Abstract
Aneuploidy is the leading cause of miscarriage and congenital birth defects, and a hallmark of cancer. Despite this strong association with human disease, the genetic causes of aneuploidy remain largely unknown. Through exome sequencing of patients with constitutional mosaic aneuploidy, we identified biallelic truncating mutations in CENATAC (CCDC84). We show that CENATAC is a novel component of the minor (U12‐dependent) spliceosome that promotes splicing of a specific, rare minor intron subtype. This subtype is characterized by AT‐AN splice sites and relatively high basal levels of intron retention. CENATAC depletion or expression of disease mutants resulted in excessive retention of AT‐AN minor introns in ˜ 100 genes enriched for nucleocytoplasmic transport and cell cycle regulators, and caused chromosome segregation errors. Our findings reveal selectivity in minor intron splicing and suggest a link between minor spliceosome defects and constitutional aneuploidy in humans.
Keywords: aneuploidy, CCDC84, CENATAC, minor spliceosome
Subject Categories: Cell Cycle; Genetics, Gene Therapy & Genetic Disease; RNA Biology
Biallelic CCDC84/CENATAC mutations identified through patient exome sequencing link altered minor intron splicing to constitutional mosaic aneuploidy in humans.
Introduction
Chromosome segregation errors in mitosis or meiosis lead to aneuploidy, a karyotype that deviates from an exact multiple of the haploid set of chromosomes. Aneuploidy is the leading cause of congenital birth defects and associated with ˜ 35% of all spontaneous human abortions (Nagaoka et␣al, 2012). Furthermore, roughly 70% of human tumors are aneuploid, making it one of the most common genomic alterations in cancer (Duijf & Benezra, 2013; Knouse et␣al, 2017). Despite this common association of aneuploidy with human disease, little is known about its genetic causes. The study of aneuploidy‐associated hereditary disorders can be instrumental in uncovering these causes.
Mosaic variegated aneuploidy (MVA; OMIM: 257300) is a rare autosomal recessive disorder characterized by mosaic aneuploidies in multiple tissues. Patients often present with microcephaly, developmental delay, various congenital abnormalities, and childhood cancers (García‐Castillo et␣al, 2008). Pathogenic mutations in BUB1B, CEP57, or TRIP13, have been identified in roughly half of all MVA patients (Hanks et␣al, 2004; Matsuura et␣al, 2006; Snape et␣al, 2011; Yost et␣al, 2017). These genes have well‐documented roles in chromosome segregation (Suijkerbuijk et␣al, 2010; Sacristan & Kops, 2015; Vader, 2015; Zhou et␣al, 2016). All three gene products (BUBR1, CEP57, and TRIP13) promote spindle assembly checkpoint (SAC) function (Wang et␣al, 2014; Musacchio, 2015; Ma et␣al, 2016; Zhou et␣al, 2016; Alfieri et␣al, 2018), and BUBR1 and CEP57 additionally ensure correct kinetochore–microtubule attachment (Emanuele & Stukenberg, 2007; Sacristan & Kops, 2015). As predicted, such mitotic processes are defective in cells from MVA patients carrying biallelic mutations in these genes, explaining the chromosomal instability (CIN) phenotype and resulting aneuploid karyotypes. CIN can also result from mutations in regulators of expression of mitotic genes. For example, mutations in the retinoblastoma gene (RB1) cause CIN by overexpression of the SAC protein MAD2 (Hernando et␣al, 2004; Sotillo et␣al, 2007; Schvartzman et␣al, 2011). In this work, we show that chromosome segregation errors can be caused by a specific defect in minor intron splicing, another process governing correct gene expression.
While the conventional, major spliceosome targets most (> 99.5%) human introns, the minor spliceosome recognizes and excises only a small subset (˜ 700 introns) (Turunen et␣al, 2013a; Moyer et␣al, 2020). These minor introns (also called U12‐type introns) have highly conserved 5′ splice site (5′ss) and branch point (BPS) sequences that are longer and differ at the sequence level from the respective sequences in major (U2‐type) introns. Most minor introns have AT‐AC or GT‐AG terminal dinucleotides (24 and 69%, respectively) (Sheth et␣al, 2006; Moyer et␣al, 2020). In addition, the 3′ terminal nucleotide can vary, thus giving rise to AT‐AN and GT‐AN classes of minor introns (Levine & Durbin, 2001; Dietrich et␣al, 2005). For simplicity, we refer to these as A‐ and G‐type introns, respectively. Thus far, there has been no indication of mechanistic or functional differences between the minor intron subtypes.
Minor intron “host” genes, the position of the minor intron within the gene, and intron subtypes, are all evolutionarily conserved (Burge et␣al, 1998; Abril et␣al, 2005; Sheth et␣al, 2006; Alioto, 2007; Moyer et␣al, 2020). Despite this high conservation, the functional significance of minor introns has remained elusive. Elevated levels of unspliced minor introns in various cell types have been reported, giving rise to the hypothesis that these are rate‐limiting controls for the expression of their host genes (Patel et␣al, 2002; Younis et␣al, 2013; Niemelä & Frilander, 2014; Niemelä et␣al, 2014). Nevertheless, the overall significance of the elevated intron retention (IR) levels has been questioned particularly at individual gene level (Singh & Padgett, 2009).
The overall architecture of the minor and major spliceosomes is highly similar. Both are composed of five small ribonucleoprotein (snRNP) complexes containing small nuclear RNA (snRNA) molecules and a large number of protein components. One of the snRNAs (U5) is shared between the spliceosomes, while U1, U2, U4, and U6 snRNAs are specific to the major spliceosome, and U11, U12, U4atac, and U6atac snRNAs to the minor spliceosome. Introns are initially recognized by the U1 and U2 snRNPs (major spliceosome) or by the U11/U12 di‐snRNP (minor spliceosome), followed by the entry of the U4/U6.U5 or U4atac/U6atac.U5 tri‐snRNP and subsequent architectural changes leading to catalytic activation of the spliceosome (Turunen et␣al, 2013a). At the protein level, the main difference between the spliceosomes is in the composition of the U11/U12 di‐snRNP that contains seven unique protein components that are needed for recognition of the unique minor intron splice sequences (Will et␣al, 2004). In contrast, the protein composition of the minor and major tri‐snRNPs appears similar, but rigorous comparative analyses have been difficult due to the ˜ 100‐fold lower cellular abundance of the minor tri‐snRNP (Schneider et␣al, 2002).
Here, we report that germline mutations in a novel component of the minor spliceosome (CENATAC/CCDC84) cause chromosomal instability in MVA patients. We identify CENATAC as a minor spliceosome‐specific tri‐snRNP subunit that promotes the splicing of A‐type minor introns, but hardly contributes to G‐type minor intron splicing. We show that CENATAC depletion or disease mutations result in increased A‐type minor IR and mitotic chromosome congression defects. Congression defects are also seen when another minor spliceosome component is depleted, suggesting that the chromosome segregation errors and aneuploidy observed in MVA patient cells are secondary effects of defective minor intron splicing.
Results
Biallelic truncating mutations in CENATAC (CCDC84) cause MVA
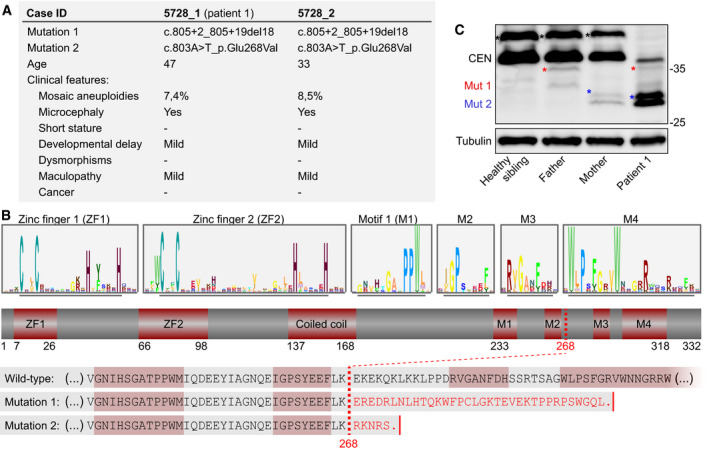
To search for additional causes of MVA, we performed exome sequencing and variant analyses on MVA patients and family members, as previously described (Yost et␣al, 2017). We identified biallelic truncating mutations in coiled‐coil domain‐containing 84 (CCDC84, hereafter named CENATAC, for centrosomal AT‐AC splicing factor, see below) in two affected siblings with 7.3 and 8.5% aneuploid blood cells, respectively (Figs 1A and EV1). Both siblings were alive at 47 and 33 years of age and had microcephaly, mild developmental delay, and mild maculopathy. Neither individual had short stature, dysmorphism, or cancer. Each parent was heterozygous for one of the mutations, and the unaffected sibling had neither mutation. Moreover, the mutations were absent from the ExAC and ICR1000 series and we estimated the chance of an individual having two truncating CENATAC mutations to be 4.8 × 10−10 (Fitzgerald et␣al, 2015). We therefore consider it very likely that the CENATAC mutations are the cause of the siblings' phenotype. The paternal and maternal mutations (mutation 1 and mutation 2, respectively) both result in the creation of novel splice sites that lead to a frameshift and the loss of the C‐terminal 64 amino acids of CENATAC (Fig 1B and Appendix␣Fig S1). Although expression of the mutant alleles was very low in the parental cells, expression of the maternal allele was elevated in the cells of patient 1 (hereafter called patient) and was responsible for the low expression of wild‐type protein in these cells due to infrequent recognition of the original splice site (Fig 1C and Appendix␣Fig S1C).
Figure 1. Biallelic truncating mutations in CENATAC (CCDC84) cause MVA.
-
AClinical phenotypes of CENATAC (CCDC84) mutant patients. See also Fig EV1.
-
BSchematic representation of CENATAC annotated with zinc fingers (ZF1 and ZF2), predicted coiled‐coil, and conserved motifs 1–4 (M1–M4). Upper: sequence logos of both zinc fingers and the conserved residues defining motifs 1–4 (underlined). See Appendix␣Fig S2 for the full‐length logo. Lower: C‐terminal protein sequences of wild‐type and MVA mutant CENATAC. The MVA truncation site is indicated by the red dotted line; the four conserved motifs are outlined in red.
-
CCENATAC and tubulin immunoblots of lysates from lymphoblasts of patient 1 and relatives. Wild‐type and truncated, mutant proteins are indicated. Wild‐type CENATAC (CEN): 38 kDa, Mut1: 34.5 kDa (father), Mut2: 31.1 kDa (mother). Phosphorylated CENATAC is indicated with asterisks: black (wild‐type), red (Mut1), or blue (Mut2) (Wang et␣al, 2019).
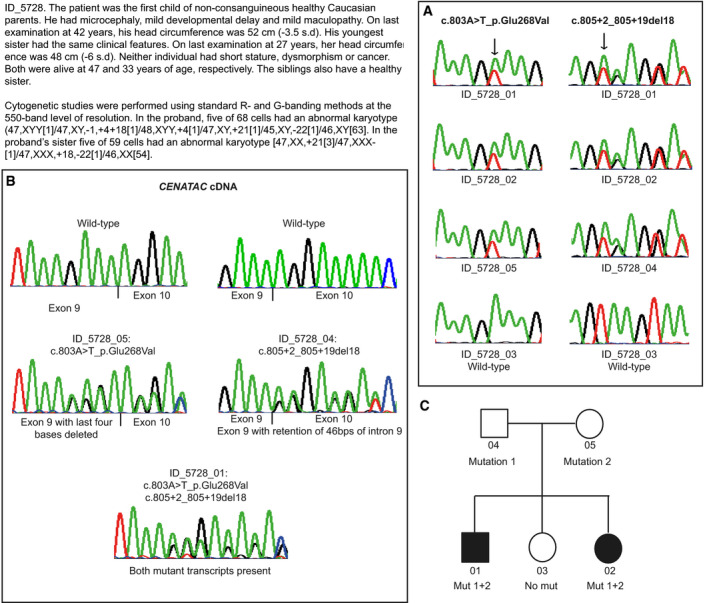
Figure EV1. Case report and chromatograms of individuals with mutations in CENATAC (CCDC84).
-
ASequencing chromatograms showing mutations in blood DNA and corresponding wild‐type sequence from a control.
-
BSequencing chromatograms from reverse transcription–PCR analysis of RNA showing the effect of CENATAC mutations. Maternal cDNA sequencing (ID_5728_05) demonstrates that c.803A>T_p.Glu268Val leads to a translational frameshift as a result of deletion of the last four bases of exon 9. Paternal cDNA sequencing (ID_5728_04) shows that c.805+2_805+19del18 results in retention of 46 bps of intron 9. The affected child's cDNA sequencing (ID_5728_01) demonstrates both mutant transcripts are present.
-
CPedigree of family (ID_5728) showing CENATAC mutation status.
CENATAC is an essential gene whose product has previously been reported to interact with pre‐mRNA splicing factors and to localize to centrosomes where it suppresses centriole over‐duplication and spindle multipolarity (Hart et␣al, 2015; Wang et␣al, 2019). Analysis of CENATAC sequence conservation in metazoan species revealed the presence of two N‐terminal C2H2 zinc fingers and four well‐conserved C‐terminal sequence motifs, of which the two most C‐terminal ones are lost as a result of the patient mutations (Fig 1B and Appendix␣Fig S2).
CENATAC promotes error‐free chromosome segregation
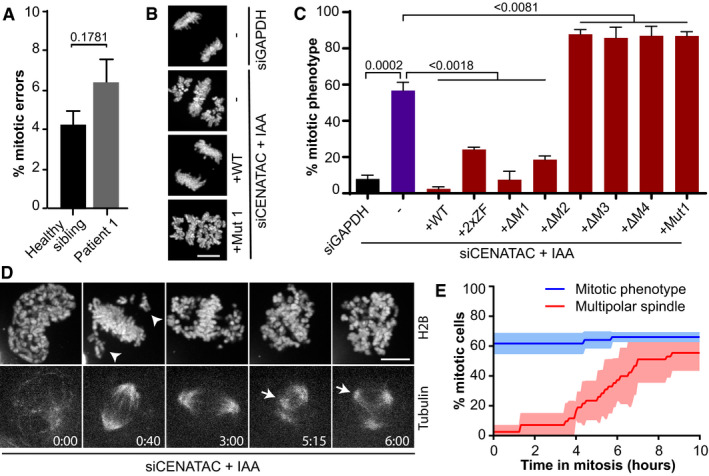
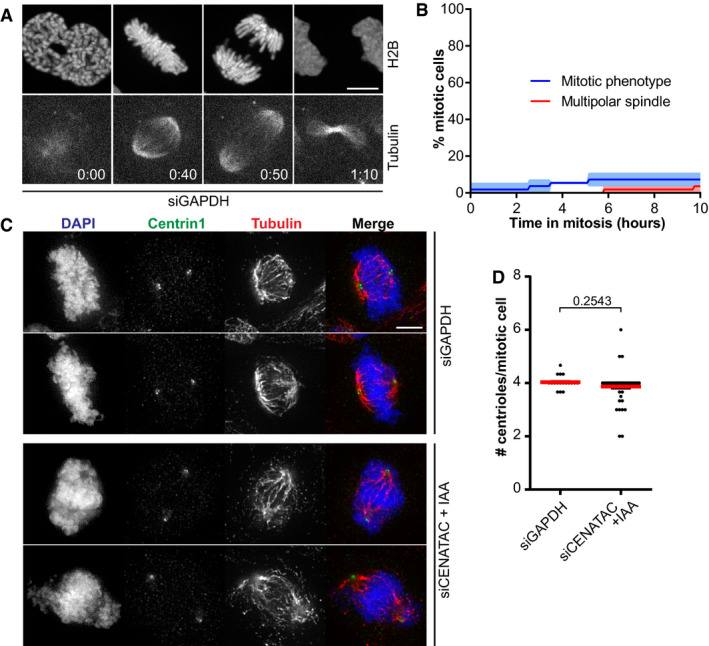
Live imaging of chromosome segregation in CENATAC mutant patient lymphoblasts stably expressing H2B‐mNeon (Yost et␣al, 2017) revealed a mild chromosomal instability phenotype, consistent with the modest levels of aneuploidy in blood cells of these patients (Figs 1A and 2A). To examine whether CENATAC patient mutations cause chromosomal instability, we expressed mutant CENATAC alleles in HeLa cells in which the endogenous loci were modified to express AID‐degron‐tagged CENATAC (HeLa EGFP‐AID‐CENATAC , Appendix␣Fig S3) (Nishimura et␣al, 2009). Efficient depletion of CENATAC through a combination of siRNA treatment and auxin addition caused chromosome congression defects and a subsequent mitotic arrest (Fig 2B and C, and Appendix␣Fig S3). This phenotype was fully rescued upon re‐expression of wild‐type but not MVA mutant CENATAC (Fig 2C and Appendix␣Figs S4 and S5), indicating that both MVA mutants are defective for CENATAC's function in mitotic chromosome congression. MVA mutant CENATAC caused a similar mitotic phenotype when expressed in near‐diploid DLD‐1 cells (Appendix␣Fig S6). CENATAC alleles missing either of the two most C‐terminal conserved motifs that are absent from MVA mutant CENATAC (Fig 1B, motifs 3 and 4) did not rescue the mitotic defects. Instead, the expression of the MVA or motif 3/4 mutants exacerbated the phenotype, suggesting that these proteins dominantly repressed the function of any residual wild‐type protein (Fig 2C). Mutations in the zinc fingers or deletion of motifs 1 or 2 only partly compromised CENATAC function (Figs 1B and 2C).
Figure 2. CENATAC promotes error‐free chromosome segregation.
-
AQuantification of chromosome segregation errors of patient and control lymphoblasts expressing H2B‐mNeon (four biological replicates, > 200 cells in total per condition).
-
BRepresentative images of H2B‐mNeon‐expressing HeLa EGFP‐AID‐CENATAC cells depleted of GAPDH (upper) or CENATAC (middle and lower) with or without re‐expression of CENATAC variants as indicated. IAA, 3‐indoleacetic acid. Scale bar, 10 μm.
-
CQuantification of mitotic defects as in (B) of H2B‐mNeon‐expressing HeLa EGFP‐AID‐CENATAC cells treated as indicated (three or five biological replicates, > 85 cells in total per condition). For 2xZF, the four zinc‐finger cysteines were mutated to alanines; for Δ1–4, the corresponding motif was removed.
-
DRepresentative stills of HeLa EGFP‐AID‐CENATAC cells expressing H2B‐mNeon and depleted of CENATAC. Microtubules were visualized with SiR‐Tubulin. Arrowheads and arrows indicate non‐congressed chromosomes and supernumerary spindle poles, respectively. Scale bar, 5 μm. Time in hours. See Fig EV2 for the control condition. See also Movies EV1 and EV2. IAA, 3‐indoleacetic acid.
-
EQuantification of the mitotic phenotype and multipolar spindle formation in time in H2B‐mNeon‐expressing HeLa EGFP‐AID‐CENATAC cells treated as in (D) (three biological replicates, > 44 cells in total per condition). See also Fig EV2.
Data information: In (A, C, E), data are presented as mean ± SEM. P‐values were calculated with unpaired Student's t‐tests.
Live imaging of HeLa EGFP‐AID‐CENATAC cells with fluorescently labeled chromatin and microtubules revealed that the chromosome congression defect upon CENATAC depletion preceded the previously described loss of spindle bipolarity (Figs 2D and E, and EV2A and B, Movies EV1 and EV2) (Wang et␣al, 2019). In addition, we did not observe centriole over‐duplication in CENATAC‐depleted cells (Fig EV2C and D). This is in contrast to what was recently reported for CENATAC knockout cells (Wang et␣al, 2019), raising the possibility that centriole over‐duplication is a cumulative effect of prolonged CENATAC loss. Our attempts to examine this failed, as we were unable to create CENATAC knockout cells, consistent with it being an essential human gene (Blomen et␣al, 2015; Hart et␣al, 2015; Wang et␣al, 2015). Taken together, these data show that CENATAC directly or indirectly promotes chromosome congression in mitosis (in a manner likely unrelated to its role in maintaining spindle bipolarity) and that MVA mutant CENATAC is a defective variant.
Figure EV2. CENATAC's congression phenotype is not the result of a multipolar mitotic spindle.
- A
-
BQuantification of the mitotic phenotype and multipolar spindle formation in time in cells treated as in (A) (three biological replicates, > 44 cells in total).
-
CRepresentative immunofluorescence images of HeLaEGFP‐AID‐CENATAC cells depleted of GAPDH or CENATAC and stained with antibodies against Centrin1 and Tubulin. IAA, 3‐indoleacetic acid. Scale bar, 5 μm.
-
DQuantification of the amount of centrioles per mitotic cell treated as in (C) (three biological replicates, > 60 cells in total).
Data information: In (B, D), data are presented as mean ± SEM. P‐values were calculated with unpaired Student's t‐tests.
CENATAC is a novel component of the minor spliceosome
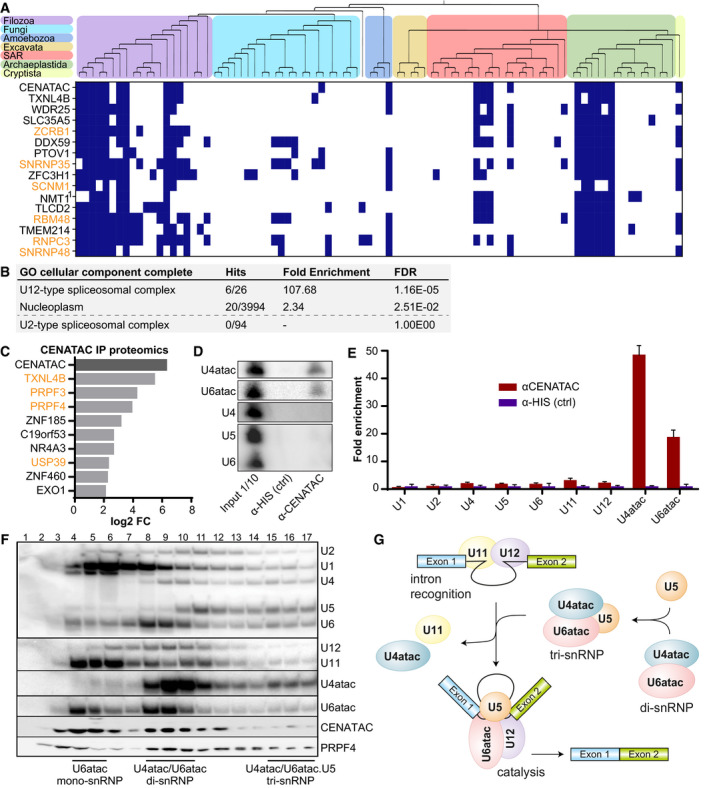
To investigate in which processes CENATAC plays a role, we performed a genome‐wide, evolutionary co‐occurrence analysis. Genes that function in the same biochemical process experience similar evolutionary pressures and therefore tend to co‐evolve, i.e., they are lost or retained in a coherent fashion (Pellegrini et␣al, 1999). Genomes from a set of 90 informative eukaryotic species (Table EV1) were mined for the presence or absence of CENATAC orthologs (Hooff et␣al, 2017). This provided a phylogenetic absence/presence profile that was used in an unbiased genome‐wide query for genes with similar phylogenetic profiles (Fig 3A). The resulting list of genes most strongly co‐occurring with CENATAC was significantly enriched for components of the minor (U12‐dependent) spliceosome complex, including the recently discovered SCNM1 (Bai et␣al, 2021) (Fig 3B, Table EV2). We thus reasoned that CENATAC may play a role in splicing by the minor spliceosome.
Figure 3. CENATAC is a novel component of the minor spliceosome.
-
APhylogenetic profiles (presences (blue) and absences (white)) of the top 15 genes co‐occurring with CENATAC in 90 eukaryotic species. Top: phylogenetic tree of the eukaryotic species (see Table EV1) with colored areas for the eukaryotic supergroups. 1For NMT1, no human ortholog was found and instead the Arabidopsis thaliana ortholog is depicted. Genes associated with the minor (U12‐dependent) spliceosome are depicted in orange. See also Table EV2.
-
BGO term analysis of the genes co‐occurring with CENATAC as in (A) with a correlation score of > 0.5. Note: The amount of hits and fold enrichment score were manually changed to accommodate the recently discovered SCNM1 (Bai et␣al, 2021).
-
CGraph of fold changes in proteins enriched (P‐value < 0.05) in proteomics analysis of CENATAC vs. control co‐immunoprecipitations of HeLa EGFP‐CENATAC cells (three biological replicates). Splicing factors are depicted in orange. See also Appendix␣Fig S7.
-
D, EExamples (D) and quantification (E) of Northern blot analyses of minor (U6atac, U4atac, U11, and U12) and major (U2, U1, U4, U5, and U6) spliceosome snRNAs in HeLa EGFP‐AID‐CENATAC cells (three biological replicates, normalized to the control). See also Appendix␣Fig S8.
-
FGlycerol gradient (10–30%) analysis of HeLa S3 nuclear extracts. snRNAs were detected by Northern blot analysis, proteins (CENATAC and PRPF4) by Western blot. Locations of the U6atac mono‐snRNP, U4atac/U6atac di‐snRNP, and U4atac/U6atac.U5 tri‐snRNP are indicated.
-
GSchematics showing key assembly stages in minor intron splicing and minor tri‐snRNP assembly: intron recognition (A complex) and the catalytic spliceosome (C complex). For simplicity, several stages of spliceosome assembly are omitted, such as the pre‐B complex, which consists of the intron recognition complex together with the tri‐snRNP before architectural changes lead to the exclusion of U11 to give rise to the B complex, after which subsequent architectural changes lead to the exclusion of U4atac to give rise to the BACT complex, which is a precursor stage for the catalytically active C complex depicted in this figure (Turunen et␣al, 2013b).
Data information: In (C), data are presented as fold change of the mean log 2‐transformed LFQ intensity. In (E), data are presented as mean ± SD.
As predicted by our co‐evolution analysis and in agreement with a previous high‐throughput screen (Hart et␣al, 2015; Hein et␣al, 2015; Huttlin et␣al, 2015, 2017), mass spectrometry analysis of proteins co‐purifying with CENATAC identified several known spliceosome components that are shared by both the major and minor spliceosomes (Fig 3C and Appendix␣Fig S7). Notably, the strongest CENATAC interactor (TXNL4B) was also the gene that showed the most significant co‐occurrence with CENATAC in eukaryotic species (Fig 3A). To determine whether CENATAC preferentially associates with major or minor spliceosome components, we analyzed CENATAC co‐immunoprecipitations by Northern blot analysis. This revealed a significant enrichment for the minor spliceosome‐specific U4atac and U6atac snRNAs (Fig 3D and E and Appendix␣Fig S8). CENATAC's association with the minor spliceosome was further supported by glycerol gradient analyses of HeLa nuclear extract preparations, which showed co‐migration of CENATAC with U6atac snRNP, U4atac/U6atac di‐snRNP, and U4atac/U6atac.U5 tri‐snRNP complexes (Fig 3F). Together, these data validate CENATAC as a bona fide functional component of the minor spliceosome and as the first identified protein component that is specific to the U4atac/U6atac and U4atac/U6atac.U5 snRNP complexes (Fig 3G).
The role of CENATAC in minor spliceosome function was further supported by the presence of evolutionarily conserved competing major (U2‐type) and minor (U12‐type) 5′ splice sequences (5′ss) in animals, that are predicted to generate productive and unproductive CENATAC mRNAs, respectively (Appendix␣Fig S9A). This configuration is indicative of an autoregulatory circuit that is conceptually similar to the previously reported autoregulation of the minor spliceosome proteins 48K and 65K (Verbeeren et␣al, 2010; Turunen et␣al, 2013b). In agreement with this, impaired minor spliceosome function, such as in Taybi–Linder syndrome (TALS/MOPD1, OMIM: 210710) patients, leads to a significant increase in the use of major 5′ss and upregulation of CENATAC mRNA levels (Cologne et␣al, 2019). Notably, evidence for a similar autoregulatory circuit is also present in plants (Appendix␣Fig S9B), where retention or splicing of a minor intron results in productive or unproductive CENATAC mRNA, respectively.
Minor intron splicing defects in CENATAC mutant cells correlate with mitotic defects
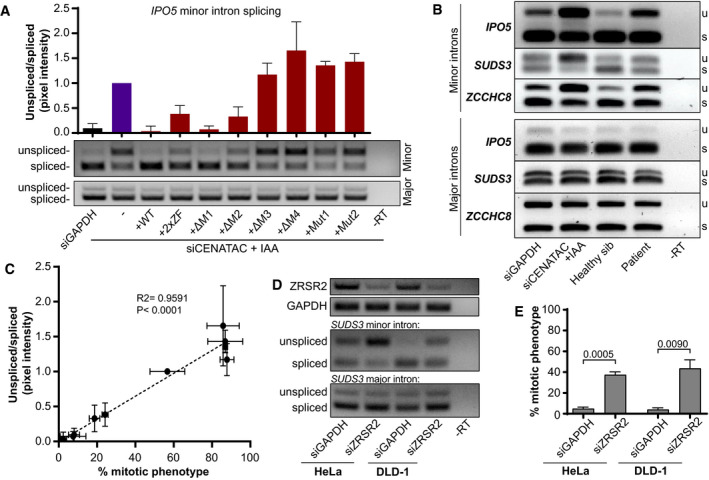
In agreement with our finding that CENATAC is a novel minor spliceosome component, splicing of several minor introns was impaired upon depletion of CENATAC in HeLa EGFP‐AID‐CENATAC cells, whereas up‐ or downstream major introns were unaffected (Fig 4A and B). This was true also for MVA patient cells and DLD‐1 cells expressing MVA mutant CENATAC (Fig 4B and Appendix␣Fig S10). Importantly, the splicing defect of minor introns in CENATAC‐depleted cells was fully rescued by re‐expression of wild‐type but not MVA mutant alleles (Fig 4A). Similar to the mitotic phenotype, the expression of the disease alleles and mutants lacking motifs 3 and 4 exacerbated the splicing defect, whereas mutations in the zinc fingers and removal of motifs 1 and 2 partially rescued it (Fig 4A and Appendix␣Fig S10). Notably, the extent of the splicing defect strongly correlated with the extent of the mitotic phenotype for all mutations (Fig 4C), supporting the possibility that impaired minor spliceosome function and the chromosome congression phenotype are causally linked. To further investigate this, we depleted ZRSR2, a component of the U11/U12 di‐snRNP functioning in 3′ss recognition of minor introns (see Fig 3G), in both HeLa EGFP‐AID‐CENATAC and DLD‐1 cells. Similar to the depletion of CENATAC, this caused significant minor IR (Fig 4D) and a chromosome congression defect (Fig 4E). We therefore consider it likely that the chromosome congression phenotype is a secondary effect of impaired minor spliceosome function.
Figure 4. Minor intron splicing defects in CENATAC mutant cells fully correlate with mitotic defects.
-
ART–PCR (middle panel) and quantification (upper panel) of IPO5 minor intron 21 (U12‐type) splicing on RNA extracted from HeLa EGFP‐AID‐CENATAC cells treated as in Fig 2C (three biological replicates, normalized to siCENATAC + IAA). The bottom panel shows RT–PCR analysis of IPO5 major intron 19 (U2‐type).
-
BRT–PCRs of IPO5, SUDS3, and ZCCHC8 minor and adjacent major introns on RNA extracted from patient lymphoblasts or HeLa EGFP‐AID‐CENATAC cells depleted of GAPDH or CENATAC as indicated. u, unspliced; s, spliced.
- C
-
DRT–PCRs of ZRSR2 and GAPDH (to visualize ZRSR2 knockdown efficiency, upper) and RT–PCRs of SUDS3 minor intron 7 (middle) and major intron 5 (lower) on RNA extracted from HeLa EGFP‐AID‐CENATAC or DLD‐1 cells depleted of GAPDH or ZRSR2 as indicated.
-
EQuantification of mitotic defects as in Appendix␣Fig S6 of H2B‐mNeon‐expressing HeLa EGFP‐AID‐CENATAC or DLD‐1 cells treated as in (D) (three biological replicates, > 103 cells in total per condition).
Data information: In (A, E), data are presented as mean ± SEM. P‐values were calculated with unpaired Student's t‐tests. In (C), the P and R 2 values were calculated with a linear regression analysis.
CENATAC promotes splicing of A‐type minor introns
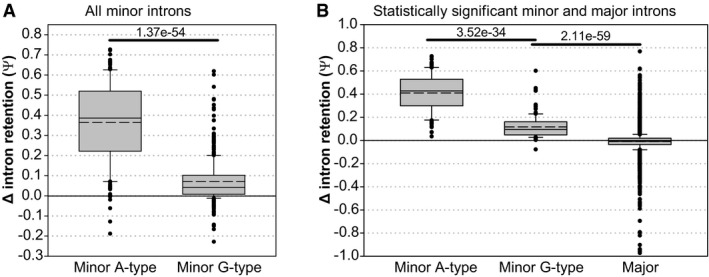
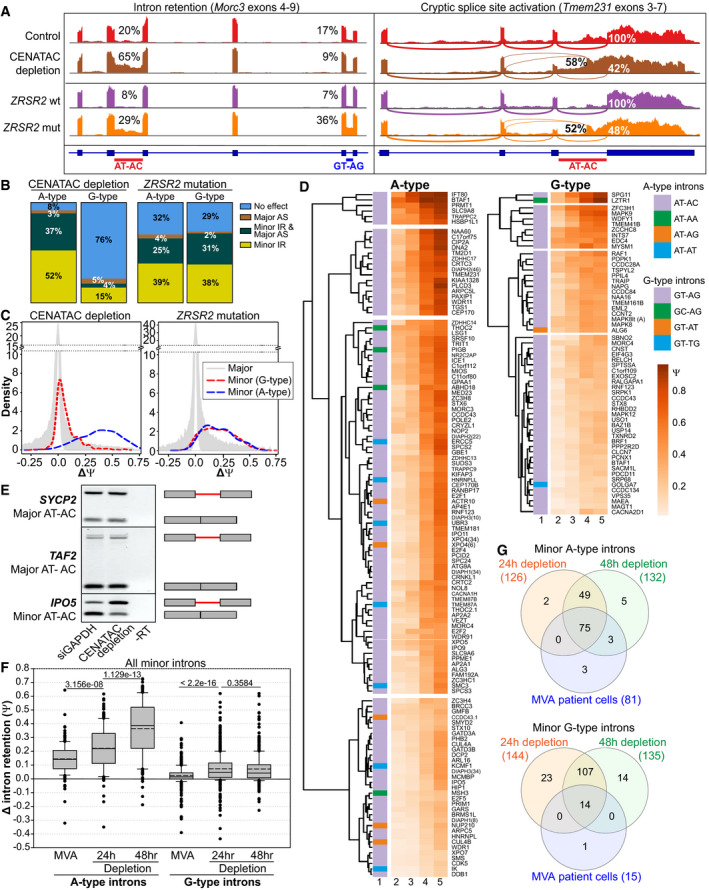
Our discovery of reduced minor spliceosome function in a constitutional aneuploidy syndrome raised the question of which introns and transcripts were affected by CENATAC malfunction. To investigate this, we compared the transcriptomes of CENATAC‐depleted HeLa EGFP‐AID‐CENATAC cells to those of control‐depleted and parental cell lines. The resulting RNAseq dataset was analyzed for changes in IR using IntEREst (Oghabian et␣al, 2018) and for alternative splicing (AS) using Whippet (Sterne‐Weiler et␣al, 2018). In agreement with our RT–PCR‐based observations (Fig 4), this analysis confirmed the significant retention of minor but not major introns after CENATAC depletion (Fig EV3). Surprisingly, it also uncovered a remarkable enrichment for a specific subclass of minor introns: While only 24% of G‐type introns (with GT‐AG, GT‐AT, GT‐TG, GC‐AG terminal dinucleotides) were affected by CENATAC depletion, virtually all (92%) of the A‐type introns (with AT‐AC 1 , AT‐AA, AT‐AG, or AT‐AT terminal dinucleotides) showed increased retention or activation of alternative major splice sites (cryptic or annotated), or both (Fig 5A and B, Appendix␣Fig S11, and Dataset EV1). For comparison, we carried out the same analysis on a previously published dataset derived from myelodysplastic syndrome (MDS) patients carrying somatic mutations in the gene encoding for the U11/12‐di‐snRNP subunit ZRSR2 (Madan et␣al, 2015). This dataset showed a nearly identical response for A‐ and G‐type introns (Fig 5B and Dataset EV2). Whereas depletion of CENATAC or mutations in␣ZRSR2 led to an average increase of approximately 36 and 19% in retention of A‐type introns, respectively (average ΔΨCENATAC = ˜ 0.36 and average ΔΨZRSR2 = ˜ 0.19), G‐type introns were only strongly affected by ZRSR2 mutations (average ΔΨCENATAC = ˜ 0.07 and average ΔΨ ZRSR2 = ˜ 0.19, Figs 5B–D and EV3). Importantly, the effect on A‐type introns was specific to minor introns as none of the 85 major AT‐AC introns or related subtypes responded to CENATAC depletion (Fig 5E and Dataset EV1). The same subtype‐specific effect on minor intron splicing was also observed in CENATAC mutant MVA patient lymphoblasts (Fig 5F, Appendix␣Fig S12A, and Dataset EV3), in which the affected introns correlated strongly with those affected by CENATAC depletion (Fig 5G, Appendix␣Fig S12B, and Dataset EV4). Notably, the strongly affected transcripts did not include any of the genes associated with MVA but did contain various mitotic regulators (Appendix␣Fig S13, Datasets EV1, EV5, and EV6).
Figure EV3. Comparison of delta‐psi values (HeLaEGFP‐AID‐CENATAC cells 48h CENATAC depletion vs. parental cell line 48h GAPDH depletion).
-
AComparison of all (statistically significant and not significant) minor A‐type (n = 179) and minor G‐type (n = 441) introns (three biological replicates).
-
BComparison of statistically significant minor A‐type introns (n = 133), minor G‐type introns (n = 130), and major introns (n = 8,818; three biological replicates). Only introns with on average at least 5 intron mapping reads were used in the analysis.
Data information: In (A, B), data are presented as median (solid line) and mean (dashed line) inside the boxes. The boundaries of the boxes indicate 25th and 75th percentiles. Whiskers indicate the 90th and 10th percentiles. P‐values were calculated with Mann–Whitney rank‐sum tests.
Figure 5. CENATAC promotes splicing of A‐type minor introns.
-
ASashimi plots showing the effect of CENATAC depletion (48hr, HeLa EGFP‐AID‐CENATAC cells) or ZRSR2 mutations (MDS patient cells) on AT‐AC and GT‐AG intron retention (left panel, Morc3), and cryptic splice site activation (right panel, Tmem231). CENATAC control represents the parental unedited HeLa cell line.
-
BTranscriptome‐wide statistics of CENATAC depletion (48hrs) and ZRSR2 mutations on G‐ and A‐type minor intron retention (U12 IR) and cryptic major splice site activation (U2 AS). Only introns showing at least 5 exon–exon junctions reads were included. For U12 IR, a statistical cutoff of P adj < 0.05 was used. For U2 AS, the probability cutoff of Pr > 0.9 was used.
-
CDensity plots showing differences in intron retention (ΔΨ) distribution after CENATAC depletion (48 h) or in samples with ZRSR2 mutations.
-
DHierarchical clustering of A‐ and G‐type intron retention in the unedited parental cell line treated with siGADPH for 48h (column 2), or in the HeLa EGFP‐AID‐CENATAC cell line treated with siGADPH for 48h (column 3) or with auxin and siCENATAC for 24 or 48 h (columns 4 and 5, respectively). Only introns showing a P adj < 0.05 and ΔΨ > 0.1 in either the 24‐h or 48‐h depletion sample were included in the analysis. The A‐type and G‐type intron terminal dinucleotide subtypes are indicated with different colors in the first column. In case the gene contained multiple introns of the same type, the intron number is indicated in parentheses.
-
ERT–PCR of major (U2‐type) AT‐AC introns in SYCP2 (intron 5) and TAF2 (intron 1), and minor (U12‐type) AT‐AC intron in IPO5 (intron 21) on RNA extracted from HeLa EGFP‐AID‐CENATAC cells depleted of GAPDH or CENATAC. Schematic representations of unspliced/spliced PCR products are depicted on the right.
-
FΔ_intron_retention values for the MVA patient cell dataset (compared with the healthy sibling) using all (significant and not significant) minor A‐type introns (n = 179 for the depletion and n = 177 for the MVA patient datasets) and minor G‐type introns (n = 441 for the depletion and n = 446 for the MVA patient datasets). Only introns with on average at least 5 intron mapping reads were used in the analysis. See also Appendix␣Fig S12.
-
GVenn diagram analysis of the MVA patient cell and HeLa EGFP‐AID‐CENATAC CENATAC depletion datasets. A‐ and G‐type minor introns showing statistically significant intron retention in each dataset were used. See also Dataset EV4.
Data information: In (F), data are presented as median (solid line) and mean (dashed line) inside the boxes. The boundaries of the boxes indicate 25th and 75th percentiles. Whiskers indicate the 90th and 10th percentiles. P‐values were calculated with two‐sided Mann–Whitney rank‐sum tests. The CENATAC depletion dataset consists of three biological replicates, the ZRSR2 mutation (MDS) dataset of eight biological replicates, and the MVA dataset of four biological replicates.
A‐type minor introns are spliced less efficiently
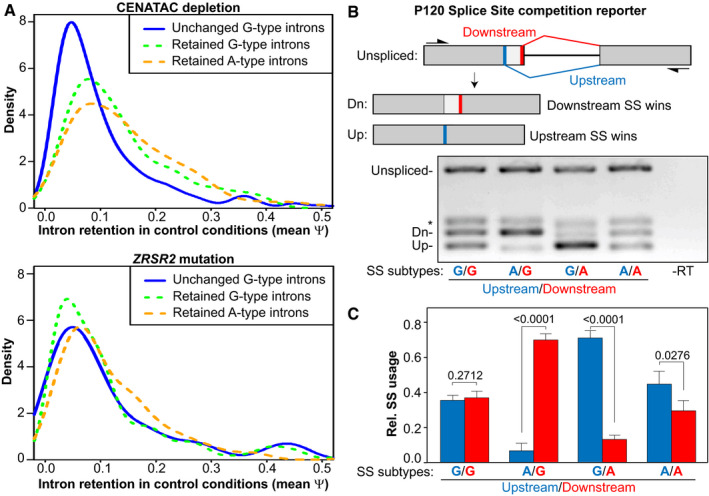
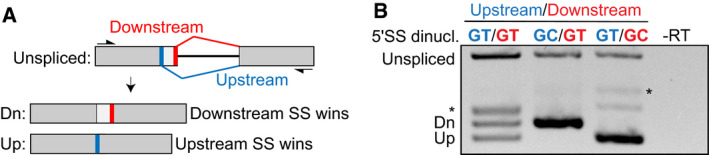
We next wished to understand the selectivity of CENATAC‐dependent splicing for A‐type minor introns. The observation that also some G‐type introns were affected by CENATAC depletion (Fig 5D) argued against a direct interaction between CENATAC and intron terminal nucleotides. Moreover, A‐ and G‐type introns that were strongly affected by CENATAC depletion had higher basal levels of IR in control conditions compared with those unaffected by CENATAC depletion (Fig 6A). This suggested that CENATAC predominantly promotes splicing of minor introns that are normally spliced less efficiently and that A‐type introns as a group belong to this category. To test this hypothesis, we engineered the widely used P120 minigene (Hall & Padgett, 1996) to contain two tandem competing A‐ or G‐type splice sites in all possible configurations (Fig 6B). Significantly, minor GT‐AG splice sites were strongly preferred over AT‐AC sites when in direct competition (Fig 6B and C), and they also outcompeted a unique GC‐AG splice site that was significantly affected by CENATAC depletion (Figs 5D and EV4, LZTR1). We thus conclude that CENATAC promotes splicing of minor introns that are recognized or spliced less efficiently, most prominently A‐type minor introns.
Figure 6. A‐type minor introns are spliced less efficiently.
-
ADensity plots showing intron retention (Ψ) values in the HeLa unedited parental control cell line (depleted of GAPDH) of A‐ and G‐type minor introns that were either unchanged or retained after CENATAC depletion (top) or ZRSR2 mutation (bottom). The median psi values of the retained G‐ and A‐type introns are significantly higher (Psi = 0.130 and Psi = 0.115, respectively; P < 0.01, Mann–Whitney rank‐sum test) compared with the unchanged introns (Psi = 0.081) in the CENATAC depletion dataset.
-
BRT–PCR P120 reporter assay (Hall & Padgett, 1996) to measure the relative usage of A‐type (AT‐AC) and G‐type (GT‐AG) 5′ splice sites in direct competition. Upper: schematic diagram showing the overall architecture of the reporter construct with its down‐ and upstream splice site (thick red and blue bars, respectively) and the products created by splicing (Dn and Up, respectively). Lower: RT–PCRs of the reporter with A‐ or G‐type splice sites in the down‐ or upstream positions as indicated below the gel. SS, splice site. *PCR product after use of a cryptic major splice site (not shown in the schematic).
-
CQuantification of relative splice site usage of A‐ and G‐type splice sites in (B) (three biological replicates).
Data information: In (C), data are presented as mean ± SEM. P‐values were calculated with unpaired Student's t‐tests. The CENATAC depletion and ZRSR2 mutation (MDS) datasets (panel (A)) consist of three and eight biological replicates, respectively.
Figure EV4. RT–PCR P120 reporter assay to measure the relative usage of GT‐AG and GC‐AG G‐type minor splice sites in direct competition.
-
ASchematic diagram showing the overall architecture of the reporter construct with its down‐ and upstream splice site (thick red and blue bars, respectively) and the products created by splicing (Dn and Up, respectively). SS, splice site.
-
BRT–PCRs of the reporter with GT‐AG or GC‐AG splice sites in the down‐ or upstream positions as indicated above the gel. 5′SS dinucl., 5′SS splice site dinucleotides. *PCR product after use of a cryptic major splice site (not shown in the schematic).
Discussion
In this work, we have uncovered a novel link between the minor spliceosome and defects in chromosome segregation in human cells. Using patient exome sequencing, evolutionary co‐occurrence analysis, and biochemistry, we identified CENATAC as a novel protein component of the U4atac/U6atac di‐snRNP and U4atac/U6atac.U5 tri‐snRNP complexes that are necessary for the formation of the catalytically active minor spliceosome. Our RNAseq analyses of CENATAC‐mutant MVA patient cells and CENATAC‐depleted HeLa EGFP‐AID‐CENATAC cells revealed widespread defects in minor intron splicing, particularly IR, but also cryptic splice site activation, indicating that CENATAC is required for proper functioning of the minor spliceosome. Unexpectedly, IR in CENATAC‐depleted cells was strongly biased for A‐type minor introns, which is a subtype that is defined by AT‐AN dinucleotide splice sites. This intron subtype‐specific function is unique among the minor spliceosome components and correlated tightly with mitotic fidelity. Furthermore, depletion of the minor spliceosome component ZRSR2 likewise caused a chromosome congression defect. Minor intron subtype mis‐splicing is therefore likely responsible, possibly in conjunction with centriolar defects (Wang et␣al, 2019), for the inefficient chromosome congression in CENATAC‐mutant cells and for the aneuploidies observed in the two MVA siblings described in this study.
The minor spliceosome was originally thought to splice only introns with AT‐AC termini (Hall & Padgett, 1994, 1996; Tarn & Steitz, 1996). Only later, it was shown that there are also major AT‐AC introns and that most of the minor introns in fact have GT‐AG termini (Dietrich et␣al, 1997; Sharp & Burge, 1997; Wu & Krainer, 1997). Additionally, minor introns have infrequent variations in the 3′ terminal nucleotide (Levine & Durbin, 2001; Dietrich et␣al, 2005), thus giving rise to the AT‐AN and GT‐AN classes of minor introns, here referred to as A‐ and G‐type introns, respectively. Significantly, in all known minor spliceosome diseases for which comprehensive transcriptome data are available, splicing defects are roughly uniformly distributed between the A‐ and G‐type introns (Argente et␣al, 2014; Madan et␣al, 2015; Merico et␣al, 2015; Cologne et␣al, 2019) (Fig 5A–C, ZRSR2 mutation). The selective A‐type IR phenotype of CENATAC can therefore not solely be explained by a general loss of minor spliceosome function but instead suggests that CENATAC has a unique function in promoting the splicing of A‐type minor introns. Nonetheless, our observation that also a subset of G‐type introns was retained upon CENATAC depletion renders it unlikely that CENATAC directly recognizes the 5′ adenosine of A‐type introns. Instead, our competition data, and the observed elevated IR baseline of the affected introns, suggest that a subset of minor introns with reduced intrinsic splicing activity (consisting not only of A‐type introns but also of a subset of G‐type introns) may be particularly dependent upon CENATAC activity.
What could be the molecular function of CENATAC? Given its participation in di‐ and tri‐snRNP complexes and potential selectivity for 5′ss identity, CENATAC may function during or after the transition from initial intron recognition (A complex) to pre‐catalytic spliceosome (B complex; Fig 3G) and may for instance participate in 5′ss recognition analogous to U11‐48K protein in the A complex (Turunen et␣al, 2008). Of particular interest are the two well‐conserved C‐terminal motifs (M3 and M4), whose deletion caused severe impairment of minor intron splicing (Figs 1 and 4). Although we have not been able to uncover their function based on sequence similarity to other motifs, our data argue they are crucial for CENATAC's role in minor intron splicing. Detailed mechanistic understanding will require cryo‐EM structures of relevant minor spliceosome assembly stages. Unlike the major spliceosome, of which high‐resolution structures are available throughout the entire spliceosome assembly/disassembly cycle (Wilkinson et␣al, 2020), of the minor spliceosome only a single high‐resolution cryo‐EM structure is available of the catalytically activated form (BACT complex) (Bai et␣al, 2021). This structure does not contain CENATAC, nor its main interactor TXNL4B (Fig 3). Even though the major spliceosome does not carry an obvious functional analog of the CENATAC protein, the major spliceosome TXNL4A/Dim1 protein is a paralog of TXNL4B, both at sequence and structural levels (Jin et␣al, 2013). High‐resolution structures of both yeast and human spliceosomal B complexes have placed TXNL4A/Dim1 in close proximity of the 5′ss and suggested a role in 5′ss recognition (Wan et␣al, 2016; Bertram et␣al, 2017). Assuming that the minor spliceosome B complex shares the molecular architecture with its major spliceosome counterpart, this could place CENATAC with TXNL4B near the 5′ss to participate in the recognition event. Furthermore, as both proteomics and structural work have shown that TXNL4A/Dim1 is released from the major spliceosome during the transition from B to BACT complex (Schmidt et␣al, 2014; Bertram et␣al, 2017; Haselbach et␣al, 2018), it is possible that both TXNL4B and CENATAC may similarly detach from the minor spliceosome prior to BACT complex formation.
Given that A‐type minor intron host genes and the locations of these introns within the host gene are evolutionarily highly conserved among metazoan species, the selectivity of CENATAC in splicing raises the possibility that minor intron subtypes are part of a conserved but unexplored regulatory mechanism for gene expression. CENATAC undergoes reversible modifications (acetylation and phosphorylation) (Wang et␣al, 2019), which may provide the means to regulate its activity (also) in the minor spliceosome.
Presently, all mutations associated with MVA have been mapped to genes that are known regulators of chromosome segregation. Our discovery of disease‐causing mutations in CENATAC extends this list for the first time with a mRNA splicing factor. Although a recent study showed that CENATAC regulates centriole duplication (Wang et␣al, 2019), we were unable to verify this. Instead, our data argue that chromosomal instability by CENATAC malfunction may instead be the result of a primary defect in splicing of A‐type minor introns. Nevertheless, it remains possible that CENATAC can also promote high‐fidelity chromosome segregation more directly, as has been suggested for several other proteins involved in splicing (Montembault et␣al, 2007; Pellacani et␣al, 2018; Somma et␣al, 2020).
Strikingly, the clinical phenotype of CENATAC mutant MVA strongly resembles that of MOPD1/TALS, Roifman and Lowry–Wood syndromes, which are caused by mutations in the U4atac snRNA component of the minor spliceosome. Patients with these syndromes likewise present with microcephaly, developmental delay, and retinal abnormalities (Farach et␣al, 2018). No aneuploidies were reported (Hallermayr et␣al, 2018; Wang et␣al, 2018), but karyotype analyses were not performed for the majority of patients. It will therefore be of interest to examine whether aneuploidies occur in some of these patients, and whether (and to what extent) the affected transcripts and the splicing defect differ between MVA and these syndromes.
Although the depletion of both ZRSR2 and CENATAC caused a chromosome congression defect in mitosis (Fig 4), patients with ZRSR2 mutations are clinically different from MVA patients with CENATAC mutations. This difference is most likely related to differences in their splice targets, such as the G‐type minor introns that are differentially affected by depletion of CENATAC vs ZRSR2 (Fig 5). Mutations in ZRSR2 are associated with MDS and clonal cytopenias of unknown significance (CCUS) (Madan et␣al, 2015; Fleischman et␣al, 2017). In line with ZRSR2's mitotic phenotype, various stable aneuploidies were observed in MDS and CCUS patients with ZRSR2 mutations (Madan et␣al, 2015; Fleischman et␣al, 2017; Hosono, 2019), though it is unclear whether mitotic defects contribute to these disease phenotypes. It would be of interest to investigate whether mitotic defects negatively impact erythropoiesis in these patients.
Materials and Methods
Samples
The MVA exome analyses were approved by the London Multicentre Research Ethics Committee (05/MRE02/17). Appropriate consent was obtained from patients and/or parents as applicable and the experiments conformed to the principles set out in the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report. DNA was extracted from whole blood using standard protocols. RNA was extracted from EBV‐transformed lymphoblastoid cell lines (LCLs) using the RNeasy Mini Kit protocol (Qiagen).
For the functional experiments, the following patient LCLs were used: ID_5728_1 (patient, biallelic CENATAC (CCDC84) mutations, ECACC ID: FACT5728DLB), ID_5728_3 (sibling, no CENATAC mutations, ECACC ID: FACT5728KC), ID_5728_4 (father, monoallelic CENATAC mutation, ECACC ID: FACT5728GLB), and ID_5728_5 (mother, monoallelic CENATAC mutation, ECACC ID: FACT5728ALB).
Lymphoblastoid cell lines were cultured in RPMI supplemented with 15% fetal bovine serum (FBS), 100 μg/ml penicillin/streptomycin, and 2 mM alanyl glutamine. Cells expressing H2B‐mNeon were created by lentiviral transduction, using standard procedures. Imaging of LCLs was performed as previously described (Yost et␣al, 2017).
Exome sequencing, alignment and variant calling, reference data sets, PTV prioritization method, recessive analysis, and Sanger sequencing: as previously described (Yost et␣al, 2017).
cDNA analysis of CENATAC (CCDC84) mutations
We synthesized cDNA using the ThermoScript RT–PCR System (Life Technologies) with random hexamers and 1 μg of total RNA. We amplified the mutation regions using cDNA‐specific primers and sequenced the PCR products as described above. Primer sequences are available on request.
Conservation logos
Hidden Markov model (HMM) profiles were created from iterative jackhmmer searches (Potter et␣al, 2018) (version: HMMER3/f [3.1b2 | January 2014]) with CENATAC's protein sequence against the sequences of all metazoan species within the UniProt database. In‐between successive iterations, non‐CENATAC sequences were manually removed. Logos were created using Skylign (Wheeler et␣al, 2014); letter height: information content above background.
Immunoblots
For Western blot samples, cells were treated as indicated and lysed in Laemmli lysis buffer (4% SDS, 120 mM Tris pH 6.8, and 20% glycerol). Lysates were processed for SDS–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Immunoblotting was performed using standard protocols. Visualization of signals was performed on an Amersham Imager 600 scanner using enhanced chemiluminescence. Primary antibodies used were rabbit anti‐CENATAC (CCDC84; Sigma, HPA071715) and mouse anti‐Tubulin (Sigma; T5168). Secondary antibodies used were goat anti‐mouse HRP (170‐6516) and goat anti‐rabbit HRP (170‐6515), both obtained from Bio‐Rad.
Cell culture
HeLa T‐REx Flp‐In osTIR‐9Myc::NEO cells (gift from Andrew Holland) were cultured in DMEM high glucose supplemented with 10% Tet‐approved FBS, 100 μg/ml penicillin/streptomycin, and 2 mM alanyl glutamine. DLD‐1 cells (ATCC CCL‐221) were cultured in DMEM/F‐12 supplemented with 10% Tet‐approved FBS, 100 μg/ml penicillin/streptomycin, and 2 mM alanyl glutamine. HeLa S3 cells (a kind gift from Dr. Joan Steitz) were cultured in suspension in 1640 RPMI supplemented with 10% FBS, 2 mM glutamine, and 100 μg/ml penicillin/streptomycin. Stable expression of H2B‐mNeon was done by lentiviral transduction using standard procedures. All cell lines were regularly tested and at all times found to be mycoplasma‐free.
Creation of HeLa EGFP‐AID‐CENATAC and HeLa EGFP ‐CENATAC cell lines
HeLa EGFP‐AID‐CENATAC and HeLa EGFP‐CENATAC cell lines were derived from HeLa T‐REx Flp‐In osTIR‐9Myc::NEO and HeLa T‐REx Flp‐In, respectively. Tagging of the endogenous locus of CENATAC was done according to the scCRISPR protocol (Arbab et␣al, 2015) using the Protospacer, HDR_insert, and HDR_ext primers in Table EV3. pcDNA5‐FRT‐TO‐EGFP‐AID (Addgene, 80075) was used as template for both the EGFP‐AID and EGFP tags. Cells were transfected with Lipofectamine LTX using standard procedures and subsequently FACS‐sorted (single cells) based on EGFP expression. Endogenous tagging was confirmed by PCR (using the Genomic primers, Appendix␣Fig S3A) and immunoblotting of CENATAC protein (Appendix␣Fig S3B and C).
Viral plasmids, cloning, and viral production
For lentiviral re‐expression of CENATAC variants, first pcDNA5 PURO FRT TO EGFP‐AID‐CENATAC was created by cloning CENATAC cDNA derived from HeLa cells into empty pcDNA5‐FRT‐TO‐EGFP‐AID (Addgene, 80075) using the cDNA PCR primers in Table EV3 and digestion of both the PCR product and the plasmid with NotI/ApaI. The CENATAC cDNA was subsequently cloned into pcDNA5 PURO FRT TO containing a LAP‐tag to create pcDNA5 PURO FRT TO LAP‐CENATAC by Gibson assembly (Gibson et␣al, 2009) with the PCR primers Gibson1 and Gibson2. Mutagenesis was then performed to make this construct resistant to CENATAC siRNA treatment (CCDC84; Dharmacon, J‐027240‐07) by Gibson assembly with PCR primers Gibson3. Next, in the siRNA‐resistant construct, CENATAC wild‐type cDNA was mutated to Mut1 (primers Gibson4), Mut2 (Gibson5), 2xZF (Gibson6; two consecutive rounds of cloning), Δ1 (Gibson7), Δ2 (Gibson8), Δ3 (Gibson9), or Δ4 (Gibson10) by Gibson assembly. Lentiviral CENATAC iresRFP constructs were derived from a lentiviral construct encoding fluorescently tagged histone 2B (H2B) and a puromycin‐resistant cassette (pLV‐H2B‐mNeon‐ires‐Puro) (Drost et␣al, 2015). First, the fluorescently tagged H2B was substituted by CENATAC derived from pcDNA5 PURO FRT TO LAP‐CENATAC (see above) by Gibson assembly with PCR primers Gibson11 and digestion by AscI/NheI. Next, the puromycin‐resistant cassette was substituted by tagRFP by Gibson assembly with PCR primers Gibson12. Finally, all siRNA‐resistant variants of CENATAC were cloned from their respective pcDNA5 PURO FRT TO LAP‐CENATAC plasmids into pLV CENATAC ires‐tagRFP by Gibson assembly with PCR primers Gibson13 and PstI digestion of the plasmid. Virions were generated by transient transfection of HEK 293T cells with the transfer vector and separate plasmids that express Gag‐Pol, Rev, Tat, and VSV‐G. Supernatants were clarified by filtration.
Immunoprecipitation
For each sample, a full 10‐cm plate of HeLa EGFP‐AID‐CENATAC cells was used, treated as indicated (Appendix␣Fig S3C). The cells were lysed in ice‐cold lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 2% NP‐40, 0.1% deoxycholate, proteasome inhibitors) and treated with benzonase for 15 min at 4°C. After centrifugation, the supernatant was incubated with beads (GFP‐Trap, Chromotek) for 2.5 h at 4°C and washed three times with ice‐cold lysis buffer. The samples were finally eluted in Laemmli sample buffer.
Live cell imaging analysis of mitotic fidelity
Lymphoblastoid cell lines were imaged as previously described (Yost et␣al, 2017). siRNA transfections (RNAiMAX, Thermo Fisher) in HeLa EGFP‐AID‐CENATAC (40 nM siRNA) and DLD‐1 cells (50 nM siRNA) were done against CENATAC (CCDC84; Dharmacon, J‐027240‐07), GAPDH (Dharmacon, D‐001830‐01‐05), or ZRSR2 (Sigma, SASI_Hs02_00338940). In the case of CENATAC depletion in HeLa EGFP‐AID‐CENATAC cells, transfections were done in the presence of 1 mM 3‐indoleacetic acid (IAA) or ethanol (IAA vehicle) for 24 h in a 24‐well plate before the cells were re‐plated to eight‐well ibidi μ‐slides with 2 mM thymidine (for early S‐phase synchronization) and 100 μl lentivirus for CENATAC re‐expression. After 18 h, the cells were released from thymidine for 6 h and imaged in CO2‐independent medium in a heated chamber (37°C), while air‐tight‐sealed in the well plate with parafilm. These cells were therefore imaged ˜ 48 h after siRNA‐mediated knockdown of CENATAC and ˜ 24 h after lentivirus addition. For CENATAC depletion and re‐expression in DLD‐1 cells, the lentivirus (150 μl) was immediately added together with the siRNA treatment (instead of 24 h later together with the 2 mM thymidine). These cells were therefore imaged ˜ 48 h after siRNA‐mediated knockdown of CENATAC and ˜ 48 h after lentivirus addition. For the experiments in Figs 2D and EV2A, the cells were additionally incubated with 200 nM SiR‐tubulin dye (Spirochrome) for 6 h prior to imaging to facilitate visualization of the mitotic spindle. For the depletion of ZRSR2 in both HeLa EGFP‐AID‐CENATAC and DLD‐1 cells, the cells were re‐plated to 8‐well ibidi μ‐slides with 2 mM thymidine 48 h (instead of 24 h) after transfection and therefore imaged ˜ 72 h after siRNA‐mediated knockdown of ZRSR2. Images were acquired every 3 or 5 min at 1 × 1 binning in 7 × 2.5 μm z‐stacks (RFP as in Appendix␣Figs S4 and S6 was imaged in only 1 z‐stack per position) and projected to a single layer by maximum intensity projection using NIS‐Elements Software 4.45. Imaging was performed with a Nikon Ti‐Eclipse wide‐field microscope equipped with an Andor Zyla 4.2 sCMOS Camera, 40× oil objective NA 1.3 WD 0.2 mm, and Lumencor SPECTRA X light engine. Analysis of these experiments was carried out with ImageJ software. When applicable, cells re‐expressing CENATAC variants were identified through co‐expression of cytosolic RFP (via ires‐tagRFP); RFP‐negative cells were omitted from the quantifications (Appendix␣Figs S4 and S6).
Immunofluorescence imaging
After treating the cells with siRNAs and IAA (see above) for 24 h in a 24‐well plate, the cells were re‐plated on round 12‐mm coverslips and treated with 2 mM thymidine (for early S‐phase synchronization) for 24 h. 10 h after release, MG132 was added for 45 min after which the cells were pre‐extracted with 0.1% Triton X‐100 in PEM (100 mM PIPES pH 6.8, 1 mM MgCl2, and 5 mM EGTA) for ± 60 s. After 60 s, 4% paraformaldehyde was added on top of the PEM in a 1:1 ratio (400 μl each) for 20 min to fixate the cells. The coverslips were subsequently washed twice with PBS and blocked with 3% BSA in PBS for 16 h at 4°C, incubated with primary antibodies for 2 h at room temperature, washed three times with PBS containing 0.1% Triton X‐100, and incubated with secondary antibodies for 1 h at room temperature. Coverslips were then washed four times with PBS/0.1% Triton X‐100 and mounted using ProLong Gold Antifade with DAPI (Molecular Probes). All images were acquired on a deconvolution system (DeltaVision Elite; Applied Precision/GE Healthcare) equipped with a 100×/1.40 NA UPlanSAPO objective (Olympus) using Softworx 6.0 software (Applied Precision/GE Healthcare). The images are maximum intensity projections of deconvoluted stacks. Random pro‐metaphase and metaphase cells were selected, and centrioles were counted by hand. Primary antibodies used were rabbit anti‐Centrin1 (Abcam, ab101332, 1/500) and mouse anti‐Tubulin (Sigma, T5168, 1/10,000). Secondary antibodies used were goat anti‐mouse 647 (A21236) and goat anti‐rabbit 568 (A11036), both obtained from Thermo Fisher.
Co‐evolution analysis
First, a phylogenetically diverse set of complete eukaryotic‐predicted proteomes was utilized. This set was previously compiled to contain the protein sequences of 90 eukaryotic species (Hooff et␣al, 2017; preprint: van Wijk & Snel, 2020). These species were selected based on their representation of eukaryotic diversity. If available, we selected two species per clade and model organisms were preferred over other species. If multiple proteomes or proteomes of different strains were available, the most complete proteome was selected. When multiple splicing variants of a single gene were annotated, the longest protein was chosen. A unique protein identifier was assigned to each protein, consisting of four letters and six numbers. The letters combine the first letter of the genus name with the first three letters of the species name. The versions and sources of the selected proteomes can be found in Table EV1.
To define phylogenetic profiles for all human proteins, we determined automatic orthologous groups (OG) across the database using information from PANTHER 9.0 (Mi et␣al, 2016). PANTHER 9.0 contains 85 genomes within total of 1,136,213 genes. Of these genes, 759,627 genes are in PANTHER families with phylogenetic trees, multiple sequence alignments, and HMM profiles. In total, there are 7,180 PANTHER families and 52,768 subfamilies. Families are groups of evolutionary‐related proteins and subfamilies are related proteins that are likely to have the same function. The division into subfamilies is done manually, by biological experts. Every subfamily of PANTHER is an OG at some taxonomic level in the tree of life. We used “hmmscan” tool from the HMMER package (Potter et␣al, 2018) (HMMER 3.1b1) to find for each protein sequence in our database, the best matching profile of a main family or subfamily in PANTHER9.0. The phylogenetic profile of panther main or subfamily was subsequently defined by utilizing the hierarchical nature of the panther classification. Specifically, the phylogenetic profile of a main or subfamily also includes all members of daughter families (and if relevant their daughter families, etc.). Note that due to the automatic nature of orthology definition and the draft quality of a few genomes, phylogenetic profiles of the human proteins are not as accurate as those defined by manual analysis (van Hooff et␣al, 2019).
To determine the phylogenetic profile similarity, Pearson's correlation (https://en.wikipedia.org/wiki/Phi_coefficient) was computed between the phylogenetic profile of the CENATAC panther (PTHR31198) and the phylogenetic profile of all other panther sub‐ and main families using in‐house scripts. To detect functional patterns in orthologous groups with similar phylogenetic profiles (correlation > 0.5), a GO enrichment analysis was performed (Ashburner et␣al, 2000; Carbon et␣al, 2019; Mi et␣al, 2019). GO cellular component overrepresentation (GO Ontology database: released 2020‐01‐03) was computed using PANTHER (test release 2019‐07‐11) with the human reference genome gene set as background. Statistical significance of overrepresented GO terms was computed using Fisher's exact test with FDR correction.
Nuclear extract and GFP pull‐down and mass spectrometry
Nuclear extract of wild‐type and HeLa EGFP ‐CENATAC cells was prepared as described earlier (Kloet et␣al, 2016). In short, cells were harvested by trypsinization and resuspended in cold hypotonic buffer (10 mM HEPES KOH pH 7.9, 1.5 mM MgCl2, 10 mM KCl). Afterward, the cell pellet was homogenized using a Douncer with type B pestle (tight) to lyse the cell membrane. After centrifuging, the nuclei were washed with cold PBS and resuspended in cold buffer for lysis (420 mM NaCl, 20 mM HEPES KOH pH 7.9, 20% v/v glycerol, 2 mM MgCl2, 0.2 mM EDTA) followed by rotation, centrifugation, and collection of the nuclear extract. 450 μl of nuclear extract was used for each GFP pull‐down using 15 μl slurry of GFP‐Trap agarose beads (Chromotek), performed in triplicate. GFP pull‐downs were done as described earlier (Smits et␣al, 2013), without the addition of EtBr during the incubation, and with an adapted buffer C (150 mM NaCl, 20 mM HEPES KOH pH 7.9, 20 % v/v glycerol, 2 mM MgCl2, 0.2 mM EDTA, complete protease inhibitors w/o EDTA, 0.5 mM DTT) for the incubation (+0.1% NP‐40) and washes (+0.5% NP‐40). Samples were digested using on‐bead digestion with trypsin overnight (Hubner & Mann, 2011). The tryptic peptides were acidified with TFA and purified on C18 StageTips (Rappsilber et␣al, 2007).
After elution from the C18 StageTips, tryptic peptides were separated on an Easy‐nLC 1000 (Thermo Scientific), connected online to a Q Exactive HF‐X Hybrid Quadrupole‐Orbitrap Mass Spectrometer (Thermo Scientific), using an acetonitrile gradient of 7–30% for 48 min followed by washes of 50–90% acetonitrile, for 60 min of total data collection. Full scans were measured with a resolution of 120,000, and the top twenty most intense precursor ions were selected for fragmentation with a resolution of 15,000 and dynamic exclusion set at 30 s. Peptides were searched against the UniProt human proteome (downloaded June 2017) using MaxQuant (Cox & Mann, 2008) (version 1.6.0.1) with default settings, and iBAQ, LFQ, and match‐between‐runs enabled. Data analysis was done using Perseus (version 1.5.5.3), and the volcano plot and stoichiometry calculations were done as described earlier (Smits et␣al, 2013) using in‐house‐made scripts for R (version 3.6.1).
Nuclear extract preparations for Northern blots
Nuclear extract from HeLa S3 suspension cells was prepared according to the protocol described by Dignam et␣al (1983) using buffer D containing 50 mM KCL in the final dialysis step.
Immunoprecipitation and Northern blots
100 µl nuclear extract diluted in lysis buffer to a final volume of 200 µl was incubated with 2 µg of anti‐CCDC84 antibody (SIGMA‐HPA071715) overnight in the cold room with end‐to‐end rotation. The following day capture of antibody–antigen complexes was done using 50 µl of resuspended Protein G Dynabeads prepared according to manufacturer's instructions and incubated with the nuclear extract antibody samples for 2 h at 4°C. Beads were then washed four times with lysis buffer lacking protease and RNase inhibitors. RNA was eluted by proteinase K treatment, extracted once with phenol:chloroform:isoamyl alcohol (25:24:1; pH 4.8) followed by ethanol precipitation. RNA was dissolved in H2O or 0.1X TE buffer.
Total volumes of 2 µl (input) and 5 µl (IP) RNA samples were separated on a 6% polyacrylamide–urea gel and analyzed by Northern blotting essentially as described by Tarn and Steitz (1996). Individual snRNAs were detected using 32P 5′‐end‐labeled DNA or LNA oligonucleotides complementary to individual snRNAs. Northern blots were exposed to image plates and visualized using Typhoon FLA‐9400 Scanner (GE Healthcare, USA) at 50‐micron resolution. The data were quantified using AIDA Software (Raytest, Germany).
Glycerol gradient and ultracentrifugation
HeLa S3 nuclear extracts were preincubated for 0–20 min at +30°C in a buffer containing 13 mM HEPES (pH 7.9), 2.4 mM MgCl2, 20 mM creatine phosphate, 2 mM DTT, 40 mM KCl, and 0.5 mM ATP. Aggregates were subsequently removed by a brief centrifugation (20,000 g, 1 min, +4°C), and the supernatant was subsequently ultracentrifuged on a linear 10–30% glycerol gradient (20 mM HEPES, pH 7.9; 40 mM KCl, 2 mM DTT, 2.4 mM MgCl2) for 18 h at 29,000 rpm, +4°C, Sorvall TH641 rotor (RCF(max) = 143,915.6 g). Following ultracentrifugation, the samples were fractionated. 20% of each fraction was deproteinized and used for RNA isolation and Northern blotting and the remaining 80% was subjected to TCA precipitation, separated on a 10% SDS–PAGE, and analyzed by Western blots. Each blot was probed for CENATAC (CCDC84‐HPA071715; Sigma‐Aldrich–Merck), PRPF4 (#HPA0221794, Sigma‐Aldrich–Merck).
RT–PCRs
For Figs 4A and 4D, and Appendix␣Fig S10: Total cellular RNA was extracted using the RNeasy Kit Protocol (Qiagen) and treated with DNase I amplification grade (Invitrogen) to remove potential genomic DNA contamination. cDNA synthesis was carried out using SuperScript™ II RT (Thermo Fisher Scientific) and Oligo(dT)18 primers. PCRs were performed with Phusion High‐Fidelity DNA Polymerase (Thermo Fisher Scientific) with the following cycling conditions: initial denaturation (98°C for 60 s), followed by 28–30 cycles of denaturing (98°C for 10 s), annealing (gene‐specific temp. for 30 s), extension (72°C for 15–20 s), and a final extension (72°C for 1 min 30 s). PCR primers and relevant annealing temperatures are listed in Table EV3. PCR products were analyzed on 2% agarose gel run using 1X TBE buffer. For Figs 4B and 5E, total RNA isolated was isolated from HeLa cells or patient/control subject lymphoblasts using TRIzol extraction followed by an additional acidic phenol (pH 5.0) extraction. 1 µg of RNA was converted to cDNA using maxima H minus reverse transcriptase (Thermo Fisher) according to the manufacturer's protocol. PCRs were performed essentially as described above, and gene‐specific primers and annealing temperatures are listed in Table EV3.
RNA isolation and high‐throughput sequencing
Total RNA isolated was isolated from HeLa EGFP‐AID‐CENATAC cells treated with siGAPDH (Dharmacon, D‐001830‐01‐05) for 48 h or with siCENATAC (CCDC84, Dharmacon, J‐027240‐07) and 1 mM 3‐indoleacetic acid (IAA) for 24 or 48 h, or unedited HeLa parental cells treated with siGAPDH for 48 h, or patient/control subject lymphoblasts using TRIzol extraction followed by an additional acidic phenol (pH 5.0) extraction. RNAseq libraries were constructed using Illumina TruSeq Stranded Total RNA Kit (Illumina) Human Ribo‐Zero rRNA Depletion Kit (Illumina). Paired‐end 150 + 150 bp sequencing was done with Illumina NextSeq 500/550 High Output Kit v2.5 for HeLa samples and with Illumina NovaSeq 6000 using partial S4 flow cell lane for patient samples.
Mapping the reads to the genome
The STAR aligner (Dobin et␣al, 2013) was used for mapping the paired sequence reads to the genome (hg38/GRCh38). Transcript annotations were obtained from GENCODE (v29). The length of genomic sequence flanking the annotated junctions (sjdbOverhang parameter) was set to 161. The Illumina adapter sequences AGATCGGAAGAGCACACGTCTGAACTCCAGTCAC and AGATCGGAAGAGCGTCGTGTAGGGAAAGAGTGTAGATCTCGGTGGTCGCCGTATCATT were, respectively, clipped from the 3′ of the first and the second pairs in the read libraries (using clip3pAdapterSeq parameter).
Differential alternative splicing analysis
Differential AS analysis was done using Whippet (v0.11) (Sterne‐Weiler et␣al, 2018). Both merged aligned reads (bam files) and AS event annotations from GENCODE (v29) were used to build the index reference for AS events. To detect the significantly differential events, probability cutoff of Pr > 0.9 and percentage spliced in deviation cutoff of |ΔΨ| > 0.1 were used.
Differential intron retention analysis
For a comprehensive and sensitive IR analysis, the IntEREst R/Bioconductor package was used (Oghabian et␣al, 2018). After reading binary alignment (.bam) files, IntEREst detects introns with significantly higher and lower number of mapped reads relative to the number of reads that span the introns. The DESeq2‐based function of IntEREst, i.e., deseqInterest(), was used for the differential IR analysis. The Benjamini–Hochberg method was used for adjusting the P‐values, and a cutoff of P adj < 0.05 was applied to extract the significantly differential IRs. The reference table was built from the NCBI RefSeq transcription annotations based on hg38/GRCh38 genome assembly.
Annotating minor introns
We used IntEREst R/Bioconductor package to annotate the minor (U12‐type) introns as described previously (Oghabian et␣al, 2018) using threshold values of 0.07 and 0.14 for 5′ss and BPS scores, respectively. BPS was identified by scanning intronic region from position −40 to position −3 upstream of the 3′ss, and the highest scoring sequence was selected as the BPS. This list was manually appended with additional introns that did not fulfill our annotation criteria (typically because of poor BPS), but have been previously identified as minor introns (Chang et␣al, 2007).
P120 minigene cloning, transfection, and analysis of RNA
The double 5´ss constructs were created by insertion mutagenesis PCR using the P120 minigene (Hall & Padgett, 1996) as a template, and further modifications of 5′ splice sites were made by PCR using mutagenic primers (for a list of primers used see Table EV3). The 3′ss was modified to accommodate for GT‐subtype splicing by insertion of a CAG trinucleotide sequence through insertion mutagenesis PCR. All mutations were confirmed by DNA sequencing. Chinese hamster ovary cells were transfected with the double 5′ss constructs (1,600 ng per well of a 12‐well plate) using Lipofectamine 2000 (Thermo Fisher Scientific), and after 24 h, total RNA was isolated using TRIzol reagent (Thermo Fisher Scientific). Following DNase treatment, a pCB6 vector‐specific oligonucleotide (ACAGGGATGCCA) was used for reverse transcription of the RNA with RevertAid (Thermo Fisher Scientific). RT–PCR was performed with primers binding exon 6 (GGATGAGGAACCATTTGTGC) and exon 7 (AGAACGAGACCGCCCTTC), and the resulting PCR products were analyzed on a 3% MetaPhor™ (Lonza) agarose gel. The gel was imaged using Fuji LAS‐3000 CCD Camera, and the band intensities were quantified using AIDA Software (Raytest, Germany). Identities of the PCR products were confirmed by DNA sequencing.
Author contributions
BW, GJPLK, MJF, NR, and ECT conceptualized the data. BW, AO, MVA, SH, JEH, SY, ECT, LV, MPAB, ECHU, JV, LER, and PP investigated the data. BW, AO, MVA, SH, JEH, SY, ECT, LV, MPAB, ECHU, JV, LER, and PP involved in formal analysis. BW, AO, MVA, SH, JEH, SY, ECT, LV, MPAB, JV, PP, and MJF designed methodology. BW, AO, MVA, SH, JEH, SY, ECT, LV, MPAB, ECHU, JV, LER, and PP validated the data. BW, AO, MVA, SH, ECT, LV, JV, and LER visualized the data. BW, AO, MVA, SH, SY, LV, JV, and LER curated the data. AO and JEH provided software. BW, GJPLK, MJF, MVA, and NR wrote the original manuscript and prepared draft. BW, GJPLK, MJF, BS, MV, NR, BI, AO, MVA, SH, JEH, SY, ECT, LV, ECHU, JV, and LER wrote, reviewed, and edited the manuscript. GJPLK, MJF, BS, MV, NR, and BI administered the project. GJPLK, MJF, BS, MV, NR, and BI supervised the data. GJPLK, MJF, BS, MV, NR, and BI provided resources. GJPLK, MJF, BS, MV, NR, and BI acquired funding.
Conflict of interest
Nazneen Rahman is a non‐executive director of AstraZeneca. The other authors declare no competing interests.
Materials and correspondence
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Geert Kops (g.kops@hubrecht.eu).
Supporting information
Appendix
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Dataset EV3
Dataset EV4
Dataset EV5
Dataset EV6
Table␣EV1
Table␣EV2
Table␣EV3
Movie EV1
Movie EV2
Review Process File
Acknowledgements
We thank the patient family members for their participation in this study. We thank Anna Zachariou for assistance with recruitment, Emma Ramsay for performing the exome sequencing, and Elise Ruark for discussions about the analyses. We thank the Kops, Frilander, Snel, and Rahman laboratories for discussions and comments on the manuscript. We thank Andrew Holland for reagents. The Kops and Vermeulen labs are part of the Oncode Institute, which is partly funded by KWF Kankerbestrijding (DCS). This study was further funded by the Dutch Research Council (NWO) (OCENW.KLEIN.182), the Cancer Genomics Center (CGC.nl), the Wellcome Trust (100210/Z/12/Z) to NR, Sigrid Jusélius Foundation (MF), Jane and Aatos Erkko Foundation (MF), Academy of Finland grant 1308657 (MF), and a Postdoctoral Research Fellowship by the Herchel Smith Fund at the University of Cambridge (ET).
The EMBO Journal (2021) 40: e106536.
Note
CENATAC was named after AT‐AC introns, which make up 84% of U12 A‐type introns
Contributor Information
Mikko J Frilander, Email: mikko.frilander@helsinki.fi.
Geert J P L Kops, Email: g.kops@hubrecht.eu.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information. The ICR1000 UK exome series data are available at the European Genome‐Phenome Archive (EGA), Reference Number EGAS00001000971 (https://ega‐archive.org/studies/EGAS00001000971). Exome data for individual patients cannot be made publicly available for reasons of patient confidentiality. Qualified researchers may apply for access to these data, pending institutional review board approval.
HeLaEGFP‐AID‐CENATAC RNAseq data were deposited in Gene Expression Omnibus GSE143392 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE143392). RNAseq data from the patient and control subject cannot be made publicly available for reasons of patient confidentiality. Qualified researchers may apply for access to these data, pending institutional review board approval.
Protein interaction AP‐MS data were deposited in PRIDE PXD024682 (https://www.ebi.ac.uk/pride/archive/projects/PXD024682).
References
- Abril JF, Castelo R, Guigo R (2005) Comparison of splice sites in mammals and chicken. Genome Res 15: 111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri C, Chang L, Barford D (2018) Mechanism for remodelling of the cell cycle checkpoint protein MAD2 by the ATPase TRIP13. Nature 559: 274–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alioto TS (2007) U12DB: a database of orthologous U12‐type spliceosomal introns. Nucleic Acids Res 35: D110–D115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab M, Srinivasan S, Hashimoto T, Geijsen N, Sherwood RI (2015) Cloning‐free CRISPR. Stem Cell Rep 5: 908–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argente J, Flores R, Gutiérrez‐Arumí A, Verma B, Martos‐Moreno GA, Cuscó I, Oghabian A, Chowen JA, Frilander MJ, Pérez‐Jurado LA (2014) Defective minor spliceosome mRNA processing results in isolated familial growth hormone deficiency. EMBO Mol Med 6: 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT et␣al (2000) Gene ontology: tool for the unification of biology. Nat Genet 25: 25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai R, Wan R, Wang L, Xu K, Zhang Q, Lei J, Shi Y (2021) Structure of the activated human minor spliceosome. Science (80‐ ) 0879: eabg0879 [DOI] [PubMed] [Google Scholar]
- Bertram K, Agafonov DE, Dybkov O, Haselbach D, Leelaram MN, Will CL, Urlaub H, Kastner B, Lührmann R, Stark H (2017) Cryo‐EM structure of a pre‐catalytic human spliceosome primed for activation. Cell 170: 701–713.e11 [DOI] [PubMed] [Google Scholar]
- Blomen Va, Majek P, Jae Lt, Bigenzahn Jw, Nieuwenhuis J, Staring J, Sacco R, van Diemen Fr, Olk N, Stukalov A et␣al (2015) Gene essentiality and synthetic lethality in haploid human cells. Science (80‐ ) 350: 1092–1096 [DOI] [PubMed] [Google Scholar]
- Burge CB, Padgett RA, Sharp PA (1998) Evolutionary fates and origins of U12‐type introns. Mol Cell 2: 773–785 [DOI] [PubMed] [Google Scholar]
- Carbon S, Douglass E, Dunn N, Good B, Harris NL, Lewis SE, Mungall CJ, Basu S, Chisholm RL, Dodson RJ et␣al (2019) The gene ontology resource: 20 years and still GOing strong. Nucleic Acids Res 47: D330–D338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WC, Chen YC, Lee KM, Tarn WY (2007) Alternative splicing and bioinformatic analysis of human U12‐type introns. Nucleic Acids Res 35: 1833–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cologne A, Benoit‐Pilven C, Besson A, Putoux A, Campan‐Fournier A, Bober MB, De Die‐Smulders CEM, Paulussen ADC, Pinson L, Toutain A et␣al (2019) New insights into minor splicing—a transcriptomic analysis of cells derived from TALS patients. RNA 25: 1130–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Mann M (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.‐range mass accuracies and proteome‐wide protein quantification. Nat Biotechnol 26: 1367–1372 [DOI] [PubMed] [Google Scholar]
- Dietrich RC, Incorvaia R, Padgett RA (1997) Terminal intron dinucleotide sequences do not distinguish between U2‐ and U12‐dependent introns. Mol Cell 1: 151–160 [DOI] [PubMed] [Google Scholar]
- Dietrich RC, Fuller JD, Padgett RA (2005) A mutational analysis of U12‐dependent splice site dinucleotides. RNA 11: 1430–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG (1983) Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11: 1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA‐seq aligner. Bioinformatics 29: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, Sachs N, Overmeer RM, Offerhaus GJ, Begthel H et␣al (2015) Sequential cancer mutations in cultured human intestinal stem cells. Nature 521: 43–47 [DOI] [PubMed] [Google Scholar]
- Duijf PHGG, Benezra R (2013) The cancer biology of whole‐chromosome instability. Oncogene 32: 4727–4736 [DOI] [PubMed] [Google Scholar]
- Emanuele MJM, Stukenberg PT (2007) Xenopus Cep57 is a novel kinetochore component involved in microtubule attachment. Cell 130: 893–905 [DOI] [PubMed] [Google Scholar]
- Farach LS, Little ME, Duker AL, Logan CV, Jackson A, Hecht JT, Bober M (2018) The expanding phenotype of RNU4ATAC pathogenic variants to Lowry Wood syndrome. Am J Med Genet Part A 176: 465–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald TW, Gerety SS, Jones WD, Van Kogelenberg M, King DA, McRae J, Morley KI, Parthiban V, Al‐Turki S, Ambridge K et␣al (2015) Large‐scale discovery of novel genetic causes of developmental disorders. Nature 519: 223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman RA, Stockton SS, Cogle CR (2017) Refractory macrocytic anemias in patients with clonal hematopoietic disorders and isolated mutations of the spliceosome gene ZRSR2. Leuk Res 61: 104–107 [DOI] [PubMed] [Google Scholar]
- García‐Castillo H, Vásquez‐Velásquez AI, Rivera H, Barros‐Núñez P (2008) Clinical and genetic heterogeneity in patients with mosaic variegated aneuploidy: delineation of clinical subtypes. Am J Med Genet A 146A: 1687–1695 [DOI] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6: 343–345 [DOI] [PubMed] [Google Scholar]
- Hall SL, Padgett RA (1994) Conserved sequences in a class of rare eukaryotic nuclear introns with non‐consensus splice‐ sites. J Mol Biol 239: 357–365 [DOI] [PubMed] [Google Scholar]
- Hall SL, Padgett RA (1996) Requirement of U12 snRNA for in␣vivo splicing of a minor class of eukaryotic nuclear pre‐mRNA introns. Science (80‐ ) 271: 1716–1718 [DOI] [PubMed] [Google Scholar]
- Hallermayr A, Graf J, Koehler U, Laner A, Schönfeld B, Benet‐Pagès A, Holinski‐Feder E (2018) Extending the critical regions for mutations in the non‐coding gene RNU4ATAC in another patient with Roifman Syndrome. Clin Case Rep 6: 2224–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S, Coleman K, Reid S, Plaja A, Firth H, FitzPatrick D, Kidd A, Méhes K, Nash R, Robin N et␣al (2004) Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat Genet 36: 1159–1161 [DOI] [PubMed] [Google Scholar]
- Hart T, Chandrashekhar M, Aregger M, Steinhart Z, Brown K, MacLeod G, Mis M, Zimmermann M, Fradet‐Turcotte A, Sun S et␣al (2015) High‐resolution CRISPR screens reveal fitness genes and genotype‐specific cancer liabilities screens reveal fitness genes. Cell 163: 1515–1526 [DOI] [PubMed] [Google Scholar]
- Haselbach D, Komarov I, Agafonov DE, Hartmuth K, Graf B, Dybkov O, Urlaub H, Kastner B, Lührmann R, Stark H (2018) Structure and conformational dynamics of the human spliceosomal Bact complex. Cell 172: 454–464.e11 [DOI] [PubMed] [Google Scholar]
- Hein M, Hubner N, Poser I, Cox J, Nagaraj N, Toyoda Y, Gak I, Weisswange I, Mansfeld J, Buchholz F et␣al (2015) A Human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell 163: 712–723 [DOI] [PubMed] [Google Scholar]
- Hernando E, Nahlé Z, Juan G, Diaz‐Rodriguez E, Alaminos M, Hemann M, Michel L, Mittal V, Gerald W, Benezra R et␣al (2004) Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature 430: 797–802 [DOI] [PubMed] [Google Scholar]
- Hooff JJ, Tromer E, Wijk LM, Snel B, Kops GJ (2017) Evolutionary dynamics of the kinetochore network in eukaryotes as revealed by comparative genomics. EMBO Rep 18: 1559–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooff JJE, Tromer E, van Dam TJP, Kops GJPL, Snel B (2019) Inferring the evolutionary history of your favorite protein: a guide for molecular biologists. BioEssays 41: 1900006 [DOI] [PubMed] [Google Scholar]
- Hosono N (2019) Genetic abnormalities and pathophysiology of MDS. Int J Clin Oncol 24: 885–892 [DOI] [PubMed] [Google Scholar]
- Hubner NC, Mann M (2011) Extracting gene function from protein‐protein interactions using quantitative BAC InteraCtomics (QUBIC). Methods 53: 453–459 [DOI] [PubMed] [Google Scholar]
- Huttlin EL, Ting L, Bruckner RJ, Gebreab F, Gygi MP, Szpyt J, Tam S, Zarraga G, Colby G, Baltier K et␣al (2015) The BioPlex network: a systematic exploration of the human interactome. Cell 162: 425–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttlin EL, Bruckner RJ, Paulo JA, Cannon JR, Ting L, Baltier K, Colby G, Gebreab F, Gygi MP, Parzen H et␣al (2017) Architecture of the human interactome defines protein communities and disease networks. Nature 545: 505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Guo F, Wang Y, Zhang Y (2013) High‐resolution crystal structure of human Dim2/TXNL4B. Acta Crystallogr Sect F 69: 223–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloet SL, Makowski MM, Baymaz HI, Van Voorthuijsen L, Karemaker ID, Santanach A, Jansen PWTC, Di Croce L, Vermeulen M (2016) The dynamic interactome and genomic targets of Polycomb complexes during stem‐cell differentiation. Nat Struct Mol Biol 23: 682–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knouse KA, Davoli T, Elledge SJ, Amon A (2017) Aneuploidy in cancer: seq‐ing answers to old questions. Annu Rev Cancer Biol 1: 335–354 [Google Scholar]
- Levine A, Durbin R (2001) A computational scan for U12‐dependent introns in the human genome sequence. Nucleic Acids Res 29: 4006–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HTT, Yat R, Poon C, Poon RYCYC, Yat R, Poon C (2016) TRIP13 regulates both the activation and inactivation of the spindle‐assembly checkpoint. Cell Rep 14: 1086–1099 [DOI] [PubMed] [Google Scholar]
- Madan V, Kanojia D, Li J, Okamoto R, Sato‐Otsubo A, Kohlmann A, Sanada M, Grossmann V, Sundaresan J, Shiraishi Y et␣al (2015) Aberrant splicing of U12‐type introns is the hallmark of ZRSR2 mutant myelodysplastic syndrome. Nat Commun 6: 6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura S, Matsumoto Y, Morishima K‐I, Izumi H, Matsumoto H, Ito E, Tsutsui K, Kobayashi J, Tauchi H, Kajiwara Y et␣al (2006) Monoallelic BUB1B mutations and defective mitotic‐spindle checkpoint in seven families with premature chromatid separation (PCS) syndrome. Am J Med Genet 140 A: 358–367 [DOI] [PubMed] [Google Scholar]
- Merico D, Roifman M, Braunschweig U, Yuen RKC, Alexandrova R, Bates A, Reid B, Nalpathamkalam T, Wang Z, Thiruvahindrapuram B et␣al (2015) Compound heterozygous mutations in the noncoding RNU4ATAC cause Roifman syndrome by disrupting minor intron splicing. Nat Commun 6: 8718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Poudel S, Muruganujan A, Casagrande JT, Thomas PD (2016) PANTHER version 10: expanded protein families and functions, and analysis tools. Nucleic Acids Res 44: D336–D342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD (2019) PANTHER version 14: more genomes, a new PANTHER GO‐slim and improvements␣in enrichment analysis tools. Nucleic Acids Res 47: D419–D426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montembault E, Dutertre S, Prigent C, Giet R (2007) PRP4 is a spindle assembly checkpoint protein required for MPS1, MAD1, and MAD2 localization to the kinetochores. J Cell Biol 179: 601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer DC, Larue GE, Hershberger CE, Roy SW, Padgett RA (2020) Comprehensive database and evolutionary dynamics of U12‐type introns. Nucleic Acids Res 48: 7066–7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A (2015) The molecular biology of spindle assembly checkpoint signaling dynamics. Curr Biol 25: R1002–R1018 [DOI] [PubMed] [Google Scholar]
- Nagaoka SI, Hassold TJ, Hunt PA (2012) Human aneuploidy: mechanisms and new insights into an age‐old problem. Nat Rev Genet 13: 493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemelä EH, Frilander MJ (2014) Regulation of gene expression through inefficient splicing of U12‐type introns. RNA Biol 11: 1325–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemelä EH, Oghabian A, Staals RHJ, Pruijn GJM, Frilander MJ (2014) Global analysis of the nuclear processing of unspliced U12‐type introns by the exosome. Nucleic Acids Res 42: 7358–7369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Fukagawa T, Takisawa H, Kakimoto T, Kanemaki M (2009) An auxin‐based degron system for the rapid depletion of proteins in nonplant cells. Nat Methods 6: 917–922 [DOI] [PubMed] [Google Scholar]
- Oghabian A, Greco D, Frilander MJ (2018) IntEREst: intron‐exon retention estimator. BMC Bioinformatics 19: 130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AA, McCarthy M, Steitz JA (2002) The splicing of U12‐type introns can be a rate‐limiting step in gene expression. EMBO J 21: 3804–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellacani C, Bucciarelli E, Renda F, Hayward D, Palena A, Chen J, Bonaccorsi S, Wakefield JG, Gatti M, Somma MP (2018) Splicing factors Sf3A2 and Prp31 have direct roles in mitotic chromosome segregation. Elife 7: e40325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Marcotte EM, Thompson MJ, Eisenberg D, Yeates TO (1999) Assigning protein functions by comparative genome analysis: protein phylogenetic profiles. Proc Natl Acad Sci USA 96: 4285–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter SC, Luciani A, Eddy SR, Park Y, Lopez R, Finn RD (2018) HMMER web server: 2018 update. Nucleic Acids Res 46: W200–W204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappsilber J, Mann M, Ishihama Y (2007) Protocol for micro‐purification, enrichment, pre‐fractionation and storage of peptides for proteomics using StageTips. Nat Protoc 2: 1896–1906 [DOI] [PubMed] [Google Scholar]
- Sacristan C, Kops GJPLPL (2015) Joined at the hip: kinetochores, microtubules, and spindle assembly checkpoint signaling. Trends Cell Biol 25: 21–28 [DOI] [PubMed] [Google Scholar]
- Schmidt C, Grønborg M, Deckert J, Bessonov S, Conrad T, Lührmann R, Urlaub H (2014) Mass spectrometry‐based relative quantification of proteins in precatalytic and catalytically active spliceosomes by metabolic labeling (SILAC), chemical labeling (iTRAQ), and label‐free spectral count. RNA 20: 406–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Will CL, Makarova OV, Makarov EM, Lührmann R, Makarov M, Lührmann R (2002) Human U4/U6.U5 and U4atac/U6atac.U5 Tri‐snRNPs exhibit similar protein compositions. Mol Cell Biol 22: 3219–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartzman JM, Duijf PHG, Sotillo R, Coker C, Benezra R (2011) Mad2 is a critical mediator of the chromosome instability observed upon Rb and p53 pathway inhibition. Cancer Cell 19: 701–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PA, Burge CB (1997) Classification of introns: U2 type or U12 type. Cell 91: 875–879 [DOI] [PubMed] [Google Scholar]
- Sheth N, Roca X, Hastings ML, Roeder T, Krainer AR, Sachidanandam R (2006) Comprehensive splice‐site analysis using comparative genomics. Nucleic Acids Res 34: 3955–3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Padgett RA (2009) Rates of in␣situ transcription and splicing in large human genes. Nat Struct Mol Biol 16: 1128–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits AH, Jansen PWTC, Poser I, Hyman AA, Vermeulen M (2013) Stoichiometry of chromatin‐associated protein complexes revealed by label‐free quantitative mass spectrometry‐based proteomics. Nucleic Acids Res 41: e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snape K, Hanks S, Ruark E, Barros‐Núñez P, Elliott A, Murray A, Lane AH, Shannon N, Callier P, Chitayat D et␣al (2011) Mutations in CEP57 cause mosaic variegated aneuploidy syndrome. Nat Genet 43: 527–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somma MP, Andreyeva EN, Pavlova GA, Pellacani C, Bucciarelli E, Popova JV, Bonaccorsi S, Pindyurin AV, Gatti M (2020) Moonlighting in mitosis: analysis of the mitotic functions of transcription and splicing factors. Cells 9: 1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillo R, Hernando E, Díaz‐Rodríguez E, Teruya‐Feldstein J, Cordón‐Cardo C, Lowe SW, Benezra R (2007) Mad2 Overexpression Promotes Aneuploidy and Tumorigenesis in Mice. Cancer Cell 11: 9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne‐Weiler T, Weatheritt RJ, Best AJ, Ha KCH, Blencowe BJ (2018) Efficient and accurate quantitative profiling of alternative splicing patterns of any complexity on a laptop. Mol Cell 72: 187–200.e6 [DOI] [PubMed] [Google Scholar]
- Suijkerbuijk SJE, Van Osch MHJ, Bos FL, Hanks S, Rahman N, Kops GJPL (2010) Molecular causes for BUBR1 dysfunction in the human cancer predisposition syndrome mosaic variegated aneuploidy. Cancer Res 70: 4891–4900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarn WY, Steitz JA (1996) A novel spliceosome containing U11, U12, and U5 snRNPs excises a minor class (AT‐AC) intron in␣vitro . Cell 84: 801–811 [DOI] [PubMed] [Google Scholar]
- Turunen JJ, Niemelä EH, Verma B, Frilander MJ (2013a) The significant other: splicing by the minor spliceosome. Wiley Interdiscip Rev RNA 4: 61–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen JJ, Verma B, Nyman TA, Frilander MJ (2013b) HnRNPH1/H2, U1 snRNP, and U11 snRNP cooperate to regulate the stability of the U11–48K pre‐mRNA. RNA 19: 380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen JJ, Will CL, Grote M, Lührmann R, Frilander MJ (2008) The U11–48K protein contacts the 5′ splice site of U12‐type introns and the U11–59K protein. Mol Cell Biol 28: 3548–3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vader G (2015) Pch2TRIP13: controlling cell division through regulation of HORMA domains. Chromosoma 124: 333–339 [DOI] [PubMed] [Google Scholar]
- Verbeeren J, Niemelä EH, Turunen JJ, Will CL, Ravantti JJ, Lührmann R, Frilander MJ (2010) An ancient mechanism for splicing control: U11 snRNP as an activator of alternative splicing. Mol Cell 37: 821–833 [DOI] [PubMed] [Google Scholar]
- Wan R, Yan C, Bai R, Wang L, Huang M, Wong CCL, Shi Y (2016) The 3.8 Å structure of the U4/U6.U5 tri‐snRNP: insights into spliceosome assembly and catalysis. Science (80‐ ) 351: 466–475 [DOI] [PubMed] [Google Scholar]
- Wang K, Sturt‐Gillespie B, Hittle JC, Macdonald D, Chan GK, Yen TJ, Liu S‐TT (2014) Thyroid hormone receptor interacting protein 13 (TRIP13) AAA‐ATPase is a novel mitotic checkpoint‐silencing protein. J Biol Chem 289: 23928–23937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, Lander ES, Sabatini DM (2015) Identification and characterization of essential genes in the human genome. Science (80‐ ) 350: 1096–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Ye, Wu X, Du L, Zheng Ju, Deng S, Bi X, Chen Q, Xie H, Férec C, Cooper DN et␣al (2018) Identification of compound heterozygous variants in the noncoding RNU4ATAC gene in a Chinese family with two successive foetuses with severe microcephaly. Hum Genomics 12: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zou Y, Huang N, Teng J, Chen J (2019) CCDC84 acetylation oscillation regulates centrosome duplication by modulating HsSAS‐6 degradation. Cell Rep 29: 2078–2091.e5 [DOI] [PubMed] [Google Scholar]
- Wheeler TJ, Clements J, Finn RD (2014) Skylign: a tool for creating informative, interactive logos representing sequence alignments and profile hidden Markov models. BMC Bioinformatics 15: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk LM, Snel B (2020) The first eukaryotic kinome tree illuminates the dynamic history of present‐day kinases. bioRxiv 10.1101/2020.01.27.920793 [PREPRINT] [DOI] [Google Scholar]
- Wilkinson ME, Charenton C, Nagai K (2020) RNA splicing by the spliceosome. Annu Rev Biochem 89: 359–388 [DOI] [PubMed] [Google Scholar]
- Will CL, Schneider C, Hossbach M, Urlaub H, Rauhut R, Elbashir S, Tuschl T, Lührmann R (2004) The human 18S U11/U12 snRNP contains a set of novel proteins not found in the U2‐dependent spliceosome. RNA 10: 929–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Krainer AR (1997) Splicing of a divergent subclass of AT‐AC introns requires the major class spliceosomal snRNAs. RNA 3: 586–601 [PMC free article] [PubMed] [Google Scholar]
- Yost S, de Wolf B, Hanks S, Zachariou A, Marcozzi C, Clarke M, de Voer RM, Etemad B, Uijttewaal E, Ramsay E et␣al (2017) Biallelic TRIP13 mutations predispose to Wilms tumor and chromosome missegregation. Nat Genet 49: 1148–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis I, Dittmar K, Wang W, Foley SW, Berg MG, Hu KY, Wei Z, Wan L, Dreyfuss G (2013) Minor introns are embedded molecular switches regulated by highly unstable U6atac snRNA. Elife 2: e00780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wang T, Zheng T, Teng J, Chen J (2016) Cep57 is a Mis12‐interacting kinetochore protein involved in kinetochore targeting of Mad1‐Mad2. Nat Commun 7: 10151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Dataset EV3
Dataset EV4
Dataset EV5
Dataset EV6
Table␣EV1
Table␣EV2
Table␣EV3
Movie EV1
Movie EV2
Review Process File
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information. The ICR1000 UK exome series data are available at the European Genome‐Phenome Archive (EGA), Reference Number EGAS00001000971 (https://ega‐archive.org/studies/EGAS00001000971). Exome data for individual patients cannot be made publicly available for reasons of patient confidentiality. Qualified researchers may apply for access to these data, pending institutional review board approval.
HeLaEGFP‐AID‐CENATAC RNAseq data were deposited in Gene Expression Omnibus GSE143392 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE143392). RNAseq data from the patient and control subject cannot be made publicly available for reasons of patient confidentiality. Qualified researchers may apply for access to these data, pending institutional review board approval.
Protein interaction AP‐MS data were deposited in PRIDE PXD024682 (https://www.ebi.ac.uk/pride/archive/projects/PXD024682).


