Abstract
Background
We aimed to determine whether Plasmodium falciparum infection affects age of Kaposi sarcoma-associated herpesvirus (KSHV) seroconversion in Kenyan children.
Methods
Kenyan children (n = 144) enrolled at age 1 month, from 2 sites with different levels of malaria transmission (stable/high vs unstable/low) were followed to age 24 months. Plasma was tested for KSHV antibodies using enzyme-linked immunosorbent assay (ELISA; K8.1 and LANA) and a multiplex bead-based assay (K8.1, K10.5, ORF38, ORF50, and LANA) and whole blood tested for P. falciparum DNA using quantitative PCR. Cox proportional hazards models were used to assess associations between P. falciparum DNA detection, malaria annualized rate (P. falciparum detections/person-years), and enrollment site (malaria-high vs malaria-low) with time to KSHV seroconversion.
Results
KSHV seroprevalence was 63% by age 2 years when assessed by multiplex assay. Children with P. falciparum were at increased hazards of earlier KSHV seroconversion and, among children with malaria, the hazard of becoming KSHV seropositive increased significantly with increasing malaria annualized rate. Children from the malaria-high transmission region had no significant difference in hazards of KSHV seroconversion at 12 months but were more likely to become KSHV seropositive by age 24 months.
Discussion
Malaria exposure increases the risk for KSHV seroconversion early in life.
Keywords: Kaposi sarcoma-associated herpesvirus, HHV-8, KSHV, sub-Saharan Africa, malaria
In Kenyan children followed to 24 months of age, children with a prior malaria infection became KSHV seropositive at a faster rate than children without malaria. Our findings will direct future work on KSHV infection prevention in sub-Saharan Africa.
Kaposi sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), is a gammaherpesvirus which varies in seroprevalence across the globe: in certain areas of sub-Saharan Africa, up to 95% of adults are seropositive [1]. Factors associated with higher KSHV seroprevalence in sub-Saharan Africa are not completely understood but the geographic differences in KSHV distribution suggest environmental factors are involved. Sub-Saharan Africa, in addition to having high KSHV seroprevalence, is also an area of high endemicity for malaria, primarily caused by the Plasmodium falciparum species [2], pointing to an association between malaria and KSHV.
Early ecological studies in Italy suggested malaria may be associated with KSHV infection and/or Kaposi sarcoma (KS) [3–6]. In Uganda, KSHV seropositive women and children were more likely to have malaria parasitemia [7–9] and higher antimalaria antibody levels [10]. Higher levels of anti-KSHV antibodies [11] and increasing KSHV DNA levels were associated with malaria [12] and in Cameroon mosquito bed net use was protective for KS [13]. However, these studies were either cross-sectional or case-control designs and thus were unable to establish a temporal association between malaria and KSHV infections. The only known longitudinal study of malaria and KSHV to date measured malaria exposure by self-report [14] but more specific measurements of malaria are required to determine whether an association truly exists. In addition, after primary infection with KSHV, anti-KSHV antibodies have been shown to decrease to levels below detection, making serodiagnosis at a single time point challenging [15–17]. To adequately evaluate KSHV antibody fluctuations, a study design that measures antibodies at multiple timepoints is needed.
KSHV seroepidemiology studies to date have also been constrained by the tools available to measure KSHV seropositivity as they have primarily used enzyme-linked immunosorbent assays (ELISAs) or immunofluorescence assays to detect antibodies to K8.1 and the ORF73 latency-associated nuclear antigen (LANA). K8.1 and LANA are well-established markers of KSHV-associated disease but the KSHV genome comprises over 85 open reading frames (ORFs), all of which are potentially antigenic at different viral stages [18]. Restricting the definition of seropositivity to detection of antibodies to just 2 antigens may underestimate KSHV seroprevalence in asymptomatic populations. More recently, we developed a bead-based multiplex assay to detect antibodies to a wider panel of KSHV antigens with a greater dynamic range than ELISA assay [19]. The ability to measure a range of anti-KSHV antibodies in small sample volumes is a potent tool to address the variability of anti-KSHV antibody responses.
The Prospective Infant Cohort (PIC) Study enrolled a cohort of infants from 2 Kenyan regions with different malaria transmission intensities [20]. We took advantage of the study’s longitudinal design and use of the multiplex assay to determine whether young children with P. falciparum infection were more susceptible to early KSHV seroconversion.
METHODS
Study Population
Between April and May 2006, 224 children born to human immunodeficiency virus (HIV)-negative mothers were enrolled at approximately 1 month of age and followed to age 2 years. Study methods have been described in detail elsewhere [20]. Briefly, children were enrolled from 2 Kenyan districts with different malaria transmission levels. Nandi district, referred to throughout as malarialo district, has unstable/low malaria transmission while Kisumu district, further referred to as malariahi district, has stable/high malaria transmission [20–22].
Informed consent was obtained from mothers or guardians of study participants. Protocol and consent forms were approved by the Kenya Medical Research Institute (KEMRI), the State University of New York (SUNY) Upstate Medical University, and the Colorado Multiple Institutional Review Board (COMIRB).
Data Collection
Child’s date of birth, sex, and number of other children living in the home were collected at enrollment. Child’s height/length (cm), weight (g), and hemoglobin (hg/dL) measures were collected at 12, 18, and 24-month follow-up visits. Child’s height/length and weight were used to calculate z-scores to determine stunting (< −2 standard deviations (SD) height-for-age for the World Health Organization [WHO] child growth standards median), underweight (< −2 SD weight for age), and wasting (< −2 SD weight for height) based on 2010 WHO guidelines [23]. Hemoglobin measures were altitude adjusted [24] and anemia (yes/no) was defined as adjusted hemoglobin levels <11.0 g/dL.
Blood was sampled monthly to age 12 months and then every 3 months to 2 years of age, as described previously [20]. P. falciparum DNA in these samples was measured by quantitative polymerase chain reaction (qPCR) as previously described [20].
Immunoglobulin G (IgG) antibodies to K8.1 and LANA (ORF73) were detected in plasma samples using ELISA and measured as optical density (OD) [25]. Plates included triplicate negative and positive controls. Cutoff values for each plate were calculated as the average background-subtracted OD of the negative control plus a constant value of 0.346 (K8.1) or 0.350 (LANA).
Serologic antibodies to 36 KSHV antigens were measured by bead-based multiplex assay as described [19]. Negative assay controls included healthy North American adult blood donors at low risk of KSHV infection. Positive assay controls included North American adults with active or history of KSHV-associated disease or detectable KSHV DNA in peripheral blood mononuclear cells (PBMCs). The median fluorescence intensity (MFI) across beads was computed for each sample and recorded after subtracting background fluorescence.
To determine KSHV seroconversion using the multiplex assay, we started with 9 KSHV antigens (K11, K10.5, K8.1, K8.1b, ORF38, ORF50, ORF65, ORF72, and ORF73) identified as good markers of steady-state infection in adults from Chulaimbo, Kenya, a population sample from the same source population as in the present study. We modeled rates of change in the log(MFI) for each KSHV antigen using piecewise linear mixed effects models with a 6-month spline. This was chosen based on the well-known decline in maternal antibodies by 6 months of age. For 5 KSHV antigens (K8.1, K10.5, ORF38, ORF50, or LANA), we saw significant decreases in log(MFI) between 1 and 6 months of age, assumed to represent maternal antibodies being lost over time and consistent with our previous observations in this same cohort for Epstein-Barr virus and malaria antibodies [20, 22, 26]. MFI values at 6 months of age were taken as the baseline level for IgG against these 5 identified KSHV antigens. Age of KSHV seroconversion was defined as a 5-fold increase in MFI compared to baseline levels to account for intrachild differences in antibody levels.
Data Analyses
Children were excluded if they did not have a clinic visit between 6 and 8 months of age (to act as baseline) and/or fewer than 3 total visits. Our primary exposure of interest was malaria as measured by qPCR. Among children who ever had a malaria infection, the malaria annualized rate was calculated as the number of malaria episodes per person-year. Enrollment site was treated as an ecological proxy of malaria transmission exposure (malariahi versus malarialo). Secondary exposures of interest included the child’s sex, number of other children in the home, hemoglobin levels, anemia, and WHO Z-scores (stunting, underweight, and wasting).
Time at risk of KSHV seroconversion was defined as months from baseline (6–8 months) to either KSHV seroconversion, censoring at 24 months of age, or censoring due to loss to follow-up (LTFU) prior to 24 months of age. Children were right censored at their last clinic visit with a KSHV result or 24 months of age, whichever came first. KSHV seroconversion was defined in 3 ways. We included the traditional criteria of KSHV seroconversion, a positive ELISA assay (ie, measuring OD levels of antibodies to K8.1 or LANA over a prespecified threshold). We also defined KSHV seroconversion as a 5-fold increase in antibody levels to K8.1 or LANA by multiplex assay, further referenced as 2Ag-multiplex, and a 5-fold increase in antibody levels to K8.1, K10.5, ORF38, ORF50, or LANA by multiplex, further referenced as 5Ag-multiplex assay.
χ 2, Fisher exact test, Student t tests, and Kaplan-Meier methods were used to produce univariate statistics, where appropriate. The association between each exposure described above and time at risk for KSHV seroconversion were modeled using extended time-dependent Cox proportional hazards regression models. P. falciparum detection by qPCR, hemoglobin levels, anemia, wasting, stunting, and underweight all had changing status over time and so were included as lagged time varying covariates to ensure we modeled exposure changes over time and also exposure prior to KSHV seroconversion. Other variables were treated as fixed effects. All models were adjusted for sex. Enrollment site, number of other children in the home, hemoglobin, anemia, malaria annualized rate, P. falciparum detection by qPCR, and WHO Z scores were examined as potential confounders for each model. Covariates were included in the final model if they were associated with both exposure and outcome at P value < .2 and changed the final estimate by ≥10%. The proportional hazards assumption was tested for covariates in the final model by plotting Schoenfeld residuals and comparing the correlation of each covariate’s Schoenfeld residuals with survival time. If a covariate did not meet the proportional hazards assumption (Pearson correlation coefficient P value < .05) we stratified by the variable or included an interaction between the covariate and time in the model [27]. All analyses were conducted using SAS 9.4.
Sensitivity Analyses
Baseline characteristics were compared between children included and excluded from our analysis and between children retained or LTFU using χ 2 test.
RESULTS
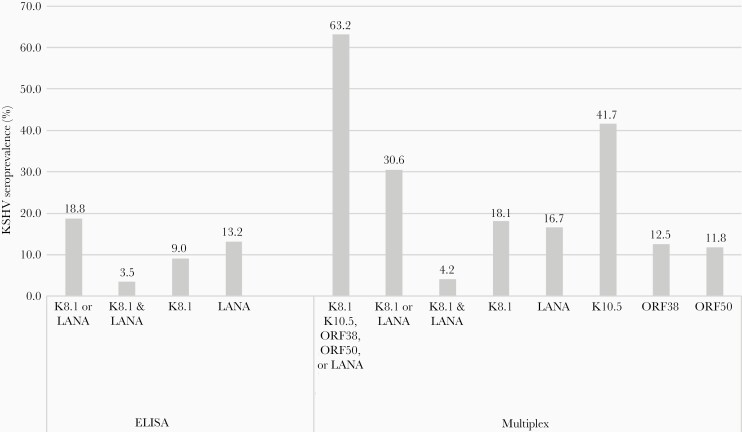
In this study, we utilized a longitudinal birth cohort study in western Kenya, the PIC cohort, that followed infants from 1 month of age to age 2 years. We included 144 study participants (64%) based on the availability of a sample obtained between 6 and 8 months of age and 3 or more total visits subsequently (Supplementary Figure 1). More children from the malariahi region were female and had stunting at 1 year (Table 1). To evaluate KSHV seroconversion by 24 months of age, we used 3 different criteria. KSHV seroconversion was 19% when measured by detection of antibodies to K8.1 or LANA using ELISA, 31% when measured by detection of antibodies to K8.1 or LANA using multiplex assay (2Ag-multiplex), and 63% when measured by detection of antibodies to K8.1, K10.5, ORF38, ORF50, or LANA using multiplex assay (5Ag-multiplex) (Figure 1). Incidence rates were 1.4 (ELISA), 2.3 (2Ag-multiplex), and 5.7 (5Ag-multiplex) KSHV seroconversions per 100 person-months. The overall mean (SD) time to KSHV seroconversion when measured by ELISA, 2Ag-multiplex, and 5Ag-multiplex was 19.9 (0.4), 20.7 (0.4), and 17.9 (0.4) months, respectively.
Table 1.
Characteristics of Children Enrolled in the Prospective Infant Cohort Study by Enrollment Region
| Characteristic | Malariahi (n = 56) | Malarialo (n = 88) | P Value |
|---|---|---|---|
| Sex | |||
| Female | 34 (60.7) | 37 (42.0) | .03a |
| Male | 22 (39.3) | 51 (58.0) | |
| Number of other children in the home | |||
| None | 15 (26.8) | 21 (23.9) | .63 |
| 1 | 11 (19.6) | 25 (28.4) | |
| 2 | 12 (21.4) | 14 (15.9) | |
| 3+ | 18 (32.1) | 28 (31.8) | |
| WHO Z-scores at 12 mo | |||
| Stunting | 21 (38.2) | 18 (20.7) | .03a |
| Underweight | 6 (10.9) | 3 (3.4) | .09 |
| Wasting | 0 (0.0) | 2 (2.3) | .52 |
| Hemoglobin levels at 12 mo, mean ± SD | 10.8 ± 1.4 | 10.3 ± 1.5 | .10 |
| Anemia at 12 mo | 30 (53.6) | 57 (64.8) | .18 |
Data are No. (%) except where indicated. Numbers may not add up to totals because of missing data. All variables had complete data except (% missing data malariahi, malarialo): stunting at 12 months (3.5%, 2.2%), underweight at 12 months (1.8%, 0.0%), and wasting at 12 months (3.5%, 1.1%)
a P values < .05 considered statistically significant.
Figure 1.
Seroprevalence of KSHV by 24 months of age comparing different definitions of KSHV seroconversion for children enrolled in the Kenyan Prospective Infant Cohort study. We evaluated plasma samples for KSHV antibodies by either ELISA to measure K8.1 and LANA, or by multiplex bead-based assay for K8.1, LANA, ORF38, ORF50, and K10.5. Children were considered KSHV seropositive if they had antibodies to the KSHV antigens at any time between 6 and 24 months of age. Abbreviations: ELISA, enzyme-linked immunosorbent assay; KSHV, Kaposi sarcoma-associated herpesvirus; LANA, latency-associated nuclear antigen.
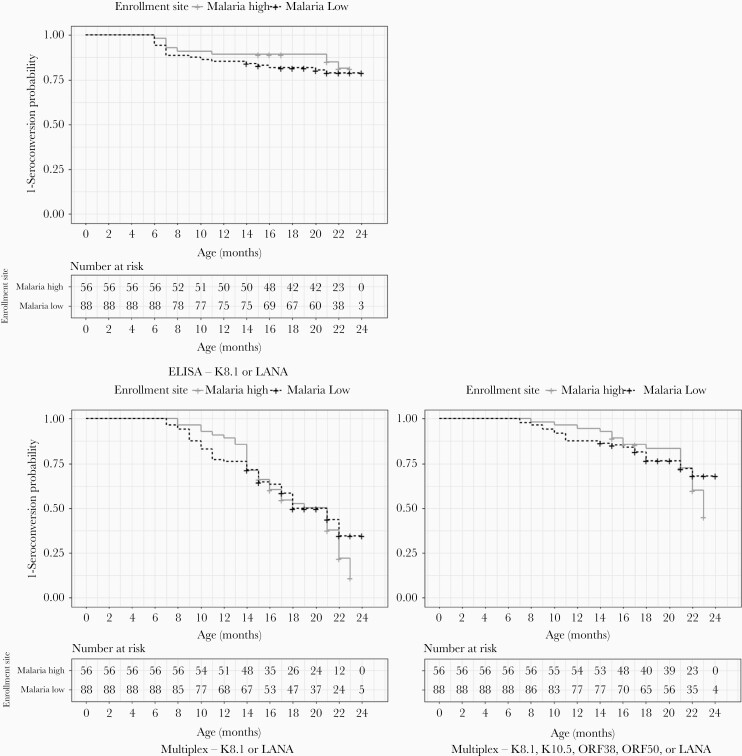
We first wanted to evaluate if an ecologic measure of malaria would affect age at KSHV seroconversion. As shown in Figure 2, Kaplan-Meier curves show a higher probability of KSHV seroconversion by 22 months of age in malariahi vs malarialo using both the 2Ag- and 5Ag-multiplex assay. In addition, there was a significant interaction between enrollment site and time to KSHV seroconversion (2Ag-multiplex P value = .02, 5Ag-multiplex P value = .01). Children from the malariahi region did not have a statistically significant difference in the hazards of KSHV seroconversion at age 12 or 18 months, regardless of seroconversion definition. However, by age 24 months, children from the malariahi region had significantly higher hazards of becoming KSHV seropositive when measured by 2Ag-multiplex and 5Ag-multiplex (Table 2). Although the number of children with available samples at 24 months of age was small, we saw similar associations between enrollment site and time to KSHV seroconversion at 22 months of age, which was significant for 5Ag-multiplex (adjusted hazard ratio = 2.5; 95% confidence interval, 1.2–5.2; P = .02). These estimates did not differ when stratified by any versus no significant antibody loss from baseline to 6 months (all interaction term P values > .05).
Figure 2.
Kaplan-Meier curves comparing probabilities of KSHV seroconversion up to 24 months of age by enrollment sites (malariahi vs malarialo) or different KSHV seroconversion definitions. Time at risk of KSHV seroconversion began at 6 months of age. Seroconversion by ELISA (A) was defined as detection of K8.1 or LANA. Seroconversion by multiplex (B and C) was defined as a 5-fold increase in any of the defined antibodies compared to antibody levels at 6 months of age, which was treated as baseline. Abbreviations: ELISA, enzyme-linked immunosorbent assay; KSHV, Kaposi sarcoma-associated herpesvirus; LANA, latency-associated nuclear antigen.
Table 2.
Crude and Adjusted Hazard Ratios of Time (Months) to KSHV Seroconversion, Using Different Definitions of KSHV Seroconversion, by Malaria Exposure and Other Exposures of Interest
| Exposure of Interest | Crude | Adjusted | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ELISA by K8.1 or LANA | Multiplex (5-Fold Increase) by K8.1 or LANA | Multiplex (5-Fold Increase) by K8.1, K10.5, ORF38, ORF50, or LANA | ELISA by K8.1 or LANA | Multiplex (5-Fold Increase) by K8.1 or LANA | Multiplex (5-Fold Increase) by K8.1 K10.5, ORF38, ORF50, or LANA | |||||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | aHR (95% CI) | P Value | |
| Malariahi vs Malarialo site | 0.8 (.3–1.7) | .47 | 1.2 (.6–2.1) | .65 | 1.2 (.8–1.8) | .41 | … | … | … | … | … | … |
| 12 mo | … | … | … | … | … | … | 0.8 (.3–1.8) | .54 | 0.6 (.2–1.4) | .21 | 0.7 (.4–1.3) | .29 |
| 18 mo | … | … | … | … | … | … | 1.1 (.3–3.9) | .85 | 1.4 (.7–2.7) | .34 | 1.5 (.9–2.5) | .09 |
| 24 mo | … | … | … | … | … | … | 1.6 (.2–11.7) | .62 | 3.4 (1.0–11.2) | .04a | 3.1 (1.2–7.9) | .02a |
| Malaria DNA | 0.8 (.2–2.5) | .67 | 1.9 (1.0–3.5) | .04a | 1.0 (.7–1.6) | .93 | 0.8 (.1–4.0) | .74 | 2.9 (1.3–6.2) | <.01a | 1.3 (.8–2.2) | .34 |
| Malaria annualized rate, P. falciparum/person-yearb | 1.2 (1.0–1.4) | .01a | 1.6 (1.3–1.9) | <.01a | 1.8 (1.5–2.1) | <.01a | 1.2 (1.1–1.4) | <.01a | 1.6 (1.3–1.9) | <.01a | 1.8 (1.5–2.1) | <.01a |
| Female vs male | 1.1 (.5–2.3) | .85 | 1.4 (.8–2.6) | .23 | 1.5 (1.0–2.3) | .04a | No additional adjustments | |||||
| No. other children in home, ref 0 | ||||||||||||
| 1 | 2.2 (.7–7.2) | .21 | 1.8 (.8–4.3) | .18 | 1.1 (.6–2.0) | .78 | 1.6 (.3–9.8) | .60 | 2.2 (.9–5.4) | .10 | 1.2 (.6–2.2) | .57 |
| 2 | 2.0 (.6–6.9) | .30 | 1.6 (.6–4.1) | .31 | 0.9 (.5–1.8) | .86 | 2.9 (.6–14.9) | .21 | 1.8 (.7–4.9) | .22 | 0.9 (.5–1.9) | .84 |
| 3+ | 1.7 (.5–5.5) | .38 | 1.0 (.4–2.4) | .95 | 1.4 (.8–2.4) | .27 | 2.1 (.4–10.5) | .36 | 1.1 (.4–2.9) | .79 | 1.5 (.9–2.7) | .16 |
| Hemoglobin, g/dL | 0.9 (.6–1.4) | .77 | 0.9 (.7–1.2) | .59 | 1.0 (.8–1.2) | .98 | 0.9 (.6–1.4) | .64 | 0.9 (.7–1.2) | .47 | 1.0 (.8–1.2) | .59 |
| Anemia | 1.5 (.4–6.5) | .56 | 1.2 (.6–2.6) | .62 | 1.0 (.6–1.6) | .92 | 1.7 (.4–7.1) | .49 | 1.3 (.6–2.8) | .51 | 1.1 (.6–1.8) | .78 |
| WHO Z-scores | ||||||||||||
| Stunting | 1.2 (.3–5.2) | .77 | 2.0 (.9–4.0) | .07 | 1.9 (1.1–3.1) | .02a | 1.0 (.2–4.4) | .98 | 2.2 (1.0–4.9) | .05a | 1.6 (.9–2.9) | .09 |
| Under weight | 5.1 (1.0–25.3) | .05a | 1.4 (.3–5.9) | .65 | 3.0 (1.2–7.6) | .02a | 4.7 (.9–25.2) | .07 | 1.3 (.3–5.4) | <.01a | 2.6 (1.0–6.7) | .05 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; KSHV, Kaposi sarcoma-associated herpesvirus; LANA, latency-associated nuclear antigen; WHO, World Health Organization.
All adjusted models included child sex. In addition, adjusted models for malariahi vs malarialo site included child sex and an interaction term between site and time; adjusted models for malaria DNA included child sex and stunting; adjusted models for number of other children in the home included child sex and malaria DNA by PCR; and adjusted models for stunting included child sex, number of children in the home, and malaria DNA by PCR and stratified by number of children in the home.
aP-value < .05 considered statistically significant.
bAnalyses of malaria annualized rate excluded children with missing or no malaria infection during the study (children excluded n = 9).
We next evaluated whether detection of any malaria infection as indicated by detection of P. falciparum DNA in peripheral blood samples was associated with risk of KSHV seroconversion. Children with P. falciparum infection had significantly higher hazards of seroconversion, defined by 2Ag-multiplex. Among children with any P. falciparum detection, there was a significant increase in the adjusted hazard of becoming KSHV seropositive for each additional P. falciparum detection per person-year (Table 2). Estimates of the association between P. falciparum detection and KSHV seroconversion were not significantly different when stratified by whether a child had any versus no significant decline in antibodies from birth to 6 months (all interaction term P values > .05).
Female children were more likely to become KSHV seropositive earlier as defined by 5Ag-multiplex. Children with stunting had an increased hazard of becoming KSHV seropositive by 2Ag-multiplex, while underweight children had an increased hazard of becoming KSHV seropositive when measured by ELISA and 5Ag-multiplex but these differences were not statistically significant at the .05 level (Table 2). Too few children were defined as wasting to be included in analyses.
We compared characteristics of children included and excluded from our analysis and found a higher percentage from the malariahi region were excluded (Supplementary Table 1). LTFU ranged from 19% to 40%, depending on KSHV seroconversion definition. Children LTFU were significantly more likely to have no other children at home (5Ag-multiplex) (Supplementary Table 2).
DISCUSSION
In this study, we found that children with malaria infection and children from regions with higher malaria transmission were more likely to seroconvert for KSHV by 24 months of age. Our results support a model where malaria infection results in early age of KSHV infection. Earlier age of KSHV seroconversion has been associated with higher anti-KSHV IgG blood levels [28], which in turn has been associated with increased KS risk [29]. In areas endemic for both KSHV and malaria, malaria infection, by promoting earlier infection with KSHV and leading to poor lifetime viral control, may be driving increased KS risk.
Malaria detection by qPCR, and among those with detectable malaria, increasing malaria annualized rate, led to increased hazards of KSHV seroconversion. This supports similar associations between malaria parasitemia or high malaria antibody titers and KSHV seropositivity in Ugandan adults [8–11] and children [7, 10] and high malaria antibody levels with KSHV seropositivity in older Kenyan children [30]. Our results agree with Ugandan studies that reported associations between increasing antibody levels to K8.1 and LANA and rapid diagnostic test-confirmed malaria [11] and, among KSHV-positive children and adults, increasing PBMC KSHV DNA levels with rapid diagnostic test-confirmed malaria [12]. A study of Brazilian non-Amerindians also reported increased odds of being KSHV seropositive among individuals reporting more than 2 malaria episodes [31].
Studies that relied on self-report of malaria did not find an association for KSHV primary infection [14], KSHV antibody titers over time following primary infection [16], nor detection of KSHV DNA in saliva or buffy coat [32]. However, because these studies relied on self-report of malaria, they likely underestimated rates of malaria in their respective study populations by only capturing symptomatic malaria. For example, in Kenyan children and adults, over 90% of malaria infections detected by PCR were asymptomatic [33]. In addition, by relying on caretaker and self-report rather than laboratory-based diagnosis, fevers unrelated to malaria may be attributed to malaria and less severe malaria episodes may not have been reported. By using qPCR, a more sensitive measure of P. falciparum exposure, both symptomatic and asymptomatic infections were captured in our analyses. Use of antimalarial antibodies to evaluate malaria only measures past exposure and cannot provide a temporal relationship between KSHV and P. falciparum infections.
How malaria affects KSHV seroconversion is unknown, but the parasite’s innate ability to alter the immune response may increase overall susceptibility to KSHV infection after exposure [34, 35]. P. falciparum, the primary agent of malaria in sub-Saharan Africa, leads to macrophage and dendritic cell dysfunction, reducing innate immunity responses [36]. Parasitic infections are known to shift the immune system towards a Th2 response [37], which in turn promotes gammaherpesvirus reactivation [10, 38]. Malaria can result in severe anemia, inducing hypoxia, a deficiency associated with KSHV reactivation and KS disease [13, 39], although we saw no association between anemia and KSHV seroconversion in this study. More research is needed to define the biological mechanisms that underlie the relationship between P. falciparum infection and KSHV.
Children in our cohort were more likely to seroconvert to KSHV if they were stunted or underweight based on WHO Z-scores, although underweight was not statistically significant at the P < .05 level. Both stunting and underweight are measures of undernutrition. Children who are undernourished are more likely to die from common infections, and malnutrition leads to both innate and adaptive immunity deficiencies [40–42], which may make children more susceptible to KSHV infection. Nutrition should be considered in the future as a potential component of primary KSHV infection and viral reactivation.
We found up to 63% KSHV seroprevalence by 24 months of age when measured by 5Ag-multiplex assay. Previous measures of seroprevalence among children by 2 years of age ranged from 16% in Uganda [43] using only K8.1 ELISA to 30% in Zambia using a monoclonal antibody enhanced immunofluorescent assay [44]. The increased sensitivity and dynamic range of the multiplex bead-based assay is one possible reason for the higher KSHV seroprevalence in our study population. In addition, our longitudinal design allowed us to measure KSHV antibodies across time. Several studies have shown that anti-KSHV antibodies fluctuate over time in an individual, sometimes to the point where they are no longer detectable [15–17]. By measuring anti-KSHV antibodies at multiple time points for each study participant, we were able to more accurately define the time of KSHV seroconversion.
While K8.1 and LANA are well-known markers of KS disease, and therefore KSHV infection, relying on only 2 of over 85 KSHV antigens to detect infection could underestimate seroconversion. We did not have data on detection of KSHV DNA and so relied on KSHV serology to define seroconversion. To determine KSHV seroconversion, we employed the multiplex bead-based assay, which has a larger dynamic range than ELISA and allowed us to look at a wider breadth of antibodies to KSHV proteins. A challenge in our study design was that the algorithm for determining multiplex assay cutoffs for seroconversion has yet to be defined. We worked with existing samples to select a number of KSHV proteins that appeared to be the best candidates for markers of primary infection and used a threshold of a 5-fold increase in antibody titers from 6 months of age, to define seroconversion. We choose a 6 months of age baseline for setting our cutoff so that our assay would not be confounded by the presence of passively transferred maternal KSHV antibodies. Maternal antibodies have been found to be detectable in infants of KSHV-positive mothers up to 6 months of age [45]. While serology status of the mothers in our study was unknown as we enrolled only infants in this study, we have found that almost 90% of pregnant women in this region were KSHV seropositive [46] and so assume maternal antibodies exist in the majority of included children.
Our study had several limitations. To be enrolled, mothers of included children had to present to the study clinic and may have differed from mothers who either did not present or refused enrollment. Children had to stay in the study for at least 3 clinic visits after they reached 6 months of age. As a consequence, our final analyses excluded a number of the enrolled children, which may reduce the generalizability of our results. However, we did not find significant differences between individuals included or excluded in our analyses. We did not have information on maternal KSHV infection, family behaviors (eg, food sharing), or whether the child had other parasitic infections, all important potential confounders of the relationship between malaria and KSHV seroprevalence. LTFU is a concern if children who drop out of the study are different from those that stay with regards to the outcome of interest. However, we saw no relevant differences in baseline characteristics for children retained or LTFU.
In summary, our data suggest malaria infection in the first 2 years of life is a driver of KSHV seroconversion in young sub-Saharan Africa children, and that primary KSHV infection is occurring at earlier ages than previously detected. Our findings highlight the importance of using sophisticated and dynamic techniques to explore KSHV seroconversion in the youngest populations in sub-Saharan Africa. Forthcoming research will need to determine the biological mechanism of interaction between P. falciparum and KSHV, as well as focus on primary infection in a younger subpopulation. Findings from these future studies will provide a base to work towards prevention of KSHV infection and subsequent KS disease in sub-Saharan Africa.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funders had no role in study design, data collection, or interpretation. The findings of this study are solely the responsibility of the author and do not necessarily represent the official views of National Cancer Institute or National Institutes of Health.
Financial support. This work was supported by the National Cancer Institute, National Institutes of Health (grant numbers R01 CA239588 and R01 CA102667 and contract number 75N91019D00024, formerly HHSN261200800001E).
Potential conflicts of interest. R. R. is a consultant for Viracta Therapeutics Inc. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 22nd International Workshop on KSHV and Related Agents, 30 June–3 July 2019, New York City, NY.
References
- 1. Newton R, Labo N, Wakeham K, et al. Kaposi sarcoma-associated herpesvirus in a rural Ugandan cohort, 1992–2008. J Infect Dis 2018; 217:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO). World malaria report 2017. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 3. Ascoli V, Belli S, Benedetti M, Trinca S, Ricci P, Comba P. High incidence of classic Kaposi’s sarcoma in Mantua, Po Valley, northern Italy (1989–1998). Br J Cancer 2001; 85:379–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crispo A, Tamburini M, De Marco MR, et al. HHV-8 prevalence, immunosuppression and Kaposi’s sarcoma in south Italy. Int J Mol Med 2001; 7:535–8. [DOI] [PubMed] [Google Scholar]
- 5. Coluzzi M, Calabro ML, Manno D, Chieco-Bianchi L, Schulz TF, Ascoli V. Reduced seroprevalence of Kaposi’s sarcoma-associated herpesvirus (KSHV), human herpesvirus 8 (HHV8), related to suppression of Anopheles density in Italy. Med Vet Entomol 2003; 17:461–4. [DOI] [PubMed] [Google Scholar]
- 6. Serraino D, Corona RM, Giuliani M, et al. Infection with human herpesvirus type 8 and Kaposi’s sarcoma in a central Italian area formerly endemic for malaria. Infection 2003; 31:47–50. [DOI] [PubMed] [Google Scholar]
- 7. Wakeham K, Webb EL, Sebina I, et al. Risk factors for seropositivity to Kaposi sarcoma-associated herpesvirus among children in Uganda. J Acquir Immune Defic Syndr 2013; 63:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wakeham K, Webb EL, Sebina I, et al. Parasite infection is associated with Kaposi’s sarcoma associated herpesvirus (KSHV) in Ugandan women. Infect Agent Cancer 2011; 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nalwoga A, Webb EL, Chihota B, et al. Kaposi’s sarcoma-associated herpesvirus seropositivity is associated with parasite infections in Ugandan fishing communities on Lake Victoria islands. PLoS Negl Trop Dis 2019; 13:e0007776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nalwoga A, Cose S, Wakeham K, et al. Association between malaria exposure and Kaposi’s sarcoma-associated herpes virus seropositivity in Uganda. Trop Med Int Health 2015; 20:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nalwoga A, Cose S, Nash S, et al. Relationship between anemia, malaria coinfection, and Kaposi sarcoma-associated herpesvirus seropositivity in a population-based study in rural Uganda. J Infect Dis 2018; 218:1061–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nalwoga A, Nakibuule M, Marshall V, et al. Risk factors for Kaposi’s sarcoma associated herpesvirus (KSHV) DNA in blood and in saliva in rural Uganda. Clin Infect Dis 2019; 71:1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stolka K, Ndom P, Hemingway-Foday J, et al. Risk factors for Kaposi’s sarcoma among HIV-positive individuals in a case control study in Cameroon. Cancer Epidemiol 2014; 38:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Minhas V, Brayfield BP, Crabtree KL, Kankasa C, Mitchell CD, Wood C. Primary gamma-herpesviral infection in Zambian children. BMC Infect Dis 2010; 10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Butler LM, Dorsey G, Hladik W, et al. Kaposi sarcoma-associated herpesvirus (KSHV) seroprevalence in population-based samples of African children: evidence for at least 2 patterns of KSHV transmission. J Infect Dis 2009; 200:430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olp LN, Minhas V, Gondwe C, et al. Longitudinal analysis of the humoral response to Kaposi’s sarcoma-associated herpesvirus after primary infection in children. J Med Virol 2016; 88:1973–81. [DOI] [PubMed] [Google Scholar]
- 17. Quinlivan EB, Wang RX, Stewart PW, et al. Longitudinal sero-reactivity to human herpesvirus 8 (KSHV) in the Swiss HIV cohort 4.7 years before KS. J Med Virol 2001; 64:157–66. [DOI] [PubMed] [Google Scholar]
- 18. Juillard F, Tan M, Li S, Kaye KM. Kaposi’s sarcoma herpesvirus genome persistence. Front Microbiol 2016; 7:1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Labo N, Miley W, Marshall V, et al. Heterogeneity and breadth of host antibody response to KSHV infection demonstrated by systematic analysis of the KSHV proteome. PLoS Pathog 2014; 10:e1004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piriou E, Asito AS, Sumba PO, et al. Early age at time of primary Epstein-Barr virus infection results in poorly controlled viral infection in infants from western Kenya: clues to the etiology of endemic Burkitt lymphoma. J Infect Dis 2012; 205:906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Malaria Control Programme (NMCP) Kenya, Kenya National Bureau of Statistics (KNBS), ICF International. Kenya malaria indicator survey 2015. Nairobi, Kenya: NMCP, KNBS, and ICF International, 2016. [Google Scholar]
- 22. Reynaldi A, Schlub TE, Piriou E, et al. Modeling of EBV infection and antibody responses in Kenyan infants with different levels of malaria exposure shows maternal antibody decay is a major determinant of early EBV infection. J Infect Dis 2016; 214:1390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization (WHO). Nutrition landscape information system (NLIS) country profile indicators interpretation guide. Geneva, Switzerland: WHO, 2010. [Google Scholar]
- 24. World Health Organization (WHO). Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva, Switzerland: WHO, 2011. [Google Scholar]
- 25. Mbisa GL, Miley W, Gamache CJ, et al. Detection of antibodies to Kaposi’s sarcoma-associated herpesvirus: a new approach using K8.1 ELISA and a newly developed recombinant LANA ELISA. J Immunol Methods 2010; 356:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reynaldi A, Dent AE, Schlub TE, Ogolla S, Rochford R, Davenport MP. Interaction between maternally derived antibodies and heterogeneity in exposure combined to determine time-to-first Plasmodium falciparum infection in Kenyan infants. Malar J 2019; 18:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xue F, Lai M. Time -dependent covariates “survival” more in PROC PHREG. PharmaSUG China 2017 Conference, Shanghai, China. 6–8 July 2017. Available at: https://www.pharmasug.org/proceedings/china2017/SP/PharmaSUG-China-2017-SP01.pdf [Google Scholar]
- 28. Nalwoga A, Miley W, Labo N, et al. Age of infection with Kaposi sarcoma-associated herpesvirus and subsequent antibody values among children in Uganda. Pediatr Infect Dis J 2018; 37:e225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wakeham K, Johnston WT, Nalwoga A, et al. Trends in Kaposi’s sarcoma-associated herpesvirus antibodies prior to the development of HIV-associated Kaposi’s sarcoma: a nested case-control study. Int J Cancer 2015; 136:2822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oluoch PO, Oduor CI, Forconi CS, et al. Kaposi sarcoma-associated herpesvirus infection and endemic Burkitt lymphoma. J Infect Dis 2020; 222:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nascimento MC. Malaria may influence the transmission of Kaposi sarcoma associated herpesvirus in endemic areas. J Acquir Immune Defic Syndr 2014; 67:e41–3. [DOI] [PubMed] [Google Scholar]
- 32. Mbulaiteye SM, Pfeiffer RM, Engels EA, et al. Detection of Kaposi sarcoma-associated herpesvirus DNA in saliva and buffy-coat samples from children with sickle cell disease in Uganda. J Infect Dis 2004; 190:1382–6. [DOI] [PubMed] [Google Scholar]
- 33. Idris ZM, Chan CW, Kongere J, et al. High and heterogeneous prevalence of asymptomatic and sub-microscopic malaria infections on islands in Lake Victoria, Kenya. Sci Rep 2016; 6:36958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Benelli G. Mosquito vectors and the spread of cancer: an overlooked connection? Parisitology Research 2016; 115:7. [DOI] [PubMed] [Google Scholar]
- 35. International Agency for Research on Cancer (IARC) Working Group on the Evaluation of Carcinogenic Risks to Humans. Malaria and some polyomaviruses (SV40, BK, JC, and Merkel cell viruses). IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 104. Lyon, France: IARC, 2014. [PMC free article] [PubMed] [Google Scholar]
- 36. Riley EM, Wahl S, Perkins DJ, Schofield L. Regulating immunity to malaria. Parasite Immunol 2006; 28:35–49. [DOI] [PubMed] [Google Scholar]
- 37. Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature 1996; 383:787–93. [DOI] [PubMed] [Google Scholar]
- 38. Reese TA, Wakeman BS, Choi HS, et al. Helminth infection reactivates latent gamma-herpesvirus via cytokine competition at a viral promoter. Science 2014; 345:573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thakker S, Verma SC. Co-infections and pathogenesis of KSHV-associated malignancies. Front Microbiol 2016; 7:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bryce J, Boschi-Pinto C, Shibuya K, Black RE, WHOCHER Group . WHO estimates of the causes of death in children. Lancet 2005; 365:1147–52. [DOI] [PubMed] [Google Scholar]
- 41. Olofin I, McDonald CM, Ezzati M, et al. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS One 2013; 8:e64636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition—a systematic review. PLoS One 2014; 9:e105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Butler LM, Were WA, Balinandi S, et al. Human herpesvirus 8 infection in children and adults in a population-based study in rural Uganda. J Infect Dis 2011; 203:625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crabtree KL, Wojcicki JM, Minhas V, et al. Risk factors for early childhood infection of human herpesvirus-8 in Zambian children: the role of early childhood feeding practices. Cancer Epidemiol Biomarkers Prev 2014; 23:300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lyall EG, Patton GS, Sheldon J, et al. Evidence for horizontal and not vertical transmission of human herpesvirus 8 in children born to human immunodeficiency virus-infected mothers. Pediatr Infect Dis J 1999; 18:795–9. [DOI] [PubMed] [Google Scholar]
- 46. Sabourin KR, Ogolla S, Daud I, et al. Malaria during pregnancy and transplacental transfer of Kaposi sarcoma-associated herpesvirus (KSHV) antibodies: a cohort study of Kenyan mother and child pairs. Infect Agent Cancer 2020; 15:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.