Tacrolimus, a calcineurin inhibitor, is commonly used to prevent rejection in kidney transplantation, but it has a narrow therapeutic window. Blood concentrations of tacrolimus ([Tac]b) are known to be affected by drug-drug interactions, diarrhea, and hepatic dysfunction.1 We observed high (Tac)b in several patients with confirmed SARS-CoV-2 infection on tacrolimus for kidney transplant immunosuppression, which could not be explained by typical interactions or any change in dosing. There is little known about the impact of SARS-CoV-2 infection on (Tac)b. This retrospective case-control study was undertaken to examine the association between SARS-CoV-2 infection and (Tac)b in our kidney transplant recipients compared with kidney transplant recipients presenting with other infections. Methods are available in the Supplementary Methods.
Results
Sixty kidney transplant recipients under follow-up by our service tested positive for SARS-CoV-2 between March 27, 2020, and January 19, 2021, inclusive. After applying exclusion criteria to ensure that patients were on a stable dose between baseline and peak (Tac)b, 27 case patients were eligible for analysis. The control group consisted of 12 patients with urinary tract infections, 9 with lower respiratory tract infections (including 3 confirmed influenza A cases), 4 bacterial infections of uncertain source, and 2 cases of gastroenteritis. Table 1 summarizes the baseline characteristics of the 2 groups. At baseline, the majority of patients were on a triple immunosuppression regimen of tacrolimus, mycophenolate, and prednisolone; further details are described in Table 1.
Table 1.
Baseline characteristics of SARS-CoV-2 cases and control groups
| SARS-CoV-2 cases |
Control |
|||||
|---|---|---|---|---|---|---|
| All (N = 27) | With GI symptoms (n = 12) | Without GI symptoms (n = 15) | All (N = 27) | With GI symptoms (n = 8) | Without GI symptoms (n = 19) | |
| Age (median) | 54.5 | 56.5 | 44 | 61 | 61 | 59 |
| Sex (% female) | 44.4 | 50 | 40 | 40.7 | 50 | 36.8 |
| Tacrolimus dose (mg/d) | 4 | 3 | 5 | 4 | 4.25 | 3 |
| Mycophenolate (mg/d, no. of patients) | 1000, n = 21 | 1000, n = 9 | 1000, n = 12 | 1000, n = 24 | 1000, n = 7 | 1000, n = 17 |
| Azathioprine (mg/d, no. of patients) | 100, n = 2 | n = 0 | 100, n = 2 | 50, n = 1 | n = 0 | 50, n = 1 |
| Prednisolone (mg/d, no. of patients) | 5, n = 26 | 5, n = 11 | 5, n = 15 | 5, n = 27 | 5, n = 8 | 5, n = 19 |
| Baseline (Tac)b (median, μg/l) | 6.7 | 5.9 | 6.8 | 5.7 | 6.1 | 5.4 |
| Time from transplant to presentation (median, months) | 61.7 | 44 | 61.7 | 67.6 | 53.7 | 68.8 |
| Time from baseline (Tac)b to peak (Tac)b (d) | 42 | 38.5 | 55 | 26 | 21 | 30 |
| Oxygen requirement (%) | 48.1 | 50 | 46.7 | 14.8 | 0 | 21.1 |
| Gastrointestinal symptoms (%) | 44.4 | 100 | 0 | 29.6 | 100 | 0 |
| Acute kidney injury (%) | 37 | 50 | 26.7 | 37 | 50 | 31.6 |
| Raised AST/ALT (%) | 22.2 | 33.3 | 13.3 | 0 | 0 | 0 |
| Managed solely as outpatient (%) | 25.9 | 8.3 | 40 | 25.9 | 12.5 | 31.6 |
| Managed on HDU/ICU (%) | 14.8 | 16.7 | 13.3 | 14.8 | 0 | 21.8 |
| Managed on general ward outside of renal unit (%) | 25.9 | 20 | 26.7 | 25.9 | 12.5 | 31.6 |
| Inpatient length of stay (d, median) | 8.5 | 12 | 8 | 7.5 | 5 | 8 |
| Antibiotics given (%) | 59.3 | 83.3 | 40 | 96.2 | 100 | 94.7 |
| 90-day mortality (%) | 22.2 | 33.3 | 13.3 | 3.7 | 0 | 5.3 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GI, gastrointestinal; HDU, high-dependency unit; ICU, intensive care unit; (Tac)b, trough tacrolimus blood concentration.
No patients were treated with hydroxychloroquine or lopinavir/ritonavir, which had only been used in the United Kingdom as part of clinical trials. From May 2020, after the benefit shown by the RECOVERY (Randomised Evaluation of COVID-19 Therapy) trial,2 hospitalized patients with an oxygen requirement were treated with dexamethasone 6 mg; 9 of the SARS-CoV-2–positive patients received this treatment. Supplementary Table S1 describes all antimicrobials that were given in 2 or more patients. No patients were on macrolide antibiotics or triazole antifungals before the peak (Tac)b.
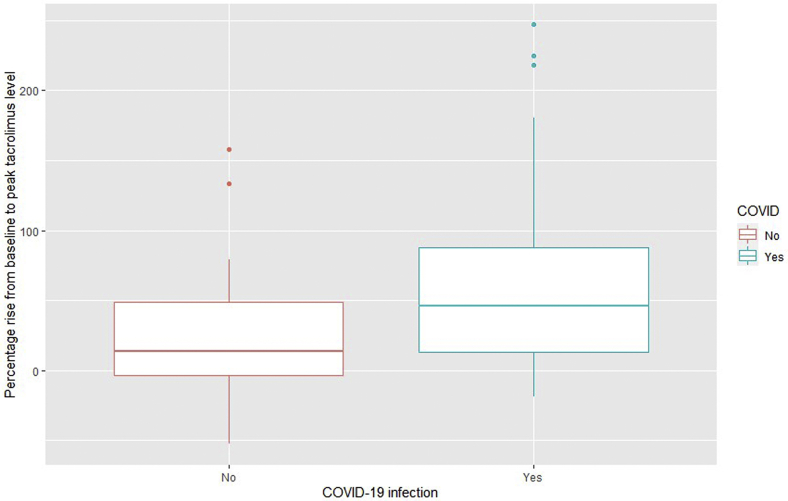
(Tac)b rose in both groups, but a significantly higher peak rise in (Tac)b was observed in the SARS-CoV-2 group, as demonstrated in Figure 1 and Table 2. This trend was observed in different subgroups, although the numbers in these groups were small (Supplementary Table S2) and did not achieve significance.
Figure 1.
The percentage rise in baseline to peak tacrolimus blood concentration in the control group and the SARS-CoV-2–positive group (229 × 145 mm [96 × 96 dots per inch]).
Table 2.
Comparison of changes in the tacrolimus blood concentration between the SARS-CoV-2 and control groups
| SARS-CoV-2 cases | Controls | P value | |
|---|---|---|---|
| Patients with observed rise in within 21 days, % | 85.2 | 70.4 | 0.18 |
| Time to first (Tac)b after presentation (median, d) | 2 | 2 | |
| First (Tac)b after presentation (median, range, μg/l) | 8.1 (4.1–26.7) | 6.1 (2.5–21) | |
| Time to peak (Tac)b from presentation (median, d)a | 6.5 | 3 | |
| Peak (Tac)b (median, range, μg/l) | 10.8 (4.4–26.7) | 7.1 (3–23.7) | |
| % Rise to peak (Tac)b from baseline (median, range) | 46.1 (−18.5 to 247) | 13.7 (−52.7 to 158) | 0.04052 |
| % Rise to peak (Tac)b from baseline (mean) | 69 | 26.5 | 0.018 |
| Time from symptom onset to presentation (median, d) | 5 | 3 | |
| Time to peak (Tac)b from symptom onset (median, d) | 11 | 8 |
(Tac)b, trough tacrolimus blood concentration.
For the SARS-CoV-2 case group, this was defined as days from the first positive test to peak (Tac)b; for the control group, this was defined as days from hospital presentation to peak (Tac)b.
The subgroup of SARS-CoV-2 cases with gastrointestinal symptoms saw the largest median rise in (Tac)b (104% vs. 24% in those with no gastrointestinal symptoms, P = 0.04144). Similar nonsignificant trends were noted in SARS-CoV-2–positive cases with raised transaminases versus those without (median rises of 85.5% vs. 39% respectively; P = 0.14) and those treated with dexamethasone 6 mg versus those who were not (median rises 73.3% vs. 32.8%, respectively; P = 0.75).
Four of the 12 (33%) patients with SARS-CoV-2 who had a (Tac)b elevation more than 50% from baseline died compared with only 2 of 15 (13%) who did not have such an observed elevation.
Discussion
This retrospective single-center case-control study suggests that SARS-CoV-2 is associated with a rise in (Tac)b beyond that seen in other infections in kidney transplant recipients. This is particularly the case for those with vomiting, diarrhea, or elevated hepatic transaminases. Diarrhea has been shown to be common in kidney transplant recipients with COVID-19 infection.3 Despite this, a trend was seen in all subgroups, even those without gastrointestinal symptoms or liver enzyme elevation. Potential theories may be symptomatic or asymptomatic alterations in bowel motility affecting absorption or subclinical alterations in hepatic enzyme metabolism, such as the cytochrome P450 pathway. Nausea and anorexia, which were not analyzed in this study because of the potential for variability in reporting, may also have affected absorption of the drug by affecting dietary intake. Antibiotic prescribing may have been a factor, but it is worth noting that fewer patients were prescribed antibiotics than in the control group.
One-third of patients in the SARS-CoV-2 group received dexamethasone 6 mg, a substantially higher dose of steroid than the typical doubling of baseline maintenance corticosteroid that occurred in hospitalizations with other infections. Dexamethasone has been shown to be a CYP3A inducer, and thus lower (Tac)b rather than a higher blood concentration would be typically expected.4,5 However, the dexamethasone-treated group was likely the sickest of the patients with SARS-CoV-2 because of the requirement to be hospitalized with an oxygen requirement before treatment initiation.
Patients with a witnessed rise in (Tac)b were more likely to die from SARS-CoV-2 infection. It is difficult to know whether this is because higher (Tac)b may have led to increased mortality due to toxicity and increased immunosuppression or if those patients who were critically ill had disordered drug metabolism leading to elevated (Tac)b.
A recent multicenter study of 102 solid organ transplant recipients from New York also identified this association, both in patients with and without diarrhea.6 Another case series from Rhode Island identified elevated tacrolimus and sirolimus blood concentrations in 66% of kidney transplant patients admitted with SARS-Cov-2 infection.7
The present study adds further information by including a significant proportion (25.9%) of nonhospitalized patients and, by the addition of a control group, aims to account for some of the natural dose fluctuations that may occur when patients are admitted to the hospital. By matching patients based on their place of care, it attempts to adjust for differences in terms of how unwell they were. Similarly, management in a specialist renal inpatient setting rather than a nonspecialist setting may have influenced how closely (Tac)b was monitored.
There was wide variation in the changes in (Tac)b, and, therefore, it is not possible based on this data to recommend a dose-reduction strategy in patients with COVID-19 disease. It is prudent to ensure that therapeutic drug monitoring is possible for those with COVID-19 infection, particularly if there are gastrointestinal symptoms. However, this would need to be balanced against the risks to the staff undertaking therapeutic monitoring and the risk to the wider population that COVID-19–positive patients traveling in the community may pose.
The limitations of this study are the relatively small numbers and its single-center, retrospective nature. More than 50% of patients had to be excluded due to a lack of contemporaneous (Tac)b or dose adjustments before (Tac)b was taken. Because of protocols in place for the protection of staff and other patients, only patients who felt unwell would have attended a hospital or clinic and had a blood test taken. Patients with mild symptoms of COVID-19 disease or an asymptomatic test would usually have had their outpatient clinic appointment postponed until they were past the window of transmissibility.
In summary, we have identified an association between SARS CoV-2 infection and an elevation in serum (Tac)b in kidney transplant recipients, which is out of keeping with that observed in other infectious presentations. Clinicians should be aware of the potential need for a temporary reduction in the tacrolimus dose during SARS CoV-2 infection.
Disclosure
All the others declared no competing interests.
Data Sharing
The data underlying this article cannot be shared publicly in order to preserve confidentiality for the individuals in the study. The data will be shared on reasonable request to the corresponding author.
Author Contributions
JM, CG, and HB were involved in conception and design of the study. The analysis was conducted by JM, LH, and CG. All authors contributed to the interpretation of the analysis and the direction of the discussion. JM wrote the first draft of the manuscript, and all authors were involved in revision, editing, and approval of the final manuscript.
Footnotes
Supplementary Methods
Table S1. Comparison of antibiotics used in the 2 groups.
Table S2. Comparison of changes in tacrolimus blood concentration between the SARS-CoV-2 and control groups in the selected subgroups.
Supplementary Material
Supplementary Methods
Table S1. Comparison of antibiotics used in the 2 groups.
Table S2. Comparison of changes in tacrolimus blood concentration between the SARS-CoV-2 and control groups in the selected subgroups.
References
- 1.Schiff J., Cole E., Cantarovich M. Therapeutic monitoring of calcineurin inhibitors. Clin J Am Soc Nephrol. 2007;2(2):374–384. doi: 10.2215/CJN.03791106. [DOI] [PubMed] [Google Scholar]
- 2.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira M., Mohan S., Cohen D. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam S., Partovi N., Ting L., Ensom M. Corticosteroid interactions with cyclosporine, tacrolimus, mycophenolate, and sirolimus: fact or fiction? Ann Pharmacol. 2008;42(7):1037–1047. doi: 10.1345/aph.1k628. [DOI] [PubMed] [Google Scholar]
- 5.Anglicheau D., Flamant M., Schlageter M.H. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant. 2003;18(11):2409–2414. doi: 10.1093/ndt/gfg381. [DOI] [PubMed] [Google Scholar]
- 6.Salerno D., Kovac D., Corbo H. SARS-CoV-2 infection increases tacrolimus concentrations in solid-organ transplant recipients. Clin Transplant. 2021;35(3):e14193. doi: 10.1111/ctr.14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardesty A., Pandita A., Vieira K. Coronavirus disease in 2019 in kidney transplant recipients: single-center experience and case-control study. Transplant Proc. 2021;53(4):1187–1193. doi: 10.1016/j.transproceed.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.