Abstract
Objective:
To determine the impact of diagnostic TORS lingual tonsillectomy (DTLT) on objective swallowing measures for carcinoma of unknown primary (CUP).
Methods:
Between 10/2016-1/2020, 27 patients with p16+ squamous cell carcinoma (SCC) level 2a nodal disease underwent DTLT and ipsilateral neck dissection for CUP. No patient had a history of cutaneous SCC. Patients participated in Modified Barium Swallow (MBS) three weeks post-TORS, which were then compared to those from a contemporaneous cohort of 40 patients with clinically-identified p16+ base of tongue (BOT) primary tumors. DIGEST scores were retrospectively calculated. Univariate and multivariate analysis performed, stratified by BOT glossectomy (n = 40) versus lingual tonsillectomy for CUP (n = 27). Radiation to the resected primary or potential primary sources was omitted if margins were ≥3 mm or if no primary identified.
Results:
Twenty-seven consecutive patients with clinical stage cT0N1 HPV-associated OPSCC had a BOT primary pathologically identified in 18/27 (67%). Univariate analysis of functional swallow assessment on MBSImP correlated with improved post-TORS DIGEST scores for CUP. On multivariate analysis (MVA) DIGEST safety scores were improved for CUP than cT1 BOT glossectomy [Odds Ratio (OR) 0.28, p = 0.038]. MVA on matched pT1 CUP (n = 27) vs. pT1 BOT (n = 19), OR of moderate/severe dysphagia for CUP was 0.54 [0.12–2.38, p = 0.417] for DIGEST safety scores and 0.27 [0.06–1.18, p = 0.082] for DIGEST efficiency scores. Moderate/severe dysphagia as determined by DIGEST overall scores for CUP compared to cT1 and pT1 yielded an OR of 0.39 (p = 0.081) and 0.42 (p = 0.195), respectively.
Twenty-six total patients received adjuvant RT, and 18 (11 with ≥3 mm margins, 9 with negative specimens) were spared intentional RT to the oropharynx. Median follow-up was 22.6 months with 100% PFS.
Conclusions:
Patients undergoing DTLT for CUP demonstrated acute swallow defecits in the post-operative setting. A comparison of long-term functional results between DTLT and elective irradiation of the primary site should be studied.
Level of evidence:
Level III.
Keywords: HPV, TORS, CUP, Unknown primary, Lingual tonsillectomy, Modified Barium Swallow Study, DIGEST
Introduction
The epidemic rise of HPV-associated oropharynx squamous cell carcinoma (OPSCC) has surpassed the incidence of HPV-associated cervical cancer [1]. Despite the availability of HPV vaccination, the vaccination rates in the United States remain insufficient to eliminate this disease in the foreseeable future. Approximately 4% of HPV-associated OPSCC cases present as carcinoma of unknown primary (CUP) [2]. As a result, the number of CUP will continue to climb.
Granular details of therapeutic recommendations for CUP continue to vary greatly and therapeutic decisions may impact functional outcomes [3]. As diagnostic TORS lingual tonsillectomy (DTLT) for CUP is increasingly used as an approach to localizing tumors [4–6], there is concern regarding the addition of treatment toxicity. On the other hand, incorporating TORS into the management of CUP may facilitate avoidance of radiation to potential oropharyngeal primary sites or allow for reduced adjuvant radiation doses/volume, thereby minimizing potential side effects that negatively impact swallowing [7]. It is important to consider that TORS for CUP brings morbidity, and the effect of DTLT remains largely unexplored. To this end, we aim to characterize swallow function after our CUP approach with diagnostic lingual tonsillectomy, hypothesizing that this strategy could potentially eliminate irradiation to the oropharynx without compromising local control.
Materials and methods
We conducted an IRB approved (IRB# 104979) retrospective electronic record review of patients previously diagnosed with stage (pT0-T1) HPV-associated oropharyngeal cancer who had bilateral lingual tonsillectomies for CUP at our center between 10/1/2016 and 1/30/2020. These patients had undergone the following diagnostic work-up: physical exam, flexible fiberoptic endoscopy, PET/CT, evaluation under anesthesia, diagnostic unilateral palatine tonsillectomy ipsilateral to the nodal diseae, and tongue base biopsy (Fig. 1). If all failed to reveal a primary site, patients diagnosed with CUP p16-positive SCC with radiographic N1 neck disease (AJCC 8th Edition) were subsequently treated with DTLT as well as a therapeutic ipsilateral neck dissection. All patients underwent dissection of levels Ib – IV, including IIb. Due to the low risk of level Ib disease, dissection is limited to the inferior aspect of the submandibular gland, including the lymph nodes along the inferior border and those lateral to the facial vein [8]. The pre- and post-vascular nodes along with the nodes anterior to the submandibular gland are not removed. All CUP patients found to have tonsil primaries were excluded. To compare objective swallow function, a cohort of patients with HPV-associated clinical T1 tongue base OPSCC treated with TORS and ipsilateral neck dissection during the same time period were identified. Final pathologic stage was reported. A subset analysis was performed with pathologically matched T1 classified tumors considering T-classification migration of the clinical T1 BOT tumors to pT2 BOT tumors post-resection.
Fig. 1.
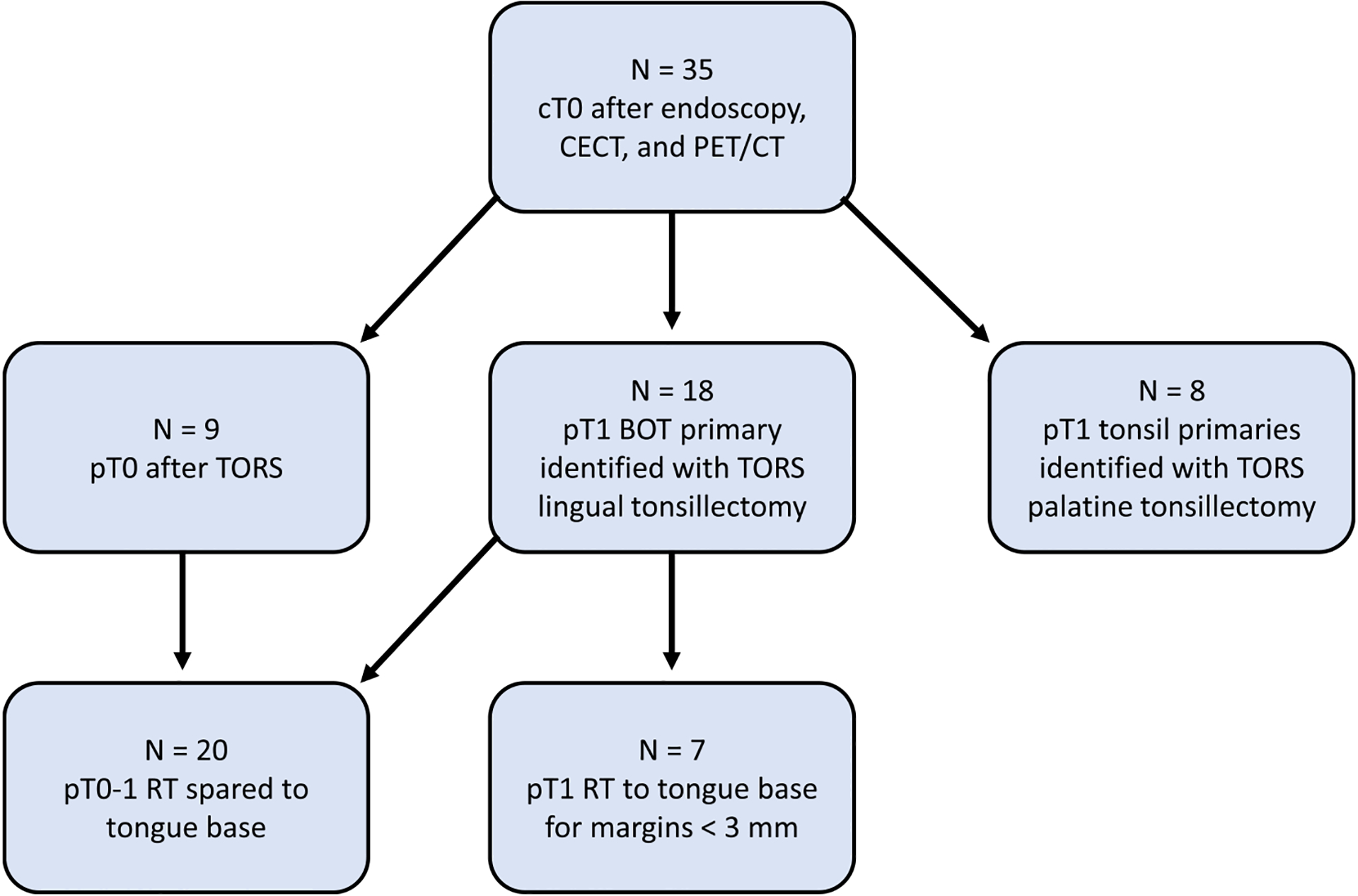
Selection criteria to identify Transoral Robotic Surgery (TORS) patients with radiation therapy (RT) sparing to the primary after lingual tonsillectomy. p: pathological staging, c: clinical staging, T: tumor stage.
Modified Barium Swallow data collection
Patients participated in a three-week post-operative Modified Barium Swallow (MBS) Study. Data from patients’ three-week post-operative MBS were collected using the Modified Barium Swallow Impairment (MBSImP), the Rosenbek 8-Point Penetration-Aspiration Scale (PAS), Functional Oral Intake Scale (FOIS), and DIGEST scores [9–12]. The MBSImP is a validated, standardized measurement tool that rates the physiological components of the oral, pharyngeal, and esophageal phases of the swallow on an ordinal scale [9]. Zero represents normal function and progression on the ordinal scale indicates greater impairment. Each MBSImP component was stratified into two groups, 1) normal to mild impairment and 2) moderate to severe impairment for the purpose of statistical analysis.
PAS is a validated scale measuring the degree to which the bolus enters the airway and the patient’s response to the penetration or aspiration event [10]. A PAS score of 1 indicates that material does not enter the airway, scores 2–5 represent laryngeal penetration to varying degrees, and scores 6–8 represent aspiration to varying degrees. Scores were dichotomized: scores of 1–2 categorized as swallowing within functional limits (WFL), while scores 3–8 indicated varying levels of impairment.
FOIS is a validated scale rating from 1 to 7: no oral intake (score = 1, tube feed dependent) to total oral intake without restrictions (score = 7) [11]. A FOIS score was assigned following an objective swallowing assessment based on the patient’s current diet level.
Videofluoroscopy-based DIGEST scores were assigned retrospectively. DIGEST is a validated method to grade pharyngeal phase dysphagia based on safety (from PAS) and efficiency (based on percentage of pharyngeal residue) of bolus clearance. [12] DIGEST was derived by independent review. DIGEST was dichotomized with grade 2 or higher as moderate-severe dysphagia based on published data [12] to suggest this is a meaningful split that correlates with diet level. No patient reported outcomes were collected.
After multidisciplinary discussion, adjuvant radiation to the primary was omitted if specimen-driven margins were ≥3 mm. All patients were treated with bilateral radiation to the cervical nodal regions in the setting of an identified or known tongue base primary. We incorporate adjuvant radiation in the setting of an ipsilateral, isolated node greater than 3 cm or if there are less than 5 ipsilateral nodes. The fileds are bilateral given that tongue base primaries have the potential for bilateral nodal drainage and 21% risk of occult disease [13]. PNI and/or LVI are not factored into our adjuvant treatment algorithm to radiate or not radiate the neck or primary site. Adjuvant chemotherapy, consisting of weekly cisplatin at 40 mg/m2 for fit patients, was incorporated for ENE > 1 mm.
Statistical analysis
Demographic data collected included patient age at diagnosis, smoking history and sex. Pathological specimen data included tumor classification (based on 8th edition AJCC), tumor size, ENE (none, <1 mm, >1 mm), LVI, and PNI. Follow-up data was recorded including follow-up time, adjuvant therapy, and cancer recurrence. The MBSImP, PAS, and FOIS were scored according to each measure’s scoring protocol. Categorical patient characteristics were compared across dichotomous PAS and DIGEST scores using chi-squared tests or Fisher’s exact tests, where appropriate, while continuous characteristics were compared using ANOVA. The swallowing parameters were compared to similar tests on 40 patients who underwent therapeutic TORS base of tongue resection for known base of tongue squamous cell cancer. Statistical significance was assessed at the 0.05 level, and the statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
From October 2016 – January 2020, 27 patients underwent DTLT after the aforementioned standard CUP work-up. Mean age at diagnosis was 63.9 years and 93% of included patients were male. Of the 27 patients, one was lost to follow-up. Pathological data was available for all patients. Details of the tumor characteristics for patients who presented with CUP are presented in Table 1a and adjuvant treatment details are presented in Table 1b. Demographic and pathologic data for CUP and known BOT primaries are presented in Table 2. The mean tumor size of the greatest measured dimension for identified CUP was 0.8 cm (range 0.3 cm – 1.9 cm), 1.25 cm (range 0.5 cm – 2.0 cm) for pT1 BOT tumors and 2.77 cm (range 2.1 cm – 3.9 cm) for pT2 BOT tumors (see Table 3).
Table 1a.
Unknown primary tumor characteristics.
| Parameter | Number of patients (%) | Mean ± SD (Range) |
|---|---|---|
| Tumor size (mm) | 18 | 8.4 ± 4.9 (2–19) |
| Therapeutic tumor excision at diagnostic lingual tonsillectomy* | 14 of 18 (78%) | |
| pT0 | 9 of 27 (33%) | |
| pT1 | 18 of 27 (67%) | |
| pN1 | 25 of 27 (93%) | |
| pN2 | 2 of 27 (7%) | |
| ENE | 8 of 27 (30%) | |
| ENE < 2 mm | 6 of 27 (22%) | |
| ENE > 2 mm | 2 of 27 (7%) | |
| PNI | 2 of 27 (7%) | |
| LVI | 4 of 27 (15%) | |
| PNI + LVI | 1 of 27 (4%) |
ENE = extra-nodal extension, PNI = peri-neural invasion, LVI = lymphovascular invasion.
4 patients with incomplete excision also had ENE on pathologic nodal assessment and were not taken for revision due to recommendation for chemoradiation.
Table 1b.
Post-operative follow-up and adjuvant treatment.
| Parameter | Number of patients (%) | Mean ± SD (Range) |
|---|---|---|
| Follow-up time (months) | 27 (100%) | 16.0 ± 9.2 (5–35) |
| Tumor Recurrence | 0 | |
| Adjuvant radiation treatment | 26 of 27 (96%) | |
| Adjuvant chemotherapy | 7 of 27 (26%) | |
| Sparing of directed radiotherapy to oropharyngeal site | 21 of 27 (78%) |
Table 2.
Patient characteristics for unknown primary and base of tongue primaries.
| Variable | Level | N = 67 | % |
|---|---|---|---|
| Sex | Female | 5 | 7.5 |
| Male | 62 | 92.5 | |
| Race | African American | 12 | 17.9 |
| Caucasian | 55 | 82.1 | |
| Smoking Status | Never Smokers | 42 | 62.7 |
| <10 pack years | 5 | 7.5 | |
| >10 pack years | 20 | 29.8 | |
| Tumor Subsite | Unknown primary | 27 | 40.3 |
| Base of tongue | 40 | 59.7 | |
| Site of TORS | Unknown primary | 8 | 11.9 |
| Base of tongue | 59 | 88.1 | |
| 8th edition - T stage | Tx | 8 | 11.9 |
| T1 | 38 | 56.7 | |
| T2 | 21 | 31.3 | |
| 8th edition - N stage | N0 | 5 | 7.5 |
| N1 | 58 | 86.6 | |
| N2 | 4 | 6.0 | |
| Margin Status | Unknown | 7 | 10.4 |
| Negative | 56 | 83.6 | |
| Positive | 4 | 6.0 | |
| PNI | Yes | 10 | 16.9 |
| No | 49 | 83.1 | |
| Missing | 8 | - | |
| LVI | Yes | 16 | 27.1 |
| No | 43 | 72.9 | |
| Missing | 8 | - | |
| ENE | Yes | 24 | 38.7 |
| No | 38 | 61.3 | |
| Missing | 5 | - | |
| Age at surgery | Mean | 61.12 | - |
| Median | 62 | - | |
| Minimum | 43 | - | |
| Maximum | 81 | - | |
| Std Dev | 9.34 | - | |
| Time to MBS | Mean | 21.64 | - |
| Median | 21 | - | |
| Minimum | 11 | - | |
| Maximum | 34 | - | |
| Std Dev | 4.06 | - |
Table 3.
Multivariate analysis of DIGEST scores for CUP compared to BOT primaries.
| Post-op DIGEST overall score = 2–4 | Post-op DIGEST safety score = 2–4 | ||||
|---|---|---|---|---|---|
| Covariate | Level | Odds Ratio (95% CI) | OR P-value | Odds Ratio (95% CI) | OR P-value |
| Tumor Subsite | Unknown primary | 0.39 (0.13–1.12) | 0.081 | 0.28 (0.08–0.93) | 0.038 |
| Base of tongue | – | – | – | – | |
| Age at surgery | 1.03 (0.98–1.09) | 0.283 | 1.04 (0.98–1.11) | 0.168 | |
Number of observations in the original data set = 67.
Number of observations used = 67.
Backward selection with an alpha level of removal of 0.05 was used. No variables were removed from the model.
3 Week post-TORS functional assessment
Post-operative FOIS scores were dichotomous with 18.2% of patients being Dobhoff tube feed-dependent (score = 1) and with the remaining 81.8% of patients on a fully oral diet (score > 6). All patients with a FOIS score of 1 at 3-weeks post-TORS were for BOT primaries.
Impairment of certain MBSImP parameters (epiglottic inversion, base of tongue retraction, pharyngeal stripping wave, laryngeal vestibular closure, pharyngeal residue, and pharyngoesophageal segment opening) correlated with severe PAS deficits in CUP and BOT primaries [p < 0.001] (Fig. 2a). When comparing CUP to only pathologically matched T1 tumors, the same aforementioned categories except for pharyngoesophageal segment opening maintained a significant correlation with severe PAS deficits (Fig. 2b).
Fig. 2.
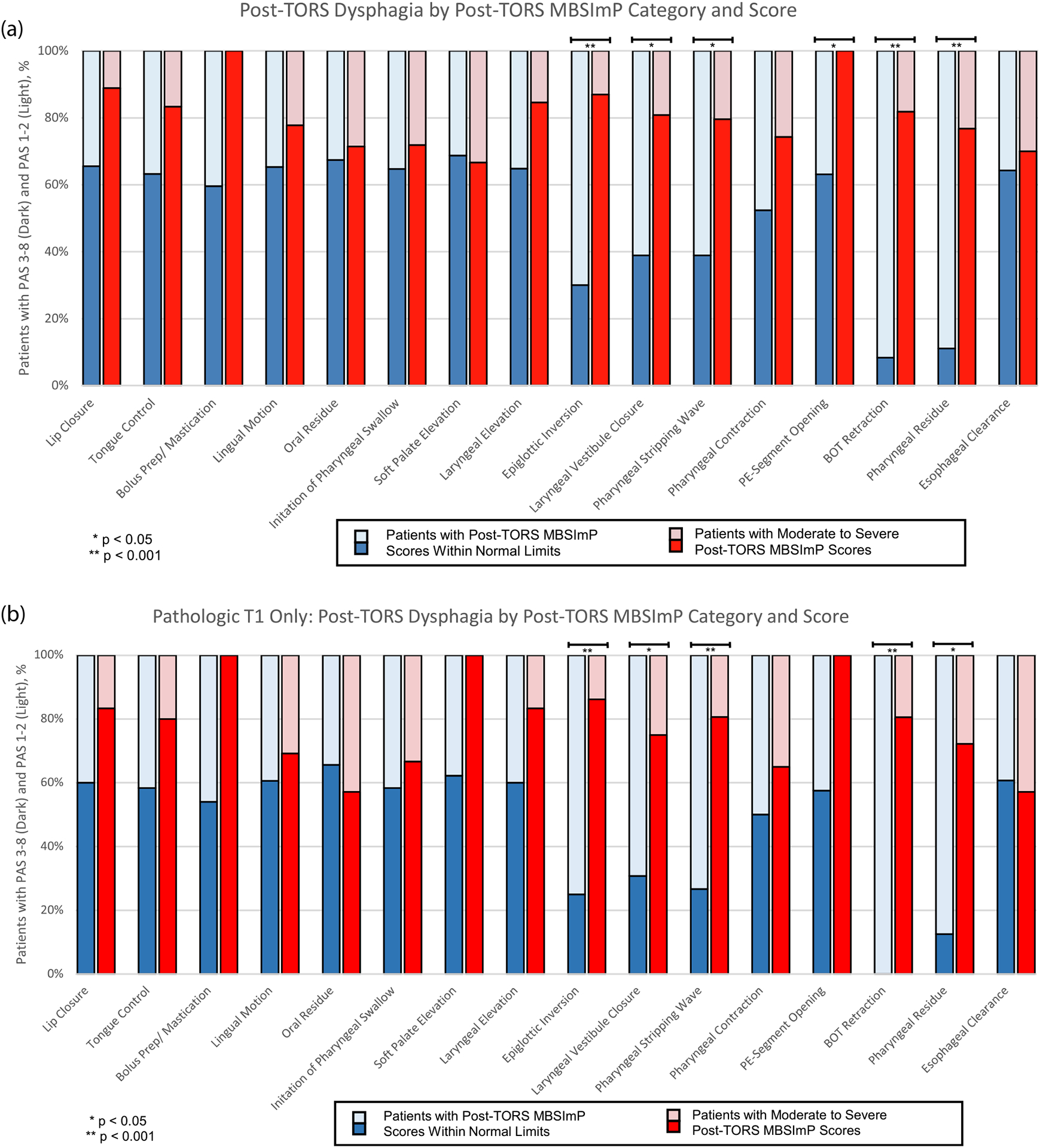
Post-TORS dysphagia as measured by PAS score stratified by post-TORS Modified Barium Swallow Impairment Profile (MBSImP) Category and Score. Each MBSImP category stratifies patients into bins with post-operative MBSImP scores either “within functional limits” or “moderate to severe”. The proportion of patients in each bin with moderate-severe PAS scores 3–8 (darker tones) are represented. Fig. 2a compares CUP patients to tongue base primaries clinically classified as T1. Fig. 2b represents the subset of pathologically matched primary classified tumors, CUP versus pT1.
3 Week post-TORS DIGEST scores
Overall DIGEST scores were not significantly different based on clinical T-classification within a site (i.e. cT1 CUP, n = 27 vs. cT1 BOT n = 40). Unknown primary (67% scored 0–1) scores indicated mild dysphagia on average compared to moderate/severe dysphagia for base of tongue resection (48% scored 0–1, p = 0.122). Subset analysis comparing pathologic matched primary classified tumors (pT1 CUP, n = 27 vs. pT1 BOT, n = 19) signaled a lower likelihood of overall DIGEST scores of 0–1 for tongue base resection (53% p = 0.337, p = 0.195) compared to unknown primary.
Post-TORS impairment (moderate to severe) was observed in >50% of both CUP and BOT primaries in multiple MBSImP categories (epiglottis inversion, laryngeal vestibule closure, pharyngeal stripping wave, pharyngeal contraction, base of tongue retraction, pharyngeal residue) (Fig. 3a and 3b). Bolus preparation/mastication category was more impaired with CUP (p = 0.048) when comparing clinically staged T1 tumors (Fig. 3a), however the relative percentage was low (14.8% vs 0%). The difference was not significant when comparing CUP to only pathologically matched T1 tumors (Fig. 3b).
Fig. 3.
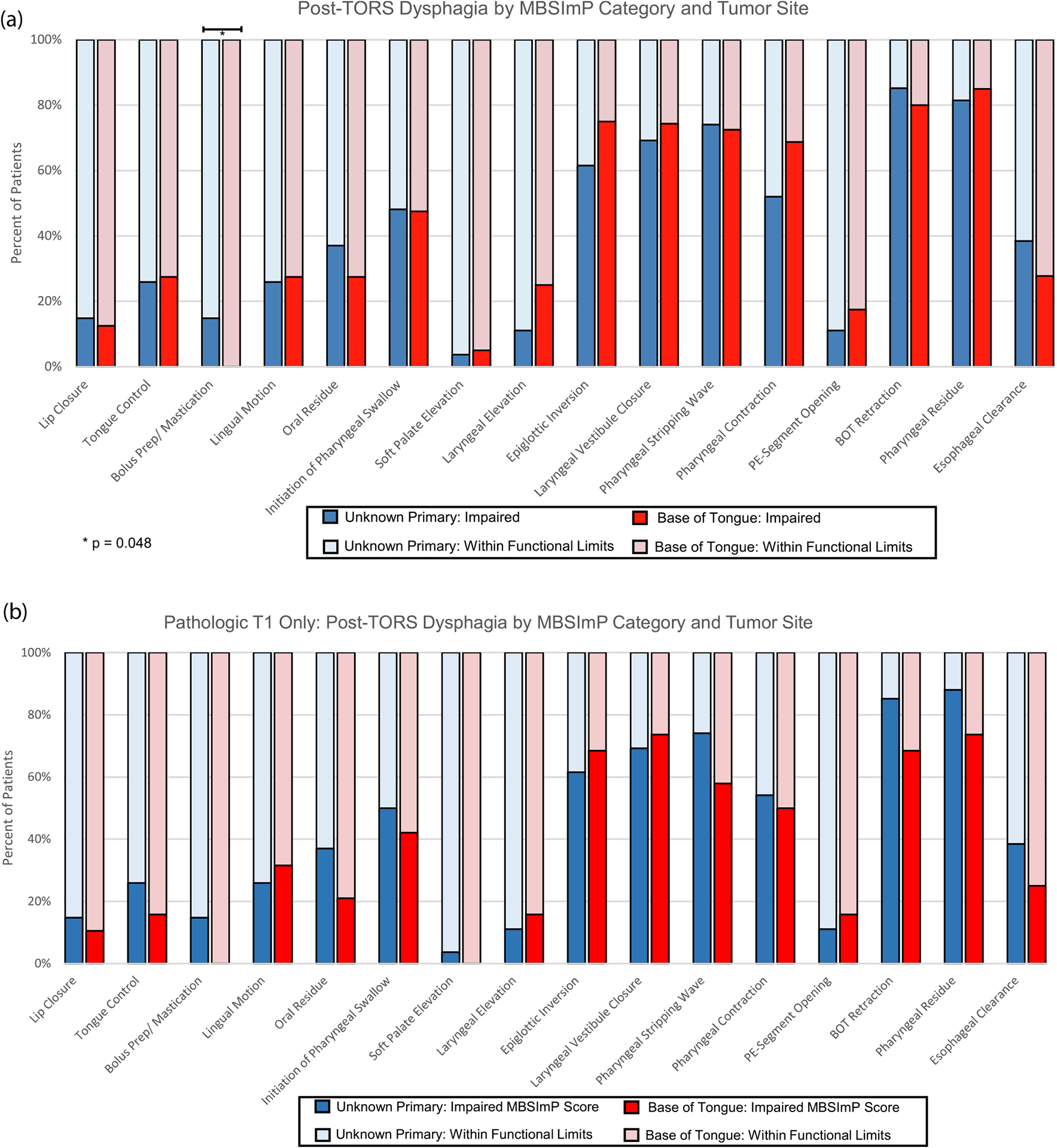
Post-TORS dysphagia as measured by percent of patients with impaired Modified Barium Swallow Impairment Profile (MBSImP) score stratified by MBSImP category and tumor subsite in clinically matched tumors. Proportion of patients with impaired MBSImP score are represented with darker tone bars (3a). The same analysis is represented comparing the subset of pathologically matched primary classified tumors (3b).
DIGEST scores at 3 weeks post-TORS demonstrated that unknown primaries, on average, were not significantly different than base of tongue glossectomy on univariate analysis. Controlling for age, multivariate analysis correlated with improved DIGEST safety scores for TORS unknown primaries (Odds Ratio = 0.28 [p = 0.038]) for clinically matched T1 classified tongue base tumors. When controlling for age while conducting multivariate analysis for pathologic T1 classification (pT1 CUP, n = 27 vs. pT1 BOT, n = 19), the Odds Ratio of moderate/severe dysphagia for CUP was 0.54 (0.12–2.38, p = 0.417) based on DIGEST safety scores and 0.27 (0.06–1.18, p = 0.082) based on DIGEST efficiency scores. Odds ratio of moderate/severe dysphagia as determined by DIGEST overall scores for CUP compared to cT1 and pT1 were 0.39 (p = 0.081) and 0.42 (p = 0.195), respectively.
Discussion
Oropharyngeal CUP poses a difficult challenge. With HPV-associated oropharyngeal cancers on the rise in younger patients with better long-term survival prognoses, the importance of improving quality of life by minimizing injury to swallow function secondary to treatment continues to drive clinical trial designs. Primary tumors in CUP for patients who have p16-positive level II nodal SCC may be small and difficult to visualize, but most often will originate in the oropharynx [14–18]. In the face of CUP, we highlight the challenges that continue to riddle decision making: swallow dysfunction versus accurately detailing subsite location. We found less morbidity following DTLT for CUP on acute swallow compared to clinically apparent T1 tongue base tumor resections. The early swallow morbidity was marginally different when comparing CUP to pT1 tongue base tumor resections. To our knowledge, this is the first study to date detailing the objective impact of TORS after lingual tonsillectomy for CUP on early swallow physiology. In addition, DTLT for p16-positive CUP eliminated intentional radiation to the oropharynx in 74% of the patients.
Through the use of MBSImP, we observed physiologic deficits following TORS that compromised swallowing in the early post-operative period for both CUP and BOT tumors. A majority of patients were found to have impairments in six specific components of pharyngeal swallow: epiglottic inversion (63.0%), laryngeal vestibular closure (69.6%), pharyngeal stripping wave (67.4%), pharyngeal contraction (52.6%), base of tongue retraction (78.3%), and pharyngeal residue (81.8%) (Fig. 3b). Despite the individual components of the MBSImP not identifying a significant difference in swallow function between CUP and BOT primaries, the combined effect on function became clear after analyzing DIGEST scores.
DIGEST safety scores were improved 3 weeks after DTLT for CUP compared to BOT glossectomy [p = 0.038]. The majority of aspiration seen on the post-TORS MBS occurred after the swallow, suggesting these are related to the impairment in driving pressure and resultant residue instead of the inability to protect the airway during the swallow. These impairments resulted in increased pharyngeal residue and necessitated strategies, including multiple swallows and often times a volitional throat clear and re-swallow to attempt to clear the residual and help eject material that invaded the airway post-swallow to prevent further penetration and aspiration events from occurring.
Importantly, longer-term morbidity after TORS lingual tonsillectomy has been studied (19). Patients treated with TORS base of tongue reduction for obstructive sleep apnea (i.e. the equivalent operation of CUP lingual tonsillectomy if bilateral lingual tonsillectomy is performed), were found to have diminished swallow function at 27 months based on findings with fiberoptic endoscopic evaluation of swallowing (FEES). Eighty-five percent of the cohort were found to have vallecular residue, 57% early spillage, and penetration in 35% [19). This study incorporated the Swallowing Performance Status Scale to describe and report swallow function, noting that all patients tested had scores translating to mild and moderate impairment [19]. These findings suggest that a FOIS score of ≥6, indicating an oral diet with no more than one restriction, may not adequately characterize swallow performance. It should be noted that not all CUP algorithms incorporate bilateral lingual tonsillectomies given the risk of a primary originating in the contralateral lingual tonsil is less than 2% [6].
Our reported differences in DIGEST safety scores could be confounded by tumor size, since cancers only found by DTLT are often small, T1 tumors (in our series of CUP, mean tumor size was 0.8 cm [range 0.3–1.9 cm]) that are difficult to detect with imaging (PET/CT) and endoscopy. An MD Anderson study did note early swallow function to negatively correlate with tumor classification, as T1 patients demonstrated better swallow function post-TORS prior to adjuvant treatment than T2 patients [20]. Tumor volume, calculated on contrast-enhanced CT imaging (CECT), correlated with swallow outcome (20). It is plausible that volume loss may be consistent with our findings that CUP DIGEST safety scores are better than scores for clinically apparent BOT tumors. Interestingly, the mean tumor volume calculated by CECT was 5.15 cm3 whereas the volume of resected lingual tissue removed, for OSA was 9.8 cm3 [19,20]. To better characterize tumor volume as a parameter of significance may require volume calculated by displacement as opposed to imaging or gross measurements on pathology reports. While we hypothesize that our findings more than likely correlate with increased loss of native tongue in DTLT for CUP compared to tongue base glossectomy, we did not incorporate tumor volume in our analysis due to lack of visualization of CUP on CECT in addition to adequate volume measurements recorded at the time of surgery.
Clinically, it could be argued that elective RT to the tongue base for CUP does cause the morbidity of DTLT to identify the T1 primary. We have characterized the acute morbidity with DTLT; however, we have no data to compare the morbidity of DTLT plus 50–60 Gy adjuvant RT versus 50–70 Gy RT to the base of tongue. When considering adjuvant therapy, we do not currently incorporate PNI or LVI into our treatment algorithm. While we do incorporate adjuvant RT for PNI for oral cavity primaries, the data regarding PNI as a biomarker for incorporating adjuvant therapy remains inconclusive. A meta-analysis evaluating PNI and LVI as a biomarker for HPV-associated oropharynx carcinoma. PNI (that included 429 patients from 3 studies) did not a yield a significant impact survival p = 0.09 while LVI demonstrated significant impact on survival (n = 305 from 2 studies; p = 0.001) [21]. A single institution study published out of the University of Pittsburgh evaluating the outcome of 201 patients also identified LVI as a negative prognostic indicator on survival (Hazard Ratio 2.54) [22]. While the two studies note LVI as a potential biomarker, caution is advised given the few events in HPV-associated disease. In fact, the aforementioned meta-analysis reported that the presence of high nodal number (>2 positive, n = 355 over 3 studies) did not have a significant impact on survival (P = 0.13) and comment that this may be due to the small number of recurrence events from their single institution series which is incorporated into the analysis (given that high positive nodal number did reach significance when excluding their series (P = 0.006) [21]. The infrequent events should also raise caution to our disease-free survival results, while promising, are limited by follow-up, cohort size, and the fact that this is early-stage disease.
In the event we do not identify the primary with bilateral lingual tonsillectomy, our approach omitted intentional radiation to the oropharynx. Our argument for this strategy is that by removing the tonsillar tissue located at the greatest potential of being the primary site, we may then eliminate irradiation to the oropharynx with the understanding that HPV-driven head and neck cancer rarely arises outside the lymphoid-rich tissues in the palatine and lingual tonsillar tissue and that a small tumor may be missed even on focused pathologic evaluation of tonsillar tissue. We have discussed limiting our diagnostic alogithm to an ipsilateral lingual tonsillectomy given only less than a 2% risk of a tongue base primary contralateral to the nodal disease [6]. If we modify our approach to unilateral lingual tonsillectomy, comparing early swallow outcomes to our current approach may be informative. It is important to emphasize we do not perform bilateral palatine tonsillectomy in the same setting as bilatral lingual tonsillectomy, given the significant risk of pharyngeal stenosis.
A limitation of our investigation is the small sample size, retrospective nature, and limited follow-up. Most importantly, we highlight the concern for selection bias of comparing pT1 to pT2. Our rationale for including this data is that pathologic tumor classification is not available at the time of work-up, rather our decision making is primarily dependent on clinical tumor classification. It is accepted that there is a degree of tumor classification migration after surgery, particularly in the oropharynx, but counseling patients and structuring follow-up based on anticipated swallow function must be done pre-operatively. For this reason, the data with respect to matched clinically classified BOT tumors is included along with pathologically classified matched BOT tumors. The admittingly small sample size lends for the comparison in DIGEST scores to shift when only looking at pathologically T1 BOT tumors versus CUP. For example, the p-value for DIGEST safety scores was no longer less than 0.05 but the odds ratio remained low (0.54). On the other hand, DIGEST efficiency scores were worse for pT1 then the cT1 and the odds ratio for moderate/severe dysphagia for CUP was 0.27 (0.06–1.18, p = 0.082) versus 0.40 (0.12–1.36, p = 0.144), respectively. The overall DIGEST scores did not demonstrate as much of a shift based on clinical T1 classification versus pathologic T1 classification. Without a pooled analysis or a prospective trial that may not be feasible to generate the necessary numbers for pathologic comparisons alone, it is difficult to completely disregard the clinical classification, particularly given the impact on the decision to move forward with surgery. We are in the process of developing a prospective trial that incorporates definitive versus adjuvant RT in pharyngeal sparing techniques to investigate local control and long-term sequelae of sparing radiation to the primary site in patients with small pathological primaries, such as a CUP, at presentation.
Conclusion
Patients undergoing DTLT for CUP have notable early swallow deficits; however, their swallowing function was better preserved compared to patients undergoing TORS for clinically identified tongue base primaries. Further studies comparing the acute and long-term morbidity of elective lingual tonsillectomy by TORS versus elective radiation of the base of tongue are needed.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].https://www.cdc.gov/cancer/hpv/statistics/cases.htm or American Cancer Society. Cancer Facts and Figures 2018; 2018. [Google Scholar]
- [2].Cheraghlou S, Torabi SJ, Husain ZA, et al. HPV status in unknown primary head and neck cancer: prognosis and treatment outcomes. Laryngoscope 2019;129: 684–91. [DOI] [PubMed] [Google Scholar]
- [3].Galloway TJ, Ridge JA. Management of squamous cancer metastatic to cervical nodes with an unknown primary site. J Clin Oncol 2015;33:3328–37. [DOI] [PubMed] [Google Scholar]
- [4].Geltzeiler M, et al. Transoral robotic surgery for management of cervical unknown primary squamous cell carcinoma: updates on efficacy, surgical technique and margin status. Oral Oncol 2017;66:9–13. [DOI] [PubMed] [Google Scholar]
- [5].Hatten KM, O’Malley BW Jr, Bur AM, et al. Transoral robotic surgery-assisted endoscopy with primary site detection and treatment in occult mucosal primaries. JAMA Otolaryngol Head Neck Surg 2017;143:267–73. [DOI] [PubMed] [Google Scholar]
- [6].Farooq S, Khandavilli S, Dretzke J, et al. Transoral tongue base mucosectomy for the identification of the primary site in the work-up of cancers of unknown origin: systematic review and meta-analysis. Oral Oncol 2019. April;1(91):97–106. [DOI] [PubMed] [Google Scholar]
- [7].Gildener-Leapman N, Kim J, Abberbock S, et al. Utility of up-front transoral robotic surgery in tailoring adjuvant therapy. Head Neck 2016. August;38(8):1201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rassekh CH, O’Malley BW Jr, Bewley AF, et al. Feasibility and relevance of level I substation node counts in oropharyngeal carcinoma. Head Neck 2016. August;38(8): 1194–200. [DOI] [PubMed] [Google Scholar]
- [9].Martin-Harris B, Brodsky MB, Michel Y, et al. MBS measurement tool for swallow impairment—MBSImp: establishing a standard. Dysphagia 2008;23:392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia 1996;77:22942301. [DOI] [PubMed] [Google Scholar]
- [11].Crary MA, Mann GC, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 2005. August 1;86(8):1516–20. [DOI] [PubMed] [Google Scholar]
- [12].Hutcheson KA, Barrow MP, Barringer DA, et al. Dynamic imaging grade of swallowing toxicity (DIGEST): scale development and validation. Cancer 2017;123 (1):62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Last AS, Pipkorn P, Chen S, et al. Risk and rate of occult contralateral nodal disease in surgically treated patients with human papillomavirus related squamous cell carcinoma of the base of the tongue. JAMA Otolaryngol-Head Neck Surg 2020. January 1;146(1):50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Klussmann JP, Gultekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol 2003;162:747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Strojan P, Ferlito A, Medina JE, et al. Contemporary management of lymph node metastases from an unknown primary to the neck: I. A review of diagnostic approaches. Head Neck 2013;35:123–32. [DOI] [PubMed] [Google Scholar]
- [16].Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res 2003. December 15;9(17):6469–75. [PubMed] [Google Scholar]
- [17].Boscolo-Rizzo P, Schroeder L, Romeo S, Pawlita M. The prevalence of human papillomavirus in squamous cell carcinoma of unknown primary site metastatic to neck lymph nodes: a systematic review. Clin Exp Metastasis 2015;32:835–45. [DOI] [PubMed] [Google Scholar]
- [18].Schroeder L, Boscolo-Rizzo P, Dal Cin E, et al. Human papillomavirus as prognostic marker with rising prevalence in neck squamous cell carcinoma of unknown primary: a retrospective multicentre study. Eur J Cancer 2017;74:73–81. [DOI] [PubMed] [Google Scholar]
- [19].Paker M, Duek I, Awwad F, et al. Long-term swallowing performance following transoral robotic surgery for obstructive sleep apnea. The Laryngoscope 2019. February; 129(2):422–8. [DOI] [PubMed] [Google Scholar]
- [20].Hutcheson KA, Warneke CL, Yao CM, et al. Dysphagia after primary transoral robotic surgery with neck dissection vs nonsurgical therapy in patients with low-to intermediate-risk oropharyngeal cancer. JAMA Otolaryngol-Head Neck Surg 2019; 145(11):1053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tassone P, Crawley M, Bovenzi C, et al. Pathologic markers in surgically treated HPV-associated oropharyngeal cancer: retrospective study, systematic review, and meta-analysis. Ann Otol, Rhinol Laryngol 2017. May;126(5):365–74. [DOI] [PubMed] [Google Scholar]
- [22].Albergotti WG, Schwarzbach HL, Abberbock S, et al. Defining the prevalence and prognostic value of perineural invasion and angiolymphatic invasion in human papillomavirus-positive oropharyngeal carcinoma. JAMA Otolaryngol-Head Neck Surg 2017. December 1;143(12):1236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]


