Abstract
Background
The current standard of treatment in primary biliary cholangitis (PBC) is ursodeoxycholic acid (UDCA), although a considerable proportion of patients show incomplete response resulting in disease progression.
Objective
This study aimed to assess the prevalence of incomplete response to UDCA and determine associated patients' characteristics.
Methods
Patients with PBC as main diagnosis were included from a national multicentric patient registry—Liver.pt. Main endpoints included incomplete response to UDCA treatment according to Barcelona, Paris I and Paris II criteria, Globe and UK PBC scores and the association between baseline characteristics and incomplete response according to Paris II criteria.
Results
A total of 434 PBC patients were identified, with a mean age of 55 years and 89.2% females. Nearly half of patients were asymptomatic at diagnosis and 93.2% had positive anti‐mitochondrial antibodies. Almost all patients (95.6%) had been prescribed at least one drug for PBC treatment. At the last follow‐up visit, 93.3% were under treatment of which 99.8% received UDCA. Incomplete response to UDCA was observed in 30.7%, 35.3%, 53.7% and 36.4% of patients according to Barcelona, Paris I, Paris II criteria and Globe score, respectively. After adjusting for age and sex, and accordingly to Paris II criteria, the risk for incomplete biochemical response was 25% higher for patients with cirrhosis at diagnosis (odds ratio [OR] = 1.25; 95% confidence interval [95%CI]: 1.02–1.54; p = 0.033) and 35% (95%CI:1.06–1.72; p = 0.016) and 5% (OR = 1.05; 95%CI:1.01–1.10; p = 0.013) for those with elevated gamma‐glutamyl transferase (GGT) and alkaline phosphatase (ALP).
Conclusion
A considerable proportion of patients showed incomplete biochemical response to UDCA treatment according to Paris II criteria. Cirrhosis, elevated GGT and ALP at diagnosis were identified as associated risk factors for incomplete response. Early identification of patients at risk of incomplete response could improve treatment care and guide clinical decision to a more careful patient monitorization.
Keywords: Barcelona, GLOBE, Paris, PBC, predictors, primary biliary cholangitis, response, score, UDCA, ursodeoxycholic acid
Key summary
Summarise the established knowledge on this subject
Primary biliary cholangitis is a liver disease that can progress to end‐stage liver disease, with premature death or need for liver transplantation.
Treatment with ursodeoxycholic acid (UDCA) significantly increases liver transplant‐free survival. However, incomplete response to UDCA reduces this beneficial effect.
What are the significant and/or new findings of this study?
By evaluating prevalence and risk factors for UDCA incomplete response through a large multicentric national registry it was found that 53.7% of patients were incomplete responders, according to Paris II criteria, with cirrhosis, elevated gamma‐glutamyl transferase and alkaline phosphatase at diagnosis as the main risk factors.
These findings suggest that patients diagnosed at an advanced stage should be closely monitored and might benefit from novel therapies to improve outcomes if incomplete response is present.
INTRODUCTION
Primary biliary cholangitis (PBC) is a chronic inflammatory autoimmune cholestatic liver disease. When untreated, PBC frequently progresses to fibrosis, end‐stage biliary cirrhosis, hepatic failure, hepatocellular carcinoma and premature death. 1 , 2 , 3 PBC is considered a rare condition, with incidence rates ranging from 0.33 to 5.8 per 100,000 inhabitants/year and prevalence rates between 1.91 and 40.2 per 100.000 inhabitants. This condition affects mainly women after 40 years of age, with a female to male ratio of approximately 10 to 1. 4−7
The majority of patients are asymptomatic at early stages, although symptoms can develop later over the course of the disease, with fatigue and pruritus being the most frequent complaints. 4 , 5 , 6
The current standard treatment for PBC is ursodeoxycholic acid (UDCA), which has been shown to delay disease progression and extend liver transplant‐free survival. 2 , 7 However, a substantial proportion of patients exhibit suboptimal response to UDCA treatment, as defined by different validated criteria. Of note, although several established binary criteria and continuous scoring systems are recommended by European Association for the Study of the Liver (EASL) guidelines to evaluate response to UDCA, namely Barcelona, Paris I and II criteria, Globe and UK PBC scores, those guidelines highlight that the two most important parameters in evaluating response to UDCA are alkaline phosphatase (ALP) and total bilirubin level. 7 Additionally, since disease stage is known to affect biochemical response to UDCA, Corpechot et al. has distinguished Paris II as the most adequate criteria to evaluate early‐stage patients, who represent the majority of the PBC patients. 8 Furthermore, recent data from the patient cohort of Global PBC study has demonstrated that attaining bilirubin levels ≤0.6 × upper limit of normal (ULN) or normal ALP are associated with the lowest risk for liver transplant or death in patients with PBC. 9 Consequently, regardless of which criteria applied, it is clear that incomplete respondent patients are at higher risk of disease progression, when compared to UDCA respondents, although still better than non‐treated, resulting in worse outcome and requiring additional treatments. 2 , 7 , 10 , 11 , 12 This becomes particularly important, since there are now available second line treatments, such as obeticholic acid, fibrates and budesonide. 5 , 7
Therefore, identifying patients at higher risk of incomplete biochemical response to UDCA treatment at diagnosis may be useful to guide clinical and therapeutic decision making. The aim of this study was to assess incomplete response to UDCA and identify potential characteristics associated.
PATIENTS AND METHODS
This was an observational cohort study based on a prospective data collection for newly diagnosed patients and retrospective data collection for patients diagnosed before the creation of patient registry and still alive—Liver.pt. Liver.pt is a Portuguese nationwide online registry database of hepatic diseases. 13 Liver.pt only includes patients under follow‐up at the time of the registry that have at least one hepatic disease as main diagnosis. Collected data included diagnosis, medical history, staging of hepatic disease, concomitant hepatic diseases and comorbidities, laboratory parameters and treatment, in real‐life context.
All Portuguese hospitals may participate in this collaborative database and include their patients voluntarily and consecutively. Currently there are 26 registered centres from 23 public and private hospitals, from several Portuguese regions. To ensure that the PBC population was representative of the entire Portuguese population, a dynamization period was taken into account, which involved numerous initiatives, namely explanation of the Liver.pt registry to several centres, presentation and awareness of the project during Portuguese Association for the Study of the Liver (APEF) congresses, and through e‐mail and telephone contacts.
This study included all adult patients (age≥18 years) with PBC as main diagnosis registered in Liver.pt, regardless of treatment or disease status. Data extraction comprised patients registered between database inception (October 2014) and March 2019. The main endpoints included the evaluation of treatment response to UDCA and the assessment of the patients' baseline characteristics associated with incomplete response. Response to treatment after 12 months of UDCA initiation was evaluated according to Barcelona, Paris I and Paris II binary criteria, and Globe and UK PBC scores in line with the recommendations of current European guidelines. 7 Patients with a GLOBE score of 0.30 or less were considered responders. 14
All patients had given informed consent to be included in the national database, aware that it was centrally anonymized.
Statistical analysis
Demographics, clinical, biochemical and therapeutic data were summarized as categorical variables and presented as frequencies and continuous variables were expressed as mean and standard deviations (SD). All analyses were conducted based only on valid answers, without missing information. ULN and lower limit of normal (LLN) from laboratory parameters were obtained from each laboratory analysis. Whenever ULN or LLN were unavailable the respective ranges from a closer laboratory analysis within the same hospital was adopted. Missing data was retrieved by the local investigator of each centre. Transplantation free‐survival according to Globe score and the risk of a liver transplant or liver‐related death according to UK PBC score were estimated. 14 , 15
A logistic regression model was used to evaluate the association between patients' baseline characteristics with incomplete biochemical response to treatment, according to Paris II criteria. Variable selection in the multivariate regression model was performed through stepwise procedure, keeping age and sex as confounding variables.
All analysis adopted a significance level of 5% and were conducted in R version 4.0.3 software. 16
Ethics
This registry was nationally approved by the Portuguese Data Protection Authority and all registered centres have approval from local Ethics Committees. All patients had given written informed consent to be included in the platform, aware that it was centrally anonymized.
RESULTS
A total of 434 patients were identified with PBC as primary diagnosis from database inception until 10 March 2019. Mean age at diagnosis was 55 years and 387 patients (89.2%) were female. Almost all patients (93.2%) had positive anti‐mitochondrial antibodies, 54.6% were also positive for anti‐nuclear antibodies (ANA). Nearly half (46.3%) of the patients were asymptomatic and 2.3% had decompensated cirrhosis. Additional information about other clinical features at diagnosis is depicted in Table 1.
TABLE 1.
PBC patients' sociodemographic and clinical characteristics at diagnosis—Liver.pt (N = 434) a
| Characteristics | Patients | |
|---|---|---|
| Sociodemographic | ||
| Female | 89.2 (387/434) | |
| Age at diagnosis, mean (SD) | 55.2 (12.8) | |
| Age at last visit, mean (SD) | 64.4 (12.8) | |
| Clinical features | ||
| Asymptomatic | 46.3 (170/367) | |
| Pruritus | 29.3 (104/355) | |
| Fatigue | 34.2 (119/348) | |
| Hyperpigmentation | 3.2 (9/283) | |
| Jaundice | 6.1 (21/345) | |
| Xanthelasmas | 1.6 (5/306) | |
| Osteopenia | 17.2 (37/215) | |
| Osteoporosis | 14.7 (34/231) | |
| Serology | ||
| Positive AMA | 93.2 (343/368) | |
| Positive AMA‐M2 | 91.7 (300/327) | |
| Positive ANA | 54.6 (155/284) | |
Abbreviations: AMA, anti‐mitochondrial antibodies; AMA‐M2, anti‐mitochondrial antibodies type 2; ANA, anti‐nuclear antibodies; PBC, primary biliary cholangitis; SD, standard deviation.
Data is presented as % (n/N), unless otherwise stated.
The mean follow‐up time was 10.8 years (SD: 6.5). During the follow‐up, the presence of cirrhosis was confirmed in 21.5% of patients and was suspected in 4.4% of patients. Diagnosis of cirrhosis was confirmed by biopsy (24.1%), elastography (9.6%), clinical or imagiological findings (55.4% or 66.3%, respectively).
The Ludwig's classification was distributed through stages 1, 2, 3, 4 with 43.4%, 28.9%, 20.4% and 7.2% of patients, respectively.
Among the 434 patients with PBC registered in the database, 191 (44.0%) presented comorbidities during follow‐up. The most frequently observed were arterial hypertension (5.8%), Sjögren‐Larsson syndrome (4.6%), type 2 diabetes mellitus (4.6%), dyslipidaemia (4.4%), arthritis (4.1%), including rheumatoid arthritis (3.0%), hypothyroidism (3.7%), thyroiditis (3.5%), including autoimmune thyroiditis (2.3%) and biliary lithiasis (3.0%). The presence of ascites was documented in 3.9% of patients.
Cirrhotic patients presented significant clinical events, namely decompensated cirrhosis (n = 4) and liver transplantation (n = 9). No occurrence of hepatocellular carcinoma was reported among these patients and four patients died, of which two present liver‐related causes. Only one liver transplantation and three non‐liver‐related deaths were reported on non‐cirrhotic patients.
At least one drug for PBC treatment was prescribed to 95.6% (n = 415) of the patients during follow‐up with all but one treated with UDCA. At the last follow‐up visit, 405 patients (93.3%) were still receiving treatment for PBC. Most of them were receiving UDCA (n = 404; 99.8%) with a mean treatment duration of 10.4 ± 6.9 years. Only 9.4% (n = 38) were under concomitant therapy for PBC, with fibrates as the most frequent combination (5.2%). The distribution of patients according to PBC treatment is shown in Table 2.
TABLE 2.
Treatment patterns of the PBC’ patients receiving treatment, at the last follow‐up visit
| Treatment | Patients, n (%) a (n = 405) |
|---|---|
| UDCA | 404 (99.8) |
| Fibrates | 21 (5.2) |
| Budesonide | 15 (3.7) |
| Obeticholic acid | 5 (1.2) |
Abbreviations: PBC, primary biliary cholangitis; UDCA, ursodeoxycholic acid.
Column add up greater than 100% because some patients received more than one drug.
Evolution of biochemical parameters and clinical features
At diagnosis, 78.2% had ALP above ULN and 25.7% over 3xULN. More than three quarters of patients had an aspartate aminotransferase (AST) and alanine aminotransferase (ALT) above ULN (76.2% and 75.5%, respectively) and gamma‐glutamyl transferase (GGT) was more than 1.5 times superior to ULN in 84.7% patients. Bilirubin was superior to ULN in 19.3% of patients (Table 3).
TABLE 3.
Biochemical parameters at diagnosis and at the last follow‐up visit
| Biochemical parameters | At diagnosis, % (n) | At last follow‐up, % (n) |
|---|---|---|
| ALP | N = 280 | N = 379 |
| ≤ULN | 21.8 (61) | 47.8 (181) |
| ULN < ALP ≤ 1.5xULN | 21.8 (61) | 26.9 (102) |
| 1.5xULN < ALP ≤ 3xULN | 30.7 (86) | 19.8 (75) |
| >3xULN | 25.7 (72) | 5.5 (21) |
| ALT | N = 253 | N = 349 |
| ≤ULN | 24.5 (62) | 68.8 (240) |
| >ULN | 75.5 (191) | 31.2 (109) |
| AST | N = 248 | N = 343 |
| ≤ULN | 23.8 (59) | 58.9 (202) |
| >ULN | 76.2 (189) | 41.1 (141) |
| GGT | N = 242 | N = 371 |
| ≤1.5xULN | 15.3 (37) | 49.3 (183) |
| >1.5xULN | 84.7 (205) | 50.7 (188) |
| Bilirubin | N = 228 | N = 363 |
| ≤ULN | 80.7 (184) | 80.7 (293) |
| >ULN | 19.3 (44) | 19.3 (70) |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; ULN, upper limit of normal.
At the last follow‐up visit, approximately half (47.8%) of PBC patients had an ALP within the normal range, despite treatment initiation. Of patients with ALP values above the ULN, 48.5% had an ALP level above 1.5xULN. The proportion of patients with liver transaminases within normal range more than doubled from diagnosis to last follow‐up date while for GGT, the proportion of patients within 1.5xULN was three times higher. The proportion of patients with bilirubin below ULN remained stable at diagnosis and last follow‐up (Table 3).
Besides biochemical parameters, clinical response was also assessed. After treatment initiation, 38.6% and 28.67% of patients with fatigue and pruritus, respectively, remained symptomatic. Also, jaundice persisted in 13.3% of patients with this condition at baseline. Overall, half (49.7%) of the symptomatic patients at baseline still reported symptoms at the last follow‐up visit, including fatigue, pruritus and jaundice.
Despite treatment initiation, 25.6% of the asymptomatic patients at baseline developed symptoms, 13.2% developed fatigue and 7.9% pruritus from treatment initiation to last follow‐up.
Response to UDCA
Among the 414 UDCA treated patients, response to the treatment was evaluated in all the patients with available data, by the Barcelona, Paris I, Paris II criteria and Globe score. Incomplete response to UDCA treatment was 30.7%, 35.3%, 53.7% and 36.4%, respectively (Figure 1).
FIGURE 1.
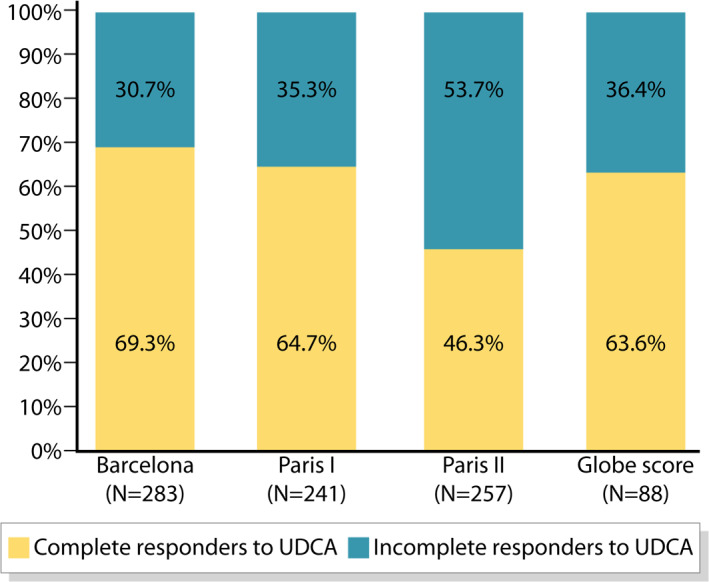
Response to UDCA treatment, according to Barcelona, Paris I and Paris II criteria and Globe score. UDCA, ursodeoxycholic acid
Transplantation free‐survival according to Globe score was 92.7 (SD: 12.6), 88.6 (SD:15.3), 76.2 (SD: 22.1) and 65.3 (SD: 25.7) at 3, 5, 10 and 15 years, respectively.
The risk of a liver transplant or liver‐related death occurring within 5, 10, or 15 years, estimated with UK PBC score, were 3.2 (SD: 5.7), 9.3 (SD: 13.7) and 15.1 (SD: 18.5), respectively.
According to Paris II criteria, the incomplete responders group had a slightly higher proportion of males (13.8% vs. 11.8%), a higher proportion of patient with cirrhosis at baseline (36.2% vs. 9.3%) and a higher proportion of patients with symptoms at baseline (62.7% vs. 51.7%), compared with responders.
We investigated whether there were any relationships between baseline characteristics, namely, sex, age, symptoms, ANA positivity, level of ALT, GGT, cirrhosis, ALP and bilirubin ratio, and incomplete response according to Paris II criteria.
The univariate analysis showed that GGT>1.5xULN, presence of cirrhosis and ALP ratio at diagnosis were independently associated with incomplete biochemical response after 1 year of UDCA treatment (Table 4).
TABLE 4.
Univariate analysis of baseline characteristics associated with incomplete biochemical response to UDCA, according to Paris II criteria
| Baseline characteristic | Univariate analysis | ||
|---|---|---|---|
| OR | 95%CI | p‐value | |
| Sex—male | 0.87 | 0.32–2.36 | 0.058 |
| Age >45 | 0.80 | 0.35–1.83 | 0.162 |
| Asymptomatic | 0.70 | 0.34–1.46 | 0.037 |
| Presence of cirrhosis at diagnosis—Yes | 4.76 | 1.53–14.85 | 0.622 |
| ANA—positive | 0.69 | 0.34–1.38 | 0.037 |
| ALT ≥2xULN | 1.41 | 0.69–2.87 | 0.407 |
| GGT >1.5xULN | 6.37 | 1.96–20.61 | 0.027 |
| ALP ratio a | 1.59 | 1.23–2.04 | 0.028 |
| Bilirubin ratio a | 1.04 | 0.78–1.40 | 0.155 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; ANA, antinuclear antibodies; GGT, gamma‐glutamyl transferase; OR, odds ratio; UDCA, ursodeoxycholic acid; ULN, upper limit of normal; 95%CI, 95% confidence interval.
ALP and bilirrubin were analysed as multiples of the upper reference level in the laboratories that measured them.
After adjusting for sex and age at diagnosis, GGT>1.5xULN, presence of cirrhosis and ALP ratio remained statistically significant in the multivariate analysis. In fact, the odds of incomplete response according to Paris II criteria was 1.25‐fold higher in patients with cirrhosis (odds ratio [OR] = 1.25; 95% confidence interval [95%CI]:1.02–1.54; p = 0.033), 1.35 times higher for patients with GGT >1.5xULN (OR = 1.35; 95%CI:1.06–1.72; p = 0.016) and 5% higher for an unit increase of ALP ratio (OR = 1.05; 95%CI:1.01–1.10; p = 0.013) at diagnosis (Figure 2).
FIGURE 2.
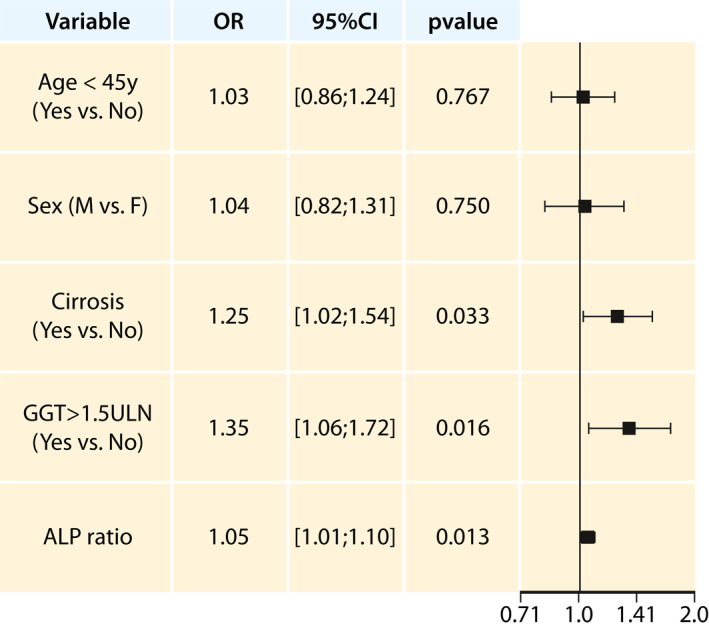
Multivariate analysis of incomplete biochemical response to UDCA, according to Paris II criteria. ALP, alkaline phosphatase; GGT, gamma‐glutamyltransferase; OR, odds ratio of incomplete response; ULN, upper limit of normal; y, years; 95%CI, 95% confidence interval
A sensitivity analysis was also performed in order assess the factors associated with incomplete response excluding cirrhotic patients. In this population, the ALP and bilirubin ratio were independently associated with incomplete biochemical response to UDCA, according to Paris II criteria in a multivariate analysis (Figure 3).
FIGURE 3.
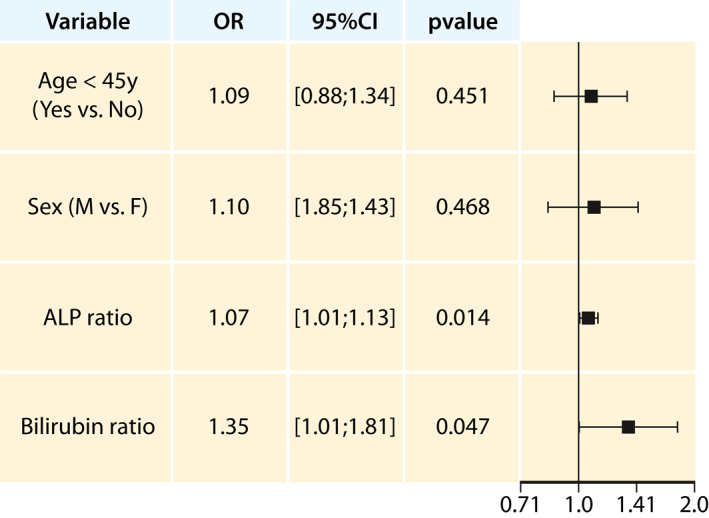
Sensitive analysis: result of the multivariate analysis of incomplete biochemical response to UDCA, according to Paris II criteria excluding cirrhotic patients. ALP, alkaline phosphatase; OR, odds ratio of incompleteresponse; ULN, upper limit of normal; y, years; 95%CI, 95% confidence interval
DISCUSSION
The results of this study shows that the proportion of incomplete biochemical response to UDCA depends on the criteria adopted, with a proportion of 30.7% incomplete response according to Barcelona criteria, 35.3% according to Paris I criteria, 53.7% according to Paris II criteria and 36.4% according to the Globe score. These results were expectable since Paris II criteria involves response to three biochemical parameters, while Globe score includes more parameters and Barcelona only evaluates ALP. Also, Paris II has lower thresholds when compared to Paris I criteria, particularly in ALP and AST parameters. The proportion of incomplete responders was in line with previously published studies. 8 , 14 , 17 , 18 Current PBC guidelines argue that ALP alone is not powerful enough to evaluate response to UDCA, thus recommending the evaluation of both ALP and total bilirubin. 7 In fact, Paris II criteria, with decreased cut‐off values for ALP and AST when compared to Paris I, was able to accurately predict long‐term survival, with higher performance and specificity. 8 Therefore, this study estimates that more than half of patients treated with UDCA are incomplete responders to treatment, according to Paris II criteria. This percentage would most probably be even larger, if the new treatment targets of Global PBC were used of bilirrubin <0.6xULN and normal ALP. 9
Several baseline characteristics were tested to ascertain which were associated with incomplete response according to Paris II criteria. This study showed that, when controlled for age and sex, cirrhosis, elevated GGT and degree of ALP elevation at diagnosis, were significantly associated with incomplete response, after 1 year of treatment with UDCA. All other characteristics, such as absence of symptoms at baseline, positive ANA and ALT level and increased bilirubin were not associated with incomplete response.
The presence of cirrhosis at diagnosis is a sign of advanced liver disease, which is a known cause for diminished treatment response to UDCA. 19 Also, an elevated GGT at diagnosis may reflect cholestatic liver injury, a recognized early marker for PBC that was found to predict a poorer treatment response. 7 , 20 This finding corroborates a previous study that identified GGT as a predictor to disease progression. 21 Also, Gerussi et al, found that higher serum levels of GGT were associated with a worse prognosis in PBC patients. 22 Regarding bilirubin, in the sensitivity analysis (excluding patients with cirrhosis), it becomes a predictor of incomplete response, what was not the case in the general group. This is probably because very slight changes of bilirubin levels become more obvious.
The results of the multivariate analysis showed that age and sex were not significantly associated with incomplete response to treatment. Previous published studies have established that patients diagnosed at a younger age had a higher risk for treatment failure, unlike the results we report. 20 , 23 , 24 Another study found that older age at diagnosis was an independent predictor of mortality. 19 Whichever is the true influence of age at diagnosis, PBC is an asymptomatic disease and only suspected after routine liver tests, therefore it is possible that our cohort had a delayed diagnosis, making age at diagnosis a misleading variable, which would explain the absence of significance in both univariate and multivariate analysis. 25 Other explanation would be that younger patients are likely to present with a more severe disease, possibly related to ductopenic phenotype, which is resistant to UDCA treatment. 23
We did not find male sex to have a negative association with response to treatment 19 , 24 , what is in line with Global PBC findings. 23 Among possible explanations to this observation, one might be that male patients are less frequently diagnosed, resulting in an insufficient power in the sample to detect a difference; another one would be that male patients develop less frequent and/or less severe symptoms thus remaining undiagnosed until a more advanced disease stage, consequently associated with a worse treatment response. 23 , 24 , 26
Possibly due to the long course of PBC, several previously published studies have investigated surrogate markers for serious clinical consequences of PBC, such as portal hypertension, oesophageal varices, hepatocellular carcinoma, liver transplantion or death. 8 , 17 , 20 , 23
As previously mentioned, earlier studies have investigated the association between symptoms and biochemical markers with overall or transplant‐free survival. 17 , 18 , 23 However, our cohort reported low proportions of both events, preventing this type of analysis. With a longer follow‐up of patients in Liver.pt it will be possible to demonstrate other surrogate markers for overall or transplant‐free survival in PBC.
All data analysed in this study was retrieved from Liver.pt. Even though it is a voluntary registry database, which may not report all affected patients from all available centres and might have some degree of missing information, we consider that it has no referral bias, since in Portugal, it is generally established that PBC patients are referred to specialized consultation. Also, we believe that this sample represents the large majority of Portuguese PBC patients. In fact, general PBC prevalence is described between 1.91 and 40.2 per 100,000 7 ; since Portuguese population is around 10,000,000, the 434 included patients represent, in the worst‐case scenario 1/10 of all Portuguese patients.
One of the major advantages of this study is that it collected data from a national registry. Although it could be arguable, 27 PBC is still generally considered a rare disease. 7 , 28 As a result, the best way to deal with the uncertainty commonly found in rare diseases care is to systematically collect data to answer the lack of solid evidence. 29 Liver.pt is the Portuguese reference registry for liver diseases that collects information directly from individual patients under follow‐up by hepatologists. As PBC is a disease with a long clinical course and often presents slow progression, other study designs, such as specifically designed observational studies with delimited periods for each phase, may fail to obtain relevant information.
Another major advantage of studies based in patient registry is that data derives from a real‐world context, reflecting current clinical practice and including several different types of patients. 30 Registries also promote collaborative networking and knowledge sharing between clinical teams, leading to a better patient care and promoting efficient public health interventions. 31
In summary, this study provides insightful information about the current landscape of PBC patients in Portugal. Although almost every patient was under treatment with UDCA, a considerable proportion had an incomplete biochemical response. Presentation with cirrhosis, elevated GGT and higher ALP at diagnosis, possibly indicating a more severe disease, were associated with worse treatment response, potentially leading to liver‐related death or transplantation. Consequently, patients with evidence of cirrhosis, should have a much closer follow‐up, being evaluated at 6 months of treatment, and if the response is incomplete they should be referred to a liver transplantation centre, in order to have an early liver transplantation whenever it will be needed. Altogether, these findings highlight the importance of careful evaluation of therapy response. While previously the absence of complete response did not change the management, since there were no alternative treatments, with the availability of second‐ and third‐line effective treatments, this is no longer true, and these patients need a closer follow‐up based on strict validated criteria to prevent liver related complications.
CONFLICT OF INTERESTS
The authors declared the following potential conflicts of interest: Alexandra Martins—Lectures and/or advisory board fees from Roche, BMS, Gilead and Abbvie; Arsénio Santos—Lectures and advisory board fees from Gilead, MSD, Intercept and Alfasigma; Filipe Calinas—Advisory fees from Intercept; Helena Cortez‐Pinto—Lectures and advisory board fees from Intercept, Genfit, Gilead and Promethera Biosciences. All other authors have nothing to declare.
AUTHOR CONTRIBUTIONS
Conception and design: Helena Cortez‐Pinto, Marta Vargas Gomes and Joana Oliveira: Acquisition of patients and/or clinical data: Helena Cortez‐Pinto, Rodrigo Liberal, Susana Lopes, Mariana V. Machado, Joana Carvalho, Teresa Dias, Arsénio Santos, Cláudia Agostinho, Pedro Figueiredo; Filipe Calinas, Rodrigo Liberal, Alexandra Martins, Gonçalo Alexandrino, Isabel Cotrim, Carina Leal, José Presa, Mónica Mesquita, Joana Nunes, Catarina Gouveia, Ana Horta e Vale, Ana Luísa Alves, Mariana Coelho, Luís Maia, Isabel Pedroto, António Banhudo, João Sebastião Pinto. Analysis and interpretation of the data: Helena Cortez‐Pinto, Marta Vargas Gomes, Joana Oliveira and Valeska Andreozzi. Draughting of the article: Helena Cortez‐Pinto, Marta Vargas Gomes, Joana Oliveira and Valeska Andreozzi. All authors critically revised the draft and gave their approval to the final version of the article.
ACKNOWLEDGEMENTS
Acknowledgements on behalf of Liver.pt registry: Adélia Simão, Alexandre Ferreira, Armando Carvalho, Carolina Palmela, Joana E. Santo, João Madaleno, Jorge Leitão, Mário Bento Miranda, Nuno Silva, Rui Santos, Rui Valente, Sara Leitão, Sofia Carvalhana, Suzana Calretas and Teresa Vaio. The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Liver.pt was developed by the Portuguese Association for the Study of the Liver (APEF) sponsored by Gilead and Intercept Pharma. This study was supported with a grant from Intercept Pharma attributed to APEF. The sponsor was not involved in the study design, data collection, analysis and interpretation of data.
Cortez‐Pinto H, Liberal R, Lopes S , Machado MV, Carvalho J, Dias T, et al. Predictors for incomplete response to ursodeoxycholic acid in primary biliary cholangitis. Data from a national registry of liver disease. United European Gastroenterol J. 2021;9(6):699–706. 10.1002/ueg2.12095
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Selmi C, Bowlus CL, Gershwin ME, Coppel RL. Primary biliary cirrhosis. Lancet. 2011;377:1600–9. [DOI] [PubMed] [Google Scholar]
- 2. Hegade VS, Khanna A, Walker LJ, Wong L‐L, Dyson JK, Jones DEJ. Long‐term fenofibrate treatment in primary biliary cholangitis improves biochemistry but not the UK‐PBC risk score. Dig Dis Sci. 2016;61:3037–44. [DOI] [PubMed] [Google Scholar]
- 3. Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DEJ, Lindor K, et al. Changing nomenclature for PBC: from ‘cirrhosis’ to ‘cholangitis’. Clin Res Hepatol Gas. 2015;39:e57–e59. [DOI] [PubMed] [Google Scholar]
- 4. Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386:1565–75. [DOI] [PubMed] [Google Scholar]
- 5. Younossi ZM, Stepanova M, Golabi P, Epstein R, Strauss M, Nader F, et al. Factors associated with potential progressive course of primary biliary cholangitis: data from real‐world US database. J Clin Gastroenterol. 2019;53:693–98. [DOI] [PubMed] [Google Scholar]
- 6. Marzioni M, Bassanelli C, Ripellino C, Urbinati D, Alvaro D. Epidemiology of primary biliary cholangitis in Italy: evidence from a real‐world database. Dig Liver Dis. 2019;51:724–9. [DOI] [PubMed] [Google Scholar]
- 7. EASL Clinical Practice Guidelines . The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145–72. [DOI] [PubMed] [Google Scholar]
- 8. Corpechot C, Chazouillères O, Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long‐term outcome. J Hepatol. 2011;55:1361–7. [DOI] [PubMed] [Google Scholar]
- 9. Murillo Perez CF, Harms MH, Lindor KD, van Buuren HR, Hirschfield GM, Corpechot C, et al. Goals of treatment for improved survival in primary biliary cholangitis: treatment target should be bilirubin within the normal range and normalization of alkaline phosphatase. Am J Gastroenterol. 2020;115:1066–74. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka A, Hirohara J, Nakanuma Y, Tsubouchi H, Takikawa H. Biochemical responses to bezafibrate improve long‐term outcome in asymptomatic patients with primary biliary cirrhosis refractory to UDCA. J Gastroenterol. 2015;50:675–82. [DOI] [PubMed] [Google Scholar]
- 11. Honda A, Tanaka A, Kaneko T, Komori A, Abe M, Inao M, et al. Bezafibrate improves GLOBE and UK‐PBC scores and long‐term outcomes in patients with primary biliary cholangitis. Hepatology. 2019;70:2035–46. [DOI] [PubMed] [Google Scholar]
- 12. Harms MH, van Buuren HR, Corpechot C, Thorburn D, Janssen HLA, Lindor KD, et al. Ursodeoxycholic acid therapy and liver transplant‐free survival in patients with primary biliary cholangitis. J Hepatol. 2019;71:357–65. [DOI] [PubMed] [Google Scholar]
- 13. Portuguese Association for the Study of the Liver (APEF) . National registry of liver diseases [Registo Nacional de Doenças Hepáticas]. Available from 2014. Liver.pt [Google Scholar]
- 14. Lammers WJ, Hirschfield GM, Corpechot C, Nevens F, Lindor KD, Janssen HLA, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015; 149:1804–12.e4. [DOI] [PubMed] [Google Scholar]
- 15. Carbone M, Sharp SJ, Flack S, Paximadas D, Spiess K, Adgey C, et al. The UK‐PBC risk scores: derivation and validation of a scoring system for long‐term prediction of end‐stage liver disease in primary biliary cholangitis. Hepatology. 2016;63:930–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. R Core Team. R : A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available from 2020. https://www.R‐project.org/.2020 [Google Scholar]
- 17. Corpechot C, Abenavoli L, Rabahi N, Chrétien Y, Andréani T, Johanet C, et al. Biochemical response to ursodeoxycholic acid and long‐term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871–7. [DOI] [PubMed] [Google Scholar]
- 18. Parés A, Caballeria L, Rodes J. Excellent long‐term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 2006;130:715–20. [DOI] [PubMed] [Google Scholar]
- 19. Myers RP, Shaheen AAM, Fong A, Burak KW, Wan A, Swain MG, et al. Epidemiology and natural history of primary biliary cirrhosis in a Canadian health region: a population‐based study. Hepatology. 2009;50:1884–92. [DOI] [PubMed] [Google Scholar]
- 20. Lammers WJ, Kowdley KV, Buuren HRv. Predicting outcome in primary biliary cirrhosis. Ann Hepatol. 2014;13:316–26. [PubMed] [Google Scholar]
- 21. Azemoto N, Abe M, Murata Y, Hiasa Y, Hamada M, Matsuura B, et al. Early biochemical response to ursodeoxycholic acid predicts symptom development in patients with asymptomatic primary biliary cirrhosis. J Gastroenterol. 2009;44:630–4. [DOI] [PubMed] [Google Scholar]
- 22. Gerussi A, Bernasconi DP, O'Donnell SE, Lammers WJ, Van Buuren H, Hirschfield G, et al. Measurement of gamma glutamyl transferase to determine risk of liver transplantation or death in patients with primary biliary cholangitis. Clin Gastroenterol Hepatol. 2020;S1542‐3565:31083–1. [DOI] [PubMed] [Google Scholar]
- 23. Cheung AC, Lammers WJ, Murillo Perez CF, van Buuren HR, Gulamhusein A, Trivedi PJ, et al. Effects of age and sex of response to ursodeoxycholic acid and transplant‐free survival in patients with primary biliary cholangitis. Clin Gastroenterol Hepatol. 2019;17: 2076–84.e2. [DOI] [PubMed] [Google Scholar]
- 24. Carbone M, Mells GF, Pells G, Dawwas MF, Newton JL, Heneghan MA, et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology. 2013;144:560‐9.e7; quiz e13‐4. [DOI] [PubMed] [Google Scholar]
- 25. Bowlus CL, Gershwin ME. The diagnosis of primary biliary cirrhosis. Autoimmun Rev. 2014;13:441–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Podda M, Selmi C, Lleo A, Moroni L, Invernizzi P. The limitations and hidden gems of the epidemiology of primary biliary cirrhosis. J Autoimmun. 2013;46:81–7. [DOI] [PubMed] [Google Scholar]
- 27. Rigopoulou EI, Bogdanos DP. Is primary biliary cirrhosis rare or common? The truth lies somewhere in between. Liver Int. 2014;34:e165–e167. [DOI] [PubMed] [Google Scholar]
- 28. Bernts LHP, Jones DEJ, Kaatee MM, Lohse AW, Schramm C, Sturm E, et al. Position statement on access to care in rare liver diseases: advancements of the European reference network (ERN) RARE‐LIVER. Orphanet J Rare Dis. 2019;14:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. D'Agnolo HM, Kievit W, Andrade RJ, Karlsen TH, Wedemeyer H, Drenth JP. Creating an effective clinical registry for rare diseases. United European Gastroenterol J. 2016;4:333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trotter JP. Patient registries: a new gold standard for “real world” research. Ochsner J. 2002;4:211–4. [PMC free article] [PubMed] [Google Scholar]
- 31. Nelson EC, Dixon‐Woods M, Batalden PB, Homa K, Van Citters A, Morgan TS, et al. Patient focused registries can improve health, care, and science. BMJ. 2016;354:i3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


