Abstract
The objective of this study was to investigate the effects of xylo-oligosaccharides (XOSs) supplementation on growth performance, serum parameters, small intestinal morphology, intestinal mucosal integrity, and immune function in weaned piglets. A total of 240 weaned piglets with an average body weight (BW) of 8.82 ± 0.05 kg (28 d of age) were assigned randomly to four dietary treatments in a 28-d trial, including a control (CON) diet and three diets with XOS supplementation at the concentration of 100 (XOS100), 500 (XOS500), and 1,000 (XOS1000) mg/kg. There were four replicates per treatment with 15 pigs per pen. From day 1 to 14, there were no differences (P > 0.05) in average daily gain (ADG), average daily feed intake, and gain to feed ratio (G:F) during the different treatments. The different doses of XOSs showed a quadratic effect on BW on day 28, ADG, and G:F on day 1 to 28 of piglets (P < 0.05). From day 15 to 28, ADG of pigs fed the XOS500 diet was higher (P < 0.05) than pigs fed the CON diet. During the overall period (day 1 to 28), pigs fed the XOS500 diet had a higher BW, ADG, and G:F than pigs fed the CON diet (P < 0.05). In addition, compared with the CON group, the XOS500 group had significantly higher serum total antioxidant capacity, total superoxide dismutase and catalase levels, and lower malondialdehyde levels on days 14 and 28 (P < 0.05). The serum immunoglobulin G (IgG) concentration in the XOS500 group was also significantly higher compared with the CON group on days 14 and 28 (P < 0.05). However, serum immunoglobulin A and immunoglobulin M were not affected by the dietary treatments. Supplementation of XOS500 to the feed significantly increased the villus height (VH) and VH to crypt depth ratio in the jejunum and ileum in comparison with the CON and XOS1000 groups. Moreover, the XOS500 group significantly elevated the expression levels of occludin and zonula occludens protein-1 in the ileum compared with the CON group. The ileal interleukin (IL)-1β, IL-8, and interferon (IFN)-γ mRNA expression levels in the XOS100 and XOS500 groups were markedly lower than in the CON group. In contrast, the ileal IL-10 mRNA expression levels were remarkably higher in the XOS500 than in the CON group. In conclusion, XOSs have a beneficial effect on growth performance by improving serum antioxidant defense system, serum IgG, small intestinal structure, and intestinal barrier function in weaned piglets.
Keywords: growth performance, serum parameters, small intestinal, weaned piglets, xylo-oligosaccharides
Introduction
The commercial practice of weaning piglets induces different levels of stress due to a new environment and nutritional challenges, including the absence of sows, new pen-mates, and changes in the source and delivery of nutrients. Weaning may cause devastating characteristics, including diarrhea, reducing feed efficiency, weight loss, and in extreme cases death (Shin et al., 2019). To overcome the weaning stress, antibiotic growth promoters (AGP) were widely used since early 1950s at a subtherapeutic dosage to improve health, optimize feed efficiency, and promote animal growth in the swine industry (Cromwell, 2002). However, their potential side effects including the increased antibiotic resistance and residues in swine products have brought about a major concern for the modern society (Teillant et al., 2015). Therefore, there has been a considerable interest in using feed additives as an alternative to AGP in recent years. Different feed additives such as prebiotics, probiotics, organic acids, exogenous enzymes, and plant extracts have been investigated (Samal and Behura, 2009; Kiarie et al., 2013; Liu et al., 2018b).
Xylo-oligosaccharides (XOSs) are carbohydrate oligomers made up of 2 to 7 xylose units linked through β-(1→4)-linkages (Aachary and Prapulla, 2011; Samanta et al., 2015). XOS has been considered as a prebiotic. XOSs have been found in some agricultural byproduct, mainly including corncob, wheat bran, sugarcane residues, and rice straw (Samanta et al., 2015). XOS has the highest stability at a pH range of 2.5 to 8 and exhibits high temperature resistance (Amorim et al., 2019). In 2015, XOSs were evaluated by the European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies requested by the European Commission, who concluded that XOSs did not present any toxicity (Turck et al., 2018). Previous reports have demonstrated that XOSs have the ability to achieve significant biological effects at low daily doses (Chen et al., 2012; Suo et al., 2015). Some studies have shown that 0.01% to 0.05% XOSs supplementation can improve performance, intestinal characteristics, and egg quality of laying hens (Zhou et al., 2009; Ding et al., 2018). In addition, some other biological benefits have been reported, such as antioxidant activity (Yu et al., 2015), immunomodulatory and anti-inflammatory properties (Ding et al., 2018), or antimicrobial effects (Liu et al., 2018a; Chen et al., 2021). However, the effects of dietary XOSs on growth performance, serum parameters, or intestinal functions have not been fully studied in weaned piglets. The optimal dose of XOSs used in the diet of weaned piglets is still unknown. Therefore, this study was to investigate the effects of dietary supplementation of different doses of XOSs, with high purity, on growth performance, serum parameters, intestinal morphology, intestinal immune function, and mucosal integrity.
Materials and Methods
Animals, diet, and experimental design
This study was approved by the Animal Welfare Committee of Institute of Animal Sciences, Chinese Academy of Agriculture Sciences (IASCAAS). All animal treatments in this study were performed according to the guidelines of the Animal Care and Use Committee of the Chinese Academy of Agriculture Sciences (CAAS). Animal care was practiced throughout the experiments and every effort was made to minimize the suffering of piglets (Ethics Approval Code: IAS2019-34).
A total of 240 healthy weaned piglets (Duroc × Landrace × Large White, weaned at 28 d of age) with an average initial body weight (BW) of 8.82 ± 0.05 kg were randomly assigned to four treatments. The control (CON) group received a basal diet without any antibiotics or prebiotics. The XOSs treatment group received 100 (XOS100), 500 (XOS500), and 1,000 (XOS1000) mg/kg corncob-derived XOS (Longlive Biotechnology Co. Ltd, Shandong, China) supplemented to the basal diet. This XOS has a purity of 95% XOS with a degree of polymerization 2 to 7 and is formed by xylose residues linked through β-(1,4)-linkages monomeric units. Prior to the trial, no clinical signs of diarrhea or other diseases were observed in the piglets. All pigs received similar husbandry practices. Each treatment had four replicate pens with 15 pigs per pen. All diets were manufactured in dry mash form and were formulated to meet or slightly exceed the nutritional requirements of the weaned pig as recommended by the NRC (2012; Table 1). The relative humidity and temperature of the piglet house were set at 60% to 65% and 25 to 28 °C, respectively. Piglets were allowed ad libitum access to feed and water throughout the experiment that lasted for 28 d.
Table 1.
Ingredient and analyzed nutrient composition of basal diets
| Item | Basal diet |
|---|---|
| Ingredients, % | |
| Corn | 59.00 |
| Soybean meal | 18.40 |
| Fermented soybean meal | 5.00 |
| Fish meal | 3.00 |
| Soybean oil | 2.50 |
| Dried whey | 5.00 |
| Sugar | 2.00 |
| Glucose | 2.00 |
| Dicalcium phosphate | 0.50 |
| Limestone | 0.50 |
| Salt | 0.30 |
| Lysine HCl | 0.40 |
| Methionine | 0.10 |
| Threonine | 0.10 |
| Choline chloride | 0.10 |
| Anti-mildew agent | 0.10 |
| Premix1 | 1.00 |
| Nutrient level | |
| Dry matter, % | 87.80 |
| Crude protein, % | 20.00 |
| Crude fiber, % | 1.60 |
| Neutral detergent fiber, % | 22.90 |
| Acid detergent fiber, % | 3.70 |
| Digestible energy, cal/g | 3,502 |
| Metabolizable energy, cal/g | 3,243 |
| Net energy, cal/g | 2,553 |
| Gross energy, cal/g | 4,563 |
1The premix provided the following per kilogram of diet: vitamin A, 13,500 IU; vitamin D3, 2,925 IU; vitamin E, 45 mg; vitamin K3, 36.75 mg; vitamin B1, 6.75 mg; vitamin B2, 11.25 mg; vitamin B6, 7.2mg; vitamin B12, 0.054 mg; nicotinamide, 54 mg; calcium pantothenate, 15.75 mg; folic acid, 1.8 mg; biotin, 0.342 mg; Fe, 140 mg; Cu, 20 mg; Zn, 100 mg; Mn, 30 mg; I, 0.4 mg; and Se, 0.4 mg.
Sample collection and measurements
Individual piglet BW was recorded initially, on days 14 and 28 of the experiment, and feed consumption per pen was recorded at the end of each phase (days 14 and 28) to calculate average daily gain (ADG), average daily feed intake (ADFI), and gain to feed ratio (G:F).
On the morning of days 14 and 28, blood samples were collected from six piglets from each group via jugular vein puncture, and 5 mL was collected into a vacutainer. After 2 h, the blood samples were centrifuged at 1,600 × g at 4 °C for 15 min to recover serum, which was stored at −20 °C until analysis.
On day 28, six piglets from each group were chosen randomly and euthanized aseptically. Afterward, the entire intestine was removed from each pig. Segments of the ileum flushed with saline were collected for morphological examination. All intestinal segments were immediately fixed in 4% paraformaldehyde solution and then embedded in paraffin for intestinal morphology observation, and mucosal samples were scraped using a scalpel blade and stored at −80 °C until further analysis.
Biochemical analysis
Serum total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD) activity, glutathione peroxidase (GSH-Px) activity, malondialdehyde (MDA), and catalase (CAT) activity were measured by biochemical methods following the instructions of the corresponding reagent kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The T-AOC was determined at 520 nm by the ferric-reducing antioxidant power assay. The activity of T-SOD was determined by the xanthine oxidase method using the T-SOD activity assay kit. The activity of GSH-Px was determined by using a GSH-Px kit. The MDA concentration was determined at 532 nm using the thiobarbituric acid method. The CAT activity was determined with CAT Assay Kit. The contents of serum immunoglobin A (IgA), immunoglobin G (IgG), and immunoglobin M (IgM) were measured by nephelometry (Beijing Kangjiahongyuan Biotechnology Institute, Beijing, P.R. China). Finally, these indices were calculated according to the formulas in the assay kits.
Morphological examination
Periodic acid–Schiff (PAS) staining was performed according to standard protocols (Shatos et al., 2003). Paraformaldehyde-fixed duodenum, jejunum, and ileum segments were dehydrated with ethanol, embedded in paraffin, and sectioned (5 μm). After dewaxing and immediately washing with distilled water for 1 min, the specimens were immersed in 0.5% periodate solution (Sigma Co.) for 5 min at room temperature in the dark. Afterward, sections were immediately washed (30 s × 2) and soaked in Schiff’s solution at 37 °C. After 60 min, sections were washed twice with a sulfuric acid solution then quickly rinsed with distilled water. The subsequent steps followed the routine protocols of the laboratory. The sections were examined using light microscopy. The villus length and crypt depth (CD) were measured by random measurement of 10 villi and 10 measurements of the crypt per section using DS-U3 (Nikon, Japan).
Quantitative real-time polymerase chain reaction
Total RNA was isolated from the ileal tissue samples with TB Green Premix Ex Taq (Tli RNaseH Plus Reagent) (Takara Biotechnology, Dalian, China). Total RNA quantification and integrity were analyzed by adding 1 µL of each sample to a Bio-Rad CFX384 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). RNA was reverse-transcribed into cDNA with a Takara PrimeScript RT Reagent Kit with gDNA Eraser (Takara Biotechnology, Dalian, China) according to the manufacturer’s protocol. The synthesized complementary deoxyribonucleic acid (cDNA) was quantified, and all tested samples were adjusted to the same concentration. The primer sequences for interleukin (IL)-1β, IL-6, IL-8, interferon (IFN)-γ, IL-10, zonula occludens protein-1 (ZO-1), occludin, claudin-2, and β-actin are presented in Table 2. β-Actin was the reference gene. Quantitative real-time RT-PCR (qRT-PCR) conditions were as follows: a single cycle of 30 s at 95 °C, followed by 40 cycles of 5 s at 95 °C, 30 s at 60 °C, and 15 s at 72 °C. Relative gene expression levels between the control and the various treatment groups were quantitated from the cycle threshold value (Livak and Schmittgen, 2002).
Table 2.
Primer sequences used for real-time PCR
| Items | Primer sequence, 5′ –3′1 |
|---|---|
| IL-1β | F: ACCTGGACCTTGGTTCTC R: GGATTCTTCATCGGCTTC |
| IL-6 | F: GCATTCCCTCCTCTGGTC R: TCTTCAAGCCGTGTAGCC |
| IL-8 | F: TACGCATTCCACACCTTTC R: GGCAGACCTCTTTTCCATT |
| IFN-γ | F: GGCCATTCAAAGGAGCATGG R: GCTCTCTGGCCTTGGAACAT |
| IL-10 | F: TCGGCCCAGTGAAGAGTTTC R: GGAGTTCACGTGCTCCTTGA |
| ZO-1 | F: CTCCAGGCCCTTACCTTTCG R: GGGGTAGGGGTCCTTCCTAT |
| Occludin | F: CAGGTGCACCCTCCAGATTG R: TATGTCGTTGCTGGGTGCAT |
| Claudin-2 | F: GCATCATTTCCTCCCTGTT R: TCTTGGCTTTGGGTGGTT |
| β-Actin | F: GCGTAGCATTTGCTGCATGA R: GCGTGTGTGTAACTAGGGGT |
1F, forward; R, reverse.
Statistical analysis
The UNIVARIATE procedure was used to confirm the homogeneity of variance and also to analyze for outliers, but no outliers were identified. Data were analyzed using one-way analysis of variance (ANOVA) with least significant difference (LSD) multiple comparison test. Linear and quadratic effects of the dietary XOS concentration were assessed by the GLM procedure (SAS Version 9.2, SAS Institute Inc., Cary, NC). The differences were considered significant if P < 0.05 and were considered a trend if the P-value was between 0.05 and 0.10.
Results
Growth performance
There was no difference in initial BW among the treatments (Table 3). The different XOSs dose groups showed a quadratic effect on BW on day 28, ADG, and G:F on day 1 to 28 of piglets (P < 0.05). Piglets in the XOS500 group had higher BW on day 28 than those in the CON and XOS1000 groups (P < 0.05). Piglets in the XOS500 group had higher ADG during day 1 to 28 than those in the CON or XOS1000 group (P < 0.05). Meanwhile, the XOS500 group had significantly better ADG and G:F in comparison with the CON group from day 1 to 28 (P < 0.05). However, there were no differences in BW, ADG, ADFI, or G:F between XOS100, XOS1000, and CON groups.
Table 3.
Effect of graded levels of XOS (mg/kg feed) on growth performance of weaned piglets
| Item | CON | XOS100 | XOS500 | XOS1000 | SEM | P-value | Linear | Quadratic |
|---|---|---|---|---|---|---|---|---|
| BW, kg | ||||||||
| Day 1 | 8.80 | 8.79 | 8.82 | 8.87 | 0.05 | 0.964 | 0.646 | 0.831 |
| Day 14 | 12.52 | 12.47 | 12.52 | 12.53 | 0.10 | 0.998 | 0.923 | 0.898 |
| Day 28 | 17.00b | 17.31ab | 17.80a | 17.24b | 0.09 | 0.024 | 0.115 | 0.017 |
| ADG, g/d | ||||||||
| Day 1 to 14 | 266 | 263 | 264 | 262 | 4 | 0.993 | 0.835 | 0.971 |
| Day 15 to 28 | 321 | 346 | 377 | 336 | 9 | 0.209 | 0.351 | 0.090 |
| Day 1 to 28 | 293b | 304b | 321a | 299b | 3 | 0.012 | 0.150 | 0.006 |
| ADFI, g/d | ||||||||
| Day 1 to 14 | 505 | 507 | 493 | 504 | 3 | 0.586 | 0.612 | 0.612 |
| Day 15 to 28 | 551 | 563 | 578 | 556 | 5 | 0.409 | 0.579 | 0.163 |
| Day 1 to 28 | 528 | 535 | 536 | 530 | 2 | 0.641 | 0.801 | 0.221 |
| G:F | ||||||||
| Day 1 to 14 | 0.53 | 0.52 | 0.54 | 0.52 | 0.01 | 0.939 | 0.999 | 0.893 |
| Day 15 to 28 | 0.58 | 0.62 | 0.65 | 0.60 | 0.02 | 0.420 | 0.446 | 0.185 |
| Day 1 to 28 | 0.56a | 0.57ab | 0.60b | 0.56a | 0.01 | 0.033 | 0.247 | 0.019 |
a,bMeans in a row with different superscripts differ significantly (P < 0.05).
Antioxidant defense system
In the present study, the effect of XOSs on the antioxidant defense system was assessed by measuring the formation of MDA and the levels of key antioxidants, such as T-AOC, T-SOD, GSH-Px, and CAT (Table 4). Compared with the CON group, the XOS500 group had higher T-SOD and CAT levels on days 14 and 28 (P < 0.05). In addition, the MDA level in the XOS500 group was lower than that of piglets in the CON group on days 14 and 28 (P < 0.05). No effect was observed for the XOS100 or XOS1000 groups compared with the CON group. The different XOS dose groups showed a quadratic effect on T-AOC, T-SOD, MDA, and CAT levels on day 28 of piglets (P < 0.05).
Table 4.
Effect of graded levels of XOS (mg/kg feed) on serum antioxidant indices of weaned piglets
| Item | CON | XOS100 | XOS500 | XOS1000 | SEM | P-value | Linear | Quadratic |
|---|---|---|---|---|---|---|---|---|
| Day 14 | ||||||||
| T-AOC, U/mL | 4.5 | 4.7 | 5.1 | 4.8 | 0.08 | 0.174 | 0.136 | 0.199 |
| T-SOD, U/mL | 136.4b | 137.8b | 143.3a | 139.9ab | 0.76 | 0.018 | 0.025 | 0.125 |
| GSH-Px, U/mL | 701.8 | 721.7 | 735.4 | 711.7 | 5.21 | 0.211 | 0.398 | 0.065 |
| MDA, nmol/mL | 4.6a | 4.5ab | 4.1b | 4.5ab | 0.08 | 0.138 | 0.354 | 0.092 |
| CAT, U/mL | 3.3b | 3.9ab | 4.2a | 3.5b | 0.10 | 0.024 | 0.338 | 0.005 |
| Day 28 | ||||||||
| T-AOC, U/mL | 4.3b | 5.2ab | 5.6a | 5.0ab | 0.14 | 0.031 | 0.065 | 0.017 |
| T-SOD, U/mL | 137.1b | 142.3ab | 144.9a | 139.6ab | 0.94 | 0.038 | 0.229 | 0.010 |
| GSH-Px, U/mL | 732.2 | 759.4 | 750.6 | 736.4 | 8.27 | 0.735 | 0.964 | 0.295 |
| MDA, nmol/mL | 4.5a | 4.0ab | 3.6b | 4.2a | 0.08 | 0.007 | 0.116 | 0.003 |
| CAT, U/mL | 3.3b | 4.2a | 4.5a | 4.3a | 0.11 | <0.001 | <0.001 | 0.002 |
a,bMeans in a row with different superscripts differ significantly (P < 0.05).
Serum immune indices
As presented in Table 5, serum IgG concentrations on day 28 were higher in the XOS500 group compared with the CON group (P < 0.05). However, no significant difference for IgA or IgM concentration was observed for the XOS100 or XOS1000 groups compared with the CON group. The different XOS dose groups showed a quadratic effect on IgG levels on day 28 of piglets (P < 0.05).
Table 5.
Effect of graded levels of XOS (mg/kg feed) on serum immunoglobulins of weaned piglets
| Item | CON | XOS100 | XOS500 | XOS1000 | SEM | P-value | Linear | Quadratic |
|---|---|---|---|---|---|---|---|---|
| Day 14 | ||||||||
| IgA, mg/mL | 1.03 | 1.10 | 1.19 | 1.14 | 0.02 | 0.149 | 0.058 | 0.241 |
| IgG, mg/mL | 7.40 | 7.87 | 8.23 | 7.95 | 0.10 | 0.068 | 0.043 | 0.088 |
| IgM, mg/mL | 0.91 | 0.91 | 0.99 | 0.99 | 0.02 | 0.300 | 0.085 | 0.962 |
| Day 28 | ||||||||
| IgA, mg/mL | 1.02 | 1.06 | 1.03 | 1.05 | 0.01 | 0.833 | 0.732 | 0.799 |
| IgG, mg/mL | 7.19c | 7.97ab | 8.20a | 7.58bc | 0.10 | 0.002 | 0.072 | <0.001 |
| IgM, mg/mL | 0.98 | 0.91 | 0.96 | 0.98 | 0.02 | 0.587 | 0.756 | 0.281 |
a–cMeans in a row with different superscripts differ significantly (P < 0.05).
Intestinal morphology
The effects of XOS on intestinal characteristics are presented in Table 6. There was no significant difference in villus height (VH), CD, or VH to CD ratio (VH:CD) of the duodenum between treatments. The VH and VH:CD in both the jejunum and ileum were increased due to XOS500 supplementation compared with the CON or XOS1000 group (P < 0.05). In addition, the XOS100 treatment improved the VH:CD in the ileum compared with the CON group (P < 0.05). The different XOS dose groups showed a quadratic effect on the VH and VH:CD in both the jejunum and ileum on day 28 of piglets (P < 0.05).
Table 6.
Effect of graded levels of XOS (mg/kg feed) on intestinal morphology of weaned piglets
| Item | CON | XOS100 | XOS500 | XOS1000 | SEM | P-value | Linear | Quadratic |
|---|---|---|---|---|---|---|---|---|
| Duodenum | ||||||||
| VH, μm | 500.1 | 507.5 | 510.8 | 505.9 | 2.94 | 0.728 | 0.499 | 0.371 |
| CD, μm | 230.5 | 222.2 | 209.2 | 223.5 | 3.46 | 0.274 | 0.325 | 0.149 |
| VH:CD | 2.21 | 2.31 | 2.39 | 2.22 | 0.03 | 0.282 | 0.767 | 0.079 |
| Jejunum | ||||||||
| VH, μm | 421.0b | 430.6ab | 442.5a | 425.5b | 2.31 | 0.014 | 0.226 | 0.007 |
| CD, μm | 196.9 | 194.9 | 192.9 | 195.5 | 1.27 | 0.815 | 0.642 | 0.442 |
| VH:CD | 2.14b | 2.25ab | 2.30a | 2.18b | 0.02 | 0.029 | 0.375 | 0.005 |
| Ileum | ||||||||
| VH, μm | 377.0c | 400.2ab | 411.8a | 387.3bc | 3.41 | 0.004 | 0.152 | <0.001 |
| CD, μm | 130.2 | 128.6 | 127.4 | 127.6 | 0.99 | 0.806 | 0.371 | 0.702 |
| VH:CD | 2.90b | 3.11a | 3.23a | 3.04ab | 0.03 | 0.001 | 0.001 | <0.001 |
a–cMeans in a row with different superscripts differ significantly (P < 0.05).
Ileal expression of genes related to barrier functions
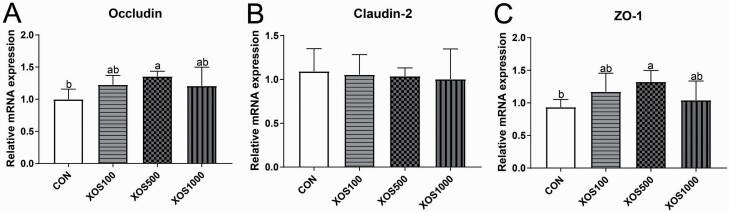
The intestinal tight junction function was tested by analyzing ileal occludin, claudin-2, and ZO-1 expressions (Figure 1). Compared with the CON group, the XOS500 group had higher expression levels of occludin and ZO-1 in the ileum (P < 0.05). The expression of occludin and ZO-1 in the ileum of the XOS100 group was numerically higher than the CON group but tend to be lower than the XOS500 group. However, dietary supplementation with XOSs failed to alter the expression of claudin-2.
Figure 1.
The relative mRNA expression of intestinal epithelium integrity-related genes in the ileal tissues of piglets. (A) Occludin, (B) claudin-2, and (C) ZO-1. Groups with no superscript letter or the same superscript letter are not significantly different (P > 0.05); those with different superscript letters are significantly different (P < 0.05).
Ileal expression of genes related to the inflammatory response
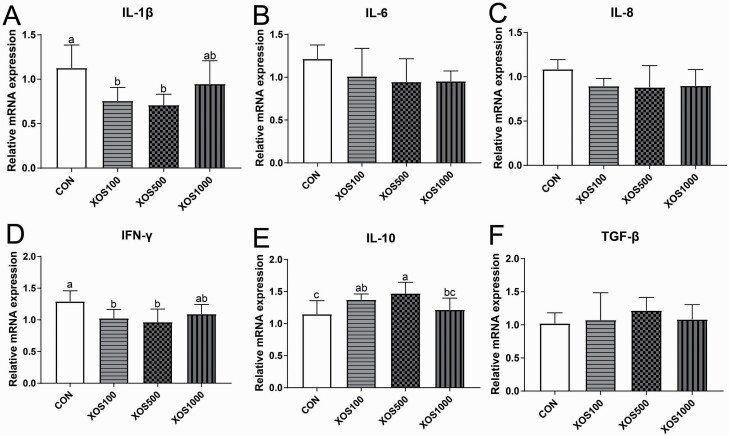
The inflammatory cytokine mRNA expression levels are shown in Figure 2. The ileal IL-1β and IFN-γ mRNA expression levels in the XOS100 or XOS500 group were lower than the CON group (P < 0.05). In contrast, the ileal IL-10 mRNA expression levels were higher in the XOS500 than the CON or XOS1000 group (P < 0.05).
Figure 2.
The relative mRNA expression of intestinal epithelium immune-related genes in the ileal tissues of piglets. (A) IL-1β, (B) IL-6, (C) IL-8, (D) IFN-γ, (E) IL-10, and (F) TGF-β. Groups with no superscript letter or the same superscript letter are not significantly different (P > 0.05); those with different superscript letters are significantly different (P < 0.05).
Discussion
XOSs are considered as promising prebiotics. In our study, XOS500 significantly increased BW, ADG, and ADFI and decreased G:F of piglets, which was consistent with previous studies. For example, Liu et al. (2018a) reported that 200 mg/kg XOS with a purity of 50% remarkably increased ADG and feed efficiency in weaned piglets. Moreover, several studies showed positive dose effects of XOSs on growth performance in broilers. Pourabedin et al. (2015) demonstrated that feed conversion ratio in broilers fed 2 g/kg XOS diets was significantly lower than those fed a control diet or 1 g/kg XOS between days 7 and 21. In contrast, Yin et al. (2019) found that 0.01% XOS with a purity of 40% had no significant improvement in the growth performance of the piglets, which might mainly be explained by the low dose. The different XOS dose groups showed a quadratic effect on BW on day 28, ADG, and G:F day 1 to 28 of piglets. The XOS1000 in our study significantly failed to improve the growth performance in weaned piglets, which may be due to an excessive dose of XOS. Therefore, we speculate that the XOS500 supplementation is the optimal dose during these three dose groups. Other oligosaccharides, for example, chitooligosaccharides have also been shown to have a dose–effect affecting the growth performance in pigs (Liu et al., 2010). These results on the growth performance indicate that there will be an optimal XOS dose for weaned piglets.
Serum biochemical parameters are often used to evaluate the physiological effects of nutrients in animals. Antioxidant parameters are regarded as important serum indices to assess animals’ health. Changes in the antioxidant defense system, mainly including T-AOC, T-SOD, CAT, and GSH-Px, may indicate oxidative stress (Zhu et al., 2012). The generation and elimination of free radicals are in a dynamic balance and can prevent diseases by maintaining a favorable and harmless level (Lobo et al., 2010). Serum T-AOC could scavenge free radicals from a specific organ or living organism, and its concentration reflects the total antioxidant ability (Wang et al., 2008). T-SOD is a well-known endogenous protective enzyme that acts as a component of the first-line defense system against reactive oxygen species. It breaks down hydrogen peroxides and hydroperoxides into less toxic molecules (H2O2/alcohol and O2) (Ighodaro and Akinloye, 2017). CAT reduces H2O2 to O2 and H2O, consequently finishing the detoxification process (Chelikani et al., 2004). GSH-Px is also an important antioxidant enzyme that converts hydrogen peroxides to water (Komatsu et al., 2003). Circulating MDA is one of the common and widely used biomarkers of oxidative stress. MDA is the most familiar degradation product of lipid peroxidation and could result in cell injury including cell senescence and even apoptosis (Han et al., 2018). In the present study, the results showed that increases of T-AOC, T-SOD, and CAT activities were found in the piglets supplemented with XOS500 on days 14 and 28 compared with the CON group. In addition, the MDA level in the XOS500 group was significantly lower than that of piglets in the CON group on days 14 and 28. These results indicate that the AOC of piglets was improved with XOS500 supplementation, while, as for the performance parameter, there seems to be an optimal dose as neither the XOS100 nor the XOS1000 obtained this positive effect on the antioxidant status. Consistent with these findings, a previous study also revealed that wheat bran xylo-oligosaccharides could increase antioxidant status in rats fed a high-fat diet (Wang et al., 2011). Additionally, the research of Jagtap et al. (2017) revealed that an XOS mixture also exhibited concentration-dependent antioxidant activity. The serum immunoglobins—IgG, IgA, and IgM—could protect the extravascular compartment against pathogenic viruses and microorganisms (Li et al., 2007). In this study, the results showed that serum IgG concentration on days 14 and 28 was significantly higher in the XOS500 group compared with the CON group. These results indicate that XOS may play a very important role in improving the immune function of piglets. Similarly, Abdelmalek et al. (2015) also reported that XOS treatment significantly increased the serum immunoglobulin compared with the control in Dicentrarchus labrax fingerlings. The increase in AOC by XOS supplementation might be responsible for the observed effects on the improvement of the immune function. As mentioned above, the underlying mechanisms for the effects of XOS are likely to be related to changes in the intestinal and systemic immune network. But further research is necessary to clarify the complete working mechanisms.
The intestinal morphology indices such as VH, CD, and VH:CD ratio are often used as a criterion to estimate the nutrient digestion and absorption capacity of the small intestine. In this study, we found that the VH and VH:CD ratio of the jejunum and ileum significantly increased in the XOS500 fed pigs compared with the CON group. However, XOS100 and XOS1000 supplementation groups could not significantly improve the intestinal structure. These results indicate that the optimal dose of XOSs may protect the intestine against villous atrophy and epithelial cell necrosis. These results are in line with previous studies, which demonstrated that using 200 mg/kg XOS with a purity of 50% not only improved the VH:CD ratio of the jejunum but also increased the apparent total tract digestibility of dry matter and gross energy on day 14 in the piglets (Liu et al., 2018a). De Maesschalck et al. (2015) also confirmed that the supplementation of 0.5% XOS with a purity of 35% to the broiler feed significantly increased the VH in the ileum. Similarly, Ding et al. (2018) demonstrated that there was a linear improvement in VH and VH:CD ratio of the jejunum as dietary XOS concentration increased in laying hens. However, the addition of 0.01% XOS with a purity of 40% in the diet of weaned piglets had little effects on the intestinal structure and villus surface area (Yin et al., 2019). In addition, Suo et al. (2015) found that the addition of XOS with a purity of 35% in the diet decreased the CD of the duodenum in broilers (Yin et al., 2019). These results suggest that an appropriate dose of XOS can be a promising approach for maintaining the intestinal epithelium in piglets. Hence, a possible explanation for the improvement of growth performance is that XOS500 supplementation improved intestinal morphology and gut absorptive function.
An intact intestinal barrier plays a crucial role in preventing luminal harmful molecules, such as pathogens, toxins, and antigens, from penetrating the mucosa (Martin-Venegas et al., 2006). Tight junctions are the crucial components of the intestinal mucosal barrier and exert a pivotal role in the maintenance of the barrier function. They are multiple protein complexes consisting of the transmembrane proteins occludin and claudin-2 and the cytosolic protein ZO-1. We found that XOS500 upregulated occludin and ZO-1 mRNA levels in the ileal mucosa in weaned pigs, indicating that dietary supplementation with XOSs enhances the intestinal barrier integrity in weaned pigs. In a recent paper, Yin et al. (2019) have shown that XOS supplementation could markedly enhance ZO-1 expression in the piglets. Gut barrier function is also tightly associated with inflammation responses. Pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and IFN-γ and anti-inflammatory cytokines including IL-10 and transforming growth factor-β (TGF-β) are essential for mediating inflammatory responses. Thus, we further determined ileal inflammatory cytokines and the results demonstrated that XOS100 and XOS500 treatments remarkably reduced the IL-1β and IFN-γ mRNA expression. Additionally, XOS500 supplementation significantly upregulated IL-10 mRNA levels. These results indicate that XOS500 supplementation may improve the inflammatory status of piglets. Similarly, Hansen et al. (2013) found that XOS could downregulate IFN-γ and low-grade inflammatory cytokine IL-1β in mice. Yuan et al. (2018) also revealed that XOS supplementation reduced mRNA expression of IFN-γ in broilers. Therefore, dietary supplementation with XOSs contributes to improve the inflammatory status. The improvement of intestinal barrier integrity and inflammatory status might be correlated with the improved intestinal morphology.
In conclusion, the results of the present study demonstrated that XOS500 supplementation could effectively increase the growth performance through enhancing the serum antioxidant defense system, elevating the serum IgG, improving the small intestinal structure, and maintaining intestinal barrier function in weaned piglets. Further studies should be done to fine-tune the optimal dose.
Acknowledgments
We are grateful for the financial support from National Natural Science Foundation of China (No. 31702119) and the Agricultural Science and Technology Innovation Program (No. CAAS-ZDRW202006-02, ASTIPIAS07) in China.
Glossary
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- AGP
antibiotic growth promoters
- BW
body weight
- CAT
catalase
- CD
crypt depth
- CON
basal diet without XOS addition
- G:F
gain to feed ratio
- GSH-Px
glutathione peroxidase
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- MDA
malondialdehyde
- qRT-PCR
quantitative real-time polymerase chain reaction
- T-AOC
total antioxidant capacity
- T-SOD
total superoxide dismutase
- VH
villus height
- VH:CD
villus height to crypt depth ratio
- XOS
xylo-oligosaccharides
Conflict of interest statement
The authors declare that there are no relevant conflicts of interest relevant to this article.
Literature Cited
- Aachary, A. A., and Prapulla S. G.. . 2011. Xylooligosaccharides (XOS) as an emerging prebiotic: microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Compr. Rev. Food Sci. F. 10(1):2–16. doi: 10.1111/j.1541-4337.2010.00135.x [DOI] [Google Scholar]
- Abdelmalek, B. E., Driss D., Kallel F., Guargouri M., Missaoui H., Chaabouni S. E., Ayadi M. A., and Bougatef A.. . 2015. Effect of xylan oligosaccharides generated from corncobs on food acceptability, growth performance, haematology and immunological parameters of Dicentrarchus labrax fingerlings. Fish Physiol. Biochem. 41:1587–1596. doi: 10.1007/s10695-015-0110-5 [DOI] [PubMed] [Google Scholar]
- Amorim, C., Silvério S. C., Prather K. L. J., and Rodrigues L. R.. . 2019. From lignocellulosic residues to market: production and commercial potential of xylooligosaccharides. Biotechnol. Adv. 37(7):107397. doi: 10.1016/j.biotechadv.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Chelikani, P., Fita I., and Loewen P. C.. . 2004. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 61:192–208. doi: 10.1007/s00018-003-3206-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. H., Chen Y. K., Chang H. C., and Su Y. L.. . 2012. Immunomodulatory effects of xylooligosaccharides[J]. Food Sci. Technol. Res. 18(2):195–199. doi: 10.3136/fstr.18.195 [DOI] [Google Scholar]
- Chen, Y. X., Xie Y. N., Zhong R. Q., Liu L., Lin C. G., Xiao L., Chen L., Zhang H. F., Yves B., and Nadia E.. . 2021. Effects of xylo-oligosaccharides on growth and gut microbiota as potential replacements for antibiotic in weaning piglets. Front. Microbiol. 12(2021). doi: 10.3389/fmicb.2021.641172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell, G. L. 2002. Why and how antibiotics are used in swine production. Anim. Biotechnol. 13:7–27. doi: 10.1081/ABIO-120005767 [DOI] [PubMed] [Google Scholar]
- De Maesschalck, C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C., De Baere S., Croubels S., Daube G., Dewulf J., . et al. 2015. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl. Environ. Microbiol. 81:5880–5888. doi: 10.1128/AEM.01616-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X. M., Li D. D., Bai S. P., Wang J. P., Zeng Q. F., Su Z. W., Xuan Y., and Zhang K. Y.. . 2018. Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poult. Sci. 97:874–881. doi: 10.3382/ps/pex372 [DOI] [PubMed] [Google Scholar]
- Han, Y. S., Tang C. H., Zhao Q. Y., Zhan T. F., Zhang K., Han Y. M., and Zhang J. M.. . 2018. Effects of dietary supplementation with combinations of organic and medium chain fatty acids as replacements for chlortetracycline on growth performance, serum immunity, and fecal microbiota of weaned piglets. Livest. Sci. 216:210–218. doi: 10.1016/j.livsci.2018.08.013 [DOI] [Google Scholar]
- Hansen, C. H. F., Frokiaer H., Christensen A. G., Bergstrom A., Licht T. R., Hansen A. K., and Metzdorff S. B.. . 2013. Dietary xylooligosaccharide downregulates IFN-[Gamma] and the low-grade inflammatory cytokine IL-1[beta] systemically in mice. J. Nutr. 143(4):533–540. doi: 10.3945/jn.112.172361 [DOI] [PubMed] [Google Scholar]
- Ighodaro, O. M., and Akinloye O. A.. . 2017. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex. J. Med.:S2090506817301550. doi: 10.1016/j.ajme.2017.09.001 [DOI] [Google Scholar]
- Jagtap, S., Deshmukh R. A., Menon S., and Das S.. . 2017. Xylooligosaccharides production by crude microbial enzymes from agricultural waste without prior treatment and their potential application as nutraceuticals. Bioresour. Technol. 245(Pt A):283–288. doi: 10.1016/j.biortech.2017.08.174 [DOI] [PubMed] [Google Scholar]
- Kiarie, E., Romero L. F., and Nyachoti C. M.. . 2013. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 26:71–88. doi: 10.1017/S0954422413000048 [DOI] [PubMed] [Google Scholar]
- Komatsu, W., Ishihara K., Murata M., Saito H., and Shinohara K.. . 2003. Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-gamma plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radic. Biol. Med. 34:1006–1016. doi: 10.1016/s0891-5849(03)00027-3 [DOI] [PubMed] [Google Scholar]
- Li, P., Yin Y. L., Li D., Kim S. W., and Wu G.. . 2007. Amino acids and immune function. Br. J. Nutr. 98:237–252. doi: 10.1017/S000711450769936X [DOI] [PubMed] [Google Scholar]
- Liu, J., Cao S., Liu J., Xie Y., and Zhang H.. . 2018a. Effect of probiotics and xylo-oligosaccharide supplementation on nutrient digestibility, intestinal health and noxious gas emission in weanling pigs. Asian-Australas. J. Anim. Sci. 31(10):1660–1669. doi: 10.5713/ajas.17.0908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Espinosa C. D., Abelilla J. J., Casas G. A., Lagos L. V., Lee S. A., Kwon W. B., Mathai J. K., Navarro D. M. D. L., Jaworski N. W., . et al. 2018b. Non-antibiotic feed additives in diets for pigs: a review. Anim. Nutr. 4:113–125. doi: 10.1016/j.aninu.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P., Piao X. S., Thacker P. A., Zeng Z. K., Li P. F., Wang D., and Kim S. W.. . 2010. Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with Escherichia coli K88. J. Anim. Sci. 88:3871–3879. doi: 10.2527/jas.2009-2771 [DOI] [PubMed] [Google Scholar]
- Livak, K. J., and Schmittgen T. D.. . 2002. Analysis of relative gene expression data using real-time quantitative PCR. Methods. 25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lobo, V., Patil A., Phatak A., and Chandra N.. . 2010. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn. Rev. 4:118–126. doi: 10.4103/0973-7847.70902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Venegas, R., S. Roig-Perez, R. Ferrer, and J. Moreno. 2006. Arachidonic acid cascade and epithelial barrier function during Caco-2 cell differentiation. J. Lipid Res. 47(7):1416–1423. doi: 10.1194/jlr.M500564-JLR200 [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC). 2012. Nutrient requirements of swine[J]. 11th rev. ed. Washington (DC): National Academies Press. [Google Scholar]
- Pourabedin, M., Guan L., and Zhao X.. . 2015. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome 3:15. doi: 10.1186/s40168-015-0079-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samal, L., and C. Behura. 2009. Prebiotics: an emerging nutritional approach for improving gut health of livestock and poultry. Asian J. Anim. Vet. Adv. 10(11):724–739. doi: 10.3923/ajava.2015.724.739 [DOI] [Google Scholar]
- Samanta, A. K., Jayapal N., Jayaram C., Roy S., Kolte A. P., Senani S., and Sridhar M.. . 2015. Xylooligosaccharides as prebiotics from agricultural by-products: production and applications. Curr. Protein Pept. Sci. 19(1):48–67. doi: 10.2174/1389203717666160923155209 [DOI] [Google Scholar]
- Shatos, M. A., Ríos J. D., Horikawa Y., Hodges R. R., Chang E. L., Bernardino C. R., Rubin P. A., and Dartt D. A.. . 2003. Isolation and characterization of cultured human conjunctival goblet cells. Invest. Ophthalmol. Vis. Sci. 44:2477–2486. doi: 10.1167/iovs.02-0550 [DOI] [PubMed] [Google Scholar]
- Shin, D., Chang S. Y., Bogere P., Won K., Choi J. Y., Choi Y. J., Lee H. K., Hur J., Park B. Y., Kim Y., . et al. 2019. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS One. 14:e0220843. doi: 10.1371/journal.pone.0220843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo, H. Q., Lin L., Xu G. H., Xiao L., Chen X. G., Xia R. R., Zhang L. Y., and Luo X. G.. . 2015. Effectiveness of dietary xylo-oligosaccharides for broilers fed a conventional corn-soybean meal diet. J. Integr. Agr. 14(010):2050–2057. doi: 10.1016/S2095-3119(15)61101-7 [DOI] [Google Scholar]
- Teillant, A., Brower C., and Laxminarayan R.. . 2015. Economics of antibiotic growth promoters in livestock. Annu. Rev. Resour. Econ. 7(1):349–374. doi: 10.1146/annurev-resource-100814-125015 [DOI] [Google Scholar]
- Turck, D., Bresson J. L., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., Hirsch-Ernst K. I., Mangelsdorf I., Mcardle H. J., Naska A., et al. . 2018. Safety of xylooligosaccharides (XOS) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 16(7). doi: 10.2903/j.efsa.2018.5361 [DOI] [Google Scholar]
- Wang, J., Cao Y., Wang C., and Sun B.. . 2011. Wheat bran xylooligosaccharides improve blood lipid metabolism and antioxidant status in rats fed a high-fat diet. Carbohyd. Polym. 86(3):1192–1197. doi: 10.1016/j.carbpol.2011.06.014 [DOI] [Google Scholar]
- Wang, Y. Z., Xu C. L., An Z. H., Liu J. X., and Feng J.. . 2008. Effect of dietary bovine lactoferrin on performance and antioxidant status of piglets. Anim. Feed Sci. Tech. 140(3–4):326–336. doi: 10.1016/j.anifeedsci.2007.02.006 [DOI] [Google Scholar]
- Yin, J., Li F., Kong X., Wen C., Guo Q., Zhang L., Wang W., Duan Y., Li T., Tan Z., . et al. 2019. Dietary xylo-oligosaccharide improves intestinal functions in weaned piglets. Food Funct. 10:2701–2709. doi: 10.1039/c8fo02485e [DOI] [PubMed] [Google Scholar]
- Yu, X., Yin J., Li L., Luan C., Zhang J., Zhao C., and Li S.. . 2015. Prebiotic potential of xylooligosaccharides derived from corn cobs and their in vitro antioxidant activity when combined with Lactobacillus. J. Microbiol. Biotechnol. 25:1084–1092. doi: 10.4014/jmb.1501.01022 [DOI] [PubMed] [Google Scholar]
- Yuan, L., Li W., Huo Q., Du C., Wang Z., Yi B., and Wang M.. . 2018. Effects of xylo-oligosaccharide and flavomycin on the immune function of broiler chickens. PeerJ. 6:e4435. doi: 10.7717/peerj.4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, E., Pan X., and Tian X.. . 2009. Application study of xylo-oligosaccharide in layer production. Mod. Appl. Sci. 3(1): 103–107. doi: 10.5539/mas.v3n1p103 [DOI] [Google Scholar]
- Zhu, L. H., Zhao K. L., Chen X. L., and Xu J. X.. . 2012. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 90: 2581–2589. doi: 10.2527/jas.2012-4444 [DOI] [PubMed] [Google Scholar]