Abstract
BACKGROUND:
“Textbook outcome” (TO) is a novel composite quality measure that encompasses multiple postoperative endpoints, representing the ideal “textbook” hospitalization for complex surgical procedures. We defined TO for kidney transplantation using a cohort from a high-volume institution.
METHODS:
Adult patients who underwent isolated kidney transplantation at our institution between 2016 and 2019 were included. TO was defined by clinician consensus at our institution to include freedom from intraoperative complication, postoperative reintervention, 30-day intensive care unit or hospital readmission, length of stay >75th percentile of kidney transplant patients, 90-day mortality, 30-day acute rejection, delayed graft function, and discharge with a Foley catheter. Recipient, operative, financial characteristics, and post-transplant patient, graft, and rejection-free survival were compared between patients who achieved and failed to achieve TO.
RESULTS:
A total of 557 kidney transplant patients were included. Of those, 245 (44%) achieved TO. The most common reasons for TO failure were delayed graft function (N=157, 50%) and hospital readmission within 30 days (N=155, 50%); the least common was mortality within 90 days (N=6, 2%). Patient, graft, and rejection-free survival were significantly improved among patients who achieved TO. On average, patients who achieved TO incurred approximately $50,000 less in total inpatient charges compared to those who failed TO.
CONCLUSIONS:
TO in kidney transplantation was associated with favorable post-transplant outcomes and significant cost-savings. TO may offer transplant centers a detailed performance breakdown to identify aspects of perioperative care in need of process improvement.
Keywords: kidney transplantation, textbook outcome, delayed graft function, patient survival, graft survival
INTRODUCTION
Kidney transplantation (KTx) is the preferred treatment for patients with end-stage renal disease and the most commonly performed solid organ transplant in the US [1]. It has become a routine procedure with improvements in patient and graft survival over time and across institutions [1–3]. While patient and graft survival are monitored and reported, there is no national system that oversees perioperative quality [4]. The Transplant National Surgical Quality Improvement Project (Transplant-NSQIP) database may eventually fill this gap [5]; however, appropriate metrics to capture perioperative quality of care remain uncertain [6].
Presently, transplant center performance is evaluated primarily using one-year patient and graft survival [7,8]. Composite measures may be more useful as they capture multiple domains of overall surgical and hospital performance [9–11]. “Textbook” outcome (TO) is a novel way to define composite measurements that reflect these domains [12–15], as it includes multiple postoperative endpoints that may represent the ideal “textbook” hospitalization. This definition generally includes important markers of perioperative quality such as perioperative morbidity, mortality, early readmissions, and procedure-specific variables, such as margin status and lymph node retrieval for cancer operations.
TO may improve understanding of perioperative quality of care for patients and transplant programs and serve as a standardized metric to aid comparison and guide quality improvement across centers. TOs have been developed for several complex procedures, especially in surgical oncology [13,16–20]. Recently, we defined TO for liver transplantation [21]. We herein defined TO for KTx using a cohort from a high-volume institution and evaluated its ability to predict clinically and financially-relevant outcomes.
METHODS
Data sources and study population
We conducted a retrospective cohort analysis using institutional and United Network for Organ Sharing data. Adult (age≥18) patients who underwent isolated KTx at Duke University Hospital between 2016 and 2019 were included. This study was approved by our Institutional Review Board (Pro00103325).
Definition and impact of textbook outcome
TO was defined by clinician consensus at our institution to include freedom from intraoperative complication, 30-day reintervention (surgical, endoscopic, radiologic), 30-day intensive care unit or hospital readmission, length of stay (LOS) >75th percentile of KTx patients, 90-day mortality, 30-day biopsy-proven acute rejection, delayed graft function (DGF), and discharge with a Foley catheter. Freedom from all listed complications constituted a TO.
Patient, graft, and rejection-free survival were compared between recipients who achieved and failed TO. As a sensitivity analysis, patient, graft, and rejection-free survival were compared among recipients with and without DGF as DGF has been associated with reduced patient and rejection-free survival [22].
Financial impact of textbook outcome
Financial data from the Duke Transplant Center was obtained for patients in the study cohort. Patient-level charge data was determined as the sum of charges billed from the date of transplant to 30 days post-discharge. Transplant-related charges were those billed to the transplant center during the same period.
Statistical analysis
Recipient, operative, and financial characteristics were compared between TO and non-TO groups using Wilcoxon rank-sum tests for continuous variables and Chi-squared and Fisher exact tests for categorical variables. Patient, graft, and rejection-free survival were estimated using the Kaplan-Meier method and compared between groups using log-rank tests.
Operative characteristics and outcomes of TO and non-TO groups were compared in aggregate and separately for living donor (LDKT) and deceased donor KTx (DDKT) recipients. The association between TO and donor type was explored using analysis of variance (ANOVA) comparing rates of TO among living, donation after brain death (DBD), and donation after circulatory death (DCD) donor kidney recipients; post-hoc analyses were conducted using pairwise student t-tests for independent samples.
Logistic regression was used to investigate associations between recipient and operative characteristics and TO. Training and validation datasets were developed by randomly sampling 2/3 and 1/3 of the dataset without replacement (Table S1). Model selection was performed on the training dataset using backward stepwise regression with an AIC criterion. The final model was used to develop a nomogram to predict the probability of TO [23]. Model discrimination was assessed using the c-statistic with 95% confidence intervals (CI) determined based on 1000 bootstrapped resamples. A two-sided p-value less than 0.05 was considered statistically significant. All analyses were performed using R version 3.6.1 (Vienna, Austria).
RESULTS
Patient and operative characteristics
Of 557 KTx recipients, 245 (44%) achieved TO. Patients who achieved TO were more likely to be female and less likely to be Black. Additional patient characteristics were similar between groups (Table 1).
Table 1.
Recipient characteristics stratified by achievement of a textbook outcome (TO).
| Characteristica | Achieved TO N = 245 (44%) |
Failed TO N = 312 (56%) |
P-value |
|---|---|---|---|
| Age (years) | 52 (43–62) | 54 (44–62) | 0.4 |
| Sex | <0.01 | ||
| Female | 123 (50%) | 116 (37%) | |
| Male | 122 (50%) | 196 (63%) | |
| Race | 0.013 | ||
| White | 124 (51%) | 116 (37%) | |
| Black | 107 (44%) | 173 (55%) | |
| Asian | 4 (2%) | 4 (1%) | |
| Other | 10 (4%) | 19 (6%) | |
| Ethnicity (Hispanic) | 6 (2%) | 12 (4%) | 0.4 |
| Body mass index (kg/m2) | 28.9 (24.8–33.0) | 28.6 (24.6–33.2) | 0.8 |
| Panel reactive antibody at transplant (%)b | |||
| Class I | 0 (0–13) | 0 (0–12) | 0.8 |
| Missing | 0 (0%) | 0 (0%) | |
| Class II | 0 (0–0) | 0 (0–0) | 0.8 |
| Missing | 0 (0%) | 1 (0.3%) | |
| Etiology of kidney disease | 0.6 | ||
| Alport syndrome | 4 (2%) | 4 (1%) | |
| Calcineurin inhibitor nephrotoxicity | 11 (5%) | 14 (5%) | |
| Chronic glomerulonephritis | 11 (5%) | 11 (4%) | |
| Chronic nephrosclerosis | 1 (0.4%) | 1 (0.3%) | |
| Congenital obstructive uropathy | 8 (3%) | 6 (2%) | |
| Focal glomerular sclerosis | 28 (11%) | 25 (8%) | |
| HIV nephropathy | 2 (0.8%) | 2 (0.6%) | |
| Hypertensive nephrosclerosis | 45 (18%) | 54 (17%) | |
| IgA nephropathy | 18 (7%) | 15 (5%) | |
| Lithium toxicity | 3 (1%) | 0 (0%) | |
| Malignant hypertension | 3 (1%) | 5 (2%) | |
| Nephrolithiasis | 3 (1%) | 0 (0%) | |
| Polycystic kidney disease | 21 (9%) | 32 (10%) | |
| Renal cell carcinoma | 1 (0.4%) | 1 (0.3%) | |
| Sickle cell anemia | 2 (0.8%) | 2 (0.6%) | |
| Systemic lupus erythematosus | 9 (4%) | 12 (4%) | |
| Type 1 diabetes mellitus | 7 (3%) | 13 (4%) | |
| Type 2 diabetes mellitus | 48 (20%) | 82 (26%) | |
| Vasculitis | 1 (0.4%) | 3 (1%) | |
| Other | 19 (8%) | 29 (9%) | |
| History of prior transplant | 37 (15%) | 46 (15%) | 0.9 |
| Kidney | 29 (12%) | 38 (12%) | 0.9 |
| Liver | 3 (1%) | 4 (1%) | >0.9 |
| Lung | 2 (0.8%) | 3 (1%) | >0.9 |
| Heart | 4 (2%) | 3 (1%) | 0.7 |
| History of diabetes | 80 (33%) | 124 (40%) | 0.08 |
| Estimated Post-Transplant Survival Score (%) | 36 (16–58) | 46 (24–72) | <0.01 |
| Pre-transplant dialysis | 178 (73%) | 273 (88%) | <0.01 |
| Dialysis duration (years) | 2.54 (1.27–4.20) | 3.83 (1.99–5.75) | <0.01 |
| Induction immunosuppression | <0.01 | ||
| Basiliximab | 38 (16%) | 72 (23%) | |
| Anti-thymocyte globulin | 89 (36%) | 114 (37%) | |
| Campath | 28 (11%) | 17 (5%) | |
| High-dose corticosteroids | 67 (27%) | 91 (29%) | |
| Other | 12 (5%) | 13 (4%) | |
| None | 11 (5%) | 4 (1%) | |
| Missing | 0 (0%) | 1 (0.3%) | |
| Initial maintenance immunosuppression | |||
| Tacrolimus | 216 (88%) | 284 (91%) | 0.3 |
| Cyclosporine | 1 (0.4%) | 3 (1%) | 0.6 |
| Mycophenolate mofetil | 213 (87%) | 286 (92%) | 0.07 |
| Azathioprine | 2 (0.8%) | 1 (0.3%) | 0.6 |
| Sirolimus | 27 (11%) | 20 (6%) | 0.052 |
| Belatacept | 30 (12%) | 22 (7%) | 0.04 |
| Corticosteroids | 217 (89%) | 289 (93%) | 0.1 |
| Other | 1 (0.4%) | 2 (0.6%) | >0.9 |
Presented as median (interquartile range) for continuous variables and frequency (proportion) for categorical variables.
Most recent pre-transplant panel reactive antibody levels.
Failure to achieve TO was associated with longer ischemic times, greater intraoperative blood loss, use of machine perfusion, receipt of a DCD donor kidney, higher Kidney Donor Profile Index (KDPI), increased human leukocyte antigen (HLA) mismatches, and intraoperative ureteral stent and drain placement. Patients who failed TO were less likely to have received living donor kidneys (Table 2).
Table 2.
Operative characteristics among all kidney transplant recipients, stratified by achievement of a textbook outcome (TO).
| Characteristica | Achieved TO N = 245 (44%) |
Failed TO N = 312 (56%) |
P-value |
|---|---|---|---|
| Cold ischemic time (minutes) | |||
| All kidneys | 868 (109–1320) | 1032 (531–1460) | <0.01 |
| Non-pumped kidneys only | 107 (66–272) | 133 (81–692) | 0.03 |
| Warm ischemic time (minutes) | 28 (23–32) | 29 (24–33) | 0.03 |
| Missing | 0 (0%) | 1 (0.3%) | |
| Total ischemic time (minutes) | 915 (136–1348) | 1058 (540–1492) | <0.01 |
| Missing | 0 (0%) | 1 (0.3%) | |
| Estimated blood loss (mL) | 100 (100–200) | 150 (100–200) | <0.01 |
| Missing | 1 (0.4%) | 3 (1.0%) | |
| Transfusion requirement (units) | |||
| Packed red blood cells | 0 (0–0) | 0 (0–0) | 0.2 |
| Missing | 0 (0%) | 1 (0.3%) | |
| Fresh frozen plasma | 0 (0–0) | 0 (0–0) | 0.2 |
| Missing | 0 (0%) | 0 (0%) | |
| Machine perfusion used | 127 (52%) | 199 (64) | <0.01 |
| Donor type | |||
| Living donor | 89 (36%) | 74 (24%) | <0.01 |
| Donation after brain death donor | 121 (50%) | 166 (53%) | 0.4 |
| Donation after circulatory death donor | 35 (14%) | 72 (23%) | <0.01 |
| US Public Health Service increased risk for disease transmission donorb | 63 (26%) | 70 (22%) | 0.02 |
| Extended criteria donorb | 19 (8%) | 36 (12%) | 0.4 |
| Donor serologies | |||
| Epstein-Barr virus positive | 235 (96%) | 297 (95%) | 0.9 |
| Missing | 0 (0%) | 2 (0.6%) | |
| Cytomegalovirus positive | 141 (58%) | 185 (59%) | 0.7 |
| Kidney Donor Profile Index (%)b | 48 (26–66) | 56 (34–71) | 0.048 |
| Human leukocyte antigen mismatch | 4 (3–5) | 4 (3–5) | <0.01 |
| Missing | 1 (0.4%) | 0 (0%) | |
| Graft laterality | 0.056 | ||
| Right | 83 (34%) | 134 (43%) | |
| Left | 160 (65%) | 173 (55%) | |
| Dual | 2 (0.8%) | 5 (2%) | |
| Ureteral stent used | 147 (60%) | 224 (72%) | <0.01 |
| Drain placed in the operating room | 46 (19%) | 84 (27%) | 0.02 |
Presented as median (interquartile range) for continuous variables and frequency (proportion) for categorical variables.
Recorded for deceased donors only.
Operative characteristics stratified by TO among LDKT and DDKT recipients are shown in Tables S2 and S3, respectively. Rates of TO were different among living, DBD, and DCD donor kidney recipients (ANOVA p<0.01). Recipients of living donor kidneys were more likely to achieve TO than recipients of DBD and DCD donor kidneys. Rates of TO were similar among DBD and DCD donor kidney recipients (Table S4).
Prevalence of events determining failure to achieve textbook outcome
Among patients who failed TO (N=312), the most common reasons for TO failure were DGF (50%) and hospital readmission (50%); the least common was mortality (2%) (Table 3; Figure 1).
Table 3.
Prevalence of events leading to textbook outcome (TO) failure among patients who failed TOa
| Characteristic (N = 312) | N (%) |
|---|---|
| Intraoperative Complicationb | 10 (3%) |
| Reintervention | 64 (21%) |
| Reoperation | 45 (14%) |
| Radiologic Intervention | 23 (7%) |
| Intensive Care Unit Readmission Within 30 Days | 32 (10%) |
| Hospital Readmission Within 30 Days | 155 (50%) |
| Post-Transplant Length of Stay >75th Percentile | 136 (44%) |
| Mortality Within 90 Days | 6 (2%) |
| Delayed Graft Function | 157 (50%) |
| Discharged With Foley Catheter | 25 (8%) |
| Acute Rejection Within 30 Days | 30 (10%) |
Failure to achieve TO was defined by the occurrence of any of the above events, however each patient who failed TO could have had multiple events therefore may be counted more than once in this table.
Intraoperative complications included renal artery thrombosis, renal artery spasm with resultant kidney allograft ischemia, severe hyperkalemia (K>8), and cardiac arrhythmia (supraventricular tachycardia: one case; asystole: one case).
Figure 1. Individual feature prevalence and cumulative achievement of textbook outcome (TO).
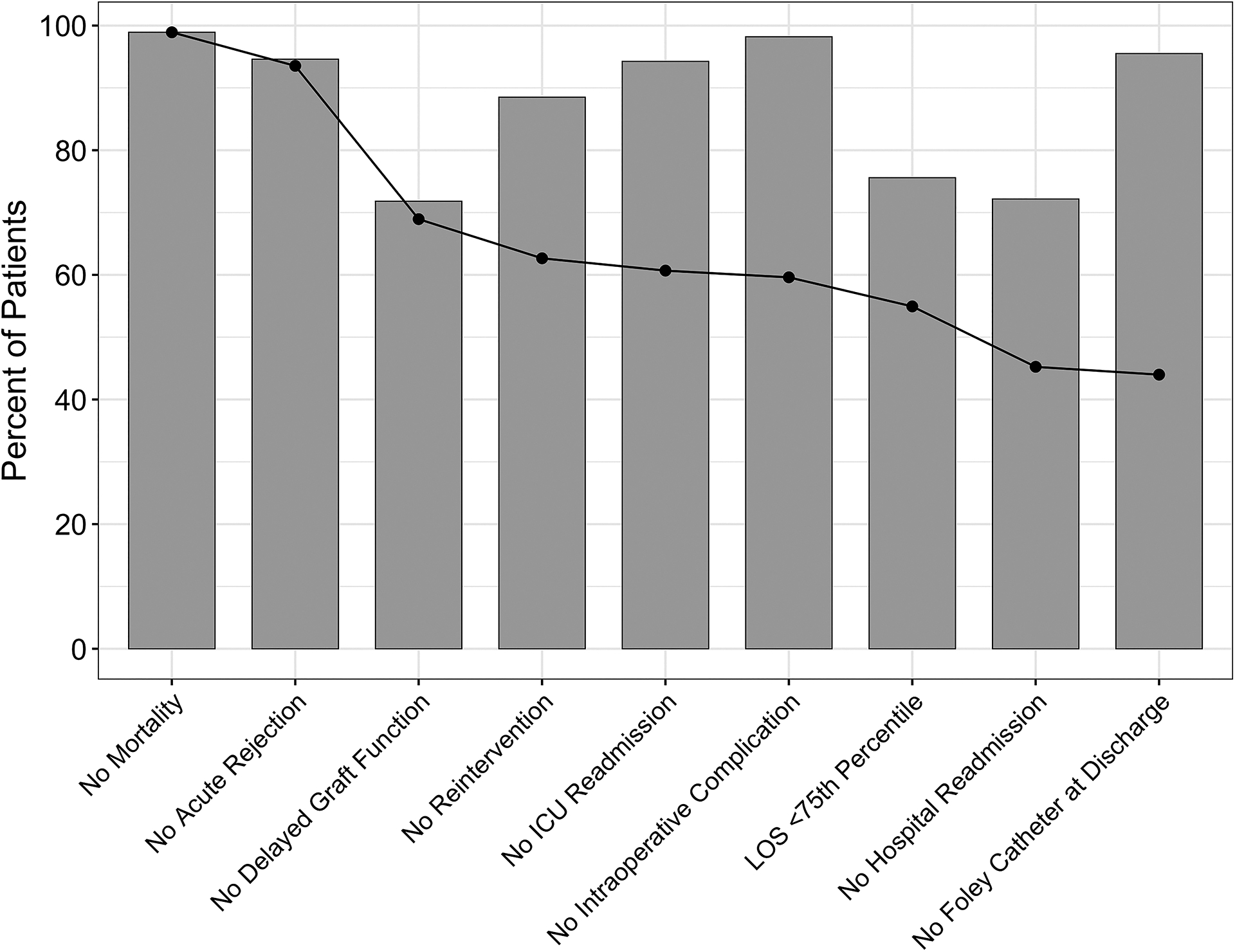
The bars represent individual features of TO ranked by severity from left to right. The bar heights correspond to freedom from those complications (i.e., taller bars indicate less complication). The black dotted line represents the cumulative achievement of TO as each feature is added. It decreases with each feature added as more patients fail to achieve TO with more specifications that must be met.
Financial implications of textbook outcome
Patients who achieved TO incurred approximately $50,000 less in total inpatient charges. Transplant-related charges accounted for most inpatient charges but were still significantly lower among patients who achieved TO (Table S5; Figure 2).
Figure 2. Financial implications of textbook outcome (TO).
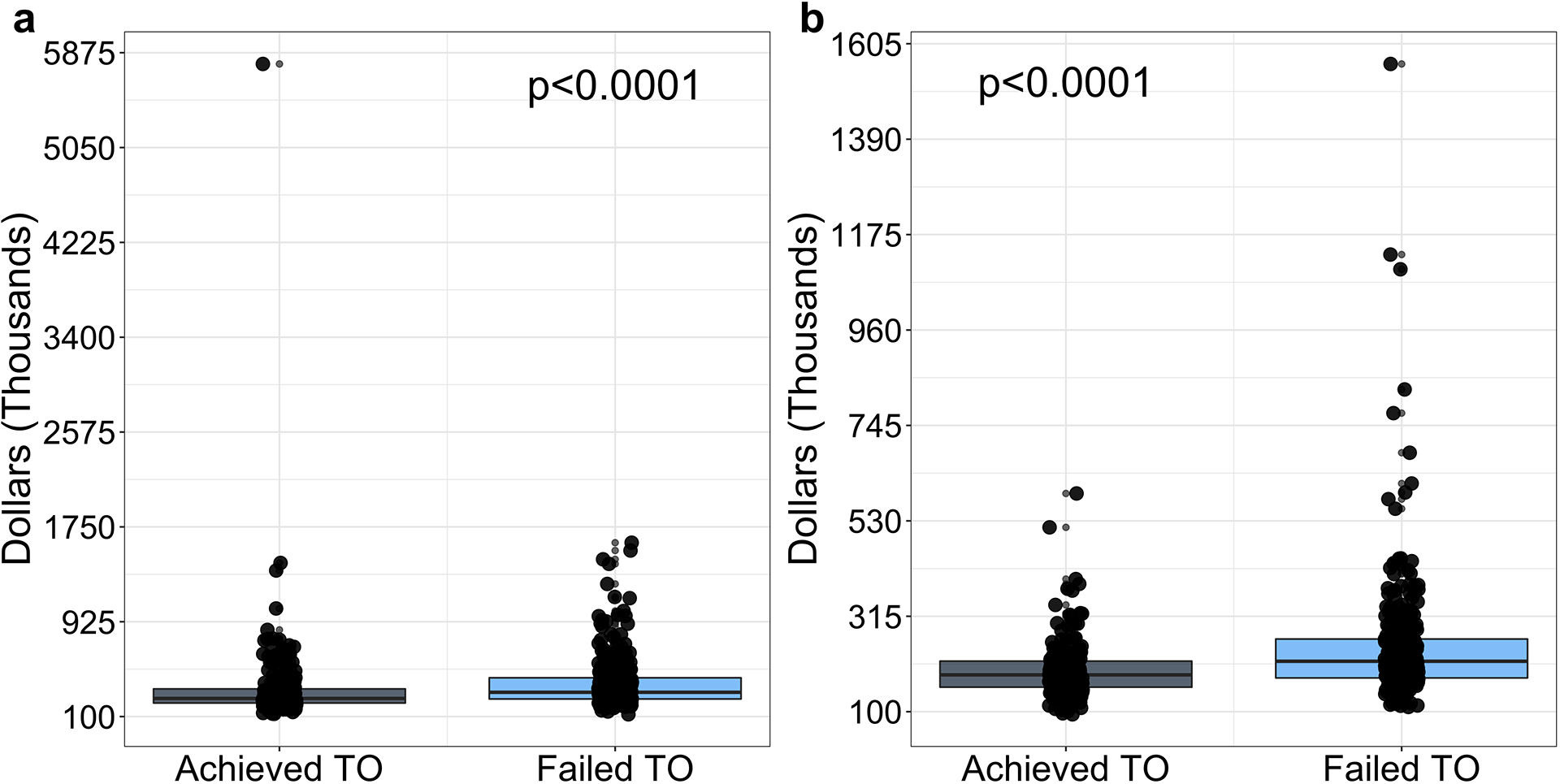
(a) Total inpatient charges during the index hospitalization. (b) Transplant-related inpatient charges during the index hospitalization.
Patient, graft, and rejection-free survival
Patient, graft, and rejection-free survival were decreased among patients who failed TO (Figure 3). In our sensitivity analysis, patient survival was similar between DGF and non-DGF groups; graft and rejection-free survival were decreased among patients with DGF compared to those without (Figure S1).
Figure 3. Kaplan-Meier survival analysis of the entire patient cohort stratified by achievement of textbook outcome (TO).
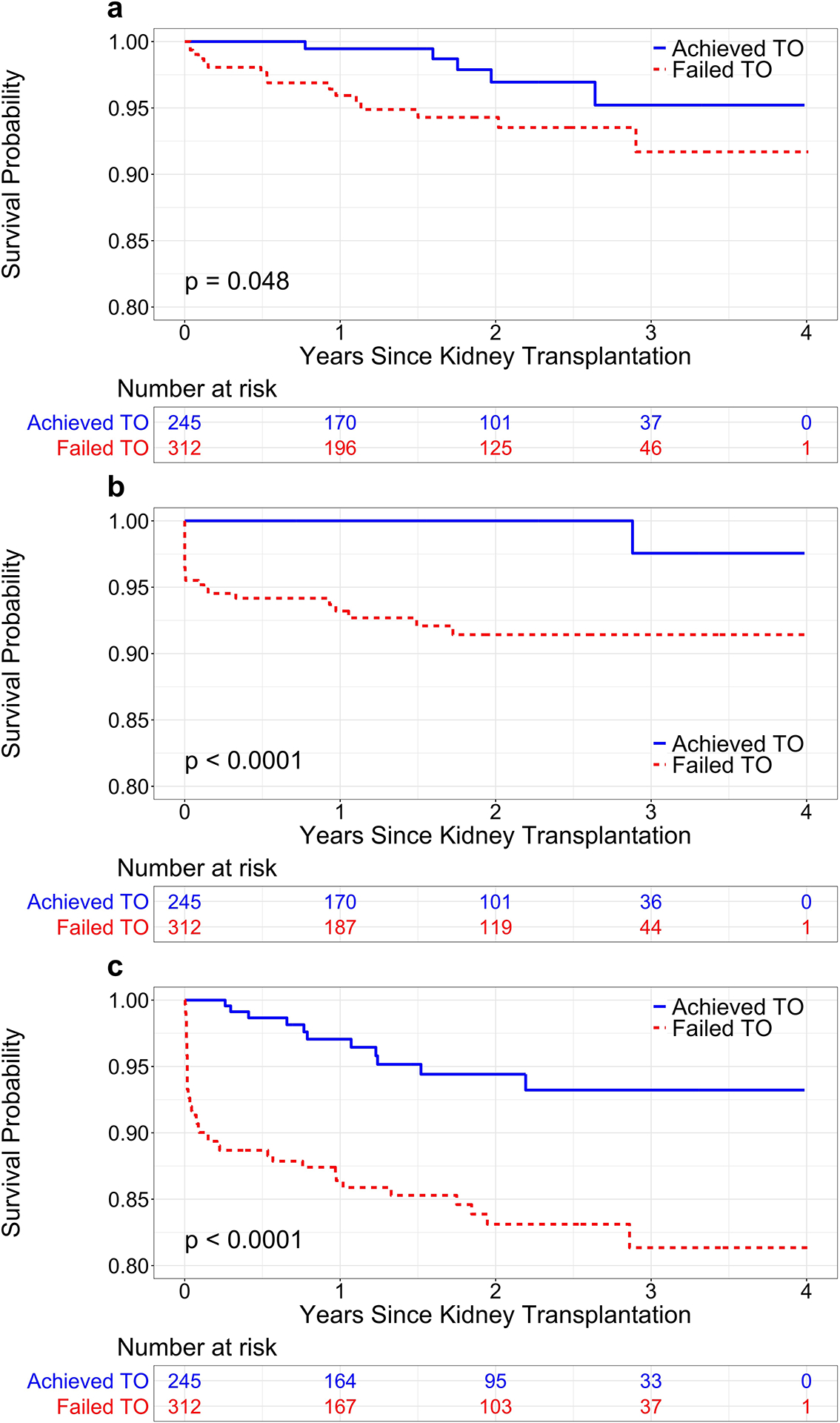
(a) Patient survival. (b) Graft survival. (c) Rejection-free survival.
Among LDKT recipients, patient and graft survival were similar between TO and non-TO groups; rejection-free survival was decreased among patients who failed TO (Figure S2). Among DDKT recipients, patient survival was similar between TO and non-TO groups; graft and rejection-free survival were decreased among those who failed TO (Figure S3).
Prediction of the probability of achieving a textbook outcome
Recipient sex, donor type, and number of HLA mismatches were independently associated with achievement of TO. Male sex was associated with 46% decreased odds of achieving TO (odds ratio [OR] 0.54, 95% CI 0.38–0.77, p<0.01); each HLA mismatch was associated with 16% decreased odds of achieving TO (OR 0.84, 95% CI 0.75–0.94, p<0.01). Receiving a DCD donor kidney decreased odds of achieving TO by 40% compared to receiving a DBD donor kidney (OR 0.60, 95% CI 0.37–0.97, p=0.042).
Recipient and operative characteristics independently associated with achievement of TO were used to construct a nomogram to predict the probability of TO (Figure 4). The logistic regression model was used to assign each factor in the nomogram a weighted point value. A patient’s probability of achieving TO is estimated by calculating the sum of points and determining its probability correlate on the nomogram. For example, a male patient (0 points) receiving a DBD donor kidney (47.5 points) with four HLA mismatches (32.5 points) would score 80 points, correlating to a TO probability of approximately 40%. A female patient (57.5 points) receiving a living donor kidney (85 points) with one HLA mismatch (82.5 points) would score 225 points corresponding to a TO probability of approximately 70%. The nomogram c-index was 0.687 (95% CI 0.611–0.729) and 0.620 (95% CI 0.525–0.661) for the training and validation sets, respectively.
Figure 4.
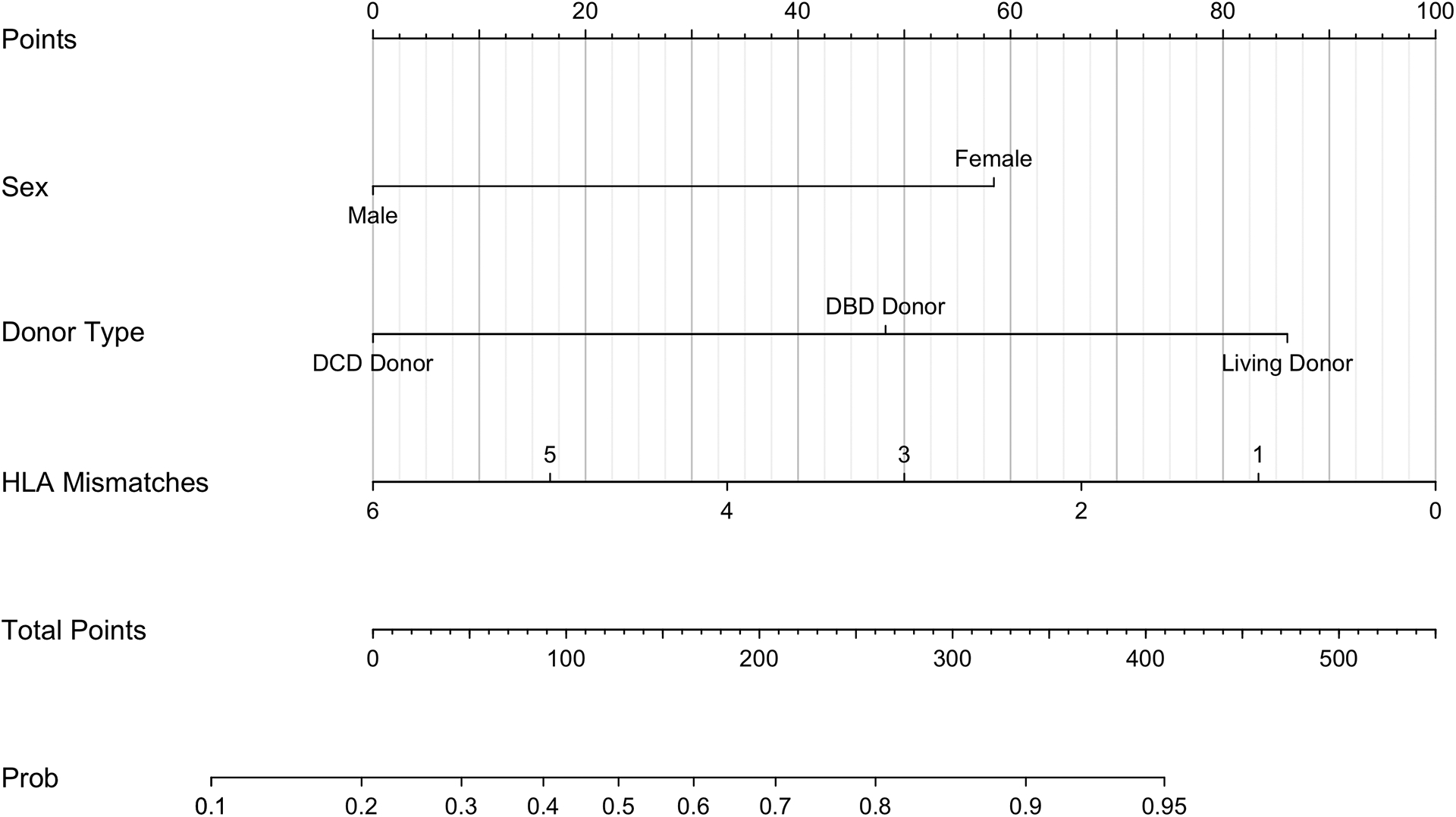
Nomogram for the prediction of the probability of achieving a textbook outcome (TO) after kidney transplantation (KTx).
DISCUSSION
In this study, we introduced TO as a novel composite quality index for KTx and demonstrated a strong association with post-transplant outcomes and cost. To define TO, we emulated existing models which include outcomes such as morbidity, mortality, LOS, and readmission [18,20,25]. We expanded the definition with KTx-specific metrics, defined by consensus among transplant clinicians at our institution.
44% of KTx patients achieved TO, similar to rates of TO for other complex surgical procedures [13,16,20,26]. Factors associated with TO failure included longer ischemic times, ureteral stent placement, and use of grafts with high KDPI. Unsurprisingly, TO failure was associated with significantly increased inpatient charges.
Transplant centers are primarily evaluated based on one-year patient and graft survival. The Centers for Medicare and Medicaid Services and some private insurance companies use publicly-reported data to determine in-network eligibility, grant transplant center certification, and flag programs with poorer patient or graft survival [27,28]. However, these metrics may no longer be best-suited to advance KTx. Currently, one-year patient and graft survival exceed 95% [7,32] with declining center-level variability amidst rising KTx volume and increasing experience nationwide [33]. In this context, there is growing recognition that longer-term outcomes may more robustly differentiate center quality, and inform ongoing management of this complex patient population that increasingly survives beyond five or ten years post-transplant [32].
In addition to highlighting long-term outcomes [7,33], a multi-faceted short-term metric such as TO may provide critical insight into care processes that can further improve perioperative care. Specific short-term outcomes are particularly important to patients and their view of the healthcare experience. Patients may be more likely to view perioperative outcomes holistically, basing transplant center quality assessments on occurrence of any complication rather than weighing performance in one domain versus another [18,20]. Unlike long-term prognosis, immediate outcomes including need for additional operations, or return to dialysis and prolonged LOS due to DGF may more directly influence patients’ decision-making and quality assessments of transplant centers. Rather than offering a new means by which to assess patient and graft survival, TO may be best understood as a patient-centered means of facilitating understanding of transplant center quality, and secondarily informing center-level initiatives to align quality improvement with care processes of greatest relevance to patients to optimize patient-centered care in KTx [34].
In contrast to prior studies identifying prolonged LOS as the primary obstacle to achievement of TO [18,20], early readmission was a leading reason for TO failure in KTx. High rates of acute care utilization and hospital readmission early post-KTx have previously been reported, portending poor post-transplant outcomes and higher healthcare costs [35–38]. While it remains difficult to identify preventable readmissions, patient factors including older age, Black race, elevated body mass index, and presence of medical comorbidities are associated with increased rates of early readmission [35,38,39], suggesting that pre-operative identification of high-risk patients may facilitate pro-active prevention of readmission through improved patient education and outreach in the perioperative period [37]. Concurrently, care processes such as intraoperative ureteral stent and drain placement, and induction and maintenance immunosuppression management may represent modifiable areas through which centers can mitigate complications necessitating readmission. At our institution, ureteral stent and drain placement vary across surgeons, for some representing routine practice, and for others potentially reflecting operative complexity. Regardless, any intervention may entail complications, potentially accounting for a higher rate of TO failure among these patients. While our findings suggest that early readmission may be the primary reflection of complications related to intraoperative or perioperative management decisions at our center, multi-institutional studies are needed to further elucidate the impact of readmissions on rates of TO nationally, and associations with early care processes, as post-transplant emergency department utilization and hospital readmission vary across centers [35,37,38].
DGF was also prevalent among patients who failed TO. It is well-established that DGF portends increased risk of graft failure and acute rejection [32,40,41]. Its occurrence may be related to use of potentially high-risk kidneys such as those from older or DCD donors [40,41], raising concern that its inclusion in TO may unintentionally deter use of these grafts. In this context, however, DGF is more appropriately viewed as a marker of center-level aggressiveness in which high-risk grafts facilitate transplantation of more waitlisted patients. Accordingly, centers that frequently manage recipients of high-risk kidneys may be equipped to offset negative impacts of DGF to achieve acceptable outcomes including short LOS, which likely indicate streamlined processes for transition between hospital-based and community dialysis centers, and mitigation of other perioperative complications to achieve preserved post-transplant survival in spite of DGF’s occurrence. While ability to investigate these associations in an institutional study is limited, future work should investigate implications of DGF within TO, and its differential impact across centers.
Several patient and procedural factors were associated with TO in KTx. Unlike donor type and number of HLA mismatches, recipient sex cannot be altered to influence odds of achieving TO. Ours is not the first study to identify a non-modifiable patient factor as a significant predictor of TO, with prior work identifying age and sex as predictors of TO in hepatopancreatic and esophagogastric surgery [16,18,20,42]. Along with our proposed nomogram, which may help pre-emptively estimate patients’ probabilities of achieving TO, understanding non-modifiable factors associated with reduced odds of achieving TO may guide resource allocation to high-risk patients, inform pre-transplant decision-making, and facilitate targeted donor-recipient matching to maximize odds of TO for all patients. While our nomogram demonstrated modest predictive ability, examination of TO in a national study may facilitate development of a more granular predictive model to improve pre-transplant prognostication and identify additional targetable areas to optimize perioperative outcomes in KTx.
Our study has several strengths and limitations. This study is the first to define TO in KTx, using data from a modern cohort at a high-volume institution. The granularity of available data allowed inclusion of particularly relevant parameters in TO, which are unavailable in national datasets. However, the single institution, retrospective nature of our analysis may decrease generalizability, particularly regarding institution-dependent practices including immunosuppression management and intraoperative ureteral stent and drain placement. Likewise, as our study reflects recipient and donor populations at a single institution, we did not adjust for case-mix heterogeneity in our assessment of TO. Future multi-institutional studies should make appropriate provisions for risk-adjustment to account for differences in recipient and donor characteristics that may influence center-level rates of TO. While we included DGF in our definition of TO due to its implications for patient experiences and outcomes, risk-adjusted multi-institutional studies are necessary to better elucidate appropriateness of its inclusion, weighing outcome optimization against inadvertently heightened center-level risk aversion. Amidst ongoing prospective data collection through the Transplant-NSQIP database, our study may inform important donor, recipient, and outcome parameters that should be included to maximize future ability to expand upon and validate novel metrics such as TO. Finally, TO was defined based on clinician consensus at a single institution. While this is consistent with processes reported in the literature [15,18,20,25,43,44], an ideal definition of TO would come from international consensus among experts in the field. Nevertheless, we demonstrate that TO in KTx as herein defined is a promising multidimensional indicator.
CONCLUSIONS
In this institutional analysis, TO in KTx was associated with favorable short-term post-transplant outcomes, and significant cost-savings. Although it remains difficult to predict TO before KTx, this composite metric may offer a powerful parameter to assess between-hospital variation, compare quality between institutions or audits, and identify actionable areas for quality improvement in KTx. Validation of this novel quality metric in a multi-institution study is warranted.
Supplementary Material
Conflicts of Interest and Sources of Funding:
The authors report no conflicts of interest. SEH is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR002555. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- ANOVA
analysis of variance
- CI
confidence interval
- DBD
donation after brain death
- DCD
donation after circulatory death
- DDKT
deceased donor kidney transplant
- DGF
delayed graft function
- HLA
human leukocyte antigen
- KDPI
kidney donor profile index
- KTx
kidney transplantation
- LDKT
living donor kidney transplant
- LOS
length of stay
- NSQIP
National Surgical Quality Improvement Project
- OR
odds ratio
- TO
textbook outcome
REFERENCES
- 1.Barbas AS, Dib MJ, Rege AS, et al. (2018) The Volume-outcome Relationship in Deceased Donor Kidney Transplantation and Implications for Regionalization. Ann Surg 267:1169–1172 [DOI] [PubMed] [Google Scholar]
- 2.Talbot D (1999) Renal transplantation: origins and future. Int Surg 84:291–296 [PubMed] [Google Scholar]
- 3.Axelrod DA, Guidinger MK, McCullough KP, et al. (2004) Association of center volume with outcome after liver and kidney transplantation. Am J Transplant 4:920–927 [DOI] [PubMed] [Google Scholar]
- 4.Sheetz KH, Englesbe MJ (2018) Rethinking performance benchmarks in kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 18:2109–2110 [DOI] [PubMed] [Google Scholar]
- 5.Parekh JR, Greenstein S, Sudan DL, et al. (2019) Beyond death and graft survival-Variation in outcomes after liver transplant. Results from the NSQIP transplant beta phase. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 19:2108–2115 [DOI] [PubMed] [Google Scholar]
- 6.Englesbe MJ, Pelletier SJ, Kheterpal S, et al. (2006) A call for a national transplant surgical quality improvement program. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 6:666–670 [DOI] [PubMed] [Google Scholar]
- 7.Chandraker A, Andreoni KA, Gaston RS, et al. (2019) Time for reform in transplant program-specific reporting: AST/ASTS transplant metrics taskforce. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 19:1888–1895 [DOI] [PubMed] [Google Scholar]
- 8.Centers for M, Medicaid Services HHS (2016) Medicare Program: Hospital Outpatient Prospective Payment and Ambulatory Surgical Center Payment Systems and Quality Reporting Programs; Organ Procurement Organization Reporting and Communication; Transplant Outcome Measures and Documentation Requirements; Electronic Health Record (EHR) Incentive Programs; Payment to Nonexcepted Off-Campus Provider-Based Department of a Hospital; Hospital Value-Based Purchasing (VBP) Program; Establishment of Payment Rates Under the Medicare Physician Fee Schedule for Nonexcepted Items and Services Furnished by an Off-Campus Provider-Based Department of a Hospital. Final rule with comment period and interim final rule with comment period. Federal register 81:79562–79892 [PubMed] [Google Scholar]
- 9.Dimick JB, Staiger DO, Baser O, et al. (2009) Composite measures for predicting surgical mortality in the hospital. Health Aff (Millwood) 28:1189–1198 [DOI] [PubMed] [Google Scholar]
- 10.Dimick JB, Welch HG, Birkmeyer JD (2004) Surgical mortality as an indicator of hospital quality: the problem with small sample size. JAMA 292:847–851 [DOI] [PubMed] [Google Scholar]
- 11.Dimick JB, Staiger DO, Osborne NH, et al. (2012) Composite measures for rating hospital quality with major surgery. Health Serv Res 47:1861–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolfschoten NE, Kievit J, Gooiker GA, et al. (2013) Focusing on desired outcomes of care after colon cancer resections; hospital variations in ‘textbook outcome’. Eur J Surg Oncol 39:156–163 [DOI] [PubMed] [Google Scholar]
- 13.van Roessel S, Mackay TM, van Dieren S, et al. (2019) Textbook Outcome: Nationwide Analysis of a Novel Quality Measure in Pancreatic Surgery. Annals of surgery [DOI] [PubMed] [Google Scholar]
- 14.Fong Y (2019) Textbook Outcome Nomograms as Multivariate Clinical Tools for Building Cancer Treatment Pathways and Prognosticating Outcomes. JAMA surgery 154:e190572. [DOI] [PubMed] [Google Scholar]
- 15.Salet N, Bremmer RH, Verhagen M, et al. (2018) Is Textbook Outcome a valuable composite measure for short-term outcomes of gastrointestinal treatments in the Netherlands using hospital information system data? A retrospective cohort study. BMJ Open 8:e019405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busweiler LA, Schouwenburg MG, van Berge Henegouwen MI, et al. (2017) Textbook outcome as a composite measure in oesophagogastric cancer surgery. Br J Surg 104:742–750 [DOI] [PubMed] [Google Scholar]
- 17.Priego P, Cuadrado M, Ballestero A, et al. (2019) Comparison of Laparoscopic Versus Open Gastrectomy for Treatment of Gastric Cancer: Analysis of a Textbook Outcome. Journal of laparoendoscopic & advanced surgical techniques Part A 29:458–464 [DOI] [PubMed] [Google Scholar]
- 18.Merath K, Chen Q, Bagante F, et al. (2019) A Multi-Institutional International Analysis of Textbook Outcomes Among Patients Undergoing Curative-Intent Resection of Intrahepatic Cholangiocarcinoma. JAMA surgery:e190571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moris D, Cerullo M, Nussbaum DP, et al. (2020) Textbook Outcomes Among Patients Undergoing Retroperitoneal Sarcoma Resection. Anticancer research 40:2107–2115 [DOI] [PubMed] [Google Scholar]
- 20.Merath K, Chen Q, Bagante F, et al. (2020) Textbook Outcomes Among Medicare Patients Undergoing Hepatopancreatic Surgery. Ann Surg 271:1116–1123 [DOI] [PubMed] [Google Scholar]
- 21.Moris D, Shaw BI, Gloria J, et al. (2020) Textbook Outcomes in Liver Transplantation. World journal of surgery [DOI] [PubMed] [Google Scholar]
- 22.Yarlagadda SG, Coca SG, Formica RN Jr., et al. (2009) Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 24:1039–1047 [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Kattan MW (2017) Drawing Nomograms with R: applications to categorical outcome and survival data. Annals of translational medicine 5:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guillamondegui OD, Gunter OL, Hines L, et al. (2012) Using the National Surgical Quality Improvement Program and the Tennessee Surgical Quality Collaborative to improve surgical outcomes. Journal of the American College of Surgeons 214:709–714; discussion 714–706 [DOI] [PubMed] [Google Scholar]
- 25.Tsilimigras DI, Mehta R, Merath K, et al. (2019) Hospital variation in Textbook Outcomes following curative-intent resection of hepatocellular carcinoma: an international multi-institutional analysis. HPB : the official journal of the International Hepato Pancreato Biliary Association [DOI] [PubMed] [Google Scholar]
- 26.van der Kaaij RT, de Rooij MV, van Coevorden F, et al. (2018) Using textbook outcome as a measure of quality of care in oesophagogastric cancer surgery. Br J Surg 105:561–569 [DOI] [PubMed] [Google Scholar]
- 27.Centers for M, Medicaid Services HHS (2007) Medicare program; hospital conditions of participation: requirements for approval and re-approval of transplant centers to perform organ transplants. Final rule. Federal register 72:15197–15280 [PubMed] [Google Scholar]
- 28.Schold JD, Miller CM, Henry ML, et al. (2017) Evaluation of Flagging Criteria of United States Kidney Transplant Center Performance: How to Best Define Outliers? Transplantation 101:1373–1380 [DOI] [PubMed] [Google Scholar]
- 29.Schold JD, Buccini LD, Srinivas TR, et al. (2013) The association of center performance evaluations and kidney transplant volume in the United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 13:67–75 [DOI] [PubMed] [Google Scholar]
- 30.Sheetz KH, Ibrahim AM, Nathan H, et al. (2019) Variation in Surgical Outcomes Across Networks of the Highest-Rated US Hospitals. JAMA surgery [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dijs-Elsinga J, Otten W, Versluijs MM, et al. (2010) Choosing a hospital for surgery: the importance of information on quality of care. Med Decis Making 30:544–555 [DOI] [PubMed] [Google Scholar]
- 32.Hart A, Smith JM, Skeans MA, et al. (2020) OPTN/SRTR 2018 Annual Data Report: Kidney. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 20 Suppl s1:20–130 [DOI] [PubMed] [Google Scholar]
- 33.Jay C, Schold JD (2017) Measuring transplant center performance: The goals are not controversial but the methods and consequences can be. Curr Transplant Rep 4:52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nolan T, Berwick DM (2006) All-or-none measurement raises the bar on performance. Jama 295:1168–1170 [DOI] [PubMed] [Google Scholar]
- 35.McAdams-Demarco MA, Grams ME, Hall EC, et al. (2012) Early hospital readmission after kidney transplantation: patient and center-level associations. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 12:3283–3288 [DOI] [PubMed] [Google Scholar]
- 36.McAdams-Demarco MA, Grams ME, King E, et al. (2014) Sequelae of early hospital readmission after kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 14:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lovasik BP, Zhang R, Hockenberry JM, et al. (2018) Emergency department use among kidney transplant recipients in the United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 18:868–880 [DOI] [PubMed] [Google Scholar]
- 38.Schold JD, Elfadawy N, Buccini LD, et al. (2016) Emergency Department Visits after Kidney Transplantation. Clinical journal of the American Society of Nephrology : CJASN 11:674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weeda ER, Su Z, Taber DJ, et al. (2019) Hospital admissions and emergency department visits among kidney transplant recipients. Clinical transplantation 33:e13522. [DOI] [PubMed] [Google Scholar]
- 40.Perico N, Cattaneo D, Sayegh MH, et al. (2004) Delayed graft function in kidney transplantation. Lancet 364:1814–1827 [DOI] [PubMed] [Google Scholar]
- 41.Bahl D, Haddad Z, Datoo A, et al. (2019) Delayed graft function in kidney transplantation. Curr Opin Organ Transplant 24:82–86 [DOI] [PubMed] [Google Scholar]
- 42.van Roessel S, Mackay TM, van Dieren S, et al. (2020) Textbook Outcome: Nationwide Analysis of a Novel Quality Measure in Pancreatic Surgery. Ann Surg 271:155–162 [DOI] [PubMed] [Google Scholar]
- 43.van der Werf LR, Wijnhoven BPL, Fransen LFC, et al. (2019) A National Cohort Study Evaluating the Association Between Short-term Outcomes and Long-term Survival After Esophageal and Gastric Cancer Surgery. Annals of surgery 270:868–876 [DOI] [PubMed] [Google Scholar]
- 44.Levy J, Gupta V, Amirazodi E, et al. (2019) Gastrectomy case volume and textbook outcome: an analysis of the Population Registry of Esophageal and Stomach Tumours of Ontario (PRESTO). Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


