Abstract
The purpose of this CPU Expert Review is to provide clinicians with guidance on the management of H. pylori after an initial attempt at eradication therapy fails, including best practice advice on specific regimen selection, and consideration of patient and systems factors that contribute to treatment efficacy.
This Expert Review is not a formal systematic review, but is based upon a review of the literature to provide practical advice. No formal rating of the strength or quality of the evidence was carried out. Accordingly, a combination of available evidence and consensus-based expert opinion were used to develop these best practice advice statements.
Introduction
Helicobacter pylori (H. pylori) infection is recognized as one of the most common chronic bacterial infections worldwide, infecting approximately half of the global population.1 H. pylori is a World Health Organization (WHO)-designated carcinogen and the strongest known risk factor for noncardia gastric adenocarcinoma, the most prevalent form of gastric cancer. It is also causally linked to peptic ulcer disease. Even though only 1–3% of infected individuals will develop malignant complications, H. pylori accounts for 15% of the total cancer burden globally, with up to 89% of all gastric cancer attributable to H. pylori infection.2 Accordingly, all major gastroenterological societies recommend that H. pylori be eradicated in individuals who test positive.
Downstream consequences of failed treatment include clinical complications related to persistent H. pylori infection and repeated exposure to antibiotics and high-dose acid suppression, generation of antibiotic resistance in H. pylori and other organisms, as well as the associated direct and indirect costs to the healthcare system. Because the likelihood of successful eradication decreases with each subsequent therapeutic attempt, every effort should be made to address factors that might contribute to eradication failure.
Several guidelines exist to help providers choose regimens to eradicate H. pylori on the first attempt; they also include advice on management after initial treatments fail. However, these guidelines are backed by limited high-quality evidence. In general, they rely heavily on trials conducted in populations that are relatively homogenous within geographic borders, albeit ethnically distinct (e.g. Asian-Pacific populations). In contrast, the United States (US) population comprises individuals with diverse ancestral backgrounds, with correspondingly diverse H. pylori strains.3 In the US, the lack of recent comparative clinical trials is coupled with limited knowledge of locoregional H. pylori antibiotic resistance patterns and of regimen-specific local cure rates, as well as limited contemporary data on temporal trends and relevant demographic details (e.g. age, race and ethnicity). Current national and international guidelines provide limited guidance on how to approach factors other than H. pylori antibiotic resistance which might also underlie eradication failure, such as host- and systems-related factors. Collectively, these issues contribute to persistent H. pylori infection (Figure 1).
Figure 1.
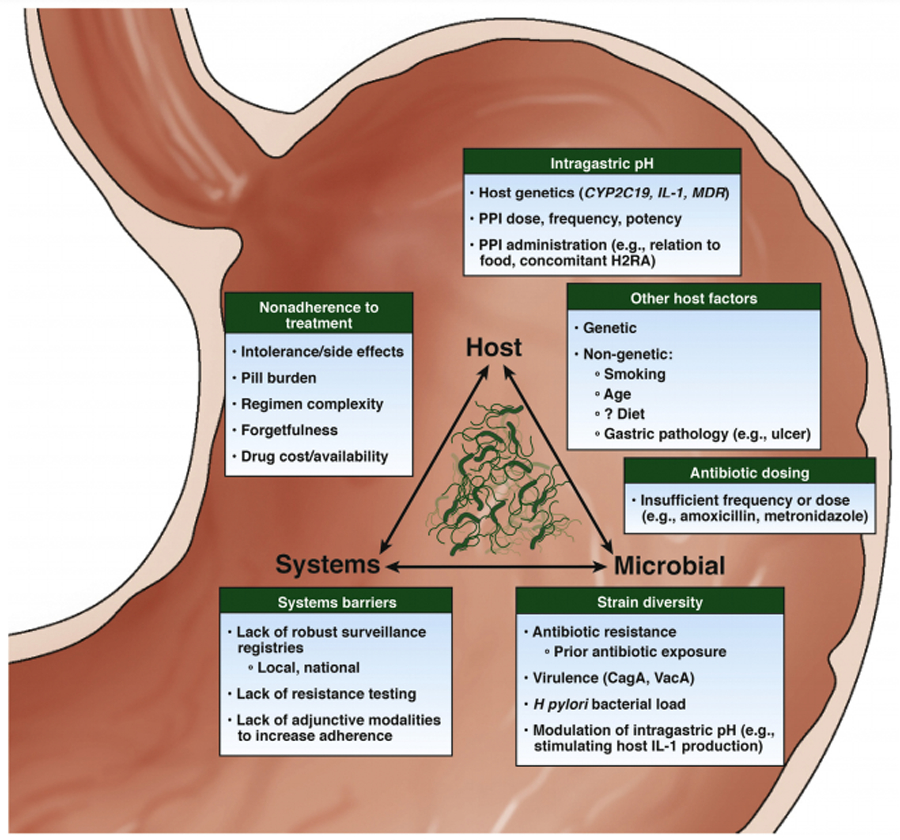
Factors impacting failure to eradicate H. pylori infection. CagA cytotoxin-associated antigen A; IL, interleukin; VacA, vacuolating cytotoxin A.
The primary objectives of this Clinical Practice Update (CPU) Expert Review are to 1) provide a salient overview of determinants of H. pylori eradication treatment failure, including host-, microbe-, and systems-related factors as they are currently understood; and, 2) leverage these data to provide clinical practitioners with evidence- and consensus-based multimodal best practice advice for treating H. pylori after the first treatment failure. We include a clinically relevant synthesis of contemporary data on the appropriateness and efficacy, or lack thereof, of specific antimicrobial treatment regimens and adjunctive therapeutic agents for this purpose. The terms “salvage” and “rescue” therapy are commonly used in the literature to describe treatment courses following the initial eradication therapy, but without a consistent definition. As such, we avoid the use of these terms. Additionally, to the extent possible, we focus on evidence from North America in order to ensure that this article is most relevant for US practitioners.
Definition of refractory infection
For the purpose of this CPU Expert Review, refractory H. pylori infection is defined by a persistently positive non-serological H. pylori test (i.e., a breath-, stool-, or gastroscopy-based test), at least 4 weeks following one or more completed course(s) of current guideline-recommended first-line H. pylori eradication therapy, and off of any medications that might impact the test sensitivity (e.g. proton-pump inhibitors (PPI)).4 Refractory H. pylori infection should be differentiated from recurrent infection—that is, a non-serological test which was initially negative after eradication therapy, but then subsequently positive at a later interval—as the latter might be the result of ongoing intrafamilial exposure and may be best addressed by testing household members and treating those who test positive.
The causes of H. pylori eradication treatment failure (Figure 1)
Failure to eradicate H. pylori results from the complex interaction of host-, microbial- and systems-related factors. Antibiotic resistance (microbial and systems) and patient nonadherence (host and systems) are the two most commonly cited reasons for eradication failure. However, because primary eradication failure still occurs despite confirmed antibiotic sensitivity and patient adherence, potentially with higher frequency in refractory H. pylori specifically, additional factors are likely also relevant. Providers should attempt to identify all contributing etiologies before simply prescribing alternative antibiotics. (BPA#1) These factors, along with antibiotic resistance and nonadherence, are described herein.
Antibiotic resistance: mechanisms and rates
Resistance to several of the antibiotics commonly used in eradication regimens has risen globally over the last 20 years. Rising rates have been linked to prior use of that specific antibiotic, or others within the same class, by the individual, as well as with widespread antibiotic consumption at the population level.5–7
Predictably, eradication failure is more likely when an antibiotic to which H. pylori demonstrates in vitro resistance is included in the regimen. Combining studies of both treatment naïve and refractory H. pylori infection, in vitro resistance to clarithromycin and levofloxacin are associated with a 7.0-fold (95% CI, 5.2–9.3) and 8.2-fold (95% CI, 3.8–17.6) significantly higher likelihood of treatment failure, respectively, in regimens containing these drugs; whereas in vitro nitroimidazole resistance has relatively less clinical impact, increasing the odds of treatment failure by 2.5-fold (95% CI, 1.8–3.5). 8 Importantly, selecting eradication therapies based on prior antibiotic exposure is not inferior to selecting therapy based on in vitro antibiotic susceptibility 9,10, and bypasses the many logistical barriers to obtaining in vitro testing. Accordingly, providers should conduct a thorough review of the medical/pharmacy record and discuss previous medication exposures with the patient and also a pharmacist11, if available. This should be done prior to the initial eradication attempt, but is especially critical for successfully treating refractory H. pylori infection.(BPA #2) A national US survey reported that only 38% of participating providers asked patients about prior antibiotic exposure12; thus, there is considerable room for improvement.
The dominant molecular mechanisms responsible for antibiotic resistance in H. pylori are well established for clarithromycin (usually due to one of three point mutations in the 23S ribosomal subunit), levofloxacin (mutations in DNA gyrase subunit A), amoxicillin (mutations in penicillin binding protein 1), tetracycline (mutations in genes encoding binding site for ribosomal 16S subunit, or increased efflux), and rifabutin (mutations in rpoB, the beta subunit of RNA polymerase gene).13 Nitroimidazole resistance is more complicated. It is usually related to mutations within rdxA, a gene encoding a nitroreductase that normally activates nitroimidazoles (e.g. metronidazole) from the prodrug state, though changes in drug uptake and efflux may also play a role. The complexity of rdxA mutations reported and possible synergy with other redox-associated H. pylori genes precludes molecular testing of any single point mutation for clinical resistance profiling. Additionally, phenotypic (culture-based) methods are not well standardized for metronidazole resistance testing and can vary by method used. This may contribute to the relatively low predictive value of in vitro metronidazole resistance testing to treatment outcome.
Based on a comprehensive systematic review and meta-analysis, including data from over 50,000 patients from 45 countries, overall primary resistance rates by global region ranged from 10–34% for clarithromycin, 11–30% for levofloxacin and 23–56% for metronidazole.8 After unsuccessful H. pylori treatment (secondary resistance) rates increased to 15–67% for clarithromycin, 19–30% for levofloxacin and 30–65% for metronidazole. In contrast, resistance rates were low for amoxicillin and tetracycline, generally occurring in less than 5% of strains, usually in the 1–2% range.8 H. pylori also demonstrates low primary and secondary resistance to rifabutin, based on other reports.14,15
Estimating H. pylori resistance rates is particularly challenging in the US because measuring resistance has been uncommon in clinical practice, ultimately equating to very limited contemporary data to guide treatment considerations. In a prospective multi-center US study of 347 strains collected from 1998 to 2002, overall H. pylori resistance rates (treatment naïve and previously treated combined) were 13% for clarithromycin and 25% for metronidazole.16 In 128 strains cultured from patients at the Houston Veterans Affairs Medical Center from 2009–2013, resistance rates in the 110 treatment-naïve patients were 15% for clarithromycin, 17% for metronidazole, and 29% for levofloxacin, with 15% of strains resistant to more than one antibiotic.17 Most recently, primary resistance rates of 345 strains collected during a multi-center clinical trial were 17% for clarithromycin and 44% for metronidazole.14 It should be recognized, however, that because H. pylori infection is most often acquired in childhood, immigrants from countries where H. pylori is endemic might exhibit antimicrobial resistance patterns characteristic of their native, as opposed to host country; this again underscores the need for robust surveillance registries that include host demographics.
Nonadherence
The level of adherence to therapy above which there is negligible incremental benefit for eradication success in refractory H. pylori is not known; however, studies demonstrate that adherence to >60% to >90% of the prescribed course might be sufficient for successful eradication, at least in primary H. pylori infection.11,18 The threshold likely varies depending on individual factors and might plausibly be higher for refractory H. pylori. Prior to prescribing therapy, barriers to adherence should be explored and addressed, and the regimen thoroughly discussed. Common barriers include complexity of eradication regimens, associated high pill burden, physical intolerance of medications, poor provider communication, and overall lack of understanding of why therapy is indicated.11,19 Based on these considerations, providers who treat H. pylori infection should provide their patients with anticipatory guidance to help ensure maximum adherence. This specifically includes explaining the rationale for therapy, dosing instructions, expected adverse events and the importance of completing the full therapeutic course (BPA #3)
Recently, two large RCTs from China demonstrated that the use of an interactive smart-phone medical application20 and text-based reminders21 during treatment improved adherence to primary therapy. These adjunctive systems are worthy of further investigation in the US for refractory H. pylori infection, and would provide information on which approaches might be more effective in certain populations compared to others, for example, based on characteristics such as age, race and ethnicity, educational level, access, and language. Pillboxes, medication calendars, medication and counseling from pharmacists may also augment patient adherence.11
Systems-related factors that contribute to refractory H. pylori infection additionally include lack of robust eradication surveillance registries, lack of widely accessible antibiotic sensitivity testing, practice pattern variability among practitioners with respect to adherence to guideline-recommended therapies12, as well as little progress in developing novel anti-H. pylori therapies.
Host genetics
Host genetics are also implicated in refractory H. pylori infection. Polymorphisms that affect intragastric pH, including those of CYP2C19, IL-1B and MDR1, are especially relevant to successful H. pylori eradication. H. pylori is most susceptible to antibiotics when intragastric pH is consistently between 6–8, since this is the optimal pH range for H. pylori replication. Some antibiotics, including clarithromycin and amoxicillin, also require intragastric acid suppression for maximum efficacy and sustained activity. For example, for gastric pH <2, the half-lives of amoxicillin and clarithromycin are approximately 15.2 (+/−0.3) hours and 1.0 (+/− 0.04) hours respectively, while for gastric pH>7, the half-lives of both antibiotics are >68 hours.22 Hence, in the absence of adequate and sustained acid suppression, H. pylori can persist despite exposure to antibiotics to which it is otherwise susceptible in vitro.23
The largest body of literature for host genetics contributing to H. pylori eradication failure is focused on CYP2C19, the cytochrome P450 gene responsible for the majority of metabolism of the earlier-generation PPIs. CYP2C19 polymorphisms giving rise to poor metabolizer phenotypes result in high plasma PPI drug concentrations.24 The metabolism-enhancing phenotypes of CYP2C19 are associated with higher rates of eradication failure when PPIs that are heavily metabolized by CYP2C19 (e.g. omeprazole, lansoprazole) are used.25 Because PPIs also have a direct antimicrobial effect and impact H. pylori bacterial load, CYP2C19-induced PPI metabolism also influences H. pylori persistence independently of intragastric pH.25
There are far less data on non-CYP2C19 genetic determinants of intragastric pH (e.g. MDR1, IL-1B) and other host genetic variants, which might contribute to refractory H. pylori infection through other mechanisms, such as H. pylori bacterial load regulation and dysregulation, evasion or alteration of mucosal immunity.
Studies evaluating CYP2C19 genotype-guided PPI selection and dosing in refractory H. pylori infection have been conducted in Asian-Pacific populations, but analogous studies in US populations are lacking. This is an important deficit since there are substantive racial and ethnic differences in the prevalence of CYP2C19 variant alleles and genotypes in the US.26,27 Caucasians, non-Hispanic African Americans, and Hispanics have a significantly higher prevalence (57%−71%) of metabolism-enhancing CYP2C19 phenotypes compared to Asian American ethnic groups (45%), even in population studies of asymptomatic individuals.28 Asian Americans also have the highest prevalence of the poor metabolism genotype.26 Furthermore, Caucasians with extensive metabolizer phenotypes might have even higher clearance of omeprazole compared to some Asian ethnic groups with the same CYP2C19 genotype28, suggesting additional genetic or gene-environment interaction determinants might be relevant.
Despite these considerations, current data are insufficient to support genetic polymorphism testing for guiding therapeutic selection in refractory (or primary) eradication therapy. Given the high population prevalence of metabolism-enhancing phenotypes of CYP2C19 at least in non-Asian groups, empiric selection of strategies that achieve greater intragastric acid suppression might be reasonable in the management of refractory H. pylori infection. These include higher dosing and/or increased frequency of first-generation PPIs; the use of later generation, more potent PPIs; and selecting potent non-PPI gastric acid suppressors, such as vonoprazan, if available. However, further population-specific data are needed, including comparisons of cost.
Other host factors
Non-genetic host-related and lifestyle factors, such as age and smoking, are also associated with eradication treatment failure. In one meta-analysis, patients who smoked vs. did not smoke were nearly twice as likely to have persistent H. pylori infection following therapy (OR 1.95, 95% CI, 1.55–2.45).29 Biological plausibility underlies this association, since smoking increases gastric acid secretion and impairs mucous secretion and gastric blood flow, thus decreasing local antibiotic delivery. Whether smoking cessation, at least while taking eradication therapy, improves eradication success in refractory H. pylori infection is not established. The data are less consistent for other host factors, such as comorbid obesity and diabetes, but are important areas for focused research.
H. pylori strain diversity
The high level of H. pylori strain-specific genetic diversity engenders microbial mechanisms that promote H. pylori persistence to variable extents. These mechanisms include manipulation and evasion of host immune responses, alteration of the gastric environment, increased bacterial load, enhanced virulence and consequent adverse gastric histopathology, as well as resistance to antimicrobials.30,31 Except for antimicrobial resistance, other H. pylori genetic constituents (e.g. cytotoxin-associated gene A, vacuolating cytotoxin A) have not been leveraged in the management of refractory H. pylori infection, but deserve attention.32,33
Proposed treatment algorithm
An algorithm for regimen considerations in refractory H. pylori cases is illustrated in Figure 2, and is based on the initial therapy used and the presence or absence of true penicillin allergy. Of these regimens, only PBMT is FDA-approved for refractory H. pylori infection. If bismuth-based quadruple therapy failed as a first-line treatment, shared decision-making between providers and patients should guide selection between a) levofloxacin- or rifabutin-based triple therapy regimens with high-dose dual proton pump inhibitor (PPI) and amoxicillin, or b) an alternative bismuth-containing quadruple therapy, as second-line options. (BPA #4) Due to rising rates of levofloxacin resistance, levofloxacin should not be considered for treatment unless the H. pylori strain is known to be sensitive to it, or if the population levofloxacin resistance rates are known to be <15% (analogous to the longstanding “rule” regarding clarithromycin usage in triple therapies). However, it is reasonable to consider rifabutin in a triple regimen without prior sensitivity testing since rifabutin and amoxicillin resistance are rare. A recent study demonstrated that the addition of rifabutin to the high dose amoxicillin, PPI dual regimen improves eradication rates significantly.14 Although the referenced study used this regimen as first-line therapy, based on these data it is reasonable to consider PAR usage with high-dose and/or high-potency PPI and amoxicillin 750mg TID over high-dose dual therapy alone. Optimal dosing of PPIs is provided in Table 1 and is described in text below. We have not included in Figure 2 several other potential regimens (such as concomitant, sequential or hybrid therapies) due to extremely limited data on their use for refractory H. pylori infection specifically.4
Figure 2.
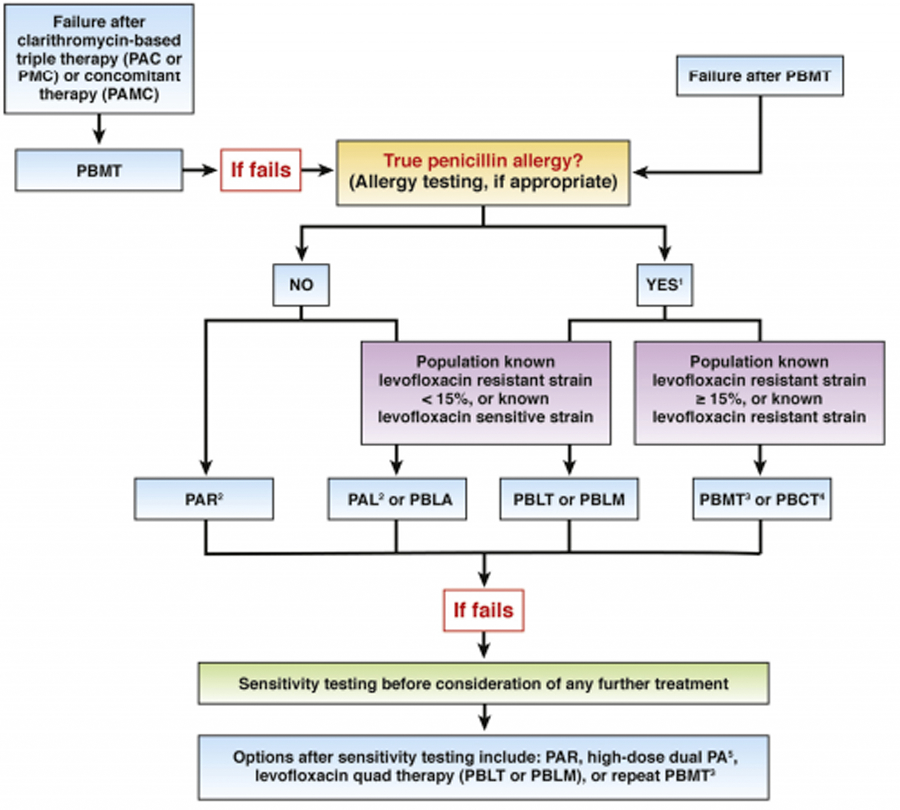
Treatment algorithm for refractory H. pylori infection.
1Limited evidence guiding therapy in individuals with true penicillin allergy
2With high-dose or high-potency PPI, amoxicillin 750 mg TID
3High-dose metronidazole (1.5–2g divided)
4Only if clarithromycin sensitive strain
5High-dose dual PA = amoxicillin 2–3g daily in 3–4 divided doses + high-dose PPI BID. PA in place of PAR may be considered, although one study from US demonstrated superiority of PAR compared to PA as first-line treatment (Graham et al. 2020); however, this has not been directly compared in refractory H pylori treatment.
P, PPI; C, Clarithromycin; A, Amoxicillin; M, Metronidazole; B, Bismuth; T, Tetracycline; R, Rifabutin; L, Levofloxacin
Table 1.
Second-line therapies for H. pylori eradication, based on selected international guidelines*
| Regimen Failures | Maastricht V/Florence Consensus Report1, 2016 | Toronto Consensus Report2, 2016 | American College of Gastroenterology Guidelines3, 2017 | Chinese National Consensus Report,4 2018 | |
|---|---|---|---|---|---|
| If clarithromycin-triple fails in 1st line |  |
• Bismuth quad • Levofloxacin-triple or quad |
• Bismuth quad • levofloxacin triple |
• Bismuth quad • Levofloxacin triple |
Not discussed |
| If bismuth quad fails in 1st line |  |
• Levofloxacin-triple or quad • In cases of high levofloxacin resistance: - Bismuth with other antibiotics - Rifabutin triple |
• Levofloxacin triple | Depending on antibiotic history: • Levofloxacin triple • Clarithromycin triple |
• Bismuth + PPI + 2 antibiotics not used in the 1st line bismuth quad treatment |
| If non-bismuth quad fails in 1st line |  |
• Bismuth quad • Levofloxacin triple or quad | • Levofloxacin triple | Not discussed | Not discussed |
| If >2 treatment failures |  |
Treatment guided by results of resistance testing | • Avoid reusing clarithromycin, levofloxacin, metronidazole • Consider rifabutin triple after >3 failures |
Depending on antibiotic history and population resistance patterns: • Concomitant • Rifabutin triple • High-dose dual |
• Bismuth + PPI + 2 antibiotics not used in 1st treatment • 2nd line bismuth quad treatments (metronidazole can be reused, but at a higher dose if not already tried) |
FOOTNOTES:
Multiple national and multinational H. pylori management guidelines exist. This table compiles data from 4 of the highest-profile recent publications.
Abbreviations: bid = two times daily; PPI = proton-pump inhibitor; qid = four times daily; tid = three times daily
Regimens (with usual doses/frequencies/durations)
Bismuth quad = bismuth ~300mg qid, metronidazole 500mg tid, tetracycline 500mg qid, PPI bid x 14 days
Concomitant = clarithromycin 500mg bid, amoxicillin 1g bid, metronidazole or tinidazole 500 mg bid, PPI bid x 14 days
Clarithromycin triple = clarithromycin 500mg bid, amoxicillin 1g bid or metronidazole 500 mg bid, PPI bid x 14 days
Levofloxacin triple = levofloxacin 500mg qd, amoxicillin 1g bid, PPI bid x 14 days
Levofloxacin quad = levofloxacin 500mg qd, PPI bid + 2 antibiotics (multiple variations exist) x 10–14 days
Rifabutin triple = rifabutin 150 or 300 mg daily, amoxicillin 1g bid, PPI bid x 10 days
High-dose dual = amoxicillin 2 −3 g daily in 3–4 split doses, PPI high-dose bid x 14 days
Note: “PPI” implies standard dose unless “high-dose” is specifically stated. Standard dose is as follows: pantoprazole 40mg, lansoprazole 30mg, omeprazole 20mg, esomeprazole 20mg, dexlansoprazole 30mg, rabeprazole 20mg. “High-dose” implies double the standard dose. Optimal dosing is 30 minutes prior to eating or drinking on an empty stomach, without concomitant use of other anti-acids (e.g. histamine-2 receptor antagonists)
Due to rising rates of levofloxacin resistance, we do not recommend levofloxacin unless the H. pylori strain is known to be sensitive to it, or if population levofloxacin resistance rates are known to be <15%. However, it is reasonable to prescribe rifabutin in a triple regimen without prior sensitivity testing since rifabutin and amoxicillin resistance are rare.
- Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017;66:6–30
- Fallone CA, Chiba N, van Zanten SV et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151:51–69.e14
- Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol 2017;112:212–39.
- Liu WZ, Xie Y, Lu H, et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475.
Considerations in regimen selection for refractory H. pylori
There is no shortage of guidelines from international authorities advising on H. pylori management. Some of the most prominent recent releases are listed in Table 1.4,34–36
Overall, the guidance for the management of refractory infection are relatively consistent among the expert groups. However, it should be emphasized that the body of evidence underlying their conclusions reflects the general low quality and heterogeneity of clinical studies conducted on refractory H. pylori infection. Furthermore, most of the trials included in metanalyses of second-line treatment have investigated treatment after failure of a first-line regimen with clarithromycin-based triple therapy, a regimen that we now appreciate should no longer be used in most regions of the world, including the US.37,38 Lastly, even though incorporation of antibiotic susceptibility testing has been advocated by the Maastricht expert consensus group since the first iteration of their guidelines in 199739, the slow uptake of resistance testing around the world persists and continues to propagate empiric selection of eradication therapy for most refractory H. pylori infections, especially in the US.
Nevertheless, several important themes have emerged for guiding treatment of refractory H. pylori infection. First, given the high resistance rates to clarithromycin and levofloxacin, these antibiotics or others in their class (macrolides, fluoroquinolones respectively) should not be repeated in subsequent treatment attempts. Based on the premise that secondary H. pylori resistance may have ensued as collateral damage, an antibiotic history of usage of any of these drug classes for other indications should be considered when selecting subsequent therapy.4 Because primary and secondary resistance to amoxicillin, tetracycline, and rifabutin are very low, these can be used in repeated regimens, even if they have been used previously for H. pylori eradication or other therapy.(BPA #2) Age, comorbidities, and concomitant medications should also guide therapeutic selection and factor into shared decision-making.
Second, resistance to nitroimidazoles, either based on in vitro testing or suspected due to prior nitroimidazole exposure, should not be considered as an ‘absolute’ preclusion for reuse of this antibiotic class for refractory H. pylori therapy, since, for reasons described above, in vitro resistance does not reliably correlate with H. pylori eradication failure associated with using this drug. Nitroimidazole resistance might be potentially overcome with dose adjustments and addition of bismuth.5 Higher doses of metronidazole, at least in the 1.5–2 g/day range, are also associated with significantly improved eradication rates.40 These higher doses might be poorly tolerated due to gastrointestinal and other side effects; thus patients should be advised to consume metronidazole in divided doses (TID to QID) with food and to avoid alcohol for the therapeutic duration due to a disulfuram-like reaction. (BPA #5)
Third, it is now increasingly appreciated that consistently achieving adequate threshold levels of amoxicillin and intragastric acid suppression are important for successful H. pylori eradication, both individually as well as concomitantly since intragastric pH affects the efficacy and half-life of amoxicillin. Drug dose, frequency, and, for acid suppression, drug potency are relevant, especially with respect to their efficacy as a dual regimen, as well as in other regimens for refractory H. pylori infection.(Figure 2) Amoxicillin was originally given twice daily in clarithromycin-based triple therapy; however, it is now recognized that dividing 2–3g amoxicillin into at least three doses daily avoids low trough levels and improves the efficacy of eradication therapy.41 (BPA #6) Given its value in treating refractory H. pylori infection, in the absence of anaphylaxis, penicillin allergy testing should be considered to delist penicillin allergy and potentially enable the use of amoxicillin.(BPA #6) Despite relatively prevalent chart documentation of penicillin allergy, true anaphylaxis to penicillin is rare.
Inadequate acid suppression may undermine eradication efforts through a variety of mechanisms, as detailed above. To this end, optimal dosing of PPIs is frequently overlooked when prescribing eradication therapy, but similar fine-tuning of the acid suppressive prescription may improve eradication outcomes in refractory H. pylori infection.(BPA #7) Providers should also confirm that patients are taking the PPI in a manner that maximizes absorption and activation; factors such as timing of PPI administration in relation to food (and types of foods) and the impact on absorption, as well as the impact of concomitant medications such as histamine H2 receptor blockers on PPI activation should be studied further. Higher dosing, greater frequency (e.g. TID or QID PPI dosing), and the use of more potent PPIs (e.g. esomeprazole or rabeprazole) may be beneficial in cases of refractory H. pylori infection and similarly warrant further investigation. Vonoprazan, a first-in-class potassium-competitive acid blocker, is a potent intragastric acid suppressor that also bypasses CYP2C19-dependent metabolism. Although not yet available in the US, trials comparing CYP2C19-metabolized PPIs versus vonoprazan are ongoing in the US (NCT04167670; clinicaltrials.gov).
Finally, longer treatment durations provide higher eradication rates; thus, a 14-day therapeutic duration should be used for refractory H. pylori infection. (BPA #8)
After multiple failed eradication attempts, the potential benefits of H. pylori eradication should be weighed carefully against the likelihood of adverse effects and inconvenience of repeated high-dose acid suppression and antibiotic exposure, particularly among individuals who are not at identifiably higher risk of complications from persistent H. pylori infection (e.g. gastric cancer, peptic ulcer disease); in such scenarios, a shared decision-making approach should be seriously considered, especially in the elderly, those with frailty, and those with intolerance to antibiotics (BPA #9)
Antibiotic Susceptibility-based Approach
Unlike most infectious diseases where therapy is guided by knowledge of antibiotic sensitivity profiling of the target organism, or at least by knowledge of strains within the relevant geographical region, H. pylori treatment has remained largely empiric. For refractory cases, it may seem obvious that sensitivity testing should be considered after two failed attempts at treatment.36 (BPA #10) However, in practice the situation is complicated by the logistical challenges of obtaining resistance profiles for H. pylori, as well as the lack of convincing data demonstrating superiority of selecting treatment based on sensitivity testing compared to empirically selecting treatment based on prior antibiotic exposure, as further discussed below.
Standard methodology to test for antibiotic sensitivity involves promptly transporting gastric biopsies in sterile containers at room temperature to the receiving microbiology laboratory where they undergo a relatively labor-intensive process to grow up the microbial colonies in micro-aerophilic conditions over several days. Once it is confirmed that the bacteria are indeed H. pylori, for example, based on morphology, urease, catalase and oxidase activity, the bacteria are then tested for viability in the presence of the relevant antibiotics. Because few hospitals or endoscopy centers in the US offer this service in-house, the biopsy specimens for sensitivity testing are instead usually sent in refrigerated packaging to a commercial laboratory. It should be emphasized, though, that in practice the success rates of obtaining a useful result are much lower than the 80–95% success usually reported in research studies. The reasons for the very low success rates outside of a clinical protocol are multifactorial and include delays and errors in sample processing and transport, compounded with the fact that H. pylori is a fastidious organism and the success rates of culturing H. pylori are further decreased by the recent use of PPIs or antibiotics. As an alternative, molecular resistance testing (using a variety of platforms) is simpler, more likely to yield results, and can also be performed on archival specimens including the formalin-fixed paraffin-embedded gastric biopsy tissue remaining after routine diagnostic histopathological testing. This obviates the need for specialized tissue handling by the endoscopist.42
Apart from the practicalities of obtaining H. pylori antibiotic sensitivity testing, especially in the US, a note of caution is still warranted when embarking on therapy directed by susceptibility testing (also referred to as “tailored therapy”); the published literature in this area, which derives almost entirely from studies conducted in the Western Pacific, provides little to no evidence that sensitivity-based treatment selection actually results in significantly improved rates of successful H. pylori eradication over empirically selected second-line regimens.34,10,43 High quality clinical trials of tailored versus empiric therapy after two or more failed attempts at eradication are unfortunately lacking in the US and should be prioritized for future research. Cost-effectiveness analyses will also be valuable, especially if non-endoscopic modalities for susceptibility testing become a viable option, such as molecular testing of stool samples. In the absence of such data, the Maastricht strategy of susceptibility testing after two unsuccessful therapies should be considered in most cases (Figure 2; BPA #10).
Strategies to advance the field and potential adjunctive therapies
The most effective strategy for managing refractory H. pylori is preventing refractory H. pylori infection by improving success rates of primary eradication therapy.
Personalizing the initial H. pylori eradication therapy by incorporating individual host genetic, host non-genetic, and, microbial factors (Figure 1) might help achieve this by shifting the paradigm away from empiric therapy alone. Population-specific research with particular attention to race, ethnic, and age groups, can indicate determinants that impact eradication success in the US. Regional information of local success rates would further refine regimen selection. The Pan-European Registry on H. pylori management44 is one prototype to emulate. Ideally, such a model would encompass systematic collection and reporting, together with periodic updates of regimen-specific local eradication rates—including regimens for primary and refractory infection—along with relevant nonidentifiable individual-level data such as demographics, smoking history, prior antibiotic exposure, and antibiotic sensitivity data if available. Aggregated data should be made publicly available to guide local selection of H. pylori eradication therapy. (BPA #11) This information could be utilized for both initial and refractory treatment choices.
Non-invasive H. pylori antibiotic sensitivity testing on stool, to supplant the current sensitivity testing using endoscopically obtained samples, would overcome many rate-limiting barriers precluding widespread uptake of sensitivity testing. Molecular testing of stool for the small number of known mutations responsible for clarithromycin of levofloxacin resistance is relatively straightforward, as there are several commercial kits for these purposes that utilize PCR. However, next generation sequencing technology offers the potential to detect the resistance footprint of all antibiotics considered in H. pylori therapy. While the clinical utility of utilizing stool-based predictions to guide antibiotic therapy remains to be fully determined, collecting this information would galvanize a data pipeline to accelerate the establishment of surveillance registries, which are immediately needed. Hand-in-hand, implementation and adherence to standard quality metrics based on current clinical guidelines, including appropriate eradication confirmation testing in all individuals treated for H. pylori would simultaneously advance surveillance programs and ideally attenuate the high practice pattern variability observed in the management of refractory H. pylori infection.
Despite rising rates of resistance, the global impact of antibiotic overuse, as well as the exorbitant cost associated with treatment failure (estimated to be at least $33 billion in the US alone45), no truly novel anti-H. pylori therapies are visible on the horizon. Development of newer antimicrobial agents against H. pylori should be fostered, as should investigation into repurposing already available antimicrobials with alternative mechanisms of action against H. pylori. For example, the addition of clavulanic acid to amoxicillin-based regimens has been associated with a 10–20% increase in eradication success46, although more rigorous studies are needed.
There are also some promising data for non-antibiotic adjuncts, such as statins47–49 and probiotics50–54. Regarding the latter, there is an increasing body of data supporting a benefit of probiotics containing Lactobacillus and Bifidobacterium on H. pylori eradication success via an inhibitory effect as well as enhanced patient tolerance of H. pylori eradication therapy resulting in improved adherence. To date, at least twenty clinical trials and several meta-analyses have evaluated the effect of probiotics on H. pylori eradication (albeit not necessarily refractory H. pylori)50–54; these are mostly positive, but there is significant trial heterogeneity and concerns over study quality. Collectively, there are limited data to guide optimal timing, formulation, dosage, duration, and appropriate patient selection for these adjunctive therapies, and their use should therefore be considered experimental. (BPA#12) Further rigorous investigation in US populations and specifically in refractory H. pylori infection would be valuable, particularly given the generally favorable side effect and cost profiles of these agents.
Conclusion
H. pylori management has become increasingly challenging due to declining eradication success rates coupled with increasing antibiotic resistance, resulting in more H. pylori infections that are now refractory to first-line therapies. Accordingly, this CPU was developed to provide practitioners with practical advice on how to manage patients whose initial H. pylori treatment was unsuccessful. When considering the major public health implications associated with persistent H. pylori infection with respect to disease- and treatment-related complications and cost, there is a clear need to prioritize systematic approaches to improve rates of successful H. pylori eradication with the least number of therapeutic attempts.
Best Practice Advice (BPA) Statements.
The usual cause of refractory H. pylori infection (persistent infection after attempting eradication therapy) is antibiotic resistance. Providers should attempt to identify other contributing etiologies, including inadequate adherence to therapy and insufficient gastric acid suppression.
Providers should conduct a thorough review of prior antibiotic exposures. If there is a history of any treatment with macrolides or fluoroquinolones, then clarithromycin- or levofloxacin-based regimens, respectively, should be avoided given the high likelihood of resistance. By contrast, resistance to amoxicillin, tetracycline and rifabutin is rare and these can be considered for subsequent therapies in refractory H. pylori infection.
Eradication regimens for H. pylori are complex and might not be fully comprehended by patients. Barriers to adherence should be explored and addressed prior to prescribing therapy. Providers should explain the rationale for therapy, dosing instructions, expected adverse events and the importance of completing the full therapeutic course.
If bismuth quadruple therapy failed as a first-line treatment, shared decision-making between providers and patients should guide selection between a) levofloxacin- or rifabutin-based triple therapy regimens with high-dose dual proton pump inhibitor (PPI) and amoxicillin, or b) an alternative bismuth-containing quadruple therapy, as second-line options.
When using metronidazole-containing regimens, providers should consider adequate dosing of metronidazole (1.5–2 g daily in divided doses) with concomitant bismuth therapy, as this may improve eradication success rates irrespective of observed in vitro metronidazole resistance.
6. In the absence of a history of anaphylaxis, penicillin allergy testing should be considered in a patient labelled as having this allergy in order to delist penicillin as an allergy and potentially enable its use. Amoxicillin should be used at a daily dose of at least 2g divided TID or QID to avoid low trough levels.
Inadequate acid suppression is associated with H. pylori eradication failure. The use of high-dose and more potent PPIs, PPIs not metabolized by CYP2C19 or potassium-competitive acid blockers if available, should be considered in cases of refractory H. pylori infection.
Longer treatment durations provide higher eradication success rates compared to shorter durations (e.g. 14 days vs 7 days). Whenever appropriate, longer treatment durations should be selected for treating refractory H. pylori infection.
In some cases, there should be shared decision-making regarding ongoing attempts to eradicate H. pylori. The potential benefits of H. pylori eradication should be weighed carefully against the likelihood of adverse effects and inconvenience of repeated exposure to antibiotics and high-dose acid suppression, particularly in vulnerable populations, such as the elderly.
After two failed therapies with confirmed patient adherence, H. pylori susceptibility testing should be considered to guide the selection of subsequent regimens.
Compiling local data on H. pylori eradication success rates for each regimen, along with patient demographic and clinical factors (including prior non-H. pylori antibiotic exposure) is important. Aggregated data should be made publicly available to guide local selection of H. pylori eradication therapy.
Proposed adjunctive therapies, including probiotics, are of unproven benefit as treatment for refractory H. pylori infection and, thus, their use should be considered experimental.
Acknowledgements
This Expert Review was commissioned and approved by the American Gastroenterological Association (AGA) Institute Clinical Practice Updates (CPU) Committee and the AGA Governing Board to provide timely guidance on a topic of high clinical importance to the AGA membership, and underwent internal peer review by the CPU Committee and external peer review through standard procedures of Gastroenterology.
Grant Support
Dr. Shah is supported by an American Gastroenterological Association Research Scholar Award (2019) and a Veterans Affairs Career Development Award ICX002027A01.
Abbreviations used in main text, tables, and figures
| Abbreviation | Full Term |
|---|---|
| AGA | American Gastroenterological Association |
| BID | Two times per day (dosing) |
| BPA | Best Practice Advice |
| CPU | Clinical Practice Update |
| H. pylori | Helicobacter pylori |
| PPI | Proton pump inhibitor |
| PAL | PPI, amoxicillin, levofloxacin |
| PAR | PPI, amoxicillin, rifabutin |
| PBCT | PPI, bismuth, clarithromycin, tetracycline |
| PBLA | PPI, bismuth, levofloxacin, amoxicillin |
| PBLT | PPI, bismuth, levofloxacin, tetracycline |
| PBLM | PPI, bismuth, levofloxacin, metronidazole |
| PBMT | PPI, bismuth, metronidazole, tetracycline |
| RCT | Randomized controlled trial |
| QID | Four times per day (dosing) |
| TID | Three times per day (dosing) |
| US | United States |
| WHO | World Health Organization |
| Figure 2 Abbreviations | Full Term |
|---|---|
| A | Amoxicillin |
| B | Bismuth |
| C | Clarithromycin |
| L | Levofloxacin |
| M | Metronidazole |
| R | Rifabutin |
| T | Tetracycline |
| PAL | PPI, amoxicillin, levofloxacin |
| PAR | PPI, amoxicillin, rifabutin |
| PBCT | PPI, bismuth, clarithromycin, tetracycline |
| PBLA | PPI, bismuth, levofloxacin, amoxicillin |
| PBLT | PPI, bismuth, levofloxacin, tetracycline |
| PBLM | PPI, bismuth, levofloxacin, metronidazole |
| PBMT | PPI, bismuth, metronidazole, tetracycline |
Footnotes
Conflict of Interest Statement
Dr. Shah has no conflicts to disclose.
Dr. Iyer receives research funding from Exact Sciences and Pentax Medical.
Dr. Moss serves on the advisory board of Redhill Biopharma and Phathom Pharmaceuticals, receives research funding from American Molecular Laboratories, and is a consultant for Takeda.
The authors report no other disclosures.
References
- 1.Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017; 153: 420–9. [DOI] [PubMed] [Google Scholar]
- 2.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer 2015; 136: 487–90. [DOI] [PubMed] [Google Scholar]
- 3.Linz B, Balloux F, Moodley Y, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature 2007; 445: 915–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol 2017; 112: 212–39. [DOI] [PubMed] [Google Scholar]
- 5.Boltin D, Levi Z, Gingold-Belfer R, et al. Effect of Previous Nitroimidazole Treatment on Helicobacter pylori Eradication Success. J Clin Gastroenterol 2020; 54: 333–7. [DOI] [PubMed] [Google Scholar]
- 6.Boltin D, Levi Z, Gingold-Belfer R, et al. Impact of Previous Exposure to Macrolide Antibiotics on Helicobacter pylori Infection Treatment Outcomes. Am J Gastroenterol 2019; 114: 900–6. [DOI] [PubMed] [Google Scholar]
- 7.Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013; 62: 34–42. [DOI] [PubMed] [Google Scholar]
- 8.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in helicobacter pylori: a systematic review and meta-analysis in world health organization regions. Gastroenterology 2018; 155: 1372–1382.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong S, Kim SE, Kim JH, et al. Helicobacter pylori eradication rates with concomitant and tailored therapy based on 23S rRNA point mutation: A multicenter randomized controlled trial. Helicobacter 2019; : e12654. [DOI] [PubMed] [Google Scholar]
- 10.Liou J-M, Chen P-Y, Luo J-C, et al. Efficacies of Genotypic Resistance-Guided vs Empirical Therapy for Refractory Helicobacter pylori Infection. Gastroenterology 2018; 155: 1109–19. [DOI] [PubMed] [Google Scholar]
- 11.Lee M, Kemp JA, Canning A, Egan C, Tataronis G, Farraye FA. A randomized controlled trial of an enhanced patient compliance program for Helicobacter pylori therapy. Arch Intern Med 1999; 159: 2312–6. [DOI] [PubMed] [Google Scholar]
- 12.Murakami TT, Scranton RA, Brown HE, et al. Management of Helicobacter Pylori in the United States: Results from a national survey of gastroenterology physicians. Prev Med 2017; 100: 216–22. [DOI] [PubMed] [Google Scholar]
- 13.Gong Y, Yuan Y. Resistance mechanisms of Helicobacter pylori and its dual target precise therapy. Crit Rev Microbiol 2018; 44: 371–92. [DOI] [PubMed] [Google Scholar]
- 14.Graham DY, Canaan Y, Maher J, Wiener G, Hulten KG, Kalfus IN. Rifabutin-Based Triple Therapy (RHB-105) for Helicobacter pylori Eradication: A Double-Blind, Randomized, Controlled Trial. Ann Intern Med 2020; 172: 795–802. [DOI] [PubMed] [Google Scholar]
- 15.Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther 2012; 35: 209–21. [DOI] [PubMed] [Google Scholar]
- 16.Duck WM, Sobel J, Pruckler JM, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerging Infect Dis 2004; 10: 1088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiota S, Reddy R, Alsarraj A, El-Serag HB, Graham DY. Antibiotic Resistance of Helicobacter pylori Among Male United States Veterans. Clin Gastroenterol Hepatol 2015; 13: 1616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham DY, Lew GM, Malaty HM, et al. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology 1992; 102: 493–6. [DOI] [PubMed] [Google Scholar]
- 19.Buring SM, Winner LH, Hatton RC, Doering PL. Discontinuation rates of Helicobacter pylori treatment regimens: a meta-analysis. Pharmacotherapy 1999; 19: 324–32. [DOI] [PubMed] [Google Scholar]
- 20.Luo M, Hao Y, Tang M, et al. Application of a social media platform as a patient reminder in the treatment of Helicobacter pylori. Helicobacter 2020; 25: e12682. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Yang X, Li Y, et al. Twice daily short-message-based re-education could improve Helicobacter pylori eradication rate in young population: A prospective randomized controlled study. Helicobacter 2019; 24: e12569. [DOI] [PubMed] [Google Scholar]
- 22.Erah PO, Goddard AF, Barrett DA, Shaw PN, Spiller RC. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrob Chemother 1997; 39: 5–12. [DOI] [PubMed] [Google Scholar]
- 23.Furuta T, Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori Infection. Gastroenterol Clin North Am 2010; 39: 465–80. [DOI] [PubMed] [Google Scholar]
- 24.Hagymási K, Müllner K, Herszényi L, Tulassay Z. Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics 2011; 12: 873–88. [DOI] [PubMed] [Google Scholar]
- 25.Kuo C-H, Lu C-Y, Shih H-Y, et al. CYP2C19 polymorphism influences Helicobacter pylori eradication. World J Gastroenterol 2014; 20: 16029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martis S, Peter I, Hulot JS, Kornreich R, Desnick RJ, Scott SA. Multi-ethnic distribution of clinically relevant CYP2C genotypes and haplotypes. Pharmacogenomics J 2013; 13: 369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Rouby N, Lima JJ, Johnson JA. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin Drug Metab Toxicol 2018; 14: 447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishizaki T, Sohn DR, Kobayashi K, et al. Interethnic differences in omeprazole metabolism in the two S-mephenytoin hydroxylation phenotypes studied in Caucasians and Orientals. Ther Drug Monit 1994; 16: 214–5. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki T, Matsuo K, Ito H, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med 2006; 119: 217–24. [DOI] [PubMed] [Google Scholar]
- 30.Mejías-Luque R, Gerhard M. Immune Evasion Strategies and Persistence of Helicobacter pylori. Curr Top Microbiol Immunol 2017; 400: 53–71. [DOI] [PubMed] [Google Scholar]
- 31.Camargo MC, Piazuelo MB, Mera RM, et al. Effect of smoking on failure of H. pylori therapy and gastric histology in a high gastric cancer risk area of Colombia. Acta Gastroenterol Latinoam 2007; 37: 238–45. [PMC free article] [PubMed] [Google Scholar]
- 32.Correa P, van Doorn LJ, Bravo JC, Ruiz B, Bravo LE, Realpe JL. Unsuccessful treatment results in survival of less virulent genotypes of Helicobacter pylori in Colombian patients. Am J Gastroenterol 2000; 95: 564–6. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T, Matsuo K, Sawaki A, et al. Systematic review and meta-analysis: importance of CagA status for successful eradication of Helicobacter pylori infection. Aliment Pharmacol Ther 2006; 24: 273–80. [DOI] [PubMed] [Google Scholar]
- 34.Fallone CA, Chiba N, van Zanten SV, et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology 2016; 151: 51–69.e14. [DOI] [PubMed] [Google Scholar]
- 35.Liu WZ, Xie Y, Lu H, et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter 2018; 23: e12475. [DOI] [PubMed] [Google Scholar]
- 36.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017; 66: 6–30. [DOI] [PubMed] [Google Scholar]
- 37.Muñoz N, Sánchez-Delgado J, Baylina M, et al. Systematic review, meta-analysis, and meta-regression: Successful second-line treatment for Helicobacter pylori. Helicobacter 2018; 23: e12488. [DOI] [PubMed] [Google Scholar]
- 38.Yeo YH, Hsu C-C, Lee C-C, et al. Systematic review and network meta-analysis: Comparative effectiveness of therapies for second-line Helicobacter pylori eradication. J Gastroenterol Hepatol 2018; published online August 31. DOI: 10.1111/jgh.14462. [DOI] [PubMed] [Google Scholar]
- 39.Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. European Helicobacter Pylori Study Group. Gut 1997; 41: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji Y, Lu H. Meta-analysis: High-dose vs. low-dose metronidazole-containing therapies for Helicobacter pylori eradication treatment. PLoS One 2018; 13: e0189888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furuta T, Sugimoto M, Yamade M, et al. Effect of dosing schemes of amoxicillin on eradication rates of Helicobacter pylori with amoxicillin-based triple therapy. J Clin Pharmacol 2014; 54: 258–66. [DOI] [PubMed] [Google Scholar]
- 42.Pohl D, Keller PM, Bordier V, Wagner K. Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J Gastroenterol 2019; 25: 4629–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.López-Góngora S, Puig I, Calvet X, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother 2015; 70: 2447–55. [DOI] [PubMed] [Google Scholar]
- 44.McNicholl AG, Gasbarrini A, Tepes B, et al. Tu1327 Pan-European Registry on H. pylori Management (Hp-EuReg): Interim Analysis of Non-Bismuth Quadruple Concomitant Treatment. Gastroenterology 2016; 150: S875. [Google Scholar]
- 45.Malnick SDH, Melzer E, Attali M, Duek G, Yahav J. Helicobacter pylori: friend or foe? World J Gastroenterol 2014; 20: 8979–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alrabadi N, Albustami IS, Abuhayyeh HA, et al. Clavulanic Acid in the Scope of Helicobacter pylori Treatment: A Literature Review and Beyond. Curr Clin Pharmacol 2020; published online July 2. DOI: 10.2174/1574884715666200702121417. [DOI] [PubMed] [Google Scholar]
- 47.Sarkeshikian SS, Ghadir MR, Alemi F, Jalali SM, Hormati A, Mohammadbeigi A. Atorvastatin in combination with conventional antimicrobial treatment of Helicobacter pylori eradication: A randomized controlled clinical trial. J Gastroenterol Hepatol 2020; 35: 71–5. [DOI] [PubMed] [Google Scholar]
- 48.Hassan AM, Shawky MAE-G, Mohammed AQ, Haridy MA, Eid KA-E-A. Simvastatin improves the eradication rate of Helicobacter pylori: upper Egypt experience. Infect Drug Resist 2019; 12: 1529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao W-C, Huang M-Z, Wang ML, et al. Statin Decreases Helicobacter pylori Burden in Macrophages by Promoting Autophagy. Front Cell Infect Microbiol 2016; 6: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng J-R, Wang F, Qiu X, et al. Efficacy and safety of probiotic-supplemented triple therapy for eradication of Helicobacter pylori in children: a systematic review and network meta-analysis. Eur J Clin Pharmacol 2017; 73: 1199–208. [DOI] [PubMed] [Google Scholar]
- 51.Lau CSM, Ward A, Chamberlain RS. Probiotics improve the efficacy of standard triple therapy in the eradication of Helicobacter pylori: a meta-analysis. Infect Drug Resist 2016; 9: 275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dang Y, Reinhardt JD, Zhou X, Zhang G. The effect of probiotics supplementation on Helicobacter pylori eradication rates and side effects during eradication therapy: a meta-analysis. PLoS One 2014; 9: e111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McFarland LV, Huang Y, Wang L, Malfertheiner P. Systematic review and meta-analysis: Multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol J 2016; 4: 546–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z-H, Gao Q-Y, Fang J-Y. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol 2013; 47: 25–32. [DOI] [PubMed] [Google Scholar]


