Abstract
RNA modification is a type of post-transcriptional modification that regulates important cellular pathways, such as the processing and metabolism of RNA. The most abundant form of methylation modification is RNA N6-methyladenine (m6A), which plays various post-transcriptional regulatory roles in cellular biological functions, including cell differentiation, embryonic development and disease occurrence. Bones play a pivotal role in the skeletal system as they support and protect muscles and other organs, facilitate movement and ensure haematopoiesis. The development and remodelling of bones require a delicate and accurate regulation of gene expression by epigenetic mechanisms that involve modifications of histone, DNA and RNA. The present review discusses the enzymes and proteins involved in mRNA m6A methylation modification and summarises current research progress and the mechanisms of mRNA m6A methylation in common orthopaedic diseases, including osteoporosis, arthritis and osteosarcoma.
Keywords: N6-methyladenine, methyltransferase, demethylase, reader proteins, common orthopaedic diseases
1. Introduction
The central dogma is one of the most important basic laws in modern biology that reveals the process by which genetic information is conveyed from DNA to RNA through transcription, and subsequently to protein through translation (1). Recent advancements in genetic technologies have revealed that genetic information encoded in the genome is not only an alignment of base sequences but is also associated with other multifarious levels of regulation, such as DNA methylation and histone modification (2). The discovery of these epigenetic research results has driven the central dogma. Increasing evidence suggest that RNA plays a vital role in the central dogma (3). Genetic material is regulated at various levels, in addition to DNA and RNA levels (4). RNA modification is a type of post-transcriptional modification that controls important pathways, such as RNA processing and metabolism (5). With the advancements in sequencing technology, the dynamics of RNA modification and its biological functions have received considerable attention from scientists and have become new research hotspots in the field of epigenetics (6-9).
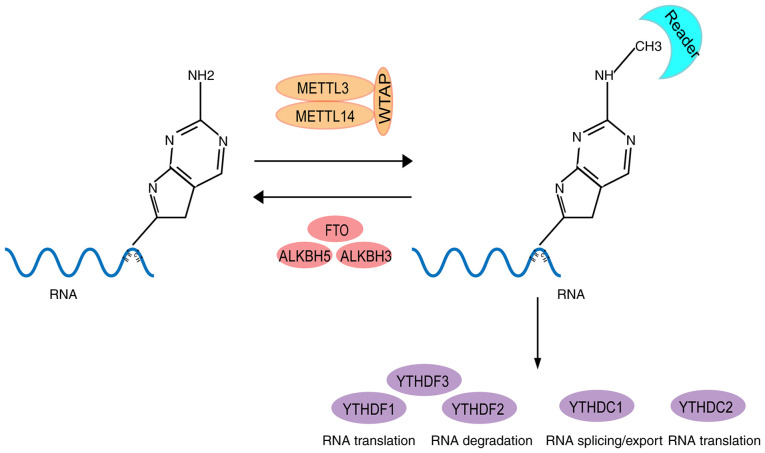
RNA methylation has been extensively identified in vertebrates, plants, yeasts, bacteria, archaea and viruses (10), and N6-methyladenine (m6A) is the most common form of RNA methylation (11). Generally, m6A modifications occur at the conserved RRACH motif (R=G or A; H=A, C or U) and are concentrated near the stop codons of mRNAs (12) (Fig. 1). Notably, with the development of enzymatic technology, m6A modification enzymes have been identified, among which, methyltransferase-like (METTL)3, METTL14 and Wilms' tumor 1-associating protein (WTAP) complexes are the main m6A methyltransferases (13-15), whereas fat-mass and obesity-associated protein (FTO) and α-ketoglutarate-dependent dioxygenase alkB homologue 5 (ALKBH5) are demethylases (16,17) (Fig. 1). Currently, m6A is the most studied RNA modification (18). m6A modification plays various post-transcriptional regulatory roles, including transcription regulation, selective splicing, stabilization and translation of RNA, by binding proteins that contain the YTH domain (19) (Fig. 1). In addition, m6a modifications regulate cellular biological functions involved in cell differentiation, embryonic development and disease occurrence (20).
Figure 1.
Dynamic regulation of RNA m6A levels by m6A and the known functions of m6A in regulation of RNA metabolism. m6A modification is a dynamic and reversible process. m6A modifications are catalysed by the methyltransferase complex, which consists of METTL3, METTL14 and WTAP (writers) and can be removed by demethylases, FTO and ALKBH5 (erasers). m6A modifications are functionally facilitated by the m6A binding proteins, YTHDF1-3 and YTHDC1-2 (readers), which leads to changes in RNA splicing, RNA stability and RNA nuclear export. m6A, RNA N6-methyladenine; METTL, methyltransferase-like; WTAP, Wilms' tumor 1-associating protein; FTO, fat-mass and obesity-associated protein; ALHBH, α-ketoglutarate-dependent dioxygenase alkB homologue; YTHDF, YTH domain family; YTHDC, YTH domain containing.
The discovery of m6A methyltransferases and demethylases proved that RNA modification is dynamic and reversible, and promoted the study of RNA modifications from micro-regulation mechanisms to epitranscriptome levels (21). However, other modification forms, including 5-methylcytosine (22) and 1-methyladenine (23) are currently at initial stages, and their modification enzymes, dynamic regulation and biological functions require further studies and development.
The main function of bones is to provide support and protection (24-26). However, bones also have other important functions, including facilitation of movement, haematopoiesis and formation of reservoirs of minerals, such as calcium (27,28). The development and remodelling of bones require an accurate regulation of gene expression in bone cells, a process that is affected by epigenetic mechanisms, such as histone modification, DNA methylation and RNA methylation (29,30). Previous studies have reported that the disruption of epigenetic processes in bone cells can notably influence the function and activity, and contribute to the pathogenesis of bone-associated diseases (31-34). The present review summarizes the latest research concerning m6A and discusses the newly identified roles that m6A plays in common orthopaedic diseases.
2. Enzymes and proteins involved in modification by m6A
Writers METTL3
Increasing evidence suggest that modification by m6A is mediated by methyltransferase complexes that are composed of several proteins (35). METTL3 was the first m6A methyltransferase that was identified (36). Methyltransferase MT-A70 was identified as part of a large protein complex that was isolated from enzymatic mammalian cell nuclear extracts (37), and is the leading catalytic enzyme of the m6A system (9,38). METTL3 contains two Cys-Cys-Cys-His (CCCH)-type zinc finger motifs at the N-terminus and one catalytic motif [D/N/S/H]PP(Y/F/W)] in the methyltransferase domain (39,40). It has functions in all the stages of the RNA lifecycle, including pre-mRNA splicing (41), nuclear export (17), translation regulation (42), mRNA decay (43) and microRNA (miRNA/miR) processing (44). Generally, METTL3 forms a stable heterodimeric complex with METTL14, which is another important methyltransferase of m6A (45), with the help of other components of writers. This methylation was reported to occur at the N6 position of adenosine on mRNA and was speculated to be a co-transcriptional methylation (46). Notably, disruption of METTL3 homologs results in severe developmental defects in yeasts, and METTL3 homologs have lethal phenotypes in Arabidopsis and mice (47-50). Recent evidence suggested that METTL3 is upregulated and plays an oncogenic role involving increased m6A expression levels in different types of cancer, such as bladder cancer (51), lung cancer (52), colorectal cancer (53), glioma (54), breast cancer (55), leukaemia (56) and other cancers including gastric cancer and melanoma (57,58). However, other studies have demonstrated opposing results from the same types of cancer (59-61). The potential molecular mechanisms have been investigated and include the regulation of downstream non-coding RNAs (62), modulation of miRNA processing via DGCR8(51), regulation of apoptosis (57) and regulation of the PI3K/AKT pathway (62,63).
METTL14
Another core writer is METTL14. Despite having ~22% sequence identity and an almost identical topological structure with the methyltransferase domains of METTL3, METTL14 is considered a pseudomethyltransferase (39,45). The function of METTL14 in the complex remains unclear. In a surface electrostatic potential analysis, RNA binding affinity and methyltransferase activity were revealed to moderately decrease in the complex with double mutations in K297E and R298E, suggesting that METTL14 may be involved in RNA interaction; however, the binding sites of S-adenosylmethionine and the DPPW functional domains of METTL14 are responsible for the catalysis of m6A formation (64). METTL14 is a vital member of the m6A methyltransferase complex (65). Notably, several studies have demonstrated that METTL3 and METTL14 are associated with each other (14,45,66). Once METTL3 and METTL14 function individually, they exhibit nearly undetectable methyltransferase activity, whereas the METTL3-METTL14 complex displays methyltransferase activity (14). However, whether METTL14 exhibits methyltransferase activity after binding to additional factors remains unknown. METTL14 is also involved in the regulation of several tumour processes (67,68). For example, the METTL14-m6A-Notch1 pathway plays a critical role in bladder tumorigenesis and bladder tumour-initiating cells (69). In haematopoietic stem cells and liver tumour cells, METTL14 is downregulated and attenuates the tumorigenesis of acute myeloid leukaemia (AML) (70).
WTAP
Another component of the human m6A methyltransferase complex is the WTAP, which plays a critical role in m6A formation via a mechanism that differs from the mechanisms observed in METTL3 and METTL14(71). In a WTAP knockdown study, global m6A levels in human cell lines were reported to be markedly decreased, which indicates its significance in producing a distinct landscape of mRNA methylation (72). WTAP predominantly acts as a regulatory subunit that initially binds to target RNA and subsequently recruits dimers formed by the catalytic subunits, METTL3 and METTL14, to perform catalytic functions owing to a lack of a catalytic domain (71). Furthermore, METTL3 levels were revealed to be important to WTAP protein homoeostasis, whereby METTL3 regulates WTAP expression at various levels via different mechanisms, including mRNA translation and stabilization (73). Taken together, these findings suggest that the components of the methyltransferase complex are essential in the formation of m6A. In addition, WTAP is overexpressed in different types of tumours, including AML (15,74,75), and interacts with different proteins associated with cell proliferation and RNA processing (75). Notably, disruption of WTAP results in embryonic lethality, which is indicative of its vital biological function in the development of vertebrates (76).
Zinc finger CCCH domain-containing protein 13 (ZC3H13)
In addition to the core complex of METTL3, METTL14 and WTAP, several other proteins have been implicated in regulating RNA m6A. For example, Virilizer and Hakai were identified as components associated with WTAP in mammalian cells. Endogenous protein complexes from different species in metazoans were studied via quantitative mass spectrometry, and ZC3H13-WTAP-Virilizer-Hakai was identified as an evolutionarily conserved complex (77). ZC3H13 plays a critical role in anchoring WTAP, Virilizer and Hakai in the nucleus to facilitate m6A methylation, and regulates mESC self-renewal (77). ZC3H13 also participates in the development and progression of different types of tumours (68,78). It has been reported that ZC3H13 expression is substantially downregulated in clear cell renal cell carcinoma tissues (79). Conversely, ZC3H13 expression is substantially upregulated in colon adenocarcinoma tumour tissues compared with adjacent mucosa (80).
Erasers FTO
FTO, also known as ALKBH9, was identified as the first RNA demethylase in 2011(81). The discovery of FTO resulted in the identification of m6A functions in a reversible and dynamic mode (82). The regulation of body mass and obesity was identified to be the primary function of FTO, as overactivation of FTO was reported to increase food intake and result in obesity, whereas an FTO disorder was reported to cause growth retardation (83,84). Increasing evidence suggest that FTO dysfunction can contribute to the development of cancer. For example, FTO expression is upregulated in breast cancer, which promotes breast cancer cell proliferation (85). FTO expression is also upregulated in hepatocellular carcinoma (HCC) tissues, which is associated with poor patient prognosis (16). Furthermore, FTO has been associated with AML (86), melanoma (87) and lung cancer (88).
ALKBH5
ALKBH5 is another m6A demethylase (89), the function of which remains partly unknown. Both FTO and ALKBH5 belong to the α-ketoglutarate-dependent dioxygenase family, which demethylate m6A in an Fe (II)- and α-ketoglutaric acid-dependent manner (90,91). Several studies have reported that ALKBH5 affects the pathogenesis and development of diseases, such as ALKBH5-deficiency, which results in testis atrophy and reduction in sperm number and motility (17,92,93). ALKBH5 is overexpressed in glioblastoma stem-like cells (GSCs), which maintains GSC tumorigenicity (94), and is involved in the proliferation and metastasis of non-small cell lung cancer (95). In addition, ALKBH5 affects the m6A levels of lncNEAT1, and subsequently promotes enhancer of zeste homolog 2 expression in gastric cancer invasion and metastasis (96).
ALKBH3
Recently, ALKBH3 was identified as another m6A demethylase (97). ALKBH3 has substrate specificity for N6-meA, 1-meA, and 3-meC, and ALKBH3-mediated tRNA demethylation has been reported to increase protein translation efficiency (98). With similar functions as other ‘eraser’ proteins, ALKBH3 mediates RNA demethylation and subsequently exerts effects on protein synthesis in cancer cells, thereby influencing tumour development and progression (99). However, further studies are required to determine substrate preference of ALKBH3 for different RNA types.
Readers
The reversible and dynamic regulation of m6A modification is mediated by the functional interaction between m6A writers and erasers; however, to identify downstream biological functions, m6A must be recognised by several readers (46,100). Several YTH domain family (YTHDF) members, including YTHDF1, YTHDF2, YTHDF3, YTH domain containing (YTHDC)1 and YTHDC2, have been identified as general ‘reader’ proteins (43).
YTHDF2
YTHDF2 was identified as the first m6A reader (101). YTHDF2 selectively recognises m6A and regulates mRNA degradation through its C-terminal region; more than 3,000 cellular RNA targets of YTHDF2, including mRNAs and non-coding RNAs, were identified (43). The binding sites of YTHDF2 are mainly in the 3'-untranslated region that is rich in GAC sequence, which is consistent with the distribution characteristics of m6A (102). Furthermore, the N-terminal region of YTHDF2 reportedly recruits the CCR4-NOT adenosylase complex, thus accelerating the degradation of substrate RNA (103). YTHDF2 has also been demonstrated to play essential roles in diverse biological processes, such as neural development, cancer progression, maternal mRNA clearance, haematopoietic stem cell expansion and male fertility (104-107).
YTHDF1
Unlike YTHDF2, YTHDF1 can bind to m6A sites around the stop codons in mRNAs (108). YTHDF1 specifically binds to m6A-containing mRNAs and accelerates cap-dependent translation by recruiting eIF3, eIF4E, eIF4G, PABP and the 40S ribosomal subunit (108). As a ‘reader’ protein, YTHDF1 is also involved in several biological processes such as enhances protein synthesis and regulate Pulmonary Hypertension (42,109). A previous study demonstrated that YTHDF1 promotes the translation of mA-methylated neuronal mRNAs, which contributes to learning and memory (110). YTHDF1 also plays a role in the malignant nature of cancer, whereby patients with colorectal cancer and upregulated YTHDF1 expression have a considerably poor prognosis (111). The cell cycle progression and metabolism of HCC are also regulated by YTHDF1(112).
YTHDF3
YTHDF3 shares >65% protein sequence identity with YTHDF1 and YTHDF2(12). In addition, YTHDF3 can interact with YTHDF1 and YTHDF2 to enhance the binding ability of YTHDF1 or YTHDF2 to RNA-containing m6A modified substrates, and thereby promote translation or degradation (107). YTHDF3 can also interact with several cellular proteins such as PABP1 and eIF4G2 to exert cell-specific regulatory functions (113). A study revealed that YTHDF3 can target YAP and participate in YAP signalling, which facilitates m6A-modified long non-coding RNA (lncRNA) GAS5 degradation, and provides insight on CRC progression (114). Furthermore, YTHDF3 is involved in the viral life cycle as it hampers interferon-dependent antiviral responses by accelerating the translation of FOXO3, and is considered a regulator of HIV (113,115).
YTHDC1
Unlike YTHDF1, YTHDF2 and YTHDF3, which are located in the cytoplasm, YTHDC1 is located in YT bodies adjacent to nuclear speckles in nucleus (116). YTHDC1 cooperates with nuclear RNA export factor 1 and the three prime repair exonuclease mRNA export complex by interacting with SRSF3 and exports m6A-methylated mRNAs from the nucleus (116). YTHDC1 interacts with metadherin and affects cancer development and progression (117). YTHDC1 also plays essential roles in the development of spermatogonia in males and the growth and maturation of oocytes in females (118).
YTHDC2
YTHDC2, the only RNA helicase-containing and multi-domain m6A reader, has been demonstrated to exhibit ATP-dependent RNA helicase activity (119,120). YTHDC2 improves the translation efficiency of target mRNA by interacting with meiosis-specific coiled-coil domain and 5'-3'exoribonuclease 1 after recognising m6A (121). A previous study reported that YTHDC2 affects the expression of drug-metabolizing P450 isoforms by mediating CYP2C8 mRNA degradation (122). YTHDC2 also plays a conserved role in mouse germ cell fate transition (123). In addition, YTHDC2 contributes to the metastasis of colon tumours and the proliferation of Huh7 HCC cells (120,124).
Other m6A readers
In addition to the members of the YTHDF, other proteins that act as m6A readers have been identified. The RNA-binding protein, HNRNPA2B1, binds to m6A-modifying RNAs, and its biochemical footprint is consistent with that of the m6A consensus motif (125). HNRNPA2B1 binds to m6A in subsets of primary miRNA transcripts, interacts with the DGCR8 microprocessor complex protein and increases primary miRNA processing (125). In addition, insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs), including IGF2BP1/2/3, can recognise m6A and improve the stability and translation of mRNAs in an m6A-dependent manner; however, this mechanism requires further investigation (126).
3. m6A and osteoporosis
METTL3 and osteoporosis
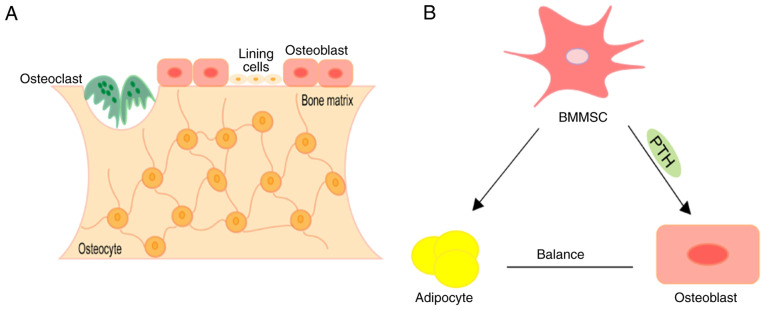
Osteoporosis is the most common bone disorder worldwide, which affects >200 million people, and is characterised by decreased bone mineral density (BMD) and increased risk of osteoporotic fracture (127,128). The main pathological changes of osteoporosis are characterised by low bone mass and excessive accumulation of adipose tissue in the bone marrow milieu (129), and bone homoeostasis plays an essential role in the pathogenesis of osteoporosis (11). Bone homoeostasis is mainly maintained by osteoblasts, osteocytes and osteoclasts (130) (Fig. 2A). Osteoblasts produce bone by synthesising extracellular matrix containing various proteins, particularly type I collagen (131,132). The extracellular matrix is deposited as osteoid and is subsequently mineralized through the accumulation of calcium phosphate in the form of hydroxyapatite (133). Conversely, osteoclasts, through the secretion of hydrochloric acid and proteolytic enzymes, can dissolve minerals and lysis the bone matrix (134). Osteocytes are the main cellular component of bone tissue (135). Osteocytes control bone homoeostasis by maintaining the balance between the function of bone-forming osteoblasts and bone-resorbing osteoclasts (136). The common progenitors for osteoblasts and marrow adipocytes are bone marrow mesenchymal stem cells (BMMSCs) (137). The osteogenic and adipogenic differentiation of BMMSCs must maintain balance under accurate spatio-temporal control to defend skeletal health (138) (Fig. 2B). With ageing or other pathological stimuluses, BMMSCs have a disposition to differentiate into adipocytes, leading to the ascendent in marrow adiposity and gradual bone loss (139,140). The variations in bone micro-architecture result in elevated skeletal fragility and an inclination to fracture (141). Recently, several studies have revealed different molecular mechanisms associated with osteoporosis, which are associated with m6A modification (142,143).
Figure 2.
Representation of bone structure and differentiation of BMMSCs. (A) During bone remodelling, osteoclasts derived from hematopoietic stem cells resorb old or damaged bone. Osteoblasts derived from mesenchymal stem cells are recruited to damaged areas to replace bone removed by osteoclasts. Osteocytes derived from osteoblasts suspend their activity when embedded in the bone matrix. (B) Mesenchymal stromal cells can differentiate into osteoblasts or adipocytes and maintain balance under accurate spatio-temporal controls to defend skeletal health. BMMSCs, bone marrow mesenchymal stem cells; PTH, parathyroid hormone.
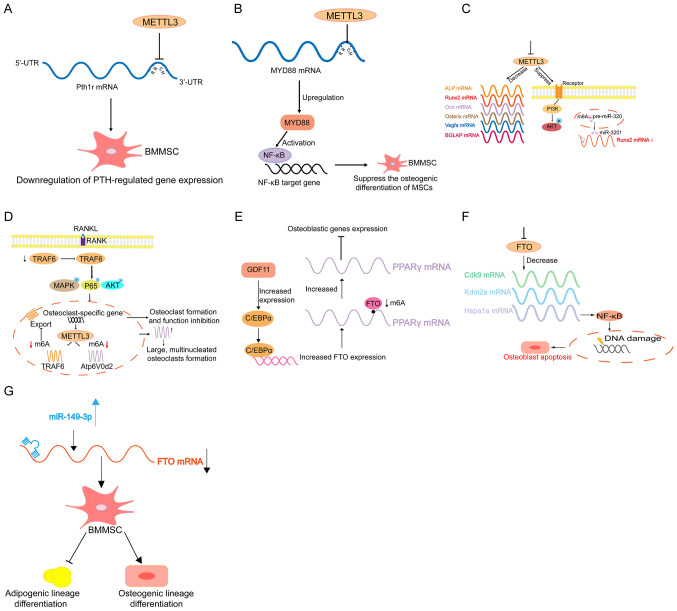
A previous study revealed that the disruption of Mettl3 in bone marrow mesenchymal stem cells (MSCs) induces pathological features of osteoporosis in mice (143). It was demonstrated that the disfunction form of METTL3 resulted in destroyed bone formation, abnormal osteogenic differentiation and improved marrow adiposity (143). However, overexpression of METTL3 in BMMSCs protects mice against osteoporosis caused by oestrogen deficiency. Mechanistically, the PTH/Pth1r signalling axis is the target downstream pathway for m6A regulation in BMMSCs (143) (Fig. 3A). However, it has also been reported that METTL3 upregulates MYD88 expression by enhancing m6A modification to MYD88-RNA, consequently leading to the activation of NF-κB, which is a repressor of osteogenesis, and inhibits osteogenic progression (144) (Fig. 3B). Furthermore, METTL3 expression increases in BMMSCs undergoing osteogenic induction, and disruption of METTL3 downregulates the expression of bone formation-related genes, such as Runx2 and Osterix (143). In addition, following METTL3 knockdown, the alkaline phosphatase activity and mineralised nodule formation also decline (145). Vascular endothelial growth factor (VEGF) plays important roles in bone formation and endothelial development (146,147). It has been reported that METTL3 knockdown decreases VEGFA expression, as well as the expression level of its splice variants, including VEGFA-164 and VEGFA-188 in BMMSCs (145). Another study revealed that METTL3 disruption decreases m6A methylation levels and hampers osteogenic differentiation of BMMSCs, thus decreasing bone mass. In addition, METTL3 disorder can affect m6A methylation of RUNX2 and precursor (pre-)miR-320 (Fig. 3C) (148). METTL3 disruption suppresses this process and thus disturbs the normal osteogenic differentiation, which results in osteoporosis (148). Recently, a study reported that METTL3 expression increases during osteoclast differentiation, whose deficiency results in increased size but decreased bone-resorbing ability of osteoclasts through the mechanism involving Atp6v0d2 mRNA degradation mediated by YTHDF2 and TRAF6 mRNA nuclear export (149). This suggests that METTL3 may contribute to osteoporosis by regulating osteoclast differentiation (Fig. 3D).
Figure 3.
Schematic model of m6A in regulating osteoporosis. (A) METTL3 knockout decreases the translation efficiency of BM. BMMSCs lineage allocator Pth1r and disrupts PTH-induced osteogenic and adipogenic responses. (B) METTL3 positively regulates the expression of MYD88 by facilitating the modification of m6A methylation to MYD88-RNA and subsequently induces the activation of NF-κB to suppress osteogenic progression. (C) METTL3 deficiency results in decreased expression levels of RUNX2, Osterix, Ocn, VEGFA, BGLAP, and ALP, and suppresses the PI3K-Akt signalling pathway. METTL3 silencing also decreases RUNX2 mRNA levels through the suppression of the m6A of precursor (pre-)miR-320, which targets RUNX2. (D) METTL3 knockdown causes the retention of TRAF6 mRNA in the nucleus, which results in the inactivation of RANKL-induced signalling pathways, suppression of osteoclast-specific gene expression and inhibition of osteoclast formation and function. METTL3 knockdown upregulates Atp6v0d2 mRNA expression and stability and leads to the formation of large, multinucleated osteoclasts. (E) GDF11 upregulates C/EBPα to promote the expression of FTO during osteoporosis. Increased FTO levels results in the demethylation of Pparg mRNA and leads to an increase in Pparg mRNA levels, which affect the differentiation of BMMSCs (10). Disruption of FTO leads to changes in the transcripts of Hspa1a and other genes in the DNA repair pathway in osteoblasts. (F) FTO-deficiency-mediated downregulation of Hspa1a in osteoblasts activates the NF-κB signalling pathway and results in the increased susceptibility of osteoblasts genotoxic agents and increased rates of apoptosis. (G) miR-149-3p represses the expression of FTO genes by binding to the 3'-UTR of the FTO mRNA to decrease the adipogenic differentiation potential of BMMSCs and increase osteogenic differentiation potential. m6A, RNA N6-methyladenine; METTL, methyltransferase-like; BMMSCs, bone marrow mesenchymal stem cells; NF, nuclear factor; Runx2, runt-related transcription factor 2; VEGF, vascular endothelial factor; ALP, alkaline phosphatase; miR, microRNA; FTO, fat-mass and obesity-associated protein; UTR, untranslated region.
Arginine-316 in human FTO corresponds to Arginine-313 in mice, which is essential for FTO catalytic activity (150). A previous study reported that FTO R313A/R313A mice not only decreased the body and bone length, which was associated with a substantial reduction in BMD and bone mineral content (BMC), but also notably abated the alkaline phosphatase activity, indicating osteoblast function (151). Peroxisome proliferator-activated receptor γ (PPARγ) is a transcriptional factor that maintains the balance between adipocyte and osteoblast differentiation from BMMSCs, that is, accelerating the differentiation of adipocytes and inhibiting osteoblast differentiation (152). It has been reported that PPARγ mRNA is targeted and demethylated by FTO, which upregulates PPARγ mRNA expression, eventually promoting the differentiation of osteoporotic BMMSCs to adipocytes, and decreases bone formation in the process of osteoporosis (153) (Fig. 3E). Another study revealed that regardless of the mouse models lacking FTO globally or selectively, both exhibited age-related decreases in bone volume, in both the trabecular and cortical compartments (154). The mechanism demonstrated that FTO disruption in osteoblasts changes the Hsp70 transcripts (Hspa1a) and other genes involved in the DNA repair pathway containing conserved m6A motifs required for demethylation by FTO, thus affecting osteoblasts (154) (Fig. 3F). Furthermore, several FTO single nucleotide polymorphisms (SNPs) are associated with BMD variations in Chinese populations (155). In addition, miR-149-3p inhibits the differentiation of the adipogenic lineage and enhances the differentiation of the osteogenic lineage by targeting FTO (156) (Fig. 3G). Thus, FTO may be a novel candidate for osteoporosis (156).
Considering that inflammatory factors can hamper osteogenesis, studies have aimed to investigate the association between m6A, inflammation and osteoporosis. Several cytokines have been identified that participate in the development of osteoporosis, including RANKL, colony-stimulating factor (CSF)-1, interleukin-34(157) and granulocyte-macrophage-CSF (158). A study revealed that osteoblast differentiation and Smad-dependent signalling is inhibited after disrupting METTL3 by stabilising Smad7 and Smurf1 mRNA transcripts, which is mediated by YTHDF2(159). In addition, the production of proinflammatory cytokines increases following METTL3 deficiency by activating the MAPK signalling pathway to promote the osteoblast inflammatory response (60). Furthermore, YTHDF2 knockdown enhances the LPS-induced expression levels of IL-6, tumor necrosis factor (TNF)-α, IL-1β and IL-12, which contributes to bone inflammation and osteoporosis through sophisticated mechanisms (160,161).
4. m6A and arthritis
Arthritis is an inflammatory disease that occurs in several human joints and surrounding tissues. It is classified into dozens of diseases that are triggered by multiple pathological conditions, such as inflammation, infection, degeneration and trauma (162). The main clinical characteristics of arthritis include red, swollen, hot, painful, dysfunctional and deformed joints, which all result in joint disability and decreased quality of life (163). Osteoarthritis (121) and rheumatoid arthritis (32) are the most common types of arthritis with different pathophysiological mechanisms and exhibit similar clinical features. The role of epigenetics, particularly of RNA modification in arthritis, has attracted great interest.
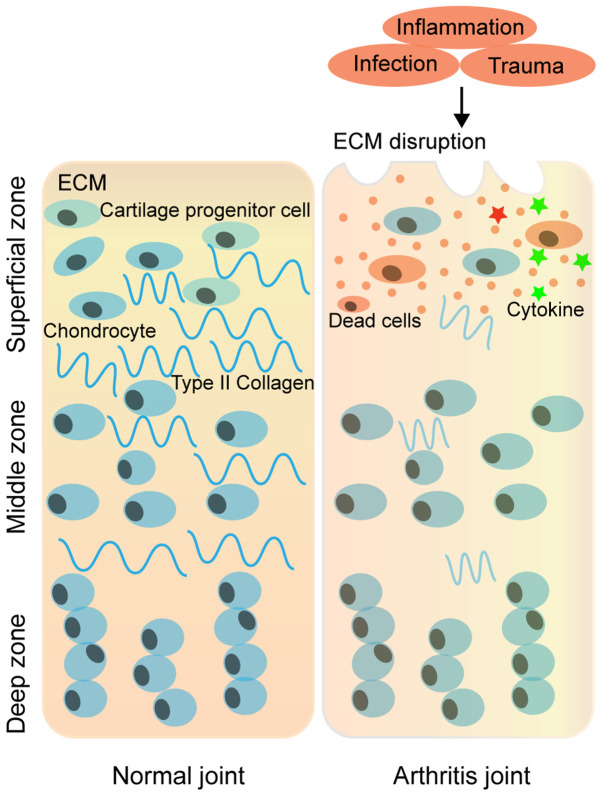
OA is the most common chronic joint disease characterised by pain, stiffness and mobility difficulties (164). OA is caused by a complex interaction among diverse molecular factors involved in integrity, genetic susceptibility, local inflammation, mechanical forces and other cellular biochemical processes. The most probable cause of OA is damage to articular cartilage via physical forces (165,166). Damage to articular cartilage may lead to degenerative OA in which several factors, including inflammatory response to various components of cartilage, are involved (167-170). Progressive cartilage degeneration is involved in chondrocyte reduction and in the variation of molecular components of chondrocytes in self-synthetized extracellular matrix (ECM) in the process of OA development (171) (Fig. 4). A study reported that following treatment of ATDC5 cells with IL-1β, both the abundance of METTL3 mRNA and the ratio of m6A methylated mRNA of total mRNA was enhanced. Although disturbance of METTL3 lowered the proportion of IL-1β-induced apoptosis, it inhibited IL-1β-induced increased levels of inflammatory cytokines and activation of NF-kB signalling in chondrocytes (172). In addition, disruption of METTL3 improves destruction of the ECM by decreasing matrix metalloproteinase-13 and collagen (Coll) X expression levels, and elevating the expression levels of Aggrecan and Coll II (172) (Fig. 5A). Simultaneously, OA is a degenerative disease of the synovial joint. The synovial membrane is responsible for the inflammatory response, which causes the release of macrophage-derived pro-inflammatory cytokines, such as RA, including IL-6(173). A study revealed that suppressing the overexpression of IL-6 in synovial fibroblasts is a prospective way to hamper the development and progression of OA (173,174). Thus, it is apparent that m6A also participates in OA by regulating several cytokines. The reasons for the occurrence of OA are complicated, and research on the association between m6A and OA is lacking; thus, further studies are required.
Figure 4.
Schematic diagram of the ECM structure of articular cartilage under normal and arthritic conditions. The cartilage ECM is a dynamic network of proteins secreted by chondrocytes. In normal joints, the ECM is composed mainly of type II collagen and proteoglycans; however, with the occurrence of inflammation, trauma and ageing, the cell density decreases and the ECM in articular cartilages degenerates. As degeneration continues, the loss of matrix leads to the propagation of cell death and tissue degeneration, which manifest as arthritis. ECM, extracellular matrix.
Figure 5.
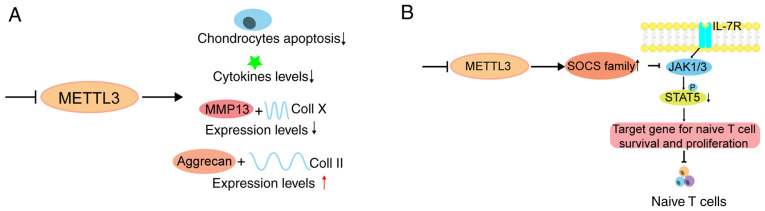
Schematic model of m6A in regulating arthritis. (A) METTL3 knockdown decreases chondrocyte apoptosis rate and inflammatory cytokine levels. In addition, METTL3 knockdown promotes degradation of the extracellular matrix by suppressing the expression levels of MMP-13 and Coll X, while elevating the expression levels of Aggrecan and Coll II. (B) METTL3-deficiency-mediated loss of m6A leads to slower SOCS mRNA degradation and increased SOCS protein levels, thereby blocking the IL-7 pathway and eventually disrupting the differentiation and proliferation of naïve T cells. m6A, RNA N6-methyladenine; METTL, methyltransferase-like; MMP, matrix metalloproteinase; Coll, collagen; IL, interleukin.
Multiple pathological factors contribute to the development of RA, including autoimmunity, various pathogen infections and genetic factors (175). A study revealed that METTL3 deficiency in mouse T cells disrupts T cell homoeostasis and differentiation (176), which implies that m6A has a potential effect on the occurrence and development of RA through the regulation of the immune system (Fig. 5B). Another study revealed that METTL3, but not other m6A methylation-related proteins, including METTL14, FTO, ALKBH5, YTHDF1 and YTHDF2, is upregulated in RA (177). Increased METTL3 expression is positively associated with CRP and ESR levels, which are the two main markers of RA disease activity. It has been reported that METTL3 participates in RA by hindering the proliferation and inflammatory response of macrophages (177). However, whether METTL3 mediates the progression and development of RA requires further investigation (177). Several studies have indicated that m6A-associated SNPs play essential roles in gene expression and mRNA homoeostasis, which may subsequently lead to the occurrence of disease (178,179). The RA GWAS dataset identified several RA-associated m6A-SNPs, which may play regulatory roles in the pathogenesis of RA, and some of them were associated with the mRNA expression of local RA-related genes (178). Taken together, these findings provide insight into the association between SNPs and RA. However, further studies are required to determine the molecular mechanisms. RA is a systemic disease with several immunological events, and the key pathogenetic changes are the production of pro-inflammatory cytokines from macrophage-like synoviocytes, including IL-1, IL-6 and TNF (180). YTHDF2 disruption notably increases the expression levels of LPS-induced IL-6, TNF-α, IL-1 β and IL-12. Thus, m6A also participates in RA pathology by regulating several cytokines (160). Furthermore, synovium inflammation is involved in extensive activated CD4+ T cells (181), suggesting that disturbed homoeostasis of CD4+ T cells plays a critical role in the development of RA (182,183). Since T cells mediate the adaptive immune response and contain several subgroups (184), the concrete details and molecular mechanisms require further investigation.
5. m6A and osteosarcoma
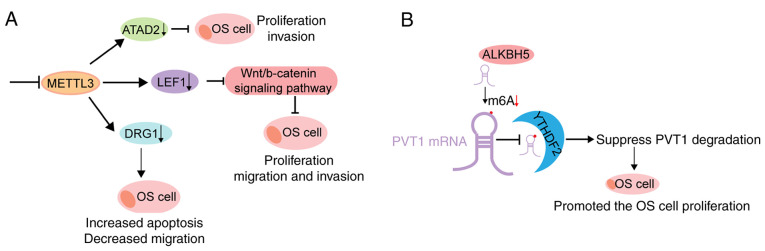
One of the most common and aggressive malignant bone tumours is OS (12), which mainly occurs in children and adolescents whose bones grow rapidly (185,186); however, the molecular mechanisms underlying OS development and progression remain unclear. A study revealed that the levels of m6A methylated RNAs are considerably higher in human OS tissues and cell lines (187). Furthermore, METTL3 disruption hinders the proliferative, migratory and invasive abilities of OS cells (187). Further mechanistic studies have reported that METTL3 deficiency decreases m6A methylation and mRNA levels of lymphoid enhancer-binding factor 1 (LEF1), a downstream factor of the Wnt signalling pathway (188), which is involved in the metastasis and chemoresistance of different types of cancer, including OS (188-190). In addition, LEF1 inhibits OS formation (187) (Fig. 6A). Knockdown of METTL3 and ELAVL1 (another m6A reader) decreases the expression levels of m6A and mRNA, and developmentally regulates GTP-binding protein 1 (DRG1), whose aberrant expression is associated with the development and progression of different tumours; attenuating DRG1 exerted OS-promoting effects (191) (Fig. 6A). Furthermore, a recent study revealed that METTL3 acts as an oncogene in the development and progression of OS (36). It has also been reported that METTL3 promotes the proliferation and metastasis of OS cells by targeting and regulating ATAD2 expression (36) (Fig. 6A). Furthermore, m6A modification may play a regulatory role by exerting biological effects on non-coding RNAs (192). In a study, ALKBH5-mediated m6A demethylation reportedly improved the stability of PVT1, a well-known oncogenic lncRNA, and accelerated the growth of OS (67) (Fig. 6B).
Figure 6.
Schematic model of m6A in regulating osteosarcoma. (A) Upper: METTL3 knockdown inhibits the expression of ATPase family ATAD2, and the proliferation and invasion of OS cells. Middle: METTL3 knockdown decreases m6A methylation and total mRNA levels of LEF1, followed by the inhibition of the activity of Wnt/b-catenin signalling pathway, such that the proliferation, migration and invasion ability of OS cells are inhibited. Lower: METTL3 knockdown decreases m6A and Drg1 mRNA levels, thereby decreasing both the mRNA and protein levels of DRG1, such that the migration and colony formation abilities of OS cells are inhibited. (B) ALKBH5 decreases the m6A modification of PVT1, thereby inhibiting the binding of reader protein YTHDF2 in PVT1 and suppressing PVT1 degradation, such that OS cell proliferation and tumour growth are promoted. m6A, RNA N6-methyladenine; METTL, methyltransferase-like; ATAD2, AAA domain-containing protein 2; OS, osteosarcoma; LEF1, lymphoid enhancer-binding factor 1; DRG1, GTP-binding protein 1; ALHBH5, α-ketoglutarate-dependent dioxygenase alkB homologue 5; YTHDF, YTH domain family.
6. Future prospects
Currently, the number of different types of chemical modifications in RNA has reached ~140(7). m6A is a pivotal epitranscriptomic modification in common orthopaedic diseases (187,193,194). Several studies have revealed the underlying molecular mechanisms of m6A modifications in cancer (51,63,195,196); however, research on the association between m6A and bone-related diseases is still lacking and should be addressed. In addition to the m6A-associated proteins mentioned, many other proteins, including writers (METTL5, METTL16, KIAA1429, RBM15, VIRMA and ZCCHC4) and readers (IGF2BP1, IGF2BP2, IGF2BP3 and eIF3), have been demonstrated to participate in the formation of m6A (19,197). However, whether these proteins are involved in the pathogenesis and development of bone-related diseases remains unknown. m6A methyltransferase can methylate non-coding RNAs (198); however, studies on the interaction between non-coding RNAs and m6A in bone-related diseases are scarce. In addition, several bone-related diseases are associated with the activities of m6A, such as epigenetic m6A modification, which plays an important role in the ossification of the ligamentum flavum (199). Recently, a review summarized the association between m6A and musculoskeletal disorders (200). However, the roles of m6A in T cell homoeostasis and differentiation remain unclear, both of which closely associated with the occurrence and development of osteoporosis, osteoarthritis, rheumatoid arthritis and osteosarcoma, which is a subject of novel and extensive research. Thus, further studies are required to determine the underlying cellular and molecular mechanisms of m6A in bone-related diseases. In conclusion, m6A may serve as a promising molecular target in regenerative medicine, bone tissue engineering and in the treatment of bone-related cancer.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
XZ conceived and designed the present review. YH and XZ performed the data analysis and interpretation. YH and XZ drafted the initial manuscript. Data authentication is not applicable. Both authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rits S, Olsen BR, Volloch V. Protein-encoding RNA to RNA information transfer in mammalian cells: RNA-dependent mRNA amplification. Identification of chimeric RNA intermediates and putative RNA end products. Ann Integr Mol Med. 2019;1:23–47. [PMC free article] [PubMed] [Google Scholar]
- 2.Howie H, Rijal CM, Ressler KJ. A review of epigenetic contributions to post-traumatic stress disorder. Dialogues Clin Neurosci. 2019;21:417–428. doi: 10.31887/DCNS.2019.21.4/kressler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt CE, Schuman EM. The central dogma decentralized: New perspectives on RNA function and local translation in neurons. Neuron. 2013;80:648–657. doi: 10.1016/j.neuron.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maydanovych O, Beal PA. Breaking the central dogma by RNA editing. Chem Rev. 2006;106:3397–3411. doi: 10.1021/cr050314a. [DOI] [PubMed] [Google Scholar]
- 5.Thakur P, Estevez M, Lobue PA, Limbach PA, Addepalli B. Improved RNA modification mapping of cellular non-coding RNAs using C- and U-specific RNases. Analyst. 2020;145:816–827. doi: 10.1039/c9an02111f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi H, Wei J, He C. Where, when, and how: Context-dependent functions of RNA methylation writers, readers, and erasers. Mol Cell. 2019;74:640–650. doi: 10.1016/j.molcel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Dou X, Chen C, Chen C, Liu C, Xu MM, Zhao S, Shen B, Gao Y, Han D, He C. N6-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science. 2020;367:580–586. doi: 10.1126/science.aay6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng C, Huang W, Li Y, Weng H. Roles of METTL3 in cancer: Mechanisms and therapeutic targeting. J Hematol Oncol. 2020;13(117) doi: 10.1186/s13045-020-00951-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinoda K, Suda A, Otonari K, Futaki S, Imanishi M. Programmable RNA methylation and demethylation using PUF RNA binding proteins. Chem Commun (Camb) 2020;56:1365–1368. doi: 10.1039/c9cc09298f. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Chen X, Zhou Z, Mao Y, Wang Y, Ma Z, Xu W, Qin A, Zhang S. Nirogacestat suppresses RANKL-Induced osteoclast formation in vitro and attenuates LPS-Induced bone resorption in vivo. Exp Cell Res. 2019;382(111470) doi: 10.1016/j.yexcr.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 13.The RNA methyltransferase METTL3 promotes oncogene translation. Cancer Discov. 2016;6(572) doi: 10.1158/2159-8290.CD-RW2016-083. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Peng C, Chen J, Chen D, Yang B, He B, Hu W, Zhang Y, Liu H, Dai L, et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol Cancer. 2019;18(127) doi: 10.1186/s12943-019-1053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Zhu L, Shi Y, Liu J, Lin L, Chen X. m6A demethylase FTO promotes hepatocellular carcinoma tumorigenesis via mediating PKM2 demethylation. Am J Transl Res. 2019;11:6084–6092. [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker MT, Knop K, Sherwood AV, Schurch NJ, Mackinnon K, Gould PD, Hall AJ, Barton GJ, Simpson GG. Nanopore direct RNA sequencing maps the complexity of Arabidopsis mRNA processing and m6A modification. eLife. 2020;9(e49658) doi: 10.7554/eLife.49658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao S, Sun H, Xu C. YTH Domain: A family of N6-methyladenosine (m6A) readers. Genomics Proteomics Bioinformatics. 2018;16:99–107. doi: 10.1016/j.gpb.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao YL, Liu YH, Wu RF, Bi Z, Yao YX, Liu Q, Wang YZ, Wang XX. Understanding m6A function through uncovering the diversity roles of YTH domain-containing proteins. Mol Biotechnol. 2019;61:355–364. doi: 10.1007/s12033-018-00149-z. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Kennedy S, Hajian T, Gibson E, Seitova A, Xu C, Arrowsmith CH, Vedadi M. A radioactivity-based assay for screening human m6A-RNA methyltransferase, METTL3-METTL14 complex, and demethylase ALKBH5. J Biomol Screen. 2016;21:290–297. doi: 10.1177/1087057115623264. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Santi DV. m5C RNA and m5C DNA methyl transferases use different cysteine residues as catalysts. Proc Natl Acad Sci USA. 2000;97:8263–8265. doi: 10.1073/pnas.97.15.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roovers M, Wouters J, Bujnicki JM, Tricot C, Stalon V, Grosjean H, Droogmans L. A primordial RNA modification enzyme: The case of tRNA (m1A) methyltransferase. Nucleic Scids Res. 2004;32:465–476. doi: 10.1093/nar/gkh191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grabowski P. Physiology of bone. Endocr Dev. 2009;16:32–48. doi: 10.1159/000223687. [DOI] [PubMed] [Google Scholar]
- 25.Scholtysek C, Kronke G, Schett G. Inflammation-associated changes in bone homeostasis. Inflamm Allergy Drug Targets. 2012;11:188–195. doi: 10.2174/187152812800392706. [DOI] [PubMed] [Google Scholar]
- 26.Suominen H. Muscle training for bone strength. Aging Clin Exp Res. 2006;18:85–93. doi: 10.1007/BF03327422. [DOI] [PubMed] [Google Scholar]
- 27.Fu R, Lv WC, Xu Y, Gong MY, Chen XJ, Jiang N, Xu Y, Yao QQ, Di L, Lu T, et al. Endothelial ZEB1 promotes angiogenesis-dependent bone formation and reverses osteoporosis. Nat Commun. 2020;11(460) doi: 10.1038/s41467-019-14076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landete-Castillejos T, Kierdorf H, Gomez S, Luna S, García AJ, Cappelli J, Pérez-Serrano M, Pérez-Barbería J, Gallego L, Kierdorf U. Antlers-Evolution, development, structure, composition, and biomechanics of an outstanding type of bone. Bone. 2019;128(115046) doi: 10.1016/j.bone.2019.115046. [DOI] [PubMed] [Google Scholar]
- 29.Hassan MQ, Tye CE, Stein GS, Lian JB. Non-coding RNAs: Epigenetic regulators of bone development and homeostasis. Bone. 2015;81:746–756. doi: 10.1016/j.bone.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bocheva G, Boyadjieva N. Epigenetic regulation of fetal bone development and placental transfer of nutrients: Progress for osteoporosis. Interdiscip Toxicol. 2011;4:167–172. doi: 10.2478/v10102-011-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adamik J, Roodman GD, Galson DL. Epigenetic-based mechanisms of osteoblast suppression in multiple myeloma bone disease. JBMR Plus. 2019;3(e10183) doi: 10.1002/jbm4.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marini F, Cianferotti L, Brandi ML. Epigenetic mechanisms in bone biology and osteoporosis: Can they drive therapeutic choices? Int J Mol Sci. 2016;17(1329) doi: 10.3390/ijms17081329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghayor C, Weber FE. Epigenetic regulation of bone remodeling and its impacts in osteoporosis. Int J Mol Sci. 2016;17(1446) doi: 10.3390/ijms17091446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montecino M, Stein G, Stein J, Zaidi K, Aguilar R. Multiple levels of epigenetic control for bone biology and pathology. Bone. 2015;81:733–738. doi: 10.1016/j.bone.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi M, Ohsugi M, Sasako T, Awazawa M, Umehara T, Iwane A, Kobayashi N, Okazaki Y, Kubota N, Suzuki R, et al. The RNA methyltransferase complex of WTAP, METTL3, and METTL14 regulates mitotic clonal expansion in adipogenesis. Mol Cell Biol. 2018;38:e00116–18. doi: 10.1128/MCB.00116-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou L, Yang C, Zhang N, Zhang X, Zhao T, Yu J. Silencing METTL3 inhibits the proliferation and invasion of osteosarcoma by regulating ATAD2. Biomed Pharmacother. 2020;125(109964) doi: 10.1016/j.biopha.2020.109964. [DOI] [PubMed] [Google Scholar]
- 37.Bujnicki JM, Feder M, Radlinska M, Blumenthal RM. Structure prediction and phylogenetic analysis of a functionally diverse family of proteins homologous to the MT-A70 subunit of the human mRNA:m(6)A methyltransferase. J Mol Evol. 2002;55:431–444. doi: 10.1007/s00239-002-2339-8. [DOI] [PubMed] [Google Scholar]
- 38.Scholler E, Weichmann F, Treiber T, Ringle S, Treiber N, Flatley A, Feederle R, Bruckmann A, Meister G. Interactions, localization, and phosphorylation of the m6A generating METTL3-METTL14-WTAP complex. RNA. 2018;24:499–512. doi: 10.1261/rna.064063.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang P, Doxtader KA, Nam Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol Cell. 2016;63:306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lence T, Paolantoni C, Worpenberg L, Roignant JY. Mechanistic insights into m6A RNA enzymes. Biochim Biophys Acta Gene Regul Mech. 2019;1862:222–229. doi: 10.1016/j.bbagrm.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Haussmann IU, Bodi Z, Sanchez-Moran E, Mongan NP, Archer N, Fray RG, Soller M. m6A potentiates Sxl alternative pre-mRNA splicing for robust drosophila sex determination. Nature. 2016;540:301–304. doi: 10.1038/nature20577. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alarcon CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519:482–485. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C, et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534:575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 46.Meyer KD, Jaffrey SR. Rethinking m6A readers, writers, and erasers. Annu Rev Cell Dev Biol. 2017;33:319–342. doi: 10.1146/annurev-cellbio-100616-060758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, Hershkovitz V, Peer E, Mor N, Manor YS, et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science. 2015;347:1002–1006. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- 48.Hongay CF, Orr-Weaver TL. Drosophila Inducer of MEiosis 4 (IME4) is required for Notch signaling during oogenesis. Proc Natl Acad Sci USA. 2011;108:14855–14860. doi: 10.1073/pnas.1111577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong S, Li H, Bodi Z, Button J, Vespa L, Herzog M, Fray RG. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20:1278–1288. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clancy MJ, Shambaugh ME, Timpte CS, Bokar JA. Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: A potential mechanism for the activity of the IME4 gene. Nucleic Acids Res. 2002;30:4509–4518. doi: 10.1093/nar/gkf573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC, Yuan WB, Lu JC, Zhou ZJ, Lu Q, et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer. 2019;18(110) doi: 10.1186/s12943-019-1036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choe J, Lin S, Zhang W, Liu Q, Wang L, Ramirez-Moya J, Du P, Kim W, Tang S, Sliz P, et al. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature. 2018;561:556–560. doi: 10.1038/s41586-018-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, Liu L, Dong Z, Li J, Yu Y, Chen X, Ren F, Cui G, Sun R. Expression patterns and prognostic value of m6A-related genes in colorectal cancer. Am J Transl Res. 2019;11:3972–3991. [PMC free article] [PubMed] [Google Scholar]
- 54.Visvanathan A, Patil V, Arora A, Hegde AS, Arivazhagan A, Santosh V, Somasundaram K. Essential role of METTL3-mediated m6A modification in glioma stem-like cells maintenance and radioresistance. Oncogene. 2018;37:522–533. doi: 10.1038/onc.2017.351. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Xu B, Shi J. N6-methyladenosine METTL3 promotes the breast cancer progression via targeting Bcl-2. Gene. 2020;722(144076) doi: 10.1016/j.gene.2019.144076. [DOI] [PubMed] [Google Scholar]
- 56.Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, Chou T, Chow A, Saletore Y, MacKay M, et al. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23:1369–1376. doi: 10.1038/nm.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin S, Liu J, Jiang W, Wang P, Sun C, Wang X, Chen Y, Wang H. METTL3 promotes the proliferation and mobility of gastric cancer cells. Open Med (Wars) 2019;14:25–31. doi: 10.1515/med-2019-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dahal U, Le K, Gupta M. RNA m6A methyltransferase METTL3 regulates invasiveness of melanoma cells by matrix metallopeptidase 2. Melanoma Res. 2019;29:382–389. doi: 10.1097/CMR.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 59.Zheng W, Dong X, Zhao Y, Wang S, Jiang H, Zhang M, Zheng X, Gu M. Multiple functions and mechanisms underlying the role of METTL3 in Human Cancers. Front Oncol. 2019;9(1403) doi: 10.3389/fonc.2019.01403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu L, Wu D, Ning J, Liu W, Zhang D. Changes of N6-methyladenosine modulators promote breast cancer progression. BMC Cancer. 2019;19(326) doi: 10.1186/s12885-019-5538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X, Tang J, Huang W, Wang F, Li P, Qin C, Qin Z, Zou Q, Wei J, Hua L, et al. The M6A methyltransferase METTL3: Acting as a tumor suppressor in renal cell carcinoma. Oncotarget. 2017;8:96103–96116. doi: 10.18632/oncotarget.21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei W, Huo B, Shi X. miR-600 inhibits lung cancer via downregulating the expression of METTL3. Cancer Manag Res. 2019;11:1177–1187. doi: 10.2147/CMAR.S181058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang C, Zhang M, Ge S, Huang W, Lin X, Gao J, Gong J, Shen L. Reduced m6A modification predicts malignant phenotypes and augmented Wnt/PI3K-Akt signaling in gastric cancer. Cancer Med. 2019;8:4766–4781. doi: 10.1002/cam4.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iyer LM, Zhang D, Aravind L. Adenine methylation in eukaryotes: Apprehending the complex evolutionary history and functional potential of an epigenetic modification. Bioessays. 2016;38:27–40. doi: 10.1002/bies.201500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X, Qin J, Gao T, Li C, Chen X, Zeng K, Xu M, He B, Pan B, Xu X, et al. Analysis of METTL3 and METTL14 in hepatocellular carcinoma. Aging (Albany NY) 2020;12:21638–21659. doi: 10.18632/aging.103959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buker SM, Gurard-Levin ZA, Wheeler BD, Scholle MD, Case AW, Hirsch JL, Ribich S, Copeland RA, Boriack-Sjodin PA. A mass spectrometric assay of METTL3/METTL14 methyltransferase activity. SLAS Discov. 2020;25:361–371. doi: 10.1177/2472555219878408. [DOI] [PubMed] [Google Scholar]
- 67.Chen X, Xu M, Xu X, Zeng K, Liu X, Pan B, Li C, Sun L, Qin J, Xu T, et al. METTL14-mediated N6-methyladenosine modification of SOX4 mRNA inhibits tumor metastasis in colorectal cancer. Mol Cancer. 2020;19(106) doi: 10.1186/s12943-020-01220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gong PJ, Shao YC, Yang Y, Song WJ, He X, Zeng YF, Huang SR, Wei L, Zhang JW. Analysis of N6-methyladenosine methyltransferase reveals METTL14 and ZC3H13 as tumor suppressor genes in breast cancer. Front Oncol. 2020;10(578963) doi: 10.3389/fonc.2020.578963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu C, Wang Z, Zhou N, Li G, Kou Y, Luo Y, Wang Y, Yang J, Tian F. Mettl14 inhibits bladder TIC self-renewal and bladder tumorigenesis through N6-methyladenosine of Notch1. Mol Cancer. 2019;18(168) doi: 10.1186/s12943-019-1084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weng H, Huang H, Wu H, Qin X, Zhao BS, Dong L, Shi H, Skibbe J, Shen C, Hu C, et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m6A modification. Cell Stem Cell. 2018;22:191–205 e9. doi: 10.1016/j.stem.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, Mertins P, Ter-Ovanesyan D, Habib N, Cacchiarelli D, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5' sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sorci M, Ianniello Z, Cruciani S, Larivera S, Ginistrelli LC, Capuano E, Marchioni M, Fazi F, Fatica A. METTL3 regulates WTAP protein homeostasis. Cell Death Dis. 2018;9(796) doi: 10.1038/s41419-018-0843-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H, Su Q, Li B, Lan L, Wang C, Li W, Wang G, Chen W, He Y, Zhang C. High expression of WTAP leads to poor prognosis of gastric cancer by influencing tumour-associated T lymphocyte infiltration. J Cell Mol Med. 2020;24:4452–4465. doi: 10.1111/jcmm.15104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bansal H, Yihua Q, Iyer SP, Ganapathy S, Proia DA, Penalva LO, Uren PJ, Suresh U, Carew JS, Karnad AB, et al. WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia. 2014;28:1171–1174. doi: 10.1038/leu.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horiuchi K, Umetani M, Minami T, Okayama H, Takada S, Yamamoto M, Aburatani H, Reid PC, Housman DE, Hamakubo T, Kodama T. Wilms' tumor 1-associating protein regulates G2/M transition through stabilization of cyclin A2 mRNA. Proc Natl Acad Sci USA. 2006;103:17278–17283. doi: 10.1073/pnas.0608357103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wen J, Lv R, Ma H, Shen H, He C, Wang J, Jiao F, Liu H, Yang P, Tan L, et al. Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69:1028–1038 e6. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu D, Zhou J, Zhao J, Jiang G, Zhang X, Zhang Y, Dong M. ZC3H13 suppresses colorectal cancer proliferation and invasion via inactivating Ras-ERK signaling. J Cell Physiol. 2019;234:8899–8907. doi: 10.1002/jcp.27551. [DOI] [PubMed] [Google Scholar]
- 79.Chen J, Yu K, Zhong G, Shen W. Identification of a m6A RNA methylation regulators-based signature for predicting the prognosis of clear cell renal carcinoma. Cancer Cell Int. 2020;20(157) doi: 10.1186/s12935-020-01238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu T, Li C, Jin L, Li C, Wang L. The prognostic value of m6A RNA methylation regulators in colon adenocarcinoma. Med Sci Monit. 2019;25:9435–9445. doi: 10.12659/MSM.920381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526:591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dina C, Meyre D, Gallina S, Durand E, Körner A, Jacobson P, Carlsson LM, Kiess W, Vatin V, Lecoeur C, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 84.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niu Y, Lin Z, Wan A, Chen H, Liang H, Sun L, Wang Y, Li X, Xiong XF, Wei B, et al. RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol Cancer. 2019;18(46) doi: 10.1186/s12943-019-1004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, Huang H, Nachtergaele S, Dong L, Hu C, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N6-Methyladenosine RNA demethylase. Cancer Cell. 2017;31:127–141. doi: 10.1016/j.ccell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang S, Wei J, Cui YH, Park G, Shah P, Deng Y, Aplin AE, Lu Z, Hwang S, He C, He YY. m6A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat Commun. 2019;10(2782) doi: 10.1038/s41467-019-10669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li J, Han Y, Zhang H, Qian Z, Jia W, Gao Y, Zheng H, Li B. The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem Biophys Res Commun. 2019;512:479–485. doi: 10.1016/j.bbrc.2019.03.093. [DOI] [PubMed] [Google Scholar]
- 89.Baltz AG, Munschauer M, Schwanhausser B, Vasile A, Murakawa Y, Schueler M, Youngs N, Penfold-Brown D, Drew K, Milek M, et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol Cell. 2012;46:674–690. doi: 10.1016/j.molcel.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 90.Fedeles BI, Singh V, Delaney JC, Li D, Essigmann JM. The AlkB family of Fe(II)/α-Ketoglutarate-dependent dioxygenases: Repairing nucleic acid alkylation damage and beyond. J Biol Chem. 2015;290:20734–20742. doi: 10.1074/jbc.R115.656462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pilzys T, Marcinkowski M, Kukwa W, Garbicz D, Dylewska M, Ferenc K, Mieczkowski A, Kukwa A, Migacz E, Wołosz D, et al. ALKBH overexpression in head and neck cancer: Potential target for novel anticancer therapy. Sci Rep. 2019;9(13249) doi: 10.1038/s41598-019-49550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo X, Li K, Jiang W, Hu Y, Xiao W, Huang Y, Feng Y, Pan Q, Wan R. RNA demethylase ALKBH5 prevents pancreatic cancer progression by posttranscriptional activation of PER1 in an m6A-YTHDF2-dependent manner. Mol Cancer. 2020;19(91) doi: 10.1186/s12943-020-01158-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shen C, Sheng Y, Zhu AC, Robinson S, Jiang X, Dong L, Chen H, Su R, Yin Z, Li W, et al. RNA demethylase ALKBH5 selectively promotes tumorigenesis and cancer stem cell self-renewal in acute myeloid leukemia. Cell Stem Cell. 2020;27:64–80.e9. doi: 10.1016/j.stem.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, Chen Y, Sulman EP, Xie K, Bögler O, et al. m6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma Stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31:591–606.e6. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu Z, Qian Q, Zhao X, Ma L, Chen P. N6-methyladenosine ALKBH5 promotes non-small cell lung cancer progress by regulating TIMP3 stability. Gene. 2020;731(144348) doi: 10.1016/j.gene.2020.144348. [DOI] [PubMed] [Google Scholar]
- 96.Zhang J, Guo S, Piao HY, Wang Y, Wu Y, Meng XY, Yang D, Zheng ZC, Zhao Y. ALKBH5 promotes invasion and metastasis of gastric cancer by decreasing methylation of the lncRNA NEAT1. J Physiol Biochem. 2019;75:379–389. doi: 10.1007/s13105-019-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ueda Y, Ooshio I, Fusamae Y, Kitae K, Kawaguchi M, Jingushi K, Hase H, Harada K, Hirata K, Tsujikawa K. AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Sci Rep. 2017;7(42271) doi: 10.1038/srep42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mohan M, Akula D, Dhillon A, Goyal A, Anindya R. Human RAD51 paralogue RAD51C fosters repair of alkylated DNA by interacting with the ALKBH3 demethylase. Nucleic Acids Res. 2019;47:11729–11745. doi: 10.1093/nar/gkz938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen Z, Qi M, Shen B, Luo G, Wu Y, Li J, Lu Z, Zheng Z, Dai Q, Wang H. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019;47:2533–2545. doi: 10.1093/nar/gky1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat Rev Genet. 2014;15:293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- 101.Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29:1343–1355. doi: 10.1101/gad.262766.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu T, Roundtree IA, Wang P, Wang X, Wang L, Sun C, Tian Y, Li J, He C, Xu Y. Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Res. 2014;24:1493–1496. doi: 10.1038/cr.2014.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee Y, Choe J, Park OH, Kim YK. Molecular mechanisms driving mRNA degradation by m6A modification. Trends Genet. 2020;36:177–188. doi: 10.1016/j.tig.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 104.Li M, Zhao X, Wang W, Shi H, Pan Q, Lu Z, Perez SP, Suganthan R, He C, Bjørås M, Klungland A. Ythdf2-mediated m6A mRNA clearance modulates neural development in mice. Genome Biol. 2018;19(69) doi: 10.1186/s13059-018-1436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang H, Zuo H, Liu J, Wen F, Gao Y, Zhu X, Liu B, Xiao F, Wang W, Huang G, et al. Loss of YTHDF2-mediated m6A-dependent mRNA clearance facilitates hematopoietic stem cell regeneration. Cell Res. 2018;28:1035–1038. doi: 10.1038/s41422-018-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67:2254–2270. doi: 10.1002/hep.29683. [DOI] [PubMed] [Google Scholar]
- 107.Huang T, Liu Z, Zheng Y, Feng T, Gao Q, Zeng W. YTHDF2 promotes spermagonial adhesion through modulating MMPs decay via m6A/mRNA pathway. Cell Death Dis. 2020;11(37) doi: 10.1038/s41419-020-2235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, Jaffrey SR. 5'UTR m(6)A promotes cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hu L, Wang J, Huang H, Yu Y, Ding J, Yu Y, Li K, Wei D, Ye Q, Wang F, et al. YTHDF1 regulates pulmonary hypertension through translational control of MAGED1. Am J Respir Crit Care Med. 2021;203:1158–1172. doi: 10.1164/rccm.202009-3419OC. [DOI] [PubMed] [Google Scholar]
- 110.Shi H, Zhang X, Weng YL, Lu Z, Liu Y, Lu Z, Li J, Hao P, Zhang Y, Zhang F, et al. m6A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature. 2018;563:249–253. doi: 10.1038/s41586-018-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nishizawa Y, Konno M, Asai A, Koseki J, Kawamoto K, Miyoshi N, Takahashi H, Nishida N, Haraguchi N, Sakai D, et al. Oncogene c-Myc promotes epitranscriptome m6A reader YTHDF1 expression in colorectal cancer. Oncotarget. 2018;9:7476–7486. doi: 10.18632/oncotarget.23554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao X, Chen Y, Mao Q, Jiang X, Jiang W, Chen J, Xu W, Zhong L, Sun X. Overexpression of YTHDF1 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Biomark. 2018;21:859–868. doi: 10.3233/CBM-170791. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Y, Wang X, Zhang X, Wang J, Ma Y, Zhang L, Cao X. RNA-binding protein YTHDF3 suppresses interferon-dependent antiviral responses by promoting FOXO3 translation. Proc Natl Acad Sci USA. 2019;116:976–981. doi: 10.1073/pnas.1812536116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, Liu J, Che L, Li J. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m6A reader YTHDF3. Mol Cancer. 2019;18(143) doi: 10.1186/s12943-019-1079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jurczyszak D, Zhang W, Terry SN, Kehrer T, Bermúdez González MC, McGregor E, Mulder LCF, Eckwahl MJ, Pan T, Simon V. HIV protease cleaves the antiviral m6A reader protein YTHDF3 in the viral particle. PLoS Pathog. 2020;16(e1008305) doi: 10.1371/journal.ppat.1008305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 117.Luxton HJ, Simpson BS, Mills IG, Brindle NR, Ahmed Z, Stavrinides V, Heavey S, Stamm S, Whitaker HC. The oncogene metadherin interacts with the known splicing proteins YTHDC1, Sam68 and T-STAR and plays a novel role in alternative mRNA splicing. Cancers (Basel) 2019;11(1233) doi: 10.3390/cancers11091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kasowitz SD, Ma J, Anderson SJ, Leu NA, Xu Y, Gregory BD, Schultz RM, Wang PJ. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet. 2018;14(e1007412) doi: 10.1371/journal.pgen.1007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mao Y, Dong L, Liu XM, Guo J, Ma H, Shen B, Qian SB. m6A in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nat Commun. 2019;10(5332) doi: 10.1038/s41467-019-13317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tanabe A, Tanikawa K, Tsunetomi M, Takai K, Ikeda H, Konno J, Torigoe T, Maeda H, Kutomi G, Okita K, et al. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1α mRNA is translated. Cancer Lett. 2016;376:34–42. doi: 10.1016/j.canlet.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 121.Kretschmer J, Rao H, Hackert P, Sloan KE, Hobartner C, Bohnsack MT. The m6A reader protein YTHDC2 interacts with the small ribosomal subunit and the 5'-3' exoribonuclease XRN1. RNA. 2018;24:1339–1350. doi: 10.1261/rna.064238.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakano M, Ondo K, Takemoto S, Fukami T, Nakajima M. Methylation of adenosine at the N6 position post-transcriptionally regulates hepatic P450s expression. Biochem Pharmacol. 2020;171(113697) doi: 10.1016/j.bcp.2019.113697. [DOI] [PubMed] [Google Scholar]
- 123.Bailey AS, Batista PJ, Gold RS, Chen YG, de Rooij DG, Chang HY, Fuller MT. The conserved RNA helicase YTHDC2 regulates the transition from proliferation to differentiation in the germline. eLife. 2017;6(e26116) doi: 10.7554/eLife.26116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tanabe A, Konno J, Tanikawa K, Sahara H. Transcriptional machinery of TNF-α-inducible YTH domain containing 2 (YTHDC2) gene. Gene. 2014;535:24–32. doi: 10.1016/j.gene.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 125.Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL, et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Srivastava M, Deal C. Osteoporosis in elderly: Prevention and treatment. Clin Geriatr Med. 2002;18:529–555. doi: 10.1016/s0749-0690(02)00022-8. [DOI] [PubMed] [Google Scholar]
- 128.Langdahl BL. Overview of treatment approaches to osteoporosis. Br J Pharmacol. 2021;178:1891–1906. doi: 10.1111/bph.15024. [DOI] [PubMed] [Google Scholar]
- 129.Rosen CJ, Bouxsein ML. Mechanisms of disease: Is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 130.Raisz LG. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Palmieri D, Valli M, Viglio S, Ferrari N, Ledda B, Volta C, Manduca P. Osteoblasts extracellular matrix induces vessel like structures through glycosylated collagen I. Exp Cell Res. 2010;316:789–799. doi: 10.1016/j.yexcr.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 132.DeNichilo MO, Shoubridge AJ, Panagopoulos V, Liapis V, Zysk A, Zinonos I, Hay S, Atkins GJ, Findlay DM, Evdokiou A. Peroxidase enzymes regulate collagen biosynthesis and matrix mineralization by cultured human osteoblasts. Calcif Tissue Int. 2016;98:294–305. doi: 10.1007/s00223-015-0090-6. [DOI] [PubMed] [Google Scholar]
- 133.Long F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13:27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 134.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 135.Villaseñor A, Aedo-Martín D, Obeso D, Erjavec I, Rodríguez-Coira J, Buendía I, Ardura JA, Barbas C, Gortazar AR. Metabolomics reveals citric acid secretion in mechanically-stimulated osteocytes is inhibited by high glucose. Sci Rep. 2019;9(2295) doi: 10.1038/s41598-018-38154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: An endocrine cell... and more. Endocr Rev. 2013;34:658–690. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016;23:1128–1139. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kawai M, Devlin MJ, Rosen CJ. Fat targets for skeletal health. Nat Rev Rheumatol. 2009;5:365–372. doi: 10.1038/nrrheum.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: Developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scheller EL, Rosen CJ. What's the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Garcia-Gomez MC, Vilahur G. Osteoporosis and vascular calcification: A shared scenario. Clin Investig Arterioscler. 2020;32:33–42. doi: 10.1016/j.arteri.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 142.Chen X, Hua W, Huang X, Chen Y, Zhang J, Li G. Regulatory role of RNA N6-methyladenosine modification in bone biology and osteoporosis. Front Endocrinol (Lausanne) 2019;10(911) doi: 10.3389/fendo.2019.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu Y, Xie L, Wang M, Xiong Q, Guo Y, Liang Y, Li J, Sheng R, Deng P, Wang Y, et al. Mettl3-mediated m6A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nat Commun. 2018;9(4772) doi: 10.1038/s41467-018-06898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yu J, Shen L, Liu Y, Ming H, Zhu X, Chu M, Lin J. The m6A methyltransferase METTL3 cooperates with demethylase ALKBH5 to regulate osteogenic differentiation through NF-κB signaling. Mol Cell Biochem. 2020;463:203–210. doi: 10.1007/s11010-019-03641-5. [DOI] [PubMed] [Google Scholar]
- 145.Tian C, Huang Y, Li Q, Feng Z, Xu Q. Mettl3 regulates osteogenic differentiation and alternative splicing of vegfa in bone marrow mesenchymal stem cells. Int J Mol Sci. 2019;20(551) doi: 10.3390/ijms20030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Busilacchi A, Gigante A, Mattioli-Belmonte M, Manzotti S, Muzzarelli RA. Chitosan stabilizes platelet growth factors and modulates stem cell differentiation toward tissue regeneration. Carbohydr Polym. 2013;98:665–676. doi: 10.1016/j.carbpol.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 147.Hu K, Olsen BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. 2016;126:509–526. doi: 10.1172/JCI82585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yan G, Yuan Y, He M, Gong R, Lei H, Zhou H, Wang W, Du W, Ma T, Liu S, et al. m6A methylation of precursor-miR-320/RUNX2 controls osteogenic potential of bone marrow-derived mesenchymal stem cells. Mol Ther Nucleic Acids. 2020;19:421–436. doi: 10.1016/j.omtn.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li D, Cai L, Meng R, Feng Z, Xu Q. METTL3 modulates osteoclast differentiation and function by controlling RNA stability and nuclear export. Int J Mol Sci. 2020;21(1660) doi: 10.3390/ijms21051660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Eyre DR. Bone biomarkers as tools in osteoporosis management. Spine (Phila Pa 1976) 1997;22 (24 Suppl):17S–24S. doi: 10.1097/00007632-199712151-00004. [DOI] [PubMed] [Google Scholar]
- 152.Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 153.Shen GS, Zhou HB, Zhang H, Chen B, Liu ZP, Yuan Y, Zhou XZ, Xu YJ. The GDF11-FTO-PPARγ axis controls the shift of osteoporotic MSC fate to adipocyte and inhibits bone formation during osteoporosis. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3644–3654. doi: 10.1016/j.bbadis.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 154.Zhang Q, Riddle RC, Yang Q, Rosen CR, Guttridge DC, Dirckx N, Faugere MC, Farber CR, Clemens TL. The RNA demethylase FTO is required for maintenance of bone mass and functions to protect osteoblasts from genotoxic damage. Proc Natl Acad Sci USA. 2019;116:17980–17989. doi: 10.1073/pnas.1905489116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guo Y, Liu H, Yang TL, Li SM, Li SK, Tian Q, Liu YJ, Deng HW. The fat mass and obesity associated gene, FTO, is also associated with osteoporosis phenotypes. PLoS One. 2011;6(e27312) doi: 10.1371/journal.pone.0027312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li Y, Yang F, Gao M, Gong R, Jin M, Liu T, Sun Y, Fu Y, Huang Q, Zhang W, et al. miR-149-3p regulates the switch between adipogenic and osteogenic differentiation of BMSCs by targeting FTO. Mol Ther Nucleic Acids. 2019;17:590–600. doi: 10.1016/j.omtn.2019.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mannerstrom B, Kornilov R, Abu-Shahba AG, Chowdhury IM, Sinha S, Seppänen-Kaijansinkko R, Kaur S. Epigenetic alterations in mesenchymal stem cells by osteosarcoma-derived extracellular vesicles. Epigenetics. 2019;14:352–364. doi: 10.1080/15592294.2019.1585177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lorenzo J. Cytokines and bone: Osteoimmunology. Handb Exp Pharmacol. 2020;262:177–230. doi: 10.1007/164_2019_346. [DOI] [PubMed] [Google Scholar]
- 159.Zhang Y, Gu X, Li D, Cai L, Xu Q. METTL3 regulates osteoblast differentiation and inflammatory response via Smad signaling and MAPK Signaling. Int J Mol Sci. 2019;21(199) doi: 10.3390/ijms21010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yu R, Li Q, Feng Z, Cai L, Xu Q. m6A reader YTHDF2 regulates LPS-induced inflammatory response. Int J Mol Sci. 2019;20(1323) doi: 10.3390/ijms20061323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang T, He C. TNF-α and IL-6: The link between immune and bone system. Curr Drug Targets. 2020;20:213–227. doi: 10.2174/1389450120666190821161259. [DOI] [PubMed] [Google Scholar]
- 162.Mathew AJ, Ravindran V. Infections and arthritis. Best Pract Res Clin Rheumatol. 2014;28:935–959. doi: 10.1016/j.berh.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 163.Harth M, Nielson WR. Pain and affective distress in arthritis: Relationship to immunity and inflammation. Expert Rev Clin Immunol. 2019;15:541–552. doi: 10.1080/1744666X.2019.1573675. [DOI] [PubMed] [Google Scholar]
- 164.Parkinson L, Waters DL, Franck L. Systematic review of the impact of osteoarthritis on health outcomes for comorbid disease in older people. Osteoarthritis Cartilage. 2017;25:1751–1770. doi: 10.1016/j.joca.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 165.Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 166.Abramoff B, Caldera FE. Osteoarthritis: Pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104:293–311. doi: 10.1016/j.mcna.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 167.Liossis SN, Tsokos GC. Cellular immunity in osteoarthritis: Novel concepts for an old disease. Clin Diagn Lab Immunol. 1998;5:427–429. doi: 10.1128/CDLI.5.4.427-429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sakata M, Tsuruha JI, Masuko-Hongo K, Nakamura H, Matsui T, Sudo A, Nishioka K, Kato T. Autoantibodies to osteopontin in patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 2001;28:1492–1495. [PubMed] [Google Scholar]
- 169.Walker J, Gordon T, Lester S, Downie-Doyle S, McEvoy D, Pile K, Waterman S, Rischmueller M. Increased severity of lower urinary tract symptoms and daytime somnolence in primary Sjogren's syndrome. J Rheumatol. 2003;30:2406–2412. [PubMed] [Google Scholar]
- 170.Kato T, Xiang Y, Nakamura H, Nishioka K. Neoantigens in osteoarthritic cartilage. Curr Opin Rheumatol. 2004;16:604–608. doi: 10.1097/01.bor.0000133661.52599.bf. [DOI] [PubMed] [Google Scholar]
- 171.Zhao W, Wang T, Luo Q, Chen Y, Leung VY, Wen C, Shah MF, Pan H, Chiu K, Cao X, Lu WW. Cartilage degeneration and excessive subchondral bone formation in spontaneous osteoarthritis involves altered TGF-β signaling. J Orthop Res. 2016;34:763–770. doi: 10.1002/jor.23079. [DOI] [PubMed] [Google Scholar]
- 172.Liu Q, Li M, Jiang L, Jiang R, Fu B. METTL3 promotes experimental osteoarthritis development by regulating inflammatory response and apoptosis in chondrocyte. Biochem Biophys Res Commun. 2019;516:22–27. doi: 10.1016/j.bbrc.2019.05.168. [DOI] [PubMed] [Google Scholar]
- 173.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;(427 Suppl):S27–S36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 174.Yang F, Zhou S, Wang C, Huang Y, Li H, Wang Y, Zhu Z, Tang J, Yan M. Epigenetic modifications of interleukin-6 in synovial fibroblasts from osteoarthritis patients. Sci Rep. 2017;7(43592) doi: 10.1038/srep43592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6(15) doi: 10.1038/s41413-018-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J, Bailis W, Cao G, Kroehling L, Chen Y, et al. m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548:338–342. doi: 10.1038/nature23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang J, Yan S, Lu H, Wang S, Xu D. METTL3 attenuates LPS-induced inflammatory response in macrophages via NF-κB signaling pathway. Mediators Inflamm. 2019;2019(3120391) doi: 10.1155/2019/3120391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mo XB, Zhang YH, Lei SF. Genome-wide identification of N6-methyladenosine (m6A) SNPs associated with rheumatoid arthritis. Front Genet. 2018;9(299) doi: 10.3389/fgene.2018.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zheng Y, Nie P, Peng D, He Z, Liu M, Xie Y, Miao Y, Zuo Z, Ren J. m6AVar: A database of functional variants involved in m6A modification. Nucleic Acids Res. 2018;46:D139–D145. doi: 10.1093/nar/gkx895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, Yamamoto K. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4(18001) doi: 10.1038/nrdp.2018.1. [DOI] [PubMed] [Google Scholar]
- 181.Kondo Y, Yokosawa M, Kaneko S, Furuyama K, Segawa S, Tsuboi H, Matsumoto I, Sumida T. Review: Transcriptional regulation of CD4+ T cell differentiation in experimentally induced arthritis and rheumatoid arthritis. Arthritis Rheumatol. 2018;70:653–661. doi: 10.1002/art.40398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13:668–677. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 183.Hunt L, Hensor EM, Nam J, Burska AN, Parmar R, Emery P, Ponchel F. T cell subsets: An immunological biomarker to predict progression to clinical arthritis in ACPA-positive individuals. Ann Rheum Dis. 2016;75:1884–1889. doi: 10.1136/annrheumdis-2015-207991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. 2018;48:202–213. doi: 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Abada A, Elazar Z. Getting ready for building: Signaling and autophagosome biogenesis. Embo Rep. 2014;15:839–852. doi: 10.15252/embr.201439076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang DW, Wu LW, Cao Y, Yang L, Liu W, E XQ, Ji G, Bi ZG. A novel mechanism of mTORC1-mediated serine/glycine metabolism in osteosarcoma development. Cell Signal. 2017;29:107–114. doi: 10.1016/j.cellsig.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 187.Miao W, Chen J, Jia L, Ma J, Song D. The m6A methyltransferase METTL3 promotes osteosarcoma progression by regulating the m6A level of LEF1. Biochem Biophys Res Commun. 2019;516:719–725. doi: 10.1016/j.bbrc.2019.06.128. [DOI] [PubMed] [Google Scholar]
- 188.Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M, Gerald WL, Massagué J. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jia P, Wei G, Zhou C, Gao Q, Wu Y, Sun X, Li X. Upregulation of miR-212 inhibits migration and tumorigenicity and inactivates Wnt/β-catenin signaling in human hepatocellular carcinoma. Technol Cancer Res Treat. 2018;17(1533034618765221) doi: 10.1177/1533034618765221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wu L, Zhao JC, Kim J, Jin HJ, Wang CY, Yu J. ERG is a critical regulator of Wnt/LEF1 signaling in prostate cancer. Cancer Res. 2013;73:6068–6079. doi: 10.1158/0008-5472.CAN-13-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ling Z, Chen L, Zhao J. m6A-dependent up-regulation of DRG1 by METTL3 and ELAVL1 promotes growth, migration, and colony formation in osteosarcoma. Biosci Rep. 2020;40(BSR20200282) doi: 10.1042/BSR20200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Coker H, Wei G, Brockdorff N. m6A modification of non-coding RNA and the control of mammalian gene expression. Biochim Biophys Acta Gene Regul Mech. 2019;1862:310–318. doi: 10.1016/j.bbagrm.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 193.Li J, Rao B, Yang J, Liu L, Huang M, Liu X, Cui G, Li C, Han Q, Yang H, et al. Dysregulated m6A-related regulators are associated with tumor metastasis and poor prognosis in osteosarcoma. Front Oncol. 2020;10(769) doi: 10.3389/fonc.2020.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fan D, Xia Y, Lu C, Ye Q, Xi X, Wang Q, Wang Z, Wang C, Xiao C. Regulatory role of the RNA N6-methyladenosine modification in immunoregulatory cells and immune-related bone homeostasis associated with rheumatoid arthritis. Front Cell Dev Biol. 2020;8(627893) doi: 10.3389/fcell.2020.627893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu T, Wei Q, Jin J, Luo Q, Liu Y, Yang Y, Cheng C, Li L, Pi J, Si Y, et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020;48:3816–3831. doi: 10.1093/nar/gkaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ma Z, Ji J. doi: 10.1002/stem.3279. N6-methyladenosine (m6A) RNA modification in cancer stem cells. Stem Cells: Sep 27, 2020 (Epub Ahead of Print). [DOI] [PubMed] [Google Scholar]
- 197.Patil DP, Pickering BF, Jaffrey SR. Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell Biol. 2018;28:113–127. doi: 10.1016/j.tcb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dai DJ, Wang HY, Zhu LY, Jin HC, Wang X. N6-methyladenosine links RNA metabolism to cancer progression. Cell Death Dis. 2018;9(124) doi: 10.1038/s41419-017-0129-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang HF, Kuang MJ, Han SJ, Wang AB, Qiu J, Wang F, Tan BY, Wang DC. BMP2 modified by the m6A demethylation enzyme ALKBH5 in the ossification of the ligamentum flavum through the AKT signaling pathway. Calcified Tissue Int. 2020;106:486–493. doi: 10.1007/s00223-019-00654-6. [DOI] [PubMed] [Google Scholar]
- 200.Zhang W, He L, Liu Z, Ren X, Qi L, Wan L, Wang W, Tu C, Li Z. Multifaceted functions and novel insight into the regulatory role of RNA N6-methyladenosine modification in musculoskeletal disorders. Front Cell Dev Biol. 2020;8(870) doi: 10.3389/fcell.2020.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.