Abstract
Mitochondria are one of the most important organelles in cells. Mitochondria are semi-autonomous organelles with their own genetic system, and can independently replicate, transcribe, and translate mitochondrial DNA. Translation initiation, elongation, termination, and recycling of the ribosome are four stages in the process of mitochondrial protein translation. In this process, mitochondrial protein translation factors and translation activators, mitochondrial RNA, and other regulatory factors regulate mitochondrial protein translation. Mitochondrial protein translation abnormalities are associated with a variety of diseases, including cancer, cardiovascular diseases, and nervous system diseases. Mutation or deletion of various mitochondrial protein translation factors and translation activators leads to abnormal mitochondrial protein translation. Mitochondrial tRNAs and mitochondrial ribosomal proteins are essential players during translation and mutations in genes encoding them represent a large fraction of mitochondrial diseases. Moreover, there is crosstalk between mitochondrial protein translation and cytoplasmic translation, and the imbalance between mitochondrial protein translation and cytoplasmic translation can affect some physiological and pathological processes. This review summarizes the regulation of mitochondrial protein translation factors, mitochondrial ribosomal proteins, mitochondrial tRNAs, and mitochondrial aminoacyl-tRNA synthetases (mt-aaRSs) in the mitochondrial protein translation process and its relationship with diseases. The regulation of mitochondrial protein translation and cytoplasmic translation in multiple diseases is also summarized.
Keywords: mitochondria, protein translation, translation factors, mitochondrial ribosome, mitoribosome assembly factors, mitochondrial aminoacyl-tRNA synthetase, translation activators, cytoplasmic translation
Introduction
Mitochondria are important, semi-autonomous organelles in eukaryotic cells. Their independent genetic system includes mitochondrial DNA (mtDNA), messenger RNA (mRNA), transfer RNA (tRNA), ribosomal RNA (rRNA), and ribosomes. Mitochondrial DNA replication, mRNA transcription, and protein translation occur independently, including the synthesis of polypeptides encoded by mtDNA. Human mtDNA comprises 16,569 base pairs and exists in multiple copies (Surovtseva et al., 2011). The mitochondrial genome lacks introns and contains only one major non-coding region, as well as a displacement loop (D-loop), which contains the promoter for transcription initiation. Mitochondrial DNA has heavy (H) and light (L) strands. The L-chain is rich in adenine and thymine, encoding one protein (ND6) and eight tRNAs. The H-strand is rich in guanine and encodes the remaining 12 proteins and 14 tRNAs (Shokolenko and Alexeyev, 2015). Despite the presence of this system, most mitochondrial proteins are encoded by nuclear DNA (nDNA). Only 13 of the proteins involved in the respiratory chain of mitochondria are encoded by mtDNA. These 13 are the core components of the mitochondrial respiratory chain complex. ND1–ND6 and ND4L are subunits of complex I. Cytochrome b (CYTB) is a subunit of complex III. COX1–COX3 are subunits of complex IV. Finally, ATP6 and ATP8 are subunits of ATPase (complex V). All complex II subunits are encoded by nDNA and are translocated through the mitochondrial membrane (Schulz et al., 2015).
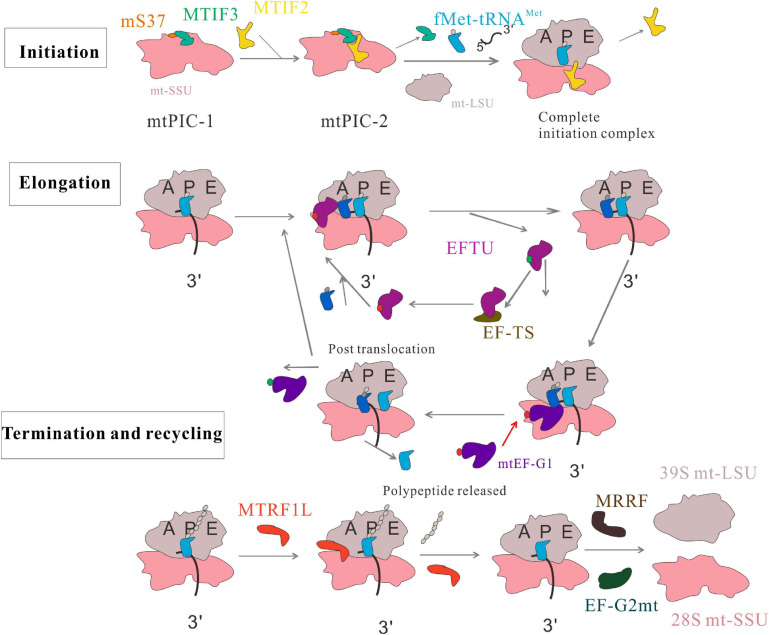
Mitochondrial translation includes initiation, elongation, termination, and ribosome-recycling stages (Figure 1). The mtDNA encodes two rRNAs, 22 tRNAs, and 13 proteins, which are important for mitochondrial function and biogenesis (Yokokawa et al., 2018). Mammalian mitochondrial ribosomes (mitoribosomes) synthesize proteins essential for ATP production via oxidative phosphorylation (OXPHOS). Mitochondrial translation mechanisms differ from those of cytoplasmic ribosomes, and are more similar to prokaryotic translation (Koripella et al., 2019a). At each stage, mitochondrial protein synthesis requires a series of mitochondrial factors (Mai et al., 2017). These include two initiation factors (MTIF2 and MTIF3), three elongation factors (EFTU, EF-TS, and mtEF-G1), a release factor (MTRF1L), and two ribosome recycling factors (MRRF and EF-G2mt). All are necessary for mitochondrial translation. Deficiency or mutation of these factors leads to abnormal mitochondrial translation and a series of metabolic disorders.
FIGURE 1.
Human mitochondrial translation. The process includes four phases: initiation, elongation, termination, and recycling. In the initiation phase, two distinct pre-initiation assembly steps, termed mitochondrial preinitiation steps 1 and 2 (mtPIC-1, mtPIC-2) are established. In the elongation phase, the aminoacyl-tRNA is transferred to the A site of mitochondrial ribosome by GTP ⋅ EFTU, and GTP ⋅ EFTU is transformed into GDP ⋅ EFTU. EF-TS converts GDP ⋅ EFTU to GTP ⋅ EFTU. The peptide-tRNA in the P site is transferred from the P site to the A site. mtEF-G1 binds to the ribosome at the A site and promotes translocation of the ribosome along the mRNA by inducing movement of A-tRNAs and P-tRNAs to the P site and E site. The tRNA at the E site leaves the monomer and this cycle continues until the polypeptide is completed and the stop codon appears at the A site. MRRF and EF-G2mt promote the separation of ribosomal subunits; MTIF3 combines with the small mitochondrial ribosomal subunits to prevent the premature reassociation of the large and small subunits.
Translation in mitochondria is carried out by specialized mitoribosomes. Mammalian mitochondrial ribosomes consist of a large subunit (39S) and a small subunit (28S). The large subunit contains a 16S rRNA and 48 proteins, while the small subunit contains a 12S rRNA and 30 proteins. To visualize the process of translation in human mitochondria, Aibara et al. (2020) determined eight cryogenic electron microscopy (cryo-EM) structures of human mitoribosomes in complex with mitochondrial mRNA (mt-mRNA), mitochondrial tRNAs (mt-tRNAs) and additional factors in different states. Most mutations in nuclear genes encoding mitoribosomal proteins, as well as mitochondrial DNA encoding tRNAs and 12S rRNA, lead to clinically and genetically heterogeneous infant multisystem diseases, such as Leigh’s syndrome, sensorineural hearing loss, encephalomyopathy, and hypertrophic cardiomyopathy (Jacobs and Turnbull, 2005; Perez-Martinez et al., 2008; Rotig, 2011).
Mitochondria are involved in many physiological and pathological processes, including electron transport, OXPHOS, fatty acid metabolism, and the tricarboxylic acid cycle. Changes in mitochondrial protein structure and function are involved in many human diseases, including nervous diseases, cardiovascular diseases, and cancers (De Silva et al., 2015; Mai et al., 2017; Ferrari et al., 2020; Kummer and Ban, 2021). Most mitochondrial diseases are characterized by oxygen- and phosphorus-related damage, affecting the OXPHOS system (Torraco et al., 2015). Many mitochondrial diseases are caused by defective mitochondrial protein synthesis. Mutations or deficiencies in mitochondrial protein translation factors (Table 1), mitochondrial tRNAs, mitochondrial aminoacyl-tRNA synthetases (mt-aaRSs), mitochondrial ribosomal proteins (MRPs), or mRNA, rRNA, and tRNA modification enzymes can lead to translation disorders and a wide range of phenotypes and diseases.
TABLE 1.
Mitochondrial translation factor mutations and related diseases.
| Gene | Protein | Related disease/clinical presentation | References |
| (1) Mitochondrial translation initiation process | |||
| MTIF2 | MTIF2 | Pathological myocardial hypertrophy | Lee et al., 2019 |
| MTIF3 | MTIF3 | Parkinson’s disease | Behrouz et al., 2010 |
| Obesity | Abadi et al., 2016 | ||
| Cardiomyopathy | Rudler et al., 2019 | ||
| (2) Mitochondrial translation elongation process | |||
| TUFM | EFTU | Lactic acidosis and fatal encephalopathy | Cao and Qin, 2016 |
| Lung cancer, colorectal carcinoma | Shi et al., 2012; He et al., 2016; Xu et al., 2019 | ||
| Hyperlactatemia | Di Nottia et al., 2017 | ||
| Metabolic cardiomyopathy | Hershkovitz et al., 2019 | ||
| MELAS | Sasarman et al., 2008 | ||
| Myocardial ischemia and reperfusion | He et al., 2001 | ||
| Polycystic encephalopathy, micropolygyria | Valente et al., 2009 | ||
| TSFM | EF-TS | Early onset encephalocardiomyopathy | Emperador et al., 2016 |
| Mitochondrial cardiomyopathy | Perli et al., 2019 | ||
| Hypertrophic or dilated cardiomyopathy | Seo et al., 2019 | ||
| Encephalomyopathy and hypertrophic cardiomyopathy | Smeitink et al., 2006 | ||
| Infant liver failure | Vedrenne et al., 2012 | ||
| GFM1 | mtEF-G1 | Early onset Leigh syndrome | Valente et al., 2007 |
| MELAS | Ahola et al., 2014; Emperador et al., 2016 | ||
| GUF1 | mtEF4 | Cancer | Zhu et al., 2018 |
| Western syndrome | Alfaiz et al., 2016 | ||
| Male infertility | Gao et al., 2016 | ||
| Peripheral neuropathy, spastic paraparesis | Temperley et al., 2003 | ||
| Axonal neuropathy and optic atrophy | Tucci et al., 2014 | ||
| Distal motor neuropathy, optic atrophy | Fang et al., 2017b | ||
| Leigh syndrome | Imagawa et al., 2016 | ||
| Leigh syndrome, optic atrophy, ophthalmoplegia | Antonicka et al., 2010 | ||
| Classical Behr’s syndrome phenotype | Pyle et al., 2014 | ||
| Optic atrophy and mild developmental delays | Heidary et al., 2014 | ||
| Spastic paraplegia and strabismus | Buchert et al., 2013 | ||
| (3) Mitochondrial termination and ribosome recycling | |||
| MRRF | RRF | Parkinson’s disease | Wu et al., 2020 |
| GFM2 | EF-G2mt | Leigh syndrome with arthrogryposis multiplex congenital | Fukumura et al., 2015 |
| MTRFR | C12orf65 | Early onset optic atrophy, progressive encephalomyopathy | Fang et al., 2017a |
| (4) Mitochondrial translational activators and disease | |||
| LRPPRC | LRPPRC | French-Canadian Leigh syndrome | Kohler et al., 2015 |
| (5) Mitochondrial miRNA | |||
| miR-181c | Heart failure | Das et al., 2012, 2014 | |
| miR-1 | Myogenesis | Das et al., 2014 | |
| miR-92a | Diabetic cardiomyopathy | Li et al., 2019 | |
| miR-21 | Myocardial hypertrophy | Li et al., 2016 |
Abnormal Mitochondrial Translation Initiation and Disease
The translation initiation process is a highly regulated and rate-limiting step in mitochondrial protein synthesis, which begins with the formation of an initiation complex. In bacteria, the first step of translation initiation is the dissociation of the ribosome into its small subunit (SSU) and large subunit (LSU). Then in the presence of mRNA, formylated methionine (fMet)-tRNAMet (fMet-tRNAMet), and three initiation factors (IF1, IF2, and IF3), the initiation complex can be formed to initiate protein synthesis. In mammalian mitochondria, the separation of the 28S and 39S mitochondrial ribosomal subunits leads to the formation of an initiation complex consisting of the 28S subunit, mRNA, fMet-tRNAMet, and initiation factors (Kuzmenko et al., 2014). These initiation factors (MTIFs) are encoded by nuclear genes that regulate the initiation of mitochondrial translation. One of these, MTIF2, closes the decoding center and stabilizes the binding of fMet-tRNAMet to leaderless mRNAs (Rudler et al., 2019). Therefore, MTIF2 promotes the binding of fMet-tRNAMet with the SSU of mitoribosomes (Gaur et al., 2008). Another important initiation factor is MTIF3, whose function has been widely studied. It enables the initiation codon (AUG) to correctly localize to the peptide (P) site of the mitoribosome and helps the mRNA bind to the mitochondrial SSU. In the absence of mRNA, fMet-tRNAMet and MTIF2 bind weakly to mitochondrial SSU; MTIF3 acts to prevent or correct the premature binding of these components (Christian and Spremulli, 2009). Furthermore, a recent paper mentioned two distinct pre-initiation assembly steps, termed mitochondrial preinitiation steps 1 and 2 (mtPIC-1, mtPIC-2). The study applied cryo-EM and fluorescence analysis to reveal that the interaction between mitochondrial-specific protein mS37 and MTIF3 keeps the mitochondrial SSU in a conformation favorable for the accommodation of MTIF2 in the second step. Then, MTIF2 produces an intermediate state mtPIC-2, which binds to the mitochondrial LSU, replaces MTIF3 with the initial tRNA, and accommodates mitochondrial leaderless mRNA, resulting in the formation of a complete elongation-competent initiation complex (Khawaja et al., 2020) (Figure 1). Some diseases are associated with abnormal mitochondrial translation initiation and mutations in the genes encoding translation initiation factors.
MTIF2
Despite its role in mitochondrial translation, MTIF2 is encoded by a nuclear gene. It participates in the activation of mitochondrial protein translation and is the main regulatory factor for initiation. It is considered to be the functional equivalent of bacterial IF1 and IF2 (Liao and Spremulli, 1990). A 37-residue sequence was inserted into the V and VI domains of MTIF2, making it substitute for the function of IF1. A cryo-EM study showed that this 37 amino acid insertion into MTIF2 produced a similar function to that of IF1, which could stereoscopically block the ribosomal A site, thus promoting the binding of the initial tRNA to the ribosomal P site during translation initiation (Yassin et al., 2011). This insert interacts with the decoding center of the small ribosomal subunit A-site and the 3′-CCA end of the sarcin/ricin loop (SRL) and fMet-tRNAMet near the large ribosome subunit peptidyltransferase center (PTC), under the action of guanosine triphosphate (GTP) and the mRNA template to generate a pre-start complex (Gaur et al., 2008). Mutation of this insertion domain seriously affects the ability of MTIF2 to bind to mitochondrial SSU and the formation of initiation complexes (Spencer and Spremulli, 2005). Similar to other protein synthesis systems, mitochondrial translation is initiated by methionine residues. However, for mitochondria, only one type of tRNAMet is used, which is in the form of fMet-tRNAMet at initiation and Met-tRNAMet during elongation. Formylation of Met-tRNAMet markedly enhances its affinity for MTIF2 (Spencer and Spremulli, 2004; Kummer et al., 2018). MTIF2 contains domains III to VI which are homologous to Escherichia coli IF2. Of these, subunit IV is the domain that mainly binds to fMet-tRNAMet (Spencer and Spremulli, 2004). Subsequently, Kummer et al. (2018) analyzed the cryo-EM structure of the complete translation initiation complex of mammalian mitochondria. They showed that the function of the additional domain insertion of MTIF2, which stabilizes the binding of leaderless mRNAs by closing the decoding center, induces the conformational changes of rRNA nucleotides involved in decoding. H678 of MTIF2 domain IV interacts with formyl of fMet-tRNAMet, while F632 interacts with methionine. The results showed that MTIF2 has a unique function in the identification of fMet-tRNAMet and the regulation of GTPase activity (Kummer et al., 2018). Thus, MTIF2 has dual functions in mammalian mitochondria (Gaur et al., 2008). Therefore, mtIF2 guides the association of fMet-tRNAMet with mRNA and the assembly of mitochondrial 55S ribosomes (D’Souza and Minczuk, 2018).
Previous studies have found a relationship between MTIF2 and cardiomyocyte death (Lee et al., 2019), but no mutation of the MTIF2 gene leading to mitochondrial diseases has been found. Specifically, in cell and animal models, MTIF2 has been linked to the oxidative capacity and redox state of cardiomyocytes (Lee et al., 2019). Its expression is reduced in the hearts of aged and obese mice, which decreases the oxidative capacity of cardiomyocytes. After in vitro hypoxic exposure, insufficient expression of MTIF2 can reduce oxygen consumption and increase cardiomyocyte death. Therefore, MTIF2 is necessary to maintain the oxidative properties of the myocardium, which plays a role in pathological myocardial hypertrophy during aging and obesity (Lee et al., 2019). The lack of MTIF2 in Saccharomyces cerevisiae reportedly results in disordered mitochondrial protein synthesis, which affects respiration. This defect was restored by either the S. cerevisiae MTIF2 gene (IFM1) or cDNA encoding bovine MTIF2 (Tibbetts et al., 2003). A recent study showed that the upregulation of MTIF2 is associated with poor prognosis of lung cell malignancy induced by inorganic arsenic (Zhang et al., 2019).
MTIF3
Mammalian MTIF3 is encoded by nuclear genes. It catalyzes the formation of initiation complexes in the presence of mitoribosomes, mRNA, mitochondrial initiator tRNA, and MTIF2 in vitro, and regulates mitochondrial protein translation. It contains N-terminal and C-terminal domains (NTD and CTD, respectively) that are separated by unstructured, flexible connection zones (Koc and Spremulli, 2002). Unlike its bacterial homolog, MTIF3 possesses unique terminal extensions on its N- and C-termini (Next and Cext, respectively). These extensions may have evolved as adaptations to the mitochondrial environment (Chicherin et al., 2019). The CTD participates in translation regulation in vitro (Haque and Spremulli, 2008). It may also play a role in the kinetics of translation initiation complex formation (Haque and Spremulli, 2008). One finding also showed that the NTD increases the fidelity function of MTIF3 in terms of the selection of the initiation codon and initiator tRNA (through its anticodon stem) (Ayyub et al., 2017). Koripella et al. (2019a) analyzed the cryo-EM structure of the mammalian mitochondrial 28S-MTIF3 complex. Unique contacts between the N-terminal domain (NTD) of MTIF3 and the 28S subunit were observed in the cryo-EM structure, which also explained the high affinity of MTIF3 for the 28S subunit. The location of mito-specific N-terminal extensions (NTE) of MTIF3 indicated the role of NTE in binding of the initial tRNA to the 28S subunit. The location of the CTD imparts anti-association activity, and the orientation of mito-specific C-terminal extensions (CTEs) explains why it can destabilize initiator tRNA in the absence of mRNA. The authors also speculate that CTD can recruit leaderless mRNAs and initiate translation. The study investigated the role of the NTD and CTD of MTIF3 in stabilization of the pre-initiator complex with mitochondrial SSU, also demonstrating the mutual binding site of MTIF3 and tRNA on the ribosome (Koripella et al., 2019a).
Mammalian MTIF3 has an affinity for small mitoribosome subunits. It locates the AUG or AUA promoter of mRNA at the P-site of 28S SSU, prevents the premature binding of 39S large subunits with the 28S SSU, and promotes the dissociation of mitoribosomes (55S) into small (28S) and large (39S) ribosomal subunits (Koc and Spremulli, 2002). It changes the equilibrium between the 55S mitoribosome and the separated 39S and 28S subunits by binding with free 28S, preventing further subunit rebinding. In addition, MTIF3 binds to the 55S mitoribosome and promotes its dissociation. This may be due to the formation of a transient intermediate that is rapidly distributed to the 28S subunit combined with MTIF3 and the free 39S subunit (Christian and Spremulli, 2009). Moreover, mammalian MTIF3 promotes the separation of the initiator tRNA from mitoribosomes with a lack of mRNA (Bhargava and Spremulli, 2005).
Another key role of MTIF3 is in mitochondrial translation initiation by regulating mitochondrial function. One study indicated that MTIF3 is essential for mitochondrial translation initiation and the coordinated assembly of respiratory complexes. Heart-specific and skeletal muscle-specific loss of MTIF3 in mice caused abnormal mitochondrial protein synthesis and induced cardiomyopathy (Rudler et al., 2019). Changes in the function or expression of MTIF3 protein may affect mitochondrial function, ATP production, or the formation of reactive oxygen species (ROS), affecting susceptibility to Parkinson’s disease (PD) and promoting its occurrence (Behrouz et al., 2010). The latter investigators also described an association of the rs7669 variant of MTIF3 with PD risk. Whether rs7669 is a functional variant remains to be confirmed. Thus, MTIF3 mutations are associated with multiple pathological processes such as PD, obesity, and diabetes. A synonymous polymorphism (Asp266Asp, caused by c.798C>T) is associated with sporadic PD, indicating that MTIF3 may be involved in the pathogenesis of PD (Anvret et al., 2010), and this single-nucleotide polymorphism (SNP, c.798C>T) in MTIF3 was shown to be strongly related to the PD allele by another group (Abahuni et al., 2007). MTIF3 is also involved in obesity (Abadi et al., 2016). It was reported that an MTIF3 SNP (rs4771122) is associated with increased body mass index (BMI) in Mexican children (Abadi et al., 2016). In addition, MTIF3 autoantibodies were found in patients with type I diabetes, indicating that MTIF3 is related to diabetes (Bian et al., 2017).
Mitochondrial Translation Elongation and Disease
Elongation is the core of protein synthesis and is the most conserved (Ott et al., 2016). In the process of protein synthesis, ribosomes move along the mRNA, which is decoded continuously through the interaction between mRNA codons and the anticodons of cognate tRNAs on the SSU. The aminoacyl (A) site, the peptidyl (P) site, and the exit (E) site are three binding sites for tRNAs in the ribosome. EFTU, EF-TS, mtEF-G1, and GUF1 are involved in the regulation of mitochondrial translation elongation. EFTU brings aminoacyl-tRNA (aa-tRNA) to the ribosomal A-site and coordinates specific codon: anticodon pairing between the mRNA and tRNA. Then, EFTU mediates hydrolysis of GTP and release of newly formed EFTU ⋅ guanosine diphosphate (GDP). EF-TS promotes GDP release from EFTU. GDP, regenerating EFTU ⋅ GTP. Next, under the catalysis of the large ribosomal subunit, peptide bonds are formed between the peptide-tRNA at the P site and aa-tRNA at the A site. mtEF-G1 (also called GFM1) then binds to the ribosomal A site and promotes ribosomal translocation along the mRNA by inducing A- and P-tRNAs to move to the P and E sites, respectively (Christian and Spremulli, 2012; Hallberg and Larsson, 2014; Ott et al., 2016) (Figure 1). The clinical expression levels of EFTU, EF-TS, and mtEF-G1 can reflect the function and translational speed of mitochondria; they can be used to evaluate the functional state of cells. Mutations in genes encoding these factors lead to mitochondrial disease (Wang X. Q. et al., 2017). Clinical manifestations include diverse diseases and phenotypes. These are usually disabling, progressive, and/or fatal, affecting the brain, liver, skeletal muscle, heart, and other organs (Boczonadi and Horvath, 2014; Diodato et al., 2014).
EFTU
A highly conserved GTPase, EFTU is encoded by the TUFM gene. EFTU is highly conserved and has 55–60% homology with bacterial EFTU (Woriax et al., 1995). In its active form (EFTU ⋅ GTP), aa-tRNA is transferred to the A-site of the mitoribosome to coordinate codon:anticodon pairing between the mRNA and tRNA through the formation of a ternary complex. Because this process requires energy, EFTU ⋅ GTP is converted to the EFTU ⋅ GDP inactive complex by EFTU-mediated GTP hydrolysis. The latter is released from ribosomes as the substrate of EF-TS, which promotes the exchange of GDP and GTP, reactivating GTP (Di Nottia et al., 2017). After correct codon:anticodon pairing, EFTU ⋅ GDP leaves the mitoribosome and aa-tRNA enters the P-site. At the P-site, the formation of peptide bonds is catalyzed by the large ribosomal subunit and the growing peptide chain is elongated (Boczonadi and Horvath, 2014). Binding at the A-site, GFM1 promotes the translocation of the ribosome along the mRNA by inducing movement of A-tRNAs and P-tRNAs to the P site and E site, respectively (Cao and Qin, 2016). The overall structure of EFTU ⋅ GTP is similar to that observed in E. coli, but the nucleotide binding domain (domain I) is in a different orientation compared with that observed in prokaryotic EFTU. In addition, domain III is followed by a short extension of 11 amino acids, forming one helical turn (Polekhina et al., 1996). It is an important step for EFTU to select the correct aa-tRNA for the ribosome A site to ensure the fidelity of translation. Using structure-based and explicit solvent molecular dynamics simulations based on recent cryo-EM reconstructions, Girodat et al. (2020) investigated the structural mechanism of how EFTU is involved in proofreading. They found that switch I of EFTU is a gate that facilitates aa-tRNA selection. Switch I of EFTU converts from an α-helix to a β-hairpin to control the movement of aa-tRNA in the accommodation corridor through steric interactions between Arg58 and the correct acceptor stem of aa-tRNA. Recent studies have also shown that EFTU plays a non-canonical role in the regulation of mitophagy mediated by PINK1. EFTU has mitochondrial-cytosolic dual localization. Ser222 of EFTU is phosphorylated by PINK1, which localizes it mainly in the cytosol and plays a role in inhibiting mitophagy (Lin et al., 2020).
Mutations in TUFM are associated with OXPHOS deficiency, which leads to lactic acidosis and fatal encephalopathy (Cao and Qin, 2016). A study described a patient with a homozygous mutation in TUFM. The patient was affected by neonatal lactic acidosis, rapidly progressive encephalopathy due to mitochondrial translation disorder, and mtDNA-related mitochondrial respiratory chain (MRC) complex deficiency (Valente et al., 2007). Another study described the case of a female infant with polycystic encephalopathy, micropolygyria, and leukodystrophy changes. An Arg336Gln substitution in EFTU was identified and associated with the failure to form active EFTU; the GTP⋅aa-tRNA ternary complex affected mitochondrial translation (Valente et al., 2009). In mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) myoblasts, an almost complete lack of respiratory chain complexes I, IV, and V has been attributed to a heteroplasmic m.3243A>G substitution in the mitochondrial tRNALeu(UUR) (Emperador et al., 2016). The authors reported that the overexpression of EFTU or EF-G2mt (also called GFM2), but not EF-TS or GFM1, partially suppressed the phenotype. The finding of EFTU phosphorylation during myocardial ischemia and reperfusion prompted the hypothesis that the phosphorylation of mitochondrial translation factors inhibits mitochondrial protein synthesis, indicating that mitochondrial protein synthesis is a decisive factor in myocardial ischemia-reperfusion injury (He et al., 2001). Therefore, TUFM may affect the function of the mitochondrial respiratory chain by regulating mitochondrial translation and may play an important role in encephalopathy and other diseases.
A novel c.964G>A mutation in TUFM changed the evolutionarily conserved EFTU-Gly322 residue to Arg, resulting in an approximately 80% decrease in expression. Patients harboring this pathological variant of TUFM displayed metabolic acidosis and hyperlactatemia. Neurological examination revealed severe encephalopathy and leukodystrophy with microlymph nodes (Di Nottia et al., 2017). The cases presented in these studies have expanded the phenotypic characteristics of TUFM-related diseases, which are characterized by lactic acidosis and dilated cardiomyopathy, without progressive encephalopathy. These findings have implicated TUFM as a candidate gene for early cardiomyopathy and in the differential diagnosis of metabolic cardiomyopathy (Hershkovitz et al., 2019).
Moreover, some studies have shown that EFTU is involved in the process of epithelial-to-mesenchymal transition (EMT); thus, it is expected to become a new prognostic indicator. In human cancer tissues, EFTU has been reported to be downregulated; moreover, EFTU knockdown induced EMT by activating the AMPK-GSK3β/β-catenin pathway (He et al., 2016). Overexpression of EFTU in colorectal carcinoma (CRC) has been described. This may be a promising new prognostic indicator for CRC (Shi et al., 2012). Another study showed that EFTU was deubiquitinated by ubiquitin-specific peptidase 5 (USP5), and its level increased in CRC (Xu et al., 2019). Moreover, EFTU knockout decreased mitochondrial respiratory chain activity, increased glycolysis, and produced ROS, inducing EMT (Samec et al., 2018). However, it has also been reported that high expression of EFTU in gastrointestinal stromal tumors (GISTs) is related to the occurrence, development, and prognosis of the tumors (Weng et al., 2020).
Some studies have shown that EFTU also participates in the process of disease by regulating oxidative stress. Silencing TUFM in Paracoccidioides brasiliensis reportedly alters translation elongation, causes respiratory defects, and increases the sensitivity of yeast cells to reactive oxygen stress, indicating the involvement of TUFM in the pathogenicity of this fungus (Marcos et al., 2019). Moreover, EFTU can physically interact with Xeroderma pigmentosum group D (XPD) protein, which is involved in mitochondrial oxidative DNA damage repair (Liu et al., 2015). Recently published articles have reported that EFTU is associated with Alzheimer’s disease (AD)-like pathologies. The expression of EFTU is decreased in the brains of AD patients. Further studies showed that EFTU participates in the pathological process of AD through ROS in Beta-secretase 1 (BACE1) translation, apoptosis, and tau phosphorylation (Zhong et al., 2021).
EF-TS
EF-TS is a guanine nucleotide exchange factor encoded by TSFM. This factor combines with the EFTU ⋅ GDP complex to promote GDP release and form a stable EFTU ⋅ EF-TS heterodimer. GTP then promotes the separation of EF-TS from this complex and regenerates EFTU ⋅ GTP, which then binds to another aa-tRNA. This ternary complex combines with the ribosome A site. When the correct codon:anticodon recognition is established, GTP is hydrolyzed, EFTU ⋅ GDP is released, and the cycle repeats (Cai et al., 2000; Mai et al., 2017). The three-dimensional crystal structure of the bovine EFTU ⋅ EF-TS complex has been determined (Jeppesen et al., 2005).
Studies have described that decreased EF-TS in fibroblasts induced the upregulation of EFTU and mitochondrial biogenesis-related genes, along with increased expression of respiratory chain subunits and an increase in normal oxygen consumption rate (Perli et al., 2019). Forced overexpression of EFTU in cells obtained from carriers of pathogenic TUFM mutations can rescue EFTU deficiency. The predicted instability of EF-TS and EFTU in a bioinformatics analysis was consistent with a significant decrease in the steady-state levels of both proteins in clinically affected myocardium (Perli et al., 2019). These findings indicate that the lack of respiratory chain enzymes leads to OXPHOS dysfunction and eventually to multiple mitochondrial diseases.
Symptoms of mitochondrial cardiomyopathy caused by mitochondrial translation disorder have been described for a patient with a new complex heterozygote variant in TSFM (Perli et al., 2019). Two novel compound heterozygous mutations, c.944G>A, p.Cys315Tyr and c.856C>T, p.Gln286Xaa, in the TSFM gene of patients with juvenile-onset Leigh disease, ataxia, neuropathy, and optic atrophy, were reported to lead to EFTU protein degradation and marginally increased mitochondrial protein translation activity (Ahola et al., 2014). Recently, the first case of a patient with a childhood-onset chorea caused by complex heterozygous mutation of TSFM (MIM∗604723) without basal ganglis lesions was reported (van Riesen et al., 2021).
A homozygous mutation of EF-TS (p.Cys997Thr) was found in both patients with encephalomyopathy and hypertrophic cardiomyopathy. The mutation resulted in mitochondrial translation defects and reduced amounts of assembled complexes I, IV, and V in fibroblasts (Smeitink et al., 2006). SNP genotyping has been used to detect homozygous TSFM mutations. The p.Arg312Trp substitution changed arginine to tryptophan, suggesting that mitochondrial translation deficiency is an increasingly serious cause of infant liver failure (Vedrenne et al., 2012). Another homozygous missense mutation was found in the mitochondrial translation elongation factor TSFM gene in a patient with slow progressive childhood ataxia and hypertrophic cardiomyopathy (Ahola et al., 2014; Emperador et al., 2016). Whole-exome sequencing (WES) was used to identify mutations in the TSFM gene leading to p.Gln111ThrfsTer5 and RNA mis-splicing in a patient with rare mitochondrial disorders (Seo et al., 2019). There is evidence that children with hypertrophic or dilated cardiomyopathy who progress slowly due to TSFM mutations develop neurological symptoms that include optic-nerve and/or peripheral neuropathy, ataxia, Leigh disease, and others, which are the main manifestations of the disease (Emperador et al., 2016). A next-generation sequencing (NGS)-based multigene panel for mitochondrial dysfunction was used to identify a TSFM homozygous variant, c.547G>A, p.Gly183Ser, associated with early onset encephalomyopathy with sensorineural hearing loss and peculiar neuroimaging features. This result showed that EF-TS-mediated mitochondrial protein translation is valuable for studies of mitochondrial diseases in children with neurological and cardiac involvement (Scala et al., 2019). A patient with a novel TSFM mutation has been reported to have an adult-onset complex generalized hyperactivity disorder (Traschutz et al., 2019). The collective findings indicate that TSFM mutations are the cause of autosomal recessive mitochondrial cardiomyopathy, encephalopathy with optic and/or peripheral neuropathy, ataxia, and Leigh syndrome.
mtEF-G1
The human genes GFM1 and GFM2 encode mtEF-G1 and EF-G2mt, respectively. Both are highly conserved homologs of bacterial translation elongation factor G (EF-G). The mtEF-G1 elongation factor displays mitoribosome translocation activity. The EF-G2mt factor disassembles the mitoribosome at the end of translation to allow a subsequent protein-synthesis cycle (Glasgow et al., 2017).
The sequence homology between the functional human mtEF-G1 and its bacterial counterpart is ∼45%. The main difference is that there is a mito-specific extension of 11 amino acids at the former’s C-terminus (Bhargava et al., 2004). It was shown that the CTE in mtEF-G1 is directly involved in the translocation of the mt-tRNA receptor arm at the A site. The complex of human 55S mitochondrial ribosome and human mtEF-G1 has three different conformational states including an intermediate state and post-translocation state (Koripella et al., 2020). mtEF-G1 is a five-domain GTPase that catalyzes the transfer of peptide-tRNA from the A site of the ribosome receptor to the P site after the formation of peptide bonds, while removing the deacylated tRNA, promoting mRNA translocation, and exposing the next codon (Brito et al., 2015). mtEF-G1, as a translational GTPase, uses the energy of GTP hydrolysis to facilitate the rearrangement of the pre-translocation ribosomes and tRNA movement, so as to accelerate the process of translocation (Chen et al., 2013). Cryo-EM structures provide insights into the structures of elongation complexes from mammalian mitochondria at two different steps of the tRNA translocation reaction using in vitro reconstitution systems. Results have shown that mtEF-G1 not only controls the conformational changes in SSU to promote the movement of tRNA, but also causes the large rearrangement of the GTPase-associated center of mitochondrial LSU. mtEF-G1 binding leads to GAC closure, which stabilizes mtEF-G1 from a weakly to a tightly bound state with translocation ability (Kummer and Ban, 2020).
A study described two new mutations in GFM1, which resulted in decreased levels of mtEF-G1, abrogated assembly of mitochondrial complexes III and V, and decreased activity of mitochondrial complexes I and IV. These changes manifested as OXPHOS defects with complex clinical manifestations (Brito et al., 2015). However, the residual steady-state level of mtEF-G1 protein found in the heart and skeletal muscle was higher than that in the liver and fibroblasts, which reduced the sensitivity of these tissues and accentuated the nerve and liver involvement. The difference in residual protein levels among cells may be due to differing regulatory and compensatory responses of the mitochondrial translation system in different tissues (Brito et al., 2015). A tissue-specific effect of novel GFM1 mutations has been described in three patients by other investigators. In these patients, the respiratory chain enzyme activity of muscle and fibroblasts decreased slightly, while the liver function was seriously deficient (Ravn et al., 2015). A GFM1 mutation was found in two siblings with serious defects in mitochondrial translation. This mutation is located in a conserved residue in the GTP binding domain of mtEF-G1 (Coenen et al., 2004). A case report showed a GFM1 mutation in a patient affected by severe, rapidly progressive mitochondrial encephalopathy. This mutation results in a p.Arg250Trp substitution in the mtEF-G1 G′ subdomain and may block ribosome-dependent GTP hydrolysis (Smits et al., 2011a). Another study showed that patients with GFM1 mutations were affected by severe lactic acidosis, rapidly progressive and fatal encephalopathy, and early onset Leigh syndrome (Valente et al., 2007). A novel intronic GFM1 mutation was described. The prognosis of patients with this mutation was poor, with death almost always occurring in infancy, although one child was still alive at 6 years of age (Simon et al., 2017). A comprehensive genomic analysis revealed that GFM1 is one gene mutation known to cause OXPHOS disease in patients with childhood-onset mitochondrial respiratory chain complex deficiencies (Kohda et al., 2016). Recently, a study reported a family that carries a novel GFM1 variant, which is associated with a rare fatal mitochondrial disease. A p.Cys1576Thr mutation in exon 13 of GFM1 resulted in a premature stop codon at amino acid position 526 (Coenen et al., 2004; Su and Wang, 2020). WES revealed a novel composition of two heterozygous mutations of GFM1 in a Chinese child with epilepsy and mental retardation (You et al., 2020). In recent years, nine unrelated children were found to carry GFM1 mutation. All of these patients presented with nervous system involvement during the neonatal period, and five of them were diagnosed with West syndrome. mtEF-G1 expression was decreased, mitochondrial translation was impaired, and OXPHOS protein levels were decreased in these patients (Barcia et al., 2020). In conclusion, GFM1 is one of the known gene mutations causing OXPHOS disease, and its mutations and abnormal expression are closely related to a series of mitochondrial diseases.
GUF1
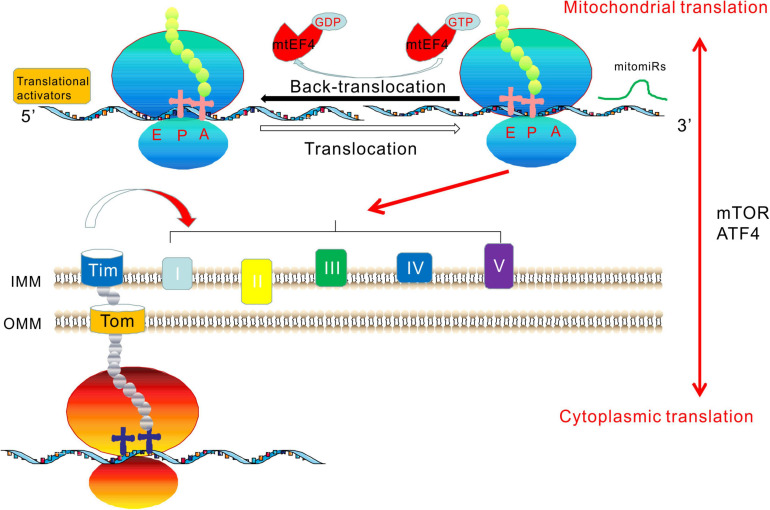
Bacterial and organellar translation employ a specific regulatory mechanism that differs from that of eukaryotes, involving the highly conserved EF4 translation elongation factor. In this phase of bacterial protein synthesis, EF4 catalyzes the back-translocation of P- and E-tRNAs to A- and P-tRNAs (Zhang et al., 2016). EF4 was originally termed lepA, because the gene encoding the protein is the first cistron of the bicistronic lep operon leader peptidase (lepB or lep) (March and Inouye, 1985). EF4 is highly conserved among all bacteria and almost all eukaryotes. In bacteria, EF4 catalyzes the translocation of peptidyl-tRNA and deacylated-tRNA in the opposite direction of EF-G catalysis. Therefore, EF4 is a back-translocase that maintains translation fidelity by back-translocating the ribosome under stress conditions (Qin et al., 2006). The E. coli lepA translation elongation factor has a mitochondrial homolog, mtEF4. Initial studies on budding yeast identified mtEF4 as an evolutionarily conserved GTPase with unknown function. Accordingly, it was also named GTPase of unknown function 1 (GUF1) (Bauerschmitt et al., 2008). In eukaryotes, the N-termini of GUF1 homologs have a mitochondria-targeting signal localized to the mitochondrial outer membrane and are considered to be mtEF4. Under suboptimal conditions, such as low temperatures and high Mg2+ concentrations, GUF1 mutant yeast displayed enhanced mitochondrial protein synthesis. At higher temperatures, the assembly of cytochrome c oxidase was shown to be defective in GUF1-deficient mutants (Bauerschmitt et al., 2008). The observations that mtEF4 ablation can reduce the mitochondrial translation rate and disrupt the assembly of complex IV-containing supercomplexes support the important roles of mtEF4 in mitochondrial translation and adaptation to stressful conditions, suggesting that mtEF4 is a key protein that maintains the fidelity of mitochondrial protein synthesis (Bauerschmitt et al., 2008; Yang et al., 2014). It has been shown that mtEF4 is essential for the quality control of respiratory chain biogenesis. Dysregulation of mitochondrial translation caused by its overexpression may be crucial in the development of human cancers. Different mtEF4 levels induce distinct bioenergetic pathways in cancer cells due to different types of speed-quality imbalances. Specific downregulation of mtEF4 expression in tumor tissue could be a promising new therapy for cancer treatment (Zhu et al., 2018). Other authors used exome sequencing to detect mutations in relatives of patients with isolated West syndrome. A homozygous variant (c.1825G>T, p.Ala609Ser) was identified in GUF1 in three affected siblings (Alfaiz et al., 2016).
In Caenorhabditis elegans, a GUF1 deletion resulted in delayed growth, and MRC complex assembly defects resulted in mitochondrial dysfunction (Yang et al., 2014). We demonstrated testis-specific dysfunction in OXPHOS by genetic ablation of mtEF4 in mice, leading to male infertility (Gao et al., 2016). Our observations demonstrated crosstalk between the mtEF4-dependent quality control in mitochondria and cytoplasmic mammalian target of rapamycin (mTOR) signaling. We showed that with mtEF4 ablation, the main feedback signal from the somatic cytoplasm was mTOR-upregulated and was accompanied by increased cytoplasmic translation, indicating that mTOR is a critical downstream effector compensating for mitochondrial translation deficiency (Gao et al., 2016). We concluded that cytoplasmic translation regulated by mTOR and mitochondrial translation involving mtEF4 have a bidirectional causal relationship. The essential function of mtEF4 provides a plausible explanation for its high degree of evolutionary conservation throughout eukaryotes. Our findings suggest a disease mechanism involving developmental decoupling of crosstalk, such as during spermatogenesis in the testis or pathological conditions in other tissues. Thus, mtEF4 could be a biomarker for human diseases and a drug target for male contraception (Gao et al., 2016).
Mitochondrial Translation Termination/Ribosome Recycling
Mitochondrial Translation Termination
Once the translation complex reaches the stop codon, the finished protein must be separated from the final tRNA, ribosome, and its homologous mRNA. In human mitochondria, UGA is not a stop codon, but a tryptophan codon. It is striking that AGA and AGG are not arginine codons, but terminating codons, only at the very end of open reading frames (ORFs) of the mitochondrial transcripts MT-CO1 (also named COX1) and MTND6 (also named ND6), respectively. This results in the possibility of a −1 frameshifting mechanism at the termination stage (Richter et al., 2010a). The remaining 11 mitochondrial ORFs are terminated by either the standard stop codon UAA or UAG. Several release factors are required during this termination. The proteins responsible for these functions are termed release factors (RFs). They recognize mRNA stop codons on the ribosome and control termination of protein synthesis (Soleimanpour-Lichaei et al., 2007). When a termination codon appears at the A site, RFs bind to the ribosome and promote ribosomal PTC-dependent hydrolysis of the ester bond of the peptide-based tRNA binding to the P site (Kisselev et al., 2003). Human mitochondria harbor four different members of the class 1 RF family: MTRF1L, MTRF1, MTRFR (previously called C12orf65), and MRPL58 (previously called ICT1) (Akabane et al., 2014). Mammalian MTRF1L is similar to the bacterial RF sequence. The absence of MTRF1L in human cells leads to growth deficiency (Soleimanpour-Lichaei et al., 2007). Changes in MTRF1L levels related to the mitochondrial inner membrane affect assembly of the respiratory complex and ROS production. MTRF1L is responsible for decoding the UAA/UAG termination codon (Kaji et al., 2001), which enables a single MTRF1L to terminate the translation of all 13 mtDNA-encoded peptides. This is sufficient to release all new human mitochondrial gene products from mitoribosomes (Rao and Varshney, 2001). Further analysis of other mitochondrial factors related to mitochondrial translation termination, such as RRFs, will help us to understand the process of mitochondrial translation termination in mammals and the role of MTRF1L (Nozaki et al., 2008). Desai et al. (2020) described the structure of MTRFR and mitochondrial LSU in rescuing the mitoribosome stalled state by analysis of cryo-EM structures of elongating mitoribosomes and revealed that MTRFR ejects the nascent chain (Desai et al., 2020).
The MRPL58 protein is a component of the human mitoribosome. It has codon-independent peptidyl-tRNA hydrolysis activity through its conserved Gly-Gly-Gln (GGQ) motif. Its function is crucial for hydrolysis of peptidyl-tRNAs that have been prematurely terminated in mitoribosomes and for cell viability (Richter et al., 2010b). MRPL58 gene knockout results in apoptosis, decreased mitochondrial membrane potential and mass, and decreased cytochrome c oxidase activity (Handa et al., 2010). The MTRFR protein is similar to MRPL58 and plays a similar role in rescuing stalled mitoribosomes. Its knockdown can increase ROS production and apoptosis, thus inhibiting cell proliferation. Compared with control cells, the mitochondrial membrane potential and mass of MTRFR knockout cells change considerably. These results indicate that the function of MTRFR is crucial for cell viability and mitochondrial function (Kogure et al., 2012). Some human diseases are caused by a disorder of translation termination in mitochondria (Temperley et al., 2003). MTRFR participates in the process of mitochondrial translation and is related to multiple phenotypes, including early onset optic atrophy, progressive encephalomyopathy, peripheral neuropathy, and spastic paraparesis (Fang et al., 2017a). Loss of MTRFR gene function causes mitochondrial translation defects, leading to encephalomyopathy (Temperley et al., 2003). Another study identified a 2-base deletion in MTRFR in a Japanese woman with mitochondrial dysfunction in choroid plexus cell bodies (Nishihara et al., 2017). Other groups identified a novel protein-truncating mutation in the MTRFR gene in a family with neuropathy and optic atrophy. Cells from these individuals exhibited mitochondrial defects, including reduced mitochondrial respiration complex activity and stability, decreased mitochondrial respiration rate, and decreased mitochondrial membrane potential (Tucci et al., 2014). Another group reported that a compound heterozygous mutation of MTRFR caused distal motor neuropathy and optic atrophy in a Chinese patient (Fang et al., 2017b). Siblings diagnosed with combined OXPHOS deficiency type 7 (COXPD7) had MTRFR compound heterozygous mutations. They displayed optic atrophy, mild developmental delays, and bilateral brainstem symmetry (Heidary et al., 2014). Homozygosity mapping was used to identify mutations in MTRFR in two patients who developed Leigh syndrome, optic atrophy, and ophthalmoplegia. Analysis of mitochondrial translation in fibroblasts from these patients revealed decreased mitochondrial translation, with considerable decreases in complexes I, IV, and V, and with a slight decrease in complex III (Antonicka et al., 2010). Finally, the analyses of two affected siblings with mild intellectual disability, spastic paraplegia, and strabismus revealed a homozygous premature stop mutation at codon 139 of MTRFR using homozygosity mapping and exome sequence analysis (Buchert et al., 2013). A homozygous nonsense mutation of MTRFR (NM_001143905:c.346delG, p.Val116∗) was described in a pair of female twins diagnosed with Leigh syndrome (Imagawa et al., 2016). Other groups described four patients with the classical Behr syndrome phenotype who had homozygous nonsense mutations in the MTRFR gene (Pyle et al., 2014). A novel MTRFR mutation was identified in seven affected individuals from two closely related families by whole-genome homozygosity mapping and exome sequencing. Disruption of the GGQ domain in the first coding exon led to a more severe phenotype (Spiegel et al., 2014). In one case, a new pathologic variant of the MTRFR gene was identified. The mutant protein lacked the GGQ domain (Perrone et al., 2020). The homozygous pathologic variant of MTRFR displayed a damaged mitochondrial OXPHOS system. The findings indicated that loss-of-function variants are more likely to lead to disease, while variations affecting the GGQ domain are associated with more severe phenotypes (Perrone et al., 2020). The results of these two cases are consistent, indicating that the GGQ domain of MTRFR is crucial for its phenotype, and its deletion causes a more severe phenotype.
Mitochondrial Translation Recycling
After the termination of protein synthesis, the mRNA and deacylated-tRNA in the peptide/exit (P/E) state remain associated with the ribosome to form the post-termination complex (PoTC) (Kaji et al., 2001). To start a new round of protein synthesis, the ligands binding to ribosomes must be removed from the PoTC and the ribosomes must separate into their two subunits. In this process, the RRF and EF-G2mt cooperate to disassemble the PoTC (Rao and Varshney, 2001), with the EF-G2mt transferred to the A site together with RRF to catalyze the release of mRNAs, deacylated-tRNA, and ribosome subunits, which are necessary for ribosome recycling (Hansen et al., 2000). The binding of EF-G2mt⋅GTP to the RRF-PoTC results in the disassembly of the 55S ribosome into two subunits during GTP hydrolysis (Peske et al., 2005). The cryo-EM structure of the human 55S mitoribosome-RRF complex revealed that the mito-specific NTE of RRF has α-helix and loop structures. These structures produce a functional key region that interacts with mitochondrial ribosomes. The structure revealed the presence of a tRNA at the P/E position and the rotation of small mitochondrial ribosomal subunits upon RRF binding. The research also revealed the interaction between P/E tRNA and mL64. These findings help to understand the unique features of the mitochondrial ribosome cycle (Koripella et al., 2019b). Furthermore, a recent paper mentioned the role of GTP binding protein 6 (GTPBP6), a homolog of the bacterial ribosome recycling factor HflX, in the division of ribosomal subunits, especially under stress conditions. This study showed that GTPBP6 plays a dual role in the ribosome cycle and biogenesis. On the one hand, it is conducive to the dissociation of ribosomes; on the other hand, it promotes the assembly of mitochondrial ribosomes. These findings contribute to our understanding of the assembly of large ribosomal subunits and the mitochondrial ribosomal recycling pathway (Lavdovskaia et al., 2020).
The EF-G2mt [also designated RRF2mt and GFM2 (Takeuchi et al., 2010)] protein mediates ribosomal recycling together with human RRF, but lacks translocation activity. The functional specificity of EF-G2mt involves domains III and IV. Therefore, EF-G2mt represents a class of guanosine triphosphate hydrolases (GTPases) involved in ribosome recycling. It cooperates with RRF and mediates ribosome dissociation during ribosome recycling, which is essential for the recycling stage.
The MRRF protein (also called RRF) was previously named mtRRF-1 (Takeuchi et al., 2010). It is a GTP-binding protein. Its binding can stabilize the rotational conformational state of mitoribosomes and has multiple weakened specific subunit bridges, preparing the complex for the dissociation of the EF-G2mt-binding subunits (Zhang and Spremulli, 1998). Deletion of MRRF in yeast does not decrease mitochondrial protein synthesis or stability of mtDNA. Thus, MRRF is involved in the coordination between yeast mitochondrial translation and OXPHOS assembly (Ostojic et al., 2016). Only the combination of EF-G2mt and MRRF can rescue the temperature-sensitive characteristics of E. coli RRF (Qin et al., 2006).
The MRRF protein is essential for the survival of human cell lines. Depletion of MRRF in human cell lines is fatal, which initially leads to severe mitochondrial abnormalities, mitoribosome aggregation, increased mitochondrial superoxide production, and eventual loss of the OXPHOS complex. Hence, MRRF loss results in decreased growth rate and cell death, leading to a variety of mitochondrial dysfunctions and diseases (Rorbach et al., 2008). Two biomarkers, RRF and ribosomal protein S18 (RPS18), distinguish early PD from normal control samples and are thus considered high-confidence biomarkers of distinct protein autoantibodies for early PD (Wu et al., 2020). These findings could help establish a timely and accurate method for the diagnosis of early PD (Wu et al., 2020). Mutations in GFM2 have been found in patients with Leigh syndrome. GFM2 mutations (c.206 + 4A>G and c.2029-1G>A) were found in both siblings, resulting in abnormal splicing of the premature stop codon (p.Gly50Glufs∗4 and p.Ala677Leufs∗2, respectively). Thus, the GFM2 mutation may be the cause of Leigh syndrome with multiple congenital arthritis phenotypes (Fukumura et al., 2015). WES was used to identify compound heterozygous (c.569G>A, p.Arg190Gln; c.636delA, p.Glu213Argfs∗3) and homozygous (c.275A>C, p.Tyr92Ser) recessive variants of GFM2 in patients presenting in early childhood with global developmental delay, elevated cerebrospinal fluid levels of lactate, and abnormalities on cranial magnetic resonance imaging (Zhang et al., 2016). Further research also identified these recessive GFM2 variants in two unrelated patients with early-onset neurological presentations of mitochondrial disease (Glasgow et al., 2017).
Mitoribosomes and Related Disease
Mammalian Mitoribosomes and Mitochondrial Ribosome Assembly
Unlike 70S ribosomes in prokaryotes and 80S ribosomes in the cytoplasm of eukaryotes, human (mammalian) mitochondria contain 55S ribosomes (O’Brien, 2003). The mitochondrial genome encodes both 12S rRNA and 16S rRNA, but all MRPs are encoded by the nuclear genome. The rRNAs have catalytic function, while ribosomal proteins have not only structural but also biological function in the process of translation. The MRPs are imported into mitochondria and assembled with the rRNAs transcribed by the mitochondria to form ribosomes responsible for translating mRNAs of 13 essential proteins in the OXPHOS system (O’Brien, 2003). The bovine 55S mitochondrial ribosome with a molecular weight of 2.71 MDa consists of two subunits of different sizes: a small subunit (28S) and large subunit (39S) (O’Brien, 1971). Compared with bacterial 30S consisting of 16S rRNA (1,542 nucleotides) and 21 proteins (S1–S21), the 28S SSU contains a 12S rRNA (950 nucleotides) and 29 proteins (Suzuki et al., 2001). Compared with bacterial 50S composed of two rRNA molecules (5S, 120 nucleotides; 23S, 2904 nucleotides) and 33 proteins (L1–L36), the 39S LSU of mitoribosomes contains a 16S rRNA (1560 nucleotides) and 48 proteins (Koc et al., 2001). Therefore, compared with bacterial ribosomes (33% protein and 67% RNA), the ratio of MRP to RNA is completely reversed, with 69% protein and 31% RNA. Of the 77 mitoribosomal component proteins, almost half are mitoribosome-specific and the remainder are bacterial protein homologs. Sharma et al. (2003) first analyzed the 3-dimensional cryo-EM map of the bovine mitochondrial 55S ribosome with a resolution of 13.5 Å. It was found that many proteins occupied a new position in ribosomes. Mitochondrial ribosomes have intersubunit bridges composed of proteins and have a gate-like structure at the mRNA entrance, which may be involved in the recruitment of unique mitochondrial mRNAs (Sharma et al., 2003). Subsequently, in 2009, the mitochondrial ribosomes of Leishmania tarentolae were reconstructed with a resolution of 14.1 Å. Greber et al. (2015) published the complete structure of the porcine 28S mitoribosome SSU and the reconstruction model of the 55S mitoribosome complexes with mRNA and tRNA. This structure revealed that the interaction between subunits in mitochondria is not as extensive as that in bacteria, and many bridges are formed by mitochondria-specific RNA and protein components. Reduced peripheral contacts may result in increased conformational flexibility of mitoribosomal subunits, including relative tilt between subunits (Greber et al., 2015). Subsequent studies confirmed the previous structure discovery that the large reduction in ribosomal RNA led to topological changes in some function-related regions in mammalian mitoribosomal structures, including the tRNA binding sites and nascent polypeptide-exit tunnels (Kaushal et al., 2015).
Despite the high-resolution mitoribosomal structures, the problem of how these macromolecular structures are assembled remains. Ribosome assembly involves the coordinated processing and modification of the time-related relationship between rRNAs and ribosomal proteins (De Silva et al., 2015). Many ribosome assembly factors act as macromolecular machines to improve efficiency and provide higher levels of control over mitochondrial translation. Mitochondrial assembly factors include GTPases, helicases, pseudouridine synthases, methyltransferases, endonucleases, and factors without known enzyme activity (Lopez Sanchez et al., 2021). To date, many mitochondrial assembly factors are RNA-binding proteins related to 12S and 16S rRNA, which have molecular chaperone activity and help them fold correctly (Lopez Sanchez et al., 2021). Studies have shown that mitoribosome assembly factors play a very important role in the process of mitochondrial translation, and are involved in diseases caused by mitochondrial translation disorders. Next-generation WES helps to identify pathogenic mutations in nuclear genes of different components of the mitochondrial protein translation machinery, including mitoribosome biogenesis and assembly. Pathogenic mutations have been identified in the RNA components of the mitoribosome (12S, 16S, and CP-tRNAVal), MRPs, and mitoribosome assembly factors. These mutations are associated with a wide range of clinical features, can be present at all stages of life, and are associated with variable tissue specificity (Lopez Sanchez et al., 2021).
MRPs Mutations
In 2004, it was reported for the first time that the nonsense mutation of mitochondrial SSU protein S16 (MRPS16) gene significantly reduced the transcription level of 12S rRNA, resulting in mitochondrial protein translation defects. This report found a case of neonatal lactic acidosis with agenesis of corpus callosum, dysmorphism, and lethality. The activities of complexes I and IV in the patient’s muscle and liver were significantly decreased, accompanied by extensive mitochondrial translation defects. Analysis of the patient showed a homozygous C-to-T substitution at nucleotide 331 of the MRPS16 cDNA (Miller et al., 2004). Similar results showed that in Drosophila, a missense mutation in mitochondrial ribosomal protein S12 prevented ribosomal proteins from assembling into active ribosomes, resulting in a significant reduction in 12S rRNA transcripts (Toivonen et al., 2001). Another mitochondrial ribosomal SSU protein mutation occurs in MRPS22. That report identified MRPS22 gene mutations in patients with antenatal skin edema, hypotonia, cardiomyopathy, and tubulopathy born to the same set of consanguineous parents. Transfection with wild-type MRPS22 cDNA could increase 12S rRNA content and normalize enzyme activity (Saada et al., 2007). Another study also reported that a patient with Cornelia de Lange-like dysmorphic features, brain abnormalities, and hypertrophic cardiomyopathy had a mutation in MRPS22. This study found that a mutation at a conserved site in the MRPS22 gene resulted in a p.Leu215Pro substitution, which seriously damaged the mitochondrial protein translation in fibroblasts and caused defects in OXPHOS complexes I, III, and IV. The amount and activity of OXPHOS complex IV and the transcription level of 12S rRNA could be restored to normal levels by transfection to increase the expression of MRPS22 in fibroblasts (Smits et al., 2011b). Researchers investigated phenotypes of mice carrying a homozygous mutation in mitochondrial ribosomal protein of small subunit 34 (MRPS34) and found that the mutant mice developed cardiac hypertrophy and liver steatosis with age. MRPS34 is one of 15 mammalian mitochondria-specific MRPs, which has not been found in the ancestors of bacterial ribosomes (Greber et al., 2015). Further studies have shown that MRPS34 is required for mitochondrial translation, stability of small ribosomal subunits, and its association with the large subunit. The MRPS34 mutation caused an obvious decrease in this protein, resulting in reduced levels of mitochondrial proteins and complexes, which led to decreased oxygen consumption and respiratory complex activity (Richman et al., 2015). The research group then identified the MRPS34 autosomal-recessive mutations in six individuals in four families with OXPHOS deficiency and Leigh syndrome or Leigh-like disease. Further investigation showed that these mutations caused reduced mitochondrial translation and combined OXPHOS deficiency by destabilizing the small mitochondrial ribosome subunit (Lake et al., 2018).
Galmiche et al. (2011) first identified mutations of MRPL3, the first large ribosomal subunit protein, through WES of individuals with multiple respiratory chain defects. The compound heterozygotes with a missense MRPL3 mutation (p.Pro317Arg) and a large-scale deletion were shown to have altered ribosome assembly and mitochondrial translation defects in skin fibroblasts, resulting in abnormal assembly of several respiratory chain complexes (RCC). The investigators also showed that these MRPL3 mutations were the cause of severe hypertrophic cardiomyopathy in four siblings born to non-consanguineous parents (Galmiche et al., 2011). NGS exome sequencing of two siblings with recessive hypertrophic cardiomyopathy uncovered a homozygous mutation (p.Leu156Arg) in the MRPL44 gene. MRPL44 is one of 20 mitochondrial ribosomal LSU proteins without a bacterial homolog and is reported to be located near the tunnel exit of the mitochondrial ribosome in yeast. Missense mutations in the MRPL44 gene affect protein stability, resulting in a severe decline in MRPL44 levels in the heart, skeletal muscle, and fibroblasts of patients. In patient fibroblasts, the reduction in MRPL44 has no effect on de novo mitochondrial translation, but seriously affects the assembly of large ribosomal subunits and the stability of 16S rRNA, resulting in the lack of complex IV. These results indicate that MRPL44 directly affects the assembly and stability of nascent mitochondrial polypeptides such as COX1 and likely interacts with chaperones or assembly factors. These studies suggest that some mitochondrial ribosomal subunit defects can produce tissue-specific phenotypes such as cardiomyopathy (Carroll et al., 2013). This conclusion was further confirmed by another study, which found that the heart muscle is particularly vulnerable to metabolic defects. This study expands the clinical spectrum of mitochondrial diseases associated with MRPL44 and indicates that the defect also leads to slowly progressive multisystem diseases, including those of skeletal muscle, liver, kidney, and the central nervous system. Therefore, MRPL44 mutations are more likely to occur in patients with slowly progressing mitochondrial multisystem diseases, especially in patients with cardiomyopathy (Distelmaier et al., 2015). In addition, one research group sequenced the mitochondrial ribosomal protein L12 (MRPL12) gene in a patient who was born to consanguineous parents and presented with growth retardation and neurological deterioration; they identified a c.542C>T missense mutation in exon 5, which converted a highly conserved alanine to valine (p.Ala181Val). This mutation led to decreased MRPL12 protein levels, which affected the assembly of large ribosomal subunits, caused overall mitochondrial translation defects, and significantly reduced the synthesis of COX1, COX2, and COX3 subunits. Studies in eubacteria have shown that eubacterial L7/L12, which are MRPL12 homologs, play an important role in protein synthesis by interacting with translation factors and regulating the speed and accuracy of protein synthesis (Pettersson and Kurland, 1980; Dey et al., 1995; Helgstrand et al., 2007). Modeling of MRPL12 showed that the p.Ala181Val change may alter the binding with elongation factor, thus reducing the affinity of mutant MRPL12 for the ribosome (Serre et al., 2013).
mt-tRNA (Mt-T) Mutations and Disease
mt-tRNA (MT-T) Overview
Among the 37 genes encoded by mtDNA, 13 encode electron transfer chain components, 2 encode mt-rRNAs, and the remaining 22 encode mt-tRNA (MT-T) genes. The MT-T gene has a unique secondary structure and forms endonuclease sites (Ojala et al., 1981; Taanman, 1999). After transcription, 14 cytosine-rich “light” mt-tRNAs and 8 guanine-rich “heavy” mt-tRNAs are released into the matrix (Anderson et al., 1981; Larsson and Clayton, 1995; Taanman, 1999). The secondary structure of tRNAs typically consists of a cloverleaf-shaped base-pairing pattern containing four domains: the acceptor stem, D-stem/loop, TψC-stem/loop, and anticodon stem/loop. Compared with cytoplasmic or bacterial tRNAs, the intrinsic thermodynamic stability of mt-tRNAs is decreased, mainly due to decreased GC content and increased non-Watson–Crick base pair frequency in the stem region. Thermodynamic instability may lead to inactivation of mt-tRNAs due to pathologic mutations; a single base substitution is more likely to destroy these weaker structures than structures with stronger contact sets (Wittenhagen and Kelley, 2003). There are more than 300 pathological mutations in the region of mtDNA encoding the MT-T gene. Although these mutations are widely distributed in the MT-T gene, three tRNAs, namely mt-tRNAIle (MT-TI), mt-tRNALeu (UUR) (MT-TL1) and mt-tRNALys (MT-TK), contain almost 50% of the known pathologic mutations (Wittenhagen and Kelley, 2003).
mt-tRNA (MT-T) Mutations
MT-T gene mutations are associated with many clinical characteristics, many of which also occur in mitochondrial diseases (Taylor and Turnbull, 2005). In general, there is little correlation between MT-T gene mutations and clinical manifestations. Mutations at different sites of the same MT-T gene may lead to completely different clinical symptoms, and the same point mutation may also lead to several different clinical phenotypes. The MITOMAP and Mamit-tRNA databases show the clinical variability of MT-T mutations, and mutations in different MT-T genes can lead to the same clinical presentation. For example, MELAS, chronic progressive external ophthalmoplegia (CPEO), and maternally inherited diabetes and deafness (MIDD) are reported clinical syndromes of m.3243A>G mutation within MT-TL1, which are also closely related to other MT-T gene mutations. Studies have identified many different possible effects of mutations, including interference of 3′ terminal maturation, prevention of aminoacylation, disruption of transcription factor binding, and codon recognition (Florentz et al., 2003). However, the basic molecular mechanism of mutation leading to disease is not well understood (Zifa et al., 2007). The only common feature of MT-T mutation is the loss of stability of MT-T. Among all the mutants, m.8344A>G and m.3243A>G are the two most common heteroplasmic MT-T genetic variants. Because pathologic changes usually occur in Watson–Crick pairs, which are structurally important in stems, it is unexpected that both mutations occur within loop structures (Yarham et al., 2010).
m.8344A>G, located in the T loop of mt-tRNALys, was the first pathologic heteroplasmic mutation of the MT-T gene to be identified (Shoffner et al., 1990). m.8344A>G is the sole cause of mitochondrial protein synthesis defects (Chomyn et al., 1991). Mutant transmitochondrial cybrids showed 10-fold reduced oxygen consumption, decreased cytochrome c oxidase activity, and respiratory defects caused by impaired protein synthesis (Chomyn et al., 1991; Masucci et al., 1995). Heteroplasmic mutation of the MT-T gene is closely related to the myoclonic epilepsy with ragged-red fiber (MERRF) phenotype. However, the mechanism of m.8344A>G mutation causing this phenotype has not been determined. One report suggested that this mutation causes a decrease in mt-tRNALys steady-state levels (Masucci et al., 1995). It was also reported that m.8344A>G causes a decrease in aminoacylation, which may be the primary cause of protein synthesis defects (Enriquez et al., 1995). Some studies have shown that m.8344A>G causes the post-transcriptional taurine modification defect of wobble position uridine in mt-tRNALys, which is also a reason for mitochondrial protein synthesis defects (Enriquez et al., 1995; Yasukawa et al., 2000a). Therefore, we suggest that the m.8344A>G mutation leads to the MERRF phenotype mainly by preventing the post-transcriptional taurine modification of wobble position uridine. m.8344A>G weakened the codon-anticodon interaction in mt-tRNALys, stalled translation, and reduced protein synthesis. Furthermore, ribosomal shifting may then occur, leading to premature translation termination and resulting in abnormal translation products. Although m.8344A>G is the most common mutation associated with MERRF (∼80% of cases) (Ozawa et al., 1997), other mutations such as m.8356T>C have also been reported (Masucci et al., 1995).
Mitochondrial disease MELAS syndrome is usually associated with many MT-T gene point mutations. Approximately 80% of MELAS syndrome patients had m.3243A>G mutations in the MT-TL1 gene (Morgan-Hughes et al., 1995). The m.3243A>G mutation occurs in the mtDNA binding site of MTERF1, which leads to a decrease in MTERF1 affinity, resulting in mitochondrial protein synthesis defects and respiratory disorders (Chomyn et al., 1992; King et al., 1992). As was previously observed with m.8344A>G, m.3243A>G mutation caused mt-tRNALeu(UUR) molecules to lack wobble position uridine modification (Yasukawa et al., 2000b, 2002). Because wobble uridine is considered to play an important role in stability, this mutation leads to protein synthesis defects, resulting in the MELAS phenotype (Yasukawa et al., 2002; Sasarman et al., 2008). Interestingly, the m.3243A>G. mutation enhanced the dimerization of mt-tRNALeu (UUR), resulting in a 10-fold reduction of aminoacylation in dimers (Wittenhagen and Kelley, 2002). Hence, decreased aminoacylation and post-transcriptional modification defects are crucial to causing respiratory deficiency and MELAS syndrome (Yasukawa et al., 2000b).
Mitochondrial Aminoacyl-tRNA Synthetases (mt-aaRss)
Aminoacyl tRNA synthetases (aaRSs) are a group of nuclear-encoded enzymes that conjugate each of the 20 amino acids to their cognate tRNA molecules to ensure correct translation of the genetic code (Sissler et al., 2017; Ognjenovic and Simonovic, 2018). mt-aaRSs are imported into the mitochondrial matrix and supply mt-tRNA conjugates for protein translation. In principle, each amino acid is recognized by its specific aaRS, resulting in 20 aaRS protein synthesis systems per cell. However, in human mitochondria, only 19 aaRSs are present, because Gln-tRNAGln is synthesized indirectly via misacylated Glu-tRNAGln through transamidation (Nagao et al., 2009). Recently, mutations in the genes encoding mt-aaRSs have been identified as a new cause of human diseases. They are believed to impair mitochondrial protein synthesis, thereby affecting the OXPHOS system and leading to surprising tissue-specific phenotypes. At present, nine gene mutations encoding mitochondria-specific aaRSs have been reported. Among these mutations, encephalopathy is the most common phenotype, while cardiomyopathy, tubulopathy, myopathy, or sensorineural neuropathy is the next most common phenotype (Konovalova and Tyynismaa, 2013). DARS2, EARS, and AARS2 are three typical genes whose mutations lead to rare and well-defined leukodystrophy (LD) syndrome. The mutations occur in mitochondrial aspartyl-tRNA synthetase, mitochondrial glutamate tRNA synthetase, and mitochondrial alanyl-tRNA synthetase, respectively (Fine et al., 2019). Mt-aaRSs as key players in the mitochondrial translation machinery play an important role in cell energy production. In AARS2 ovarian LD, leukoencephalopathy with thalamus and brainstem involvement and high lactate (LTBL), and leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL), protein and enzyme activities are reduced to varying degrees, but are not completely lacking. For AARS2 ovario-LD and LTBL, RCC dysfunction occurred, which was not detected in LBSL patient cells (Scheper et al., 2007; Mikhailova et al., 2009; Synofzik et al., 2011; Taskin et al., 2016). In addition, in LTBL, the oxygen consumption rate decreased significantly (Fine et al., 2019). Moreover, AARS2 was found to be the disease gene for early-onset fatal hypertrophic cardiomyopathy with lactic acidosis (Gotz et al., 2011). The patients died during the perinatal period or within 10 months after birth. Cardiomyopathy was a prominent clinical manifestation, but in addition to the heart, OXPHOS deficiency in the brain and muscle was observed at autopsy. The patients, however, did not show OXPHOS deficiency in fibroblasts or myoblasts. AARS2 mutations have been identified as homozygous or heterozygous, but to date, all the patients described have a mutation leading to p.Arg592Trp in mitochondrial alanyl-tRNA synthetase (Gotz et al., 2011; Calvo et al., 2012). Mt-aaRSs not only promote the translation of proteins forming the mitochondrial respiratory chain complex, but they also affect cell signaling, transcription, and RNA biological genesis in neurons (Sissler et al., 2017). At present, many studies have reported the non-canonical effects of mt-aaRSs, such as the angiogenic function of rat mitochondrial tryptophanyl-tRNA synthetase (WARS2) and the possible role of mt-aaRS mutations in the integrated stress response (ISR) (Agnew et al., 2018). Complete inhibition of mitochondrial translation in DARS2 knockout mice leads to the accumulation of unassembled nuclear-encoded respiratory chain subunits, resulting in severe protein homeostasis stress and mitochondrial unfolded protein response (UPRmt)-dependent ISR activation. The ISR does not reach steady state, which leads to severe heart disease and decreased survival (Dogan et al., 2014). In addition to mt-aaRS mutations that affect the pathology of the central nervous system, mt-AlaRS, mt-GlyRS, and mt-LysRS mutations are known to cause cardiomyopathies (Hei et al., 2019; Sommerville et al., 2019); mt-TyrRS mutations caused myopathy, lactic acidosis, and sideroblastic anemia (MLASA syndrome) (Shahni et al., 2013); and mt-SerRS mutations caused hyperuricemia, pulmonary hypertension, renal failure in infancy, and alkalosis (HUPRA syndrome) (Rivera et al., 2013). Generally speaking, the characteristics of mt-aaRSs are relatively poorly understood, and future research will provide important knowledge regarding the function of these synthetases. The detection of identified AARS2 mutations provides some clues for understanding the molecular mechanism of these rare diseases.
Mitochondrial Translational Activators
Mitochondrial translational activators are nuclear-encoded proteins. A yeast translational activator was proposed in the late 1980s. These activators generally interact with the 5′-untranslated region (UTR) of mitochondrial mRNA. They may also bind to nascent proteins or interact with mitoribosomes. Translation activators are often bound to the membrane, which may limit translation to the region close to the inner membrane (Fox, 2012). It is not clear whether all translational activators exhibit all of these activities. Unfortunately, due to the lack of an in vitro translation system, analyzing their activities in the process of translation initiation and elongation is not yet possible. Some protein factors, such as translational activator of cytochrome oxidase I (TACO1), mitochondrial translation regulation assembly intermediate of cytochrome c oxidase (MITRAC), and COX14 (previously called C12orf62), are recruited to bind directly to mitochondrial transcripts and regulate the translation of MT-CO1 (Szklarczyk et al., 2012; Weraarpachai et al., 2012; Richter-Dennerlein et al., 2016). In yeast, the translational activator of COX2 (MT-CO2) is PET111 (Sanchirico et al., 1998), which is necessary for COX2 mRNA translation (Fiori et al., 2005). PET54, PET122, and PET494 are translational activators of COX3 (MT-CO3). They interact with the 5′-UTR of COX3 mRNA, the small ribosomal subunit protein, and the inner membrane to promote the synthesis of COX3 (Naithani et al., 2003). The mitochondrial translation activators CBS1 and CBS2 interact specifically with the cytochrome b gene (MT-CYB or COB) mRNA via its 5′-UTR (Mittelmeier and Dieckmann, 1995). Both activators participate in translation initiation and promote the synthesis of MT-CYB by binding to mRNA-ribosome complexes (Rodel, 1986). CBP1 is another protein that binds to the 5′-UTR of MT-CYB mRNA, which is necessary for MT-CYB mRNA translation (Dieckmann et al., 1984; Islas-Osuna et al., 2002). In addition, CBP3 and CBP6 participate in the translation of MT-CYB (Gruschke et al., 2011).
Most yeast genes involved in the translation of mitochondria-encoded proteins lack mammalian homologs due to the lack of 5′-UTRs in mammalian mitochondrial mRNAs (Fontanesi et al., 2006). To date, TACO1 is the only specific mitochondrial mammalian translation activator. Expression of TACO1 in fibroblasts rescued the COX1 (MT-CO1) synthesis and assembly defects (Weraarpachai et al., 2009). TACO1 is necessary for the efficient translation of COX1. Investigations on TACO1mut/mut mice showed that TACO1 is required for COX1 translation through its specific binding of MT-CO1 mRNA and association with mitochondrial ribosomes. These mutant mice developed a late-onset syndrome similar to human patients with visual impairment, motor dysfunction, and cardiac hypertrophy (Richman et al., 2016). MITRAC is a complex IV assembly intermediate that regulates mitochondrial translation. MITRAC was found to interact with multiple assembly factors and efficiently translate COX1 mRNA (Mick et al., 2012). Lrpl30 and leucine rich pentatricopeptide repeat containing (LRPPRC) are translation activators of the human cytochrome c oxidase subunits (Debray et al., 2011). Both have multiple targets, including COX1 and COX3. Mutations result in a deficiency of cytochrome c oxidase, leading to French–Canadian Leigh syndrome (Kohler et al., 2015).
Regulation of Mitochondrial microRNAs (mitomiRs) in Mitochondrial Translation and Their Role in Disease
MicroRNAs (miRNAs) are single-stranded non-coding RNAs that are 18–23 nucleotides in length. They can translocate into the mitochondria and regulate mitochondrial translation (Bandiera et al., 2013). MitomiRs regulate mitochondrial gene expression and function under physiological and pathological conditions (Paramasivam and Vijayashree Priyadharsini, 2020). Several mitomiRs may be derived from mitochondrial genomic mRNA. MitomiRs post-translationally regulate gene expression in the mitochondria (Das et al., 2012; Fan et al., 2019). Most importantly, differentially expressed mitomiRs were observed in heart failure (Pinti et al., 2017; Wang X. et al., 2017). However, the translocation mechanism of nuclear-encoded miRNAs into mitochondria is not clear. MitomiR transport is one of the most controversial fields in mitochondrial research. Researchers have questioned the transport of RNA into mitochondria. In recent years, many miRNA processing proteins, including Ago, Dicer and RISC, have been found in mitochondria (Chen et al., 2010; Wang et al., 2015). Knowledge of the mechanism of miRNA transport to the mitochondrial matrix may provide important insights into the pathophysiology of disease and may become a new target for therapeutic intervention.
MicroRNAs miR-1, miR-210, and miR-338 can enhance mitochondrial translation and regulate mitochondrial proteomics and mitochondrial bioenergetics in myocytes (Aschrafi et al., 2008; Colleoni et al., 2013; Zhang et al., 2014; Srinivasan and Das, 2015). In mitochondria, miR-1 unexpectedly stimulates, rather than inhibits, translation of mitochondrial genome-encoded transcripts. The observed positive role of mitomiRs in mitochondrial translation suggests that miR-1 regulates the myogenic program by mediating mitochondrial translational activation and inhibiting cytoplasmic translation. Thus, mitomiRs play an important role in the crosstalk between mitochondrial and cytoplasmic translation. However, another study showed that hypoxia induced the expression of miR-210 in fibroblasts, while the expression of its downstream targets, iron-sulfur cluster assembly enzyme (ISCU) and the COX10 cytochrome c oxidase assembly protein, decreased. Moreover, miR-210 inhibited protein synthesis, thus reducing the level of the electron transport system (ETS) complex protein (Colleoni et al., 2013). MicroRNA-181c (miR-181c) inhibits the translation of COX1, resulting in the remodeling of complex IV and enhancement of mitochondrial function in ventricular myocytes. It regulates the mitochondrial genome and bioenergy and may potentially regulate heart failure in vivo (Das et al., 2012, 2014). The microRNA miR-762 translocates into mitochondria and is upregulated during hypoxia/reoxygenation in cardiomyocytes. Therefore, miR-762 can directly reduce MT-ND2 translation, mitochondrial complex I enzyme activity, and ATP levels, and increase ROS levels and cardiomyocyte apoptosis (Yan et al., 2019). It can also be translocated to the mitochondria to inhibit the downregulation of MT-CYB. Overexpression of miR-92a enhances mitochondrial translation and reduces ROS production and lipid deposition, thus improving diabetic cardiomyopathy (Li et al., 2019). Another study reported significant upregulation of miR-21 in spontaneously hypertensive rats. Computational prediction and biochemical analyses revealed that miR-21 directly targets MT-CYB and positively regulates its mitochondrial translation. miR-21 also reduces blood pressure and myocardial hypertrophy in spontaneously hypertensive rats by upregulating the mitochondrial translation level of MT-CYB (Li et al., 2016). Therefore, some mitomiRs promote mitochondrial translation, while others inhibit it. The mechanism of mitomiRs regulating mitochondrial translation needs further study.
Crosstalk Between Mitochondrial and Cytoplasmic Protein Synthesis
OXPHOS subunits are encoded by both the nuclear and mitochondrial genomes. However, the co-regulation of OXPHOS subunit genes remains poorly understood. This cooperative translation program provides one-way control through complex and dynamic cytoplasmic translation control. Therefore, the nuclear genome precisely guides the coordination of mitochondrial and cytoplasmic translation to coordinate the synchronous synthesis of the OXPHOS complex (Tang et al., 2020). Recently, Guo et al. (2020) identified the molecular pathway of the mitochondrial stress signal relayed to the cytoplasm. In mammalian cells, mitochondrial dysfunction caused by mitochondrial translation disorders leads to an ISR. This ISR is mediated through eIF2α phosphorylation. Phosphorylation of eIF2α decreases overall protein synthesis, but increases the translation of only ATF4, the master transcriptional regulator of ISR. Mitochondrial stress stimulates a mitochondrial stress-activated protease (OMA1)-dependent cleavage of DAP3 binding cell death enhancer 1 (DELE1), a protein found to be associated with the inner mitochondrial membrane. Hence, DELE1 accumulates in the cytosol and interacts with eukaryotic translation initiation factor 2 alpha kinase 1 (HRI or EIF2AK1), an eIF2α kinase necessary for activating eIF2α kinase (Guo et al., 2020). Another investigation further uncovered the OMA1-DELE1-HRI signaling axis that constitutes a link between mitochondrial perturbation and the cytosolic ISR. Moreover, this research combined genome engineering and haploid genetics to identify a suite of additional regulators in ISR. Therefore, this pathway is a potential therapeutic target, which can fine-tune ISR and obtain beneficial results in treating diseases involving mitochondrial dysfunction (Fessler et al., 2020). The serine/threonine kinase mTOR integrates extracellular and intracellular signals to drive growth and proliferation. mTORC1 is an important upstream regulator of integrated mitochondrial stress response (ISRmt). Khan et al. (2017) reported that mTORC1 is activated by mitochondrial DNA replication defects, which drive ISRmt through ATF4 activation. mTORC1 activation induces the mitochondrial one-carbon cycle, fibroblast growth factor 21 (FGF21), and the UPRmt. Downregulation of this response by rapamycin reverts progression of mitochondrial myopathy in mice (Khan et al., 2017). In mammals, mTOR coordinates the energy consumption of mRNA translation mechanisms with mitochondrial energy production by stimulating the synthesis of nuclear-encoded mitochondrial-related proteins, including mitochondrial transcription factor A (TFAM), mitochondrial ribosomal protein, and components of complexes I and V (Chapman et al., 2018). We have shown that mTOR, which is the upstream regulator of mitochondria, can sense mitochondrial translation defects and subsequently activate cytoplasmic translation to compensate for them (Gao et al., 2016). We recently found that mTOR adapts cytoplasmic translation to mitochondrial translation defects caused by mtEF4 ablation in C. elegans and mice (Gao et al., 2016) (Figure 2). Another example of mTOR regulation by mitochondrial stress is the ubiquitination of mTOR by Parkin, an E3 ubiquitin ligase located in mitochondria. Parkin is required to maintain mTORC1 activity during mitochondrial stress (Park et al., 2014). Thus, mTOR may be important in sensing mitochondrial translation defects and subsequently activating cytoplasmic translation. It is conceivable that cytoplasmic translation regulated by mTOR and mitochondrial translation controlled by mitochondrial translation factors, such as mtEF4, have a bidirectional causal relationship (Gao et al., 2016).
FIGURE 2.
Crosstalk between mitochondrial and cytoplasmic translation. mtEF4, translation activators, and mitomiRs are important regulators that directly affect mitochondrial translation. The components of mitochondrial complexes I, II, III, IV, and V are produced by both mitochondrial and cytoplasmic translation, requiring coordination between the two translation systems. mtEF4 has a back-translocation effect and catalyzes the back-translocation of the P- and E-tRNAs to A- and P-tRNAs. Mitochondrial translation activator promotes mRNA-specific translation initiation. MitomiRs function at the 3′ end of mRNA. mTOR and ATF4 play an important role in the crosstalk between mitochondrial and cytoplasmic translation.
RNA interference to reduce expression of mitochondrial ribosomal protein MRPS5 leads to an imbalance of OXPHOS subunits (mitonuclear protein imbalance) encoded by nDNA and mtDNA. Expression of the mtDNA-encoded MT-CO1 homolog MTCE.26 and nDNA-encoded ATP5PF homolog H28O16.1 can be unbalanced (Houtkooper et al., 2013). In mammalian cells, MRP knockout results in mitochondrial protein imbalance, decreased mitochondrial respiration, and activation of the UPRmt. Specific antibiotics or ethidium bromide, targeting mitochondrial translation, can extend lifespan by inducing mitonuclear protein imbalance (Houtkooper et al., 2013). Silencing miRNA-382-5p significantly increases the expression of genes related to mitochondrial dynamics and biogenesis. Conventional microarray analysis revealed the downregulation of MRPs and respiratory chain proteins in C2C12 myotubes upon silencing of miRNA-382-5p. This effect was accompanied by an imbalance between mitochondrial proteins encoded by nDNA and mtDNA and induction of heat shock protein 60 (HSP60), indicating that UPRmt was activated and that silencing of miR-382-5p resulted in mitonuclear protein imbalance and activated UPRmt in skeletal muscle (Dahlmans et al., 2019). Chloramphenicol and other antibiotics inhibit mitochondrial protein translation, effectively decreasing the synthesis of mitochondrial proteins in INS-1E cells and reducing the expression of mtDNA encoding the COX1 subunit of the respiratory chain, rather than the ATP synthase subunit ATP5PF. Although expression of the important respiratory chain subunit COX1 was significantly reduced, the INS-1E cells maintained a normal respiratory rate, indicating that inhibition of mitochondrial protein translation caused mitonuclear protein imbalance. However, in insulin-secreting cells, a compensatory mechanism effectively maintained a normal respiratory rate and even increased ATP synthase-dependent respiration and calcium signaling pathways (Santo-Domingo et al., 2017).
Conclusion
Mitochondria participate in important life activities of cells and are important in the study of evolution. Mitochondrial protein translation is an important and unique mitochondrial function, essential for the biogenesis of mitochondrial OXPHOS, cellular energy supply, and other mitochondrial functions. Mitochondrial protein translation is directly regulated by mitochondrial translation initiation, elongation, termination factors, translation activators, and mitomiRs. Mitochondrial translation and cytoplasmic translation are regulated by mTOR and other signaling pathways. Mitochondrial translation defects are the main causes of devastating human diseases. In this group of diseases, most mutations in mtDNA-encoding tRNAs, as well as mutations in nuclear genes encoding mitoribosomal proteins, translation initiation factors, and elongation factors, are the causes of clinical and genetic heterogeneity of infant multisystem diseases, such as Leigh syndrome, sensorineural hearing loss, encephalomyopathy, and hypertrophic cardiomyopathy (Perez-Martinez et al., 2008; Rotig, 2011). Many patients with mitochondrial diseases have multiple OXPHOS defects of unknown genetic causes, which indicates that many genes related to the biogenesis and function of mitochondrial translation mechanisms still need to be identified. Comprehensive clinical diagnosis and treatment based on mitochondrial translation defects remain challenging. With the advent of a boom in mitochondrial research and the progress in modern sequencing technology, the study of protein function will be more in-depth, and research on mitochondrial protein translation regulation will increase in importance. Research on mitochondrial protein translation will enhance our understanding of the pathogenesis and early diagnosis of human diseases.
Author Contributions
YG conceived of the manuscript. YG, FW, DYZ, DJZ, and PL drafted the manuscript, constructed the figures, and revised the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
- AD
Alzheimer’s disease
- aa-tRNA
aminoacyl-tRNA
- CTD
C-terminal domain
- COXPD7
OXPHOS deficiency type 7
- CPEO
chronic progressive external ophthalmoplegia
- CRC
colorectal carcinoma
- CTEs
mito-specific C-terminal extensions
- MT-CYB
mitochondrial cytochrome b
- EMT
epithelial-to-mesenchymal transition
- ETS
electron transport system
- fMet
formyl-methionine
- GDP
guanosine diphosphate
- GGQ
Gly-Gly-Gln
- GTP
guanosine triphosphate
- GTPBP6
GTP binding protein 6
- GUF1
GTPase of unknown function 1
- HUPRA syndrome
hyperuricemia, pulmonary hypertension, renal failure in infancy, and alkalosis
- ISCU
iron-sulfur cluster assembly enzyme
- ISR
integrated stress response
- LBSL
leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation
- LRPPRC
leucine rich pentatricopeptide repeat containing
- ISR
integrated stress response
- LSU
large subunit
- LTBL
leukoencephalopathy with thalamus and brainstem involvement and high lactate
- MELAS
mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes
- MERRF
myoclonic epilepsy with ragged-red fiber
- MIDD
maternally inherited diabetes and deafness
- miRNA
microRNA
- mitomiRs
mitochondrial miRNAs
- MITRAC
mitochondrial translation regulation assembly intermediate of cytochrome c oxidase
- MLASA, syndrome
myopathy, lactic acidosis, and sideroblastic anemia
- mt-tRNA
mitochondrial transfer RNA
- MT-TI
mt-tRNAIle
- MT-TL1
mt-tRNALeu
- MT-TK
mt-tRNALys
- mt-aaRSs
mitochondrial aminoacyl-tRNA synthetases
- mitoribosomes
mitochondrial ribosomes
- mRNA
messenger RNA
- mtDNA
mitochondrial DNA
- mtPIC
mitochondrial preinitiation step
- mTOR
mammalian target of rapamycin
- MTRF
mitochondrial release factor
- nDNA
nuclear DNA
- NGS
next-generation sequencing
- NTD
N-terminal domain
- NTEs
mito-specific N-terminal extensions
- OXPHOS
oxidative phosphorylation
- PD
Parkinson’s disease
- PoTC
post-termination complex
- PTC
peptidyltransferase center
- RCC
respiratory chain complexes
- ROS
reactive oxygen species
- RRF
mitochondrial ribosome recycling factor
- rRNA
ribosomal RNA
- SNP
single nucleotide polymorphism
- SRL
sarcin/ricin loop
- SSU
small subunit
- TACO1
translational activator of cytochrome oxidase I
- TFAM
mitochondrial transcription factor A
- tRNA
transfer RNA
- UPRmt
mitochondrial unfolded protein response
- USP5
ubiquitin-specific peptidase 5
- UTR
untranslated region
- WES
whole-exome sequencing.
Footnotes
Funding. This study was funded by grants from the National Natural Science Foundation of China (Nos. 81602353 and 31870816), Major Research Program of the National Natural Science Foundation of China (No. 91849209), the Natural Science Foundation of Jiangsu Province (BK20171145), the China Postdoctoral Science Foundation (2019M652314 and 2020T130333), the Qingdao Applied Basic Research Project (19-6-2-39-cg), and the Qingdao Science and Technology Plan Fund (18-6-1-63-nsh).
References
- Abadi A., Peralta-Romero J., Suarez F., Gomez-Zamudio J., Burguete-Garcia A. I., Cruz M., et al. (2016). Assessing the effects of 35 European-derived BMI-associated SNPs in Mexican children. Obesity (Silver Spring) 24 1989–1995. 10.1002/oby.21590 [DOI] [PubMed] [Google Scholar]
- Abahuni N., Gispert S., Bauer P., Riess O., Kruger R., Becker T., et al. (2007). Mitochondrial translation initiation factor 3 gene polymorphism associated with Parkinson’s disease. Neurosci. Lett. 414 126–129. 10.1016/j.neulet.2006.12.053 [DOI] [PubMed] [Google Scholar]
- Agnew T., Goldsworthy M., Aguilar C., Morgan A., Simon M., Hilton H., et al. (2018). A Wars2 mutant mouse model displays OXPHOS deficiencies and activation of tissue-specific stress response pathways. Cell Rep. 25 3315–3328.e6. 10.1016/j.celrep.2018.11.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahola S., Isohanni P., Euro L., Brilhante V., Palotie A., Pihko H., et al. (2014). Mitochondrial EFTs defects in juvenile-onset Leigh disease, ataxia, neuropathy, and optic atrophy. Neurology 83 743–751. 10.1212/WNL.0000000000000716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aibara S., Singh V., Modelska A., Amunts A. (2020). Structural basis of mitochondrial translation. Elife 9:e58362. 10.7554/eLife.58362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akabane S., Ueda T., Nierhaus K. H., Takeuchi N. (2014). Ribosome rescue and translation termination at non-standard stop codons by ICT1 in mammalian mitochondria. PLoS Genet. 10:e1004616. 10.1371/journal.pgen.1004616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaiz A. A., Muller V., Boutry-Kryza N., Ville D., Guex N., De Bellescize J., et al. (2016). West syndrome caused by homozygous variant in the evolutionary conserved gene encoding the mitochondrial elongation factor GUF1. Eur. J. Hum. Genet. 24 1001–1008. 10.1038/ejhg.2015.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., De Bruijn M. H., Coulson A. R., Drouin J., et al. (1981). Sequence and organization of the human mitochondrial genome. Nature 290 457–465. 10.1038/290457a0 [DOI] [PubMed] [Google Scholar]
- Antonicka H., Ostergaard E., Sasarman F., Weraarpachai W., Wibrand F., Pedersen A. M., et al. (2010). Mutations in C12orf65 in patients with encephalomyopathy and a mitochondrial translation defect. Am. J. Hum. Genet. 87 115–122. 10.1016/j.ajhg.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anvret A., Ran C., Westerlund M., Thelander A. C., Sydow O., Lind C., et al. (2010). Possible involvement of a mitochondrial translation initiation factor 3 variant causing decreased mRNA levels in Parkinson’s disease. Parkinsons Dis. 2010:491751. 10.4061/2010/491751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschrafi A., Schwechter A. D., Mameza M. G., Natera-Naranjo O., Gioio A. E., Kaplan B. B. (2008). MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. J. Neurosci. 28 12581–12590. 10.1523/JNEUROSCI.3338-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyub S. A., Dobriyal D., Varshney U. (2017). Contributions of the N- and C-terminal domains of initiation factor 3 to its functions in the fidelity of initiation and antiassociation of the ribosomal subunits. J. Bacteriol. 199 e51–e17. 10.1128/JB.00051-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandiera S., Mategot R., Girard M., Demongeot J., Henrion-Caude A. (2013). MitomiRs delineating the intracellular localization of microRNAs at mitochondria. Free Radic. Biol. Med. 64 12–19. 10.1016/j.freeradbiomed.2013.06.013 [DOI] [PubMed] [Google Scholar]
- Barcia G., Rio M., Assouline Z., Zangarelli C., Gueguen N., Dumas V. D., et al. (2020). Clinical neuroimaging and biochemical findings in patients and patient fibroblasts expressing ten novel GFM1 mutations. Hum. Mutat. 41 397–402. 10.1002/humu.23937 [DOI] [PubMed] [Google Scholar]
- Bauerschmitt H., Funes S., Herrmann J. M. (2008). The membrane-bound GTPase Guf1 promotes mitochondrial protein synthesis under suboptimal conditions. J. Biol. Chem. 283 17139–17146. 10.1074/jbc.M710037200 [DOI] [PubMed] [Google Scholar]
- Behrouz B., Vilarino-Guell C., Heckman M. G., Soto-Ortolaza A. I., Aasly J. O., Sando S., et al. (2010). Mitochondrial translation initiation factor 3 polymorphism and Parkinson’s disease. Neurosci. Lett. 486 228–230. 10.1016/j.neulet.2010.09.059 [DOI] [PubMed] [Google Scholar]
- Bhargava K., Spremulli L. L. (2005). Role of the N- and C-terminal extensions on the activity of mammalian mitochondrial translational initiation factor 3. Nucleic Acids Res. 33 7011–7018. 10.1093/nar/gki1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava K., Templeton P., Spremulli L. L. (2004). Expression and characterization of isoform 1 of human mitochondrial elongation factor G. Protein Expr. Purif. 37 368–376. 10.1016/j.pep.2004.06.030 [DOI] [PubMed] [Google Scholar]
- Bian X., Wasserfall C., Wallstrom G., Wang J., Wang H., Barker K., et al. (2017). Tracking the antibody immunome in type 1 diabetes using protein arrays. J. Proteome Res. 16 195–203. 10.1021/acs.jproteome.6b00354 [DOI] [PubMed] [Google Scholar]
- Boczonadi V., Horvath R. (2014). Mitochondria: impaired mitochondrial translation in human disease. Int. J. Biochem. Cell Biol. 48 77–84. 10.1016/j.biocel.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito S., Thompson K., Campistol J., Colomer J., Hardy S. A., He L., et al. (2015). Long-term survival in a child with severe encephalopathy, multiple respiratory chain deficiency and GFM1 mutations. Front. Genet. 6:102. 10.3389/fgene.2015.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchert R., Uebe S., Radwan F., Tawamie H., Issa S., Shimazaki H., et al. (2013). Mutations in the mitochondrial gene C12ORF65 lead to syndromic autosomal recessive intellectual disability and show genotype phenotype correlation. Eur. J. Med. Genet. 56 599–602. 10.1016/j.ejmg.2013.09.010 [DOI] [PubMed] [Google Scholar]
- Cai Y. C., Bullard J. M., Thompson N. L., Spremulli L. L. (2000). Interaction of mitochondrial elongation factor Tu with aminoacyl-tRNA and elongation factor Ts. J. Biol. Chem. 275 20308–20314. 10.1074/jbc.M001899200 [DOI] [PubMed] [Google Scholar]
- Calvo S. E., Compton A. G., Hershman S. G., Lim S. C., Lieber D. S., Tucker E. J., et al. (2012). Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci. Transl. Med. 4:118ra10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Qin Y. (2016). Mitochondrial translation factors reflect coordination between organelles and cytoplasmic translation via mTOR signaling: implication in disease. Free Radic. Biol. Med. 100 231–237. 10.1016/j.freeradbiomed.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Carroll C. J., Isohanni P., Poyhonen R., Euro L., Richter U., Brilhante V., et al. (2013). Whole-exome sequencing identifies a mutation in the mitochondrial ribosome protein MRPL44 to underlie mitochondrial infantile cardiomyopathy. J. Med. Genet. 50 151–159. 10.1136/jmedgenet-2012-101375 [DOI] [PubMed] [Google Scholar]
- Chapman N. M., Zeng H., Nguyen T. M., Wang Y., Vogel P., Dhungana Y., et al. (2018). mTOR coordinates transcriptional programs and mitochondrial metabolism of activated Treg subsets to protect tissue homeostasis. Nat. Commun. 9:2095. 10.1038/s41467-018-04392-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Feng S., Kumar V., Ero R., Gao Y. G. (2013). Structure of EF-G-ribosome complex in a pretranslocation state. Nat. Struct. Mol. Biol. 20 1077–1084. 10.1038/nsmb.2645 [DOI] [PubMed] [Google Scholar]
- Chen Z., Li Y., Zhang H., Huang P., Luthra R. (2010). Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 29 4362–4368. 10.1038/onc.2010.193 [DOI] [PubMed] [Google Scholar]
- Chicherin I. V., Baleva M. V., Levitskii S. A., Dashinimaev E. B., Krasheninnikov I. A. (2019). Mitochondrial translation initiation factor 3: structure, functions, interactions, and implication in human health and disease. Biochemistry (Mosc) 84 1143–1150. 10.1134/S0006297919100031 [DOI] [PubMed] [Google Scholar]
- Chomyn A., Martinuzzi A., Yoneda M., Daga A., Hurko O., Johns D., et al. (1992). MELAS mutation in mtDNA binding site for transcription termination factor causes defects in protein synthesis and in respiration but no change in levels of upstream and downstream mature transcripts. Proc. Natl. Acad. Sci. U.S.A. 89 4221–4225. 10.1073/pnas.89.10.4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. (1991). In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol. Cell Biol. 11 2236–2244. 10.1128/mcb.11.4.2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian B. E., Spremulli L. L. (2009). Evidence for an active role of IF3mt in the initiation of translation in mammalian mitochondria. Biochemistry 48 3269–3278. 10.1021/bi8023493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian B. E., Spremulli L. L. (2012). Mechanism of protein biosynthesis in mammalian mitochondria. Biochim. Biophys. Acta 1819 1035–1054. 10.1016/j.bbagrm.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen M. J., Antonicka H., Ugalde C., Sasarman F., Rossi R., Heister J. G., et al. (2004). Mutant mitochondrial elongation factor G1 and combined oxidative phosphorylation deficiency. N. Engl. J. Med. 351 2080–2086. 10.1056/NEJMoa041878 [DOI] [PubMed] [Google Scholar]
- Colleoni F., Padmanabhan N., Yung H. W., Watson E. D., Cetin I., Tissot Van Patot M. C., et al. (2013). Suppression of mitochondrial electron transport chain function in the hypoxic human placenta: a role for miRNA-210 and protein synthesis inhibition. PLoS One 8:e55194. 10.1371/journal.pone.0055194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlmans D., Houzelle A., Andreux P., Wang X., Jorgensen J. A., Moullan N., et al. (2019). MicroRNA-382 silencing induces a mitonuclear protein imbalance and activates the mitochondrial unfolded protein response in muscle cells. J. Cell Physiol. 234 6601–6610. 10.1002/jcp.27401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Bedja D., Campbell N., Dunkerly B., Chenna V., Maitra A., et al. (2014). miR-181c regulates the mitochondrial genome, bioenergetics, and propensity for heart failure in vivo. PLoS One 9:e96820. 10.1371/journal.pone.0096820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Ferlito M., Kent O. A., Fox-Talbot K., Wang R., Liu D., et al. (2012). Nuclear miRNA regulates the mitochondrial genome in the heart. Circ. Res. 110 1596–1603. 10.1161/CIRCRESAHA.112.267732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva D., Tu Y. T., Amunts A., Fontanesi F., Barrientos A. (2015). Mitochondrial ribosome assembly in health and disease. Cell Cycle 14 2226–2250. 10.1080/15384101.2015.1053672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debray F. G., Morin C., Janvier A., Villeneuve J., Maranda B., Laframboise R., et al. (2011). LRPPRC mutations cause a phenotypically distinct form of Leigh syndrome with cytochrome c oxidase deficiency. J. Med. Genet. 48 183–189. 10.1136/jmg.2010.081976 [DOI] [PubMed] [Google Scholar]
- Desai N., Yang H., Chandrasekaran V., Kazi R., Minczuk M., Ramakrishnan V. (2020). Elongational stalling activates mitoribosome-associated quality control. Science 370 1105–1110. 10.1126/science.abc7782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey D., Oleinikov A. V., Traut R. R. (1995). The hinge region of Escherichia coli ribosomal protein L7/L12 is required for factor binding and GTP hydrolysis. Biochimie 77 925–930. 10.1016/0300-9084(95)80003-4 [DOI] [PubMed] [Google Scholar]
- Di Nottia M., Montanari A., Verrigni D., Oliva R., Torraco A., Fernandez-Vizarra E., et al. (2017). Novel mutation in mitochondrial Elongation Factor EF-Tu associated to dysplastic leukoencephalopathy and defective mitochondrial DNA translation. Biochim. Biophys. Acta Mol. Basis Dis. 1863 961–967. 10.1016/j.bbadis.2017.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann C. L., Koerner T. J., Tzagoloff A. (1984). Assembly of the mitochondrial membrane system. CBP1, a yeast nuclear gene involved in 5″ end processing of cytochrome b pre-mRNA. J. Biol. Chem. 259, 4722–4731. 10.1016/S0021-9258(17)42907-3 [DOI] [PubMed] [Google Scholar]
- Diodato D., Ghezzi D., Tiranti V. (2014). The mitochondrial aminoacyl tRNA synthetases: genes and syndromes. Int. J. Cell Biol. 2014:787956. 10.1155/2014/787956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelmaier F., Haack T. B., Catarino C. B., Gallenmuller C., Rodenburg R. J., Strom T. M., et al. (2015). MRPL44 mutations cause a slowly progressive multisystem disease with childhood-onset hypertrophic cardiomyopathy. Neurogenetics 16 319–323. 10.1007/s10048-015-0444-2 [DOI] [PubMed] [Google Scholar]
- Dogan S. A., Pujol C., Maiti P., Kukat A., Wang S., Hermans S., et al. (2014). Tissue-specific loss of DARS2 activates stress responses independently of respiratory chain deficiency in the heart. Cell Metab. 19 458–469. 10.1016/j.cmet.2014.02.004 [DOI] [PubMed] [Google Scholar]
- D’Souza A. R., Minczuk M. (2018). Mitochondrial transcription and translation: overview. Essays Biochem. 62 309–320. 10.1042/EBC20170102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emperador S., Bayona-Bafaluy M. P., Fernandez-Marmiesse A., Pineda M., Felgueroso B., Lopez-Gallardo E., et al. (2016). Molecular-genetic characterization and rescue of a TSFM mutation causing childhood-onset ataxia and nonobstructive cardiomyopathy. Eur. J. Hum. Genet. 25 153–156. 10.1038/ejhg.2016.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez J. A., Chomyn A., Attardi G. (1995). MtDNA mutation in MERRF syndrome causes defective aminoacylation of tRNA(Lys) and premature translation termination. Nat. Genet. 10 47–55. 10.1038/ng0595-47 [DOI] [PubMed] [Google Scholar]
- Fan S., Tian T., Chen W., Lv X., Lei X., Zhang H., et al. (2019). Mitochondrial miRNA determines chemoresistance by reprogramming metabolism and regulating mitochondrial transcription. Cancer Res. 79 1069–1084. 10.1158/0008-5472.CAN-18-2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F., Liu Z., Fang H., Wu J., Shen D., Sun S., et al. (2017a). The clinical and genetic characteristics in children with mitochondrial disease in China. Sci. China Life Sci. 60 746–757. 10.1007/s11427-017-9080-y [DOI] [PubMed] [Google Scholar]
- Fang X. J., Zhang W., Lyu H., Wang Z. X., Wang W. W., Yuan Y. (2017b). Compound heterozygote mutation of C12orf65 causes distal motor neuropathy and optic atrophy. Chin. Med. J. (Engl) 130 242–244. 10.4103/0366-6999.198019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A., Del’olio S., Barrientos A. (2020). The diseased mitoribosome. FEBS Lett. 595 1025–1061. 10.1002/1873-3468.14024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler E., Eckl E. M., Schmitt S., Mancilla I. A., Meyer-Bender M. F., Hanf M. (2020). A pathway coordinated by DELE1 relays mitochondrial stress to the cytosol. Nature 579 433–437. 10.1038/s41586-020-2076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine A. S., Nemeth C. L., Kaufman M. L., Fatemi A. (2019). Mitochondrial aminoacyl-tRNA synthetase disorders: an emerging group of developmental disorders of myelination. J. Neurodev. Disord. 11:29. 10.1186/s11689-019-9292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori A., Perez-Martinez X., Fox T. D. (2005). Overexpression of the COX2 translational activator, Pet111p, prevents translation of COX1 mRNA and cytochrome c oxidase assembly in mitochondria of Saccharomyces cerevisiae. Mol. Microbiol. 56 1689–1704. 10.1111/j.1365-2958.2005.04658.x [DOI] [PubMed] [Google Scholar]
- Florentz C., Sohm B., Tryoen-Toth P., Putz J., Sissler M. (2003). Human mitochondrial tRNAs in health and disease. Cell Mol. Life Sci. 60 1356–1375. 10.1007/s00018-003-2343-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanesi F., Soto I. C., Horn D., Barrientos A. (2006). Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process. Am. J. Physiol. Cell Physiol. 291 C1129–C1147. 10.1152/ajpcell.00233.2006 [DOI] [PubMed] [Google Scholar]
- Fox T. D. (2012). Mitochondrial protein synthesis, import, and assembly. Genetics 192 1203–1234. 10.1534/genetics.112.141267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura S., Ohba C., Watanabe T., Minagawa K., Shimura M., Murayama K., et al. (2015). Compound heterozygous GFM2 mutations with Leigh syndrome complicated by arthrogryposis multiplex congenita. J. Hum. Genet. 60 509–513. 10.1038/jhg.2015.57 [DOI] [PubMed] [Google Scholar]
- Galmiche L., Serre V., Beinat M., Assouline Z., Lebre A. S., Chretien D., et al. (2011). Exome sequencing identifies MRPL3 mutation in mitochondrial cardiomyopathy. Hum. Mutat. 32 1225–1231. 10.1002/humu.21562 [DOI] [PubMed] [Google Scholar]
- Gao Y., Bai X., Zhang D., Han C., Yuan J., Liu W., et al. (2016). Mammalian elongation factor 4 regulates mitochondrial translation essential for spermatogenesis. Nat. Struct. Mol. Biol. 23 441–449. 10.1038/nsmb.3206 [DOI] [PubMed] [Google Scholar]
- Gaur R., Grasso D., Datta P. P., Krishna P. D., Das G., Spencer A., et al. (2008). A single mammalian mitochondrial translation initiation factor functionally replaces two bacterial factors. Mol. Cell 29 180–190. 10.1016/j.molcel.2007.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girodat D., Blanchard S. C., Wieden H. J., Sanbonmatsu K. Y. (2020). Elongation Factor Tu Switch I Element is a gate for aminoacyl-tRNA selection. J. Mol. Biol. 432 3064–3077. 10.1016/j.jmb.2020.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R. I. C., Thompson K., Barbosa I. A., He L., Alston C. L., Deshpande C., et al. (2017). Novel GFM2 variants associated with early-onset neurological presentations of mitochondrial disease and impaired expression of OXPHOS subunits. Neurogenetics 18 227–235. 10.1007/s10048-017-0526-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz A., Tyynismaa H., Euro L., Ellonen P., Hyotylainen T., Ojala T., et al. (2011). Exome sequencing identifies mitochondrial alanyl-tRNA synthetase mutations in infantile mitochondrial cardiomyopathy. Am. J. Hum. Genet. 88 635–642. 10.1016/j.ajhg.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber B. J., Bieri P., Leibundgut M., Leitner A., Aebersold R., Boehringer D., et al. (2015). Ribosome. The complete structure of the 55S mammalian mitochondrial ribosome. Science 348 303–308. 10.1126/science.aaa3872 [DOI] [PubMed] [Google Scholar]
- Gruschke S., Kehrein K., Rompler K., Grone K., Israel L., Imhof A., et al. (2011). Cbp3-Cbp6 interacts with the yeast mitochondrial ribosomal tunnel exit and promotes cytochrome b synthesis and assembly. J. Cell Biol. 193 1101–1114. 10.1083/jcb.201103132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Aviles G., Liu Y., Tian R., Unger B. A., Lin Y. T., et al. (2020). Mitochondrial stress is relayed to the cytosol by an OMA1-DELE1-HRI pathway. Nature 579 427–432. 10.1038/s41586-020-2078-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg B. M., Larsson N. G. (2014). Making proteins in the powerhouse. Cell Metab. 20 226–240. 10.1016/j.cmet.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Handa Y., Hikawa Y., Tochio N., Kogure H., Inoue M., Koshiba S., et al. (2010). Solution structure of the catalytic domain of the mitochondrial protein ICT1 that is essential for cell vitality. J. Mol. Biol. 404 260–273. 10.1016/j.jmb.2010.09.033 [DOI] [PubMed] [Google Scholar]
- Hansen L. L., Jorgensen R., Justesen J. (2000). Assignment of the human mitochondrial translational release factor 1 (MTRF1) to chromosome 13q14.1– > q14.3 and of the human mitochondrial ribosome recycling factor (MRRF) to chromosome 9q32–>q34.1 with radiation hybrid mapping. Cytogenet. Cell Genet. 88 91–92. 10.1159/000015494 [DOI] [PubMed] [Google Scholar]
- Haque M. E., Spremulli L. L. (2008). Roles of the N- and C-terminal domains of mammalian mitochondrial initiation factor 3 in protein biosynthesis. J. Mol. Biol. 384 929–940. 10.1016/j.jmb.2008.09.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Chen M., Scheffler N. K., Gibson B. W., Spremulli L. L., Gottlieb R. A. (2001). Phosphorylation of mitochondrial elongation factor Tu in ischemic myocardium: basis for chloramphenicol-mediated cardioprotection. Circ. Res. 89 461–467. 10.1161/hh1701.096038 [DOI] [PubMed] [Google Scholar]
- He K., Guo X., Liu Y., Li J., Hu Y., Wang D., et al. (2016). TUFM downregulation induces epithelial-mesenchymal transition and invasion in lung cancer cells via a mechanism involving AMPK-GSK3beta signaling. Cell Mol. Life Sci. 73 2105–2121. 10.1007/s00018-015-2122-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hei Z., Wu S., Liu Z., Wang J., Fang P. (2019). Retractile lysyl-tRNA synthetase-AIMP2 assembly in the human multi-aminoacyl-tRNA synthetase complex. J. Biol. Chem. 294 4775–4783. 10.1074/jbc.RA118.006356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidary G., Calderwood L., Cox G. F., Robson C. D., Teot L. A., Mullon J., et al. (2014). Optic atrophy and a Leigh-like syndrome due to mutations in the c12orf65 gene: report of a novel mutation and review of the literature. J. Neuroophthalmol. 34 39–43. 10.1097/WNO.0000000000000076 [DOI] [PubMed] [Google Scholar]
- Helgstrand M., Mandava C. S., Mulder F. A., Liljas A., Sanyal S., Akke M. (2007). The ribosomal stalk binds to translation factors IF2, EF-Tu, EF-G and RF3 via a conserved region of the L12 C-terminal domain. J. Mol. Biol. 365 468–479. 10.1016/j.jmb.2006.10.025 [DOI] [PubMed] [Google Scholar]
- Hershkovitz T., Kurolap A., Gonzaga-Jauregui C., Paperna T., Mory A., Wolf S. E., et al. (2019). A novel TUFM homozygous variant in a child with mitochondrial cardiomyopathy expands the phenotype of combined oxidative phosphorylation deficiency 4. J. Hum. Genet. 64 589–595. 10.1038/s10038-019-0592-6 [DOI] [PubMed] [Google Scholar]
- Houtkooper R. H., Mouchiroud L., Ryu D., Moullan N., Katsyuba E., Knott G., et al. (2013). Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497 451–457. 10.1038/nature12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa E., Fattal-Valevski A., Eyal O., Miyatake S., Saada A., Nakashima M., et al. (2016). Homozygous p.V116∗ mutation in C12orf65 results in Leigh syndrome. J. Neurol. Neurosurg. Psychiatry 87 212–216. 10.1136/jnnp-2014-310084 [DOI] [PubMed] [Google Scholar]
- Islas-Osuna M. A., Ellis T. P., Marnell L. L., Mittelmeier T. M., Dieckmann C. L. (2002). Cbp1 is required for translation of the mitochondrial cytochrome b mRNA of Saccharomyces cerevisiae. J. Biol. Chem. 277 37987–37990. 10.1074/jbc.M206132200 [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Turnbull D. M. (2005). Nuclear genes and mitochondrial translation: a new class of genetic disease. Trends Genet. 21 312–314. 10.1016/j.tig.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Jeppesen M. G., Navratil T., Spremulli L. L., Nyborg J. (2005). Crystal structure of the bovine mitochondrial elongation factor Tu.Ts complex. J. Biol. Chem. 280 5071–5081. 10.1074/jbc.M411782200 [DOI] [PubMed] [Google Scholar]
- Kaji A., Kiel M. C., Hirokawa G., Muto A. R., Inokuchi Y., Kaji H. (2001). The fourth step of protein synthesis: disassembly of the posttermination complex is catalyzed by elongation factor G and ribosome recycling factor, a near-perfect mimic of tRNA. Cold Spring Harb. Symp. Quant. Biol. 66 515–529. 10.1101/sqb.2001.66.515 [DOI] [PubMed] [Google Scholar]
- Kaushal P. S., Sharma M. R., Agrawal R. K. (2015). The 55S mammalian mitochondrial ribosome and its tRNA-exit region. Biochimie 114 119–126. 10.1016/j.biochi.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. A., Nikkanen J., Yatsuga S., Jackson C., Wang L., Pradhan S., et al. (2017). mTORC1 regulates mitochondrial integrated stress response and mitochondrial myopathy progression. Cell Metab. 26 419–428.e5. 10.1016/j.cmet.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Khawaja A., Itoh Y., Remes C., Spahr H., Yukhnovets O., Hofig H., et al. (2020). Distinct pre-initiation steps in human mitochondrial translation. Nat. Commun. 11:2932. 10.1038/s41467-020-16503-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. P., Koga Y., Davidson M., Schon E. A. (1992). Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol. Cell Biol. 12 480–490. 10.1128/mcb.12.2.480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselev L., Ehrenberg M., Frolova L. (2003). Termination of translation: interplay of mRNA, rRNAs and release factors? EMBO J. 22 175–182. 10.1093/emboj/cdg017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc E. C., Burkhart W., Blackburn K., Moyer M. B., Schlatzer D. M., Moseley A., et al. (2001). The large subunit of the mammalian mitochondrial ribosome. Analysis of the complement of ribosomal proteins present. J. Biol. Chem. 276 43958–43969. 10.1074/jbc.M106510200 [DOI] [PubMed] [Google Scholar]
- Koc E. C., Spremulli L. L. (2002). Identification of mammalian mitochondrial translational initiation factor 3 and examination of its role in initiation complex formation with natural mRNAs. J. Biol. Chem. 277 35541–35549. 10.1074/jbc.M202498200 [DOI] [PubMed] [Google Scholar]
- Kogure H., Hikawa Y., Hagihara M., Tochio N., Koshiba S., Inoue Y., et al. (2012). Solution structure and siRNA-mediated knockdown analysis of the mitochondrial disease-related protein C12orf65. Proteins 80 2629–2642. 10.1002/prot.24152 [DOI] [PubMed] [Google Scholar]
- Kohda M., Tokuzawa Y., Kishita Y., Nyuzuki H., Moriyama Y., Mizuno Y., et al. (2016). A comprehensive genomic analysis reveals the genetic landscape of mitochondrial respiratory chain complex deficiencies. PLoS Genet. 12:e1005679. 10.1371/journal.pgen.1005679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler F., Muller-Rischart A. K., Conradt B., Rolland S. G. (2015). The loss of LRPPRC function induces the mitochondrial unfolded protein response. Aging (Albany NY) 7 701–717. 10.18632/aging.100812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konovalova S., Tyynismaa H. (2013). Mitochondrial aminoacyl-tRNA synthetases in human disease. Mol. Genet. Metab. 108 206–211. 10.1016/j.ymgme.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Koripella R. K., Sharma M. R., Bhargava K., Datta P. P., Kaushal P. S., Keshavan P., et al. (2020). Structures of the human mitochondrial ribosome bound to EF-G1 reveal distinct features of mitochondrial translation elongation. Nat. Commun. 11:3830. 10.1038/s41467-020-17715-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koripella R. K., Sharma M. R., Haque M. E., Risteff P., Spremulli L. L., Agrawal R. K. (2019a). Structure of human mitochondrial translation initiation factor 3 bound to the small ribosomal subunit. iScience 12 76–86. 10.1016/j.isci.2018.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koripella R. K., Sharma M. R., Risteff P., Keshavan P., Agrawal R. K. (2019b). Structural insights into unique features of the human mitochondrial ribosome recycling. Proc. Natl. Acad. Sci. U.S.A. 116 8283–8288. 10.1073/pnas.1815675116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer E., Ban N. (2020). Structural insights into mammalian mitochondrial translation elongation catalyzed by mtEFG1. EMBO J. 39:e104820. 10.15252/embj.2020104820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer E., Ban N. (2021). Mechanisms and regulation of protein synthesis in mitochondria. Nat. Rev. Mol. Cell Biol. 22 307–325. 10.1038/s41580-021-00332-2 [DOI] [PubMed] [Google Scholar]
- Kummer E., Leibundgut M., Rackham O., Lee R. G., Boehringer D., Filipovska A., et al. (2018). Unique features of mammalian mitochondrial translation initiation revealed by cryo-EM. Nature 560 263–267. 10.1038/s41586-018-0373-y [DOI] [PubMed] [Google Scholar]
- Kuzmenko A., Atkinson G. C., Levitskii S., Zenkin N., Tenson T., Hauryliuk V., et al. (2014). Mitochondrial translation initiation machinery: conservation and diversification. Biochimie 100 132–140. 10.1016/j.biochi.2013.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake N. J., Webb B. D., Stroud D. A., Richman T. R., Ruzzenente B., Compton A. G., et al. (2018). Biallelic mutations in MRPS34 lead to instability of the small mitoribosomal subunit and Leigh syndrome. Am. J. Hum. Genet. 102:713. 10.1016/j.ajhg.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson N. G., Clayton D. A. (1995). Molecular genetic aspects of human mitochondrial disorders. Annu. Rev. Genet. 29 151–178. 10.1146/annurev.ge.29.120195.001055 [DOI] [PubMed] [Google Scholar]
- Lavdovskaia E., Denks K., Nadler F., Steube E., Linden A., Urlaub H., et al. (2020). Dual function of GTPBP6 in biogenesis and recycling of human mitochondrial ribosomes. Nucleic Acids Res. 48 12929–12942. 10.1093/nar/gkaa1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. E., Perry R. A., Jr., Brown J. L., Rosa-Caldwell M. E., Brown L. A., Haynie W. S., et al. (2019). Mitochondrial mRNA translation initiation contributes to oxidative metabolism in the myocardia of aged, obese mice. Exp. Gerontol. 121 62–70. 10.1016/j.exger.2019.03.009 [DOI] [PubMed] [Google Scholar]
- Li H., Dai B., Fan J., Chen C., Nie X., Yin Z., et al. (2019). The different roles of miRNA-92a-2-5p and let-7b-5p in mitochondrial translation in db/db mice. Mol. Ther. Nucleic Acids 17 424–435. 10.1016/j.omtn.2019.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Zhang X., Wang F., Zhou L., Yin Z., Fan J., et al. (2016). MicroRNA-21 Lowers blood pressure in spontaneous hypertensive rats by upregulating mitochondrial translation. Circulation 134 734–751. 10.1161/CIRCULATIONAHA.116.023926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H. X., Spremulli L. L. (1990). Identification and initial characterization of translational initiation factor 2 from bovine mitochondria. J. Biol. Chem. 265 13618–13622. 10.1016/s0021-9258(18)77393-6 [DOI] [PubMed] [Google Scholar]
- Lin J., Chen K., Chen W., Yao Y., Ni S., Ye M., et al. (2020). Paradoxical mitophagy regulation by PINK1 and TUFm. Mol. Cell 80:e612. 10.1016/j.molcel.2020.10.007 [DOI] [PubMed] [Google Scholar]
- Liu J., Fang H., Chi Z., Wu Z., Wei D., Mo D., et al. (2015). XPD localizes in mitochondria and protects the mitochondrial genome from oxidative DNA damage. Nucleic Acids Res. 43 5476–5488. 10.1093/nar/gkv472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Sanchez M. I. G., Kruger A., Shiriaev D. I., Liu Y., Rorbach J. (2021). Human mitoribosome biogenesis and its emerging links to disease. Int. J. Mol. Sci. 22:3827. 10.3390/ijms22083827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai N., Chrzanowska-Lightowlers Z. M., Lightowlers R. N. (2017). The process of mammalian mitochondrial protein synthesis. Cell Tissue Res. 367 5–20. 10.1007/s00441-016-2456-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March P. E., Inouye M. (1985). Characterization of the lep operon of Escherichia coli. Identification of the promoter and the gene upstream of the signal peptidase I gene. J. Biol. Chem. 260 7206–7213. 10.1016/s0021-9258(17)39594-7 [DOI] [PubMed] [Google Scholar]
- Marcos C. M., Tamer G., De Oliveira H. C., Assato P. A., Scorzoni L., Santos C. T., et al. (2019). Down-regulation of TUFM impairs host cell interaction and virulence by Paracoccidioides brasiliensis. Sci. Rep. 9:17206. 10.1038/s41598-019-51540-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci J. P., Davidson M., Koga Y., Schon E. A., King M. P. (1995). In vitro analysis of mutations causing myoclonus epilepsy with ragged-red fibers in the mitochondrial tRNA(Lys)gene: two genotypes produce similar phenotypes. Mol. Cell Biol. 15 2872–2881. 10.1128/mcb.15.5.2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick D. U., Dennerlein S., Wiese H., Reinhold R., Pacheu-Grau D., Lorenzi I., et al. (2012). MITRAC links mitochondrial protein translocation to respiratory-chain assembly and translational regulation. Cell 151 1528–1541. 10.1016/j.cell.2012.11.053 [DOI] [PubMed] [Google Scholar]
- Mikhailova S. V., Zakharova E., Banin A. V., Demushkina A. A., Petrukhin A. S. (2009). Clinical and molecular genetic diagnosis of leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation in children. Zh Nevrol. Psikhiatr. Im. S S Korsakova 109 16–22. [PubMed] [Google Scholar]
- Miller C., Saada A., Shaul N., Shabtai N., Ben-Shalom E., Shaag A., et al. (2004). Defective mitochondrial translation caused by a ribosomal protein (MRPS16) mutation. Ann. Neurol. 56 734–738. 10.1002/ana.20282 [DOI] [PubMed] [Google Scholar]
- Mittelmeier T. M., Dieckmann C. L. (1995). In vivo analysis of sequences required for translation of cytochrome b transcripts in yeast mitochondria. Mol. Cell Biol. 15 780–789. 10.1128/mcb.15.2.780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Hughes J. A., Sweeney M. G., Cooper J. M., Hammans S. R., Brockington M., Schapira A. H., et al. (1995). Mitochondrial DNA (mtDNA) diseases: correlation of genotype to phenotype. Biochim. Biophys. Acta 1271 135–140. 10.1016/0925-4439(95)00020-5 [DOI] [PubMed] [Google Scholar]
- Nagao A., Suzuki T., Katoh T., Sakaguchi Y., Suzuki T. (2009). Biogenesis of glutaminyl-mt tRNAGln in human mitochondria. Proc. Natl. Acad. Sci. U.S.A. 106 16209–16214. 10.1073/pnas.0907602106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naithani S., Saracco S. A., Butler C. A., Fox T. D. (2003). Interactions among COX1, COX2, and COX3 mRNA-specific translational activator proteins on the inner surface of the mitochondrial inner membrane of Saccharomyces cerevisiae. Mol. Biol. Cell 14 324–333. 10.1091/mbc.e02-08-0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara H., Omoto M., Takao M., Higuchi Y., Koga M., Kawai M., et al. (2017). Autopsy case of the C12orf65 mutation in a patient with signs of mitochondrial dysfunction. Neurol. Genet. 3:e171. 10.1212/NXG.0000000000000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki Y., Matsunaga N., Ishizawa T., Ueda T., Takeuchi N. (2008). HMRF1L is a human mitochondrial translation release factor involved in the decoding of the termination codons UAA and UAG. Genes Cells 13 429–438. 10.1111/j.1365-2443.2008.01181.x [DOI] [PubMed] [Google Scholar]
- O’Brien T. W. (1971). The general occurrence of 55 S ribosomes in mammalian liver mitochondria. J. Biol. Chem. 246 3409–3417. 10.1016/s0021-9258(18)62239-2 [DOI] [PubMed] [Google Scholar]
- O’Brien T. W. (2003). Properties of human mitochondrial ribosomes. IUBMB Life 55 505–513. 10.1080/15216540310001626610 [DOI] [PubMed] [Google Scholar]
- Ognjenovic J., Simonovic M. (2018). Human aminoacyl-tRNA synthetases in diseases of the nervous system. RNA Biol. 15 623–634. 10.1080/15476286.2017.1330245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala D., Montoya J., Attardi G. (1981). tRNA punctuation model of RNA processing in human mitochondria. Nature 290 470–474. 10.1038/290470a0 [DOI] [PubMed] [Google Scholar]
- Ostojic J., Panozzo C., Bourand-Plantefol A., Herbert C. J., Dujardin G., Bonnefoy N. (2016). Ribosome recycling defects modify the balance between the synthesis and assembly of specific subunits of the oxidative phosphorylation complexes in yeast mitochondria. Nucleic Acids Res. 44 5785–5797. 10.1093/nar/gkw490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M., Amunts A., Brown A. (2016). Organization and regulation of mitochondrial protein synthesis. Annu. Rev. Biochem. 85 77–101. 10.1146/annurev-biochem-060815-014334 [DOI] [PubMed] [Google Scholar]
- Ozawa M., Nishino I., Horai S., Nonaka I., Goto Y. I. (1997). Myoclonus epilepsy associated with ragged-red fibers: a G-to-A mutation at nucleotide pair 8363 in mitochondrial tRNA(Lys) in two families. Muscle Nerve 20 271–278. [DOI] [PubMed] [Google Scholar]
- Paramasivam A., Vijayashree Priyadharsini J. (2020). MitomiRs: new emerging microRNAs in mitochondrial dysfunction and cardiovascular disease. Hypertens Res. 43 851–853. 10.1038/s41440-020-0423-3 [DOI] [PubMed] [Google Scholar]
- Park D., Lee M. N., Jeong H., Koh A., Yang Y. R., Suh P. G., et al. (2014). Parkin ubiquitinates mTOR to regulate mTORC1 activity under mitochondrial stress. Cell Signal. 26 2122–2130. 10.1016/j.cellsig.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Perez-Martinez X., Funes S., Camacho-Villasana Y., Marjavaara S., Tavares-Carreon F., Shingu-Vazquez M. (2008). Protein synthesis and assembly in mitochondrial disorders. Curr. Top Med. Chem. 8 1335–1350. 10.2174/156802608786141124 [DOI] [PubMed] [Google Scholar]
- Perli E., Pisano A., Glasgow R. I. C., Carbo M., Hardy S. A., Falkous G., et al. (2019). Novel compound mutations in the mitochondrial translation elongation factor (TSFM) gene cause severe cardiomyopathy with myocardial fibro-adipose replacement. Sci. Rep. 9:5108. 10.1038/s41598-019-41483-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone E., Cavole T. R., Oliveira M. G., Virmond L. D. A., Silva M. F. B., Soares M. F. F., et al. (2020). Leigh syndrome in a patient with a novel C12orf65 pathogenic variant: case report and literature review. Genet. Mol. Biol. 43:e20180271. 10.1590/1678-4685-GMB-2018-0271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peske F., Rodnina M. V., Wintermeyer W. (2005). Sequence of steps in ribosome recycling as defined by kinetic analysis. Mol. Cell 18 403–412. 10.1016/j.molcel.2005.04.009 [DOI] [PubMed] [Google Scholar]
- Pettersson I., Kurland C. G. (1980). Ribosomal protein L7/L12 is required for optimal translation. Proc. Natl. Acad. Sci. U.S.A. 77 4007–4010. 10.1073/pnas.77.7.4007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinti M. V., Hathaway Q. A., Hollander J. M. (2017). Role of microRNA in metabolic shift during heart failure. Am. J. Physiol. Heart Circ. Physiol. 312 H33–H45. 10.1152/ajpheart.00341.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polekhina G., Thirup S., Kjeldgaard M., Nissen P., Lippmann C., Nyborg J. (1996). Helix unwinding in the effector region of elongation factor EF-Tu-GDP. Structure 4 1141–1151. 10.1016/s0969-2126(96)00122-0 [DOI] [PubMed] [Google Scholar]
- Pyle A., Ramesh V., Bartsakoulia M., Boczonadi V., Gomez-Duran A., Herczegfalvi A., et al. (2014). Behr’s syndrome is typically associated with disturbed mitochondrial translation and mutations in the C12orf65 gene. J. Neuromuscul. Dis. 1 55–63. 10.3233/JND-140003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Polacek N., Vesper O., Staub E., Einfeldt E., Wilson D. N., et al. (2006). The highly conserved LepA is a ribosomal elongation factor that back-translocates the ribosome. Cell 127 721–733. 10.1016/j.cell.2006.09.037 [DOI] [PubMed] [Google Scholar]
- Rao A. R., Varshney U. (2001). Specific interaction between the ribosome recycling factor and the elongation factor G from Mycobacterium tuberculosis mediates peptidyl-tRNA release and ribosome recycling in Escherichia coli. EMBO J. 20 2977–2986. 10.1093/emboj/20.11.2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravn K., Schonewolf-Greulich B., Hansen R. M., Bohr A. H., Duno M., Wibrand F., et al. (2015). Neonatal mitochondrial hepatoencephalopathy caused by novel GFM1 mutations. Mol. Genet. Metab. Rep. 3 5–10. 10.1016/j.ymgmr.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman T. R., Ermer J. A., Davies S. M., Perks K. L., Viola H. M., Shearwood A. M., et al. (2015). Mutation in MRPS34 compromises protein synthesis and causes mitochondrial dysfunction. PLoS Genet. 11:e1005089. 10.1371/journal.pgen.1005089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman T. R., Spahr H., Ermer J. A., Davies S. M., Viola H. M., Bates K. A., et al. (2016). Loss of the RNA-binding protein TACO1 causes late-onset mitochondrial dysfunction in mice. Nat. Commun. 7:11884. 10.1038/ncomms11884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter R., Pajak A., Dennerlein S., Rozanska A., Lightowlers R. N., Chrzanowska-Lightowlers Z. M. (2010a). Translation termination in human mitochondrial ribosomes. Biochem. Soc. Trans. 38 1523–1526. 10.1042/BST0381523 [DOI] [PubMed] [Google Scholar]
- Richter R., Rorbach J., Pajak A., Smith P. M., Wessels H. J., Huynen M. A., et al. (2010b). A functional peptidyl-tRNA hydrolase, ICT1, has been recruited into the human mitochondrial ribosome. EMBO J. 29 1116–1125. 10.1038/emboj.2010.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Dennerlein R., Oeljeklaus S., Lorenzi I., Ronsor C., Bareth B., Schendzielorz A. B., et al. (2016). Mitochondrial protein synthesis adapts to influx of nuclear-encoded protein. Cell 167 471–483.e10. 10.1016/j.cell.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera H., Martin-Hernandez E., Delmiro A., Garcia-Silva M. T., Quijada-Fraile P., Muley R., et al. (2013). A new mutation in the gene encoding mitochondrial seryl-tRNA synthetase as a cause of HUPRA syndrome. BMC Nephrol. 14:195. 10.1186/1471-2369-14-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodel G. (1986). Two yeast nuclear genes, CBS1 and CBS2, are required for translation of mitochondrial transcripts bearing the 5″-untranslated COB leader. Curr. Genet. 11 41–45. 10.1007/BF00389424 [DOI] [PubMed] [Google Scholar]
- Rorbach J., Richter R., Wessels H. J., Wydro M., Pekalski M., Farhoud M., et al. (2008). The human mitochondrial ribosome recycling factor is essential for cell viability. Nucleic Acids Res. 36 5787–5799. 10.1093/nar/gkn576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotig A. (2011). Human diseases with impaired mitochondrial protein synthesis. Biochim. Biophys. Acta 1807 1198–1205. 10.1016/j.bbabio.2011.06.010 [DOI] [PubMed] [Google Scholar]
- Rudler D. L., Hughes L. A., Perks K. L., Richman T. R., Kuznetsova I., Ermer J. A., et al. (2019). Fidelity of translation initiation is required for coordinated respiratory complex assembly. Sci. Adv. 5:eaay2118. 10.1126/sciadv.aay2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saada A., Shaag A., Arnon S., Dolfin T., Miller C., Fuchs-Telem D., et al. (2007). Antenatal mitochondrial disease caused by mitochondrial ribosomal protein (MRPS22) mutation. J. Med. Genet. 44 784–786. 10.1136/jmg.2007.053116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samec N., Jovcevska I., Stojan J., Zottel A., Liovic M., Myers M. P., et al. (2018). Glioblastoma-specific anti-TUFM nanobody for in-vitro immunoimaging and cancer stem cell targeting. Oncotarget 9 17282–17299. 10.18632/oncotarget.24629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchirico M. E., Fox T. D., Mason T. L. (1998). Accumulation of mitochondrially synthesized Saccharomyces cerevisiae Cox2p and Cox3p depends on targeting information in untranslated portions of their mRNAs. EMBO J. 17 5796–5804. 10.1093/emboj/17.19.5796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo-Domingo J., Chareyron I., Broenimann C., Lassueur S., Wiederkehr A. (2017). Antibiotics induce mitonuclear protein imbalance but fail to inhibit respiration and nutrient activation in pancreatic beta-cells. Exp. Cell Res. 357 170–180. 10.1016/j.yexcr.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Sasarman F., Antonicka H., Shoubridge E. A. (2008). The A3243G tRNALeu(UUR) MELAS mutation causes amino acid misincorporation and a combined respiratory chain assembly defect partially suppressed by overexpression of EFTu and EFG2. Hum. Mol. Genet. 17 3697–3707. 10.1093/hmg/ddn265 [DOI] [PubMed] [Google Scholar]
- Scala M., Brigati G., Fiorillo C., Nesti C., Rubegni A., Pedemonte M., et al. (2019). Novel homozygous TSFM pathogenic variant associated with encephalocardiomyopathy with sensorineural hearing loss and peculiar neuroradiologic findings. Neurogenetics 20 165–172. 10.1007/s10048-019-00582-5 [DOI] [PubMed] [Google Scholar]
- Scheper G. C., Van Der Klok T., Van Andel R. J., Van Berkel C. G., Sissler M., Smet J., et al. (2007). Mitochondrial aspartyl-tRNA synthetase deficiency causes leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. Nat. Genet. 39 534–539. 10.1038/ng2013 [DOI] [PubMed] [Google Scholar]
- Schulz C., Schendzielorz A., Rehling P. (2015). Unlocking the presequence import pathway. Trends Cell Biol. 25 265–275. 10.1016/j.tcb.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Seo G. H., Oh A., Kim E. N., Lee Y., Park J., Kim T., et al. (2019). Identification of extremely rare mitochondrial disorders by whole exome sequencing. J. Hum. Genet. 64 1117–1125. 10.1038/s10038-019-0660-y [DOI] [PubMed] [Google Scholar]
- Serre V., Rozanska A., Beinat M., Chretien D., Boddaert N., Munnich A., et al. (2013). Mutations in mitochondrial ribosomal protein MRPL12 leads to growth retardation, neurological deterioration and mitochondrial translation deficiency. Biochim. Biophys. Acta 1832 1304–1312. 10.1016/j.bbadis.2013.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahni R., Wedatilake Y., Cleary M. A., Lindley K. J., Sibson K. R., Rahman S. (2013). A distinct mitochondrial myopathy, lactic acidosis and sideroblastic anemia (MLASA) phenotype associates with YARS2 mutations. Am. J. Med. Genet. A 161A 2334–2338. 10.1002/ajmg.a.36065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M. R., Koc E. C., Datta P. P., Booth T. M., Spremulli L. L., Agrawal R. K. (2003). Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell 115 97–108. 10.1016/s0092-8674(03)00762-1 [DOI] [PubMed] [Google Scholar]
- Shi H., Hayes M., Kirana C., Miller R., Keating J., Macartney-Coxson D., et al. (2012). TUFM is a potential new prognostic indicator for colorectal carcinoma. Pathology 44 506–512. 10.1097/PAT.0b013e3283559cbe [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. (1990). Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell 61 931–937. 10.1016/0092-8674(90)90059-n [DOI] [PubMed] [Google Scholar]
- Shokolenko I. N., Alexeyev M. F. (2015). Mitochondrial DNA: a disposable genome? Biochim. Biophys. Acta 1852 1805–1809. 10.1016/j.bbadis.2015.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. T., Ng B. G., Friederich M. W., Wang R. Y., Boyer M., Kircher M., et al. (2017). Activation of a cryptic splice site in the mitochondrial elongation factor GFM1 causes combined OXPHOS deficiency. Mitochondrion 34 84–90. 10.1016/j.mito.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissler M., Gonzalez-Serrano L. E., Westhof E. (2017). Recent advances in mitochondrial aminoacyl-tRNA synthetases and disease. Trends Mol. Med. 23 693–708. 10.1016/j.molmed.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Smeitink J. A., Elpeleg O., Antonicka H., Diepstra H., Saada A., Smits P., et al. (2006). Distinct clinical phenotypes associated with a mutation in the mitochondrial translation elongation factor EFTs. Am. J. Hum. Genet. 79 869–877. 10.1086/508434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P., Antonicka H., Van Hasselt P. M., Weraarpachai W., Haller W., Schreurs M., et al. (2011a). Mutation in subdomain G’ of mitochondrial elongation factor G1 is associated with combined OXPHOS deficiency in fibroblasts but not in muscle. Eur. J. Hum. Genet. 19 275–279. 10.1038/ejhg.2010.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P., Saada A., Wortmann S. B., Heister A. J., Brink M., Pfundt R., et al. (2011b). Mutation in mitochondrial ribosomal protein MRPS22 leads to Cornelia de Lange-like phenotype, brain abnormalities and hypertrophic cardiomyopathy. Eur. J. Hum. Genet. 19 394–399. 10.1038/ejhg.2010.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimanpour-Lichaei H. R., Kuhl I., Gaisne M., Passos J. F., Wydro M., Rorbach J., et al. (2007). mtRF1a is a human mitochondrial translation release factor decoding the major termination codons UAA and UAG. Mol. Cell 27 745–757. 10.1016/j.molcel.2007.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville E. W., Zhou X. L., Olahova M., Jenkins J., Euro L., Konovalova S., et al. (2019). Instability of the mitochondrial alanyl-tRNA synthetase underlies fatal infantile-onset cardiomyopathy. Hum. Mol. Genet. 28 258–268. 10.1093/hmg/ddy294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer A. C., Spremulli L. L. (2004). Interaction of mitochondrial initiation factor 2 with mitochondrial fMet-tRNA. Nucleic Acids Res. 32 5464–5470. 10.1093/nar/gkh886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer A. C., Spremulli L. L. (2005). The interaction of mitochondrial translational initiation factor 2 with the small ribosomal subunit. Biochim. Biophys. Acta 1750 69–81. 10.1016/j.bbapap.2005.03.009 [DOI] [PubMed] [Google Scholar]
- Spiegel R., Mandel H., Saada A., Lerer I., Burger A., Shaag A., et al. (2014). Delineation of C12orf65-related phenotypes: a genotype-phenotype relationship. Eur. J. Hum. Genet. 22 1019–1025. 10.1038/ejhg.2013.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan H., Das S. (2015). Mitochondrial miRNA (MitomiR): a new player in cardiovascular health. Can. J. Physiol. Pharmacol. 93 855–861. 10.1139/cjpp-2014-0500 [DOI] [PubMed] [Google Scholar]
- Su C., Wang F. (2020). Clinical and molecular findings in a family expressing a novel heterozygous variant of the G elongation factor mitochondrial 1 gene. Exp. Ther. Med. 20:173. 10.3892/etm.2020.9303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surovtseva Y. V., Shutt T. E., Cotney J., Cimen H., Chen S. Y., Koc E. C., et al. (2011). Mitochondrial ribosomal protein L12 selectively associates with human mitochondrial RNA polymerase to activate transcription. Proc Natl Acad Sci U S A 108 17921–17926. 10.1073/pnas.1108852108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Terasaki M., Takemoto-Hori C., Hanada T., Ueda T., Wada A., et al. (2001). Proteomic analysis of the mammalian mitochondrial ribosome. Identification of protein components in the 28 S small subunit. J. Biol. Chem. 276 33181–33195. 10.1074/jbc.M103236200 [DOI] [PubMed] [Google Scholar]
- Synofzik M., Schicks J., Lindig T., Biskup S., Schmidt T., Hansel J., et al. (2011). Acetazolamide-responsive exercise-induced episodic ataxia associated with a novel homozygous DARS2 mutation. J. Med. Genet. 48 713–715. 10.1136/jmg.2011.090282 [DOI] [PubMed] [Google Scholar]
- Szklarczyk R., Wanschers B. F., Cuypers T. D., Esseling J. J., Riemersma M., Van Den Brand M. A., et al. (2012). Iterative orthology prediction uncovers new mitochondrial proteins and identifies C12orf62 as the human ortholog of COX14, a protein involved in the assembly of cytochrome c oxidase. Genome Biol. 13:R12. 10.1186/gb-2012-13-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taanman J. W. (1999). The mitochondrial genome: structure, transcription, translation and replication. Biochim. Biophys. Acta 1410 103–123. 10.1016/s0005-2728(98)00161-3 [DOI] [PubMed] [Google Scholar]
- Takeuchi N., Tomita N., Ueda T. (2010). EF-G2mt is an exclusive recycling factor in mammalian mitochondrial protein synthesis. Seikagaku 82 825–831. [PubMed] [Google Scholar]
- Tang J. X., Thompson K., Taylor R. W., Olahova M. (2020). Mitochondrial OXPHOS biogenesis: Co-regulation of protein synthesis, import, and assembly pathways. Int. J. Mol. Sci. 21:3820. 10.3390/ijms21113820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskin B. D., Karalok Z. S., Gurkas E., Aydin K., Aydogmus U., Ceylaner S., et al. (2016). Early-onset mild type leukoencephalopathy caused by a homozygous EARS2 mutation. J. Child Neurol. 31 938–941. 10.1177/0883073816630087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. W., Turnbull D. M. (2005). Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 6 389–402. 10.1038/nrg1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temperley R. J., Seneca S. H., Tonska K., Bartnik E., Bindoff L. A., Lightowlers R. N., et al. (2003). Investigation of a pathogenic mtDNA microdeletion reveals a translation-dependent deadenylation decay pathway in human mitochondria. Hum. Mol. Genet. 12 2341–2348. 10.1093/hmg/ddg238 [DOI] [PubMed] [Google Scholar]
- Tibbetts A. S., Oesterlin L., Chan S. Y., Kramer G., Hardesty B., Appling D. R. (2003). Mammalian mitochondrial initiation factor 2 supports yeast mitochondrial translation without formylated initiator tRNA. J. Biol. Chem. 278 31774–31780. 10.1074/jbc.M304962200 [DOI] [PubMed] [Google Scholar]
- Toivonen J. M., O’dell K. M., Petit N., Irvine S. C., Knight G. K., Lehtonen M., et al. (2001). Technical knockout, a Drosophila model of mitochondrial deafness. Genetics 159 241–254. 10.1093/genetics/159.1.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torraco A., Peralta S., Iommarini L., Diaz F. (2015). Mitochondrial diseases Part I: mouse models of OXPHOS deficiencies caused by defects in respiratory complex subunits or assembly factors. Mitochondrion 21 76–91. 10.1016/j.mito.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traschutz A., Hayer S. N., Bender B., Schols L., Biskup S., Synofzik M. (2019). TSFM mutations cause a complex hyperkinetic movement disorder with strong relief by cannabinoids. Parkinsonism Relat. Disord. 60 176–178. 10.1016/j.parkreldis.2018.09.031 [DOI] [PubMed] [Google Scholar]
- Tucci A., Liu Y. T., Preza E., Pitceathly R. D., Chalasani A., Plagnol V., et al. (2014). Novel C12orf65 mutations in patients with axonal neuropathy and optic atrophy. J. Neurol. Neurosurg. Psychiatry 85 486–492. 10.1136/jnnp-2013-306387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente L., Shigi N., Suzuki T., Zeviani M. (2009). The R336Q mutation in human mitochondrial EFTu prevents the formation of an active mt-EFTu.GTP.aa-tRNA ternary complex. Biochim. Biophys. Acta 1792 791–795. 10.1016/j.bbadis.2009.06.002 [DOI] [PubMed] [Google Scholar]
- Valente L., Tiranti V., Marsano R. M., Malfatti E., Fernandez-Vizarra E., Donnini C., et al. (2007). Infantile encephalopathy and defective mitochondrial DNA translation in patients with mutations of mitochondrial elongation factors EFG1 and EFTu. Am. J. Hum. Genet. 80 44–58. 10.1086/510559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riesen A. K., Biskup S., Kuhn A. A., Kaindl A. M., van Riesen C. (2021). Novel mutation in the TSFM gene causes an early-onset complex chorea without basal ganglia lesions. Mov. Disord. Clin. Pract. 8 453–455. 10.1002/mdc3.13144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedrenne V., Galmiche L., Chretien D., De Lonlay P., Munnich A., Rotig A. (2012). Mutation in the mitochondrial translation elongation factor EFTs results in severe infantile liver failure. J. Hepatol. 56 294–297. 10.1016/j.jhep.2011.06.014 [DOI] [PubMed] [Google Scholar]
- Wang W. X., Visavadiya N. P., Pandya J. D., Nelson P. T., Sullivan P. G., Springer J. E. (2015). Mitochondria-associated microRNAs in rat hippocampus following traumatic brain injury. Exp. Neurol. 265 84–93. 10.1016/j.expneurol.2014.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Song C., Zhou X., Han X., Li J., Wang Z., et al. (2017). Mitochondria associated microRNA expression profiling of heart failure. Biomed. Res. Int. 2017:4042509. 10.1155/2017/4042509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Q., Zhang X. D., Han Y. M., Shi X. F., Lan Z. B., Men X. X., et al. (2017). Clinical efficacy of gamma knife and surgery treatment of mesial temporal lobe epilepsy and their effects on EF-Tumt and EF-Tsmt expression. Eur. Rev. Med. Pharmacol. Sci. 21 1774–1779. [PubMed] [Google Scholar]
- Weng X., Zheng S., Shui H., Lin G., Zhou Y. (2020). TUFM-knockdown inhibits the migration and proliferation of gastrointestinal stromal tumor cells. Oncol. Lett. 20:250. 10.3892/ol.2020.12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weraarpachai W., Antonicka H., Sasarman F., Seeger J., Schrank B., Kolesar J. E., et al. (2009). Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset Leigh syndrome. Nat. Genet. 41 833–837. 10.1038/ng.390 [DOI] [PubMed] [Google Scholar]
- Weraarpachai W., Sasarman F., Nishimura T., Antonicka H., Aure K., Rotig A., et al. (2012). Mutations in C12orf62, a factor that couples COX I synthesis with cytochrome c oxidase assembly, cause fatal neonatal lactic acidosis. Am. J. Hum. Genet. 90 142–151. 10.1016/j.ajhg.2011.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenhagen L. M., Kelley S. O. (2002). Dimerization of a pathogenic human mitochondrial tRNA. Nat. Struct. Biol. 9 586–590. 10.1038/nsb820 [DOI] [PubMed] [Google Scholar]
- Wittenhagen L. M., Kelley S. O. (2003). Impact of disease-related mitochondrial mutations on tRNA structure and function. Trends Biochem. Sci. 28 605–611. 10.1016/j.tibs.2003.09.006 [DOI] [PubMed] [Google Scholar]
- Woriax V. L., Burkhart W., Spremulli L. L. (1995). Cloning, sequence analysis and expression of mammalian mitochondrial protein synthesis elongation factor Tu. Biochim. Biophys. Acta 1264 347–356. 10.1016/0167-4781(95)00176-x [DOI] [PubMed] [Google Scholar]
- Wu Y., Yao Q., Jiang G. X., Wang G., Cheng Q. (2020). Identification of distinct blood-based biomarkers in early stage of Parkinson’s disease. Neurol. Sci. 41 893–901. 10.1007/s10072-019-04165-y [DOI] [PubMed] [Google Scholar]
- Xu X., Huang A., Cui X., Han K., Hou X., Wang Q., et al. (2019). Ubiquitin specific peptidase 5 regulates colorectal cancer cell growth by stabilizing Tu translation elongation factor. Theranostics 9 4208–4220. 10.7150/thno.33803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K., An T., Zhai M., Huang Y., Wang Q., Wang Y., et al. (2019). Mitochondrial miR-762 regulates apoptosis and myocardial infarction by impairing ND2. Cell Death Dis. 10:500. 10.1038/s41419-019-1734-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Gao Y., Li Z., Chen L., Xia Z., Xu T., et al. (2014). Mitochondrial EF4 links respiratory dysfunction and cytoplasmic translation in Caenorhabditis elegans. Biochim. Biophys. Acta 1837 1674–1683. 10.1016/j.bbabio.2014.05.353 [DOI] [PubMed] [Google Scholar]
- Yarham J. W., Elson J. L., Blakely E. L., Mcfarland R., Taylor R. W. (2010). Mitochondrial tRNA mutations and disease. Wiley Interdiscip. Rev. RNA 1 304–324. 10.1002/wrna.27 [DOI] [PubMed] [Google Scholar]
- Yassin A. S., Haque M. E., Datta P. P., Elmore K., Banavali N. K., Spremulli L. L., et al. (2011). Insertion domain within mammalian mitochondrial translation initiation factor 2 serves the role of eubacterial initiation factor 1. Proc. Natl. Acad. Sci. U.S.A. 108 3918–3923. 10.1073/pnas.1017425108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa T., Suzuki T., Ishii N., Ueda T., Ohta S., Watanabe K. (2000a). Defect in modification at the anticodon wobble nucleotide of mitochondrial tRNA(Lys) with the MERRF encephalomyopathy pathogenic mutation. FEBS Lett. 467 175–178. 10.1016/s0014-5793(00)01145-5 [DOI] [PubMed] [Google Scholar]
- Yasukawa T., Suzuki T., Ueda T., Ohta S., Watanabe K. (2000b). Modification defect at anticodon wobble nucleotide of mitochondrial tRNAs(Leu)(UUR) with pathogenic mutations of mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes. J. Biol. Chem. 275 4251–4257. 10.1074/jbc.275.6.4251 [DOI] [PubMed] [Google Scholar]
- Yasukawa T., Suzuki T., Watanabe K., Yasukawa T., Ohta S. (2002). Wobble modification defect suppresses translational activity of tRNAs with MERRF and MELAS mutations. Nihon. Rinsho. 60 Suppl 4 197–201. [PubMed] [Google Scholar]
- Yokokawa T., Kido K., Suga T., Isaka T., Hayashi T., Fujita S. (2018). Exercise-induced mitochondrial biogenesis coincides with the expression of mitochondrial translation factors in murine skeletal muscle. Physiol. Rep. 6:e13893. 10.14814/phy2.13893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C., Xu N., Qiu S., Li Y., Xu L., Li X., et al. (2020). A novel composition of two heterozygous GFM1 mutations in a Chinese child with epilepsy and mental retardation. Brain Behav. 10:e01791. 10.1002/brb3.1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Yan K., Liu G., Song G., Luo J., Shi Y., et al. (2016). EF4 disengages the peptidyl-tRNA CCA end and facilitates back-translocation on the 70S ribosome. Nat. Struct. Mol. Biol. 23 125–131. 10.1038/nsmb.3160 [DOI] [PubMed] [Google Scholar]
- Zhang L., Huang Y., Ling J., Xiang Y., Zhuo W. (2019). Screening of key genes and prediction of therapeutic agents in Arsenic-induced lung carcinoma. Cancer Biomark 25 351–360. 10.3233/CBM-182333 [DOI] [PubMed] [Google Scholar]
- Zhang X., Zuo X., Yang B., Li Z., Xue Y., Zhou Y., et al. (2014). MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell 158 607–619. 10.1016/j.cell.2014.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Spremulli L. L. (1998). Identification and cloning of human mitochondrial translational release factor 1 and the ribosome recycling factor. Biochim. Biophys. Acta 1443 245–250. 10.1016/s0167-4781(98)00223-1 [DOI] [PubMed] [Google Scholar]
- Zhong B. R., Zhou G. F., Song L., Wen Q. X., Deng X. J., Ma Y. L., et al. (2021). TUFM is involved in Alzheimer’s disease-like pathologies that are associated with ROS. FASEB J. 35:e21445. 10.1096/fj.202002461R [DOI] [PubMed] [Google Scholar]
- Zhu P., Liu Y., Zhang F., Bai X., Chen Z., Shangguan F., et al. (2018). Human elongation factor 4 regulates cancer bioenergetics by acting as a mitochondrial translation switch. Cancer Res. 78 2813–2824. 10.1158/0008-5472.CAN-17-2059 [DOI] [PubMed] [Google Scholar]
- Zifa E., Giannouli S., Theotokis P., Stamatis C., Mamuris Z., Stathopoulos C. (2007). Mitochondrial tRNA mutations: clinical and functional perturbations. RNA Biol. 4 38–66. [DOI] [PubMed] [Google Scholar]