Abstract
Introduction
Pregnant women with covid-19 are more likely to experience preterm birth. The virus seems to be associated with a wide range of placental lesions, none of them specific.
Method
We collected cases of Covid-19 maternal infection during pregnancy associated with poor pregnancy outcomes, for which we received the placenta. We studied clinical data and described pathological findings of placenta and post-mortem examination of fetuses. We performed an immunohistochemical study and RT-PCR of SARS-Cov-2 on placenta samples.
Results
We report 5 cases of poor fetal outcome, 3 fetal deaths and 2 extreme premature neonates, one with growth restriction, without clinical and biological sign of SARS-Cov-2 infection. All placenta presented massive perivillous fibrin deposition and large intervillous thrombi associated with strong SARS-Cov-2 expression in trophoblast and SARS-CoV-2 PCR positivity in amniotic fluid or on placenta samples. Chronic histiocytic intervillositis was present in 4/5 cases. Placental ultrasound was abnormal and the sFLT1-PIGF ratio was increased in one case. Timing between mothers’ infection and the poor fetal outcome was ≤10 days in 4 cases. The massive placental damage are directly induced by the virus whose receptors are expressed on trophoblast, leading to trophoblast necrosis and massive inflammation in villous chamber, in a similar way it occurs in diffuse alveolar damage in adults infected by SARS-Cov-2.
Discussion
SARS-Cov-2 can be associated to a rare set of placental lesions which can lead to fetal demise, preterm birth, or growth restriction. Stronger surveillance of mothers infected by SARS-Cov-2 is required.
Keywords: SARS-Cov-2, Fetal demise, Placenta, COVID-19, Prenatal ultrasounds, sFLT1/PIGF, Histopathology
1. Introduction
Since late 2019, SARS-Cov-2 has been circulating all over the world infecting more than 70 million people and causing more than 2 million deaths [1]. Since the beginning of the pandemic, some authors tried to assess the transplacental transmission of the virus and its effects on both pregnancy and fetus [2,3]. It is now known that SARS-Cov-2 requires 2 receptors to infect a cell: the ACE2 and the TMPRSS2. Both are expressed in the syncytiotrophoblast. According to some authors, the low co-expression profile of those two receptors in the placenta probably explain the low risk of vertical transmission of SARS-CoV-2 from mother to fetus during pregnancy [4]. However the potential adverse effects of the virus on maternal and perinatal outcomes are of concern [5]. Indeed, pregnant women with covid-19 are at increased risk of admission to an intensive care unit and are more likely to experience preterm birth [6]. There are growing arguments for an increased risk of fetal death (2) and hypertensive complications of pregnancy [7]. Intra-uterine growth restriction (IUGR) seems to be three times more frequent than in COVID negative patients [5]. Furthermore there is an increased risk of abnormalities in fetal oxygenation that could induce cardiac and neurological disorders in fetus and neonates, including in patients without particular risk factors for COVID19 [8]. For these reasons, the International Society of Ultrasound in Obstetrics & Gynecology recommends monitoring the fetuses of infected mothers closely [9]. These anomalies could be explained by placental damage. Regarding placental lesions in SARS-Cov-2 infection, some authors found no significant placental histopathological changes [10] whereas other reported various lesions such as villositis, chorangiosis, chorioamniotis, fetal vascular malperfusion or fetal vascular thrombosis [11,12], villous edema and retroplacental hematoma [13] as well as massive fibrin deposition along with chronic histiocytic intervillositis [14,15]. None of them seem to be specific of SARS-Cov-2 infection [6]. However, some authors noticed that placentas from SARS‐CoV‐2‐positive women significantly harbor more villous agglutination and subchorionic thrombi than placentas from SARS‐CoV‐2‐negative women. Fetal deaths issued from Covid 19 infected mothers have been reported in a few isolated cases to date [14,[16], [17], [18]] and some authors suggested a link with placental lesions. We report here 5 cases of massive placental damage due to SARS-Cov-2 infection, harboring all similar pathological findings, which rapidly led to 3 fetal deaths and 2 extreme premature births requiring intensive care unit hospitalization. We clearly attribute this poor fetal outcome to this massive placental damage. We aim to alert clinicians of potential poor outcomes of SARS-cov-2 maternal infection during pregnancy and to better understand the pathophysiology of this infection.
2. Materials and method
In this study, from March 2020 to March 2021, we collected all cases of Covid-19 maternal infection during pregnancy associated with poor pregnancy outcomes including fetal death (FD) and prematurity, for which we received the placenta.
We assessed clinical and biological data of mothers and pregnancies: maternal age, ethnicity, past medical history, presence of predisposing factor of severe COVID-19 (hypertension, gestational diabetes, thrombophilia, auto-immune and thyroid disorders, smoking, drug and alcohol consumption and obesity or high Body Mass Index (BMI), prenatal ultrasounds findings, delivery mode, term of pregnancy, delay between maternal COVID-19 with PCR positivity from nasopharyngeal swab and adverse fetal outcome, and clinical signs which led to perform nasopharyngeal tests.
Data from postmortem examination and neonates’ outcome were also collected.
Macroscopic and microscopic features of placentas were described. Placental sampling, gross and microscopic examinations were performed according to the Amsterdam Consensus statement [19]. Samples of placenta were fixed in 4% buffered formalin and paraffin embedded (FFPE) and stained with Hematoxylin Eosin Saffron (HES). An immunohistochemical study was performed on FFPE placenta tissue with VENTANA™ OptiView DAB automat using the Bond Polymer Refine Detection kit (Leica™ DS9800) with the following antibodies: CD68 (Dako PG-M1, 1:200), CD3 (Dako F7.2.38, 1:50), CD4 (Ventana™, 1F6, prediluated), CD15 (LeuM1, Becton Dicikinson), CD8 (Dako, C8/144 B, 1:50), SARS-CoV-2 (Diagomics, rabbit pAB, 2019-nCoV N Protein, 1:2400). Negative controls for SARS-CoV-2 immunohistochemistry were performed on SARS-CoV-2 negative placental specimen with similar pre-analytic conditions.
RNA extraction from nasopharyngeal RT-PCR swabs, placentas and amniotic fluid was realized using MagNA Pure 24 from ROCHE Diagnostics™, according to GISAID database of January 2020, primers and probes (nCoV_IP2 and nCoV_IP4) were designed to target the RdRp gene spanning nt 12,621–12727 and 14,010–14116 (positions according to SARS-CoV, NC_004718). The same block of FFPE placenta was used to perform the Sars-Cov2 PCR and the immunostaining.
3. Results
From March 2020 to March 2021, 5 out of 82 placentas of Covid-19 infected mothers during pregnancy were associated with an adverse pregnancy outcome (3 FD and 2 extreme preterm births). One case occurred during the first French lockdown and 4 during the second one. All results are summarized in Table 1 .
Table 1.
Summary of our fives cases and comparison with similar cases.
| 1st case | 2nd case | 3rd case | 4th case | 5th case | Richtmann et al. (1st case) [16] | Richtmann et al. (2nd case) [16] | Pulinx et al. [17] | Schwartz et al. [18] | Hosier et al. [14] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Date of SARS-Cov-2 infection | April 2020 | September 2020 | November 2020 | December 2020 | December 2020 | NC | NC | NC | NC | March 2020 |
| Maternal age | 35 | 26 | 33 | 30 | 43 | 32 | 35 | 32 | 32 | 35 |
| Mother BMI (beginning at of the pregnancy) | 19,6 | 20.1 | 21 | 20.3 | 26.8 | 30.5 | 25.7 | NC | NC | NC |
| Ethnicity | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | NC | NC | Asian American |
| Obstetrical background | G2P1 | G1P0 | G2P1 | G1P0 | G11P8 | G1P0 | G2P1 | G2P1 | NC | G3P1 |
| Other background | Genital Herpes | None | None | None | Bypass surgery | None | None | NC | NC | None |
| Risk factors | None | None | None | None | Smoking (10 cigarettes a day before pregnancy) | None | None | Diabetes mellitus | NC | Psoriasis (without current symptoms) Gestational hypertension |
| Pregnancy follow up | Normal | Normal | Normal | Normal | IUGR at 26 GW | Normal | Normal | Normal | Normal | Normal |
| Clinical signs leading to SARS-Cov-2 RT-PCR | Fever | Fever | Fever | Ageusia and fever | Fever | Rhinorrhea, myalgia and fever | Fever, dry cough | Rhinitis and fever | None | Fever, cough, myalgia, nausea, anorexia, diarrhea |
| Delay between COVID PCR swab and onset of the Poor outcome | COVID day 6 | COVID day 10 | COVID day 10 | COVID day 4 | COVID day 28 | COVID day 4 | COVID day 6 | COVID day 15 | Unknown | COVID day 10 |
| Delivery mode | C-section | Natural | Natural | Natural | C-section | Natural | Natural | Natural | NC | Dilatation and evacuation (Under general anesthesia) |
| Term (weeks) | 26 + 5 | 34 + 5 | 30 | 22 + 4 | 26 + 5 | 28 | 21 | 24 | 39 | 22 |
| Sex | Female | Female | Male | Male | Female | Female | Male | NC | Male | Unknown |
| Fetal weight (g) | 680 | 2410 | 1210 | 465 | 630 | 1070 | 329 | NC | NC | 485 |
| Fetal outcome | Neonatal intensive unit care | Fetal death | Fetal Death | Fetal death | Neonatal intensive care unit | Fetal death | Fetal death | Fetal death | Fetal death | Termination of pregnancy (Severe Pre-eclampsia) |
| Autopsy results | Not concerned | No abnormality | No abnormality | No abnormality | Not concerned | NP | No abnormality | NP | NP | No abnormality |
| Placental weight (g) | 187 (<10th p+) | 346 (<10thp+) | 255 (<10th+) | 105 (<10thp+) | 196 (<10thp+) | NC | NC | NC | NC | NC |
| Ratio of fetal-placental weight ratio percentiles | 3.63 (25-50thp+) | 6.96 (50-75thp+) | 4.74 (25-50thp+) | 4.42 (>95thp+) | 3.21 (10-25thp+) | NC | NC | NC | NC | NC |
| Placental histology | CHI | CHI | CHI | CHI | Focal CHI | CHI | CHI | CHI | CHI | CHI |
| MPFD | MPFD | MPFD | MPFD | MPFD | MPFD | MPFD | MPFD | MPFD | MPFD | |
| LIT | LIT | LIT | LIT | VUE | ||||||
| SARS-Cov-2 RT-PCR on placenta (CT) | 23 | + (38.7) | + (27.59) | – | + (30.5) | + (NC) | NP | 33 | + (NC) | + (NC) |
| SARS-Cov-2 immunostaining | + | + | + | + | + | NP | NP | + | + | + |
3.1. Clinical and biological data of mothers and pregnancies
Mean age of mothers was 36,4-year-old (26–43 years old). All were Caucasians and had no peculiar past medical history, except one who had a gastric bypass surgery for obesity (case 5). Three were primiparous. Regarding obstetrical history, one had a background of genital herpes (case 1) which was absent at the time of the delivery, and one had 3 previous unexplained first trimester miscarriage (case 5). No mother presented with predisposing factor of severe COVID-19, except case 5 who still had a high BMI despite gastric bypass surgery.
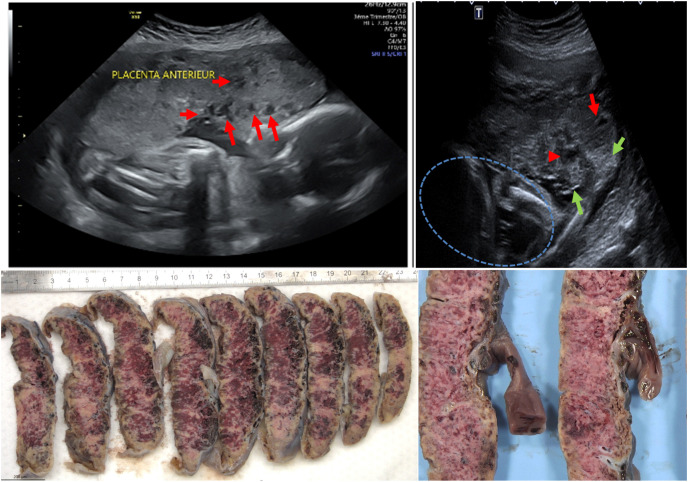
Prenatal US findings: second trimester prenatal US suggested fetal growth restriction in case 5 and were normal in other cases. Furthermore, an abnormal heterogeneous aspect of the placenta was noted in case 5 at 26 GW placenta with coexistence of hypo and hyperechoic areas, suggestive of infarction and calcification respectively. (Figs. .1 A and 1 B). This lead to test sFlt-1/PIGF which was increased (116 pg/mL). None of the five mothers presented with preeclampsia or HELLP syndrome. Coagulation tests were normal and Factor V Leiden mutation has been investigated in one patient (case number 1) and was negative.
Delivery mode and term of pregnancy: a C-section was performed in cases 1 and 5, at 26 + 5 GW because of fetal heart rate anomalies in case 1 and pathologic Doppler ultrasound of fetal vessels in case 5. Intrauterine fetal demises were observed in the 3 other cases and led to vaginal delivery at 34 W G, 30 W G, and 22 W G (case 2, 3 and 4 respectively). Parents gave their consents for post-mortem examination.
Delay between maternal nasopharyngeal swab PCR positivity and adverse fetal outcome or premature delivery was ≤10 days in 4 cases out of 5.
Fever was the only clinical sign which led to perform nasopharyngeal tests in mothers 1, 2, 3. Mother 4 presented also with ageusia, and mother 5 with fever, myalgia and rhinopharyngitis.
3.2. Neonates’ outcome
The 2 cases born prematurely by C-section were admitted and followed in a level III Neonatal Intensive Care Unit (NICU) for 3 months. They presented neither fever nor sign suggestive of infection. Nasopharyngeal swabs for SARS-CoV-2 RT-PCR performed at admission were negative in case 1 and was not performed in case 5. Apgar scores were 2-3-6-8 and 7-9-10-10 at 1, 3, 5 and 10 min, respectively. Both received basic cares of premature neonates including support for thermal, respiratory and nutrition. Both are now doing well with normal physical exam and normal development, after respectively 11 and 3 months of follow-up since NICU discharge.
3.3. Post-mortem examination
An autopsy was performed in the 3 fetal demises (cases 2, 3 and 4), 2 males and 1 female, and revealed neither gross nor microscopic abnormality, especially no IUGR, no sign suggestive of diabetes, no thrombosis, no malformation, no signs of vertically transmitted infection. We only noticed signs suggestive of chronic hypoxemia with lymphoid depletion in thymus and spleen in case 2.
3.4. Placenta findings
All 5 placentas had the same macroscopic features (Fig. 1 C and D) with whitish mottled net-like appearance involving at least 80% of parenchyma suggestive of massive fibrin deposition.
Fig. 1.
Prenatal ultrasounds and gross features of placenta (case 5): A: prenatal ultrasounds at 26 GW: hypoechoic lacunae (red arrows)
B: prenatal ultrasounds at 26 GW: coexistence of hyperechoic lesion (green arrows) and hypoechoic lesion (red arrows) suggestive of calcification and infarction respectively. Blue circle: Fetus
C: gross features on cut sections after formalin fixation: whitish mottled net-like appearance involving at least 80% of parenchyma suggestive of massive fibrin deposition
D: gross features on cut sections after formalin fixation: same anomalies on higher magnification.
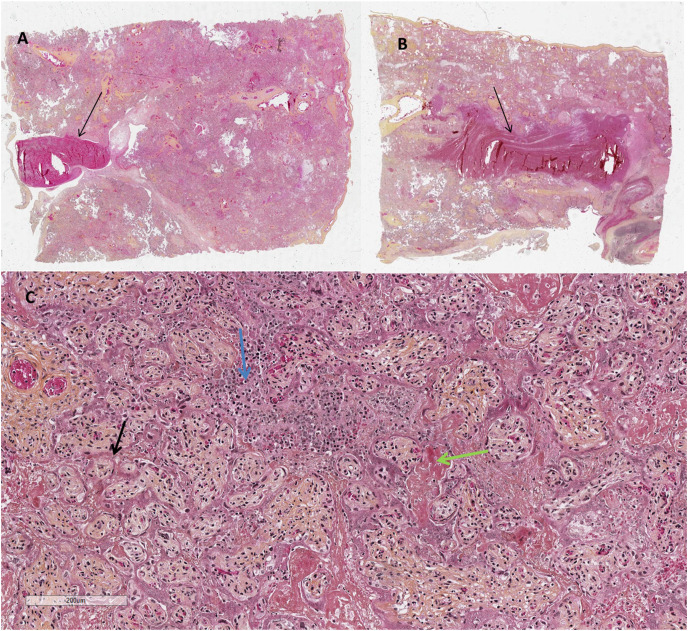
On microscopic examination, all placentas presented (Fig. 2 ) massive perivillous fibrin deposition (MPFD) and numerous large intervillous thrombi (LIT). Among areas of MPFD, trophoblast was necrotic and sometimes replaced by neutrophils or infiltrated by histiocytes and/or lymphocytes. Severe chronic histiocytic intervillositis (CHI) was also observed in all cases except one (case 5), which was the one with the largest delay between SarsCov2 infection and the poor outcome. The large intervillous thrombi were disseminated throughout all the placental parenchyma and were not only confined to the basal plate. The features were not those of infarction-hematoma or rounded intraplacental hematoma [[20], [21], [22]]. Furthermore there was no lesion suggestive of maternal vascular malperfusion such as infarct or decidual vasculopathy. We did not observe other lesions such as villositis, chorangiosis, chorioamniotis, or features suggestive of fetal vascular malperfusion.
Fig. 2.
Microscopic features of the placenta on HES (Hematoxylin Eosin Saffron)
A & B: x 5: 2 different cut sections showing large intervillous thrombi (black arrows)
C: x 200: MPFD with trophoblast necrosis (black arrow), fibrin deposits (green arrow) along with CHI (blue arrow).
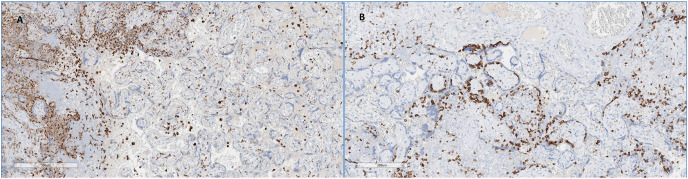
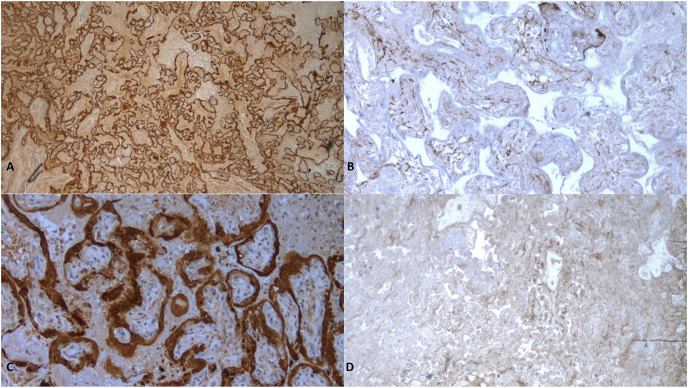
Immunohistochemistry (Fig. 3 ) showed that severe chronic histiocytic intervillositis was mainly composed of CD68+ macrophages along with lymphocytes T CD3+ CD8+. Neutrophils in areas of trophoblast necrosis expressed CD15. SARS-Cov-2 was diffusely expressed in trophoblast of all placentas (Fig. 4 ).
Fig. 3.
Immunohistochemical study
A: CD68 antibody x 80: numerous histiocytes in the intervillous chamber
B: CD3 antibody x 80: numerous lymphocytes inside or adjacent to the trophoblast.
Fig. 4.
Immunohistochemical study of Sars-Cov2 on placenta
A: case 2 (34+5 GW) x 25: diffuse and strong expression on trophoblast
B: negative control x 25: placenta from annon-infected mother at 34 GW: no expression on trophoblast
C: case 4 × 200 (22 +4 GW): diffuse and strong expression on trophoblast
D: negative control x 200: placenta from a non-infected mother at 22 GW: no expression on trophoblast.
SARS-Cov-2 RT-PCR from FFPE placenta samples was positive for case 1, 2, 3 and was negative for case 4. In case 5, RT-PCR was positive on both amniotic fluid and placenta sample.
3.5. Comment
3.5.1. Principal findings
We report 5 cases of poor fetal outcomes due to SARS-Cov-2 maternal infection during pregnancy leading to 3 fetal deaths and 2 extreme premature neonates who required NICU hospitalization. All cases have in common MPFD with LIT and strong SARS-Cov-2 expression in trophoblast. MPFD and LIT corresponded respectively to hypo and hyperechoic areas seen on prenatal US in case 5 at 26 GW. MPFD was associated with CHI in 4 cases out of 5. These placental lesions, especially MPFD and CHI, explain the poor fetal outcome as oxygen and nutriments exchange between mother and fetus are highly compromised, leading in turn to IUGR or FD.
3.5.2. Results
MPFD is a classical cause of fetal demises. The fundamental pathogenesis of this lesion remains unknown. Different hypotheses have been suggested: trophoblast anomaly, blood stasis in intervillous space or inflammatory cause [23]. Indeed, MPFD has been reported in Coxsackie virus, CMV and syphilis infections during pregnancy [[24], [25], [26], [27]]. Occasionally this disorder overlaps with maternal floor infarction. Focal PFD is reported in diabetes but no mother presented with diabetes in our series [23].
Numerous and large intervillous thrombi (LIT) were also present in 4 cases out of 5 in our series. LIT may develop in case of maternal thrombophilia or pre-eclampsia [23], which was not present in our series. According to Mendoza et al. numerous LIT can be related to a pre-eclampsia-like syndrome [28]. Indeed, Mendoza et al. reported pre-eclampsia-like syndrome in women with severe COVID19 pneumonia [28], with an increased sFlt1/PIGF ratio before the clinical onset of preeclampsia in 1 out of 42 mothers included in their series [28,29]. In our series, we only assessed sFlt1/PIGF ratio in one case which was >85 pg/mL, threshold beyond which the positive predictive value at admission for indicated delivery within 2 weeks is 91%[30]. As the mother did no present clinical signs of PE, increased sFLT1/PIGF ratio associated with placental US anomalies could be a way of foreseeing PE-like syndrome among COVID-19 pregnancies.
The increased sFLT1/PIGF ratio could be explained by trophoblast infected cells inducing cell apoptosis, thus in turn leading to massive release of anti-angiogenic factors.
Chronic histiocytic intervillositis (CHI) is a rare idiopathic inflammatory lesion distinct from villitis of unknown origin (VUE) and associated lesions. It is characterized by maternal histiocytic infiltrate in the intervillous space without villitis. CHI is usually highly suggestive of an immunological conflict between mother and fetus [23]. However CHI can be observed in some infections such as Chagas’ disease, malaria and cytomegalovirus [[31], [32], [33]]. Like MPFD, chronic histiocytic intervillositis is strongly associated with miscarriage, IUGR, preterm birth, and early intrauterine fetal demise. It has the highest recurrence rate of any placental lesion, sometimes affecting 10 or more consecutive pregnancies [12,13]. Interestingly, the only case without CHI in our series was the one with the largest delay (28 days) between infection and the poor outcome. Inflammatory elements might have been present in the intervillous space at the beginning of the infection of this case and then might have disappeared. This case is also the only one with IUGR. In the other cases of our series, the delay between infection and poor outcome might have been too short to allow IUGR to settle.
Twelve cases with MPFD/CIH dyad and Sars-cov2 infection during pregnancy have been reported in the literature, 8 of whom during the third trimester and 3 during the second one. Five cases out of 12 were associated with fetal demises (included in our table), including 1 termination of pregnancy at 22 GW for severe PE [3,14,[16], [17], [18],[34], [35], [36]].
Compared to our cases, predisposing factors for severe COVID 19 were present in 5/12 (3 BMI>25, 1 diabetes mellitus, 1 gestational hypertension), corresponding to the 5 cases with fetal demises. Four newborns were preterm and three of them were SARS-Cov-2 positive. One presented with neurological signs at physical examination and MRI [3,14,18,37].
We also report the 4th case in literature [3,16,38] of SARS-Cov-2 positive amniotic fluid. However, RT-PCR performed from placenta was negative, whereas SARS-Cov-2 was strongly expressed in trophoblast on immunohistochemistry. This mismatch could be explained by a false negative. SARS-Cov-2 mother-to-child transmission can be secondary to amniotic fluid inhalation [39]. However, we did not find any sign of mother to fetus infection on post-mortem examination in our case with RT-PCR amniotic fluid positivity. Microscopic examination of the fetus was normal without any neutrophils in lungs as reported in one case of FD by Richtmann et al. [16]. It is interesting to note that ACE-2 receptors are not expressed in fetal lungs whereas they are in adults [4,40].
3.6. Clinical implications
Finding such lesions on placental gross and microscopic examination should suggest the diagnosis of SARS-Cov-2 infection during pregnancy when COVID-19 has not been previously diagnosed. MPFD and LIT could be evoked on prenatal ultrasounds and this should alert clinicians in order they harder follow the pregnancy, look for IUGR as well as an increased sFlt1/PLG1 ratio suggestive of PE [29] and eventually process to premature delivery, especially as COVID-19 is an insidious disease and as poor outcome can happen in a short lapse of time.
3.7. Research implications
As we did not find any sign of mother to fetus infection on post-mortem examination as well as in the 2 premature neonates, the poor fetal outcome cannot be explained by the virus transmissibility to the fetus but rather by the massive placental damage.
Severe lung lesions with diffuse alveolar damage observed in infected adults are mainly caused by the cytokine storm and the exaggerate immune response [41]. The diffuse placental damage might be rather a consequence of the exaggerate mother inflammation (CHI) in the placenta than a consequence of the virus itself, just as it happens in lungs. Inflammatory elements involved in diffuse alveolar damage are the same we observed in intervillous spaces: CD68+ macrophages along with lymphocytes T CD3+ CD8+. Inflammation could lead in turn to perivillous deposition and villous necrosis, in parallelism with the fibrin deposition along the alveolar spaces. Trophoblastic necrosis could be either a direct cause of the virus or a consequence of the inflammation surrounding the trophoblast in reaction of the virus infection.
SARS-Cov-2 infection is also known to cause severe thrombosis [42] that might be explained by Tissue Factor activation by the virus itself. This could explain LIT as well as some cases of maternal vascular malperfusion observed in previous reports of maternal Covid 19 infection during pregnancy [2,14,15].
3.8. Strengths and limitations
Numerous pregnant women were infected by SARS-Cov-2 during the pandemic, but only a small fraction ended up in maternal or NICU hospitalization or fetal demise [43]. According to the literature and to our cases, this poor fetal outcome is linked to massive placental damage with MPFD, LIT and CHI. This massive placental damage could be compared to the one that occurs in lungs. In adult's severe form of COVID-19, there is some evidence of disturbed interferon profile in severe inflammatory reaction to SARS-Cov-2 [44]. Unfortunately, interferon profile was not realized in our series as it requires special blood sample conditioning which was not available at time of delivery. We could also not eliminate the role of mutants could have played in the poor prognosis of our cases, as mutations strengthened SARS-CoV-2 transmissibility and mortality [45].
4. Conclusions
We reported 5 cases of massive placental damages due to SARS-Cov-2 infection during pregnancy which led to 3 fetal deaths and 2 extreme premature births requiring NICU hospitalization. The poor outcome is explained by diffuse placental damage due to SARS-Cov-2 infection without transmission of the virus to the fetuses and neonates. The massive placental damage with MPFD, CHI and LIT are directly induced by placental infection by the virus whose receptors are expressed on trophoblast leading to inflammatory reaction in the villous chamber. This leads to trophoblast necrosis, massive fibrin deposition, and decreased exchange between mother and fetus in a short lapse of time. As some mothers did not present predisposing factor of severe COVID-19 or severe clinical sign of COVID-19, these findings should alert clinicians and entail them to look for IUGR and placental lesions on prenatal US and test sFlt1/PIGF ratio to predict possible pre-eclampsia-like syndrome.
Ethics declaration
Written informed consent was obtained from the woman for the publication of this report. According to French regulation, institutional review board (IRB) approval is not required for case reports, if patients’ written consent is obtained. The case study was performed in agreement with principles of the Declaration of Helsinki and CARE guidelines [46].
Contributions
Amine BOUACHBA, Fabienne ALLIAS, Beatrice NADAUD, Alexis TRECOURT and Sophie COLLARDEAU-FRACHON have gathered clinical data, performed gross and microscopic examination of fetuses and placentas and written the pathological description and discussion. They declare no conflict of interest.
Jerome MASSARDIER, Benoit DE LA FOURNIERE and Cyril HUISSOUD have followed the pregnancies, gathered clinical data and contributed to the discussion. They declare no conflict of interest.
Yahia MEKKI and Maude BOUSCAMBERT DUCHAMP performed viral analyses and contributed to the discussion. They declare no conflict of interest.
We also thank Pr. Sophie Prevot from Hôpital Antoine-Béclère, AP-HP, for her help in immunohistochemical techniques.
Conflict of interest statement
Amine BOUACHBA, Fabienne ALLIAS, Beatrice NADAUD, Alexis TRECOURT and Sophie COLLARDEAU-FRACHON declare no conflict of interest.
Jerome MASSARDIER, Benoit DE LA FOURNIERE and Cyril HUISSOUD declare no conflict of interest.
Yahia MEKKI and Maude BOUSCAMBERT DUCHAMP declare no conflict of interest.
Declaration of competing interest
None.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Inst. Pasteur.; 2020. Maladie Covid-19 (nouveau coronavirus)https://www.pasteur.fr/fr/centre-medical/fiches-maladies/maladie-covid-19-nouveau-coronavirus [Google Scholar]
- 2.Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab Med. 2020 doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 3.Transplacental transmission of SARS-CoV-2 infection | Nat. Commun., (n.d.). https://www.nature.com/articles/s41467-020-17436-6 (accessed February 3, 2021). [DOI] [PMC free article] [PubMed]
- 4.Lü M., Qiu L., Jia G., Guo R., Leng Q. Single-cell expression profiles of ACE2 and TMPRSS2 reveals potential vertical transmission and fetus infection of SARS-CoV-2. Aging. 2020;12:19880–19897. doi: 10.18632/aging.104015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., Debenham L., Llavall A.C., Dixit A., Zhou D., Balaji R., Lee S.I., Qiu X., Yuan M., Coomar D., Sheikh J., Lawson H., Ansari K., van Wely M., van Leeuwen E., Kostova E., Kunst H., Khalil A., Tiberi S., Brizuela V., Broutet N., Kara E., Kim C.R., Thorson A., Escuriet R., Oladapo O.T., Mofenson L., Zamora J., Thangaratinam S. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smithgall M.C., Liu‐Jarin X., Hamele‐Bena D., Cimic A., Mourad M., Debelenko L., Chen X. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology. 2020;77:994–999. doi: 10.1111/his.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenbloom J.I., Raghuraman N., Carter E.B., Kelly J.C. Coronavirus disease 2019 infection and hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 2021 doi: 10.1016/j.ajog.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raschetti R., Vivanti A.J., Vauloup-Fellous C., Loi B., Benachi A., De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat. Commun. 2020;11:5164. doi: 10.1038/s41467-020-18982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Mascio D., Buca D., Berghella V., Khalil A., Rizzo G., Odibo A., Saccone G., Galindo A., Liberati M., D'Antonio F. Counseling in maternal-fetal medicine: SARS-CoV-2 infection in pregnancy, Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2021 doi: 10.1002/uog.23628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulersen M., Prasannan L., Tam Tam H., Metz C.N., Rochelson B., Meirowitz N., Shan W., Edelman M., Millington K.A. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am. J. Obstet. Gynecol. Mfm. 2020;2:100211. doi: 10.1016/j.ajogmf.2020.100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baergen R.N., Heller D.S. Placental pathology in covid-19 positive mothers: preliminary findings. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2020;23:177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhu M., Cagino K., Matthews K.C., Friedlander R.L., Glynn S.M., Kubiak J.M., Yang Y.J., Zhao Z., Baergen R.N., DiPace J.I., Razavi A.S., Skupski D.W., Snyder J.R., Singh H.K., Kalish R.B., Oxford C.M., Riley L.E. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG An Int. J. Obstet. Gynaecol. 2020;127:1548–1556. doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosier H., Farhadian S.F., Morotti R.A., Deshmukh U., Lu-Culligan A., Campbell K.H., Yasumoto Y., Vogels C.B.F., Casanovas-Massana A., Vijayakumar P., Geng B., Odio C.D., Fournier J., Brito A.F., Fauver J.R., Liu F., Alpert T., Tal R., Szigeti-Buck K., Perincheri S., Larsen C., Gariepy A.M., Aguilar G., Fardelmann K.L., Harigopal M., Taylor H.S., Pettker C.M., Wyllie A.L., Cruz C.D., Ring A.M., Grubaugh N.D., Ko A.I., Horvath T.L., Iwasaki A., Reddy U.M., Lipkind H.S. SARS–CoV-2 infection of the placenta. J. Clin. Invest. 2020;130:4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menter T., Mertz K.D., Jiang S., Chen H., Monod C., Tzankov A., Waldvogel S., Schulzke S.M., Hösli I., Bruder E. Placental pathology findings during and after SARS-CoV-2 infection: features of villitis and malperfusion. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2021;88:69–77. doi: 10.1159/000511324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: A case series, (n.d.). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7354271/(accessed February 3, 2021). [DOI] [PMC free article] [PubMed]
- 17.Pulinx B., Kieffer D., Michiels I., Petermans S., Strybol D., Delvaux S., Baldewijns M., Raymaekers M., Cartuyvels R., Maurissen W. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur. J. Clin. Microbiol. Infect. Dis. 2020:1–5. doi: 10.1007/s10096-020-03964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz D.A., Morotti D. Placental pathology of COVID-19 with and without fetal and neonatal infection: trophoblast necrosis and chronic histiocytic intervillositis as risk factors for transplacental transmission of SARS-CoV-2. Viruses. 2020;12 doi: 10.3390/v12111308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khong T.Y., Mooney E.E., Ariel I., Balmus N.C.M., Boyd T.K., Brundler M.-A., Derricott H., Evans M.J., Faye-Petersen O.M., Gillan J.E., Heazell A.E.P., Heller D.S., Jacques S.M., Keating S., Kelehan P., Maes A., McKay E.M., Morgan T.K., Nikkels P.G.J., Parks W.T., Redline R.W., Scheimberg I., Schoots M.H., Sebire N.J., Timmer A., Turowski G., van der Voorn J.P., van Lijnschoten I., Gordijn S.J. Sampling and definitions of placental lesions: Amsterdam placental workshop group Consensus statement. Arch. Pathol. Lab Med. 2016;140:698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald B., Shannon P., Kingdom J., Keating S. Rounded intraplacental haematomas due to decidual vasculopathy have a distinctive morphology. J. Clin. Pathol. 2011;64:729–732. doi: 10.1136/jcp.2010.087916. [DOI] [PubMed] [Google Scholar]
- 21.Aurioles-Garibay A., Hernandez-Andrade E., Romero R., Qureshi F., Ahn H., Jacques S.M., Garcia M., Yeo L., Hassan S.S. Prenatal diagnosis of a placental infarction hematoma associated with fetal growth restriction, preeclampsia and fetal death: clinicopathological correlation. Fetal Diagn. Ther. 2014;36:154–161. doi: 10.1159/000357841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bendon R.W. Nosology: infarction hematoma, a placental infarction encasing a hematoma. Hum. Pathol. 2012;43:761–763. doi: 10.1016/j.humpath.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Kraus F.T. first ed. American Registry of Pathology; Washington, DC: 2005. Placental Pathology. [Google Scholar]
- 24.Yu W., Tellier R., Wright J.R. Coxsackie virus A16 infection of placenta with massive perivillous fibrin deposition leading to intrauterine fetal demise at 36 Weeks gestation. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2015;18:331–334. doi: 10.2350/15-01-1603-CR.1. [DOI] [PubMed] [Google Scholar]
- 25.Sanjita Ravishankar; 2021. Massive perivillous fibrin deposition in congenital cytomegalovirus infection: a case report - catherine K gestrich, yi yuan zhou.https://journals.sagepub.com/doi/abs/10.1177/1093526620961352 (n.d.) [DOI] [PubMed] [Google Scholar]
- 26.Nutchanok Thawornwong, Paul Scott Thorner; 2021. Massive perivillous fibrin deposition associated with placental syphilis: a case report - mana taweevisit.https://journals.sagepub.com/doi/abs/10.1177/1093526620957523 (n.d.) [DOI] [PubMed] [Google Scholar]
- 27.Redline R.W. Extending the spectrum of massive perivillous fibrin deposition (maternal floor infarction) Pediatr. Dev. Pathol. 2021;24:10–11. doi: 10.1177/1093526620964353. [DOI] [PubMed] [Google Scholar]
- 28.Mendoza M., Garcia-Ruiz I., Maiz N., Rodo C., Garcia-Manau P., Serrano B., Lopez-Martinez R.M., Balcells J., Fernandez-Hidalgo N., Carreras E., Suy A. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG An Int. J. Obstet. Gynaecol. 2020;127:1374–1380. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeisler H., Llurba E., Chantraine F., Vatish M., Staff A.C., Sennström M., Olovsson M., Brennecke S.P., Stepan H., Allegranza D., Dilba P., Schoedl M., Hund M., Verlohren S. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N. Engl. J. Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 30.Baltajian K., Bajracharya S., Salahuddin S., Berg A.H., Geahchan C., Wenger J.B., Thadhani R., Karumanchi S.A., Rana S. Sequential plasma angiogenic factors levels in women with suspected preeclampsia. Am. J. Obstet. Gynecol. 2016;215 doi: 10.1016/j.ajog.2016.01.168. 89.e1-89.e10. [DOI] [PubMed] [Google Scholar]
- 31.Altemani A.M., Bittencourt A.L., Lana A.M. Immunohistochemical characterization of the inflammatory infiltrate in placental Chagas' disease: a qualitative and quantitative analysis. Am. J. Trop. Med. Hyg. 2000;62:319–324. doi: 10.4269/ajtmh.2000.62.319. [DOI] [PubMed] [Google Scholar]
- 32.Ordi J., Ismail M.R., Ventura P.J., Kahigwa E., Hirt R., Cardesa A., Alonso P.L., Menendez C. Massive chronic intervillositis of the placenta associated with malaria infection. Am. J. Surg. Pathol. 1998;22:1006–1011. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Taweevisit M., Sukpan K., Siriaunkgul S., Thorner P.S. Chronic histiocytic intervillositis with cytomegalovirus placentitis in a case of hydrops fetalis. Fetal Pediatr. Pathol. 2012;31:394–400. doi: 10.3109/15513815.2012.659405. [DOI] [PubMed] [Google Scholar]
- 34.Mongula J.E., Frenken M.W.E., van Lijnschoten G., Arents N.L.A., de Wit‐Zuurendonk L.D., Kok A.P.A.S., Heimel P.J. van R., Porath M.M., Goossens S.M.T.A. COVID-19 during pregnancy: non-reassuring fetal heart rate, placental pathology and coagulopathy. Ultrasound Obstet. Gynecol. 2020;56:773–776. doi: 10.1002/uog.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valk J.E., Chong A.M., Uhlemann A.-C., Debelenko L. Detection of SARS-CoV-2 in placental but not fetal tissues in the second trimester. J. Perinatol. 2020:1–3. doi: 10.1038/s41372-020-00877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linehan L., O'Donoghue K., Dineen S., White J., Higgins J.R., Fitzgerald B. SARS-CoV-2 placentitis: an uncommon complication of maternal COVID-19. Placenta. 2021;104:261–266. doi: 10.1016/j.placenta.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirtsman M., Diambomba Y., Poutanen S.M., Malinowski A.K., Vlachodimitropoulou E., Parks W.T., Erdman L., Morris S.K., Shah P.S. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. Can. Med. Assoc. J. 2020;192 doi: 10.1503/cmaj.200821. E647–E650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamaniyan M., Ebadi A., Aghajanpoor S., Rahmani Z., Haghshenas M., Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat. Diagn. 2020;40:1759–1761. doi: 10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa S., Posteraro B., Marchetti S., Tamburrini E., Carducci B., Lanzone A., Valentini P., Buonsenso D., Sanguinetti M., Vento G., Cattani P. Excretion of SARS-CoV-2 in human breast milk. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020;26:1430–1432. doi: 10.1016/j.cmi.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology - EBioMedicine, (n.d.). https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(20)30480-1/fulltext (accessed February 3, 2021). [DOI] [PMC free article] [PubMed]
- 41.Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eslamifar Z., Behzadifard M., Soleimani M., Behzadifard S. Coagulation abnormalities in SARS-CoV-2 infection: overexpression tissue factor. Thromb. J. 2020;18:38. doi: 10.1186/s12959-020-00250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C., O'Brien P., Quigley M., Brocklehurst P., Kurinczuk J.J. UK Obstetric Surveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group, Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., Manry J., Shaw E., Haljasmägi L., Peterson P., Lorenzo L., Bizien L., Trouillet-Assant S., Dobbs K., de Jesus A.A., Belot A., Kallaste A., Catherinot E., Tandjaoui-Lambiotte Y., Pen J.L., Kerner G., Bigio B., Seeleuthner Y., Yang R., Bolze A., Spaan A.N., Delmonte O.M., Abers M.S., Aiuti A., Casari G., Lampasona V., Piemonti L., Ciceri F., Bilguvar K., Lifton R.P., Vasse M., Smadja D.M., Migaud M., Hadjadj J., Terrier B., Duffy D., Quintana-Murci L., van de Beek D., Roussel L., Vinh D.C., Tangye S.G., Haerynck F., Dalmau D., Martinez-Picado J., Brodin P., Nussenzweig M.C., Boisson-Dupuis S., Rodríguez-Gallego C., Vogt G., Mogensen T.H., Oler A.J., Gu J., Burbelo P.D., Cohen J.I., Biondi A., Bettini L.R., D'Angio M., Bonfanti P., Rossignol P., Mayaux J., Rieux-Laucat F., Husebye E.S., Fusco F., Ursini M.V., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Castagnoli R., Montagna D., Licari A., Marseglia G.L., Duval X., Ghosn J., Lab H., N.-U.I.R. to C. Group§. Clinicians C., Clinicians C.-S., Group§ I.C., Group§ F.C.C.S., Consortium T.M.I., Cohort C.-C., 19 Biobank§ A.U.C., Effort§ C.H.G., Tsang J.S., Goldbach-Mansky R., Kisand K., Lionakis M.S., Puel A., Zhang S.-Y., Holland S.M., Gorochov G., Jouanguy E., Rice C.M., Cobat A., Notarangelo L.D., Abel L., Su H.C., Casanova J.-L. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J., Wang R., Wang M., Wei G.-W. Mutations strengthened SARS-CoV-2 infectivity. J. Mol. Biol. 2020;432:5212–5226. doi: 10.1016/j.jmb.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.79(4)373.pdf, (n.d.). https://www.who.int/bulletin/archives/79(4)373.pdf (accessed February 3, 2021).