ABSTRACT
Endophytic fungi usually establish a symbiotic relationship with the host plant and affect its growth. In order to evaluate the impact of endophytic fungi on the Chinese herbal medicinal plant Houttuynia cordata Thunb., three endophytes isolated from the rhizomes of H. cordata, namely Ilyonectria liriodendra (IL), unidentified fungal sp. (UF), and Penicillium citrinum (PC), were co-cultured individually with H. cordata in sterile soil for 60 days. Analysis of the results showed that the endophytes stimulated the host plant in different ways: IL increased the growth of rhizomes and the accumulation of most of the phenolics and volatiles, UF promoted the accumulation of the medicinal compounds afzelin, decanal, 2-undecanone, and borneol without influencing host plant growth, and PC increased the fresh weight, total leaf area and height of the plants, as well as the growth of the rhizomes, but had only a small effect on the concentration of major secondary metabolites. Our results proved that the endophytic fungi had potential practical value in terms of the production of Chinese herbal medicines, having the ability to improve the yield and accumulation of medicinal metabolites.
KEYWORDS: Endophytic fungi, chinese medicinal plant, medicinal metabolites, phenolics, volatile compounds
Introduction
Plants usually contain a number of fungal species within their tissues during part or all of their life cycle, and the complex relationship between host plants and their endophytic fungi has aroused considerable research interest.1 Host plants usually provide shelter and nutrients for endophytic fungi, protecting them from the harsh natural environment. In return, many endophytic fungi have the ability to enhance the host plant’s tolerance to biotic or abiotic stresses, including attack by phytophagous insects or pathogens, or stressful conditions, such as salinity or drought.2–5 Fungal endophytes can also produce various products, such as phytohormones, siderophores, hydrogen cyanide, phosphate-solubilizing agents, and hydrolytic enzymes.6 These metabolites can play a role in promoting plant growth and the accumulation of bioactive metabolites, affecting the uptake or redistribution of resources,7 which can greatly improve the vigor of the host plant.
Currently, there is considerable active research into fungal endophytes from medicinal plants, and the medicinal potential of fungal endophytes has been widely reported.8 To adapt to the internal environment of the host plant tissue, endophytes may biosynthesize the same metabolites as the host, such as paclitaxel from Taxus baccata, or isocoumarin flavonoids from Ginkgo biloba.9,10 On the other hand, natural products from endophytes, such as alkaloids, phenolics, peptides, steroids, flavonoids, and quinines, can also exhibit medicinal activities, including antimicrobial properties and cytotoxicity against cancer cells.11,12 Fungal metabolites of potential medicinal value and growth-promoting effects of the endophytes at the host seedling stage have been widely reported [13, Jian et al., 2017, 14], although very little attention has yet been paid as to whether a single endophyte fungal species can contribute significantly to the yield of bioactive metabolites by medicinal plants.
Houttuynia cordata Thunb. is a traditional Chinese herbal ethnomedicinal plant with a long history, as well as being a popular condiment in Southwest China due to its special aroma. It is also distributed in countries in Southeast Asia, where it is eaten by the locals.15 H. cordata has been confirmed to exhibit a number of pharmaceutical activities, including antibacterial, antiviral, anti-inflammatory, immunological, anticancer, and anti-mutagenic effects,16 with volatile oils and flavonoids being reported to be particularly active medicinal metabolites.17 Many species of endophytic fungi have been isolated and identified from healthy host plant tissues of H. cordata.18 However, the role of single endophytic fungal species in H. cordata has rarely been directly examined. Revealing the effects of individual endophytic fungi in H. cordata and tapping into their value in the traditional Chinese herbal medicine industry may provide a novel way by which to solve the current disconnect between production capacity and consumer demand.
Taking into consideration the widely accepted view that many endophytes are beneficial for the host plants, we hypothesized that endophytic fungi positively affect plant growth and the accumulation of secondary metabolites. Seedlings grown in vitro are an ideal experimental material as they contain very few native or opportunistic endophytic microorganisms. To test our hypothesis, we inoculated the seedlings individually with three endophytic fungi, the fungi used having been isolated from rhizome tissue of H. cordata and identified in our previous work, namely Ilyonectria liriodendra, an unidentified fungal sp., and Penicillium citrinum. Then, we evaluated and identified any significant differences among treatments, including non-inoculated control seedlings, using morphological parameters, antioxidant enzyme activities, and concentrations of phenolics and volatile secondary metabolites, to assess the ability of those endophytic fungi to promote plant growth and the yield of bioactive secondary metabolism.
Materials and methods
Fungal strains and in-vitro grown seedlings.
Three endophytic fungi had been isolated from healthy H. cordata rhizomes and identified in our previous work. Freshly harvested, healthy H. cordata rhizomes were rinsed with tap water for 6 h and then cut into segments 6‒8 cm long. The rhizomes were then surface sterilized by immersion in 75% (v/v) ethanol for 3 min, followed by 0.2% (w/v) HgCl2 for 5 min, and finally rinsed five times in sterile water for 2 min each time. The rhizome segments were cut longitudinally and incubated on potato dextrose agar medium (PDA; potato 200 g/L, agar 20 g/L, glucose 20 g/L) containing 30 μg/mL streptomycin at 28°C for 10 days in the dark. Each fungal colony obtained was purified on PDA, and were identified (100% identity) as Ilyonectria liriodendra, an unidentified fungal sp., and Penicillium citrinum by comparing nuclear rRNA ITS DNA sequences with those on the GenBank database (https://www.ncbi.nlm.nih.gov/).
Seeds of H. cordata were sterilized in 75% ethanol for 30 s, followed by 0.2% HgCl2 (w/v) for 5 min, and cultured on half-strength Murashige & Skoog (1/2 MS) medium containing 0.5 mg/L GA3 (gibberellin A3) for 60 d. The seedlings were then transplanted to 1/2 MS medium + 0.1 mg/L KT (kinetin) + 0.3 mg/L NAA (naphthalene acetic acid) + 0.2 mg/L GA3 for 40 d. The culture conditions were temperature 23°C – 25°C, light intensity 1,500–2,000 lx, under a 12-h light/12-h dark photoperiod.19
Spore or hyphal suspension preparations
A sterilized coverslip was used to scrape the spores of IL and PC growing on the PDA culture medium into a 50-mL beaker, after which 30 mL of sterile water were added to rinse material from the coverslip. The mixture was stirred and filtered to obtain a pure spore suspension of each isolate. UF did not produce spores on PDA, so a fungal colony (6-cm diameter) was scraped from PDA into fragments, which were added to 30 mL sterile water in a 50-mL beaker, and the resulting hyphal fragment suspension was collected. Mycelial fragments of UF with a length of more than 25 µm were regarded as active fragments, and the spore or hyphal fragment concentration was adjusted to 1.0 × 103 colony-forming units (CFU)/mL by the aid of a hemocytometer viewed under a microscope.20,21
Establishment of a co-culture system between H. cordata and individual endophytic fungi in soil.
Yellow soil was air dried and passed through a 2-mm sieve to remove stones and plant debris, then packed into glass bottles for high-temperature sterilization at 121°C for 60 min.22 After cooling, the soil was transferred into sterile plastic seedling bags (10 × 15 cm). The agar medium attached to the roots of in vitro-grown seedlings of H. cordata was washed away, and the seedlings were planted into the soil in the seedling bags, one seedling per bag. After 14 days of acclimation, the spore or hyphal suspension (20 mL) of one of the three fungi were irrigated into the soil at 3 cm around the seedling, with the same volume of sterile water being used instead with the control (CON) seedlings, with a further 20 mL fungal suspension sterile or water added every seven days. Three replicates were set up for each treatment (and the corresponding control). This experiment ran for 60 d, from July to September, at temperatures of 20 ± 5°C, and 60–90% relative humidity, under natural light conditions.23
Determination of morphological parameters
After 60 d, seedlings were washed under running water to remove soil, and surface moisture was absorbed using filter paper. The total seedling fresh weight, rhizome length, and seedling height were measured. The total area of leaves was determined by an indirect representation as leaf length × leaf width × 0.7.2425 All three replicate seedlings were measured for each treatment.
Determination of chlorophyll and malondialdehyde concentrations in H. cordata
All extractions and determinations followed the methods described in 25, and were carried out separately on the three replicate seedlings for each treatment.
The third leaf from the top of each plant was collected and cut into pieces. A 100-mg subsample of leaf tissue was ground together with quartz and calcium carbonate powder and placed into a 20-mL brown glass bottle containing 15 mL 80% (v/v) acetone, ultrasonic extracted for 15 minutes and filtered. The chlorophyll concentration was determined from the absorbance values at 645 and 663 nm. The total chlorophyll = [(20.2 × A645) + (8.02 × A663)]
The level of lipid peroxidation was expressed as the concentration of malondialdehyde (MDA). A sample of 200 mg leaf fresh weight was homogenized in 10 mL of 10% trichloroacetic acid, followed by centrifugation at 3,000 rpm for 10 min, after which 2 mL supernatant was added to 2 mL of 0.6% thiobarbituric acid. The mixture was heated at 100°C for 15 min, then quickly cooled on ice and centrifuged as before. The absorbances of the supernatant at 532 nm, 600 nm, and 450 nm were recorded, and the concentration of MDA (CMDA) in extract was determined from the equation:
CMDA (μmol/L) = [6.45 × (A532−A600) − [0.56 ×A450)]
Determination of the activities of antioxidant enzymes in H. cordata
All extractions and enzyme assays were carried out according to the methods described by 25. Three biological replicates were measured for each treatment, one per replicate seedling.
Superoxide dismutase (SOD] activity was quantified by the method using nitroblue tetrazolium (NBT). A sample (200 mg) of sliced fresh leaf tissue was ground with 0.05 mM phosphate-buffered saline (PBS) at pH 7.8, and the extract was centrifuged at 4000 rpm for 10 min before a 50 μL aliquot of the supernatant was added to the reaction mixture containing 13 mM methionine, 75 μM NBT, 2 μM riboflavin, and 10 μM EDTA-Na2, adjusted to a 3-mL reaction volume. After exposing the reaction mixture to a fluorescent lamp for 20 min (4000 lx), the absorbance of the reaction mixture was measured at 560 nm to measure the SOD activity, in terms of suppression ratio. Suppression ratio (%) = [(A0 – Ai)/(A0)] × 100%, where A0 is the absorbance of the control reaction volume in the absence of the leaf extract, and Ai is the absorbance of the treatment reaction volume in the presence of the leaf extract. One unit (1 U) of SOD activity was described as the 50% inhibitory concentration.
Peroxidase (POD) activity was assayed by grinding 100 mg of leaf fresh weight with 5 mL 40 mM PBS at pH 6.0 and centrifuging the homogenate at 4000 rpm for 15 min. A 50 μL aliquot of supernatant was added to a reaction mixture consisting of 2 μM H2O2 and 9 mM guaiacol, adjusted to a total volume of 5 mL with PBS at pH 6.0. Changes of absorbance at 470 nm were recorded every 30 s for 3 min. POD enzyme activity was presented in terms of activity units, where one unit of the enzyme (U) was represented by an increase in A470 of 0.01 per min.
HPLC analysis of phenolics
Phenolics in H. cordata rhizomes were extracted, identified and quantified using the methodology of.2627 Fresh rhizomes were washed and cut into segments, 0.5 g of which were placed into a 20-mL glass bottle containing 5 mL methanol, then subjected to ultrasonic extraction for 30 min. The extract was filtered immediately through a 0.44-μm pore size membrane filter, with the filtrate collected into a 2-mL brown glass bottle. All bottles were sealed and stored at 4°C until analysis was carried out.
Chlorogenic acid, rutin, afzelin, isoquercitrin, quercitin, and quercetin were analyzed using high-performance liquid chromatography (HPLC) on the same extracts. The system consisted of an HPLC instrument (LC-20AT; Shimadzu, Kyoto, Japan), a SHIM-PACK C18 CLC ODS reversed-phase chromatographic column (150 × 6.0 mm ID), solvent A (acetonitrile: methanol = 11: 5 (v/v)) and solvent B (0.1% formic acid (v/v). The gradient elution program was set up as follows: 6% ~ 18% A (0 ~ 8 min), 8 ~ 26% A (8 ~ 26 min), 34 ~ 40% A (26 ~ 35 min), 40 ~ 100% A (35 ~ 44 min), 100% A (45 ~ 55 min), 100 ~ 6% A (55 ~ 66 min), and 6% A (66 ~ 75 min). The flow rates were 1.4 mL/min (0 ~ 15 min), 0.8 mL/min (15 ~ 35 min) and 1.2 mL/min (35 ~ 75 min). The detection wavelength was 345 nm and the column temperature was 40°C. The concentration of each phenolic compound was calculated based on the peak area in the HPLC chromatogram file of the phenolic compound, using the external standard method, and the standard phenolics are from Sigma [USA). Extracts from three replicate seedlings were used for each treatment.
Extraction, identification, and quantification of major volatile compounds
The extraction and detection of volatile compounds followed the method of 27. The cleaned rhizomes were cut into small pieces (≈0.2 g] and transferred to a brown glass bottle containing 2 mL dichloromethane, sealed and subjected to ultrasonic extraction for 30 minutes. All extracts were stored at 4°C until measured. Gas chromatography–mass spectrometry (GC–MS) analyses were carried out with a GC-MS QP2010 instrument (Shimadzu, Kyoto, Japan). Extract components were separated on capillary columns (Factor Four TM: capillary column, VF-WAXms, 30 m × 0.25 mm × 0.25 µm, Varian Medical System, Palo Alto, CA, USA). The GC conditions were as follows: injector temperature: 250°С; carrier gas: helium (99.999%); flow rate: 1.10 mL/min; injection method: not split. The column temperature program was 35°C maintained for 3 min, increasing by 5.6°C/min to 100°C, then maintained for 1 min, then increasing by 2.65°C/min to 125°C, and held at 15°C/min to 230°C. The MS conditions included: ion source: EI; ionization voltage: 70 eV; ion source temperature: 210 °С; interface temperature: 250 °С; detection voltage: 1.2 kV; scan mode: selected ion monitoring (SIM); solvent delay time: 4.5 min. Naphthalene was added to both standard solutions and to sample extracts in equal amounts as an internal standard to allow calculation of the correction factor. The concentrations of individual volatile compounds were calculated by comparing the peak area of quantifier ions. A total of 19 volatile compounds were detected, namely α-pinene, camphene, β-pinene, β-myrcene, limonene, trans-2-hexenal, γ-terpinene, cis-3-hexeneyl-acetate, 1-hexanol, cis-3-hexen-1-ol, trans-2-hexen-1-ol, decanal, linalool, bornyl acetate, β-caryophyllene, 2-undecanone, borneol, decanol and 3-tetradecanone, standard volatile compounds are from Sigma (USA). Three replicate samples (one from each replicate seedling) were measured for each treatment.
Statistical analysis
Microsoft Office Excel 2010 was used to analyze the raw data from each treatment, with each parameter being measured in three independent replicate samples. Summary statistics were the mean ± standard deviation (SD). Data analysis was carried out by one-way analysis of variance (ANOVA), followed Tukey test to identify significant differences between individual samples, using the R programming language.
Results
Effects of inoculation with endophytic fungi on H. cordata morphology.
Inoculation with different species of fungal endophytes resulted in different morphological effects on seedlings (Figure 1). PC significantly increased fresh weight (FW) accumulation, with H. cordata seedlings inoculated with PC weighing on average 4.6 g FW, more than 50% heavier than the average weight of the control (CON) seedlings (3.0 g). IL and UF seedlings weighed, on average, 3.1 g and 2.6 g, respectively, and their impacts on seedling fresh weight compared to CON seedlings were not significant (Figure 2a). Meanwhile, PC also increased mean total leaf area (6072 mm2), which was 58% higher than that of the control samples (3839 mm2), while the effects of IL and UF on mean total leaf area, which were 4505 mm2 and 3432 mm2, respectively, were not significantly different from one another or CON seedlings (Figure 2b). Our results showed that co-culture of H. cordata with IL or PC increased the length of rhizomes significantly, with H. cordata seedlings achieving a maximum total rhizome length of 552 mm when inoculated with IL, 521 mm when inoculated with PC (both significantly greater than those of the CON seedlings (301 mm)), while UF had no significant effect on rhizome length (Figure 2c). There was no significant difference in seedling height of H. cordata seedlings inoculated with the three different endophytes, with the mean heights ranging from 49 ± 1.2 mm (CON) to 65 ± 15 mm (PC) (Figure 2d).
Figure 1.

Effect of endophytic fungi as bioinoculants on houttuynia cordata thunb. seedings were transplanted into sterilized soil, spore or hyphal suspensions were inoculated six times every 7 d. the images were taken on the 60th day. the scale bars represent a length of 3 cm. CON = control, IL = Ilyonectria liriodendra, UF = unidentified fungal sp., PC = Penicillium citrinum.
Figure 2.
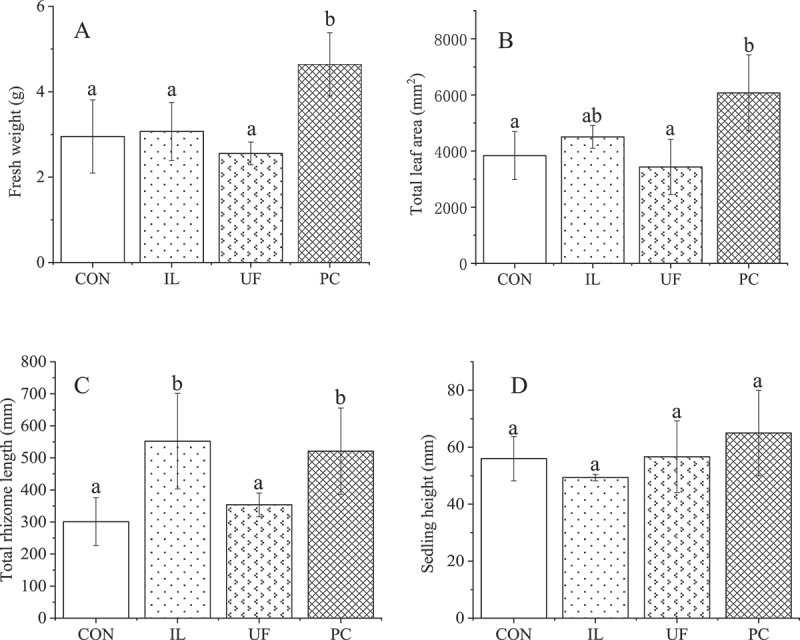
Effects on morphology of houttuynia cordata thunb. inoculated with endophytic fungi. values represent means ± standard deviation (n = 3). any two samples in the same bar chart with a different lowercase letter on the bar have significantly different mean values (P< .05), using tukey test. CON = control, IL = Ilyonectria liriodendra, UF = unidentified fungal sp., PC = Penicillium citrinum.
Physiological effects on H. cordata seedlings inoculated with endophytic fungi
There was no significant difference in the MDA concentrations, reflecting that lipid peroxidation was a consequence of oxidative stress between the treatment and CON seedlings, although the MDA concentration of seedlings inoculated with PC was significantly (64%) higher than that of IL-inoculated seedlings, reaching 1.8 μmol·g−1 (Figure 3a). The activities of the antioxidant enzyme SOD in the IL-, UF- and PC-inoculated seedlings were 51%, 88% and 177% higher (P< .05) than that in CON seedlings (18.8 U·g−1 FW·h−1), respectively, with significant differences between each treatment and CON (Figure 3b). The same pattern was repeated with respect to the activities of the other antioxidant enzyme, POD, the activities of which increased significantly in IL-, UF- and PC-inoculated seedlings by 132%, 194% and 212%, compared with that of the CON seedlings (125.2 U·g−1·min−1), respectively (Figure 3c). On the other hand, total chlorophyll concentration did not differ significantly between seedlings from the different treatments, including the control (Figure 3d), and the mean value was 1.2 mg·g−1.
Figure 3.
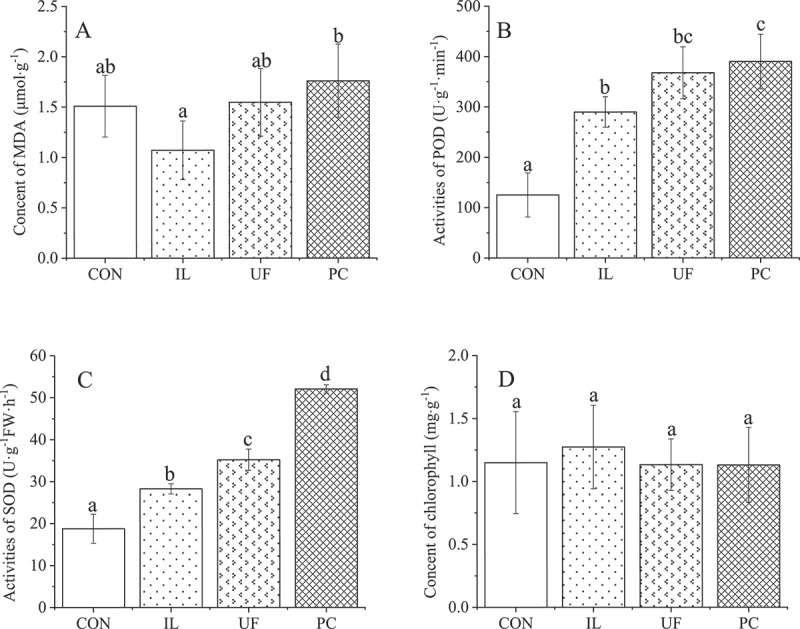
Effects on physiological of H. cordata inoculated with endophytic fungi. Values represent means ± standard deviation (n = 3). Any two samples in the same bar chart with a different lowercase letter on the bar have significantly different mean values (P< .05), using Tukey test. CON = control, IL = Ilyonectria liriodendra, UF = unidentified fungal sp., PC = Penicillium citrinum.
Effects on the concentrations of phenolics of H. cordata inoculated with endophytic fungi.
The effect on phenolic concentrations in H. cordata seedlings in response to inoculation with different endophytic fungi differed between the various treatments. In seedlings inoculated with IL, the concentrations of chlorogenic acid and quercitrin were 49% and 240% higher than in CON seedlings, which were 88.3 μg·g−1 and 1.8 μg·g−1, respectively (P< .05), although no significant difference was observed among CON, UF and PC with respect to the concentrations of these two phenolics (Figure 4a, Figure 4b). Compared with CON, all endophytes enhanced the accumulation of isoquercitrin (P< .05), with the mean concentrations following co-culture with IL being 7.1 μg·g−1, with UF being 6.9 μg·g−1, and with PC being 6.3 μg·g−1, corresponding to concentrations that were 206%, 194% and 168% higher than that observed in CON seedlings, respectively (Figure 4c). Similarly, the concentration of afzelin was increased most by inoculation with UF (633% that of CON). followed by PC and IL (535% and 326%, respectively) (Figure 4d). However, there was no significant difference among the treatments with respect to the mean concentration of rutin, which ranged marginally from 3.1 μg·g−1 to 4.5 μg·g−1 (Figure 4e).
Figure 4.
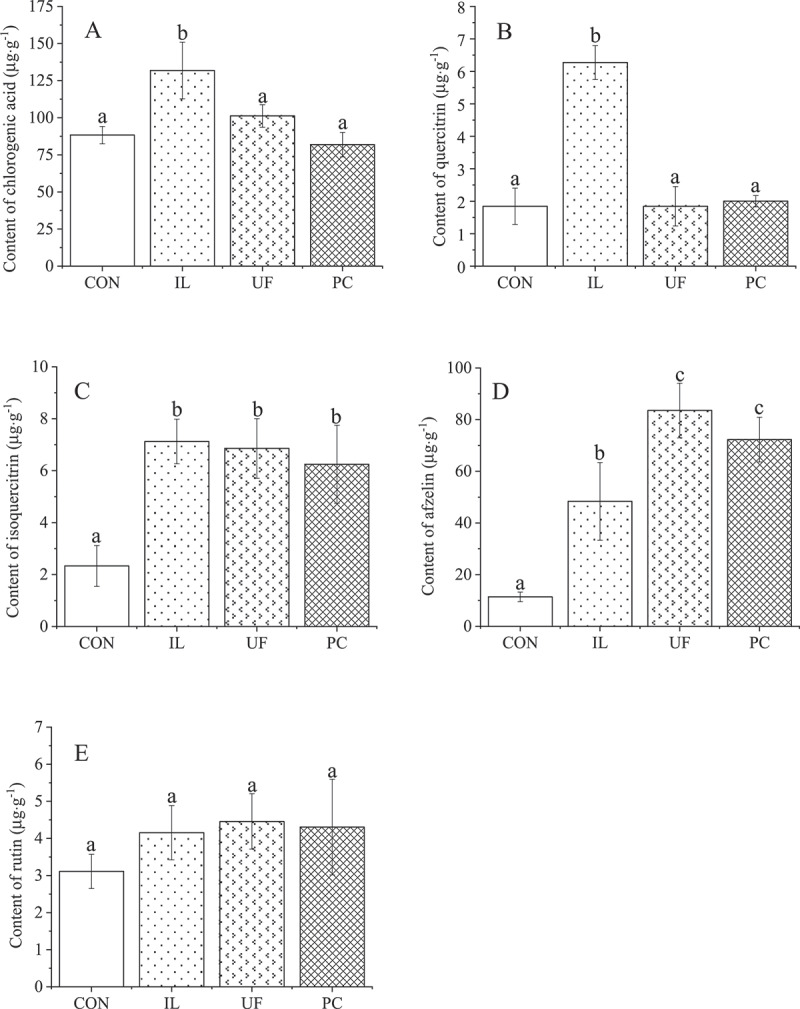
Effects on phenolics of houttuynia cordata thunb. inoculated with endophytic fungi. values represent means ± standard deviation (n = 3). any two samples in the same bar chart with a different lowercase letter on the bar have significantly different mean values (P< .05), using tukey test. CON = control, IL = Ilyonectria liriodendra, UF = unidentified fungal sp., PC = Penicillium citrinum.
Effects on the concentrations of volatile compounds of H. cordata inoculated with endophytic fungi.
Eleven of the 19 volatile compounds were detected, with trans-2-hexenal, cis-3-hexeneyl-acetate, 1-hexanol, cis-3-hexen-1-ol, trans-2-hexen-1-ol, linalool, decanol, and 3-tetradecanone not being detected. IL had a broad promotional effect on the concentration of volatile compounds compared to CON, with α-pinene increasing by 60%, camphene by 48%, β-pinene by 59%, β-myrcene by 75%, limonene by 62%, decanal by 70%, bornyl acetate by 55%, β-caryophyllene by 57% and 2-undecanone by 70% in IL-inoculated seedlings, compared with CON seedlings (all P< .05) (Figure 5a). However, the effect of IL inoculation on borneol concentration was significantly negative, with its concentration decreasing by 44% (P< .05) (Figure 5a). UF also made a positive contribution, and the concentrations of β-myrcene, decanal, β-caryophyllene, and 2-undecanone in UF-inoculated seedlings were 59%, 76%, 38%, and 77% higher, respectively, than those in CON seedlings (P< .05) (Figure 5a). Conversely, the effect of PC on volatile compounds was not significant, except for the concentration of borneol, which decreased significantly by 53% compared to CON seedlings (Figure 5b). This finding was similar to the situation with IL-inoculated seedlings. Furthermore, the concentration of γ-terpinene in H. cordata was not significantly affected by inoculation with any fungal endophyte (Figure 5b).
Figure 5a.
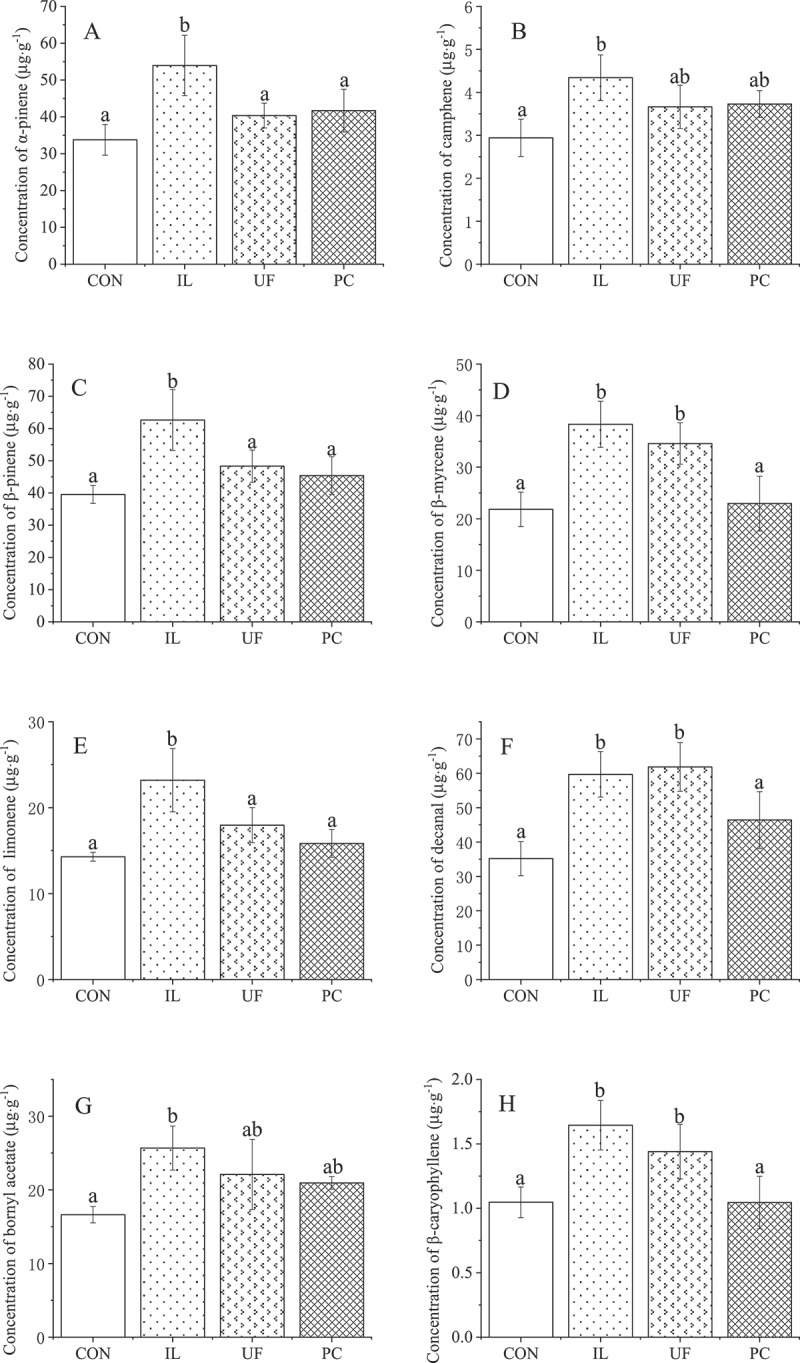
Effects on volatile compounds of houttuynia cordata thunb. inoculated with endophytic fungi
Figure 5b.
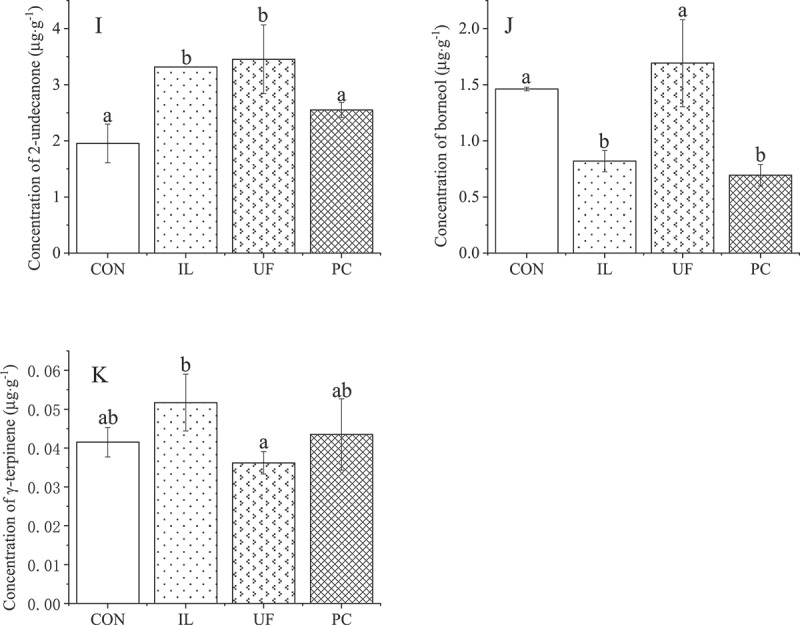
Values represent means ± standard deviation (n = 3). any two samples in the same bar chart with a different lowercase letter on the bar have significantly different mean values (P< .05), using tukey test. CON = control, IL = Ilyonectria liriodendra, UF = unidentified fungal sp., PC = Penicillium citrinum.
Discussion
Chinese traditional medicine is a highly precious resource for the world, not only providing abundant medicinal resources, but also helping to treat major human diseases.28 Increasing the yield of pharmaceutically active metabolites of Chinese herbal medicinal plants continues to be studied by researchers, where success could bring huge economic and medicinal benefits in the future.29 However, most researchers focus on the effects of planting conditions on the concentrations of pharmaceutically active metabolites,30,31 with biological factors, such as the impact of endophytic fungi generally being ignored. Our results proved that endophytic fungi can positively affect medicinal plants and their bioactive secondary metabolites, findings which confirmed our original hypothesis.
Ilyonectria spp. have been reported to be notorious phytopathogens, including but not limited to, the causative agents of black foot disease of grapevine, ginseng root-rot disease and some rust diseases,32,33 which pose serious threats to economically important crops. However, plant pathogens always face multiple stresses, such as the plant’s immune system as well as plant microbiota, which contribute to the observation that most plants are resistant to most plant pathogens.34,35 The chemical substances produced by plant metabolism can directly prevent attack by pathogens or can mediate plant microbiota to achieve a similar effect,36,37 with phenolics and volatile secondary metabolite compounds being among the important bioactive substances.38,39 When H. cordata seedlings were inoculated with IL, the fungus was recognized by the host plant’s defense system, resulting in increased activities of the antioxidant enzymes, POD and SOD, to mitigate the toxic accumulation of reactive oxygen species (ROS),40 thus, preventing oxidative stress. Simultaneously, the concentrations of phenolics and volatile compounds increased to relieve biological stress, and these metabolites might also inhibit the pathogenicity of IL,39,41 resulting in the greater accumulation of H. cordata medicinal compounds and the greater length of the rhizomes, the main organ storing these compounds. Additionally, we found that the growth of the rhizomes in IL-inoculated seedlings was significantly increased, although the total seedling fresh weight was not affected, a response which may involve hormone signaling in the defense responses.42
Although the activities of antioxidant enzymes in UF-inoculated seedlings were much higher than those in the CON seedlings, UF had a lower stimulatory effect on the yield of medicinal metabolites of H. cordata. On the other hand, UF inoculation caused the greatest concentrations of afzelin, decanal, 2-undecanone and borneol, which are amongst the most effective medicinal compounds from H. cordata.43 This selective promotional effect should be emphasized, as specific plant secondary chemistry is a bridge between the endophytic fungi and the host plant, it was produced under microbial activities and regulated the structure of microbial community conversely.44 The specificity of this effect would make it possible to meet specific needs, e.g., increases in particular chemicals, during metabolite production. There has been complex coevolution between fungal endophytes and their host, each partner being interdependently mutualistic with the other, with the benefits for the fungus being more obvious when the plants are under stress.45 According to the growth-differentiation balance hypothesis (GDBH), which predicts a trade-off between the costs of secondary metabolism and the demands for photosynthate to achieve growth, plants must maintain a balance between growth and differentiation. Limited resources would be allocated for secondary metabolite production to achieve the best defense state.46 This may explain why UF had a selective promotional effect on pharmaceutically active H. cordata metabolites.
We noted that the activities of POD and SOD in PC-inoculated seedlings were markedly increased (about three times that in CON seedlings), and this might reflect intense fungal activity around the roots. PC has been reported to be a beneficial endophytic fungus, with plant growth-promoting activity associated with its gibberellin-producing ability.47 Penicillium sp. can also increase nutrient uptake by producing the auxin indole-3-acetic acid (IAA), which can also stimulate plant growth.48 In our experiment, inoculation with PC gave the greatest H. cordata fresh weight, total leaf area, and seedling height. It also significantly promoted the growth of rhizomes, the organs that are particularly rich in bioactive secondary metabolites. However, the effect on the concentrations of secondary metabolites was very low, with only quercitrin and afzelin increasing significantly, whereas the concentration of borneol actually decreased, relative to CON seedlings. PC may change the resource allocation strategy of the host plant, with more resources being distributed to growth rather than to the accumulation of secondary metabolites.49
The genus Penicillium has a large number of species, which usually establish mutually beneficial relationships with plants, alleviating negative effects, such as stresses caused by cold, heavy metal, or pathogens.50–52 Researchers continue to explore the potential value of endophytic fungi in the fields of agriculture, biotechnology, and pharmaceuticals.53 However, we do not think that it is safe to inoculate PC onto young seedlings. Many Penicillium species can produce mycotoxins, which may increase the health risk to humans.54
The phenolics and volatile compounds we analyzed in this study are among the most important pharmaceutically active compounds of H. cordata, and have been reported to exhibit anti-cancer, anti-inflammatory, antibacterial and antiviral activity.55,56 Many fungal endophytes have been reported to actively promote the growth of, or metabolite accumulation in, the host plants, revealing their potential value in the production of Chinese herbal medicines.57,58 However, previous research on the endophytic fungi from H. cordata focused on the secondary metabolites and their antimicrobial activities, whereas research on their potential value as biofertilizers, to stimulate plant growth, is lacking.59
In summary, we have reported in the current study that inoculation of H. cordata seedlings with the endophytic fungi I. liriodendra, an unidentified fungal sp., and P. citrinum, isolated from H. cordata rhizomes, had positive effects on the yield and concentration of the plant’s medicinal metabolites. However, we only tested the effects of each fungus individually under ideal conditions, so that many mechanisms were still unclear. In our future work, we will expand our research to explore the role of fungal endophytes in complex situations, including natural planting conditions, different microbial colonization patterns, and under different stresses. We will also evaluate the feasibility and safety of fungal inoculation and identify the optimal cultivation conditions.
Supplementary Material
Acknowledgments
This research was funded by the National Natural Science Foundation of China (41761010); Science and Technology Foundation of Guizhou Province of China (QianKeHeJiChu[2017]1121).
Funding Statement
This work was supported by the National Natural Science Foundation of China [41761010]; Guizhou Science and Technology Department [QianKeHeJiChu[2017]1121].
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Chen L, Wang YC, Qin LY, He HY, Yu XL, Yang MZ, Zhang HB.. Dynamics of fungal communities during Gastrodia elata growth. BMC Microbiol. 2019;19:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamisile BS, Dash CK, Akutse KS, Keppanan R, Wang LD.. Fungal endophytes: Beyond herbivore management. Front Microbiol. 2018;9:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S, Schillaci M, Walker R, Smith PMC, Watt M, Roessner U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: current knowledge, perspectives and future directions. Plant Soil. 2021;461:219–244. 10.1007/s11104-020-04618-w [DOI] [Google Scholar]
- 4.Gonzalez TM, Urzua A, Plaza P, Bascunan GL. Effects of root endophytic fungi on response of Chenopodium quinoa to drought stress. Plant Ecol. 2018;219(3):231–11. doi: 10.1007/s11258-017-0791-1. [DOI] [Google Scholar]
- 5.Latz MAC, Jensen B, Collinge DB, Jorgensen HJL. Endophytic fungi as biocontrol agents: elucidating mechanisms in disease suppression. Plant Ecol Divers. 2018;11(5–6):555–567. doi: 10.1080/17550874.2018.1534146. [DOI] [Google Scholar]
- 6.Rana KL, Kour D, Kaur T, Devi R, Yadav AN, Yadav N, Dhaliwal HS, Saxena AK. Endophytic microbes: biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Van Leeuwenhoek Int J Gen Mol Microbiol. 2020;113:1075–1107. [DOI] [PubMed] [Google Scholar]
- 7.Christian N, Ea H, Clay K. Foliar endophytic fungi alter patterns of nitrogen uptake and distribution in Theobroma cacao. New Phytol. 2019;222(3):1573–1583. doi: 10.1111/nph.15693. [DOI] [PubMed] [Google Scholar]
- 8.Singh A, Singh DK, Kharwar RN, White JF, Gond SK. Fungal endophytes as efficient sources of plant-derived bioactive compounds and their prospective applications in natural product drug discovery: Insights, avenues, and challenges. Microorganisms. 2021;9(1):197. doi: 10.3390/microorganisms9010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noor AO, Almasri DM, Bagalagel AA, Abdallah HM, Mohamed SGA, Mohamed GA, Ibrahim SRM. Naturally occurring isocoumarins derivatives from endophytic fungi: Sources, isolation, structural characterization, biosynthesis, and biological activities. Molecules. 2020;25(2):25. doi: 10.3390/molecules25020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu M, Xie RS, Shi Y, Zhang H, Chen, HM. Isolation and identification of two flavonoid-producing endophytic fungi from Ginkgo biloba L. Annals of Microbiology, 2010;60(1):143-150. doi: 10.1007/s13213-010-0016-5 [DOI] [Google Scholar]
- 11.Bibi SN, Gokhan Z, Rajesh J, Mf M. Fungal endophytes associated with mangroves – chemistry and biopharmaceutical potential. S Afr J Bot. 2020;134:187–212. doi: 10.1016/j.sajb.2019.12.016. [DOI] [Google Scholar]
- 12.Majoumouo MS, Tincho MB, Kouipou Toghueo RM, Morris T, Hiss DC, Boyom FF, Mandal C. Cytotoxicity potential of endophytic fungi extracts from terminalia catappa against human cervical cancer cells. J Toxicol. 2020;2020:8871152. doi: 10.1155/2020/8871152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aboobaker Z, Viljoen A, Chen W, Crous PW, Maharaj VJ, Van Vuuren S. Endophytic fungi isolated from Pelargonium sidoides DC: antimicrobial interaction and isolation of a bioactive compound. S Afr J Bot. 2019;122:535–542. doi: 10.1016/j.sajb.2019.01.011. [DOI] [Google Scholar]
- 14.Ye BZ, Wu YB, Zhai X, Zhang RQ, Wu JZ, Zhang C, Rahman K, Qin LP, Han T, Zheng CJ. Beneficial effects of endophytic fungi from the anoectochilus and ludisia species on the growth and secondary metabolism of anoectochilus roxburghii. Acs Omega. 2020;5(7):3487–3497. doi: 10.1021/acsomega.9b03789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebickova K, Bajer T, Silha D, Houdkova M, Ventura K, Bajerova P. Chemical composition and determination of the antibacterial activity of essential oils in liquid and vapor phases extracted from two different southeast asian herbs-houttuynia cordata (saururaceae) and persicaria odorata (polygonaceae). Molecules. 2020;25(10):25. doi: 10.3390/molecules25102432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Jiang JG. Bioactive components and functional properties of Hottuynia cordata and its applications. Pharm Biol. 2009;47(12):1154–1161. doi: 10.3109/13880200903019200. [DOI] [Google Scholar]
- 17.Lj L, Lu Y, Yy Z, Hy Z, Tu P, Li H, Chen DF. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine. 2020;67:67. doi: 10.1016/j.phymed.2019.153150. [DOI] [PubMed] [Google Scholar]
- 18.Rajreepa T, Sudem W, Chiranjib M, Subham R, Kumananda T. Antimicrobial secondary metabolites obtained from endophytic fungi inhabiting healthy leaf tissues of Houttuynia cordata Thunb., an ethnomedicinal plant of Northeast India. J Appl Pharm Sci. 2020. doi: 10.7324/JAPS.2020.10912. [DOI] [Google Scholar]
- 19.Ye HT, Luo SQ, Zn Y, Ys W, Ding Q. Establishment of rapid propagation system on houttuynia cordata thunb. Mol Plant Breed. 2020;1-18. [Google Scholar]
- 20.Theodorou C, Bowen GD. Inoculation of seeds and soil with basidiospores of mycorrhizal fungi. Soil Biol Biochem. 1973;5(6):765–771. doi: 10.1016/0038-0717(73)90021-7. [DOI] [Google Scholar]
- 21.Yang ZZ, L X LZ, Jz P, Qiu J, Yang WY. Effect of Bacillus subtilis SY1 on antifungal activity and plant growth. Int J Agric Biol Eng. 2010;2:55–61. [Google Scholar]
- 22.Delmont TO, Francioli D, Jacquesson S, Laoudi S, Mathieu CMT, Nannipieri P, Simonet P, Vogel TM. Microbial community development and unseen diversity recovery in inoculated sterile soil. Biol Fertil Soils. 2014;50(7):1069–1076. doi: 10.1007/s00374-014-0925-8. [DOI] [Google Scholar]
- 23.Liu B, Liu X, Liu F, Ma H, Ma B, Zhang W, Peng L. Growth improvement of lolium multiflorum lam. induced by seed inoculation with fungus suspension of xerocomus badius and serendipita indica. AMB Express. 2019;9(1):1–11. doi: 10.1186/s13568-019-0865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Meng F. Comparitive study on the measure methods of the plant leaf area (in chinese). Agri Network Inf. 2008;2008:14–15+31. [Google Scholar]
- 25.Xiao JX. Experiment of Plant Physiology. Anhui Peoples Publishing; 2010. Anhui. [Google Scholar]
- 26.Yang ZN, Sun YM, Luo SQ, Chen JW, Chen JW, Yu ZW, Sun M. Quality evaluation of Houttuynia cordata Thunb. by high performance liquid chromatography with photodiode-array detection (HPLC-DAD). Pak J Pharm Sci. 2014;27:223–231. [PubMed] [Google Scholar]
- 27.Yang ZN, Luo SQ, Ma J, Wu D, Hong L, Yu ZW. GC-MS analyses of the volatiles of Houttuynia cordata Thunb. Pak J Pharm Sci. 2016;29:1591–1600. [PubMed] [Google Scholar]
- 28.Mirzaie A, Halaji M, Dehkordi FS, Ranjbar R, Noorbazargan H. A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19). Complement Ther Clin Pract. 2020;40:101214. doi: 10.1016/j.ctcp.2020.101214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu L, Deng Z, Cao L, Meng L. Effect of plant density on yield and Quality of perilla sprouts. Sci Rep. 2020;10(1):9937. doi: 10.1038/s41598-020-67106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez Del Campo M, Dj C, Trentacoste ER. Long-term effect of intra-row spacing on growth and productivity of super-high density hedgerow olive orchards (cv. arbequina). Front Plant Sci. 2017;8:1790. doi: 10.3389/fpls.2017.01790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Niu M, Zhu S, Zhang F, Liu Q, Liu Y, Liu R, Zhang Y. Effect study of continuous monoculture on the quality of salvia miltiorrhiza bge roots. Biomed Res Int. 2020;2020:4284385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabrala A, Rego C, Crous PW, Oliveira H. Virulence and cross-infection potential of Ilyonectria spp. to grapevine. Phytopathol Mediterr. 2012;51(2):340–354. [Google Scholar]
- 33.Farh MEA, Kim YJ, Kim YJ, Yang DC. Cylindrocarpon destructans/Ilyonectria radicicola-species complex: causative agent of ginseng root-rot disease and rusty symptoms. J Ginseng Res. 2018;42(1):9–15. doi: 10.1016/j.jgr.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cesari S. Multiple strategies for pathogen perception by plant immune receptors. New Phytol. 2018;219(1):17–24. doi: 10.1111/nph.14877. [DOI] [PubMed] [Google Scholar]
- 35.Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK. Plant–microbiome interactions: from community assembly to plant health. Nat Rev Microbiol. 2020;18(11):607–621. doi: 10.1038/s41579-020-0412-1. [DOI] [PubMed] [Google Scholar]
- 36.Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. nature. 2001;411:826–833. [DOI] [PubMed] [Google Scholar]
- 37.Teixeira PJP, Colaianni NR, Fitzpatrick CR, Dangl JL. Beyond pathogens: microbiota interactions with the plant immune system. Curr Opin Microbiol. 2019;49:7–17. doi: 10.1016/j.mib.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Gharbi Y, Barkallah M, Bouazizi E, Hibar K, Gdoura R, Ma T. Lignification, phenols accumulation, induction of PR proteins and antioxidant-related enzymes are key factors in the resistance of Olea europaea to Verticillium wilt of olive. Acta Physiol Plant. 2017;39(2):43. doi: 10.1007/s11738-016-2343-z. [DOI] [Google Scholar]
- 39.Tilocca B, Cao A, Migheli Q. Scent of a killer: microbial volatilome and its role in the biological control of plant pathogens. Front Microbiol. 2020;11:11. doi: 10.3389/fmicb.2020.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang LB, Feng MG. Antioxidant enzymes and their contributions to biological control potential of fungal insect pathogens. Appl Microbiol Biotechnol. 2018;102(12):4995–5004. doi: 10.1007/s00253-018-9033-2. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Yang Y, Mei X, Li Y, Wu J, Li Y, Wang H, Huang H, Yang M, He X. Phenolic acids released in maize rhizosphere during maize-soybean intercropping inhibit Phytophthora blight of soybean. Front Plant Sci. 2020;11:886. doi: 10.3389/fpls.2020.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhar N, Chen JY, Subbarao KV, Klosterman SJ. Hormone signaling and its interplay with development and defense responses in Verticillium-plant interactions. Front Plant Sci. 2020;11:11. doi: 10.3389/fpls.2020.584997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shingnaisui K, Dey T, Manna P, Kalita J. Therapeutic potentials of Houttuynia cordata Thunb. against inflammation and oxidative stress: a review. J Ethnopharmacol. 2018;220:35–43. doi: 10.1016/j.jep.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christian N, Sedio BE, Florez‐Buitrago X, Ramírez‐Camejo LA, Rojas EI, Mejía LC, Palmedo S, Rose A, Schroeder JW, Herre EA. Host affinity of endophytic fungi and the potential for reciprocal interactions involving host secondary chemistry. Am J Bot. 2020;107(2):219–228. doi: 10.1002/ajb2.1436. [DOI] [PubMed] [Google Scholar]
- 45.Brundrett MC. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002;154:275–304. [DOI] [PubMed] [Google Scholar]
- 46.Barto EK, Cipollini D. Testing the optimal defense theory and the growth-differentiation balance hypothesis in Arabidopsis thaliana. Oecologia. 2005;146(2):169–178. doi: 10.1007/s00442-005-0207-0. [DOI] [PubMed] [Google Scholar]
- 47.Khan SA, Hamayun M, Yoon H, Kim H-Y, Suh S-J, Hwang S-K, Kim J-M, Lee I-J, Choo Y-S, Yoon U-H. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008;8(1):1–10. doi: 10.1186/1471-2180-8-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikram M, Ali N, Jan G, Jan FG, Rahman IU, Iqbal A, Hamayun M. IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS One. 2018;13(11):e0208150. doi: 10.1371/journal.pone.0208150. Jian ZY, Meng L, Hu XQ. 2017. An endophytic fungus efficiently producing paclitaxel isolated from Taxus wallichiana var. mairei. Medicine, 96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu F, Zhang H, Fang F, Liu H, Tang M. Arbuscular mycorrhizal fungi alter nitrogen allocation in the leaves of Populus× canadensis ‘Neva’. Plant Soil. 2017;421(1–2):477–491. doi: 10.1007/s11104-017-3461-0. [DOI] [Google Scholar]
- 50.Chang J, Shi Y, Si G, Yang Q, Dong J, Chen J. The bioremediation potentials and mercury (II)-resistant mechanisms of a novel fungus Penicillium spp. DC-F11 isolated from contaminated soil. J Hazard Mater. 2020;396:122638. doi: 10.1016/j.jhazmat.2020.122638. [DOI] [PubMed] [Google Scholar]
- 51.Gómez‐Muñoz B, Lekfeldt JD, Magid J, Jensen LS, De Neergaard A. Seed treatment with Penicillium sp. or Mn/Zn can alleviate the negative effects of cold stress in maize grown in soils dependent on soil fertility. J Agron Crop Sci. 2018;204(6):603–612. doi: 10.1111/jac.12288. [DOI] [Google Scholar]
- 52.Ikram M, Ali N, Jan G, Hamayun M, Jan FG, Iqbal A. Novel antimicrobial and antioxidative activity by endophytic Penicillium roqueforti and Trichoderma reesei isolated from Solanum surattense. Acta Physiol Plant. 2019;41(9):1–11. doi: 10.1007/s11738-019-2957-z. [DOI] [Google Scholar]
- 53.Toghueo RMK, Boyom FF. Endophytic Penicillium species and their agricultural, biotechnological, and pharmaceutical applications. 3 Biotech. 2020;10:1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perrone G, Susca A. Penicillium species and their associated mycotoxins. Mycotoxigenic Fungi. 2017;1542:107–119. [DOI] [PubMed] [Google Scholar]
- 55.Chiow KH, Phoon MC, Putti T, Tan BK, Chow VT. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac J Trop Med. 2016;9(1):1–7. doi: 10.1016/j.apjtm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khamsan S, Intecha N, Inpeng P, Wongwan W, Phintakul S, Jitmanee C. Chemical compositions and anticancer activity of essential oil from houttuynia cordata thunb. NU. Int J Sci. 2020;17:23–31. [Google Scholar]
- 57.Dai CC, Yu BY, Li X. Screening of endophytic fungi that promote the growth of Euphorbia pekinensis. African J Biotechnol. 2008;7:19. [Google Scholar]
- 58.Yuan J, Zhou JY, Li X, Dai CC. The primary mechanism of endophytic fungus Gilmaniella sp. AL12 promotion of plant growth and sesquiterpenoid accumulation in Atractylodes lancea. Plant Cell, Tissue and Organ Culture (PCTOC). 2016;125(3):571–584. doi: 10.1007/s11240-016-0971-z. [DOI] [Google Scholar]
- 59.Wary S TR, Mili C, Roy S, Tayung K. Antimicrobial secondary metabolites obtained from endophytic fungi inhabiting healthy leaf tissues of Houttuynia cordata Thunb., an ethnomedicinal plant of Northeast India. J Appl Pharm Sci. 2020;10:99–106. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


