Abstract
Background
The human immunodeficiency virus (HIV)-1 latent reservoir (LR) in resting CD4+ T cells is a barrier to cure. LR measurements are commonly performed on blood samples and therefore may miss latently infected cells residing in tissues, including lymph nodes.
Methods
We determined the frequency of intact HIV-1 proviruses and proviral inducibility in matched peripheral blood (PB) and lymph node (LN) samples from 10 HIV-1-infected patients on antiretroviral therapy (ART) using the intact proviral DNA assay and a novel quantitative viral induction assay. Prominent viral sequences from induced viral RNA were characterized using a next-generation sequencing assay.
Results
The frequencies of CD4+ T cells with intact proviruses were not significantly different in PB versus LN (61/106 vs 104/106 CD4+ cells), and they were substantially lower than frequencies of CD4+ T cells with defective proviruses. The frequencies of CD4+ T cells induced to produce high levels of viral RNA were not significantly different in PB versus LN (4.3/106 vs 7.9/106), but they were 14-fold lower than the frequencies of cells with intact proviruses. Sequencing of HIV-1 RNA from induced proviruses revealed comparable sequences in paired PB and LN samples.
Conclusions
These results further support the use of PB as an appropriate proxy for the HIV-1 LR in secondary lymphoid organs.
Keywords: HIV latency, intact provirus, latent reservoir, next-generation sequencing, T cell
This study found that the levels of intact and inducible HIV proviruses found in the blood and the lymph node are similar, and supports the continued use of blood to monitor the status of the HIV latent reservoir.
Human immunodeficiency virus (HIV)-1 persists in a latent reservoir (LR) of stably integrated, transcriptionally quiescent proviruses in resting CD4+ T cells [1–4]. The pool of latently infected cells remains a barrier to eliminating infection, and accurate measurement of the LR is important for assessing potential HIV-1 cure interventions. Multiple approaches to quantifying the HIV-1 LR have been described [2, 5–7]. Quantifying the LR is complicated by the heterogeneous nature of integrated proviruses, many of which are defective and therefore not capable of re-establishing viremia when antiretroviral therapy (ART) is interrupted [8–10]. Even within the intact proviral population, many proviruses may not have the potential for induction [8, 11]. Inducibility is an important consideration because proviruses not subject to induction are not a barrier to cure, and many cure strategies require induction of latent HIV-1 proviruses [12–14].
In addition to these difficulties, concerns have arisen regarding the measurement of latently infected cells resident in tissues. The vast majority of LR measurements are done using peripheral blood (PB) samples, although other lymphoid tissues clearly harbor latently infected cells [15–17]. Specifically, lymph nodes (LN) may be of concern because subsets of CD4+ T cells found in B cell follicles may have higher levels of infection [18–23], due in part to inability of virus-specific cytolytic T lymphocytes to localize there [19, 20]. In addition, HIV-1 infection alters the structural and immunological characteristics of LNs that do not fully resolve even on long-term ART [15]. Furthermore, previous studies have suggested that concentrations of antiretroviral drugs reaching the LN are suboptimal, potentially allowing de novo infection of cells and subsequent viral evolution [24]. Other studies have challenged this hypothesis [25], and multiple studies have demonstrated that viral evolution does not continue during ART. In fact, identical HIV-1 sequences have been detected in the PB and LN [26–28], consistent with the known ability of infected cells to undergo clonal expansion in vivo. The proviral sequence similarities in PB and LN cells are also consistent with constant recirculation of lymphocytes, which typically spend only hours in a given LN unless antigen is encountered [29]. Nevertheless, a potential sanctuary for viral replication in LN would hinder cure efforts, which depend on a reduction in total number or long-term silencing of latent HIV-1 proviruses. Therefore, it is important to understand the total number and relative inducibility of latent proviruses in LN, and how this compares to the levels observed in PB.
To further clarify the relationship between the LR sampled in PB and LN, we have obtained simultaneous paired PB and LN samples from patients on ART and used the recently described intact proviral DNA assay (IPDA) to estimate the frequency of cells carrying proviruses lacking overt fatal defects [7]. We also examined the frequency of cells that can express HIV-1 RNA following T-cell activation using the novel quantitative viral induction assay (QVIA).
METHODS
HIV-1 Study Participants
HIV-1-infected individuals scheduled to receive a kidney and/or liver transplant under the HOPE in Action pilot and clinical trial protocols were included in the study (ClinicalTrials.gov Identifier NCT02602262). All participants were on ART with plasma HIV-1 RNA levels below the limit of detection (<50 copies/mL) for >6 months prior to inclusion in this study. On the day of transplant and before surgery, PB was collected and processed. During surgery, mesenteric LN removed as part of the normal surgical procedure were collected and processed. The study was approved by the Johns Hopkins University Institutional Review Board. All participants provided written informed consent.
Peripheral Blood and Lymph Node Mononuclear Cell Isolation
PBMC were isolated from whole blood by Ficoll density gradient centrifugation. Lymph nodes removed during surgery were immediately placed in RPMI medium (Thermo Fisher Scientific) and processed. Single-cell suspensions were generated by manually disrupting the nodes with scissors and a syringe. The suspension was then passed through 100-µm cell strainers (Thermo Fisher Scientific). Lymph node mononuclear cells (LNMCs) were isolated from this filtered suspension by Ficoll density gradient centrifugation. For both IPDA and the QVIA, CD4+ T cells were isolated from PBMC/LNMC using negative selection (CD4+ T Cell Isolation Kit; Miltenyi Biotec).
Intact Proviral DNA Assay
DNA was extracted from 2–5 × 106 CD4+ T cells using the QIAamp DNA Mini kit (QIAGEN). DNA concentration was measured by NanoDrop 2000 (Thermo Fisher Scientific). The IPDA was performed by digital droplet PCR (ddPCR) as previously described [7] (Figure 1). The ddPCR analysis of a cellular gene (RPP30) was used to quantify input cell equivalents and to control for DNA shearing [7]. The ddPCR output was analyzed using QuantaSoft Analysis-Pro (Bio-Rad). The DNA Shearing Index (DSI) was calculated as previously described [7]. Values for intact and defective proviruses were normalized to copies per 106 input cells (determined by RPP30) and adjusted for shearing using the DSI as previously described.
Figure 1.
Analysis of intact proviral frequency and inducibility in paired PB and LN samples. (A) Map of the HIV-1 proviral genome and HIV-1 RNA species indicating positions of the IPDA and QVIA amplicons. For the IPDA, there are 2 amplicons: 1 in the packaging signal (Ψ, blue arrow), and 1 in the conserved Rev response element in the env gene (green arrow). Intact proviruses give amplification at both sites, whereas most defective proviruses (>90%) fail to give amplification at one or the other position [12]. The QVIA amplicon covers the shared 3’ end of all HIV-1 mRNAs (unspliced, singly spliced, and multiply spliced) and partially overlaps the polyA tail [30]. The transcription start site (right arrow and dotted line) and the polyadenylation signal (dashed line) are indicated. (B) Methods of analysis. The PB and LN samples were processed to PB mononuclear cells and LN mononuclear cells, respectively, and then CD4+ T cells were isolated from each preparation. CD4+ T cells from each sample were then analyzed with the IPDA to detect cells with intact proviruses [12] and the QVIA to detect inducible proviruses. In the IPDA, individual proviruses distribute into nanoliter-sized droplets are interrogated with duplex PCRs amplifying the Ψ signal region and a conserved region of the env gene. Most defective proviruses fail to give amplification at one or both sites due to large deletions and/or hypermutation, whereas intact proviruses can be directly counted as double-positive droplets [12]. Separate ddPCR analysis of a cellular gene (RPP30) allows for quantitation of input cell number and for correction for DNA shearing [12]. The QVIA is a limiting dilution RT-PCR assay that detects cells expressing polyadenylated HIV-1 RNA after activation with PMA and ionomycin [30]. The precise quantitation afforded by the use of a single round of PCR distinguishes cells with high and low levels of HIV-1 RNA, typically separated by 1–2 cycle threshold values (Supplementary Figure 1). The RT-PCR analysis of induced RNA indicated that cells expressing only low levels of HIV-1 RNA typically contained defective proviruses (Supplementary Figure 1). These cells were excluded from the analysis. DMSO, dimethyl sulfoxide; LTR, long terminal repeat.
Quantitative Viral Induction Assay
Induction of HIV-1 gene expression was measured using a limiting dilution modification of a previously described assay for HIV-1 RNA [31]. In brief, up to 4 × 106 isolated CD4+ T cells were plated using a limiting dilution strategy, treated for 18 hours with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) and 1 µM ionomycin or with dimethyl sulfoxide (DMSO) alone, and then lysed (Figure 1). mRNA was isolated using Dynabeads mRNA Direct Purification kit (Thermo Fisher Scientific). cDNA was synthesized from a portion of extracted RNA using the bead-conjugated oligo-dT primer and random hexamers (qScript Flex cDNA Kit; Quanta Biosciences). The final readout for the QVIA was determined using a quantitative PCR (qPCR) amplifying the poly-A region of HIV mRNA, carried out on an AB ViiA instrument (Thermo Fisher Scientific). Extended protocol and validation can be found in Supplemental Materials.
Next-Generation Sequencing of Prominent Induced Viral Sequences
RNA isolated from qPCR-positive wells with high HIV-1 RNA levels was diluted and used for sequencing of a portion of the viral envelope (env) and reverse-transcriptase (RT) genes using a validated site-directed next-generation sequencing (NGS) Nextera MiSeq-based assay (Illumina Inc., San Diego, CA) [32]. In brief, RNA was reversed transcribed, amplified using a nested PCR format for each genetic region (gp41 and pol), labeled with barcodes, and sequenced (Illumina Inc.). Prominent consensus viral sequences were selected (>2.5% of the total sequence reads) for further analysis. In addition, sequences one nucleotide away from and comprising <10% of the reads of the most prominent species in the same well were excluded. Sequence variation was displayed with neighbor-joining phylogenetic trees.
Bayesian Estimation of Frequency of Individual Sequence Variants
When samples had >75% of qPCR+ wells successfully sequenced for gp41 (no samples had >75% of wells successfully sequence pol) from both PB and LN, further analysis was performed using a Bayesian model to re-estimate the level of inducible proviruses for each individual variant in both LN and PB separately [32]. As part of this analysis, the Bayesian model provides estimated levels of each proviral isolate found in a given sample. These individual variant point estimates were used to calculate the proportion of the inducible LR for each variant by dividing the variant point estimate by the Bayesian-derived total sample point estimate. The pattern of the variant proportions for each individual with qualifying sequences were compared between each compartment.
Statistical Analysis
Within-patient PB and LN samples were compared by Wilcoxon matched-pairs rank tests. Correlations between values within samples, or between PB and LN samples within patients, were calculated using Spearman’s r.
RESULTS
Measurement of the Frequency of Cells With Intact and Inducible Proviruses
Matched PB and LN samples were obtained from HIV-1-infected patients undergoing kidney transplantation (n = 10) (Table 1). All patients had stable suppression of viremia on ART at the time of transplantation, and had been suppressed for a minimum of 6 months. Mesenteric LNs were removed from graft recipients as part of standard transplant protocols and analyzed along with blood samples taken just before surgery. To compare the LR in PB and LN samples, we measured intact and defective proviruses in purified CD4+ T cells from paired samples using the IPDA [7] and simultaneously evaluated proviral inducibility using a novel QVIA (Figure 1, Supplementary Table S2). In the IPDA, DNA from PB or LN CD4+ T cells was analyzed by duplex ddPCR. The assay specifically distinguishes intact from defective proviruses by simultaneously amplifying packaging signal (Ψ) sequence and non-hypermutated env sequences within individual proviruses (Figure 1A). For most defective proviruses, one of these regions will fail to amplify [7]. Results are displayed on a two-dimensional dot plot with fluorescence from the Ψ and env probes on the y and x axes, respectively (Figure 1B) [7].
Table 1.
Characteristics of Study Participants
| Patient ID | Age | Sex | Race | ART Regimen | CD4 Counta | Time on ART Before Transplant | Reason for Transplant |
|---|---|---|---|---|---|---|---|
| 1 | 55 | F | AA | TDF/3TC/RAL | 506 | >1 year | Hypertensive nephrosclerosis |
| 2 | 32 | M | AA | ABC/3TC/DTG | 649 | >1 year | HIVAN |
| 3 | 41 | M | AA | ABC/3TC/DTG | 410 | >1 year | HIVAN |
| 4 | 41 | F | AA | ABC/3TC/DTG | 481 | >1 year | HIVAN |
| 5 | 38 | M | AA | TDF/3TC/DTG | 790 | >6 months | Hypertensive nephrosclerosis/ possible HIVAN |
| 6 | 54 | M | AA | ETR/DRV/DTG | 244 | >1 year | Hypertensive nephrosclerosis |
| 7 | 66 | M | AA | TDF/3TC/DTG | 425 | >6 months | Hypertensive nephrosclerosis /HIVAN |
| 8 | 59 | M | AA | ABC/DRV/r/RAL | 565 | >1 year | FSGS |
| 9 | 57 | M | AA | ETR/3TC/RAL | 528 | >1 year | FSGS |
| 10 | 45 | M | AA | TDF/MVC/DTG | 490 | >6 months | HIVAN |
Abbreviations: AA, African American; ABC, abacavir; ART, antiretroviral therapy; DRV, darunavir; DRV/r, ritonavir-boosted darunavir; DTG, dolutegravir; ETR, etravirine; FSGS, focal segmental glomerulosclerosis; HIV, human immunodeficiency virus; HIVAN, HIV-associated nephropathy; ID, identification; MVC, maraviroc; RAL, raltegravir; TDF, tenofovir; 3TC, lamivudine.
aMeasured within 16 weeks of transplant.
Recent work has shown that not all intact proviruses are readily induced to produce infectious virus after cellular activation [8, 11, 33]. Therefore, with purified CD4+ cells from the same paired PB and LN samples, we also measured the number of cells that could be induced to produce HIV-1 RNA after stimulation with PMA and ionomycin, a drug combination that mimics many aspects of T-cell activation and robustly activates HIV-1 gene expression [34, 35]. Viral RNA was quantitated using a previously published quantitative RT-PCR method that amplifies the extreme 3’ end of all spliced and unspliced HIV-1 RNAs [31]. The reverse primer for this amplicon partially overlaps the poly-A tail (Figure 1A), giving the assay high specificity for HIV-1 RNA relative to DNA and the sensitivity to detect viral RNA in individual cells [31]. When carried out in a limiting dilution format, this QVIA allows detection and quantification of HIV-1 RNA-positive cells (Figure 1B). Unlike other induction assays that rely on nested PCR [6], the QVIA uses only a single round of PCR for more direct and accurate quantitation. This makes it possible to distinguish cells with different levels of HIV-1 RNA. In limiting dilution QVIA assays, we typically observed two families of amplification curves from positive wells that were separated by at least 1–2 cycle threshold values (Figure 1B, Supplemental Figure 1). We therefore explored the possibility that cells with lower levels of induced HIV-1 RNA might carry defective proviruses. Many defective proviruses have deletions or hypermutation affecting the tat gene [7, 8] and therefore are unlikely to give rise to high levels of viral RNA. Other defective proviruses have hypermutation affecting NFκB sites in the long terminal repeat that prevent effective induction of viral RNA [8]. Thus, it is expected that many defective proviruses would produce only low levels of HIV-1 RNA after induction. To test the hypothesis that low-level HIV-1 RNA expression was associated with proviral defects, we used the same informative amplicons selected for the IPDA [7]. When cDNA from QVIA+ wells was amplified with IPDA primers, cells with low levels of induced RNA most often showed defects as indicted by failure of one or both IPDA amplicons (Supplemental Figure 1B and C). In addition, all samples had cells treated with DMSO as a control; these wells sometimes amplified for these low levels of RNA (<60 copies), suggesting that cells carrying a noninduced provirus generally do not express “high” levels of RNA by this assay design. Therefore, in the subsequent analysis, we only considered cells with higher levels of HIV-1 RNA after induction to be contain inducible latent proviruses.
Frequencies of Cells With Intact Proviruses in Peripheral Blood and Lymph Nodes
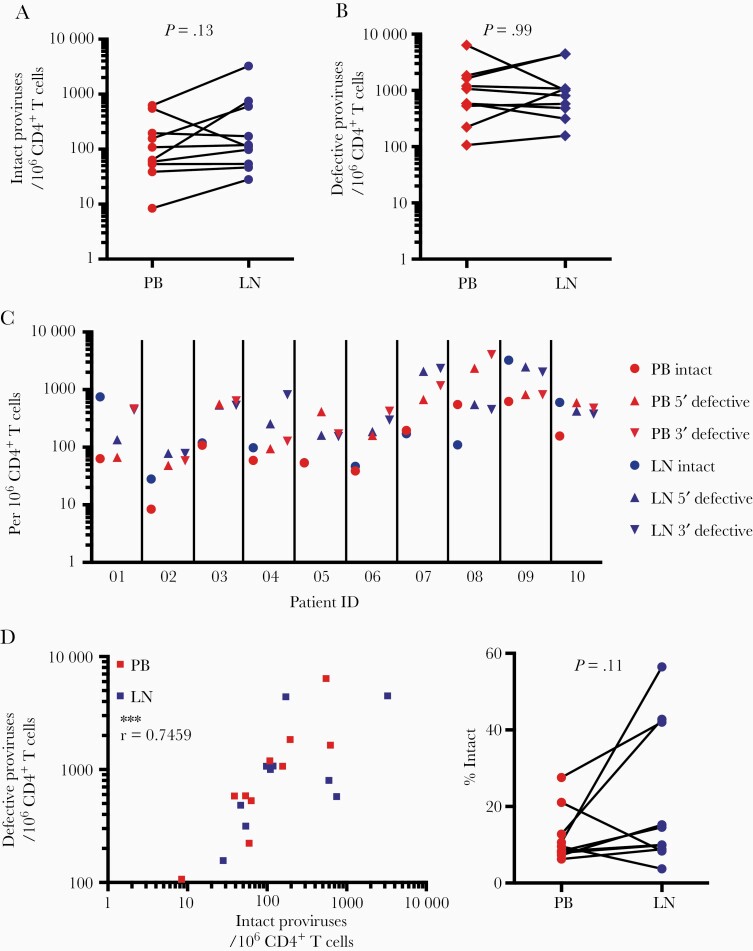
Using the IPDA, we detected a median of 61 intact proviruses per 106 CD4+ T cells in PB (interquartile range [IQR] = 43–440) and 104 intact proviruses per 106 CD4+ T cells in LN (IQR = 48–591), which was not significantly different (P = .13) (Figure 2A). There was no difference in the number of defective proviruses per 106 CD4+ T cells between PB and LN (median = 789 [IQR = 357–3642] vs median = 584 [IQR = 299–1530], respectively) (Figure 2B). Frequencies of intact and defective proviruses in PB and LN were comparable to those observed in PB samples in two large cohort studies, indicating that the clinical condition of the patients in this study (ie, dialysis) did not have a major effect on reservoir size. In both PB and LN samples, defective proviruses were present at higher frequency than intact proviruses (Figure 2C). Levels of intact and defective provirus within samples were correlated in both PB and LN (Figure 2D). This corresponded to a relatively consistent ratio of intact to defective proviruses in both compartments (median = 9.9% intact) (Figure 2E).
Figure 2.
Intact and defective proviral DNA frequencies are similar in PB and LN CD4+ T cells. (A) Intact proviral frequencies per 106 CD4+ T cells as determined by IPDA for cells from paired simultaneous PB and LN samples from 10 transplant recipients on ART.. (B) Defective proviruses per 106 CD4+ T cells as determined by IPDA for cells from paired simultaneous PB and LN samples. Defective proviral frequencies were computed from the sum of proviruses with 5′ defects and proviruses with 3’ defects. The 3′ defects include both deletions and APOBEC3G-mediated hypermutation [12]. (C) Intact and defective proviral frequencies by anatomical site and study participant. (D) Correlation between intact and combined defective (5’ defective + 3’ defective) proviral frequencies for PB and LN, as well as the fraction of proviruses that are intact. Data are shown as percentage of all detected proviruses that were intact by IPDA analysis (n.s., nonsignificant, by Wilcoxon matched-pairs signed rank test; ***, P < .001, by Spearman’s r).
Frequencies of Cells With Inducible Proviruses in Peripheral Blood and Lymph Nodes
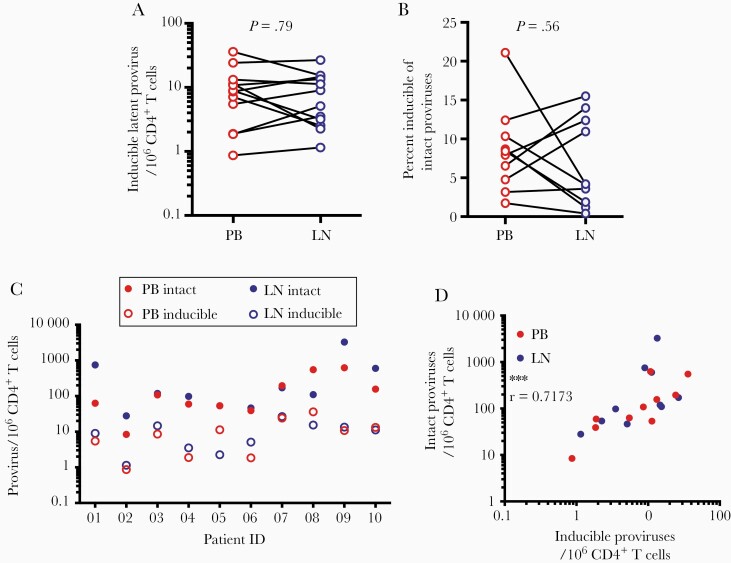
Using the QVIA, we found no difference in the frequency of cells with inducible proviruses between PB and LN samples (median values = 7.9 and 4.3 per 106 CD4+ T cells, respectively (Figure 3A). To look for potential differences in proviral inducibility between anatomical sites, we then examined the ratio between inducible and intact proviruses in PB and LN. The ratio of induced to intact proviruses was low in both PB and LN (median ratio = 0.081 and 0.041, respectively, in PB and LN) (Figure 3B and C). There was no significant difference in the ratio of inducible to intact proviruses in PB versus LN. Intact provirus levels determined by IPDA correlated with the inducible provirus levels determined by QVIA in both PB and LN (Figure 3D). Overall, there was no measurable difference in HIV-1 inducibility between sites, indicating that neither PB nor LN harbors an environment especially conducive to HIV-1 latency reversal in CD4+ T cells.
Figure 3.
Inducible proviruses are present at similar frequencies in PB and LN CD4+ T cells. (A) Frequencies of cells with inducible proviruses among 106 CD4+ T cells as determined by QVIA analysis of paired simultaneous PB and LN samples from 10 transplant recipients on antiretroviral therapy (nonsignificant, by Wilcoxon matched-pairs signed rank test). (B) Ratio between frequencies of CD4+ T cells with inducible vs intact proviruses in paired PB and LN samples (nonsignificant, by Wilcoxon matched-pairs signed rank test). (C) Frequencies of inducible and intact proviruses by anatomical site and study participant. (D) Correlation between intact and inducible proviruses in CD4+ T cells from PB and LN (***, P < .001, by Spearman’s r).
Sequence Analysis of Induced Proviruses in Peripheral Blood and Lymph Nodes
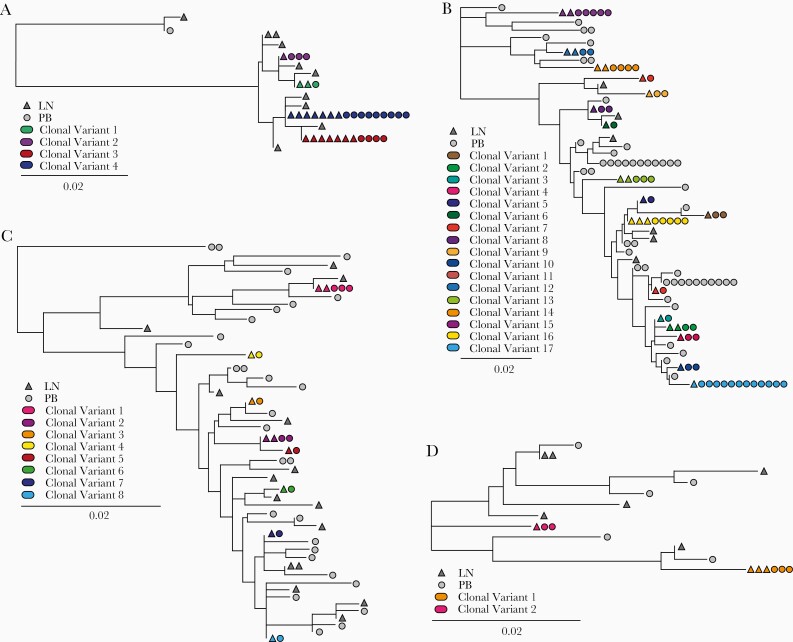
We also examined sequence similarities between inducible proviruses in PB and LN sites. RNA from QVIA wells with high RNA levels containing induced proviruses was analyzed using an NGS-based assay to identify prominent viral species [32]. Two regions, a 320-nucleotide portion of gp41 and a 500-nucleotide portion of RT, were chosen for sequence analysis. These regions represent dynamic sequences in the HIV-1 genome and can predict clonality between proviruses with 83% and 91% accuracy, respectively [36]. Initial phylogenetic analyses identified these 10 recipients as being infected with subtype B (Supplementary Figure S3). For four study participants, gp41 sequencing was successful for >75% of QVIA wells containing an induced provirus from both PB and LN, allowing for a relatively complete view of the isolates sampled from each compartment (Supplemental Table 1). This cutoff was used to ensure that any differences seen between the two compartments was not due to any bias caused by limited sampling. In all of these cases, multiple identical sequences were shared between PB and LN compartments (Figure 4). Some wells above the limiting dilution contained multiple unique viral sequences, indicating, as expected, that for some patients, wells with higher cell numbers contained multiple latently infected cells. The majority of sequences had no detectable defects; however, a small number of hypermutated sequences were found in wells containing other intact proviral sequences or in low RNA signal wells, which are considered negative in the assay. This suggests that these defective proviruses were capable of producing low levels of RNA.
Figure 4.
Despite substantial intraparticipant sequence diversity, identical sequences can be found in PB and LN. Phylogenetic of induced viral RNA populations from QVIA-positive wells. The prominent gp41 NGS-derived sequence data for PB (circles) and LN (triangles) are shown for patients with >75% sequence detection: patient 10 (A), patient 8 (B), patient 9 (C), and patient 4 (D). Identical sequences shown originated from different culture wells, and they represent multiple infected cells carrying proviruses with sequence identity in the regions analyzed. Identical sequences shared between PB and LN are shown in color; sequences appearing in only one compartment are shown in gray. Genetic distance in substitutions/site is indicated by the scale in the key.
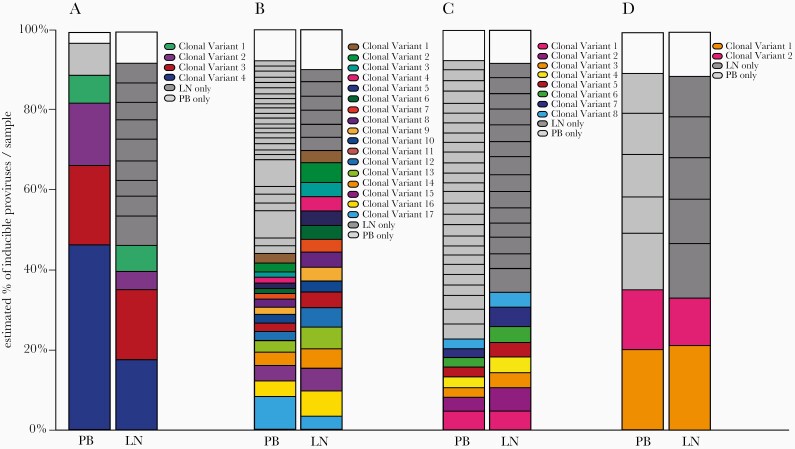
To estimate and compare the proportion that each individual viral variant contributed to the overall LR landscape, a Bayesian analysis was used to account for cell input and number of wells containing a particular sequence [32]. These proportional analyses, were comparable between compartments (Figure 5). Although integration site or T-cell receptor sequencing are required to provide a definitive demonstration of clonality, these results are consistent with sharing of sequence variants between PB and LN.
Figure 5.
Distinct and shared viral sequences between PB and LN compartments. Bars show the estimated proportion of each detected variant relative to total sequenced RNA in patient 10 (A), patient 8 (B), patient 9 (C), and patient 4 (D). Colored sequences shared between compartments correspond to those in Figure 4; gray sequences were detected in only PB or LN. White space at the top of the graphs is due to differences in median calculations of total and combined variant IUPM.
DISCUSSION
These data demonstrate that HIV-1 proviral populations are similar between PB and LN with respect to the frequency intact and defective proviruses, proviral inducibility, and the distribution of viral variants. The median frequencies of cells with intact proviruses among CD4+ T cells as measured by the IPDA were 61/106 in PB and 104/106 in LN. These values agree well with previous studies of intact proviral frequencies in PB [37]. We show that in LN, cells with defective proviruses greatly outnumber cells with intact proviruses (by ~6:1), consistent with previous characterization of the HIV-1 proviral landscape in PB [7–10, 30]. These results, taken together, suggest that accurate measurement of proviruses capable of causing viral rebound depends less on whether samples are obtained from PB or LN, but more on whether the assay method distinguishes intact from defective proviruses.
The frequencies of CD4+ T cells with intact proviruses as measured by IPDA in PB and LN were 1–2 logs higher than the frequencies of resting PB CD4+ T cells that can be induced to produce infectious virus as measured by the quantitative viral outgrowth assay (QVOA) [2, 7, 38]. The IPDA detects common fatal defects such as large deletions and APOBEC3G-mediated hypermutation, and some of the proviruses classified as intact by this assay have minor defects that could affect viral fitness [7]. However, the major factor seems to be that many apparently intact proviruses are not induced following T-cell activation or release an insufficient amount of infection virus to be detected in the QVOA [8, 11]. Recent studies have shown that after latency reversal, some infected cells release virus but in an amount that is insufficient to establish a spreading infection [39].
To look more directly at the issue of inducibility, we used a sensitive assay that detects all HIV-1 RNA species in a limiting dilution format [31]. We quantified inducible proviruses at median frequencies of 7.9 and 4.3 per 106 CD4+ T cells, respectively, in PB and LN, which were not significantly different. Several factors may explain why these values are higher than those generally seen in viral outgrowth assays. First, although the more precise quantitation afforded by single-round RT-PCR in the QVIA allowed us to exclude some cells with defective proviruses, it is possible that some of the RNA+ cells detected carried defective proviruses. Second, as mentioned above, some cells may not release a sufficient amount of infectious virus to initiate a spreading infection [39]. Finally, the stimulation used here to induce RNA production is significantly stronger than strategies used when performing long-term outgrowth assays.
For both PB and LN, the frequencies of cells with inducible proviruses were substantially lower than the frequencies of cells with intact proviruses; however, the ratio of inducible to intact proviruses was not significantly different between PB and LN. This low general level of inducibility has been noted in previous studies with the viral outgrowth assay [8, 11]. It is not due to incomplete T-cell activation in the culture system [11]. Some intact, noninduced proviruses can be induced with additional rounds of T-cell activation [8, 11, 33]. Surprisingly, inducibility does not appear to be related to conventionally defined memory T-cell subsets [33]. Rather, it is possible that in some latently infected cells, epigenetic silencing prevents viral gene expression even in the setting of full T-cell activation [40]. Determining whether intact, noninduced proviruses can be induced in vivo and contribute to viral rebound is a critical question in cure research.
One limitation of our study is analysis of only two anatomical sites where latently infected cells reside. The LN is a site of particular interest because it is a major site of T-cell activation, and some studies have suggested a higher frequency of infection in particular subsets of LN CD4+ T cells, particularly T follicular helper cells [18–23]. However, our analysis of total LN CD4+ T cells did not reveal a significantly higher frequency of intact or inducible proviruses. In addition, sequence analysis provided evidence consistent with the free movement of infected cells between PB and LN. Although, it should be noted that our site-directed sequencing method examined two relatively short areas of the viral genome, and therefore it may be overestimating the sequence similarity between the two compartments. We also did not examine the gut-associated lymphoid tissue (GALT), a site where CD4+ T-cell levels drastically decline during active HIV-1 infection and do not fully recover even after years of ART treatment. One study that directly compared infected cell frequencies in PB, LN, and GALT samples suggested a larger amount of both HIV-1 DNA and inducible HIV-1 RNA in GALT compared with both PB and LN [41]. However, this analysis was done by a less accurate measurement using nested gag PCR, which cannot distinguish between intact and defective proviral populations. Further studies on the potential of GALT, as well as other tissues, to harbor higher levels of intact or inducible HIV-1 provirus than represented in PB and LN are warranted. Finally, as with many detailed studies of the LR, these analyses were done on a relatively small sample size of 10 individuals, which limited our ability to identify more subtle differences between the two compartments, especially given that some of our comparisons were trending towards a significant difference.
CONCLUSIONS
When assessing a potential cure strategy, it is vital to know how and to what extent the inducible LR has been truly altered throughout the whole body. Therefore, if a larger or distinctly different population of latently infected cells was found in LN or other tissue sites, assessment in those locations would be critical, which would further complicate HIV-1 cure efforts. However, our data expands on the findings of others and suggest that there is no significant difference in intact proviral DNA or the inducible proviruses between PB and LN. These data support the hypothesis that circulating latently infected cells containing HIV-1 proviruses can make their way between these sites, and that PB is an appropriate proxy for latent HIV-1 in LNs.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgements. We thank the recipients for their generous participation in the HOPE pilot study. We also thank the HOPE in Action clinical and laboratory teams.
Author contributions. A. R. M. and A. M. B. performed laboratory experiments, data analysis, and wrote the manuscript. J. H., B. A. L., D. B., and C. M. generated next-generation sequencing data and performed analysis. S. S. F., N. D., D. S., A. A. R. T., and C. M. D. oversaw clinical study and patient care. K. J. K., S. B., G. M. L., and J. D. S. performed laboratory experiments. T. C. Q., R. F. S., and A. D. R. managed the study team, oversaw data analysis, and wrote the manuscript.
Financial support. This study was funded by the National Institute of Allergy and Infectious Diseases ([NIAID] Grant Numbers 1R01AI120938, U01AI138897, and U01AI134591), the Howard Hughes Medical Institute, Gilead HIV Cure Grants Program (90072171), and in part by the Division of Intramural Research, NIAID, National Institutes of Health.
Potential conflicts of interest. C. M. D. reports serving on a grant review committee for Gilead Sciences as well as research grants paid to the institution from Abbvie, Bristol Meyers Squibb, GlaxoSmithKline, Merck Dohme & Sharp Corporation, and Viiv Healthcare. D. S. reports speaking honoraria from Novartis and Sanofi and consulting for Sanofi, Novartis, and CSL Behring. R. F. S. is an inventor on a patent application dealing with the intact proviral DNA assay filed by Johns Hopkins University and licensed by AccelevirDx. He holds no equity interest in AccelevirDx. He consults for Merck and Abbvie on human immunodeficiency virus cure-related issues. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med 1995; 1:1284–90. [DOI] [PubMed] [Google Scholar]
- 2. Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278:1295–300. [DOI] [PubMed] [Google Scholar]
- 3. Wong JK, Hezareh M, Günthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997; 278:1291–5. [DOI] [PubMed] [Google Scholar]
- 4. Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 1997; 94:13193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Doherty U, Swiggard WJ, Jeyakumar D, McGain D, Malim MH. A sensitive, quantitative assay for human immunodeficiency virus type 1 integration. J Virol 2002; 76:10942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Procopio FA, Fromentin R, Kulpa DA, et al. A novel assay to measure the magnitude of the inducible viral reservoir in HIV-infected individuals. EBioMedicine 2015; 2:872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruner KM, Wang Z, Simonetti FR, et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019; 566:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013; 155:540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hiener B, Horsburgh BA, Eden JS, et al. Identification of genetically intact HIV-1 proviruses in specific CD4+ T cells from effectively treated participants. Cell Rep 2017; 21:813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee GQ, Orlova-Fink N, Einkauf K, et al. Clonal expansion of genome-intact HIV-1 in functionally polarized Th1 CD4+ T cells. J Clin Invest 2017; 127:2689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hosmane NN, Kwon KJ, Bruner KM, et al. Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: potential role in latent reservoir dynamics. J Exp Med 2017; 214:959–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Archin NM, Liberty AL, Kashuba AD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012; 487:482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spivak AM, Planelles V. HIV-1 eradication: early trials (and tribulations). Trends Mol Med 2016; 22:10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deeks SG, Lewin SR, Ross AL, et al. International AIDS society global scientific strategy: towards an HIV cure 2016. Nat Med 2016; 22:839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pantaleo G, Graziosi C, Demarest JF, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 1993; 362:355–8. [DOI] [PubMed] [Google Scholar]
- 16. Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 1997; 387:183–8. [DOI] [PubMed] [Google Scholar]
- 17. Shen A, Zink MC, Mankowski JL, et al. Resting CD4+ T lymphocytes but not thymocytes provide a latent viral reservoir in a simian immunodeficiency virus-Macaca nemestrina model of human immunodeficiency virus type 1-infected patients on highly active antiretroviral therapy. J Virol 2003; 77:4938–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perreau M, Savoye AL, De Crignis E, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med 2013; 210:143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Connick E, Folkvord JM, Lind KT, et al. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol 2014; 193:5613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukazawa Y, Lum R, Okoye AA, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 2015; 21:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deleage C, Wietgrefe SW, Del Prete G, et al. Defining HIV and SIV reservoirs in lymphoid tissues. Pathog Immun 2016; 1:68–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Banga R, Procopio FA, Noto A, et al. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med 2016; 22:754–61. [DOI] [PubMed] [Google Scholar]
- 23. McGary CS, Deleage C, Harper J, et al. CTLA-4(+)PD-1(-) memory CD4(+) T cells critically contribute to viral persistence in antiretroviral therapy-suppressed, SIV-infected rhesus macaques. Immunity 2017; 47:776–88.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lorenzo-Redondo R, Fryer HR, Bedford T, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016; 530:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenbloom DIS, Hill AL, Laskey SB, Siliciano RF. Re-evaluating evolution in the HIV reservoir. Nature 2017; 551:E6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. von Stockenstrom S, Odevall L, Lee E, et al. Longitudinal genetic characterization reveals that cell proliferation maintains a persistent HIV type 1 DNA pool during effective HIV therapy. J Infect Dis 2015; 212:596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McManus WR, Bale MJ, Spindler J, et al. HIV-1 in lymph nodes is maintained by cellular proliferation during antiretroviral therapy. J Clin Invest 2019; 129; 4629–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vibholm LK, Lorenzi JCC, Pai JA, et al. Characterization of intact proviruses in blood and lymph node from HIV-infected individuals undergoing analytical treatment interruption. J Virol 2019; 93: doi: 10.1128/JVI.01920-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee M, Mandl JN, Germain RN, Yates AJ. The race for the prize: T-cell trafficking strategies for optimal surveillance. Blood 2012; 120:1432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imamichi H, Dewar RL, Adelsberger JW, et al. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc Natl Acad Sci U S A 2016; 113:8783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shan L, Rabi SA, Laird GM, et al. A novel PCR assay for quantification of HIV-1 RNA. J Virol 2013; 87:6521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poon AFY, Prodger JL, Lynch BA, et al. Quantitation of the latent HIV-1 reservoir from the sequence diversity in viral outgrowth assays. Retrovirology 2018; 15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kwon KJ, Timmons AE, Sengupta S, et al. Different human resting memory CD4(+) T cell subsets show similar low inducibility of latent HIV-1 proviruses. Sci Transl Med 2020; 12: doi: 10.1126/scitranslmed.aax6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med 2014; 20:425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laird GM, Bullen CK, Rosenbloom DI, et al. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest 2015; 125:1901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laskey SB, Pohlmeyer CW, Bruner KM, Siliciano RF. Evaluating clonal expansion of HIV-infected cells: optimization of PCR strategies to predict clonality. PLoS Pathog 2016; 12:e1005689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simonetti FR, White JA, Tumiotto C, et al. Intact proviral DNA assay analysis of large cohorts of people with HIV provides a benchmark for the frequency and composition of persistent proviral DNA. Proc Natl Acad Sci U S A 2020; 117:18692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crooks AM, Bateson R, Cope AB, et al. Precise quantitation of the latent HIV-1 reservoir: implications for eradication strategies. J Infect Dis 2015; 212:1361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hataye JM, Casazza JP, Best K, et al. Principles governing establishment versus collapse of HIV-1 cellular spread. Cell Host Microbe 2019; 26:748–63.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pearson R, Kim YK, Hokello J, et al. Epigenetic silencing of human immunodeficiency virus (HIV) transcription by formation of restrictive chromatin structures at the viral long terminal repeat drives the progressive entry of HIV into latency. J Virol 2008; 82:12291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khoury G, Fromentin R, Solomon A, et al. Human immunodeficiency virus persistence and T-cell activation in blood, rectal, and lymph node tissue in human immunodeficiency virus-infected individuals receiving suppressive antiretroviral therapy. J Infect Dis 2017; 215:911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nettles RE, Kieffer TL, Kwon P, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA 2005; 293:817–29. [DOI] [PubMed] [Google Scholar]
- 43. Josefsson L, von Stockenstrom S, Faria NR, et al. The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc Natl Acad Sci U S A 2013; 110:4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bozzi G, Simonetti FR, Watters SA, et al. No evidence of ongoing HIV replication or compartmentalization in tissues during combination antiretroviral therapy: implications for HIV eradication. Sci Adv 2019; 5:eaav2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bailey JR, Sedaghat AR, Kieffer T, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol 2006; 80:6441–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maldarelli F, Wu X, Su L, et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014; 345:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wagner TA, McLaughlin S, Garg K, et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 2014; 345:570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lorenzi JC, Cohen YZ, Cohn LB, et al. Paired quantitative and qualitative assessment of the replication-competent HIV-1 reservoir and comparison with integrated proviral DNA. Proc Natl Acad Sci U S A 2016; 113:E7908–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bui JK, Sobolewski MD, Keele BF, et al. Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog 2017; 13:e1006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang Z, Gurule EE, Brennan TP, et al. Expanded cellular clones carrying replication-competent HIV-1 persist, wax, and wane. Proc Natl Acad Sci U S A 2018; 115:E2575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.