Abstract
Study objectives were to determine the effects of continuously infusing glucose (GLC) or casein (CAS) into the terminal ileum on biomarkers of metabolism, inflammation, and intestinal morphology in growing pigs. Crossbred gilts (n = 19; 81 ± 3 kg body weight [BW]) previously fitted with T-cannulas at terminal ileum were used in the current experiment. Following 4 d of acclimation, pigs were enrolled in 2 experimental 4-d periods (P). During P1, pigs were housed in individual pens and fed ad libitum for collection of baseline parameters. At the beginning of P2, pigs were assigned to 1 of 3 infusion treatments: 1) control (CON; water; 3 liters/d; n = 7), 2) GLC (dextrose 50%; 500 g/d; n = 6;), or 3) CAS (casein sodium salt; 300 g/d; n = 6). Water, GLC, and CAS solutions were continuously infused at a rate of 125 mL/h for the entirety of P2. Animals were euthanized at the end of P2, and intestinal tissue was collected. During P2, average daily feed intake differed across treatments and was reduced in GLC compared with CON pigs (14%), while CAS pigs consumed an intermediate amount (P = 0.05). Average daily gain and final BW were similar across treatments. A treatment by time interaction was observed for blood urea nitrogen (BUN; P < 0.01), as it decreased in GLC (21%) while it gradually increased in CAS (76%) pigs relative to CON pigs. Mild hyperthermia occurred with both GLC and CAS infusions relative to CON (+0.3 and 0.2 °C, respectively; P < 0.01). Blood neutrophils increased in CAS relative to CON pigs (26%) but remained similar between CON and GLC treatments (P < 0.01). Blood monocytes decreased in GLC relative to CON pigs (24%) while CAS pigs had an intermediate value (P = 0.03). Circulating lipopolysaccharide binding protein tended to decrease in GLC (29%) relative to CON pigs but remained similar between CON and CAS pigs (P = 0.10). Plasma tumor necrosis factor-alpha was similar across treatments. Ileum villus height:crypt depth was increased in CAS compared with CON pigs (33%; P = 0.05) while GLC pigs had an intermediate value. Colon myeloperoxidase-stained area increased in CAS compared with CON pigs (45%; P = 0.03) but remained similar between GLC and CON pigs. In summary, continuously infusing GLC or CAS into the terminal ileum appeared to stimulate a mild immune response and differently altered BUN patterns but had little or no effects on blood inflammatory markers, intestinal morphology, or key production parameters.
Keywords: carbohydrate, fermentation, hindgut, ileal infusion, inflammation, protein
Introduction
Although monogastric digestion and absorption of nonstructural carbohydrates and proteins in the small intestine are typically efficient, numerous factors, including sudden dietary changes, decreased digestibility, increased passage rate, small intestinal malabsorption, etc., can negatively influence nutrient assimilation in the proximal gastrointestinal tract (Pieper et al., 2016). Increased passage of simple carbohydrates and soluble proteins into the large intestine are susceptible to fermentation by cecum and colonic bacteria. Rapid fermentation of undigested carbohydrates leads to the increased rate and extent of short-chain fatty acid (SCFA) and lactic acid production, exceeding the large intestine’s capacity for their absorption. Excessive accumulation of organic acids and the subsequent decrease in digesta pH creates an acidic environment, a condition known as hindgut acidosis (Lin, 2004; Gressley et al., 2011). Akin to carbohydrate fermentation, increased microbial digestion of nitrogenous compounds in the large intestine results in the production of various metabolites (i.e., SCFA and branch-chain fatty acids [BCFA]) as well as other potentially toxic compounds, including ammonia, biogenic amines, and hydrogen sulfide; this process is referred to as protein putrefaction (Blachier et al., 2007; Pieper et al., 2016).
Various gastrointestinal disorders in both ruminants and monogastrics have been associated with increased rates of carbohydrate or protein fermentation in the hindgut (Pluske et al., 1996; Gressley et al., 2011; Gilbert et al., 2018). Although the etiology of these disorders remains to be fully elucidated, excessive production and accumulation of fermentation end-products in the large intestine and the ensuing increase in intestinal luminal osmolarity ostensibly damages the intestinal epithelium (Lin, 2004; Blachier et al., 2007; Grauso et al., 2019). Compromised intestinal barrier integrity and the subsequent translocation of luminal contents into the circulation could stimulate local and systemic inflammatory responses (Plaizier et al., 2008). Once activated, the immune system causes hypophagia and utilizes a substantial amount of nutrients that would have otherwise been directed for anabolic purposes (i.e., growth, reproduction; Kvidera et al., 2017a, 2017b; Huntley et al., 2018).
Owing to the obvious implications to animal health and productivity, it is important to better understand how rapid and excessive soluble carbohydrate and protein fermentation contributes to intestinal pathologies. Therefore, study objectives were to evaluate the effects of continuously infusing glucose (a simple carbohydrate) or casein (a soluble protein) into the terminal ileum on biomarkers of metabolism, inflammation, and intestinal morphology in growing pigs. We hypothesized that the appearance of glucose and casein into the large intestine would increase hindgut fermentation and result in altered circulating leukocyte dynamics, inflammatory biomarkers, intestinal morphology, and ultimately decrease growth.
Materials and Methods
All experimental procedures followed the guidelines for the ethical and humane use of animals for research according to the Guide for the Care and Use of Agricultural Animals in Research and Teaching (FASS, 2010) and were approved by the Iowa State University Institutional Animal Care and Use Committee (#9-17-8614-S).
Animals and Experimental Design
Nineteen crossbred gilts (81 ± 3 kg body weight; [BW]) previously fitted with a simple T-cannula at the distal ileum according to the procedures described by Petry et al. (2020) were utilized in the current experiment. Pigs were allowed to acclimate to individual pens for 4 d during which jugular catheters were surgically implanted. Following acclimation, pigs were enrolled in 2 experimental periods (P). Period 1 (4 d) served for the collection of baseline measurements. At the beginning of P2 (4 d), pigs were randomly assigned to 1 of 3 infusion treatments: 1) control (CON; water; 3 liters/d; n = 7), 2) glucose (GLC; 500 g/d dextrose 50%; n = 6), or 3) casein (CAS; 300 g/d casein sodium salt; n = 6). Pigs were housed in individual crates (57 × 221 cm) equipped with a stainless-steel feeder and a nipple drinker. Pigs were fed ad libitum a standard diet formulated to meet or exceed the requirements for growing pigs for essential amino acids, minerals, and vitamins (NRC, 2012; Table 1). Water was provided ad libitum during the entire experiment.
Table 1.
Ingredient and chemical composition of diet (as-fed basis)
| Item | % |
|---|---|
| Ingredient composition | |
| Corn | 63.98 |
| Soybean meal, CP 46% | 13.67 |
| Corn DDGS1 | 20.21 |
| Lysine HCl | 0.29 |
| Limestone | 1.26 |
| NaCl | 0.43 |
| Vitamin-mineral premix2 | 0.13 |
| Ronozyme (500 FTU/kg)3 | 0.02 |
| Chemical composition | |
| Starch4 | 42.22 |
| Crude protein5 | 17.60 |
| Acid hydrolyzed ether extract4 | 4.51 |
| Ca4 | 0.54 |
| P4 | 0.38 |
1Corn distillers dried grains with solubles.
2Vitamin–mineral premix provided the following (per kilogram diet): 8,400 IU/kg of vitamin A, 1,540 IU/kg of vitamin D3, 45 IU of vitamin E, 0.03 mg of vitamin B12, 2.2 mg of menadione, 4.2 mg of riboflavin, 17 mg of D-pantothenic acid, 21 mg of niacin, 1.9 mg of ethoxyquin.112 mg of Fe (ferrous sulfate), 112 mg of Zn (zinc sulfate), 51 mg of Mn (manganous oxide), 20 mg of Cu (copper chloride), 0.78 mg of I (calcium iodate), and 0.17 mg of Se (sodium selenite).
3DSM Nutritional Products Ltd, Basel, Switzerland.
4Calculated value.
5Assayed value.
Ileal Infusions
Approximately 5% of dietary starch and 14% of dietary N are assumed to reach the large intestine in the growing pig fed a typical corn-soybean meal diet (Acosta et al., 2017; Rosenfelder-Kuon et al., 2017). Based on this premise and a baseline average daily feed intake (ADFI) of 3 kg, a total of 63 g of dietary starch/d and 11.8 g of dietary N/d was assumed to reach the large intestine in the current trial. In an attempt to induce unhealthy carbohydrate and protein fermentation, an 8-fold increment (63 vs. 500 g, in the form of dextrose) was implemented for the GLC pigs, while a 4-fold increase (11.8 vs. 44 g of N; in the form of casein sodium salt) was applied in the CAS treatment. We are unaware of previous experiments evaluating the safety threshold of large intestine infused GLC and CAS, but we theorized that the amounts and rates we selected would cause a pathological response. Two solutions were prepared according to the following specifications: 1) a GLC solution (50% dextrose; AgriLabs, St. Joseph, MO) dissolved in tap water for a final concentration of 166.6 g GLC/L; and 2) a CAS solution (casein sodium salt; 14.75% N; Thermo Fisher Scientific Chemicals Inc., Ward Hill, MA) dissolved in tap water for a final concentration of 100 g CAS/L. Tap water was used as the CON treatment. Water, GLC, and CAS solutions were kept at room temperature and continuously infused via the ileocecal cannula at a rate of 125 mL/h during the entirety of P2. The infusion rate was set using a modular pump (Deltec 3000, Deltec Inc., St. Paul, MN), and the total volume infused per pig was approximately 3 liters/d. Each respective infusion treatment was administered at 0600 h on day 1 of P2 (immediately following the 0 h blood sample collection).
Production Parameters
Average daily feed intake (on an ad libitum basis) was measured during P1 and P2 as feed disappearance. Body weights were obtained at the end of acclimation, P1, and P2. Average daily gain (ADG) was calculated for both P1 and P2.
Rectal Temperature
Rectal temperature (TR) was measured daily at 1800 h during P1 and at 0, 6, 12, 24, 36, 48, 72, and 96 h relative to infusion during P2 using a calibrated electronic thermometer (SureTemp Plus 590; accuracy: ± 0.1 ºC; WelchAllyn, Skaneateles Falls, NY, USA).
Blood Sampling and Analysis
An indwelling jugular catheter was surgically inserted on day 4 of acclimation using a percutaneous technique as previously described (Sanz Fernandez et al., 2015). All pigs received antibiotics (Ceftiofur, Excede, Pfizer Animal Health, New York, NY) and non-steroidal anti-inflammatory drugs (Flunixin Meglumine, Banamine-S, Schering-Plough Animal Health Corp., Whitehouse Station, NJ) during surgery. Blood samples in a non-fasted state were obtained from the jugular catheter on day 4 of P1 and at 0, 6, 12, 24, 36, 48, 72, and 96 h relative to infusion during P2. Blood samples were collected into disposable tubes (plasma, K2EDTA tube, BD vacutainers, Franklin Lakes, NJ) and catheters were flushed afterwards with heparinized saline (100 IU/mL). Plasma samples were harvested by centrifugation at 1500 × g for 15 min at 4 ºC, aliquoted into 2.0 mL microcentrifuge tubes, and stored at -20 ºC until analysis. A second set of plasma samples was collected from the same time points, stored at 4 ºC, and sent later to the Iowa State Department of Veterinary Pathology for hematology analysis.
Plasma glucose, non-esterified fatty acids (NEFA), and blood urea nitrogen (BUN) concentrations were measured enzymatically (glucose, Wako Chemicals USA, Inc., Richmond, VA; NEFA, Wako Chemicals USA, Inc., Richmond, VA; BUN, Teco Diagnostics, Anaheim, CA). The intra- and inter-assay coefficients of variation for glucose, NEFA, and BUN were 4.0% and 6.3%, 3.9% and 6.5%, and 6.1% and 12.0%, respectively. ELISA kits were used to determine plasma insulin (Mercodia Porcine Insulin ELISA; Mercodia AB, Uppsala, Sweden), lipopolysaccharide binding protein (LBP; Hycult Biotech, Uden, The Netherlands) and tumor necrosis factor-alpha (TNFα; R&D Systems, Inc., Minneapolis, MN) concentrations at time 0, 24, and 96 h relative to infusion. The intra- and inter-assay coefficients of variation for insulin, LBP, and TNFα were 3.6% and 3.0%, 4.0% and 6.2%, and 3.5% and 5.9%, respectively.
Fecal pH
During P1 and P2, fresh fecal samples were collected twice daily (~0600 and 1800 h) via grab sampling from each pen. Samples were stabilized at room temperature and then homogenized with distilled water in a 1:1 ratio. Fecal pH was measured using a hand-held pH meter (Oakton Instruments, Vernon Hills, IL). Values were averaged by day for statistical analysis.
Tissue Collection and Analysis
Pigs were euthanized at the end of P2 with the captive bolt technique followed by exsanguination. Intestinal tissues were immediately harvested following euthanasia. A jejunum segment measuring about 20 cm long was collected approximately 90 cm distal to the pyloric sphincter. An ileum segment measuring about 20 cm long was obtained approximately 30 cm proximal to the ileocecal junction (~15 cm proximal to the T-cannula). A colon section measuring about 20 cm was obtained approximately 30 cm proximal to the rectum. Colon digesta contents were collected, snap frozen in liquid nitrogen, and stored at -80 ºC until analysis. Subsequently, intestinal segments from the jejunum, ileum, and colon were flushed with sterile saline to remove luminal contents. A transversal section was collected from each intestinal segment, fixed in 10% neutral buffered formalin for 24 h, and then transferred into 70% ethanol. Fixed intestinal samples were submitted to the Iowa State University Veterinary Diagnostic Laboratory for sectioning and hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and myeloperoxidase (MPO) staining. One slide per pig per intestinal segment was generated for each stain. Using a microscope (Leica DMI3000 B Inverted Microscope, Bannockburn, IL) with an attached camera (QImaging 12-bit QICAM Fast 1394; Surrey, BC, Canada), five intact intestinal segment images per pig were obtained at 50× (H&E and PAS slides) and at 200× magnification (MPO slides) with the Q-capture Pro 6.0 software (QImaging; Surrey, BC, Canada). Image processing and quantification were completed using ImageJ 1.49v (National Institutes of Health, USA). For intestinal morphology, villus height was measured from the villus tip to the villus-crypt interface, villus width was measured at mid-villus height, and crypt depth was measured from the villus-crypt opening to the lamina propria. Goblet cell area was quantified as a percentage of the total mucosal area stained by PAS. Similarly, MPO was expressed as a percentage of positive MPO relative to total stained area.
Volatile Fatty Acid (VFA) Analysis
Volatile fatty acid concentrations in colon digesta were determined using gas chromatography. Briefly, 1 g of digesta was weighed in a 15 mL conical tube and mixed with 2.5 mL of deionized water. A portion of the mixture (~1 mL) was transferred into a microcentrifuge tube and centrifuged at 9,000 rpm for 15 min at 4 ºC. A total of 0.5 mL of the resultant supernatant was transferred into microcentrifuge tubes and mixed with 0.1 mL of 25% metaphosphoric acid and 0.05 mL of internal standard solution (4-methyl-valeric acid, S381810, Sigma-Aldrich) for a final dilution of 1:3. Standards and samples were centrifuged at 12,000 rpm for 25 min at 4 ºC. The resultant supernatant was analyzed for VFA concentration (i.e., acetic, propionic, butyric, isobutyric, valeric, and isovaleric acids) using a Varian model 3800 Gas Chromatograph (Varian, Analytical Instruments, Walnut Creek, CA) with a Nukol capillary column (Supelco 24106-U, Bellefonte, PA). A flame-ionization detector was used with an injector temperature of 200 ºC and a detector temperature of 200 ºC.
Statistical Analysis
Data from P2 were statistically analyzed using SAS version 9.4 (SAS Inst. Inc., Cary, NC). Average daily feed intake, fecal pH, TR, and blood metabolites were analyzed using the MIXED procedure with autoregressive (for ADFI and fecal pH) or with spatial power covariance structure (for TR and blood metabolites), and time as the repeated factor. When available, each specific variable’s P1 value served as a covariate. The model included treatment, time, and treatment × time interaction as fixed effects; pig was used as a random effect. Body weight, ADG, VFA concentrations, and intestinal measurements were analyzed using PROC MIXED with a diagonal covariance structure. The model included treatment as a fixed effect. Data are reported as least squares means and considered significant if P ≤ 0.05 and a tendency if 0.05 < P ≤ 0.10.
Results
Average daily feed intake differed across treatments and was reduced by 14% in GLC compared to CON pigs, while CAS pigs had an intermediate value (P = 0.05; Table 2). No differences in ADG or final BW were observed across treatments.
Table 2.
Effects of continuously infusing glucose or casein at the terminal ileum on production parameters
| Parameter | Treatment1 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| CON | GLC | CAS | Trt2 | Time | Trt × Time3 | ||
| ADFI4, kg | 2.96a | 2.56b | 2.71ab | 0.11 | 0.05 | 0.28 | 0.83 |
| ADG5, kg | 0.83 | 0.91 | 0.86 | 0.17 | 0.96 | - | - |
| Final BW6, kg | 94.1 | 93.9 | 94.8 | 0.9 | 0.82 | - | - |
1CON, Control, tap water, 3 liters/d; GLC, Glucose, 500 g/d; CAS, Casein, 300 g/d.
2Treatment.
3Treatment by time interaction.
4Average daily feed intake.
5Average daily gain.
6Final body weight.
a,bMeans with different superscripts significantly differ (P ≤ 0.05).
Blood glucose and NEFA levels were similar among treatments. However, there was a tendency for a treatment × time interaction for insulin as it decreased over time in CON pigs, while it remained almost unchanged in GLC and CAS treatments during P2 (P = 0.07; Table 3). Circulating BUN was decreased in GLC relative to CON pigs during P2 (21%), whereas CAS pigs had progressively increased BUN levels from 6 to 96 h post-infusion when compared with their CON counterparts (76%, P < 0.01; Figure 1).
Table 3.
Effects of continuously infusing glucose or casein at the terminal ileum on blood metabolites and inflammatory biomarkers
| Parameter | Treatment1 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| CON | GLC | CAS | Trt2 | Time | Trt × Time3 | ||
| Metabolism | |||||||
| Glucose, mg/dL | 100 | 101 | 101 | 2 | 0.99 | 0.32 | 0.52 |
| Insulin, μg/L | 0.21 | 0.15 | 0.17 | 0.03 | 0.45 | 0.04 | 0.07 |
| NEFA, μEq/L | 78 | 86 | 77 | 7 | 0.67 | 0.31 | 0.61 |
| Immune Metrics | |||||||
| WBC4, × 103/μL | 20.7 | 20.1 | 20.2 | 0.7 | 0.79 | 0.03 | 0.19 |
| Lymphocytes, × 103/μL | 12.9 | 12.0 | 12.2 | 0.5 | 0.41 | 0.17 | 0.82 |
| Eosinophils, × 103/μL | 0.82 | 0.91 | 0.84 | 0.07 | 0.74 | <0.01 | 0.68 |
| Basophils, × 103/μL | 0.16 | 0.14 | 0.14 | 0.02 | 0.53 | <0.01 | 0.73 |
| Platelets, × 103/μL | 333 | 297 | 350 | 25 | 0.35 | <0.01 | 0.43 |
| LBP5, μg/L | 16.4x | 11.6y | 14.8x | 1.5 | 0.10 | 0.19 | 0.72 |
| TNFα 6, pg/dL | 53 | 57 | 55 | 3 | 0.52 | <0.01 | 0.62 |
1CON, Control, tap water, 3 liters/d; GLC, Glucose, 500 g/d; CAS, Casein, 300 g/d.
2Treatment.
3Treatment by time interaction.
4White blood cells.
5Lipopolysaccharide binding protein.
6Tumor necrosis factor-alpha.
x,yMeans with different superscripts tend to differ (0.05 < P ≤ 0.10).
Figure 1.
Effects of continuously infusing glucose or casein at the terminal ileum on blood urea nitrogen (BUN). Treatments: CON = Control, tap water, 3 liters/d; GLC = Glucose, 500 g/d; CAS = Casein, 300 g/d. Hour 0 (0600 h) was utilized as a covariate. Results are expressed as least squares means ± SEM.
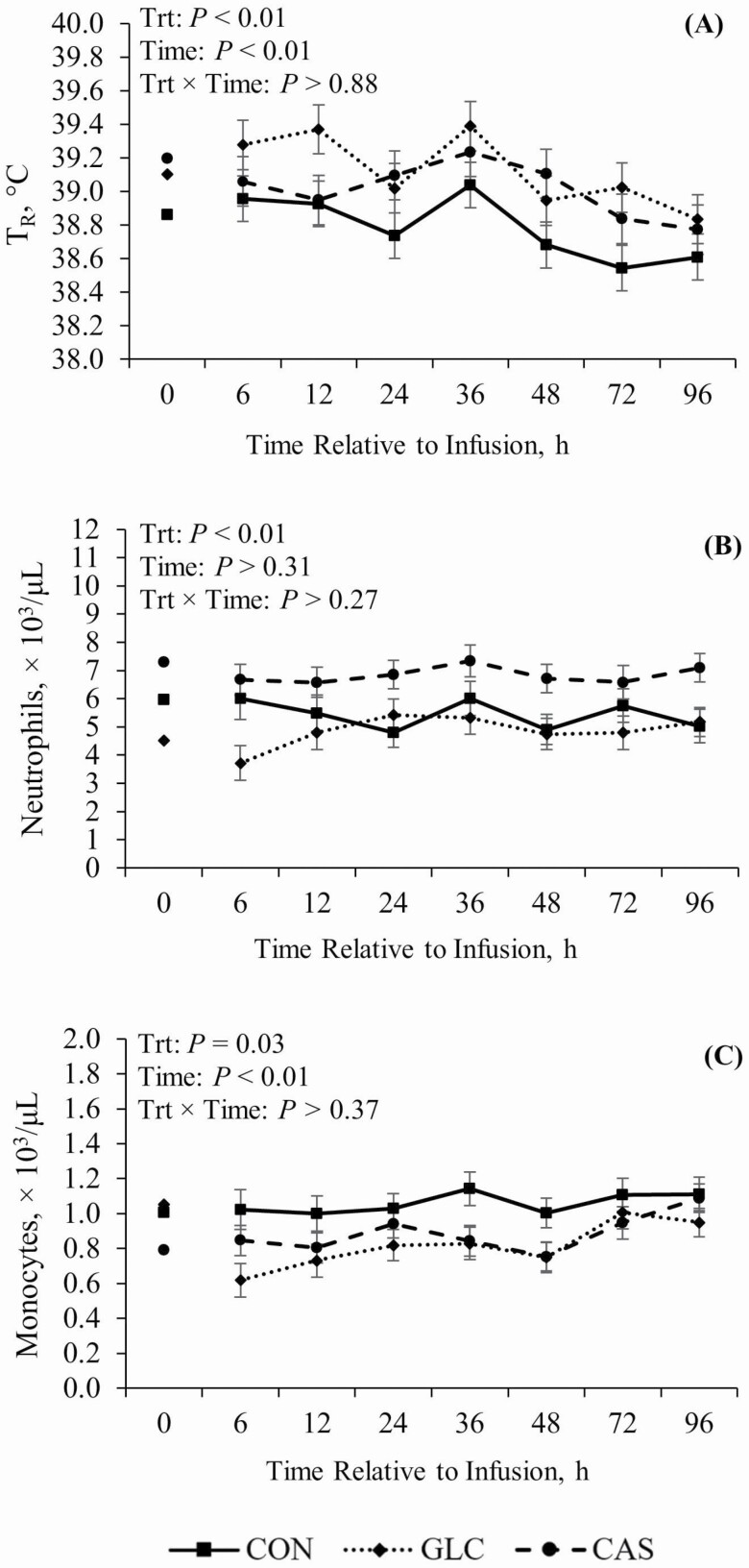
Mild hyperthermia was observed during P2 in both GLC and CAS treatments when compared to CON (+0.3 and 0.2 ºC, respectively; P < 0.01; Figure 2A). Plasma neutrophils increased in CAS relative to their CON counterparts (26%; P < 0.01) but remained similar between GLC and CON pigs (Figure 2B). Circulating monocytes decreased in GLC relative to CON pigs (24%) while CAS pigs had an intermediate value (P = 0.03; Figure 2C). No other treatment differences were observed in circulating white blood cells, lymphocytes, eosinophils, basophils, or platelets (Table 3).
Figure 2.
Effects of continuously infusing glucose or casein at the terminal ileum on (A) rectal temperature (TR) and on circulating (B) neutrophils and (C) monocytes. Treatments: CON = Control, tap water, 3 liters/d; GLC = Glucose, 500 g/d; CAS = Casein, 300 g/d. Hour 0 (0600 h) was utilized as a covariate. Results are expressed as least squares means ± SEM.
Circulating LBP tended to differ across treatments as it decreased in GLC relative to CON pigs (29%) but remained similar between CON and CAS pigs (P = 0.10; Table 3). However, no differences were observed in TNFα levels among treatments during P2.
There was no treatment effect for all measured parameters of intestinal histology (Table 4), except for ileum villus height to crypt depth ratio (P = 0.05) and colon MPO stained area (P = 0.03).
Table 4.
Effects of continuously infusing glucose or casein at the terminal ileum on intestinal morphology
| Parameter | Treatment1 | SEM | P | ||
|---|---|---|---|---|---|
| CON | GLC | CAS | Trt2 | ||
| Jejunum | |||||
| Villus height, μm | 359 | 378 | 391 | 25 | 0.67 |
| Villus width, μm | 85.3 | 81.0 | 82.1 | 4.50 | 0.70 |
| Crypt depth, μm | 171 | 170 | 172 | 11 | 0.99 |
| Villus height:crypt depth | 2.11 | 2.26 | 2.30 | 0.16 | 0.68 |
| Goblet cell area, %3 | 3.41 | 2.58 | 2.97 | 0.34 | 0.22 |
| MPO4 area, %5 | 2.49 | 2.52 | 2.51 | 0.07 | 0.94 |
| Ileum | |||||
| Villus height, μm | 220 | 243 | 274 | 19 | 0.20 |
| Villus width, μm | 89.4 | 73.5 | 74.8 | 6.9 | 0.25 |
| Crypt depth, μm | 161 | 155 | 139 | 9 | 0.27 |
| Villus height:crypt depth | 1.48b | 1.62ab | 1.97a | 0.12 | 0.05 |
| Goblet cell area, %3 | 5.26 | 6.93 | 5.10 | 0.77 | 0.21 |
| MPO4 area, %5 | 2.95 | 2.90 | 2.84 | 0.14 | 0.85 |
| Colon | |||||
| Crypt depth, μm | 242 | 276 | 249 | 14 | 0.23 |
| Goblet cell area, %3 | 7.95 | 6.31 | 7.35 | 0.99 | 0.51 |
| MPO4 area, %5 | 0.67b | 0.80ab | 0.97a | 0.07 | 0.03 |
1CON, Control, tap water, 3 liters/d; GLC, Glucose, 500 g/d; CAS, Casein, 300 g/d.
2Treatment.
3Expressed as a percentage of epithelial area.
4Myeloperoxidase.
5Expressed as a percentage of positive myeloperoxidase relative to total stained area.
a,bMeans with different superscripts significantly differ (P ≤ 0.05).
Fecal pH remained similar across treatments during P2 (Table 5). Infusing GLC at the terminal ileum increased propionic and valeric acid (34% and 70%, respectively; P ≤ 0.04; Table 5), and decreased butyric, isobutyric, and isovaleric acid (24%, 54%, and 39%, respectively; P ≤ 0.08; Table 5) concentrations in colon digesta when compared with CON pigs. Conversely, when pigs were continuously infused with CAS, acetic and propionic acid concentrations decreased in colon digesta (20% and 31%, respectively; P ≤ 0.01; Table 5), while butyric, isobutyric, isovaleric, and valeric acid concentrations remained unchanged relative to CON pigs. Total VFA concentrations were similar between CON and GLC treatments but were reduced in CAS relative to both CON and GLC pigs (18% and 19%, respectively; P = 0.03; Table 5).
Table 5.
Effects of continuously infusing glucose or casein at the terminal ileum on fecal pH and colon volatile fatty acids1
| Parameter | Treatment2 | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| CON | GLC | CAS | Trt3 | Time | Trt × Time4 | ||
| Fecal pH | 6.33 | 6.24 | 6.32 | 0.07 | 0.62 | 0.54 | 0.46 |
| Short chain fatty acids | |||||||
| Acetic acid, mMol/g | 331a | 310a | 255b | 14 | 0.01 | - | - |
| Propionic acid, mMol/g | 146b | 197a | 101c | 15 | <0.01 | - | - |
| Butyric acid, mMol/g | 62.4x | 47.5y | 59.2xy | 4.6 | 0.08 | - | - |
| Total, mMol/g | 540a | 554a | 425b | 28 | 0.01 | - | - |
| Branched chain fatty acids | |||||||
| Isobutyric acid, mMol/g | 17.5a | 8.1b | 17.8a | 1.7 | <0.01 | - | - |
| Isovaleric acid, mMol/g | 27.4a | 16.7b | 29.6a | 2.6 | 0.01 | - | - |
| Valeric acid, mMol/g | 20.9b | 35.5a | 26.6b | 3.7 | 0.04 | - | - |
| Total, mMol/g | 65.8 | 60.3 | 74.0 | 5.8 | 0.29 | - | - |
| Total VFA, mMol/g | 605a | 614a | 499b | 31 | 0.03 | - | - |
1Expressed as mMol/g of colon digesta on dry matter basis.
2CON, Control, tap water, 3 liters/d; GLC, Glucose, 500 g/d; CAS, Casein, 300 g/d.
3Treatment.
4Treatment by time interaction.
a-cMeans with different superscripts significantly differ (P ≤ 0.05).
x,yMeans with different superscripts tend to differ (0.05 < P ≤ 0.10).
Discussion
Excessive carbohydrate or protein fermentation in the hindgut has been associated with the onset and exacerbation of different gastrointestinal disorders in ruminants and monogastrics (Pluske et al., 1996; Gressley et al., 2011; Gilbert et al., 2018). Although hindgut acidosis is apparently more common in ruminants, especially growing steers, and dairy cows (Gressley et al., 2011), evidence suggests that monogastrics, including horses (Garner et al., 1977; Crawford et al., 2007) and pigs (Argenzio and Meuten, 1991; Pluske et al., 1996, 1998) are also prone to acidotic conditions in the hindgut. Furthermore, excessive protein fermentation in the large intestine has been associated with post-weaning diarrhea in pigs and with the incidence of wet-litter in poultry (as reviewed by Collett, 2012; Gilbert et al., 2018). Thus, there is increasing recognition that lower intestine digestion dysfunction contributes to nutritional pathologies and compromises farm animal productivity.
The underlying mechanisms by which excessive carbohydrate or protein fermentation in the large intestine causes intestinal dysfunction are not fully understood. Nevertheless, changes in fermentation patterns, shifts in microbial populations, the accumulation of potentially toxic and inflammatory compounds (i.e., SCFA, lactic acid, BCFA, phenols, indols) in combination with the resultant osmotic stress can damage the gut epithelium (Lin et al., 2002; Hughes et al., 2008; Gressley et al., 2011; Grauso et al., 2019). In both scenarios, altered intestinal barrier integrity and the subsequent translocation of luminal contents into circulation result in a local and systemic inflammatory response that has consequences to animal health and productivity. Therefore, understanding the role these fermentation processes and their associated metabolites play in the onset of intestinal dysfunction and systemic inflammation is a prerequisite to developing strategies that improve overall gut function and animal performance.
Circulating Metabolites
Altered post-absorptive metabolism is characteristic of animals undergoing an inflammatory state (Spurlock, 1997; McGuiness, 2005; Klasing, 2007), as nutrients, especially glucose, are diverted to support the increased energetic requirements of the immune system (Elsasser et al., 2008; Kvidera et al., 2017a, 2017b; Huntley et al., 2018). Because altered fermentation dynamics have been associated with an inflammatory response (Plaizier et al., 2008; Pieper et al., 2016), we anticipated differences in these metabolic biomarkers following GLC or CAS infusions. However, circulating glucose, NEFA, and insulin did not differ across treatments, suggesting both GLC or CAS fermentation did not meaningfully influence overall carbohydrate or lipid metabolism in this study.
Changes in BUN were observed in the current study as circulating BUN remained decreased in GLC, whereas it increased over time in CAS when compared with CON pigs. Reduced BUN in GLC pigs ostensibly indicates that microbial proliferation in the large intestine was stimulated and that ammonia was used for de novo synthesis of bacterial protein rather than being absorbed (Misir and Sauer, 1982). This agrees with previous studies where supplying dietary fermentable carbohydrates resulted in decreased BUN in pigs (Mosenthin et al., 1992; Li et al., 2011), rats (Younes et al., 1997), sheep (Thornton et al., 1970), and dairy cows (Gressley and Armentano, 2007). In contrast, when casein was infused into the large intestine, ammonia production exceeded the capacity of growing bacteria to assimilate it, and the enhanced nitrogen absorption increased circulating BUN. These results are similar to those reported in pigs fed high-dietary protein diets (Heo et al., 2008, 2009; Jeaurond et al., 2008). Altogether, altered BUN dynamics support the fact that our current model accurately resembled conditions of increased fermentation in the large intestine in GLC and CAS pigs.
Fever, Circulating Markers of Inflammation, and Intestinal Morphology
Infusing GLC and CAS in the current trial induced a mild fever response that was sustained throughout P2. Additionally, slight changes in circulating leukocytes were detected herein; increased neutrophils were observed in CAS, while monocytes decreased in both GLC and CAS pigs relative to CON. Nevertheless, variations in other circulating markers of inflammation were minimal. For instance, LBP mildly decreased in GLC relative to CON, but remained similar between CAS and CON pigs. Whereas no changes in circulating TNFα were detected across treatments.
Thus, while our data validate altered fermentation patterns in both GLC and CAS pigs, it is likely that the infusion challenge was not severe enough to elicit a systemic inflammatory response. Although surprising, previous reports investigating the effects of excessive fermentation in the hindgut on systemic inflammation have also been inconclusive. Accordingly, Mainardi et al. (2011) observed no changes in rectal temperature or circulating acute-phase proteins when hindgut fermentation was experimentally induced with a pulse-dose of oligofructose in growing steers. Similarly, despite marked reductions in fecal pH, inflammatory markers were unaltered in cows abomasally infused with either 500 g/d resistant starch (Piantoni et al., 2018), 4 kg/d of starch (Abeyta et al., 2019a, 2019b), or 3 kg/d of ground corn (van Gastelen et al., 2021). On the other hand, the literature regarding the effects of protein fermentation on circulating markers of inflammation is scarce, mainly because previous studies have focused on local (as discussed below) rather than systemic inflammatory responses. We used relatively heavy pigs (>80 kg) and maybe the fermentative and absorptive capacity of the large intestine was able to safely adjust to the continuous infusion. Regardless, the lack of observable differences in systemic inflammation in GLC and CAS clearly demonstrates that there was not a robust immune response.
Compromised intestinal integrity has been previously demonstrated in animals fed high carbohydrate or protein diets (Krueger et al., 1986; Andriamihaja et al., 2010; Tao et al., 2014a, 2014b; Ye et al., 2016). Furthermore, in vivo and in vitro studies have reported local inflammatory responses, including epithelial cell damage, altered tight-junction profile, increased expression of inflammatory mediators, and immune cell infiltration associated with mucosal exposure to organic acids (i.e., SCFA) or excessive protein fermentation in the lower gut (Argenzio and Meuten, 1991; Hughes et al., 2008; Richter et al., 2014). Therefore, in an attempt to evaluate the effects of GLC or CAS infusion in both the small and large intestine, markers of villi morphology, mucosal area, and immune cell infiltration were assessed herein. Contrary to our hypothesis, GLC or CAS infusion did not seem to alter intestinal morphology or mucosal surface area in the current model; however, we observed increased MPO stained area in the colon of CAS relative to CON pigs. Increased MPO stained area could be interpreted as increased neutrophil infiltration in the colonic tissue of CAS pigs due to immune activation; however, the biological relevance of this observation must be interpreted with caution as no major alterations in neither intestinal morphology nor circulating inflammatory biomarkers were observed by the continuous CAS infusion.
Fecal pH and VFA Concentrations
Fecal pH was similar in both GLC and CAS treatments when compared with CON pigs. This is unexpected as excessive carbohydrate fermentation in the hindgut has been shown to induce marked reductions in colonic pH (Lin, 2004; Ye et al., 2016; Abeyta et al., 2019a, 2019b), while fermentation of nitrogenous compounds in the lower gut concurs with a more alkaline intestinal environment (Rist et al., 2013; Wang et al., 2018). Although fecal pH has been previously assessed in different studies as an indirect marker of colonic fermentation (Yao et al., 2016), its values might resemble conditions in the distal rather than the proximal colon, where most of the fermentation is presumably taking place (Cummings and Macfarlane, 1991; Macfarlane et al., 1992; Williams et al., 2005).
Despite no treatment differences observed in fecal pH, VFA concentrations in the colon were altered. Increased propionate and decreased butyrate concentrations were observed in GLC pigs when compared with their CON counterparts. Along with acetate, propionate and butyrate are the main SCFA produced from microbial fermentation of carbohydrates and are known to play an important role as metabolic substrates, regulators of gene expression, and signaling molecules (as reviewed by Tan et al., 2014). In particular, butyrate is a key fuel for intestinal epithelium and has been shown to improve barrier dysfunction (Leonel and Alvarez-Leite, 2012). Furthermore, although BCFA concentrations did not differ between CAS and CON pigs, CAS infusion coincided with increased isobutyric and isovaleric concentrations relative to GLC-infused pigs. Both isobutyric and isovaleric fatty acids are produced exclusively upon branched-chain amino acid fermentation, specifically valine, leucine, and isoleucine, which corroborates the fact that conditions for protein fermentation were favored in the CAS pigs during the current study (Smith and Macfarlane, 1997; Andriamihaja et al., 2010). Unlike other fermentation end-products, no adverse effects of BCFA on colonic epithelium have been previously reported (Gilbert et al., 2018).
Production Parameters
We hypothesized that both GLC and CAS pig would be immune stimulated and thus experience inflammation-induced anorexia. Immune activation decreased appetite is a highly conserved species response and occurs even in insects (Adamo, 2005). A slight decrease in ADFI was observed in GLC relative to CON pigs, while no differences were detected between CAS and CON pigs. Considering that healthy animals regulate ADFI to meet their energy requirements, it is likely that GLC pigs decreased their nutrient intake when an additional energy source (in the form of GLC infusions) was supplied (Conrad et al., 1964; Li and Patience, 2017). Regardless, this difference was moderate and had no overall influence on other productive parameters, including ADG and final BW. Although measuring production parameters was not the main objective of this study, this allows us to put our results into context, indicating that both GLC and CAS infusions had little to no detrimental effects on overall pig performance.
Limitations and Further Directions
Although our observations validate that altered fermentation patterns occurred in GLC and CAS pigs, our current model did not effectively stimulate a robust inflammatory response. The lack of changes in biomarkers of inflammation or intestinal morphology in this study suggests the large intestine is highly adaptable to large variations in diet constituents. Consequently, other mechanisms might exist that facilitate adaptation to these fermentation processes and their associated metabolites in the hindgut. For instance, colonic ion transporters (i.e., bicarbonate, Na+/H+ exchangers) are secreted in the lumen of the large intestine in exchange with SCFA, which may contribute to colonic alkalinity by regulating luminal pH and by reducing the accumulation of organic acids produced during hindgut acidosis (Musch et al., 2001; Binder et al., 2005). Similarly, increased colonocyte expression of a gene involved in the detoxification of hydrogen sulfide, a bacterial metabolite produced during protein fermentation, was observed in rats exposed to a high protein diet, suggesting an adaptive response to the increased production of this toxic compound (Beaumont et al., 2016). Regardless, investigating the mechanisms by which the large intestine maintains colonic homeostasis is warranted.
Unfortunately, a limitation of our study is the lack of measurements of other bacterial metabolites (i.e., lactic acid, ammonia, phenols, indols, amines, hydrogen sulfide) produced during excessive fermentation in the hindgut. The ability of these bacterial products to exert adverse effects on epithelial health has been extensively studied (Pieper et al., 2016; Gilbert et al., 2018); however, their deleterious effects seem to be directly associated with their luminal concentrations (Blachier et al., 2010; Beaumont et al., 2016). Thus, when present in low concentrations, end-fermentation products might be easily handled by the colonic environment, at least in healthy animals (Beaumont et al., 2016). Consequently, it is likely that hindgut acidosis or protein putrefaction alone does not necessarily result in an inflammatory state. Rather, the presence of other intrinsic (i.e., stress, viral/bacterial infections, nutrient malabsorption) and/or extrinsic factors (i.e., dietary/environmental changes, off-feed events) may contribute to an increased flow of undigested nutrients to the large intestine, promote excessive fermentation, cause dysbiosis, and exceed the large intestine’s capacity to detoxify harmful metabolites (Pieper et al., 2016). Additional research is needed to better understand the association between these intrinsic/extrinsic factors and the incidence of hindgut acidosis or protein putrefaction.
Conclusion
We continuously infused GLC or CAS in the terminal ileum in growing pigs in an attempt to model “unhealthy” hindgut acidosis and protein fermentation. Decreased ADFI and mild hyperthermia were observed in GLC and CAS-infused pigs, along with minor changes in circulating leukocytes. Additionally, changes in BUN patterns and in the concentration of VFA in the colon suggests our model successfully resembled increased carbohydrate and protein fermentation patterns in the hindgut. However, contrary to our hypothesis, excessive fermentation did not seem to stimulate an immune response nor alter markers of intestinal morphology. Furthermore, no phenotypic responses or changes in production parameters were detected, suggesting other mechanisms might exist that facilitate the animals’ adaptation to these fermentation processes and their associated metabolites in the hindgut. What is clear is that fermenting large amounts of soluble carbohydrates and proteins in the large intestine does not appear to be pathological, at least not under the conditions of this trial. Therefore, hindgut acidosis and protein putrefaction may not be causal to alimentary disorders as many predict, but merely symptoms of an underlying intestinal disorder. Regardless, further research is warranted to better understand how hindgut acidosis or protein putrefaction causes (or it is caused by) intestinal dysfunction.
Acknowledgments
Results reported herein were supported by the Norman Jacobson Endowment (Iowa State University).
Glossary
Abbreviations
- ADFI
Average daily feed intake
- ADG
Average daily gain
- BCFA
Branched-chain fatty acids
- BUN
Blood urea nitrogen
- BW
Body weight
- H&E
Hematoxylin and eosin
- LBP
Lipopolysaccharide binding protein
- MPO
Myeloperoxidase
- NEFA
Non-esterified fatty acids
- PAS
Periodic acid-Schiff
- SCFA
Short-chain fatty acids
- TNFα
Tumor necrosis factor-alpha
- TR
Rectal temperature
- VFA
Volatile fatty acids
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Abeyta, M. A., Horst E. A., Mayorga E. J., Goetz B. M., Al-Qaisi M., McCarthy C. S., O’Neil M. R., Dooley B. C., Piantoni P., Schroeder G. F., . et al. 2019a. Effects of hindgut acidosis on metabolism, inflammation, and production in dairy cows consuming a standard lactation diet. J. Dairy Sci. 102:270. (Abstr.) [DOI] [PubMed] [Google Scholar]
- Abeyta, M. A., Horst E. A., Rodriguez-Jimenez S. J., Mayorga E. J., Goetz B. M., Al-Qaisi M., McCarthy C. S., O’Neil M. R., Dooley B. C., Piantoni P., . et al. 2019b. Effects of hindgut acidosis on metabolism, inflammation, and production in dairy cows acclimated to a low-starch diet. J. Dairy Sci. 102:402. (Abstr.) [DOI] [PubMed] [Google Scholar]
- Acosta, J. A., Boyd R. D., and Patience J. F.. . 2017. Digestion and nitrogen balance using swine diets containing increasing proportions of coproduct ingredients and formulated using the net energy system. J. Anim. Sci. 95:1243–1252. doi: 10.2527/jas.2016.1161 [DOI] [PubMed] [Google Scholar]
- Adamo, S. A. 2005. Parasitic suppression of feeding in the tobacco hornworm, Manduca sexta: parallels with feeding depression after an immune challenge. Arch. Insect Biochem. Physiol. 60:185–197. doi: 10.1002/arch.20068 [DOI] [PubMed] [Google Scholar]
- Andriamihaja, M., Davila A. M., Eklou-Lawson M., Petit N., Delpal S., Allek F., Blais A., Delteil C., Tomé D., and Blachier F.. . 2010. Colon luminal content and epithelial cell morphology are markedly modified in rats fed with a high-protein diet. Am. J. Physiol. Gastrointest. Liver Physiol. 299:G1030–G1037. doi: 10.1152/ajpgi.00149.2010 [DOI] [PubMed] [Google Scholar]
- Argenzio, R. A., and Meuten D. J.. . 1991. Short-chain fatty acids induce reversible injury or porcine colon. Dig. Dis. Sci. 36:1459–1468. doi: 10.1007/bf01296816 [DOI] [PubMed] [Google Scholar]
- Beaumont, M., Andriamihaja M., Lan A., Khodorova N., Audebert M., Blouin J. M., Grauso M., Lancha L., Benetti P. H., Benamouzig R., . et al. 2016. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: the adaptive response. Free Radic. Biol. Med. 93:155–164. doi: 10.1016/j.freeradbiomed.2016.01.028 [DOI] [PubMed] [Google Scholar]
- Binder, H. J., Rajendran V., Sadasivan V., and Geibel J. P.. . 2005. Bicarbonate secretion: a neglected aspect of colonic ion transport. J. Clin. Gastroenterol. 39:S53–S58. doi: 10.1097/01.mcg.0000155521.81382.3a [DOI] [PubMed] [Google Scholar]
- Blachier, F., Davila A. M., Mimoun S., Benetti P. H., Atanasiu C., Andriamihaja M., Benamouzig R., Bouillaud F., and Tomé D.. . 2010. Luminal sulfide and large intestine mucosa: friend or foe? Amino Acids 39:335–347. doi: 10.1007/s00726-009-0445-2 [DOI] [PubMed] [Google Scholar]
- Blachier, F., Mariotti F., Huneau J. F., and Tomé D.. . 2007. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 33:547–562. doi: 10.1007/s00726-006-0477-9 [DOI] [PubMed] [Google Scholar]
- Collett, S. R. 2012. Nutrition and wet litter problems in poultry. Anim. Feed Sci. Technol. 173:65–75. doi: 10.1016/j.anifeedsci.2011.12.013 [DOI] [Google Scholar]
- Conrad, H. R., Pratt A. D., and Hibbs J. W.. . 1964. Regulation of feed intake in dairy cows. 1. Change in importance of physical and physiological factors with increasing digestibility. J. Dairy Sci. 47:54–62. doi: 10.3168/jds.S0022-0302(64)88581-7 [DOI] [Google Scholar]
- Crawford, C., Sepulveda M. F., Elliott J., Harris P. A., and Bailey S. R.. . 2007. Dietary fructan carbohydrate increases amine production in the equine large intestine: implications for pasture-associated laminitis. J. Anim. Sci. 85:2949–2958. doi: 10.2527/jas.2006-600 [DOI] [PubMed] [Google Scholar]
- Cummings, J. H., and Macfarlane G. T.. . 1991. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 70:443–459. doi: 10.1111/j.1365-2672.1991.tb02739.x [DOI] [PubMed] [Google Scholar]
- Elsasser, T. H., Caperna T. J., Li C. L., Kahl S., and Sartin J. L.. . 2008. Critical points in the impact of the proinflammatory immune response on growth and metabolism. J. Anim. Sci. 86:E105–E125. doi: 10.2527/jas.2007-0634 [DOI] [PubMed] [Google Scholar]
- FASS . 2010. Guide for the care and use of agricultural animals in research and teaching. 3rd ed. Champaign (IL):Federation of Animal Science Societies. [Google Scholar]
- Garner, H. E., Hutcheson D. P., Coffman J. R., Hahn A. W., and Salem C.. . 1977. Lactic acidosis: a factor associated with equine laminitis. J. Anim. Sci. 45:1037–1041. doi: 10.2527/jas1977.4551037x [DOI] [PubMed] [Google Scholar]
- van Gastelen, S., Dijkstra J., Nichols K., and Bannink A.. . 2021. Abomasal infusion of ground corn and ammonium chloride in early-lactating Holstein-Friesian dairy cows to induce hindgut and metabolic acidosis. J. Dairy Sci. 104:4174–4191. doi: 10.3168/jds.2020-19300 [DOI] [PubMed] [Google Scholar]
- Gilbert, M. S., Ijssennagger N., Kies A. K., and van Mil S. W. C.. . 2018. Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry. Am. J. Physiol. Gastrointest. Liver Physiol. 315:G159–G170. doi: 10.1152/ajpgi.00319.2017 [DOI] [PubMed] [Google Scholar]
- Grauso, M., Lan A., Andriamihaja M., Bouillaud F., and Blachier F.. . 2019. Hyperosmolar environment and intestinal epithelial cells: impact on mitochondrial oxygen consumption, proliferation, and barrier function in vitro. Sci. Rep. 9:11360. doi: 10.1038/s41598-019-47851-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressley, T. F., and Armentano L. E.. . 2007. Effects of low rumen-degradable protein or abomasal fructan infusion on diet digestibility and urinary nitrogen excretion in lactating dairy cows. J. Dairy Sci. 90:1340–1353. doi: 10.3168/jds.S0022-0302(07)71621-1 [DOI] [PubMed] [Google Scholar]
- Gressley, T. F., Hall M. B., and Armentano L. E.. . 2011. Ruminant Nutrition Symposium: Productivity, digestion, and health responses to hindgut acidosis in ruminants. J. Anim. Sci. 89:1120–1130. doi: 10.2527/jas.2010-3460 [DOI] [PubMed] [Google Scholar]
- Heo, J. M., Kim J. C., Hansen C. F., Mullan B. P., Hampson D. J., and Pluske J. R.. . 2008. Effects of feeding low protein diets to piglets on plasma urea nitrogen, faecal ammonia nitrogen, the incidence of diarrhoea and performance after weaning. Arch. Anim. Nutr. 62:343–358. doi: 10.1080/17450390802327811 [DOI] [PubMed] [Google Scholar]
- Heo, J. M., Kim J. C., Hansen C. F., Mullan B. P., Hampson D. J., and Pluske J. R.. . 2009. Feeding a diet with decreased protein content reduces indices of protein fermentation and the incidence of postweaning diarrhea in weaned pigs challenged with an enterotoxigenic strain of Escherichia coli. J. Anim. Sci. 87:2833–2843. doi: 10.2527/jas.2008-1274 [DOI] [PubMed] [Google Scholar]
- Hughes, R., Kurth M. J., McGilligan V., McGlynn H., and Rowland I.. . 2008. Effect of colonic bacterial metabolites on Caco-2 cell paracellular permeability in vitro. Nutr. Cancer 60:259–266. doi: 10.1080/01635580701649644 [DOI] [PubMed] [Google Scholar]
- Huntley, N. F., Nyachoti C. M., and Patience J. F.. . 2018. Lipopolysaccharide immune stimulation but not β-mannanase supplementation affects maintenance energy requirements in young weaned pigs. J. Anim. Sci. Biotech. 9:901–916. doi: 10.1186/s40104-018-0264-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeaurond, E. A., Rademacher M., Pluske J. R., Zhu C. H., and de Lange C. F. M.. . 2008. Impact of feeding fermentable proteins and carbohydrates on growth performance, gut health and gastrointestinal function of newly weaned pigs. Can. J. Anim. Sci. 88:271–281. doi: 10.4141/CJAS07062 [DOI] [Google Scholar]
- Klasing, K. C. 2007. Nutrition and the immune system. Br. Poult. Sci. 48:525–537. doi: 10.1080/00071660701671336 [DOI] [PubMed] [Google Scholar]
- Krueger, A. S., Kinden D. A., Garner H. E., and Sprouse R. F.. . 1986. Ultrastructural study of the equine cecum during onset of laminitis. Am. J. Vet. Res. 47:1804–1812. [PubMed] [Google Scholar]
- Kvidera, S. K., Horst E. A., Abuajamieh M., Mayorga E. J., Fernandez M. V., and Baumgard L. H.. . 2017a. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 100:2360–2374. doi: 10.3168/jds.2016-12001 [DOI] [PubMed] [Google Scholar]
- Kvidera, S. K., Horst E. A., Mayorga E. J., Sanz-Fernandez M. V., Abuajamieh M., and Baumgard L. H.. . 2017b. Estimating glucose requirements of an activated immune system in growing pigs. J. Anim. Sci. 95:5020–5029. doi: 10.2527/jas2017.1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonel, A. J., and Alvarez-Leite J. I.. . 2012. Butyrate: implications for intestinal function. Curr. Opin. Nutri. Metab. Care. 15:474–479. doi: 10.1097/MCO.0b013e32835665fa [DOI] [PubMed] [Google Scholar]
- Li, Q., and Patience J. F.. . 2017. Factors involved in the regulation of feed and energy intake of pigs. Anim. Feed. Sci. Technol. 233:22–33. doi: 10.1016/j.anifeedsci.2016.01.001 [DOI] [Google Scholar]
- Li, P. F., Xue L. F., Zhang R. F., Piao X. S., Zeng Z. K., and Zhan J. S.. . 2011. Effects of fermented potato pulp on performance, nutrient digestibility, carcass traits and plasma parameters of growing-finishing pigs. Asian-Aust. J. Anim. Sci. 24:1456–1463. doi: 10.5713/ajas.2011.11169 [DOI] [Google Scholar]
- Lin, J. 2004. Too much short chain fatty acids cause neonatal necrotizing enterocolitis. Med. Hypotheses 62:291–293. doi: 10.1016/S0306-9877(03)00333-5 [DOI] [PubMed] [Google Scholar]
- Lin, J., Nafday S. M., Chauvin S. N., Magid M. S., Pabbatireddy S., Holzman I. R., and Babyatsky M. W.. . 2002. Variable effects of short chain fatty acids and lactic acid in inducing intestinal mucosal injury in newborn rats. J. Pediatr. Gastroenterol. Nutr. 35:545–550. doi: 10.1097/00005176-200210000-00016 [DOI] [PubMed] [Google Scholar]
- Macfarlane, G. T., Gibson G. R., and Cummings J. H.. . 1992. Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol. 72:57–64. doi: 10.1111/j.1365-2672.1992.tb04882.x [DOI] [PubMed] [Google Scholar]
- Mainardi, S. R., Hengst B. A., Nebzydoski S. J., Nemec L. M., and Gressley T. F.. . 2011. Effects of abomasal oligofructose on blood and feces of Holstein steers. J. Anim. Sci. 89:2510–2517. doi: 10.2527/jas.2010-3348 [DOI] [PubMed] [Google Scholar]
- McGuinness, O. P. 2005. Defective glucose homeostasis during infection. Annu. Rev. Nutr. 25:9–35. doi: 10.1146/annurev.nutr.24.012003.132159 [DOI] [PubMed] [Google Scholar]
- Misir, R., and Sauer W. C.. . 1982. Effect of starch infusion at the terminal ileum on nitrogen balance and apparent digestibilities of nitrogen and amino acids in pigs fed meat-and-bone and soybean meal diets. J. Anim. Sci. 55:599–607. doi: 10.2527/jas1982.553599x [DOI] [PubMed] [Google Scholar]
- Mosenthin, R., Sauer W. C., Henkel H., Ahrens F., and de Lange C. F.. . 1992. Tracer studies of urea kinetics in growing pigs: II. The effect of starch infusion at the distal ileum on urea recycling and bacterial nitrogen excretion. J. Anim. Sci. 70:3467–3472. doi: 10.2527/1992.70113467x [DOI] [PubMed] [Google Scholar]
- Musch, M. W., Bookstein C., Xie Y., Sellin J. H., and Chang E. B.. . 2001. SCFA increase intestinal NA absorption by induction on NHE3 in rat colon and human intestinal C2/bbe cells. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G687–G693. doi: 10.1152/ajpgi.2001.280.4.G687 [DOI] [PubMed] [Google Scholar]
- NRC . 2012. Nutrient requirements of swine: eleventh revised edition. 11th ed.Washington, DC:Natl. Acad. Press. [Google Scholar]
- Petry, A. L., Gould S. A., and Patience J. F.. . 2020. A procedure for dual simple-T cannulation in the small intestine of pigs using a single right flank laparotomy. J. Appl. Anim. Nutr. 8:135–141. doi: 10.3920/JAAN2020.0008 [DOI] [Google Scholar]
- Piantoni, P., Abeyta M. A., Schroeder G. F., Ramírez-Ramírez H. A., Tucker H. A., and Baumgard L. H.. . 2018. Induction of leaky gut through feed restriction or abomasal infusion of resistant starch in healthy post-peak lactating cows. J. Dairy Sci. 101:228. (Abstr.) [DOI] [PubMed] [Google Scholar]
- Pieper, R., Villodre Tudela C., Taciak M., Bindelle J., Pérez J. F., and Zentek J.. . 2016. Health relevance of intestinal protein fermentation in young pigs. Anim. Health Res. Rev. 17:137–147. doi: 10.1017/S1466252316000141 [DOI] [PubMed] [Google Scholar]
- Plaizier, J. C., Krause D. O., Gozho G. N., and McBride B. W.. . 2008. Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. Vet. J. 176:21–31. doi: 10.1016/j.tvjl.2007.12.016 [DOI] [PubMed] [Google Scholar]
- Pluske, J. R., Durmic Z., Pethick D. W., Mullan B. P., and Hampson D. J.. . 1998. Confirmation of the role of rapidly fermentable carbohydrates in the expression of swine dysentery in pigs after experimental infection. J. Nutr. 128:1737–1744. doi: 10.1093/jn/128.10.1737 [DOI] [PubMed] [Google Scholar]
- Pluske, J. R., Siba P. M., Pethick D. W., Durmic Z., Mullan B. P., and Hampson D. J.. . 1996. The incidence of swine dysentery in pigs can be reduced by feeding diets that limit the amount of fermentable substrate entering the large intestine. J. Nutr. 126:2920–2933. doi: 10.1093/jn/126.11.2920 [DOI] [PubMed] [Google Scholar]
- Richter, J. F., Pieper R., Zakrzewski S. S., Günzel D., Schulzke J. D., and Van Kessel A. G.. . 2014. Diets high in fermentable protein and fibre alter tight junction protein composition with minor effects on barrier function in piglet colon. Br. J. Nutr. 111:1040–1049. doi: 10.1017/S0007114513003498 [DOI] [PubMed] [Google Scholar]
- Rist, V. T., Weiss E., Eklund M., and Mosenthin R.. . 2013. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: a review. Animal 7:1067–1078. doi: 10.1017/S1751731113000062 [DOI] [PubMed] [Google Scholar]
- Rosenfelder-Kuon, P., Strang E. J. P., Spindler H. K., Eklund M., and Mosenthin R.. . 2017. Ileal starch digestibility of different cereal grains fed to growing pigs. J. Anim. Sci. 95:2711–2717. doi: 10.2527/jas.2017.1450 [DOI] [PubMed] [Google Scholar]
- Sanz Fernandez, M. V., Johnson J. S., Abuajamieh M., Stoakes S. K., Seibert J. T., Cox L., Kahl S., Elsasser T. H., Ross J. W., Isom S. C., . et al. 2015. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 3:e12315. doi: 10.14814/phy2.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, E. A., and Macfarlane G. T.. . 1997. Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe 3:327–337. doi: 10.1006/anae.1997.0121 [DOI] [PubMed] [Google Scholar]
- Spurlock, M. E. 1997. Regulation of metabolism and growth during immune challenge: an overview of cytokine function. J. Anim. Sci. 75:1773–1783. doi: 10.2527/1997.7571773x [DOI] [PubMed] [Google Scholar]
- Tan, J., McKenzie C., Potamitis M., Thorburn A. N., Mackay C. R., and Macia L.. . 2014. The role of short chain fatty acids in health and disease. Adv. Immunol. 121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9 [DOI] [PubMed] [Google Scholar]
- Tao, S. Y., Duanmu Y. Q., Dong H. B., Ni Y. D., Chen J., Shen X. Z., and Zhao R. Q.. . 2014a. High concentrate diet induced mucosal injuries by enhancing epithelial apoptosis and inflammatory response in the hindgut of goats. PLoS One. 9:e111596. doi: 10.1371/journal.pone.0111596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, S. Y., Duanmu Y. Q., Dong H. B., Tian J., Ni Y. D., and Zhao R. Q.. . 2014b. A high concentrate diet induced colonic epithelial barrier disruption is associated with the activating of cell apoptosis in lactating goats. BMC Vet. Res. 10:235. doi: 10.1186/s12917-014-0235-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton, R. F., Bird P. R., Somers M., Moir R. J.. . 1970. Urea excretion in ruminants. III. The role of the hind-gut (caecum and colon). Aust. J. Agric. Res. 21:345–354. doi: 10.1071/AR9700345 [DOI] [Google Scholar]
- Wang, Y., Zhou J., Wang G., Cai S., Zeng X., and Qiao S.. . 2018. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 9:60. doi: 10.1186/s40104-018-0276-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, B. A., Bosch M. W., Awati A., Kostantinov S. R., Smidt H., Akkermans A. D. L., Verstegen M. W. A., and Tamminga S.. . 2005. In vitro assessment of gastrointestinal tract (GIT) fermentation in pigs: fermentable substrates and microbial activity. Anim. Res. 54:191–201. doi: 10.1051/animres:2005011 [DOI] [Google Scholar]
- Yao, C. K., Muir J. G., and Gibson P. R.. . 2016. Review article: insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 43:181–196. doi: 10.1111/apt.13456 [DOI] [PubMed] [Google Scholar]
- Ye, H., Liu J., Feng P., Zhu W., and Mao S.. . 2016. Grain-rich diets altered the colonic fermentation and mucosa-associated bacterial communities and induced mucosal injuries in goats. Scient. Reports. 6:20329. doi: 10.1038/srep20329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes, H., Rémésy C., Behr S., and Demigné C.. . 1997. Fermentable carbohydrate exerts a urea-lowering effect in normal and nephrectomized rats. Am. J. Physiol. 272:G515–G521. doi: 10.1152/ajpgi.1997.272.3.G515 [DOI] [PubMed] [Google Scholar]