ABSTRACT
Campylobacter spp. are the leading cause of bacterium-derived gastroenteritis worldwide, impacting 96 million individuals annually. Unlike other bacterial pathogens of the gastrointestinal tract, Campylobacter spp. lack many of the classical virulence factors that are often associated with the ability to induce disease in humans, including an array of canonical secretion systems and toxins. Consequently, the clinical manifestations of human campylobacteriosis and its resulting gastrointestinal pathology are believed to be primarily due to the host immune response toward the bacterium. Further, while gastrointestinal infection is usually self-limiting, numerous postinfectious disorders can occur, including the development of Guillain-Barré syndrome, reactive arthritis, and irritable bowel syndrome. Because gastrointestinal disease likely results from the host immune response, the development of these postinfectious disorders may be due to dysregulation or misdirection of the same inflammatory response. As a result, it is becoming increasingly important to the Campylobacter field, and human health, that the cellular immune responses toward Campylobacter be better understood, including which immunological events are critical to the development of disease and the postinfectious disorders mentioned above. In this review, we collectively cover the cellular immune responses across susceptible hosts to Campylobacter jejuni infection, along with the tissue pathology and postinfectious disorders which may develop.
KEYWORDS: Campylobacter, gastrointestinal infection, immune response, infectious disease
INTRODUCTION
Campylobacter spp. are Gram-negative gastrointestinal pathogens that are projected to cause 96 million annual infections worldwide (1, 2). Campylobacter jejuni and C. coli are the leading causes of these infections, accounting for approximately 90% and 10%, respectively (3). While the bacteria are predominantly commensal in numerous species of livestock, including poultry and cattle, infection in humans and other hosts can lead to gastroenteritis (3–5). In the developed world, infection most often occurs through consumption of undercooked, contaminated animal products, while in the developing world, infections are believed to arise from contaminated drinking water (6, 7). Once ingested, the bacterium infects the mucosal surface of intestinal crypts, where it can lead to pronounced inflammation and gastrointestinal pathology (8, 9). Clinical symptoms of acute gastrointestinal infection typically include bloody diarrhea, abdominal pain, fever, and weight loss, which last for an average of 6 days in immunocompetent individuals (10).
While most infections in the developed world are self-limiting, numerous postinfectious disorders can occur. Several Campylobacter spp. have been associated with such disorders, including C. coli, C. concisus, C. curvus, C. gracilis, C. hominis, C. jejuni, C. rectus, C. showae, C. sputorum, and C. ureolyticus (4). Postinfectious disorders associated with Campylobacter infections include Guillain-Barré syndrome (GBS), reactive arthritis (ReA), and irritable bowel syndrome (IBS) (11, 12). Among patients that develop GBS, C. jejuni can be attributed to as many as 40% of all cases, with seropositivity toward C. jejuni occurring in up to 76% of patients (13). This results in total annual productivity losses and medical costs up to $1.8 billion per year (14, 15). The outdated nature of these data, combined with observations that infections are increasing in prevalence, suggests that the current economic burden of this disease is currently far more than those previous estimates. Further, in the first year following Campylobacter infection, patients have a greater risk of developing IBS than uninfected individuals (16). Finally, it is estimated that 18% of infected individuals develop ReA, which can result in potent joint inflammation and reduced range of motion (12). Despite the health and financial impacts of these disorders, understanding of the immunological basis for their onset and progression is far from complete.
Because gastrointestinal infection results in several hallmarks of inflammation and that most Campylobacter spp. lack many of the classical virulence factors possessed by bacterial pathogens of the gastrointestinal tract, the disease and intestinal pathology that result are likely due to the host’s own immune response (3, 17, 18). For example, during human infection, there is a potent induction of proinflammatory cytokine production, including interleukin 1β (IL-1β), IL-8, IL-6, and gamma interferon (IFN-γ) (19). Unfortunately, the consistency with which these responses occur and the downstream effects that result in both acute disease and the development of postinfectious disorders are poorly understood, especially compared to the case with less prevalent gastrointestinal pathogens (20). This deficiency is primarily due to the lack of an immunocompetent small-animal model that develops clinical symptoms similar to those in human infection (17). Beyond the gastrointestinal disease and postinfectious disorders mentioned above, Campylobacter spp. are increasingly associated with long-term health consequences in the developing world, particularly in pediatric populations, in which persistent intestinal colonization is associated with enteric dysfunction and decreased development (4). Taking this all together, it is becoming increasingly apparent that Campylobacter colonization can be more than a simple, transient gastrointestinal infection: it can be an inflammatory event that has lasting impacts on diverse hosts. This observation makes it particularly urgent that the cellular immune response during infection be better understood, including how it affects extraintestinal tissues and the long-term health of the host gastrointestinal tract.
This review highlights cellular immunity during campylobacteriosis by combining mouse, ferret, human, and other host studies to understand how mammalian host cells respond to Campylobacter spp. and how these may drive the acute and chronic diseases mentioned above. It is worth noting that because C. jejuni is the predominant cause of diarrheal infections in the developed world, many of these studies focus on that species. We hope to bring light to the host inflammatory responses and the potential links to the development of autoimmune diseases and tissue pathology. While highlighting what is currently known, we also call attention to the large gaps in knowledge that exist regarding the cellular immune responses during campylobacteriosis.
EPITHELIAL CELLS
Adhesion and extracellular sensing.
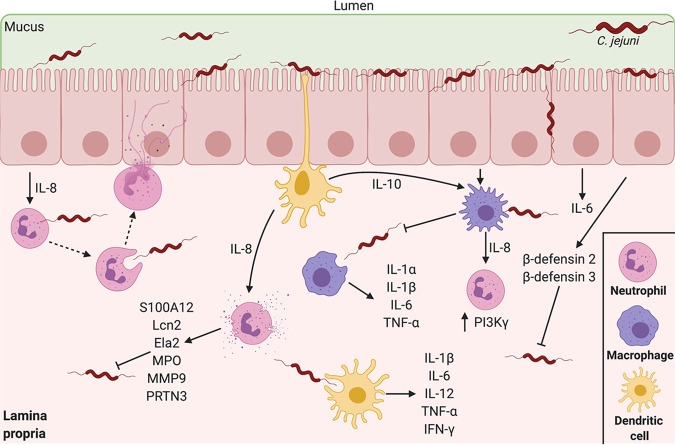
The gastrointestinal tract has been referred to as the largest immune organ in the body, as 65% to 80% of the body’s total immune cells are associated with it (21). Gastrointestinal epithelial cells not only serve as a physical barrier but are also equipped with extracellular and intracellular receptors that can sample the gut lumen and sense invasive pathogens, respectively (22). After being consumed in a relatively low infectious dose from contaminated food or drinking water, C. jejuni is able to penetrate the mucus layer of the distal intestine and proximal colon to reach the apical surface of the intestinal epithelial cells (IECs) (23, 24). To reach the IECs, C. jejuni resists acidic stomach pH conditions through the upregulation of numerous acid stress responses and downregulation of protein synthesis (25). Mucus is crucial in the colonization of C. jejuni, as mucin is a chemoattractant for C. jejuni and facilitates the increased flagellar gene expression and motility that is required to reach the underlying epithelium (26, 27). Once C. jejuni has transited through the mucus layer, the bacterium is able to adhere to and invade into the IECs, which has been reviewed elsewhere (28, 29). To sense the bacterium, Toll-like receptor (TLR) reporter HeLa cells have been found to be stimulated by lysed C. jejuni through the sensing activities of various TLRs, including TLR1/2/6 and TLR4, which detect bacterial lipoproteins and lipopolysaccharides, respectively. Stimulation of these TLRs is transduced through the MyD88 signaling cascade and leads to activation of NF-κB, which drives the production and secretion of IL-8, tumor necrosis factor alpha (TNF-α), IL-1β, monocyte chemoattractant protein 1 (MCP-1), GRO-α, and IL-12p42 (30). The Toll/IL-1R domain-containing adaptor-inducing IFN-β (TRIF) signaling cascade is also activated by TLR4 stimulation, resulting in the production of IFN-β (31–33). Secretion of IL-8 from human IECs stimulated by C. jejuni then promotes chemoattraction and recruitment of abundant neutrophils to the site of infection (34–36). In addition to IL-8, stimulation of IEC TLR1/2/6 results in secretion of IL-6, a proinflammatory cytokine necessary to mount an adaptive immune response (37). C. jejuni adheres to chicken epithelial cells but does not invade them, resulting in chicken CXCLi2 (chCXCLi2) and chCXCLi1 induction; however, their levels were significantly lower than those of human IL-8 (38). Interestingly, C. jejuni is able to evade flagellum-dependent TLR5 recognition through mutations in flagellin that are recognized by the immune system, allowing the bacterium to become highly motile and evasive (39, 40). When TLR2 and −4 are knocked down in IL-10−/− mice and the mice are subsequently infected with C. jejuni, the levels of cytokines TNF-α, IFN-γ, and IL-6 and T lymphocyte recruitment are markedly decreased, demonstrating TLR-dependent responses to animal infection (41). IECs have also been shown to produce beta-defensins 2 and 3 in response to stimulation by C. jejuni; however, the stimulus required for induction remains unknown (42). Beta-defensins are secreted cationic antimicrobial peptides which can bind to negatively charged bacterial membranes, thus driving bacterial cell death and leukocyte chemoattraction (43). These molecules have been shown to have potent anti-Campylobacter activities in vitro.
Invasion and intracellular responses.
Once C. jejuni is at the apical surface, the bacterium invades into IECs, which is dependent upon the secretion of Campylobacter invasion antigen (Cia) proteins, the translocation of which is believed to be through the flagellar type III secretion system (Table 1) (44–46). In addition to promoting cellular invasion, Cia proteins can stimulate p38 mitogen-activated protein (MAP) kinase and extracellular signal-regulated kinases (ERK) pathways to drive further IL-8 secretion from IECs, which results in potent neutrophil chemotaxis to the site of infection (47, 48) (Fig. 1). Ultimately, C. jejuni uses the remodeling of host actin and microtubules to invade IECs, though it does not appear to form actin tails to traffic intracellularly, suggesting that C. jejuni remains confined within a Campylobacter-containing vesicle (CCV) (48, 49).
TABLE 1.
List of C. jejuni effector proteins which influence immune signaling and viability of IECs
| C. jejuni protein(s) | Influence on epithelial cells |
|---|---|
| Cia proteins | CCV formation, MAPK/ERK signaling activation, and IL-8 secretion |
| CDT proteins | Cell cycle arrest, cell distension, and cell swelling; apoptosis; villous widening during IBD formation; and DNA damage |
| Cas9 nuclease | DNA damage, apoptosis, and NF-κB signaling upregulation |
| HtrA serine protease | Occludin and claudin-8 tight junction cleavage; possible upregulation of MCP-1, IL-6, IFN-γ, TNF-α, IL-13, and IL-1β; and epithelial cell adherence |
FIG 1.
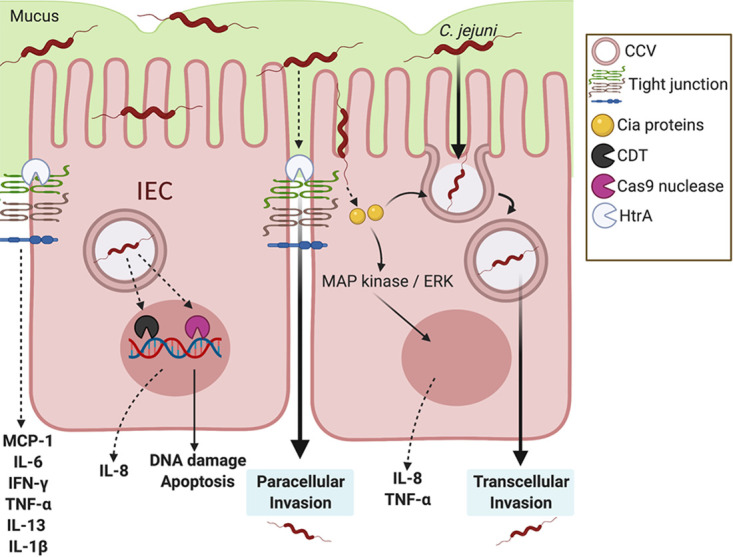
Influence of C. jejuni on intestinal epithelial cell immune signaling. For DNA damage, instead of canonical gastrointestinal effector proteins, C. jejuni is able to secrete the CDT genotoxin and Cas9 nuclease. Both result in DNA damage, apoptosis, and potential upregulation of the neutrophil chemoattractant IL-8. For transcellular invasion: C. jejuni secretes Campylobacter invasion antigens (Cia proteins) possibly through a type III flagellar secretion system, in which it activates the MAPK/ERK pathway. C. jejuni enters the intestinal epithelial cell and is bound within a Campylobacter-containing vesicle (CCV) as it travels through the cell. Microtubules and actin are utilized by C. jejuni to travel from the apical side toward the nucleus and basolateral side of the cell. For paracellular invasion, C. jejuni is able to reach the basolateral side of the IECs by passing between cells through HtrA tight junction cleavage, rather than through the cell itself. This tight junction disruption results in the upregulation of proinflammatory cytokines.
Once intracellular, some C. jejuni strains produce a genotoxin called cytolethal distending toxin (CDT) which can cause cell cycle arrest, cell distension, and cell swelling (Table 1) (50, 51). This cellular response is predicted to result in the disruption of the epithelial barrier and impair signaling pathways that alter the host immune response (52). Using the rat IBS model, CDT was shown to not be necessary for IBS development, but it was involved in villous widening, a characteristic additionally noted in C. jejuni infection of the gnotobiotic piglet model, further demonstrating a potential role for CDT during and after infection (53, 54). Following the release of CDT, the toxin is processed by host Rab7, which has also been shown to be an essential component of the CCV (55). As a result, CDT may have an important role in the development of the CCV in IECs. While the genotoxin has not been fully investigated for its role in disease, it was recently shown that Helicobacter hepaticus CDT leads to the development of nucleoplasmic reticulum, a common feature in cancer cells (56, 57). Indeed, there is some work in germfree ApcMin/+ mice which indicates a potential correlation between tumorigenesis and C. jejuni (58). Therefore, if C. jejuni CDT is capable of the same activity, it may influence colorectal cancer development following infection. Further, because C. jejuni strains lacking CDT still induce DNA damage and disease, the bacterium may employ additional strategies to target host DNA. For example, C. jejuni was recently found to elaborate clustered regularly interspaced palindromic repeat (CRISPR)-associated gene 9 (CjeCas9), associated with outer membrane vesicles, while in IECs (Table 1). Once released, CjeCas9 can target host DNA and cause epithelial cell death and upregulation of proinflammatory gene expression (59, 60). Additionally, other studies have demonstrated that C. jejuni activates caspase-3-dependent apoptosis of IECs; however, the mechanism behind this response remains unknown (61). Since it appears that C. jejuni utilizes numerous systems to damage host DNA and that those responses may promote inflammation, more research should be conducted to comprehensively identify these systems and determine how they influence inflammation and tissue pathology.
In addition to responding to extracellular bacteria, IECs are also capable of sensing intracellular C. jejuni. While intracellular, C. jejuni is capable of activating TLR9, which recognizes intracellular DNA (31). Furthermore, intracellular C. jejuni appears to be sensed through nucleotide-binding oligomerization protein (NOD) receptors. For example, when NOD2−/− mice are infected by C. jejuni, increased bacterial loads and reduced colonic leukocytes are observed (62). While NOD2 is expressed in other immune cells, including macrophages and dendritic cells (DCs), the absence of NOD2 in colonocytes could dampen the host immune response, resulting in increased bacterial burden (63). Indeed, NOD2 results in activation of antibacterial function in IECs and specifically against C. jejuni to some extent (64). NOD1 is also activated in response to C. jejuni and results in decreased intracellular C. jejuni presence and increased IL-8 and hBD2 (65). Interestingly, when C. jejuni transitions from helical to coccoid peptidoglycan, NOD1 and NOD2 have reduced activation and inflammatory signaling (66). As there is a close relationship between NOD stimulation and cytotoxicity, epithelial NOD signaling can be hypothesized to result in tissue pathology within infected individuals (67). By understanding the critical role of epithelial cells in coordinating the inflammatory response classically observed during campylobacteriosis, targeted therapies can be developed to reduce inflammation-driven tissue pathology. While in the CCV, the bacterium can traffic to the basolateral side of the colonocyte and exocytose to the underlying colonic tissue to encounter chemoattracted leukocytes. The exact mechanism of this intracellular trafficking remains poorly understood and is one area of C. jejuni infection biology that needs to be elucidated (49).
C. jejuni has also been observed passing between IECs to reach the basolateral side (68, 69). It was found that barrier dysfunction caused by C. jejuni-induced tight junction disruption results in signaling of proinflammatory cytokines, including MCP-1, IL-6, IFN-γ, TNF-α, IL-13, and IL-1β (70). Specifically, at high temperatures, C. jejuni secretes a serine protease, HtrA, which cleaves occludin and claudin-8 found within tight junctions (Table 1) (71, 72). HtrA has additionally been found to be necessary for increased adherence to avian epithelial cells compared to human epithelial cells, demonstrating the host specificity of HtrA activity (73). As tight junction proteins are essential for regulating intestinal inflammation upon injury, this virulence factor needs to be further investigated for influencing inflammation during campylobacteriosis (74).
As mentioned above, IECs are vital for coordinating the host immune response to Campylobacter infection, which can be inhibited in individuals that are immunosuppressed or have poor nutrition and can result in the inability to combat the infection (75). The role of nutrition in the gastrointestinal response to Campylobacter infection is particularly interesting, since IECs are the point whether nutrients and the bacterium intersect. A potential result of this fact is that Campylobacter infections have been shown to be more prevalent and persistent in malnourished children (6). Related to this, in developing regions, Campylobacter infection is endemic; however, children are more likely to display symptoms than adults, possibly due to the protective immunity resulting from the early exposure to the bacterium (4, 76). In contrast, in the developed world, campylobacteriosis is an acute, inflammatory illness with a greater incidence of the postinfectious disorders mentioned above. A potential dietary driver of these differences is the amount of fiber consumption. Fiber-rich diets allow for greater short-chain fatty acid (SCFA) production by microbial fermentation in the colon, and it has been shown that diets in developing countries are more fiber rich. As a result, patients in these regions may experience less inflammation but greater persistence because of the anti-inflammatory effects of SCFAs, most notably, butyrate (77). Beyond effects on the host, butyrate abundance may also promote colonization by C. jejuni, as the BumSR two-component system has recently been shown to indirectly sense butyrate and upregulate genes essential for colonization of avian and human hosts (78). In addition to SCFA abundance, vitamin C treatment of IL-10−/− mice can decrease C. jejuni loads and significantly reduce the number of apoptotic cells in the colon and secretion of proinflammatory cytokines (TNF-α, IFN-γ, and IL-6) (79). Interestingly, vitamin C deficiencies are more prevalent in developing nations than in developed regions, which suggests that the differences in clinical manifestations between these regions may not be due to vitamin C abundance (80). For the above reasons, there has been an emerging interest in developing dietary strategies that can promote an adequate immune response to eliminate the bacterium while at the same time preventing tissue damaging inflammation. Within C. jejuni-infected intestinal tissue, enteroendocrine cells increase in prevalence 5-fold (81). As these cells have previously been associated with IBS development, this area of research needs to be further investigated (82). As mentioned earlier, an understanding of the intersecting responses of the IECs and the pathogen to their nutritional environments is required to develop such targeted therapies to reduce disease and tissue pathology.
INNATE IMMUNE CELL RESPONSES
Neutrophils.
After Campylobacter successfully breaches the epithelial barrier, neutrophils are the first innate immune cells recruited to the site of infection (83). Neutrophils are produced at high numbers in humans, with around 1011 cells daily, accounting for 50% to 70% of the leukocytes in circulation (84). Neutrophils possess three main antibacterial mechanisms: phagocytosis of microbes, degranulation of antimicrobial proteins, and extrusion of neutrophil extracellular traps (NETs) (85, 86). While these cells have long been noted as simple, transcriptionally inert phagocytes, current research demonstrated their multifunctionality and transcriptional diversity (87). Using human, ferret, cat, porcine ileal loop, and the IL-10−/− mouse models of campylobacteriosis, neutrophils have been consistently shown to migrate and accumulate within the gastrointestinal tissue of infected hosts (10, 88–91). As a result of this trafficking, several indicators of neutrophil involvement during infection have been identified using these models. Furthermore, recent evidence has demonstrated that neutrophil-to-lymphocyte ratios of 3.05 correlate with GBS onset and hyperinflammation (normal ratio is 1.51) (92). As neutrophils are the most numerous leukocytes within colonic tissue during C. jejuni infection and are incredibly proinflammatory, they need to be considered a potential source for acute and chronic diseases and tissue pathology.
Within colonic crypts, neutrophils transmigrate from the basolateral to the apical side of the epithelium, which is dependent on bacterially sourced n-formyl peptides and the host-derived enzyme, 12-lipoxygenase (12-LOX) (93). Furthermore, IEC-dependent secretion of IL-8 results in neutrophil chemotaxis and peaked within the blood and colon at 3 days postinfection, which correlated with the height of C. jejuni fecal loads in the ferret model of campylobacteriosis (90). Using green fluorescent protein (GFP)-labeled C. jejuni in the IL-10−/− mouse model, 99.7% to 100% of CD11b+ Gr-1+ peritoneal neutrophils were found to have engulfed C. jejuni by 4 h postinfection (94). During Campylobacter infection of cats, there appeared to be a close association with neutrophil elastase within the colon and the development of neutrophilic irritable bowel disease (IBD) (95).
At the molecular level, phosphatidylinositol 3-kinase-γ (PI3K-γ)-dependent signaling leads to the recruitment of neutrophils into colonic crypts during C. jejuni infection of IL-10−/− mice, which leads to the development of colitis (89) (Fig. 2). PI3K-γ is highly expressed in numerous immune cells and, via actin polymerization, mediates chemotaxis through G protein-coupled receptors (96, 97). Inhibition of PI3K-γ activity by the pharmacological inhibitor AS252424 resulted in reduced inflammation, neutrophil accumulation, NF-κB activity, and transcript levels of IL-1β, CXCL2, and IL-17α during C. jejuni infection (89). This C. jejuni-induced inflammatory cascade was found to be dependent on mTOR activation, which is a signaling event downstream of PI3K-γ. Further, inactivation of mTOR signaling using rapamycin, a pharmacological inhibitor, led to attenuation of C. jejuni-induced inflammation (98).
FIG 2.
Innate leukocyte responses during early stages of campylobacteriosis. During infection, neutrophils are recruited to the site of infection as a result of IEC IL-8 secretion, leading to elaboration of NETs and degranulation. Macrophages and dendritic cells are then recruited to the site of infection and perform both inflammatory and anti-inflammatory signaling. Within these first days of infection, macrophages, dendritic cells, neutrophils, and colonocyte antimicrobial proteins reduce C. jejuni levels within the infected host. These innate immune responses classically peak on day 3 postinfection, the heightened day of infection in hosts.
Once neutrophils and C. jejuni interact, complement-opsonized cells are phagocytosed, which leads to reactive oxygen species (ROS) generation, resulting in direct bacterial killing and localized tissue damage (99). Interestingly, the ability of neutrophils to kill C. jejuni varies, as some bacteria can escape these bactericidal effects (99). In addition to phagocytosis and direct killing, numerous neutrophil-derived antimicrobial proteins are released into the surrounding tissue and accumulate in the feces of C. jejuni-infected humans, including calgranulin C (S100A12), lipocalin-2 (Lcn2), myeloperoxidase (MPO), and neutrophil elastase (Ela2) (90, 100). Within the C. jejuni-infected porcine ligated loop, numerous neutrophil-derived markers were shown to increase, including matrix metallopeptidase 9 (MMP9), Lcn2, Ela2, and proteinase 3 (PRTN3) (91). Based on the activities of these antimicrobial proteins, their release during infection is likely to contribute to C. jejuni growth restriction, which is supported by recent data showing that these purified components reduced growth in vitro (90, 100). While these antimicrobial proteins are likely released as a result of degranulation, MPO, and Ela2 were also found to colocalize with NETs induced by C. jejuni. Additionally, NET-like structures were found within crypt abscesses of colon tissue isolated from ferrets infected with C. jejuni (100). As the presence of Ela2 and MPO correlates with colonic tissue damage and IBD due to protease and ROS-generating activities, respectively, more research needs to be done on their roles during campylobacteriosis (101, 102). Due to the cytotoxic nature of NETs, it has been hypothesized that NETs contribute to crypt abscess formation and intestinal pathology during campylobacteriosis. As NETs are also associated with numerous autoimmune diseases, these structures could have tremendous influence on the development of the postinfectious disorders mentioned above (103–106). Because of the association between neutrophil activity, inflammation, pathology, and autoimmune development, more research needs to be conducted on C. jejuni-neutrophil interactions.
Eosinophils.
Eosinophils account for 1% to 4% of bone marrow nucleated cells and have been investigated extensively as drivers of asthma and parasite immunity (107, 108). Eosinophils display a wide array of antibacterial activities against both Gram-positive and Gram-negative organisms (109). C. jejuni is a potent activator of eosinophils in vitro, resulting in chemotaxis, a respiratory burst, degranulation, and the release of eosinophil cationic proteins (ECP); however, there has been little direct evidence of eosinophil involvement during campylobacteriosis (110, 111). Interestingly, eosinophils play an important role in the development of IBS and functional dyspepsia, which are both postinfectious disorders associated with C. jejuni infection (4, 54, 112–114). While eosinophils are not abundant, the response of these cells to C. jejuni and their role in gastrointestinal inflammation provide the foundation for hypotheses that eosinophils may contribute to inflammation during infection and/or the development of postinfectious disorders.
Mast cells.
Mast cells are inflammatory granulocytes responsible for the release of histamine and a variety of cytokines (115). While mast cells have been observed in the stool of Campylobacter-infected individuals, it is suspected that they play only a minimal role during infection (28). Interestingly, other gastrointestinal diseases have mast cell involvement, including IBS, a postinfectious disorder that can occur following Campylobacter infection. For example, mast cell proximity to enteric nerves was found to correlate with abdominal pain during IBS (116). Consequently, while mast cells do not appear to be directly involved in campylobacteriosis, their role in gastroenteritis cannot be entirely ignored.
BRIDGING THE GAP BETWEEN INNATE AND ADAPTIVE IMMUNE RESPONSES
Monocytes/macrophages.
In circulation, monocytes make up 2% to 8% of total leukocytes. Monocytes are produced at a rate of 3 × 108 cells/liter of blood per day, maintaining a half-life of around 1 day (117). Once reaching the colon, monocytes can develop into tissue resident macrophages, possessing a half-life of 4 to 6 weeks within the tissue (118). These cells are mononuclear phagocytes with significant roles in tissue homeostasis and inflammation. While monocytes are involved in inflammation and pathogen recognition, monocyte-derived tissue resident memory macrophages provide crucial immunological functions, including tissue repair and promotion of anti-inflammatory signaling pathways (119). Specifically, tissue resident macrophages are responsible for ingesting and degrading dead cells, debris, and foreign material, while also serving as professional antigen presenters and orchestrating the inflammatory immune response within the tissue (120). Within C. jejuni-infected ferrets, mononuclear cell chemotaxis is observed within the blood and colon, where it peaks at day 3 postinfection (90). During IL-10−/− murine infection, 77.0% to 80.0% of CD11b+ Gr-1− peritoneal macrophages had engulfed C. jejuni at 4 h postinfection (94). Interestingly, macrophage uptake of C. jejuni appears to vary among hosts, as both chicken and human macrophages can internalize the bacterium, while mouse and guinea pig macrophages exhibit a reduced capacity to phagocytose C. jejuni (121–123). In contrast, another study determined that acidified nitrite within bone marrow-derived murine macrophages could kill C. jejuni in a nitric oxide synthase 2 (NOS2)-dependent manner (124). As a result, C. jejuni catalase (KatA) activity is essential for intramacrophage persistence, as it is required for ROS detoxification (125). In response to infection, human peripheral blood mononuclear cells (PBMC) were found to secrete elevated levels of IL-8 and IL-6 (126). Secretion of IL-8 was also demonstrated using macrophage-like differentiated THP-1 cells, further supporting the role of neutrophil chemotaxis during infection (127). To sense the bacterium, murine macrophages are activated by hypoacylated C. jejuni LOS via TLR4, which results in secretion of IL-6 and TNF-α (128). Further phagocytosis of the bacterium leads to the secretion of additional proinflammatory cytokines, including IL-1α, IL-1β, IL-6, IL-8, and TNF-α (90, 122, 127). Interestingly, C. jejuni lacking a capsule and O-methyl phosphoramidate (MeOPN) modification elicited enhanced IL-6 and IL-10 transcripts, suggesting that the C. jejuni capsule and modification are involved in immune evasion (122). Further, C. jejuni lacking a capsule resulted in increased TLR4 activation and more severe gastroenteritis in Sigirr−/− mice (129). The result of this stimulation was also observed during ferret infection with C. jejuni, in which numerous macrophage-dependent cytokines were found to be upregulated, including TNF-α and IL-10 (90, 130). Once internalized, C. jejuni activates NOD-1 in macrophages, resulting in enhanced activation markers and potent IL-1β secretion via inflammasome-dependent signaling pathways (131). Additionally, there was a notable positive correlation between intracellular bacteria and NLRP3 activation via Campylobacter LOS; however, lactate dehydrogenase (LDH) secretion was absent, indicating that C. jejuni can activate macrophage inflammasomes without inducing cell death (132, 133). While some strains of C. jejuni are capable of surviving intracellularly within monocytes and inducing apoptosis, differentiated macrophages are efficient at killing intracellular bacteria due to the inability of C. jejuni to avoid delivery to lysosomes (49, 134, 135). Interestingly, Campylobacter DNA is present in CD14+ CD33+ mononuclear cells from C. jejuni-infected GBS patients (136). Additionally, monocyte-to-macrophage ratios are unbalanced during colonic inflammation, with increased monocyte presence and subsequent tissue pathology (137). Recently, macrophage infiltration into peripheral nerves has been strongly associated with GBS development; however, this phenomenon has yet to be investigated during campylobacteriosis (138). As C. jejuni-infected monocytes and macrophages undergo proinflammatory switches, more research is needed to understand the molecular mechanisms of this event.
Dendritic cells.
Monocytes can additionally develop into dendritic cells (DCs), which act as professional antigen-presenting cells that activate the adaptive immune response (139). During infection, DCs likely encounter Campylobacter in the lamina propria intraluminally, as these cells transcytose and sample the intestinal lumen (140). In the colonic lamina propria of C. jejuni-infected mice, anti-inflammatory Siglec-10-expressing CD11c+ CD103+ DCs were found to express IL-10. While IL-10 plays a vital role in resolving intestinal inflammation, Siglec-10-expressing DCs may play an anti-inflammatory role in C. jejuni mucosal immunity; however, the role of these cells shaping campylobacteriosis has yet to be elucidated (141). Once encountered, C. jejuni activates DCs through a unique TLR4-MyD88/TLR4-TRIF cooperative signaling mechanism that is driven by C. jejuni LOS sialylation, which demonstrates that the carbohydrate moiety can modulate DC activation and drive B cell proliferation and T cell polarization (142–144). Further, C. jejuni-stimulated DCs secrete NF-κB-dependent chemokines, including macrophage inflammatory protein 1α (MIP-1α), MIP-1β, RANTES, growth-related oncogene α (GRO-α), IP-10, and monokine induced by gamma interferon (MIG) (145). In order to stimulate secretion of cytokines and chemokines, C. jejuni induces phosphorylation of P38, P44/42, stress-activated protein kinase/Jun N-terminal protein kinase (SAPK/JNK), and mitogen-activated protein kinases (MAPKs) (146). Once activated, DCs efficiently internalize and kill C. jejuni, resulting in significant upregulation of mature phenotype cell surface major histocompatibility complex class II (MHC-II), CD40, CD80, and CD86 (146) (Table 2). Although DCs efficiently secrete cytokines during infection, C. jejuni with capsule and capsule modifications, including O-methyl phosphoramidate modifications, result in diminished cytokine secretion (147). While DCs appear to have anti-inflammatory activities during campylobacteriosis, proinflammatory DCs have been shown to be in significant quantities within damaged colonic tissue in response to pathogen-associated molecular patterns (PAMPs), along with their involvement in the development in GBS and IBS (148–151). Through the secretion of both inflammatory and anti-inflammatory cytokines, along with antigen presentation, DCs play a critical role in shaping campylobacteriosis and setting the stage for postinfection activities.
TABLE 2.
List of cell surface markers characterizing leukocytes and lymphocytes during campylobacteriosis
| Immune cell type | Cell surface markers | Reference(s) |
|---|---|---|
| Neutrophils | CD11b+, CD63+, Gr-1+, CD177+ | 90, 91, 94, 100 |
| Natural killer lymphocytes | CD19-, NKp46+, Siglec-7+, KIR2DS4+ | 154, 157 |
| Monocytes/macrophages | CD11b+, Gr-1−, CD14+, CD33+ | 94, 122, 136 |
| Dendritic cells | CD11c+, CD103+, Siglec-10+, MHC-II+, CD40+, CD80+, CD86+ | 141, 145, 146 |
| T lymphocytes | CD3+, CD19-, CD4+ (Th1, Th17), CD90+, CD8+ | 154 |
| B lymphocytes | CD11b−, CD45R+ | 94 |
NK cells.
Natural killer (NK) cells are large granular lymphocytes that possess an expansive arsenal of cytotoxic and chemoattractant effector functions (152). Within the epithelium and stroma, NK cells interact with antigens of pathogenic and commensal bacteria along with several other host cell types, including epithelial cells, fibroblasts, macrophages, dendritic cells, and T lymphocytes (153). During infection of IL-10−/− mice with C. jejuni, NK cells (CD19− NKp46+ [Table 2]) increased in the colon and mesenteric lymph nodes at days 7 and 11 postinfection but returned to preinfection levels at day 21 (154). Relevant to this timing, it is important to note that in many IL-10−/− mouse studies, C. jejuni infection is persistent and often not self-limiting. During this infection, NK cells secreted IL-22 and IFN-γ, which should result in tissue regeneration, cellular defense, and inflammation (154). NK cells bind to C. jejuni LOS using Siglec-7 molecules, which leads to the promotion of host inflammation and immunity (155). Siglec-7 dampens NK cell activation pathways and cytotoxicity, resulting in reduced inflammation (156). In addition to LOS binding, conserved C. jejuni RecA epitopes presented by HLA-C*05:01 bound strongly to the killer cell immunoglobulin-like receptor KIR2DS4, which led to stimulation of KIR2DS4+ NK cells (157). Taken together, the above responses indicate that NK cells coordinate T lymphocyte responses through antigen presentation and dampen the immune system to benefit the host following C. jejuni infection.
ADAPTIVE IMMUNE RESPONSES
T lymphocyte response and subtype switching.
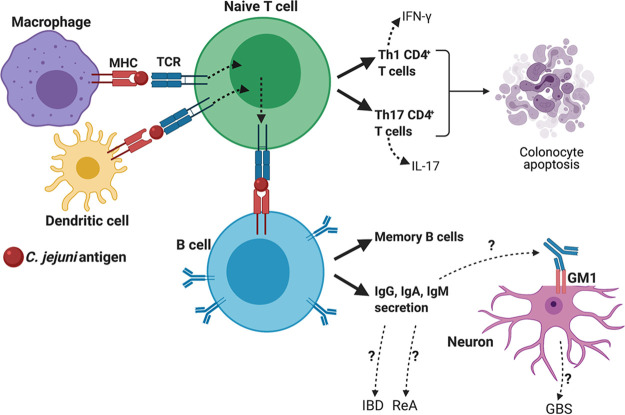
During late stages of infection, T lymphocytes coordinate numerous aspects of the adaptive immune response, including responses to pathogens, allergens, and tumorigenesis. From CD4+ helper T lymphocytes to CD8+ cytotoxic T lymphocytes, these cells play an enormous role in combating infections and developing memory to fight future infections (158, 159). In IL-10−/− mice infected with C. jejuni, the number of T lymphocytes (CD3+ CD19− [Table 2]) increases within the colon and mesenteric lymph nodes at days 7 and 11 postinfection (154). In C. jejuni-infected gnotobiotic IL-10−/− TLR2−/− TLR4−/− mice, significant decreases in both apoptotic cells and T lymphocytes within the colonic tissue were observed, indicating a TLR-dependent mechanism for T lymphocyte recruitment and activation (160). Furthermore, infected IL-10−/− mice treated with a Thy-1 antibody to deplete innate lymphocytes and T lymphocytes developed lower histopathology scores, indicating a potential link between inflammatory T lymphocyte functions and tissue pathology (154). During the later stages of infection, mature dendritic cells secrete IL-12 to promote naive T cells to differentiate into T helper 1 (Th1) cells to secrete IFN-γ (144, 146). Once differentiated into Th1 lymphocytes, Th1-derived cytokines peaked 7 to 14 days postinfection, with IFN-γ+ CD4+ T cells being the most abundant lymphocyte in C. jejuni-infected humans (161) (Fig. 3). Furthermore, infected IL-10−/− mice exhibited significantly higher levels of type 1 and 17 cytokines, but not type 2 cytokines, within the colonic tissue. Based on these observations, it can be hypothesized that campylobacteriosis is primarily a Th1 lymphocyte disease; however, Th17 lymphocytes additionally develop. This is supported by data showing that C. jejuni capsular mutants exhibit elevated IL-17 secretion due to increased recognition by CD4+ Th17 cells in the lamina propria in infected mice; this response was also observed in C. jejuni-colonized chickens (162–164). Within the lumen of C. jejuni-infected porcine intestinal loops, IL-17A was detected at significantly higher concentrations than in the control loop (91). This demonstrates that there is a specific and localized Th17 lymphocyte response during campylobacteriosis. Of the T cells produced during human Campylobacter infection, patients can possess more Vδ1 γδ (Vδ1) CD8+ T cells, which is particularly interesting because these cell types are associated with cytotoxicity and autoimmunity, including Guillain-Barré syndrome and IBD (165–167). Within the intestines and colon, Vδ1 T cell receptor (TCR) can be activated by proinflammatory cytokines and activation of Vδ1 cells by DCs is achieved using microbial antigens, especially lipid extracts from Gram-negative bacteria. This recognition is key for the potent host defense and immunoregulation attributed to Vδ1 T lymphocytes (168). Furthermore, T lymphocytes may be able to recognize C. jejuni LOS via TLR4, an antigen associated with the GM1 ganglioside mimicry mentioned earlier (169). Therefore, T lymphocytes may play a crucial role in the tissue pathology and the development of autoantibodies following campylobacteriosis.
FIG 3.
Generation of the adaptive immune response through antigen presentation during campylobacteriosis. During infection, macrophages and dendritic cells present processed C. jejuni antigens to naive T lymphocytes. Through T cell differentiation, naive T cells develop into Th1 and Th17 CD4+ T lymphocytes, resulting in IFN-γ and IL-17 secretion, respectively. These activated T lymphocytes can then cause the upregulation of proapoptotic pathways within colonocytes. Additionally, T cells present processed C. jejuni antigen to B cells, leading to proliferation of memory B cells and C. jejuni-specific IgG, IgA, and IgM secreting plasma cells. These antibodies have been hypothesized to target self-GM1 gangliosides on neurons, leading to the generation of numerous autoimmune diseases, such as Guillain-Barré syndrome.
B cell response and antibody production.
Initiation of humoral immune responses requires that antigen-reactive B lymphocytes come into contact with antigens. These interactions occur within secondary lymphoid organs, where B cells are trained by antigen-presenting lymphoid tissue (165). Once in the periphery, these stimulated B cells produce a diverse array of antibodies (approximately 1012 variants) (170, 171). During human infection with C. jejuni, titers of serum IgG, IgA, and IgM antibodies specific to bacterial epitopes peak around 11 days postinfection (10). These results were supported using the ferret model of campylobacteriosis in which IgA antigen-secreting cells (ASC) were found to increase during infection, which correlated with increased serum and fecal IgA and IgG levels at 9 days postinfection (88). In terms of immunodominant epitopes, human serum antibodies were detected to be specific to 62-kDa flagellin, an uncharacterized 40-kDa antigen, and an uncharacterized 29-kDa antigen, while human salivary antibodies were specific to flagellin, a major outer membrane protein (MOMP), and the same uncharacterized 40-kDa antigen. These antibodies were able to be detected for up to a year postinfection (172). Of the antibodies produced, autoreactive IgG1 antibodies are the most numerous subtype following campylobacteriosis (154). Because there is a positive correlation between GBS severity and IgG1 levels, it has been hypothesized that this response is important to the development of GBS following C. jejuni infection, which can occur in 1/900 individuals. This hypothesis is largely due to the observation that of the IgA and IgG antibodies that are produced during infection, several can be cross-reactive to the human GM1 gangliosides in neurons (173, 174). This response is also likely due to some C. jejuni LOS core oligosaccharides mimicking human ganglioside GM1 structures (175–178) (Table 3). Furthermore, antibody cross-reactivity has been reported in a recent case in which an individual with C. jejuni gastroenteritis developed encephalopathy (179). As encephalopathy is also associated with autoantibodies toward GM1 gangliosides, it can be hypothesized that this target is a critical component of developing postinfectious neurological conditions (180). Because of these findings, more research needs to be conducted to understand the molecular and genetic bases of these responses at the gut-neuron axis.
TABLE 3.
List of potential factors of campylobacteriosis leading to the generation of postinfection disorders
| Postinfection disorder | Factors during campylobacteriosis | Reference(s) |
|---|---|---|
| Guillain-Barré syndrome | Self-reactive antibody generation from LOS-ganglioside mimics, neutrophil-to-lymphocyte ratio | 154 |
| Colorectal cancer | Cytolethal distending toxin, NET formation | 58, 100 |
| Irritable bowel syndrome | NET formation, CDT-dependent villous widening | 53, 100 |
| Reactive arthritis | Interaction with host HLA-B27 | 12 |
CONCLUSIONS AND FUTURE RESEARCH
Although Campylobacter is the leading cause of bacterium-mediated gastroenteritis in humans, the host immune response remains poorly understood (181, 182). Despite C. jejuni lacking classical virulence factors possessed by better-studied gastrointestinal pathogens, it still colonizes the human gastrointestinal tract and induces a robust immune response that appears to be responsible for pronounced colonic and extraintestinal site immunopathology. While C. jejuni is considered a commensal organism within chickens, recent findings demonstrate that this paradigm is much more complicated than previously described (183). In line with this, there is a large gap in knowledge for the immune responses of chicken heterophils in response to C. jejuni, as they undergo numerous processes similar to those in human neutrophils (184, 185). Over the last 2 decades, there has been a tremendous increase in our understanding of both innate and adaptive immunity regarding bacterial pathogens. As a result, the C. jejuni field is well positioned to begin understanding the bacterial and host factors that lead to both colonic and systemic inflammation, as well as what strategies and therapies may be effective at reducing these effects. For example, the recent discovery of innate memory may provide insights into the autoimmunity that is characteristic of the postinfectious disorders mentioned above (186, 187). Further, there have also been advances in our understanding of neutrophil biology, including transcriptional and epigenetic changes that lead to neutrophil subtype diversity (188–190). Neutrophil subtype diversity has been demonstrated during infection with Helicobacter pylori, which is closely related to C. jejuni, and this finding may therefore have tremendous implications for the development of campylobacteriosis (191). Along this line of inquiry, because campylobacteriosis appears to be an inflammatory disease, there is a need to further understand immune signaling pathways and transcriptional changes in leukocytes that lead to the onset of inflammation. With tremendous advances in sequencing, these effects can now be understood in both in vitro and in vivo systems. In addition, since C. jejuni colonizes numerous mammals with various clinical signs, understanding the response of each host to the bacterium may provide insights into the shared or divergent evolution of immune mechanisms in hosts. Furthermore, because symptoms and disease progression may vary depending on diet, as is observed in the developed versus developing worlds, it is necessary that we understand how dietary or microbiome variations affect the immunological processes mentioned above. By advancing our understanding of cellular immunity during and after infection, the field can begin devising approaches that allow for antibacterial levels of inflammation without the levels or specificities that lead to immunopathology.
ACKNOWLEDGMENTS
Support was provided by the University of Tennessee as start-up funds to J.G.J. We thank Trevor Hancock for his assistance with the preparation of this review. Figures were created with BioRender software.
Contributor Information
Jeremiah G. Johnson, Email: jjohn358@utk.edu.
Karen M. Ottemann, University of California, Santa Cruz
REFERENCES
- 1.Man SM. 2011. The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol 8:669–685. 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 2015. WHO estimates of the global burden of foodborne diseases. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Janssen R, Krogfelt KA, Cawthraw SA, van Pelt W, Wagenaar JA, Owen RJ. 2008. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin Microbiol Rev 21:505–518. 10.1128/CMR.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. 2015. Global epidemiology of Campylobacter infection. Clin Microbiol Rev 28:687–720. 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damborg P, Olsen KEP, Moller Nielsen E, Guardabassi L. 2004. Occurrence of Campylobacter jejuni in pets living with human patients infected with C. jejuni. J Clin Microbiol 42:1363–1364. 10.1128/JCM.42.3.1363-1364.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platts-Mills JA, Kosek M. 2014. Update on the burden of Campylobacter in developing countries. Curr Opin Infect Dis 27:444–450. 10.1097/QCO.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Facciolà A, Riso R, Avventuroso E, Visalli G, Delia SA, Laganà P. 2017. Campylobacter: from microbiology to prevention. J Prev Med Hyg 58:E79–E92. [PMC free article] [PubMed] [Google Scholar]
- 8.Stahl M, Vallance BA. 2015. Insights into Campylobacter jejuni colonization of the mammalian intestinal tract using a novel mouse model of infection. Gut Microbes 6:143–148. 10.1080/19490976.2015.1016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stahl M, Frirdich E, Vermeulen J, Badayeva Y, Li X, Vallance BA, Gaynor EC. 2016. The helical shape of Campylobacter jejuni promotes in vivo pathogenesis by aiding transit through intestinal mucus and colonization of crypts. Infect Immun 84:3399–3407. 10.1128/IAI.00751-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. 1988. Experimental Campylobacter jejuni infection in humans. J Infect Dis 157:472–479. 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 11.Nachamkin I, Allos BM, Ho T. 1998. Campylobacter species and Guillain-Barré syndrome. Clin Microbiol Rev 11:555–567. 10.1128/CMR.11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pope JE, Krizova A, Garg AX, Thiessen-Philbrook H, Ouimet JM. 2007. Campylobacter reactive arthritis: a systematic review. Semin Arthritis Rheum 37:48–55. 10.1016/j.semarthrit.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyati KK, Nyati R. 2013. Role of Campylobacter jejuni infection in the pathogenesis of Guillain-Barré syndrome: an update. Biomed Res Int 2013:852195. 10.1155/2013/852195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scallan Walter EJ, Crim SM, Bruce BB, Griffin PM. 2020. Incidence of Campylobacter-associated Guillain-Barré syndrome estimated from health insurance data. Foodborne Pathog Dis 17:23–28. 10.1089/fpd.2019.2652. [DOI] [PubMed] [Google Scholar]
- 15.Buzby JC, Allos BM, Roberts T. 1997. The economic burden of Campylobacter‐associated Guillain‐Barré syndrome. J Infect Dis 176:S192–S197. 10.1086/513785. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen HL, Dalager-Pedersen M, Nielsen H. 2020. High risk of microscopic colitis after Campylobacter concisus infection: population-based cohort study. Gut 69:1952–1958. 10.1136/gutjnl-2019-319771. [DOI] [PubMed] [Google Scholar]
- 17.Giallourou N, Medlock GL, Bolick DT, Medeiros PH, Ledwaba SE, Kolling GL, Tung K, Guerry P, Swann JR, Guerrant RL. 2018. A novel mouse model of Campylobacter jejuni enteropathy and diarrhea. PLoS Pathog 14:e1007083. 10.1371/journal.ppat.1007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson SA, Gaynor EC. 2008. Campylobacter jejuni host tissue tropism: a consequence of its low-carb lifestyle? Cell Host Microbe 4:409–410. 10.1016/j.chom.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowdhury MN. 1984. Campylobacter jejuni enteritis; a review. Trop Geogr Med 36:215–222. [PubMed] [Google Scholar]
- 20.Young KT, Davis LM, DiRita VJ. 2007. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol 5:665–679. 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 21.Brandtzaeg P, Halstensen TS, Kett K, Krajči P, Kvale D, Rognum TO, Scott H, Sollid LM. 1989. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology 97:1562–1584. 10.1016/0016-5085(89)90406-X. [DOI] [PubMed] [Google Scholar]
- 22.Tang X, Liu H, Yang S, Li Z, Zhong J, Fang R. 2016. Epidermal growth factor and intestinal barrier function. Mediators Inflamm 2016:1927348. 10.1155/2016/1927348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang C, Miller JF. 2006. Campylobacter jejuni colonization of mice with limited enteric flora. Infect Immun 74:5261–5271. 10.1128/IAI.01094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teunis PFM, Bonačić Marinović A, Tribble DR, Porter CK, Swart A. 2018. Acute illness from Campylobacter jejuni may require high doses while infection occurs at low doses. Epidemics 24:1–20. 10.1016/j.epidem.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Mills DC, Gundogdu O, Elmi A, Bajaj-Elliott M, Taylor PW, Wren BW, Dorrell N. 2012. Increase in Campylobacter jejuni invasion of intestinal epithelial cells under low-oxygen coculture conditions that reflect the in vivo environment. Infect Immun 80:1690–1698. 10.1128/IAI.06176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid AN, Pandey R, Palyada K, Naikare H, Stintzi A. 2008. Identification of Campylobacter jejuni genes involved in the response to acidic pH and stomach transit. Appl Environ Microbiol 74:1583–1597. 10.1128/AEM.01507-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao R, Burr DH, Doig P, Trust TJ, Niu H, Guerry P. 1994. Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells. Mol Microbiol 14:883–893. 10.1111/j.1365-2958.1994.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 28.Hendrixson DR, DiRita VJ. 2004. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract: C. jejuni commensal colonization. Mol Microbiol 52:471–484. 10.1111/j.1365-2958.2004.03988.x. [DOI] [PubMed] [Google Scholar]
- 29.Lugert R, Gross U, Zautner AE. 2015. Campylobacter jejuni: components for adherence to and invasion of eukaryotic cells. Berl Munch Tierarztl Wochenschr 128:90–97. [PubMed] [Google Scholar]
- 30.Konkel ME, Talukdar PK, Negretti NM, Klappenbach CM. 2020. Taking control: Campylobacter jejuni binding to fibronectin sets the stage for cellular adherence and invasion. Front Microbiol 11:564. 10.3389/fmicb.2020.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Zoete MR, Keestra AM, Roszczenko P, van Putten JPM. 2010. Activation of human and chicken Toll-like receptors by Campylobacter spp. Infect Immun 78:1229–1238. 10.1128/IAI.00897-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu S, Gao N. 2015. Compartmentalizing intestinal epithelial cell Toll-like receptors for immune surveillance. Cell Mol Life Sci 72:3343–3353. 10.1007/s00018-015-1931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu L, Hickey TE. 2005. Campylobacter jejuni induces secretion of proinflammatory chemokines from human intestinal epithelial cells. Infect Immun 73:4437–4440. 10.1128/IAI.73.7.4437-4440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hickey TE, Baqar S, Bourgeois AL, Ewing CP, Guerry P. 1999. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infect Immun 67:88–93. 10.1128/IAI.67.1.88-93.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Köhidai L, Csaba G. 1998. Chemotaxis and chemotactic selection induced with cytokines (IL-8, RANTES and TNF-alpha) in the unicellular Tetrahymena pyriformis. Cytokine 10:481–486. 10.1006/cyto.1997.0328. [DOI] [PubMed] [Google Scholar]
- 36.Hickey TE, McVeigh AL, Scott DA, Michielutti RE, Bixby A, Carroll SA, Bourgeois AL, Guerry P. 2000. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect Immun 68:6535–6541. 10.1128/iai.68.12.6535-6541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friis LM, Keelan M, Taylor DE. 2009. Campylobacter jejuni drives MyD88-independent interleukin-6 secretion via Toll-like receptor 2. Infect Immun 77:1553–1560. 10.1128/IAI.00707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larson CL, Shah DH, Dhillon AS, Call DR, Ahn S, Haldorson GJ, Davitt C, Konkel ME. 2008. Campylobacter jejuni invade chicken LMH cells inefficiently and stimulate differential expression of the chicken CXCLi1 and CXCLi2 cytokines. Microbiology (Reading) 154:3835–3847. 10.1099/mic.0.2008/021279-0. [DOI] [PubMed] [Google Scholar]
- 39.de Zoete MR, Keestra AM, Wagenaar JA, van Putten JPM. 2010. Reconstitution of a functional Toll-like receptor 5 binding site in Campylobacter jejuni flagellin. J Biol Chem 285:12149–12158. 10.1074/jbc.M109.070227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreutzberger MAB, Ewing C, Poly F, Wang F, Egelman EH. 2020. Atomic structure of the Campylobacter jejuni flagellar filament reveals how ε Proteobacteria escaped Toll-like receptor 5 surveillance. Proc Natl Acad Sci U S A 117:16985–16991. 10.1073/pnas.2010996117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haag L-M, Fischer A, Otto B, Grundmann U, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM. 2012. Campylobacter jejuni infection of infant mice: acute enterocolitis is followed by asymptomatic intestinal and extra-intestinal immune responses. Eur J Microbiol Immunol (Bp) 2:2–11. 10.1556/EuJMI.2.2012.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zilbauer M, Dorrell N, Boughan PK, Harris A, Wren BW, Klein NJ, Bajaj-Elliott M. 2005. Intestinal innate immunity to Campylobacter jejuni results in induction of bactericidal human beta-defensins 2 and 3. Infect Immun 73:7281–7289. 10.1128/IAI.73.11.7281-7289.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cobo E, Chadee K. 2013. Antimicrobial human β-defensins in the colon and their role in infectious and non-infectious diseases. Pathogens 2:177–192. 10.3390/pathogens2010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konkel ME, Klena JD, Rivera-Amill V, Monteville MR, Biswas D, Raphael B, Mickelson J. 2004. Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J Bacteriol 186:3296–3303. 10.1128/JB.186.11.3296-3303.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grant CC, Konkel ME, Cieplak W, Tompkins LS. 1993. Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect Immun 61:1764–1771. 10.1128/IAI.61.5.1764-1771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buelow DR, Christensen JE, Neal-McKinney JM, Konkel ME. 2011. Campylobacter jejuni survival within human epithelial cells is enhanced by the secreted protein CiaI. Mol Microbiol 80:1296–1312. 10.1111/j.1365-2958.2011.07645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samuelson DR, Eucker TP, Bell JA, Dybas L, Mansfield LS, Konkel ME. 2013. The Campylobacter jejuni CiaD effector protein activates MAP kinase signaling pathways and is required for the development of disease. Cell Commun Signal 11:79. 10.1186/1478-811X-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watson RO, Galán JE. 2008. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog 4:e14. 10.1371/journal.ppat.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopecko DJ, Hu L, Zaal KJM. 2001. Campylobacter jejuni—microtubule-dependent invasion. Trends Microbiol 9:389–396. 10.1016/S0966-842X(01)02107-2. [DOI] [PubMed] [Google Scholar]
- 50.Lara-Tejero M, Galán JE. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354–357. 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- 51.Lara-Tejero M, Galán JE. 2001. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect Immun 69:4358–4365. 10.1128/IAI.69.7.4358-4365.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scuron MD, Boesze-Battaglia K, Dlakić M, Shenker BJ. 2016. The cytolethal distending toxin contributes to microbial virulence and disease pathogenesis by acting as a tri-perditious toxin. Front Cell Infect Microbiol 6:168. 10.3389/fcimb.2016.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morales W, Pimentel M, Hwang L, Kunkel D, Pokkunuri V, Basseri B, Low K, Wang H, Conklin JL, Chang C. 2011. Acute and chronic histological changes of the small bowel secondary to C. jejuni infection in a rat model for post-infectious IBS. Dig Dis Sci 56:2575–2584. 10.1007/s10620-011-1662-6. [DOI] [PubMed] [Google Scholar]
- 54.de Vries SP, Gupta S, Baig A, Wright E, Wedley A, Jensen AN, Lora LL, Humphrey S, Skovgård H, Macleod K, Pont E, Wolanska DP, L’Heureux J, Mobegi FM, Smith DGE, Everest P, Zomer A, Williams N, Wigley P, Humphrey T, Maskell DJ, Grant AJ. 2017. Genome-wide fitness analyses of the foodborne pathogen Campylobacter jejuni in in vitro and in vivo models. Sci Rep 7:1251. 10.1038/s41598-017-01133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DiRienzo J. 2014. Uptake and processing of the cytolethal distending toxin by mammalian cells. Toxins (Basel) 6:3098–3116. 10.3390/toxins6113098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azzi-Martin L, He W, Péré-Védrenne C, Korolik V, Alix C, Prochazkova-Carlotti M, Morel J-L, Le Roux-Goglin E, Lehours P, Djavaheri-Mergny M, Grosset CF, Varon C, Dubus P, Ménard A. 2019. Cytolethal distending toxin induces the formation of transient messenger-rich ribonucleoprotein nuclear invaginations in surviving cells. PLoS Pathog 15:e1007921. 10.1371/journal.ppat.1007921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malhas A, Goulbourne C, Vaux DJ. 2011. The nucleoplasmic reticulum: form and function. Trends Cell Biol 21:362–373. 10.1016/j.tcb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 58.He Z, Gharaibeh RZ, Newsome RC, Pope JL, Dougherty MW, Tomkovich S, Pons B, Mirey G, Vignard J, Hendrixson DR, Jobin C. 2019. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut 68:289–300. 10.1136/gutjnl-2018-317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saha C, Horst-Kreft D, Kross I, van der Spek PJ, Louwen R, van Baarlen P. 2020. Campylobacter jejuni Cas9 modulates the transcriptome in Caco-2 intestinal epithelial cells. Genes 11:1193. 10.3390/genes11101193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saha C, Mohanraju P, Stubbs A, Dugar G, Hoogstrate Y, Kremers G-J, van Cappellen WA, Horst-Kreft D, Laffeber C, Lebbink JHG, Bruens S, Gaskin D, Beerens D, Klunder M, Joosten R, Demmers JAA, van Gent D, Mouton JW, van der Spek PJ, van der Oost J, van Baarlen P, Louwen R. 2020. Guide-free Cas9 from pathogenic Campylobacter jejuni bacteria causes severe damage to DNA. Sci Adv 6:eaaz4849. 10.1126/sciadv.aaz4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butkevych E, Lobo de Sá FD, Nattramilarasu PK, Bücker R. 2020. Contribution of epithelial apoptosis and subepithelial immune responses in Campylobacter jejuni-induced barrier disruption. Front Microbiol 11:344. 10.3389/fmicb.2020.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bereswill S, Grundmann U, Alutis ME, Fischer A, Heimesaat MM. 2017. Campylobacter jejuni infection of conventionally colonized mice lacking nucleotide-oligomerization-domain-2. Gut Pathog 9:5. 10.1186/s13099-017-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moreira LO, Zamboni DS. 2012. NOD1 and NOD2 signaling in infection and inflammation. Front Immunol 3:328. 10.3389/fimmu.2012.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnich N, Aguirre JE, Reinecker H-C, Xavier R, Podolsky DK. 2005. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor–κB activation in muramyl dipeptide recognition. J Cell Biol 170:21–26. 10.1083/jcb.200502153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zilbauer M, Dorrell N, Elmi A, Lindley KJ, Schüller S, Jones HE, Klein NJ, Núnez G, Wren BW, Bajaj-Elliott M. 2007. A major role for intestinal epithelial nucleotide oligomerization domain 1 (NOD1) in eliciting host bactericidal immune responses to Campylobacter jejuni. Cell Microbiol 9:2404–2416. 10.1111/j.1462-5822.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- 66.Frirdich E, Biboy J, Pryjma M, Lee J, Huynh S, Parker CT, Girardin SE, Vollmer W, Gaynor EC. 2019. The Campylobacter jejuni helical to coccoid transition involves changes to peptidoglycan and the ability to elicit an immune response. Mol Microbiol 112:280–301. 10.1111/mmi.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heim VJ, Stafford CA, Nachbur U. 2019. NOD signaling and cell death. Front Cell Dev Biol 7:208. 10.3389/fcell.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Konkel ME, Mead DJ, Hayes SF, Cieplak W. 1992. Translocation of Campylobacter jejuni across human polarized epithelial cell monolayer cultures. J Infect Dis 166:308–315. 10.1093/infdis/166.2.308. [DOI] [PubMed] [Google Scholar]
- 69.Hatayama S, Shimohata T, Amano S, Kido J, Nguyen AQ, Sato Y, Kanda Y, Tentaku A, Fukushima S, Nakahashi M, Uebanso T, Mawatari K, Takahashi A. 2018. Cellular tight junctions prevent effective Campylobacter jejuni invasion and inflammatory barrier disruption promoting bacterial invasion from lateral membrane in polarized intestinal epithelial cells. Front Cell Infect Microbiol 8:15. 10.3389/fcimb.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmidt A-M, Escher U, Mousavi S, Boehm M, Backert S, Bereswill S, Heimesaat MM. 2019. Protease activity of Campylobacter jejuni HtrA modulates distinct intestinal and systemic immune responses in infected secondary abiotic IL-10 deficient mice. Front Cell Infect Microbiol 9:79. 10.3389/fcimb.2019.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harrer A, Bücker R, Boehm M, Zarzecka U, Tegtmeyer N, Sticht H, Schulzke JD, Backert S. 2019. Campylobacter jejuni enters gut epithelial cells and impairs intestinal barrier function through cleavage of occludin by serine protease HtrA. Gut Pathog 11:4. 10.1186/s13099-019-0283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharafutdinov I, Esmaeili DS, Harrer A, Tegtmeyer N, Sticht H, Backert S. 2020. Campylobacter jejuni serine protease HtrA cleaves the tight junction component claudin-8. Front Cell Infect Microbiol 10:590186. 10.3389/fcimb.2020.590186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simson D, Boehm M, Backert S. 2020. HtrA‐dependent adherence and invasion of Campylobacter jejuni in human vs avian cells. Lett Appl Microbiol 70:326–330. 10.1111/lam.13277. [DOI] [PubMed] [Google Scholar]
- 74.Slifer ZM, Blikslager AT. 2020. The integral role of tight junction proteins in the repair of injured intestinal epithelium. Int J Mol Sci 21:972. 10.3390/ijms21030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Molina J-M, Casin I, Hausfater P, Giretti E, Welker Y, Decazes J-M, Garrait V, Lagrange P, Modaï J. 1995. Campylobacter infections in HIV-infected patients: clinical and bacteriological features. AIDS 9:881–886. 10.1097/00002030-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 76.McMurry TL, McQuade ETR, Liu J, Kang G, Kosek MN, Lima AAM, Bessong PO, Samie A, Haque R, Mduma ER, Leite JP, Bodhidatta L, Iqbal NT, Page N, Kiwelu I, Bhutta ZA, Ahmed T, Houpt ER, Platts-Mills JA. 9 October 2020. Duration of postdiarrheal enteric pathogen carriage in young children in low-resource settings. Clin Infect Dis 10.1093/cid/ciaa1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. 2012. Diversity, stability and resilience of the human gut microbiota. Nature 489:220–230. 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goodman KN, Powers MJ, Crofts AA, Trent MS, Hendrixson DR. 2020. Campylobacter jejuni BumSR directs a response to butyrate via sensor phosphatase activity to impact transcription and colonization. Proc Natl Acad Sci U S A 117:11715–11726. 10.1073/pnas.1922719117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mousavi S, Escher U, Thunhorst E, Kittler S, Kehrenberg C, Bereswill S, Heimesaat MM. 2020. Vitamin C alleviates acute enterocolitis in Campylobacter jejuni infected mice. Sci Rep 10:2921. 10.1038/s41598-020-59890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rowe S, Carr AC. 2020. Global vitamin C status and prevalence of deficiency: a cause for concern? Nutrients 12:2008. 10.3390/nu12072008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spiller RC. 2000. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47:804–811. 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Worthington JJ, Reimann F, Gribble FM. 2018. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol 11:3–20. 10.1038/mi.2017.73. [DOI] [PubMed] [Google Scholar]
- 83.Kolaczkowska E, Kubes P. 2013. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13:159–175. 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 84.Teng Y, Luo HR, Kambara H. 2017. Heterogeneity of neutrophil spontaneous death. Am J Hematol 92:E156–E159. 10.1002/ajh.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hidalgo A, Chilvers ER, Summers C, Koenderman L. 2019. The neutrophil life cycle. Trends Immunol 40:584–597. 10.1016/j.it.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 86.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535. 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 87.Thomas CJ, Schroder K. 2013. Pattern recognition receptor function in neutrophils. Trends Immunol 34:317–328. 10.1016/j.it.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 88.Nemelka KW, Brown AW, Wallace SM, Jones E, Asher LV, Pattarini D, Applebee L, Gilliland TC, Guerry P, Baqar S. 2009. Immune response to and histopathology of Campylobacter jejuni infection in ferrets (Mustela putorius furo). Comp Med 59:363–371. [PMC free article] [PubMed] [Google Scholar]
- 89.Sun X, Liu B, Sartor RB, Jobin C. 2013. Phosphatidylinositol 3-kinase-γ signaling promotes Campylobacter jejuni–induced colitis through neutrophil recruitment in mice. J Immunol 190:357–365. 10.4049/jimmunol.1201825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shank JM, Kelley BR, Jackson JW, Tweedie JL, Franklin D, Damo SM, Gaddy JA, Murphy CN, Johnson JG. 2018. The host antimicrobial protein calgranulin C participates in the control of Campylobacter jejuni growth via zinc sequestration. Infect Immun 86:e00234-18. 10.1128/IAI.00234-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Negretti NM, Ye Y, Malavasi LM, Pokharel SM, Huynh S, Noh S, Klima CL, Gourley CR, Ragle CA, Bose S, Looft T, Parker CT, Clair G, Adkins JN, Konkel ME. 2020. A porcine ligated loop model reveals new insight into the host immune response against Campylobacter jejuni. Gut Microbes 12:1–25. 10.1080/19490976.2020.1814121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang Y, Ying Z, Quan W, Xiang W, Xie D, Weng Y, Li X, Li J, Zhang X. 2018. The clinical significance of neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio in Guillain–Barré syndrome. Int J Neurosci 128:729–735. 10.1080/00207454.2017.1418342. [DOI] [PubMed] [Google Scholar]
- 93.Murphy H, Cogan T, Humphrey T. 2011. Direction of neutrophil movements by Campylobacter-infected intestinal epithelium. Microbes Infect 13:42–48. 10.1016/j.micinf.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 94.Mixter PF, Klena JD, Flom GA, Siegesmund AM, Konkel ME. 2003. In vivo tracking of Campylobacter jejuni by using a novel recombinant expressing green fluorescent protein. Appl Environ Microbiol 69:2864–2874. 10.1128/aem.69.5.2864-2874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maunder CL, Reynolds ZF, Peacock L, Hall EJ, Day MJ, Cogan TA. 2016. Campylobacter species and neutrophilic inflammatory bowel disease in cats. J Vet Intern Med 30:996–1001. 10.1111/jvim.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P, Schmid MC, Pink M, Winkler DG, Rausch M, Palombella VJ, Kutok J, McGovern K, Frazer KA, Wu X, Karin M, Sasik R, Cohen EEW, Varner JA. 2016. PI3Kγ is a molecular switch that controls immune suppression. Nature 539:437–442. 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rickert P, Weiner OD, Wang F, Bourne HR, Servant G. 2000. Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products. Trends Cell Biol 10:466–473. 10.1016/S0962-8924(00)01841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun X, Threadgill D, Jobin C. 2012. Campylobacter jejuni induces colitis through activation of mammalian target of rapamycin signaling. Gastroenterology 142:86–95.e5. 10.1053/j.gastro.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walan A, Dahlgren C, Kihlström E, Stendahl O, Lock R. 1992. Phagocyte killing of Campylobacter jejuni in relation to oxidative activation. APMIS 100:424–430. 10.1111/j.1699-0463.1992.tb00893.x. [DOI] [PubMed] [Google Scholar]
- 100.Callahan S, Doster RS, Jackson JW, Kelley BR, Gaddy JA, Johnson JG. 2020. Induction of neutrophil extracellular traps by Campylobacter jejuni. Cell Microbiol 22:e13210. 10.1111/cmi.13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barry R, Ruano-Gallego D, Radhakrishnan ST, Lovell S, Yu L, Kotik O, Glegola-Madejska I, Tate EW, Choudhary JS, Williams HRT, Frankel G. 2020. Faecal neutrophil elastase-antiprotease balance reflects colitis severity. Mucosal Immunol 13:322–333. 10.1038/s41385-019-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chami B, Martin NJJ, Dennis JM, Witting PK. 2018. Myeloperoxidase in the inflamed colon: a novel target for treating inflammatory bowel disease. Arch Biochem Biophys 645:61–71. 10.1016/j.abb.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 103.Papayannopoulos V. 2018. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18:134–147. 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 104.Dinallo V, Marafini I, Di Fusco D, Laudisi F, Franzè E, Di Grazia A, Figliuzzi MM, Caprioli F, Stolfi C, Monteleone I, Monteleone G. 2019. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohns Colitis 13:772–784. 10.1093/ecco-jcc/jjy215. [DOI] [PubMed] [Google Scholar]
- 105.Euler M, Hoffmann MH. 2019. The double-edged role of neutrophil extracellular traps in inflammation. Biochem Soc Trans 47:1921–1930. 10.1042/BST20190629. [DOI] [PubMed] [Google Scholar]
- 106.Li T, Wang C, Liu Y, Li B, Zhang W, Wang L, Yu M, Zhao X, Du J, Zhang J, Dong Z, Jiang T, Xie R, Ma R, Fang S, Zhou J, Shi J. 2020. Neutrophil extracellular traps induce intestinal damage and thrombotic tendency in inflammatory bowel disease. J Crohns Colitis 14:240–253. 10.1093/ecco-jcc/jjz132. [DOI] [PubMed] [Google Scholar]
- 107.Ramirez GA, Yacoub M-R, Ripa M, Mannina D, Cariddi A, Saporiti N, Ciceri F, Castagna A, Colombo G, Dagna L. 2018. Eosinophils from physiology to disease: a comprehensive review. Biomed Res Int 2018:9095275. 10.1155/2018/9095275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McBrien CN, Menzies-Gow A. 2017. The biology of eosinophils and their role in asthma. Front Med (Lausanne) 4:93. 10.3389/fmed.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Persson T, Andersson P, Bodelsson M, Laurell M, Malm J, Egesten A. 2001. Bactericidal activity of human eosinophilic granulocytes against Escherichia coli. Infect Immun 69:3591–3596. 10.1128/IAI.69.6.3591-3596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Svensson L, Wennerås C. 2005. Human eosinophils selectively recognize and become activated by bacteria belonging to different taxonomic groups. Microbes Infect 7:720–728. 10.1016/j.micinf.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 111.Hogan SP, Waddell A, Fulkerson PC. 2013. Eosinophils in infection and intestinal immunity. Curr Opin Gastroenterol 29:7–14. 10.1097/MOG.0b013e32835ab29a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walker MM, Warwick A, Ung C, Talley NJ. 2011. The role of eosinophils and mast cells in intestinal functional disease. Curr Gastroenterol Rep 13:323–330. 10.1007/s11894-011-0197-5. [DOI] [PubMed] [Google Scholar]
- 113.Mehta P, Furuta GT. 2015. Eosinophils in gastrointestinal disorders. Immunol Allergy Clin North Am 35:413–437. 10.1016/j.iac.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Loktionov A. 2019. Eosinophils in the gastrointestinal tract and their role in the pathogenesis of major colorectal disorders. World J Gastroenterol 25:3503–3526. 10.3748/wjg.v25.i27.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krystel-Whittemore M, Dileepan KN, Wood JG. 2015. Mast cell: a multi-functional master cell. Front Immunol 6:620. 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Eslick G. 2019. Gastrointestinal diseases and their associated infections. Elsevier, Amsterdam, the Netherlands. [Google Scholar]
- 117.He Z, Allers C, Sugimoto C, Ahmed N, Fujioka H, Kim W-K, Didier ES, Kuroda MJ. 2018. Rapid turnover and high production rate of myeloid cells in adult rhesus macaques with compensations during aging. J Immunol 200:4059–4067. 10.4049/jimmunol.1800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ginhoux F, Guilliams M. 2016. Tissue-resident macrophage ontogeny and homeostasis. Immunity 44:439–449. 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 119.Ginhoux F, Jung S. 2014. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 14:392–404. 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 120.Varol C, Mildner A, Jung S. 2015. Macrophages: development and tissue specialization. Annu Rev Immunol 33:643–675. 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 121.Myszewski MA, Stern NJ. 1991. Phagocytosis and intracellular killing of Campylobacter jejuni by elicited chicken peritoneal macrophages. Avian Dis 35:750–755. 10.2307/1591606. [DOI] [PubMed] [Google Scholar]
- 122.Kim S, Vela A, Clohisey SM, Athanasiadou S, Kaiser P, Stevens MP, Vervelde L. 2018. Host-specific differences in the response of cultured macrophages to Campylobacter jejuni capsule and O-methyl phosphoramidate mutants. Vet Res 49:3. 10.1186/s13567-017-0501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Banfi E, Cinco M, Zabucchi G. 1986. Phagocytosis of Campylobacter jejuni and C. coli by peritoneal macrophages. J Gen Microbiol 132:2409–2412. 10.1099/00221287-132-8-2409. [DOI] [PubMed] [Google Scholar]
- 124.Iovine NM, Pursnani S, Voldman A, Wasserman G, Blaser MJ, Weinrauch Y. 2008. Reactive nitrogen species contribute to innate host defense against Campylobacter jejuni. Infect Immun 76:986–993. 10.1128/IAI.01063-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Day WA, Sajecki JL, Pitts TM, Joens LA. 2000. Role of catalase in Campylobacter jejuni intracellular survival. Infect Immun 68:6337–6345. 10.1128/iai.68.11.6337-6345.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hamza E, Kittl S, Kuhnert P. 2017. Temporal induction of pro-inflammatory and regulatory cytokines in human peripheral blood mononuclear cells by Campylobacter jejuni and Campylobacter coli. PLoS One 12:e0171350. 10.1371/journal.pone.0171350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jones MA, Tötemeyer S, Maskell DJ, Bryant CE, Barrow PA. 2003. Induction of proinflammatory responses in the human monocytic cell line THP-1 by Campylobacter jejuni. Infect Immun 71:2626–2633. 10.1128/iai.71.5.2626-2633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Korneev KV, Kondakova AN, Sviriaeva EN, Mitkin NA, Palmigiano A, Kruglov AA, Telegin GB, Drutskaya MS, Sturiale L, Garozzo D, Nedospasov SA, Knirel YA, Kuprash DV. 2018. Hypoacylated LPS from foodborne pathogen Campylobacter jejuni induces moderate TLR4-mediated inflammatory response in murine macrophages. Front Cell Infect Microbiol 8:58. 10.3389/fcimb.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stahl M, Ries J, Vermeulen J, Yang H, Sham HP, Crowley SM, Badayeva Y, Turvey SE, Gaynor EC, Li X, Vallance BA. 2014. A novel mouse model of Campylobacter jejuni gastroenteritis reveals key pro-inflammatory and tissue protective roles for Toll-like receptor signaling during infection. PLoS Pathog 10:e1004264. 10.1371/journal.ppat.1004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Canonico B, Di Sario G, Cesarini E, Campana R, Luchetti F, Zamai L, Ortolani C, Nasoni M, Baffone W, Papa S. 2018. Monocyte response to different Campylobacter jejuni lysates involves endoplasmic reticulum stress and the lysosomal–mitochondrial axis: when cell death is better than cell survival. Toxins 10:239. 10.3390/toxins10060239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Karaffová V, Revajová V, Koščová J, Gancarčíková S, Nemcová R, Ševčíková Z, Herich R, Levkut M. 2020. Local intestinal immune response including NLRP3 inflammasome in broiler chicken infected with Campylobacter jejuni after administration of Lactobacillus reuteri B1/1. Food Agric Immunol 31:954–966. 10.1080/09540105.2020.1788516. [DOI] [Google Scholar]
- 132.Hameed A, Machado L, Woodacre A, Marsden G. 2019. Induction of inflammasome-dependent signalling in the human monocytic cell line THP-1 by Campylobacter lipooligosaccharides. Access Microbiol 10.1099/acmi.ac2019.po0564. [DOI] [Google Scholar]
- 133.Bouwman LI, de Zoete MR, Bleumink-Pluym NMC, Flavell RA, van Putten JPM. 2014. Inflammasome activation by Campylobacter jejuni. J Immunol 193:4548–4557. 10.4049/jimmunol.1400648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hickey TE, Majam G, Guerry P. 2005. Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytholethal distending toxin. Infect Immun 73:5194–5197. 10.1128/IAI.73.8.5194-5197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wassenaar TM, Engelskirchen M, Park S, Lastovica A. 1997. Differential uptake and killing potential of Campylobacter jejuni by human peripheral monocytes/macrophages. Med Microbiol Immunol 186:139–144. 10.1007/s004300050056. [DOI] [PubMed] [Google Scholar]
- 136.Van Rhijn I, Bleumink‐Pluym NMC, Van Putten JPM, Van den Berg LH. 2002. Campylobacter DNA is present in circulating myelomonocytic cells of healthy persons and in persons with Guillain‐Barré syndrome. J Infect Dis 185:262–265. 10.1086/338264. [DOI] [PubMed] [Google Scholar]
- 137.Jones G-R, Bain CC, Fenton TM, Kelly A, Brown SL, Ivens AC, Travis MA, Cook PC, MacDonald AS. 2018. Dynamics of colon monocyte and macrophage activation during colitis. Front Immunol 9:2764. 10.3389/fimmu.2018.02764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leonhard SE, Mandarakas MR, Gondim FAA, Bateman K, Ferreira MLB, Cornblath DR, van Doorn PA, Dourado ME, Hughes RAC, Islam B, Kusunoki S, Pardo CA, Reisin R, Sejvar JJ, Shahrizaila N, Soares C, Umapathi T, Wang Y, Yiu EM, Willison HJ, Jacobs BC. 2019. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol 15:671–683. 10.1038/s41582-019-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patente TA, Pinho MP, Oliveira AA, Evangelista GCM, Bergami-Santos PC, Barbuto JAM. 2018. Human dendritic cells: their heterogeneity and clinical application potential in cancer immunotherapy. Front Immunol 9:3176. 10.3389/fimmu.2018.03176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker H-C. 2005. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307:254–258. 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 141.Stephenson HN, Mills DC, Jones H, Milioris E, Copland A, Dorrell N, Wren BW, Crocker PR, Escors D, Bajaj-Elliott M. 2014. Pseudaminic acid on Campylobacter jejuni flagella modulates dendritic cell IL-10 expression via Siglec-10 receptor: a novel flagellin-host interaction. J Infect Dis 210:1487–1498. 10.1093/infdis/jiu287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bax M, Kuijf ML, Heikema AP, van Rijs W, Bruijns SCM, García-Vallejo JJ, Crocker PR, Jacobs BC, van Vliet SJ, van Kooyk Y. 2011. Campylobacter jejuni lipooligosaccharides modulate dendritic cell-mediated T cell polarization in a sialic acid linkage-dependent manner. Infect Immun 79:2681–2689. 10.1128/IAI.00009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kuijf ML, Samsom JN, van Rijs W, Bax M, Huizinga R, Heikema AP, van Doorn PA, van Belkum A, van Kooyk Y, Burgers PC, Luider TM, Endtz HP, Nieuwenhuis EES, Jacobs BC. 2010. TLR4-mediated sensing of Campylobacter jejuni by dendritic cells is determined by sialylation. J Immunol 185:748–755. 10.4049/jimmunol.0903014. [DOI] [PubMed] [Google Scholar]
- 144.Rathinam VAK, Appledorn DM, Hoag KA, Amalfitano A, Mansfield LS. 2009. Campylobacter jejuni-induced activation of dendritic cells involves cooperative signaling through Toll-like receptor 4 (TLR4)-MyD88 and TLR4-TRIF axes. Infect Immun 77:2499–2507. 10.1128/IAI.01562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hu L, Bray MD, Geng Y, Kopecko DJ. 2012. Campylobacter jejuni-mediated induction of CC and CXC chemokines and chemokine receptors in human dendritic cells. Infect Immun 80:2929–2939. 10.1128/IAI.00129-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu L, Bray MD, Osorio M, Kopecko DJ. 2006. Campylobacter jejuni induces maturation and cytokine production in human dendritic cells. Infect Immun 74:2697–2705. 10.1128/IAI.74.5.2697-2705.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rose A, Kay E, Wren BW, Dallman MJ. 2012. The Campylobacter jejuni NCTC11168 capsule prevents excessive cytokine production by dendritic cells. Med Microbiol Immunol 201:137–144. 10.1007/s00430-011-0214-1. [DOI] [PubMed] [Google Scholar]
- 148.Stagg AJ. 2018. Intestinal dendritic cells in health and gut inflammation. Front Immunol 9:2883. 10.3389/fimmu.2018.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Coutant F, Miossec P. 2016. Altered dendritic cell functions in autoimmune diseases: distinct and overlapping profiles. Nat Rev Rheumatol 12:703–715. 10.1038/nrrheum.2016.147. [DOI] [PubMed] [Google Scholar]
- 150.Press R, Nennesmo I, Kouwenhoven M, Huang Y-M, Link H, Pashenkov M. 2005. Dendritic cells in the cerebrospinal fluid and peripheral nerves in Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Neuroimmunol 159:165–176. 10.1016/j.jneuroim.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 151.Li M, Zhang L, Lu B, Chen Z, Chu L, Meng L, Fan Y. 2015. Role of dendritic cell-mediated abnormal immune response in visceral hypersensitivity. Int J Clin Exp Med 8:13243–13250. [PMC free article] [PubMed] [Google Scholar]
- 152.Vivier E, Ugolini S. 2011. Natural killer cells: from basic research to treatments. Front Immunol 2:18. 10.3389/fimmu.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Poggi A, Benelli R, Venè R, Costa D, Ferrari N, Tosetti F, Zocchi MR. 2019. Human gut-associated natural killer cells in health and disease. Front Immunol 10:961. 10.3389/fimmu.2019.00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Malik A, Sharma D, St Charles J, Dybas LA, Mansfield LS. 2014. Contrasting immune responses mediate Campylobacter jejuni-induced colitis and autoimmunity. Mucosal Immunol 7:802–817. 10.1038/mi.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Avril T, Wagner ER, Willison HJ, Crocker PR. 2006. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect Immun 74:4133–4141. 10.1128/IAI.02094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Daly J, Carlsten M, O’Dwyer M. 2019. Sugar free: novel immunotherapeutic approaches targeting Siglecs and sialic acids to enhance natural killer cell cytotoxicity against cancer. Front Immunol 10:1047. 10.3389/fimmu.2019.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sim MJW, Rajagopalan S, Altmann DM, Boyton RJ, Sun PD, Long EO. 2019. Human NK cell receptor KIR2DS4 detects a conserved bacterial epitope presented by HLA-C. Proc Natl Acad Sci U S A 116:12964–12973. 10.1073/pnas.1903781116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kumar BV, Connors TJ, Farber DL. 2018. Human T cell development, localization, and function throughout life. Immunity 48:202–213. 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Farber DL. 2020. Form and function for T cells in health and disease. Nat Rev Immunol 20:83–84. 10.1038/s41577-019-0267-8. [DOI] [PubMed] [Google Scholar]
- 160.Haag L-M, Fischer A, Otto B, Plickert R, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM. 2012. Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10−/− mice via Toll-like-receptor-2 and −4 signaling. PLoS One 7:e40761. 10.1371/journal.pone.0040761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fimlaid KA, Lindow JC, Tribble DR, Bunn JY, Maue AC, Kirkpatrick BD. 2014. Peripheral CD4+ T cell cytokine responses following human challenge and re-challenge with Campylobacter jejuni. PLoS One 9:e112513. 10.1371/journal.pone.0112513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maue AC, Mohawk KL, Giles DK, Poly F, Ewing CP, Jiao Y, Lee G, Ma Z, Monteiro MA, Hill CL, Ferderber JS, Porter CK, Trent MS, Guerry P. 2013. The polysaccharide capsule of Campylobacter jejuni modulates the host immune response. Infect Immun 81:665–672. 10.1128/IAI.01008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reid WDK, Close AJ, Humphrey S, Chaloner G, Lacharme-Lora L, Rothwell L, Kaiser P, Williams NJ, Humphrey TJ, Wigley P, Rushton SP. 2016. Cytokine responses in birds challenged with the human food-borne pathogen Campylobacter jejuni implies a Th17 response. R Soc Open Sci 3:150541. 10.1098/rsos.150541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Flaujac Lafontaine GM, Richards PJ, Connerton PL, O’Kane PM, Ghaffar NM, Cummings NJ, Fish NM, Connerton IF. 2019. Prebiotic driven increases in IL-17A do not prevent Campylobacter jejuni colonization of chickens. Front Microbiol 10:3030. 10.3389/fmicb.2019.03030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lo Presti E, Corsale AM, Di Simone M, Dieli F, Meraviglia S. 31 July 2020. Characterisation of γδ T cells infiltrating colorectal cancer. Gut 10.1136/gutjnl-2020-322101. [DOI] [PubMed] [Google Scholar]
- 166.Scelsa SN, Ghali V, Herskovitz S, Bieri P, Shank DL, MacGowan DDJ, Liau S. 2004. Blood γδ T cells, Campylobacter jejuni, and GM1 titers in Guillain-Barré syndrome. Muscle Nerve 30:423–432. 10.1002/mus.20105. [DOI] [PubMed] [Google Scholar]
- 167.Ben-Smith A, Goodall JC, Gaston JSH, Winer JB. 1997. Stimulation of peripheral blood lymphocytes with Campylobacter jejuni generates a γδ T cell response in patients with Guillain-Barre syndrome. Clin Exp Immunol 109:121–126. 10.1046/j.1365-2249.1997.4221318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Das H, Sugita M, Brenner MB. 2004. Mechanisms of Vδ1 γδ T cell activation by microbial components. J Immunol 172:6578–6586. 10.4049/jimmunol.172.11.6578. [DOI] [PubMed] [Google Scholar]
- 169.Cutillo G, Saariaho A-H, Meri S. 2020. Physiology of gangliosides and the role of antiganglioside antibodies in human diseases. Cell Mol Immunol 17:313–322. 10.1038/s41423-020-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cyster JG, Allen CDC. 2019. B cell responses: cell interaction dynamics and decisions. Cell 177:524–540. 10.1016/j.cell.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Briney B, Inderbitzin A, Joyce C, Burton DR. 2019. Commonality despite exceptional diversity in the baseline human antibody repertoire. Nature 566:393–397. 10.1038/s41586-019-0879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cawthraw SA, Feldman RA, Sayers AR, Newell DG. 2002. Long-term antibody responses following human infection with Campylobacter jejuni. Clin Exp Immunol 130:101–106. 10.1046/j.1365-2249.2002.01966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ketley JM. 1997. Pathogenesis of enteric infection by Campylobacter. Microbiology 143:5–21. 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- 174.Masanta WO, Heimesaat MM, Bereswill S, Tareen AM, Lugert R, Groß U, Zautner AE. 2013. Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin Dev Immunol 2013:526860–526810. 10.1155/2013/526860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ang CW, Noordzij PG, de Klerk MA, Endtz HP, van Doorn PA, Laman JD. 2002. Ganglioside mimicry of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity in rabbits. Infect Immun 70:5081–5085. 10.1128/iai.70.9.5081-5085.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Godschalk PCR, Heikema AP, Gilbert M, Komagamine T, Ang CW, Glerum J, Brochu D, Li J, Yuki N, Jacobs BC, van Belkum A, Endtz HP. 2004. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barré syndrome. J Clin Invest 114:1659–1665. 10.1172/JCI200415707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guerry P, Szymanski CM, Prendergast MM, Hickey TE, Ewing CP, Pattarini DL, Moran AP. 2002. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect Immun 70:787–793. 10.1128/iai.70.2.787-793.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yuki N, Susuki K, Koga M, Nishimoto Y, Odaka M, Hirata K, Taguchi K, Miyatake T, Furukawa K, Kobata T, Yamada M. 2004. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barre syndrome. Proc Natl Acad Sci U S A 101:11404–11409. 10.1073/pnas.0402391101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huayanay I, Pozo L, Bangash S, Ramirez D, Rosas L, Arauco Brown R. 2020. An unusual case of Campylobacter jejuni gastroenteritis presenting with acute reversible encephalopathy in an immunocompetent host. Case Rep Infect Dis 2020:9603428. 10.1155/2020/9603428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tatsumoto M, Koga M, Gilbert M, Odaka M, Hirata K, Kuwabara S, Yuki N. 2006. Spectrum of neurological diseases associated with antibodies to minor gangliosides GM1b and GalNAc-GD1a. J Neuroimmunol 177:201–208. 10.1016/j.jneuroim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 181.Crofts AA, Poly FM, Ewing CP, Kuroiwa JM, Rimmer JE, Harro C, Sack D, Talaat KR, Porter CK, Gutierrez RL, DeNearing B, Brubaker J, Laird RM, Maue AC, Jaep K, Alcala A, Tribble DR, Riddle MS, Ramakrishnan A, McCoy AJ, Davies BW, Guerry P, Trent MS. 2018. Campylobacter jejuni transcriptional and genetic adaptation during human infection. Nat Microbiol 3:494–502. 10.1038/s41564-018-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu MM, Boinett CJ, Chan ACK, Parkhill J, Murphy MEP, Gaynor EC. 2018. Investigating the Campylobacter jejuni transcriptional response to host intestinal extracts reveals the involvement of a widely conserved iron uptake system. mBio 9:e01347-18. 10.1128/mBio.01347-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Humphrey S, Chaloner G, Kemmett K, Davidson N, Williams N, Kipar A, Humphrey T, Wigley P. 2014. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. mBio 5:e01364-14. 10.1128/mBio.01364-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chuammitri P, Ostojić J, Andreasen CB, Redmond SB, Lamont SJ, Palić D. 2009. Chicken heterophil extracellular traps (HETs): novel defense mechanism of chicken heterophils. Vet Immunol Immunopathol 129:126–131. 10.1016/j.vetimm.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 185.Bennoune O, Melizi M, Khazal K, Bourouba R, Ayachi A. 2009. Chicken heterophils: a model for non-oxidative antimicrobial activity. Worlds Poult Sci J 65:625–632. 10.1017/S0043933909000439. [DOI] [Google Scholar]
- 186.Van Belleghem JD, Bollyky PL. 2018. Macrophages and innate immune memory against Staphylococcus skin infections. Proc Natl Acad Sci U S A 115:11865–11867. 10.1073/pnas.1816935115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dai H, Lan P, Zhao D, Abou-Daya K, Liu W, Chen W, Friday AJ, Williams AL, Sun T, Chen J, Chen W, Mortin-Toth S, Danska JS, Wiebe C, Nickerson P, Li T, Mathews LR, Turnquist HR, Nicotra ML, Gingras S, Takayama E, Kubagawa H, Shlomchik MJ, Oberbarnscheidt MH, Li XC, Lakkis FG. 2020. PIRs mediate innate myeloid cell memory to nonself MHC molecules. Science 368:1122–1127. 10.1126/science.aax4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mistry P, Nakabo S, O’Neil L, Goel RR, Jiang K, Carmona-Rivera C, Gupta S, Chan DW, Carlucci PM, Wang X, Naz F, Manna Z, Dey A, Mehta NN, Hasni S, Dell’Orso S, Gutierrez-Cruz G, Sun H-W, Kaplan MJ. 2019. Transcriptomic, epigenetic, and functional analyses implicate neutrophil diversity in the pathogenesis of systemic lupus erythematosus. Proc Natl Acad Sci U S A 116:25222–25228. 10.1073/pnas.1908576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P. 2019. Neutrophil diversity in health and disease. Trends Immunol 40:565–583. 10.1016/j.it.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ng LG, Ostuni R, Hidalgo A. 2019. Heterogeneity of neutrophils. Nat Rev Immunol 19:255–265. 10.1038/s41577-019-0141-8. [DOI] [PubMed] [Google Scholar]
- 191.Whitmore LC, Weems MN, Allen L-AH. 2017. Cutting Edge: Helicobacter pylori induces nuclear hypersegmentation and subtype differentiation of human neutrophils in vitro. J Immunol 198:1793–1797. 10.4049/jimmunol.1601292. [DOI] [PMC free article] [PubMed] [Google Scholar]