Abstract

Tin chalcogenides are considered as promising anode materials for lithium-ion batteries (LIBs) due to their high theoretical lithium-storage capacity. Herein, we have successfully synthesized the composites of tin chalcogenides and graphite, that is, SnS/C, SnSe/C, and SnS0.5Se0.5/C, via a simple one-pot solid-state method. During the electrochemical test, they exhibit excellent lithium-storage ability and cyclic performance as the anode electrodes of LIBs due to the introduction of carbon. In particular, (i) SnS/C displayed a high specific capacity of 875 mAh g–1 at 0.2 A g–1 over 200 cycles; (ii) SnSe/C presents 734 mAh g–1 at 0.2 A g–1 after 100 cycles, and it delivers 690 mAh g–1 at 1.0 A g–1 over 300 cycles; and (iii) the SnS0.5Se0.5/C composite electrode delivers a specific capacity of 643 mAh g–1 at 0.5 A g–1 over 150 cycles. Furthermore, another series of tin-based composites have also been successfully fabricated (i.e., Sn/C, SnS2/C, SnSe2/C, and SnTe/C), showing the general applicability of the synthetic route applied here. Our synthetic approach demonstrates a promising route for the large-scale production of high-performance tin chalcogenides/C anode materials for LIBs and other battery systems (e.g., Na-ion and K-ion batteries).
Introduction
Due to the shortage of fossil energy and the environmental pollution caused by fossil energy, electric vehicles powered by lithium-ion batteries (LIBs) are more and more popular in the auto market.1−3 However, facing the competition of internal combustion engines, there is an urgent demand for the improvement of energy densities of LIBs. Nowadays, the widely used anode material of LIBs is graphite, owing to its low cost and high chemical stability. However, the theoretical specific capacity of graphite is relatively low, only 372 mAh g–1.4−7 Thus, massive research works have been done to develop alternative anodes with enhanced specific capacities.8−10 Tin-based compounds such as tin metal,11,12 tin oxides,13,14 tin chalcogenides,15,16 and tin phosphides17,18 have been considered as a promising family for alternative anodes owing to their high theoretical specific capacity, cost-effectiveness, and eco-friendliness. However, these materials have huge volume change problems during the charging/discharging cycling, causing poor stability. Over the last decade, a lot of efforts have been dedicated to solve these problems. These efforts can be generally categorized into two types: (i) decreasing the particle size into nanoscale to protect the structural integrity and (ii) combining tin-based compounds with conductive materials (e.g., graphite) to alleviate volume expansion and particle aggregation.19−23 Based on these efforts, a large number of literature about carbon-modified tin or tin chalcogenides, for example, Sn/C,24,25 SnS/C,26,27 and SnSe/C,28,29 had been reported and they exhibited improved electrochemical properties for Li-ion batteries.
Up to now, the reported pathways for the synthesis of tin chalcogenides mainly include the solvothermal method,26,28 hydrothermal method,30 solution method,31 coprecipitation method,32 and chemical vapor transport.33 However, these synthetic approaches have shown several drawbacks at present, such as complicated reaction conditions and relatively poor repeatability. Herein, we developed a simple one-pot route by employing tetraphenyltin as the tin source and successfully synthesized Sn(S, Se)/C composites without any complex equipment, harsh reagents, and reaction conditions. Elemental analysis revealed that the carbon contents of the as-synthesized SnS/C, SnSe/C, and SnS0.5Se0.5/C were 32.8, 31.6, and 36.8 wt %, respectively. During the electrochemical test, the as-synthesized Sn(S, Se)/C composites exhibited excellent electrochemical properties, which were comparable to the best properties of Sn(S, Se) anode materials reported in the literature. Furthermore, we extended the one-pot route to synthesize other series of tin-based composites, demonstrating the general applicability for the synthesis of high-performance tin-based anode materials.
Experimental section
Materials
Tetraphenyltin (Sn(C6H5)4, 97% purity), sublimate sulfur (99.95% purity), selenium powder (99.9% purity), tellurium powder (99.9% purity), dibenzyl disulfide (98% purity), and dibenzyl diselenide (95% purity) were purchased from Aladdin Company. No further purification of reagents was done.
Synthetic Methods
For the typical synthesis of SnS/C and SnSe/C composites, powders of 0.2 g of tetraphenyltin and 0.015 g of sublimate sulfur or 0.037 g of selenium powder were mixed together and sealed in a vacuumized quartz tube. Then, the quartz tube was annealed at 873 K for 10 h. The SnS0.5Se0.5/C composite was prepared from a stoichiometric mixture of tetraphenyltin, sublimate sulfur, and selenium powder. The mixture was then sealed in a vacuumized quartz tube and annealed at 873 K for 10 h. Scheme 1 schematically illustrates the synthetic process for Sn(S,Se)/C composites. Then, the as-prepared product was collected, washed several times with DI water and absolute alcohol, and dried in a vacuum oven at 60 °C.
Scheme 1. Schematic Illustration of Synthetic Routes for Sn(S,Se)/C Composites.

Structural Characterization
The purity of the samples was examined by powder XRD on a Philips X’Pert diffractometer with a Cu Kα radiation (λ = 1.5418 Å). Field emission scanning electron microscopy (SEM, JEOLJSM-6700F) was used for the morphological studies of the prepared samples. X-ray photoelectron spectral studies were performed on Thermo ESCALAB 250. All spectra were calibrated by the C 1s peak resulting from the graphite. The stoichiometric ratios of tin chalcogenides were derived from the XPS data. TGA measurements were performed in an air environment from room temperature (RT) to 800 °C with a heating rate of 10 °C min–1. Raman spectroscopy was performed on a JY LABRAM-HR confocal laser micro-Raman spectrometer utilizing Ar+ laser excitation (λ = 514.5 nm). The carbon content was examined by elemental analysis (EA, Elemental vario EL cube, Thermal Conductivity Detector) under an oxygen atmosphere. Energy-dispersive spectrometer (EDS) images were taken at an acceleration voltage of 200 kV (JEOL-2010 HRTEM).
Electrochemical Characterization
An argon-filled glovebox (O2, H2O < 1 ppm) enclosing a counter electrode (lithium foil), a separator (Celgard 2400), and 1.0 M LiPF6 in ethylene carbonate (EC)/diethyl carbonate (DEC) in the volume ratio of 1:1 as the electrolyte was used to keep assembled coin-type 2016 cells. For the preparation of the working electrode, active material, polyvinylidene fluoride (PVDF), and carbon black at a weight ratio of 6:2:2 were mixed in N-methyl-2-pyrrolidone. The found mixture was ball-milled (10 h) to mix uniformly, and the obtained slurry was spread on the substrate (Cu foil) and dried (80 °C) in a vacuum oven (10 h). Cyclic voltammetric (CV) experiments were conducted at a scan rate of 0.1 mV s–1 (electrochemical workstation, CHI660E). Galvanostatic measurements were examined using a LAND-CT2001A instrument in the potential range of 0.01–3 V (vs Li/Li+) at a selected current density and constant RT. Here, the specific capacities and currents were calculated based on the total mass of the anode materials.
Results and Discussion
The phases of the as-prepared samples are examined by powder XRD, and the results are displayed in Figures 1a and 2a: orthorhombic SnS/C (JCPDS card no. 39-0354, Pmcn, a = 3.98 Å, b = 4.33 Å, c = 11.18 Å) and orthorhombic SnSe/C (JCPDS card no. 65-3811, Pnma, a = 11.59 Å, b = 4.21 Å, c = 4.41 Å), respectively. The morphologies and microstructures of the as-synthesized products are characterized by SEM, TEM, high-resolution TEM (HRTEM), high-angle annular dark-field STEM (HAADF-STEM), and EDX mapping. Figures 1b and S1 are the SEM images of the as-prepared SnS/C sample, revealing the uneven aggregation of particles. For the identification of the microstructure of the SnS/C sample, TEM characterization was conducted with the results shown in Figure 1c–h. In Figure 1c, the TEM image demonstrates the combination of SnS nanoparticles and carbon layers and the irregular morphology of the SnS nanoparticles marked with green circles. Figure 1d shows the HRTEM image of the SnS/C sample, and the selected lattice fringe is about 0.289 nm wide, which corresponds to the d-spacing of the (101) plane of SnS. The HAADF-STEM image in Figure 1e and the corresponding EDX images in Figure 1f–h further confirmed the distribution of SnS nanoparticles on carbon layers and the compositional elements (i.e., Sn, S, and C) of the SnS/C sample. The EDX (Figure S4) indicates that this composite is composed of C, Sn, and S elements and the signal of Cu arises from the TEM grid.
Figure 1.
(a) Powder XRD pattern of the SnS/C sample. (b) SEM, (c) TEM, and (d) HRTEM images of the SnS/C sample. (e–h) HAADF-STEM and corresponding EDX images of the SnS/C sample.
Figure 2.
(a) Powder XRD pattern of the SnSe/C sample. (b) SEM, (c) TEM, and (d) HRTEM images of the SnSe/C sample. (e–h) HAADF-STEM and the corresponding EDX images of the SnSe/C sample.
From the SEM images in Figures 2b and S2, it could be found that the SnSe/C sample shows an unfixed lumpy morphology similar to that of the SnS/C sample. The TEM images in Figures 2c and S3 reveal that the diameter of the SnSe nanoparticles is around a few to tens of nanometers and the SnSe nanoparticles disperse over the carbon layer without severe agglomeration. The lattice fringe in the HRTEM image (Figure 2d) of the SnSe/C sample is about 0.304 nm wide, which matches the d-spacing of the (011) plane of SnSe. Similar to the SnS/C sample, the HAADF-STEM image in Figure 2e and the corresponding EDX images in Figure 2f–h further confirmed the distribution of SnSe nanoparticles on carbon layers and the compositional elements (i.e., Sn, Se, and C) of the SnSe/C sample. The EDX spectra in Figure S5 shows that the SnSe/C composite is composed of C, Sn, and Se elements and note that the trace oxygen signal may be caused by slight surface oxidation.
The images in Figure 3 reveal the XPS characterization results of the SnS/C and SnSe/C samples. In Figure 3a, the two peaks at around 487.4 and 495.9 eV correspond to Sn 3d5/2 and 3d3/2, respectively.34,35 The S 2p3/2 at 162.4 eV and S 2p1/2 at 163.9 eV shown in Figure 3b are ascribed to the binding energies of SnS, which suggests that no elemental sulfur (165.1 eV in binding energy) remains in the composite.36,37 The C 1s peak at 284.7 eV corresponding to the C–C bonding in carbon is displayed in Figure S6.38 The peaks at 487.6 and 496.1 eV in Figure 3c can be related to Sn 3d5/2 and 3d3/2 of SnSe.34,35 As shown in Figure 3d, the peaks at 55.5 and 56.6 eV denote the spin–orbit splitting of 3d5/2 and 3d3/2 of Se.39 The peak at 284.8 eV corresponds to the C 1s peak (Figure S7).38
Figure 3.
XPS for (a) Sn 3d and (b) S 2p levels of the SnS/C sample. XPS for (c) Sn 3d and (d) Se 3d levels of the SnSe/C sample.
For electrochemical tests, CV was first utilized to understand the dynamic process of the SnS/C sample for lithiation/delithiation. Figure 4a reveals the CV curves of the first five cycles of the SnS/C sample. From the negative scanning curve of the first cycle, two reduction peaks located at 1.24 V could be detected, which are in connection with the irreversible decomposition of SnS into Sn and Li2S (1).30 The reduction peak at 0.75 V is related to the reactions between the electrode materials and the electrolyte, which cause the formation of the SEI membrane.9,10 The reduction peak at 0.2 V represents the Li-ion alloying with Sn (2).31,40
| 1 |
| 2 |
Figure 4.
(a) CV curves of the SnS/C sample. (b) Galvanostatic charging–discharging curves of the SnS/C sample at 0.2 A g–1. (c) Rate capability of the SnS/C sample from 0.2 to 2.0 A g–1. (d) Cycling performance and Coulombic efficiency (CE) of the SnS/C sample at 0.2 A g–1.
In the positive scanning curve of the first cycle, the two broad oxidation peaks at 0.51 and 0.65 V are in relation with the delithiation process of LixSn. The oxidation peaks located at 1.87 V originated from the reversible conversion reaction of SnS.
The charging–discharging curves for the 1st, 2nd, 3rd, 10th, and 200th cycles at a current density of 0.2 A g–1 of the SnS/C sample are presented in Figure 4b. Consistent with the CV, an obvious platform around 1.3 V can be observed in the initial discharging process. Note that in the subsequent cycles, the platform around 1.3 V gradually weakens due to the irreversible decomposition of SnS. During the charging process, the platform located at around 0.55 V is ascribed to the extraction of Li ions from the discharged SnS/C sample. The charging–discharging curves in Figure 4c exhibit the excellent rate performance of the SnS/C sample. The specific capacities at the currents of 0.2, 0.5, 1.0, and 2.0 A g–1 are found to be 1076, 958, 837, and 661 mAh g–1, respectively, and after charging/discharging at high current densities, the specific capacity can recover to 886 mAh g–1 at 0.2 A g–1. Figure 4d displays the excellent cyclic performance of the SnS/C sample at 0.2 A g–1. In the first cycle, the specific capacities are found to be 3164 and 1693 mAh g–1 during the discharging/charging process, respectively, suggesting an initial Coulombic efficiency (CE) of 53.5% due to the irreversible decomposition of SnS. Then, the CE of the SnS/C sample increases to 98% and remains almost unchanged during the subsequent cycles. After cycling 200 times at 0.2 A g–1, the specific capacity of the SnS/C sample can still remain around 875 mAh g–1. Since the specific capacity of graphite is lower than that of SnS, the specific capacity of bare SnS should be higher than the value of the SnS/C sample obtained here. Considering the theoretical specific capacity (i.e., 372 mAh g–1) of graphite and the 32.8 wt % carbon content of the SnS/C sample measured here, the specific capacity of the SnS nanoparticles synthesized through the one-pot route could be roughly estimated to be about 1120 mAh g–1 at 0.2 A g–1. The SnS without carbon showed a rapid and continuous capacity attenuation during the electrochemistry cycling process and decreased to 163 mAh g–1 after 50th cycles.33 In comparison to the pure SnS electrode, the SnS/C electrode presented here evidently displays a better Li-storage ability due to the introduction of carbon material.
The electrochemical properties of the SnSe/C sample were measured in the same way as that for the SnS/C sample. Figure 5b presents the 1st, 2nd, 3rd, 10th, and 100th charging–discharging curves of the SnSe/C sample at the current of 0.2 A g–1. It could be observed that the SnSe/C sample also shows a discharging platform observed at 1.4 V in the first charging/discharging cycle, and the platform disappears in the subsequent cycles, which is assigned to the irreversible degradation of SnSe into metallic tin and Li2Se:28,29
| 3 |
Figure 5.
(a) CV curves of the SnSe/C sample. (b) Galvanostatic charging–discharging curves of the SnSe/C sample at 0.2 A g–1. (c) Rate capability of the SnSe/C sample from 0.2 to 2.0 A g–1. (d) Cycling performance and Coulombic efficiency (CE) of the SnSe/C sample at 1.0 A g–1. (e) Cycling performance and CE of the SnSe/C sample at 0.2 A g–1.
During the subsequent charging processes, the two platforms located at 0.5 and 1.8 V originate from the dealloying reaction of Sn with Li (Figure 5a, discussed later). The first CV curve of the SnSe/C sample in Figure 5a was also collected, in which the redox peaks are in accordance with the charging–discharging curves in Figure 5b. Obviously, the reduction peak around 0.25 V represents the Li alloying with Sn:28,29
| 4 |
As for the rate capabilities of the SnSe/C sample, the specific capacities at 0.2, 0.5, 1.0, 2.0, and 0.2 A g–1 estimated from Figure 5c are around 861, 789, 702, 593, and 738 mAh g–1, respectively. They only undergo a capacity loss of 31.1% with the current density increasing from 0.2 to 2.0 A g–1, suggesting a relatively good rate capability. Figure 5e displays the cyclic performance of the SnSe/C sample at the current density of 0.2 A g–1. After the first cycle, the SnSe/C sample shows a high specific capacity with the CE nearly 100%, indicating an excellent ability of energy storage. After 100 cycles at 0.2 A g–1, the specific capacity of the SnSe/C sample maintains around 734 mAh g–1. Moreover, the SnSe/C sample displays a specific capacity of nearly 690 mAh g–1 over 300 cycles at 1.0 A g–1, as shown in Figure 5d. In the same way, for estimating the specific capacity of the SnS nanoparticles, the specific capacity of the SnSe nanoparticles is estimated to be around 901 mAh g–1 at 0.2 A g–1 and 837 mAh g–1 at 1.0 A g–1. The SnSe without carbon maintained a specific capacity of only 271 mAh g–1 at 0.5 A g–1 over 100th cycles,28 indicating that the carbon-modified SnSe/C electrode demonstrated in this work delivers a significantly enhanced cycling property than the pure SnSe electrode.
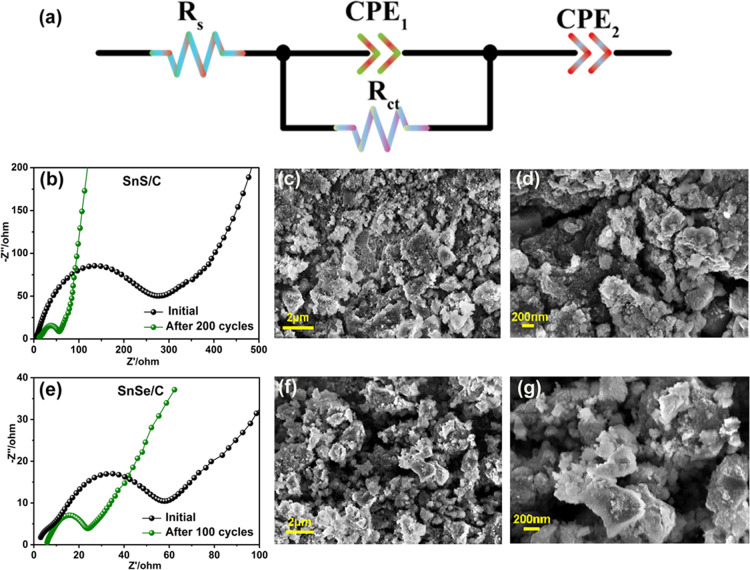
To obtain further insight into intrinsic electrochemical mechanisms of composite electrodes, electrochemical impedance spectroscopy (EIS) was used to study the conductivity of the SnS/C and SnSe/C samples before and after electrochemical cycling. Figure 6a displays the equivalent circuit model, where Rs is the internal resistance between the electrolyte and electrode and CPE and Rct correspond to the constant phase element and charge transfer resistance, respectively. The Nyquist plots of the two samples are displayed in Figure 6b,e. The semicircle at the medium-frequency region represents the internal resistances in the electrode.41 We find that SnS/C and SnSe/C electrodes show observable impedance decrease after electrochemical processes compared to impedance values before cycling, demonstrating that two composites retain good electron transport during the cycled process, which may be ascribed to the carbon introduction.42,43 On the other hand, the ex situ SEM was carried out to study the morphologies of the two samples after cycling. These two composites maintain similar irregular morphologies before and after cycling, which were considered to be advantageous to the cycling stability due to the good electron transport between the carbon and corresponding matrix, as displayed in Figures 6c,d,f,g.44,45
Figure 6.
(a) Equivalent circuit model to fit the Nyquist plots. Rs is the contact resistance between the electrode and electrolyte; Rct is charge transfer resistance; and CPE is the constant phase element. (b) EIS of the SnS/C sample before cycling and after 200 cycles at 0.2 A g–1, (c) Low-magnified and (d) high-magnified SEM images of the SnS/C composite electrode after 200 cycles at 0.2 A g–1. (e) EIS of the SnSe/C composite electrode before cycling and after 100 cycles at 0.2 A g–1. (f) Low-magnified and (g) high-magnified SEM images of the SnSe/C composite electrode after 200 cycles at 0.2 A g–1.
As discussed above, SnS/C, SnSe/C, and SnS0.5Se0.5/C composites were successfully synthesized via a simple and cost-effective one-pot route and displayed superior electrochemical properties when utilized as anode materials for LIBs due to the hybridizations of carbon. It can be clearly observed from Table 1 and Figure S11 that the as-synthesized SnS/C, SnSe/C, and SnS0.5Se0.5/C composites deliver outstanding lithium-storage capabilities exceeding or comparable to those of the Sn(S,Se)-based anode materials that have been reported in the literature, demonstrating the feasibility of the one-pot route developed in this work for the synthesis of high-performance Sn-based anode materials. The structure and electrochemical performances of other tin-based anode materials are presented in Figures S12–S15.
Table 1. Summary of Electrochemical Performance of Sn(S,Se)/C Anodes in This Work and That of the Previously Typical Literature.
| materials | cycled capacities | cycle number | reference |
|---|---|---|---|
| SnS/C composite | 875 mAh g–1 at 0.2 A g–1 | 200 | this work |
| SnSe/C composite | 734 mAh g–1 at 0.2 A g–1 | 100 | this work |
| 690 mAh g–1 at 1.0 A g–1 | 300 | ||
| SnS0.5Se0.5/C composite | 643 mAh g–1 at 0.5 A g–1 | 150 | this work |
| SnS@rGO | 791 mAh g–1 at 0.1 A g–1 | 100 | (26) |
| SnS/C hybrid | 466 mAh g–1 at 0.05 A g–1 | 50 | (27) |
| SnS nanoflower | 580 mAh g–1 at 0.05 A g–1 | 30 | (31) |
| SnS/polypyrrole nanosheet | 703 mAh g–1 at 1.0 A g–1 | 500 | (32) |
| SnS nanosheet | 560 mAh g–1 at 0.78 A g–1 | 50 | (33) |
| SnS@polypyrrole-nanobelt/carbon nanotube | 757 mAh g–1 at 1.0 A g–1 | 500 | (46) |
| SnS nanocrystal | 867 mAh g–1 at 0.1 A g–1 | 70 | (47) |
| net-like SnS/C film | 540 mAh g–1 at 0.04 A g–1 | 40 | (48) |
| SnS/C nanofibers | 648 mAh g–1 at 0.2 A g–1 | 500 | (40) |
| 548 mAh g–1 at 0.5 A g–1 | 500 | ||
| SnS/C nanosphere | 936 mAh g–1 at 0.1 A g–1 | 50 | (49) |
| 830 mAh g–1 at 0.5 A g–1 | 250 | ||
| SnS/TiO2@C nanosphere | 672 mAh g–1 at 0.5 A g–1 | 100 | (50) |
| NG–SnS composite | 790 mAh g–1 at 0.5 A g–1 | 900 | (51) |
| SnS/C nanocomposite | 696 mAh g–1 at 0.5 A g–1 | 200 | (52) |
| 423 mAh g–1 at 2.0 A g–1 | 200 | ||
| SnS/graphene nanosheet | 840 mAh g–1 at 0.1 A g–1 | 150 | (53) |
| YDSC-SnS@NSC | 561 mAh g–1 at 1.0 A g–1 | 100 | (54) |
| 344 mAh g–1 at 2.0 A g–1 | 600 | ||
| SnS@C nanoparticle | 484 mAh g–1 at 0.04 A g–1 | 40 | (55) |
| Se-doped SnS/C nanofiber | 700 mAh g–1 at 0.2 A g–1 | 100 | (56) |
| SnS/C nanocomposite | 607 mAh g–1 at 1.0 A g–1 | 200 | (57) |
| SnS/NC nanocomposite | 832 mAh g–1 at 0.1 A g–1 | 100 | (58) |
| SnS/graphene | 535 mAh g–1 at 0.05 A g–1 | 50 | (59) |
| flower-likeC@SnS | 594 mAh g–1 at 0.5 A g–1 | 100 | (60) |
| SnS/spherical grapheme framework | 800 mAh g–1 at 0.078 A g–1 | 100 | (61) |
| 527 mAh g–1 at 0.78 A g–1 | 300 | ||
| SnSe/C nanocomposite | 633 mAh g–1 at 0.5 A g–1 | 100 | (28) |
| SnSe@CNF composite | 840 mAh g–1 at 0.2 A g–1 | 100 | (29) |
| SnSe nanocrystal | 510 mAh g–1 at 0.1 A g–1 | 70 | (47) |
| SnSe–MWCNT hybrid | 651 mAh g–1 at 0.05 A g–1 | 50 | (62) |
| SnSe quantum dot | 550 mAh g–1 at 0.5 A g–1 | 1500 | (63) |
| SnSe/C nanofiber | 405 mAh g–1 at 1.0 A g–1 | 500 | (64) |
| SnS0.5Se0.5 nanocomposite | 625 mAh g–1 at 0.5 A g–1 | 1000 | (34) |
| SnS0.5Se0.5 nanoplate | 681 mAh g–1 at 0.5 A g–1 | 200 | (65) |
Conclusions
Tin chalcogenide-based composites, that is, Sn(S, Se)/C, were successfully synthesized by a facile one-pot route. During the electrochemical test, they displayed excellent lithium-storage capabilities, revealing their potential as the anode materials for LIBs. In particular, the SnS/C composite delivers a specific capacity of 875 mAh g–1 after 200 cycles at a current density of 0.2 A g–1, the SnSe/C composite electrode delivers a specific capacity of 734 mAh g–1 over 100 cycles at 0.2 A g–1 and 690 mAh g–1 at 1.0 A g–1 after 300 cycles, and the SnS0.5Se0.5/C composite electrode delivers a capacity of 643 mAh g–1 at 0.5 A g–1 over 150 cycles, respectively. The excellent lithium-storage capabilities could be ascribed to the synergistic effect of the nanometer-scale size of the tin chalcogenide particles and the conductivity of carbon. In addition, other series of tin-based composites, that is, Sn/C, SnS2/C, SnSe2/C, and SnTe/C, were also synthesized in a similar way, demonstrating the general applicability of the one-pot route developed in this work.
Acknowledgments
We acknowledge support from the Doctoral Research Fund of Lanzhou City University (LZCU-BS2020-03).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01647.
Figures showing additional characterization data, including SEM, TEM, XPS, XRD, EDX mapping, Raman spectrum, TGA, and Li-storage performances, for tin chalcogenide anodes (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Lee A.; Vörös M.; Dose W. M.; Niklas J.; Poluektov O.; Schaller R. D.; Iddir H.; Maroni V. A.; Lee E.; Ingram B.; Curtiss L. A.; Johnson C. S. Photo-accelerated fast charging of lithium-ion batteries. Nat. Commun. 2019, 10, 4946 10.1038/s41467-019-12863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q.; Li J.-Y.; Sun J.-K.; Yin Y.-X.; Wan L.-J.; Guo Y.-G. Watermelon-inspired Si/C microspheres with hierarchical buffer structures for densely compacted lithium-ion battery anodes. Adv. Energy Mater. 2017, 7, 1601481 10.1002/aenm.201601481. [DOI] [Google Scholar]
- Manthiram A.; Song B.; Li W. A perspective on nickel-rich layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2017, 6, 125–139. 10.1016/j.ensm.2016.10.007. [DOI] [Google Scholar]
- Tarascon J. M.; Armand M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. 10.1038/35104644. [DOI] [PubMed] [Google Scholar]
- Wu Z.-S.; Ren W.; Wen L.; Gao L.; Zhao J.; Chen Z.; Zhou G.; Li F.; Cheng H.-M. Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano 2010, 4, 3187–3194. 10.1021/nn100740x. [DOI] [PubMed] [Google Scholar]
- Han X.; Qing G.; Sun J.; Sun T. How many lithium ions can be inserted onto fused C6 aromatic ring systems?. Angew. Chem., Int. Ed. 2012, 51, 5147–5151. 10.1002/anie.201109187. [DOI] [PubMed] [Google Scholar]
- Huo K.; Wang L.; Peng C.; Peng X.; Li Y.; Li Q.; Jin Z.; Chu P. K. Crumpled N-doped carbon nanotubes encapsulated with peapod-like Ge nanoparticles for high-rate and long-life Li-ion battery anodes. J. Mater. Chem. A 2016, 4, 7585–7590. 10.1039/C6TA01972B. [DOI] [Google Scholar]
- Liang X.; Chen M.; Zhu H.; Zhu H.; Cui X.; Yan J.; Chen Q.; Xia X.; Liu Q. Unveiling the solid-solution charge storage mechanism in 1T vanadium disulfide nanoarray cathodes. J. Mater. Chem. A 2020, 8, 9068–9076. 10.1039/D0TA02922J. [DOI] [Google Scholar]
- Zhang K.; Zhang T.; Liang J.; Zhu Y.; Lin N.; Qian Y. A potential pyrrhotite (Fe7S8) anode material for lithium storage. RSC Adv. 2015, 5, 14828–14831. 10.1039/C4RA14819C. [DOI] [Google Scholar]
- Xue H.; Yu D. Y. W.; Qing J.; Yang X.; Xu J.; Li Z.; Sun M.; Kang W.; Tang Y.; Lee C.-S. Pyrite FeS2 microspheres wrapped by reduced graphene oxide as high-performance lithium-ion battery anodes. J. Mater. Chem. A 2015, 3, 7945–7949. 10.1039/C5TA00988J. [DOI] [Google Scholar]
- Grugeon S.; Laruelle S.; Herrera-Urbina R.; Dupont L.; Poizot P.; Tarascon J. M. Particle size effects on the electrochemical performance of copper oxides toward lithium. J. Electrochem. Soc. 2001, 148, A285 10.1149/1.1353566. [DOI] [Google Scholar]
- Shembel E.; Apostolova R.; Nagirny V.; Kirsanova I.; Grebenkin P.; Lytvyn P. Electrolytic molybdenum oxides in lithium batteries. J. Solid State Electrochem. 2005, 9, 96–105. 10.1007/s10008-004-0565-2. [DOI] [Google Scholar]
- Lin Y.-S.; Duh J.-G.; Hung M.-H. Shell-by-shell synthesis and applications of carbon-coated SnO2 hollow nanospheres in lithium-ion battery. J. Phys. Chem. C 2010, 114, 13136–13141. 10.1021/jp1042624. [DOI] [Google Scholar]
- Chen M.; Chao D.; Liu J.; Yan J.; Zhang B.; Huang Y.; Lin J.; Shen Z. Rapid Pseudocapacitive Sodium-Ion Response Induced by 2D Ultrathin Tin Monoxide Nanoarrays. Adv. Funct. Mater. 2017, 27, 1606232 10.1002/adfm.201606232. [DOI] [Google Scholar]
- Kim T.-J.; Kim C.; Son D.; Choi M.; Park B. Novel SnS2-nanosheet anodes for lithium-ion batteries. J. Power Sources 2007, 167, 529–535. 10.1016/j.jpowsour.2007.02.040. [DOI] [Google Scholar]
- Qu B.; Ma C.; Ji G.; Xu C.; Xu J.; Meng Y. S.; Wang T.; Lee J. Y. Layered SnS2-reduced graphene oxide composite - a high-capacity, high-rate, and long-cycle life sodium-ion battery anode material. Adv. Mater. 2014, 26, 3854–3859. 10.1002/adma.201306314. [DOI] [PubMed] [Google Scholar]
- Ueda A.; Nagao M.; Inoue A.; Hayashi A.; Seino Y.; Ota T.; Tatsumisago M. Electrochemical performance of all-solid-state lithium batteries with Sn4P3 negative electrode. J. Power Sources 2013, 244, 597–600. 10.1016/j.jpowsour.2013.01.061. [DOI] [Google Scholar]
- Kim Y.; Kim Y.; Choi A.; Woo S.; Mok D.; Choi N.-S.; Jung Y. S.; Ryu J. H.; Oh S. M.; Lee K. T. Tin Phosphide as a promising anode material for Na-ion batteries. Adv. Mater. 2014, 26, 4139–4144. 10.1002/adma.201305638. [DOI] [PubMed] [Google Scholar]
- Park M.-H.; Kim K.; Kim J.; Cho J. Flexible Dimensional control of high-capacity Li-ion-battery anodes: From 0D hollow to 3D porous germanium nanoparticle assemblies. Adv. Mater. 2010, 22, 415–418. 10.1002/adma.200901846. [DOI] [PubMed] [Google Scholar]
- Wang X.-L.; Han W.-Q.; Chen H.; Bai J.; Tyson T. A.; Yu X.-Q.; Wang X.-J.; Yang X.-Q. Amorphous hierarchical porous GeOx as high-capacity anodes for Li ion batteries with very long cycling life. J. Am. Chem. Soc. 2011, 133, 20692–20695. 10.1021/ja208880f. [DOI] [PubMed] [Google Scholar]
- Jo G.; Choi I.; Ahn H.; Park M. J. Binder-free Ge nanoparticles-carbon hybrids for anode materials of advanced lithium batteries with high capacity and rate capability. Chem. Commun. 2012, 48, 3987–3989. 10.1039/c2cc30294b. [DOI] [PubMed] [Google Scholar]
- Xue D.-J.; Xin S.; Yan Y.; Jiang K.-C.; Yin Y.-X.; Guo Y.-G.; Wan L.-J. Improving the electrode performance of Ge through Ge@C core-shell nanoparticles and graphene networks. J. Am. Chem. Soc. 2012, 134, 2512–2515. 10.1021/ja211266m. [DOI] [PubMed] [Google Scholar]
- Seng K. H.; Park M.-H.; Guo Z. P.; Liu H. K.; Cho J. Self-assembled germanium/carbon nanostructures as high-power anode material for the lithium-ion battery. Angew. Chem., Int. Ed. 2012, 51, 5657–5661. 10.1002/anie.201201488. [DOI] [PubMed] [Google Scholar]
- Cui G.; Hu Y.-S.; Zhi L.; Wu D.; Lieberwirth I.; Maier J.; Müllen K. A one-step approach towards carbon-encapsulated hollow tin nanoparticles and their application in lithium batteries. Small 2007, 3, 2066–2069. 10.1002/smll.200700350. [DOI] [PubMed] [Google Scholar]
- Zhao Z. W.; Yao Z. P. G. P.; Liu H. K. Mesoporous carbon-tin nanocomposites as anode materials for Li-ion battery. J. Mater. Sci. Technol. 2008, 24, 657–660. [Google Scholar]
- Li S.; Zheng J.; Zuo S.; Wu Z.; Yan P.; Pan F. 2D hybrid anode based on SnS nanosheet bonded with graphene to enhance electrochemical performance for lithium-ion batteries. RSC Adv. 2015, 5, 46941–46946. 10.1039/C5RA07292A. [DOI] [Google Scholar]
- Cai J.; Li Z.; Shen P. K. Porous SnS nanorods/carbon hybrid materials as highly stable and high capacity anode for Li-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 4093–4098. 10.1021/am300873n. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Zhao X.; Li J. SnSe/carbon nanocomposite synthesized by high energy ball milling as an anode material for sodium-ion and lithium-ion batteries. Electrochim. Acta 2015, 176, 1296–1301. 10.1016/j.electacta.2015.07.140. [DOI] [Google Scholar]
- Zhang L.; Lu L.; Zhang D.; Hu W.; Wang N.; Xu B.; Li Y.; Zeng H. Dual-buffered SnSe@CNFs as negative electrode with outstanding lithium storage performance. Electrochim. Acta 2016, 209, 423–429. 10.1016/j.electacta.2016.05.106. [DOI] [Google Scholar]
- Lu J.; Nan C.; Li L.; Peng Q.; Li Y. Flexible SnS nanobelts: Facile synthesis, formation mechanism and application in Li-ion batteries. Nano Res. 2013, 6, 55–64. 10.1007/s12274-012-0281-7. [DOI] [Google Scholar]
- Vaughn D. D.; Hentz O. D.; Chen S.; Wang D.; Schaak R. E. Formation of SnS nanoflowers for lithium ion batteries. Chem. Commun. 2012, 48, 5608–5610. 10.1039/c2cc32033a. [DOI] [PubMed] [Google Scholar]
- Liu J.; Gu M.; Ouyang L.; Wang H.; Yang L.; Zhu M. Sandwich-like SnS/polypyrrole ultrathin nanosheets as high-performance anode materials for Li-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 8502–8510. 10.1021/acsami.6b00627. [DOI] [PubMed] [Google Scholar]
- Kang J.-G.; Park J.-G.; Kim D.-W. Superior rate capabilities of SnS nanosheet electrodes for Li ion batteries. Electrochem. Commun. 2010, 12, 307–310. 10.1016/j.elecom.2009.12.025. [DOI] [Google Scholar]
- Tang Q.; Cui Y.; Wu J.; Qu D.; Baker A. P.; Ma Y.; Song X.; Liu Y. Ternary tin selenium sulfide (SnSe0.5S0.5) nano alloy as the high-performance anode for lithium-ion and sodium-ion batteries. Nano Energy 2017, 41, 377–386. 10.1016/j.nanoen.2017.09.052. [DOI] [Google Scholar]
- Xia W.; Wang H.; Zeng X.; Han J.; Zhu J.; Zhou M.; Wu S. High-efficiency photocatalytic activity of type II SnO/Sn3O4 heterostructures via interfacial charge transfer. CrystEngComm 2014, 16, 6841–6847. 10.1039/C4CE00884G. [DOI] [Google Scholar]
- Kim Y.; Hwang H.; Lawler K.; Martin S. W.; Cho J. Electrochemical behavior of Ge and GeX2 (X = O, S) glasses: Improved reversibility of the reaction of Li with Ge in a sulfide medium. Electrochim. Acta 2008, 53, 5058–5064. 10.1016/j.electacta.2007.12.015. [DOI] [Google Scholar]
- Wei Y.; He J.; Zhang Q.; Liu C.; Wang A.; Li H.; Zhai T. Synthesis and investigation of layered GeS as a promising large capacity anode with low voltage and high efficiency in full-cell Li-ion batteries. Mater. Chem. Front. 2017, 1, 1607–1614. 10.1039/C7QM00060J. [DOI] [Google Scholar]
- Liao H. M.; Sodhi R. N. S.; Coyle T. W. Surface composition of AlN powders studied by x-ray photoelectron spectroscopy and bremsstrahlung-excited Auger electron spectroscopy. J. Vac. Sci. Technol., A 1993, 11, 2681–2686. 10.1116/1.578626. [DOI] [Google Scholar]
- Pu Z.; Wei S.; Chen Z.; Mu S. 3D flexible hydrogen evolution electrodes with Se-promoted molybdenum sulfide nanosheet arrays. RSC Adv. 2016, 6, 11077–11080. 10.1039/C5RA28078H. [DOI] [Google Scholar]
- Xia J.; Liu L.; Jamil S.; Xie J.; Yan H.; Yuan Y.; Zhang Y.; Nie S.; Pan J.; Wang X.; Cao G. Free-standing SnS/C nanofiber anodes for ultralong cycle-life lithium-ion batteries and sodium-ion batteries. Energy Storge Mater. 2019, 17, 1–11. 10.1016/j.ensm.2018.08.005. [DOI] [Google Scholar]
- Wen Z.; Cui S.; Kim H.; Mao S.; Yu K.; Lu G.; Pu H.; Mao O.; Chen J. Binding Sn-based nanoparticles on graphene as the anode of rechargeable lithium-ion batteries. J. Mater. Chem. 2012, 22, 3300–3306. 10.1039/c2jm14999k. [DOI] [Google Scholar]
- Zhang J.; Cui R.; Li X. A.; Liu X.; Huang W. A nanohybrid consisting of NiPS3 nanoparticles coupled with defective graphene as a pH-universal electrocatalyst for efficient hydrogen evolution. J. Mater. Chem. A 2017, 5, 23536–23542. 10.1039/C7TA07672J. [DOI] [Google Scholar]
- Wang Z.; Wang Z.; Liu W.; Xiao W.; Lou X. W. Amorphous CoSnO3@C nanoboxes with superior lithium storage capability. Energy Environ. Sci. 2013, 6, 87–91. 10.1039/C2EE23330D. [DOI] [Google Scholar]
- Deng D.; Novoselov K. S.; Fu Q.; Zheng N.; Tian Z.; Bao X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218–230. 10.1038/nnano.2015.340. [DOI] [PubMed] [Google Scholar]
- Deng J.; Deng D.; Bao X. Robust catalysis on 2D materials encapsulating metals: Concept, application, and perspective. Adv. Mater. 2017, 29, 1606967 10.1002/adma.201606967. [DOI] [PubMed] [Google Scholar]
- Liu J.; Wen Y.; Van Aken P. A.; Maier J.; Yu Y. In situ reduction and coating of SnS2 nanobelts for free-standing SnS@polypyrrole-nanobelt/carbon-nanotube paper electrodes with superior Li-ion storage. J. Mater. Chem. A 2015, 3, 5259–5265. 10.1039/C5TA00431D. [DOI] [Google Scholar]
- Im H. S.; Lim Y. R.; Cho Y. J.; Park J.; Cha E. H.; Kang H. S. Germanium and tin selenide nanocrystals for high-capacity lithium ion batteries: Comparative phase conversion of germanium and tin. J. Phys. Chem. C 2014, 118, 21884–21888. 10.1021/jp507337c. [DOI] [Google Scholar]
- Li Y.; Tu J. P.; Huang X. H.; Wu H. M.; Yuan Y. F. Net-like SnS/carbon nanocomposite film anode material for lithium ion batteries. Electrochem. Commun. 2007, 9, 49–53. 10.1016/j.elecom.2006.08.019. [DOI] [Google Scholar]
- Deng Z.; Jiang H.; Hu Y.; Li C.; Liu Y.; Liu H. Nanospace-confined synthesis of coconut-like SnS/C nanospheres for high-rate and stable lithium-ion batteries. AIChE J. 2018, 64, 1965–1974. 10.1002/aic.16068. [DOI] [Google Scholar]
- Zhang Y.; Su H.; Wang C.; Yang D.; Li Y.; Zhang W.; Wang H.; Zhang J.; Li D. Heterostructured SnS/TiO2@C hollow nanospheres for superior lithium and sodium storage. Nanoscale 2019, 11, 12846–12852. 10.1039/C9NR04015C. [DOI] [PubMed] [Google Scholar]
- Mei S.; An W.; Fu J.; Guo W.; Feng X.; Li X.; Gao B.; Zhang X.; Huo K.; Chu P. K. Hierarchical micro-flowers self-assembled from SnS monolayers and nitrogen-doped graphene lamellar nanosheets as advanced anode for lithium-ion battery. Electrochim. Acta 2020, 331, 135292 10.1016/j.electacta.2019.135292. [DOI] [Google Scholar]
- Zheng J.; Luo Y.; Xie D.; Xiong X.; Lin Z.; Wang G.; Yang C.; Liu M. One-pot synthesis of SnS/C nanocomposites on carbon paper as a high-performance free-standing anode for lithium ion batteries. J. Alloys Compd. 2019, 779, 67–73. 10.1016/j.jallcom.2018.11.259. [DOI] [Google Scholar]
- Ma T.; Sun L.; Niu Q.; Xu Y.; Zhu K.; Liu X.; Guo X.; Zhang J. N-doped carbon-coated Tin sulfide/graphene nanocomposite for enhanced lithium storage. Electrochim. Acta 2019, 300, 131–137. 10.1016/j.electacta.2019.01.104. [DOI] [Google Scholar]
- Chen M.; Zhang Z.; Si L.; Wang R.; Cai J. Engineering of yolk-double shell cube-like SnS@N-S codoped carbon as a high-performance anode for Li- and Na-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 35050–35059. 10.1021/acsami.9b14287. [DOI] [PubMed] [Google Scholar]
- Li Y.; Tu J. P.; Huang X. H.; Wu H. M.; Yuan Y. F. Nanoscale SnS with and without carbon-coatings as an anode material for lithium ion batteries. Electrochim. Acta 2006, 52, 1383–1389. 10.1016/j.electacta.2006.07.041. [DOI] [Google Scholar]
- Lu L.; Zhang L.; Zeng H.; Xu B.; Wang L.; Li Y. Enhanced cycling performance of Se-doped SnS carbon nanofibers as negative electrode for lithium-ion batteries. J. Alloys Compd. 2017, 695, 1294–1300. 10.1016/j.jallcom.2016.10.259. [DOI] [Google Scholar]
- Zhu C.; Kopold P.; Li W.; Van Aken P. A.; Maier J.; Yu Y. A general strategy to fabricate carbon-coated 3D porous interconnected metal sulfides: Case study of SnS/C nanocomposite for high-performance lithium and sodium ion batteries. Adv. Sci. 2015, 2, 1500200 10.1002/advs.201500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Ma L.; Zhou X.; Ling Y.; Wang X.; Chen M. Hierarchical flower-like SnS grafted with glucosamine-derived nitrogen-doped carbon with enhanced reversible Li-storage performance. Appl. Surf. Sci. 2018, 458, 86–94. 10.1016/j.apsusc.2018.07.082. [DOI] [Google Scholar]
- Tao H.-C.; Yang X.-L.; Zhang L.-L.; Ni S.-B. One-step in situ synthesis of SnS/graphene nanocomposite with enhanced electrochemical performance for lithium ion batteries. J. Electroanal. Chem. 2014, 728, 134–139. 10.1016/j.jelechem.2014.07.004. [DOI] [Google Scholar]
- Lian Q.; Zhou G.; Liu J.; Wu C.; Wei W.; Chen L.; Li C. Extrinsic pseudocapacitve Li-ion storage of SnS anode via lithiation-induced structural optimization on cycling. J. Power Sources 2017, 366, 1–8. 10.1016/j.jpowsour.2017.09.009. [DOI] [Google Scholar]
- Zhao B.; Wang Z.; Chen F.; Yang Y.; Gao Y.; Chen L.; Jiao Z.; Cheng‡ L.; Jiang Y. Three-dimensional interconnected spherical graphene framework/SnS nanocomposite for anode material with superior lithium storage performance: Complete reversibility of Li2S. ACS Appl. Mater. Interfaces 2017, 9, 1407–1415. 10.1021/acsami.6b10708. [DOI] [PubMed] [Google Scholar]
- Gurung A.; Naderi R.; Vaagensmith B.; Varnekar G.; Zhou Z.; Elbohy H.; Qiao Q. Tin selenide - multi-walled carbon nanotubes hybrid anodes for high performance lithium-ion batteries. Electrochim. Acta 2016, 211, 720–725. 10.1016/j.electacta.2016.06.065. [DOI] [Google Scholar]
- Li J.; Liu W.; Chen C.; Zhao X.; Qiu Z.; Xu H.; Sheng F.; Hu Q.; Zheng Y.; Lin M.; Pennycook S. J.; Su C.; Lu J. High yield electrochemical exfoliation synthesis of tin selenide quantum dots for high-performance lithium-ion batteries. J. Mater. Chem. A 2019, 7, 23958–23963. 10.1039/C9TA04643G. [DOI] [Google Scholar]
- Xia J.; Yuan Y.; Yan H.; Liu J.; Zhang Y.; Liu L.; Zhang S.; Li W.; Yang X.; Shu H.; Wang X.; Cao G. Electrospun SnSe/C nanofibers as binder-free anode for lithium-ion and sodium-ion batteries. J. Power Sources 2020, 449, 227559 10.1016/j.jpowsour.2019.227559. [DOI] [Google Scholar]
- Tang Q.; Su H.; Cui Y.; Baker A. P.; Liu Y.; Lu J.; Song X.; Zhang H.; Wu J.; Yu H.; Qu D. Ternary tin-based chalcogenide nanoplates as a promising anode material for lithium-ion batteries. J. Power Sources 2018, 379, 182–190. 10.1016/j.jpowsour.2018.01.051. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.