Coronavirus disease 2019 (COVID-19) can lead to long-term health effects. This article systematically characterizes the prevalence and longitudinal evolution of COVID-19 symptoms more than 7 months after diagnosis among 410 relatively healthy, nonhospitalized patients.
Visual Abstract. Prevalence of Symptoms More Than 7 Months After Symptomatic COVID-19.
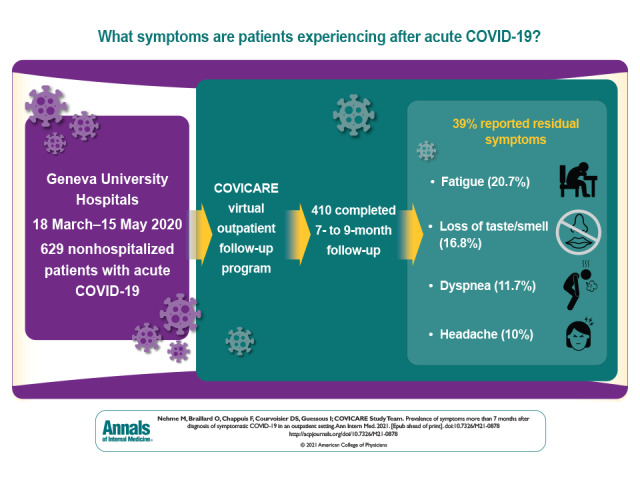
Coronavirus disease 2019 (COVID-19) can lead to long-term health effects. This article systematically characterizes the prevalence and longitudinal evolution of COVID-19 symptoms more than 7 months after diagnosis among 410 relatively healthy, nonhospitalized patients.
Abstract
Background:
With millions of SARS-CoV-2 infections worldwide, increasing numbers of patients are coming forward with long-term clinical effects of the disease lasting several weeks to months.
Objective:
To characterize symptoms 7 to 9 months after diagnosis of COVID-19.
Design:
Self-reported surveys and semistructured telephone interviews at enrollment and 30 to 45 days and 7 to 9 months from diagnosis.
Setting:
From 18 March to 15 May 2020, symptomatic persons who tested positive for SARS-CoV-2 at the Geneva University Hospitals were followed by CoviCare, a virtual, clinical, outpatient follow-up program. Persons were contacted again at 30 to 45 days and 7 to 9 months from diagnosis.
Participants:
Persons who were a part of the CoviCare program from 18 March to 15 May 2020.
Measurements:
A standardized interview of symptoms consistent with COVID-19, with grading of intensity.
Results:
Of the 629 participants in the study who completed the baseline interviews, 410 completed follow-up at 7 to 9 months after COVID-19 diagnosis; 39.0% reported residual symptoms. Fatigue (20.7%) was the most common symptom reported, followed by loss of taste or smell (16.8%), dyspnea (11.7%), and headache (10.0%).
Limitation:
Limitations include generalizability and missing data for 34.8% of participants.
Conclusion:
Residual symptoms after SARS-CoV-2 infection are common among otherwise young and healthy persons followed in an outpatient setting. These findings contribute to the recognition of long-term effects in a disease mostly counted by its death toll to date by promoting communication on postacute sequelae of SARS-CoV-2 and encouraging physicians to continue long-term monitoring of their patients.
Primary Funding Source:
None.
Long COVID, postacute sequelae of SARS-CoV-2 infection, or post–COVID-19 condition are terms introduced in the literature (1–5) to describe illness in persons who continue to report lasting effects several weeks to months after SARS-CoV-2 infection. Several months after the first cases of COVID-19, physicians, scientists, and patients alike started reporting unrelenting symptoms associated with COVID-19 that could be continuous or fluctuating in nature (6–8). The National Institutes of Health and World Health Organization have recently led international workshops to describe and recognize long-term effects and a lengthy course of the disease (1, 5). To date, no clear predictors, pathophysiology, or even disease course has been identified. Recent studies show persistent symptoms after hospitalization (7, 9) and in outpatient settings (10–14). Patient-led advocacy groups and the scientific community are trying to shed more light on this condition by providing suggested definitions (15, 16) and guidance for management (17) and follow-up (18). The message so far has been consistent in suggesting that symptoms and postacute sequelae of SARS-CoV-2 can persist weeks to months after the infection, and potentially years, in both mild to moderate and severe disease. The frequency of postacute sequelae of SARS-CoV-2 infection should be recognized as such to facilitate information, management, and follow-up (19).
Recently, we reported the prevalence and distribution of symptoms at 30 to 45 days from diagnosis in a cohort of 669 outpatient persons with mostly mild to moderate COVID-19 (11). Our earlier study showed that even in a largely young and otherwise healthy population with mild initial symptoms, at least a third of persons reported persistent symptoms at 30 to 45 days, highlighting the importance of monitoring for long-term COVID-19–related symptoms even in nonhospitalized patients. Other studies showed that age and sex could be associated with postacute sequelae of SARS-CoV-2 infection (18), although this association remains unclear, and any generalization should be considered carefully.
The time course and evolution of post–COVID-19 symptoms are key elements for communication and health care planning as well as to inform public health decisions based on the overall burden of COVID-19 on survivors. Identifying any potential risk factors for the persistence of symptoms could allow the health care system, patients, and physicians alike to be better prepared in managing and following this disease.
This study aims to characterize the prevalence and longitudinal evolution of COVID-19 symptoms more than 7 months after diagnosis.
Methods
Study Design
After the enforced closure of primary care practices in Geneva, Switzerland, on 18 March 2020 during the first COVID-19 lockdown, persons testing positive for SARS-CoV-2 at the Geneva University Hospitals were eligible for virtual ambulatory care follow-up (CoviCare). The CoviCare program was initially set up as a clinical program to follow patients' symptoms during the first 10 days from diagnosis (date of positive result on reverse transcriptase polymerase chain reaction test). The first 10 days were considered the acute phase of the disease, as defined by the Swiss Federal Office of Public Health (20), with the first contact considered as the baseline interview. The role of CoviCare during that phase was to look for deteriorating clinical signs and treat patients quickly and appropriately (full description of CoviCare processes available at www.covicare24.com). Participants were contacted via telephone or video consultation every 2 days during the first 10 days (with the potential for missing data if not reached at any time during the first 10 days) and again at 30 to 45 days; details and results were previously published (11). The same participants were recontacted at 7 to 9 months from diagnosis. Persons were first sent an online questionnaire, and those who did not respond within 2 weeks were then called individually. With their consent and according to their preferences, the questionnaire was then completed by telephone or an e-mail was sent again for completion. Participants who completed the interview did so within a 2-month time frame.
The study was approved by the Cantonal Research Ethics Commission of Geneva (CER 2020-01273).
Study Setting
Geneva is a state (canton) of about 500 000 residents, with 40% of the population made up of foreigners from different nationalities. About 73% of foreigners are European, 9.1% are from Africa, 9.0% are from the Americas, and 8.5% are from Asia. From 18 March to 15 May 2020, the Geneva University Hospitals were 1 of the 5 available testing centers for the whole Geneva population. Being the only public university hospital and the largest health care facility in the canton, the Geneva University Hospitals did more than 50% of the total number of SARS-CoV-2 tests for persons tested during that period. Between 13 March and 29 March 2020, only symptomatic patients with risk factors were tested. (Of note, if patients with no risk factors presented to the Geneva University Hospitals, they were still tested.) As of 30 March 2020, all symptomatic patients were tested. Since March 2020, all test dates, results (positive and negative), hospitalizations, and deaths for persons positive for SARS-CoV-2 were recorded in the cantonal Actionable Register of Geneva Outpatients with SARS-CoV-2. During the study period, 30 557 symptomatic persons were tested for SARS-CoV-2. Among them, 18.1% tested positive (n = 5534), and 22.2% of these persons were hospitalized (n = 1229) (21).
Participants
Persons benefiting from the CoviCare follow-up between 18 March and 15 May 2020 were included in this study. Patients were excluded if they were younger than 18 years, declined to provide consent, or lived outside the Geneva canton. Although none of our participants were hospitalized at baseline, 40 persons were hospitalized during follow-up. To characterize the evolution of symptoms in an outpatient setting, we excluded the 40 hospitalized persons from the analysis.
Measurements
A standardized interview was used to inquire about the presence or absence of several symptoms (Supplement). Approximately every 2 days during the first 10 days of enrollment or the “baseline” period, participants were asked to report any of the following symptoms: fatigue, anosmia or ageusia, dyspnea, headache, cough, nausea or vomiting, abdominal pain, diarrhea, and fever (temperature >38 °C). At the 30- to 45-day and 7- to 9-month follow-ups, participants were first asked whether they still had symptoms related to COVID-19 (yes or no). Those who reported the presence of COVID-19–related symptoms were then asked about the presence and intensity of each symptom. The list of symptoms asked at the 30- to 45-day and 7- to 9-month follow-ups included all of the symptoms elicited at baseline in addition to symptoms of myalgia, arthralgia, palpitations, chest pain, lack of equilibrium, neuropathy, back pain, throat pain, loss of appetite, and rash (Supplement). The symptoms of anosmia and ageusia, combined at baseline, were separated at the 30- to 45-day and 7- to 9-month follow-ups. At the 7- to 9-month follow-up, patients were also asked about difficulty concentrating (brain fog), memory loss, insomnia, and hair loss. The symptoms of nausea, vomiting, abdominal pain, and diarrhea were combined as “digestive symptoms.” Symptoms that were asked about at all 3 time points (fatigue, anosmia or ageusia, dyspnea, headache, cough, digestive symptoms, and fever) were categorized as “any symptoms” at each time point.
A Likert scale (mild, moderate, or severe) was used to grade the intensity of each symptom, except for back pain. The Eastern Cooperative Oncology Group (ECOG) performance scale (22) was used for fatigue and the modified Medical Research Council (mMRC) scale (23) for dyspnea. The ECOG performance scale describes the degree of functional status in terms of daily activities and physical performance (22). In this study, the ECOG performance scale was rated as 0 (no limitation in activities of daily living), 1 (restricted in physically strenuous activity), 2 (unable to carry out any work activities, but up and about more than 50% of waking hours), 3 (capable of only limited self-care, confined to bed or chair more than 50% of waking hours), and 4 (completely disabled, totally confined to bed or chair). The mMRC dyspnea scale describes the degree of disability linked to shortness of breath in terms of daily activities and physical performance (23). In this study, the mMRC scale was rated as 0 (breathless with strenuous exercise), 1 (breathless walking up a slight hill or hurrying on level ground), 2 (walks slower than persons of the same age or has to stop for breath when walking at own pace), 3 (stops for breath after about 100 yd [90 m] or after a few minutes on level ground), and 4 (too breathless to leave the house or breathless when dressing).
Statistical Analysis
We used the statistical software Stata, version 15.1 (StataCorp), and RStudio, version 4.0.2 (R Foundation). Age was categorized on the basis of the distribution in our sample and previous studies suggesting that middle age, defined as between 40 and 60 years, may be a predictor of persistent symptoms (24). Standardized differences were used to compare the characteristics of groups of participants versus nonparticipants at each time point. We used the cantonal Actionable Register of Geneva Outpatients with SARS-CoV-2 to compare the characteristics of our cohort with those of persons who tested positive in the canton of Geneva, outside the Geneva University Hospitals, during the same time period.
Two estimates of the prevalence of symptoms at 7 to 9 months, stratified by sex (male or female) and age (18 to 39 years, 40 to 59 years, and ≥60 years), were computed. The first was obtained by calculating the proportion of symptoms of participants still followed at 7 to 9 months and reported with 95% CIs. The second was obtained using the marginal prediction of a logistic regression adjusted for age, sex, and the number of symptoms at baseline (<2 symptoms or ≥2 symptoms), corrected for attrition using inverse probability weighting. Inverse probability weights were calculated considering baseline characteristics (age; sex; profession, defined as health care professional vs. non–health care professional; and risk factors for complications from COVID-19, including cardiovascular disease, hypertension, diabetes, respiratory disease, and cancer). We computed CIs using bootstrap with 10 000 samples.
Role of the Funding Source
This study received no external funding.
Results
Of the 703 persons from the initial cohort, 629 were included in the study (Figure 1). The mean age was 42.1 years (SD, 13.5), and the median age was 41 years; 60.9% were women, 25.3% were health care workers, and 70.7% had no underlying risk factors (Table 1). Using the cantonal register, outpatients who tested positive for SARS-CoV-2 elsewhere in the Geneva canton during the same time period (n = 2272) had a mean age of 47.7 years (SD, 17); 56.6% of them were women, and 67.3% had no underlying risk factors.
Figure 1. Flow chart describing participation rate at each follow-up.
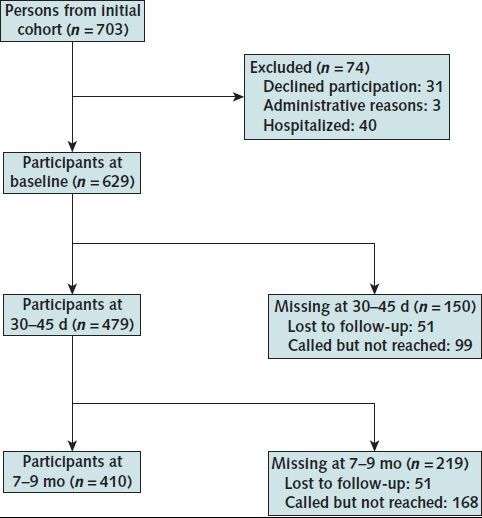
Only outpatients were included. Missing participants were called but not reached at 30 to 45 d or 7 to 9 mo, and 51 were completely lost to follow-up at 30 to 45 d and 7 to 9 mo.
Table 1.
Participant Characteristics at Baseline, 30- to 45-Day Follow-up, and 7- to 9-Month Follow-up
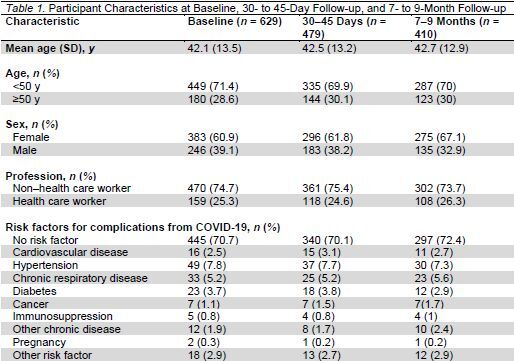
Of the 629 participants, 410 answered the questionnaire at 7 to 9 months (65.2% response rate). Participants who were lost to follow-up had overall similar baseline characteristics, except that men were more likely to be lost to follow-up (Appendix Table 1).
Appendix Table 1.
Characteristics of Participants and Nonparticipants at Baseline, 30- to 45-Day Follow-up, and 7- to 9-Month Follow-up
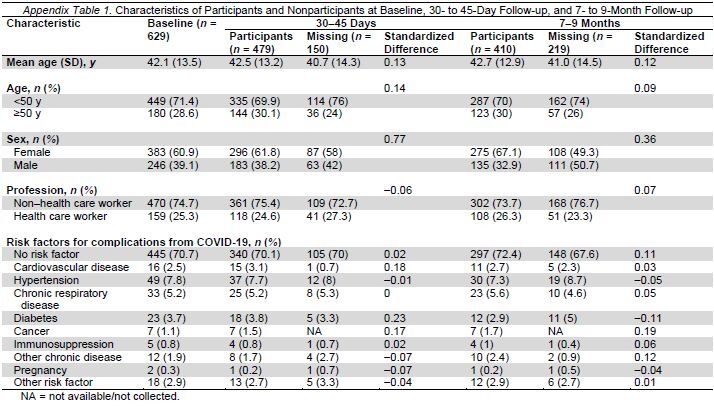
Table 2 presents the prevalence of COVID-19–related symptoms at all 3 time points. Of the participants who answered the questionnaire at 7 to 9 months, 39.0% reported some symptoms, with the most common being fatigue, followed by loss of taste or smell, dyspnea, and headache. Of the 174 participants who reported symptoms at 7 to 9 months, 27.6% had only 1 symptom, 26.4% had 2 symptoms, 18.4% had 3 symptoms, 10.9% had 4 symptoms, 4.0% had 5 symptoms, and 12.6% had 6 or more symptoms. Appendix Table 2 presents the prevalence of symptoms at all 3 time points stratified by sex (male or female) and age (18 to 39 years, 40 to 59 years, and ≥60 years). Table 3 presents the symptoms by sex, age, and the number of symptoms at baseline (<2 symptoms or ≥2 symptoms) after inverse probability weighting.
Table 2.
Prevalence of Symptoms Among Participants at Each Follow-up*
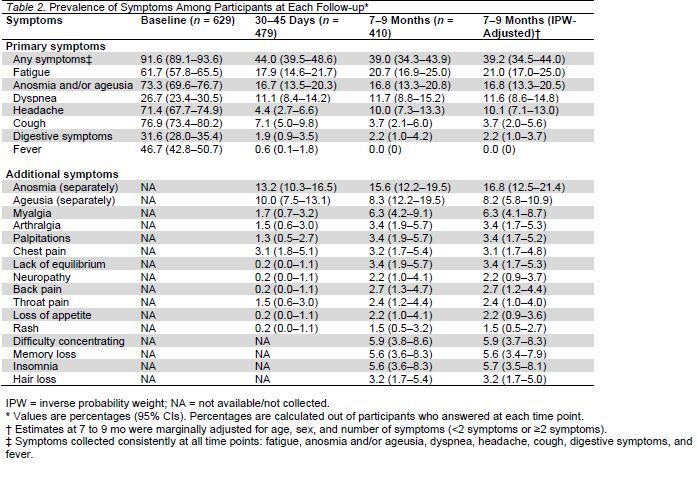
Appendix Table 2.
Prevalence of Symptoms at Baseline, 30 to 45 Days, and 7 to 9 Months, Stratified by Sex and Age Group*
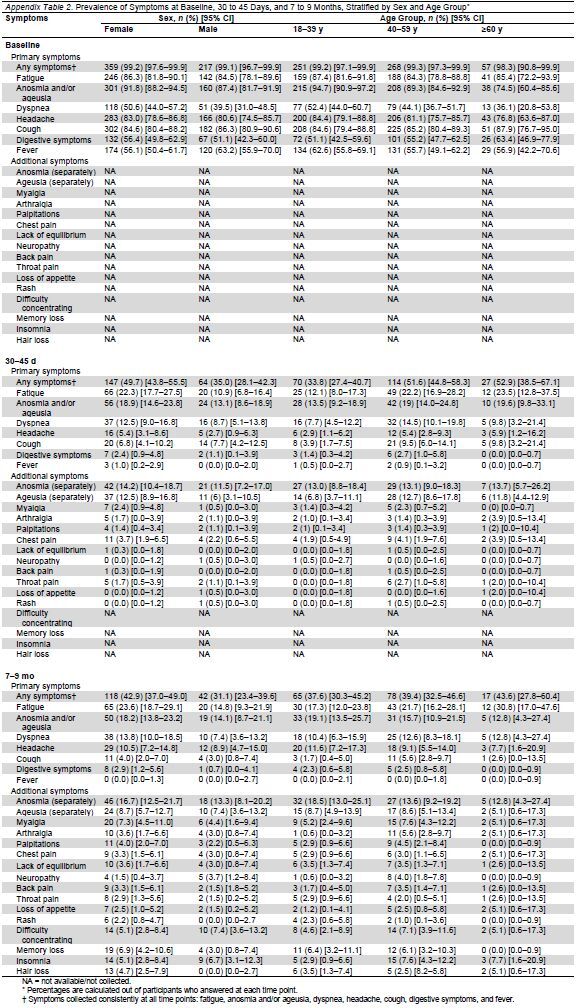
Table 3.
Prevalence of Symptoms at 7 to 9 Months After Adjustment and Inverse Probability Weighting for Missing Data
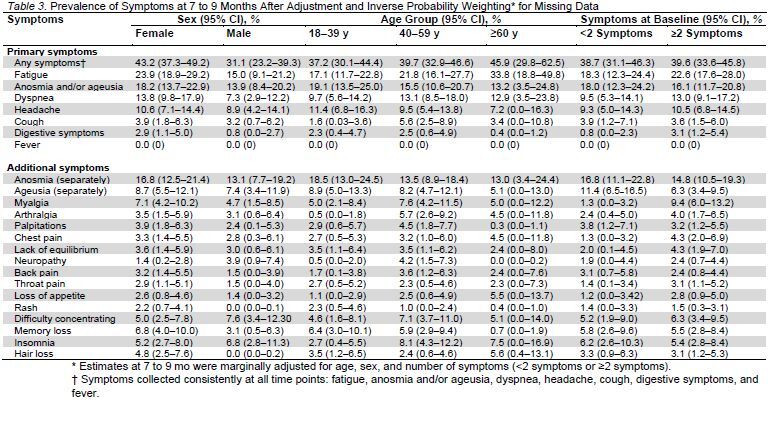
Figure 2 presents the evolution of symptoms in our study sample. Of the 311 participants with data at all 3 follow-up time points, 37% reported symptom resolution by 30 to 45 days and an additional 19% reported symptom resolution by 7 to 9 months (total of 56% with symptom resolution). On the other hand, 27% had symptoms at all 3 time points, and 10% reported symptoms at baseline and at 7 to 9 months but not at 30 to 45 days. Finally, 4% of participants reported no symptoms at all 3 time points, 2% had no symptoms at baseline but had symptoms at 30 to 45 days and 7 to 9 months, and 1 person had no symptoms at baseline and 30 to 45 days but had symptoms at 7 to 9 months.
Figure 2. Evolution of COVID-19 symptoms over time for all persons included in the study (n = 629).
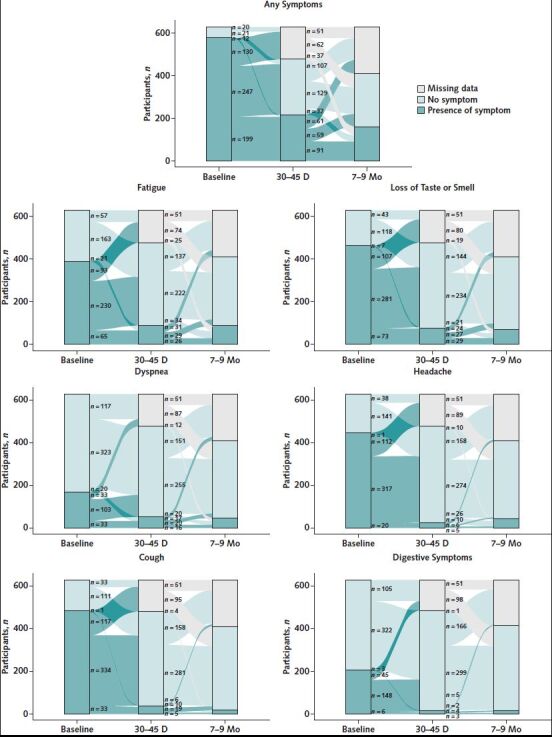
Symptoms included in “any symptoms” are those collected consistently at all time points: fatigue, anosmia and/or ageusia, dyspnea, headache, cough, digestive symptoms, and fever.
Figure 3 presents the severity of symptoms at 7 to 9 months. Of the participants who reported fatigue, 70.6% (60 of 85) graded their fatigue on the ECOG performance scale as 0 (no limitations) and 27.0% (23 of 85) graded their fatigue as 1 (restricted in physically strenuous activity). Dyspnea was graded as 0 (breathless with strenuous exercise) on the mMRC dyspnea scale (22) in 29.2% (14 of 48) of cases and as 1 (breathless walking up a slight hill or hurrying on ground level) in 60.4% (29 of 48) of cases. Most patients who reported loss of smell, headache, loss of taste, insomnia, myalgia, hair loss, cough, or loss of appetite reported their symptoms as at least moderate. Most participants with remaining symptoms rated them as mild.
Figure 3. Symptoms of COVID-19, with the severity of each symptom in absolute numbers at 7 to 9 months from diagnosis.
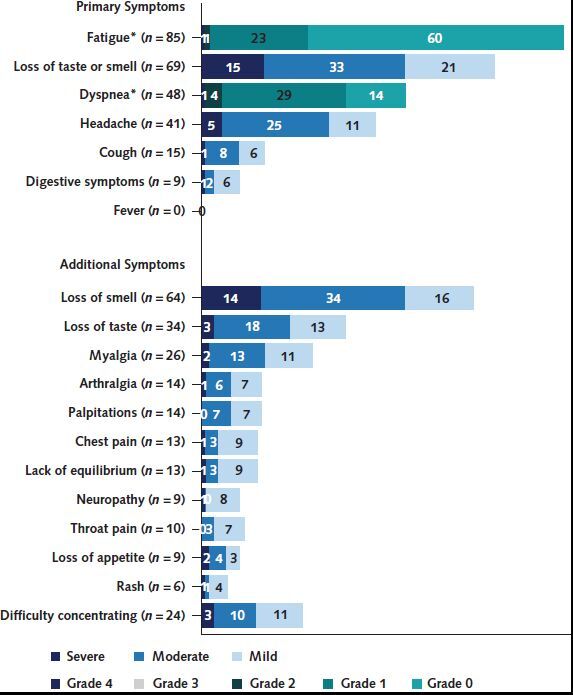
* Fatigue intensity is based on the Eastern Cooperative Oncology Group performance scale (17), and dyspnea intensity is based on the modified Medical Research Council scale (18).
Discussion
Almost a third of persons with mild to moderate, acute COVID-19 in an outpatient setting have long-term symptoms at 30 to 45 days and 7 to 9 months from diagnosis. Persons are most likely to report fatigue, loss of taste or smell, dyspnea, and headache. Our study shows that even though the prevalence of overall symptoms decreased with time (37% had resolution of symptoms at 30 to 45 days and an additional 19% at 7 to 9 months), several symptoms are still reported, with a high prevalence at 7 to 9 months. When self-reporting symptom severity, participants in our cohort indicated mostly mild to moderate symptoms at 7 to 9 months from diagnosis.
A recent viewpoint (25) described mechanisms that could lead to long-term symptoms. These mechanisms include direct tissue invasion, profound inflammation, and a related immune system response (25). Sequelae have been identified in several organ systems, including the pulmonary, cardiac, renal, and central nervous systems (2, 8, 26).
The long-lasting nature of symptoms we report is in line with the results of recent studies (27, 28). A survey of 234 participants with COVID-19 showed persisting symptoms in 32.7% of outpatient cases at 6 months from diagnosis. A meta-analysis of 15 studies on postacute sequelae of SARS-CoV-2 showed that an estimated 80% of patients (aged 17 to 87 years) developed 1 or more long-term symptoms, with mostly fatigue, attention disorder, hair loss, and dyspnea being reported (13). Another meta-analysis recently showed the persistence of symptoms in 73% of persons, with varying follow-up times (14). A patient-led online survey found that several patients had persistent symptoms at 7 months, experiencing relapses with exercise, physical or mental activity, and stress (29); 45.2% of participants required a reduced work schedule compared with before their illness, and 22.3% were not working at the time of the survey because of their health conditions (29). Our study adds to the ongoing research on postacute sequelae of SARS-CoV-2 by following outpatients prospectively over time, with a long follow-up period. More than half of participants have symptom resolution by 7 to 9 months; however, 11% reported worsening symptoms after initially reporting resolution of symptoms, and 39% of participants had symptoms at 7 to 9 months from diagnosis. Our study also indicates the need to determine the severity of symptoms because mild, moderate, and severe symptoms could have a varying effect on daily life. Finally, predictors of symptom persistence are still unclear, with several studies showing different results.
Persons treated on an outpatient basis should be informed about the potential long-term effects of COVID-19. Those more at risk for persistent symptoms should be followed regularly and provided support and management. To date, treatment includes a multidisciplinary approach with physical therapy, respiratory therapy, and nonpharmaceutical interventions (2, 17), and long-term studies are needed to prove treatment efficacy as well as to elucidate the pathophysiology of the disease to provide the appropriate management. Physicians should also be aware of other causes of such symptoms as fatigue, cognitive and neurologic symptoms, and dyspnea and look for a differential diagnosis to avoid misinterpretation (30).
This study has several limitations. First, at baseline, only symptomatic patients had access to testing and were subsequently included in the study. Including asymptomatic persons can give a more thorough picture of the disease spectrum over time. The persistence of symptoms at 30 days from diagnosis has now been seen even in initially asymptomatic persons (28). Second, ascertainment bias is possible. This was mitigated by accounting for informative missingness; in addition, baseline characteristics were similar overall between persons lost to follow-up at 7 months and those participating. Third, although participants were asked whether their symptoms were related to COVID-19, we cannot exclude the possibility that these symptoms were related to other conditions that may have occurred in the months after diagnosis, especially for symptoms that were not asked about at baseline. Fourth, more women participated in the questionnaire than men, raising the issue of generalizability; this limitation was partly addressed by adjusting prevalence and stratification for differential attrition. Fifth, we did not have socioeconomic or race/ethnicity information on participants. Of note, all participants resided in Geneva, were tested at the same testing center, and had a similar follow-up, thus mitigating differential access to care as a potential confounder leading to symptom persistence.
Future research should continue studying the prevalence of postacute sequelae of SARS-CoV-2 and their potential effect on functioning and quality of life.
In conclusion, our study suggests that COVID-19–related symptoms persist more than 7 months after diagnosis in a large subset of ambulatory patients. Recognition of postacute sequelae of SARS-CoV-2 and potential predictors is important in reaching a more thorough understanding of the disease and its long-term effects. With a high prevalence of symptoms at 7 to 9 months, physicians should continue to monitor patients with COVID-19 over the long term.
Supplementary Material
Appendix: CoviCare Study Team
Mayssam Nehme*, Idris Guessous*, François Chappuis*, Olivia Braillard*, Delphine S. Courvoisier*, Sigiriya Aebischer Perone†, Gabriel Alcoba†, Frederic Lador†, Garance Kopp†, Lamyae Benzakour†, Marwene Grira†, Giovanni Innaurato†, Jennifer Socquet†, Hervé Spechbach†, Julien Salamun†, Frederique Jacquerioz†, Thomas Agoritsas†, Gilles Allali†, Chloé Chevallier-Lugon†, Stéphanie Baggio†, Luisa Carnino†, Bruno Lab†, Jose Sandoval†, Silvia Stringhini†, Pierre Chopard†, Simon Regard†, Camille Genecand†, Flora Koegler†, Dan Lebowitz†, and Aglaé Tardin†.
* Author.
† Nonauthor contributor.
Footnotes
This article was published at Annals.org on 6 July 2021.
* For members of the CoviCare Study Team, see the Appendix
References
- 1. Lerner AM , Robinson DA , Yang L , et al. Toward understanding COVID-19 recovery: National Institutes of Health workshop on postacute COVID-19. Ann Intern Med. 2021. [PMID: ]. doi: 10.7326/M21-1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laine C , Cotton D . COVID-19: evaluation and care of patients with persistent symptoms following acute SARS-CoV-2 infection. Ann Intern Med. 2021. [PMID: ]. doi: 10.7326/M21-2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahase E . Covid-19: what do we know about “long Covid”. BMJ. 2020;370:m2815. [PMID: ] doi: 10.1136/bmj.m2815 [DOI] [PubMed] [Google Scholar]
- 4. Nabavi N . Long Covid: how to define it and how to manage it. BMJ. 2020;370:m3489. [PMID: ] doi: 10.1136/bmj.m3489 [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Expanding our understanding of post COVID-19 condition: report of a WHO webinar - 9 February 2021. 24 April 2021. Accessed at www.who.int/publications/i/item/9789240025035 on 13 May 2021.
- 6. Garner P. Paul Garner: for 7 weeks I have been through a roller coaster of ill health, extreme emotions, and utter exhaustion. BMJ Opinion. 5 May 2020. Accessed at https://blogs.bmj.com/bmj/2020/05/05/paul-garner-people-who-have-a-more-protracted-illness-need-help-to-understand-and-cope-with-the-constantly-shifting-bizarre-symptoms on 26 July 2020.
- 7. Carfì A , Bernabei R , Landi F ; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603-605. [PMID: ] doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C , Huang L , Wang Y , et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. [PMID: ] doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang Y , Tan C , Wu J , et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21:163. [PMID: ] doi: 10.1186/s12931-020-01429-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tenforde MW , Rose EB , Lindsell CJ , et al; CDC COVID-19 Response Team. Characteristics of adult outpatients and inpatients with COVID-19—11 academic medical centers, United States, March–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:841-846. [PMID: ] doi: 10.15585/mmwr.mm6926e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nehme M , Braillard O , Alcoba G , et al; COVICARE TEAM. COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med. 2021;174:723-725. [PMID: ]. doi: 10.7326/M20-5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lund LC , Hallas J , Nielsen H , et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis. 2021. [PMID: ] doi: 10.1016/S1473-3099(21)00211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv. Preprint posted online 30 January 2021. doi:10.1101/2021.01.27.21250617 [DOI] [PMC free article] [PubMed]
- 14. Nasserie T , Hittle M , Goodman SN . Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4:e2111417. [PMID: ] doi: 10.1001/jamanetworkopen.2021.11417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long COVID: let patients help define long-lasting COVID symptoms [Editorial]. Nature. 2020;586:170. [PMID: ] doi: 10.1038/d41586-020-02796-2 [DOI] [PubMed] [Google Scholar]
- 16. Callard F , Perego E . How and why patients made long Covid. Soc Sci Med. 2021;268:113426. [PMID: ] doi: 10.1016/j.socscimed.2020.113426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sivan M , Taylor S . NICE guideline on long Covid [Editorial]. BMJ. 2020;371:m4938. [PMID: ] doi: 10.1136/bmj.m4938 [DOI] [PubMed] [Google Scholar]
- 18. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv. Preprint posted online 19 December 2020. doi:10.1101/2020.10.19.20214494
- 19. Briggs A , Vassall A . Count the cost of disability caused by COVID-19. Nature. 2021;593:502-505. [PMID: ] doi: 10.1038/d41586-021-01392-2 [DOI] [PubMed] [Google Scholar]
- 20. Federal Office of Public Health. Coronavirus: isolation and quarantine. Accessed at www.bag.admin.ch/bag/en/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/novel-cov/isolation-und-quarantaene.html on 7 June 2021.
- 21. Directorate General of Health in Geneva. COVID19 in Geneva. Cantonal data. Accessed at https://infocovid.smc.unige.ch on 1 June 2021.
- 22. Buccheri G , Ferrigno D , Tamburini M . Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A:1135-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 23. Mahler DA , Wells CK . Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 24. Carvalho-Schneider C , Laurent E , Lemaignen A , et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258-263. [PMID: ] doi: 10.1016/j.cmi.2020.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubin R . As their numbers grow, COVID-19 “long haulers” stump experts. JAMA. 2020;324:1381-1383. [PMID: ] doi: 10.1001/jama.2020.17709 [DOI] [PubMed] [Google Scholar]
- 26. Nath A . Long-haul COVID [Editorial]. Neurology. 2020;95:559-560. [PMID: ] doi: 10.1212/WNL.0000000000010640 [DOI] [PubMed] [Google Scholar]
- 27. Logue JK , Franko NM , McCulloch DJ , et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4:e210830. [PMID: ] doi: 10.1001/jamanetworkopen.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cirulli ET, Schiabor Barrett KM, Riffle S, et al. Long-term COVID-19 symptoms in a large unselected population. medRxiv. Preprint posted online 1 December 2020. doi:10.1101/2020.10.07.20208702
- 29. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. medRxiv. Preprint posted online 5 April 2021. doi:10.1101/2020.12.24.20248802 [DOI] [PMC free article] [PubMed]
- 30. Greenhalgh T , Knight M , A'Court C , et al. Management of post-acute Covid-19 in primary care. BMJ. 2020;370:m3026. [PMID: ] doi: 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


