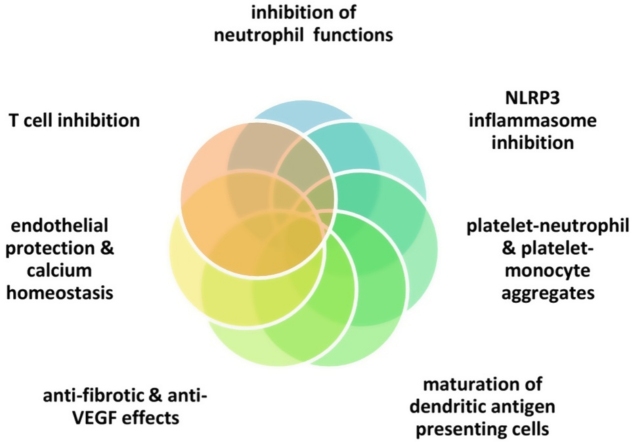
Graphical abstract
Abbreviations: ACS, acute coronary syndrome; CAD, coronary artery disease; COVID-19, coronavirus disease - 19; eGFR, estimated glomerular filtration rate; DOACs, direct oral anticoagulants; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; ROCK, Rho-associated protein kinase; Lp-PLA2, Lipoprotein-associated phospholipase A2
Keywords: Colchicine, History, Pharmacokinetics, Inflammation, Safety, Repurposing
Highlights
-
•
Colchicine is among the oldest drug molecules in medical history.
-
•
Clinicians should always check for drug to drug interactions and modify colchicine’s dose according to published guidelines.
-
•
Beyond known indications (e.g. pericarditis, gout), current evidence implies favorable colchicine effects in CAD, stroke and even in COVID19.
Abstract
Colchicine’s medical evolution is historically bound to the Mediterranean basin, since remarkable researchers from this region underscored its valuable properties. With the passing of years colchicine became an essential pharmaceutical substance for the treatment of rheumatologic and cardiovascular diseases. In light of recent findings, the therapeutic value of colchicine has grown. In clinical practice, colchicine remains underutilized in view of its proven efficacy and safety. Its complex pharmacokinetics and multifaceted anti-inflammatory role remain under investigation. The current review addresses the safe administration of colchicine in view of key drug to drug interactions. Finally, we are briefly presenting colchicine’s future potential applications.
1. Introduction
Since its discovery, colchicine has proven to be a potent anti-inflammatory agent [1]. Although current evidence have established colchicine as a useful therapeutic medication in many cardiovascular and non-cardiovascular disorders with a major role in auto-inflammatory disease [2,3], misconceptions about its side effects and safety need to clarified [4,5]. In this article, we are presenting the main historical and pharmacological aspects regarding colchicine focusing on drug-to-drug interactions and drug safety. We are also aiming to illuminate the drug repurposing potential of colchicine [6].
1.1. Historical aspects
Colchicine is extracted from the plant Autumn Crocus (Colchicum autumnale). According to German archeologist Ebers and the medical papyrus named after him, the first clinical application of colchicine dates back to 16th century BC, when Egyptians used the herb for the treatment of swelling [7]. Ancient Greeks used colchicine as a purgative more than 2000 years ago [8] and the eminent Greek philosopher and botanist Theophrastus also described the therapeutic potential of colchicine [9]. Another distinguished Greek botanist Pedanius Dioscorides (40–90 AD), who served for Roman Emperor Nero, published a thorough pharmacology textbook describing among others the role of colchicine in gout [10,11]. During Byzantine times, Alexander of Tralles selectively chose colchicine for the treatment of gout in 6th century AD, but the renowned English physician Thomas Sydenham undermined colchicine’s potential since he deemed all drugs with gastrointestinal side effects as too toxic for clinical application [12].
In 1763, the Austrian physician Anton von Störck, who would later treat Empress Maria Theresa for smallpox, published his work about Colchicum autumnale root and suggested that it is both safe and effective in managing many incurable maladies [12]. 19th century scientists established some modern era knowledge about colchicine and introduced the opportunity for future research [13]. In 1833, German pharmacist and chemist Philipp Lorenz Geiger, based on previous work from French scientists Pierre-Joseph Pelletier and Joseph Bienaimé Caventou, analyzed the molecule of colchicine and coined its current name [13]. Half a century later in 1884, French Alfred Houde was the first pharmacist to produce a pure crystallized form for clinical usage [13].
It took another century for colchicine to enter the cardiovascular pharmacopeia, since in 1987 Rodriguez de la Serna A and a team of Spanish researchers reported the benefits of colchicine in managing recurrent pericarditis [8]. Noteworthy, Katsilabros investigated the inhibitory effects of colchicine in a variety of viral disease (herpes zoster virus, mumps, echovirus and coxsackievirus among others) in 1955 [14]. From antiquity to nowadays, researchers and physicians have investigated colchicine’s pleiotropic effects and ultimately broaden its therapeutic indications, while new data are constantly coming to light. It is currently known that gout, pericarditis, familial Mediterranean fever, Adamantiades-Behcet’s disease and post-operative pericarditis syndrome prevention can be effectively treated with colchicine [2,[15], [16], [17]].
1.2. Colchicine pharmacology
Colchicine is a lipophilic alkaloid derived from the plant Colchicum autumnale and absorbed by the small intestine (jejunum and ileum). Within one hour of administration colchicine achieves significant serum levels, while the immunomodulatory action may take up to two days [18]. Bioavailability is approximately 50 %, with 40 % of colchicine bound to albumin [19]. The drug has a hepatic metabolism, 80 % of its excretion occurs via the gastrointestinal (GI) tract, and the estimated half-life ranges from 26 to 31 h [18]. To avoid side effects and minimize drug-to-drug interactions, clinicians should carefully examine co-administration of medications that are substrates of CYP3A4 enzyme and P-glycoprotein transporter. Colchicine is neither an inhibitor nor an inducer of these two enzymatic systems. Pharmaceutical agents affecting those two important metabolic pathways can alter the plasma concentration of colchicine and induce toxic effects [17]. The P-glycoprotein efflux system is involved both in renal and hepatobiliary clearance of colchicine. Concurrent use of CYP3A4 inhibitors can result in a two-fold increase of colchicine levels while administration of drugs that moderately or severely inhibit the P-glycoprotein system can quadruple colchicine levels [17]. GI side effects (namely diarrhea, nausea and vomiting) are commonly encountered when colchicine is used at high dosages, but most cases can be adequately managed since it is a dose-related phenomenon. Transient myelosuppression and myopathy have rarely been described with high-dose colchicine [20].
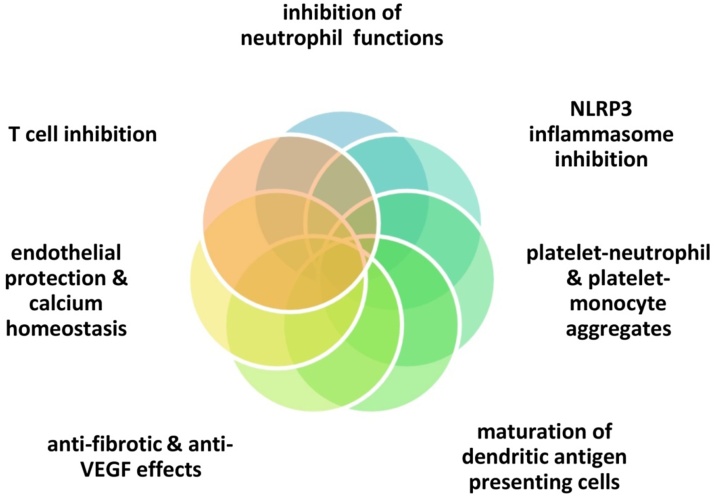
Colchicine is only known to bind to tubulin, which appears to lead to multiple pleiotropic effects [7,18,[20], [21], [22]] (Fig. 1): 1) inhibition of the NLRP3 inflammasome in neutrophils and macrophages, with reduced secretion of IL-1 β, 2) inhibition of neutrophil chemotaxis, extravasation and degranulation, 3) inhibition of platelet-neutrophil and platelet-monocyte aggregates (notably, platelet to platelet interactions are spared and normal coagulation mechanisms remain intact) 4) maturation of dendritic antigen presenting cell forms 5) induction of Bcl-2 gene expression and down-regulation of the ROCK pathway as well as transforming growth factor-β1 with anti-fibrotic effects 6) reduction of Lp-PLA2 activity and increase in nitric oxide levels, 7) T cell inhibition via downregulation of L-selectin [23], 8) inhibition of vascular endothelial growth factor [24,25], and 9) regulation of calcium homeostasis in cardiomyocytes [26]. Regarding COVID-19 infection, clathrin-mediated endocytocis (at least partly regulated via tubulin polymerization) might be invovled in colchicine’s potentially favorable effects [27]
Fig. 1.
Colchicine exerts many pleiotropic actions which affect critical stages of inflammatory reaction (innate, adaptive immunity, angiogenesis, fibrosis) as well as cardiomycte and endothelial function.
1.3. Colchicine and drug-to-drug interactions
1.3.1. Co-administration with statins or fibrates
A retrospective study carried out in South Korea suggested that co-administration of statins and colchicine in 674 patients suffering from both dyslipidemia and gout is safe, with no apparent increase in the incidence of myopathy. Indeed, statins and colchicine do not exhibit synergistic deleterious effects on myopathy [28]. In contrast, the investigators found that chronic kidney disease (estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2), liver cirrhosis, high colchicine dose (≥1.2 mg daily) and CYP3A4 inhibitors (diltiazem, cyclosporine, verapamil and amiodarone) could increase the risk of myopathy. Additionally, two large RCTs of colchicine having followed a total of more than 10,000 patients with coronary artery disease for more than 2 years clearly support the safety of the concomitant use of statins and colchicine. Specifically, the LoDoCo-2 and COLCOT trials reported no differences in the incidence of myopathy and rhabdomyolysis between statin-treated patients receiving colchicine or placebo [29,30].
Statins demonstrate varying P-glycoprotein and CYP3A4 effects, hence specific recommendations have been reported concerning colchicine co-administration. Rosuvastatin, pitavastatin, fluvastatin and pravastatin show minimal to no interaction with the above-mentioned enzymes and can be safely prescribed with colchicine [31]. Although atorvastatin, simvastatin and lovastatin appear to affect the activity of these enzymes, chronic co-administration with colchicine was safe in LoDoCo-2 and COLCOT [31,32]. A modified dosing scheme has been suggested for those statins only, with a loading dose not exceeding 1.2 mg and a daily maintenance scheme not exceeding 0.6 mg qd [31].
Among fibric acid derivatives, fenofibrate is known to inhibit P-glycoprotein as potently as simvastatin does, thus the risk-benefit balance should be carefully evaluated when patients are also treated with colchicine [33,34].
1.3.2. Other cardiovascular medications of interest
Although a soft recommendation was made for the avoidance of ticagrelor in patients on colchicine, 98 % of patients received 2 anti-platelet agents (including ticagrelor) in the colchicine arm of COLCOT and no safety issue was observed [17]. Verapamil and diltiazem also constitute inhibitors of both P-glycoprotein and CYP3A4, and a reduction in the dose of colchicine should be considered in patients receiving moderate to high dosages of these non-dihydropyridine calcium channel blockers [17]. If the use of an agent from this class is of importance, the dihydropyridine agent amlodipine could be safely used because it shows only weak inhibitory effect on CYP3A4 [17]. Although the beta-blockers propranolol, carvedilol and bisoprolol exhibit P-glycoprotein inhibitory effects [32], no safety issue was observed with the concomitant use of colchicine in COLCOT. Renin-angiotensin-aldosterone system inhibitors (RAASi) seem to be deprived of P-glycoprotein effects, apart from telmisartan [17,35,36]. Regarding the coadministration of colchicine with anti-arrhythmic drugs (AADs), amiodarone and dronaderone inhibit the P-glycoprotein efflux system [37]. In contrast, the class III β-blocking AAD sotalol can be safely administered with colchicine [17,32]. Finally, DOACs (direct oral anticoagulants) have no clinically important effect on CYP3A4 and P-glycoprotein activity, while warfarin is considered to be a P-glycoprotein inhibitor warranting colchicine dose reduction if high-dose colchicine is used [17,32].
Current evidence suggests that patients treated with P-glycoprotein and or CYP3A4 inhibitors should have their colchicine regimen modified to 0.6 mg qd. In the presence of severe liver or renal disease, co-administration of colchicine and enzyme inhibitors should be avoided [17]. Drug-drug interaction studies concluded that the colchicine dosage for acute gout episode or prophylaxis should be reduced to 1/3 or 1/4 of the recommended posology when administered with strong P-glycoprotein or CYP3A4 inhibitors. Furthermore, patients on moderate to strong CYP3A4 inhibitors (diltiazem, verapamil, clarithromycin, ketoconazole, ritonavir) should be given a reduced colchicine regimen by 1/3 to 1/2 for acute flares or gout prophylaxis, respectively [38].
In terms of Human Immunodeficiency Virus patients taking protease inhibitors, the United States Food and Drug Administration endorsed revised guidelines regarding colchicine co-administration with CYP3A4 or P-glycoprotein inhibitors [39]. Specifically, concurrent prescription of colchicine and protease inhibitors is contraindicated in patient suffering from renal or hepatic impairment. If colchicine is combined with potent CYP3A4 inhibitors, the gout flare prophylaxis regimen should be modified to 0.3 mg qd or every other day instead of 0.6 mg bid or qd. Furthermore, colchicine prescription for acute gout flares should be done with a dose reduction of 0.6 mg, followed by 0.3 mg one hour after, as opposed to the classic scheme of a 1.2 mg starting dose and an additional 0.6 mg. Potent CYP3A4 inhibitors include protease inhibitors (atazanavir, indinavir, nelfinavir, saquinavir, and ritonavir), antifungals (ketoconazole, itraconazole) and antibacterial (telithromycin, clarithromycin). Moderate CYP3A4 inhibitors should also dictate dose reductions of colchicine to 0.3 mg qd or bid when given for gout prophylaxis; for acute gout flares, a single dose of 1.2 mg of colchicine should be administered with at least three days washout before repetition. Moderate CYP3A4 inhibitors include some protease inhibitors (amprenavir and fosamprenavir), antiemetic aprepitant, the calcium channel blockers diltiazem and verapamil, as well as the antibiotics erythromycin and clarithromycin and the anti-yeast agent fluconazole. Physicians should keep in mind that grapefruit is a moderate inhibitor of CYP3A4 and the combination of two moderate inhibitors is equivalent to utilizing one strong CYP3A4 inhibitor. Finally, P-glycoprotein inhibitors (such as ranolazine and cyclosporine) should dictate colchicine dose adjustments analogous to strong CYP3A4 inhibitors [39].
Pericarditis management guidelines recommend dose adjustment according to body weight and specifically a dose reduction to 0.6 mg qd if weight <70 kg [17]. Additionally, severe liver dysfunction and severe renal dysfunction (eGFR below 10 mL/min/1.73m2) represent contraindications for colchicine utilization [17]. Table 1 summarizes the relevant pharmacokinetic interactions discussed here.
Table 1.
Clinically relevant drug to drug interaction concerning colchicine.
| Medication | Drug Class | Type of interaction with colchicine | Alternatives |
|---|---|---|---|
| Verapamil | Calcium Channel Blockers | P-gp & CYP3A4 inhibition | Amlodipine |
| Diltiazem | |||
| Telmisartan | ACE inhibitor | P-gp inhibition | Other RAAS inhibitors |
| Amiodarone | Class III AADs | P-gp inhibition | Sotalol |
| Dronaderone | |||
| Warfarin | Anticoagulant | P-gp inhibition | DOACs |
| Atazanavir | Protease Inhibitors | CYP3A4 inhibition | Colchicine dose adjustment |
| Indinavir | |||
| Nelfinavir | |||
| Saquinavir | |||
| Ritonavir | |||
| Amprenavir | |||
| Fosamprenavir | |||
| Itraconazole | Antifungal | CYP3A4 inhibition | Colchicine dose adjustment |
| Ketoconazole | |||
| Fluconazole | |||
| Clarithromycin | Macrolides | CYP3A4 inhibition | Azithromycin |
| Erythromycin | |||
| Ranolazine | Antianginal | P-gp inhibition | Colchicine dose adjustment |
| Cyclosporine | Immunosuppressant | P-gp inhibition | Colchicine dose adjustment |
| Aprepitant | Antiemetic | CYP3A4 inhibition | Colchicine dose adjustment |
| P-gp: P-glycoprotein | |||
1.4. Emerging clinical data on the efficacy and safety of colchicine
1.4.1. Prevention of atherothrombotic events in patients with coronary artery disease (CAD)
In a pilot study from our research group, colchicine seemed to reduce infarct size in STEMI patients, demonstrated by CK-MB levels and cardiovascular magnetic resonance imaging [40]. The large LoDoCo-2 [30] and COLCOT [29] trials have established the cardioprotective effects of colchicine in patients with stable CAD and after myocardial infarction, respectively. LoDoCo-2 showed that low-dose colchicine (0.5 mg once daily) reduces adverse cardiovascular events by 31 %, which was primarily driven by a reduction in myocardial infarction. COLCOT investigators demonstrated that low-dose colchicine, initiated within 30 days after myocardial infarction, decreases the risk of first and total ischemic cardiovascular events by 23 % and 34 %, respectively. Interestingly, in a post-hoc analysis of COLCOT, investigators found that early initiation of colchicine before hospital discharge (within 3 days) following myocardial infarction resulted in even greater benefits [41,42].
A recent meta-analysis of studies in patients with coronary artery disease showed that low-dose colchicine reduces the incidence of myocardial infarction bv 38 %, that of stroke by 62 % and of urgent coronary revascularization by 44 % compared to placebo [43]. A similar MI risk reduction (RR 0.77; 95 % CI 0.64−0.94) was also reported by Xiang et al. (11 RCTs included) [44]. Interestingly a meta-analysis by Tien et al. found that colchicine administration after percutaneous coronary intervention reduces the risk of restenosis (OR 0.46, 95 % CI 0.23−0.92) [45]. In the bare metal stent era, colchicine seemed to prevent restenosis in patients with diabetes [46].
1.4.2. Stroke
Three meta-analyses demonstrated the benefit of colchicine on stroke reduction. Specifically, Masson et al. reported a greatly reduced odds ratio (OR 0.33; 95 % CI, 0.15−0.70) after analyzing data from six studies [47]. Another meta-analysis conducted in 2020 by Katsanos et al. (four trials reporting on stroke incidence included) also supports stroke risk reduction via colchicine (RR 0.31, 95 % CI 0.13−0.71) [48]. Interestingly, our group has shown that colchicine can potentially be effectively in reducing atrial fibrillation recurrences after pulmonary vein isolation, hence favoring a lower arrhythmic burden [49,50].
1.4.3. COVID-19
Colchicine is being vigorously investigated in the context of the COVID-19 pandemic. Colchicine has been proposed as a potential means of alleviating the cytokine storm and hence preventing COVID-19 complications [51,52]. GRECCO-19 [15] was the first published open-label, randomized controlled study in this field [53]. Deftereos et al. observed improved time to clinical deterioration and a lower level of d-Dimer in hospitalized COVID-19 patients treated with colchicine [53]. These markers of better outcome were consistent in a series of studies in hospitalized patients which were meta-analyzed and showed a mortality risk reduction in COVID-19 patients treated with colchicine (OR 0.35; 95 % CI 0.24−0.52) [54].
Recently, the COLCORONA double-blind, randomized controlled trial included 4488 non-hospitalized patients with COVID-19 [55]. The primary endpoint (death or hospitalization due to COVID-19), while numerically lower in favor of colchicine did not reach statistical significance (p = 0.08) for the whole population. However, in a prespecified analysis of patients with PCR-proven diagnosis (4159 patients), colchicine significantly reduced the occurrence of the primary endpoint (4.6 % vs. 6.0 %, respectively; p = 0.04). It should be noted that this was primarily driven by hospitalizations rather than deaths. A series of studies in the field are ongoing and their results are awaited.
1.4.4. Side effects
As already stated, colchicine is efficacious for the treatment of several cardiovascular and non-cardiovascular diseases. Nevertheless, some clinicians still limit its use, mainly in the fear of side effects. In 2018, Indraranta et al. reviewed the safety of colchicine administration during pregnancy and found no statistical significant increase in fetal malformations or miscarriage [56]. Stewart et al. published a meta-analysis of 8659 participants (35 RCTs) and concluded that apart from increased diarrhea (RR 2.4, 95 % CI 1.6–3.7) colchicine does not increase the incidence of other adverse events (hepatologic, neuromuscular, infectious or hematologic side effects) or mortality [57].
Recently, Andreis et al. reported that colchicine increases the risk for both diarrhea (RR 2.77, 95 % CI 1.55–4.94, P < 0.001) and myalgia (RR 1.16, 95 % CI 1.02–1.32, P = 0.03). Drug discontinuation was 4.8 % in colchicine versus 3.4 % in placebo (RR 1.54, 95 % CI 1.20–1.99, P < 0.001). Interestingly, these investigators also found that lower doses and longer treatment duration were associated with fewer GI side effects [58].
The aforementioned data in combination with the results deriving from the large randomized controlled trials in CAD (in which colchicine was co-administered with statins), indicate that colchicine is a safe drug. Furthermore, the vast majority of adverse events are restricted to gastrointestinal intolerance or diarrhea, which are reversible with drug discontinuation.
2. Conclusion
Colchicine exhibits multiple pleiotropic anti-inflammatory effects and seems to affect positively both the early and late stages of inflammation, which is integral in the pathophysiology of numerous disorders. Pharmacokinetic considerations and specifically interactions with potent P-glycoprotein and CYP3A4 inhibitors should not hinder clinicians from reaping colchicine’s benefits.
Declaration of Competing Interest
The authors report no declarations of interest.
Handling Editor: Dr. Aristidis Tsatsakis
References
- 1.Tong D.C., Wilson A.M., Layland J. Colchicine in cardiovascular disease: an ancient drug with modern tricks. Heart. 2016;102:995–1002. doi: 10.1136/heartjnl-2015-309211. [DOI] [PubMed] [Google Scholar]
- 2.Vrachatis D.A., Giannopoulos G., Deftereos S.G. Editorial: colchicine: conventional and contemporary indications. Curr. Pharm. Des. 2018;24 doi: 10.2174/138161282406180510170818. 647–647. [DOI] [PubMed] [Google Scholar]
- 3.Liantinioti G., Argyris A.A., Protogerou A.D., Vlachoyiannopoulos P. The role of colchicine in the treatment of autoinflammatory diseases. Curr. Pharm. Des. 2018;24:690–694. doi: 10.2174/1381612824666180116095658. [DOI] [PubMed] [Google Scholar]
- 4.Roubille F., Kritikou E., Busseuil D., Barrere-Lemaire S., Tardif J.-C. Colchicine: an old wine in a new bottle? Antiinflamm. Antiallergy Agents Med. Chem. 2013;12:14–23. doi: 10.2174/1871523011312010004. [DOI] [PubMed] [Google Scholar]
- 5.Tsatsakis A.M., Vassilopoulou L., Kovatsi L., Tsitsimpikou C., Karamanou M., Leon G. The dose response principle from philosophy to modern toxicology: the impact of ancient philosophy and medicine in modern toxicology science. Toxicol. Rep. 2018;5:1107–1113. doi: 10.1016/j.toxrep.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misra D.P., Gasparyan A.Y., Zimba O. Benefits and adverse effects of hydroxychloroquine, methotrexate and colchicine: searching for repurposable drug candidates. Rheumatol. Int. 2020;40:1741–1751. doi: 10.1007/s00296-020-04694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slobodnick A., Shah B., Krasnokutsky S., Pillinger M.H. Update on colchicine, 2017. Rheumatology (Oxford) 2018;57:i4–11. doi: 10.1093/rheumatology/kex453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayes-Genis A., Adler Y., De Luna A.B., Imazio M. Colchicine in pericarditis. Eur. Heart J. 2017;38:1706–1709. doi: 10.1093/eurheartj/ehx246. [DOI] [PubMed] [Google Scholar]
- 9.Tsoucalas G., Papaioannou T., Panayiotakopoulos G., Saridaki Z., Vrachatis D.A., Karamanou M. Colchicum genus in the writings of ancient Greek and Byzantine physicians. Curr. Pharm. Des. 2018;24 doi: 10.2174/1381612824666180115111546. [DOI] [PubMed] [Google Scholar]
- 10.Haas L.F. Pedanius Dioscorides (born about AD40, died about AD90) J. Neurol. Neurosurg. Psychiatry. 1996;60 doi: 10.1136/jnnp.60.4.427. 427–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laios K., Lytsikas-Sarlis P., Manes K., Kontaxaki M.-I., Karamanou M., Androutsos G. Drugs for mental illnesses in ancient Greek medicine. Psychiatriki. 2019;30:58–65. doi: 10.22365/jpsych.2019.301.58. [DOI] [PubMed] [Google Scholar]
- 12.Nuki G., Simkin P.A. A concise history of gout and hyperuricemia and their treatment. Arthritis Res. Ther. 2006;8:1–5. doi: 10.1186/ar1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karamanou M., Tsoucalas G., Pantos K., Androutsos G. Isolating colchicine in 19th century: an old drug revisited. Curr. Pharm. Des. 2018;24:654–658. doi: 10.2174/1381612824666180115105850. [DOI] [PubMed] [Google Scholar]
- 14.Katsilabros L. La colchicine et ses dérivés contre les viroses. Arch. Inst. Pasteur Hell. 1958;4:139–145. [Google Scholar]
- 15.Deftereos S.G., Siasos G., Giannopoulos G., Vrachatis D.A., Angelidis C., Giotaki S.G. The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): rationale and study design. Hellenic J. Cardiol. 2020 doi: 10.1016/j.hjc.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deftereos S.G.S.G., Vrachatis D.A.D.A., Angelidis C., Vrettou A.-R.A.-R., Sarri E.K.E.K., Giotaki S.G.S.G. The role of colchicine in treating postoperative and post-catheter ablation atrial fibrillation. Clin. Ther. 2019;41:21–29. doi: 10.1016/j.clinthera.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Schenone A.L., Menon V. Colchicine in pericardial disease: from the underlying biology and clinical benefits to the drug-drug interactions in cardiovascular medicine. Curr. Cardiol. Rep. 2018;20 doi: 10.1007/s11886-018-1008-5. [DOI] [PubMed] [Google Scholar]
- 18.Angelidis C., Kotsialou Z., Kossyvakis C., Vrettou A.-R., Zacharoulis A., Kolokathis F. Colchicine pharmacokinetics and mechanism of action. Curr. Pharm. Des. 2018;24:659–663. doi: 10.2174/1381612824666180123110042. [DOI] [PubMed] [Google Scholar]
- 19.Chen K., Schenone A.L., Borges N., Militello M., Menon V. Teaching an old dog new tricks: colchicine in cardiovascular medicine. Am. J. Cardiovasc. Drugs. 2017;17:347–360. doi: 10.1007/s40256-017-0226-3. [DOI] [PubMed] [Google Scholar]
- 20.Leung Y.Y., Yao Hui L.L., Kraus V.B. Colchicine-update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015;45:341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah B., Allen N., Harchandani B., Pillinger M., Katz S., Sedlis S.P. Effect of colchicine on platelet-platelet and platelet-leukocyte interactions: a pilot study in healthy subjects. Inflammation. 2016;39:182–189. doi: 10.1007/s10753-015-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deftereos S., Giannopoulos G., Papoutsidakis N., Panagopoulou V., Kossyvakis C., Raisakis K. Colchicine and the heart: pushing the envelope. J. Am. Coll. Cardiol. 2013;62:1817–1825. doi: 10.1016/j.jacc.2013.08.726. [DOI] [PubMed] [Google Scholar]
- 23.Perico N., Ostermann D., Bontempeill M., Morigi M., Amuchastegui C.S., Zoja C. Colchicine interferes with L-selectin and leukocyte function-associated antigen-1 expression on human T lymphocytes and inhibits T cell activation. J. Am. Soc. Nephrol. 1996;7:594–601. doi: 10.1681/ASN.V74594. [DOI] [PubMed] [Google Scholar]
- 24.Atta H.M., El-Rehany M.A., Abdel Raheim S.R., Fouad R., Galal A.M.F. Colchicine inhibits intimal hyperplasia and leukocyte VEGF expression in dogs. J. Surg. Res. 2008;146:184–189. doi: 10.1016/j.jss.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Marzo-Mas A., Falomir E., Murga J., Carda M., Marco J.A. Effects on tubulin polymerization and down-regulation of c-Myc, hTERT and VEGF genes by colchicine haloacetyl and haloaroyl derivatives. Eur. J. Med. Chem. 2018;150:591–600. doi: 10.1016/j.ejmech.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y.Y., Chen Y.C., Kao Y.H., Lin Y.K., Yeh Y.H., Chen S.A. Colchicine modulates calcium homeostasis and electrical property of HL-1 cells. J. Cell. Mol. Med. 2016;20:1182–1190. doi: 10.1111/jcmm.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaksonen M., Roux A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018;19:313–326. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- 28.Kwon O.C., Hong S., Ghang B., Kim Y.-G., Lee C.-K., Yoo B. Risk of colchicine-associated myopathy in gout: influence of concomitant use of statin. Am. J. Med. 2017;130:583–587. doi: 10.1016/j.amjmed.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Tardif J.C., Kouz S., Waters D.D., Bertrand O.F., Diaz R., Maggioni A.P. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 30.Nidorf S.M., Eikelboom J.W., Budgeon C.A., Thompson P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Wiggins B.S., Saseen J.J., Page R.L., Reed B.N., Sneed K., Kostis J.B. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2016;134:e468–95. doi: 10.1161/CIR.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 32.Al-Khazaali A., Arora R. P-Glycoprotein: a focus on characterizing variability in cardiovascular pharmacotherapeutics. Am. J. Ther. 2014;21:2–9. doi: 10.1097/MJT.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 33.Ehrhardt M., Lindenmaier H., Burhenne J., Haefeli W.E., Weiss J. Influence of lipid lowering fibrates on P-glycoprotein activity in vitro. Biochem. Pharmacol. 2004;67:285–292. doi: 10.1016/j.bcp.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki M., Li B., Louie S.W., Pudvah N.T., Stocco R., Wong W. Effects of fibrates on human organic anion-transporting polypeptide 1B1-, multidrug resistance protein 2- and P-glycoprotein-mediated transport. Xenobiotica. 2005;35:737–753. doi: 10.1080/00498250500136676. [DOI] [PubMed] [Google Scholar]
- 35.Weiss J., Sauer A., Divac N., Herzog M., Schwedhelm E., Böger R.H. Interaction of angiotensin receptor type 1 blockers with ATP-binding cassette transporters. Biopharm. Drug Dispos. 2010;31:150–161. doi: 10.1002/bdd.699. [DOI] [PubMed] [Google Scholar]
- 36.Kamiyama E., Nakai D., Mikkaichi T., Okudaira N., Okazaki O. Interaction of angiotensin II type 1 receptor blockers with P-gp substrates in Caco-2 cells and hMDR1-expressing membranes. Life Sci. 2010;86:52–58. doi: 10.1016/j.lfs.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Ledwitch K.V., Roberts A.G. Cardiovascular ion channel inhibitor drug-drug interactions with P-glycoprotein. AAPS J. 2017;19:409–420. doi: 10.1208/s12248-016-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terkeltaub R.A., Furst D.E., Digiacinto J.L., Kook K.A., Davis M.W. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis Rheum. 2011;63:2226–2237. doi: 10.1002/art.30389. [DOI] [PubMed] [Google Scholar]
- 39.FDA Approves New Dosing Guidelines for Colchicine Taken With Protease Inhibitors - Medscape - May 11, 2010 (n.d.).
- 40.Deftereos S., Giannopoulos G., Angelidis C., Alexopoulos N., Filippatos G., Papoutsidakis N. Anti-inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. Circulation. 2015;132:1395–1403. doi: 10.1161/CIRCULATIONAHA.115.017611. [DOI] [PubMed] [Google Scholar]
- 41.Bouabdallaoui N., Tardif J.-C., Waters D.D., Pinto F.J., Maggioni A.P., Diaz R. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT) Eur. Heart J. 2020;41:4092–4099. doi: 10.1093/eurheartj/ehaa659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vrachatis D.A., Giannopoulos G.V., Deftereos S.G. Colchicine administered early in acute myocardial infarction: ready, set … go? Eur. Heart J. 2021 doi: 10.1093/eurheartj/ehab010. [DOI] [PubMed] [Google Scholar]
- 43.Samuel M., Tardif J.-C., Bouabdallaoui N., Khairy P., Dubé M.-P., Blondeau L. Colchicine for secondary prevention of cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Can. J. Cardiol. 2020 doi: 10.1016/j.cjca.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Xiang Z., Yang J., Yang J., Zhang J., Fan Z., Yang C. Efficacy and safety of colchicine for secondary prevention of coronary heart disease: a systematic review and meta-analysis. Intern. Emerg. Med. 2021 doi: 10.1007/s11739-020-02606-7. [DOI] [PubMed] [Google Scholar]
- 45.Tien Y.Y., Huang H.K., Shih M.C., Tu Y.K. Drug repurposing? Cardiovascular effect of colchicine on patients with coronary artery disease: a systematic review and meta-analysis. J. Cardiol. 2020 doi: 10.1016/j.jjcc.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Deftereos S., Giannopoulos G., Raisakis K., Kossyvakis C., Kaoukis A., Panagopoulou V. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. J. Am. Coll. Cardiol. 2013;61:1679–1685. doi: 10.1016/j.jacc.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 47.Masson W., Lobo M., Molinero G., Masson G., Lavalle-Cobo A. Role of colchicine in stroke prevention: an updated meta-analysis. J. Stroke Cerebrovasc. Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.104756. [DOI] [PubMed] [Google Scholar]
- 48.Katsanos A.H., Palaiodimou L., Price C., Giannopoulos S., Lemmens R., Kosmidou M. Colchicine for stroke prevention in patients with coronary artery disease: a systematic review and meta-analysis. Eur. J. Neurol. 2020;27:1035–1038. doi: 10.1111/ene.14198. [DOI] [PubMed] [Google Scholar]
- 49.Deftereos S., Giannopoulos G., Kossyvakis C., Efremidis M., Panagopoulou V., Kaoukis A. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J. Am. Coll. Cardiol. 2012;60:1790–1796. doi: 10.1016/j.jacc.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 50.Deftereos S., Giannopoulos G., Efremidis M., Kossyvakis C., Katsivas A., Panagopoulou V. Colchicine for prevention of atrial fibrillation recurrence after pulmonary vein isolation: mid-term efficacy and effect on quality of life. Hear Rhythm. 2014;11:620–628. doi: 10.1016/j.hrthm.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Deftereos S., Giannopoulos G., Vrachatis D.A., Siasos G., Giotaki S.G., Cleman M. Colchicine as a potent anti-inflammatory treatment in COVID-19: can we teach an old dog new tricks? Eur. Hear J. Cardiovasc. Pharmacother. 2020:255. doi: 10.1093/ehjcvp/pvaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giannopoulos G., Vrachatis D.A., Deftereos S.G. Myocardial injury in COVID-19-can we successfully target inflammation? JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.2569. [DOI] [PubMed] [Google Scholar]
- 53.Deftereos S.G., Giannopoulos G., Vrachatis D.A., Siasos G.D., Giotaki S.G., Gargalianos P. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vrachatis D.A., Giannopoulos G.V., Giotaki S.G., Raisakis K., Kossyvakis C., Iliodromitis K.E. Impact of colchicine on mortality in patients with COVID-19: a meta-analysis. Hellenic J. Cardiol. 2021 doi: 10.1016/j.hjc.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tardif J.-C., Bouabdallaoui N., L'Allier P.L., Daudet D., Shah B., Pillinger M.H. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00222-8. S2213-2600(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Indraratna P.L., Virk S., Gurram D., Day R.O. Use of colchicine in pregnancy: a systematic review and meta-analysis. Rheumatology (United Kingdom) 2018;57:382–387. doi: 10.1093/rheumatology/kex353. [DOI] [PubMed] [Google Scholar]
- 57.Stewart S., Yang K.C.K., Atkins K., Dalbeth N., Robinson P.C. Adverse events during oral colchicine use: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 2020;22 doi: 10.1186/s13075-020-2120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andreis A., Imazio M., Avondo S., Casula M., Paneva E., Piroli F. Adverse events of colchicine for cardiovascular diseases. J. Cardiovasc. Med. 2021 doi: 10.2459/jcm.0000000000001157. Publish Ah. [DOI] [PubMed] [Google Scholar]