Abstract
Purpose
To evaluate local control and functional and cosmetic outcomes of postoperative high-dose-rate interventional radiotherapy (HDR-IRT) in patients affected by conjunctival melanoma with orbit invasion.
Methods
A retrospective study was conducted in 2 patients affected by conjunctival melanoma infiltrating the orbit, treated with surgical excision and HDR-IRT. The treatment procedures consisted of surgical excision of the orbital infiltrating nodule followed, 1 month after surgery, by adjuvant HDR-IRT. A target dose of 34 Gy was delivered in 10 twice-a-day fractions over 5 consecutive days. Data analysis included local tumor control and metastatic rate, acute and late toxicity, functional and aesthetic results.
Results
In both patients, treatment was well tolerated, and there was no orbital recurrence at a median follow-up of 37–40 months. There was an excellent functional outcome, without no significant acute or late side effects.
Conclusions
HDR-IRT could be considered a promising, feasible, successful, and well-tolerated option for selected patients affected by ocular tumors with orbital invasion.
Keywords: Brachytherapy, Conjunctival melanoma, Ocular oncology, Ocular tumors, Ophthalmic oncology
Established Facts
Conjunctival malignant melanoma is a rare but potentially life-threatening cancerous growth of the eye.
Conjunctival malignant melanoma is a rare but potentially life-threatening cancerous growth of the eye. Excision without additional treatment is associated with more local recurrences.
Novel Insights
Postoperative high-dose-rate interstitial brachytherapy (interventional radiotherapy) for conjunctival melanoma with extension in anterior orbit could be considered a promising, feasible, successful, and well-tolerated option for selected patients affected by ocular tumors with orbital invasion.
Introduction
Conjunctival malignant melanoma (CMM) is a rare but potentially life-threatening cancerous growth of the eye. It comprises about 5% of all ocular melanoma [1] with an incidence of 0.2 − 0.8 case per million in the Caucasian population [2].
Although a rare diagnosis, CMM has serious implications with respect to recurrence, metastasis, and mortality. Despite treatment of the primary lesion, metastatic disease can develop and can be fatal with a 10-year melanoma-related mortality of up to 29% [3]. Local recurrence is reported to be 36–56% at 5 year, depending on the treatment, and it has been associated with increased mortality [4, 5]. Risk factors for recurrence include thickness of the primary tumor, incomplete excision, and non-limbal tumor location [6].
The current therapeutic strategy for CMM consists of local excision in combination with adjuvant therapy: cryotherapy, topical chemotherapy, and/or radiotherapy. Excision without additional treatment is associated with more local recurrences [7].
Orbital metastases from CMM are rare. In a series of 89 patients with secondary orbital melanoma, Rose et al. [8] found that the commonest primary source was choroidal melanoma (54% of cases), while conjunctival melanoma accounted only for 17% of cases. According to other reviews, secondary orbital metastasis from conjunctival tumors represent 1.1–26% of all metastatic tumors to the orbit [9, 10, 11, 12].
Treatment of secondary orbital melanoma remains controversial and it can change according to the degree of orbit invasion [13]. Orbital exenteration and/or external beam irradiation remains the mainstay of treatment for extensive CMM invading the orbit [14] although previous studies have shown that life prognosis does not improve after exenteration compared to other therapeutic options [15].
External beam radiotherapy (EBRT) has been used as adjuvant therapy or as an alternative to exenteration in high-risk CMM (increased thickness, recurrent, multifocal, orbital involvement, unfavorable location, and ineligible for brachytherapy) [16]. Proton radiotherapy approach demonstrated similar results to exenteration regarding local relapse and metastatic disease for T2 and T3 conjunctival melanomas [17, 18].
In cases with minimal residual tumor after surgical excision and excellent vision in the affected eye, brachytherapy with a custom-designed iodine-125 plaque has been proposed as a reasonable alternative to exenteration and external irradiation [19]. Combining multiple conservative treatments has demonstrated to enable local control in selected cases of invasive conjunctival melanoma. Recent studies have demonstrated favorable outcomes when managing diffuse orbito-conjunctival melanoma with tumor debulking, brachytherapy (using custom unshielded radioactive device), and adjuvant ipilimumab [20, 21].
High-dose-rate interventional radiotherapy (HDR-IRT) is a technique widely used in the treatment of head and neck tumors [22, 23]. It is ideally suited to deliver a high dose exclusively to the primary tumor volume, thus minimizing treatment related toxicity to the nearby structures. In this retrospective study we report our experience in the use of HDR-IRT as adjuvant therapy, after surgical excision, in 2 cases of anterior orbital metastasis from conjunctival melanoma.
Case Report/Case Presentation
Patient 1
In 2014, a 50-year-old woman underwent surgical excision and adjuvant topical Mitomycin C (0.02%, 1 drop 4 times daily for 4 weeks) for a limbal conjunctival melanoma (T1a) in her right eye. The patient was followed up regularly every 6 months. After 2 years, although the conjunctiva was clear, the whole-body PET revealed mild fluorodeoxyglucose (FDG) uptake in a nodular mass lesion located in the anterior right orbit, later confirmed by orbital ultrasound and CT (Fig. 1). An en bloc excisional biopsy of the orbital lesion was performed (Fig. 2) and after 1 month the patient underwent HDR-IRT to prevent further recurrence. Histopathologic examination revealed a mixed-type cell melanoma. Subsequent PET/CT findings have been normal until July 2018, when an intense FDG uptake in the lymph nodes above and below the diaphragm was recorded, without pathological uptake in the orbital region. Therefore, the patient was subjected to 4 cycles of the immune checkpoint inhibitor ipilimumab, from August to September 2018. After 40 months from surgery and HDR-IRT, no local recurrences were documented, and a stabilization of the secondary lesions was recorded. The cosmetic assessment is very good as shown in Figure 5.
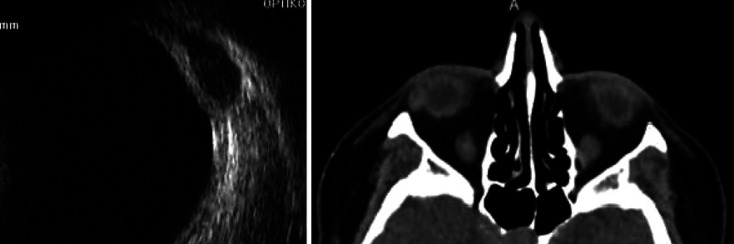
Fig. 1.
Orbital ultrasound and CT showing orbital nodule in the first case.
Fig. 2.
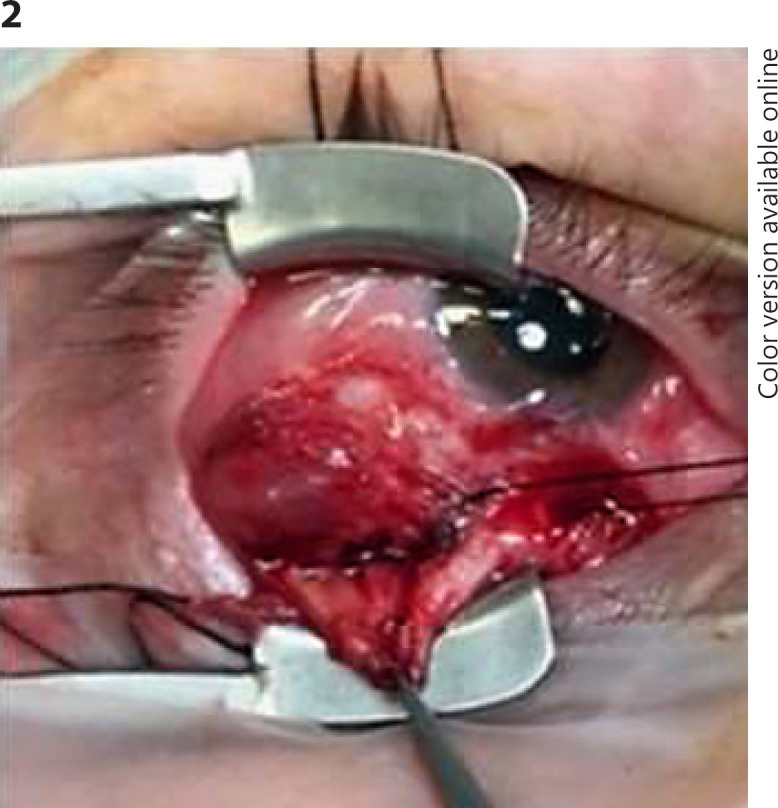
Excisional biopsy of the orbital nodule in the first case.
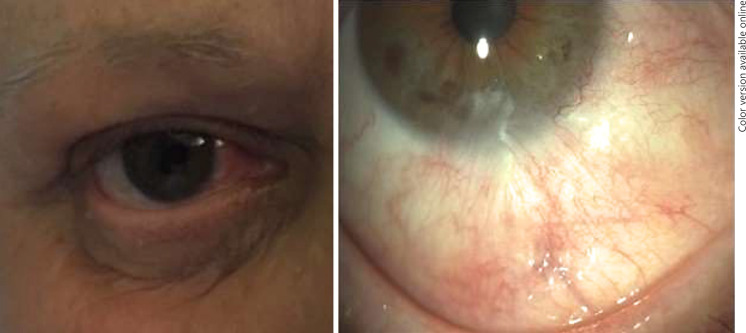
Fig. 5.
Cosmetic results after HDR-BT treatment.
Patient 2
In 2014, a 71-year-old woman was referred to our center because of a recurrent conjunctival melanoma involving the fornix in her right eye (T2a). A wide surgical excision, followed by adjuvant topical Mitomycin C drops, was performed. The patient was followed up regularly every 6 months. After 1 year a MRI of the orbit was required as symblepharon prevented the observation of the inferior fornix. MRI showed a nodular mass lesion located in the inferior fornix and extended to the anterior right orbit. Whole-body PET/CT documented FDG uptake just in the anterior right orbit. An en bloc excisional biopsy of the forniceal/orbital lesion was performed, and after 1 month the patient underwent HDR-IRT. Subsequent MRI showed the complete resolution of the orbital lesion and PET/CT documented no pathological uptake. Regular follow-ups over 3 years (every 3 months for the first year, then every 6 months) documented no evidence of local recurrence.
Procedure Description
The 2 patients described with secondary orbital localization from conjunctival melanoma underwent surgical excision of the orbital nodule under general anesthesia; after 1 month from surgery, they were planned for HDR-IRT. Both patients were staged T3cN0MO at the time of HDR-IRT procedure.
The procedure involved 3 phases as reported in Table 1: the pre-planning, the implantation, and treatment planning/delivery [24]. The implant procedures were carried out under general anesthesia. Needle entry points were extrapolated and marked on the skin as determined from the pre-planning CT scan.
Table 1.
Procedure steps
|
Step 1: pre-planning a. CT defined the ideal position and depth of insertion for each catheter b. CT based pre-planning and optimization c. Catheter insertion coordinates with reference to bony landmarks |
|
Step 2: implantation technique a. Bimanual catheter implantation was carried out under general anesthesia b. Catheters inserted carefully to avoid injury, especially to prevent extension beyond the orbit c. The catheters were sewn to the skin for stability |
|
Step 3: treatment planning and delivery a. CT defined the actual catheter position and depth of insertion for 3D treatment planning b. CT based BT planning and optimization c. Treatment delivery (34 Gy in 10 fractions twice a day) |
Transcutaneous incisions were performed, then catheters containing metal trocars with needle tip were introduced into and passaged through the adnexal and orbital soft tissue (Fig. 3). The depth of each catheter had been previously marked following the CT orbit scan in order to avoid deeper implantation. Safe penetration depth for each catheter was determined on the axial CT scans. Once in place, each central metal catheter trocar was removed, a plastic button was advanced onto the catheter, then vycril 5-0 was sutured to sew the button to the skin.
Fig. 3.
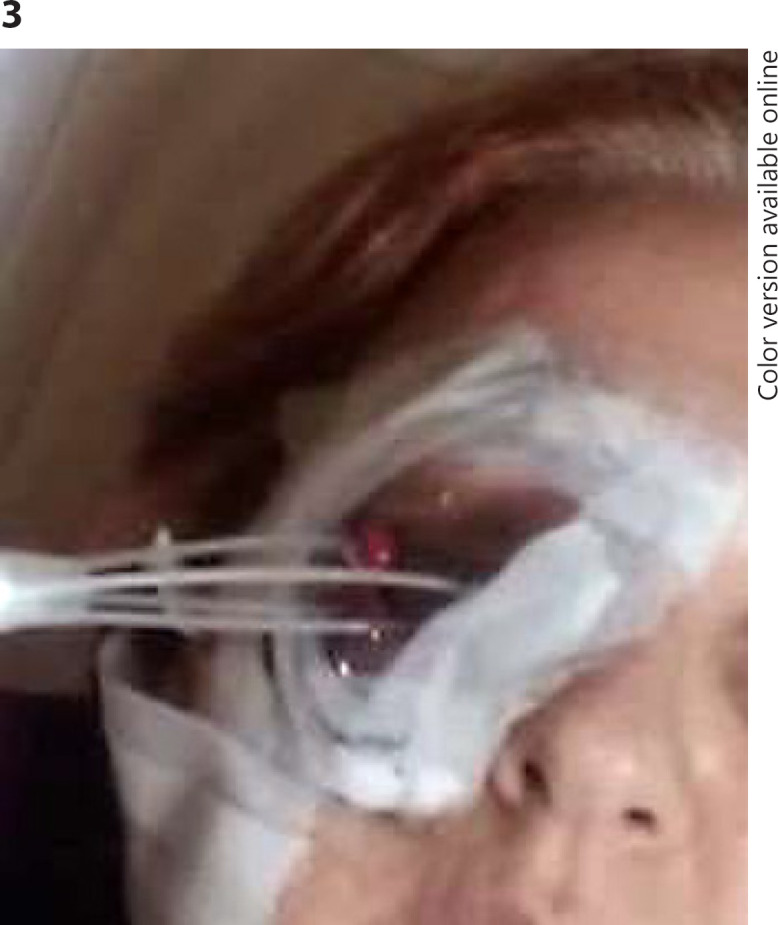
Catheters introduction.
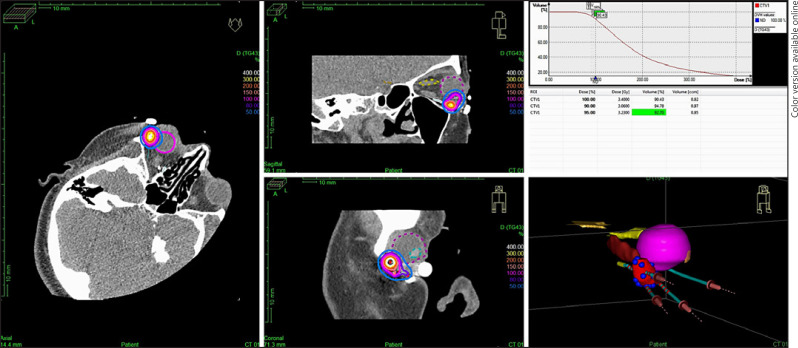
After the procedure in the operating room, the patient was transferred to the CT suite and a CT planning was done on the Oncentra Brachy (Nucletron, an Elekta company, Elekta AB, Stockholm, Sweden) console. Clinical target volume was delineated on the disease extent visible on the presurgery CT images with a margin of 3–5mm with modification if required. Iridium-192 HDR-IRT plan was generated using Oncentra Brachytherapy planning system. The dose distribution was evaluated and optimized to ensure coverage of the target volume by the reference isodose. The plan was evaluated using dose volume histogram for target volume coverage and dose to the critical structures (Fig. 4). After treatment completion, the tubes were removed under aseptic conditions.
Fig. 4.
Dose distribution.
The dosimetric analysis of the organs at risk close to the catheters, such as cornea, lens, and optic nerve, was carried out. The maximum dose delivered to these structures was noted. The maximum dose for the cornea was 4.31 Gy for the first patient and 14.65 Gy for the second patient. For the lens it was 9.2 and 24.2 Gy and for the optic nerve 4 and 12.59 Gy respectively for the first and second patient.
The total dose delivered was 34 Gy, using 10 twice-daily fractions, over 5 days. The number of catheters implanted was 4 for the first patient and 6 for the second.
Clinical Outcomes
The patients are currently at 37–40-months post-radiation treatment showing excellent local tumor control. MRI scan, performed every 6 months at follow-up, did not show orbital recurrence and PET/CT documented no pathological uptake in the orbital region.
The treatment was well tolerated, and there were no significant side effects; both patients experienced dry eye syndrome which resolved with topical lubricants. Best corrected visual acuity was stable, without decrease with respect to the baseline, and was 20/20 in the first case and 20/40 in the second case due to a cataract. Excellent cosmesis was achieved, without any restriction of ocular motility or eyelid scar from catheter insertion (Fig. 5).
Fundus exam remained normal; neither of the patients developed optic disc neuropathy, radiation retinopathy, iris neovascularization, or secondary glaucoma.
The first case described that she presented local lymph node metastases in 2018, 2 years after HDR-IRT.
Due to the presence of metastatic lymph nodes, she started immunotherapy with single-agent pembrolizumab for eighteen cycles, then, following the appearance of distant lymph node metastases, she was switched to ipilimumab for 4 cycles. After 24 months, there is no evidence of recurrence, and a stabilization of metastatic lesions has been documented.
For the second case, subsequent MRI showed the complete resolution of the orbital lesion and PET/CT showed no pathological uptake. Follow-up controls in the following 3 years (every 3 months for the first year, then every 6 months) documented a complete resolution, with neither local nor systemic recurrence.
Discussion/Conclusion
The use of radiotherapy as adjuvant treatment after conjunctival melanoma excision has been shown to reduce the local recurrence rate. Misotten found trend toward increased local tumor recurrence after excision without additional treatment compared with excision and ruthenium plaque or additional external radiotherapy. A very low recurrence rate of 1.5% is reported at a median of 2.2 years after local excision and radiotherapy [25, 26, 27].
Adjuvant brachytherapy for conjunctival melanoma involves the use of plaque or seeds or ring and can be used for bulbar conjunctival melanoma or may be reverse mounted to administer radiation to tarsal conjunctival melanoma.
For non-bulbar conjunctival melanoma, adjuvant treatments with EBRT or proton beam radiotherapy (PBRT) are indicated.
EBRT has been reported as adjuvant treatment after conjunctival melanoma excision in 51 patients and has been associated with numerous adverse effects: dry eye syndrome in 96% of patients, local eyelash loss in 77%, cataracts in 27%, limbal stem cell deficiency with corneal vascularization in 15% of cases, and keratinization of the conjunctiva in 4% [28, 29].
The use of PBRT was described in different cases series: Wuestemeyer treated 20 patients with adjuvant PBRT for conjunctival melanoma with a diffuse lesion. Scholz treated 89 patients with extended conjunctival melanoma and multifocal bulbar located tumors. Cajoulle treated 92 patients with conservative surgery and proton therapy; 72% of stage T1 and 28% T2.
The main complications reported were sicca syndrome, cataract, secondary glaucoma, conjunctiva and corneal thinning, a scleral perforation, and decrease of visual acuity [30, 31, 32]. For orbital interventional radiotherapy implants, there are 3 general indications found in the literature where such kind of procedure has been described: orbital soft tissue sarcoma in infancy, enucleation for extrascleral extension of uveal melanoma, and recurrent retinoblastoma [33, 34].
The major advantage of HDR-IRT is the rapid falloff. It means that the primary tumor volume receives a total dose which could not be safely delivered by EBRT alone and the rapid falloff allows relative sparing of critical normal tissues and, above all, less posterior irradiation to the brain and optic chiasm [35]. Even with the best of EBRT technique such as intensity modulate radiotherapy or PBRT therapy, a significant dose is received by surrounding orbital structure.
The dose delivered to the lens was equal to TD 5/5 of 10 Gy for the first patient and greater than this for the second patient. Only the second patient developed cataract and underwent surgery for this.
Because of the low dose delivered to the critical structure of the orbit, the HDR-IRT procedure was well tolerated and the 2 patients treated complained only of a mild transient dry eye syndrome. After a follow-up of 37–40 months, no late side effects were registered, the visual acuity remained stable, and they did not develop radiation retinopathy, optic neuropathy, or neovascular glaucoma.
The cosmetic result was excellent; the introduction of catheters with a diameter of 6 French through the eyelid did not cause any skin scar, epiphora, or lagophthalmos. Another advantage of the HDR-IRT is that an equivalent dose can be delivered over a short period of time; the total dose delivered in our study was 34 Gy, in 10 fractions of 3.4 Gy administered twice a day for only 5 days.
HDR-IRT is ideally suited to deliver a high dose to a small volume. For this reason, it is indicated as postoperative adjuvant treatment or in presence of a microscopic residue after tumor excision.
In the cases described in this study, HDR-IRT has been used to treat anterior orbital localization of CMM; neverthless, it is indicated as adjuvant treatment of all CMM located at unfavorable sites, such as fornix, caruncle, or palpebral conjunctiva, not eligible for applicator brachytherapy with non-customized plaque.
The first patient presented with a local recurrence, and 2 years later, she developed regional lymph nodes metastases; therefore, she was treated with 18 plus 4 cycles of different immune checkpoint inhibitors.
Since no local recurrence was registered for a long follow-up, and there is a stabilization of the distant metastases, we may suppose that immunotherapy also had a beneficial effect on local disease. As reported by Sagiv et al. [36], 2 cases of recurrent conjunctival melanoma with metastasis were successfully treated with systemic PD-1 inhibitors (nivolumab and pembrolizumab), while Shields [37] described the resolution of an orbito-conjunctival melanoma mass and regression of liver metastasis after 14 cycles of systemic immunotherapy.
Conclusion
In this retrospective study, the authors presented 2 cases of orbital metastasis from conjunctival melanoma treated with surgical excision and, 1 month later, with HDR-IRT. The combined treatment carried out allowed a complete local control after 40 months of follow-up, preservation of visual function, and excellent cosmetic result.
The major limitation of this work is the small number of patients described. The treatment of choice of diffuse orbital invasion of malignant tumor is exenteration; however, if the tumoral tissue is well circumscribed and orbital extension is limited, a wide surgical excision with adjuvant radiotherapy is likely to be curative, thus highlighting the possibility of personalized medicine.
Although HDR-IRT procedure requires special skills and a multidisciplinary approach [38, 39, 40], being extremely conformal and targeting only the tumor bed, it can spare a considerable amount of normal tissue and provides an important alternative opportunity for function preservation in these cases.
Statement of Ethics
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. All subjects have given their written informed consent to publish their case including publication of images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors did not receive any funding.
Author Contributions
All authors contributed substantially to this work and have read and approved the final manuscript.
References
- 1.Isager P, Engholm G, Overgaard J, Storm H. Uveal and conjunctival malignant melanoma in Denmark 1943–97:observed and relative survival of patients followed through 2002. Ophthalmic Epidemiol. 2006;13:85–96. doi: 10.1080/09286580600553330. [DOI] [PubMed] [Google Scholar]
- 2.Kastelan S, Antunica AG, Oreskovic LB, Rabatic JS, Kasun B, Bakija I. Conjunctival melanoma, epidemiological trends and features. Pathol Oncol Res. 2018;24((4)):787–96. doi: 10.1007/s12253-018-0419-3. [DOI] [PubMed] [Google Scholar]
- 3.Brouwer NJ, Marinkovic M, Luyten GPM, Shields CL, Jager MJ. Pigmentation of conjunctival melanoma recurrences and outcome. Graefes Arch Clin Exp Ophthalmol. 2019;257((8)):1783–8. doi: 10.1007/s00417-019-04342-x. [DOI] [PubMed] [Google Scholar]
- 4.De Potter P, Shields CL, Shields JA, Menduke H. Clinical predictive factor for development of recurrence and metastasis in conjunctival melanoma : a review of 68 casese. Br J Ophthalmol. 1993 Oct;77((10)):624–30. doi: 10.1136/bjo.77.10.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esmaeli B, Wang X, Youssef A, Gershenwald JE. “Pattern of regional and distant metastasis in patients with conjunctival melanoma” Ophthalmology 2001,108;2101-2105)(Kuiala E, Tuomaala S, Eskelin S, Kivela T. Mortality after uveal and conjunctival melanoma:which tumour is more deadly? Ophthalmology. 2009;87:149–53. [Google Scholar]
- 6.Wong JR, Nanji AA, Galor A, Karp CL. Management of conjunctival malignant melanoma: a review and update. Expert Rev Ophthalmol. 2014;9((3)):185–204. doi: 10.1586/17469899.2014.921119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lommatzsch PK, Werschnik C. Das maligne melanom der bind-ehaut. Klinische Ubersich mit Empfehlungen zur diagnose, therapie und nachsorge. Klin Monatsbl Augenheilkd. 2002;219:710–21. doi: 10.1055/s-2002-35693. [DOI] [PubMed] [Google Scholar]
- 8.Rose AM, Cowen S, Jayasena CN, Verity DH, Rose GE. Presentation, treatment, and prognosis of secondary melanoma within the orbit. Front Oncol. 2017;7:125. doi: 10.3389/fonc.2017.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soysal HG, Ardic F. Malignant conjunctival tumors invading the orbit. Ophthalmologica. 2008;222:338–43. doi: 10.1159/000146079. [DOI] [PubMed] [Google Scholar]
- 10.Shields JA, Bakewell B, Augsburger JJ, Flanagan JC. Classification and incidence of space occupying lesions of the orbit: a survey of 645 biopsies. Arch Ophthalmol. 1984;102:1606–11. doi: 10.1001/archopht.1984.01040031296011. [DOI] [PubMed] [Google Scholar]
- 11.Gunalp I, Gunduz K. Secondary orbital tumors. Ophthalmic Plast Reconstr Surg. 1997;13:31–5. doi: 10.1097/00002341-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Rahman I, Cook AE, leatherbarrow B. Orbital exenteration: a 13 year Manchester experience. Br J Ophthalmol. 2005;89:1335–40. doi: 10.1136/bjo.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polito E. Leccisotti a “primary and secondary orbital melanomas: a clinical and prognostic study”. Ophthalmic Plast Reconstr Surg. 1995 Sep;11((3)):169–81. doi: 10.1097/00002341-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Shields JA, Shields CL, Freire JE, Brady LW, Komarnicky L. Plaqeu radiotherapy for selected orbital malignancies: preliminary observations. Ophthalmic Plast Reconstr Surg. 2003;19((2)):91–5. doi: 10.1097/01.IOP.0000056020.66654.33. [DOI] [PubMed] [Google Scholar]
- 15.Paridaens AD, McCartney AC, Minassian DC, Hungerford JL. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78((7)):520–8. doi: 10.1136/bjo.78.7.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong JR, Nanji AA, Galor A, Karp CL. Management of conjunctival malignant melanoma: a review and update. Expert Rev Ophthalmol. 2014;9((3)):185–204. doi: 10.1586/17469899.2014.921119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholz SL, Hérault J, Stang A, Griewank KG, Meller D, Thariat J, et al. Proton radiotherapy in advanced malignant melanoma of the conjunctiva. Graefes Arch Clin Exp Ophthalmol. 2019;257((6)):1309–18. doi: 10.1007/s00417-019-04286-2. [DOI] [PubMed] [Google Scholar]
- 18.Stannard CE, Sealy GRH, Hering ER, Pereira SB, Knowles R, Hill JC. Malignant melanoma of the eyelid and palpebral conjunctiva treated with iodine-125 brachytherapy. Ophthalmology. 2000;107((5)):951–8. doi: 10.1016/s0161-6420(00)00061-0. [DOI] [PubMed] [Google Scholar]
- 19.Shields JA, Shields CL, Freire JE, Brady LW, Komarnicky L. Plaqeu radiotherapy for selected orbital malignancies: preliminary observations. Ophthalmic Plast Reconstr Surg. 2003;19((2)):91–5. doi: 10.1097/01.IOP.0000056020.66654.33. [DOI] [PubMed] [Google Scholar]
- 20.Chaves LJ, Huth B, Augsburger JJ, Correa ZM. Eye-sparing treatment for diffuse invasive conjunctival melanoma. Ocul Oncol Pathol. 2018 Jun;4((4)):261–6. doi: 10.1159/000485978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang M, Lally SE, Dalvin LA, Orloff MM, Shields CL. Conjunctival melanoma with orbital invasion and liver metastasis managed with systemic immune checkpoint inhibitor therapy. Indian J Ophthalmol. 2019 Dec;67((12)):2071–3. doi: 10.4103/ijo.IJO_663_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tagliaferri L, Bussu F, Rigante M, Gambacorta MA, Autorino R, Mattiucci GC, et al. Endoscopy-guided brachytherapy for sinonasal and nasopharyngeal recurrences. Brachytherapy. 2015;14((3)):419–25. doi: 10.1016/j.brachy.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Tagliaferri L, Bussu F, Fionda B, Catucci F, Rigante M, Gambacorta MA, et al. Perioperative HDR brachytherapy for reirradiation in head and neck recurrences: single-institution experience and systematic review. Tumori. 2017;103((6)):516–24. doi: 10.5301/tj.5000614. [DOI] [PubMed] [Google Scholar]
- 24.Finger PT. Radiation therapy for orbital tumors: concepts, current use, and ophthalmic radiation side effects. Surv Ophthalmol. 2009;54((5)):545–68. doi: 10.1016/j.survophthal.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Misotten GS, Keijer S, De Keizer RJW, De Wolff-Rouendaal D. Conjunctival melanoma in the Netherlands: a nationwide study. Invest Ophthalmol Vis sci. 2005;46:75–82. doi: 10.1167/iovs.04-0344. [DOI] [PubMed] [Google Scholar]
- 26.Kenawy N, Lake SL, Coupland SE, Damato BE. Conjunctival melanoma and melanocytic intra-epithelial neoplasia. Eye. 2013;27((2)):142–52. doi: 10.1038/eye.2012.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stannard C, Maree G, Munro R, Lecuona K, Sauerwein W. Iodine-125 orbital brachytherapy with a prosthetic implant in situ. Strahlenther Onkol. 2011;187((5)):322–7. doi: 10.1007/s00066-011-2177-y. [DOI] [PubMed] [Google Scholar]
- 28.Hsu A, Frank SJ, Ballo MT, Garden AS, Morrison WH, Rosenthal DI, et al. Postoperative adjuvant external-beam radiation therapy for cancers of the eyelid and conjunctiva. Ophthalmic Plast Reconstr Surg. 2008;24((6)):444–9. doi: 10.1097/IOP.0b013e31818be098. [DOI] [PubMed] [Google Scholar]
- 29.Norregaard JC, Gerner N, Jensen OA, JU P. Malignant melanoma of the conjunctiva:occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234((9)):569–72. doi: 10.1007/BF00448801. [DOI] [PubMed] [Google Scholar]
- 30.Wuestemeyer H, Sauerwein W, Meller D, Chauvel P, Schueler A, Steuhl KP, et al. Proton radiotherapy as an alternative to exenteration in the management of extended conjunctival melanoma. Graefes Arch Clin Exp Ophthalmol. 2005;244((4)):438–46. doi: 10.1007/s00417-005-0093-5. [DOI] [PubMed] [Google Scholar]
- 31.Scholz SL, Hérault J, Stang A, Griewank KG, Meller D, Thariat J, et al. Proton radiotherapy in advanced malignant melanoma of the conjunctiva. Graefes Arch Clin Exp Ophthalmol. 2019;257((6)):1309–18. doi: 10.1007/s00417-019-04286-2. [DOI] [PubMed] [Google Scholar]
- 32.Thariat J, Salleron J, Maschi C, Fevrier E, Lassalle S, Gastaud L, et al. Oncologic and visual outcomes after postoperative proton therapy of localized conjunctival melanomas. Radiat Oncol. 2019;14((1)):239. doi: 10.1186/s13014-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finger PT, Tena LB, Semenova E, Aridgides P, Choi WH. Extrascleral extension of choroidal melanoma: post -enucleation high-dose-rate interstitial brachytherapy of the orbit. Brachytherapy. 2014;13((3)):275–80. doi: 10.1016/j.brachy.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Kovàcs G, Rochels R, Mehdorn HM, Werner J, Wilhelm R, Kohr P, et al. Eye preservation brachytherapy for orbital and adjacent tumors: preliminary results. Front Radiat Ther Oncol. 1997;30:56–64. doi: 10.1159/000425688. [DOI] [PubMed] [Google Scholar]
- 35.Mazeron JJ, Noël G, Simon JM. Head and neck brachytherapy. Semin Radiat Oncol. 2002;12((1)):95–108. doi: 10.1053/srao.2002.28668. [DOI] [PubMed] [Google Scholar]
- 36.Sagiv O, Thakar SD, Kandl TJ, Ford J, Sniegowski MC, Hwu W, et al. Immunotherapy with programmed cell death 1 inhibitors for 5 patients with conjunctival melanoma. JAMA Ophthalmol. 2018;136((11)):1236–41. doi: 10.1001/jamaophthalmol.2018.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang M, Lally SE, Dalvin LA, Orloff MM, Shields CL. Conjunctival melanoma with orbital invasion and liver metastasis managed with systemic immune checkpoint inhibitor therapy. Indian J Ophthalmol. 2019Dec;67((12)):2071–3. doi: 10.4103/ijo.IJO_663_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin KL, Tena LB, Finger PT. Orbital brachytherapy for extrascleral extension of choroidal melanoma. Br J Ophthalmol. 2006;90((11)):1438–9. doi: 10.1136/bjo.2006.097139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laskar S, Pilar A, Khanna N, Ghadi Y. Interstitial brachytherapy for orbital soft tissue sarcoma: an innovative technique. J Contemp Brachytherapy. 2017;9((5)):466–71. doi: 10.5114/jcb.2017.70957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovács G. Modern head and neck brachytherapy: from radium towards intensity modulated interventional brachytherapy. J Contemp Brachytherapy. 2015;6((4)):404–16. doi: 10.5114/jcb.2014.47813. [DOI] [PMC free article] [PubMed] [Google Scholar]