ABSTRACT
Cytolethal distending toxin (CDT) is a bacterial genotoxin that causes host cell cycle arrest and death. We previously employed a Saccharomyces cerevisiae model with inducible expression of the CDT catalytic subunit from Aggregatibacter actinomycetemcomitans, AaCdtB, and showed that a wide variety of host factors play a role in facilitating the activity of CdtB. Our observation that a yeast H2B mutant defective in chromatin condensation was partially resistant to CdtB implies that chromatin structure may affect CDT function. In this study, we identified host chromatin regulatory genes required for CdtB cytotoxicity. We found that the deletion of HTZ1 or certain subunits of SWR, INO80, and SIR complexes increased cellular resistance to CdtB. We hypothesized that CdtB may interact with Htz1 or the chromatin, but immunoprecipitation experiments failed to detect physical interaction between CdtB and Htz1 or the chromatin. However, we observed reduced nuclear localization of CdtB in several mutants, suggesting that impaired nuclear translocation may, at least partly, explain the mechanisms of CdtB resistance. In addition, mutations in chromatin regulatory genes induce changes in the global gene expression profile, and these may indirectly affect CdtB toxicity. Our results suggest that decreased expression of endoplasmic reticulum (ER)-Golgi transport-related genes that may be involved in CdtB transport and/or increased expression of DNA repair genes may contribute to CdtB resistance. These results suggest that the functions of chromatin regulators may contribute to the activity of CDT in host cells.
KEYWORDS: Aggregatibacter actinomycetemcomitans, chromatin, cytolethal distending toxin, genotoxin, Saccharomyces cerevisiae
INTRODUCTION
Several species of Gram-negative pathogenic bacteria, including Aggregatibacter actinomycetemcomitans, a periodontopathic bacterium associated with aggressive periodontitis, express cytolethal distending toxin (CDT), which may be involved in the pathogenesis of chronic infections (1, 2). CDT is considered a virulence factor in A. actinomycetemcomitans, and its expression significantly increased upon interaction with epithelial cells (3, 4). Clinical isolates of A. actinomycetemcomitans from patients with periodontitis show a high frequency of CDT production, and topical application of A. actinomycetemcomitans CDT (AaCDT) alone could induce gingival inflammation and destruction in a rat model (5–8). In addition, AaCDT has been shown to induce cell death in periodontal and immune cells (9–11). Together, current evidence suggests that AaCDT could contribute to pathogenesis through periodontal tissue destruction and immune evasion.
CDT is a heterotrimeric protein complex composed of surface-binding subunits, CdtA and CdtC, and the catalytic subunit, CdtB. CdtB is a mammalian DNase I homologue (12). Genotoxic activity of CDT activates DNA damage response (DDR) and induces cell cycle arrest and eventually death in mammalian cells in a cell type-dependent manner; for example, epithelial and endothelial cell lines undergo G2/M arrest, fibroblasts undergo G1/S or G2/M arrest, and hematopoietic cells undergo G2 arrest (1). A live-cell imaging study showed that, after binding to the host cell membrane, AaCdtB-CdtC heterodimers are internalized, while CdtA remains on the cell surface (13). Upon endocytosis, CdtB-CdtC was shown to pass through the Golgi apparatus and ER via retrograde transport (14). CdtC was proposed to be removed by endoplasmic reticulum-associated degradation (ERAD) (15), while CdtB is translocated to the nucleus via its nuclear localization signal (16). A study in haploid human cells has implicated several host membrane proteins, including Golgi glycoprotein 1 (GLG1) and vacuolar ATPase subunit 2 (ATP6V0A2), in facilitating AaCDT toxicity (17). Additionally, our recent genome-wide analysis of host genes required for AaCdtB toxicity in yeast also suggested the roles of genes related to ER-Golgi and organic anion transport in nuclear translocation and cytotoxic activity of CdtB (18).
We and others have employed the budding yeast Saccharomyces cerevisiae as a model to investigate the molecular mechanisms of CdtB-induced DNA damage and cell death (18–21). We previously showed that DNA breaks caused by AaCdtB require homologous recombination repair, and a wide variety of host genes are required for AaCdtB toxicity (18, 21). Interestingly, a yeast H2B (S10A) mutant defective in chromatin condensation during apoptosis showed increased resistance to CdtB toxicity, suggesting that chromatin structure may affect CdtB function (21). Furthermore, the in vitro DNase activity of CdtB was lower than in vivo cytotoxicity, suggesting that in vivo host factors may help facilitate CdtB activity (22). Since CdtB requires access to DNA to exert its activity, we hypothesize that chromatin regulators may directly or indirectly affect CdtB activity in vivo.
Chromatin structure is regulated by chromatin remodeling complexes and histone-modifying enzymes to control DNA-related processes. The SWR complex (SWR-C) is required for the incorporation of Htz1, a histone H2A variant, into the nucleosome, locating around promoters and transcription start sites, whereas the INO80 complex (INO80-C) is required for Htz1 removal (23). The silent information regulator (SIR) complex regulates heterochromatin structure at the telomere, ribosomal DNA (rDNA), and mating-type loci in yeast (24). Several histone-modifying enzymes contribute to chromatin regulation; for example, histone acetyltransferases (HATs) are associated with transcriptionally active euchromatin, while histone deacetylases (HDACs) are associated with heterochromatin formation and silencing (25).
In this study, we aimed to examine if host chromatin regulators may be involved in regulating CdtB activity. We identified the chromatin regulatory genes required for CdtB cytotoxicity using plate sensitivity and survival plating assays. We further examined protein-protein interaction, nuclear localization, DNA damage, and changes in gene expression to determine the mechanisms by which these chromatin regulators could affect CdtB function.
RESULTS
Identification of chromatin regulatory genes required for CdtB cytotoxicity.
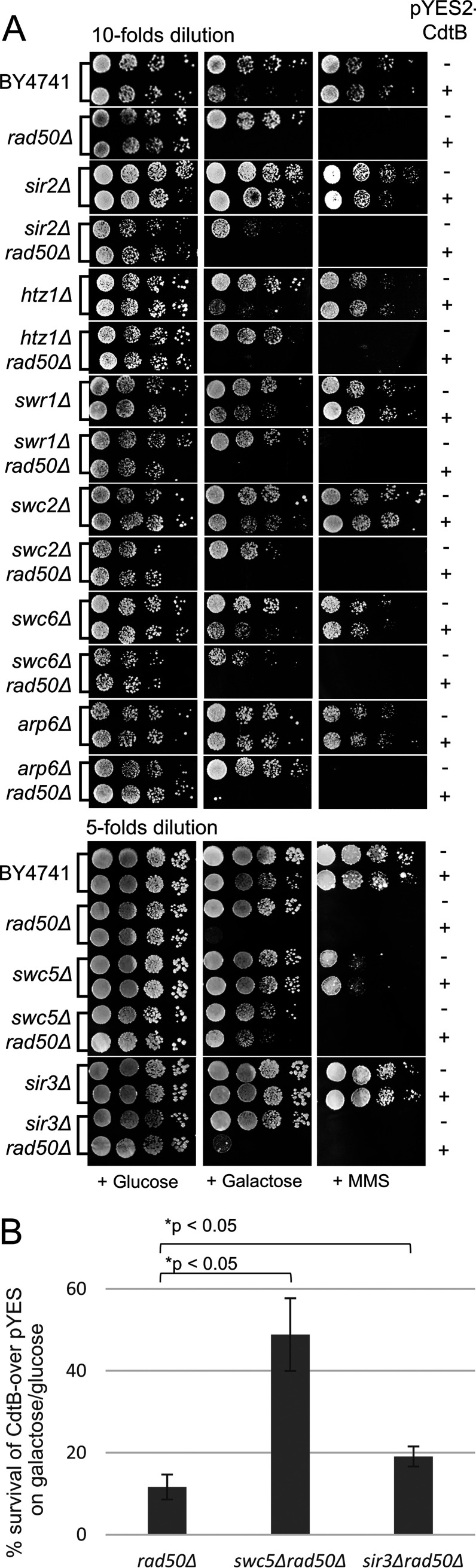
To explore the role of chromatin structure in CdtB cytotoxicity, we first examined the CdtB susceptibility of various yeast strains with chromatin regulatory gene deletion. The deletion of any chromatin regulatory genes that leads to CdtB resistance suggests that their normal functions are required for CdtB toxicity. We examined several components of chromatin regulatory enzymes/complexes, including SWR-C, histone-modifying enzymes, the SIR complex, and INO80-C. Since some of these complexes shared certain subunits with other complexes, unique subunits were chosen to represent the functions of each complex. These genes have well-established roles in chromatin regulation, and their deletions have been shown previously to affect chromatin structure and DNA accessibility (26–32). We employed the plate susceptibility test (spot test) and survival plating assay in yeast strains harboring plasmids with CdtB under the control of a galactose-inducible promoter compared to a vector control. The expression of CdtB was induced in the presence of galactose in the medium, while it was repressed in medium supplemented with glucose, which served as a control. The survival plating assay showed that CdtB expression led to approximately 60% cell death (40% survival) in the wild-type (WT) yeast strain (Fig. 1A). For the spot test, yeast mutants that showed more growth than the WT under CdtB-inducing conditions were classified as CdtB resistant. The results showed that the deletion of HTZ1 and deletion of certain subunits of SWR-C (SWR1, SWC2, SWC5, SWC6, and ARP6), INO80-C (ARP5), and SIR complex (SIR2 and SIR3) led to various degrees of CdtB resistance (Fig. 1A). In contrast, the deletion of other subunits of these complexes and histone-modifying enzymes tested did not confer CdtB resistance. In addition, we performed growth curve analyses of planktonic cultures, and the results were similar to those of spot tests and survival plating assays in that growth retardation was observed in WT organisms expressing CdtB but not in CdtB-resistant mutants (data not shown).
FIG 1.
CdtB susceptibility and survival rate of the chromatin regulatory gene deletion strains upon CdtB expression. (A) Representative photographs of 10-fold serial dilutions of various yeast mutants carrying pYES2-CdtB (+) in comparison to a pYES2 control (−) grown on glucose (repressing medium) and galactose (inducing medium). Images were taken after approximately 40 h of incubation at 30°C. Survival percentages were calculated from the number of CFU on galactose plates relative to that on glucose plates and normalized to the survival rate of the pYES2 control in the same strain. The experiments were performed with three transformants in three independent experiments. Data are means ± SE. The vertical dashed line showed the WT susceptibility level. The asterisks represent a statistically significant difference between mutants and the WT strain analyzed by the Mann-Whitney U test (P < 0.05). (B) CdtB expression in chromatin regulatory gene mutants was examined in whole-cell extracts by immunoblotting with anti-CdtB serum and anti-IgG with HRP-conjugated secondary antibody. The level of Pgk1 was used as a loading control.
To rule out the possibility that CdtB resistance was due to a failure to induce CdtB expression in the mutant strains, immunoblot assays were performed to examine the levels of CdtB expression, with Pgk1 as a loading control. The results showed that, upon induction with galactose, CdtB expression in the mutants was similar to that in WT (Fig. 1B). Thus, the CdtB-resistant phenotype was not due to a defect in CdtB expression.
Interaction between CdtB and Htz1.
The major function of SWR-C is to deposit Htz1 into the nucleosomes (33). Our observations that HTZ1 and subunits of SWR-C are required for CdtB activity suggest that Htz1 may facilitate CdtB interaction with chromatin. To test this hypothesis, the physical interaction between Htz1 and CdtB was examined by a coimmunoprecipitation assay using whole-cell extract from a yeast strain with hemagglutinin (HA)-tagged Htz1. With this method, we could not detect coimmunoprecipitation of Htz1 with anti-CdtB antibody-coated beads, or vice versa (data not shown). To detect the interaction of CdtB with chromatin, we used formaldehyde cross-linking and performed chromatin immunoprecipitation (ChIP). However, no coprecipitation was observed (data not shown). These results suggest that CdtB and Htz1 may not have direct and stable physical interaction that can be detected under these experimental conditions.
Nuclear localization of CdtB was decreased in certain chromatin regulatory gene mutants.
Translocation into the nucleus is an essential step for AaCdtB to exert its enzymatic activity on host DNA, including in yeast (16, 21). Besides, we observed a decrease in nuclear localization in several AaCdtB-resistant yeast mutants in our previous genome-wide study (18). Hence, we hypothesized that CdtB resistance in chromatin regulatory gene mutants may also result from a reduction in nuclear localization. To test this hypothesis, we observed the enhanced green fluorescent protein (EGFP)-tagged CdtB signal in mutant strains compared to the wild type using fluorescence microscopy (Fig. 2). Interestingly, we found reduced nuclear localization in the htz1Δ mutant and certain SWR-C mutants, including swr1Δ, swc2Δ, swc6Δ, arp6Δ, and arp5Δ strains, but not in a swc5Δ strain and strains with the deletion of the SIR complex.
FIG 2.
CdtB nuclear localization was reduced in certain chromatin regulatory gene deletion mutants. (A) Representative fluorescent images of CdtB-EGFP localization and EGFP control in the WT and chromatin regulatory gene deletion mutants with the CdtB-resistant phenotype. The nuclei were stained using DAPI and observed with a confocal fluorescence microscope at ×600 magnification. (B) Manders’ coefficient was used to calculate the relative CdtB nuclear localization ratio between CdtB-EGFP and EGFP control. Data from 36 randomly selected cells of each sample were obtained and are shown as a box plot (10th to 90th percentile; gray dots are outliers), and a Mann-Whitney U test was used to analyze the data in comparison to the WT. **, P < 0.01; ***, P < 0.001. The gray dashed line represents the background level of EGFP in the nucleus.
CdtB induced DNA damage in CdtB-resistant strains.
Although we found reduced nuclear localization of CdtB in certain CdtB resistant mutants, CdtB was not completely excluded from the nuclei (Fig. 2A). Therefore, we asked whether it could still cause DNA breaks. The deletion of RAD50, a component of the MRX DNA break sensor, leads to hypersensitivity to DNA damaging agents (such as methyl methanesulfonate [MMS]) and CdtB (21, 34). Thus, we exploited this phenotype as an indicator of DNA damage in CdtB-resistant mutants. RAD50 was deleted in these strains to generate double-deletion strains. If any double-deletion strain remains resistant to CdtB, it would indicate that CdtB could not induce DNA damage in that strain. Interestingly, we found that only swc5Δ rad50Δ and sir3Δ rad50Δ strains were more resistant to CdtB than the rad50Δ strain, while the others showed levels of hypersensitivity similar to those of the rad50Δ strain (Fig. 3A and B).
FIG 3.
CdtB could induce DNA damage in most CdtB-resistant mutants. (A) Deletion of RAD50 leads to hypersensitivity to DNA damage, so it was used to monitor DNA damage in CdtB-resistant strains. Representative images of 10-fold or 5-fold dilutions of CdtB resistant mutants with or without rad50Δ carrying pYES2-CdtB (+) or pYES2 control (−) grown on medium containing glucose or galactose. Growth on medium containing MMS (DNA alkylating agent) was shown to verify the hypersensitivity to DNA-damaging agents of the rad50Δ strain. (B) A survival plating assay showed increased survival of the swc5Δ rad50Δ and sir3Δ rad50Δ strains compared to the rad50Δ strain. Survival percentages were calculated from the number of CFU on galactose plates relative to that on glucose plates and normalized to the ratio of the pYES2 control. The experiment was done with three transformants in three independent experiments. Data are means ± SE. The asterisks represent statistically significant differences between double mutants and the rad50Δ strain, analyzed by a Mann-Whitney U test (P < 0.05).
Downregulation of genes required for CdtB toxicity in chromatin regulatory gene mutants.
Since chromatin regulators play an important role in controlling gene expression, the changes in expression of genes required for CdtB activity may indirectly affect CdtB susceptibility of the mutant strains. We had previously identified 243 genes whose deletions led to CdtB resistance in a genome-wide screen (18); thus, the reduction of expression of these genes may also lead to CdtB resistance. The data on expression of these genes in chromatin regulatory gene deletion strains, including htz1Δ, swr1Δ, swc2Δ, swc5Δ, sir2Δ, and sir3Δ strains, were obtained from publicly available microarray data sets (31, 35). We found that 63 of the 243 genes showed significant changes in the level of expression (≥1.5-fold; false discovery rate [FDR]-adjusted P value < 0.05) in at least one of these mutant strains (Fig. 4A and B). Hierarchical clustering showed consistent downregulation of a subset of genes among all SWR subunit deletion mutants and the htz1Δ strain, but a distinct subset of genes was downregulated in strains with the deletion of SIR subunits. Genes associated with ER-Golgi transport and with the nucleus, which potentially play a role in CdtB translocation and function in the nucleus, respectively, were also identified in these lists. To confirm the findings from the public data sets, we examined the expression levels of these genes in CdtB-resistant mutant strains using reverse transcription-quantitative PCR (RT-qPCR) (Fig. 4C). The results are mostly consistent with the microarray data. We observed a downregulation (>1.5-fold) of SPO1, COS10, and LDB18 genes in all mutants tested, except SPO1 and LDB18 in the swc2Δ mutant. Interestingly, three genes related to organic anion transport (MVB12, PDR17, and YMC1), whose deletions were previously shown to reduce nuclear localization of CdtB (18), were also downregulated (>1.5-fold) in the htz1Δ and swr1Δ mutants. This also correlated well with the microarray data.
FIG 4.
Expression of genes required for CdtB function in chromatin regulatory gene deletion mutants. (A) Heat map of expression changes of 63 of 243 genes required for CdtB function from a previous genome-wide screen that showed a ≥1.5-fold change and an FDR-adjusted P value of <0.05 in the mutants with deletions of SWR-C subunits and HTZ1 (A) and SIR complex subunits (B). Data were hierarchically clustered with Pearson’s correlation distance matrix. ER-associated genes are in bold, and nucleus-associated genes are underlined. The expression data sets used were GSE21571 (mutations with “a”) and GSE25909 (mutations with “b”). (C) The level of expression of certain genes associated with ER-Golgi transport and nucleus were measured in chromatin regulatory gene deletion mutants using RT-qPCR. Relative expression (log2) was calculated from mutants’ 2−ΔΔCT values compared to WT values, using ACT1 as a housekeeping gene.
Upregulation of DNA repair genes in chromatin regulatory gene mutants.
Global gene expression changes in chromatin regulatory gene deletion mutants may also lead to upregulation of DNA repair genes that enhance protection against CdtB cytotoxicity. As we found that CdtB could still damage DNA in several mutants (Fig. 3), we hypothesized that DNA repair genes may be upregulated to cope with DNA damage. The upregulation of certain DNA repair genes in CdtB-resistant mutants may poise the cells to be able to readily repair the DNA damages induced by CdtB. To test this hypothesis, we examined the gene expression profile of 258 DNA repair genes as annotated by the Gene Ontology term GO:0006281 (Saccharomyces Genome Database [SGD]) retrieved from publicly available data sets of chromatin regulatory gene deletion strains (31, 35) and a wild-type strain treated with DNA-damaging agents, including MMS and gamma radiation (36–38). DNA repair genes that were upregulated ≥1.5-fold with an FDR-adjusted P value of <0.05 in at least one of the mutants and DNA damaging conditions were selected (Fig. 5A). Since these DNA repair genes were upregulated under various DNA damaging conditions, we first examined the expression levels of 6 DNA repair genes upon CdtB expression in the wild-type strain. The levels of mRNA were measured at 0, 2, 4, and 6 h after CdtB induction by RT-qPCR. The results show that these genes were upregulated within 2 to 4 h upon CdtB induction in the WT (Fig. 5B). We investigated the expression of these genes in CdtB resistant mutants. We found that SSL2, DEF1, EPL1, and EAF1 were upregulated in htz1Δ, swr1Δ, and swc5Δ mutants compared to the WT. In contrast, we found downregulation of RFA1 and DNA2 in all the mutants tested (Fig. 5C).
FIG 5.
Expression of DNA repair genes in chromatin regulatory gene deletion mutants. The 258 genes annotated in the GO term “DNA repair” were analyzed in both chromatin regulatory gene deletion mutants and DNA damaging agent-treated yeast gene expression data sets, indicated with superscript letters (GSE21571 [a], GSE25909 [b], GSE6018 [c], GSE2224 [d], and GSE5301 [e]). (A) Expression profiles of DNA repair genes that are upregulated under at least one condition of the treatment were selected. The heat map indicates down-regulated (blue) and up-regulated (red) genes with FDR-adjusted P values of <0.05 and a Z-score cutoff of ±1 (approximately ≥1.5-fold changes). (B) DNA repair gene expression levels at various time points after CdtB induction. (C) DNA repair gene expression levels in chromatin regulatory gene deletion mutants without CdtB expression.
DISCUSSION
In this study, we showed that the functions of certain chromatin regulators, including SWR-C, Htz1, and SIR complex, may help facilitate CdtB cytotoxicity in a Saccharomyces cerevisiae model. We investigated several possible underlying mechanisms and propose that the reduction in nuclear localization and/or changes in gene expression may, at least partly, explain how gene deletion of these chromatin regulators leads to CdtB resistance.
Chromatin remodeling complexes are critical for the dynamics of chromatin structure. Our results suggest the role of SWR-C and Htz1 in CdtB cytotoxicity. Intriguingly, CdtB resistance conferred by deletion of certain SWR-C components correlates well with the subunit requirements for complex integrity and enzymatic activity of the complex (Fig. 1). Only SWR-C subunits that are necessary for Htz1 deposition, including Swr1, Swc2, Swc5, Swc6, and Arp6 (39, 40) and Htz1 itself, are required for CdtB toxicity. In contrast, subunits dispensable for Htz1 deposition, i.e., Swc3 and Swc7, are not required for CdtB toxicity (41, 42). This suggests that the activity of SWR-C and incorporation of Htz1 in the chromatin is crucial for CdtB activity. The functions of the critical subunits are as follows. Swr1 is the ATPase catalytic subunit and serves as a scaffold protein for the binding of the N module, the C module, Swc5, and RvB1/2 (33, 41). Swc2 directly binds to Htz1 and may prevent Htz1 removal from the nucleosomes (41, 43). It also binds to DNA and recruits SWR-C to nucleosome-free regions at yeast promoters (44). Swc6 and Arp6 are required for Swc2 stable association with SWR-C and nucleosome binding (41). Swc5 is required for Htz1 deposition through Swr1 ATPase activation upon substrate recognition, although it is not required for Htz1 binding, nucleosome binding, and complex assembly (41, 45). In contrast, Swc3 and Swc7 are dispensable for Htz1 incorporation in vitro (41, 42). Our results show that the lack of Swc3 or Swc7 does not lead to CdtB resistance. Therefore, Htz1 deposition appears to be critical in facilitating CdtB activity. A possible explanation for the requirement for Htz1 deposition for CdtB activity could be related to protein-protein interaction. However, we could not detect any physical interaction between Htz1 and CdtB using coimmunoprecipitation and chromatin immunoprecipitation assays (data not shown). Nevertheless, these experimental conditions may not be able to capture weak/dynamic/transient interactions, and further investigations with more sensitive methods are warranted.
Because CdtB needs to be translocated to the nucleus to target host DNA and decreased nuclear localization may be a mechanism underlying CdtB resistance, we investigated intracellular localization of CdtB-EGFP in these CdtB-resistant mutants. We observed a decrease in nuclear localization of CdtB in most resistant mutants (Fig. 2A and B), suggesting that this could be a factor that reduces CdtB cytotoxicity. In mammalian cells, a live-cell imaging study showed that while the AaCdtB-CdtC complex was internalized into host cells, only CdtB was translocated to the nucleus (13). CdtB has been shown to be translocated via Golgi-ER retrograde transport and to the nucleus (14). An alternative pathway where CdtB is transported from ER to cytosol and delivered to the nucleus via the nuclear pore complex has also been proposed (46). A screen in human haploid cells indicated that several membrane proteins, including sphingomyelin synthase 1 (SGMS1), Golgi glycoprotein 1 (GLG1), vacuolar ATPase subunit 2 (ATP6V0A2), and synaptogyrin 2 (SYNGR2), were required for AaCDT toxicity (17). In the yeast model, although CdtB is expressed intracellularly, CdtB still requires its nuclear localization signal for cytotoxicity, suggesting that it uses conserved mechanisms for nuclear entry (21). Moreover, our genome-wide screen showed that several genes related to ER and Golgi transport are required for CdtB nuclear localization in yeast (18).
Interestingly, several studies suggested a role of Htz1 and SWR-C in ER homeostasis and protein transport pathways (47, 48). Several subunits of SWR-C play a role in vacuolar protein sorting related to the carboxypeptidase Y (CPY) pathway, a major transport pathway of newly synthesized proteins from the trans-Golgi network to the prevacuolar compartment (49). Aberrant secretion of CPY protein has been observed in several mutants, including htz1Δ, swr1Δ, swc2Δ/vps72Δ, swc5Δ, swc6Δ/vps71Δ, arp6Δ, and arp5Δ mutants (47). These mutants also showed CdtB resistance, and most had reduced nuclear localization of CdtB in this study (Fig. 1 and 2). In addition, yeast lacking Htz1 or important SWR-C components showed defects in ER homeostasis and the ER protein retrieval pathway (48). These studies suggest that Htz1 and SWR-C may regulate intracellular protein trafficking through the Golgi apparatus and ER, which could affect CdtB translocation to the nucleus. We hypothesize that Htz1 and SWR-C may affect these processes by regulating the expression of genes involved in ER-Golgi transport. Analysis of available transcriptomic data of these mutant strains showed significant downregulation of several ER-Golgi transport-related genes that are required for CdtB toxicity (Fig. 4). For example, SPO1 was downregulated in the certain SWR-C subunit mutants and the htz1Δ strain. Interestingly, a null mutant of SPO1 also showed increased cellular resistance to tirapazamine, a topoisomerase inhibitor that causes DNA breaks similarly to CdtB (50). Cos10 is involved in the endomembrane system for vacuolar protein transport and ubiquitin-dependent protein catabolic process via the multivesicular body (MVB) sorting pathway (51). Furthermore, MVB12, PDR17, and YMC1, whose deletions were previously shown to decrease CdtB localization to the nucleus and increase CdtB resistance (18), were downregulated in most mutants tested. Thus, decreased expression of these genes may contribute to CdtB resistance. In addition, Ldb18 is a component of the dynactin complex that is required for retrograde cargo activity (52), which may also affect CdtB transport into the nucleus.
Another hypothesis that could explain CdtB resistance in chromatin regulatory gene mutants is their effects on DNA repair gene expression that could enhance DNA repair capacity. We examined DNA repair gene expression under DNA-damaging conditions and in CdtB-resistant mutants from publicly available data sets. Several DNA repair genes were upregulated under these conditions, and a subset of them were also responsive to CdtB expression (Fig. 5A and B). Interestingly, SSL2 was upregulated in the SWR-C mutant data sets, and we also observed upregulation in response to CdtB and htz1Δ and swc5Δ by RT-qPCR (Fig. 5C). Overexpression of SSL2 was previously shown to increase resistance to doxorubicin (Adriamycin), a topoisomerase II inhibitor whose activity leads to DNA breaks similarly to CdtB (53). Moreover, the helicase function of Ssl2 has been shown to rescue yeast from doxorubicin toxicity (54). Therefore, increased expression of SSL2 in htz1Δ and swc5Δ strains may also increase resistance to CdtB. Furthermore, we found DEF1, EAF1, and EPL1 upregulation in the swr1Δ mutant. Def1 plays a role in the degradation of stalled RNA polymerase II at UV-induced DNA lesions (55). It is also recruited to double-strand breaks (DSBs) and required for DSB repair (56). Eaf1 and Epl1 are subunits of the NuA4 HAT complex, which is recruited to HO-induced DSBs upon H2A-S129 phosphorylation and required for efficient DSB repair (57). We speculate that upregulation of these genes may aid in the repair of CdtB-induced DNA damages and render the cells more tolerant to CdtB.
In addition, we examined whether CdtB could still damage DNA in the CdtB-resistant mutants using the hypersensitivity of the rad50Δ mutant to DNA damage as an in vivo indicator. We found that when RAD50 was deleted, swr1Δ, swc2Δ, swc6Δ, arp6Δ, and htz1Δ strains were as hypersensitive to CdtB as the rad50Δ mutant (Fig. 3A), suggesting that CdtB could still damage DNA in these mutants. Thus, the mechanisms underlying CdtB resistance may occur after DNA damage, such as the upregulation of DNA repair genes. Furthermore, the loss of resistance in chromatin regulatory gene mutants that lack RAD50 also suggests that the mechanism for CdtB resistance likely depends on the role of DNA double-strand break repair. Interestingly, only the swc5Δ rad50Δ mutant showed more CdtB resistance than the rad50Δ control (Fig. 3B), implying that CdtB DNA-damaging activity may be reduced. A previous study showed that Swc5 may have other functions independent of SWR-C because it is not restricted to +1 nucleosome but is distributed together with Swc4 (58). Our results also suggest that the mechanism for how the lack of Swc5 leads to CdtB resistance appears to be distinct from that of the main SWR-C. Taken together, these results indicate that there may be several possible mechanisms underlying CdtB resistance in yeast lacking critical SWR-C components and Htz1. These mechanisms are not mutually exclusive, and they may concomitantly contribute to CdtB resistance.
In contrast to the requirement for SWR-C, the related chromatin remodeler INO80-C, which plays a reciprocal role relative to SWR-C in Htz1 exchange, did not seem to affect CdtB activity. The nhp10Δ and arp8Δ strains were not CdtB resistant, but the arp5Δ mutant was (Fig. 1). Arp5 has been shown to be required for Htz1 eviction and nucleosome remodeling activity of INO80-C (58, 59). Arp8 is also required for nucleosome remodeling, while INO80-C lacking Nhp10 was able to remodel the nucleosomes, although it is required for nucleosome binding (59, 60). Interestingly, Arp5 is also found as a distinct subcomplex with Ies6 in vivo; this may partly explain the difference in CdtB resistance between the arp5Δ and arp8Δ mutants (61). Intriguingly, INO80-C also plays critical roles in genome stability and DNA damage checkpoint responses (62, 63). Thus, the deletion of INO80-C subunits may affect both DNA accessibility and the repair of CdtB-induced damages. The level of sensitivity to CdtB that we observed in the mutant strains likely results from the combination of these various effects on the cells.
We also observed CdtB resistance in strains with the deletion of SIR2 and SIR3, but not SIR4, subunits of the SIR complex, which play critical roles in heterochromatin formation (Fig. 1). Interestingly, Sir4 plays a role in the key initial step in heterochromatin formation at telomere or mating type loci but not the rDNA locus (24, 64). This suggests that silencing at rDNA may be more important for CdtB function. Moreover, CdtB nuclear localization in sir2Δ and sir3Δ mutants was similar to that in the wild type (Fig. 2A and B). CdtB could damage DNA in the sir2Δ strain, but its activity was slightly reduced in the sir3Δ strain (Fig. 3A and B). Available transcriptomics data suggest that candidate DNA repair genes were not upregulated in the sir2Δ and sir3Δ mutants (Fig. 5A), suggesting that the CdtB-resistant phenotype of the sir2Δ and sir3Δ mutants may not be promoted by enhanced DNA repair.
We did not observe CdtB resistance in strains lacking the histone-modifying enzyme Ste20, Gcn5, or Dot1 and the linker histone Hho1. Ste20 is a kinase that phosphorylates the H2B histone tail for chromatin condensation during apoptosis (65). Although the H2B (S10A) mutant was partially resistant to CdtB, the ste20Δ mutant was as sensitive to CdtB as the wild type (Fig. 1). This could be due to other functions of Ste20 besides H2B phosphorylation. Gcn5 is the histone acetyltransferase (HAT) subunit of SAGA and ADA transcription coactivator complexes (66). Although Gcn5 could acetylate Htz1, Htz1 is also acetylated by Esa1 (NuA4 complex) (67). Thus, it is unclear if acetylation of the Htz1 tail affects CdtB toxicity. Dot1 is an H3K79 methyltransferase important for gene silencing. The deletion of DOT1 decreased the occupancy of SIR subunits at the telomere, while in the H3 (K79A) mutant, silencing was lost at the telomere and mating type loci but only slightly decreased at the rDNA region (68). Because the dot1Δ mutant was not resistant to CdtB, this may further support the notion that silencing at rDNA loci may be more important for CdtB activity.
Taken together, our results suggest a complex interplay of multiple host factors with CdtB that help to facilitate its activity, either directly or indirectly. Further investigations into the interactions between CdtB and host factors are needed to devise novel strategies to control bacterial genotoxic activity in infections and/or to employ the toxin in other applications, such as in cancer therapy (69). Human orthologues of the genes identified in this study also play important evolutionarily conserved roles in chromatin regulation (70, 71). Thus, our findings are likely applicable to human cells, and future studies are warranted.
MATERIALS AND METHODS
Plasmids and yeast strains.
The pYES2 vector carrying wild-type cdtB of Aggregatibacter actinomycetemcomitans (pYES2-CdtB) and CdtB-EGFP plasmids were previously described (18, 21). The plasmid was transformed using the lithium acetate method into wild-type BY4741 yeast and the chromatin regulatory gene deletion mutants (Euroscarf deletion library; Invitrogen, USA) according to standard protocols (72). A PCR-based gene deletion strategy was used to generate rad50Δ in the chromatin regulatory gene deletion mutants. A LEU2 marker was amplified from pRS315 using the RAD50 upstream primer (5′-AACCATTGAGAGGCAAAAACAAGGGAACGACGGAAAGCAGGCATGAGATTGTACTGAGAGTGCAC-3′) and the RAD50 downstream primer (5′-ATCAATCAAAGTCTATCCCTTCGTAGATATTATGGGGTCTTTTCACTGTGCGGTATTTCACACCG-3′). Transformants were selected on synthetic complete medium without leucine (SC−Leu; ingredients from Difco [BD, USA] and Sigma, Germany).
CdtB susceptibility, survival plating assay, and growth curve analysis.
Overnight yeast culture in SC−Ura with 2% glucose was adjusted to an optical density at 600 nm (OD600) of 0.1 and grown in 2% sucrose to reach an OD600 of 0.4 to 0.6. A CdtB susceptibility test was performed by spotting 10-fold serial dilutions of early-log-phase culture on plates containing SC−Ura with 2% galactose (Fluka, Sigma-Aldrich, Germany) as the CdtB-inducing medium and with 2% glucose as control and incubated at 30°C for 2 days. For survival plating assay, approximately 500 cells/sample were plated on medium containing both carbon sources. We counted the number of CFU after 3 to 4 days of incubation. The survival percentage of yeast under CdtB expression condition was calculated from the ratio of the number of CFU on 2% galactose versus that on 2% glucose and normalized to that of the same strain carrying the pYES2 vector control. The Mann-Whitney U test (SPSS Statistics 20) was used to analyze the data in comparison to those for the wild-type strain from biological triplicates of three transformants. For growth curve analysis, early-log-phase yeast cultures were inoculated in SC−Ura broth with 2% galactose and incubated at 30°C with 180-rpm shaking. The OD600 was measured every 4 h until the 12th hour and every 2 h thereafter. The means and standard errors (SE) for three transformants in three independent experiments were calculated.
Cell lysate preparation and immunoblotting assay.
The whole-cell lysate was extracted by the glass bead disruption method (73). Briefly, the yeast pellet was resuspended in lysis buffer (20 mM HEPES [pH 7.6], 200 mM potassium acetate, 10% glycerol, 1 mM EDTA, and protease inhibitors, which included 1 μM pepstatin A [Sigma, Germany], 0.1 mM phenylmethylsulfonyl fluoride [PMSF] [OmniPur; Calbiochem, USA], 1 μM E-64 protease inhibitor [Calbiochem, USA], and 1 mM 1,10-phenanthroline [Sigma, Germany]) and vortexed with glass beads. Protein samples were separated by SDS-PAGE and transferred onto nitrocellulose membranes according to the standard protocol (74). Antibodies were used to detect the proteins of interest, including 1:5,000 rabbit anti-AaCdtB serum (2), 1:5,000 rabbit anti-HA polyclonal antibody (ab9110; Abcam, UK), 1:5,000 mouse anti-Pgk1 IgG (ab113687; Abcam, UK), 1:5,000 goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP) (ADI-SAB-300; Enzo, USA), and 1:5,000 rabbit anti-mouse IgG conjugated with HRP (AP160P; Sigma, Germany).
Recombinant CdtB expression and purification.
The plasmid pET28a carrying CdtB with a 6-histidine tag (2) was transformed into E. coli BL21(DE3) competent cells by heat shock transformation and plated on LB agar supplemented with 30 μg/ml kanamycin. CdtB expression was induced by adding 250 μM IPTG (isopropyl-β-d-thiogalactopyranoside) to the mid-log-phase culture for 4 h at 37°C. Cells were lysed by sonication, and the soluble fraction was collected. Nickel-nitrilotriacetic acid (Ni-NTA) beads (catalog no. 30210; Qiagen, Germany) were used to purified 6×His-CdtB protein. Samples were washed and eluted with various concentrations of imidazole in purification buffer (50 mM NaH2PO4, 300 mM NaCl, protease inhibitors). Protein samples were analyzed using SDS-PAGE and Coomassie blue staining.
Pulldown assay and immunoprecipitation.
Recombinant 6-histidine-tagged CdtB was captured on protein A beads (Sigma, Germany) by rabbit anti-CdtB serum in binding buffer (20 mM Na3PO4 [pH 7.0], 50 mM Tris-Cl [pH 7.0]). Whole-cell lysate of the HA-tagged Htz1 yeast strain (gift from M. C. Keogh [75]) was coincubated with CdtB-protein A bead complex in buffer (20 mM HEPES [pH 7.6], 10% glycerol, 200 mM potassium acetate, 1 mM EDTA, and protease inhibitors; modified from reference 76) overnight with gentle agitation at 4°C. The immunoprecipitated samples were examined by SDS-PAGE and immunoblotting as described above.
Chromatin immunoprecipitation.
Yeast containing Htz1–HA tag with or without CdtB expression was used for chromatin immunoprecipitation as previously described with minor modification (76). Briefly, protein A beads were coated with 1:1,000 rabbit anti-AaCdtB serum or 1:1,000 anti-HA antibody. CdtB was induced by 2% galactose for 8 h before 1% (final concentration) formaldehyde was added to cross-link the samples. Formaldehyde solution was prepared in the diluent (100 mM NaCl, 1 mM EDTA, 50 mM HEPES-KOH [pH 7.5]). Samples were resuspended in the lysis buffer (20 mM HEPES-KOH [pH 7.5], 1 mM EDTA, 2% Triton X-100, 10 mM potassium acetate, and protease inhibitors), and then glass beads and vortexing were applied for yeast extraction. The chromatin preparation was sonicated twice at 10% output with a 2-s pulse cycle for 40 s. Samples were incubated with anti-CdtB and anti-HA-coated beads at 4°C under rotary agitation for 4-h. The immunoprecipitated samples were examined by SDS-PAGE and immunoblotting as described above.
Nuclear localization of CdtB.
Yeast strains carrying pYES2-CdtB-EGFP or pYES2-EGFP control were incubated in medium containing 2% galactose for 8 h, and nuclear localization of EGFP was observed as previously described (18). Briefly, samples were fixed in formaldehyde and permeabilized with 1% Triton X-100. DAPI (4′,6-diamidino-2-phenylindole) staining was performed for nucleus detection. Cells were pipetted onto a 1% agarose patch on glass slides. Fluorescent images were captured with a confocal fluorescence microscope (FV10i-ASW model; Olympus, Japan) at an optical magnification of ×600.
Fluorescent images were analyzed by Manders’ coefficient function of Just Another Co-localization Plug-in (JACoP) in the ImageJ 1.46r program with Java 1.6.0_20 (64 bit) (77). Mean fluorescent intensity plus 1 standard deviation (SD) and plus 2 SD were set as the threshold for DAPI (area of nuclei) and EGFP signal, respectively. The analyses were performed in a blind fashion. The relative percentage of CdtB localization in the nucleus was calculated by comparing the percentage of EGFP-positive pixel numbers in the nucleus over the total in yeast cells expressing CdtB-EGFP versus the EGFP control. The data were analyzed with the Mann-Whitney U test with a significant level at a P value of <0.05.
In silico analysis of microarray expression data.
Microarray data were retrieved from Gene Expression Omnibus (NCBI; https://www.ncbi.nlm.nih.gov/geo/). Gene expression data sets included those for chromatin regulatory gene mutants, including GSE21571 (31) and GSE25909 (35), and treatment with methyl methanesulfonate (MMS) or gamma radiation, including GSE6018 (36), GSE2224 (37), and GSE5301 (38). Gene selection criteria were set as an FDR-adjusted P value of <0.05 and a Z-score cutoff of ±1 (approximately ≥1.5-fold changes). Genes that are required for CdtB function from previous yeast genome-wide screening (18) and DNA repair from SGD annotation (GO:0006281) were selected. Hierarchical clustering was performed using the clusterMaker2 version 1.3.1 plug-in running on Cytoscape version 3.8.0 (78). Clustering was calculated by an uncentered Pearson’s correlation distance matrix with pairwise average linkage.
RNA extraction and quantitative real-time PCR.
CdtB expression was induced for 2 to 4 h with 2% galactose in log-phase cultures. Cultures were collected and resuspended in 350 μl lysis buffer (0.5 M NaCl, 10 mM EDTA, 1% SDS, and 0.2 M Tris-Cl [pH 7.6]). Glass beads and 350 μl of an acid phenol-chloroform-isoamyl alcohol (25:24:1) mixture was added. The maximum speed of the vortex was applied for 1.5 min (twice). The aqueous phase was separated by centrifugation at 16,000 × g for 15 min, and nucleic acid was precipitated with ethanol. The pellet was dried at 65°C. Afterward, samples were treated with DNase (1 U per 5 μg sample) in 37°C for 120 min. Acid phenol-chloroform extraction and ethanol precipitation were repeated to remove DNase. RNA quality and quantification were analyzed by 1.5% agarose gel electrophoresis (100 V, 70 min) and Nanodrop (2000/c; Thermo Scientific). The gel was stained with ethidium bromide and was observed under UV light.
DNA-free mRNA samples were converted to cDNA using the ImProm-II kit (Promega, USA) with an oligo(dT) primer at 42°C for 1 h. cDNA samples were amplified with the qPCR master mix (Luna M3003S; New England Biolabs [NEB], USA). The primers used are shown in Table 1. Gene expression level was calculated by the difference between cycle threshold values for the WT and the mutants, using ACT1 as a housekeeping gene.
TABLE 1.
Primers for gene expression analysis by RT-qPCR in this studya
| Name | Sequences | Reference |
|---|---|---|
| ACT1-F | 5′-TGCCGAAAGAATGCAAAAGG-3′ | 79 |
| ACT1-R | 5′-TCTGGAGGAGCAATGATCTTGA-3′ | |
| SPO1-F | 5′-CCACTTATTAGGGAGGCCA-3′ | This study |
| SPO1-R | 5′-GAGGATCTTTCAACACGCG-3′ | |
| COS10-F | 5′-GCAGTGAGATTCGCAACTC-3′ | This study |
| COS10-R | 5′-GCAGTGAGATTCGCAACTC-3′ | |
| MVB12-F | 5′-TACGGCGTATTCCCCTCTA-3′ | This study |
| MVB12-R | 5′-CAACAATTCCCGGTGGTTT-3′ | |
| PDR17-F | 5′-CCTGCTGTGCCTAAAGAGA-3′ | This study |
| PDR17-R | 5′-TCGCGGTGTTCCATTTATTG-3′ | |
| YMC1-F | 5′-CCAGCCCTTTGATACGACA-3′ | This study |
| YMC1-R | 5′-ACATGCGTAATACTGTGGC-3′ | |
| LDB18-F | 5′-GAATTCCGATGTTTCAGTGC-3′ | This study |
| LDB18-R | 5′-GCGTCTTTGAAGGAAATCTG-3′ | |
| RFA1-F | 5′-AAATCTGATGGGGCTAACAG-3′ | This study |
| RFA1-R | 5′-TGACTGGAACTTGGATGCA-3′ | |
| DNA2-F | 5′-TGCGAAGAAGACAGAGGA-3′ | This study |
| DNA2-R | 5′-GGACACTTGCATTGGATACC-3′ | |
| SSL2-F | 5′-TGATGCAGAGATCGATGAGA-3′ | This study |
| SSL2-R | 5′-GAGGCTTCTTCAACCCTGT-3′ | |
| DEF1-F | 5′-TCCTGCGCTAAAGTCCAA-3′ | This study |
| DEF1-R | 5′-TCCCATCTTGTCACTGCG-3′ | |
| EPL1-F | 5′-AAAGGCGATTCAGGTGCT-3′ | This study |
| EPL1-R | 5′-CCACCTCTCTCTGTTGCA-3′ | |
| EAF1-F | 5′-CTGACCGAACTATACTGCGT-3′ | This study |
| EAF1-R | 5′-CGCCTCGTCAAACCTTATAC-3′ |
All primer sequences and specificities were designed by Primer3-py package (https://www.yeastgenome.org/primer3) and checked by BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
ACKNOWLEDGMENTS
We thank G. R. Fink, S. Buratowski, E. J. Cho, C. Boonchird, and M. C. Keogh for kindly providing strains and plasmids. We are also grateful to members of the RU on Oral Microbiology and Immunology and Oral Biology Research Center of Faculty of Dentistry, Chulalongkorn University, and Laboratory of Biotechnology, Chulabhorn Research Institute, for assistance and suggestions. We also thank the Oral Biology Research Center, Faculty of Dentistry, Chulalongkorn University, for facility support.
This work was supported by Thailand Research Fund (grant RSA5480007 to O.M.) and the Royal Golden Jubilee Ph.D. Program (grant PHD/0133/2554 to S.D. and O.M.), Chulabhorn Research Institute (to S.M. and O.M.), and the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University (Research Unit on Oral Microbiology and Immunology).
Contributor Information
Oranart Matangkasombut, Email: oranart.m@chula.ac.th.
Andreas J. Bäumler, University of California, Davis
REFERENCES
- 1.Scuron MD, Boesze-Battaglia K, Dlakic M, Shenker BJ. 2016. The cytolethal distending toxin contributes to microbial virulence and disease pathogenesis by acting as a tri-perditious toxin. Front Cell Infect Microbiol 6:168. 10.3389/fcimb.2016.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugai M, Kawamoto T, Peres SY, Ueno Y, Komatsuzawa H, Fujiwara T, Kurihara H, Suginaka H, Oswald E. 1998. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun 66:5008–5019. 10.1128/IAI.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson B, Nair SP, Ward JM, Wilson M. 2003. Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu Rev Microbiol 57:29–55. 10.1146/annurev.micro.57.030502.090908. [DOI] [PubMed] [Google Scholar]
- 4.Umeda JE, Longo PL, Simionato MR, Mayer MP. 2013. Differential transcription of virulence genes in Aggregatibacter actinomycetemcomitans serotypes. J Oral Microbiol 5:21473. 10.3402/jom.v5i0.21473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawamoto D, Ando ES, Longo PL, Nunes AC, Wikstrom M, Mayer MP. 2009. Genetic diversity and toxic activity of Aggregatibacter actinomycetemcomitans isolates. Oral Microbiol Immunol 24:493–501. 10.1111/j.1399-302X.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 6.Ohara M, Miyauchi M, Tsuruda K, Takata T, Sugai M. 2011. Topical application of Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces cell cycle arrest in the rat gingival epithelium in vivo. J Periodontal Res 46:389–395. 10.1111/j.1600-0765.2011.01348.x. [DOI] [PubMed] [Google Scholar]
- 7.Tan KS, Song KP, Ong G. 2002. Cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Occurrence and association with periodontal disease. J Periodontal Res 37:268–272. 10.1034/j.1600-0765.2002.01618.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Li L, Yang M, Geng Y, Chen H, Xu Y, Sun Y. 2014. Prevalence and distribution of Aggregatibacter actinomycetemcomitans and its cdtB gene in subgingival plaque of Chinese periodontitis patients. BMC Oral Health 14:37. 10.1186/1472-6831-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belibasakis G, Johansson A, Wang Y, Claesson R, Chen C, Asikainen S, Kalfas S. 2002. Inhibited proliferation of human periodontal ligament cells and gingival fibroblasts by Actinobacillus actinomycetemcomitans: involvement of the cytolethal distending toxin. Eur J Oral Sci 110:366–373. 10.1034/j.1600-0722.2002.21350.x. [DOI] [PubMed] [Google Scholar]
- 10.Ohara M, Hayashi T, Kusunoki Y, Miyauchi M, Takata T, Sugai M. 2004. Caspase-2 and caspase-7 are involved in cytolethal distending toxin-induced apoptosis in Jurkat and MOLT-4 T-cell lines. Infect Immun 72:871–879. 10.1128/iai.72.2.871-879.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shenker BJ, Hoffmaster RH, Zekavat A, Yamaguchi N, Lally ET, Demuth DR. 2001. Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J Immunol 167:435–441. 10.4049/jimmunol.167.1.435. [DOI] [PubMed] [Google Scholar]
- 12.Elwell CA, Dreyfus LA. 2000. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol Microbiol 37:952–963. 10.1046/j.1365-2958.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- 13.Damek-Poprawa M, Jang JY, Volgina A, Korostoff J, DiRienzo JM. 2012. Localization of Aggregatibacter actinomycetemcomitans cytolethal distending toxin subunits during intoxication of live cells. Infect Immun 80:2761–2770. 10.1128/IAI.00385-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerra L, Teter K, Lilley BN, Stenerlow B, Holmes RK, Ploegh HL, Sandvig K, Thelestam M, Frisan T. 2005. Cellular internalization of cytolethal distending toxin: a new end to a known pathway. Cell Microbiol 7:921–934. 10.1111/j.1462-5822.2005.00520.x. [DOI] [PubMed] [Google Scholar]
- 15.DiRienzo JM. 2014. Uptake and processing of the cytolethal distending toxin by mammalian cells. Toxins (Basel) 6:3098–3116. 10.3390/toxins6113098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikubo S, Ohara M, Ueno Y, Ikura M, Kurihara H, Komatsuzawa H, Oswald E, Sugai M. 2003. An N-terminal segment of the active component of the bacterial genotoxin cytolethal distending toxin B (CDTB) directs CDTB into the nucleus. J Biol Chem 278:50671–50681. 10.1074/jbc.M305062200. [DOI] [PubMed] [Google Scholar]
- 17.Carette JE, Guimaraes CP, Wuethrich I, Blomen VA, Varadarajan M, Sun C, Bell G, Yuan B, Muellner MK, Nijman SM, Ploegh HL, Brummelkamp TR. 2011. Global gene disruption in human cells to assign genes to phenotypes by deep sequencing. Nat Biotechnol 29:542–546. 10.1038/nbt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denmongkholchai S, Katare P, Choochuay S, Thanyasrisung P, Tsuruda K, Sugai M, Mongkolsuk S, Matangkasombut O. 2019. Genome-wide identification of host genes required for toxicity of bacterial cytolethal distending toxin in a yeast model. Front Microbiol 10:890. 10.3389/fmicb.2019.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassane DC, Lee RB, Pickett CL. 2003. Campylobacter jejuni cytolethal distending toxin promotes DNA repair responses in normal human cells. Infect Immun 71:541–545. 10.1128/iai.71.1.541-545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitagawa T, Hoshida H, Akada R. 2007. Genome-wide analysis of cellular response to bacterial genotoxin CdtB in yeast. Infect Immun 75:1393–1402. 10.1128/IAI.01321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matangkasombut O, Wattanawaraporn R, Tsuruda K, Ohara M, Sugai M, Mongkolsuk S. 2010. Cytolethal distending toxin from Aggregatibacter actinomycetemcomitans induces DNA damage, S/G2 cell cycle arrest, and caspase-independent death in a Saccharomyces cerevisiae model. Infect Immun 78:783–792. 10.1128/IAI.00857-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lara-Tejero M, Galan JE. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354–357. 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- 23.Gerhold CB, Gasser SM. 2014. INO80 and SWR complexes: relating structure to function in chromatin remodeling. Trends Cell Biol 24:619–631. 10.1016/j.tcb.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Kueng S, Oppikofer M, Gasser SM. 2013. SIR proteins and the assembly of silent chromatin in budding yeast. Annu Rev Genet 47:275–306. 10.1146/annurev-genet-021313-173730. [DOI] [PubMed] [Google Scholar]
- 25.Bannister AJ, Kouzarides T. 2011. Regulation of chromatin by histone modifications. Cell Res 21:381–395. 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellahi A, Thurtle DM, Rine J. 2015. The chromatin and transcriptional landscape of native Saccharomyces cerevisiae telomeres and subtelomeric domains. Genetics 200:505–521. 10.1534/genetics.115.175711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fritze CE, Verschueren K, Strich R, Easton Esposito R. 1997. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J 16:6495–6509. 10.1093/emboj/16.21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaglio D, D'Alfonso A, Camilloni G. 2013. Functional complementation of sir2Delta yeast mutation by the human orthologous gene SIRT1. PLoS One 8:e83114. 10.1371/journal.pone.0083114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gartenberg MR, Smith JS. 2016. The nuts and bolts of transcriptionally silent chromatin in Saccharomyces cerevisiae. Genetics 203:1563–1599. 10.1534/genetics.112.145243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottlieb S, Esposito RE. 1989. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell 56:771–776. 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- 31.Morillo-Huesca M, Clemente-Ruiz M, Andujar E, Prado F. 2010. The SWR1 histone replacement complex causes genetic instability and genome-wide transcription misregulation in the absence of H2A.Z. PLoS One 5:e12143. 10.1371/journal.pone.0012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santisteban MS, Hang M, Smith MM. 2011. Histone variant H2A.Z and RNA polymerase II transcription elongation. Mol Cell Biol 31:1848–1860. 10.1128/MCB.01346-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuguchi G, Shen X, Landry J, Wu WH, Sen S, Wu C. 2004. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303:343–348. 10.1126/science.1090701. [DOI] [PubMed] [Google Scholar]
- 34.Steininger S, Ahne F, Winkler K, Kleinschmidt A, Eckardt-Schupp F, Moertl S. 2010. A novel function for the Mre11-Rad50-Xrs2 complex in base excision repair. Nucleic Acids Res 38:1853–1865. 10.1093/nar/gkp1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenstra TL, Benschop JJ, Kim T, Schulze JM, Brabers NA, Margaritis T, van de Pasch LA, van Heesch SA, Brok MO, Groot Koerkamp MJ, Ko CW, van Leenen D, Sameith K, van Hooff SR, Lijnzaad P, Kemmeren P, Hentrich T, Kobor MS, Buratowski S, Holstege FC. 2011. The specificity and topology of chromatin interaction pathways in yeast. Mol Cell 42:536–549. 10.1016/j.molcel.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benton MG, Somasundaram S, Glasner JD, Palecek SP. 2006. Analyzing the dose-dependence of the Saccharomyces cerevisiae global transcriptional response to methyl methanesulfonate and ionizing radiation. BMC Genomics 7:305. 10.1186/1471-2164-7-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caba E, Dickinson DA, Warnes GR, Aubrecht J. 2005. Differentiating mechanisms of toxicity using global gene expression analysis in Saccharomyces cerevisiae. Mutat Res 575:34–46. 10.1016/j.mrfmmm.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Fry RC, DeMott MS, Cosgrove JP, Begley TJ, Samson LD, Dedon PC. 2006. The DNA-damage signature in Saccharomyces cerevisiae is associated with single-strand breaks in DNA. BMC Genomics 7:313. 10.1186/1471-2164-7-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krogan NJ, Keogh MC, Datta N, Sawa C, Ryan OW, Ding H, Haw RA, Pootoolal J, Tong A, Canadien V, Richards DP, Wu X, Emili A, Hughes TR, Buratowski S, Greenblatt JF. 2003. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol Cell 12:1565–1576. 10.1016/s1097-2765(03)00497-0. [DOI] [PubMed] [Google Scholar]
- 40.Tramantano M, Sun L, Au C, Labuz D, Liu Z, Chou M, Shen C, Luk E. 2016. Constitutive turnover of histone H2A.Z at yeast promoters requires the preinitiation complex. Elife 5:e14243. 10.7554/eLife.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu WH, Alami S, Luk E, Wu CH, Sen S, Mizuguchi G, Wei D, Wu C. 2005. Swc2 is a widely conserved H2AZ-binding module essential for ATP-dependent histone exchange. Nat Struct Mol Biol 12:1064–1071. 10.1038/nsmb1023. [DOI] [PubMed] [Google Scholar]
- 42.Wu WH, Wu CH, Ladurner A, Mizuguchi G, Wei D, Xiao H, Luk E, Ranjan A, Wu C. 2009. N terminus of Swr1 binds to histone H2AZ and provides a platform for subunit assembly in the chromatin remodeling complex. J Biol Chem 284:6200–6207. 10.1074/jbc.M808830200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe S, Radman-Livaja M, Rando OJ, Peterson CL. 2013. A histone acetylation switch regulates H2A.Z deposition by the SWR-C remodeling enzyme. Science 340:195–199. 10.1126/science.1229758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ranjan A, Mizuguchi G, FitzGerald PC, Wei D, Wang F, Huang Y, Luk E, Woodcock CL, Wu C. 2013. Nucleosome-free region dominates histone acetylation in targeting SWR1 to promoters for H2A.Z replacement. Cell 154:1232–1245. 10.1016/j.cell.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun L, Luk E. 2017. Dual function of Swc5 in SWR remodeling ATPase activation and histone H2A eviction. Nucleic Acids Res 45:9931–9946. 10.1093/nar/gkx589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guerra L, Nemec KN, Massey S, Tatulian SA, Thelestam M, Frisan T, Teter K. 2009. A novel mode of translocation for cytolethal distending toxin. Biochim Biophys Acta 1793:489–495. 10.1016/j.bbamcr.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonangelino CJ, Chavez EM, Bonifacino JS. 2002. Genomic screen for vacuolar protein sorting genes in Saccharomyces cerevisiae. Mol Biol Cell 13:2486–2501. 10.1091/mbc.02-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Copic A, Dorrington M, Pagant S, Barry J, Lee MC, Singh I, Hartman JLt, Miller EA. 2009. Genomewide analysis reveals novel pathways affecting endoplasmic reticulum homeostasis, protein modification and quality control. Genetics 182:757–769. 10.1534/genetics.109.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conibear E, Stevens TH. 1998. Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim Biophys Acta 1404:211–230. 10.1016/s0167-4889(98)00058-5. [DOI] [PubMed] [Google Scholar]
- 50.Hellauer K, Lesage G, Sdicu AM, Turcotte B. 2005. Large-scale analysis of genes that alter sensitivity to the anticancer drug tirapazamine in Saccharomyces cerevisiae. Mol Pharmacol 68:1365–1375. 10.1124/mol.105.012963. [DOI] [PubMed] [Google Scholar]
- 51.MacDonald C, Payne JA, Aboian M, Smith W, Katzmann DJ, Piper RC. 2015. A family of tetraspans organizes cargo for sorting into multivesicular bodies. Dev Cell 33:328–342. 10.1016/j.devcel.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amaro IA, Costanzo M, Boone C, Huffaker TC. 2008. The Saccharomyces cerevisiae homolog of p24 is essential for maintaining the association of p150Glued with the dynactin complex. Genetics 178:703–709. 10.1534/genetics.107.079103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furuchi T, Nitta K, Takahashi T, Naganuma A. 2004. Overexpression of Ssl2p confers resistance to adriamycin and actinomycin D in Saccharomyces cerevisiae. Biochem Biophys Res Commun 314:844–848. 10.1016/j.bbrc.2003.12.160. [DOI] [PubMed] [Google Scholar]
- 54.Furuchi T, Takahashi T, Tanaka S, Nitta K, Naganuma A. 2004. Functions of yeast helicase Ssl2p that are essential for viability are also involved in protection from the toxicity of adriamycin. Nucleic Acids Res 32:2578–2585. 10.1093/nar/gkh582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woudstra EC, Gilbert C, Fellows J, Jansen L, Brouwer J, Erdjument-Bromage H, Tempst P, Svejstrup JQ. 2002. A Rad26-Def1 complex coordinates repair and RNA pol II proteolysis in response to DNA damage. Nature 415:929–933. 10.1038/415929a. [DOI] [PubMed] [Google Scholar]
- 56.Wang P, Byrum S, Fowler FC, Pal S, Tackett AJ, Tyler JK. 2017. Proteomic identification of histone post-translational modifications and proteins enriched at a DNA double-strand break. Nucleic Acids Res 45:10923–10940. 10.1093/nar/gkx844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Downs JA, Allard S, Jobin-Robitaille O, Javaheri A, Auger A, Bouchard N, Kron SJ, Jackson SP, Cote J. 2004. Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol Cell 16:979–990. 10.1016/j.molcel.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Yen K, Vinayachandran V, Pugh BF. 2013. SWR-C and INO80 chromatin remodelers recognize nucleosome-free regions near +1 nucleosomes. Cell 154:1246–1256. 10.1016/j.cell.2013.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tosi A, Haas C, Herzog F, Gilmozzi A, Berninghausen O, Ungewickell C, Gerhold CB, Lakomek K, Aebersold R, Beckmann R, Hopfner KP. 2013. Structure and subunit topology of the INO80 chromatin remodeler and its nucleosome complex. Cell 154:1207–1219. 10.1016/j.cell.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 60.Shen X, Ranallo R, Choi E, Wu C. 2003. Involvement of actin-related proteins in ATP-dependent chromatin remodeling. Mol Cell 12:147–155. 10.1016/s1097-2765(03)00264-8. [DOI] [PubMed] [Google Scholar]
- 61.Yao W, King DA, Beckwith SL, Gowans GJ, Yen K, Zhou C, Morrison AJ. 2016. The INO80 complex requires the Arp5-Ies6 subcomplex for chromatin remodeling and metabolic regulation. Mol Cell Biol 36:979–991. 10.1128/MCB.00801-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morrison AJ. 2017. Genome maintenance functions of the INO80 chromatin remodeller. Philos Trans R Soc Lond B Biol Sci 372:20160289. 10.1098/rstb.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papamichos-Chronakis M, Watanabe S, Rando OJ, Peterson CL. 2011. Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell 144:200–213. 10.1016/j.cell.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith JS, Brachmann CB, Pillus L, Boeke JD. 1998. Distribution of a limited Sir2 protein pool regulates the strength of yeast rDNA silencing and is modulated by Sir4p. Genetics 149:1205–1219. 10.1093/genetics/149.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahn SH, Cheung WL, Hsu JY, Diaz RL, Smith MM, Allis CD. 2005. Sterile 20 kinase phosphorylates histone H2B at serine 10 during hydrogen peroxide-induced apoptosis in S. cerevisiae. Cell 120:25–36. 10.1016/j.cell.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 66.Helmlinger D, Tora L. 2017. Sharing the SAGA. Trends Biochem Sci 42:850–861. 10.1016/j.tibs.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Millar CB, Xu F, Zhang K, Grunstein M. 2006. Acetylation of H2AZ Lys 14 is associated with genome-wide gene activity in yeast. Genes Dev 20:711–722. 10.1101/gad.1395506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Leeuwen F, Gafken PR, Gottschling DE. 2002. Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell 109:745–756. 10.1016/s0092-8674(02)00759-6. [DOI] [PubMed] [Google Scholar]
- 69.Lin HJ, Liu HH, Lin CD, Kao MC, Chen YA, Chiang-Ni C, Jiang ZP, Huang MZ, Lin CJ, Lo UG, Lin LC, Lai CK, Lin H, Hsieh JT, Chiu CH, Lai CH. 2017. Cytolethal distending toxin enhances radiosensitivity in prostate cancer cells by regulating autophagy. Front Cell Infect Microbiol 7:223. 10.3389/fcimb.2017.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaquero A. 2009. The conserved role of sirtuins in chromatin regulation. Int J Dev Biol 53:303–322. 10.1387/ijdb.082675av. [DOI] [PubMed] [Google Scholar]
- 71.Willhoft O, Wigley DB. 2020. INO80 and SWR1 complexes: the non-identical twins of chromatin remodelling. Curr Opin Struct Biol 61:50–58. 10.1016/j.sbi.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gietz RD, Woods RA. 2002. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol 350:87–96. 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- 73.Keogh MC, Cho EJ, Podolny V, Buratowski S. 2002. Kin28 is found within TFIIH and a Kin28-Ccl1-Tfb3 trimer complex with differential sensitivities to T-loop phosphorylation. Mol Cell Biol 22:1288–1297. 10.1128/mcb.22.5.1288-1297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith BJ. 1994. SDS polyacrylamide gel electrophoresis of proteins. Methods Mol Biol 32:23–34. 10.1385/0-89603-268-X:23. [DOI] [PubMed] [Google Scholar]
- 75.Keogh MC, Mennella TA, Sawa C, Berthelet S, Krogan NJ, Wolek A, Podolny V, Carpenter LR, Greenblatt JF, Baetz K, Buratowski S. 2006. The Saccharomyces cerevisiae histone H2A variant Htz1 is acetylated by NuA4. Genes Dev 20:660–665. 10.1101/gad.1388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keogh MC, Podolny V, Buratowski S. 2003. Bur1 kinase is required for efficient transcription elongation by RNA polymerase II. Mol Cell Biol 23:7005–7018. 10.1128/mcb.23.19.7005-7018.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bolte S, Cordelieres FP. 2006. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224:213–232. 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 78.Morris JH, Apeltsin L, Newman AM, Baumbach J, Wittkop T, Su G, Bader GD, Ferrin TE. 2011. clusterMaker: a multi-algorithm clustering plugin for Cytoscape. BMC Bioinformatics 12:436. 10.1186/1471-2105-12-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mizuno T, Masuda Y, Irie K. 2015. The Saccharomyces cerevisiae AMPK, Snf1, negatively regulates the Hog1 MAPK pathway in ER stress response. PLoS Genet 11:e1005491. 10.1371/journal.pgen.1005491. [DOI] [PMC free article] [PubMed] [Google Scholar]