Abstract
Background:
Childhood cancer survivors are at risk for chronic pain, yet the impact of pain on daily functioning is not well understood.
Methods:
2,836 survivors (mean[SD] age 32.2[8.5] years; time since diagnosis 23.7[8.2] years) and 343 non-cancer community controls (age 35.5[10.2] years) underwent comprehensive medical, neurocognitive, and physical performance assessments, and completed measures of pain, health-related quality of life (HRQoL), and social functioning. Multinomial logistic regression models, using odds ratios (OR) and 95% confidence intervals (95% CI), examined associations between diagnosis, treatment exposures, chronic health conditions and pain. Relative risks between pain and neurocognition, physical performance, social functioning, and HRQoL were examined using modified Poisson regression.
Results:
18% (95% CI: 16.1–18.9) of survivors versus 8% (95% CI: 5.0–10.9) of controls reported moderate to very severe pain with moderate to extreme daily interference (P<0.001). Severe and life-threatening chronic health conditions were associated with increased likelihood of pain with interference (OR=2.03; 95% CI: 1.62–2.54). Pain with daily interference was associated with an increased risk of impaired neurocognition (attention: RR=1.88, 95% CI: 1.46–2.41; memory: RR=1.65, 95% CI: 1.25–2.17), physical functioning (aerobic capacity: RR=2.29, 95% CI: 1.84–2.84; mobility: RR=1.71; 95% CI: 1.42–2.06), social functioning (inability to hold a job/attend school: RR=4.46, 95% CI: 3.45–5.76; assistance with routine/personal care needs: RR=5.64, 95% CI: 3.92–8.10), and HRQoL (physical: RR=6.34, 95% CI: 5.04–7.98; emotional: RR=2.83, 95% CI: 2.28–3.50).
Conclusions:
Childhood cancer survivors are at risk for pain and associated functional impairments. Survivors should be routinely screened for pain and interventions targeting pain interference are needed.
Keywords: pain, childhood cancer, survivorship, neurocognition, physical function, quality of life
Precis:
Adult survivors of childhood cancer are at increased risk of moderate to very severe pain compared to community controls. In long-term survivors, pain significantly interferes with multiple aspects of daily functioning.
Introduction
Advances in treatment and supportive care have dramatically improved childhood cancer survival such that 84% of children and adolescents diagnosed today survive beyond five-years from diagnosis.1 However, 95% of survivors develop physical, neurocognitive, and/or psychosocial impairments by 45 years of age.2, 3 Of these late-effects, pain has been reported by up to 59% of adult survivors of childhood cancer.4–6 Among survivors, 9% report persistent pain over a 5-year period, 9% worsened pain, 8% persistent pain interference, and 11% worsened pain interference, suggesting the potential chronicity of pain in this population.7
Established risk factors for pain in childhood cancer survivors include a past diagnosis of sarcoma, treatment with amputation or hemi-abdominal radiation,8 history of relapse,9 older age at assessment,10 and female sex.5 Among adult survivors of childhood cancer, pain has been associated with perceived physical performance and cognitive problems,11, 12 psychological distress,12 and diminished health-related quality of life (HRQoL).6 However, less is known about the impact of pain on clinically assessed neurocognitive functioning, measured physical performance abilities, or social functioning among survivors. Because childhood cancer survivors are at-risk for neurocognitive deficits, physical performance impairments, and reduced social attainment secondary to their primary cancer diagnosis and its treatment,13–15 pain may exacerbate the underlying risk of impairment thereby further compromising functional outcomes and quality of life in adulthood.
Given the intrusive and potentially disabling nature of pain, understanding the experience of pain in childhood cancer survivors and its potential impact on daily functioning is important. The aims of this study were to: (1) examine the prevalence of pain and perceived pain interference in a clinically assessed cohort of adult survivors of childhood cancer, (2) identify cancer-related factors associated with pain, and (3) examine associations between pain and neurocognition, physical performance, social functioning, and HRQoL.
Methods
Participants
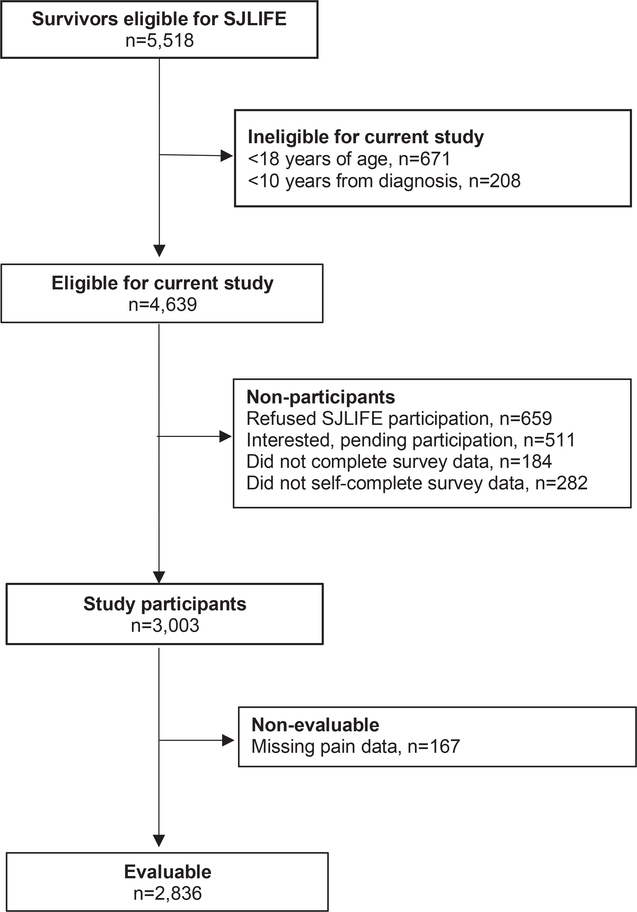
Study participants were enrolled in the St. Jude Lifetime Cohort (SJLIFE), a retrospective cohort with prospective longitudinal clinical assessments of five-year survivors treated for childhood cancer at St. Jude Children’s Research Hospital.16, 17 Participants in the current analysis included 2,836 survivors who were ≥10 years from diagnosis and ≥18 years of age (Figure 1). Survivors were excluded if they did not self-complete study questionnaires. A comparison group of 343 community controls without a history of childhood cancer who were non first-degree relatives of St. Jude patients was included in the current study.
Figure 1.
Participant flow diagram.
Primary Outcomes: Pain and Pain Interference
Pain was assessed using a single item from the Medical Outcomes Survey Short Form survey (SF-36);18 “How much bodily pain have you had during the past 4 weeks?”, and two questions regarding headaches; “Have you ever been told by a doctor or other health care professional that you have or have had: 1) migraines and 2) other severe headaches?” The SF-36 pain item was rated on a 6-point Likert scale anchored by none and very severe. Three response options for the questions on headaches and migraines included: “No”, “Yes, and the condition is still present”, and “Yes, but the condition is no longer present”. Survivors were considered to have pain if they reported either moderate to very severe bodily pain or headaches/migraines with the condition still present. Pain interference was classified using a single item from the SF-36: “During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?”. Reponses of moderate to extreme interference were classified as representing daily interference. The two pain items from the SF-36 have a reported internal consistency of 0.83.19
Secondary Outcomes: Neurocognition, Physical Function, Social Functioning, HRQoL
Neurocognition was assessed across four domains: 1) attention (Conners’ Continuous Performance Test II:20 omissions, variability, and detectability), 2) memory (California Verbal Learning Test – 2nd edition:21 total recall on trials 1–5, short-delay free recall, and long-delay free recall), 3) executive function (Trail Making Test Part B;22 Controlled Oral Word Association Test;23 Wechsler Adult Intelligence Scale – 3rd edition (WAIS-III)24 Digit Span Backward), and 4) processing speed (Trail Making Test Part A;22 WAIS-III24 Coding and Symbol Search). Impairment was considered present if, within a single domain, any one of the three tests yielded a score ≥2.0 standard deviations (SD) below the normative mean.
Physical function was assessed using the following tests: 1) aerobic capacity (6-minute walk test, distance [meters],25 impairment ≤10th percentile of controls), 2) flexibility (sit and reach test,26 [centimeters], impairment ≤10th percentile of controls), 3) strength (three measures; average of best trials for right and left hand/knee/ankle separately, impairment ≤10th percentile of controls adjusted for age and sex; maximal hand grip in kilograms,27 knee extension strength [Newton-meters/kg at 60 degrees/s], and ankle dorsiflexion strength [Newton-meters/kg at 90 degrees/s]), 4) balance (Sensory Organization Test28, 29 [percentage of time spent inside a 12.5° sway envelope, impairment=composite score<70]), 5) mobility (Timed Up and Go [TUG] test30 [time in seconds to complete the test], impairment ≤10th percentile of controls), and 6) adaptive physical function (Physical Performance Test,31 [total score, maximum 28], impairment = lowest tertile of survivors).
Social functioning was assessed via self-report: education (college graduate or higher or <college graduate), employment (full time employment/student/caring for home, part-time employment, or no employment), unable to hold job/attend school because of a health condition (yes/no), marital status (never married, widowed/divorced/separated, or married/living as married), independent living (yes/no), assistance with personal care needs and/or assistance with routine needs (yes/no).
Health-related quality of life was assessed using the SF-3618 subscales of physical functioning, role limitations due to physical problems, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. T-scores ≤40 on any subscale represented reduced HRQoL.
Covariates
Cancer directed treatment exposures within five years from diagnosis were abstracted from medical records. Major treatment-related surgeries were classified as amputation, limb-sparing, or other invasive or radical surgeries. Chronic health conditions were considered by organ system (cardiovascular, endocrine, gastrointestinal, musculoskeletal, neurologic, and respiratory) and graded per a modified version of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE v4.03: mild [grade 1], moderate [grade 2], severe or disabling [grade 3], life-threatening [grade 4]), based on a comprehensive clinical assessment including a complete medical history and physical examination, laboratory tests, and risk-based diagnostic imaging.17
Emotional distress was assessed using the Brief Symptom Inventory 18 (BSI-18).32 The BSI-18 provides subscales for anxiety, depression, and somatization; however, for purpose of this study only the subscales of anxiety and depression were used, because there is considerable overlap between the symptoms assessed on the somatization scale and pain. T-scores ≥63 or more were considered to represent elevated anxiety/depression.
Statistical Analyses
Descriptive data were compared between survivors and controls using χ2-test for nominal variables and Student’s t-test for continuous variables. Multinomial logistic regression was used to compare the prevalence of pain (with or without daily interference) in survivors and controls, adjusting for age at evaluation, sex, and race/ethnicity. Separate multinomial logistic regression analyses were used to examine associations between pain (no pain; pain without daily interference; pain with daily interference) and 1) treatment exposures 2) primary cancer diagnosis, and 3) chronic conditions. All models were adjusted for age at diagnosis and assessment, race/ethnicity, and sex. For functional outcomes, Poisson regression models were used for dichotomous outcomes and multinomial logistic regression models were used for trichotomous outcomes (i.e., employment, marital status). All multivariable models were adjusted for anxiety, depression, sex, and race/ethnicity. Neurocognitive outcome models were adjusted for age at diagnosis, educational attainment, methotrexate (high dose intravenous and/or intrathecal), and cranial radiation therapy. Other models were adjusted for age at evaluation and chronic health conditions. Because of the number of observations, when chronic health conditions were considered by individual organ system, Grades 2–4 were utilized; however, when considered collectively, Grades 3–4 were utilized. Level of significance was set at P<0.05 with no adjustment for multiple comparisons given our a priori hypotheses.33 Analyses were conducted using SAS (SAS Institute, Cary, NC).
Results
Survivors were assessed at an average of (mean(SD) 23.7(8.2) years from diagnosis, at an age of 32.2(8.5) years (Table 1). Thirty-six percent were survivors of leukemia, 9% central nervous system tumors, 21% lymphoma, 7% Ewing sarcoma or osteosarcoma, and 25% other solid tumors. Survivors were treated with surgery (62%), chemotherapy (85%), cranial radiation (32%), and non-cranial radiation (25%).
Table 1.
Demographic, treatment, and clinical characteristics of study sample
| Survivors N=2,836 | Controls N=343 | P-value | |
|---|---|---|---|
| M (SD) | M (SD) | ||
| Age at evaluation, years | 32.2 (8.5) | 35.5 (10.2) | <0.001 |
| Age at diagnosis, years | 8.6 (5.6) | ||
| Time since diagnosis, years | 23.7 (8.2) | ||
| N (%) | N (%) | ||
| Sex | 0.07 | ||
| Male | 1403 (49.5) | 152 (44.3) | |
| Female | 1433 (50.5) | 191 (55.7) | |
| Race/Ethnicity | 0.03 | ||
| White, non-Hispanic | 2354 (83.2) | 288 (87.8) | |
| Other | 474 (16.8) | 40 (12.2) | |
| Diagnosis | |||
| Acute lymphoblastic leukemia | 907 (32.0) | ||
| Acute myeloid leukemia | 103 (3.6) | ||
| CNS tumors | 249 (8.8) | ||
| Non-Hodgkin lymphoma | 213 (7.5) | ||
| Hodgkin lymphoma | 371 (13.1) | ||
| Wilms, neuroblastoma, retinoblastoma, rhabdomyosarcoma | 504 (17.8) | ||
| Soft tissue sarcoma | 78 (2.8) | ||
| Ewing, osteosarcoma | 199 (7.0) | ||
| Other non-CNS solid tumors | 135 (4.8) | ||
| Others | 77 (2.7) | ||
| Radiation | |||
| None | 1218 (43.0) | ||
| Cranial radiation | 899 (31.8) | ||
| Non-cranial radiation | 714 (25.2) | ||
| Radiation dose (cGy; median, range) | |||
| Cranial | 2400 (390–10600) | ||
| Chest/neck | 2600 (300–8000) | ||
| Abdomen/pelvis | 2600 (300–7600) | ||
| Chemotherapy (median, range) | |||
| Vinblastine (mg/m2) | 46.1 (10–1735.4) | ||
| Vincristine (mg/m2) | 24.2 (1.5–152.6) | ||
| Carboplatin (mg/m2) | 2770 (402.6–11058) | ||
| Cisplatin (mg/m2) | 400 (42.9–997.0) | ||
| High dose intravenous methotrexate (mg/m2) | 15587 (829.6–211900) | ||
| Intrathecal methotrexate (mg/m2) | 150 (5.6–797.1) | ||
| Corticosteroids (PED mg/m2) | 3360 (98.0–26961) | ||
| Surgery | |||
| Major therapeutic surgery | 1745 (61.5) | ||
| Amputation/limb-sparing | 147 (5.2) | ||
| Subsequent malignancies | 695 (24.5) | ||
| Body mass index | |||
| Underweight (<18.5) | 91 (3.5) | ||
| Normal (18.5–24.9) | 864 (33.0) | ||
| Overweight (25–29.9) | 751 (28.7) | ||
| Obese (≥30) | 910 (34.8) | ||
| Chronic health conditions (Grades 2–4)* | |||
| Cardiovascular | 871 (32.9) | ||
| Endocrine† | 551 (20.8) | ||
| Gastrointestinal | 758 (28.7) | ||
| Musculoskeletal | 623 (23.6) | ||
| Neurological‡ | 441 (16.7) | ||
| Pulmonary | 542 (20.5) | ||
| Any Grade 3–4 chronic health condition | 1046 (39.5) | ||
| Analgesic use | |||
| Non-opioid analgesics | 408 (14.4) | ||
| Opioid analgesics | 169 (6.0) | ||
Based on Common Terminology Criteria for Adverse Events.
Excluding obesity.
Excluding headaches.
PED = prednisone equivalent dose.
Prevalence of pain
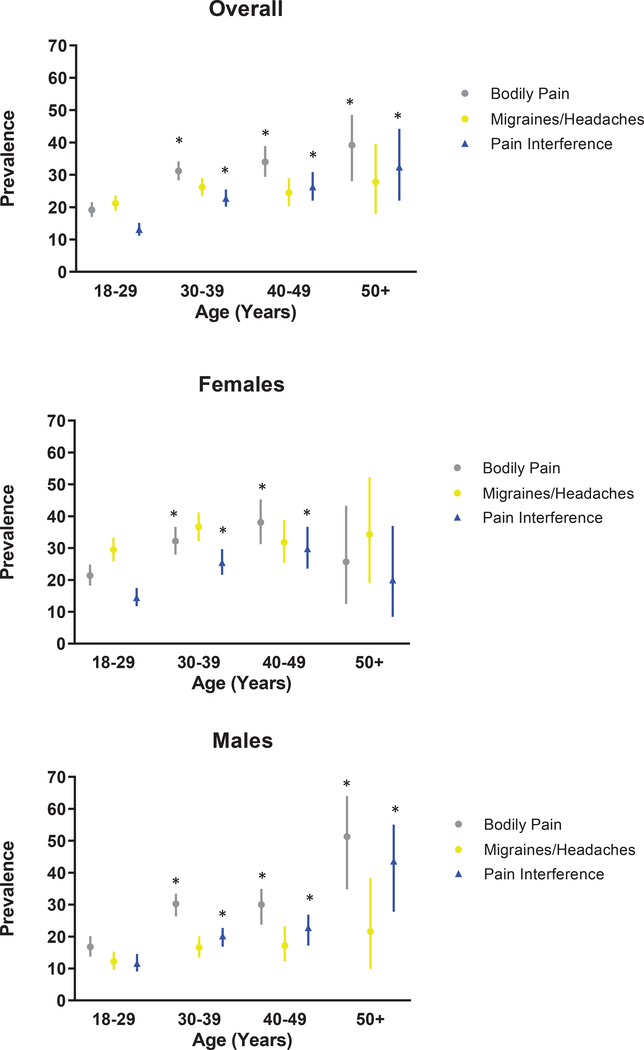
Twenty-seven percent of survivors (95% CI, 24.9–28.2) reported moderate to very severe bodily pain and 24% (95%CI, 22.0–25.2) reported migraines or other severe headaches (Table 2). Compared to non-cancer community controls, with adjustment for age, sex, and race/ethnicity, survivors were 3.4 times more likely to report pain with daily interference (17.5% vs. 7.6%; OR=3.4, 95% CI, 2.2–5.2), while there was no significant difference in the prevalence of pain without daily interference. The prevalence of bodily pain and pain interference was significantly higher among survivors >30 years of age compared to survivors 18–29 years of age, except for women >50 years of age (Figure 2; Supplemental Table 1). Supplemental Figure 1 shows the overlap between pain, pain interference, and analgesic use in our sample. Among survivors who reported analgesic use, 65% reported pain with or without pain interference. Characteristics of survivors who reported taking analgesics by pain status are shown in Supplemental Table 2.
Table 2.
Prevalence of pain in survivors and non-cancer community controls
| Survivors | Controls | P-value* | |
|---|---|---|---|
| N (%; 95% CI) | N (%; 95% CI) | ||
| Pain | <0.001 | ||
| Bodily pain (moderate to very severe) | 750 (26.5; 24.9–28.2) | 50 (14.6; 11.0–18.8) | |
| Migraines or other severe headaches (yes, still present) | 656 (23.6; 22.0–25.2) | 65 (19.2; 15.1–23.8) | |
| Pain interference (moderate to extreme) | 544 (19.2; 17.8–20.7) | 30 (8.8; 6.0–12.3) | |
| Pain and interference | <0.001 | ||
| Pain with daily interference† | 496 (17.5; 16.1–18.9) | 26 (7.6; 5.0–10.9) | |
| Pain without daily interference‡ | 607 (21.4; 19.9–23.0) | 73 (21.3; 17.1–26.0) | |
| No pain | 1733 (61.1; 59.3–62.9) | 244 (71.1; 66.0–75.9) | |
P-value calculated using Chi-square test.
Moderate to very severe bodily pain or migraines or other severe headaches that are still present AND moderate to extreme pain interference.
Moderate to very severe bodily pain or migraines or other severe headaches that are still present AND less than moderate to extreme pain interference.
Figure 2.
Prevalence of pain overall and by sex and age among adult survivors of childhood cancer.
P-values calculated for each age group vs. 18–29 years, separately for bodily pain, migraines/headaches, and pain interference.
*Indicates P<0.001.
Diagnosis and treatment exposures associated with pain
Survivors of soft tissue sarcoma (OR=9.25, 95% CI, 4.80–17.8), non-Hodgkin lymphoma (OR=4.13, 95% CI, 2.40–7.10), and Ewing/osteosarcoma (OR=3.93, 95% CI, 2.23–6.92) had the highest odds of pain with daily interference compared to controls (Supplemental Table 3). In multivariable models including treatment exposures, history of amputation (OR=1.89, 95% CI, 0.90–3.94) and limb sparing surgery (OR=2.30, 95% CI, 1.03–5.17) were associated with increased odds of pain with daily interference (Supplemental Table 4).
Chronic health conditions associated with pain
Survivors with any grade 3 to 4 chronic health condition had 2-fold increased odds of pain with daily interference and 1.6-fold increased odds of pain without daily interference compared to survivors without severe to life-threatening chronic health conditions (Supplemental Table 5). Survivors who were overweight or obese also had increased odds of pain with daily interference (OR=1.60, 95% CI, 1.26–2.03). Associations between individual organ systems (e.g., cardiac, musculoskeletal, neurological) and pain are presented in Supplemental Table 6. Specifically, grade 2–4 musculoskeletal, neurologic, gastrointestinal, cardiovascular, and pulmonary conditions were associated with increased risk of pain with interference.
Neurocognitive functioning
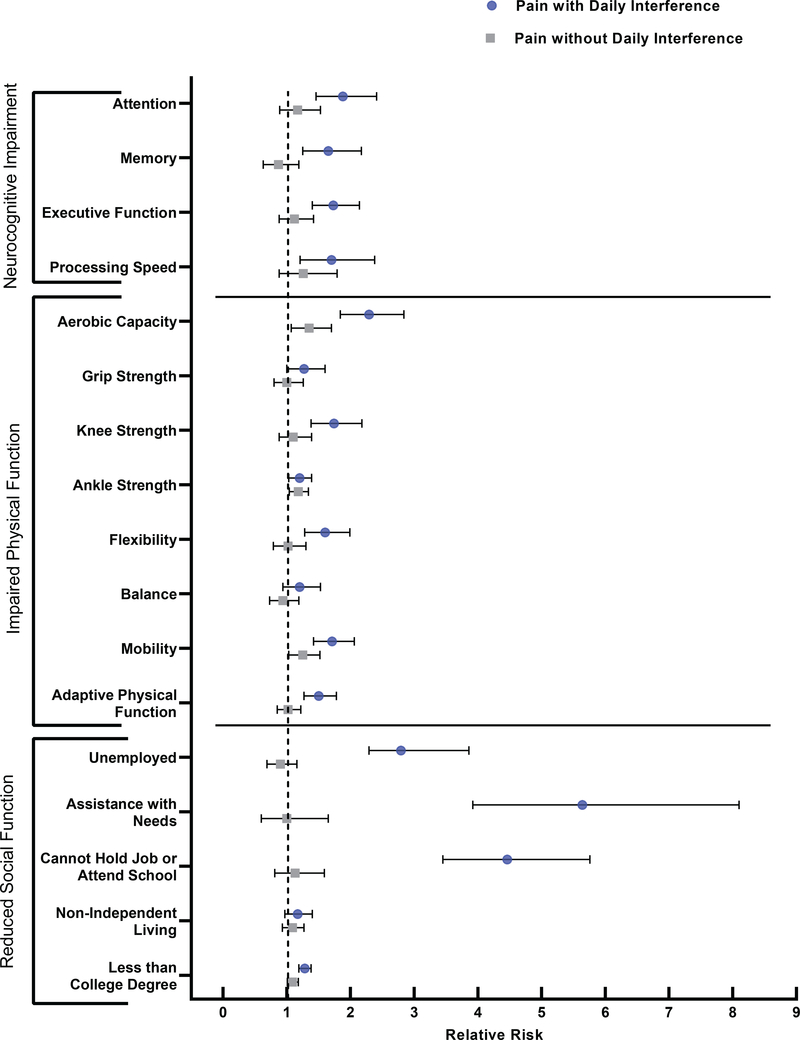
In multivariable models adjusted for neurotoxic treatment exposures, survivors who reported pain with daily interference were at 65 to 90% increased risk of impaired attention (RR=1.88, 95% CI, 1.46–2.41), memory (RR=1.65, 95% CI, 1.25–2.17), executive function (RR=1.73, 95% CI, 1.40–2.14), and processing speed (RR=1.70, 95% CI, 1.21–2.38) compared to survivors with no pain (Figure 3 & Supplemental Table 7). Pain in the absence of daily interference was not associated with increased risk of neurocognitive impairment.
Figure 3.
Pain and relative risk of impaired functional outcomes.
Relative Risks (RRs) and 95% Confidence Intervals (CIs) shown. RRs for pain with daily interference and pain without daily interference compared to no pain.
Physical performance outcomes
Pain with daily interference was associated with impaired performance on nearly all measures of physical function (Figure 3 & Supplemental Table 8). In multivariable models adjusted for chronic health conditions and obesity, pain with daily interference was associated with impaired aerobic capacity (RR=2.29, 95% CI, 1.84–2.84), strength (knee: RR=1.52, 95% CI, 1.21–1.92; ankle: RR=1.20, 95% CI, 1.04–1.99), flexibility (RR=1.60, 95% CI, 1.28–1.99), mobility (RR=1.71, 95% CI, 1.42–2.06), and adaptive physical function (RR=1.50, 95% CI, 1.27–1.78).
Social functioning
Survivors who reported pain with daily interference were at increased risk of unemployment (RR=2.97, 95% CI, 2.29–3.86) or part-time work (RR=1.99, 95% CI, 1.32–2.99) compared to working full time and earning less than a college degree (RR=1.28, 95% CI, 1.19–1.38) (Figure 3 & Supplemental Table 9). Although pain was not significantly associated with survivors’ risk of living non-independently, survivors with pain with daily interference were at nearly 6-fold increased risk of needing assistance with personal and/or routine care needs (RR=5.64, 95% CI, 3.92–8.10).
Health-related quality of life
In multivariable models adjusted for chronic health conditions and psychological distress, survivors who reported pain with daily interference were at significantly increased risk of reduced physical and mental HRQoL compared to survivors with no pain (Supplemental Table 10). This risk ranged from a 2-fold increased risk for reduced mental health (RR=1.90, 95% CI, 1.62–2.23) to a 6-fold increased risk for difficulty completing activities because of physical role limitations (RR=6.34, 95% CI, 5.04–7.98).
Discussion
In this large, prospectively assessed cohort of childhood cancer survivors, we observed a high prevalence of pain associated with clinically relevant decrements in physical, cognitive, and social functioning and HRQoL. To our knowledge, this is the first study to comprehensively quantify multiple clinically assessed functional morbidities associated with pain in adult survivors of childhood cancer. These results are clinically actionable, as pain and pain interference are behaviorally modifiable constructs that can serve as key intervention targets to optimize functional outcomes in survivors.
We observed a greater prevalence of pain in survivors compared with community controls, with 39% of survivors reporting moderate to very severe pain in the past month, with (18%) or without (21%) moderate to extreme daily interference. While past studies have also reported greater pain among survivors than siblings,5 our results expand upon those data through consideration of pain severe enough to impact daily functioning. This is a more clinically relevant phenotype as interference with daily functioning would very likely necessitate the need for intervention. Moreover, our results demonstrate that the prevalence of bodily pain and pain interference is higher among older survivors. This may suggest worsening of persistent pain or new onset of pain as survivors age and develop chronic health conditions.7
Our results indicate that severe to life-threatening chronic health conditions were associated with 2-fold increased risk of pain, specifically, musculoskeletal, neurologic, gastrointestinal, and cardio-pulmonary conditions. These associations are not particularly surprising but highlight the importance of considering survivors’ current health status, in addition to distal treatment exposures, when anticipating survivors’ risk of developing pain. Our results are consistent with recent conceptualizations of pain among childhood cancer survivors, which highlight the role that chronic health conditions may play in contributing to the development and maintenance of persistent or chronic pain.34
Pain was associated with several functional outcomes in our study. After adjustment for neurotoxic treatment exposures, pain with daily interference was associated with >50% increased risk of impaired performance on measures of attention, memory, executive function, and processing speed. In non-cancer populations, both acute and chronic pain have been associated with reduced neurocognitive performance.35, 36 In a review of both clinical and preclinical research, Moriarty, et al. 36 suggests three potential mechanisms: 1) competing or limited cognitive resources during the experience of pain, 2) neuroplastic changes in the brain secondary to pain interfering with cognition, and/or 3) neurochemical responses to pain that may adversely affect cognition. Future research is needed to better understand potential mechanisms underlying these associations in childhood cancer survivors.
We also observed that pain with daily interference was associated with reduced aerobic capacity, muscle strength, flexibility, mobility, and adaptive physical function. Importantly, associations between pain and physical function are likely to be bidirectional,10 such that increased pain or fear of pain may limit one’s ability to engage in physical activities, and greater sedentary behavior, may in turn, exacerbate existing pain or result in the development of new pain. Physical performance interventions delivered early in survivorship may be of particular benefit to prevent the onset of or break negative reciprocal interactions between pain and physical activity.37
We observed striking associations between pain and engagement in or attainment of excepted adult social outcomes. For example, survivors who reported pain with daily interference were at 4.5-fold increased risk of not being able to hold a job or attend school and 5.6-fold increased risk of needing assistance with routine or personal care needs. These results suggest the potential for significant disability and/or disease burden resulting from pain among survivors. While this increased burden associated with pain is consistent with results from the general population,38 it is particularly concerning for a medically vulnerable population of survivors who face a substantial burden of chronic health conditions secondary to their childhood cancer treatment.39 Lastly, pain was associated with reduced HRQoL in survivors. These results underscore the adverse impact of pain on the quality of survivors physical and mental health several decades following their diagnosis and treatment.
Our results highlight the need for heightened surveillance via the administration of developmentally informed pain assessments at all stages of the survivorship trajectory. We observed that a large proportion of survivors who reported prescription analgesic use also reported moderate to very severe pain, suggesting that medication management alone may not be sufficient treatment. Strong evidence exists for the efficacy of cognitive behavioral therapy (CBT) among adults in the general population with chronic pain40 and evidence is beginning to emerge for third wave CBT approaches such as acceptance and commitment therapy (ACT).41 These interventions may also benefit survivors, however; it should be noted that no randomized trials targeting chronic pain among survivors of childhood currently exist.
The current study should be considered in the context of several limitations. Apart from diagnosis and treatment data, all data are cross-sectional and preclude inference of causal or temporal relations. Survivors were asked to report their experience of pain over the past month and therefore our results do not speak to the impact of chronic pain on functional outcomes. Because only a small number of our survivors reported use of opioid analgesics (6%) the independent impact of these medications on functional outcomes could not be reliably assessed in multivariable models (e.g., neurocognitive function).12 Moreover, non-pharmacologic treatments for pain such as CBT, ACT, yoga, and physical therapy were not assessed. Nonetheless, our results demonstrate that a substantial proportion of adult survivors of childhood cancer experience pain that adversely impacts multiple aspects of daily functioning. Results also highlight the need for routine assessment of pain and related factors within the context of survivorship care. The need for interventions that target both pain and pain interference while considering the complex burden of late effects experienced by many long-term survivors of childhood cancer is evident. Future research should incorporate multidimensional measures of pain, including assessment of pain duration, location and specific causes (e.g., pelvic pain, neuropathic pain in diabetes, postoperative pain) as well as factors associated with aging-related changes in pain among survivors. It will be important for future research to consider factors associated with resilience in relation to the experience of pain in survivors, including pain acceptance, positive affect, and adaptive pain beliefs. Finally, potential mediating factors, including aspects of behavioral health, such as sleep and sedentary behavior should be considered when examining associations between pain and functional outcomes.
Supplementary Material
Supplemental Figure 1. Relative proportions and overlap among pain, pain interference, and analgesic use. Analgesic use includes opioid and non-opioid analgesics.
Acknowledgments
Funding: This study was supported by the National Cancer Institute (CA195547, M. Hudson and L. Robison, Principal Investigators). Support to St. Jude Children’s Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1): 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22): 2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15): 1572–82. [DOI] [PubMed] [Google Scholar]
- 4.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12): 1583–92. [DOI] [PubMed] [Google Scholar]
- 5.Lu Q, Krull KR, Leisenring W, et al. Pain in long-term adult survivors of childhood cancers and their siblings: a report from the childhood cancer survivor study. Pain. 2011;152(11): 2616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang IC, Brinkman TM, Kenzik K, et al. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort study. J Clin Oncol. 2013;31(33): 4242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlson CW, Alberts NM, Liu W, et al. Longitudinal pain and pain interference in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. Cancer. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crom DB, Chathaway DK, Tolley EA, Mulhern RK, Hudson MM. Health status and health-related quality of life in long-term adult survivors of pediatric solid tumors. Int J Cancer Suppl. 1999;12: 25–31. [DOI] [PubMed] [Google Scholar]
- 9.Essig S, von der Weid NX, Strippoli MP, et al. Health-related quality of life in long-term survivors of relapsed childhood acute lymphoblastic leukemia. PLoS One. 2012;7(5): e38015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeltzer LK, Lu Q, Leisenring W, et al. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2008;17(2): 435–46. [DOI] [PubMed] [Google Scholar]
- 11.Ness KK, Hudson MM, Ginsberg JP, et al. Physical performance limitations in the childhood cancer survivor study cohort. J Clin Oncol. 2009;27(14): 2382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinkman TM, Zhang N, Ullrich NJ, et al. Psychoactive medication use and neurocognitive function in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor study. Pediatr Blood Cancer. 2013;60(3): 486–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurney JG, Krull KR, Kadan-Lottick N, et al. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14): 2390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krull KR, Hardy KK, Kahalley LS, Schuitema I, Kesler SR. Neurocognitive Outcomes and Interventions in Long-Term Survivors of Childhood Cancer. J Clin Oncol. 2018;36(21): 2181–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ness KK, Mertens AC, Hudson MM, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med. 2005;143(9): 639–47. [DOI] [PubMed] [Google Scholar]
- 16.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56(5): 825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(5): 666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware JE Jr. SF-36 health survey update. Spine (Phila Pa 1976). 2000;25(24): 3130–9. [DOI] [PubMed] [Google Scholar]
- 19.Hays RD, Sherbourne CD, Mazel R. User’s Manual for the Medical Outcomes Study (MOS) Core Measures of Health-Related Quality of Life. Available from URL: https://www.rand.org/pubs/monograph_reports/MR162.html [accessed 09/01/2020, 2020].
- 20.Conners CK. Conners Continuous Performance Test II. North Tonawanda, NY: Multi-Health Systems Inc., 2001. [Google Scholar]
- 21.Delis DC, Kramer JH, Kaplan E, Ober. California Verbal Learning Test - Second Edition. 2 ed. San Antonio, TX, 2000. [Google Scholar]
- 22.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2): 203–14. [DOI] [PubMed] [Google Scholar]
- 23.Strauss E, Sherman EMS, Spreen OA. A compendium of neuropsychological tests: administration, norms and commentary. Third ed. Oxford: Oxford University Press, 2006. [Google Scholar]
- 24.Wechsler D Wechsler Adult Intelligence Scale - third edition, technical manual. San Antonio, TX: The Psychological Corporation, 1997. [Google Scholar]
- 25.American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1): 111–7. [DOI] [PubMed] [Google Scholar]
- 26.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription Baltimore, MD: Lippincott Williams & Wilkins, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9(2): 222–6. [DOI] [PubMed] [Google Scholar]
- 28.Ford-Smith CD, Wyman JF, Elswick RK Jr., Fernandez T, Newton RA. Test-retest reliability of the sensory organization test in noninstitutionalized older adults. Arch Phys Med Rehabil. 1995;76(1): 77–81. [DOI] [PubMed] [Google Scholar]
- 29.Nashner LM. Computerized dynamic posturography. In: Jacobson GP, Newman CW, Kartush JM, editors. Handbook of Balance Function Testing. Norwich, UK: Singular, 1993:280–307. [Google Scholar]
- 30.Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1): 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc. 1990;38(10): 1105–12. [DOI] [PubMed] [Google Scholar]
- 32.Derogatis L Brief Symptom Inventory (BSI): administration, scoring, and procedures manual. Minneapolis, MN: NCS Pearson, 2000. [Google Scholar]
- 33.Rothman KJ. Six persistent research misconceptions. J Gen Intern Med. 2014;29(7): 1060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alberts NM, Gagnon MM, Stinson JN. Chronic pain in survivors of childhood cancer: a developmental model of pain across the cancer trajectory. Pain. 2018;159(10): 1916–27. [DOI] [PubMed] [Google Scholar]
- 35.Attridge N, Eccleston C, Noonan D, Wainwright E, Keogh E. Headache impairs attentional performance: a conceptual replication and extension. J Pain. 2017;18(1): 29–41. [DOI] [PubMed] [Google Scholar]
- 36.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93(3): 385–404. [DOI] [PubMed] [Google Scholar]
- 37.Mustian KM, Sprod LK, Janelsins M, Peppone LJ, Mohile S. Exercise recommendations for cancer-related fatigue, cognitive impairment, sleep problems, depression, pain, anxiety, and physical dysfunction: a review. Oncol Hematol Rev. 2012;8(2): 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray CJ, Barber RM, Foreman KJ, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386(10009): 2145–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St. Jude lifetime cohort study (SJLIFE). Lancet. 2017;390(10112): 2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol. 2014;69(2): 153–66. [DOI] [PubMed] [Google Scholar]
- 41.Hughes LS, Clark J, Colclough JA, Dale E, McMillan D. Acceptance and commitment therapy (ACT) for chronic pain: a systematic review and meta-analyses. Clin J Pain. 2017;33(6): 552–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Relative proportions and overlap among pain, pain interference, and analgesic use. Analgesic use includes opioid and non-opioid analgesics.