Abstract
Background:
To evaluate the clinical characteristics, treatment patterns, and clinical effectiveness and safety of high doses of metformin (1500-2500 mg/day) in Indian adults with type 2 diabetes mellitus (T2DM).
Materials and methods:
A retrospective, multicentric (n = 241), real-world study included patients with T2DM (aged >18 years) receiving high doses of metformin. Details were retrieved from patient’s medical records.
Results:
Out of 5695 patients, 62.7% were men with median age was 50.0 years. Hypertension (67.5%) and dyslipidemia (48.7%) were the prevalent comorbidities. Doses of 2000 mg (57.4%) and 1500 mg (29.1%) were the most commonly used doses of metformin and median duration of high-dose metformin therapy was 24.0 months. Metformin twice daily was the most frequently used dosage pattern (94.2%). Up-titration of doses was done in 96.8% of patients. The mean HbA1c levels were significantly decreased post-treatment (mean change: 1.08%; P < .001). The target glycemic control was achieved in 91.2% patients. A total of 83.0% had decreased weight. Adverse events were reported in 156 patients. Physician global evaluation of efficacy and tolerability showed majority of patients on a good to excellent scale (98.2% and 97.7%).
Conclusion:
Clinical effectiveness and safety of a high-dose metformin was demonstrated through significant improvement in HbA1c levels and weight reduction.
Keywords: HbA1c reduction, obesity, treatment patterns, up-titration
Introduction
Guidelines published by the national and international diabetes associations have unequivocally supported the use of metformin as the first-line therapy along with lifestyle changes for the management of adults with newly diagnosed type 2 diabetes mellitus (T2DM) with or without any risk of cardiovascular diseases (CVDs) or CKD.1-3 Furthermore, metformin has several additional benefits that include improvements in lipid profiles, fat redistribution, insulin resistance, endothelial dysfunction, hemostasis, and oxidative stress. These pleiotropic effects of metformin may aid in reducing adverse cardiovascular outcomes in patients with T2DM. 4 Therefore, metformin can be considered as an initial therapy across various subgroups of diabetes including patients with T2DM without CVD but at moderate cardiovascular risk, patients with T2DM and heart failure, and certain patients with mild and moderate renal impairment.5,6
Several clinical studies and meta-analyses have reported that metformin is an effective and safe first-line therapy for the management of T2DM.7-9 Up-titration of metformin dose is another strategy that has been shown to be beneficial in achieving glycemic control at 6-months follow-up after titration of metformin dose and may be preferred as an initial intensification strategy before switching to second-line therapy in patients not responding to initial metformin monotherapy. 10
There is a scarcity of real-world data on the usage, treatment pattern, and dosing frequency of high-dose metformin in an Indian scenario. Hence, the present real-world study aimed to evaluate the demographics, clinical characteristics, and treatment patterns (including dosage and duration, with or without other antidiabetic therapy) in adults with T2DM receiving a high-dose of metformin (1500-2500 mg/day) therapy. Additionally, the clinical effectiveness and safety of high-dose metformin therapy were also assessed.
Subjects, Materials, and Methods
This was a retrospective, multicentric, and real-world study conducted at 241 healthcare centers across India. The study was conducted in accordance with the ethical principles that are consistent with the Declaration of Helsinki, International Conference on Harmonization-Good Clinical Practices, and the applicable legislation on non-interventional studies. The study protocol was approved by an Independent Ethics Committee (CLINICOM Independent Ethics Committee for Evaluation of Protocols for Clinical Research: 01625/26.11.2019; 01593/24.09.2019; 01579/03.09.2019; and 01658/14.01.2020). Considering the retrospective nature of the study the consent was waived by the ethics committee as all the identifying information was removed.
Medical records of patients of either sex, aged >18 years, diagnosed with T2DM and receiving treatment with high-dose of metformin (1500-2500 mg/day) therapy were analyzed in this study from July 2019 to March 2020. Patients having incomplete data files or with any condition that according to the discretion of the investigator indicated that the patient was not suitable for inclusion in the study were excluded.
Information on baseline characteristics, risk factors, medical history, metformin dosages, glycated hemoglobin (HbA1c) status, and weight changes pre- and post-therapy were retrieved from patient’s medical records available at outpatient clinics.
The endpoints were to determine
Dosage patterns of high-dose metformin treatment
Up-titration or down-titration of metformin
Effects of high-dose metformin on HbA1c and weight change between the current visit and prior visit
Adverse events within the past year of high-dose metformin therapy
Percentage of patients receiving concomitant medications (other anti-diabetic therapies with or without insulin or non-diabetic medication/s)
Percentage of patients presenting with any comorbidities
Data were analyzed using Statistical Package for the Social Sciences (SPSS) software, version 23.0 and GraphPad Prism 8. Demographic characteristics were summarized with descriptive statistics, including median and interquartile range (IQR) for continuous variables, and frequency and percentages for categorical variables. A comparison of qualitative and quantitative variables between the groups was done using the chi-square test and Mann-Whitney U test, respectively. A paired sample t-test was used for comparing the pre- and post-treatment HbA1c levels. A P < .05 was considered statistically significant.
Results
Demographics
A total of 5695 patients with T2DM were included, majority being from urban and semi-urban areas. The median (IQR) age was 50.0 (42.0-60.0) years and 57.7% of patients were from the age group of >40 to ⩽60 years. Proportion of male patients was higher than female patients (62.7% vs 37.3%). The median BMI of overall population was 27.7 kg/m2. Peripheral neuropathy was most commonly observed complication (43.2%) followed by CAD (25.9%) and nephropathy (22.8%) in the overall population. The most commonly observed comorbidities were hypertension (67.5%), dyslipidemia (48.7%) and obesity (46.9%) respectively (Table 1).
Table 1.
Patient demographics and treatment related observations.
| Parameters | Number of patients (N = 5695)* |
|---|---|
| Age (years), median (IQR) [n = 5688] | 50.0 (42.0-60.0) |
| Age group (years), n (%) | |
| ⩾20-⩽40 | 1217 (21.4) |
| >40-⩽60 | 3282 (57.7) |
| >60-⩽80 | 1188 (20.9) |
| Sex, n (%) [n = 5548] | |
| Men | 3480 (62.7) |
| Women | 2068 (37.3) |
| Height (cm), median (IQR) [n = 5394] | 164.0 (158.0-170.0) |
| Weight (kg), median (IQR) [n = 5627] | 74.0 (67.0-82.0) |
| BMI (kg/m2), median (IQR) [n = 5043] | 27.7 (25.1-30.4) |
| Duration of diabetes (months), median (IQR) [n = 5654] | 36.0 (12.0-72.0) |
| Locality, n (%) [n = 4175] | |
| Urban | 2196 (52.6) |
| Semi-urban | 1097 (26.3) |
| Rural | 678 (16.2) |
| Semi-rural | 204 (4.9) |
| Biochemical investigations, median (IQR) | |
| FPG (mg/dL), [n = 3955] | 113.0 (99.0-134.0) |
| PPG (mg/dL), [n = 3356] | 176.0 (148.0-205.0) |
| Total cholesterol (mg/dL), [n = 1893] | 186.0 (165.0-210.0) |
| HDL-C (mg/dL), [n = 1846] | 42.0 (38.0-48.0) |
| LDL-C (mg/dL), [n = 1776] | 105.0 (90.0-129.0) |
| Triglyceride (mg/dL), [n = 1700] | 164.0 (135.0-193.0) |
| Serum creatinine (mg/dL), [n = 1258] | 0.9 (0.8-1.1) |
| Urine albumin (mg/g), [n = 212] | 10.0 (1.5-30.0) |
| Complications, n (%) [n = 2654] | |
| Peripheral neuropathy | 1147 (43.2) |
| CAD | 688 (25.9) |
| Nephropathy | 605 (22.8) |
| Autonomic neuropathy | 429 (16.2) |
| Retinopathy | 410 (15.4) |
| PAD | 92 (3.5) |
| Stroke/TIA | 57 (2.1) |
| Others | 12 (0.4) |
| Comorbidity, n (%) [n = 4801] | |
| Hypertension | 3241 (67.5) |
| Dyslipidemia | 2341 (48.7) |
| Obesity | 2253 (46.9) |
| NAFLD | 187 (3.9) |
| Risk factors, median (IQR) [n = 5492] | |
| Obesity | 2830 (51.5) |
| Sedentary lifestyle | 2811 (51.2) |
| Family history of DM | 2448 (44.6) |
| Smoking | 2083 (37.9) |
| Intake of excess salt | 1585 (28.9) |
| Alcohol consumption | 993 (18.1) |
| Tobacco chewing | 792 (14.4) |
| Emotional stress | 1633 (29.7) |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; HDL, high density lipoprotein; IQR, interquartile range; LDL, low density lipoprotein; NAFLD, nonalcoholic fatty liver disease; PAD, peripheral artery disease; TIA, transient ischemic attack.
N = 5695, unless otherwise specified.
Metformin dosage pattern
The majority of patients received metformin 2000 mg (57.4%) and metformin 1500 mg (29.1%) while remaining patients received other doses of metformin such as 1700 mg (6.7%), 2500 mg (2.8%), 1850 mg (2.4%), and 2250 mg (1.6%). Metformin twice daily was the most frequently used dosage pattern (94.2%). The median duration of high-dose metformin therapy administered to the patients was 24.0 months (Table 2).
Table 2.
Treatment patterns of metformin and concomitant therapies.
| Parameters | Number of patients (N = 5695)* |
|---|---|
| Metformin drug dosage per day, n (%) | |
| Metformin 1500 mg | 1656 (29.1) |
| Metformin 1700 mg | 386 (6.7) |
| Metformin 1850 mg | 136 (2.4) |
| Metformin 2000 mg | 3267 (57.4) |
| Metformin 2250 mg | 90 (1.6) |
| Metformin 2500 mg | 159 (2.8) |
| Metformin: Frequency of dose, n (%) [n = 5574] | |
| OD | 244 (4.4) |
| BD | 5252 (94.2) |
| QID | 78 (1.4) |
| Metformin: Duration of treatment (mo), median (IQR) [n = 5092] | 24.0 (7.0-36.0) |
| Concomitant anti-diabetic medication, n (%) [n = 4667] | |
| Sulfonylureas | 3123 (66.9) |
| DPP4i | 2429 (52.0) |
| SGLT2i | 554 (11.8) |
| AGIs | 350 (7.5) |
| Thiazolidinedione | 327 (7.0) |
| Insulin | 287 (6.1) |
| GLP1 agonist | 25 (0.5) |
| Concomitant non-diabetic medications, n (%) [n = 6022] | |
| Antihypertensives | 2978 (49.5) |
| Statins | 1476 (24.5) |
| Non-steroidal anti-inflammatory | 354 (5.9) |
| Non-statin lipid lowering agents | 56 (0.9) |
| Others | 874 (14.5) |
Abbreviations: AGIs, alpha-glucosidase inhibitors; BD, twice a day; DPP4i, dipeptidyl peptidase-4 inhibitors; FPG, fasting plasma glucose; GLP1, glucagon-like peptide-1; IQR, interquartile range; OD, once a day; PPG, postprandial plasma glucose; QID, quater in die; SGLT2i, sodium-glucose co-transporter-2 inhibitor.
Others, patients who were on concomitant non-diabetic medication including antiallergic, antianxiety, antibiotic, anticonvulsant, antiemetic, antihistamine, antiplatelet, diuretic, hyperthyroidism, hypothyroidism, antiasthmatic, antacid, neuropathic pain, vitamins, and multivitamins.
N = 5695, unless otherwise specified.
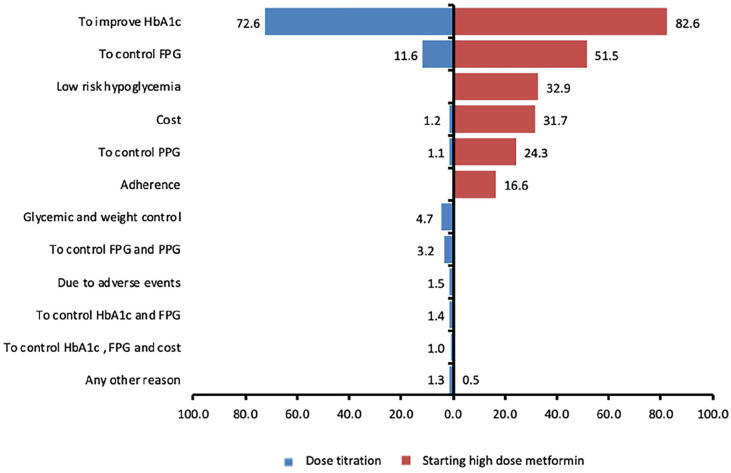
The most common reasons for selecting high-dose metformin (opinion of treating physicians from the study) were to improve current HbA1c levels (82.6%), to control fasting plasma glucose (51.5%), low risk of hypoglycemia (32.9%), and cost (31.7%) (Figure 1).
Figure 1.
Reasons for starting high dose-metformin (1500-2500 mg) and dose titration.
Abbreviations: FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; PPG, postprandial plasma glucose.
Glycemic control
Prior to treatment initiation, 41.3% and 23.6% of patients had HbA1c level in the range of 7.5% to 8.0% and 8.0% to 8.5%, respectively (Table 3). Figure 2A shows the trend of metformin dosages with respect to HbA1c levels across the study population which indicates that the most commonly prescribed dosage of metformin was 2000 mg/day in the patient population across a wide range of HbA1c levels.
Table 3.
Observations related to glycemic control and weight alterations.
| Parameters | Number of patients (N = 5695)* |
|---|---|
| Up-titration or down-titration of metformin done during treatment, n (%) [n = 5693] | 2370 (41.6) |
| Dosage up-titration | 2295 (96.8) |
| Dosage down-titration | 75 (3.2) |
| HbA1c level before treatment initiation, n (%) [n = 5543] | |
| <7.5 | 835 (15.1) |
| 7.5-8.0 | 2289 (41.3) |
| 8.0-8.5 | 1307 (23.6) |
| 8.5-9.0 | 518 (9.3) |
| 9.0-9.5 | 341 (6.1) |
| 9.5-10.0 | 185 (3.3) |
| <10.0 | 68 (1.2) |
| Patients with glycemic goal achieved, n (%) [n = 5693] | 5193 (91.2) |
| Patients with weight changes during the therapy, n (%) [n = 5672] | 4196 (73.9) |
| (a) Decreased weight (kg) | |
| 0-2 | 2415 (57.6) |
| 2-4 | 905 (21.6) |
| >4 | 161 (3.8) |
| (b) Increased weight (kg) | |
| 0-2 | 453 (10.8) |
| 2-4 | 239 (5.7) |
| >4 | 23 (0.5) |
| Adverse events reported, n | [n = 156] |
| Gastritis | 50 |
| Dyspepsia | 22 |
| GI disease | 19 |
| Nausea | 18 |
| Diarrhea | 17 |
| Vomiting | 14 |
| Dizziness | 10 |
| Others | 6 |
Abbreviations: FPG, fasting plasma glucose; GI, gastrointestinal; HbA1c, glycated hemoglobin; PPG, postprandial plasma glucose.
Other adverse events include abdominal discomfort and inadequate bowel movement, heart burn, constipation.
N = 5695, unless otherwise specified. Other reasons include combination of HbA1c, FPG, PPG, weight, and cost.
Figure 2.
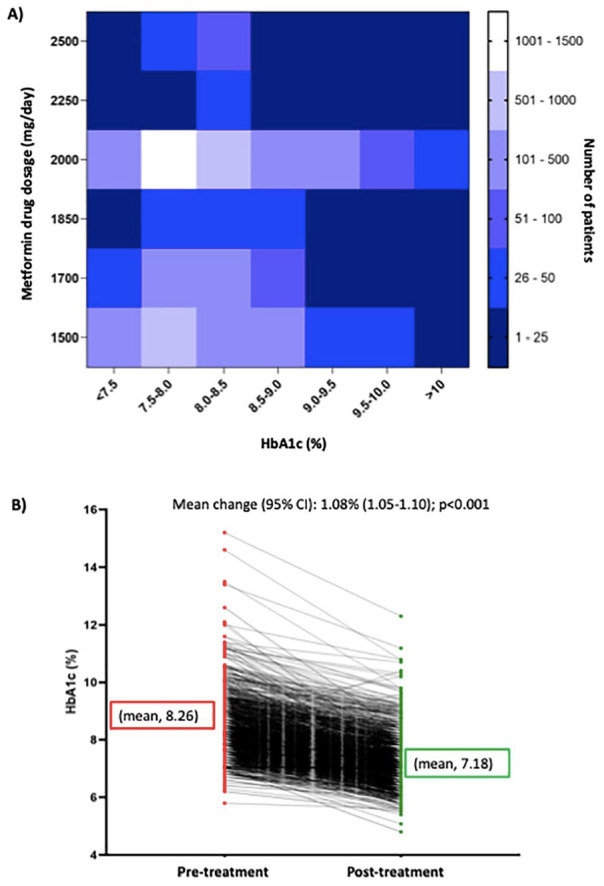
(A) The trend of metformin dosages with respect to HbA1c levels and (B) mean change in HbA1c levels from pre-treatment to post-treatment.
Abbreviations: CI, confidence interval; HbA1c, glycated hemoglobin.
Dose titration
A total of 2370 patients required metformin dose up-titration or down-titration during the treatment and out of these, 96.8% of patients required dosage up-titration (Table 3). The most common reason given for titration was to improve HbA1c level (72.6%) (Figure 1).
The mean HbA1c levels significantly decreased (8.26% vs 7.18%) with high-dose metformin with mean change of 1.08% (95% CI [1.05-1.10]; P < .001) (Figure 2B).
Concomitant medications
Proportion of patients receiving sulfonylureas was 66.9% followed by dipeptidyl peptidase-4 inhibitors (DPP4i) (52.0%), sodium-glucose co-transporter-2 inhibitors (SGLT2i) (11.8%), alpha-glucosidase inhibitors (7.5%), thiazolidinedione (TZD) (7.0%), insulin (6.1%), and glucagon-like peptide-1 (GLP1) agonist (0.5%). In concomitant non-diabetic medications, most common class of drugs (other than supplements) were antihypertensives (49.5%) followed by statins (24.5%), non-steroidal anti-inflammatory drugs (5.9%), and non-statin lipid-lowering drugs (0.9%) (Table 2).
Weight change
A total of 73.9% of patients experienced weight changes during the therapy. Of these, majority of patients had decreased weight (83.0%) in the range of 0 to >4 kg while remaining 17.0% of patients had increased weight (Table 3).
HbA1c level-wise analysis
The median duration of high-dose metformin treatment was significantly higher in patients with HbA1c >8.5% and ⩾7.5% to ⩽8.5% than those with <7.5% (24.0 and 20.0 months vs 12.0 months, respectively; P < .001 and P = .005). The mean HbA1c levels significantly decreased post-treatment with high-dose metformin (1500-2500 mg/day) therapy with mean change of 0.9%, 1.0%, and 1.4% (P < .001) in patients with <7.5%, ⩾7.5% to ⩽8.5%, and >8.5%, respectively (Table 4).
Table 4.
HbA1c level wise treatment distribution.
| Characteristics | Group A, <7.5% (n = 835)* | Group B, ⩾7.5 to ⩽8.5% (n = 3605)** | Group C, >8.5% (n = 1119) # | P value |
|---|---|---|---|---|
| Age (y) | 49.0 (42.0-59.0) | [n = 3599] 49 (41.0-58.0) | 47.0 (55.0-63.0) | .092 a , .001b,c |
| Age group (y), n (%) | ||||
| ⩾20-⩽40 | 179 (21.4) | 889 (24.7) | 128 (11.4) | <.001a,b,c |
| >40-⩽60 | 490 (58.7) | 2067 (57.4) | 648 (57.4) | |
| >60-⩽80 | 166 (19.9) | 643 (17.9) | 343 (30.7) | |
| Sex, n (%) | [n = 803] | [n = 3518] | [n = 1102] | .186 |
| Men | 480 (59.8) | 2216 (63.0) | 700 (63.5) | |
| Women | 323 (40.2) | 1302 (37.0) | 402 (36.5) | |
| BMI (kg/m2) | [n = 742] 26.7 (24.3-29.5) | [n = 3183] 27.7 (25.3-30.4) | [n = 998] 28.7 (26.0-31.0) | <.001a,b,c |
| Location, n (%) | [n = 600] | [n = 2736] | [n = 760] | .137 |
| Urban | 124 (20.7) | 598 (21.9) | 141 (18.6) | |
| Rural | 476 (79.3) | 2138 (78.1) | 619 (81.4) | |
| Duration of diabetes (mo) | [n = 829] 36.0 (12.0-60.0) | [n = 3581] 36.0 (12.0-72.0) | [n = 1112] 60.0 (24.0-96.0) | <.001a,b,c |
| Duration of high-dose treatment (mo) | [n = 692] 12.0 (6.0-24.0) | [n = 3397] 20.0 (6.0-36.0) | [n = 987] 24.0 (12.0-48.0) | .005 a , <0.001b,c |
| Mean HbA1c level (%) | [n = 301] | [n = 1481] | [n = 417] | — |
| Previous | 7.5 (0.6) | 8.1 (0.6) | 9.3 (0.8) | |
| Current | 6.6 (0.5) | 7.1 (0.6) | 7.8 (0.8) | |
| Mean change [95% CI]; P value | 0.9 [0.8-0.9]; <.001 | 1.0 [1.0-1.1]; <.001 | 1.4 [1.3-1.4]; <.001 | |
| Weight change (kg), n (%) | [n = 599] | [n = 2728] | [n = 793] | |
| Increase | ||||
| 0-2 | 109 (18.2) | 239 (8.7) | 96 (12.9) | — |
| 2-4 | 28 (4.7) | 151 (5.5) | 54 (6.8) | |
| >4 | 0 | 13 (0.5) | 10 (1.3) | |
| Decrease | ||||
| 0-2 | 314 (52.4) | 1708 (62.6) | 349 (44.0) | — |
| 2-4 | 134 (22.4) | 541(19.8) | 215 (27.1) | |
| >4 | 14 (2.3) | 76 (2.7) | 69 (8.7) | |
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin; IQR, interquartile range.
Data shown as median (IQR), unless otherwise specified.
Group A versus B; bgroup A versus C; cgroup B versus C.
n = 835. **n = 3605. #n = 1119, unless otherwise specified.
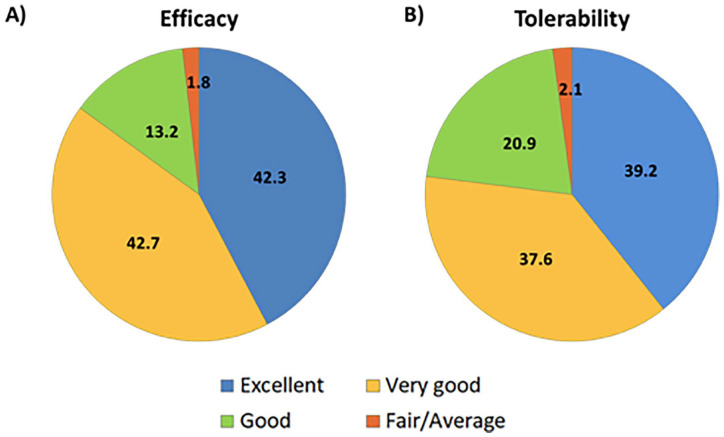
Physicians global evaluation of efficacy and tolerability showed majority of patients on a good to excellent scale (98.2% and 97.7%, respectively) (Figure 3). A total of 156 patients (2.7%) reported adverse events and gastritis was the most common among them (Table 3).
Figure 3.
Physicians global evaluation for: (A) efficacy and (B) tolerability of the treatment.
Data shown as %.
Discussion
Literature supports the safety and effectiveness of metformin in lowering levels of HbA1c and blood glucose including other beneficial effects such as weight stabilization or weight loss, improved lipid profile, decreased cardiovascular risk markers, and diabetic complications. Higher metformin doses can be prospectively used to enhance glycemic control in patients with T2DM with minimal hypoglycemic and gastrointestinal effects. 11 This real-world study mainly assessed the treatment patterns of high-dose metformin therapy (1500-2500 mg/day) in adult patients with T2DM across 241 study centers in India. In addition, this study also evaluated the clinical effectiveness and safety of high-dose metformin therapy for the management of T2DM. Majority of patients (71%) were administered with metformin dosage above 1700 mg to improve levels of HbA1c and FPG.
Metformin is recommended as the first-line treatment for the T2DM in all guidelines, and it is typically administered in the range of doses from 500 to 2550 mg per day.1,3,12 The latest ICMR guidelines recommend the initiation of metformin therapy at 500 mg OD post meals and intensification of dose by 500 mg fortnightly to achieve the required glycemic targets or until the highest daily doses of 2500 mg are reached. 13 Thus, up-titration in a gradual fashion will help to prevent gastrointestinal side effects. The most commonly used daily regimen in the present study was 2000 mg followed by 1500 mg. Also, an increasing trend was observed in the duration of high-dose metformin therapy according to the increased levels of HbA1c indicating an association between poor glycaemic control and longer duration of metformin therapy. Several real-world studies in India demonstrated that metformin was the commonly used medication in T2DM, with 1000 mg per day as the maximum dose used either once or as two divided doses.14-16 The findings from a 14-week, multicenter double-blind study suggest that most patients achieved maximal therapeutic efficacy with a daily dose of 2000 mg. 17 In patients who do not tolerate higher doses, a daily dose of 1000 to 2000 mg should be considered effective. 17 However, in a separate study conducted at a tertiary care center in India, metformin monotherapy was prescribed as an initial treatment with varying doses of 500 to 1500 mg/day. 18 In another prospective study from India on the pattern of metformin dosages, the daily dose of metformin was 1000 mg in almost half of the patients. The remaining patients received 500, 1350, 1500, 2000, or 2500 mg. 19 Metformin requires dose titration to achieve the desired glycemic effect while maintaining optimal tolerability. Up-titration needs to be done with care, as per patient tolerability. Similarly, down-titration may be considered, as needed to achieve a balance between glucose levels and safety of high-dose metformin therapy.20,21 Among the 41.6% of patients requiring dose titration in the present study, the majority had up-titration of the metformin doses. The most common reason for selecting high-dose metformin therapy was to improve the HbA1c and FPG level.
In the present study, more than 80% of patients received other anti-diabetic medications along with metformin. Sulfonylureas and DPP-4 inhibitors were the most commonly prescribed anti-diabetic medications. In the real-world TIGHT (The Investigation of Glycosylated Hemoglobin on Therapy in Indian diabetics) study, metformin as monotherapy or combination therapy was prescribed in 83% of patients. Sulfonylureas, DPP4i, alpha-glucosidase inhibitors, and thiazolidinediones were the most commonly prescribed oral anti-hyperglycemic drug classes along with metformin in 60%, 53%, 15.5%, and 10% of patients, respectively. 22 Thus, metformin can be safely combined with most of the oral anti-hyperglycemic drug and effectively used. 23
These observations corroborate the previous study findings indicating effectiveness of high-dose metformin in achieving HbA1c reduction and weight reduction.11,24 The landmark UK Prospective Diabetes Study (UKPDS) evaluated a median daily metformin dose of 2550 mg/day in people with newly diagnosed T2DM and reported a median HbA1c of 7.4% compared with 8.0% in the conventional group. 8 In the Diabetes Prevention Program study, during the unblinded follow-up, weight loss was significantly greater in the metformin group compared to the placebo group (−2.0 vs −0.2%, P < .001). 25 A meta-analysis by Maruthur et al 26 have also reported beneficial effects of metformin as first-line therapy on HbA1C, weight, and cardiovascular mortality. Metformin decreases the glucose production in patients with diabetes by lowering gluconeogenesis. Additionally, it is reported to activate the 5′-AMP-activated protein kinase in different tissues, chiefly in liver leading to enhanced insulin sensitivity.27,28
Role of metformin in reducing blood pressure levels in patients with T2DM is variable; however, a potential of metformin in lowering blood pressure in patients with impaired glucose tolerance or impaired fasting glucose is an established evidence.4,29,30 In addition, metformin therapy is also linked to improvements in lipoprotein metabolism, as well as in cardiovascular mechanisms such as endothelial dysfunction, oxidative stress, and fat redistribution. 4 A cohort study from United Kingdom 31 showed that dosing of metformin monotherapy in patients with T2DM is dependent on HbA1c levels. They observed that up-titration of metformin was infrequent in the first year after the commencement of therapy with age and higher HbA1c levels as the key predictors of up-titration. Another cohort study from United States reported that metformin dosage up-titration was equally effective as adding another anti-diabetic drug. 10 The addition of a DPP4i to alpha-glucosidase inhibitors therapy in patients with T2DM enabled to achieve a better glycemic control. 32 Similarly, metformin up-titration or adding another anti-diabetic drug to metformin monotherapy in patients who cannot tolerate higher doses is likely to show enhanced glycemic control.
There are few studies which have established good tolerance of high dose metformin.8,25 Our study results are in line with previous observations that a large number of patients being compliant to high doses of metformin without any adverse events. The majority of the physicians in this study rated “Very Good” followed by “Excellent” and “Good” scores, both for the efficacy and safety of high-dose metformin. As the patients tolerated 1000 mg dose, the doses were gradually increased to 2000 mg during the treatment; hence, patients may have better tolerated the higher dosage.
The present study is not without limitations. Details about the duration of therapy was not captured. Due to the retrospective nature of the study, several parameters such as the antidiabetic regimen used prior to high-dose metformin and time of the previous visit could not be captured. The large-scale, prospective, well-designed studies with high-dose metformin therapy will be very important.
Conclusion
This nationwide, retrospective real-world study demonstrated the clinical effectiveness and safety of high-dose metformin for the management of adult patients with T2DM in India. A high-dose metformin therapy was beneficial in achieving significant improvements in HbA1c reductions along with weight loss benefits in majority of patients. The clinical characteristics and treatment patterns of high-dose metformin therapy described in this study may be beneficial in identifying and treating the key risk factors and proposing optimal strategies for better management of T2DM in India.
Acknowledgments
We acknowledge Ms. Farida Hussain, Ms. Monal Patil, Mr. Smitabrata Dasgupta, and Ms. Annsusan Renji from USV Pvt. Ltd. for their assistance in carrying out the project. The medical writing support was provided by Dr. Sona Warrier from the scientific services team of USV Pvt. Ltd. and Ms. Snehal Khanolkar and Dr. Tejal Vedak from Sqarona Medical Communications LLP (Mumbai). We acknowledge BioQuest Solutions Private Limited for their services in the conduction of the real-world study.
Footnotes
Author Contributions: Dr. A Chandrashekar, Dr. AK Malhotra, Dr. A Kamlesh, Dr. AM Rao, Dr. A Balachandran, Dr. Abhijit Jadhav, Dr. Abishekh Bhattacharya, Dr. Aghosh Pasricha, Dr. Ajay Bhandari, Dr. M Ajay Reddy, Dr. Ajay Yadav, Dr. Ak Chapariya, Dr. Alam Nawaz, Dr. KA Amarnath, Dr. Amit Gupta, Dr. Amit Kumar Mittal, Dr. Amit Maheshwari, Dr. Amitesh Chatterjee, Dr. Anand Swaroop Meenawat, Dr. Ankur Sinha, Dr. Anurag Srivastava, Dr. Arijit Roy Chowdhury, Dr. Arun Bajaj, Dr. Arun Kalra, Dr. Arun Kumar, Dr. Arvind Kumar Mishra, Dr. Ashok B Malipatil, Dr. Ashok Jadhav, Dr. Ashok Kumar Yadav, Dr. Ashok Pansari, Dr. Ashwani Kumar Jain, Dr. Atul Khanna, Dr. Atul Saxena, Dr. BB Mittal, Dr. BG Baliga, Dr. BKS Murthy, Dr. BK Sundar, Dr. B Ramulu, Dr. BS Shivakumar, Dr. BS Sudhir, Dr. BV Manjunath, Dr. BVR Kumar, Dr. Bikash Bhattacharjee, Dr. MS Brunda, Dr. Bvs Ravi Shankar, Dr. CD Chapadia, Dr. Chayan Bhattacharjee, Dr. Chetan Gupta, Dr. SC Chidanand, Dr. Deepak Gauba, Dr. Dheeraj Verma, Dr. Dnyanoba Bhaskar, Dr. Keerthy Shetty, Dr. Shardul Kothary, Dr. Shubhashree Patil, Dr. E Arunachalam, Dr. EM Surendra, Dr. S Eswar Prasad, Dr. Eswaran Thangavel, Dr. G Aravindan, Dr. G Balaraju, Dr. GS Ramkumar, Dr. Ghan Shyam Goyal, Dr. Gyanendra Mohan Rohatgi, Dr. Haridas Upadhyay, Dr. Harshd Shah, Dr. Hem Kumar, Dr. Himanshu Mehta, Dr. JK Sharma, Dr. J Nagaraju, Dr. Javed Shaikh, Dr. Jyothi Prakash, Dr. K Hari Babu, Dr. KO Joseph, Dr. KR Ravish, Dr. K Rajesh, Dr. K Rina, Dr. KSS Bhat, Dr. K Sai, Dr. KM Jeyabalaji, Dr. Kabir Das Purohit, Dr. Kabir Dutta, Dr. Kalpana S Mehta, Dr. Karthik, Dr. S Karthik, Dr. Kasargaon Paten Vitthal Rao, Dr. Kashi Nath Padhiari, Dr. Katakam Narender, Dr. Krishna Prasad Kotha, Dr. L Gopal Naik, Dr. Lakshmi Vinutha Reddy, Dr. M Ashok Kumaar, Dr. M Chellamariappan, Dr. M Harinath Reddy, Dr. M Leelavathi, Dr. M Madhu, Dr. M Shyam Prasad, Dr. M Sudhir, Dr. Madan Pal Singh, Dr. Mahendra Mahadev Deshmane, Dr. Mahendra Singh Suri, Dr. Mahesh Kumar Marda, Dr. M Manjanna, Dr. Manoj Kumar Sharma, Dr. Manoj Rawat, Dr. Margam Kiran Kumar, Dr. Masood Batin, Dr. Mathew Jacob, Dr. Mohan Lal Jain, Dr. Mukesh Budhwani, Dr. Munna Sherpa, Dr. S Murthy, Dr. BR Nagarjun, Dr. Navneet Agarwal, Dr. Nipun Gupta, Dr. Niran Uthaiah, Dr. Nitin B Agarwal, Dr. PN Deokate, Dr. P Ravikaladhar Reddy, Dr. P Chandramohan, Dr. Parvez Khan, Dr. Patnala Chakradhar, Dr. Pawan Goyal, Dr. Philips Routray, Dr. Prabhat Pandey, Dr. Pradip Gupta, Dr. Pranab Ranjan Majumdar, Dr. Prasanth Sankar, Dr. G Praveen, Dr. Praveen Kumar Gupta, Dr. Praveenkumar R Jarag, Dr. S Preeti, Dr. R Manjunath, Dr. R Srinivasan, Dr. J Raghu, Dr. Rahul Jain, Dr. Rahul Kapur, Dr. Rajesh Agrawal, Dr. Rajesh Regonda, Dr. Rajpreet Singh, Dr. Rakesh Agarwal, Dr. Ramesh Kumar, Dr. Ranjith Shetty, Dr. Ratan Saha, Dr. SC Das, Dr. SK Nagrani, Dr. S Muni Bala Krishna, Dr. SN Jayaprakash, Dr. S Nanda Kumar, Dr. SS Annamalaisamy, Dr. Sagar Rakecha, Dr. Sai Pradeep, Dr. C Sanil, Dr. Sanjay Dhall, Dr. Sanjay Jain, Dr. Sanjay Reddy, Dr. KM Santosh, Dr. Satish Sutradhar, Dr. Saumitra Ray, Dr. Savita Agarwal, Dr. Shaik Ahmed, Dr. Y Shekhar, Dr. Shiladitya Nandi, Dr. Somnath Mitra, Dr. Soumitra Ghosh, Dr. Sradhananda Mahapatra, Dr. Srinivas Sahu, Dr. Srinivas Yogan, Dr. Subhash Saxena, Dr. Subodh Chandra, Dr. Sudhir Jambagi, Dr. Suhas Erande, Dr. Sukhen Kumar Saha, Dr. Suman Kotwal, Dr. Sunil Bhardwaj, Dr. Sunil Kumar Choudhary, Dr. Sunil R Karande, Dr. Surekha B Shetty, Dr. Surendra Bhakal, Dr. Suresh Mittal, Dr. Syed Nazim Uddin Ahmed, Dr. T Suman, Dr. Tapas Roy, Dr. Uday Lal, Dr. Umesh Kumar, Dr. Umesh Masand, Dr. V Elil Saravanan, Dr. VN Selvam, Dr. Vaibhav Chakkarwar, Dr. Vijay Shankar Upadhyay, Dr. Vikas Desle, Dr. Vipin Porwal, Dr. Vivek Bhosale, Dr. Vivek Jha, Dr. Vivek M S, Dr. Vivek Mehta, and Dr. Yogesh Patil.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Mahesh Abhyankar and Dr. Santosh Revankar are employees of USV Pvt. Ltd. All other authors have no conflicts of interest to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project has been funded by USV Pvt. Ltd.
ORCID iD: Mahesh Abhyankar  https://orcid.org/0000-0003-3127-0575
https://orcid.org/0000-0003-3127-0575
References
- 1. American Diabetes Association. Standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S98-S110. [DOI] [PubMed] [Google Scholar]
- 2. Bajaj S. RSSDI clinical practice recommendations for the management of type 2 diabetes mellitus. Int J Diabetes Dev Ctries. 2017;38:S1-S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rojas LB, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr. 2013;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. Accessed July 20, 2020. http://www.fda.gov/Drugs/DrugSafety/ucm493244.htm
- 6. Cosentino F, Grant PJ, Aboyans V, et al. ; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255-323. [DOI] [PubMed] [Google Scholar]
- 7. Chakraborty A, Chowdhury S, Bhattacharyya M. Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res Clin Pract. 2011;93:56-62. [DOI] [PubMed] [Google Scholar]
- 8. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865. [PubMed] [Google Scholar]
- 9. Palmer SC, Mavridis D, Nicolucci A, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA. 2016;316:313-324. [DOI] [PubMed] [Google Scholar]
- 10. Mahabaleshwarkar R, Liu TL, Mulder H. Comparative effectiveness of metformin dosage uptitration versus adding another antihyperglycemic medication on glycemic control in type 2 diabetes patients failing initial metformin monotherapy: a retrospective cohort study. Popul Health Manag. 2019;22:457-463. [DOI] [PubMed] [Google Scholar]
- 11. Hirst JA, Farmer AJ, Ali R, Roberts NW, Stevens RJ. Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care. 2012;35:446-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corcoran C, Jacobs TF. Metformin. In: StatPearls. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 13. ICMR guidelines for management of diabetes II [13 April 2018]. Accessed May 8, 2020. http://icmr.nic.in/guidelines_diabetes/guide_diabetes.htm
- 14. Singla R, Bindra J, Singla A, Gupta Y, Kalra S. Drug prescription patterns and cost analysis of diabetes therapy in India: audit of an endocrine practice. Indian J Endocrinol Metab. 2019;23:40-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dutta D, Jaisani R, Khandelwal D, Ghosh S, Malhotra R, Kalra S. Role of metformin, Sodium-Glucose Cotransporter-2 (SGLT2) inhibitors, Glucagon-Like Peptide-1 (GLP-1) receptor agonists, and orlistat based multidrug therapy in glycemic control, weight loss, and euglycemia in diabesity: a real-world experience. Indian J Endocrinol Metab. 2019;23:460-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mokta J, Mokta K, Ranjan A, Joshi I, Garg M. Diabetes drug prescription pattern and awareness among health care providers in sub-Himalayan region of India: a population based study. J Assoc Physicians India. 2017;65:50-54. [PubMed] [Google Scholar]
- 17. Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am J Med. 1997;103:491-497. [DOI] [PubMed] [Google Scholar]
- 18. Srivastava SS, Roy V, Mohanty A, Mohanty M. Prescribing pattern of antidiabetic drugs amongst pre-obese diabetic patients in a tertiary care hospital-an observational study. Int J Sci Res. 2019;8:1-4. [Google Scholar]
- 19. Joshi DB, Lakhani JD, Siddhpuria RY, Tandel HP, Hajariwala NR. A study on drug utilization pattern of metformin and its different formulations used in patients with type-2 diabetes mellitus in tertiary care teaching hospital. J Integr Health Sci. 2018;6:22-26. [Google Scholar]
- 20. Kaiser Permanente. Type 2 diabetes screening and treatment guidelines. 2019. Accessed May 8, 2020. https://wa.kaiserpermanente.org/static/
- 21. Kalra S, Gupta Y. Starting titrating and intensifying metformin. J Pak Med Assoc. 2015;65:799-800. [PubMed] [Google Scholar]
- 22. Borgharkar SS, Das SS. Real-world evidence of glycemic control among patients with type 2 diabetes mellitus in India: the TIGHT study. BMJ Open Diabetes Res Care. 2019;7:e000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ICMR guidelines for management of type 2 diabetes mellitus. Accessed October 6, 2020. https://main.icmr.nic.in/sites/default/files/guidelines/ICMR_GuidelinesType2diabetes2018_0.pdf
- 24. Sivitz WI, Phillips LS, Wexler DJ, et al. ; GRADE Research Group. Optimization of metformin in the GRADE cohort: effect on glycemia and body weight. Diabetes Care. 2020;43:940-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164:740-751. [DOI] [PubMed] [Google Scholar]
- 27. Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. González-Ortiz M, Martínez-Abundis E, Robles-Cervantes JA, Ramos-Zavala MG, Barrera-Durán C, González-Canudas J. Effect of metformin glycinate on glycated hemoglobin A1C concentration and insulin sensitivity in drug-naive adult patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2012;14:1140-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fontbonne A, Diouf I, Baccara-Dinet M, Eschwege E, Charles A. Effects of 1-year treatment with metformin on metabolic and cardiovascular risk factors in non-diabetic upper-body obese subjects with mild glucose anomalies: a post-hoc analysis of the BIGPRO1 trial. Diabetes Metab. 2009;35:385-391. [DOI] [PubMed] [Google Scholar]
- 30. Zhou L, Liu H, Wen X, Peng Y, Tian Y, Zhao L. Effects of metformin on blood pressure in nondiabetic patients: a meta-analysis of randomized controlled trials. J Hypertens. 2017;35:18-26. [DOI] [PubMed] [Google Scholar]
- 31. Iglay K, Sawhney B, Fu AZ, et al. Dose distribution and up-titration patterns of metformin monotherapy in patients with type 2 diabetes. Endocrinol Diabetes Metab. 2019;3:e00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Min SH, Yoon JH, Hahn S, Cho YM. Efficacy and safety of combination therapy with an α-glucosidase inhibitor and a dipeptidyl peptidase-4 inhibitor in patients with type 2 diabetes mellitus: a systematic review with meta-analysis. J Diabetes Investig. 2018;9:893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]