Key Points
Question
Does treatment with paroxetine mitigate adverse left ventricular remodeling in patients presenting with acute anterior myocardial infarction?
Findings
In this double-blind, placebo-controlled randomized clinical trial allocating patients after acute anterior myocardial infarction to treatment with paroxetine or placebo, no difference was found in recovery of left ventricular ejection fraction at 12 weeks. In contrast, patients in the experimental arm experienced a greater reduction in late gadolinium enhancement compared with those receiving placebo, indicating attenuation of myocardial fibrosis.
Meaning
In this trial, in patients with acute myocardial infarction, treatment with paroxetine did not improve left ventricular ejection fraction compared with placebo.
This double-blind, placebo-controlled randomized clinical trial investigates the efficacy of paroxetine-mediated G-protein–coupled receptor kinase 2 inhibition to mitigate adverse left ventricular remodeling in patients with acute myocardial infarction.
Abstract
Importance
Left ventricular remodeling following acute myocardial infarction results in progressive myocardial dysfunction and adversely affects prognosis.
Objective
To investigate the efficacy of paroxetine-mediated G-protein–coupled receptor kinase 2 inhibition to mitigate adverse left ventricular remodeling in patients presenting with acute myocardial infarction.
Design, Setting, and Participants
This double-blind, placebo-controlled randomized clinical trial was conducted at Bern University Hospital, Bern, Switzerland. Patients with acute anterior ST-segment elevation myocardial infarction with left ventricular ejection fraction (LVEF) of 45% or less were randomly allocated to 2 study arms between October 26, 2017, and September 21, 2020.
Interventions
Patients in the experimental arm received 20 mg of paroxetine daily; patients in the control group received a placebo daily. Both treatments were provided for 12 weeks.
Main Outcomes and Measures
The primary end point was the difference in patient-level improvement of LVEF between baseline and 12 weeks as assessed by cardiac magnetic resonance tomography. Secondary end points were changes in left ventricular dimensions and late gadolinium enhancement between baseline and follow-up.
Results
Fifty patients (mean [SD] age, 62 [13] years; 41 men [82%]) with acute anterior myocardial infarction were randomly allocated to paroxetine or placebo, of whom 38 patients underwent cardiac magnetic resonance imaging both at baseline and 12 weeks. There was no difference in recovery of LVEF between the experimental group (mean [SD] change, 4.0% [7.0%]) and the control group (mean [SD] change, 6.3% [6.3%]; mean difference, −2.4% [95% CI, −6.8% to 2.1%]; P = .29) or changes in left ventricular end-diastolic volume (mean difference, 13.4 [95% CI, −12.3 to 39.0] mL; P = .30) and end-systolic volume (mean difference, 11.4 [95% CI, −3.6 to 26.4] mL; P = .13). Late gadolinium enhancement as a percentage of the total left ventricular mass decreased to a larger extent in the experimental group (mean [SD], −13.6% [12.9%]) compared with the control group (mean [SD], −4.5% [9.5%]; mean difference, −9.1% [95% CI, −16.6% to −1.6%]; P = .02).
Conclusions and Relevance
In this trial, treatment with paroxetine did not improve LVEF after myocardial infarction compared with placebo.
Trial Registration
ClinicalTrials.gov Identifier: NCT03274752
Introduction
Left ventricular remodeling after acute myocardial infarction is the result of a cascade of intracellular signaling processes modulating adaptive and reparative changes that invokes scar formation, ventricular dilatation, and deterioration of contractile function.1 Dysregulation of G-protein–coupled receptor kinases (GRK) plays a central role in the progression of left ventricular remodeling after ischemic injury.2,3 Competitive inhibition of GRK2 has been associated with attenuation of the maladaptive response, reduced myocardial fibrosis, and improved left ventricular function in animal studies, thus offering a potential target for pharmacologic intervention.4,5,6
The selective serotonin reuptake inhibitor paroxetine has been shown to selectively inhibit GRK2 as an off-target effect.7 In a mouse model,8 paroxetine treatment has been demonstrated to mitigate sympathetic overdrive, reverse myocardial remodeling, and improve left ventricular function. The aim of the present study was to prospectively investigate the efficacy of paroxetine-mediated GRK2 inhibition to mitigate adverse left ventricular remodeling in patients presenting with acute myocardial infarction.
Methods
Study Design and Participants
The Paroxetine-Mediated GRK2 Inhibition to Reduce Cardiac Remodeling After Acute Myocardial Infarction (CARE-AMI) trial was an investigator-initiated, double-blind, placebo-controlled randomized clinical trial that compared treatment with paroxetine with placebo in addition to guideline-directed medical treatment in patients with acute anterior ST-segment elevation myocardial infarction with a left ventricular ejection fraction (LVEF) of 45% or less. Detailed inclusion and exclusion criteria are listed in the eMethods in Supplement 1. All patients gave written informed consent for participation in the trial before randomization. The study protocol complied with the Declaration of Helsinki and was approved by the cantonal ethics committee of Bern. The research team of Bern University Hospital conducted the study and managed all study data. The Clinical Trials Unit Bern (University of Bern, Switzerland) performed statistical analyses. Additional information can be found in the Trial Protocol in Supplement 2.
Randomization and Masking
Participants were randomly allocated between October 26, 2017, and September 21, 2020, in a 1:1 ratio by use of sequentially numbered boxes to daily treatment with 20 mg of paroxetine or a placebo. Placebo tablets were specifically prepared for this trial and identical to the real drug in color, appearance, smell, and taste as well as packaging and labeling. The allocation schedule was based on computer-generated random numbers. Study participants, treating physicians (T.P., R.V., S.D., S.S., M.F., G.C.M.S., and J.L.), outcome assessors (C.G., A.W.S., S.A.E., and K.F.), and data analysts (F.B.B. and D.H.) were blinded to treatment allocation.
Investigational Product and Study Procedures
Paroxetine is a selective serotonin reuptake inhibitor approved for the treatment of major depressive disorder. As an off-target effect, paroxetine directly binds to the catalytic domain of GRK2 and increases β-adrenergic receptor–mediated myocardial contractility.7
Left ventricular ejection fraction was assessed at baseline and 12 weeks by use of transthoracic echocardiography and cardiac magnetic resonance (CMR) imaging. Methods used for CMR measurements are provided in the eMethods in Supplement 1. A telephone follow-up was performed at 4 weeks, and a clinical visit including measurement of paroxetine levels was conducted at 12 weeks.
Study End Points and Definitions
The primary end point was the difference in mean patient-level change in LVEF between baseline and follow-up in the experimental and control arms as assessed by CMR imaging. The primary analysis was performed in the intention-to-treat population. Secondary end points were the change in left-ventricular end-diastolic and end-systolic volumes and the change in late gadolinium enhancement between baseline and 12 weeks. A secondary, per-protocol analysis included all participants who underwent baseline and follow-up CMR imaging and had paroxetine levels of 6 nmol/L or more or returned less than 20% of the study medication.
Statistical Analysis
We hypothesized that paroxetine is superior to placebo with respect to the primary end point of LVEF improvement between baseline and 12 weeks. Based on preclinical results, we assumed a change in LVEF of 10% and anticipated a study attrition of 10%.8 A sample of 50 individuals was required to show superiority of paroxetine with greater than 90% power and a 2-sided type I error of .05.
Continuous variables were summarized as means (SDs) and categorical variables as frequencies (percentages), as appropriate. The P values have been computed using χ2 or Fisher tests for categorical variables and t tests for normally distributed continuous variables. Patient-level changes in values within each group were compared using paired t tests, with equal variances assumed. The comparisons of paroxetine vs placebo arm are reported using mean differences with 95% CIs and P values (2 sided) of unpaired t tests, with equal variances assumed. Data were analyzed with R software version 4.0.3 (R Foundation for Statistical Computing). Additional details are in the Statistical Analysis Plan in Supplement 3.
Results
Baseline Characteristics
A total of 50 patients (mean [SD] age, 62 [13] years; 41 men [82%]) presenting with acute anterior myocardial infarction were randomly assigned to the experimental group or control group, of whom 38 patients underwent CMR imaging both at baseline and 12 weeks (eFigure 1 in Supplement 1). Selected baseline characteristics are provided in Table 1; comprehensive characteristics, including information on discharge medications and treatment regimen at 12 weeks, are summarized in eTables 1, 2, and 3 in Supplement 1. Door-to-balloon times and intervals between symptom onset and first balloon inflation were comparable between the groups (eTable 2 in Supplement 1). Intervals between primary percutaneous coronary intervention and CMR imaging at baseline were similar (Table 1). There were no significant differences in baseline characteristics between patients who underwent CMR imaging at follow-up compared with those who did not (eTable 4 in Supplement 1).
Table 1. Selected Baseline Characteristics.
| Characteristic | Patients, No. (%)a | |
|---|---|---|
| Paroxetine | Placebo | |
| Demographics | ||
| No. | 25 | 25 |
| Age, mean (SD), y | 62.2 (12.9) | 61.4 (12.5) |
| Sex | ||
| Female | 5 (20) | 4 (16) |
| Male | 20 (80) | 21 (84) |
| Medical history | ||
| Hypertension | 11 (44) | 12 (48) |
| Hypercholesterolemia | 11 (44) | 9 (36) |
| Diabetes | 2 (8) | 6 (24) |
| Glomerular filtration rate <60 mL/min | 0 | 4 (16) |
| History of atrial fibrillation or atrial flutter | 1 (4) | 0 |
| Characteristics of myocardial infarction | ||
| Killip class III or IV | 4 (16) | 4 (16) |
| Culprit vessel | ||
| Left main artery | 0 | 1 (4) |
| Left anterior descending artery | 25 (100) | 24 (96) |
| Multivessel coronary artery disease | 12 (48) | 12 (48) |
| Complete revascularization during index PCI | 0 | 1 (4) |
| Complete revascularization during staged PCI | 7 (28) | 9 (36 |
| No revascularization of nonculprit arteriesb | 5 (20) | 2 (8) |
| Mean (SD) peak creatine kinase, U/L | 3604 (2454) | 3541 (2383) |
| Medication at discharge | ||
| Aspirin | 25 (100) | 25 (100) |
| Clopidogrel | 5 (20) | 6 (24) |
| Prasugrel | 6 (24) | 4 (16) |
| Ticagrelor | 14 (56) | 15 (60) |
| Vitamin K antagonist | 1 (4) | 0 |
| Non–vitamin K antagonist | 4 (16) | 6 (24) |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker antagonist | 25 (100) | 25 (100) |
| β-Blocker | 25 (100) | 25 (100) |
| Aldosterone antagonist | 10 (40) | 11 (44) |
| Angiotensin receptor neprilysin inhibitor | 3 (12) | 3 (12) |
| Sodium glucose cotransporter 2 inhibitor | 3 (12) | 4 (16) |
| Imaging at baseline, mean (SD), d | ||
| Interval between PCI and cardiac magnetic resonance | 2.7 (1.4) | 3.2 (1.5) |
| Interval between PCI and transthoracic echocardiography | 1.5 (0.8) | 1.7 (1.1) |
Abbreviation: PCI, percutaneous coronary intervention.
SI conversion factor: To convert creatine kinase to μkat/L, multiply by 0.0167.
Data are presented stratified by allocated study drug according to the intention-to-treat principle.
Because of the absence of viability in respective territory, based on cardiac magnetic resonance imaging (n = 6) or small vessel disease (n = 1).
Primary and Secondary End Points
Between baseline and follow-up at 12 weeks, mean LVEF as assessed by CMR imaging improved in both the experimental group (mean [SD] change, 4.0% [7.0%]) and the control group (mean [SD] change, 6.3% [6.3%]), with no difference between the 2 treatment arms (mean difference, −2.4% [95% CI, −6.8% to 2.1%]; P = .29; Figure, A). There were no differences in the change in left ventricular end-diastolic diameter (mean difference, −0.2 [95% CI, −4.8 to 4.3] mm; P = .92) or end-systolic diameter (mean difference, 3.6 [95% CI, −1.7 to 8.8] mm; P = .18) between baseline and follow-up (Table 2). Late gadolinium enhancement as a percentage of total left-ventricular mass decreased more in the experimental group (mean [SD], −13.6% [12.9%]) compared with the control group (mean [SD], −4.5% [9.5%]; mean difference, −9.1% [95% CI, −16.6% to −1.6%]; P = .02; Figure, B). Illustrative examples of temporal changes in late gadolinium enhancement observed by CMR imaging are provided in eFigure 2 in Supplement 1.
Figure. Patient-Level Changes in Left Ventricular (LV) Ejection Fraction and Late Gadolinium Enhancement.
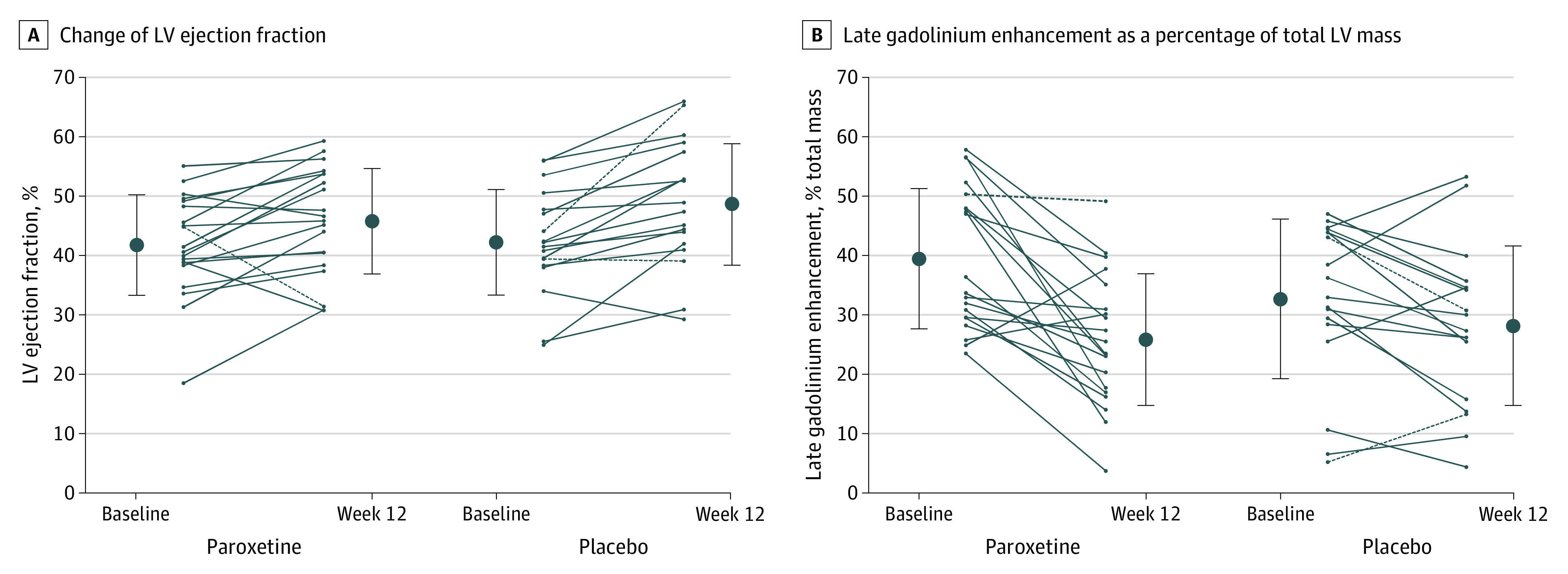
Line graphs illustrate patient-level changes in LV ejection fraction (A) and late gadolinium enhancement as a percentage of total left ventricular mass (B) between baseline and follow-up at 12 weeks in patients in the paroxetine and the placebo groups. Additionally, mean values and SDs indicated by error bars are shown at each point. Results are presented by intention-to-treat analysis; dashed lines indicate patients who were not part of the per-protocol population. A, Mean (SD) patient-level changes: paroxetine group (n = 20), 4.0% (7.0%); placebo group (n = 18), 6.3% (6.3%); difference, −2.4% (95% CI, −6.8% to 2.1%); P = .29. B, Mean (SD) patient-level changes: paroxetine group (n = 20), −13.6% (12.9%); placebo group (n = 18), −4.5% (9.5%); difference, −9.1% (95% CI, −16.6% to −1.6%); P = .02.
Table 2. Left Ventricular (LV) Dimension, LV Function, and Late Gadolinium Enhancement Between Baseline and 12 Weeks, as Assessed by Cardiac Magnetic Resonance Imaginga.
| Characteristic | Mean (SD) | Paroxetine vs placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Paroxetine | Placebo | |||||||||
| Baseline | Week 12 | Change | P value | Baseline | Week 12 | Change | P value | Difference in the change, mean (95% Cl) | P value | |
| No. of participants | 24 | 20 | 20 | NA | 24 | 18 | 18 | NA | 38 | NA |
| Primary end point | ||||||||||
| LV ejection fraction, % | 41.1 (7.9) | 45.7 (8.9) | 4.0 (7.0) | .02 | 39.5 (10.3) | 48.5 (10.2) | 6.3 (6.3) | .001 | −2.4 (−6.8 to 2.1) | .29 |
| LV dimensions and function | ||||||||||
| End-diastolic diameter, mm | 51.7 (6.5) | 53.9 (9.4) | 2.0 (6.24) | .18 | 49.0 (7.4) | 50.9 (6.8) | 2.2 (7.6) | .24 | −0.2 (−4.8 to 4.3) | .92 |
| End-systolic diameter, mm | 34.6 (7.6) | 38.4 (10.4) | 2.6 (8.3) | .17 | 33.7 (7.7) | 32.8 (8.9) | −0.9 (7.5) | .60 | 3.6 (−1.7 to 8.8) | .18 |
| End-diastolic volume, mL | 192.7 (44.9) | 210.3 (65.7) | 17.4 (45.7) | .11 | 174.9 (41.7) | 177.2 (51.5) | 4.0 (29.5) | .57 | 13.3 (−12.3 to 39.0) | .30 |
| End-systolic volume, mL | 114.2 (33.8) | 116.8 (47.8) | 3.52 (26.9) | .57 | 105.7 (30.7) | 93.3 (37.2) | −7.9 (17.2) | .07 | 11.4 (−3.6 to 26.4) | .13 |
| Stroke volume, mL | 78.5 (22.4) | 93.4 (27.3) | 13.9 (28.3) | .04 | 69.2 (21.6) | 83.6 (22.2) | 11.6 (17.3) | .01 | 2.3 (−13.4 to 17.9) | .77 |
| Mass, g | 140.0 (40.7) | 123.2 (34.9) | −12.9 (26.3 | .04 | 126.1 (25.8) | 111.2 (21.9) | −15.1 (13.0) | <.001 | 2.2 (−11.7 to 16.1) | .75 |
| Cardiac output, L/min | 5.5 (1.4) | 5.5 (1.8) | 0.2 (1.8) | .65 | 5.1 (1.5) | 5.4 (1.3) | 0.2 (1.0) | .33 | 0.0 (−1.0 to 0.9) | .92 |
| Late gadolinum enhancement, 5 SDs, g | ||||||||||
| Total | 53.1 (22.9) | 33.2 (18.2) | −19.3 (16.7) | <.001 | 42.4 (20.0) | 29.5 (16.1) | −9.4 (12.2) | .005 | −9.9 (−19.6 to −0.2) | .047 |
| % of Total | 39.4 (11.1) | 25.9 (11.1) | −13.6 (12.9) | <.001 | 34.8 (13.8) | 28.2 (13.3) | −4.5 (9.5) | .06 | −9.1 (−16.6 to −1.6) | .02 |
Abbreviation: NA, not applicable.
Data are presented stratified by allocated study drug according to the intention-to-treat principle.
Echocardiographic data at baseline and follow-up (eTable 5 in Supplement 1) and clinical end points (eTable 6 in Supplement 1) were comparable between the experimental and control groups. None of the study participants died. Reported adverse effects are provided in eTable 7 in Supplement 1.
Per-Protocol Analysis
Thirty-five patients were considered for the per-protocol analysis. The results of the per-protocol analysis were consistent with the intention-to-treat analysis and are summarized in eTable 8 in Supplement 1.
Discussion
In this double-blind, placebo-controlled randomized clinical trial, the extent of LVEF recovery 12 weeks after acute anterior myocardial infarction was comparable in patients treated with paroxetine or placebo. There were no significant differences in changes of left ventricular dimensions and volumes. In contrast, patients treated with paroxetine had greater reduction in late gadolinium enhancement, indicating attenuation of myocardial fibrosis and scar formation after myocardial infarction.
Preclinical evidence8 has suggested a causal role of GRK2 in maladaptive cardiac remodeling and proposed it as a potential target for pharmacologic intervention after myocardial infarction. In an animal model,8 wild-type mice started on paroxetine at a dosage of 5 mg/kg/day after induced myocardial infarction exhibited robust improvement of left ventricular function and structure compared with mice treated with a control drug or fluoxetine. In contrast, in the present study, which is to our knowledge the first conducted in humans, we found no difference in CMR imaging–determined LVEF at 3 months after myocardial infarction in patients with paroxetine treatment compared with control participants.
The observation of reduced late gadolinium enhancement, a marker for scar formation, in patients treated with paroxetine is consistent with data from preclinical studies8 indicating a reduction of fibrosis in the border zone of the infarct area. The finding is relevant against the background of an established association9,10 of infarct size as determined by CMR imaging with an increased risk of death at 1 year and warrants further validation in larger studies. In contrast with the mouse model, in which treatment was started 2 weeks after inducing acute myocardial infarction, patients in this study were included 48 to 96 hours after primary percutaneous coronary revascularization and paroxetine was started on top of guideline-directed optimal medical therapy less than 7 days after percutaneous coronary revascularization. Furthermore, study medication may have been underdosed, because GRK2 inhibition mediated by paroxetine is an off-target effect; however, serum levels in the experimental arm were comparable with those observed in studies with positive results conducted in animal models.8
Limitations
Our findings need to be interpreted in light of several limitations. First, only 38 patients underwent CMR imaging at the time of follow-up, and the actual attrition rate (24%) was larger than anticipated. Hence, the study was underpowered, resulting in an increased likelihood of both type I and type II errors. As a consequence, the observed reduction in late gadolinium enhancement in patients treated with paroxetine is hypothesis generating. Second, despite confining the study population to patients with anterior wall myocardial infarction, study participants had on average only a moderately reduced LVEF. This limited the sensitivity of the primary end point and further decreased the chances to demonstrate a difference in LVEF recovery between groups.
Conclusions
In conclusion, in this double-blind, placebo-controlled randomized clinical trial, a 3-month course of paroxetine treatment following acute myocardial infarction did not lead to an improvement in left ventricular ejection fraction compared with placebo. Further studies are needed to investigate the effect of paroxetine-mediated GRK2 inhibition on the development of myocardial fibrosis after myocardial infarction.
eMethods.
eReferences.
eTable 1. Baseline Characteristics
eTable 2. Procedural Characteristics
eTable 3. Medication
eTable 4. Baseline characteristics in patients with versus without CMR at follow-up
eTable 5. Echocardiography
eTable 6. Clinical Events
eTable 7. Reported Side Effects
eTable 8. CMR Results in Per-Protocol Population
eFigure 1. Flowchart according to CONSORT statement
eFigure 2. Examples of LGE in CMR
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981-2988. doi: 10.1161/01.CIR.101.25.2981 [DOI] [PubMed] [Google Scholar]
- 2.Brinks H, Boucher M, Gao E, et al. Level of G protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circ Res. 2010;107(9):1140-1149. doi: 10.1161/CIRCRESAHA.110.221010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfleger J, Gresham K, Koch WJ. G protein-coupled receptor kinases as therapeutic targets in the heart. Nat Rev Cardiol. 2019;16(10):612-622. doi: 10.1038/s41569-019-0220-3 [DOI] [PubMed] [Google Scholar]
- 4.Rengo G, Lymperopoulos A, Zincarelli C, et al. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119(1):89-98. doi: 10.1161/CIRCULATIONAHA.108.803999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White DC, Hata JA, Shah AS, Glower DD, Lefkowitz RJ, Koch WJ. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci U S A. 2000;97(10):5428-5433. doi: 10.1073/pnas.090091197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodall MC, Woodall BP, Gao E, Yuan A, Koch WJ. Cardiac fibroblast GRK2 deletion enhances contractility and remodeling following ischemia/reperfusion injury. Circ Res. 2016;119(10):1116-1127. doi: 10.1161/CIRCRESAHA.116.309538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thal DM, Homan KT, Chen J, et al. Paroxetine is a direct inhibitor of G protein-coupled receptor kinase 2 and increases myocardial contractility. ACS Chem Biol. 2012;7(11):1830-1839. doi: 10.1021/cb3003013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher SM, Gao E, Zhu W, et al. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci Transl Med. 2015;7(277):277ra31. doi: 10.1126/scitranslmed.aaa0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eitel I, de Waha S, Wöhrle J, et al. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64(12):1217-1226. doi: 10.1016/j.jacc.2014.06.1194 [DOI] [PubMed] [Google Scholar]
- 10.Stone GW, Selker HP, Thiele H, et al. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J Am Coll Cardiol. 2016;67(14):1674-1683. doi: 10.1016/j.jacc.2016.01.069 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eReferences.
eTable 1. Baseline Characteristics
eTable 2. Procedural Characteristics
eTable 3. Medication
eTable 4. Baseline characteristics in patients with versus without CMR at follow-up
eTable 5. Echocardiography
eTable 6. Clinical Events
eTable 7. Reported Side Effects
eTable 8. CMR Results in Per-Protocol Population
eFigure 1. Flowchart according to CONSORT statement
eFigure 2. Examples of LGE in CMR
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement


