Abstract
The goal of the present work was to evaluate the additive effects of biochar and chicken manure on maize growth in Pb-contaminated soils. In this study, we conducted a pot experiment to investigate how biochar in soil (20, 40 g·kg−1), chicken manure in soil (20, 40 g·kg−1), or a combination of biochar and chicken manure in soil (each at 20 g·kg−1) effect maize growth, Pb uptake, leaves’ antioxidant enzymatic activities, and soil enzyme activities under artificial conditions to simulate moderate soil pollution (800 Pb mg·kg−1). The results showed that all biochar and/or chicken manure treatments significantly (P < 0.05) increased maize plant height, biomass, and superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) activity but decreased the malondialdehyde (MDA) content. These results indicated that amending the soil with biochar and/or chicken manure could alleviate Pb’s phytotoxicity. The biochar and/or chicken manure treatments remarkably decreased the Pb concentration in maize roots, stems, leaves, bioconcentration factor (BCF), translocation factor (TF), and available Pb concentration in the soil. Amending the soil with chicken manure alone was more effective at increasing maize growth and antioxidant enzymatic activity; the biochar treatment alone was more effective at inducing soil alkalinization and contributing to Pb immobilization. The combined use of biochar and chicken manure had an additive effect and produced the largest increases in maize growth, leaves’ antioxidant enzymatic activity, and soil enzyme activity. Their combined use also led to the most significant decreases in maize tissues Pb and soil available Pb. These results suggest that a combination of biochar and chicken manure was more effective at reducing soil Pb bioavailability and uptake by maize tissues, and increasing maize growth. This combination increased plant height by 43.23% and dry weight by 69.63% compared to the control.
Keywords: Lead uptake, Biochar, Chicken manure, Maize growth, Antioxidant enzymatic activities, Bioconcentration factor
Introduction
Recently, soil contamination of agricultural land throughout the world by heavy metals has become a serious problem. Heavy metals are toxic for plants and can inhibit plant growth, development, and productivity (Chernyshuk et al., 2020; Zhang et al., 2018). Lead (Pb) is a classically deleterious heavy metal that threatens agro-ecosystem sustainability through anthropogenic activities such as mining, waste disposal, and the intensive use of pesticides and fertilizers (Adler et al., 2016; Fu & Wang, 2011). Pb is easily accumulated in soil and is readily absorbed by plants, which may cause harm to human health through the food chain (Deng et al., 2014; Ma et al., 2016). Therefore, the remediation of Pb contaminated soil is critical to ensure soil security and the sustainable development of agriculture (García-Delgado et al., 2019). Modern remediation approaches, including physical extraction, chemical immobilization, and bioremediation have been used to alleviate heavy metal toxicity in soils and improve plant performance (Diaconu et al., 2020; Hu et al., 2019; Meng et al., 2020). Previous studies have shown that organic soil amendments may immobilize heavy metals, and are regarded as an environmentally friendly and economically feasible process (Jiang et al., 2012; Lahori et al., 2017; Zama et al., 2018). Adding organic amendments may change heavy metal speciation by precipitation, adsorption, and ion exchange reactions (Li et al., 2017; Liu et al., 2012). Organic amendments may also decrease heavy metal concentrations in plants and available heavy metal contents in soils through different mechanisms, such as metal immobilization in the soil, improve soil fertility and enzyme activity (Al-Wabel et al., 2015). Soil enzymes are involved in nutrient cycling and availability to plants, and the enzymes activity can be used as an indicator of soil health (Zeng et al., 2007).
Biochar is a carbon-rich by-product resulting from the pyrolysis of biological residues in an oxygen-free environment (Meng et al., 2013; Ahmad et al., 2014). Biochar is characterized by an alkaline pH, large surface area, high porosity, high stability, and high amounts of oxygen-containing functional groups on its surface. Biochar contains valuable macro- and micro-nutrients (Lebrun et al., 2021; Liu et al., 2017). It has been used to absorb and immobilize trace element (TE) contaminants, including Pb, and alleviate the phytotoxicity in TE-contaminated soils (Ippolito et al., 2012; Li et al., 2017; Luo et al., 2014; Zwieten et al., 2010). Biochar influences heavy metals speciation and reduces metal phytoavailability through precipitation, sorption, ion exchange reactions, and alteration of soil microbial activity and community structure (Hammer et al., 2014; Liu et al., 2018). Bian et al. (2014) reported that wheat straw biochar significantly reduced Pb mobility and uptake by rice plants. Gao et al. (2020a) found that biochar transformed the heavy metals in soil from available speciation to stable speciation. Biochar may improve soil fertility and plant growth due to its prominent properties of water holding capacity, nutrition retention and soil enzyme activity (Hossain et al., 2010; Spokas et al., 2012).
Biochar has a limited immobilization capacity for heavy metals and cannot release an adequate amount of nutrients to effect soil fertility and plant growth (Gao et al., 2020b; Lehmann, 2007). Farm manure is an organic material that has attracted attention recently for its higher levels of fertility (Liang et al., 2017; Wang et al., 2020). It is a stabilized soil amendment that can enhance and restore soil organic matter, improve water retention and soil structure, and provide available nutrients, including nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulphur (S) and essential TE to promote plant growth (Gajalakshmi & Abbasi, 2008; Kumpiene, Lagerkvist & Maurice, 2008; Lannan, Erich & Ohno, 2013). Applying farm manure can also decrease the availability of heavy metals in soils and plants (Pichtel & Bradway, 2008). Numerous studies have shown that farm manure may reduce the exchangeable fraction of Pb in soil due to Pb ions’ strong affinity for organic complexation sites. Farm manure may reduce Pb phytoavailability by decreasing the soil bulk density to dilute Pb concentration, improve plants’ nutrient uptake, and form immobilized complex between humic acids and Pb (Caballero et al., 2009; Fleming et al., 2013; Kumpiene, Lagerkvist & Maurice, 2008).
Some studies have reported on the additive effects of biochar and other organic amendments to improve soil quality and plant performance in degraded or contaminated soil (Kammann, Glaser & Schmidt, 2016; Głąb et al., 2018). Biochar and farm manure retain nutrition and may stabilize inorganic contaminants through adsorption, binding, and co-precipitation (Karami et al., 2011; Kumpiene, Lagerkvist & Maurice, 2008; Cui et al., 2020; Wang et al., 2020). Chicken excrement is widely used as farm manure; however, there are few studies that compare biochar, chicken manure and their combined effects as a soil amendment in Pb contaminated soil. In this study, a pot culture experiment was conducted to investigate the effects of biochar and chicken manure, individually or in combination, on maize growth, leaves’ antioxidant enzyme activity, Pb uptake, and soil enzyme activities in 800 g·kg−1 Pb contaminated soil. The objective of the present study was to evaluate the additive effects of biochar in combination with chicken manure on maize growth and alleviating Pb stress.
Materials and Methods
Soil sampling and soil amendment materials
We sampled the surface soil at a depth of 0–20 cm from an agricultural field at the Henan University of Science and Technology, Luoyang, China. Soil samples were air-dried and crushed to pass through a 2-mm mesh. The soil was classified as Aquic Ustochrept (US soil taxonomy), and the soil texture was loamy (sand 48.95%, silt 32.24%, clay 18.81%). The soil had a pH of 7.6, 12.62 g·kg−1 organic matter, 0.56 g·kg−1 total N, 46.66 mg·kg−1 available N, 16.13 mg·kg−1 available P, 106.52 mg·kg−1 available K, and 10.23 mg·kg−1 total Pb.
Biochar was provided by the Sanli New Energy Company, Henan Province, China. It was derived from wheat straw pyrolyzed at 450 °C in an oxygen-free environment for 2 h. We analyzed the biochar’s properties according to Lu (2000)’s methodology. Basic biochar’s properties were: 46.8% organic C content, 5.9 g·kg−1 total N, 23.2 g·kg−1 K, 0.89 g·kg−1 available P, 0.53 g·kg−1 dissolved organic carbon, pH of 10.4, and 8.92 m2·g−1 surface area.
Dried, decomposed chicken manure was provided by Luoyang Qihe Ecological Agriculture Science and Technology Co. Ltd, Henan Province, China. Chicken manure’s properties were: pH of 6.7, 41.6% organic C content, 24.3 g·kg−1 total N, 34.3g·kg−1 K, 20.5 g·kg−1 available P, and 0.53 g·kg−1 dissolved organic carbon (DOC).
Pot experiment
The pot experiment included six treatments: the control (CK, no amendment), low chicken manure (LM, chicken manure in soil at 20 g·kg−1), high chicken manure (HM, chicken manure in soil at 40 g·kg−1), low biochar (LB, biochar in soil at 20 g·kg−1), high biochar (HB, biochar in soil at 40 g·kg−1), and a combination of chicken manure and biochar (BM, biochar and chicken manure in soil each at 20 g·kg−1). Each plastic pot (20 cm diameter × 25 cm depth) contained 4 kg of sampling soil. All the amendments were thoroughly mixed into the soil. Pb was added to a level of 800 mg·kg−1 from a Pb (CH3COO)2 solution to simulate moderately Pb contaminated soil. To achieve an equilibrium condition, the simulated contaminated soil was incubated at room temperature for 2 months and irrigated with deionized water to maintain the water holding capacity at about 60%.
Three maize (Zea mays L. cv. Keda No. 16) seeds per plot were sown on May 10, 2019. At 10 days after germination, seedlings were hand-thinned to one plant per plot. There were no additional fertilizers were applied and watered regularly to maintain a soil moisture content of 60–70% of water holding capacity during the growth period. The pots were randomly arranged in the greenhouse, having eight replications of each treatment. Four replicates of each treatment were selected to measure the antioxidant enzyme activities in the leaves 45 days after sowing. At 80 days after sowing, the other four replicates were used to measure maize height, biomass, and Pb content in maize tissues. Soil samples were collected for analysis of soil available Pb, pH, and soil enzyme activity.
Assays of antioxidant enzymatic activities in leaves
Leaf samples (0.5 g) from plants of each treatment group were collected and homogenized in 8 mL of 50 mM potassium phosphate buffer (pH 7.8) under ice-cold conditions to measure MDA content (Salah et al., 2015). MDA was tested using the thiobarbituric acid (TBA) reaction (Heath & Packer, 1968). Superoxide dismutase (SOD) activity levels were determined based on their ability to inhibit nitroblue tetrazolium (NBT) reduction by O−2 radicals (Madhava Rao & Sresty, 2000). Peroxidase (POD) activity levels were assessed using guaiacol as the substrate in 3 mL total volume (Ghosh & Singh, 2005). Catalase (CAT) activity levels were determined using the ultraviolet absorption method according to the H2O2 consumption rate at 240 nm by (Cakmak & Marschner, 1992).
Plant analysis methods
Plant height was recorded with a ruler before harvesting. After plant harvest, roots, shoots, and leaves were separated, and were carefully washed with tap water followed by distilled water. The samples were put in an electric oven until a constant weight to record the dried roots, stems, and leaves biomass and stored for further analysis. The dried plant samples were ground to <0.25 mm in a Retsch MM 400 ball-mill and acid-digested to determine plant Pb concentration by inductively coupled plasma atomic emission spectrometry (ICP-AES, Varian AA240).
Soil analysis methods
After harvesting, maize roots were shaken by hand to remove the loosely adhered soil to allow for the collection of rhizosphere soil. The rhizosphere soil samples were dried, ground, and sieved (2-mm sieve) to test for soil physicochemical parameters, diethylene triaminepentaacetic acid (DTPA) extractable Pb, and soil enzymes activity. Soil pH was measured using an acidity agent (soil water ration of 1: 5) (PHS-3C pH acidometer, China). Soil organic carbon was determined using the potassium dichromate oxidation method after digestion with concentrated sulfuric acid (Kalembasa & Jenkinson, 1973). The available N content in soil was alkali dispersed by 1 mol l−1 NaOH. The available P content was measured with by 0.5 mol l−1 NaHCO3 followed by the molybdenum blue colorimetry method using a UV-2300 spectrophotometer (Tianmei Technology Company, Jiaxing City, China). The available K content was determined using a flame photometer (M410, Sherwood, England). To determine the diethylene triaminepentaacetic acid (DTPA) extractable Pb concentration, we extracted 20 g dried soil with 50 mL of DTPA-TEA (triethanolamine) solution that had a pH of 7.3. The suspension was extracted and the Pb content was measured using inductively coupled plasma atomic emission spectrometry (ICP-AES, Varian AA240) (Lindsay & Norvell, 1978). Urease and sucrase activity were measured using a Perkin-Elmer Lambda 25 spectrophotometer (MA, USA) at a wavelength (λ) of 578 nm and 508 nm, respectively. Catalase activity was determined by hydrogen peroxide decomposition using potassium permanganate (Borowik, Wyszkowska & Wyszkowski, 2017).
Calculating of bioconcentration factor and transfer factor
Two indices were used to evaluate an individual plant’s ability to accumulate and translocate Pb. The bioconcentration factor (BCF) was calculated as: BCF = Caboveground/Csoil, where Caboveground is the average concentration of Pb in stem and leaf tissues and Csoil is the Pb concentration in the soil. The transfer Factor (TF) was calculated using the equation: TF = Caboveground/Croot, where Caboveground is the average concentration of Pb in stem and leaf tissues and Croot is the Pb concentration in the roots (Cakmak & Marschner, 1992).
Statistical analysis
Statistical analyses were conducted with one-way ANOVA using the least significant difference (LSD) test to determine whether the means were significantly different, P < 0.05 was considered to be significant. All experimental data were analyzed using SPSS software (ver. 22.0, SPSS Inc., Chicago, IL, USA), and all bar graphs were drawn using the Origin software (ver. 8.0).
Results
Maize plant height and dry weight
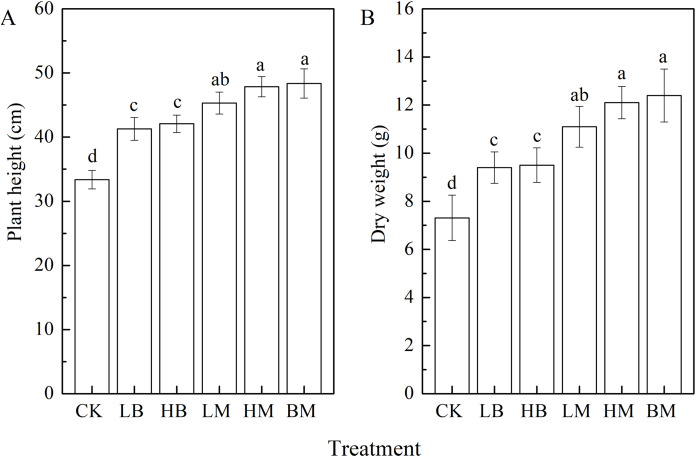
Plant height and dry weight reflected the growth difference between the control and the five amended contaminated soils (Fig. 1). The control treatment, without biochar or chicken manure application, showed the shortest plant height and smallest dry weight. All five biochar and chicken manure treatments significantly improved maize height and dry weight (P < 0.05) compared to the control. Furthermore, chicken manure had a greater effect than biochar, and biochar together with chicken manure had the strongest effect of all. Compared to the control, LB, HB, LM, HM, and BM improved the plant height by 23.67%, 26.06%, 35.80%, 43.23%, and 44.894, respectively (Fig. 1A). They improved the dry weight by 28.59%, 29.96%, 51.84%, 65.53%, and 69.63%, respectively (Fig. 1B).
Figure 1. Plant height (A) and dry weight (B) of maize grown at Pb contaminated soil.
CK, LB, HB, LM, HM, BM represent the control, biochar application of 20 g·kg−1 soil, biochar application of 40 g·kg−1 soil, chicken manure application of 20 g·kg−1 soil, chicken manure application of 40 g·kg−1 soil, a combination of biochar and chicken manure (each at 20 g·kg−1 soil), respectively. Values are means (±SD) of four replicates; different lowercase letters indicate significant differences among different soil amendment treatments according to LSD at P < 0.05.
MDA contents and antioxidant enzymatic activities
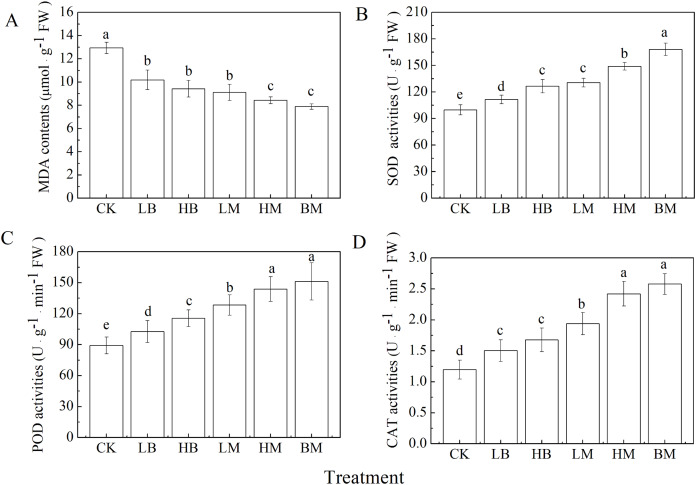
All five biochar and chicken manure treatments significantly decreased MDA contents and increased antioxidant enzymatic activities as compared to the control (Fig. 2) (P < 0.05). The lowest MDA content was observed in the BM treatment, but there was no significant difference with the HM treatment (P > 0.05). No significant differences were found between the LB, HB, and LM treatments (P > 0.05).
Figure 2. Malondialdehyde (MDA) contents (A) and activities of superoxide dismutase (SOD) (B), peroxidases (POD) (C), catalase (CAT) (D) in maize leaves grown at Pb contaminated soil.
CK, LB, HB, LM, HM, BM represent the control, biochar application of 20 g·kg−1 soil, biochar application of 40 g·kg−1 soil, chicken manure application of 20 g·kg−1 soil, chicken manure application of 40 g·kg−1 soil, a combination of biochar and chicken manure (each at 20 g·kg−1 soil), respectively. Values are means (±SD) of four replicates; different lowercase letters indicate significant differences among different soil amendment treatments according to LSD at P < 0.05
The BM treatment demonstrated the most significant (P < 0.05) increase in SOD, POD, and CAT activities by 68.44%, 69.41%, and 115.27%, respectively, when compared to the control. Application of biochar and chicken manure at higher rates significantly (P < 0.05) increased SOD, POD, and CAT activities compared to the lower amendment application rate (Fig. 2). The exception was the biochar only amendment on CAT activities. Furthermore, chicken manure had a greater effect on antioxidant enzymatic activity than biochar.
Pb concentration in maize
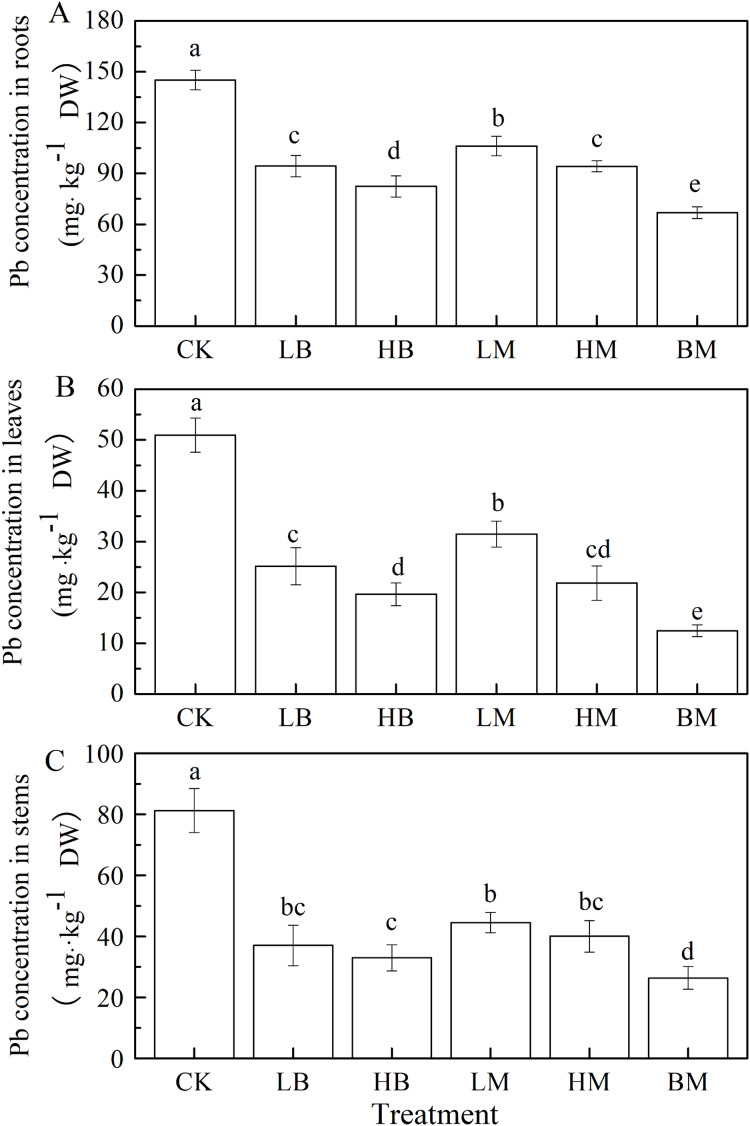
Pb concentrations in maize tissue trended as root>stem>leave (Fig. 3). Amendments of LB, HB, LM, HM, and BM all significantly (P < 0.05) decreased the Pb concentrations in maize tissues. The combined biochar and chicken manure treatment was most effective in decreasing Pb concentration in maize roots, stems and leaves with 53.93%, 75.53% and 67.54%, respectively. The higher application rate of biochar and chicken manure treatments significantly decreased (P < 0.05) the Pb concentration in roots and leaves, compared to the lower application rate. However, no significant difference was found in the stems (P > 0.05). The HB treatment decreased the Pb concentration of the roots, stems and leaves by 43.24%, 61.45%, and 59.39%, respectively. The HM treatment also decreased the Pb concentration of the roots, stems, and leaves by 35.06%, 57.15%, and 50.74%, respectively.
Figure 3. Pb concentrations in roots (A), leaves (B) and stems (C) of maize grown at Pb contaminated soil.
CK, LB, HB, LM, HM, BM represent the control, biochar application of 20 g·kg−1 soil, biochar application of 40 g·kg−1 soil, chicken manure application of 20 g·kg−1 soil, chicken manure application of 40 g·kg−1 soil, a combination of biochar and chicken manure (each at 20 g·kg−1 soil), respectively. Values are means (±SD) of four replicates; different lowercase letters indicate significant differences among different soil amendment treatments according to LSD at P < 0.05.
Effects of different soil amendments on soil physicochemical properties and available Pb content
Applying of biochar and chicken manure amendments alone or in combination all significantly (P < 0.05) decreased the soil DTPA extractable Pb concentrations (Table 1). The trend of DTPA extractable Pb concentrations in the soil was BM<HB<HM<LB<LM<CK (Table 1), and there were significant differences among the different treatments (P < 0.05). These results showed that biochar had a greater effect than chicken manure on decreasing the soil available Pb. The higher application rate of biochar and chicken manure treatments significantly decreased (P < 0.05) the soil DTPA extractable Pb concentration. Biochar and chicken manure in combination had the greatest impact on the reduction in Pb bioavailability.
Table 1. Effect of different treatments on soil physicochemical properties of Pb contaminated soil.
| Treatments | pH | Soil organic carbon (g·kg−1) |
Available N (mg·kg−1) |
Available P (mg·kg−1) |
Available K (mg·kg−1) |
Available Pb (mg·kg−1) |
|---|---|---|---|---|---|---|
| CK | 7.79 ± 0.09c | 11.34 ± 0.22e | 61.51 ± 1.49e | 10.23 ± 0.78d | 106.24 ± 4.23e | 133.21 ± 11.63a |
| LB | 8.21 ± 0.13b | 12.28 ± 0.16d | 73.47 ± 1.78d | 13.85 ± 0.67c | 115.07 ± 4.35d | 103.52 ± 6.24c |
| HB | 8.45 ± 0.14a | 15.23 ± 0.21c | 79.01 ± 1.88c | 17.45 ± 1.21b | 131.48 ± 5.12b | 80.56 ± 4.30e |
| LM | 7.57 ± 0.16d | 16.37 ± 0.25c | 82.01± 0.98b | 15.45 ± 0.91c | 121.28 ± 4.35c | 115.32 ± 7.25b |
| HM | 7.51 ± 0.09d | 18.56 ± 0.34b | 92.34 ± 4.12a | 19.57 ± 1.12a | 145.23 ± 5.07a | 93.65 ± 4.14d |
| BM | 7.91 ± 0.14c | 19.23 ± 0.27a | 91.23 ± 4.78a | 19.43 ± 1.03a | 143.46 ± 4.67a | 64.35 ± 3.22f |
Soil pH significantly (P < 0.05) increased due to amendments with HB and LB, but decreased with the application of HM and LM. No significant difference was found between BM and CK treatment (Table 1). As compared to the lower rate addition of biochar or chicken manure, higher rate of biochar significantly (P < 0.05) increased soil pH, while no significant decreased (P > 0.05) with higher rate of chicken manure. The application with higher rate of biochar showed the highest increase in soil pH by 8.4% compared to the control. All five biochar and chicken manure treatments significantly (P < 0.05) increased soil organic carbon, available nitrogen, available P, and available K contents compared to the control (Table 1). Furthermore, the HB and HM treatments increased the soil organic carbon, available nitrogen, available P, and available K content more than the LB and LM treatments.
Effects of different soil amendments on soil enzyme activities
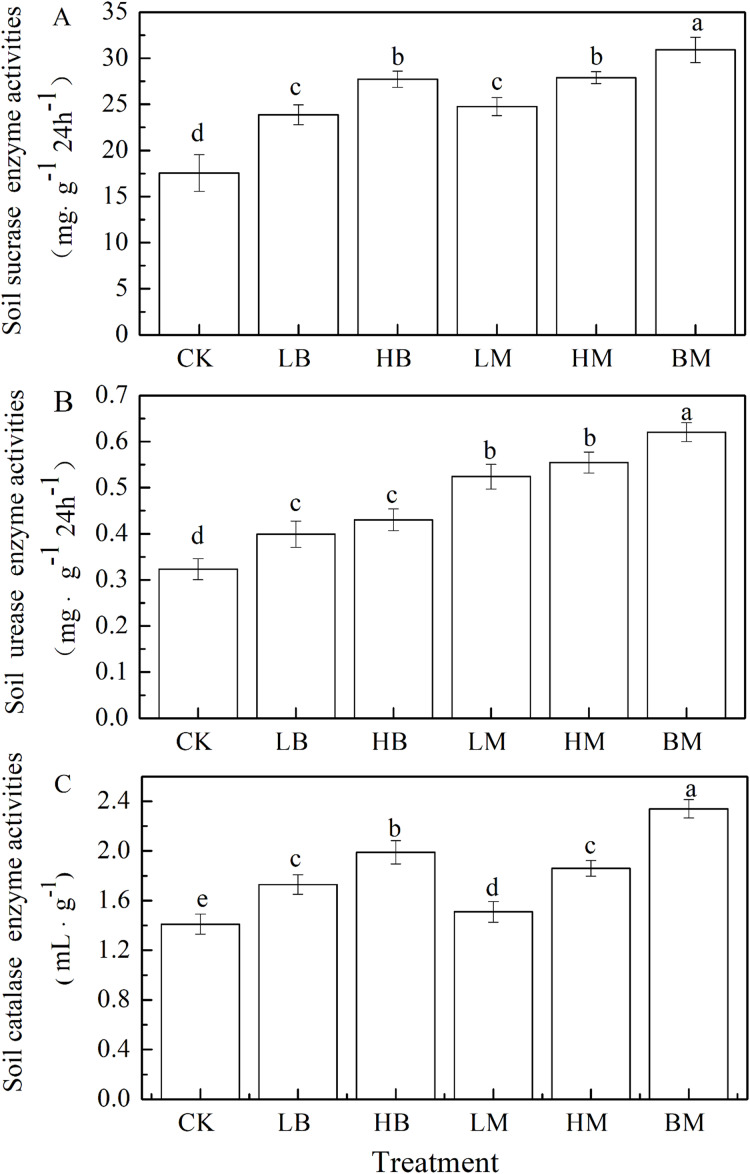
Soil amended with LB, HB, LM, HM, and BM significantly increased sucrase, urease, and catalase activities by 34.21%, 53.02%, 40.95%, 58.81%, and 75.91% (Fig. 4A), 23.33%, 33.03%, 51.03%, 66.35%, and 81.82% (Fig. 4B) and 21.42%, 41.13%, 15.17%, 36.57%, and 65.75% (Fig. 4C), respectively, compared to the control. Biochar amendments were more effecting in catalase activity while chicken manure amendments had a greater impact on the urease activity. There were no significant (P > 0.05) differences on sucrase activity between the biochar and chicken manure amendments. Biochar and chicken manure in combination showed the largest increase in sucrase, urease and catalase activities in Pb-contaminated soil.
Figure 4. Soil enzyme activities of sucrase (A), urease (B) and catalase (C) of maize grown at Pb contaminated soil.
CK, LB, HB, LM, HM, BM represent the control, biochar application of 20 g·kg−1 soil, biochar application of 40 g·kg−1 soil, chicken manure application of 20 g·kg−1 soil, chicken manure application of 40 g·kg−1 soil, a combination of biochar and chicken manure (each at 20 g·kg−1 soil), respectively. Values are means (±SD) of four replicates; different lowercase letters indicate significant differences among different soil amendment treatments according to LSD at P < 0.05.
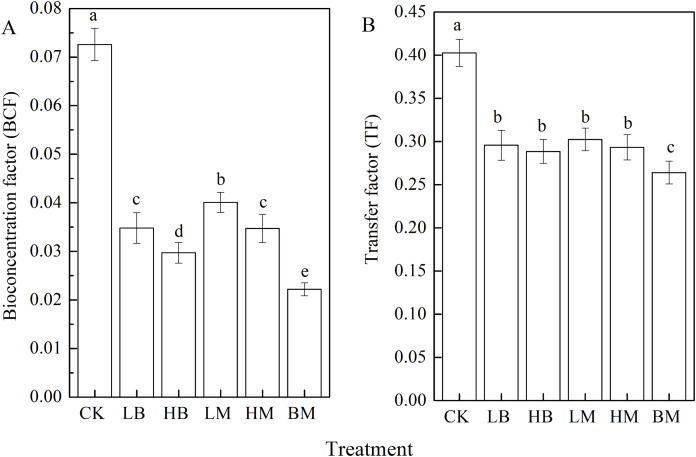
Effects of different soil amendments on BCF and TF of Pb in maize
The five soil amendments significantly (P < 0.05) decreased Pb’s BCF and TF in maize (Fig. 5). Adding LB, HB, LM, HM, and BM decreased the BCF by 52.0%, 59.09%, 44.76%, 52.20%, and 69.42%, respectively, compared to the control. The most significant (P < 0.05) decrease in TF was observed in BM. In BM, TF decreased by 34.42% compared to the control, but no difference was found between LM, HM, LB, and HB (Fig. 5). These results showed that biochar and chicken manure in combination had the best effect, and that biochar was more effective than chicken manure in decreasing Pb translocation from the soil to maize.
Figure 5. Bioconcentration factors (BCF) (A) and transfer factors (TF) (B) of maize grown at Pb contaminated soil.
CK, LB, HB, LM, HM, BM represent the control, biochar application of 20 g·kg−1 soil, biochar application of 40 g·kg−1 soil, chicken manure application of 20 g·kg−1 soil, chicken manure application of 40 g·kg−1 soil, a combination of biochar and chicken manure (each at 20 g·kg−1 soil), respectively. Values are means (±SD) of four replicates; different lowercase letters indicate significant differences among different soil amendment treatments according to LSD at P < 0.05.
Discussion
The present study evaluated the effectiveness of applying biochar and/or chicken manure to improving physicochemical properties of soil, promoting plant growth, and reducing Pb uptake. The results showed that all of the biochar and chicken manure amendments improved the maize height and dry weight under Pb stress (Fig. 1). These results are congruent with previous studies which found that organic amendments increased plant growth and biomass under heavy metal stress (Baker, White & Pierzynski, 2011; Khan et al., 2013; Kiran & Prasad, 2019; Liu et al., 2018; Zhou et al., 2005). The chicken manure amendment alone was more effective at facilitating maize growth and increasing antioxidant enzyme activities in leaves compared to the addition of only biochar. Chicken manure was better able to provide essential nutrients directly to maize, improving the growth of the plant.
Soil organic carbon, N, P, and K are the major limiting factors for crop growth (Kumar, Swaminathan & Kumar, 2009). In this study, all soil amendments significantly increased soil organic carbon, available N, available P, and available K contents while decreasing the available Pb content in the soil as compared to the control (Table 1). Biochar contains numerous carbons, valuable macro-nutrients (especially N, P and K), and some metal ions (e.g., Ca2+ and Mg 2+) (Oustriere et al., 2016) which provide nutrients for plant growth. The surface charge and functional groups of biochar are conducive to the retention of soil nutrients. Biochar has a porous structure, large surface area, and high surface charge density, all of which are favorable to accumulating soil moisture, increasing soil porosity, reducing bulk density, and providing beneficial environment for plant growth. Chicken manure also contains numerous nutrients, which may increase soil nutrient content, improve the structure and stability of soil aggregates, and enhance soil microbial activity (Fleming et al., 2013).
The results showed that maize leaves’ SOD, POD, and CAT activities increased with the application of biochar and chicken manure, alone or in combination (Fig. 2). These amendments scavenged reactive oxygen species (ROS) and alleviated the oxidative damage to biomolecules (Wang et al., 2016). Chicken manure alone was more effective at increasing antioxidant enzyme activities in leaves than the addition of only biochar. Biochar in combination with chicken manure had the greatest effect on increasing antioxidant enzyme activities in leaves. The application of biochar and chicken manure improved the antioxidant enzymatic activity of maize by improving the physiochemical properties of the soil and decreasing Pb uptake (Table 1) (Lu et al., 2014; Walker, Clemente & Bernal, 2004; Wang et al., 2013; Xu et al., 2018).
Soil enzymatic activity was the most important factor of soil fertility and soil amendments (Kong & Chu, 2018; Ouyang et al., 2014). Soil microbial communities secrete intracellular and extracellular enzymes that play a role in the biogeochemical cycle of nutrients in the soil and contribute to the fertility and health of the soil (Mierzwa-Hersztek, Gondek & Baran, 2016). The present experiment demonstrated that biochar and chicken manure amendments significantly increased soil sucrase, urease, and catalase activities under Pb stress (Fig. 4), which are in agreement with previous studies (Awasthi et al., 2017; Bandara et al., 2017; Meng et al., 2018). The combination of biochar and chicken manure application showed the topmost activities of sucrose, urease and catalase in the Pb-contaminated soil, compared to the control. Increased enzymatic activities in the soil may be due to the reduction in Pb stress on microbiota. This may have been achieved by binding Pb in soil and transforming Pb into unavailable speciation (Khan et al., 2020; Naeem et al., 2021). Biochar and chicken manure may improve soil nutrients, water retention capacity, and porosity to create a suitable environment for the soil microbiota. The presence of compounds in biochar and chicken manure, including free radicals, minerals, volatile organics, and labile substrates, also reshape the microbial community to positively influence the enzymatic activities in the soil (Foster et al., 2016; Ge et al., 2010; Mierzwa-Hersztek, Gondek & Baran, 2016; Paz-Ferreiro et al., 2012).
The results showed that applying biochar and/or chicken manure amendments decreased Pb uptake by maize (Figs. 3, 5). Combining biochar and chicken manure decreased Pb uptake by maize most significantly. The biochar amendment had a greater effect than chicken manure on lowering Pb concentrations in maize tissues. Al-Wabel et al. (2015) found that applying biochar decreased Pb concentrations in maize plants. Gul et al. (2016) reported that livestock manure decreased Pb, Zn, Cr, Cu, and Cd concentrations in the roots and shoots of maize. Pb reduction in maize after applying biochar may be due to biochar’s ability to absorb heavy metals. This is possible due to biochar’s large surface area, porous structure, and high surface charge density (Liu et al., 2018; Xu, Cao & Zhao, 2013). The biochar amendment was more effective at increasing soil pH compared to the control. Increasing the soil pH alters heavy metals to their less mobile ionic form and controls heavy metal’s bioavailability in the soil, reducing the heavy metal ionic translocation from soil to maize tissues (Liu et al., 2018; Nie et al., 2018; Wang et al., 2013). The effects of chicken manure amendments in decreasing Pb concentration in maize tissues may be related to the surface charge, metal-binding compounds, humic substances, all of which can produce the adsorption, complexation, precipitation reactions with heavy metals (Park et al., 2011; Wang et al., 2013; Wu et al., 2017).
In the study, the combination of biochar and chicken manure was most effecting in decreasing Pb uptake by maize. There was an additive interaction between biochar and chicken manure to alleviate Pb toxicity and improve maize growth under Pb stress. There were several possible mechanisms for the additive effects of the combined application of biochar and chicken manure. On one hand, the addition of chicken manure compensates for the lack of nutrients in biochar (Sorrenti & Toselli, 2016). Applying biochar increases nutrient retention and prolongs the release period of the nutrients in chicken manure, effectively improving the utilization of organic fertilizer (Foster et al., 2016; Jones et al., 2016; Rehman et al., 2018). A combination of biochar and chicken manure can enhance soil organic carbon, pH, physical adsorption, and surface precipitation ability, of which can sorb and immobilize heavy metals (Belyaeva & Haynes, 2012; Karer et al., 2015; Liang et al., 2017). On the other hand, there is a positive interaction between biochar and chicken manure resulting in crop benefits. Biochar has an impact on the process of manure humification (Zeng et al., 2015) and its surface can be oxidized by the humus and microorganisms found in organic manure (Jones et al., 2016; Wu et al., 2017). The humic matter and mineral oxides in the biochar and manure mixture could produce heavy metals complex (Meng et al., 2018; Oustriere et al., 2016; Zeng et al., 2015). Moreover, biochar can provide an extra source of energy, microporous space and act as a carrier for microorganisms in manure and soil, thus providing a favorable environment for microbial growth to immobilize heavy metal (Liu et al., 2018; Liu et al., 2020; Nawab et al., 2018; Rehman et al., 2016). In brief, biochar and chicken manure are involved in reducing Pb uptake and improving maize growth and there is an additive effect when they are combined. The application ratio of biochar and chicken manure and the mechanisms underlying the effects of the combination of biochar and chicken manure should be investigated in more detail.
Conclusions
The application of biochar or chicken manure alone or in combination, all improved maize growth, antioxidant enzymatic activities and soil enzymatic activities under Pb stress. These soil additives decreased Pb concentrations in maize plants and available Pb concentrations in the soil. The addition of biochar alone was more effective at increasing soil pH, decreasing Pb translocation from soil to maize and contributing to Pb immobilization. Chicken manure alone had a greater effect in promoting maize growth and antioxidant enzymatic activities in leaves. Where biochar and manure were applied as solo amendment, higher application rate was more effective at improving maize growth, antioxidant enzymatic activities and decreasing Pb concentrations in maize tissues and soil. Generally, biochar showed an additive effect with chicken manure for plant growth, antioxidant enzymatic activities and Pb uptake under Pb stress. Higher soil pH, lower available Pb concentrations, and stronger soil enzymatic activities under biochar and chicken manure applications could serve as tactics to be more widely adopted to bind/immobilize Pb. These findings suggest that biochar in combination with chicken manure may be an effective method for remediating Pb-contaminated soil and to promote plant growth.
Supplemental Information
CK, LB, HB, LM, HM, BM represent the control, biochar application of 20 g·kg−1 soil, biochar application of 40 g·kg−1 soil, chicken manure application of 20 g·kg−1 soil, chicken manure application of 40 g·kg−1 soil, a combination of biochar and chicken manure (each at 20 g·kg−1 soil), respectively. Values are means (±SD) of four replicates; different lowercase letters indicate significant differences among different soil amendment treatments according to LSD at P < 0.05
CK, LB, HB, LM, HM, BM represent the control, biochar application of 20 g·kg−1 soil, biochar application of 40 g·kg−1 soil, chicken manure application of 20 g·kg−1 soil, chicken manure application of 40 g·kg−1 soil, a combination of biochar and chicken manure (each at 20 g·kg−1 soil), respectively. Values are means (±SD) of four replicates; different lowercase letters indicate significant differences among different soil amendment treatments according to LSD at P < 0.05
Funding Statement
This work was supported by the National Natural Science foundation of China (31700367 and 31200332). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Guanghai Wu and Hongtao Shen are employed by China Tobacco Henan Industrial Limited Company.
Author Contributions
Ling Liu conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Jiwei Li performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Guanghai Wu performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Hongtao Shen performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Guozhan Fu conceived and designed the experiments, prepared figures and/or tables, and approved the final draft.
Yanfang Wang conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Raw measurements are available in the Supplemental Files.
References
- Adler et al. (2016).Adler A, Devarajan N, Wildi W, Poté J. Metal distribution and characterization of cultivable lead-resistant bacteria in shooting range soils. Soil & Sediment Contamination. 2016;25(4):378–394. doi: 10.1080/15320383.2016.1138929. [DOI] [Google Scholar]
- Ahmad et al. (2014).Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere. 2014;99(09):19–33. doi: 10.1016/j.chemosphere.2013.10.071. [DOI] [PubMed] [Google Scholar]
- Al-Wabel et al. (2015).Al-Wabel MI, Usman ARA, El-Naggar AH, Aly AA, Ibrahim HM, Elmaghraby S, Al-Omran A. Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi Journal of Biological Sciences. 2015;22(4):503–511. doi: 10.1016/j.sjbs.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi et al. (2017).Awasthi MK, Wang Q, Chen HY, Awasthi SK, Wang MJ, Ren XN, Zhao JC, Zhang ZQ. Beneficial effect of mixture of additives amendment on enzymatic activities, organic matter degradation and humification during biosolids co-composting. Bioresource Technology. 2017;247(10):138–146. doi: 10.1016/j.biortech.2017.09.061. [DOI] [PubMed] [Google Scholar]
- Baker, White & Pierzynski (2011).Baker LR, White PM, Pierzynski GM. Changes in microbial properties after manure, lime, and bentonite application to a heavy metal-contaminated mine waste. Applied Soil Ecology. 2011;48(1):1–10. doi: 10.1016/j.apsoil.2011.02.007. [DOI] [Google Scholar]
- Bandara et al. (2017).Bandara T, Herath I, Kumarathilaka P, Seneviratne M, Seneviratne G, Rajakaruna N, Vithanage M, Yong SO. Role of woody biochar and fungal-bacterial co-inoculation on enzyme activity and metal immobilization in serpentine soil. Journal of Soils and Sediments. 2017;17(3):665–673. doi: 10.1007/s11368-015-1243-y. [DOI] [Google Scholar]
- Belyaeva & Haynes (2012).Belyaeva ON, Haynes RJ. Comparison of the effects of conventional organic amendments and biochar on the chemical, physical and microbial properties of coal fly ash as a plant growth medium. Environmental Earth Sciences. 2012;66(7):1987–1997. doi: 10.1007/s12665-011-1424-y. [DOI] [Google Scholar]
- Bian et al. (2014).Bian RJ, Joseph S, Cui LQ, Pan GX, Li LQ, Liu XY, Zhang AF, Rutlidge H, Wong S, Chia C, Marjo C, Gong B, Munroe P, Donn S. A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. Journal of Hazardous Materials. 2014;272:121–128. doi: 10.1016/j.jhazmat.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Borowik, Wyszkowska & Wyszkowski (2017).Borowik A, Wyszkowska J, Wyszkowski M. Resistance of aerobic microorganisms and soil enzyme response to soil contamination with Ekodiesel Ultra fuel. Environmental Science and Pollution Research. 2017;24(31):24346–24363. doi: 10.1007/s11356-017-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero et al. (2009).Caballero R, Pajuelo P, Carmona E, Delgado A. Evaluation and correction of nutrient availability to Gerbera jamesonii H. Bolus in various compost-based growing media. Scientia Horticulturae. 2009;122(2):244–250. doi: 10.1016/j.scienta.2009.05.010. [DOI] [Google Scholar]
- Cakmak & Marschner (1992).Cakmak I, Marschner H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiology. 1992;98:1222–1227. doi: 10.1104/pp.98.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyshuk et al. (2020).Chernyshuk DK, Ivachenko LY, Dogan H, Raza G, Ali MA, Golokhvast KS, Nawaz MA. Dihydroquercetin increases the adaptive potential of wild soybean against copper sulfate and cadmium sulfate toxicity. Turkish Journal of Agriculture and Forestry. 2020;44:492–499. [Google Scholar]
- Cui et al. (2020).Cui H, Ou Y, Wang LX, Yan BX, Li YX, Ding DW. The passivation effect of heavy metals during biochar-amended composting: emphasize on bacterial communities. Waste Management. 2020;118:360–368. doi: 10.1016/j.wasman.2020.08.043. [DOI] [PubMed] [Google Scholar]
- Deng et al. (2014).Deng ZJ, Cao LX, Zhang RD, Wang WF, Shi Y, Tan HM, Wang ZY, Cao LX. Enhanced phytoremediation of multi-metal contaminated soils by interspecific fusion between the protoplasts of endophytic Mucor sp. CBRF59 and Fusarium sp. CBRF14. Soil Biology & Biochemistry. 2014;77:31–40. [Google Scholar]
- Diaconu et al. (2020).Diaconu M, Pavel LV, Hlihor RM, Rosca M, Fertu DI, Lenz M, Corvini PX, Gavrilescu M. Characterization of heavy metal toxicity in some plants and microorganisms-A preliminary approach for environmental bioremediation. New Biotechnology. 2020;56:130–139. doi: 10.1016/j.nbt.2020.01.003. [DOI] [PubMed] [Google Scholar]
- Fleming et al. (2013).Fleming M, Tai Y, Ping Z, Mcbride MB. Extractability and bioavailability of Pb and As in historically contaminated orchard soil: effects of compost amendments. Environmental Pollution. 2013;177:90–97. doi: 10.1016/j.envpol.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster et al. (2016).Foster EJ, Hansen N, Wallenstein M, Cotrufo MF. Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agriculture Ecosystems & Environment. 2016;233(3):404–414. doi: 10.1016/j.agee.2016.09.029. [DOI] [Google Scholar]
- Fu & Wang (2011).Fu FL, Wang Q. Removal of heavy metal ions from wastewaters: a review. Journal of Environmental Management. 2011;92(3):407–418. doi: 10.1016/j.jenvman.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Gajalakshmi & Abbasi (2008).Gajalakshmi S, Abbasi SA. Solid waste management by composting: state of the art. Critical Reviews in Environmental Science and Technology. 2008;38(5):311–400. doi: 10.1080/10643380701413633. [DOI] [Google Scholar]
- Gao et al. (2020a).Gao RL, Hu HQ, Fu QL, Li ZH, Xing ZQ, Ali U, Zhu J, Liu YH. Remediation of Pb, Cd, and Cu contaminated soil by co-pyrolysis biochar derived from rape straw and orthophosphate: speciation transformation, risk evaluation and mechanism inquiry. Science of the Total Environment. 2020a;730(2):139119. doi: 10.1016/j.scitotenv.2020.139119. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2020b).Gao RL, Xiang L, Hu HQ, Fu QL, Zhu J, Liu YH, Huang GY. High-efficiency removal capacities and quantitative sorption mechanisms of Pb by oxidized rape straw biochars. Science of the Total Environment. 2020b;699(5):134262. doi: 10.1016/j.scitotenv.2019.134262. [DOI] [PubMed] [Google Scholar]
- García-Delgado et al. (2019).García-Delgado C, Fresno T, Rodríguez-Santamaría JJ, Diaz E, Mohedano AF, Moreno-Jimenez E. Co-application of activated carbon and compost to contaminated soils: toxic elements mobility and PAH degradation and availability. International Journal of Environmental Science and Technology. 2019;16(2):1057–1068. doi: 10.1007/s13762-018-1751-6. [DOI] [Google Scholar]
- Ge et al. (2010).Ge GF, Li ZJ, Fan FL, Chu GX, Hou ZN, Liang YC. Soil biological activity and their seasonal variations in response to long-term application of organic and inorganic fertilizers. Plant and Soil. 2010;326(1–2):31–44. doi: 10.1007/s11104-009-0186-8. [DOI] [Google Scholar]
- Ghosh & Singh (2005).Ghosh M, Singh SP. A comparative study of cadmium phytoextraction by accumulator and weed species. Environmental Pollution. 2005;133(2):365–371. doi: 10.1016/j.envpol.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Głąb et al. (2018).Głąb T, Żabiński A, Sadowska U, Gondek K, Kopeć M, Mierzwa-Hersztek M, Tabor S. Effects of co-composted maize, sewage sludge, and biochar mixtures on hydrological and physical qualities of sandy soil. Geoderma. 2018;315(3):27–35. doi: 10.1016/j.geoderma.2017.11.034. [DOI] [Google Scholar]
- Gul et al. (2016).Gul S, Naz A, Khan A, Nisa S, Irshad M. Phytoavailability and leachability of heavy metals from contaminated soil treated with composted livestock manure. Journal of Soil Contamination. 2016;25:181–194. [Google Scholar]
- Hammer et al. (2014).Hammer EC, Balogh-Brunstad Z, Jakobsen I, Olsson PA, Stipp SLS, Rillig MC. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biology & Biochemistry. 2014;77:252–260. [Google Scholar]
- Heath & Packer (1968).Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hossain et al. (2010).Hossain MK, Strezov V, Chan KY, Nelson PF. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum) Chemosphere. 2010;78:1167–1171. doi: 10.1016/j.chemosphere.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2019).Hu ZH, Zhuo F, Jing SH, Li X, Yan TX, Lei LL, Lu RR, Zhang XF, Jing YX. Combined application of arbuscular mycorrhizal fungi and steel slag improves plant growth and reduces Cd, Pb accumulation in Zea mays. International Journal of Phytoremediation. 2019;21:857–865. doi: 10.1080/15226514.2019.1577355. [DOI] [PubMed] [Google Scholar]
- Ippolito et al. (2012).Ippolito JA, Strawn DG, Scheckel KG, Novak JM, Ahmedna M, Niandou MAS. Macroscopic and molecular investigations of copper sorption by a steam-activated biochar. Journal of Environmental Quality. 2012;41(4):1150–1156. doi: 10.2134/jeq2011.0113. [DOI] [PubMed] [Google Scholar]
- Jiang et al. (2012).Jiang J, Xu RK, Jiang TY, Li Z. Immobilization of Cu(II), Pb(II) and Cd(II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. Journal of Hazardous Materials. 2012;229:145–150. doi: 10.1016/j.jhazmat.2012.05.086. [DOI] [PubMed] [Google Scholar]
- Jones et al. (2016).Jones S, Bardos RP, Kidd PS, Mench M, De LF, Hutchings T, Cundy A, Joyce C, Soja G, Friesl-Hanl W. Biochar and compost amendments enhance copper immobilisation and support plant growth in contaminated soils. Journal of Environmental Management. 2016;171(3):101–112. doi: 10.1016/j.jenvman.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Kalembasa & Jenkinson (1973).Kalembasa SJ, Jenkinson DS. A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. Journal of the Science of Food and Agriculture. 1973;24:1085–1090. doi: 10.1002/(ISSN)1097-0010. [DOI] [Google Scholar]
- Kammann, Glaser & Schmidt (2016).Kammann CI, Glaser B, Schmidt HP. Combining biochar and organic amendments. London: routledge; 2016. [Google Scholar]
- Karami et al. (2011).Karami N, Clemente R, Moreno-Jiménez E, Lepp NW, Beesley L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. Journal of Hazardous Materials. 2011;191(1–3):41–48. doi: 10.1016/j.jhazmat.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Karer et al. (2015).Karer J, Wawra A, Zehetner F, Dunst G, Wagner M, Pavel PB, Puschenreiter M, Friesl-Hanl W, Soja G. Effects of biochars and compost mixtures and inorganic additives on immobilisation of heavy metals in contaminated soils. Water Air and Soil Pollution. 2015;226:342. [Google Scholar]
- Khan et al. (2020).Khan MA, Mahmood-ur-Rahman, Ramzani PMA, Zubair M, Rasool B, Khan MK, Ahmed A, Khan SA, Turan V, Iqbal M. Associative effects of lignin-derived biochar and arbuscular mycorrhizal fungi applied to soil polluted from Pb-acid batteries effluents on barley grain safety. Science of the Total Environment. 2020;710:136294. doi: 10.1016/j.scitotenv.2019.136294. [DOI] [PubMed] [Google Scholar]
- Khan et al. (2013).Khan S, Chao C, Waqas M, Arp HP, Zhu YG. Sewage sludge biochar influence upon rice (Oryza sativa L) yield, metal bioaccumulation and greenhouse gas emissions from acidic paddy soil. Environmental Science & Technology. 2013;47:8624–8632. doi: 10.1021/es400554x. [DOI] [PubMed] [Google Scholar]
- Kiran & Prasad (2019).Kiran BR, Prasad MNV. Biochar and rice husk ash assisted phytoremediation potentials of Ricinus communis L. for lead-spiked soils. Ecotoxicology and Environmental Safety. 2019;183:109574. doi: 10.1016/j.ecoenv.2019.109574. [DOI] [PubMed] [Google Scholar]
- Kong & Chu (2018).Kong L, Chu LM. Subtropical urban turfs: carbon and nitrogen pools and the role of enzyme activity. Journal of Environmental Sciences. 2018;65:18–28. doi: 10.1016/j.jes.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Kumar, Swaminathan & Kumar (2009).Kumar TS, Swaminathan V, Kumar S. Influence of nitrogen, phosphorus and biofertilizers on growth, yield and essential oil constituents in ratoon crop of davana (Artemisia pallens Wall.) Electronic Journal of Environmental, Agricultural and Food Chemistry. 2009;8:86–95. [Google Scholar]
- Kumpiene, Lagerkvist & Maurice (2008).Kumpiene J, Lagerkvist A, Maurice C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments-a review. Waste Management. 2008;28:215–225. doi: 10.1016/j.wasman.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Lahori et al. (2017).Lahori AH, Guo Z, Zhang Z, Li R, Mahar A, Awasthi MK, Shen F, Kumbhar F, Sial TA, Kumbhar F, Wang P, Jiang S. Use of biochar as an amendment for remediation of heavy metal contaminated soils: prospects and challenges. Pedosphere. 2017;27:991–1014. [Google Scholar]
- Lannan, Erich & Ohno (2013).Lannan AP, Erich MS, Ohno T. Compost feedstock and maturity level affect soil response to amendment. Biology & Fertility of Soils. 2013;49:273–285. [Google Scholar]
- Lebrun et al. (2021).Lebrun M, Nandillon R, Miard F, Bourgerie S, Visser R, Morabito D. Biochar application modifies soil properties of a former mine technosol: SEM/EDS study to investigate Pb and As speciation. Biomass Conversion and Biorefinery. 2021. Epub ahead of print 15 January 2021. [DOI]
- Lehmann (2007).Lehmann J. Bio-energy in the black. Frontiers in Ecology & the Environment. 2007;5:381–387. [Google Scholar]
- Li et al. (2017).Li H, Dong X, da Silva EB, de Oliveira LM, Chen Y, Ma LQ. Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere. 2017;178:466–478. doi: 10.1016/j.chemosphere.2017.03.072. [DOI] [PubMed] [Google Scholar]
- Liang et al. (2017).Liang J, Li XM, Yu ZG, Zeng GM, Luo Y, Jiang LB, Yang ZX, Qian YY, Wu HP. Amorphous MnO2 Modified Biochar derived from aerobically composted swine manure for adsorption of Pb(II) and Cd(II) ACS Sustainable Chemistry & Engineering. 2017;5:5049–5058. [Google Scholar]
- Lindsay & Norvell (1978).Lindsay WL, Norvell WA. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Science Society of America Journal. 1978;42:421–428. [Google Scholar]
- Liu et al. (2012).Liu L, Hu LL, Tang JJ, Li YF, Zhang Q, Chen X. Food safety assessment of planting patterns of four vegetable-type crops grown in soil contaminated by electronic waste activities. Journal of Environmental Management. 2012;93:22–30. doi: 10.1016/j.jenvman.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2020).Liu L, Li D, Ma YL, Shen HT, Zhao SM, Wang YF. Combined application of arbuscular mycorrhizal fungi and exogenous melatonin alleviates drought stress and improves plant growth in tobacco seedlings. Journal of Plant Growth Regulation. 2020;40(3):1074–1087. doi: 10.1007/s00344-020-10165-6. [DOI] [Google Scholar]
- Liu et al. (2018).Liu L, Li JW, Yue FX, Yan XW, Wang FT, Bloszies S, Wang YF. Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere. 2018;194:495–503. doi: 10.1016/j.chemosphere.2017.12.025. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2017).Liu L, Wang YF, Yan XW, Li J, Jiao NY, Hu SJ. Biochar amendments increase the yield advantage of legume-based intercropping systems over monoculture. Agriculture Ecosystems & Environment. 2017;237:16–23. doi: 10.1016/j.agee.2016.12.026. [DOI] [Google Scholar]
- Lu et al. (2014).Lu KP, Yang X, Shen JJ, Robinson B, Huang HG, Liu D, Bolan N, Pei JC, Wang HL. Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agriculture Ecosystems & Environment. 2014;191(Suppl.):124–132. doi: 10.1016/j.agee.2014.04.010. [DOI] [Google Scholar]
- Lu (2000).Lu RK. Methods of soil and agro-chemical analysis. Beijing: China Agricultural Science Technology Press; 2000. [Google Scholar]
- Luo et al. (2014).Luo F, Song J, Xia WX, Dong MG, Chen MF, Soudek P. Characterization of contaminants and evaluation of the suitability for land application of maize and sludge biochars. Environmental Science and Pollution Research. 2014;21(14):8707–8717. doi: 10.1007/s11356-014-2797-8. [DOI] [PubMed] [Google Scholar]
- Ma et al. (2016).Ma XL, Zuo H, Tian MJ, Zhang LY, Meng J, Zhou XN, Min N, Chang XY, Liu Y. Assessment of heavy metals contamination in sediments from three adjacent regions of the Yellow River using metal chemical fractions and multivariate analysis techniques. Chemosphere. 2016;144:264–272. doi: 10.1016/j.chemosphere.2015.08.026. [DOI] [PubMed] [Google Scholar]
- Madhava Rao & Sresty (2000).Madhava Rao KV, Sresty TVS. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Science. 2000;157:113–128. doi: 10.1016/s0168-9452(00)00273-9. [DOI] [PubMed] [Google Scholar]
- Meng et al. (2020).Meng J, Cui ZH, Zhang HL, Zhang J, Tang XJ, Wong MH, Shan SD. Combined effects of arbuscular mycorrhizae fungus and composted pig manure on the growth of ryegrass and uptake of Cd and Zn in the soil from an e-waste recycling site. Environmental Science and Pollution Research. 2020;28(10):12677–12685. doi: 10.1007/s11356-020-11215-y. [DOI] [PubMed] [Google Scholar]
- Meng et al. (2018).Meng J, Tao M, Wang L, Liu X, Xu J. Changes in heavy metal bioavailability and speciation from a Pb-Zn mining soil amended with biochars from co-pyrolysis of rice straw and swine manure. Science of the Total Environment. 2018;633:300–307. doi: 10.1016/j.scitotenv.2018.03.199. [DOI] [PubMed] [Google Scholar]
- Meng et al. (2013).Meng J, Wang LL, Liu XM, Wu JJ, Brookes PC, Xu JM. Physicochemical properties of biochar produced from aerobically composted swine manure and its potential use as an environmental amendment. Bioresource Technology. 2013;142:641–646. doi: 10.1016/j.biortech.2013.05.086. [DOI] [PubMed] [Google Scholar]
- Mierzwa-Hersztek, Gondek & Baran (2016).Mierzwa-Hersztek M, Gondek K, Baran A. Effect of poultry litter biochar on soil enzymatic activity, ecotoxicity and plant growth. Applied Soil Ecology. 2016;105:144–150. [Google Scholar]
- Naeem et al. (2021).Naeem I, Masood N, Turan V, Iqbal M. Prospective usage of magnesium potassium phosphate cement combined with Bougainvillea alba derived biochar to reduce Pb bioavailability in soil and its uptake by Spinacia oleracea L. Ecotoxicology and Environmental Safety. 2021;208:111723. doi: 10.1016/j.ecoenv.2020.111723. [DOI] [PubMed] [Google Scholar]
- Nawab et al. (2018).Nawab J, Ghani J, Khan S, Khan S, Wang XP. Minimizing the risk to human health due to the ingestion of arsenic and toxic metals in vegetables by the application of biochar, farmyard manure and peat moss. Journal of Environmental Management. 2018;214:172–183. doi: 10.1016/j.jenvman.2018.02.093. [DOI] [PubMed] [Google Scholar]
- Nie et al. (2018).Nie C, Yang X, Niazi NK, Xu X, Wen Y, Rinklebe J, Yong SO, Xu S, Wang H. Impact of sugarcane bagasse-derived biochar on heavy metal availability and microbial activity: a field study. Chemosphere. 2018;200:274–282. doi: 10.1016/j.chemosphere.2018.02.134. [DOI] [PubMed] [Google Scholar]
- Oustriere et al. (2016).Oustriere N, Marchand L, Galland W, Gabbon L, Lottier N, Motelica M, Mench M. Influence of biochars, compost and iron grit, alone and in combination, on copper solubility and phytotoxicity in a Cu-contaminated soil from a wood preservation site. Science of the Total Environment. 2016;566:816–825. doi: 10.1016/j.scitotenv.2016.05.091. [DOI] [PubMed] [Google Scholar]
- Ouyang et al. (2014).Ouyang L, Tang Q, Yu LQ, Zhang RD. Effects of amendment of different biochars on soil enzyme activities related to carbon mineralisation. Soil Research. 2014;52:706–716. [Google Scholar]
- Park et al. (2011).Park JH, Lamb D, Paneerselvam P, Choppala G, Bolan N, Chung JW. Role of organic amendments on enhanced bioremediation of heavy metal (loid) contaminated soils. Journal of Hazardous Materials. 2011;185:549–574. doi: 10.1016/j.jhazmat.2010.09.082. [DOI] [PubMed] [Google Scholar]
- Paz-Ferreiro et al. (2012).Paz-Ferreiro J, Gascó G, Gutiérrez B, Méndez A. Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biology and Fertility of Soils. 2012;48:511–517. [Google Scholar]
- Pichtel & Bradway (2008).Pichtel J, Bradway D. Conventional crops and organic amendments for Pb, Cd and Zn treatment at a severely contaminated site. Bioresource Technology. 2008;99:1242–1251. doi: 10.1016/j.biortech.2007.02.042. [DOI] [PubMed] [Google Scholar]
- Rehman et al. (2016).Rehman MZU, Rizwan M, Ali S, Fatima N, Yousaf B, Naeem A, Sabir M, Ahmad HR, Ok YS. Contrasting effects of biochar, compost and farm manure on alleviation of nickel toxicity in maize (Zea mays L.) in relation to plant growth, photosynthesis and metal uptake. Ecotoxicology & Environmental Safety. 2016;133:218–225. doi: 10.1016/j.ecoenv.2016.07.023. [DOI] [PubMed] [Google Scholar]
- Rehman et al. (2018).Rehman MZU, Rizwan M, Khalid H, Ali S, Naeem A, Yousaf B, Liu GJ, Sabir M, Farooq M. Farmyard manure alone and combined with immobilizing amendments reduced cadmium accumulation in wheat and rice grains grown in field irrigated with raw effluents. Chemosphere. 2018;199:468–476. doi: 10.1016/j.chemosphere.2018.02.030. [DOI] [PubMed] [Google Scholar]
- Salah et al. (2015).Salah SM, Guan YJ, Cao DD, Li J, Aamir N, Hu QJ, Hu WM, Ning MY, Hu J. Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Scientific Reports. 2015;5:14278. doi: 10.1038/srep14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrenti & Toselli (2016).Sorrenti G, Toselli M. Soil leaching as affected by the amendment with biochar and compost. Agriculture Ecosystems & Environment. 2016;226:56–64. [Google Scholar]
- Spokas et al. (2012).Spokas KA, Cantrell KB, Novak JM, Archer DW, Ippolito JA, Collins HP, Collins HP, Boateng AA, Lima IM, Lamb MC, Mcaloon AJ. Biochar: a synthesis of its agronomic impact beyond carbon sequestration. Journal of Environmental Quality. 2012;41:973–989. doi: 10.2134/jeq2011.0069. [DOI] [PubMed] [Google Scholar]
- Walker, Clemente & Bernal (2004).Walker DJ, Clemente R, Bernal MP. Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in a soil contaminated by pyritic mine waste. Chemosphere. 2004;57:215–224. doi: 10.1016/j.chemosphere.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2016).Wang FY, Liu XQ, Shi ZY, Tong RJ, Adams CA, Shi XJ. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants-a soil microcosm experiment. Chemosphere. 2016;147:88–97. doi: 10.1016/j.chemosphere.2015.12.076. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2013).Wang FY, Shi ZY, Xu XF, Wang XG, Li YJ. Contribution of AM inoculation and cattle manure to lead and cadmium phytoremediation by tobacco plants. Environmental Science Processes & Impacts. 2013;15(4):794–801. doi: 10.1039/c3em30937a. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2020).Wang QQ, Huang Q, Guo GM, Qin JM, Luo JY, Zhu ZQ, Hong Y, Xu YX, Hu S, Hu W, Yang C, Wang JF. Reducing bioavailability of heavy metals in contaminated soil and uptake by maize using organic-inorganic mixed fertilizer. Chemosphere. 2020;261:128122. doi: 10.1016/j.chemosphere.2020.128122. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2017).Wu H, Lai C, Zeng G, Liang J, Chen J, Xu J, Dai J, Li X, Liu J, Chen M, Lu L, Hu L, Wan J. The interactions of composting and biochar and their implications for soil amendment and pollution remediation: a review. Critical Reviews in Biotechnology. 2017;37(6):754–764. doi: 10.1080/07388551.2016.1232696. [DOI] [PubMed] [Google Scholar]
- Xu, Cao & Zhao (2013).Xu XY, Cao XD, Zhao L. Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: role of mineral components in biochars. Chemosphere. 2013;92(8):955–961. doi: 10.1016/j.chemosphere.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2018).Xu YL, Seshadri B, Sarkar B, Wang HL, Rumpel C, Sparks D, Farrell M, Hall T, Yang XD, Bolan N. Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Science of the Total Environment. 2018;621(2):148–159. doi: 10.1016/j.scitotenv.2017.11.214. [DOI] [PubMed] [Google Scholar]
- Zama et al. (2018).Zama EF, Reid BJ, Arp HPH, Sun GX, Yuan HY, Zhu YG. Advances in research on the use of biochar in soil for remediation: a review. Journal of Soils and Sediments. 2018;18:2433–2450. [Google Scholar]
- Zeng et al. (2015).Zeng GM, Wu HP, Liang J, Guo SL, Huang L, Xu P, Liu YY, Yuan YJ, He XX, He Y. Efficiency of biochar and compost (or composting) combined amendments for reducing Cd, Cu, Zn and Pb bioavailability, mobility and ecological risk in wetland soil. RSC Advances. 2015;5:34541–34548. [Google Scholar]
- Zeng et al. (2007).Zeng LS, Liao M, Chen CL, Huang CY. Effects of lead contamination on soil enzymatic activities, microbial biomass, and rice physiological indices in soil-lead–rice (Oryza sativa L.) system. Ecotoxicology and Environmental Safety. 2007;67:67–74. doi: 10.1016/j.ecoenv.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2018).Zhang L, Zhu G, Ge X, Xu G, Guan Y. Novel insights into heavy metal pollution of farmland based on reactive heavy metals (RHMs): pollution characteristics, predictive models, and quantitative source apportionment. Journal of Hazardous Materials. 2018;360:32–42. doi: 10.1016/j.jhazmat.2018.07.075. [DOI] [PubMed] [Google Scholar]
- Zhou et al. (2005).Zhou DM, Hao XZ, Wang YJ, Dong YH, Cang L. Copper and Zn uptake by radish and pakchoi as affected by application of livestock and poultry manures. Chemosphere. 2005;59:167–175. doi: 10.1016/j.chemosphere.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Zwieten et al. (2010).Zwieten LV, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant and Soil. 2010;327(1–2):235–246. doi: 10.1007/s11104-009-0050-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CK, LB, HB, LM, HM, BM represent the control, biochar application of 20 g·kg−1 soil, biochar application of 40 g·kg−1 soil, chicken manure application of 20 g·kg−1 soil, chicken manure application of 40 g·kg−1 soil, a combination of biochar and chicken manure (each at 20 g·kg−1 soil), respectively. Values are means (±SD) of four replicates; different lowercase letters indicate significant differences among different soil amendment treatments according to LSD at P < 0.05
CK, LB, HB, LM, HM, BM represent the control, biochar application of 20 g·kg−1 soil, biochar application of 40 g·kg−1 soil, chicken manure application of 20 g·kg−1 soil, chicken manure application of 40 g·kg−1 soil, a combination of biochar and chicken manure (each at 20 g·kg−1 soil), respectively. Values are means (±SD) of four replicates; different lowercase letters indicate significant differences among different soil amendment treatments according to LSD at P < 0.05
Data Availability Statement
The following information was supplied regarding data availability:
Raw measurements are available in the Supplemental Files.