Key Points
Question
Is Toxoplasma gondii seropositivity in otherwise healthy people associated with alterations in cognitive function?
Findings
In this systematic review and meta-analysis of 13 studies comprising 13 289 healthy individuals, a modest but significant association was observed between T gondii seropositivity and impaired performance on cognitive tests in all analyzed domains (processing speed, working memory, short-term verbal memory, and executive functioning).
Meaning
This study’s findings suggest that, given the high global prevalence of T gondii infection, the consequences of these associated cognitive impairments for global mental health could be substantial.
Abstract
Importance
The parasite Toxoplasma gondii has been associated with behavioral alterations and psychiatric disorders. Studies investigating neurocognition in people with T gondii infection have reported varying results. To systematically analyze these findings, a meta-analysis evaluating cognitive function in healthy people with and without T gondii seropositivity is needed.
Objective
To assess whether and to what extent T gondii seropositivity is associated with cognitive function in otherwise healthy people.
Data Sources
A systematic search was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. A systematic search of PubMed, MEDLINE, Web of Science, PsycInfo, and Embase was performed to identify studies from database inception to June 7, 2019, that analyzed cognitive function among healthy participants with available data on T gondii seropositivity. Search terms included toxoplasmosis, neurotoxoplasmosis, Toxoplasma gondii, cognition disorder, neuropsychological, and psychomotor performance.
Study Selection
Studies that performed cognitive assessment and analyzed T gondii seroprevalence among otherwise healthy participants were included.
Data Extraction and Synthesis
Two researchers independently extracted data from published articles; if needed, authors were contacted to provide additional data. Quantitative syntheses were performed in predefined cognitive domains when 4 independent data sets per domain were available. Study quality, heterogeneity, and publication bias were assessed.
Main Outcomes and Measures
Performance on neuropsychological tests measuring cognitive function.
Results
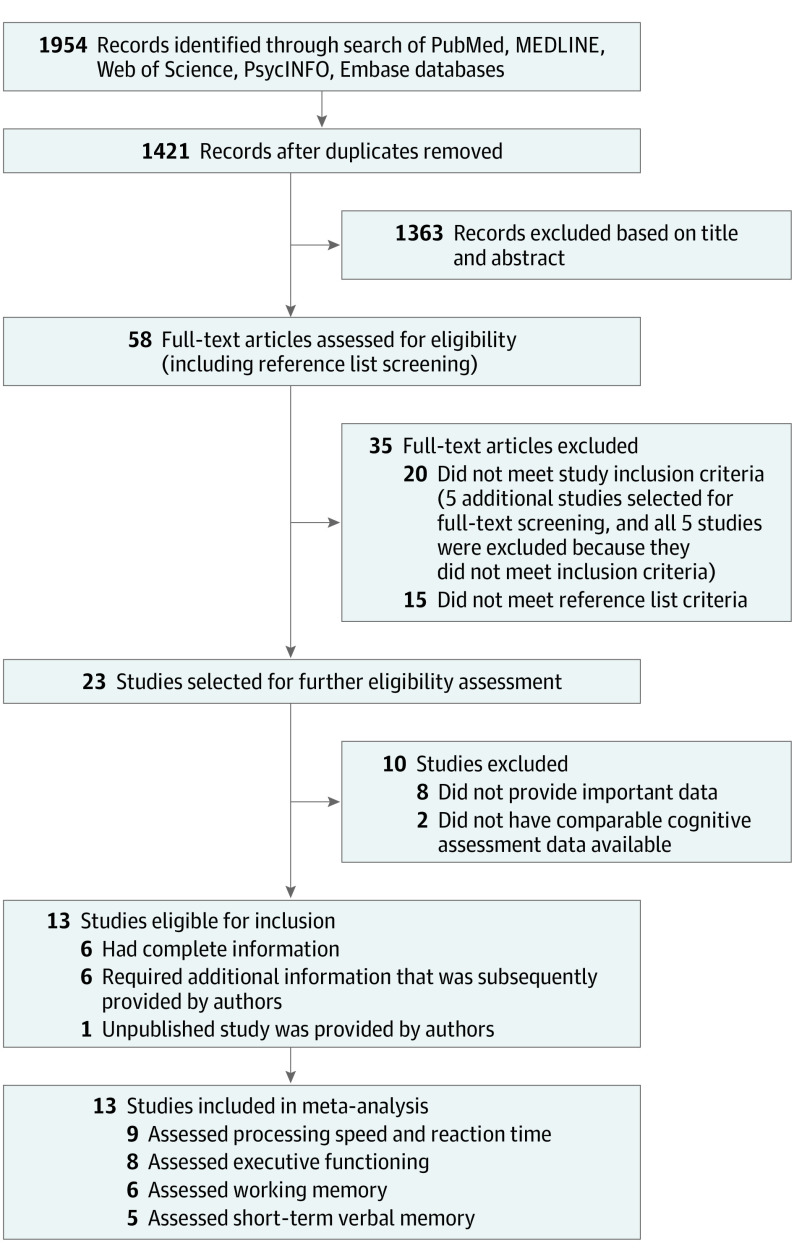
The systematic search yielded 1954 records. After removal of 533 duplicates, an additional 1363 records were excluded based on a review of titles and abstracts. A total of 58 full-text articles were assessed for eligibility (including reference list screening); 45 articles were excluded because they lacked important data or did not meet study inclusion or reference list criteria. The remaining 13 studies comprising 13 289 healthy participants (mean [SD] age, 46.7 [16.0] years; 6586 men [49.6%]) with and without T gondii seropositivity were included in the meta-analysis. Participants without T gondii seropositivity had favorable functioning in 4 cognitive domains: processing speed (standardized mean difference [SMD], 0.12; 95% CI, 0.05-0.19; P = .001), working memory (SMD, 0.16; 95% CI, 0.06-0.26; P = .002), short-term verbal memory (SMD, 0.18; 95% CI, 0.09-0.27; P < .001), and executive functioning (SMD, 0.15; 95% CI, 0.01-0.28; P = .03). A meta-regression analysis found a significant association between older age and executive functioning (Q = 6.17; P = .01). Little suggestion of publication bias was detected.
Conclusions and Relevance
The study’s findings suggested that T gondii seropositivity was associated with mild cognitive impairment in several cognitive domains. Although effect sizes were small, given the ubiquitous prevalence of this infection globally, the association with cognitive impairment could imply a considerable adverse effect at the population level. Further research is warranted to investigate the underlying mechanisms of this association.
This systematic review and meta-analysis uses data from observational studies to examine the association between Toxoplasma gondii infection and cognitive function among otherwise healthy individuals.
Introduction
Toxoplasma gondii is an intracellular parasite that produces quiescent infection in approximately 30% of humans worldwide. Toxoplasmosis prevalence varies depending on geographic location and increases with age. The course of the infection is usually asymptomatic or occasionally symptomatic with nonspecific symptoms.1 Reproduction of T gondii is possible in the intestines of felids only, but a wide range of intermediate hosts is known to carry the infection.2 Hosts acquire toxoplasmosis by ingesting oocysts or cysts of the parasite via contaminated water or food. The T gondii parasite is able to permeate the blood-brain barrier and can settle as a quiescent infection in muscle, brain, and liver tissue.3,4,5,6
Toxoplasmosis may have consequences for the behavior of intermediate hosts. Infected rodents have exhibited more risk-taking and impulsive behavior compared with uninfected rodents,7,8 and infected mice have exhibited impaired reaction times,9 reduced learning capacity,10 and decreased motor performance.11,12 However, these observations have not been consistently reported in all studies.13
Several observational studies have reported neurocognitive changes associated with toxoplasmosis in humans; however, effect sizes and directions varied.14,15,16,17 Meta-analyses have suggested an association between T gondii and neuropsychiatric disorders, particularly schizophrenia.18,19,20 Although meta-analytic findings have not found an association between T gondii and attention-deficit/hyperactivity disorder,21 1 recent case-control study did report an association.22 Furthermore, exposure to T gondii has been associated with an increased number of motor vehicle crashes and suicide attempts.23
To identify potential adverse consequences of T gondii seropositivity, it is important to evaluate whether the highly prevalent T gondii parasite is associated with cognition in humans and, if so, to examine which specific cognitive functions are impaired, to what extent they are impaired, and whether participant or study characteristics have consequences for any association between seropositivity and cognitive performance. The findings of the literature thus far have not conclusively answered these questions. Therefore, we conducted a systematic review and meta-analysis to examine whether T gondii seropositivity was associated with alterations in cognitive function among otherwise healthy people.
Methods
Search Strategy and Selection Criteria
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines.24,25 The study was registered on PROSPERO (CRD42020154860). A systematic search of PubMed, MEDLINE, Web of Science, PsycInfo, and Embase was performed by a trained researcher (A.S.) to identify studies from database inception to June 7, 2019, that analyzed cognitive function among healthy participants with available data on T gondii seropositivity. Search terms included toxoplasmosis, Toxoplasma gondii, cognition disorder, neuropsychological, and psychomotor performance (the full search strategy is available in eMethods 1 in the Supplement).
Titles, abstracts, and full-text articles were independently screened by 2 researchers (Lies H. and J.S.), and differences in study selection were resolved by consensus. Reference lists of all reviews pertaining to toxoplasmosis and brain involvement were hand searched for eligible studies. In cases of overlapping participants, the largest cohort was included. Studies were included if (1) they enrolled a group of healthy individuals among whom antibodies to T gondii were assessed, (2) neuropsychological tests were used to assess cognitive function, and (3) the means and SDs of the cognitive test scores were described, calculable, or made available by the authors. Studies were excluded if participants (1) were immunocompromised or had severe complications associated with toxoplasmosis, (2) had congenital toxoplasmosis, or (3) had a psychiatric disorder. Corresponding authors were approached to request additional data if needed.
The following data were collected: year of publication, study design, inclusion and exclusion criteria, sample size, age presented in means and SDs, male to female ratio, assay used for T gondii detection, IgG threshold for T gondii seropositivity, and cognitive outcomes presented in means and SDs. Both cross-sectional and longitudinal studies were included; for longitudinal studies, the baseline assessments were used. Some studies enrolled a healthy population sample, whereas in other studies, patients with psychiatric disorders were compared with healthy individuals. Only data concerning healthy participants were extracted.
Data Extraction
Data (summary estimates) were extracted from published articles, and 2 researchers (Lies H. and J.S.) requested additional (raw) data from the corresponding authors of selected studies26,27,28,29,30,31,32 when the available published results were not usable for meta-analysis. Data provided were used in the meta-analysis with written permission from the authors of the following studies: Cobia et al27 (D. Cobia, PhD, written communication, April 9, 2020), El-Hadidy et al28 (M. A. El-Hadidy, MD, written communication, May 11, 2020), Gajewski et al29 (P. D. Gajewski, PhD, written communication, April 3, 2020), Guenter et al30 (W. Guenter, PhD, written communication, April 30, 2020), Nimgaonkar et al31 (V. J. Nimgaonkar, MD, PhD, written communication, May 12, 2020), and Torniainen-Holm et al32 (M. Torniainen-Holm, PhD, written communication, May 8, 2020). Partial data from the Cobia et al27 study were published as a conference abstract in a journal supplement. Unpublished data from Cobia et al27 and other studies28,29,30,31,32 were used in the following calculations: mean age; male to female ratio; seropositivity to seronegativity ratio; overall SMD of processing speed, working memory, short-term verbal memory, and executive functioning; and corresponding sensitivity analyses, meta-regression analyses, and funnel plots.
Cognitive tests were classified into corresponding cognitive domains according to the Compendium of Neuropsychological Tests: Administration, Norms, and Commentary of Strauss et al,33 Neuropsychological Assessment by Lezak et al,34 and the MATRICS Consensus Cognitive Battery part 135 (eMethods 2 and eTable 1 in the Supplement). When a study used multiple neuropsychological tests pertaining to the same cognitive domain, we included only the data from the test that was applied most frequently across the included studies to minimize heterogeneity.
Statistical Analysis
We calculated standardized mean differences (SMDs) by dividing mean differences by SDs. Effect directions of all available tests were adjusted so that a positive SMD represented favorable cognitive performance. The significance threshold was set at 2-sided P = .05.
The quality of the included studies was assessed by 2 researchers (Lies H. and J.S.) using the case-control form of the Newcastle-Ottawa Scale for quality assessment (score range, 0-9 stars, with 0 indicating lowest quality and 9 indicating highest quality).36 Differences in judgment of quality were discussed with a third researcher (A.S.) and resolved by consensus.
A random-effects model was applied because heterogeneity was expected. A minimum of 4 studies was required per domain to allow data to be pooled for the meta-analysis. An overview of outcome measures that could not be pooled in the meta-analysis because they were reported in an insufficient number of studies is available in eTable 2 in the Supplement. Publication bias was assessed using the Egger test (with a significance threshold of 1-sided P < .10) and visual inspection of funnel plots. The Duval and Tweedie trim-and-fill method37 was applied if suggestions of publication bias were detected.
The I2 statistic was used to measure heterogeneity.38 If heterogeneity was detected, the following moderating factors were assessed: mean age, sex, type of test, cutoff values for seropositivity, study sample (population sample or healthy control group), and study quality. Subgroup analyses (for categorical variables) or meta-regression analyses (for continuous variables) were performed to assess the variable’s consequences for heterogeneity. Comprehensive Meta-analysis, version 3 was used for the analyses.39
Results
Study Characteristics
The systematic search yielded 1954 total records. After removal of 533 duplicates, an additional 1363 records were excluded based on a review of titles and abstracts. A total of 58 full-text articles were assessed for eligibility (including reference list screening); 45 articles were excluded because they lacked important data or did not meet study inclusion or reference list criteria. Two of those articles26,40 reported outcomes in which fewer than 4 studies were available and were therefore excluded. Thirteen studies16,27,28,29,30,31,32,41,42,43,44,45,46 met eligibility criteria and were included in the meta-analysis (Figure 1).
Figure 1. Flow Diagram of Study Selection Process.
Partial data from the unpublished study were published as a conference abstract in a journal supplement.27 The authors provided additional (raw) data and written permission to use these data in the meta-analysis (D. Cobia, PhD, written communication, April 9, 2020).
The 13 studies16,27,28,29,30,31,32,41,42,43,44,45,46 included in the meta-analysis comprised 13 289 healthy participants (mean [SD] age, 46.7 [16.0] years based on 8 studies29,31,32,41,42,43,44,46 reporting complete age data; 6586 men [49.6%]); of those, 3006 participants (22.6%) had antibodies against T gondii (Table). All studies used the enzyme-linked immunosorbent assay to detect T gondii antibodies.
Table. Study Characteristics.
| Source | Country | Study design | Inclusion criteria of healthy population | Total healthy participants, No. | Age of healthy participants, mean (SD), y | Cutoff value for seropositivity, IU/mL | Participant infection status, No. | Male participants, No. (%) | Cognitive tests administered | |
|---|---|---|---|---|---|---|---|---|---|---|
| Seropositive | Seronegative | |||||||||
| Berrett et al,41 2017 | US | Cross-sectional case-control | NHANES III adults | 2037 | 37.3 (24.5) | 7 | 417 | 1620 | 1012 (49.7) | SRTT |
| Cobia et al,27 2017 | US | Cross-sectional case-control | Healthy control group | 25 | 43.5 (NR)a | 35a | 11 | 14 | 14 (56.0)a | TMT-A; digit span forward; TMT-B |
| El-Hadidy et al,28 2013 | Egypt | Cross-sectional case-control | Healthy control group | 124 | 35.7 (NR)b | 50 | 34 | 90 | 90 (72.6)b | TMT-A; TMT-B |
| Ene et al,42 2016 | Romania | Cross-sectional case-control | Healthy control group with ≥8 y of education | 51 | 24.2 (2.4) | NR | 18 | 33 | 28 (55.0) | TMT-A; VLT; TMT-B |
| Gajewski et al,29 2014 | Germany | Cross-sectional case-control | Healthy adults aged ≥65 y living independently | 84 | 70.5 (4.5)c | 50 | 42c | 42c | 28 (33.3)c | TMT-A; digit span forward; VLMT; TMT-B |
| Guenter et al,30 2012 | Poland | Case-control | Healthy control group with ≥12 y of education | 70 | 27.3 (NR) | 35 | 26 | 44 | 14 (20.0) | TMT-A; digit span forward; TMT-B |
| Hamdani et al,43 2017 | France | Cross-sectional case-control | Healthy control group | 180 | 40.1 (13.8) | NR | 105 | 75 | 98 (54.4) | CVLT; digit span backward |
| Mendy et al,44 2015 | US | Cross-sectional case-control | NHANES III children | 1755 | 13.97 (2.5) | 6 | 135 | 1620 | 818 (46.6) | Digit span scaled |
| Nimgaonkar et al,31 2016 | US | Cohort | MYHAT older adults | 1022 | 77.5 (7.5) | NR | 489 | 533 | 409 (40.0) | TMT-B; VFT; clock drawing |
| Novotna,45 2008d | ||||||||||
| Cohort 1 | Czech Republic | Cross-sectional case-control | Voluntary blood donors | 114 | 36.4 (NR) | 10 | 41 | 73 | 69 (60.5) | SRTT |
| Cohort 2 | Voluntary blood donors | 439 | NR | 10 | 151 | 288 | 310 (70.6) | SRTT | ||
| Cohort 3 | Military service members | 315 | 19.7 (NR) | 10 | 95 | 220 | 315 (100) | SRTT | ||
| Stock et al,46 2014 | Germany | Case-control | Healthy control group (right-handed) | 36 | 24.6 (2.6) | 15 | 18 | 18 | 13 (36.1) | Go reaction time test |
| Sugden et al,16 2016 | New Zealand | Cohort | Birth cohort | 837 | 38 (NR)e | 50 | 236 | 601 | 423 (50.5) | Arithmetic subtest and digit span; AVLT; TMT-B |
| Torniainen- Holm et al,32 2019 | Finland | Cohort | Finnish population sample | 6200 | 54.2 (16.0)f | 50 | 1188f | 5012f | 2945 (47.5)f | SRTT; VLMT; VFT |
Abbreviations: AVLT, Auditory Verbal Learning Test; CVLT, California Verbal Learning Test; MYHAT, Monongahela-Youghiogheny Healthy Aging Team; NHANES, National Health and Nutrition Examination Survey; NR, not reported; SRTT, Simple Reaction Time Test; TMT-A, Trail-Making Test Part A; TMT-B, Trail-Making Test Part B; VFT, verbal fluency test; VLMT, Verbal Learning and Memory Test; VLT, verbal learning test.
Unpublished data were provided (with written permission to use these data in the meta-analysis) by D. Cobia, PhD (written communication, April 14, 2020).
Unpublished data were provided (with written permission to use these data in the meta-analysis) by M. A. El-Hadidy, MD (written communication, May 11, 2020).
Unpublished data were provided (with written permission to use these data in the meta-analysis) by P. D. Gajewski, PhD (written communication, April 3, 2020).
The Novotna et al45 study was split into 3 cohorts because the original study was divided into 3 phases with different participants. Novotna et al cohort 1 comprised blood donors from 1998 to 2000, Novotna et al cohort 2 comprised blood donors from 2002 to 2006, and Novotna et al cohort 3 comprised military service members.
All participants received assessment at age 38 years.
Unpublished data were provided (with written permission to use these data in the meta-analysis) by M. Torniainen-Holm, PhD (written communication, May 8, 2020).
Data eligible for meta-analysis comprised 4 cognitive domains: processing speed, working memory, short-term verbal memory, and executive functioning (eMethods 2 in the Supplement). None of the included studies received fewer than 5 stars during the quality assessment. Eleven studies controlled for 1 or more important factor. All 13 studies16,27,28,29,30,31,32,41,42,43,44,45,46 used independent validation of cases and ascertained outcomes using a validated test. Detailed results of the quality assessment are available in eTable 3 and eTable 4 in the Supplement.
Processing Speed
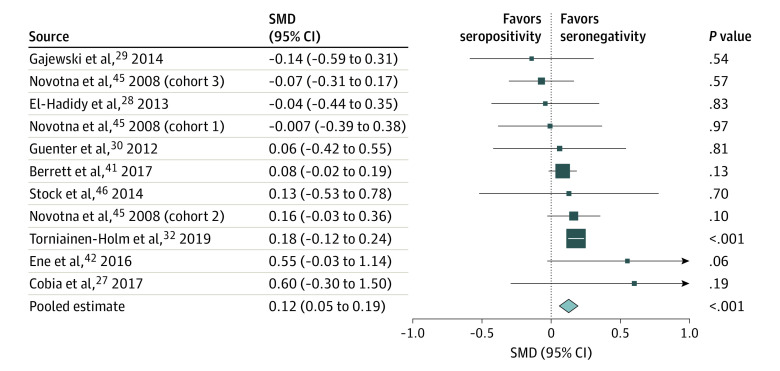
A total of 9 studies27,28,29,30,32,41,42,45,46 reported data on processing speed that could be aggregated. In these studies, processing speed was measured by the Trail Making Test Part A, the Serial Reaction Time Test, or go/no-go reaction time tests. An overall SMD of 0.12 (95% CI, 0.05-0.19; P = .001) was found, indicating a small but statistically significant association between T gondii seropositivity and decreased processing speed (Figure 2; eTable 5A in the Supplement). The Egger test (intercept, −0.40; P = .21) and funnel plot detected no publication bias (eFigure 5 in the Supplement). Heterogeneity was low (I2 = 13%; P = .32). No significant consequences for heterogeneity by the available moderating factors were found (eFigures 9-11 in the Supplement). A sensitivity analysis, in which the study with the lowest quality27 was excluded, did not significantly change the results (SMD, 0.13; 95% CI, 0.07-0.19; P < .001) (eFigure 2 in the Supplement).
Figure 2. Forest Plot of Processing Speed.
A random-effects model was used. Data markers represent standardized mean differences (SMDs), and horizontal lines represent 95% CIs. The diamond represents the pooled summary estimate, and the horizontal extremes of the diamond represent its 95% CI. The arrows pointing right on the forest plot represent the upper limit of the 95% CI exceeding 1.0. Data on overall heterogeneity are available in eTable 5A in the Supplement. Unpublished (raw) data were requested from the authors of several studies because the available published results were not usable for meta-analysis; these data were used in the calculations with written permission from the authors of the following studies: Cobia et al27 (D. Cobia, PhD, April 9, 2020), El-Hadidy et al28 (M. A. El-Hadidy, MD, written communication, May 11, 2020), Gajewski et al29 (P. D. Gajewski, PhD, written communication, April 3, 2020), Guenter et al30 (W. Guenter, PhD, written communication, April 30, 2020), and Torniainen-Holm et al32 (M. Torniainen-Holm, PhD, written communication, May 8, 2020).
Working Memory
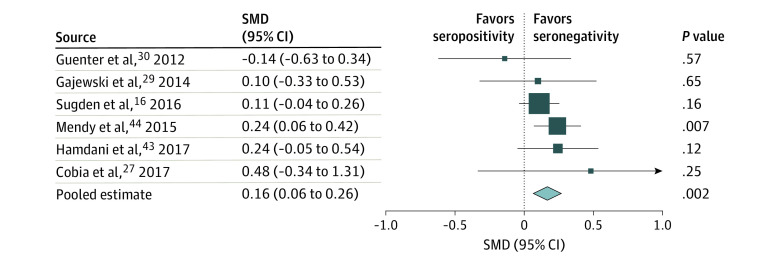
Six studies16,27,29,30,43,44 provided results from the Wechsler Adult Intelligence Scale or the Wechsler Intelligence Scale for Children digit span test measuring working memory. An overall SMD of 0.16 (95% CI, 0.06-0.26; P = .002) was found, which represented a small but significant association between T gondii seropositivity and working memory impairment (Figure 3; eTable 5B in the Supplement). The Egger test (intercept, −0.01; P = .50) and funnel plot did not detect any publication bias (eFigure 6 in the Supplement). There was no evidence of heterogeneity (I2 = 0%), and all included studies were of fair quality. Therefore, no sensitivity analyses were performed, and meta-regression analyses revealed no significant change in results (eFigure 12 and eFigure 13 in the Supplement).
Figure 3. Forest Plot of Working Memory.
A random-effects model was used. Data markers represent standardized mean differences (SMDs), and horizontal lines represent 95% CIs. The diamond represents the pooled summary estimate, and the horizontal extremes of the diamond represent its 95% CI. The arrow pointing right on the forest plot represents the upper limit of the 95% CI exceeding 1.0. Data on overall heterogeneity are available in eTable 5B in the Supplement. Unpublished (raw) data were requested from the authors of several studies because the available published results were not usable for meta-analysis; these data were used in the calculations with written permission from the authors of the following studies: Cobia et al27 (D. Cobia, PhD, written communication, April 9, 2020), Gajewski et al29 (P. D. Gajewski, PhD, April 3, 2020), and Guenter et al30 (W. Guenter, PhD, written communication, April 30, 2020).
Short-term Verbal Memory
Five studies16,29,32,42,43 provided results from the Auditory Verbal Learning Test, the California Verbal Learning Test, or the Verbal Learning and Memory Test measuring short-term verbal memory. A statistically significant association was found between T gondii seropositivity and verbal memory impairment, with an SMD of 0.18 (95% CI, 0.09-0.27; P < .001) (eFigure 1 and eTable 5C in the Supplement). The Egger test (intercept, 1.10; P = .09) and funnel plot suggested some publication bias (eFigure 7 in the Supplement). The Duval and Tweedie trim-and-fill method imputed 1 study to the right of the mean. This result suggested some publication bias, causing the unadjusted effect size to appear more convincing compared with the real effect size. However, the adjusted effect size remained significant (SMD, 0.17; 95% CI, 0.06-0.29). Heterogeneity was moderate (I2 = 22%; P = .28), and an analysis of moderating factors did not provide an explanation for this heterogeneity (eFigure 14 in the Supplement).
Executive Functioning
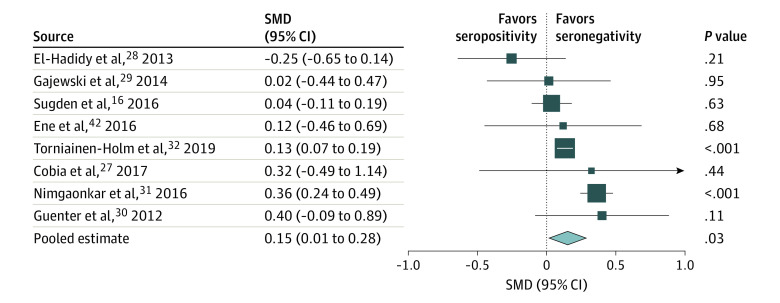
Eight studies16,27,28,29,30,31,32,42 reported results from the Trail Making Test Part B, verbal fluency tests, or clock drawing tests measuring executive functioning. An association between T gondii seropositivity and worse executive functioning was observed, with an SMD of 0.15 (95% CI, 0.01-0.28; P = .03) (Figure 4; eTable 5D in the Supplement). Both the funnel plot and the Egger test (intercept, −0.10; P = .46) did not detect publication bias (eFigure 8 in the Supplement). Significant heterogeneity was found (I2 = 63%; P = .008). An analysis of moderating factors found no significant associations between heterogeneity and study design, sex, type of test, cutoff values, or study quality (eFigures 4, 16, and 17 in the Supplement). A sensitivity analysis, in which the study with the lowest quality27 was excluded, did not substantially change the findings (SMD, 0.15; 95% CI, 0.004-0.29; P = .04) (eFigure 3 in the Supplement).29 A meta-regression analysis of mean age revealed significantly greater effect sizes as age increased [Q = 6.17; R2 = 81%; P = .01) (eTable 6 and eFigure 15 in the Supplement).
Figure 4. Forest Plot of Executive Functioning.
A random-effects model was used. Data markers represent standardized mean differences (SMDs), and horizontal lines represent 95% CIs. The diamond represents the pooled summary estimate, and the horizontal extremes of the diamond represent its 95% CI. The arrow pointing right on the forest plot represents the upper limit of the 95% CI exceeding 1.0. Data on overall heterogeneity are available in eTable 5D in the Supplement. Unpublished (raw) data were requested from the authors of several studies because the available published results were not usable for meta-analysis; these data were used in the calculations with written permission from the authors of the following studies: Cobia et al27 (D. Cobia, PhD, written communication, April 9, 2020), El-Hadidy et al28 (M. A. El-Hadidy, MD, written communication, May 11, 2020), Gajewski et al29 (P. D. Gajewski, PhD, written communication, April 3, 2020), Guenter et al30 (W. Guenter, PhD, written communication, April 30, 2020), Nimgaonkar et al31 (V. J. Nimgaonkar, MD, PhD, written communication, May 12, 2020), and Torniainen-Holm et al32 (M. Torniainen-Holm, PhD, written communication, May 8, 2020).
We conducted 1 post hoc sensitivity analysis that excluded data from Nimgaonkar et al31 because this study reported that some participants may have provided their blood samples 1 year after study entry (when baseline cognition had already been assessed), which presented a risk of bias. This analysis did not substantially change the results (SMD, 0.11; 95% CI, 0.03-0.18; P = .005) (eFigure 4 in the Supplement).
Discussion
To our knowledge, this meta-analysis is the first to evaluate the association between T gondii seroprevalence and cognitive performance in otherwise healthy individuals. The results suggested that T gondii seropositivity was significantly associated with worse cognitive function in all analyzed domains, which comprised processing speed, working memory, verbal short-term memory, and executive functioning. Although the extent of the associations was modest, the ubiquitous prevalence of the quiescent infection worldwide (approximately 30%)1 suggests that the consequences for cognitive function of the population as a whole may be substantial, although it is difficult to quantify the global impact.
Infection with T gondii has thus far been associated with several behavioral changes and psychiatric disorders.18,19,20,23 Furthermore, a recent meta-analysis47 found a marginally significant association between T gondii seroprevalence and Alzheimer disease. These findings have mainly been associated with the manipulation hypothesis, which asserts that the T gondii parasite invades the brain of warm-blooded animals and alters their behavior to render them easier prey for felids, thereby increasing the likelihood of the parasite completing its life cycle.7,8,9,10,11,12 The findings of the current meta-analysis are consistent with this hypothesis, as worse cognitive function was observed in otherwise healthy people with T gondii seropositivity. However, because these associations were largely derived from cross-sectional studies, it remains uncertain whether cognitive impairment may be causally linked to T gondii infection. Reverse causation (ie, people with more cognitive or psychiatric problems acquire infection at a higher rate) or another factor (eg, poverty) that may increase the likelihood of both T gondii infection and cognitive or psychiatric problems cannot be excluded. Nevertheless, the association between T gondii infection and cognitive impairment in otherwise healthy people and the potential for increased morbidity and mortality rates among humans is concerning.18,19,20,23 It is therefore important to further explore this association by examining consistency, temporality, dose response, experimental association, and the possibility of a biological explanation.48
Consistency
The findings of this meta-analysis suggested that T gondii seropositivity was consistently associated with worse cognitive function in several domains. Moreover, there was little suggestion of publication bias. As expected, some heterogeneity between studies was found. A meta-regression analysis of executive functioning by mean age revealed a positive association between mean age and the extent of cognitive function deficits, which may suggest that cognitive impairment increases as exposure increases. Dichotomizing for mean age did not yield any new insights (eTable 7 in the Supplement).
Temporality
A temporal association could not be inferred from the present study’s findings. However, 1 longitudinal study reported that T gondii seropositivity preceded more rapid decreases in executive functioning and Mini-Mental State Examination scores.31
Dose Response
Although the present study was not able to evaluate whether serointensity had potential implications for the extent of association between T gondii infection and cognition, an association between T gondii seropositivity and cognitive impairment among adults was noted in a previous study, and anxiety and depression in pregnant women were observed as anti–T gondii titers increased.49,50,51 However, 1 study reported a more substantial association between T gondii seropositivity and reaction time among participants with lower titers, and another study reported no association with anxiety and depression.52,53 Evaluating the association of serointensity with the severity of cognitive impairment is hampered by varying or unknown cutoff plasma IgG values for T gondii seropositivity. In the sensitivity analysis, the cutoff values of the different studies did not alter the overall results (eTable 7 in the Supplement).
Experimental Association
Studies of rodents found inhibition of neuronal functioning, suggesting neuronal pathogenicity of the T gondii parasite.2,54,55 Infection with T gondii was followed by behavioral and cognitive changes, including impaired reaction time, motor performance, memory, and learning.7,8,9,10,11,12 However, the consistency and specificity of these findings have been debated.13,56
Biological Explanation
Several neurobiological mechanisms underlying the association between T gondii seropositivity and cognition may provide a plausible explanation at a biological level. Mice with T gondii infection have exhibited increased dopamine release.57,58,59 Moreover, T gondii infection has been reported to be associated with increased dopaminergic release in neurons in vitro.59,60 A critical range of dopamine turnover for optimal cognitive function has been found, with excessive dopamine turnover being associated with cognitive impairment.61,62,63 Dysregulated dopamine may also have implications for neuronal plasticity in the hippocampus, a brain structure that is indispensable for memory function and spatial orientation.64,65 Reduced memory capacity in otherwise healthy older adults with T gondii seroprevalence was reported.29,49
The tryptophan pathway is another possible mechanism by which T gondii seroprevalence could alter cognition. As a defense mechanism to infection with the T gondii parasite, hosts rapidly break down tryptophan, which is necessary for duplication of the parasite; this breakdown produces increased levels of kynurenine and quinolinic acid.66 Increased levels of these substances could alter neurotransmitter signaling, and seropositivity and higher levels of kynurenic acid have been associated with suicide attempts.67,68,69 Higher rates of neurotoxic effects and impulsive behavior associated with increased levels of dopamine, kynurenine, and quinolinic acid together have been reported.70 In addition, the T gondii parasite enters most hosts through the gastrointestinal tract and initiates a proinflammatory immune response that activates complement component 1q, which also functions in the brain as a selective marker for clearance of excessive synapses. It is conceivable that increased expression of complement component 1q is associated with excessive clearance of synapses.71
Infection with T gondii has also been associated with the presence of dysbiotic intestinal flora in mice,72 which could increase the permeability of the gut-blood barrier; this increased permeability has been associated with psychiatric disorders and altered behavior.73,74 In the central nervous system of mice, T gondii initiates microglial activation and an increased number of leukocytes interacting with the cerebral capillary endothelium. Infected mice were less capable of vasodilatation compared with uninfected mice, suggesting vascular dysfunction.75 The eventual consequences of neurotransmitter disruption (direct implications) or neurodegeneration (indirect implications) may include cognitive impairment and susceptibility to psychiatric disorders.41,76,77
Future Directions
Further research is needed given the substantial prevalence of T gondii infection worldwide and the consistent modest association between T gondii seropositivity and less favorable cognitive function. Future studies may examine potential moderating factors, such as socioeconomic status, strain type, serointensity, possible coinfections, and duration of infection. Moreover, development of improved laboratory methods is needed not only to distinguish strain types78 but also to detect bradyzoites and tachyzoites to assess the stage of infection and measure T-cell–based immunity.
More studies of individuals from non-Western countries and more longitudinal studies are needed to investigate causality and pathophysiological mechanisms. It may also be valuable to perform studies that focus on the improvement of anti-Toxoplasma medications and the development of a vaccine because current treatments for complicated T gondii infection have been associated with several adverse effects, limited therapeutic benefits, and drug resistance.29,79 Further research into alternative antiparasitic drugs is warranted.
Strengths and Limitations
This study has strengths. The aggregation of neuropsychological test results into predefined cognitive domains reduced the risk of chance findings. Furthermore, most of the included studies were of fair to high quality, suggesting a low risk of bias, and the evidence of publication bias was low.
This study also has limitations. First, the study used only cross-sectional data; therefore, causality could not be established. The findings did not allow us to examine whether the association between T gondii seropositivity and cognition was altered by prolonged exposure to the parasite, recent exposure in which the IgG immune response just began, or reverse causation.
Second, heterogeneity could be only partially explained. Some potential moderating factors, such as the strain of T gondii and genetic variation in hosts, could not be evaluated. Toxoplasma strain type 1 and strain types with divergent alleles appear to be the most virulent and might underlie the association with schizophrenia, depression, or anxiety.51,78,80 Positivity for the RHD gene may offer protection against Toxoplasma-induced motor performance impairment in humans.45,78,81 Some polymorphisms of the MMP-9 gene have been associated with the development of neurological disorders. One particular polymorphism of MMP-9 has been found more frequently among patients with schizophrenia and T gondii seropositivity.82 Many other genes are reported to be involved in susceptibility to infection or immune response to T gondii.83 The studies included in the current meta-analysis did not examine strain types or genetic factors among participants with seropositivity, hindering exploration of these associations.
Third, coinfection is another possible moderating factor that could not be evaluated. The presence of multiple neurotropic infections has been associated with schizophrenia, presumably because coinfections may moderate the immune response more substantially.84,85 Other potential moderating factors that could not be evaluated were serointensity and socioeconomic status. In addition, most included studies were performed in Western countries and comprised a predominantly White study population. Our findings are therefore not necessarily representative of the global population.
Conclusions
Quiescent infection with T gondii is highly prevalent and may be associated with cognitive function, including processing speed, working and verbal memory, and executive functioning, in healthy people. Based on the present findings and those of previous meta-analyses18,19,20,23 examining the association of T gondii seropositivity with motor vehicle crashes, suicide attempts, and the prevalence of psychiatric disorders, public health programs to prevent T gondii infection are warranted. These programs might, at a minimum, consist of hygienic measures, especially after human contact with contaminated sources.56 Hygienic measures are often already undertaken to prevent other infectious diseases. However, these measures are not sufficient to prevent quiescent T gondii infection. It may be wise to consider further research into the development of a vaccine against infection with T gondii in either humans or felids.29
eMethods 1. Search Strategy
eMethods 2. Aggregation of Results
eTable 1. Aggregation of Tests in Cognitive Domains
eTable 2. Insufficient Reported Outcome Measures
eTable 3. Newcastle-Ottawa Scale Quality Assessment: Male and Female Participants Combined
eTable 4. Newcastle-Ottawa Scale Quality Assessment: Male and Female Participants Separated
eTable 5. Results From Meta-analyses and Subgroup Analysis
eTable 6. Results From Meta-regression Analyses
eTable 7. Meta-regression Analysis Dichotomized by Mean Age
eFigure 1. Forest Plot for Short-term Verbal Memory
eFigure 2. Forest Plot for Processing Speed Without Ene et al Study (Low Quality)
eFigure 3. Forest Plot for Executive Functioning Without Ene et al Study (Low Quality)
eFigure 4. Forest Plot for Executive Functioning Without Nimgaonkar et al Study
eFigure 5. Funnel Plot for Processing Speed
eFigure 6. Funnel Plot for Working Memory
eFigure 7. Funnel Plot for Short-term Verbal Memory
eFigure 8. Funnel Plot for Executive Functioning
eFigure 9. Meta-regression Analysis of Processing Speed: Mean Age
eFigure 10. Meta-regression Analysis of Processing Speed: Study Quality
eFigure 11. Meta-regression Analysis of Processing Speed: Seropositivity Cutoff
eFigure 12. Meta-regression Analysis of Working Memory: Mean Age
eFigure 13. Meta-regression Analysis of Working Memory: Seropositivity Cutoff
eFigure 14. Meta-regression Analysis of Short-term Verbal Memory: Mean Age
eFigure 15. Meta-regression Analysis of Executive Functioning: Mean Age
eFigure 16. Meta-regression Analysis of Executive Functioning: Study Quality
eFigure 17. Meta-regression Analysis of Executive Functioning: Seropositivity Cutoff
eReferences
References
- 1.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363(9425):1965-1976. doi: 10.1016/S0140-6736(04)16412-X [DOI] [PubMed] [Google Scholar]
- 2.Afonso C, Paixao VB, Costa RM. Chronic Toxoplasma infection modifies the structure and the risk of host behavior. PLoS One. 2012;7(3):e32489. doi: 10.1371/journal.pone.0032489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maubon D, Ajzenberg D, Brenier-Pinchart MP, Darde ML, Pelloux H. What are the respective host and parasite contributions to toxoplasmosis? Trends Parasitol. 2008;24(7):299-303. doi: 10.1016/j.pt.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 4.Dubey JP. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol. 1998;28(7):1019-1024. doi: 10.1016/S0020-7519(98)00023-X [DOI] [PubMed] [Google Scholar]
- 5.Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophr Bull. 2007;33(3):745-751. doi: 10.1093/schbul/sbm008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matta SK, Rinkenberger N, Dunay IR, Sibley LD. Toxoplasma gondii infection and its implications within the central nervous system. Published online February 24, 2021. Nat Rev Microbiol. 2021. doi: 10.1038/s41579-021-00518-7 [DOI] [PubMed] [Google Scholar]
- 7.Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci. 2000;267(1452):1591-1594. doi: 10.1098/rspb.2000.1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster JP. The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizophr Bull. 2007;33(3):752-756. doi: 10.1093/schbul/sbl073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hrda S, Votypka J, Kodym P, Flegr J. Transient nature of Toxoplasma gondii–induced behavioral changes in mice. J Parasitol. 2000;86(4):657-663. doi: 10.1645/0022-3395(2000)086[0657:TNOTGI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 10.Witting PA. Learning capacity and memory of normal and Toxoplasma-infected laboratory rats and mice. Z Parasitenkd. 1979;61(1):29-51. doi: 10.1007/BF00927085 [DOI] [PubMed] [Google Scholar]
- 11.Hutchison WM, Aitken PP, Wells BW. Chronic Toxoplasma infections and motor performance in the mouse. Ann Trop Med Parasitol. 1980;74(5):507-510. doi: 10.1080/00034983.1980.11687376 [DOI] [PubMed] [Google Scholar]
- 12.Hay J, Aitken PP, Hutchison WM, Graham DI. The effect of congenital and adult-acquired Toxoplasma infections on the motor performance of mice. Ann Trop Med Parasitol. 1983;77(3):261-277. doi: 10.1080/00034983.1983.11811707 [DOI] [PubMed] [Google Scholar]
- 13.Johnson HJ, Koshy AA. Latent toxoplasmosis effects on rodents and humans: how much is real and how much is media hype? mBio. 2020;11(2):02164-19. doi: 10.1128/mBio.02164-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickerson F, Stallings C, Origoni A, et al. Antibodies to Toxoplasma gondii and cognitive functioning in schizophrenia, bipolar disorder, and nonpsychiatric controls. J Nerv Ment Dis. 2014;202(8):589-593. doi: 10.1097/NMD.0000000000000166 [DOI] [PubMed] [Google Scholar]
- 15.Gale SD, Brown BL, Erickson LD, Berrett A, Hedges DW. Association between latent toxoplasmosis and cognition in adults: a cross-sectional study. Parasitology. 2015;142(4):557-565. doi: 10.1017/S0031182014001577 [DOI] [PubMed] [Google Scholar]
- 16.Sugden K, Moffitt TE, Pinto L, Poulton R, Williams BS, Caspi A. Is Toxoplasma gondii infection related to brain and behavior impairments in humans? evidence from a population-representative birth cohort. PLoS One. 2016;11(2):e0148435. doi: 10.1371/journal.pone.0148435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng X, Brenner LA, Mathai AJ, et al. Moderation of the relationship between Toxoplasma gondii seropositivity and trait impulsivity in younger men by the phenylalanine-tyrosine ratio. Psychiatry Res. 2018;270:992-1000. doi: 10.1016/j.psychres.2018.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutterland AL, Fond G, Kuin A, et al. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta Psychiatr Scand. 2015;132(3):161-179. doi: 10.1111/acps.12423 [DOI] [PubMed] [Google Scholar]
- 19.Snijders GJLJ, van Mierlo HC, Boks MP, et al. The association between antibodies to neurotropic pathogens and bipolar disorder: a study in the Dutch Bipolar (DB) Cohort and meta-analysis. Transl Psychiatry. 2019;9(1):311. doi: 10.1038/s41398-019-0636-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arias I, Sorlozano A, Villegas E, et al. Infectious agents associated with schizophrenia: a meta-analysis. Schizophr Res. 2012;136(1-3):128-136. doi: 10.1016/j.schres.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 21.Nayeri T, Sarvi S, Moosazadeh M, Hosseininejad Z, Amouei A, Daryani A. Toxoplasma gondii infection and risk of attention-deficit hyperactivity disorder: a systematic review and meta-analysis. Pathog Glob Health. 2020;114(3):117-126. doi: 10.1080/20477724.2020.1738153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam AP, de Sordi D, Muller HHO, et al. Aggravation of symptom severity in adult attention-deficit/hyperactivity disorder by latent Toxoplasma gondii infection: a case-control study. Sci Rep. 2020;10(1):14382. doi: 10.1038/s41598-020-71084-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutterland AL, Kuin A, Kuiper B, et al. Driving us mad: the association of Toxoplasma gondii with suicide attempts and traffic accidents—a systematic review and meta-analysis. Psychol Med. 2019;49(10):1608-1623. doi: 10.1017/S0033291719000813 [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroup DF, Berlin JA, Morton SC, et al. ; Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group . Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 26.Wyman CP, Gale SD, Hedges-Muncy A, Erickson LD, Wilson E, Hedges DW. Association between Toxoplasma gondii seropositivity and memory function in nondemented older adults. Neurobiol Aging. 2017;53:76-82. doi: 10.1016/j.neurobiolaging.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cobia D, Perry C, Gale S, et al. Toxoplasma gondii affects posterior association cortex and related functions in healthy, but not schizophrenia, individuals. Schizophr Bull. 2017;43(suppl 1):S141. doi: 10.1093/schbul/sbx023.077 [DOI] [Google Scholar]
- 28.El-Hadidy MA, Elemshaty W, Othman W. Could infection effect cognitive function in schizophrenia? one Egyptian center study. Arab Journal of Psychiatry. 2013;24(2):85-92. doi: 10.12816/0001365 [DOI] [Google Scholar]
- 29.Gajewski PD, Falkenstein M, Hengstler JG, Golka K. Toxoplasma gondii impairs memory in infected seniors. Brain Behav Immun. 2014;36:193-199. doi: 10.1016/j.bbi.2013.11.019 [DOI] [PubMed] [Google Scholar]
- 30.Guenter W, Bielinski M, Deptuła A, et al. Does Toxoplasma gondii infection affect cognitive function? a case control study. Folia Parasitol (Praha). 2012;59(2):93-98. doi: 10.14411/fp.2012.014 [DOI] [PubMed] [Google Scholar]
- 31.Nimgaonkar VL, Yolken RH, Wang T, et al. Temporal cognitive decline associated with exposure to infectious agents in a population-based, aging cohort. Alzheimer Dis Assoc Disord. 2016;30(3):216-222. doi: 10.1097/WAD.0000000000000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torniainen-Holm M, Suvisaari J, Lindgren M, Harkanen T, Dickerson F, Yolken RH. The lack of association between herpes simplex virus 1 or Toxoplasma gondii infection and cognitive decline in the general population: an 11-year follow-up study. Brain Behav Immun. 2019;76:159-164. doi: 10.1016/j.bbi.2018.11.016 [DOI] [PubMed] [Google Scholar]
- 33.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. Oxford University Press; 2006. [Google Scholar]
- 34.Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. 4th ed. Oxford University Press; 2004. [Google Scholar]
- 35.Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203-213. doi: 10.1176/appi.ajp.2007.07010042 [DOI] [PubMed] [Google Scholar]
- 36.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Accessed September 27, 2019. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 37.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 38.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis Version 3. Biostat; 2013. https://www.meta-analysis.com/downloads/Meta-Analysis%20Manual%20V3.pdf [Google Scholar]
- 40.Holub D, Motlova L, Dragomirecka E, et al. Vigilance/sustained attention abnormalities in subjects with latent toxoplasmosis. Psychiatrie. 2008;12(suppl 3):21-25. [Google Scholar]
- 41.Berrett AN, Gale SD, Erickson LD, Brown BL, Hedges DW. Toxoplasma gondii moderates the association between multiple folate-cycle factors and cognitive function in U.S. adults. Nutrients. 2017;9(6):564. doi: 10.3390/nu9060564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ene L, Marcotte TD, Umlauf A, et al. Latent toxoplasmosis is associated with neurocognitive impairment in young adults with and without chronic HIV infection. J Neuroimmunol. 2016;299:1-7. doi: 10.1016/j.jneuroim.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamdani N, Daban-Huard C, Godin O, et al. Effects of cumulative Herpesviridae and Toxoplasma gondii infections on cognitive function in healthy, bipolar, and schizophrenia subjects. J Clin Psychiatry. 2017;78(1):e18-e27. doi: 10.4088/JCP.15m10133 [DOI] [PubMed] [Google Scholar]
- 44.Mendy A, Vieira ER, Albatineh AN, Gasana J. Toxoplasma gondii seropositivity and cognitive functions in school-aged children. Parasitology. 2015;142(9):1221-1227. doi: 10.1017/S0031182015000505 [DOI] [PubMed] [Google Scholar]
- 45.Novotna M, Havlicek J, Smith AP, et al. Toxoplasma and reaction time: role of toxoplasmosis in the origin, preservation and geographical distribution of Rh blood group polymorphism. Parasitology. 2008;135(11):1253-1261. doi: 10.1017/S003118200800485X [DOI] [PubMed] [Google Scholar]
- 46.Stock AK, von Heinegg EH, Kohling HL, Beste C. Latent Toxoplasma gondii infection leads to improved action control. Brain Behav Immun. 2014;37:103-108. doi: 10.1016/j.bbi.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 47.Bayani M, Riahi SM, Bazrafshan N, Gamble HR, Rostami A. Toxoplasma gondii infection and risk of Parkinson and Alzheimer diseases: a systematic review and meta-analysis on observational studies. Acta Trop. 2019;196:165-171. doi: 10.1016/j.actatropica.2019.05.015 [DOI] [PubMed] [Google Scholar]
- 48.Antonelli G, Cutler S. Evolution of the Koch postulates: towards a 21st-century understanding of microbial infection. Clin Microbiol Infect. 2016;22(7):583-584. doi: 10.1016/j.cmi.2016.03.030 [DOI] [PubMed] [Google Scholar]
- 49.Mendy A, Vieira ER, Albatineh AN, Gasana J. Immediate rather than delayed memory impairment in older adults with latent toxoplasmosis. Brain Behav Immun. 2015;45:36-40. doi: 10.1016/j.bbi.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 50.Gale SD, Erickson LD, Thacker EL, Mitchell EL, Brown BL, Hedges DW. Toxoplasma gondii seropositivity and serointensity and cognitive function in adults. PLoS Negl Trop Dis. 2020;14(10):e0008733. doi: 10.1371/journal.pntd.0008733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Groer MW, Yolken RH, Xiao JC, et al. Prenatal depression and anxiety in Toxoplasma gondii–positive women. Am J Obstet Gynecol. 2011;204(5):433.e1-433.e7. doi: 10.1016/j.ajog.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Havlícek J, Gasova ZG, Smith AP, Zvara K, Flegr J. Decrease of psychomotor performance in subjects with latent ‘asymptomatic’ toxoplasmosis. Parasitology. 2001;122(Pt 5):515-520. doi: 10.1017/S0031182001007624 [DOI] [PubMed] [Google Scholar]
- 53.Markkula N, Lindgren M, Yolken RH, Suvisaari J. Association of exposure to Toxoplasma gondii, Epstein-Barr virus, herpes simplex virus type 1 and cytomegalovirus with new-onset depressive and anxiety disorders: an 11-year follow-up study. Brain Behav Immun. 2020;87:238-242. doi: 10.1016/j.bbi.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 54.Henriquez SA, Brett R, Alexander J, Pratt J, Roberts CW. Neuropsychiatric disease and Toxoplasma gondii infection. Neuroimmunomodulation. 2009;16(2):122-133. doi: 10.1159/000180267 [DOI] [PubMed] [Google Scholar]
- 55.Haroon F, Handel U, Angenstein F, et al. Toxoplasma gondii actively inhibits neuronal function in chronically infected mice. PLoS One. 2012;7(4):e35516. doi: 10.1371/journal.pone.0035516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao J. Toxoplasma-induced behavioral changes: an aspecific consequence of neuroinflammation. Trends Parasitol. 2020;36(4):317-318. doi: 10.1016/j.pt.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 57.McConkey GA, Martin HL, Bristow GC, Webster JP. Toxoplasma gondii infection and behaviour—location, location, location? J Exp Biol. 2013;216(Pt 1):113-119. doi: 10.1242/jeb.074153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, McConkey GA. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One. 2011;6(9):e23866. doi: 10.1371/journal.pone.0023866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skallova A, Kodym P, Frynta D, Flegr J. The role of dopamine in Toxoplasma-induced behavioural alterations in mice: an ethological and ethopharmacological study. Parasitology. 2006;133(Pt 5):525-535. doi: 10.1017/S0031182006000886 [DOI] [PubMed] [Google Scholar]
- 60.Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS One. 2009;4(3):e4801. doi: 10.1371/journal.pone.0004801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67(1):53-83. doi: 10.1016/S0301-0082(02)00011-4 [DOI] [PubMed] [Google Scholar]
- 62.Murphy BL, Arnsten AF, Jentsch JD, Roth RH. Dopamine and spatial working memory in rats and monkeys: pharmacological reversal of stress-induced impairment. J Neurosci. 1996;16(23):7768-7775. doi: 10.1523/JNEUROSCI.16-23-07768.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11(2):151-162. doi: 10.1177/026988119701100208 [DOI] [PubMed] [Google Scholar]
- 64.Backman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci Biobehav Rev. 2010;34(5):670-677. doi: 10.1016/j.neubiorev.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 65.Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69(6):375-390. doi: 10.1016/S0301-0082(03)00085-6 [DOI] [PubMed] [Google Scholar]
- 66.Miller CM, Boulter NR, Ikin RJ, Smith NC. The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol. 2009;39(1):23-39. doi: 10.1016/j.ijpara.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 67.Sublette ME, Galfalvy HC, Fuchs D, et al. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav Immun. 2011;25(6):1272-1278. doi: 10.1016/j.bbi.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steiner J, Bogerts B, Sarnyai Z, et al. Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. World J Biol Psychiatry. 2012;13(7):482-492. doi: 10.3109/15622975.2011.583941 [DOI] [PubMed] [Google Scholar]
- 69.Erhardt S, Lim CK, Linderholm KR, et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. 2013;38(5):743-752. doi: 10.1038/npp.2012.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barake M, Evins AE, Stoeckel L, et al. Investigation of impulsivity in patients on dopamine agonist therapy for hyperprolactinemia: a pilot study. Pituitary. 2014;17(2):150-156. doi: 10.1007/s11102-013-0480-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Severance EG, Yolken RH, Eaton WW. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: more than a gut feeling. Schizophr Res. 2016;176(1):23-35. doi: 10.1016/j.schres.2014.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang S, El-Fahmawi A, Christian DA, et al. Infection-induced intestinal dysbiosis is mediated by macrophage activation and nitrate production. mBio. 2019;10(3):00935-19. doi: 10.1128/mBio.00935-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol. 2014;817:373-403. doi: 10.1007/978-1-4939-0897-4_17 [DOI] [PubMed] [Google Scholar]
- 74.Severance EG, Xiao J, Jones-Brando L, et al. Toxoplasma gondii—a gastrointestinal pathogen associated with human brain diseases. Int Rev Neurobiol. 2016;131:143-163. doi: 10.1016/bs.irn.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Estato V, Stipursky J, Gomes F, et al. The neurotropic parasite Toxoplasma gondii induces sustained neuroinflammation with microvascular dysfunction in infected mice. Am J Pathol. 2018;188(11):2674-2687. doi: 10.1016/j.ajpath.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 76.Parlog A, Schluter D, Dunay IR. Toxoplasma gondii–induced neuronal alterations. Parasite Immunol. 2015;37(3):159-170. doi: 10.1111/pim.12157 [DOI] [PubMed] [Google Scholar]
- 77.Colzato L, Zhang W, Beste C, Stock AK. Dissociating direct and indirect effects: a theoretical framework of how latent toxoplasmosis affects cognitive profile across the lifespan. Neurobiol Aging. 2021;102:119-128. doi: 10.1016/j.neurobiolaging.2021.02.007 [DOI] [PubMed] [Google Scholar]
- 78.Xiao J, Yolken RH. Strain hypothesis of Toxoplasma gondii infection on the outcome of human diseases. Acta Physiol (Oxf). 2015;213(4):828-845. doi: 10.1111/apha.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Azami SJ, Rahimi HM, Mirjalali H, Zali MR. Unravelling Toxoplasma treatment: conventional drugs toward nanomedicine. World J Microbiol Biotechnol. 2021;37(3):48. doi: 10.1007/s11274-021-03000-x [DOI] [PubMed] [Google Scholar]
- 80.Xiao J, Buka SL, Cannon TD, et al. Serological pattern consistent with infection with type I Toxoplasma gondii in mothers and risk of psychosis among adult offspring. Microbes Infect. 2009;11(13):1011-1018. doi: 10.1016/j.micinf.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 81.Flegr J, Novotna M, Lindova J, Havlicek J. Neurophysiological effect of the Rh factor: protective role of the RhD molecule against Toxoplasma-induced impairment of reaction times in women. Neuro Endocrinol Lett. 2008;29(4):475-481. [PubMed] [Google Scholar]
- 82.El Mouhawess A, Hammoud A, Zoghbi M, et al. Relationship between Toxoplasma gondii seropositivity and schizophrenia in the Lebanese population: potential implication of genetic polymorphism of MMP-9. BMC Psychiatry. 2020;20(1):264. doi: 10.1186/s12888-020-02683-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang AW, Avramopoulos D, Lori A, et al. Genome-wide association study in two populations to determine genetic variants associated with Toxoplasma gondii infection and relationship to schizophrenia risk. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:133-147. doi: 10.1016/j.pnpbp.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 84.Li Y, Weber NS, Fisher JA, et al. Association between antibodies to multiple infectious and food antigens and new onset schizophrenia among US military personnel. Schizophr Res. 2013;151(1-3):36-42. doi: 10.1016/j.schres.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 85.Krause D, Matz J, Weidinger E, et al. The association of infectious agents and schizophrenia. World J Biol Psychiatry. 2010;11(5):739-743. doi: 10.3109/15622971003653246 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Search Strategy
eMethods 2. Aggregation of Results
eTable 1. Aggregation of Tests in Cognitive Domains
eTable 2. Insufficient Reported Outcome Measures
eTable 3. Newcastle-Ottawa Scale Quality Assessment: Male and Female Participants Combined
eTable 4. Newcastle-Ottawa Scale Quality Assessment: Male and Female Participants Separated
eTable 5. Results From Meta-analyses and Subgroup Analysis
eTable 6. Results From Meta-regression Analyses
eTable 7. Meta-regression Analysis Dichotomized by Mean Age
eFigure 1. Forest Plot for Short-term Verbal Memory
eFigure 2. Forest Plot for Processing Speed Without Ene et al Study (Low Quality)
eFigure 3. Forest Plot for Executive Functioning Without Ene et al Study (Low Quality)
eFigure 4. Forest Plot for Executive Functioning Without Nimgaonkar et al Study
eFigure 5. Funnel Plot for Processing Speed
eFigure 6. Funnel Plot for Working Memory
eFigure 7. Funnel Plot for Short-term Verbal Memory
eFigure 8. Funnel Plot for Executive Functioning
eFigure 9. Meta-regression Analysis of Processing Speed: Mean Age
eFigure 10. Meta-regression Analysis of Processing Speed: Study Quality
eFigure 11. Meta-regression Analysis of Processing Speed: Seropositivity Cutoff
eFigure 12. Meta-regression Analysis of Working Memory: Mean Age
eFigure 13. Meta-regression Analysis of Working Memory: Seropositivity Cutoff
eFigure 14. Meta-regression Analysis of Short-term Verbal Memory: Mean Age
eFigure 15. Meta-regression Analysis of Executive Functioning: Mean Age
eFigure 16. Meta-regression Analysis of Executive Functioning: Study Quality
eFigure 17. Meta-regression Analysis of Executive Functioning: Seropositivity Cutoff
eReferences