Abstract
Purpose:
The purpose of this study was to compare the retinal vascular caliber of COVID-19 patients with that of healthy subjects.
Methods:
This was a prospective case–control study. Forty-six patients who had COVID-19 were successfully treated, and 38 age- and gender-matched healthy subjects were enrolled in this study. Fundus photography was taken using fundus fluorescein angiography (FA; Visucam 500; Carl Zeiss Meditec, Jena, Germany). Retinal vascular caliber was analyzed with IVAN, a semi-automated retinal vascular analyzer (Nicole J. Ferrier, College of Engineering, Fundus Photography Reading Center, University of Wisconsin, Madison, WI, USA). Central retinal artery equivalent (CRAE), central retinal vein equivalent (CRVE), and artery–vein ratio (AVR) were compared between groups.
Results:
The mean age was 37.8 ± 9.5 years in the COVID-19 group (n = 46) and 40 ± 8 years in the control group (n = 38) (p = 0.45). The mean CRAE was 181.56 ± 6.40 in the COVID-19 group and 171.29 ± 15.06 in the control group (p = 0.006). The mean CRVE was 226.34 ± 23.83 in the COVID-19 group and 210.94 ± 22.22 in the control group (p = 0.044). AVR was 0.81 ± 0.09 in the COVID-19 group and 0.82 ± 0.13 in the control group (p = 0.712).
Conclusion:
Patients who had COVID-19 have vasodilation in the retinal vascular structure after recovery. As they may be at risk of retinal vascular disease, COVID-19 patients must be followed after recovery.
Keywords: COVID-19, retinal vascular caliber, retinal vasodilation
Introduction
On 29 December 2019, the Chinese government reported a severe and acute respiratory pneumonia outbreak of unknown cause. China immediately investigated to characterize and control the disease. In January 2020, Chinese scientists isolated a beta coronavirus strain, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), and the illness became known as Coronavirus Disease 2019 (COVID-19). 1 The disease soon spread throughout the world, and in March 2020, the World Health Organization declared it a pandemic.
In the current literature, seven types of coronavirus are known to infect humans, and most clinical symptoms of these infections are in the respiratory and gastrointestinal systems.2,3 Thus far, coronavirus has only been shown to cause conjunctivitis in humans, but it has caused retinal disorders such as vasculitis and retinal degeneration in animal experiments.4,5 In light of this information, we aimed to investigate the possible effects of COVID-19 on human retinal vascular structures.
Methods
This prospective case-control study was carried out in the Department of Ophthalmology at Ulucanlar Eye Research and Training Hospital. The institutional board of the Ankara Research and Training Hospital ethics committee approved the study protocol (approval number: 304/2020, approval date: 25.06.2020). Included in this study were 46 patients who had survived COVID-19 infection as well as 38 healthy controls. Informed written consent was obtained from the participants before their admission into the study.
In the COVID-19 group, subjects with clinical symptoms of COVID-19 and positive SARS-CoV-2 test results in their sputum swab specimens were examined. For ethical and clinical reasons, patients were evaluated after being discharged from the hospital and completing a period of isolation. Due to the potential effects of drugs on retinal vascular structure, only patients who received anti-viral and anti-coagulation treatments were included. None of the patients were admitted to the intensive care unit. One month after complete recovery, all patients were evaluated. For the control group, age- and gender-matched healthy subjects were selected. Patients with any ocular or systemic diseases such as hypertension and diabetes mellitus were excluded.
All patients underwent a complete ophthalmic examination involving best-corrected visual acuity (BCVA), slit-lamp bio-microscopy, intraocular pressure (IOP) values with Goldman applanation tonometry, and posterior segment examination with dilated pupillary. Fundus images of both groups were taken with a fundus camera system (fluorescein angiography; Visucam 500; Carl Zeiss Meditec, Jena, Germany). Participants’ blood pressure was measured before and after examining the fundus images. Only the subjects’ right eyes were photographed. Retinal vascular caliber was analyzed with IVAN, a semi-automated system that measures the width of retinal vessels using a digital retinal image (with permission from Dr Nicola Ferrier of the University of Wisconsin–Madison School of Engineering and the Department of Ophthalmology and Visual Sciences, University of Wisconsin–Madison).6–8
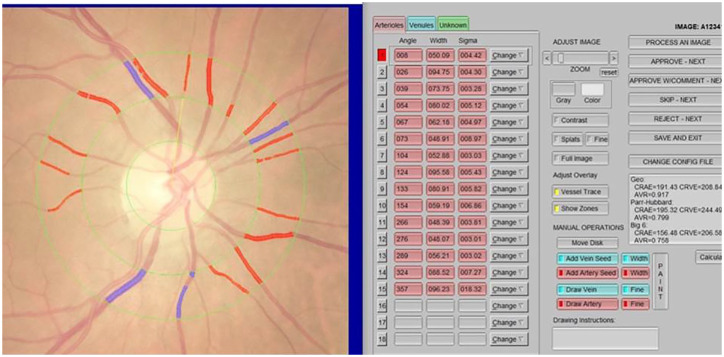
Three concentric rings were placed on the fundus images to determine the vascular measurement field, forming two zones. The area from the disc margin to a half-disc diameter was defined as Zone A, whereas the area from a half-disc diameter to one disc diameter was defined as Zone B. All vessels coursing through Zone B were measured. Central retinal artery equivalent (CRAE) and central retinal vessel equivalent (CRVE) measurements were calculated using the formula created by Hubbard and colleagues 7 and revised by Knudtson and colleagues 8 (Figure 1).
Figure 1.
Measurement of retinal vascular caliber in IVAN.
Statistical analyses were performed using SPSS software version 25. Descriptive analyses were presented using means and standard deviations for normally distributed variables. A normality assessment was done using the Kolmogorov–Smirnov test. The independent-samples t, Mann–Whitney U, and Chi-square tests were used for analyses. A p-value of less than 0.05 was considered a statistically significant result.
Results
The right eyes of 46 COVID-19 patients and 38 age- and gender-matched healthy subjects were examined in this prospective comparative study. The mean age was 37.8 ± 9.5 years in the COVID-19 group (n = 46) and 40 ± 8 years in the control group (n = 38) (p = 0.45). The COVID-19 group consisted of 25 women and 21 men, and the control group consisted of 19 women and 19 men (p = 0.691). The mean spherical equivalent was 0.08 ± 1.35 for the COVID-19 patients and 0.11 ± 1.42 for the control group (p = 0.952)
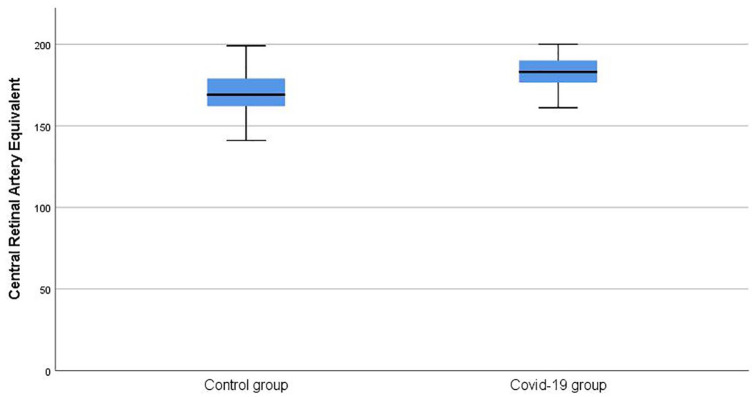
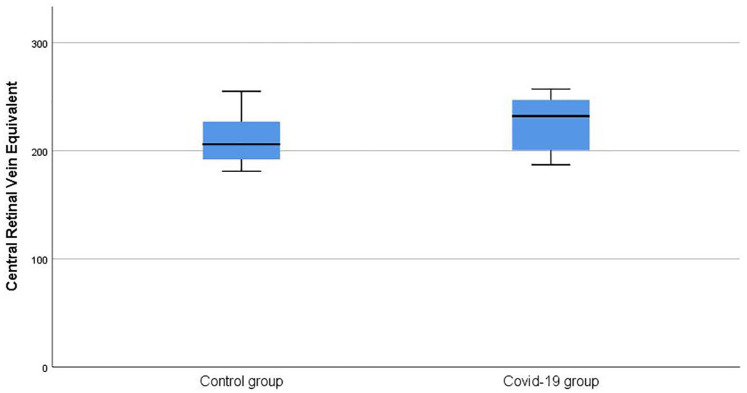
The mean CRAE was 181.56 ± 6.40 in the COVID-19 group, whereas it was 171.29 ± 15.06 in the control group (p = 0.006) (Figure 2). The mean CRVE was 226.34 ± 23.83 in the COVID-19 group and 210.94 ± 22.22 in the control group (p = 0.044) (Figure 3). The artery–vein ratio (AVR) was 0.81 ± 0.09 in the COVID-19 group and 0.82 ± 0.13 in the control group (p = 0.712).
Figure 2.
Comparison of central retinal artery equivalent values between groups.
Figure 3.
Comparison of central retinal vein equivalent values between groups.
Discussion
While the COVID-19 pandemic continues affecting the entire world, scientists are investigating the effects of COVID-19 on multiple systems. Although coronavirus most often causes acute respiratory distress syndrome, conjunctivitis was reported in some patients. 9 It appears the virus must bind to the angiotensin-converting enzyme 2 (ACE2) receptor to enable it to infect host cells. 10 In an experimental study, the presence of the ACE2 receptor was shown in the ciliary body, retina, vitreous body, and inner nuclear layer body, 11 which indicates that the virus can appear in ocular tissue. As evidence, Casagrande and colleagues 12 found the virus in the retina of a deceased person with confirmed COVID-19. In addition, Marinho and colleagues 13 reported the retinal optical coherence tomography (OCT) and optical coherence tomography angiography (OCT-A) findings of 12 subjects with COVID-19. In their study, all patients had hyper-reflective lesions at the level of the ganglion cell and inner plexiform layers that were more prominent in the papillomacular bundle in both eyes in OCT images. Furthermore, 4 of the 12 subjects had cotton wool spots and microhemorrhages in the retina upon fundus examination, color fundus photography, and red-free imaging, yet there were no changes in OCT-A. These studies confirmed that COVID-19 infection might affect patients’ retina segments. Considering these results, we aimed to investigate whether COVID-19 affects retinal vascular structure.
In our study, retinal arteries and venules were larger in COVID-19 patients than in healthy subjects. Only one study has aimed to explain the effects of COVID-19 on retinal vascular structure: In the Screening the retina in patients with COVID-19 (SERPICO-19) study, Invernizzi and colleagues 14 reported the retinal findings of 54 patients with COVID-19 and 133 healthy subjects. Compared with healthy subjects, COVID-19 patients had larger retinal arteries and veins, and the vein diameter was correlated with the severity of a patient’s disease. They reported that these findings are secondary to the inflammatory process. Our study yielded similar results, finding vasodilation in arteries and venules in COVID-19 patients. However, the authors of the SERPICO study 14 and Marinho and colleagues 13 found cotton wool spots and microhemorrhages in fundus examinations and retinal images. Cotton wool spots and microhemorrhages are common signs of retinal microangiopathies. 15 Also, in the SERPICO study, some patients had diabetes mellitus and hypertension, so it is unclear whether the dilation in the vascular structure was due to COVID-19 or another systemic disease. We did not find any hemorrhages or cotton wool spots in our study, nor did we enroll patients with systemic diseases such as hypertension and diabetes mellitus due to their potential effects on vascular structure. Furthermore, we examined the patients 1 month after they recovered fully, while patients in the SERPICO study were examined 1 month after their first symptoms. This may indicate that, even after a full recovery, COVID-19 patients have an inflammatory process in their retinas.
One strength of our study is that it is the first to explain the later effects of COVID-19 on retinal vascular structure. However, our study also has limitations such as a small sample size and a lack of follow-up data. To confirm our results, a larger sample size and long-term follow-up studies are needed. In conclusion, retinal vascular structure can be affected even after a patient recovers fully from COVID-19. These retinal vascular changes may also be one of the long-term effects of COVID-19.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Emre Aydemir  https://orcid.org/0000-0001-6969-0095
https://orcid.org/0000-0001-6969-0095
Alper Halil Bayat  https://orcid.org/0000-0003-1827-968X
https://orcid.org/0000-0003-1827-968X
Yasin Şakir Göker  https://orcid.org/0000-0001-6908-4888
https://orcid.org/0000-0001-6908-4888
Contributor Information
Emre Aydemir, Department of Ophthalmology, Adiyaman University Training and Research Hospital, 02100 Adiyaman, Turkey.
Alper Halil Bayat, Department of Ophthalmology, Esenler Hospital, Medipol University, Istanbul, Turkey.
Burak Ören, Department of Ophthalmology, Adiyaman University Training and Research Hospital, Adiyaman, Turkey.
Halil Ibrahim Atesoglu, Department of Ophthalmology, Ulucanlar Eye Training and Research Hospital, Ankara, Turkey.
Yasin Şakir Göker, Department of Ophthalmology, Ulucanlar Eye Training and Research Hospital, Ankara, Turkey.
Kazım Çağlar Özçelik, Surgical Oncology Department, Yıldırım Beyazıt University, Ankara, Turkey.
References
- 1. Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet 2020; 395: 470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corman VM, Muth D, Niemeyer D, et al. Hosts and sources of endemic human coronaviruses. Adv Virus Res 2018; 100: 163–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi X, Gong E, Gao D, et al. Severe acute respiratory syndrome associated coronavirus is detected in intestinal tissues of fatal cases. Am J Gastroenterol 2005; 100: 169–176. [DOI] [PubMed] [Google Scholar]
- 4. Chin MS, Hooper LC, Hooks JJ, et al. Identification of α-fodrin as an autoantigen in experimental coronavirus retinopathy (ECOR). J Neuroimmunology 2014; 272: 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hooks JJ, Wang Y, Detrick B. The critical role of IFN-gamma in experimental coronavirus retinopathy. Invest Ophthalmol Vis Sci 2003; 44: 3402–3408. [DOI] [PubMed] [Google Scholar]
- 6. Wong TY, Knudtson MD, Klein R, et al. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology 2004; 111: 1183–1190. [DOI] [PubMed] [Google Scholar]
- 7. Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 1999; 106: 2269–2280. [DOI] [PubMed] [Google Scholar]
- 8. Knudtson MD, Lee KE, Hubbard LD, et al. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 2003; 27: 143–149. [DOI] [PubMed] [Google Scholar]
- 9. Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol 2020; 138: 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luhtala S, Vaajanen A, Oksala O, et al. Activities of angiotensin-converting enzymes ACE1 and ACE2 and inhibition by bioactive peptides in porcine ocular tissues. J Ocul Pharmacol Ther 2009; 25: 23–28. [DOI] [PubMed] [Google Scholar]
- 12. Casagrande M, Fitzek A, Püschel K, et al. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm 2020; 28: 721–725. [DOI] [PubMed] [Google Scholar]
- 13. Marinho PM, Marcos AAA, Romano AC, et al. Retinal findings in patients with COVID-19. Lancet 2020; 395: 1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Invernizzi A, Torre A, Parrulli S, et al. Retinal findings in patients with COVID-19: results from the SERPICO-19 study. EClinicalMedicine 2020; 27: 100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong TY, McIntosh R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. Br Med Bull 2005; 73–74: 57–70. [DOI] [PubMed] [Google Scholar]