Abstract
Background:
While current human papillomavirus (HPV) genotype screening tests identify genotypes 16 and 18 and do not specifically identify other high-risk types; a new extended genotyping test identifies additional individual (31, 45, 51, and 52) and groups (33/58, 35/39/68, and 56/59/66) of high-risk genotypes.
Methods:
We developed a Markov model of the HPV disease course and evaluated the clinical and economic value of HPV primary screening with Onclaritya capable of extended genotyping in a cohort of women ≥30 years old. Women with certain genotypes were later rescreened instead of undergoing immediate colposcopy and varied which genotypes were rescreened, disease progression rate, and test cost.
Results:
Assuming 100% compliance with screening, HPV primary screening using current tests resulted in 25,194 invasive procedures and 48 invasive cervical cancer (ICC) cases per 100,000 women. Screening with extended genotyping (100% compliance) and later rescreening women with certain genotypes averted 903-3,163 invasive procedures and resulted in 0-3 more ICC cases compared to current HPV primary screening tests. Extended genotyping was cost-effective [$2,298-$7,236/quality-adjusted life year (QALY)] when costing $75 and cost saving (median: $0.3-$1.0 million) when costing $43. As the probabilities of disease progression increased (2-4 times), extended genotyping was not cost-effective as it resulted in more ICC cases and accrued fewer QALYs.
Conclusions:
Our study identified the conditions under which extended genotyping was cost-effective and even cost saving compared to current tests. A key driver of cost-effectiveness is the risk of disease progression, which emphasizes the need to better understand such risks in different populations.
Keywords: HPV, Extended Genotyping, Cost-Effectiveness, Cost
Short Summary:
HPV primary screening with extended genotyping could be cost-effective and even cost saving (median ≤$1.0 million at equal genotype test costs) compared to current tests.
INTRODUCTION
Current human papillomavirus (HPV) genotype screening tests in the US only identify genotypes 16 and 18 and do not specifically identify the other 12 high-risk HPV (hrHPV) genotypes;1–3 a new HPV assay (BD Onclarity™) identifies additional individual genotypes (31, 45, 51, and 52) and groups other genotypes (33/58, 35/39/68, and 56/59/66)4. The question is, what is the potential clinical and economic value of this additional information.
While these genotypes are all hrHPV, they have varying risks of cervical cancer and some are considered lower risk than others.5–7 Since current tests group all 12 non-16/18 genotypes together, women infected with other genotypes are managed the same regardless of their cancer risk.3 Furthermore, studies have shown that some hrHPV genotypes (e.g., 66, 68) carry little to no risk of cervical cancer.7,8 As women with lower risk genotypes often spontaneously clear infection,9 identifying which non-16/18 genotypes a woman is infected with may allow some to wait and be rescreened instead of undergoing immediate procedures. This may allow enough time for spontaneous clearance, thereby avoiding invasive procedures (e.g., colposcopy, biopsy). Alternatively, delaying treatment may allow some women to progress to later disease states, thereby requiring additional or more invasive procedures (i.e., hysterectomy). Thus, there is a tradeoff between the time until rescreening and the potential for the disease to progress to later stages.
An extended genotyping test (which individually identifies ≥5 genotypes while reporting the others as a group10), such as Onclarity, could provide additional information to allow time for spontaneous clearance versus partial genotyping tests (which individually identifies only 16 and 1810). To understand the tradeoff, we developed a computational Markov model simulating HPV’s disease course in a cohort of US women to determine the potential clinical and economic value of using Onclarity’s capability of extended genotyping to wait and rescreen women with lower risk genotypes.
MATERIALS and METHODS
Model Structure
Using Microsoft Excel 2016 (Redmond, WA), we developed a Markov model that simulated a cohort of US women ≥30 years old moving through HPV’s disease course to determine the potential clinical and economic value of HPV screening utilizing extended genotyping to wait and rescreen some women from the third-party payer perspective. Figure 1 outlines the model, while the Appendix describes our data sources, and Table A1 shows the model input parameters, values, distribution types, and sources. The model consisted of seven mutually exclusive states: healthy (not infected with hrHPV); infected with hrHPV (any hrHPV), cervical intraepithelial neoplasia (CIN) 1, CIN 2, and CIN 3; invasive cervical cancer (ICC); and death. We staged women with ICC based on the Fédération Internationale de Gynécologie et d'Obstétrique (FIGO) classification and divided into three groups based on treatment: FIGO IA, FIGO IB/IIA, and FIGO IIB+ (FIGO IIB and worse). The model ran in one-year cycle lengths. At the end of each cycle, HPV-infected women could remain in the same disease state (i.e., persist), progress to a later disease state, or regress to an earlier disease state (Figure 1a), based on annual genotype-specific probabilities of progression, regression, and persistence for a given disease state (Table A1). Women left the model once they developed ICC or died (i.e., annual probability of all-cause mortality).
Figure 1.
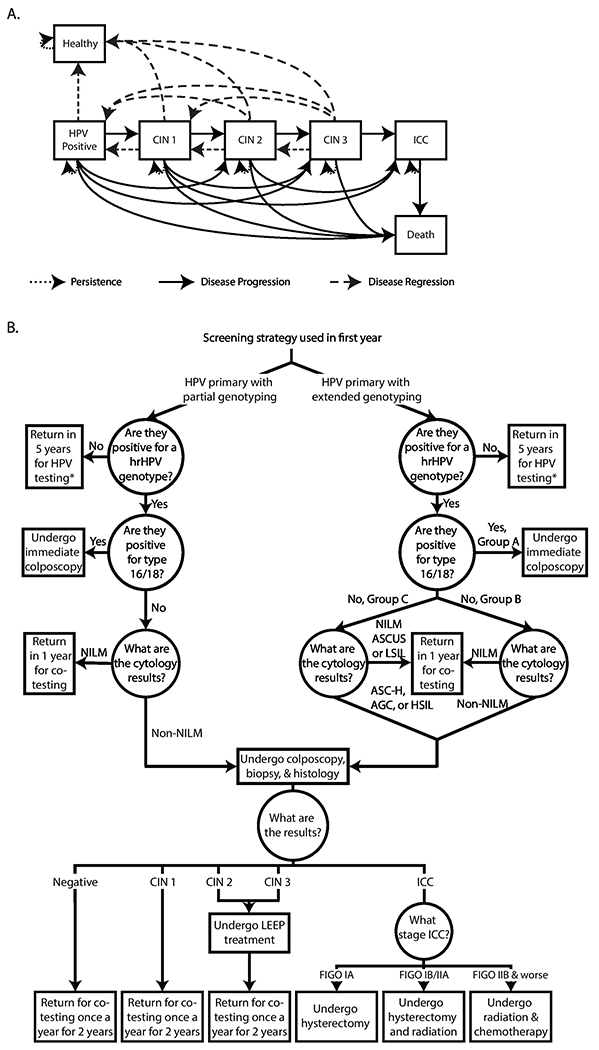
Model structure. A) Human papillomavirus (HPV) model states. B) HPV primary with and without extended genotyping patient management strategies. Note: AGC=atypical glandular cells; ASC-H=atypical squamous cells: cannot exclude high-grade squamous intraepithelial lesion; ASCUS= atypical squamous cells of undetermined significance; CIN=cervical intraepithelial neoplasia; FIGO=Fédération Internationale de Gynécologie et d'Obstétrique; hrHPV=high-risk HPV; HSIL=high-grade squamous intraepithelial lesion; ICC=invasive cervical cancer; LEEP=loop electrosurgical excision procedure; LSIL=low-grade squamous intraepithelial lesion; NILM=negative for intraepithelial lesion or malignancy. Co-testing consists of both HPV primary testing (with or without extended genotyping) and cytology. * indicates that women will undergo the same genotype test (with or without extended genotyping) every 5 years.
Modeled Screening Strategies
Figure 1b outlines our modeled screening strategies. In the first simulated year, all women underwent HPV primary screening (genotyping first, followed by cytology as needed). The first screening strategy used HPV primary screening with partial (16/18) genotyping, following US screening guidelines.3,11,12 These guidelines were set forth by the US Preventive Services Task Force and various official societies (e.g., American Cancer Society) and were based on reviewing scientific evidence, working groups, and symposiums/panel discussions.3,11,12 Women were deemed hrHPV-positive or hrHPV-negative based on genotype test sensitivity and specificity and if they were infected with a hrHPV genotype. Those testing negative returned in five years to undergo partial genotyping. Those testing positive (true and false positives) for 16/18, underwent colposcopy, while those positive for a non-16/18 hrHPV genotype had their initial samples undergo cervical cytology. Women had an abnormal cytology result based on cervical cytology sensitivity and specificity and the probability of abnormal cells. Abnormal cytology results include: atypical glandular cells (AGC), atypical squamous cells: cannot exclude high-grade squamous intraepithelial lesion (ASC-H), a high-grade squamous intraepithelial lesion (HSIL), atypical squamous cells of undetermined significance (ASC-US) or a low-grade squamous intraepithelial lesion (LSIL). Additionally, the cytology sample had a probability of being inadequate and another sample was collected. Women with any abnormal cytology results underwent colposcopy. Those who were negative for intraepithelial lesion or malignancy (NILM) returned in one year for co-testing (i.e., HPV test and cytology performed concurrently).
The second screening strategy used HPV primary screening with extended genotyping (individually detects: 16, 18, 31, 45, 51, 52; groups: 33/58, 35/39/68, 56/59/664; Figure 1b). Women testing hrHPV-positive were divided into three management strategies (group A, B, or C) depending on genotype (described below). Women who tested positive (based on test sensitivity and specificity) for genotypes 16/18 (group A) underwent colposcopy. Those testing positive for a non-16/18 hrHPV genotype (groups B and C) underwent cytology. Women with group B genotypes and any abnormal cytology results (AGC, ASC-H, HSIL, ASC-US, or LSIL) underwent colposcopy, those with NILM cytology results returned for co-testing in one year. Women with group C genotypes and cytology results of AGC, ASC-H, or HSIL underwent colposcopy, those with NILM, ASC-US, or LSIL cytology results return for co-testing in one year (similar to current US guidelines for women aged 21-2912).
Diagnostic Investigation and Treatment
Based on genotype and cytology results, women had a colposcopy, biopsy, and histology. Women without CIN or with CIN 1 returned once a year for the next two years for co-testing and women with CIN 2 or CIN 3 underwent a loop electrosurgical excision procedure (LEEP), which had an associated probability of being curative. Those with a successful procedure moved to the healthy state, while those with an unsuccessful procedure could progress to a later disease state. Women with FIGO IA had a hysterectomy, FIGO IB/IIA had a hysterectomy and radiation, and FIGO IIB+ received radiation and chemotherapy.
Simulations and Model Outcomes
We populated the model with a cohort of 100,000 divided into the healthy, infected, and CIN 1-3 states (no women started in the ICC state) and assigned one of the 14 hrHPV genotypes using US population estimates.4 While it is possible that women can be co-infected with more than one HPV genotype at a time, we assigned women to just one genotype, as treatment in cases of co-infection is based on the single genotype with the highest risk of progressing through the CIN stages to cancer. Each scenario ran for 40 years13 such that women were screened through age 65 (recommended age for stopping cervical cancer screening for women without any HPV infection during their lifetime).11
For each simulation, we calculated the incremental cost-effectiveness ratio (ICER) as: (CostExtendedGenotyping–CostPartialGenotyping)/(EffectivenessExtendedGenotyping–EffectivenessPartialGenotyping) where effectiveness was measured in quality-adjusted life years (QALYs) and costs and QALYs were only captured during the 40-year duration. Women accrued QALYs based on the age-dependent healthy QALY value attenuated by the utility weight associated with each disease state for the duration spent in that state (described in detail in Appendix). Women with undiagnosed CIN did not experience a reduction in QALYs. A half-cycle correction accounted for women changing disease states during a cycle (i.e., at any point during the year). For example, when CIN is diagnosed, women received the CIN-adjusted QALY value for half a year.
The third-party payer perspective included direct medical costs (e.g., diagnostics, procedures). All costs and QALYs were in net present value, 2018 $US, with all past and future scosts and future QALYs discounted with a 3% rate. Extended genotyping was considered cost-effective if ICERs were ≤$50,000/QALY14.
Scenarios and Senstivity Analyses
Our initial scenario used HPV primary screening with partial genotyping following the August 2018 US Preventive Services Task Force guidelines.11 Experimental scenarios used extended genotyping and varied which hrHPV genotypes were in groups B and C (Table 1). In all scenarios, genotypes 31, 33, and 45 remained in group B given their risk,7,15 while, 56/59/66 remained in group C (as genotype 66 is considered to have little or no carcinogenicity6,7).
Table 1.
Genotype groupings for the different patient management strategies
| Group A | Group B | Group C | |
|---|---|---|---|
| Patient Management Strategy 1 | 16, 18 | 31, 45, 33/58, 51, 52, 35/39/68 | 56/59/66 |
| Patient Management Strategy 2 | 16, 18 | 31, 45, 33/58, 52 | 51, 35/39/68, 56/59/66 |
| Patient Management Strategy 3 | 16, 18 | 31, 45, 33/58 | 51, 52, 35/39/68, 56/59/66 |
Group A patients undergo immediate colposcopy
Group B patients with any abnormal cytology results will go to colposcopy
Group C patients with atypical glandular cells (AGC), atypical squamous cells: cannot exclude high-grade squamous intraepithelial lesion (ASC-H), or a high-grade squamous intraepithelial lesion (HSIL) on cytology will go to colposcopy
To account for variability in model inputs, each experiment consisted of running Monte Carlo simulations (i.e., probabilistic sensitivity analyses) of 1,000 trials, varying each parameter throughout their ranges (Table A1). Sensitivity analyses varied the key parameters of extended genotyping assay cost ($43.3316-$100) and the probability of progression. The values for the probability of progression reported in the literature may be underestimated as most studies measuring progression do not include women with higher grade lesions, which may increase the rate at which a woman progresses17, and some include women who are HPV-negative or NILM.8,18–21 Thus, sensitivity analyses varied these reported values from 1 to 2 to 4 times the reported values, thereby progressively increasing these probabilities to explore the relationship between the sprogression rate and the value of extended genotyping. Additional scenarios increased the amount of time a woman waits after a negative cytology (i.e., NILM for group B or NILM, ASC-US, or LSIL for group C) to undergo co-testing in 2 years.
RESULTS
Impact of HPV Primary Screening with Partial Genotyping
As Table 2 shows, partial genotyping for 100,000 women over a 40-year period resulted in 25,194 invasive procedures (i.e., colposcopies and LEEPs) and 48 cases of ICC. Figure 2 shows the number of times women did or did not undergo a colposcopy by disease state. There were 5,189 times where women with CIN or ICC did not undergo colposcopy and 14,629 times healthy or infected women unnecessarily underwent a colposcopy (i.e., avoidable colposcopy). Partial genotyping resulted in a median of $30.4 million over the 40-year period (Table 2).
Table 2.
Clinical and economic (net present value 2018 $US) outcomes [median (95% uncertainty interval)] of human papillomavirus (HPV) primary screening with partial genotyping or extended genotyping (patient management strategies 1-3) over 40 simulated years in a cohort of 100,000 US women
| Scenario | Number of genotype tests | Number of cytologies | Number of colposcopies | Number of LEEPs | Number of ICC | Quality-adjusted life years (QALYs) | Cost of genotype tests (in millions) | Total cost (in millions) | ICER ($/QALY)* |
|---|---|---|---|---|---|---|---|---|---|
| HPV primary with partial genotyping | 575,598 | 144,332 | 23,583 | 1,611 | 48 | 1,240,569 | 17.6 | 30.4 | - |
| (410,407 - 738,479) | (99,611 - 190,494) | (19,223 - 27,799) | (1,473 - 1,763) | (35 - 64) | (923,530 - 1,543,255) | (13.7 - 21.1) | (24.6 - 35.9) | ||
| $43.33 Onclarity test | |||||||||
| Patient management strategy 1 | 573,020 | 144,375 | 22,699 | 1,592 | 48 | 1,242,093† | 17.5 | 30.1 | Dominant |
| (408,505 - 739,428) | (99,045 - 193,070) | (18,252 - 26,905) | (1,453 - 1,747) | (34 - 65) | (918,894 - 1,532,973) | (13.6 - 21.2) | (24.3 - 35.4) | ||
| Patient management strategy 2 | 574,712 | 143,971 | 21,323 | 1,501 | 50 | 1,243,995† | 17.5 | 29.6 | Dominant |
| (413,495 - 738,657) | (99,670 - 191,619) | (17,022 - 25,516) | (1,359 - 1,632) | (36 - 67) | (922,480 - 1,528,628) | (13.8 - 21.1) | (23.8 - 35.0) | ||
| Patient management strategy 3 | 573,808 | 143,249 | 20,572 | 1,442 | 50 | 1,242,874† | 17.5 | 29.2 | Dominant |
| (399,578 - 733,365) | (97,581 - 191,642) | (16,161 - 24,626) | (1,302 - 1,576) | (37 - 65) | (901,701 - 1,537,171) | (13.4 - 21.0) | (23.2 - 34.7) | ||
| $75 Onclarity test | |||||||||
| Patient management strategy 1 | 573,599 | 143,458 | 22,610 | 1,593 | 48 | 1,242,835† | 30.3 | 42.8 | 5,472 |
| (407,922 - 728,811) | (98,368 - 188,653) | (18,157 - 26,837) | (1,450 - 1,727) | (35 - 62) | (916,214 - 1,524,269) | (23.5 - 36.2) | (34.1 - 50.8) | ||
| Patient management strategy 2 | 572,730 | 143,181 | 21,352 | 1,502 | 49 | 1,242,246† | 30.2 | 42.5 | 7,236 |
| (404,442 - 735,325) | (98,279 - 193,460) | (16,982 - 25,524) | (1,360 - 1,635) | (37 - 66) | (908,881 - 1,529,568) | (23.4 - 36.5) | (33.7 - 50.2) | ||
| Patient management strategy 3 | 573,372 | 144,205 | 20,656 | 1,443 | 50 | 1,242,143† | 30.3 | 42.1 | 2,298 |
| (401,296 - 728,829) | (97,365 - 190,506) | (16,123 - 24,466) | (1,308 - 1,579) | (38 - 64) | (905,372 - 1,522,290) | (23.3 - 36.2) | (33.1 - 50.0) | ||
Note: Patient management strategy ordered by increasing number of high-risk HPV genotypes for which cytology results of negative for intraepithelial lesion or malignancy (NILM), atypical squamous cells of undetermined significance (ASC-US), or low-grade squamous intraepithelial lesion (LSIL) were deferred to co-testing instead of colposcopy (i.e., in Group C). Patient management strategy 1: 31, 45, 33/58, 51, 52, 35/39/68 with any abnormal cytology results will go to colposcopy; and 56/59/66 with atypical glandular cells (AGC), atypical squamous cells: cannot exclude high-grade squamous intraepithelial lesion (ASC-H), or a high-grade squamous intraepithelial lesion (HSIL) on cytology will go to colposcopy
Patient management strategy 2: 31, 45, 33/58, 52 with any abnormal cytology results will go to colposcopy; and 51, 35/39/68, 56/59/69 with AGC, ASC-H, HSIL will go to colposcopy
Patient management strategy 3: 31, 45, 33/58 with any abnormal cytology results will go to colposcopy; and 51, 52, 35/39/68, 56/59/66 with AGC, ASC-H, HSIL will go to colposcopy
ICC=invasive cervical cancer; ICER=incremental cost-effectiveness ratio; LEEP=loop electrosurgical excision procedure
Dominant indicates HPV primary screening with extended genotyping is less costly and more effective than HPV primary screening
Incremental cost-effectiveness ratio (ICER) of HPV primary screening with extended genotyping compared to partial genotyping. QALYs and total cost values in the table are rounded to millions and cannot be used to reconstruct ICERs reported in the tables and text, which used unrounded values.
Compared to HPV primary screening with partial genotyping, increases in QALYs with extended genotyping were not statistically significant, with wide ranges due to variability in the model.
Figure 2.

Number of times women did or did not undergo a colposcopy in different health states for human papillomavirus (HPV) primary screening with partial genotyping and extended genotyping (patient management strategies 1-3). A) Number of times women with cervical intraepithelial neoplasia (CIN) 1-3 undergoes a colposcopy; B) Number of times women without CIN 1-3 (healthy or HPV-positive) undergoes a colposcopy; C) Number of times women with CIN 1-3 did not undergo a colposcopy. Patient management strategy 1: 31, 45, 33/58, 51, 52, 35/39/68 with any abnormal cytology results will go to colposcopy; and 56/59/66 with atypical glandular cells (AGC), atypical squamous cells: cannot exclude high-grade squamous intraepithelial lesion (ASC-H), or a high-grade squamous intraepithelial lesion (HSIL) on cytology will go to colposcopy. Patient management strategy 2: 31, 45, 33/58, 52 with any abnormal cytology results will go to colposcopy; and 51, 35/39/68, 56/59/69 with AGC, ASC-H, HSIL will go to colposcopy. Patient management strategy 3: 31, 45, 33/58 with any abnormal cytology results will go to colposcopy; and 51, 52, 35/39/68, 56/59/66 with AGC, ASC-H, HSIL will go to colposcopy.
Impact of HPV Primary Screening with Extended Genotyping
When genotypes 56/59/66 with cytology results less than AGC were deferred to co-testing instead of colposcopy (i.e., patient management strategy 1), there were a median of 903 fewer invasive procedures [mean: 968; 95% confidence interval (CI): 752-1,182] compared to partial genotyping (Table 2). As Figure 2 shows, there were 396 fewer avoidable colposcopies, but 375 more times women with CIN or ICC did not undergo colposcopy. Compared to partial genotyping, extended genotyping accrued a mean of 2,971 (95% CI: -12,063-18,006) additional QALYs and saved a mean of $285,785 (95% CI: $14,829-$556,741) when $43.33; while costing a mean of $12.4 million (95% CI: $12.1 million-$12.7 million) more when $75, it was cost-effective (Table 2). At $100, extended genotyping was cost-effective ($12,111/QALY), costing a median of $23.0 million more compared to partial genotyping. Figure 3 plots acceptability curves, which show the percent of Monte Carlo trials with an ICER value less than or equal to a given willingness-to-pay threshold for extended genotyping. For example, using patient management strategy 1, with a $500 willingness-to-pay threshold, 96.8% of ICERs were cost-effective when screening cost $43.33 and 65.3% were when costing $75 (Figure 3a–b).
Figure 3.
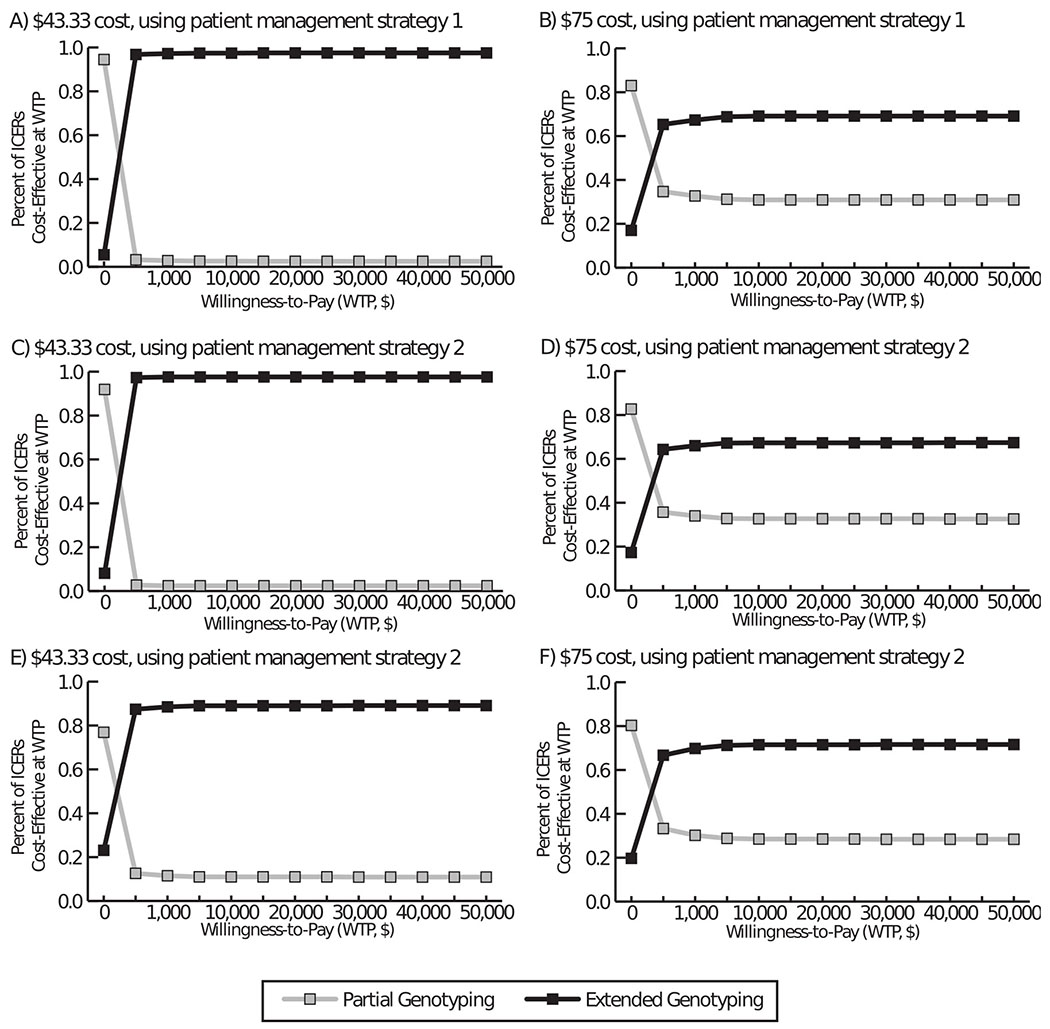
Acceptability curves of the cost-effectiveness of extended genotyping compared to partial genotyping. Plots show the percent of Monte Carlo trials with an incremental cost-effectiveness ratio (ICER) value less than or equal to a given willingness-to-pay threshold for extended genotyping when costing A) $43.33 and using patient management strategy 1, B) $75 and using patient management strategy 1, C) $43.33 and using patient management strategy 2, D) $75 and using patient management strategy 2, E) $43.33 and using patient management strategy 3, and F) $75 and using patient management strategy 3. Patient management strategy 1: 31, 45, 33/58, 51, 52, 35/39/68 with any abnormal cytology results will go to colposcopy; and 56/59/66 with atypical glandular cells (AGC), atypical squamous cells: cannot exclude high-grade squamous intraepithelial lesion (ASC-H), or a high-grade squamous intraepithelial lesion (HSIL) on cytology will go to colposcopy. Patient management strategy 2: 31, 45, 33/58, 52 with any abnormal cytology results will go to colposcopy; and 51, 35/39/68, 56/59/69 with AGC, ASC-H, HSIL will go to colposcopy. Patient management strategy 3: 31, 45, 33/58 with any abnormal cytology results will go to colposcopy; and 51, 52, 35/39/68, 56/59/66 with AGC, ASC-H, HSIL will go to colposcopy.
Using patient management strategy 2, there were a median of 2,370 fewer invasive procedures (mean: 2,386; 95% CI: 2,173-2,597) compared to partial genotyping (Table 2); however, more women progressed to ICC (mean: 2.1, 95% CI: 1.4-2.81). While there were a median of 957 fewer avoidable colposcopies, there were 994 more times those with CIN or ICC did not undergo colposcopy (Figure 2). Compared to partial genotyping, extended genotyping was cost saving (mean: $769,991; 95% CI: $501,277-$1,039,704)) at $43.33 and was cost-effective at $75 (Table 2, Figure 3) and $100 ($8,687/QALY).
With patient management strategy 3, there were a median 3,180 (mean: 3,288; 95% CI: 3,075-3,501) fewer invasive procedures, but more ICC cases (mean: 2.9; 95% CI: 2.2-3.5) compared to partial genotyping. There were a median of 1,242 fewer avoidable colposcopies, but 1,413 more times those with CIN or ICC did not undergo colposcopy (Figure 2). Compared to partial genotyping, extended genotyping accrued more QALYs despite additional ICC cases (increases were not significant); it saved a mean of $1.1 million (95% CI: $0.8-$1.3 million) when $43.33, but cost a mean of $11.8 million (95% CI: $11.5-$12.2 million) more when$75 (Table 2, Figure 3). Although costing a median of $22.0 million more than partial genotyping, extended genotyping was still cost-effective ($3,541/QALY) at $100.
Impact of Increasing the Probability of Progression
Table 3 and Figure 4 show what happens when increasing the probability of progression 2 times. Women screened using partial genotyping underwent 25,871 invasive procedures (Table 3) and there were 6,092 times women with CIN or ICC did not have a colposcopy and 13,997 avoidable colposcopies (Figure 4). Compared to partial genotyping, extended genotyping using any of the patient management strategies resulted in a mean of 977 (95% CI: 760-1,194) to 3,415 (95% CI: 3,203-3,626) fewer invasive procedures, but a mean of 1.6 (95% CI: 0.3-3.0) to 8 (95% CI: 6.7-9.5) more ICC cases (Table 3). As 4 Figure shows, there were 418-1,269 fewer avoidable colposcopies but 424-1,611 more times those with CIN or ICC did not undergo colposcopy. Despite fewer avoidable colposcopies, extended genotyping was not cost-effective (Table 3) and was even dominated (i.e., costs more and less effective) when costing $75 or more compared to partial genotyping.
Table 3.
Clinical and economic (net present value 2018 $US) outcomes [median (95% uncertainty interval)] of human papillomavirus (HPV) primary screening with partial genotyping or extended genotyping (patient management strategies 1-3) over 40 simulated years in a cohort of 100,000 US women when increasing the probability of progression 2 times
| Scenario | Number of genotype tests | Number of cytologies | Number of colposcopies | Number of LEEPs | Number of ICC | Quality-adjusted life years (QALYs) | Cost of genotype tests (in millions) | Total cost (in millions) | ICER ($/QALY)* |
|---|---|---|---|---|---|---|---|---|---|
| HPV primary with partial genotyping | 578,101 | 144,476 | 23,868 | 2,003 | 110 | 1,249,382 | 17.6 | 33.6 | -† |
| (405,336 - 735,958) | (98,165 - 13,104) | (19,254 - 28,022) | (1,822 - 2,185) | (82 - 143) | (913,440 - 1,538,147) | (13.6 - 21.1) | (27.0 - 39.9) | ||
| $43.33 Onclarity test | |||||||||
| Patient management strategy 1 | 578,474 | 144,685 | 22,889 | 1,987 | 112 | 1,244,871 | 17.6 | 33.3 | Less costly, but less effective |
| (407,614 - 739,989) | (98,307 - 191,417) | (18,304 - 27,057) | (1,780 - 2,156) | (83 - 143) | (915,578 - 1,532,741) | (13.6 - 21.1) | (26.5 - 39.8) | ||
| Patient management strategy 2 | 574,269 | 143,440 | 21,355 | 1,859 | 114 | 1,241,198 | 17.5 | 32.6 | Less costly, but less effective |
| (405,894 - 727,248) | (99,343 - 191,244) | (17,093 - 25,464) | (1,675 - 2,052) | (86 - 149) | (916,383 - 1,517,023) | (13.6 - 20.9) | (26.2 - 39.4) | ||
| Patient management strategy 3 | 574,520 | 143,734 | 20,617 | 1,801 | 118 | 1,242,745 | 17.5 | 32.6 | Less costly, but less effective |
| (409,897 - 737,930) | (99,761 - 190,989) | (16,394 - 24,519) | (1,601 - 1,980) | (91 - 152) | (924,928 - 1,536,586) | (13.7 - 21.1) | (25.9 - 39.1) | ||
Note: Patient management strategy ordered by increasing number of high-risk HPV genotypes for which cytology results of negative for intraepithelial lesion or malignancy (NILM), atypical squamous cells of undetermined significance (ASC-US), or low-grade squamous intraepithelial lesion (LSIL) were deferred to co-testing instead of colposcopy (i.e., in Group C).
Patient management strategy 1: 31, 45, 33/58, 51, 52, 35/39/68 with any abnormal cytology results will go to colposcopy; and 56/59/66 with atypical glandular cells (AGC), atypical squamous cells: cannot exclude high-grade squamous intraepithelial lesion (ASC-H), or a high-grade squamous intraepithelial lesion (HSIL) on cytology will go to colposcopy
Patient management strategy 2: 31, 45, 33/58, 52 with any abnormal cytology results will go to colposcopy; and 51, 35/39/68, 56/59/69 with AGC, ASC-H, HSIL will go to colposcopy
Patient management strategy 3: 31, 45, 33/58 with any abnormal cytology results will go to colposcopy; and 51, 52, 35/39/68, 56/59/66 with AGC, ASC-H, HSIL will go to colposcopy
ICC=invasive cervical cancer; ICER=incremental cost-effectiveness ratio; LEEP=loop electrosurgical excision procedure
Incremental cost-effectiveness ratio (ICER) of HPV primary screening with extended genotyping compared to partial genotyping.
ICERs of partial genotyping compared to extended genotyping with patient management strategies 1, 2, and 3 are $61/QALY, $119/QALY, and $158/QALY, respectively.
Figure 4.
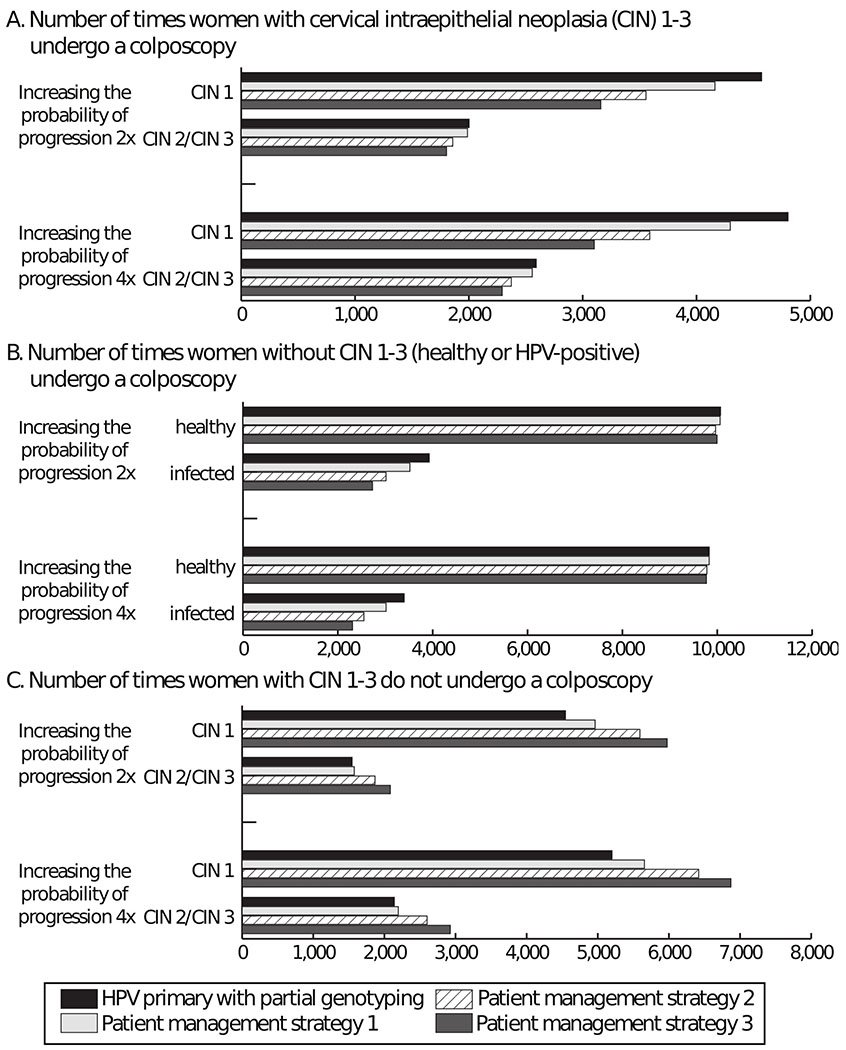
Number of times women did or did not undergo a colposcopy in different health states for human papillomavirus (HPV) primary screening with partial genotyping and extended genotyping (patient management strategies 1-3) when increasing the probability of progression 2-4 times. A) Number of times women with cervical intraepithelial neoplasia (CIN) 1-3 undergoes a colposcopy; B) Number of times women without CIN 1-3 (healthy or HPV-positive) undergoes a colposcopy; C) Number of times women with CIN 1-3 did not undergo a colposcopy. Patient management strategy 1: 31, 45, 33/58, 51, 52, 35/39/68 with any abnormal cytology results will go to colposcopy; and 56/59/66 with atypical glandular cells (AGC), atypical squamous cells: cannot exclude high-grade squamous intraepithelial lesion (ASC-H), or a high-grade squamous intraepithelial lesion (HSIL) on cytology will go to colposcopy. Patient management strategy 2: 31, 45, 33/58, 52 with any abnormal cytology results will go to colposcopy; and 51, 35/39/68, 56/59/69 with AGC, ASC-H, HSIL will go to colposcopy. Patient management strategy 3: 31, 45, 33/58 with any abnormal cytology results will go to colposcopy; and 51, 52, 35/39/68, 56/59/66 with AGC, ASC-H, HSIL will go to colposcopy.
When increasing the probability of progression 4 times, partial genotyping resulted in a median of 26,698 [95% uncertainty interval (UI): 22,139-30,793] invasive procedures, 257 (95% UI: 200-325) ICC cases, and cost $40.3 million (95% UI: $31.6-$50.5 million). Compared to partial genotyping, extended genotyping resulted in a mean of 1,099 (95% CI: 892-1,307) to 3,802 (95% CI: 3,600-4,004) fewer invasive procedures and up to a mean of 21 (95% CI: 18-24) more ICC cases, varying with patient management strategy, and was not cost-effective.
Impact of Delaying Co-testing
Delaying co-testing for 2 years for HPV-positive, cytology-negative women resulted in a median of 20,125-21,734 colposcopies, 1,369-1,496 LEEPs, and 54-56 ICC cases, varying with patient management strategy. Compared to partial genotyping, extended genotyping was cost saving at $43.33 (mean: ≥$3.3 million; 95% CI: $3.0-$3.5 million) and cost-effective at $75-$100 (≤$10,308/QALY).
DISCUSSION
Our results show that using HPV primary screening with extended genotyping to wait and rescreen women with lower risk genotypes resulted in a lower number of invasive procedures (≤3,163/100,000 women screened); however, more women developed ICC (≤3/100,000) compared to partial genotyping. Increasing the number of genotypes where women waited and were rescreened later, led to fewer invasive procedures and lower costs, but allowed more women to progress to ICC. Extended genotyping was cost-effective and could save costs (≤$1.0 million when costing $43.33). However, as the disease progression rate increased, extended genotyping resulted in higher costs (despite decreasing invasive procedures) and worse health outcomes because more women with CIN did not undergo colposcopy leading to more ICC cases.
Our study shows the rate at which women progress is a key driver of extended genotyping’s value when waiting and rescreening women with lower risk genotypes. With a higher progression risk, more women may advance to later disease states while waiting to be rescreened, thereby delaying treatment. This leads to more ICC cases when waiting and rescreening women. Given the probability of progression is a key driver and current literature on genotype- and cytology-specific probabilities is limited, having more robust data would be beneficial, especially since the progression rate may vary with CIN state severity. In fact, the progression rate may be underestimated as many studies only include healthy and HPV-infected women without cytology results or only those who are NILM or LSIL.8,18–21 Additionally, if disease progression risk is higher than understood from the literature this may result in adjustments to the interval between screenings (i.e., frequency of HPV screening) recommended by guidelines.
Extended genotyping’s value depends on how the additional information is used (e.g., how patients are managed). While saving an immediate invasive procedure, waiting and resecreening could allow women to progress to later disease states or potentially need more invasive or additional procedures later. Thus, there is a tradeoff between rescreening women and disease progression. Patient management strategies for women identified with non-16/18 genotypes (e.g., rescreening and delaying treatment as explored here), are still under investigation. Therefore, we varied which genotypes undergo rescreening and the time before rescreening to determine the impact on the value of identifying these genotypes. Our study shows that increasing the number of genotypes that delayed treatment led to fewer invasive procedures and lower costs, but more women progressed.
Our results can help a variety of decisionmakers.. For example, clinicians can use our results to help weigh clinical risk and benefits of extended genotyping to allow for more patient-specific management and treatment options. Healthcare administrators can make decisions regarding its use in their facilities and investment in equipment to perform such testing. Third-party payers can determine reimbursement. Policy makers can use our results when considering testing strategies in future cervical cancer screening guidelines. While preventing thousands of colposcopies, waiting to treat resulted in a few more ICC cases, thus decisionmakers should take this tradeoff into consideration in the context of their own circumstances.
While our results show that extended genotyping may provide health benefits (i.e., increased QALYs), these differences were not statistically significant due to the wide variability in the progression risk through the CIN stages. Progression risk varies by factors such as co-mobidities, co-infection with other sexually transmitted infections, having multiple births, and use of hormonal oral contraceptive.22 Thus, a better understanding of these values for all genotypes may reduce variability in modeled results. However, this does not mean that extended genotyping would not be clinically useful and this study can begin to inform how it may be used in practice. For example, correct identification of genotypes is important to determine treatment, given many infections naturally clear, but delayed treatment for those at high-risk of ICC could prove fatal.23–25 Patient management options for those with individually identified, non-16/18 genotypes are not well established and identifying additional genotypes could lead to other management strategies (e.g., more aggressive treatment for other high-risk genotypes), which may increase extended genotyping’s value. These options and the detection of non-vaccine genotypes may become more important as HPV vaccine coverage increases, potentially chaning genotype prevalence.26,27 Extended genotyping may be even more favorable in certain subpopulations that are known to be previously vaccinated or have more transient infections.
Limitations
By definition, models are simplifications of real-life and cannot represent every event or outcome. Our model drew from data of varying quality and results may change as better and more data becomes available (e.g., age- and genotype-specific progression and regression probabilities). As both genotype and cytology results determine patient management, we note that cytology’s sensitivity and specificity are variable and dependent upon how they were measured. Our model does not capture all possible negative impacts of colposcopies (e.g., preterm labor28). As we aimed to explore the full potential value of extended genotyping to wait and rescreen women in the absence of other factors that can affect their management, we assumed 100% compliance with screening strategies; however, it can vary from 50-80% depending on the population characteristics (e.g., marital status, age).29,30 In our scenarios, women received the same HPV test throughout the modeled duration and did not change tests, which may occur in real-life. While we did not consider infection with more than one genotype at a time, our results would be reasonably similar had they been included, as treatment is based on the genotype with the highest progression risk (using the hierarchical methodology typically used in the literature), which is captured in our risk estimates from the literature (Appendix). As we evaluated the value of extended genotyping from third-party payer perspective, we did not include indirect patient costs (e.g., travel costs and time spent on rescreening).
Additionally, we did not consider HPV transmission, which would increase the number of HPV-infected women who are potentially detected by screening. However, as both strategies rescreen women at the same intervals (e.g., every 5 years for HPV-negative women) and the next screening interval would not be changed by acquiring HPV until they are identified, the difference in results between screening strategies would remain the same. New HPV infections would be caught at the same rate for both partial and extended genotyping, given extended genotyping can delay treatment but not the frequency of screening. Moreover, as the probability of HPV infection does not depend on the screening strategy and subsequent treatment received (e.g., treatment for previous HPV infection does not change risk of re-acquiring HPV), the modeled number of new infections would be similar in both the partial and extended genotyping scenarios. However, extended genotyping’s relative value would vary with transmission, depending on the genotype. For example, if more women acquire genotypes 16/18 (for which patent management is not altered by extended genotyping) the value would decrease, whereas acquisition of non-16/18 (for which patient management can be altered) would increase the value. As 16/18 are more prevalent, transmission may reduce extended genotyping’s value, nonetheless, these types are targeted by vaccines, thus, vaccination may increase the value of extended genotyping. Future studies could evaluate the value and use of extended genotyping when considering transmission and vaccination.
Conclusions
Screening with extended genotyping to wait and rescreen women with lower risk genotypes was cost-effective and even cost saving, averting ≤3,163 colposcopies but resulting in ≤3 more ICCs, compared to current HPV primary tests. The value varies with the rate at which women progress; at higher rates, extended genotyping did not provide clinical or economic value.
Supplementary Material
Acknowledgments
Conflicts of Interest and Source of Funding: This work was supported by Becton, Dickinson and Company, the Agency for Healthcare Research and Quality (AHRQ) via grant R01HS023317, and the National Institute of General Medical Sciences (NIGMS) via MIDAS grant U24GM110707. The funders did not in any way restrict our ability or right to publish any analyses, results, or interpretation of results that emerged from this study. CH, JA, and CA are employees of Becton, Dickinson and Company, the sponsor of the study. The remaining authors report no conflicts of interest.
Footnotes
Becton Dickenson (BD) Diagnostics, 1 Becton Drive, Franklin Lakes, New Jersey, 07417, USA
REFERENCES
- 1.Szarewski A, Mesher D, Cadman L, et al. Comparison of seven tests for high-grade cervical intraepithelial neoplasia in women with abnormal smears: the Predictors 2 study. J Clin Microbiol. 2012;50(6):1867–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuzick J, Cadman L, Mesher D, et al. Comparing the performance of six human papillomavirus tests in a screening population. Br J Cancer. 2013;108(4):908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182. [DOI] [PubMed] [Google Scholar]
- 4.Stoler MH, Wright TC Jr., Parvu V, et al. The Onclarity Human Papillomavirus Trial: Design, methods, and baseline results. Gynecol Oncol. 2018;149(3):498–505. [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J, Ho L, Terry G, et al. Individual detection of 14 high risk human papilloma virus genotypes by the PapType test for the prediction of high grade cervical lesions. J Clin Virol. 2014;60(1):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100(Pt B):1–441. [PMC free article] [PubMed] [Google Scholar]
- 7.Cuzick J, Wheeler C. Need for expanded HPV genotyping for cervical screening. Papillomavirus Res. 2016;2:112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiffman M, Burk RD, Boyle S, et al. A study of genotyping for management of human papillomavirus-positive, cytology-negative cervical screening results. J Clin Microbiol. 2015;53(1):52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulkmans NW, Berkhof J, Bulk S, et al. High-risk HPV type-specific clearance rates in cervical screening. Br J Cancer. 2007;96(9):1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonde J, Ejegod DM, Cuschieri K, et al. The Valgent4 protocol: Robust analytical and clinical validation of 11 HPV assays with genotyping on cervical samples collected in SurePath medium. J Clin Virol. 2018;108:64–71. [DOI] [PubMed] [Google Scholar]
- 11.U. S. Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(7):674–686. [DOI] [PubMed] [Google Scholar]
- 12.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542. [DOI] [PubMed] [Google Scholar]
- 13.Huh WK, Williams E, Huang J, Bramley T, Poulios N. Cost effectiveness of human papillomavirus-16/18 genotyping in cervical cancer screening. Appl Health Econ Health Policy. 2015;13(1):95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146(4):473–481. [PMC free article] [PubMed] [Google Scholar]
- 15.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare & Medicaid Services. Clinical Laboratory Fee Schedule: 18CLABQ3. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/18CLABQ3.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Accessed 10 Oct 2018.
- 17.Schlecht NF, Platt RW, Duarte-Franco E, et al. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst. 2003;95(17):1336–1343. [DOI] [PubMed] [Google Scholar]
- 18.Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;102(19):1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomsen LT, Frederiksen K, Munk C, Junge J, Iftner T, Kjaer SK. Long-term risk of cervical intraepithelial neoplasia grade 3 or worse according to high-risk human papillomavirus genotype and semi-quantitative viral load among 33,288 women with normal cervical cytology. Int J Cancer. 2015;137(1):193–203. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler CM, Hunt WC, Cuzick J, et al. The influence of type-specific human papillomavirus infections on the detection of cervical precancer and cancer: A population-based study of opportunistic cervical screening in the United States. Int J Cancer. 2014;135(3):624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto K, Oki A, Furuta R, et al. Predicting the progression of cervical precursor lesions by human papillomavirus genotyping: a prospective cohort study. Int J Cancer. 2011;128(12):2898–2910. [DOI] [PubMed] [Google Scholar]
- 22.Tulay P, Serakinci N. The Route to HPV-Associated Neoplastic Transformation: A Review of the Literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27–39. [DOI] [PubMed] [Google Scholar]
- 23.Gradissimo A, Burk RD. Molecular tests potentially improving HPV screening and genotyping for cervical cancer prevention. Expert Rev Mol Diagn. 2017;17(4):379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCredie MR, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–434. [DOI] [PubMed] [Google Scholar]
- 25.Sudenga SL, Shrestha S. Key considerations and current perspectives of epidemiological studies on human papillomavirus persistence, the intermediate phenotype to cervical cancer. Int J Infect Dis. 2013;17(4):e216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berenson AB, Laz TH, Rahman M. Reduction in Vaccine-Type Human Papillomavirus Prevalence Among Women in the United States, 2009-2012. J Infect Dis. 2016;214(12):1961–1964. [DOI] [PubMed] [Google Scholar]
- 27.Kahn JA, Brown DR, Ding L, et al. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics. 2012;130(2):e249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruinsma FJ, Quinn MA. The risk of preterm birth following treatment for precancerous changes in the cervix: a systematic review and meta-analysis. BJOG. 2011;118(9):1031–1041. [DOI] [PubMed] [Google Scholar]
- 29.Paynter CA, Van Treeck BJ, Verdenius I, et al. Adherence to cervical cancer screening varies by human papillomavirus vaccination status in a high-risk population. Prev Med Rep. 2015;2:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health. BRFSS Prevalence &Trends Data. 2015; https://nccd.cdc.gov/BRFSSPrevalence/rdPage.aspx?rdReport=DPH_BRFSS.ExploreByTopic&irbLocationType=StatesAndMMSA&islClass=CLASS18&islTopic=TOPIC42&islYear=2016&rdRnd=72619, 11 Dec 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


