In patients recovering from coronavirus disease 2019 (COVID-19), specific T-cell responses against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been detected [1]. These responses targeted not only the spike (S) protein but also the membrane (M), nucleocapsid (N), and other open reading frames (ORFs) [2]. A licensed inactivated COVID-19 vaccine in China, BBIBP-CorV, induced good humoral responses [3]. However, whether virus-specific T cell immunity could be induced by BBIBP-CorV has not yet been clarified. Given that recent studies have demonstrated that mRNA (BNT162b2, mRNA-1273) and viral vector vaccines elicit strong T cell responses as well as neutralizing Abs against SARS-CoV-2 [4], we conducted a study to evaluate the T-cell response to SARS-CoV-2 before and after receiving BBIBP-CorV.
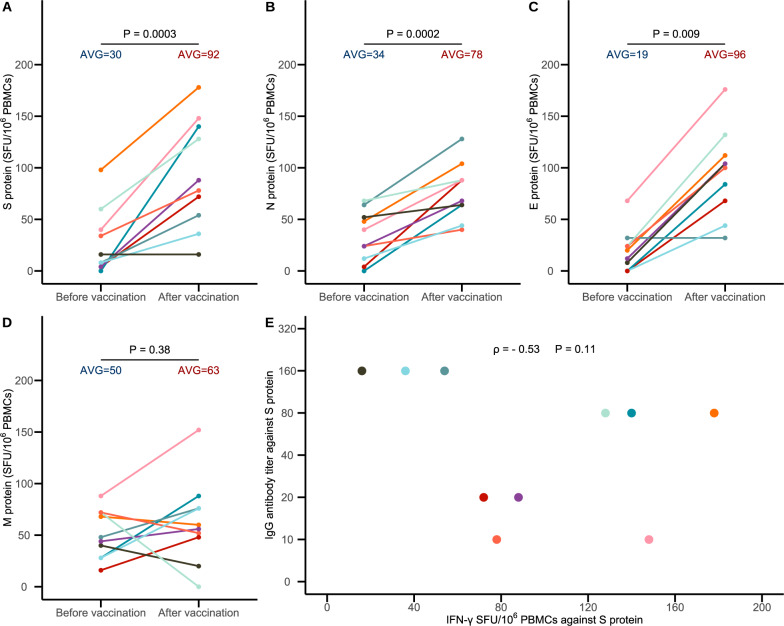
The study was approved by the Institutional Review Board of the China CDC. All vaccinees provided written informed consent. We collected blood samples from 10 healthy individuals before and after vaccination (median age, 35.5 years; range, 23–51; 3 males and 7 females) who received BBIBP-CorV containing 4 μg of total protein on days 0 and 21. Postvaccination blood was collected 12 weeks after the second dose. We performed IFN-y ELISpot assays on PBMCs to quantify the frequency of virus-specific T cells (details are provided in the Supplementary Appendix, available with the full text of this letter). A significant increase in IFN-γ spot-forming units (SFU) per million peripheral blood mononuclear cells (PBMCs) was observed against the S, N and E peptide pools of SARS-CoV-2 after vaccination (Fig. 1A–C). Nevertheless, the increase was not statistically significant for the M peptide pools, although the increase in IFN-γ SFU per million PBMCs was observed in six out of the 10 study participants (Fig. 1D). Given the presence of a certain degree of homology in the amino acid sequence of epitope peptides between SARS-CoV-2 and endemic HCoVs, preexisting SARS-CoV-2-reactive T cells or cross-reactivity with HCoVs might be detected in certain individuals; [5] this was observed in the significant SFU background in the prevaccination samples. We also tested postvaccination serum samples for IgG antibodies against the S protein (details on the laboratory assays are provided in the Supplementary Appendix). All study participants had detectable IgG antibodies with a median titer of 1:80 (range: 1:10 to 1:160). There was no significant correlation between the IgG antibody titer and IFN-γSFU against the S protein (Spearman’s correlation coefficient ρ = −0.53, P = 0.11).
Fig. 1.
T-cell responses against SARS-CoV-2 peptide pools and IgG antibody titers against the SARS-CoV-2 S protein in BBIBP-CorV recipients. A–D The T-cell responses against SARS-CoV-2 spike (S), nucleocapsid (N), envelope (E), and membrane (M) peptide pools, respectively. The T-cell response was measured as IFN-γ spot forming units (SFU) per 106 peripheral blood mononuclear cells (PBMCs). The mean IFN-γ SFU per 106 PBMCs was obtained and compared before and after vaccination. The differences were tested using paired t tests (A, B, and D) or Wilcoxon signed-rank tests (C). The P value is given (horizontal line) for each comparison. Panel E shows the correlation between T-cell responses and IgG antibody titers against the SARS-CoV-2 S protein in BBIBP-CorV recipients 12 weeks after the second dose. Spearman’s correlation coefficient was used to determine the correlation between the IgG antibody titer and IFN-γ SFU against the S protein. All the data in Fig. 1A–E from the 10 persons are labeled with different colors
Our findings demonstrate that specific T cell responses to multiple structural proteins (S, N, and E proteins) of SARS-CoV-2 were elicited in BBIBP-CorV recipients. This suggests that inactivated vaccines against SARS-CoV-2 may elicit T-cell responses in addition to humoral responses, and cellular responses may play a role in the protection offered by inactivated vaccines. Our findings also suggest that there is likely no association between vaccine-induced T cell responses and humoral immunity. The limitations of this study were the limited number of study participants and lack of a phenotypical characterization of the SARS-CoV-2 T-cell response. Further experiments will be very helpful for characterizing the T-cell subsets and their responses to SARS-CoV-2 in a larger sample of participants. It is important to determine the immune correlates of protection against SARS-CoV-2 infection.
Supplementary information
Acknowledgements
We thank all vaccine recipients for providing blood and PBMC samples. This work was supported by grants from the National Natural Science Foundation of China (82041041, 82061138008).
Author contributions
WJT and YD conceived of the study. YD and RY performed the experiment; YD, YL, and WJT analyzed the data; WJT and YL drafted the manuscript. All authors reviewed and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yao Deng, Yu Li.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-021-00730-8.
References
- 1.Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020;20:529–36. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassaniti I, Percivalle E, Bergami F, A Piralla, G Comolli, R Bruno, et al. SARS-CoV-2 specific T cell immunity in COVID-19 convalescent patients and unexposed controls measured by ex-vivo ELISpot assay. Clin Microbiol Infect. 2021. S1198-743X (21)00145-2. [DOI] [PMC free article] [PubMed]
- 3.Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2020;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prendecki M, Clarke C, Brown J, Cox A, Gleeson S, Guckian M, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–81. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun J, Loyal L, Frentsch M, Wendisch D, Georg P, Kurth F, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–4. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.