I, Starace M, Patrizi A, Balestri R. Corkscrew hair: a trichoscopy marker of tinea capitis in an adult white patient. JAMA Dermatol. 2013;149((8)):990. doi: 10.1001/jamadermatol.2013.4352. [DOI] [PubMed] [Google Scholar]
- 16.Olsen EA. The midline part: an important physical clue to the clinical diagnosis of androgenetic alopecia in women. J Am Acad Dermatol. 1999;40((1)):106–9. doi: 10.1016/s0190-9622(99)70539-6. [DOI] [PubMed] [Google Scholar]
- 17.Gajula N, Vumma N, Rohit V, Kalikota A. A clinico-epidemiological study of tinea capitis in children attending a Tertiary Care Hospital in Karimnagar. Indian J Paediatr Dermatol. 2019;20:332–7. [Google Scholar]
- 18.Hubbard TW. The predictive value of symptoms in diagnosing childhood tinea capitis. Arch Pediatr Adolesc Med. 1999;153((11)):1150. doi: 10.1001/archpedi.153.11.1150. [DOI] [PubMed] [Google Scholar]
- 19.Ayanlowo O, Akinkugbe A, Oladele R, Balogun M. Prevalence of Tinea capitis infection among primary school children in a rural setting in south-west Nigeria. J Public Health Africa. 2014;5((1)):14–8. doi: 10.4081/jphia.2014.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatia VK, Sharma PC. Epidemiological studies on dermatophytosis in human patients in Himachal Pradesh, India. Springerplus. 2014;3:134. doi: 10.1186/2193-1801-3-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takwale A, Agarwal S, Holmes SC, Berth-Jones J. Tinea capitis in two elderly women: transmission at the hairdresser. Br J Dermatol. 2001;144((4)):898–900. doi: 10.1046/j.1365-2133.2001.04154.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee JY, Hsu ML. Tinea capitis in adults in Southern Taiwan. Int J Dermatol. 1991;30((8)):572–5. doi: 10.1111/j.1365-4362.1991.tb02643.x. [DOI] [PubMed] [Google Scholar]