Abstract
Toxoplasma gondii is remarkably unique in its ability to successfully infect vertebrate hosts from multiple phyla and can successfully infect most cells within these organisms. The infection outcome in each of these species is determined by the complex interaction between parasite and host genotype. As techniques to quantify global changes in cell function become more readily available and precise, new data are coming to light about how i) different host cell types respond to parasitic infection and ii) different parasite species impact the host. Here we focus on recent studies comparing the response to intracellular parasitism by different cell types and insights into understanding host-parasite interactions from comparative studies on T. gondii and its close extant relatives.
Keywords: Toxoplasma gondii, Hammondia hammondi, Neospora caninum, Host response, Chemokines, Placenta
Graphical Abstract
1. Toxoplasma gondii
Toxoplasma gondii, a member of the phylum Apicomplexa, has major implications in public health as it is estimated that one-third of the world’s population is infected with this parasite (Halonen and Weiss, 2013). Toxoplasma gondii is an obligate intracellular parasite that can infect all warm-blooded animals and felines are the definitive host for sexual reproduction. Toxoplasma gondii infection can occur by ingestion of food or water contaminated with T. gondii tissue cysts or oocysts. The life cycle of T. gondii involves multiple hosts and developmental stages (reviewed in Hutchison, 1965; see also Dubey, 2009a). Given this complexity, like most parasites T. gondii encounters multiple host cell and tissue types during its life cycle. Following excystation of the oocyst, sporozoites primarily infect gut epithelial cells, and then differentiate into tachyzoites which disseminate throughout the organism after infecting circulating cells such as dendritic cells, natural killer cells, monocytes and macrophages (Dubey et al., 1997; Courret et al., 2006; Persson et al., 2009) Throughout this dissemination process, a robust immune response to the parasite is generated, ultimately leading to mobilization of cytotoxic T-cells and production of protective antibodies (Suzuki et al., 1988; Parker et al., 1991; Khan et al., 1994; Ely et al., 1999) Coincident with the emergence of this host response, some tachyzoites differentiate into slow-growing bradyzoites which eventually become encased in a cyst wall. These tissue cysts can be found in a variety of tissues including heart, skeletal muscle, lung and the brain (Remington and Cavanaugh, 1965; Di Cristina et al., 2008). The adaptations that underlie the ability of T. gondii to replicate within, and persist in, such a wide variety of cell types is poorly understood, but this feature is critical for its ability to cause disease in humans. The bradyzoite-containing tissue cyst can effectively de-differentiate to the proliferative tachyzoite form in immunocompromised individuals such as those with HIV/AIDS and organ transplant patients (Luft et al., 1984; Gazzinelli et al., 1992; Derouin et al., 2008), causing disseminated disease and/or lethal encephalitis. The fact that T. gondii thrives in such a wide variety of tissues and cell types provides a unique opportunity to examine how different host cells respond to infection with the same parasite, and ultimately how these responses impact parasite growth, stage conversion, and survival.
2. Toxoplasma gondii modulation of host responses
Studies on T. gondii-mediated host gene expression regulation were first explored using cDNA microarrays (Blader et al., 2001; Gail et al., 2001; Chaussabel et al., 2003), and more recently RNA-sequencing (seq) (Garfoot et al., 2019; Li et al., 2019; Lu et al., 2019; Panas et al., 2019; Seizova et al., 2019). Genes found to be regulated by T. gondii, according to these studies, include genes encoding for many different processes including inflammation, apoptosis, metabolism, cell growth and differentiation (reviewed in Blader and Saeij, 2009). As an intracellular parasite, T. gondii is not able to replicate extracellularly. For invasion, T. gondii relies on the secretion of multiple effectors from the microneme and rhoptries (Alexander et al., 2005; Lebrun et al., 2005; Besteiro et al., 2009; Lamarque et al., 2011; Tyler and Boothroyd, 2011; Guerin et al., 2017), which are localized anteriorly for polarized anterior secretion (Nichols et al., 1983; Carruthers and Sibley, 1997). In addition to microneme and rhoptry proteins, the dense granules secrete proteins that can be found in multiple locations outside of the parasite, including soluble proteins in the parasitophorous vacuole (PV; Henriquez et al., 2005), associated with the PV tubulovesicular network (Labruyere et al., 1999), and the host cell cytoplasm and nucleus (Bonhomme et al., 1998; Rosowski et al., 2011; Bougdour et al., 2013; Braun et al., 2013; Ma et al., 2014).
Several parasite secreted effectors such as rhoptry- (ROP) and dense granules- (GRA) proteins are identified to be major players involved in interacting with host signaling pathways. Some T. gondii ROPs are essential for invasion of the host cell while several other ROPs have been shown to be important for T. gondii virulence and/or to co-opt host gene regulation (Taylor et al., 2006; Saeij et al., 2007). The ROP5/ROP18/GRA7 complex plays an important role in parasite virulence in vivo (Saeij et al., 2006; Taylor et al., 2006; Behnke et al., 2011; Reese and Boothroyd, 2011; Reese et al., 2011; Behnke et al., 2015; Hermanns et al., 2016). In murine hosts, TgROP18 from type 1 T. gondii strains (such as RH and GT-1) can disrupt the host interferon-gamma (IFN-γ) response by inhibiting the loading of immune-related GTPases (IRGs) onto the PV (Fentress et al., 2010; Khaminets et al., 2010). Interestingly T. gondii ROP18 was originally discovered as a virulence effector based on quantitative trait locus mapping in progeny derived from sexual crosses between either type 1 or type 2 strains and derivatives of the same type 3 isolate (CTG; (Taylor et al., 2006; Saeij et al., 2007)). The basis for this virulence Quantitative trait locus (QTL) was found to be an insertion/deletion in the ROP18 promoter (Boyle et al., 2008) that was associated with dramatically reduced ROP18 transcript abundance in type 3 strains. This suggests that ROP18 itself is not required for T. gondii infection and its ability to infect a wide variety of hosts (since type 3) strains are found at a similar prevalence across the globe as other strain types; (Lehmann et al., 2006; Lorenzi et al., 2016)), but that it does have a dramatic influence on pathogenesis in the mouse model. Similarly, ROP5 was also identified based on the same or similar genetic crosses as those that led to the identification of ROP18, with the type 2 allele of ROP5 being associated with reduced virulence in the mouse model compared with the type 1 and type 3 alleles (Behnke et al., 2011; Reese et al., 2011). Interestingly, both ROP5 and ROP18 play critical roles in mouse virulence in South American T. gondii strains (Behnke et al., 2015), suggesting that their function in virulence is ancestral to the T. gondii lineage and that they have been subject to selection driven diversification and/or inactivation (in the case of type 3 ROP18) in canonical North American lineages (types 1, 2 and 3). We also discuss this issue below in regard to what is known about these genes in Hammondia hammondi and Neospora caninum below.
In addition to host-targeting ROPs that are mostly derived from the rhoptry bulb (rather than the rhoptry neck), parasites secrete proteins from the GRAs during and after invasion (Bonhomme et al., 1998). Effector GRA proteins (GRA15 (Rosowski et al., 2011), GRA16 (Bougdour et al., 2013), GRA24 (Braun et al., 2013), GRA28 (Ander et al., 2017), TgIST (Gay et al., 2016; Olias et al., 2016) and HCE1/TEEGR (Braun et al., 2019; Panas et al., 2019)) modulate multiple host pathways including necrosis factor (NF)-κB, p53, mitogen-activated protein kinase (MAPK), cytokine signaling, signal transducer and activator of transcripton (STAT)-regulated gene expression and the host cell cycle. What is particularly interesting about these effectors is that many of them have overlapping effects on the same host cell pathways.
During T. gondii infection, Types I and II IFN signaling are suppressed. Type II (IFN-γ)-dependent signaling is down-regulated by T. gondii Inhibition of STAT transcription (TgIST; (Gay et al., 2016; Olias et al., 2016; Matta et al., 2019)). The transcription factor STAT1 is the main signal transducer of the IFN-γ response to T. gondi infection (Zimmermann et al., 2006; Kim et al., 2007; Lang et al., 2012; Schneider et al., 2013; Rosowski et al., 2014) and subsequently impeding expression of genes for major histocompatibility (MHC) class II, inducible nitric oxide synthase (NOS2), class II transactivator (CIITA), IFN-inducible GTPases and chemokines such as CXCL9 and CXCL10 (Scharton-Kersten et al., 1997; Lüder et al., 2003; Kim et al., 2007; Lang et al., 2012; Rosowski and Saeij, 2012). When TgIST is secreted into the host cell, it translocates into the host cell nucleus and interacts with gamma-activated sequences (GASs) in the promoters of IFN-stimulated genes (ISGs) via an interaction with STAT1 homodimers. STAT1-mediated transcription of target genes is inhibited by IST due to its recruitment of the nucleosome remodeling and repressive (NuRD) complex (Gay et al., 2016; Olias et al., 2016). Recently, it was discovered that TgIST also associates with phosphorylated STAT2 and recruits the NuRD complex in response to IFN-β treatment, and represses a subset of Type I IFN response genes. Growth of parasites lacking TgIST is also restricted in host cells treated with IFN-β (Matta et al., 2019), suggesting that TgIST disrupts host responses to both Type I and Type II interferons.
In addition to TgIST, T. gondii HCE1/TEEGR also suppresses host responses during infection. HCE1/TEEGR partners with host E2F3/E2F4 transcription factors (Panas et al., 2019) and suppresses NF-κB regulated TNF-α-cytokine signaling via interactions with the polycomb repressive complex 2 (PRC2) subunit EZH2 (Braun et al., 2019). Among these effectors only GRA15 has a strain-specific function, where Type II T. gondii induces an high level of NF-κB activation compared with Type 1 and Type 3 parasites (Rosowski et al., 2011) and IL-1β secretion in inflammatory monocytes (Gov et al., 2013). Interestingly HCE1/TEEGR of Type II T. gondii was found to not disrupt the activity of T. gondii type 2 strain GRA15 (Braun et al., 2019), suggesting that they are driving NF-κB activation in distinct ways.
Interestingly, HCE1/TEEGR seems to have another role in modulating the host cell cycle by direct interaction with cyclins. Toxoplasma gondii HCE1/TEEGR associates with cyclin E (CCNE) by partnering with cell cycle transcription factor dimerization partner 1 (DP1) and ultimately forming a complex with E2F3/E2F4 proteins (Braun et al., 2019; Panas et al., 2019). DP1 and E2F proteins are part of the DREAM complex which plays a role in regulating host cell cycle regulation (reviewed in Engeland, 2018). The DP1/E2F complex binds to E2F binding sites to repress transcription during the early G0/G1 phase (Litovchick et al., 2007). Repression of transcription is released when the FOXM1 and B-MYB are recruited to the MuvB core when E2F4–5/DP and p107/p130 (pRB-like proteins) are dissociated from the DNA binding site (Mannefeld et al., 2009; Quaas et al., 2012; Sadasivam et al., 2012; Chen et al., 2013).
The ability of T. gondii to modulate the host cell cycle is not surprising as it has also been shown that T. gondii infection induces quiescent host cells to reenter the cell cycle (Holmes et al., 2019), or host cells in G1 phase to enter into the S phase and arrest host cells at the G2/M phase (Brunet et al., 2008; Molestina et al., 2008; Wong et al., 2019). While the impact of T. gondii-mediated host cell cycle modulation is poorly understood, early studies show that T. gondii may have a preference for infecting cells in the S phase (Lavine and Arrizabalaga, 2008) and it has been speculated that this is due to the fact that the microtubule-organizing centers (MTOCs) that are actively remodeled by T. gondii during an infection are not accessible at other host cell cycle stages (Coppens, 2006; Walker et al., 2008). In addition to the CCNE/E2F/DP1 complexes, other host factors have also been shown to be involved in T. gondii-mediated cell cycle regulation, including UHRF1 (Brunet et al., 2008), p53 and CDKN1A (Bougdour et al., 2013). Toxoplasma gondii-mediated UHRF1 gene expression causes host cells to arrest in the G1 phase. When expression of UHFR1 was suppressed using small interfering RNA (siRNA), the growth of T. gondii was reduced in BeWo and normal human dermal fibroblast (NHDF) cells (Brunet et al., 2008). While the parasite effector responsible for host cell cycle regulation is unknown, the T. gondii-secreted effector GRA16 increases p53 and p21 levels in human host cells (Bougdour et al., 2013). The host transcription factor P53 can induce cell cycle arrest at either G1/S or G2/M checkpoints (Agarwal et al., 1995; Bunz et al., 1998) and p21 (gene product of CDKN1A) was the first transcriptional target identified for p53 (el-Deiry et al., 1993). While the exact mechanism of these parasite effector(s) in mediating host cell cycle regulation is not fully understood, these data suggest that T. gondii could also be modulating the p53-p21-DREAM-E2F pathway as a parallel means to disrupt host cell cycle progression (more on this pathway in the section below)
An important recent advance in understanding of T. gondii manipulation of the host cell is the discovery of a complex of proteins that are required for secretion of multiple dense granule-derived effectors (including those described above). This complex, named after its founding member “Myc Regulation 1” (“MYR1”), was identified in a mutagenesis screen for T. gondii parasites that were deficient in inducing c-Myc upregulation in the host cell (Franco et al., 2016). This complex is now known to contain at least six dense granule proteins (Gay et al., 2016; Olias et al., 2016; Ander et al., 2017; He et al., 2018; Marino et al., 2018; Braun et al., 2019; Panas et al., 2019) and T. gondii ΔMYR1 parasites are less able to regulate the host cell cycle compared with wild type parasites (Franco et al., 2016). Importantly, transcriptome data from host cells infected with wild type (WT) and ΔMYR1 parasites suggests that MYR1-dependent effectors can have opposing effect son the same processes, including the cell cycle (Franco et al., 2016; Panas et al., 2019). The sum total of the response of a given host cell will depend on its sensitivity to each of these effectors, and this could provide a unique means for T. gondii to use the same effectors to mediate distinct outcomes in different cell types.
3. Response to intracellular parasitism by different cell types
Despite the fact that T. gondii resides in a variety of host cell types during infection of the definitive and intermediate host, including epithelial, endothelial, immune and neuronal cells, genome-wide data comparing the host response in different cell types are mostly lacking. Given their ease of cultivation, primary human foreskin fibroblasts (HFFs) are most commonly used in the study of many aspects of T. gondii biology including host responses to infection. TgGRA-mediated host modulation has been thoroughly investigated in HFFs and yielded important insights (Bougdour et al., 2013; Franco et al., 2016; Gay et al., 2016; Olias et al., 2016; Naor et al., 2018; Braun et al., 2019; Panas et al., 2019). However, in the few studies that have been performed there can be remarkably different responses to T. gondii between cell types. For example, T. gondii suppresses IL-1β and lipopolysaccharide (LPS)-induced IL-1β production in neutrophils, but fails to do so in monocytes (Lima et al., 2018). In our work, we have compared infection of HFFs with primary human trophoblast (PHT) cells, and discovered a cluster of genes that are induced in PHT cells but not in HFFs, including the transcription factor IRF4, the chemokines CCL22, CCL17, CCL20 and CCL1, and the chemokine receptor CCR7 (Ander et al., 2017). Importantly, some of these chemokines (CCL22 and CCL17, in particular) are also induced to be expressed in a variety of mouse macrophage cell types (He et al., 2018).
During the later stages of infection, parasite tissue cysts have a higher propensity to be found in neurons and muscle (Remington and Cavanaugh, 1965; Ferguson and Hutchison, 1987; Halonen et al., 1996; Fisher et al., 1997; Lüder et al., 1999, 2003; Dubey, 2009b; Cabral et al., 2016), although the reason for this is unknown. It has been speculated that i) the condition of immunity-related stress factors such as reactive oxygen and nitrogen species or nutrient depletion (Bohne et al., 1994; Bohne and Roos, 1997; Fox et al., 2004) and ii) the absence of exogenous stressors in neuronal and muscular cells provide a suitable microenvironment for the development of bradyzoites and tissue cysts (Lüder et al., 1999; Ferreira-da-Silva Mda et al., 2008). Genome-wide transcriptomic comparative studies were performed on murine cells and identified highly divergent responses to T. gondii in different cell types (skeletal muscle cells (SkMCs), neurons, astrocytes and fibroblasts). Intriguingly, with only a small number of genes commonly regulated in these cell types (including only a few immune response-related genes), none of these genes were commonly regulated in all four cell types, suggesting that the host transcriptomic profile in relation to T. gondii infection is host cell type-specific rather than parasite-driven host cell manipulation (Swierzy et al., 2017).
In addition to differences between cell types within the same host, differences in expression profiles between different host cells (humans and mice) were also recognized (Channon et al., 2000; Blader et al., 2001; Chaussabel et al., 2003; Swierzy et al., 2017). Toxoplasma gondii infection in dendritic cells (DCs) and macrophages have a greater number of inflammation- and immunity-related genes being more prominently regulated (Chaussabel et al., 2003). Despite the differences in immune-related responses, in both human and mouse host cells, genes involved in translation and host cell cycle regulation were commonly regulated by T. gondii. In murine fibroblasts and astrocytes as well as human fibroblasts, T. gondii infection induces expression of genes involved in translation (Blader et al., 2001; Swierzy et al., 2017). Host cell cycle-related genes were regulated in human fibroblasts as well as murine SkMCs by T. gondii, with a lesser impact on murine neurons (Swierzy et al., 2017).
While this data will be helpful for investigation of T. gondii-mediated responses in the human host cells, a similar study would be useful to dissect the common and different responses in different human host cells and we have seen differential responses in human and mouse host cells by T. gondii, specifically in the regulation of IL-12 and IFN-γ (reviewed in Pifer and Yarovinsky, 2011). We expect new data examining transcriptomic and proteomic responses of different cell types to T. gondii to continue to emerge as RNAseq (both bulk and single cell) and label-free quantitative proteomics become more readily available to most research groups.
4. Host-pathogen interaction comparisons between T. gondii and its close relatives
Toxoplasma gondii is closely related to N. caninum and H. hammondi, and T. gondii is more closely related to H. hammondi than N. caninum. Toxoplasma gondii shares a high degree of gene-by-gene synteny with both species (>81% between T. gondii and N. caninum and >95% between T. gondii and H. hammondi) (Walzer et al., 2013; Adomako-Ankomah et al., 2014; Lorenzi et al., 2016). As tissue-dwelling coccidia, these parasite species share a number of life cycle features, with sexual stages occurring in either canine (for N. caninum) or feline (for T. gondii and H. hammondi) gut epithelial cells, and asexual reproduction and encystment occurring in intermediate host species. However, these species are divergent in a number of important phenotypes for which no molecular mechanisms are known. For example, N. caninum is not naturally transmitted by rodents and experimental infection has shown that N. caninum is significantly less pathogenic in the mouse model compared with T. gondii, despite displaying highly similar growth profiles in HFFs in vitro (English et al., 2015; Coombs et al., 2020). Hammondia hammondi is naturally transmitted by rodents but is avirulent in laboratory mice, including mice that lack IFNγ-driven host responses. Hammondia hammondi also has a host range that is comparatively restricted compared with T. gondii as it is only known to naturally infect rodents, roe deer, and goats. However, experimentally H. hammondi is also capable of infecting dogs, pigs, monkeys and rabbits but fails to infect birds (Dubey and Sreekumar, 2003). Understanding the molecular mechanisms driving these phenotypic differences could provide new insights into host range determinants and virulence mechanisms that may be undetectable when studying only a single species. To this end, in laboratory settings these species can infect most, if not all, of the same cell types, allowing for rigorous interspecies comparisons of parasite modulation of host cell biology and responses to infection (Reid et al., 2012; Sokol et al., 2018). The outcomes of these infections are driven by the complex interplay between introduction of effectors into the host cell during infection and the host response. Here we focus on what is known regarding the T. gondii common and/or distinct impacts (as compared to H. hammondi or N. caninum) of parasite infection on the modulation of host signaling pathways, with a focus on IFN signaling and the host cell cycle.
4.1. Neospora caninum
Neospora caninum was first described in 1984 (Bjerkas et al., 1984) and is a major cause of abortion in cattle and therefore a significant threat to the cattle industry (Almería et al., 2017; Dubey et al., 2007). Similar to T. gondii, N. caninum can be transmitted by ingestion of sporulated oocysts from the environment or tissues harboring bradyzoite-containing tissue cysts. In bovines, N. caninum can also be transmitted vertically through the placenta during pregnancy (Dubey et al., 2007). The genomes of T. gondii and N. caninum are largely syntenic (DeBarry and Kissinger, 2011; Reid et al., 2012). In addition, corresponding orthologous genes are found elsewhere in the genome in regions where synteny is disrupted (Reid et al., 2012). Comparisons of transcript abundance between T. gondii and N. caninum tachyzoites show that SAG1-Related Sequences (SRSs), ROPs and AP2s are among the genes with higher expression in T. gondii relative to N. caninum (Reid et al., 2012).
In contrast to suppression of Type I and Type II IFN signaling pathway genes by T. gondii, N. caninum infection has been shown to induce robust Type I (IFN-α and −β; (Beiting et al., 2014)) and Type II (IFN-γ) interferon signaling during infection (Baszler et al., 1999; Long and Baszler, 2000; Nishikawa et al., 2003). Neospora caninum infection induces a more potent expression of Type I IFN signaling pathway genes compared with T. gondii in vitro, and it appears that this response is actively suppressed by T. gondii since media from T. gondii-infected host cells can suppress N. caninum-induced Type I IFN responses (Beiting et al., 2014).
4.2. Hammondia hammondi
Hammondia hammondi is the closest known extant relative of T. gondii. Unlike N. caninum, both H. hammondi and T. gondii complete their sexual life cycle stage in the small intestine of cats. While T. gondii is identified as a major threat to human public health and animals, as with N. caninum, H. hammondi has not been shown to be associated with any human clinical diseases (Dubey and Sreekumar, 2003). Despite having differential impact on parasite pathogenesis and infection outcomes, genetically H. hammondi is very similar to T. gondii with approximately 4% of molecular divergence in the first internal transcribed spacers (ITS-1) of rDNA (Ellis et al., 1999). Hammondia hammondi has also been previously referred to as Toxoplasma hammondi (Levine, 1977, 1985). A recent comparative analysis of the transcriptomic profiles of T. gondii and H. hammondi identified genetic profiles that might underlie differences in the in vitro developmental program and life cycle flexibility of these two parasites (Sokol et al., 2018).
Unlike T. gondii, H. hammondi replicates more slowly and is unable to be subcultured indefinitely in vitro in a variety of different host cells. Ultimately H. hammondi parasites spontaneously form bradyzoite-containing cysts that are infectious only to the definitive host (Sheffield et al., 1976; Riahi et al., 1995; Sokol et al., 2018). However, H. hammondi can infect and replicate in new intermediate host cells (in vitro and in vivo) for a limited time before the parasite begins to spontaneously form tissue cysts in vitro. Furthermore, H. hammondi and T. gondii have differential gene expression in vitro following sporozoite-initiated infections. One striking difference in gene expression between T. gondii and H. hammondi was the enrichment of merozoite and bradyzoite-related genes in both early tachyzoite (day 4) and late-tachy-early-bradyzoite life (day 15) in H. hammondi (Sokol et al., 2018). In T. gondii these transcriptional profiles are uniquely expressed in T. gondii bradyzoites and alkaline pH-treated T. gondii for tissue cyst formation induction (Jerome et al., 1998; Lyons et al., 2002; Behnke et al., 2008; Croken et al., 2014a, 2014b; Sokol et al., 2018). Despite being genetically closely related, the differential growth and virulence of H. hammondi in comparison to T. gondii has made T. gondii/H. hammondi a promising comparative model system to understand T. gondii virulence and pathogenesis.
Heterologous expression of orthologs of H. hammondi virulence factors in T. gondii has contributed to the understanding of some of the most important T. gondii virulence factors. When HhROP18/ROP5 are expressed heterologously in T. gondii, the orthologs are functional. Specifically HhROP52–1 ortholog expression in TgROP5 knockout mice caused higher mortality than the expression TgROP5 in the knockout mice (Reese et al., 2011; Walzer et al., 2013). Despite the fact that the HhROP18/ROP5 orthologs are i) expressed in T. gondii, and ii) can complement virulence defects in T. gondii knockouts, the fact that H. hammondi is not as virulent as T. gondii suggests that the differences in gene expression as these parasite species develop in vitro might be one of the contributing factors to the differential virulence observed between T. gondii and H. hammondi (Walzer et al., 2013). It is likely that H. hammondi is pre-programed to develop into a bradyzoite as it appears to have a highly predicable window of infectivity and replicative capacity prior to terminally differentiating into tissue cysts. This type of strict, pre-defined development program resulting in 100% tissue cyst formation could underlie why H. hammondi is unable to be continually grown in cell culture or in intermediate hosts.
While transcriptomic studies on parasites at different life cycle stages have given insights into the biology of the parasites, the dynamic changes in host responses during infections of a virulent (T. gondii) and avirulent (H. hammondi) parasite could also contribute to understanding of the host responses that T. gondii has to overcome and/or manipulate to ensure its intracellular survival. We have recently taken a comparative transcriptomic approach to analyze the global host response to parasite infections using dual RNA-seq to understand the interplay between parasite and host transcript regulatory network in parallel.
It was initially thought that H. hammondi and T. gondii would elicit similar host responses as these parasites are very similar genetically and morphologically, with cross-reactivity in serological tests (Frenkel and Dubey, 1975; Weiland et al., 1979), cross-reactivity of poly- and monoclonal antibodies (Araujo et al., 1984; Riahi et al., 1998; Riahi et al., 1999, 2000; Dumetre and Darde, 2007) and cross-protection against these two parasites in animals (Dubey, 1981; Munday and Dubey, 1988; Reddacliff et al., 1993). However, in the human acute monocytic leukemia THP-1 cell line (THP-1) (Tsuchiya et al., 1980), while both T. gondii (Type I-GT1, Type II-ME49, and Type III-VEG) and H. hammondi (HhEth1 and HhAmer) elicit a potent host response, H. hammondi induces it at a much higher magnitude than T. gondii. During T. gondii and H. hammondi sporozoite-initiated infections, the majority of the host responses are commonly regulated in both T. gondii and H. hammondi infection. As in N. caninum infection, some of the important T. gondii-initiated host responses such as the IFNγ and the TNF-α response(Suzuki et al., 1988; Yap and Sher, 1999) were more highly induced during H. hammondi infection in the monocytes compared with T. gondii infection (Wong et al., 2019).
Strikingly, the study showed that differential regulation of cell cycle-related control responses by T. gondii and H. hammondi might be one of the key players in determining parasite replication in vitro (Wong et al., 2019). Although both T. gondii and H. hammondi arrested host cell cycle progression, T. gondii-infected THP-1 cells were arrested at the G2/M phase while H. hammondi-infected cells were arrested at G1/S and, in some replicates, resembled cells without parasite infection. In addition, differential regulation of Myc and cell cycle pathways target genes, and activation of the forkhead transcription factor (FOXM1) during T. gondii infection also supports the host cell cycle state being at the G2/M phase. TgGRA16 is shown to increase p53 and p21 levels in HFFs (Bougdour et al., 2013), and THP-1 cells infected with T. gondii show decent levels of transcripts for these genes. However, H. hammondi infection induces higher levels of p53 and CDKN1A gene transcripts relative to T.gondii infection (Wong et al., 2019). As E2F/DP1 and p53 are components of the p53-p21-DREAM-E2F pathway, in which activation of p53 increases p21-induced suppression of the transcription of DREAM target genes (reviewed in Engeland, 2018), it is likely that altering transcription of these genes is one of the mechanisms that T. gondii uses to regulate host cell cycle arrest. It is therefore likely that during H. hammondi infection the FOXM1-MMB complex was displaced during p53-mediated increased p21/CDKN1A expression during the DNA damage response and subsequently caused the formation of DREAM complex and p53-mediated G1/S cell cycle arrest (Litovchick et al., 2007; Mannefeld et al., 2009; Quaas et al., 2012; Chen et al., 2013). While it was not identified whether formation of the DREAM complex during parasite infection is i) mediated by a H. hammondi secreted effector that is not functional in T. gondii or ii) a host response mechanism that is counter-balanced by T. gondii effector(s), both hypotheses are plausible as induction of the CDKN1A gene requires direct H. hammondi invasion and/or infection. It is also likely that interaction between E2F1/3/4/6 and DP-1 with the T. gondii effector HCE1/TEEGR (Braun et al., 2019; Panas et al., 2019) might prevent formation of the DREAM complex (Wong et al., 2019).
4.3. An example head-to-head comparison examining species- and cell type-specific responses to infection
To further explore the impact of cell type and parasite species host transcriptional responses to intracellular parasitism, we performed head-to-head comparisons of the host transcriptional responses to N. caninum and T. gondii using two cell types: THP-1 cells and PHT cells cultivated from term placentas; (Ander et al., 2017). Note: T. gondii and N. caninum infection data in PHTs have been published previously (Ander et al., 2017) and are analyzed here in a different context. Our data show a clear difference in the response of each cell type to infection, as THP-1 cells appear to be much more responsive to T. gondii and N. caninum infection compared with PHTs (Fig. 1A). In both of these cell types, the chemokine CCL22 is one of the genes with the highest transcript abundance after T. gondii infection. Furthermore, T. gondii infection results in a much greater number of genes that have increased transcript abundance compared with N. caninum infection (Fig. 1A, left). In contrast, THP-1 cells showed a much more robust response to T. gondii and N. caninum infection, with each parasite species inducing changes in its own unique set of genes (Fig. 1A, right). Gene set enrichment analysis (Fig. 1B) provides clear evidence for cell type-specific responses to infection by T. gondii and N. caninum. For example, the cell cycle-related gene sets (p53 and MYC targets v1 and v2) were enriched in T. gondii-infected THP-1 cells but not PHTs, while N. caninum induced changes in multiple inflammation-related genes sets (Inflammatory response; Complement, and IL6 JakStat3 signaling; Fig 1B) in THP-1 cells but not PHT cells.
Fig. 1.
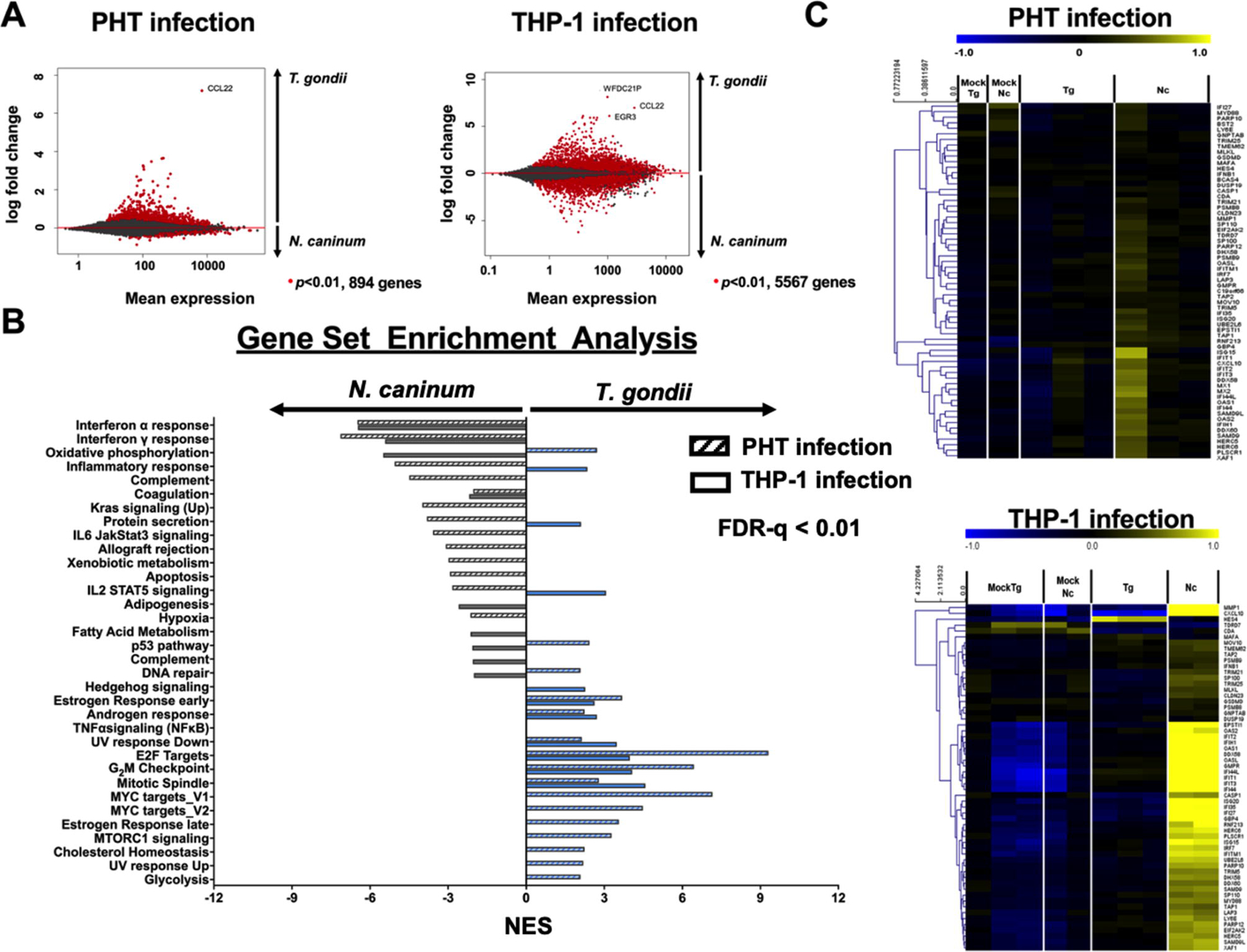
Differential expression analysis of human trophoblast cells (PHTs) against Toxoplasma gondii and Neospora caninum tachyzoite infection. (A) Plots showing mean expression against log fold change of the transcriptomic profile of PHTs during T. gondii and N. caninum infections. Each dot represents a host gene and genes that are significantly different in response to the parasitic infection (in comparison to mock infection) are represented by ● (P<0.01). (B) Gene set enrichment analysis of the PHT (solid bar, either blue or gray) and THP-1 (hatched bar, either blue or gray) transcriptomes in response to T. gondii (blue) and N. caninum (gray) infection. Schown are Hallmark gene sets that are significantly enriched (false discovery rate (FDR-q) < 0.01 (computed with 1000 Monte-Carlo simulations); positive and negative values show up- and down-regulated gene sets, respectively). (C) Heatmaps showing log2-transformed expression of gene clusters during T. gondii and N. caninum infection. Shown are Type I Interferon (IFN) that were specifically induced by N. caninum in human foreskin fibroblasts (HFFs) (Beiting et al., 2014). Genes were mean-centered and hierarchically-clustered.
In both THP-1 and PHTs, T. gondii induced changes in the abundance of more transcripts compared with N. caninum, although this difference was much more pronounced in PHT cells (Fig. 1 A,B). In both cell types, T. gondii induced the CCL22 gene in high abundance. Despite having a small number of genes differentially regulated in PHTs compared with THP-1, gene set enrichment analysis revealed similar enrichment of IFNα (Type 1) responses in PHTs infected with N. caninum compared with T. gondii-infected PHTs (Fig. 1B,C). These data are consistent with prior work in HFFs showing induction of the type I IFN pathway in N. caninum-infected HFFs compared with T. gondii; (Beiting et al., 2014). This also suggests that T. gondii suppression of Type I (and possibly Type II; Fig. 1B) IFN signaling pathways could be a common adaptation strategy that may be evident in multiple cell types. In contrast, the cell cycle-related gene sets E2F targets and G2M checkpoints are more robustly altered by T. gondii compared with N. caninum in both cell types (Fig. 1B), suggesting that manipulation of the host cell cycle may be a species-specific trait in T. gondii. Overall, transcriptome datasets such as these from multiple parasite species and cell types can provide new insights into the evolution of unique traits within one parasite lineage compared with another and identify how cell types respond uniquely to species-specific mechanisms of immune suppression and/or activation.
5. Summary and conclusions
Toxoplasma gondii is an obligate intracellular parasite that infects virtually all mammalian cells (Sibley, 2003). The fact that T. gondii is able to naturally infect multiple cell types in multiple species and its near relatives are less able to do so provides a unique opportunity to study the evolution of both host and cell type specificity in tissue-dwelling coccidia, about which very little is known at the molecular level. Throughout its life within a given host, T. gondii finds itself in contact with a wide variety of cell and tissue types, and must be able to counteract and/or survive unique aspects of host defenses in each of these replication sites. By comparing closely related species with different degrees of adaptation to a given host species and/or tissue, we can better identify the host barriers to infection that exist, and ultimately how compatible hosts have superseded these restrictions.
Highlights.
Toxoplasma gondii has a very wide host range, and a remarkable ability within those hosts to infect a wide variety of cells
Here we review the literature as it pertains to the host response of different cell types to Toxoplasma gondii
We also review how relatives of T. gondii with more restricted host ranges may induce divergent changes in the host cell
Acknowledgements
Work described in this manuscript was supported by the National Institutes of Health (NIH; USA) Grant AI116855 to JPB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adomako-Ankomah Y, Wier GM, Borges AL, Wand HE, Boyle JP, 2014. Differential locus expansion distinguishes Toxoplasmatinae species and closely related strains of Toxoplasma gondii. mBio 5, e01003–01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal ML, Agarwal A, Taylor WR, Stark GR, 1995. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci U S A 92, 8493–8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC, 2005. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. Plos Pathogen 1, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almería S, Serrano-Pérez B, F L-G, 2017. Immune response in bovine neosporosis: protection or contribution to the pathogenesis of abortion. Microb. Pathog 109, 177–182. [DOI] [PubMed] [Google Scholar]
- Ander SE, Rudzki EN, Arora N, Sadovsky Y, Coyne CB, Boyle JP, 2017. Human placental syncytiotrophoblasts restrict Toxoplasma gondii attachment and replication and respond to infection by producing immunomodulatory chemokines. mBio 9, e01678–01617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo FG, Dubey JP, Remington JS, 1984. Antigenic similarity between the coccidian parasites Toxoplasma gondii and Hammondia hammondi. J. Protozool 31, 145–147. [DOI] [PubMed] [Google Scholar]
- Baszler TV, Long MT, McElwain TF, Mathison BA, 1999. Interferon-gamma and interleukin-12 mediate protection to acute Neospora caninum infection in BALB/c mice. Int. J. Parasitol 29, 1635–1646. [DOI] [PubMed] [Google Scholar]
- Behnke MS, Khan A, Lauron EJ, Jimah JR, Wang Q, Tolia NH, Sibley LD, 2015. Rhoptry proteins ROP5 and ROP18 are major murine virulence factors in genetically divergent South American strains of Toxoplasma gondii. PLoS Genet. 11, e1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke MS, Khan A, Wootton JC, Dubey JP, Tang K, Sibley LD, 2011. Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proc. Natl. Acad. Sci. U. S. A 108, 9631–9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke MS, Radke JB, Smith AT, Sullivan WJ, White MW, 2008. The transcription of bradyzoite genes in Toxoplasma gondii is controlled by autonomous promoter elements. Mol. Microbiol 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiting DP, Peixoto L, Akopyants NS, Beverley SM, Wherry EJ, Christian DA, Hunter CA, Brodsky IE, Roos DS, 2014. Differential induction of TLR3-dependent innate immun signaling by closely related parasite species. PLoS One 9, e88398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besteiro S, Michelin A, Poncet J, Dubremetz JF, Lebrun M, 2009. Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. Plos Pathogen 5, e1000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerkas I, Mohn SF, Presthus J, 1984. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z Parasitenkd 70, 271–274. [DOI] [PubMed] [Google Scholar]
- Blader IJ, Manger ID, Boothroyd JC, 2001. Micro-array analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J Biol Chem 276, 24223–24231. [DOI] [PubMed] [Google Scholar]
- Blader IJ, Saeij JP, 2009. Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion and virulence. J Pathol, Microbiol Immunol, doi: 10.1111/j.1600-0463.2009.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne W, Heesemann J, Gross U, 1994. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun 62, 1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne W, Roos DS, 1997. Stage-specific expression of a selectable marker in Toxoplasma gondii permits selective inhibition of either tachyzoites or bradyzoites. Mol Biochem Parasitol 88, 115–126. [DOI] [PubMed] [Google Scholar]
- Bonhomme A, Maine GT, Beorchia A, Burlet H, Aubert D, Villena I, Hunt J, Chovan L, Howard L, Brojanac S, Sheu M, Tyner J, Pluot M, Pinon JM, 1998. Quantitative immunolocalization of a P29 protein (GRA7), a new antigen of Toxoplasma gondii. J. Histochem. Cytochem 46, 1411–1422. [DOI] [PubMed] [Google Scholar]
- Bougdour A, Durandau E, Brenier-Pinchart MP, Ortet P, Barakat M, Kieffer-Jaquinod S, Curt-Varesano A, Curt-Bertini RL, Bastien O, Coute Y, Pelloux H, Hakimi MA, 2013. Host cell subversion by Toxoplasma GRA16, an exported dense granule protein that targets the host cell nucleus and alters gene expression. Cell Host Microbe 13, 489–500. [DOI] [PubMed] [Google Scholar]
- Boyle JP, Saeij JP, Harada SY, Ajioka JW, Boothroyd JC, 2008. Expression quantitative trait locus mapping of Toxoplasma genes reveals multiple mechanisms for strain-specific differences in gene expression. Eukaryot Cell 7, 1403–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L, Brenier-Pinchart MP, Curt-Varesano A, Curt-Bertini RL, Hussain T, Kieffer-Jaquinod S, Coute Y, Pelloux H, Tardieux I, Sharma A, Belrhali H, Bougdour A, Hakimi MA, 2013. A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J. Exp. Med 210, 2071–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L, Brenier-Pinchart MP, Hammoudi P, Cannella D, Kieffer-Jaquinod S, Vollaire J, Josserand V, Touquet B, Coute Y, Tardieux I, Bougdour A, Hakimi M, 2019. The Toxoplasma effector TEEGR promotes parasite persistence by modulating NF-κB signaling via EZH2. Nature Microbiol 4, 1208–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet J, Pfaff AW, Abidi A, Unoki M, Nakamura Y, Guinard M, Klein JP, Candolfi E, Mousli M, 2008. Toxoplasma gondii exploits UHRF1 and induces host cell cycle arrest at G2 to enable its proliferation. Cell. Microbiol 10, 908–920. [DOI] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B, 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282, 1497–1501. [DOI] [PubMed] [Google Scholar]
- Cabral CM, Tuladhar S, Dietrich HK, Nguyen E, MacDonald WR, Trivedi T, Devineni A, Koshy AA, 2016. Neurons are the primary target cell for the brain-tropic intracellular parasite Toxoplasma gondii. Plos Pathogen 12, e1005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers VB, Sibley LD, 1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol 73, 114–123. [PubMed] [Google Scholar]
- Channon JY, Seguin RM, Kasper LH, 2000. Differential infectivity and division of Toxoplasma gondii in human peripheral blood leukocytes. Infect. Immun 68, 4822–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussabel D, Semnani RT, McDowell MA, Sacks D, Sher A, Nutman TB, 2003. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 102, 672–681. [DOI] [PubMed] [Google Scholar]
- Chen X, Muller GA, Quaas M, Fischer M, Han N, Stutchburry B, Sharrocks AD, Engeland K, 2013. The forkhead transcription factor FOXM1 controls cell cycle-dependent gene expression through an atypical chromatin binding mechanism. Mol. Cell. Biol 33, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs RS, Blank ML, English ED, Adomako-Ankomah Y, Urama IS, Martin AT, Yarovinsky F, Boyle JP, 2020. Immediate interferon gamma induction determines murine host compatibility differences between Toxoplasma gondii and Neospora caninum. Infect. Immun 88, e00027–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens I, D.J., Romano JD, Pypaert M, Zhang H, Boothroyd JC, Joiner KA, 2006. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125, 261–274. [DOI] [PubMed] [Google Scholar]
- Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gatel D, Tardieux I, 2006. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107, 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croken MM, Ma Y, Markillie LM, Taylor RC, Orr G, Weiss LM, Kim K, 2014a. Distinct strains of Toxoplasma gondii feature divergent transcriptomes regardless of developmental stage. PLoS One 9, e111297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croken MM, Qiu W, White MW, Kim K, 2014b. Gene Set Enrichment Analysis (GSEA) of Toxoplasma gondii expression datasets links cell cycle progression and the bradyzoite developmental program. BMC Genomics 15, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBarry JD, Kissinger JC, 2011. Jumbled genomes: missing Apicomplexan synteny. Mol. Biol. Evol 28, 2855–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouin F, Pelloux H, Parasitology, E.S.G.o.C., 2008. Prevention of toxoplasmosis in transplant patients. Clin Microbiol Infect 14, 1089–1101. [DOI] [PubMed] [Google Scholar]
- Di Cristina M, Marocco D, Galizi R, Proietti C, Spaccapelo R, Crisanti A, 2008. Temporal and spatial distribution of Toxoplasma gondii differentiation into Bradyzoites and tissue cyst formation in vivo. Infect. Immun 76, 3491–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J, Schares G, Ortega-Mora L, 2007. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev 20, 323–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, 1981. Protective immunity against clinical toxoplasmosis in dairy goats vaccinated with Hammondia hammondi and Hammondia heydorni. Am. J. Vet. Res 42, 2068–2070. [PubMed] [Google Scholar]
- Dubey JP, 2009a. History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol 39, 877–882. [DOI] [PubMed] [Google Scholar]
- Dubey JP, 2009b. Toxoplasmosis of animals and humans. CRC Press. [Google Scholar]
- Dubey JP, Speer CA, Shen SK, Kwok OC, Blixt JA, 1997. Oocyst-induced murine toxoplasmosis: life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii oocysts. J. Parasitol 83, 870–882. [PubMed] [Google Scholar]
- Dubey JP, Sreekumar C, 2003. Redescription of Hammondia hammondi and its differentiation from Toxoplasma gondii. Int. J. Parasitol 33, 1437–1453. [DOI] [PubMed] [Google Scholar]
- Dumetre A, Darde ML, 2007. Detection of Toxoplasma gondii in water by an immunomagnetic separation method targeting the sporocysts. Parasitol. Res 101, 989–996. [DOI] [PubMed] [Google Scholar]
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B, 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825. [DOI] [PubMed] [Google Scholar]
- Ellis JT, Morrison DA, Liddell S, Jenkins MC, Mohammed OB, Ryce C, Dubey JP, 1999. The genus Hammondia is paraphyletic. Parasitology 118, 357–362. [DOI] [PubMed] [Google Scholar]
- Ely KH, Kasper LH, Khan IA, 1999. Augmentation of the CD8+ T cell response by IFN-gamma in IL-12-deficient mice during Toxoplasma gondii infection. J. Immunol 162, 5449–5454. [PubMed] [Google Scholar]
- Engeland K, 2018. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell Death Differ. 25, 114–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English ED, Adomako-Ankomah Y, Boyle JP, 2015. Secreted effectors in Toxoplasma gondii and related species: determinants of host range and pathogenesis? Parasite Immunol. 37, 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentress SJ, Behnke MS, Dunay IR, Mashayekhi M, Rommereim LM, Fox BA, Bzik DJ, Taylor GA, Turk BE, Lichti CF, Townsend RR, Qiu W, Hui R, Beatty WL, Sibley LD, 2010. Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe 8, 484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DJ, Hutchison WM, 1987. The host-parasite relationship of Toxoplasma gondii in the brains of chronically infected mice. Virchows. Arch. A Pathol. Anat. Histopathol 411, 39–43. [DOI] [PubMed] [Google Scholar]
- Ferreira-da-Silva Mda F, Barbosa HS, Gross U, Lüder CG, 2008. Stress-related and spontaneous stage differentiation of Toxoplasma gondii. Mol. Biosyst 4, 824–834. [DOI] [PubMed] [Google Scholar]
- Fisher HG, Nitzgen B, Reichmann G, Gross U, Hadding U, 1997. Host cells of Toxoplasma gondii encystation in infected primary culture from mouse brain. Parasitol. Res 83, 637–641. [DOI] [PubMed] [Google Scholar]
- Fox BA, Gigley JP, Bzik DJ, 2004. Toxoplasma gondii lacks the enzymes required for de novo arginine biosynthesis and arginine starvation triggers cyst formation. Int J Parasitol 34, 323–331. [DOI] [PubMed] [Google Scholar]
- Franco M, Panas MW, Marino ND, Lee MC, Buchholz KR, Kelly FD, Bednarski JJ, Sleckman BP, Pourmand N, Boothroyd JC, 2016. A novel secreted protein, MYR1, is central to Toxoplasma’s manipulation of host cells. mBio 7, e02231–022315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel JK, Dubey JP, 1975. Hammondia hammondi: A new coccidium of cats producing cysts in muscle of other mammals. Science 189, 222–224. [DOI] [PubMed] [Google Scholar]
- Gail M, Gross U, Bohne W, 2001. Transcriptional profile of Toxoplasma gondii-infected human fibroblasts as revealed by gene-array hybridization. Mol. Genet. Genomics 265, 905–912. [DOI] [PubMed] [Google Scholar]
- Garfoot AL, Cervantes PW, Knoll LJ, 2019. Transcriptional analysis shows a robust host responses to Toxoplasma gondii during early and late chronic infection in both male and female mice. Infect. Immun, doi: 10.1128/IAI.00024-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay G, Braun L, Brenier-Pinchart MP, Vollaire J, Josserand V, Bertini R, Varesano A, Touquet B, De Bock P, Coute Y, Tardieux I, Bougdour A, Hakimi M, 2016. Toxoplasma gondii TgIST co-opts host chromatin repressors dampening STAT1-dependent gene regulation and IFN-γ–mediated host defenses. J. Exp. Med 213, 1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli RT, Xu Y, Hieny S, Cheever A, Sher A, 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol 149, 175–180. [PubMed] [Google Scholar]
- Gov L, Larimzadeh A, Ueno N, Lodoen MB, 2013. Human innate immunity to Toxoplasma gondii is mediated by host caspase-1 and ASC and parasite GRA15. mBio 4, e002505–002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin A, El Hajj H, Penarete-Vargas D, Besteiro S, Lebrun M, 2017. RON4L1 is a new member of the moving junction complex in Toxoplasma gondii. Sci. Rep 7, 17907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen SK, Lyman WD, Chiu FC, 1996. Growth and development of Toxoplasma gondii in human neurons and astrocytes. J Neuropathol 55, 1150–1156. [DOI] [PubMed] [Google Scholar]
- Halonen SK, Weiss LM, 2013. Toxoplasmosis. Handb. Clin. Neurol 114, 125–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Brenier-Pinchart MP, Braun L, Kraut A, Touquet B, Coute Y, Tardieux I, Hakimi M, Bougdour A, 2018. Characterization of a Toxoplasma effector uncovers an alternative GSK3/β-catenin-regulatory pathway of inflammation. eLife 7, e39887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez FL, Nickdel MB, McLeod R, Lyons RE, Lyons K, Dubremetz JF, Grigg ME, Samuel BU, Roberts CW, 2005. Toxoplasma gondii dense granule protein 3 (GRA3) is a type I transmembrane protein that possesses a cytoplasmic dilysine (KKXX) endoplasmic reticulum (ER) retrieval motif. Parasitology 131, 169–179. [DOI] [PubMed] [Google Scholar]
- Hermanns T, Müller UB, Konen-Waisman S, Howard JC, Steinfeldt T, 2016. The Toxoplasma gondii rhotry protein ROP18 is an Irga6-specific kinase and regulated by the dense granule protein GRA7. Cell Microbiology 18, 244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MJ, Shah P, Wek RC, Sullivan WJ, 2019. Simultaneous ribosome profiling of human host cells infected with Toxoplasma gondii. mSphere 4, e00292–00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison WM, 1965. Experimental transmission of Toxoplasma gondii. Nature 206, 961–962. [DOI] [PubMed] [Google Scholar]
- Jerome ME, Radke JR, Bohne W, Roos DS, White MW, 1998. Toxoplasma gondii bradyzoites form spontaneously during sporozoite initiated development. Infect. Immun 66, 4838–4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaminets A, Hunn JP, Könen-Waisman S, Zhao YO, Preukschat D, Coers J, Boyle JP, Ong YC, Boothroyd JC, Reichmann G, Howard JC, 2010. Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole. Cell Microbiology 12, 939–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IA, Matsuura T, Kasper LH, 1994. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect. Immun 62, 1639–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Fouts AE, Boothroyd JC, 2007. Toxoplasma gondii dysregulates IFN-gamma-inducible gene expression in human fibroblasts: insights from a genome-wide transcriptional profiling. J. Immunol 178, 5154–5165. [DOI] [PubMed] [Google Scholar]
- Labruyere E, Lingnau M, Mercier C, Sibley LD, 1999. Differential membrane targeting of the secretory proteins GRA4 and GRA6 within the parasitophorous vacuole formed by Toxoplasma gondii. Mol. Biochem. Parasitol 102, 311–324. [DOI] [PubMed] [Google Scholar]
- Lamarque M, Besteiro S, Papoin D, Roques M, Vulliez-Le Normand B, Morlon-Guyot J, Dubremetz JF, Fauquenoy S, Tomavo S, Faber BW, Kocken CH, Thomas AW, Boulanger MJ, Bentley GA, Lebrun M, 2011. The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. Plos Pathogen 7, e1001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Hildebrandt A, Brand F, Opitz L, Dihazi H, Luder CGK, 2012. Impaired chromatin remodelling at STAT1-regulated promoters leads to global unresponsiveness of Toxoplasma gondii-infected macrophages to IFN-γ. PLoS Pathogen 8, e1002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine MD, Arrizabalaga G, 2008. Induction of mitotic S-phase of host and neighboring cells by Toxoplasma gondii enhances parasite invasion. Mol. Biochem. Parasitol 164, 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun M, Michelin A, El Hajj H, Poncet J, Bradley PJ, Vial H, Dubremetz JF, 2005. The rhoptry neck protein RON4 re-localizes at the moving junction during Toxoplasma gondii invasion. Cell Microbiol 7, 1823–1833. [DOI] [PubMed] [Google Scholar]
- Lehmann T, Marcet PL, Graham DH, Dahl ER, Dubey JP, 2006. Globalization and the population structure of Toxoplasma gondii. Proc. Natl. Acad. Sci. U. S. A 103, 11423–11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ND, 1977. Tazonomy of Toxoplasma. J. Protozool 24, 36–41. [DOI] [PubMed] [Google Scholar]
- Levine ND, 1985. Veterinary protozoology, 1st ed. Iowa State University Press, Ames. [Google Scholar]
- Li J, He J, Elsheikha HM, Chen D, Zhai B, Zhu X, Yan H, 2019. Toxoplasma gondii ROP17 inhibits the innate immune response of HEK293T cells to promote its survival. Parasitol. Res 118, 783–792. [DOI] [PubMed] [Google Scholar]
- Lima TS, Gov L, Lodoen MB, 2018. Evasion of human neutrophil-mediated host defense during Toxoplasma gondii infection. mBio 9, e02027–02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovchick L, Sadasivam S, Florens L, Zhu X, Swanson SK, Velmurugan S, Chen R, Washburn MP, Liu XS, DeCaprio JA, 2007. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell 26, 539–551. [DOI] [PubMed] [Google Scholar]
- Long MT, Baszler TV, 2000. Neutralization of maternal IL-4 modulates confenital protozoal transmission: comparison of innate versus acquired immune responses. J. Immunol 164, 4768–4774. [DOI] [PubMed] [Google Scholar]
- Lorenzi H, Khan A, Behnke MS, Namasivayam S, Swapna LS, Hadjithomas M, Karamycheva S, Pinney D, Brunk BP, Ajioka JW, Ajzenberg D, Boothroyd JC, Boyle JP, Dardé ML, Diaz-Miranda MA, Dubey JP, Fritz HM, Gennari SM, Gregory BD, Kim K, Saeij JP, Su C, White MW, Zhu X, Howe DK, Rosenthal BM, Grigg ME, Parkinson J, Liu L, Kissinger JC, Roos DS, Sibley LD, 2016. Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nature Communications 7, 10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Zhou J, Zhao YH, Li QL, Gao YY, Wang L, 2019. Transcriptome sequencing investigated the tumor-related factors changes after T. gondii infection. Front. Microbiol 10, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüder CG, Giraldo-Velasquez M, Sendtner M, Gross U, 1999. Toxoplasma gondii in primary rat CNS cells: differential contribution of neurons, astrocytes, and microglial cells for the intracerebral development and stage differentiation. Exp. Parasitol 93, 23–32. [DOI] [PubMed] [Google Scholar]
- Lüder CG, Lang C, Giraldo-Velasquez M, Algner M, Gerdes J, Gross U, 2003. Toxoplasma gondii inhibits MHC class II expression in neural antigen-presenting cells by down-regulating the class II transactivator CIITA. J. Neuroimmunol 134, 12–24. [DOI] [PubMed] [Google Scholar]
- Luft BJ, Brooks RG, Conley FK, McCabe RE, Remington JS, 1984. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA 252, 913–917. [PubMed] [Google Scholar]
- Lyons RE, McLeod R, Roberts CW, 2002. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol 18. [DOI] [PubMed] [Google Scholar]
- Ma JS, Sasai M, Ohshima J, Lee Y, Bando H, Takeda K, Yamamoto M, 2014. Selective and strain-specific NFAT4 activation by the Toxoplasma gondii polymorphic dense granule protein GRA6. J Exp Med 211, 2013–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannefeld M, Klassen E, Gaubatz S, 2009. B-MYB is required for recovery from the DNA damage-induced G2 checkpoint in p53 mutant cells. Cancer Res. 69, 4073–4080. [DOI] [PubMed] [Google Scholar]
- Marino N, Panas MW, Franco M, Theisen T, Naor A, Rastogi S, Buchholz KR, Lorenzi H, Boothroyd JC, 2018. Identification of a novel protein complex essential for effector translocation across the parasitophorous vacuole membrane of Toxoplasma gondii. Plos Pathogen, 1006828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SK, Olias P, Huang Z, Wang Q, Park E, Yokoyama WM, Sibley LD, 2019. Toxoplasma gondii effector TgIST blocks type I interferon signaling to promote infection. Proc. Natl. Acad. Sci. U. S. A 116, 17480–17491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molestina RE, El-Guendy N, Sinai AP, 2008. Infection with Toxoplasma gondii results in dysregulation of the host cell cycle. Cell. Microbiol 10, 1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday BL, Dubey JP, 1988. Prevention of Toxoplasma gondii abortion in goats by vaccination with oocysts of Hammondia hammondi. Aust. Vet. J 65, 150–153. [DOI] [PubMed] [Google Scholar]
- Naor A, Panas MW, Marino N, Coffey MJ, Tonkin CJ, Boothroyd JC, 2018. MYR1-dependent effectors are the major drivers of a host cell’s early response to Toxoplasma, including counteracting MYR1-independent effects. mBio 9, e02401–02417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BA, Chiappino ML, O’Connor GR, 1983. Secretion from the rhoptries of Toxoplasma gondii during host-cell invasion. J Ultrastruct Res 83, 85–98. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Inoue N, Makala L, Nagasawa H, 2003. A role for balance of interferon-gamma and interleukin-4 production in protective immunity against Neospora caninum infection. Vet. Parasitol 116, 175–184. [DOI] [PubMed] [Google Scholar]
- Olias P, Etheridge RD, Zhang Y, Holtzman MJ, Sibley LD, 2016. Toxoplasma effector recruits the Mi-2/NuRD complex to repress STAT1 transcription and block IFN-γ-dependent gene expression. Cell Host Microbe 20, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas MW, Naor A, Cygan AM, Boothroyd JC, 2019. Toxoplasma controls host cyclin E expression through the use of a novel MYR-1 dependent effector protein, HCE1. mBio, doi: 10.1128/mBio.00674-00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SJ, Roberts CW, Alexander J, 1991. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin. Exp. Immunol 84, 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson CM, Lambert H, Vutova PP, Dellacasa-Lindberg I, Nederby J, Yagita H, Ljunggren H, Grandien A, Barragan A, Chambers BJ, 2009. Transmission of Toxoplasma gondii from infected dendritic cells to natural killer cells. Infect. Immun 77, 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifer R, Yarovinsky F, 2011. Innate responses to Toxoplasma gondii in mice and humans. Trends iParasitol 27, 388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaas M, Muller GA, Engeland K, 2012. p53 can repress transcription of cell cycle genes through a p21 (WAF1/CIP1)-dependent switch from MMB to DREAM protein complex binding at CHR promoter elements. Cell Cycle 11, 4661–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddacliff GL, Parker SJ, Dubey JP, Nicholls PJ, Johnson AM, Cooper DW, 1993. An attempt to prevent acute toxoplasmosis in macropods by vaccination with Hammondia hammondi. Aust. Vet. J 70, 33–35. [DOI] [PubMed] [Google Scholar]
- Reese ML, Boothroyd JC, 2011. A conserved non-canonical motif in the pseudoactive site of the ROP5 pseudokinase domain mediates its effect on Toxoplasma virulence. J. Biol. Chem 286, 29366–29375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP, 2011. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc. Natl. Acad. Sci. U. S. A 23, 9625–9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AJ, Vermont SJ, Cotton JA, Harris D, Hill-Cawthorne GA, Konen-Waisman S, Latham SM, Mourier T, Norton R, Quail MA, Sanders M, Shanmugam D, Sohal A, Wasmuth JD, Brunk B, Grigg ME, Howard JC, Parkinson J, Roos D, Trees AJ, Berriman M, Pain A, Wastling JM, 2012. Comparative genomics of the Apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy. Plos Pathogen 8, e1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington JS, Cavanaugh EN, 1965. Isolation of the encysted form of Toxoplasma gondii from human skeletal muscle and brain. New England Jf Med 273, 1308–1310. [DOI] [PubMed] [Google Scholar]
- Riahi H, Bouteille B, Darde ML, 1998. Antigenic similarity between Hammondia hammondi and Toxoplasma gondii tachyzoites. J. Parasitol 84, 651–653. [PubMed] [Google Scholar]
- Riahi H, Dardé ML, Bouteille B, Leboutet MJ, Prestre-Alexandre M, 1995. Hammondia hammondi cysts in cell cultures. J Parasitol 81, 821–824. [PubMed] [Google Scholar]
- Riahi H, Leboutet M, Labrousse F, Bouteille B, Darde ML, 2000. Monoclonal antibodies to Hammondia hammondi allowing immunological differentiation from Toxoplasma gondii. J. Parasitol 86, 1362–1366. [DOI] [PubMed] [Google Scholar]
- Riahi H, Leboutet MJ, Bouteille B, Dubremetz JF, Darde ML, 1999. Hammondia hammondi organelle proteins are recognized by monoclonal antibodies directed against organelles of Toxoplasma gondii. J. Parasitol 85, 580–583. [PubMed] [Google Scholar]
- Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD, Saeij JP, 2011. Strain-specific activation of NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J. Exp. Med 208, 195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski EE, Nguyen QP, Camejo A, Spooner E, Saeij JP, 2014. Toxoplasma gondii inhibits gamma interferon (IFNγ)- and IFN-β-induced host cell STAT1 transcriptional activity by increasing association of STAT1 with DNA. Infect. Immun 82, 706–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski EE, Saeij JP, 2012. Toxoplasma gondii clonal strains all exhibit STAT1 transcriptional activity but differentially modulate IFN gamma induced gene expression and STAT1 phosphorylation. PLoS One 7, e51448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivam S, Duan S, DeCaprio JA, 2012. The MuvB complex sequentially recruits B-Myb and FoxM1 to promote mitotic gene expression. Genes Dev 26, 474–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij JP, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, Ajioka JW, Boothroyd JC, 2006. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 15, 1780–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij JPJ, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC, 2007. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445, 324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharton-Kersten TM, Yap G, Magram J, Sher A, 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med 185, 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider AG, Abi Abdallah DS, Butcher BA, Denkers EY, 2013. Toxoplama gondii triggers phosphorylation and nuclear translocation of dendritic cell STAT1 while simultaneously blocking IFNγ-induced STAT1 transcriptional activity. PLoS One 8, e60215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seizova S, Garnham AL, Coffey MJ, Whitehead LW, Rogers KL, Tonkin CJ, 2019. Toxoplasma gondii bradyzoites induce transcriptional changes to host cells and prevent IFNγ-mediated cell death. bioRxiv, doi: 10.1101/669689. [DOI] [PubMed] [Google Scholar]
- Sheffield HG, Melton ML, Neva FA, 1976. Development of Hammondia hammondi in cell cultures. Helminthol. Soc. Washington 41, 218–225. [Google Scholar]
- Sibley LD, 2003. Toxoplasma gondii: perfecting an intracellular life style. Traffic 4, 581–586. [DOI] [PubMed] [Google Scholar]
- Sokol SL, Primack AS, Nair SC, Wong ZS, Tembo M, Verma SK, Cerqueira-Cezar C, Dubey JP, Boyle JP, 2018. Dissection of the in vitro developmental program of Hammondia hammondi reveals a link between stress sensitivity and life cycle flexibility in Toxoplasma gondii. eLife 7, e36491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Orellana MA, Schreiber RD, Remington JS, 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240, 516–518. [DOI] [PubMed] [Google Scholar]
- Swierzy IJ, Handel U, Kaever A, Jarek M, Scharfe M, Schluter D, Luder GK, 2017. Divergent co-transcriptomes of different host cells infected with Toxoplasma gondii reveal cell type-specific host-parasite interactions. Sci. Rep 7, 7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, Beatty WL, Hajj HE, Jerome M, Behnke MS, White M, Wootton JC, Sibley LD, 2006. A secreted serine-threnine kinase determines virulance in the eukaryotic pathogen Toxoplasma gondii. Science 314, 1776–1780. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K, 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26, 171–176. [DOI] [PubMed] [Google Scholar]
- Tyler JS, Boothroyd JC, 2011. The C-terminus of Toxoplasma RON2 provides the crucial link between AMA1 and the host-associated invasion complex. Plos Pathogen 7, e1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ME, Hjort EE, Smith SS, Tripathi A, Hornick JE, Hinchcliffe EH, Archer W, Hager KM, 2008. Toxoplasma gondii actively remodels the microtubule network in host cells. Microbes Infect 10, 1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer KA, Adomako-Ankomah Y, Dam RA, Herrmann DC, Schares G, Dubey JP, Boyle JP, 2013. Hammondia hammondi, an avirulent relative of Toxoplasma gondii, has functional orthologs of known T. gondii virulence genes. Proc. Natl. Acad. Sci. U. S. A 110, 7446–7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland G, Rommel M, von Seyerl F, 1979. Serological cross-reactions between Toxoplasma and Hammondia. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene, Series A. 244, 391–393. [PubMed] [Google Scholar]
- Wong ZS, Sokol SL, Dubey JP, Boyle JP, 2020. Dramatic differences in the host response to Toxoplasma gondii and its near relative Hammondia hammondi reveal new means of immune suppression and effectors with species-specific functions. PLoS Pathog. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap GS, Sher A, 1999. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J. Exp. Med 189, 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Murray PJ, Heeg K, Dalpke AH, 2006. Induction of suppressor of cytokin signaling-1 by Toxoplasma gondii contributes to immune evasion in macrophages by blocking IFN-γ signaling. J. Immunol 176, 1840. [DOI] [PubMed] [Google Scholar]


