ABSTRACT
In this issue of Clinical Kidney Journal, Van der Willik et al. report findings from a pilot study where they introduced collection of patient-reported outcome measures (PROMs) into routine kidney care in Dutch dialysis centres. It is comparable to a registry-led PROMs initiative in Sweden, published in Clinical Kidney Journal in 2020. Both studies reported low average PROMs response rates with substantial between-centre variation, and both identified suboptimal patient and staff engagement as a key barrier to implementing PROMs in routine care for people with chronic kidney disease (CKD). This suggests that national kidney registries could be well placed to facilitate large-scale collection of PROMs data, but that they may require additional guidance on how to do this successfully. In this editorial, we discuss the current state-of-play of PROMs collection by kidney registries and provide an overview of what is (un)known about the feasibility and effectiveness of PROMs in CKD and other conditions. We anticipate that the fast-growing evidence base on whether, and how, PROMs can be of value in CKD settings will expedite registry-based PROMs collection, which will ultimately lead to more valuable and person-centred services and to enhanced health and well-being of people with CKD.
Keywords: chronic renal insufficiency, patient-centred care, patient-generated health data, patient-reported outcome measures, registries
BACKGROUND
In this issue of Clinical Kidney Journal, Van der Willik et al. share their initial experiences of introducing collection of patient-reported outcome measures (PROMs) into routine kidney care in the Netherlands [1]. With the Dutch kidney registry providing a national infrastructure to collect and feedback PROMs data, they conducted a pilot study in 16 renal centres. Patients on any type of dialysis were invited, at the discretion of their nephrologist, to report their health-related quality of life (HRQoL) and symptom burden at baseline and 3 and 6 months. Centres could organize the data collection process in line with their workflows and they received individual-level reports for a random selection of patients who consented to their PROMs data being shared with the clinical team. On average, the PROMs response rate was low (36% of eligible patients) but varied widely between centres (from 6% to 70%). The authors concluded that achieving high response rates in routine care settings was challenging and that it remains unclear how best to encourage patients and healthcare professionals to collect and use PROMs data.
In 2019, Clinical Kidney Journal published results from a comparable initiative by Pagels et al. in Sweden [2]. As in Van der Willik’s study, the national kidney registry provided the infrastructure to capture and report HRQoL data in people with chronic kidney disease (CKD) treated in one of 68 Swedish renal centres. Despite offering centres more implementation support compared with Van der Willik, Pagels similarly reported a low average PROMs response rate (39%), with substantial between-centre variation (2–98%) and suboptimal patient and staff engagement as a key barrier to successfully introducing PROMs in routine kidney care.
These initiatives suggest that national kidney registries may be willing and able to facilitate large-scale collection of renal PROMs data, but that this requires an enhanced understanding of how to do this successfully. This editorial, therefore, gives an overview of the current state-of-play of registry-based PROMs collection and of the evidence base to support and expedite it.
PROMs FOR VALUABLE AND PERSON-CENTRED KIDNEY CARE
The value-based healthcare movement, popularized by Porter [3], defines value as health outcomes achieved relative to costs. It squarely places outcomes that matter to patients, such as survival, HRQoL and treatment-related discomfort, at the centre. Value-based, or valuable, healthcare aims to create sustainable health systems that are personalized, invest in wellness and strive to deliver and continuously improve outcomes important to patients and the community, such as effectiveness, efficiency and experiences of receiving and providing care. Porter proposes that the only way to accurately assess value is by tracking patient-important outcomes and costs [3].
To operationalize valuable healthcare, the International Consortium for Health Outcomes Measurement (ICHOM) develops standardized outcome sets for a broad range of conditions. Integrating these outcome sets in routine clinical practice would allow value-based comparisons across treatments, institutions and systems, while also supporting patients and clinicians with shared decision-making. The ICHOM set for CKD consists of 12 outcome domains [4]. Four are PROMs (HRQoL, fatigue, pain and physical functioning), which are included in the outcome set’s ‘essential tier’ and were rated as most important by patients [5]. Similarly, PROMs have been included in standardized outcomes sets for kidney research [6], as well as in quality improvement programmes [7].
Van der Willik et al. [1] and others [8–10] advocated that collecting PROMs as part of clinical practice, research and policy-making is essential for making kidney care more person-centred. They argue it has the potential to bring novel insights at patient, provider and systems level that would otherwise remain unrevealed. To unlock this potential, PROMs need to be collected repeatedly and sustainably over time across kidney service providers and patient groups. This requires large-scale infrastructure for collecting, storing, analysing and reporting PROMs data [11]. The Dutch and Swedish pilot studies [1, 2] published in Clinical Kidney Journal suggest that national kidney registries may be well placed to provide such infrastructure.
EXTENDING THE ROLE OF KIDNEY REGISTRIES TO COLLECT PROMs
Kidney registries are organizations or initiatives that systematically collect, store, analyse and report information about kidney disease in a standardized way. They primarily focus on people undergoing renal replacement therapy (RRT) [12], with some extending their target population to include people with earlier stages of CKD or acute kidney injury [13]. Traditionally, kidney registries aimed to describe the characteristics and epidemiology of kidney disease and the spatial distribution and temporal trends in treatment and outcomes [14], which in turn might support assessment of treatment effectiveness [15]. But over time, kidney registries have broadened their remit to include, for example, driving service improvements, whereby they systematically monitor kidney care quality, define benchmarks, identify unwarranted variation in health outcomes or unaddressed patient needs and report this back to providers and policy makers [16]. Other additional registry roles may include: informing public health strategies [17]; enabling pay-for-performance models for financing care [14] and conducting economic evaluation studies [18].
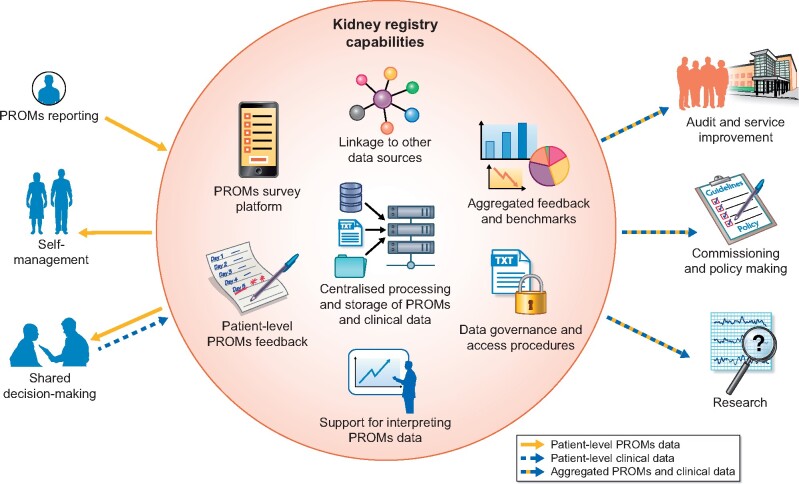
More recently, there has been a call for registries to start collecting data on patient-reported aspects of care alongside conventional clinical data [19, 20]. In keeping with their broadening remit, registries could employ their capabilities to collect PROMs once for multiple purposes to maximize patient benefit (see Figure 1). Van der Willik’s pilot study [1] illustrates that, with registries already longitudinally tracking patient outcomes and feeding this back to providers, they are suited to collect, process and report PROMs for supporting individual patient management. In a 2017 survey, 78% of kidney care providers in Australia and New Zealand said they were interested in extending the Australia and New Zealand Dialysis and Transplant (ANZDATA) registry to collect PROMs [21]. In keeping with international figures [13], a similar percentage indicated they were already collecting some form of patient-reported data. Yet, the instruments, mode and frequency of collection, and patient populations varied greatly between providers [21]. Registry-based PROMs collection would enable harmonizing this across centres and patient groups, for example by implementing standardized outcome sets [4, 6, 22, 23]. It could also facilitate linkage of PROMs data to clinical and administrative data sources [24], and integration of PROMs as an outcome in registry-based trials [25]. The latter may be particularly relevant for treatments where there is a trade-off between clinical efficacy and adverse effects, or where patient attitudes and perceptions might influence the outcome [26].
FIGURE 1:
How kidney registries could use their capabilities to provide an infrastructure for facilitating large-scale collection of PROMs to support individual patient management and other purposes for multiple stakeholders.
THE CURRENT STATE-OF-PLAY OF REGISTRY-BASED PROMs COLLECTION
In 2015, Breckenridge et al. surveyed 45 kidney registries in Europe [27]. Of the 23 respondents, 2 had experience with collecting patient-reported data and 3 were actively considering collection. More recently, global surveys reported that up to 25% of kidney registries in middle and high-income countries [13, 28] and 50% in low-income countries [13] collected PROMs, either routinely or incidentally. Table 1 lists examples of studies from nine different countries that described PROMs data collected by or with support from kidney registries [1, 2, 29–35]. They were mostly conducted in people on RRT, with two also including people with CKD [2, 30]. All nine studies collected data on QoL using seven different instruments; three studies [2, 30, 33] used instruments recommended by ICHOM [5]. All studies conducted in non-English speaking countries used translated versions of the instruments.
Table 1.
Examples of studies reporting PROMs data collected by or with support of kidney registries
| References | Country | Study population | Instruments | Frequency | Collection modes | Support for collection and use | Feedback to patients and clinicians | Number of participating centres | Number of returns |
|---|---|---|---|---|---|---|---|---|---|
| Duncanson et al. [29] | Australia and New Zealand | In-centre HD |
EQ-5D-5L IPOS-Renal |
0, 3 and 6 months (IPOS); 0 and 6 months (EQ-5D) |
Online |
Patient-specific quick response codes for easy login Tablets and user guides with completion instructions Training sessions for nursing staff (e.g. on purpose of PROMs, interpretation of IPOS symptom scores) |
IPOS symptom scores sent to nephrologist and dialysis nurse manager via tailored emails. High scores are flagged and evidence-based symptom management guidelines attached. Clinicians are encouraged to discuss result at next clinical encounter | 6 | NRa |
| Gentile et al. (2013) [30] | Franceb | Transplant recipients with functioning graft for ≥1 year |
SF-36c ReTransQoL |
Once | Paper | French questionnaires were sent to patients’ home address. Non-respondents were reminded by a second letter three weeks later and contacted by phone | NR | NAd | 1061 of 1462 (73%) invited patients responded |
| Romano-Zelekha et al. (2017) [31] | Israel | In-centre HD | KDQOL-36 | Once | Interview | After receiving a detailed explanation about the study, participants were interviewed face-to-face while on dialysis and in their native language | NR | 64 | 1102 of 1444 (76%) patients from 64 centres were interviewed |
| Lim et al. (2008) [32] | Malaysiae | Any type of RRT | Spitzer’s QoL index | Annually | NR | NR | NR | NR | NR |
| Van der Willik et al. (2021) [1] | Netherlandse | Any type of dialysis |
SF-12 DSI |
0, 3 and 6 months | Online |
Centres developed their own process of inviting and motivating patients Questionnaires available in Dutch, English, Turkish and Arabic |
Digital patient-level report sent to nephrologist at 3 months for random sample of consented patients | 16 | 512 of 1415 invited (36%) patients responded across time points; centre-level response rates varied from 6% to 70% |
| Lægreid et al. (2014) [33] | Norwayb | Any type of dialysis; aged ≥75 years |
SF-36c EORTC QLQ-C30f SGAf |
Once | Paper | Questionnaires in Norwegian were sent to patients’ home address. Non-respondents were reminded by a second letter 2 weeks later | NR | NAd | 233 of 320 (73%) invited patients responded |
| Nimmo et al. (2018) [34] | Scotland | In-centre HD | KDQOL-36 | Annually | Paper | Patients requiring assistance or translation were encouraged to ask a friend or relative to help | NR | 35 | 896 (48% of the total HD population)g |
| Pagels et al. (2020) [2] | Swedenb | CKD 3–5; any type of RRT | RAND-36c | At least annually | Paper and online |
Questionnaires in Swedish Patient information pack (e.g. response instructions, objectives) Implementation pack for renal centres (e.g. checklists, office support) Promotion at professional meetings and through Swedish Kidney Patient Association |
Instant online patient-level report available for patient and renal team Instant online aggregated report |
68 | 1378 from 26 centres; in 23 HD centres, 474 of 1220 (39%) invited patients responded; centre-level response rates varied from 2% to 98% |
| Gair et al. (2019) [35] | UKe | Treated in participating renal centres |
EQ-5D-5L IPOS-Renal PAM |
Once, with some centres re-surveying | Paper |
Training and engagement events Person-centred care facilitator |
Real-time calculation of PAM scores via macro-based excel file requiring manual input Online patient-level report available for patient List with patient-level results provided to renal centres Substantial delays in patient-level feedback due to time needed to process paper-based forms |
14 | 3325 patients from 12 centres returned at least one questionnaire |
DSI, Dialysis Symptom Index; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer core Quality of Life questionnaire; EQ-5D-5L, EuroQol five-dimensional five-level version; IPOS-Renal, Integrated Palliative Outcome Scale—renal; KDQOL-36, Kidney Disease Quality of Life instrument; NA, not applicable; NR, not reported; PAM, patient activation measure; ReTransQoL, Renal Transplant Quality of Life; SGA, Subjective Global; SF-12/36, 12/36-item short-form health survey.
Results from feasibility study not yet available.
Country with registry-based PROMs collection in 2015 Breckenridge survey [27].
PROMs instruments recommended by ICHOM [5].
Questionnaires were sent directly to patients’ home address via the kidney registry, so renal centres were not involved in the data collection.
Country with registry-based PROMs collection in 2018 SharE-RR survey [28].
Three items (nausea, vomiting and appetite) from the EORTC QLQ-C30 were used to assess symptom severity; the SGA measures nutritional status.
Unclear how many centres were represented.
Five of nine studies in Table 1 collected data once or annually through paper questionnaires or bedside interviews. For collecting PROMs data more frequently, such as every 3 months in Van der Willik et al. [1] and Duncanson et al. [29], online collection modes are more suitable, if not essential. To support online collection and avoid exclusion of patients without access to digital technology, renal centres in Duncanson’s study were provided with tablet computers to facilitate PROMs completion during dialysis sessions. In addition, and similar to Pagels et al. [2] and Gair et al. [35], they provided training sessions and materials, as well as online, patient-level PROMs feedback to clinicians.
Six of nine studies reported a PROMs response rate. Three, all of which used paper or interview-based collection modes, had response rates >70% [30, 31, 33]. Response rates for the other three ranged from 36% to 48% [1, 2, 34], with the one study using paper-based PROMs having the highest response rate [34]. Together with the relatively low percentage of kidney registries currently collecting PROMs [13, 28], this suggests that incorporating this new type of data alongside traditional clinical measures may not be straightforward, particularly if collected digitally.
FEASIBILITY OF REGISTRY-BASED PROMs COLLECTION
In a review of reviews on PROMs implementation, Foster et al. [36] recommended that organizations should invest time and resources in designing PROMs processes (what to measure, how to collect data and how to use PROMs for clinical purposes), as well as in preparing the implementation (i.e. explaining validity and potential value, staff training and integrating PROMs data into electronic systems). Others have additionally highlighted that patients’ and clinicians’ needs and experiences regarding PROMs do not always align [37, 38]. For example, while patients may consider PROMs helpful for reflecting on and drawing attention to problems, clinicians worry it may constrain patient–clinician conversations [37], or while patients may expect a rapid response to their PROMs results, providers fear this could disrupt workflows [38]. Such diverging views need to be identified and addressed for PROMs collection to be acceptable and feasible.
In keeping with the recommendations from Foster’s review [36] and Pagels’ Swedish registry-based PROMs pilot [2], Van der Willik et al. [1] concluded that motivating healthcare professionals and involving them in PROMs implementation was a key success factor. This was supported by the finding that response rates were highest in centres where professionals indicated they had invested substantial effort in informing and inviting patients. Patients, in turn, reported being satisfied with the content, length and structure of the PROMs, and confirmed the crucial role of clinicians in providing individual feedback on PROMs results. They also indicated, however, that communication about the content and the purpose of PROMs was not always clear to them [1]. Experiences from the Scottish Renal Registry suggested that enhanced patient information letters may address this issue [34].
Two other findings from Van der Willik are worth mentioning when considering the feasibility of registry-based on PROMs collection. (i) Over 40% of PROMs respondents indicated they had had some kind of support with PROMs completion, e.g. by having someone reading out the questions to them. If such support appears to be essential for patient engagement and renal centres are expected to use their existing resources to provide it, it may end up forming a critical barrier to successful PROMs implementation [21]. (ii) People who started RRT more recently were more likely to complete a PROM compared with those on renal replacement for longer. Registry-based PROMs initiatives in other conditions found similar response patterns [39], suggesting that additional strategies may be needed to sustain CKD patients’ engagement in PROMs collection throughout their disease trajectory.
A realist synthesis (PROSPERO CRD42017056063) is underway that will shed light on how PROMs collection and feedback may enhance person-centred care and improve outcomes for people with CKD [40]. Together with ongoing feasibility studies in Australia [29] and the UK [41], this will complement existing guidance [42] and further enhance our understanding of how kidney registries and healthcare providers could collect and use PROMs successfully as part of routine care.
STRENGTHENING THE EVIDENCE TO SUPPORT ROUTINE COLLECTION OF PROMs IN CKD
Implementing routine collection, analysis and interpretation of PROMs and integrating them in clinical decision and policy-making requires investing in infrastructure and skilled personnel by governments and health service providers. Despite the broad consensus that PROMs are crucial for making kidney care more person-centred [1, 8, 9, 21], these investments warrant high-quality evidence of the positive effect of PROMs on the health, care and wellbeing of kidney patients [21].
Studies in other clinical areas have shown clear benefits of PROMs collection. For example, a review in oncology concluded that PROMs improved patient–clinician communication, patient satisfaction, monitoring of treatment response and detection of unrecognized problems [43]. More recently, a landmark randomized trial found that monitoring patient-reported side effects of chemotherapy improved HRQoL, acute hospital admissions and survival [44]. However, asking people to collect PROMs for a short period to inform chemotherapy prescriptions is different from inviting people to do this for the rest of their lives to guide a more complex treatment regime. It is therefore unknown to what extent the evidence from oncology settings generalizes to CKD and other long-term conditions.
In their Discussion section, Van der Willik et al. highlighted that it is still largely unknown if routine PROMs collection improves kidney patients’ outcomes [1]. There are indeed few published studies that investigated the effectiveness of PROMs in CKD. One cluster-randomized trial of 288 people on haemodialysis (HD) compared two symptom management strategies based on monthly patient-reported symptoms. In one arm, the symptom reports were sent to the renal team with management left at their discretion, while in the other arm trained nurses assessed and followed up the reports guided by treatment recommendations. After 12 months, both strategies showed a similar, modest reduction in symptoms compared with baseline [45]. In another cluster-randomized trial in people with three or more long-term conditions, >90% of 1546 participants had CKD or cardiovascular disease. The study compared usual care with 6-month comprehensive patient-centred reviews focusing on dimensions of health, depression and drugs informed by patient-reported outcomes and priorities, but found no difference between groups [46].
There are several pragmatic, randomized studies in progress that will further strengthen the evidence of benefits of routine PROMs collection in CKD. These studies include the following:
Symptom monitoring WIth Feedback Trial (SWIFT; ACTRN12618001976279) [29]; a multicentre cluster-randomized trial of >3000 participants in Australia and New-Zealand, led by the ANZDATA registry. SWIFT will evaluate the effect on HRQoL after 1 year of 3-monthly patient-reported symptom scores with feedback to clinicians and provision of evidence-based guidance for managing severe or overwhelming symptoms (intervention) compared with usual care (control). Secondary outcomes include dialysis withdrawal, clearance of uraemic toxins, hospitalization, quality-adjusted survival and cost-effectiveness;
Evaluation of Routinely Measured PATient Reported Outcomes in HaemodialYsis Care trial (EMPATHY; NCT03535922) [47]; a cluster-randomized trial across three regions in Canada among >4000 in people on HD. This four-arm trial will compare the impact on patient–clinician communication after 1 year of 2-monthly completion and feedback of a disease-specific PROM, a generic PROM, a combination of both or usual care. All arms will be provided with symptom management aids;
Follow-up Using PRO Measures in Patients With Chronic Kidney Disease trial (PROKID; NCT03847766) [48]; a single-centre, non-inferiority randomized trial in Denmark in people with newly diagnosed advanced CKD. PROKID has three arms comparing PROMs-based remote follow-up (i.e. need for outpatient visit determined based on PROMs, clinical data and patient preference, with PROMs guiding patient–clinician discussions during visits), PROMs-based telephone consultations (fixed-frequency telephone consultations with PROMs guiding patient–clinician discussions) and usual care (control). After 18 months, they will evaluate the effect on loss of renal function as the primary outcome. Secondary outcomes include initiation of acute dialyses, hospitalization, mortality, resource use, HRQoL and illness perception;
Renal ePROM pilot trial (RePROM; ISRCTN12669006) [49]; a single-centre pilot trial in 66 people with advanced CKD approaching end-stage kidney disease in the UK. RePROM will inform the design of a multicentre trial to evaluate the effect after 1 year of monthly patient reports of health status with self-management advice and symptom severity-based clinician alerts (intervention) compared with usual care (control). Possible outcome measures for the main trial include HRQoL, laboratory tests, progression to end-stage kidney disease and resource use.
It has been estimated that trials embedded within clinical registries can be conducted more efficiently than standard trials [50]. In many countries, kidney registries have a robust and longstanding infrastructure that facilitates large-scale dissemination of health service interventions and long-term follow-up of bigger samples of patients, reducing the risk of underpowered trials. The SWIFT trial is an example of how kidney registries could harness this infrastructure to support pragmatic comparative effectiveness studies to evaluate the benefits of PROMs-based interventions.
IN SUMMARY
Routinely collecting and using PROMs as part of clinical practice has clear potential for improving the care and lives of people with CKD. National kidney registries are in a strong position to help unlock this potential by employing their existing infrastructure for longitudinal and sustainable PROMs collection across service providers and patient groups, and making the data available for individual patient care, research and policy making. The Dutch kidney registry and several others mentioned in this editorial can be considered trailblazers in this area. We anticipate that the fast-growing evidence base on whether, and how, PROMs can be of value in CKD settings will expedite the uptake of routine PROMs collection by other kidney registries and healthcare providers across the globe. Ultimately, this will lead to more valuable and person-centred services, and enhanced health and wellbeing of people with CKD.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare. The results presented in this article have not been published previously in whole or part.
REFERENCES
- 1. van der Willik EM, Hemmelder MH, Bart HAJ. et al. Routinely measuring symptom burden and health-related quality of life in dialysis patients: first results from the Dutch registry of patient-reported outcome measures. Clin Kidney J 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pagels AA, Stendahl M, Evans M.. Patient-reported outcome measures as a new application in the Swedish Renal Registry: health-related quality of life through RAND-36. Clin Kidney J 2020; 13: 442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Porter ME. What is value in health care? N Engl J Med 2010; 363: 2477–2481 [DOI] [PubMed] [Google Scholar]
- 4.International Consortium of Health Outcome Measurement (ICHOM). The ICHOM Standard Set for Chronic Kidney Disease [Internet].https://www.ichom.org/portfolio/chronic-kidney-disease/ (16 February 2021, date last accessed)
- 5. Verberne WR, Das-Gupta Z, Allegretti AS. et al. Development of an international standard set of value-based outcome measures for patients with chronic kidney disease: a report of the international consortium for health outcomes measurement (ICHOM) CKD working group. Am J Kidney Dis 2019; 73: 372–384 [DOI] [PubMed] [Google Scholar]
- 6. Tong A, Manns B, Hemmelgarn B. et al. ; SONG-HD Investigators. Establishing core outcome domains in hemodialysis: report of the Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) consensus workshop. Am J Kidney Dis 2017; 69: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nissenson AR. Improving outcomes for ESRD patients: shifting the quality paradigm. Clin J Am Soc Nephrol 2014; 9: 430–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nair D, Wilson FP.. Patient-reported outcome measures for adults with kidney disease: current measures, ongoing initiatives, and future opportunities for incorporation into patient-centered kidney care. Am J Kidney Dis 2019; 74: 791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aiyegbusi OL, Kyte D, Cockwell P. et al. A patient-centred approach to measuring quality in kidney care: patient-reported outcome measures and patient-reported experience measures. Curr Opin Nephrol Hypertens 2017; 26: 442–449 [DOI] [PubMed] [Google Scholar]
- 10. van der Veer SN, Aresi G, Gair R.. Incorporating patient-reported symptom assessments into routine care for people with chronic kidney disease. Clin Kidney J 2017; 10: 783–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calvert M, Thwaites R, Kyte D. et al. Putting patient-reported outcomes on the “Big Data Road Map”. J R Soc Med 2015; 108: 299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu FX, Rutherford P, Smoyer-Tomic K. et al. A global overview of renal registries: a systematic review epidemiology and health outcomes. BMC Nephrol 2015; 16: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bello A, Levin A, Lunney M. et al. Global Kidney Health Atlas: A report by the International Society of Nephrology on the Global Burden of End-stage Kidney Disease and Capacity for Kidney Replacement Therapy and Conservative Care across World Countries and Regions [Internet]. 2019. International Society of Nephrology, Brussels, Belgium
- 14. Foley RN, Collins AJ.. The USRDS: what you need to know about what it can and can’t tell us about ESRD. Clin J Am Soc Nephrol 2013; 8: 845–851 [DOI] [PubMed] [Google Scholar]
- 15. Jansz TT, Noordzij M, Kramer A. et al. Survival of patients treated with extended-hours haemodialysis in Europe: an analysis of the ERA-EDTA registry. Nephrol Dial Transplant 2020; 35: 488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kasza J, Polkinghorne KR, Wolfe R. et al. Comparing dialysis centre mortality outcomes across Australia and New Zealand: identifying unusually performing centres 2008–2013. BMC Health Serv Res 2018; 18: 1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jager KJ, Stel VS, Branger P. et al. The effect of differing kidney disease treatment modalities and organ donation and transplantation practices on health expenditure and patient outcomes. Nephrol Dial Transplant 2018; 33: 560–562 [DOI] [PubMed] [Google Scholar]
- 18. Couchoud C, Couillerot AL, Dantony E. et al. Economic impact of a modification of the treatment trajectories of patients with end-stage renal disease. Nephrol Dial Transplant 2015; 30: 2054–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson EC, Dixon-Woods M, Batalden PB. et al. Patient focused registries can improve health, care, and science. BMJ 2016; 354: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nilsson E, Orwelius L, Kristenson M.. Patient-reported outcomes in the Swedish National Quality Registers. J Intern Med 2016; 279: 141–153 [DOI] [PubMed] [Google Scholar]
- 21. Morton RL, Lioufas N, Dansie K. et al. Use of patient-reported outcome measures and patient-reported experience measures in renal units in Australia and New Zealand: a cross-sectional survey study. Nephrology (Carlton) 2020; 25: 14–21 [DOI] [PubMed] [Google Scholar]
- 22. Tong A, Gill J, Budde K. et al. ; SONG-Tx Investigators. Toward establishing core outcome domains for trials in kidney transplantation: report of the Standardized Outcomes in Nephrology - Kidney Transplantation (SONG-Tx) consensus workshops. Transplantation 2017; 101: 1887–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manera KE, Johnson D, Craig J. et al. ; SONG-PD Workshop Investigators. Establishing a core outcome set for peritoneal dialysis: report of the SONG-PD (Standardized Outcomes in Nephrology–Peritoneal Dialysis) consensus workshop. Am J Kidney Dis 2020; 75: 404–412 [DOI] [PubMed] [Google Scholar]
- 24. Pagels AA, Soderkvist BK, Medin C. et al. Health-related quality of life in different stages of chronic kidney disease. Health Qual Life Outcomes 2012; 10: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crossnohere NL, Brundage M, Calvert MJ. et al. International guidance on the selection of patient-reported outcome measures in clinical trials: a review. Qual Life Res 2021; 30: 21–40 [DOI] [PubMed] [Google Scholar]
- 26. Anker SD, Agewall S, Borggrefe M. et al. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J 2014; 35: 2001–2009 [DOI] [PubMed] [Google Scholar]
- 27. Breckenridge K, Bekker HL, Gibbons E. et al. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting. Nephrol Dial Transplant 2015; 30: 1605–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hole BD, Evans KM, Pyart R. et al. International collaborative efforts to establish kidney health surveillance systems. Kidney Int 2020; 98: 812–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duncanson E, Bennett PN, Viecelli A. et al. ; Symptom monitoring WIth Feedback Trial (SWIFT) Investigators. Feasibility and acceptability of e-PROMs data capture and feedback among patients receiving haemodialysis in the Symptom monitoring WIth Feedback Trial (SWIFT) pilot: protocol for a qualitative study in Australia. BMJ Open 2020; 10: e039014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gentile S, Beauger D, Speyer E. et al. Factors associated with health-related quality of life in renal transplant recipients. Health Qual Life Outcomes 2013; 11: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Romano-Zelekha O, Golan E, Ifrah A. et al. Differences in quality of life between Jewish and Arab patients on hemodialysis. Qual Life Res 2017; 26: 3343–3352 [DOI] [PubMed] [Google Scholar]
- 32. Lim Y, Lim T, Lee D. et al. A report of the Malaysian dialysis registry of the national renal registry, Malaysia. Med J Malaysia 2008; 63: 5–8 [PubMed] [Google Scholar]
- 33. Lægreid IK, Aasarod K, Bye A. et al. The impact of nutritional status, physical function, comorbidity and early versus late start in dialysis on quality of life in older dialysis patients. Ren Fail 2014; 36: 9–16 [DOI] [PubMed] [Google Scholar]
- 34. Nimmo A, Bell S, Brunton C. et al. ; Scottish Renal Registry. Collection and determinants of patient reported outcome measures in haemodialysis patients in Scotland. QJM 2018; 111: 15–21 [DOI] [PubMed] [Google Scholar]
- 35. Gair R, Stanndard C, Wong E. et al. Transforming Participation in Chronic Kidney Disease - Programme Report [Internet]. 2019. https://www.thinkkidneys.nhs.uk/ckd/resources/reports/ (4 March 2021, date last accessed)
- 36. Foster A, Croot L, Brazier J. et al. The facilitators and barriers to implementing patient reported outcome measures in organisations delivering health related services: a systematic review of reviews. J Patient Rep Outcomes 2018; 2: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Greenhalgh J, Dalkin S, Gooding K. et al. Functionality and feedback: a realist synthesis of the collation, interpretation and utilisation of patient-reported outcome measures to improve patient care. Health Serv Deliv Res 2017; 5: 1–280 [PubMed] [Google Scholar]
- 38. Reading MJ, Merrill JA.. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc 2018; 25: 759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ashley L, Jones H, Thomas J. et al. Integrating patient reported outcomes with clinical cancer registry data: a feasibility study of the electronic patient-reported outcomes from cancer survivors (ePOCS) system. J Med Internet Res 2013; 15: e230–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schick-Makaroff K, Thummapol O, Thompson S. et al. Strategies for incorporating patient-reported outcomes in the care of people with chronic kidney disease (PRO kidney): a protocol for a realist synthesis. Syst Rev 2019; 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van der Veer SN, Ercia A, Caskey FJ. et al. Developing an intervention to implement electronic patient - reported outcomes in renal services in the UK. Stud Health Technol Inform 2020; 270: 936–940 [DOI] [PubMed] [Google Scholar]
- 42. Gliklich R, Dreyer N, Leavy M.. Chapter 5: use of pa tient-reported outcomes in registries. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville, MD: Agency for Healthcare Research and Quality (US; ), 2014 [PubMed] [Google Scholar]
- 43. Chen J, Ou L, Hollis SJ.. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res 2013; 13: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Basch E, Deal AM, Kris MG. et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016; 34: 557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weisbord SD, Mor MK, Green JA. et al. Comparison of symptom management strategies for pain, erectile dysfunction, and depression in patients receiving chronic hemodialysis: a cluster randomized effectiveness trial. Clin J Am Soc Nephrol 2013; 8: 90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salisbury C, Man M, Bower P. et al. Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet 2018; 392: 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnson JA, Al Sayah F, Buzinski R. et al. A cluster randomized controlled trial for the Evaluation of routinely Measured PATient reported outcomes in HemodialYsis care (EMPATHY): a study protocol. BMC Health Serv Res 2020; 20: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grove BE, Ivarsen P, De Thurah A. et al. Remote follow-up using patient-reported outcome measures in patients with chronic kidney disease: the PROKID study-study protocol for a non-inferiority pragmatic randomised controlled trial. BMC Health Serv Res 2019; 19: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kyte D, Bishop J, Brettell E. et al. Use of an electronic patient-reported outcome measure in the management of patients with advanced chronic kidney disease: the RePROM pilot trial protocol. BMJ Open 2018; 8: e026080–e026087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. James S, Rao SV, Granger CB.. Registry-based randomized clinical trials—a new clinical trial paradigm. Nat Rev Cardiol 2015; 12: 312–316 [DOI] [PubMed] [Google Scholar]