Abstract
Background
Recently, three updated guidelines for post‐polypectomy colonoscopy surveillance (PPCS) have been published. These guidelines are based on a comprehensive summary of the literature, while some recommendations are similar, different surveillance intervals are recommended after detection of specific types of polyps.
Aim
In this review, we aimed to compare and contrast these recommendations.
Methods
The updated guidelines for PPCS were reviewed and the recommendations were compared.
Results
For patients with 1–4 adenomas <10 mm with low‐grade dysplasia, irrespective of villous components, or 1–4 serrated polyps <10 mm without dysplasia, the European Society of Gastrointestinal Endoscopy (ESGE) and British Society of Gastroenterology (BSG), the Association of Coloproctology of Great Britain and Ireland (ACPGBI) and Public Health England (PHE) (BSG/ACPGBI/PHE) guidelines do not recommend colonoscopic surveillance and instead recommend that the participate in routine CRC screening program (typically based on the fecal immunochemical test), while the USMSTF recommends surveillance colonoscopies 7–10 years after diagnosis of 1–2 tubular adenomas <10 mm and 3–5 years for 3–4 tubular adenomas of the same size. The USMSTF define adenomas with tubulovillous or villous histology as high‐risk adenomas; thus, surveillance colonoscopy is recommended after 3 years. However, the ESGE and BSG do not consider such histology as a criterion for repeating colonoscopy at this short interval. For patients with 1–2 sessile serrated polyps (SSPs) <10 mm and those with 3–4 SSPs <10 mm, the USMSTF recommends surveillance colonosocopy after 5–10 and 3–5 years, respectively.
Keywords: colonoscopy, guidelines, polypectomy, surveillance
INTRODUCTION
Adenomas are neoplastic lesions of the colonoic epithelia tissue considered to be a precursor for colorectal cancer (CRC). Risk for metachronous adenomas and neoplasia after removal of colonic polyps is stratified based on the size, histology, and number of polyps detected and removed. The aim of post‐polypectomy colonoscopy surveillance (PPCS) is to reduce CRC incidence and mortality. 1 Removal of missed or new polyps prevents progression of these lesions to advanced adenomas or CRC and decreases CRC incidence and mortality. 1 Both removal of precancerous adenomatous polyps at the time of index colonoscopy and early detection of curable CRC have been shown to reduce CRC mortality. 2
Guidelines for PPCS were recently updated by the US Multi‐Society Task Force (USMSTF) on CRC, 3 the European Society of Gastrointestinal Endoscopy (ESGE), 4 and the British Society of Gastroenterology (BSG), the Association of Coloproctology of Great Britain and Ireland (ACPGBI), and Public Health England (PHE) (BSG/ACPGBI/PHE). 5
The aim of surveillance guidelines is to determine the appropriate course of follow‐up for patients after index colonoscopy, that strikes a balance between the risk of further development of neoplasia and the burden of colonoscopy, directing surveillance resources to patients at increased risk of developing advanced neoplasia post‐polypectomy while minimizing the burden of surveillance colonoscopy on low‐risk patients. As new data on long‐term CRC incidence and mortality after polypectomy was published, an update of the guidelines was necessary.
This review serves to compare and contrast the recommendations of these recently published guidelines.
DEFINITIONS
Table 1 summarizes the important definitions as published by the three guidelines. While the USMSTF defines in detail the different polyp types as low‐risk adenoma, high‐risk adenoma, advanced adenoma, and advanced neoplasia, the ESGE guidelines divide polyps into those requiring or not requiring surveillance. With respect to terminology, the ESGE guidelines use the term “polyp” rather than “lesion” or “neoplasia”. The BSG/ACPGBI/PHE guidelines use the term “premalignant polyp”, to include serrated and adenomatous polyps and defines high risk findings as one of the following:
≥2 premalignant polyps, of which at least one is a serrated polyp ≥10 mm or with dysplasia, or an adenoma ≥10 mm or with high‐grade dysplasia (HGD).
≥5 premalignant polyps.
TABLE 1.
Definitions used in the guidelines
| Description/Term | Definition | ||
|---|---|---|---|
| USMSTF 3 | ESGE 4 | BSG/ACPGBI/PHE 5 | |
| 1–2 non‐advanced adenomas <10 mm in size | Low‐risk adenoma | Polyp not requiring surveillance | Premalignant polyp (not requiring surveillance) |
| Advanced adenoma/advanced adenomatous polyp |
|
|
|
|
|
|
|
|
|
||
| Advanced neoplasia | Advanced adenoma CRC | This term has been used historically to describe the combination of advanced adenomas and colorectal cancers. It is considered outmoded because the serrated pathway is not included. | |
| High‐risk adenoma | Advanced neoplasia ≥3 adenomas | ||
| Serrated polyp | Hyperplastic polyps (HPs), sessile serrated lesions (SSLs), SSLs with dysplasia (SSLd), traditional serrated adenomas (TSA) and mixed polyps. | ||
| Premalignant polyp | Serrated polyps and adenomatous polyps (excluding diminutive [1–5 mm] and rectal HPs) | ||
| Advanced serrated polyp | A serrated polyp ≥10 mm or with any grade of dysplasia. | ||
| Advanced colorectal polyp | The term includes both advanced serrated and advanced adenomatous polyps. | ||
Abbreviations: ACPGBI, Association of Coloproctology of Great Britain and Ireland; BSG, British Society of Gastroenterology; CRC, colorectal cancer; ESGE, European Society of Gastrointestinal Endoscopy; PHE, Public Health England; USMSTF, US Multi‐Society Task Force.
Low‐risk adenoma
The low‐risk adenoma term was used by the USMSTF guideline and refers to having 1–2 tubular adenomas with low‐grade dysplasia, each <10 mm in size.
High‐risk adenoma
The USMSTF considers high‐risk adenoma to be adenoma ≥10 mm or with tubulovillous/villous histology or HGD.
Polyps not requiring surveillance
According to the ESGE, polyps not requiring surveillance include 1‐4 <10 mm adenomas with low‐grade dysplasia, irrespective of villous components or any serrated polyp <10 mm without dysplasia.
Polyps requiring surveillance
According to the ESGE, polyps requiring surveillance include adenoma ≥10 mm or with HGD or ≥5 adenomas, or any serrated polyp that is either ≥10 mm or with dysplasia.
METHODS FOR GUIDELINE UPDATES
The key concepts in all guidelines were designed to answer questions relevant to clinicians within the framework of the patient, intervention, comparison and outcomes model. The different guidelines were updated after reviewing of the newly published literature in the last years and since the previous update.
MAIN RECOMMENDATIONS IN THE POST‐POLYPECTOMY COLONOSCOPY SURVEILLANCE GUIDELINES
The updated recommendations published in 2020 represent a significant departure from previous guidelines with regards to specific polyp subgroups (such as low‐risk adenomas). As a number of cohort studies have shown, the risk of long‐term CRC following polypectomy of low‐risk adenoma is low; therefore, the surveillance interval has been extended in all guidelines, and villous histology is no longer a criterion for high‐risk findings in the ESGE guidelines. Since additional evidence‐based data regarding serrated polyps are available, updated recommendations were included in the present guidelines. These updated recommendations for surveillance intervals also differ between the different societies as described below, causing inconsistencies and a potential source of confusion for clinicians/endoscopists.
However, all guidelines consider size, histology, and number of polyps to be the determining factors for surveillance intervals. USMSTF and ESGE recommend a surveillance interval of 3 years in cases of adenoma/serrated polyp ≥10 mm, adenoma <10 mm with HGD, serrated polyp <10 mm with dysplasia or 5–10 adenoma/serrated polyps, while the BSG/ACPGVI/PHE recommend the 3 years interval only in cases of two or more premalignant polyps with one of the above‐mentioned criteria but not in cases of a single premalignant polyp. In the following sections, we discuss the main differences between the guidelines regarding the subtypes of polyps. A summary of the updated recommendations is presented in Table 2 and Figure 1.
TABLE 2.
Comparison of the main recommendations of the three guidelines
| USMSTF 3 | ESGE 4 | BSG/ACPGBI/PHE 5 | |
|---|---|---|---|
| 1–2 tubular adenomas <10 mm | 7–10 years | No surveillance/return to screening | No surveillance/return to screening when invited |
| 3–4 tubular adenomas <10 mm | 3–5 years | No surveillance/return to screening | No surveillance/return to screening when invited |
| 5–10 tubular adenomas <10 mm | 3 years | 3 years | 3 years |
| Adenoma ≥10 mm | 3 years | 3 years | 3 years b |
| Adenoma with tubulovillous or villous histology, <10 mm, low‐grade dysplasia | 3 years | No surveillance/return to screening | No surveillance/return to screening when invited |
| Adenoma with high‐grade dysplasia | 3 years | 3 years | 3 years b |
| >10 adenomas on single examination | 1 year and genetic counseling | Genetic counseling | Referred to BSG hereditary CRC guidelines |
| Piecemeal resection of adenoma/SSP >20 mm | 6 m | 3–6 m | 2–6 m a |
| ≤20 HPs in rectum or sigmoid colon or proximal to sigmoid colon and <10 mm | 10 years | No specific recommendation | No specific recommendation |
| HP > 10 mm | 3–5 years | No specific recommendation | No specific recommendation |
| 1–2 SSPs <10 mm | 5–10 years | No surveillance/return to screening | No surveillance/return to screening |
| 3–4 SSPs <10 mm | 3–5 years | No surveillance/return to screening | No surveillance/return to screening |
| 5–10 SSPs <10 mm | 3 years | No specific recommendation | 3 years |
| SSP with dysplasia | 3 years | 3 years | 3 years b |
| SSP ≥ 10 mm | 3 years | 3 years | 3 years b |
| Traditional serrated adenoma (TSA) | 3 years | 3 years | 3 years b |
Abbreviations: ACPGBI, Association of Coloproctology of Great Britain and Ireland; BSG, British Society of Gastroenterology; CRC, colorectal cancer; ESGE, European Society of Gastrointestinal Endoscopy; PHE, Public Health England; SSP, sessile serrated polyp; USMSTF, US Multi‐Society Task Force.
The BSG/ACPGBI/PHE recommend a second site check 18 months after the original resection.
Surveillance at 3 years is recommended if there are two or more premalignant polyps, of which at least one is advanced (surveillance at 3 years would not be recommended if the patient has only one of these adenomas/SSPs).
FIGURE 1.
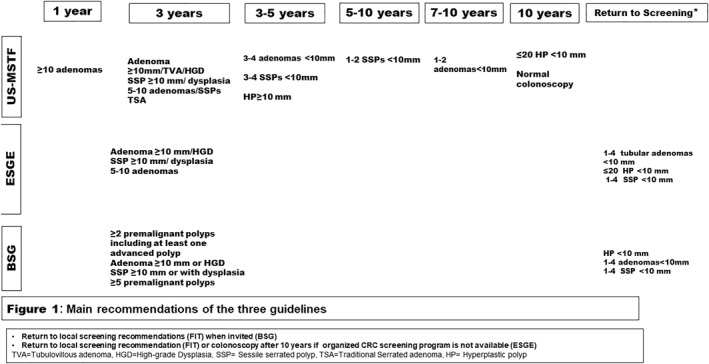
Main recommendations of the three guidelines
Tubular adenomas <10 mm
In all three guidelines, surveillance intervals for tubular adenomas <10 mm were extended from that of previous guidelines. However, the exact timing of these intervals is not unanimous. The USMSTF recommends an interval of 7–10 years for patients with 1–2 tubular adenomas <10 mm and 3–5 years for patients with 3–4 tubular adenomas <10 mm, compared with 5–10 and 3 years in the 2012 guidelines, respectively. On the other hand, the ESGE concludes that individuals with 1–4 adenomas <10 mm have the same risk of CRC incidence and mortality as the general population 6 ; as such, the ESGE recommends treating patients with these adenomas as an average‐risk population and returning them to the local screening programs (most commonly fecal immunochemical test [FIT]). In countries without an organized screening program, colonoscopy at 10‐year intervals is a secondary option. This is an update on their 2013 guidelines, which recommend 3‐year surveillance intervals for patients with ≥3 adenomas.
The BSG/ACPGVI/PHE do not recommend colonic surveillance, rather participation in a national bowel screening program (FIT) when invited. This is in contrast to the previous 2010 guidelines, which recommended surveillance intervals of 3 years in cases of 3–4 small adenomas.
Adenomas ≥10 mm or with high‐grade dysplasia
The USMSTF and ESGE guidelines are in agreement that adenomas ≥10 mm or HGD warrant a repeat colonoscopy after 3 years, but the BSG/ACPGVI/PHE guidelines recommend surveillance intervals of 3 years for patients with adenoma ≥10 mm or with HGD in cases of two or more premalignant polyps, though not in cases of a single premalignant polyp.
Tubulovillous/villous histology
There is substantial disagreement between guidelines regarding adenomas <10 mm with a villous component (tubulovillous/villous histology). Only the USMSTF recommends repeating colonoscopy after 3 years, while ESGE and BSG/ACPGBI/PHE do not recommend surveillance colonoscopy and refer patients to average‐risk CRC screening when invited.
Number of adenomas
The number of adenomas is a major point of contention among the guidelines.
As mentioned above, different intervals are recommended in cases of 1–4 adenomas <10 mm. However, according to the USMSTF, patients with more than 10 adenomas detected during a single colonoscopy should undergo colonoscopy surveillance after 1 year as well as genetic counseling. The ESGE guidelines make a recommendation of genetic counseling for patients with 10 or more adenomas, while the BSG/ACPGBI/PHE guidelines refers to the BSG hereditary CRC guidelines for the management of these patients.
HYPERPLASTIC AND SESSILE SERRATED POLYPS
All three guidelines divided hyperplastic polyps (HPs) and sessile serrated polyps (SSPs) based on number and histology. The ESGE recommend surveillance at 3 years for high‐risk SSP (SSP with dysplasia, SSPs ≥10 mm, or traditional serrated adenomas), while the USMSTF recommends a distinction between the number of lesions and provides detailed recommendations for cases of 1–2, 3–4 and 5–10 SSPs. The BSG/ACPGBI/PHE guidelines recommend 3‐year intervals only for high‐risk SSPs in case of two premalignant polyps and no in case of single polyp. Recommendation of returning to general national screening program when invited was made by the ESGE and BSG/ACPGBI/PHE for patients with serrated polyps <10 mm without dysplasia. The USMSTF provides a low‐quality evidence recommendation for repeat colonoscopy after 5–10 years for 1–2 SSPs <10 mm, and 3–5 years for 3–4 SSPs <10 mm. Notably, the USMSTF recommends repeating colonoscopy 3–5 years after removal of HPs ≥10 mm, while there is no mention of HPs in the ESGE guidelines.
AGE TO STOP SURVEILLANCE
For surveillance, the USMSTF guidelines also discuss age as a contributing factor to their recommendations. They highlight the need for more research to determine whether the benefits of potential cancer prevention and early detection of CRC by way of surveillance outweigh the short‐term procedure‐related risks for individuals older than age 75 years.
The ESGE recommends discontinuing post‐polypectomy surveillance at the age of 80 or earlier if life expectancy is significantly limited by comorbidities. In a similar vein, the BSG/ACPGBI/PHE advise against routine post‐polypectomy surveillance on patients older than age 75 years or patients with comorbidities that limit their life expectancy to less than 10 years.
DISCUSSION
In the current review, we have elucidated the main discrepancies between the three most recently updated guidelines published by the leading gastroenterological societies.
In all three updated guidelines, surveillance intervals for patients with 1–4 adenomas <10 mm were extended. The USMSTF is more conservative, recommending colonoscopy surveillance after 7–10 years in cases of 1–2 tubular adenomas <10 mm, and after 3–5 years for 3–4 tubular adenomas <10 mm, compared with 5–10 years and 3‐year that were recommended previously, respectively. In contrast, the ESGE and BSG/ACPGBI/PHE recommend returning patients to routine national CRC screening when invited, and not colonoscopic surveillance. This is updated from previous guidelines that recommended 3‐year intervals for 3–4 adenomas <10 mm.
The ESGE and BSG/ACPGBI/PHE recommendations for no colonoscopic surveillance for non‐advanced adenomas are based on evidence that the long‐term risk for CRC incidence and mortality are lower than or similar to that of people without adenomas at baseline or the risk of the general population. 6 , 7 , 8 , 9 The USMSTF guidelines cite literature showing a lower‐than‐average risk for incident and fatal CRC among patients with low‐risk adenomas. 9 , 10 , 11 However, the USMSTF ultimately advises 7–10‐year intervals because of the uncertainty and the possibility that the lower risk profile of this group may be related to exposure to surveillance. 9 , 10 , 12 Similarly, the USMSTF recommends 10‐years intervals for normal colonoscopy (where no adenoma, SSP, TSA, HP > 10 mm, or CRC was found). There is no mention of a recommendation for “normal colonoscopy” in the ESGE and BSG/ACPGBI/PHE guidelines because the guidelines are concerned with the management of patients who have polyps detected and removed at baseline colonoscopy.
USMSTF and ESGE agree that adenomas ≥10 mm or HGD should be criteria for repeating colonoscopy after 3 years, but only the USMSTF considers tubulovillous/villous adenoma as a criterion for colonoscopic surveillance after 3 years. This recommendation is based on studies showing an increased risk for advanced adenomas and CRC after the removal of tubulovillous/villous adenoma. 13 , 14 The ESGE considers tubulovillous/villous adenoma as a “no surveillance” group based on the assumption that tubulovillous/villous histology alone without HGD is rare in polyps <10 mm and does not independently increase the long‐term risk of CRC incidence and mortality. 6 , 8 , 15 , 16 The BSG/ACPGBI/PHE guidelines did consider tubulovillous/villous histology but, after reviewing the evidence, did not include adenomas with tubulovillous/villous histology in the classification of high‐risk patients requiring surveillance. The reason for this exclusion is the disagreement among histopathologists in the assessment of villous architecture. 17 , 18
Regarding HPs and SSPs, the USMSTF and ESGE agree that SSP with dysplasia or ≥10 mm warrant surveillance colonoscopy 3 years after index colonoscopy. BSG/ACPGBI/PHE recommend 3 years interval in cases of two premalignant polyps, one of them SSP with dysplasia or ≥10 mm but not in a single SSP. However, regarding 1–4 small (<10 mm) SSPs, the USMSTF guidelines recommend a repeat colonoscopy, while the ESGE and BSG ACPGBI/PHE recommend no surveillance.
Taken together, there is remarkable variation among the updated guidelines regarding time intervals for surveillance in specific conditions. While on one hand, these differences give gastroenterologists more flexibility in decision‐making; on the other hand, this may be a source of miscommunication among colleagues in the international arena. In addition, several other factors may affect the variability in intervals for colonoscopic surveillance recommended by the endoscopist. In all guidelines, quality of colonoscopy depends on thoroughness of preparation, cecal intubation rates, and adenoma detection rates, which can vary from one clinical setting to another. Another factor is polyp size, as there may be significant measurement bias. 19 Differences in quality of the colonoscopic procedure itself, adenoma detection rate, availability of screening programs, and patient reimbursement for colonoscopy may also affect intervals for PPCS.
Moreover, adherence to the guidelines is problematic, low adherence rate was found with a tendency to recommend PPCS more frequent than recommended in the previous guidelines in Korea, Japan and the USA. 20 , 21 Our previous report showed that 57.4% of clinician recommendations for coloonoscopy interval was in accordance with guidelines and 37.2% of interval recommendations was shorter. 22 A recently published systematic review and meta‐analysis analyzing 16 studies from different countries and investigated adherence for different guidelines found an adherence rate of 48.4%. 23 For cases of low‐risk and high‐risk lesions, guideline adherence to the recommended surveillance intervals was 24.4% and 73.6%, respectively. 23 Factors that may contribute to low adherence for guidelines include disagreement with the recommendations or clinical studies, concerns of liability reimbursement issues, patients’ race, the quality of bowel preparation and unavailability of resources. 22
Guidelines for PPCS published by national and international gastroenterological societies have a substantial impact on the decisions of gastroenterologists to perform surveillance future colonoscopies, which in turn, influence CRC prevention and the burden on health care systems. However, inconsistencies can cause suboptimal compliance with these guidelines, be it under‐ or over‐utilization of resources. The consequences of under‐utilization could include an increase of CRC incidence and mortality as CRC can be missed among high‐risk people, who should be more rigorously surveilled. On the other hand, over‐utilization in low‐risk cases significantly increases the burden on the surveillance services and exposes patients unnecessarily to invasive surveillance procedures that carry a small but real risk of serious complications.
While it is understandable that surveillance recommendations differ between the guidelines, it has been suggested that inconsistencies among the guidelines may be a result of a lack of data, varying interpretations of published studies, differences in clinical experience, and availability of resources. 24 Additionally, different health care system structures, availability of local CRC screening programs and colonoscopy capacity are others health system related causes for inconsistencies between the guidelines. The consequences could involve suboptimal clinical practices/quality of care, uncertainty for the clinician as to which guidelines to adopt, increased utilization of limited resources and inconsistency of definitions and terms causing ambiguity.
We believe that coordination between associations and societies when formulating guidelines is vital. To help the gastroenterology community establish standards of care, guidelines should use universal definitions and terms. In addition, consistency in the recommendations can be achieved in the future by further data on long‐term post‐polypectomy CRC incidence and mortality, in accordance to the baseline polyp characteristics.
In summary, there is a prominent divergence between guidelines regarding recommendations for PPCS after detection of 1–4 adenomas <10 mm, adenomas with villous components, and SSPs. The lengthening of surveillance intervals after polyp removal can have a notable effect on the health system, such as a decline in the number of colonoscopies performed for these specific cases but increased availability of colonoscopies for other indications (patients with positive FIT, investigation of abdominal symptoms or inflammatory bowel diseases). In light of the fundamental differences in recommendations discussed in this review, we suggest that local health care systems adhere to a single set of guidelines (local or one in this review), based on local data regarding quality of colonoscopy, availability of colonoscopy, and clinician adherence to guidelines.
CONFLICT OF INTERESTS
There are no conflicts of interest for any of the authors, and no financial disclosures. We transfer the copyright to the publisher where the article be published.
AUTHOR CONTRIBUTION
All authors have contributed significantly to the work and have approved the final version of the manuscript.
Abu‐Freha N, Katz LH, Kariv R , Vainer E, Laish I, Gluck N, et al. Post‐polypectomy surveillance colonoscopy: comparison of the updated guidelines. United European Gastroenterol J. 2021;9(6):681–87. 10.1002/ueg2.12106
Elizabeth E. Half and Zohar Levi have contributed equally to this study.
The manuscript has not been published and is not been considered for publication elsewhere.
DATA AVAILABILITY STATEMENT
The guidelines were published by the organizations and there are no additional data for sharing.
REFERENCES
- 1. Zauber AG, Winawer SJ, O'Brien MJ Lansdrop‐Vogellar I, van Ballegooijen M, Hankey BF, Hankey BF, et al. Colonoscopic polypectomy and long‐term prevention of colorectal cancer deaths. N Engl J Med. 2012;366:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7:770–5. [DOI] [PubMed] [Google Scholar]
- 3. Gupta S, Lieberman D, Anderson C, Burke CA, Dominitz JA, Kaltenbach T, et al. Recommendations for follow‐up after colonoscopy and polypectomy: a consennsus update by US Multi‐Society Task Force on colorectal cancer. Gastroenterology. 2020;158:1131–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hassan C, Giulio A, Dumoncreau JM. Post‐polepectomy colonoscopy surveillance: Europena Society of Gastrointestinal Endoscopy (ESGE) guideline‐update 2020. Endoscopy. 2020;52(8):687–700. [DOI] [PubMed] [Google Scholar]
- 5. Rutter M, East J, Rees C, Cripps N, Docherty J, Dolwani S, et al. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post‐polypectomy and post‐colorectal cancer resection surveillance guidelines. Gut. 2020;69:201–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee JK, Jensen CD, Levin TR, Doubeni CA, Zauber AG, Chubak J, et al. Long‐term risk of colorectal cancer and related death after adenoma removal in a large community based population. Gastroenterology. 2020;158:884–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atkin W, Wooldrage K, Breener A, Martin J, Shah U, Perera S, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, mutlicentre, cohort sutdy. Lancet Oncol. 2017;18:823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wieszczy P, Kaminski MF, Franczyk R, Loberg M, Kobiela J, Rupinska M, et al. Colorectal cancer incidence and mortality after removal of adenomas during screening colonoscopies. Gastroenterology. 2020;158:875–83. [DOI] [PubMed] [Google Scholar]
- 9. Cottet V, Jooste V, Fournel L, Bouvier A‐M, Faivre J, Bonithon‐Kopp C. Long‐term risk of colorectal cancer after adenoma removal: a population based cohort study. Gut. 2012;61:1180–6. [DOI] [PubMed] [Google Scholar]
- 10. Click B, Pinsky PF, Hickey T, Doroudi M, Schoen RE. Association of colonoscopy adenoma findings with long‐term colorectal cancer. J Am Med Assoc. 2018;319:2021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta S, Jacobs ET, Baron JA, Lieberman DA, Murphy G, Ladabaum U, et al. Risk stratification of individuals with low‐risk colorectal adenomas using clinical characteristics: a pooled analysis. Gut. 2017;66:446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loberg M, Kalager M, Holme O, Hoffe G, Adami H‐O, Bretthauer M. Long‐term colorctal cancer mortality after adenoma removal. N Engl J Med. 2014;371:799–807. [DOI] [PubMed] [Google Scholar]
- 13. Fairley KJ, Li J, Komer M, Steigerwalt N, Erlich P. Predicting the risk of recurrent adenoma and incident colorectal cancer based on findings of the baseline colonoscopy. Clin Transl Gastroenterol. 2014;5:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Heijningen EM, Lansdrop‐Vogelaar I, Kuipers EJ, Dekker E, Lesterhuis W, Ter Borg F, et al. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community based study. Gastroenterology. 2013;144:1410–8. [DOI] [PubMed] [Google Scholar]
- 15. Saini SD, Kim HM, Schoenfel P. Incidence of advacned adenomas at surveillance colonoscopy in patients with personal history of colono adenomas: a meta‐analysis and systematic review. Gastrointest Endosc. 2006;64:614–26. [DOI] [PubMed] [Google Scholar]
- 16. de Jonge V, Sint Nicolass J, van leerdam M, Kuipers E, Veldhuyzen van Zanten S. Systematic literature review and pooled analysis of risk factors for finding adenomas at surveillance colonoscopy. Endoscopy. 2011;43:560–74. [DOI] [PubMed] [Google Scholar]
- 17. Foss FA, Milkins S, McGregor AH. Inter‐observer variability in the histological assessment of colorectal polyps deteced throught the NHS bowel cancer screening programme. Histopathology. 2012;61:47–52. [DOI] [PubMed] [Google Scholar]
- 18. Mahajan D, Downs‐Kelly E, Liu X, Pai RK, Patil DT, Rybicki L, et al. Reproducibility of the villous commpnent and high‐grade dysplasia in colorectal adenomas <1cm: implication for endoscopic surveillance. Am J Surg Pathol. 2013;37:427–33. [DOI] [PubMed] [Google Scholar]
- 19. Sakata S, Klein K, Stevenson ARL, Hewett DG. Measurement bias of polyp size at colonoscopy. Dis Colon Rectum. 2017;60:987–91. [DOI] [PubMed] [Google Scholar]
- 20. Hong S, Suh M, Son Choi K, Park B, Cha JM, Kim H‐S, et al. Guideline adherence to colonoscopic surveillance intervals after polypectomy in Korea: resutls from a Nationwide Survey. Gut Liver. 2018;4:426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanaka S, Obata D, Chinzei R, Yoshida S, Sanuki T, Morita Y, et al. Surveillance after colorectal polypectomy; comparison between Japan and U.S. Kobe. J Med Sci. 2011;2 (56):204–13. [PubMed] [Google Scholar]
- 22. Abu Freha N, Abu Tailakh J, Elkrinawi J, Abu Kaf H, Philip A, Schwartz D, et al. Post‐polypecotmy surveillance colonoscopy: are we following the guidelines? Int J Colorectal Dis. 2020;35 (6):1343–6. [DOI] [PubMed] [Google Scholar]
- 23. Djinbachian R, Dube AJ, Durand M, Camara LR, Panzini B, Bouchard S, et al. Adherence to post‐polypectomy surveillance guideline: a systematic review and meta‐analysis. Endoscopy. 2019;51 (7):673–83. [DOI] [PubMed] [Google Scholar]
- 24. Woolf S, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318:527–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The guidelines were published by the organizations and there are no additional data for sharing.


