Key Points
Question
What is the association between risk-enhancing factors and incident atherosclerotic cardiovascular disease by coronary artery calcium burden among intermediate-risk individuals?
Findings
In this cross-sectional study of a multiethnic sample of 1688 adults with an intermediate risk of atherosclerotic cardiovascular disease, most risk-enhancing factors (individual and combined) had an absolute atherosclerotic cardiovascular disease incidence rate of less than 7.5 events per 1000 person-years in the absence of subclinical atherosclerosis (coronary artery calcium score of 0). The use of coronary artery calcium scoring was associated with significant improvements in the reclassification and discrimination of atherosclerotic cardiovascular events.
Meaning
The study’s findings suggested that, among individuals at intermediate risk with risk-enhancing factors, atherosclerotic cardiovascular event rates were generally lower than the recommended threshold to initiate statin therapy when the coronary artery calcium score was 0; for cases in which additional risk stratification is required or uncertainty exists regarding the utility of statin therapy, coronary artery calcium scoring may be used to further guide the clinician-patient risk discussion.
Abstract
Importance
The 2018 American Heart Association/American College of Cardiology Guideline on the Management of Blood Cholesterol recommends the use of risk-enhancing factor assessment and the selective use of coronary artery calcium (CAC) scoring to guide the allocation of statin therapy among individuals with an intermediate risk of atherosclerotic cardiovascular disease (ASCVD).
Objective
To examine the association between risk-enhancing factors and incident ASCVD by CAC burden among those at intermediate risk of ASCVD.
Design, Setting, and Participants
The Multi-Ethnic Study of Atherosclerosis is a multicenter population-based prospective cross-sectional study conducted in the US. Baseline data for the present study were collected between July 15, 2000, and July 14, 2002, and follow-up for incident ASCVD events was ascertained through August 20, 2015. Participants were aged 45 to 75 years with no clinical ASCVD or diabetes at baseline, were at intermediate risk of ASCVD (≥7.5% to <20.0%), and had a low-density lipoprotein cholesterol level of 70 to 189 mg/dL.
Exposures
Family history of premature ASCVD, premature menopause, metabolic syndrome, chronic kidney disease, lipid and inflammatory biomarkers, and low ankle-brachial index.
Main Outcomes and Measures
Incident ASCVD over a median follow-up of 12.0 years.
Results
A total of 1688 participants (mean [SD] age, 65 [6] years; 976 men [57.8%]). Of those, 648 individuals (38.4%) were White, 562 (33.3%) were Black, 305 (18.1%) were Hispanic, and 173 (10.2%) were Chinese American. A total of 722 participants (42.8%) had a CAC score of 0. Among those with 1 to 2 risk-enhancing factors vs those with 3 or more risk-enhancing factors, the prevalence of a CAC score of 0 was 45.7% vs 40.3%, respectively. Over a median follow-up of 12.0 years (interquartile range [IQR], 11.5-12.6 years), the unadjusted incidence rate of ASCVD among those with a CAC score of 0 was less than 7.5 events per 1000 person-years for all individual risk-enhancing factors (with the exception of ankle-brachial index, for which the incidence rate was 10.4 events per 1000 person-years [95% CI, 1.5-73.5]) and combinations of risk-enhancing factors, including participants with 3 or more risk-enhancing factors. Although the individual and composite addition of risk-enhancing factors to the traditional risk factors was associated with improvement in the area under the receiver operating curve, the use of CAC scoring was associated with the greatest improvement in the C statistic (0.633 vs 0.678) for ASCVD events. For incident ASCVD, the net reclassification improvement for CAC was 0.067.
Conclusions and Relevance
In this cross-sectional study, among participants with CAC scores of 0, the presence of risk-enhancing factors was generally not associated with an overall ASCVD risk that was higher than the recommended treatment threshold for the initiation of statin therapy. The use of CAC scoring was associated with significant improvements in the reclassification and discrimination of incident ASCVD. The results of this study support the utility of CAC scoring as an adjunct to risk-enhancing factor assessment to more accurately classify individuals with an intermediate risk of ASCVD who might benefit from statin therapy.
This cross-sectional study examines the association between risk-enhancing factors and incident atherosclerotic cardiovascular disease by coronary artery calcium burden among intermediate-risk adults who participated in the Multi-Ethnic Study of Atherosclerosis.
Introduction
The 2018 American Heart Association/American College of Cardiology (AHA/ACC) Cholesterol Guideline on the Management of Blood Cholesterol1 recommends assessment of the 10-year risk of developing atherosclerotic cardiovascular disease (ASCVD) as a first step in the clinician-patient risk discussion. The AHA/ACC guideline introduced the assessment of ASCVD risk-enhancing factors as a means to guide discussion regarding the initiation of statin therapy among individuals with intermediate or borderline risk (class 2a [indicating that the weight of evidence and opinion is in favor of the usefulness and/or efficacy of statin therapy] for those with intermediate risk and class 2b [indicating that the usefulness and/or efficacy is less well established by evidence and opinion] for those with borderline risk) when the decision to treat is uncertain.1
The presence of risk-enhancing factors (including a family history of premature ASCVD, lipid and inflammatory biomarkers, chronic kidney disease [CKD], chronic inflammatory conditions, premature menopause or preeclampsia, South Asian ethnicity, and low ankle-brachial index [ABI]) is considered to be associated with increased risk, leading to reclassification of individuals with borderline or intermediate risk into a higher risk category that favors the addition of statin therapy.2 If the decision to initiate treatment with statin therapy remains uncertain, testing for coronary artery calcium (CAC) may then be considered to further reclassify risk (ie, class 2a).1
Coronary artery calcium is a marker of subclinical atherosclerosis that correlates with total coronary plaque burden and is associated with ASCVD events.3 A CAC score of 100 or higher has been reported to equate to observed 10-year ASCVD incidence rates of 7.5 or more events per 1000 person-years, regardless of sex, race and ethnicity, or age.4 In contrast, the absence of CAC (ie, a CAC score of 0) is associated with a low risk of future ASCVD events over the next 10 years, even in the presence of multiple traditional ASCVD risk factors.4,5,6,7 In addition, despite an increased risk of short-term ACSVD events among those with vs without risk-enhancing factors, data suggest that certain risk-enhancing factors (family history of premature ASCVD, dyslipidemia, diabetes, and metabolic syndrome) are frequently associated with low short-term rates of ASCVD events (ie, <7.5 events per 1000 person-years) among those with CAC scores of 0.6,7,8
The 2018 AHA/ACC cholesterol guideline acknowledged difficulty in assessing the extent to which an individual risk-enhancing factor may quantitatively change the 10-year risk estimate for an individual patient.1,9 In addition, it remains unknown how frequently risk-enhancing factors are present when the CAC score is 0. Given these considerations, we sought to evaluate the association of risk-enhancing factors and clinically relevant CAC categories with incident ASCVD events in the context of the 2018 AHA/ACC cholesterol guideline.
Methods
Study Population
Methods used in the Multi-Ethnic Study of Atherosclerosis (MESA) have been described previously.10 In brief, MESA enrolled 6814 participants between July 2000 and July 2002 across 6 field sites in the US (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York, New York; and St Paul, Minnesota). Participants were aged 45 to 84 years and of White, Black, Hispanic, and Chinese American race or ethnicity. All participants were free of clinical ASCVD at baseline. Institutional review boards at each site approved the study, and all participants provided written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Inclusion and Exclusion Criteria
Baseline data for the present study were collected between July 15, 2000, and July 14, 2002, and follow-up for incident events was ascertained through August 20, 2015. We included MESA participants with an intermediate risk of ASCVD, which was defined using the 2013 pooled cohort equations as a 10-year ASCVD risk between 7.5% and less than 20.0%.1 Individuals with Hispanic and Chinese American ancestry were categorized as other race/ethnicity per the pooled cohort equations algorithm. We excluded those with low (<7.5%) and high (>20.0%) 10-year estimated ASVCD risk (3180 individuals); those with missing data on low-density lipoprotein cholesterol (LDL-C) levels, LDL-C levels less than 70 mg/dL, or LDL-C levels of 190 mg/dL or higher (573 individuals); those older than 75 years (741 individuals); and those with diabetes (632 individuals, of whom 86 were receiving insulin and 7 had type 1 diabetes) (eFigure 1 in the Supplement). After exclusions, 1688 participants were included in the study sample.
Coronary Artery Calcium
Details pertaining to the MESA cardiac computed tomography protocol have been published in detail elsewhere.11 In brief, CAC was measured using either electron-beam computed tomography or multidetector-row helical computed tomography, depending on the field site. Additional information is available in the eMethods in the Supplement.
Risk-Enhancing Factors
Biochemistry assays were performed on serum markers of lipids and lipid metabolism, systemic inflammation, and insulin resistance and on urinary markers of kidney function at a central laboratory (University of Vermont).10 Age, sex, race and ethnicity, reproductive history, and lifestyle factors were ascertained using validated questionnaires.10 Additional details pertaining to serum and urinary laboratory data assessment as well as anthropometric, demographic, medical history, and clinical variables in MESA are available in the eMethods in the Supplement.
Available risk-enhancing factors in MESA were defined according to the 2018 AHA/ACC Cholesterol Guideline on the Management of Blood Cholesterol.1 South Asian ethnicity is a risk-enhancing factor; however, MESA did not recruit any individuals of South Asian descent.10 Details are available in eFigure 1 in the Supplement.
Ascertainment of Incident ASCVD
A detailed description of the event adjudication process has been previously published.10,12 In brief, ASCVD events included coronary heart disease (CHD) events (myocardial infarction, resuscitated cardiac arrest, or CHD-associated death) and fatal and nonfatal hemorrhagic or ischemic stroke (eMethods in the Supplement).
Statistical Analysis
The baseline characteristics of study participants in each of 3 clinically relevant categories (ie, CAC scores of 0, 1-99, and ≥100) were compared using an analysis of variance for continuous variables, a χ2 test for categorical variables, and age-standardized proportions.1,4 In addition, high-sensitivity C-reactive protein (hsCRP), lipoprotein a (Lp[a]), apolipoprotein B100 (apo B100), and triglycerides were examined as continuous variables; hsCRP, Lp(a), and CAC were log-transformed to ensure normality of the data.
Risk-enhancing factors were examined individually and in combination (categorized as 0 factors, 1-2 factors, and ≥3 factors). We also distinguished between 2 groups of risk-enhancing factors (basic and advanced) depending on how readily information about these factors was likely to be available to the clinician. Basic risk-enhancing factors included a family history of premature ASCVD and the presence of CKD, metabolic syndrome, premature menopause, or elevated triglycerides. Advanced risk-enhancing factors included elevated hsCRP, Lp(a), and apo B100 and low ABI.
Unadjusted incidence rates of ASCVD were calculated as the number of events per 1000 person-years. Kaplan-Meier curves were constructed to graphically describe ASCVD-free cumulative survival by risk-enhancing factor burden and CAC group.
We used Cox proportional hazards regression models to examine the association between risk-enhancing factors (single or combined) and incident ASCVD stratified by baseline CAC group (CAC score of 0, 1-99, or ≥100). The proportionality assumption was tested using graphical models (log-log plots). Models were adjusted for age, sex, race and ethnicity, MESA site, educational level, presence of hypertension, receipt of statin medication, LDL-C level, and cigarette smoking status.
Receiver operating characteristic curves were constructed to assess discrimination (using the C statistic) of ASCVD by CAC group and risk-enhancing factors (modeled as continuous and binary variables) when added to traditional risk factors (age, sex, race and ethnicity, systolic blood pressure level, LDL-C level, high-density lipoprotein cholesterol level, receipt of antihypertensive medication, presence of diabetes, and cigarette smoking status).
Improvement in reclassification was assessed using the net reclassification index for the addition of risk-enhancing factors and CAC groups to the pooled cohort equations. In this analysis, we used the entire MESA study population to assess reclassification to low (<5.0%) and high (≥20.0%) risk among those who did and did not experience ASCVD events.
All statistical analyses were performed using Stata software, version 13 (StataCorp LLC). A 2-tailed P < .05 was considered statistically significant.
Results
Baseline Demographic Characteristics
The study population consisted of 1688 participants (mean [SD] age, 65 [6] years; 976 men [57.8%]). Of those, 648 individuals (38.4%) were White, 562 (33.3%) were Black, 305 (18.1%) were Hispanic, and 173 (10.2%) were Chinese American. A total of 722 participants (42.8%) had a CAC score of 0, 532 (31.5%) had a CAC score of 1 to 99, and 434 (25.7%) had a CAC score of 100 or more. Compared with participants with a CAC score of 0, those with CAC scores of 1 to 99 and CAC scores of 100 or greater were more likely to be male (367 men [50.8%] vs 532 men [59.6%] vs 292 men [67.3%], respectively; P < .001) and White (190 individuals [26.3%] vs 212 individuals [39.8%] vs 246 individuals [56.7%]; P < .001) and to have higher receipt of lipid-lowering medications (99 individuals [13.7%] vs 101 individuals [19.0%] vs 100 individuals [23.0%]; P < .001) and higher 10-year ASCVD risk (mean [SD], 11.9% [3.4%] vs 12.6% [3.5%] vs 13.4% [3.4%]; P < .001) but lower rates of current cigarette smoking (135 individuals [18.7%] vs 83 individuals [15.6%] vs 54 individuals [12.4%]; P < .001) and lower Lp(a) levels (median, 21.1 mg/dL [interquartile range (IQR), 9.3-48.8 mg/dL] vs 19.2 mg/dL [IQR, 7.9-41.0 mg/dL] vs 15.9 mg/dL [IQR, 7.8-35.3 mg/dL]; P = .003) (Table).
Table. Baseline Participant Characteristics and Prevalence of Available Risk-Enhancing Factors Stratified by CAC Scores.
| Characteristic | Participants, No./total No. (%)a | P value | |||
|---|---|---|---|---|---|
| Total | CAC score group | ||||
| 0 | 1-99 | ≥100 | |||
| Participants, No. | 1688 | 722 | 532 | 434 | NA |
| Age, mean (SD), y | 65 (6) | 63 (7) | 65 (6) | 66 (6) | <.001b |
| Sex | |||||
| Male | 976/1688 (57.8) | 367/722 (50.8) | 317/532 (59.6) | 292/434 (67.3) | <.001b |
| Female | 712/1688 (42.2) | 355/722 (49.2) | 215/532 (40.4) | 142/434 (32.7) | |
| Race/ethnicity | |||||
| White | 648/1688 (38.4) | 190/722 (26.3) | 212/532 (39.8) | 246/434 (56.7) | <.001b |
| Chinese | 173/1688 (10.2) | 74/722 (10.2) | 59/532 (11.1) | 40/434 (9.2) | |
| Black or African American | 562/1688 (33.3) | 321/722 (44.5) | 155/532 (29.1) | 86/434 (19.8) | |
| Hispanic | 305/1688 (18.1) | 137/722 (19.0) | 106/532 (19.9) | 62/434 (14.3) | |
| >Graduate school educational level | 321/1688 (19.0) | 126/722 (17.5) | 104/532 (19.5) | 91/434 (21.0) | .31 |
| Hypertension | 1210/1688 (71.7) | 530/722 (73.4) | 376/532 (70.7) | 304/434 (70.0) | .39 |
| Antihypertensive medication | 710/1688 (42.1) | 309/722 (42.8) | 212/532 (39.8) | 189/434 (43.5) | .44 |
| Lipid-lowering medication | 300/1688 (17.8) | 99/722 (13.7) | 101/532 (19.0) | 100/434 (23.0) | <.001a |
| Current cigarette smoking | 272/1688 (16.1) | 135/722 (18.7) | 83/532 (15.6) | 54/434 (12.4) | <.001a |
| BMI, mean (SD) | 28.4 (5.2) | 28.5 (5.2) | 28.4/532 (5.2) | 28.4 (5.2) | .91 |
| Cholesterol, mean (SD), mg/dL | |||||
| Total | 197 (30) | 197 (29) | 196 (30) | 198 (30) | .80 |
| HDL-C | 50 (14) | 51 (14) | 49 (14) | 50 (15) | .06 |
| LDL-C | 121 (26) | 120 (26) | 122 (27) | 122 (27) | .71 |
| Apo B100, mean (SD), mg/dL | 112 (24) | 111 (23) | 113 (23) | 114 (27) | .09 |
| Lp(a), median (IQR), mg/dL | 19.2 (8.5-42.7) | 21.1 (9.3-48.8) | 19.2 (7.9-41.0) | 15.9 (7.8-35.3) | .003b |
| Triglycerides, mean (SD), mg/dL | 128 (64) | 126 (64) | 128 (63) | 130 (67) | .64 |
| Non-HDL cholesterol, mean (SD), mg/dL | 147 (30) | 146 (29) | 147 (31) | 148 (30) | .54 |
| Estimated GFR, mean (SD), mL/min | 76 (15) | 78 (14) | 76 (15) | 75 (14) | .002b |
| hsCRP, median (IQR), mg/L | 1.95 (0.92-4.18) | 2.04 (0.95-4.02) | 1.96 (0.91-4.35) | 1.78 (0.81-4.11) | .34 |
| ABI, mean (SD) | 1.12 (0.11) | 1.12 (0.10) | 1.12 (0.10) | 1.12 (0.13) | .33 |
| 10-y ASCVD risk, mean (SD), % | 12.5 (3.5) | 11.9 (3.4) | 12.6 (3.5) | 13.4 (3.4) | <.001b |
| Prevalence of individual risk-enhancing factorsc | |||||
| Family history of premature ASCVD | 258/1334 (19.3) | 99/1334 (17.3) | 91/1334 (21.6) | 68/1334 (20.1) | .21 |
| Chronic kidney disease | 194/1688 (11.5) | 72/722 (10.0) | 69/532 (13.0) | 53/434 (12.2) | .22 |
| Metabolic syndrome | 594/1688 (35.2) | 247/722 (34.2) | 190/532 (35.7) | 157/434 (36.2) | .76 |
| Premature menopause | 77/1688 (4.6) | 34/722 (4.7) | 24/532 (4.5) | 19/434 (4.4) | .96 |
| Triglycerides | 329/1688 (19.5) | 133/722 (18.4) | 109/532 (20.5) | 87/434 (20.0) | .62 |
| hsCRP | 832/1688 (49.3) | 369/722 (51.1) | 262/532 (49.2) | 201/434 (46.3) | .29 |
| High Lp(a) | 364/1688 (21.6) | 180/722 (24.9) | 105/532 (19.7) | 79/434 (18.2) | .01 |
| High apo B100 | 792/1688 (46.9) | 307/722 (42.5) | 257/532 (48.3) | 228/434 (52.5) | .003 |
| Low ABI | 42/1688 (2.5) | 10/722 (1.4) | 8/532 (1.5) | 24/434 (5.5) | <.001 |
| Prevalence of combined risk-enhancing factorsc | |||||
| 0 Factors | 175/1334 (13.1) | 70/1334 (12.2) | 60/1334 (14.2) | 45/1334 (13.3) | .37 |
| 1-2 Factors | 678/1334 (50.8) | 310/1334 (54.0) | 203/1334 (48.2) | 165/1334 (48.7) | |
| ≥3 Factors | 481/1334 (36.1) | 194/1334 (33.8) | 158/1334 (37.5) | 129/1334 (38.1) | |
| Basic factors | 938/1688 (56.0) | 385/1688 (53.3) | 302/1688 (56.8) | 251/1688 (55.6) | .26 |
| Advanced factors | 1301/1688 (77.1) | 565/1688 (78.3) | 411/1688 (77.3) | 325/1688 (74.9) | .42 |
Abbreviations: ABI, ankle-brachial index; apo B100, apolipoprotein B100; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAC, coronary artery calcium; hsCRP, high-sensitivity C-reactive protein; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein a.
Continuous variables are reported as mean (SD) or median (IQR), depending on the normality of data. Categorical variables are reported as number (%).
Statistically significant.
Percentages are relative to CAC score.
Risk-Enhancing Factors and CAC Scores
The prevalence of high Lp(a) was significantly greater among individuals with CAC scores of 0 (180 participants [24.9%] compared with individuals with scores of 1 to 99 (105 participants [19.7%]) or scores of 100 or more (79 participants [18.2%]; P = .01), whereas the prevalence of low ABI was significantly lower among those with CAC scores of 0 (10 participants [1.4%]) vs those with CAC scores of 1 to 99 (8 participants [1.5%]) or scores of 100 or more (24 participants [5.5%]; P < .001) (Table). No significant differences by CAC category were observed when risk-enhancing factors were assessed in combination. The prevalence of risk-enhancing factors individually and in combination, stratified by CAC category, is shown in eTable 1 in the Supplement.
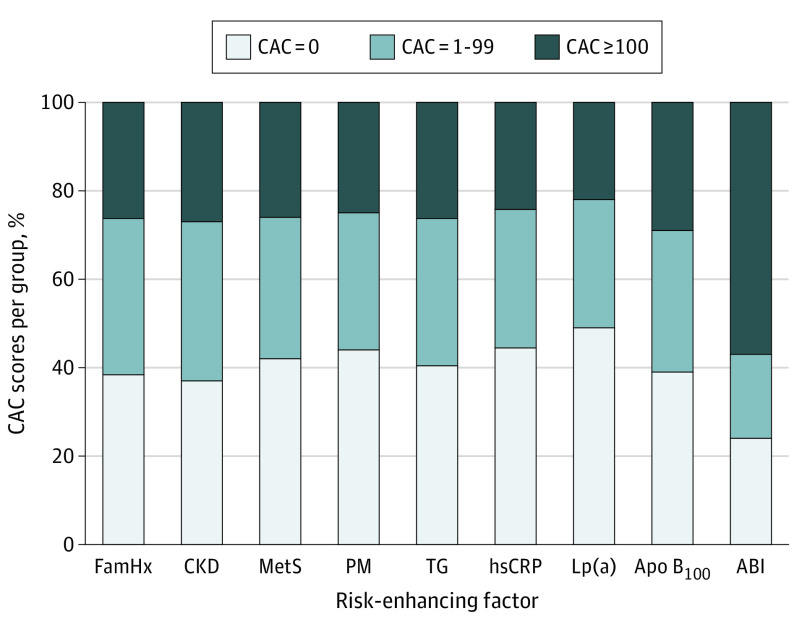
The distribution of CAC scores among those with each of the individual risk-enhancing factors is shown in Figure 1. The prevalence of CAC scores of 0 was approximately 40% across all of the individual risk-enhancing factors, ranging from 37.1% among those with CKD to 49.5% among those with high Lp(a) (with the exception of low ABI, for which the prevalence was 23.8%). In the age-standardized analysis, there was no notable difference in the prevalence of CAC across individual risk-enhancing factor categories. For example, the prevalence of a CAC score of 0 was 45.7% vs 40.3% among those with 1 to 2 risk-enhancing factors vs 3 or more risk-enhancing factors, respectively (P = .11) (eFigure 2 in the Supplement). When stratified by sex, the prevalence of a CAC score of 0 was greater than 50% for all risk-enhancing factors (with the exception of low ABI) among women (eTable 2 in the Supplement).
Figure 1. Distribution of Coronary Artery Calcium Scores at Baseline by Risk-Enhancing Factor Group.
Coronary artery calcium scores based on 2018 American Heart Association/American College of Cardiology Guideline on the Management of Blood Cholesterol.1 ABI indicates ankle-brachial index; Apo B100, apolipoprotein B100; CAC, coronary artery calcium; CKD, chronic kidney disease; FamHx, family of history of premature atherosclerotic cardiovascular disease; hsCRP, high-sensitivity C-reactive protein; Lp(a), lipoprotein a; MetS, metabolic syndrome; PM, premature menopause; and TG, triglycerides.
Risk-Enhancing Factors, CAC Scores, and Cardiovascular Events
The number of events by individual and composite risk-enhancing factors, stratified by CAC score, is shown in eTable 3 in the Supplement. Over a median follow-up of 12.0 years (IQR, 11.5-12.6 years), 145 first-time ASCVD events occurred (92 participants experienced CHD events, 58 experienced strokes, and 5 experienced both CHD and cardiovascular events). Among participants with CAC scores of 0, there were 40 ASCVD events (19 individuals experienced CHD events, 23 experienced strokes, and 2 experienced both CHD events and strokes). Of all stroke events, 11 were hemorrhagic (5 events among participants with CAC scores of 0, and 5 events among participants with CAC scores >0), and 7 of these events were fatal (1 participant with a CAC score of 0 and 6 participants with CAC scores >0).
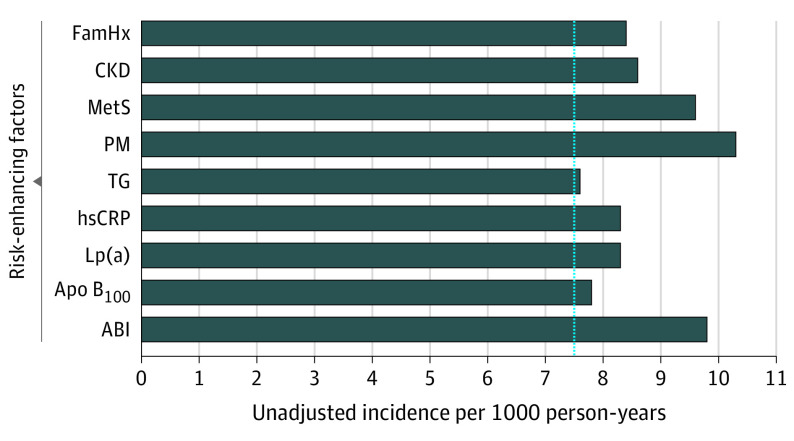
The unadjusted incidence rates for individual and combined risk-enhancing factors are shown in Figure 2. For all individual risk-enhancing factors, the unadjusted incidence rate for each factor was 7.5 or more events per 1000 person-years, with the highest incidence rate occurring among those who experienced premature menopause (10.3 events per 1000 person-years [95% CI, 5.1-20.6]).
Figure 2. Unadjusted Incidence Rates of Individual Risk-Enhancing Factors.
Incidence rates among individuals with an intermediate risk of ASCVD. The dashed line represents the reference line (7.5%) above which statin therapy is recommended. ABI indicates ankle-brachial index; Apo B100, apolipoprotein B100; CAC, coronary artery calcium; CKD, chronic kidney disease; FamHx, family of history of premature atherosclerotic cardiovascular disease; hsCRP, high-sensitivity C-reactive protein; Lp(a), lipoprotein a; MetS, metabolic syndrome; PM, premature menopause; and TG, triglycerides.
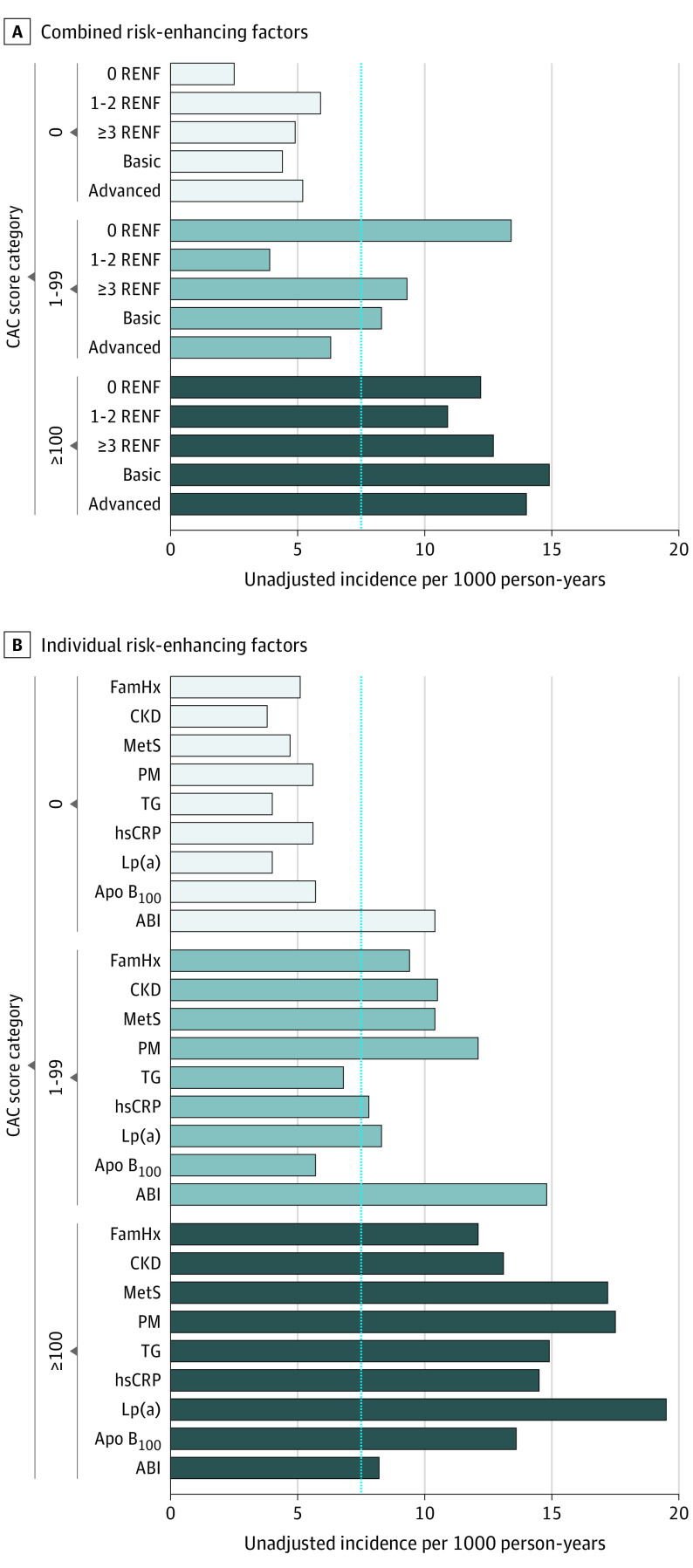
Overall, when risk-enhancing factors were assessed in combination (using the categories of 0 factors, 1-2 factors, and ≥3 factors), the highest incidence rate among risk-enhancing factor groups was observed in those who had CAC scores of 100 or greater and 3 or more risk-enhancing factors (12.7 events per 1000 person-years [95% CI, 7.9-20.4]) (Figure 3A). Similar results were found when risk-enhancing factors were combined into basic and advanced groups (incidence rate, 14.9 [95% CI, 10.8-20.4] and 14.0 [95% CI, 10.5-18.7], respectively, among those with CAC scores ≥100 and ≥3 risk-enhancing factors) (Figure 3A). Kaplan-Meier estimates of ASCVD event–free survival by risk-enhancing factor burden according to CAC groups are shown in eFigure 3 in the Supplement, and unadjusted incidence rates for each risk-enhancing factor according to CAC categories are shown in Figure 3B. Overall, the incidence rate among those with a CAC score of 0 was less than 7.5 events per 1000 person-years for all risk-enhancing factors, with the exception of low ABI (10.4 events per 1000 person-years [95% CI, 1.5-73.5]). Unadjusted incidence rates ranged from 3.8 events per 1000 person-years among those with CKD and CAC scores of 0 (95% CI, 1.2-11.7) to 19.5 events per 1000 person-years among those with high Lp(a) and CAC scores ≥100 (95% CI, 11.8-32.4).
Figure 3. Unadjusted Incidence Rates for Risk-Enhancing Factors Across Coronary Artery Calcium Categories.
A, Combined risk-enhancing factors with low ABI excluded. B, Individual risk-enhancing factors. The dashed line represents the reference line (7.5%) above which statin therapy is recommended. ABI indicates ankle-brachial index; Apo B100, apolipoprotein B100; CAC, coronary artery calcium; CKD, chronic kidney disease; FamHx, family of history of premature atherosclerotic cardiovascular disease; hsCRP, high-sensitivity C-reactive protein; Lp(a), lipoprotein a; MetS, metabolic syndrome; PM, premature menopause; RENF, risk-enhancing factors; and TG, triglycerides.
Risk-Enhancing Factors and ASCVD Risk
Hazard ratios (HRs) for the association between individual risk-enhancing factors and incident ASCVD were nonsignificant in the overall population (eTable 3 in the Supplement). When stratified by CAC score, the presence of metabolic syndrome was significantly associated with incident ASCVD among those with a CAC score of 100 or higher (eTable 3 in the Supplement). Among those with a CAC score of 1 to 99, the presence of 1 to 2 risk-enhancing factors and an advanced risk-enhancing factor burden was significantly associated with ASCVD events.
Discrimination and Reclassification of ASCVD Events
For composite ASCVD events, the addition of CAC (modeled continuously) to the pooled cohort equations compared with all risk-enhancing factors was associated with the greatest increase in the C statistic (from 0.633 at baseline to 0.678) (eTable 4 in the Supplement).
The addition of CAC as a continuous variable to the pooled cohort equations produced the highest net reclassification index value (0.067). The presence of CAC (when modeled as a continuous variable) was also associated with the greatest number of correctly reclassified participants to the high-risk category (eTable 5 in the Supplement). When modeled as a binary variable, an ABI of less than 0.9 had the highest net reclassification index value; however, these results were not significant (0.017; 95% CI, −0.001 to 0.035) (eTable 5 in the Supplement).
Discussion
This prospective multiethnic population-based cross-sectional study found that all risk-enhancing factors had an absolute incidence rate of 7.5 or more events per 1000 person-years over a median follow-up of 12.0 years among individuals with an intermediate risk of ASCVD. However, substantial discordance existed based on CAC group, whereby most risk-enhancing factors had incidence rates that were less than 7.5 events per 1000 person-years (95% CI, 1.4-73.5) among those with CAC scores of 0 (with the exception of low ABI, for which the incidence rate was 10.4 events per 1000 person-years). The use of CAC scoring was associated with significant improvement in discrimination and risk reclassification for ASCVD events.
Risk-Enhancing Factors
The 2018 AHA/ACC blood cholesterol guideline1 introduced the concept of risk-enhancing factors to further guide the discussion on the initiation of statin therapy among individuals with an intermediate risk of ASCVD. The guideline posits that these factors could be used to confirm a theoretically higher risk state, which could support the addition of a moderate-intensity statin therapy.1 However, the guideline acknowledges that “in the general population, [risk-enhancing factors] may or may not predict risk independently of PCE”1(p3180) and suggests that future research is needed to examine the efficacy of moderate-intensity statin medications in the presence of risk-enhancing factors. Nonetheless, the rationale and data broadly supporting each risk-enhancing factor have been described, highlighting the fact that, although short- and intermediate-term risk may be low, the presence of risk-enhancing factors may be associated with an increase in the lifetime risk of ASCVD and could therefore inform a more personalized risk discussion.13 This finding may be especially relevant for those with low ABI, hsCRP, and a family history of premature ASCVD because these risk-enhancing factors have been reported to be independently associated with incident ASCVD events.14,15 To our knowledge, the present study is the first to report that, among individuals at intermediate risk, each risk-enhancing factor was associated with observed incident rates that exceeded the threshold favoring the initiation of statin therapy (≥7.5 events per 1000 person-years in the present study or 7.5% in the 2018 AHA/ACC cholesterol guideline1). Therefore, those individuals with at least 1 risk-enhancing factor would generally be expected to derive benefit from statin therapy.16,17
It is important to consider that the presence of low ABI was the only risk-enhancing factor in which estimation of future ASCVD events was not clinically reclassified among those with concurrent CAC scores of 0. In addition, compared with available (non-CAC) risk-enhancing factors, low ABI values were associated with improved risk reclassification. Low ABI is the only risk-enhancing factor that serves as a surrogate for atherosclerotic disease, considering that it is a direct measure of vascular health given its ability to identify atherosclerosis in peripheral circulation; the presence and severity of low ABI is significantly associated with the presence of CAC.18 In the current study, only 1.4% of patients with CAC scores of 0 had low ABI, suggesting that, for most patients with low ABI, the assessment of CAC for risk estimation would have low clinical utility given the low prevalence of CAC scores of 0 in this group. Furthermore, ASCVD event rates were high (9.8 events per 1000 person-years [95% CI, 3.7-26.2]) in the presence of low ABI. Therefore, when present, clinical peripheral artery disease appears to be an important factor associated with ASCVD risk, favoring the initiation of preventive pharmacotherapy. Thus, the results of the present study support the initiation of statin therapy among those with peripheral artery disease, as recommended by current professional society guidelines.19,20
Coronary Artery Calcium and Incidence Rates
According to the 2018 AHA/ACC blood cholesterol guideline,1 CAC scoring is an optional third step after 10-year pooled cohort equation risk estimation and risk-enhancing factor assessment to be used in the clinician-patient risk discussion. Previous studies evaluating the association of individual risk-enhancing factors (such as family history of premature ASCVD, metabolic syndrome, CKD, lipid parameters, and hsCRP) and CAC with incident CHD and ASCVD events among asymptomatic participants have found low 10-year event rates among those with CAC scores of 0.6,7,21,22,23,24 For example, low CHD event rates have been described in MESA and the Dallas heart study among those with a family history of premature ASCVD and a CAC score of 0.8,22 In MESA, individuals with metabolic syndrome and CAC scores of 0 had CHD and ASCVD event rates of less than 5 events per 1000 person-years.21
The current study found lower absolute short-term event rates for individual (with the exception of low ABI) and combined risk-enhancing factors among those with CAC scores of 0. These findings have several implications. The identification of this low-risk population can aid the clinician-patient discussion and may result in more selective clinical use of statin and aspirin therapies for primary prevention. Notably, a CAC score of 0 was associated with the greatest downward change in risk estimation.15,25 Statin therapy for the treatment of primary ASCVD was not associated with a lower risk of ASCVD in the absence of CAC over a median of 9.4 years.26 In addition, the 10-year incident ASCVD rate among those with CAC scores of 0 was less than 5 events per 1000 person-years over a median follow-up of 11.1 years.4
The absence of CAC as it pertains to specific lipid and inflammatory risk-enhancing factors is not unexpected. For example, in asymptomatic populations, the presence of hsCRP, triglycerides, and Lp(a), is not associated with the presence or burden of CAC, suggesting distinct pathophysiologic characteristics with regard to ASCVD risk.27,28,29 However, studies directly comparing the risk estimation ability of hsCRP values vs CAC scoring have reported that CAC scoring is superior in estimating future CHD events.24,30 A plausible explanation for this finding is that CAC is a direct measure of atherosclerosis rather than a risk factor for atherothrombosis.24,30
The presence of a CAC score of 0 does not indicate lifetime low-risk status. The natural history of atherosclerosis is dynamic, with a tendency to progress over time. Therefore, a CAC score of 0 is likely to convert to a CAC score higher than 0, especially in the presence of traditional cardiovascular risk factors or risk-enhancing factors.31 The initial finding of a CAC score of 0 may encourage the patient to continue to maintain a healthy lifestyle. Clinicians can continue to review lifestyle-associated factors (such as diet, physical activity, weight or body mass index, tobacco use, and blood glucose level) and assess risk factor burden over time following appropriate guidelines. If clinically indicated, repeated CAC scanning among those with CAC scores of 0 can be considered 5 years after the initial CAC scan to assess the progression of CAC.9 Given that previous studies have reported that CAC progression is associated with a higher risk of ASCVD events over medium- to long-term follow-up,32 individuals with CAC progression could be considered for treatment with statin therapy to mitigate the risk of ASCVD.
Implications for Risk Assessment and Therapy
Given the imprecision of multivariable risk estimation scores, clinicians and patients may encounter uncertainty regarding the utility of statin medications or concerns about treatment intolerance. Clinical judgment that incorporates the individual patient’s preference, the presence of risk-enhancing factors, and the selective use of CAC scoring can help to appropriately reclassify risk to guide the allocation of statin therapy.31
The present study’s finding that the presence of risk-enhancing factors suggests that individuals with an intermediate risk of ASCVD are of sufficiently high risk to justify the initiation of statin therapy. However, even in the presence of risk-enhancing factors (single or multiple), individuals at intermediate risk with CAC scores of 0 have a low risk of experiencing short-term ASCVD events (<7.5 events per 1000 person-years). Notably, CAC scoring was associated with significant improvement in the reclassification of ASCVD risk.15,33,34 Thus, these analyses suggest that the information offered by CAC testing may be clinically useful to guide the clinician-patient risk discussion after the performance of ASCVD risk estimation and the consideration of the presence of risk-enhancing factors.
Central to this discussion is also patient health literacy and financial burden, particularly as hospitals and health care systems continue to develop strategies for the appropriate use of medical resources. Understandable concern exists that CAC testing may add to health care costs; however, compared with an approach in which all patients are prescribed treatment with statins, CAC testing is less costly, particularly when considerations are made for adverse events and disutility associated with statin therapy and noncardiac findings requiring follow-up imaging.35,36,37 Radiation exposure from computed tomographic scans is minimal and can be discussed with the patient. When considering large cohorts, the expected mean radiation exposure is less than 1.05 mSv, which is equivalent to the radiation risk encountered during a screening mammogram.38
Limitations
This study has limitations. The study included a relatively small cohort, which may have underpowered the analyses and likely explains the lack of association observed between risk-enhancing factors and ASCVD outcomes. The study did not account for longitudinal changes in preventive therapy because knowledge of CAC scores may have influenced the prescription rates for aspirin or statin therapies. However, the availability of imaging (including CAC scores) and laboratory data from MESA39 indicated a low overall number of individuals who reported initiation of statin therapy (10%), blood pressure–lowering therapy (13%), and aspirin therapy (18%) at a mean follow-up of 1.6 years after the baseline MESA visit.
Because risk-enhancing factors and CAC were assessed at the same time, we could not evaluate a sequential testing strategy of risk-enhancing factors followed by CAC, which is endorsed by the guidelines.1 We were not able to evaluate all 2018 AHA/ACC cholesterol guideline–defined risk-enhancing factors because of constraints within MESA, including the lack of individuals with South Asian ancestry and exclusion of individuals with preeclampsia or additional specific inflammatory conditions. However, available data suggest that individuals with South Asian ancestry have CAC profiles similar to those of White individuals.40 In addition, because LDL-C values were obtained at the baseline visit (ie, a single measurement), it was not possible to assess the consequences of persistently high LDL-C levels of 160 mg/dL or greater (which would require at least 3 measurements). Although we attempted to adjust for all possible confounders, the possibility of residual confounding remains.
Conclusions
In this cross-sectional study, in individuals with risk-enhancing factors, the presence of CAC was associated with ASCVD event rates of 7.5 or more events per 1000 person-years, which exceeded the threshold used to initiate statin therapy, whereas ASCVD event rates were less than 7.5 events per 1000 person-years when CAC scores were 0. An exception was observed among individuals with low ABI, for whom ASCVD event rates were 7.5 or more events per 1000 person-years, even in the absence of CAC. The use of CAC scoring was associated with significant improvements in the reclassification and discrimination of incident ASCVD and may therefore be useful as an adjunct to risk-enhancing factors to identify individuals with intermediate risk of ASCVD who would benefit from statin therapy.
eMethods. Coronary Artery Calcium, Risk-Enhancing Factors Assessment, and Ascertainment of Incident ASCVD
eTable 1. Prevalence of CAC Among Those With ASCVD Risk-Enhancing Factors
eTable 2. Age-Standardized Prevalence of CAC According to Presence of ASCVD Risk-Enhancing Factors
eTable 3. Prevalence of Events and Unadjusted and Multivariable-adjusted Hazard Ratios (95% CIs) for the Association of Risk-Enhancing Factors With Incident Cardiovascular Disease Stratified by Baseline Coronary Artery Calcium
eTable 4. Adjusted Area Under the Curve (AUC) for Different Models With 95% CIs for the Difference in Adjusted AUC for the Addition of Risk-Enhancing Factors and Coronary Artery Calcium (Modeled as Continuous and Binary) to Traditional Risk Factors
eTable 5. Reclassification of Incident Cardiovascular Disease Events With Addition of Risk-Enhancing Factors and Coronary Artery Calcium (Modeled as Continuous and Binary) to the Pooled Cohort Equations
eFigure 1. Derivation of Study Population
eFigure 2. Age-standardized Distribution of CAC by Each Individual and Cumulative Risk-Enhancing Factors in the Overall Population
eFigure 3. Kaplan-Meier Survival Curves Free of ASCVD Events Among the Population by Risk-Enhancing Factor Burden According to CAC Groups
eReferences
References
- 1.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168-3209. doi: 10.1016/j.jacc.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 2.Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15):1657-1668. doi: 10.1016/j.jacc.2015.07.066 [DOI] [PubMed] [Google Scholar]
- 3.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345. doi: 10.1056/NEJMoa072100 [DOI] [PubMed] [Google Scholar]
- 4.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the Multi-Ethnic Study of Atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401-2408. doi: 10.1093/eurheartj/ehy217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverman MG, Blaha MJ, Krumholz HM, et al. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2014;35(33):2232-2241. doi: 10.1093/eurheartj/eht508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin SS, Blaha MJ, Blankstein R, et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the Multi-Ethnic Study of Atherosclerosis. Circulation. 2014;129(1):77-86. doi: 10.1161/CIRCULATIONAHA.113.003625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675-688. doi: 10.1016/j.jcmg.2008.12.031 [DOI] [PubMed] [Google Scholar]
- 8.Patel J, Al Rifai M, Blaha MJ, et al. Coronary artery calcium improves risk assessment in adults with a family history of premature coronary heart disease: results from Multiethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8(6):e003186. doi: 10.1161/CIRCIMAGING.115.003186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Braun LT, Ndumele CE, et al. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2019;73(24):3153-3167. doi: 10.1016/j.jacc.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871-881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 11.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35-43. doi: 10.1148/radiol.2341040439 [DOI] [PubMed] [Google Scholar]
- 12.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi: 10.1016/0735-1097(90)90282-T [DOI] [PubMed] [Google Scholar]
- 13.Agarwala A, Liu J, Ballantyne CM, Virani SS. The use of risk enhancing factors to personalize ASCVD risk assessment: evidence and recommendations from the 2018 AHA/ACC multi-society cholesterol guidelines. Curr Cardiovasc Risk Rep. 2019;13(7):18. doi: 10.1007/s12170-019-0616-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308(8):788-795. doi: 10.1001/jama.2012.9624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849-858. doi: 10.1161/CIRCULATIONAHA.115.018524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 17.Mihaylova B, Emberson J, Blackwell L, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaborators . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581-590. doi: 10.1016/S0140-6736(12)60367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tullos BW, Sung JH, Lee JE, Criqui MH, Mitchell ME, Taylor HA. Ankle-brachial index (ABI), abdominal aortic calcification (AAC), and coronary artery calcification (CAC): the Jackson heart study. Int J Cardiovasc Imaging. 2013;29(4):891-897. doi: 10.1007/s10554-012-0145-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(12):e726-e779. doi: 10.1161/CIR.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aboyans V, Ricco JB, Bartelink MLEL, et al. ; ESC Scientific Document Group . 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European Stroke Organization (ESO), the Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC), and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39(9):763-816. doi: 10.1093/eurheartj/ehx095 [DOI] [PubMed] [Google Scholar]
- 21.Malik S, Zhao Y, Budoff M, et al. Coronary artery calcium score for long-term risk classification in individuals with type 2 diabetes and metabolic syndrome from the Multi-Ethnic Study of Atherosclerosis. JAMA Cardiol. 2017;2(12):1332-1340. doi: 10.1001/jamacardio.2017.4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paixao ARM, Berry JD, Neeland IJ, et al. Coronary artery calcification and family history of myocardial infarction in the Dallas heart study. JACC Cardiovasc Imaging. 2014;7(7):679-686. doi: 10.1016/j.jcmg.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Budoff MJ, Reilly MP, et al. ; CRIC Investigators . Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol. 2017;2(6):635-643. doi: 10.1001/jamacardio.2017.0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684-692. doi: 10.1016/S0140-6736(11)60784-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortensen MB, Fuster V, Muntendam P, et al. Negative risk markers for cardiovascular events in the elderly. J Am Coll Cardiol. 2019;74(1):1-11. doi: 10.1016/j.jacc.2019.04.049 [DOI] [PubMed] [Google Scholar]
- 26.Mitchell JD, Fergestrom N, Gage BF, et al. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol. 2018;72(25):3233-3242. doi: 10.1016/j.jacc.2018.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamirani YS, Pandey S, Rivera JJ, et al. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis. 2008;201(1):1-7. doi: 10.1016/j.atherosclerosis.2008.04.045 [DOI] [PubMed] [Google Scholar]
- 28.Paramsothy P, Knopp RH, Bertoni AG, et al. Association of combinations of lipid parameters with carotid intima-media thickness and coronary artery calcium in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2010;56(13):1034-1041. doi: 10.1016/j.jacc.2010.01.073 [DOI] [PubMed] [Google Scholar]
- 29.Guerra R, Yu Z, Marcovina S, Peshock R, Cohen JC, Hobbs HH. Lipoprotein(a) and apolipoprotein(a) isoforms: no association with coronary artery calcification in the Dallas heart study. Circulation. 2005;111(12):1471-1479. doi: 10.1161/01.CIR.0000159263.50305.BD [DOI] [PubMed] [Google Scholar]
- 30.Mohlenkamp S, Lehmann N, Moebus S, et al. ; Heinz Nixdorf Recall Study Investigators . Quantification of coronary atherosclerosis and inflammation to predict coronary events and all-cause mortality. J Am Coll Cardiol. 2011;57(13):1455-1464. doi: 10.1016/j.jacc.2010.10.043 [DOI] [PubMed] [Google Scholar]
- 31.Gassett AJ, Sheppard L, McClelland RL, et al. Risk factors for long-term coronary artery calcium progression in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4(8):e001726. doi: 10.1161/JAHA.114.001726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67(18):2118-2130. doi: 10.1016/j.jacc.2016.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeboah J, Polonsky TS, Young R, et al. Utility of nontraditional risk markers in individuals ineligible for statin therapy according to the 2013 American College of Cardiology/American Heart Association cholesterol guidelines. Circulation. 2015;132(10):916-922. doi: 10.1161/CIRCULATIONAHA.115.016846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeboah J, Young R, McClelland RL, et al. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67(2):139-147. doi: 10.1016/j.jacc.2015.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts ET, Horne A, Martin SS, et al. Cost-effectiveness of coronary artery calcium testing for coronary heart and cardiovascular disease risk prediction to guide statin allocation: the Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One. 2015;10(3):e0116377. doi: 10.1371/journal.pone.0116377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57(15):1622-1632. doi: 10.1016/j.jacc.2011.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Kempen BJH, Spronk S, Koller MT, et al. Comparative effectiveness and cost-effectiveness of computed tomography screening for coronary artery calcium in asymptomatic individuals. J Am Coll Cardiol. 2011;58(16):1690-1701. doi: 10.1016/j.jacc.2011.05.056 [DOI] [PubMed] [Google Scholar]
- 38.Patel AA, Fine J, Naghavi M, Budoff MJ. Radiation exposure and coronary artery calcium scans in the society for heart attack prevention and eradication cohort. Int J Cardiovasc Imaging. 2019;35(1):179-183. doi: 10.1007/s10554-018-1431-0 [DOI] [PubMed] [Google Scholar]
- 39.Nasir K, McClelland RL, Blumenthal RS, et al. Coronary artery calcium in relation to initiation and continuation of cardiovascular preventive medications: the Multi-Ethnic Study of Atherosclerosis (MESA). Circ Cardiovasc Qual Outcomes. 2010;3(3):228-235. doi: 10.1161/CIRCOUTCOMES.109.893396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanaya AM, Kandula NR, Ewing SK, et al. Comparing coronary artery calcium among U.S. South Asians with four racial/ethnic groups: the MASALA and MESA studies. Atherosclerosis. 2014;234(1):102-107. doi: 10.1016/j.atherosclerosis.2014.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Coronary Artery Calcium, Risk-Enhancing Factors Assessment, and Ascertainment of Incident ASCVD
eTable 1. Prevalence of CAC Among Those With ASCVD Risk-Enhancing Factors
eTable 2. Age-Standardized Prevalence of CAC According to Presence of ASCVD Risk-Enhancing Factors
eTable 3. Prevalence of Events and Unadjusted and Multivariable-adjusted Hazard Ratios (95% CIs) for the Association of Risk-Enhancing Factors With Incident Cardiovascular Disease Stratified by Baseline Coronary Artery Calcium
eTable 4. Adjusted Area Under the Curve (AUC) for Different Models With 95% CIs for the Difference in Adjusted AUC for the Addition of Risk-Enhancing Factors and Coronary Artery Calcium (Modeled as Continuous and Binary) to Traditional Risk Factors
eTable 5. Reclassification of Incident Cardiovascular Disease Events With Addition of Risk-Enhancing Factors and Coronary Artery Calcium (Modeled as Continuous and Binary) to the Pooled Cohort Equations
eFigure 1. Derivation of Study Population
eFigure 2. Age-standardized Distribution of CAC by Each Individual and Cumulative Risk-Enhancing Factors in the Overall Population
eFigure 3. Kaplan-Meier Survival Curves Free of ASCVD Events Among the Population by Risk-Enhancing Factor Burden According to CAC Groups
eReferences