Abstract
Background: The associations of whole grain and refined grain consumption with metabolic syndrome (MetS) has been evaluated in several epidemiological studies with conflicting results. This meta-analysis was therefore employed to further investigate the above associations.
Method: We searched the PubMed, Web of Science and Embase database until March 2021 (without restriction for inclusion time), for observational studies on the associations of whole grain and refined grain consumption with MetS. The pooled relative risk (RR) of MetS for the highest vs. lowest category of whole grain and refined grain consumption, as well as their corresponding 95% confidence interval (CI) were calculated.
Results: A total of 14 observational studies, which involved seven cross-sectional and seven prospective cohort studies, were identified. Specifically, nine studies were related to whole grain consumption, and the overall multi-variable adjusted RR demonstrated that the whole grain consumption was inversely associated with MetS (RR = 0.80, 95%CI: 0.67–0.97; P = 0.021). With regard to refined grain consumption, 13 studies were included. The overall multi-variable adjusted RR indicated that refined grain consumption was positively associated with MetS (RR = 1.37, 95%CI: 1.02–1.84; P = 0.036).
Conclusions: The existing evidence suggests that whole grain consumption is negatively associated with MetS, whereas refined grain consumption is positively associated with MetS. Our result might be helpful to better consider the diet effect on MetS. However, more well-designed prospective cohort studies are needed to elaborate the concerned issues further.
Keywords: whole grain, refined grain, metabolic syndrome, meta-analysis, observational study
Introduction
The metabolic syndrome is a complex of interrelated risk factors for cardiovascular disease (CVD), diabetes and all-cause mortality. These factors include dysglycemia, raised blood pressure, elevated triglyceride levels, low high-density lipoprotein cholesterol levels, and obesity (particularly central adiposity) (1). Affecting about 25% of the population in developed countries in parallel with obesity and diabetes, MetS has been known as an important public health issue in the 21st century (2). Although the etiology of MetS is still not well-understood yet, dietary factors are considered to be associated with MetS (3–5).
Grain, a small, hard and dry seed, is composed of the endosperm, germ, and bran (6). Grain is served as the primary source of carbohydrates and global staples of diets (7). Whole grain contains more abundant and diverse nutrients with potential health benefits (fiber, vitamins, and minerals) than refined grains (8). Indeed, a potential different biological effect of whole grain and refined grain on health issues has been reported, e.g., gastric cancer (6), chronic kidney disease (9) or all-cause mortality (10), etc. Moreover, whole grain consumption was indicated to be associated with a lower risk of hypertension (11) and diabetes (12, 13), whereas refined grain consumption was associated with higher risk of diabetes (13). Moreover, some randomized control trials demonstrated that whole grain (replacing refined grain) within a weight-loss diet could reduce blood glucose (14). A whole grain-based diet could also lower the postprandial plasma insulin and triglyceride level in MetS (15). With regard to the fundamental research, the pre-germinated brown rice extract was also proved to ameliorate MetS model (16, 17).
As far as we know, the associations of whole grain and refined grain consumption with MetS has been investigated by numerous observational studies (18–31). However, their results are still controversial. The present meta-analysis of observational studies was therefore employed to examine the issues further. It was hypothesized that whole grain consumption was inversely associated with MetS, whereas refined grain consumption was positively associated with MetS.
Materials and Methods
Search Strategy
We conducted this meta-analysis according to the Preferred Reporting Items for Systematic review and Meta-analyses (PRISMA) guidelines (32). The PubMed, Web of Science and Embase database were searched until March 2021 (without restriction for inclusion time), by a series of logic combinations of keywords related to metabolic syndrome (“metabolic syndrome”) and grain (“grain,” “grains,” “rice,” “bread,” “breads,” “wheat,” “wheats,” “rye,” “cereal,” “cereals”). No language restrictions were set in the search strategy. We first screened the titles and abstracts of all of the articles to identify eligible studies and then read the full articles to include eligible studies. Moreover, the reference lists from retrieved articles were reviewed to identify additional studies.
Study Selection
Two researchers (YZ and JD) reviewed the titles, abstracts and full texts of all retrieved studies independently. Disagreements were resolved by discussions and mutual-consultations. The potentially eligible articles were selected through full text review in line with the inclusion and exclusion criteria according to PICOS strategy. The included studies were required to meet the following criteria: (1) the participants were general population; (2) the exposure of interest was the consumption of whole grain or refined grain; (3) the comparison was the highest vs. lowest category of exposure; (4) the outcomes included the MetS; (5) observational studies in general population. The exclusion criteria were as follows: (1) duplicated or irrelevant articles; (2) reviews, letters or case reports; (3) randomized controlled trials; (4) non-human studies.
Data Extraction
Two researchers extracted the data (YZ and JD) independently. Disagreements were resolved by consensus. The information about first author, year of publication, location, age, gender, sample size, study design, exposure assessment, category of exposure, effect estimates for MetS, adjustments, and diagnostic criteria of MetS was collected. The corresponding effect estimates adjusted for the maximum number of confounding variables with corresponding 95% CIs for the highest vs. lowest level were extracted. For the studies that reported the effect estimates by gender, the pooled effect estimates were calculated. In addition, Huang presented the data as southern and northern China, and they were processed independently (30). Rice/white rice was also processed as refined grain (23, 25–27).
Quality Assessment
Quality assessment was conducted according to the Newcastle-Ottawa (NOS) criteria for non-randomized studies, which is based on three broad perspectives: the selection process of study cohorts, the comparability among different cohorts, and the identification of either the exposure or outcome of study cohorts. Disagreements with respect to the methodological quality were resolved by discussion and mutual-consultation. In the current study, we considered a study awarded seven or more stars as a high-quality study (33).
Statistical Analyses
RR was considered as the common measure of the associations of whole grain or refined grain consumption with MetS, and OR and HR was directly converted into RR. The I2 statistic, which measures the percentage of the total variation across studies due to heterogeneity, was also examined (I2 > 50% was considered heterogeneity). If significant heterogeneity was observed among studies, the random-effects model was used; otherwise, the fixed effects model was utilized. Begg's tests were performed to assess the publication bias (34), and statistical analyses were performed using STATA version 11.0 (StataCorp LP, College Station, Texas). A p-value < 0.05 was accepted as statistically significant. Subgroup analysis for study design, diagnostic criteria of MetS, sample size, exposure assessment, study quality and adjustment of BMI and energy, were conducted.
Results
Study Identification and Selection
The detailed flow diagram of study identification and selection was presented in Figure 1. A total of 998 potentially relevant articles (PubMed 252, Embase 321 and Web of Science 425) were retrieved during the initial literature search. After eliminating 513 duplicated articles, 485 articles were screened by titles and abstracts. Three hundred fifty six irrelevant studies were excluded thereafter. Then, 33 reviews, case reports or letters, 29 non-human studies, and 53 randomized control trials were removed. Eventually, a total of 14 studies were identified for this meta-analysis.
Figure 1.
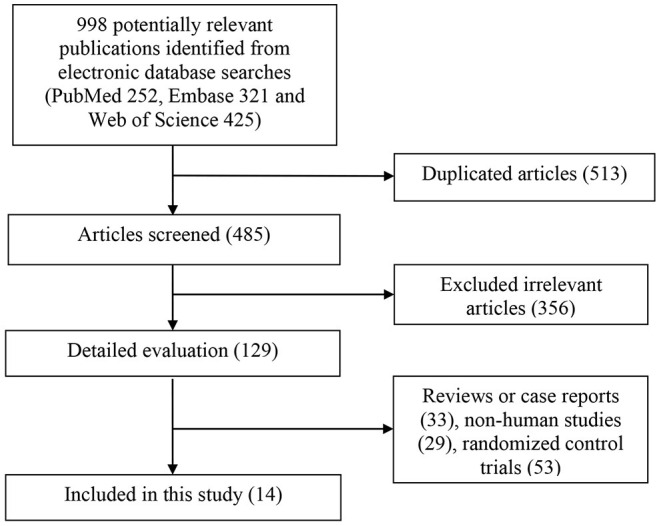
Flow chart for the identification of studies that were included in this meta-analysis.
Study Characteristics
Table 1 shows the main characteristics of the included studies. These studies were published between 2004 and 2020, which involved seven cross-sectional and seven prospective cohort studies. Ten studies were performed in the Asian countries [Korea (24, 25, 28, 31), Iran (19, 27), Japan (26), India (22) and China (23, 30)], three studies were conducted in US (18, 20, 21), and one study was conducted in Chile (29). Thirteen articles included both male and female participants (18–25, 27–31), whereas 1 study included only male participants (26). The sample size ranged from 535 to 15,972 for a total number of 61,431. Food frequency questionnaire (FFQ) was utilized in 11 studies (18, 19, 21–24, 26, 27, 29–31) and three studies employed recall record (24 h or 3 days) (20, 25, 28). Ten (18–22, 24, 25, 28, 30, 31) and four (23, 26, 27, 29) studies were defined as high and low-quality study, respectively. The diagnostic criteria for MetS were National Cholesterol Education Program-Adult Treatment Panel III (NCEP ATP III) in nine (18–20, 22, 26–29, 31), International Diabetes Foundation (IDF) in three (23, 24, 30), American Heart Association (AHA) (21, 25) in two studies, respectively.
Table 1.
Characteristics of the individual studies included in this meta-analysis.
| First authoryear of publication | Location | Ageyears | Gender | Sample Size | Study design | Exposure assessment | Category of Exposure | Effect estimates for MetS (95%CI) | Adjustments | Diagnostic criteria of MetS | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mckeown (18) | US | 26–82 | Both | 2,834 | Cross-sectional | 126-item FFQ | Whole grain | Sex, age, cigarette dose, total energy intake, alcohol intake, percentage saturated fat, percentage polyunsaturated fat, multivitamin use, and physical activity |
NCEP ATP III | 7 | |
| Quintile 1Quintile 2Quintile 3Quintile 4Quintile 5 | 1.00.81 (0.60, 1.08)1.09 (0.82, 1.44)0.82 (0.61, 1.10)0.67 (0.48, 0.91) | ||||||||||
| Refined grain | |||||||||||
| Quintile 1Quintile 2Quintile 3Quintile 4Quintile 5 | 1.01.13 (0.84, 1.52)1.01 (0.74, 1.38)1.03 (0.75, 1.42)0.76 (0.53, 1.09) | ||||||||||
| Esmaillzadeh (19) | Iran | 18–74 | Both | 827 | Cross-sectional | 132-item FFQ | Whole grain | Age, total energy intake, energy from fat, use of blood pressure medication, use of estrogen, smoking, physical activity, consumption of meats and fish, fruit and vegetables |
NCEP ATP III | 8 | |
| Quartile 1Quartile 2Quartile 3Quartile 4 | 1.00.84 (0.79, 0.89)0.76 (0.69, 0.82)0.68 (0.60, 0.78) | ||||||||||
| Refined grain | |||||||||||
| Quartile 1Quartile 2Quartile 3Quartile 4 | 1.01.68 (1.26, 2.31)1.92 (1.48, 2.58)2.25 (1.80, 2.84) | ||||||||||
| Sahyoun (20) | US | 60–98 | Both | 535 | Cross-sectional | 3-day food record (single recall) | Whole grain | Age, sex, race, educational attainment, marital status, smoking, alcohol intake, exercise, BMI, energy intake, percentage saturated fatty acid intake and use of antihypertensive or lipid-lowering medication |
NCEP ATP III | 7 | |
| Quartile 1Quartile 2Quartile 3Quartile 4 | 1.00.58 (0.35, 0.97)0.41 (0.24, 0.69)0.46 (0.27, 0.79) | ||||||||||
| Refined grain |
|||||||||||
| Quartile 1Quartile 2Quartile 3Quartile 4 | 1.01.17 (0.69, 1.97)1.57 (0.91, 2.68)2.16 (1.20, 3.87) | ||||||||||
| Lutsey (21) | US | 45–64 | Both | 15,972 | Cohort | 66-item FFQ | Whole grain | Age, sex, race, education, center, and total calories, smoking status, pack-years, physical activity, meat, dairy, fruits and vegetables. | AHA | 8 | |
| Quintile 1Quintile 2Quintile 3Quintile 4Quintile 5 | 1.01.02 (0.92, 1.13)1.06 (0.96, 1.18)1.02 (0.92, 1.14)1.02 (0.92, 1.14) | ||||||||||
| Refined grain |
|||||||||||
| Quintile 1Quintile 2Quintile 3Quintile 4Quintile 5 | 1.00.92 (0.83, 1.02)0.95 (0.86, 1.06)0.95 (0.85, 1.06)0.89 (0.78, 1.01) | ||||||||||
| Radhika (22) | India | ≥20 | Both | 2,042 | Cross-sectional | 222-item FFQ | Refined grain | Age, sex, smoking, alcohol, BMI, total energy, legumes, visible fats and oils, dairy products, sugars, fruits and vegetables, tubers, fish and seafoods, and nuts and oil seeds |
NCEP ATP III | 8 | |
| Quartile 1Quartile 2Quartile 3Quartile 4 | 1.03.37 (2.13, 5.31)4.33 (2.72, 6.90)7.83 (4.72, 12.99) | ||||||||||
| Shi (23) | China | >20 | Both | 1,231 | Cohort | 33-item FFQ | Refined grain | Smoking, drinking, active commuting, leisure time physical activity, education, occupation, energy intake | IDF | 6 | |
| <200 g201–400 g>401 g | 1.00.70 (0.39, 1.26)0.76 (0.43, 1.36) | ||||||||||
| Baik (24) | Korea | 40–69 | Both | 5,251 | Cohort | 103-item FFQ | Whole grain | Age, sex, income, occupation, education, smoking status, alcohol intake, quartiles of MET-hours/day, study sites, FTO genotypes, quartiles of energy intake | IDF | 7 | |
| Quintile 1Quintile 2Quintile 3Quintile 4Quintile 5 | 1.0NA0.96 (0.81, 1.14)1.10 (0.84, 1.45)0.96 (0.75, 1.22) | ||||||||||
| Refined grain | |||||||||||
| Quintile 1Quintile 2Quintile 3Quintile 4Quintile 5 | 1.00.82 (0.68, 0.99)1.00 (0.80, 1.25)0.87 (0.68, 1.12)0.79 (0.59, 1.04) | ||||||||||
| Son (25) | Korea | 20–64 | Both | 5,830 | Cross-sectional | 24 h recall (single recall) | Refined grain | Age, energy, sex, BMI, alcohol, smoke, income, activity | AHA | 7 | |
| Quartile 1Quartile 2Quartile 3Quartile 4 | 1.01.02 (0.73, 1.42)1.01 (0.73, 1.40)1.09 (0.77, 1.53) | ||||||||||
| Watanabe (26) | Japan | 40–74 | Male | 6,095 | Cohort | 29-item FFQ | Refined grain <300 g/day 300–450 g/day >450 g/day |
1.01.05 (0.92, 1.21)1.19 (0.79, 1.81) |
Age, egg intake, vegetable intake, milk intake, sugary beverage intake, alcoholic beverage intake, family structure, daily physical activity, checking body weight <3 times per week, a gain of ≥10 kg in body weight since the age of 20, eating quickly, having dinner within 2 h of going to bed more than three times a week, snacking after dinner three or more times per week and skipping breakfast three or more times per week |
NCEP ATP III | 5 |
| Bahadoran (27) | Iran | 19–70 | Both | 2,799 | Cohort | 168-item FFQ | Refined grain | Age, sex, BMI, energy intake, carbohydrate, protein and fiber | NCEP ATP III | 6 | |
| Quartile 1Quartile 2Quartile 3Quartile 4 | 1.01.11 (0.72, 1.72)1.23 (0.79, 1.89)1.66 (1.04, 2.66) | ||||||||||
| Song (28) | Korea | 30–65 | Both | 6,845 | Cross-sectional | 24 h recall (single recall) | Whole grain | Age, living area, education, smoking status, current alcohol intake, vigorous physical activity and total energy intake. | NCEP ATP III | 8 | |
| Quintile 1Quintile 2Quintile 3Quintile 4Quintile 5 | 1.00.99 (0.70, 1.41)1.26 (0.88, 1.79)1.25 (0.88, 1.76)1.15 (0.82, 1.61) | ||||||||||
| Refined grain | |||||||||||
| Quintile 1Quintile 2Quintile 3Quintile 4Quintile 5 | 1.00.87 (0.62, 1.21)1.12 (0.80, 1.56)1.21 (0.87, 1.70)1.02 (0.75, 1.40) | ||||||||||
| Dussaillant (29) | Chile | >18 | Both | 2,561 | Cross-sectional | FFQ | Whole grain | Age, gender, education, physical activity, BMI | NCEP ATP III | 6 | |
| <1 serving/day≥1 serving/day | 1.01.78 (1.09, 2.92) | ||||||||||
| Huang South (30) | China | 18–75 | Both | 1,804 | Cohort | 74-item FFQ | Whole grain | Gender, age, marital status, income level, urbanicity index, BMI, smoking, alcohol, physical activity, TEI, vegetable, fruit, red meat consumption | IDF | 8 | |
| Quartile 1Quartile 2Quartile 3Quartile 4 | 1.01.02 (0.70,1.47)1.19 (0.82,1.72)1.28 (0.87,1.90) | ||||||||||
| Refined grain | |||||||||||
| Quartile 1Quartile 2Quartile 3Quartile 4 | 1.01.01 (0.41, 2.49)1.05 (0.53, 2.06)1.18 (0.44, 3.16) | ||||||||||
| HuangNorth (30) | China | 18–75 | Both | 1,088 | Cohort | 74-item FFQ | Whole grain | Gender, age, marital status, income level, urbanicity index, BMI, smoking, alcohol, physical activity, TEI, vegetable, fruit, red meat consumption | IDF | 8 | |
| Quartile 1Quartile 2Quartile 3Quartile 4 | 1.01.06 (0.71,1.58)0.77 (0.50,1.14)0.72 (0.47,1.11) | ||||||||||
| Refined grain | |||||||||||
| Quartile 1Quartile 2Quartile 3Quartile 4 | 1.00.80 (0.59, 1.07)0.93 (0.69, 1.26)0.95 (0.68, 1.33) | ||||||||||
| Kang (31) | Korea | 40–69 | Both | 5,717 | Cohort | 103-item FFQ | Male | Age, education level, household income, smoking status, alcohol intake, physical activity, BMI, LDL cholesterol, total energy intake, vegetable intake, fruit intake, meat intake and dairy intake |
NCEP ATP III | 9 | |
| Whole grain | |||||||||||
| <1 servings/day1–3 servings/day≥3 servings/day | 1.00.51 (0.43, 0.61)0.51 (0.41, 0.63) | ||||||||||
| Refined grain | |||||||||||
| <1 servings/day1–3 servings/day≥3 servings/day | 1.01.15 (0.98, 1.36)1.63 (1.31, 2.03) | ||||||||||
| Female | |||||||||||
| Whole grain | |||||||||||
| <1 servings/day1-3 servings/day≥3 servings/day | 1.00.58 (0.49, 0.68)0.73 (0.60, 0.90) | ||||||||||
| Refined grain | |||||||||||
| <1 servings/day1-3 servings/day≥3 servings/day | 1.00.96 (0.82, 1.12)2.25 (1.82, 2.78) |
Association Between Whole Grain Consumption and MetS
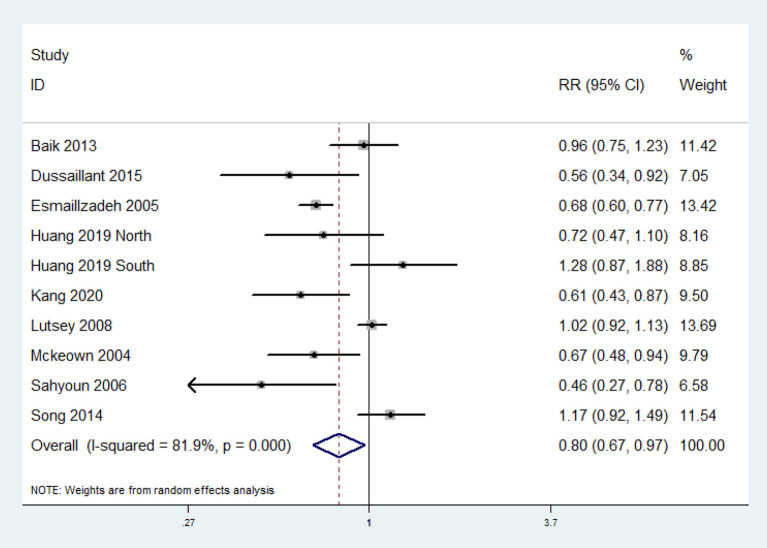
The overall multi-variable adjusted RR evidenced an inverse association between whole grain consumption and MetS (RR = 0.80, 95%CI: 0.67–0.97; P = 0.02) (Figure 2). A substantial level of heterogeneity was observed among studies (P < 0.001, I2 = 81.9%). No evidence of publication bias was observed among the included studies according to the Begg rank-correlation test (P = 0.152). In addition, such findings were obtained only in cross-sectional (RR = 0.71, 95%CI: 0.53–0.95; P = 0.02), NCEP ATP III (RR = 0.69, 95%CI: 0.54–0.89; P = 0.004), FFQ (RR = 0.80, 95%CI: 0.65–0.97; P = 0.03), adjustment of BMI (RR = 0.69, 95%CI: 0.49–0.98; P = 0.04) and energy intake (RR = 0.75, 95%CI: 0.59–0.96; P = 0.01) studies (Table 2).
Figure 2.
Forest plot of meta-analysis: Overall multi-variable adjusted RR of MetS for the highest vs. the lowest category of whole grain consumption.
Table 2.
Subgroup analyses of whole grain consumption and MetS.
| Stratification | Number of studies | Pooled RR | 95% CI | P-value | Heterogeneity |
|---|---|---|---|---|---|
| All | 9 | 0.80 | 0.67, 0.97 | P = 0.02 | P < 0.001; I2 = 82% |
| Study design | |||||
| Cross-sectional | 5 | 0.71 | 0.53, 0.95 | P = 0.02 | P < 0.001; I2 = 80% |
| Cohort | 4 | 0.91 | 0.74, 1.12 | P = 0.37 | P = 0.02; I2 = 65% |
| Diagnostic criteria of MetS | |||||
| NCEP ATP III | 6 | 0.69 | 0.54, 0.89 | P = 0.004 | P = 0.001; I2 = 76% |
| Other | 3 | 1.01 | 0.92, 1.10 | P = 0.85 | P = 0.25; I2 = 26% |
| Exposure assessment | |||||
| FFQ | 7 | 0.80 | 0.65, 0.97 | P = 0.03 | P < 0.001; I2 = 82% |
| Other | 2 | 0.76 | 0.30, 1.89 | P = 0.55 | P = 0.002; I2 = 90% |
| Study quality | |||||
| High-quality | 8 | 0.83 | 0.68, 1.00 | P = 0.05 | P < 0.001; I2 = 83% |
| Low-quality | 1 | 0.56 | 0.34, 0.92 | / | / |
| Adjustment of BMI | |||||
| Adjusted | 4 | 0.69 | 0.49, 0.98 | P = 0.04 | P = 0.01; I2 = 69% |
| Unadjusted | 5 | 0.88 | 0.70, 1.11 | P = 0.29 | P < 0.001; I2 = 88% |
| Adjustment of energy intake | |||||
| Adjusted | 6 | 0.75 | 0.59, 0.96 | P = 0.01 | P < 0.001; I2 = 79% |
| Unadjusted | 3 | 0.90 | 0.67, 1.20 | P = 0.45 | P = 0.03; I2 = 67% |
Association Between Refined Grain Consumption and MetS
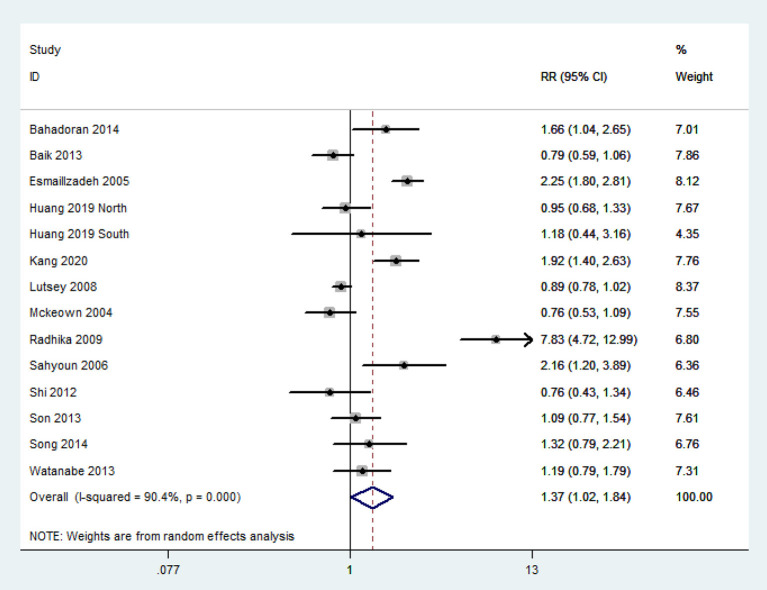
The overall multi-variable adjusted RR demonstrated that refined grain consumption was positively associated with MetS (RR = 1.37, 95%CI: 1.02–1.84 P = 0.036) (Figure 3). A substantial level of heterogeneity was observed among studies (P < 0.001, I2 = 90.4%). No evidence of publication bias was observed among the included studies according to the Begg rank-correlation test (P = 0.324). In addition, such findings were obtained only in cross-sectional (RR = 1.84, 95%CI: 1.03–3.28; P = 0.04), NCEP ATP III (RR = 1.84, 95%CI: 1.22–2.79; P = 0.004), high-quality studies (RR = 1.44 95%CI: 1.01–2.04; P = 0.04), adjustment of BMI (RR = 1.82, 95%CI: 1.10–3.02; P = 0.02) and energy intake (RR = 1.54, 95%CI: 1.04–2.28; P = 0.02) studies (Table 3).
Figure 3.
Forest plot of meta-analysis: Overall multi-variable adjusted RR of MetS for the highest vs. the lowest category of refined grain consumption.
Table 3.
Subgroup analyses of refined grain consumption and MetS.
| Stratification | Number of studies | Pooled RR | 95% CI | P-value | Heterogeneity |
|---|---|---|---|---|---|
| All studies | 13 | 1.37 | 1.02, 1.84 | P = 0.04 | P < 0.001; I2 = 90% |
| Study design | |||||
| Cross-sectional | 6 | 1.84 | 1.03, 3.28 | P = 0.04 | P < 0.001; I2 = 93% |
| Cohort | 7 | 1.10 | 0.86, 1.40 | P = 0.46 | P < 0.001; I2 = 75% |
| Diagnostic criteria of MetS | |||||
| NCEP ATP III | 8 | 1.84 | 1.22, 2.79 | P = 0.004 | P < 0.001; I2 = 89% |
| Other | 5 | 1.02 | 1.00, 1.04 | P = 0.12 | P = 0.22; I2 = 27% |
| Exposure assessment | |||||
| FFQ | 10 | 1.36 | 0.95, 1.93 | P = 0.09 | P < 0.001; I2 = 92% |
| Other | 3 | 1.31 | 1.01, 1.69 | P = 0.04 | P = 0.15; I2 = 48% |
| Study quality | |||||
| High-quality | 10 | 1.44 | 1.01, 2.04 | P = 0.04 | P < 0.001; I2 = 92% |
| Low-quality | 3 | 1.18 | 0.78, 1.76 | P = 0.43 | P = 0.12; I2 = 54% |
| Adjustment of BMI | |||||
| Adjusted | 6 | 1.82 | 1.10, 3.02 | P = 0.02 | P < 0.001; I2 = 89% |
| Unadjusted | 7 | 1.06 | 0.75, 1.51 | P = 0.74 | P < 0.001; I2 = 90% |
| Adjustment of energy intake | |||||
| Adjusted | 10 | 1.54 | 1.04, 2.28 | P = 0.03 | P < 0.001; I2 = 91% |
| Unadjusted | 3 | 0.92 | 0.82, 1.04 | P = 0.17 | P = 0.56; I2 = 0% |
Discussions
In this study, a total of 14 observational studies were identified. Our pooled analysis showed that whole grain consumption was negatively associated with MetS, whereas refined grain consumption was positively associated with MetS.
The opposite results with regard to the whole grain and refined grain consumption could be explained by several biological mechanisms. First, the glycemic index (GI) and the glycemic load (GL), which are both determined by the amount of carbohydrates consumed, contribute to the glycemic response directly (35). It was reported that GIs and GLs were associated with a higher risk of MetS, which was independent of diabetes mellitus (36). Compared with refined grain, whole grain tends to be absorbed slowly with a relatively low GI. On the contrary, refined grain is abundant in carbohydrate content, which leads to a higher dietary GL (37). Second, whole grain is rich in dietary fiber, trace minerals, and phytochemicals (37). These nutrients and food components were considered to be beneficial for MetS (38, 39). However, the nutrient components of refined grain were lost during the refining process (22).
Our results were supported by several randomized control trials directly. Jackson et al. (14) showed that the replacing refined grain by whole grain in weight-loss diet could reduce glucose directly. Moreover, Giacco et al. (15) further indicated that a 12-weeks of whole grain intervention could reduce postprandial insulin and triglycerides responses in MetS (also compared to refined grain) (15). On the other hand, some fundamental experimental study should also be emphasized. Both Hao et al. (16) and Yen et al. (17) demonstrated that pre-germinated brown rice extract could ameliorate high-fat diet-induced MetS model. Above all, the results of our study were partly supported by the corresponding clinical and experimental evidence.
Generally speaking, whole grain referred to barley, multigrain and ground mixed grain, whereas refined grain included white rice, noodles and bread. Interestingly, several studies have considered the grain consumption as a whole (without subtype specification). Unsurprisingly, no significant relationship was obtained in these studies (40–43). It could be attributed to the synergistic effect of whole grain and refined grain consumption on MetS. Of note, our result was only confirmed in cross-sectional studies both for whole grain and refined grain. Although the reliability of our results may be influenced by the substantial level of heterogeneity, the potential different effect of grain consumption on the prevalence or risk of MetS could not be ignored, it was still an open question for MetS prevention. Moreover, the inconsistent result with regard to diagnostic criteria of MetS, exposure assessment and study quality (for refined grain) was also acquired. FFQ seems to be more reliable and precise for dietary assessment, and the NCEP ATP III criteria may be suitable for considering the effect of grain consumption. In addition, the quality of study may also influence the results. High consumption of whole-grain foods is associated with lower BMI in a dose-dependent manner (44), and BMI is considered as an important factor in MetS (1). Moreover, grain consumption is closely associated with appetite and energy intake (45), and a low reported energy intake is also reported to be associated with MetS (46). Indeed, the inconsistent result with regard to the adjustment of BMI and energy intake was obtained in our study. Therefore, further studies are required to consider BMI and energy intake as confounding factors. It should also be noted that the heterogeneity of our study cannot be ignored, especially for the exposure assessment. A semi-quantitative FFQ was utilized in most studies (18, 19, 21–24, 26, 27, 29–31), whereas several studies used recall record (20, 25, 28). On the other hand, the definition of whole grain or refined grain may vary greatly among individuals. For example, refined grain always refers to rice, noodles or bread etc, but several studies only considered rice/white rice (23, 25–27). These inconsistent exposure assessments could cause significant heterogeneity and inaccuracies in the interpretation of the results. Of note, the plasma level of alkylresorcinols, which was served as a reliable marker of whole grain consumption (14), was unfortunately ignored in all the included studies. Therefore, more well-designed prospective cohort studies are still needed.
Our study has several strengths. First, this is the first meta-analysis of observational study aiming at the associations of whole grain and refined grain consumption with MetS. Second, the included studies were analyzed based on adjusted results and large samples. Third, our result might be helpful to better consider the diet effect on MetS. We should also acknowledge the limitations of the present study. First, the substantial level of heterogeneity might have distorted the results. Second, due to the limitation of relevant literature, only a limited number of observational studies were identified for this meta-analysis. Third, the classification of exposure may vary greatly among individuals. Fourth, the diagnostic criteria of MetS and the selection of adjusted factors were not uniform. Last but not the least, a subgroup for gender could not be performed since very limited study specified the effect estimates by gender. These limitations might weaken the significance of this study.
Conclusions
The existing evidence suggests that whole grain consumption is negatively associated with MetS, whereas refined grain consumption is positively associated with MetS. More well-designed prospective cohort studies are needed to elaborate the concerned issues further.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
YZ conceived the idea and performed the statistical analysis. YZ, HG, and JD drafted this meta-analysis. HG and JD selected retrieved relevant papers. HG and JL assessed each study. YZ was the guarantor of the overall content. All authors revised and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the Young Investigator Grant of Xiangya Hospital, Central South University (2020Q14).
References
- 1.Alberti K, Eckel R, Grundy S, Zimmet P, Cleeman J, Donato K, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. (2009) 120:1640–5. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 2.Athyros V, Ganotakis E, Elisaf M, Mikhailidis D. The prevalence of the metabolic syndrome using the National Cholesterol Educational Program and International Diabetes Federation definitions. Curr Med Res Opin. (2005) 21:1157–9. 10.1185/030079905X53333 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Zhang D. Associations of vegetable and fruit consumption with metabolic syndrome. A meta-analysis of observational studies. Public Health Nutr. (2018) 21:1693–703. 10.1017/S1368980018000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhang D. Relationship between nut consumption and metabolic syndrome: a meta-analysis of observational studies. J Am Coll Nutr. (2019) 38:499–505. 10.1080/07315724.2018.1561341 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Zhang D. Relationship between serum zinc level and metabolic syndrome: a meta-analysis of observational studies. J Am Coll Nutr. (2018) 37:708–15. 10.1080/07315724.2018.1463876 [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, Yang J, Du L, Li K, Zhou Y. Association of whole grain, refined grain, and cereal consumption with gastric cancer risk: a meta- analysis of observational studies. Food Sci Nutr. (2018) 7:256–65. 10.1002/fsn3.878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slavin J. Whole grains and human health. Nutr Res Rev. (2004) 17:99–110. 10.1079/NRR200374 [DOI] [PubMed] [Google Scholar]
- 8.Kashino I, Eguchi M, Miki T, Kochi T, Nanri A, Kabe I, et al. Prospective association between whole grain consumption and hypertension: the Furukawa Nutrition and Health Study. Nutrients. (2020) 12:902. 10.3390/nu12040902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazidi M, Katsiki N, Mikhailidis D, Banach M. A higher ratio of refined grain to whole grain is associated with a greater likelihood of chronic kidney disease: a population-based study. Br J Nutr. (2019) 121:1294–302. 10.1017/S0007114518003124 [DOI] [PubMed] [Google Scholar]
- 10.Steffen L, Jacobs D, Stevens J, Shahar E, Carithers T, Folsom A. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. (2003) 78:383–90. 10.1093/ajcn/78.3.383 [DOI] [PubMed] [Google Scholar]
- 11.Schwingshackl L, Schwedhelm C, Hoffmann G, Knüppel S, Iqbal K, Andriolo V, et al. Food groups and risk of hypertension: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. (2017) 8:793–803. 10.3945/an.117.017178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aune D, Norat T, Romundstad P, Vatten L. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol. (2013) 28:845–58. 10.1007/s10654-013-9852-5 [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Manson J, Stampfer M, Hu F, Giovannucci E, Colditz G, et al. A prospective study of whole-grain intake and risk of type 2 diabetes mellitus in US women. Am J Public Health. (2000) 90:1409–15. 10.2105/AJPH.90.9.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson K, West S, Heuvel J, Jonnalagadda S, Ross A, Hill A, et al. Effects of whole and refined grains in a weight-loss diet on markers of metabolic syndrome in individuals with increased waist circumference: a randomized controlled-feeding trial. Am J Clin Nutr. (2014) 100:577–86. 10.3945/ajcn.113.078048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giacco R, Costabile G, Pepa G, Anniballi G, Griffo E, Mangione A, et al. A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutr Metab Cardiovasc Dis. (2014) 24:837–44. 10.1016/j.numecd.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 16.Hao C, Lin H, Ke L, Yen H, Shen K. Pre-germinated brown rice extract ameliorates high-fat diet-induced metabolic syndrome. J Food Biochem. (2019) 43:e12769. 10.1111/jfbc.12769 [DOI] [PubMed] [Google Scholar]
- 17.Yen H, Lin H, Hao C, Chen F, Chen C, Chen J, et al. Effects of pre-germinated brown rice treatment high-fat diet-induced metabolic syndrome in C57BL/6J mice. Biosci Biotechnol Biochem. (2017) 81:979–86. 10.1080/09168451.2017.1279848 [DOI] [PubMed] [Google Scholar]
- 18.McKeown N, Meigs J, Liu S, Saltzman E, Wilson P, Jacques P. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the framingham offspring cohort. Diabetes Care. (2004) 27:538–46. 10.2337/diacare.27.2.538 [DOI] [PubMed] [Google Scholar]
- 19.Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain consumption and the metabolic syndrome: a favorable association in Tehranian adults. Eur J Clin Nutr. (2005) 59:353–62. 10.1038/sj.ejcn.1602080 [DOI] [PubMed] [Google Scholar]
- 20.Sahyoun N, Jacques P, Zhang X, Juan W, McKeown N. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am J Clin Nutr. (2006) 83:124–31. 10.1093/ajcn/83.1.124 [DOI] [PubMed] [Google Scholar]
- 21.Lutsey P, Steffen L, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. (2008) 117:754–61. 10.1111/j.0030-1299.2008.16455.x [DOI] [PubMed] [Google Scholar]
- 22.Radhika G, Dam R, Sudha V, Ganesan A, Mohan V. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57). Metabolism. (2009) 58:675–81. 10.1016/j.metabol.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 23.Shi Z, Taylor A, Hu G, Gill T, Wittert G. Rice intake, weight change and risk of the metabolic syndrome development among Chinese adults: the Jiangsu Nutrition Study (JIN). Asia Pac J Clin Nutr. (2012) 21:35–43. [PubMed] [Google Scholar]
- 24.Baik I, Lee M, Jun N, Lee J, Shin C. A healthy dietary pattern consisting of a variety of food choices is inversely associated with the development of metabolic syndrome. Nutr Res Pract. (2013) 7:233–41. 10.4162/nrp.2013.7.3.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son S, Lee H, Park K, Ha T, Seo J. Nutritional evaluation and its relation to the risk of metabolic syndrome according to the consumption of cooked rice and cooked rice with multi-grains in Korean adults: based on 2007-2008 Korean National Health and Nutrition Examination Survey. Korean J Commun Nutr. (2013) 18:77–87. 10.5720/kjcn.2013.18.1.77 [DOI] [Google Scholar]
- 26.Watanabe Y, Saito I, Asada Y, Kishida T, Matsuo T, Yamaizumi M, et al. Daily rice intake strongly influences the incidence of metabolic syndrome in japanese men aged 40–59 years. J Rural Med. (2013) 8:161–70. 10.2185/jrm.8.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahadoran Z, Mirmiran P, Delshad H, Azizi F. White rice consumption is a risk factor for metabolic syndrome in Tehrani adults: a prospective approach in Tehran lipid and glucose study. Arch Iran Med. (2014) 17:435–40. [PubMed] [Google Scholar]
- 28.Song S, Lee J, Song W, Paik H, Song Y. Carbohydrate intake and refined-grain consumption are associated with metabolic syndrome in the Korean adult population. J Acad Nutr Diet. (2014) 114:54–62. 10.1016/j.jand.2013.08.025 [DOI] [PubMed] [Google Scholar]
- 29.Dussaillant C, Echeverría G, Villarroel L, Marin P, Rigotti A. Uuhealthy food intake is linked to higher prevalence of metabolic syndrome in children adult population: cross-sectional study in 2009-2010 national health survey. Nutr Hosp. (2015) 32:2098–104. 10.3305/nh.2015.32.5.9657 [DOI] [PubMed] [Google Scholar]
- 30.Huang L, Wang H, Wang Z, Zhang J, Zhang B, Ding G. Regional disparities in the association between cereal consumption and metabolic syndrome: results from the china health and nutrition survey. Nutrients. (2019) 11:764. 10.3390/nu11040764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang Y, Lee K, Lee J, Kim J. Grain subtype and the combination of grains consumed are associated with the risk of metabolic syndrome: analysis of a community-based prospective cohort. J Nutr. (2020) 150:118–27. 10.1093/jn/nxz179 [DOI] [PubMed] [Google Scholar]
- 32.Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuhara H, Steinmaus C, Cohen S, Corley D, Tei Y, Buffler P. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. (2011) 106:1911–22. 10.1038/ajg.2011.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 35.Augustin L, Kendall C, Jenkins D, Willett W, Astrup A, Barclay A, et al. Glycemic index, glycemic load and glycemic response: an International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis. (2015) 25:795–815. 10.1016/j.numecd.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 36.Juanola-Falgarona M, Salas-Salvadó J, Buil-Cosiales P, Corella D, Estruch R, Ros E, et al. Dietary glycemic index and glycemic load are positively associated with risk of developing metabolic syndrome in middle-aged and elderly adults. J Am Geriatr Soc. (2015) 63:1991–2000. 10.1111/jgs.13668 [DOI] [PubMed] [Google Scholar]
- 37.Slavin J, Martini M, Jacobs D, Marquart L. Plausible mechanisms for the protectiveness of whole grains. Plausible mechanisms for the protectiveness of whole grains. Am J Clin Nutr. (1999) 70:459S−63S. 10.1093/ajcn/70.3.459s [DOI] [PubMed] [Google Scholar]
- 38.Grooms K, Ommerborn M, Pham D, Djoussé L, Clark C. Dietary fiber intake and cardiometabolic risks among US adults, NHANES 1999-2010. Am J Med. (2013) 126:1059–67.e1-4. 10.1016/j.amjmed.2013.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ju S, Choi W, Ock S, Kim C, Kim D. Dietary magnesium intake and metabolic syndrome in the adult population: dose-response meta-analysis and meta-regression. Nutrients. (2014) 6:6005–19. 10.3390/nu6126005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruidavets J, Bongard V, Dallongeville J, Arveiler D, Ducimetière P, Perret B, et al. High consumptions of grain, fish, dairy products and combinations of these are associated with a low prevalence of metabolic syndrome. J Epidemiol Commun Health. (2007) 61:810–7. 10.1136/jech.2006.052126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung H, Han S, Song S, Paik H, Baik H, Joung H. Association between adherence to the Korean Food Guidance System and the risk of metabolic abnormalities in Koreans. Nutr Res Pract. (2011) 5:560–8. 10.4162/nrp.2011.5.6.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliveira E, McLellan K, Silveira L, Burini R. Dietary factors associated with metabolic syndrome in Brazilian adults. Nutr J. (2012) 11:13. 10.1186/1475-2891-11-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasdar Y, Moradi S, Moludi J, Darbandi M, Niazi P, Nachvak S, et al. Risk of metabolic syndrome in non-alcoholic fatty liver disease patients. Mediterr J Nutr Metab. (2019) 12:353–63. 10.3233/MNM-190290 [DOI] [Google Scholar]
- 44.McKeown N, Yoshida M, Shea M, Jacques P, Lichtenstein A, Rogers G, et al. Whole-grain intake and cereal fiber are associated with lower abdominal adiposity in older adults. J Nutr. (2009) 139:1950–5. 10.3945/jn.108.103762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanders L, Zhu Y, Wilcox M, Koecher K, Maki K. Effects of whole grain intake, compared with refined grain, on appetite and energy intake: a systematic review and meta-analysis. Adv Nutr. (2021) nmaa178. 10.1093/advances/nmaa178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buscemi S, Verga S, Donatelli M, D'Orio L, Mattina A, Tranchina M, et al. A low reported energy intake is associated with metabolic syndrome. J Endocrinol Invest. (2009) 32:538–41. 10.1007/BF03346503 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.