Summary:
Relapsed or refractory non-Hodgkin lymphomas (NHLs) often carry poor prognosis and pose management challenges. We evaluated the safety and efficacy of dasatinib, a broad-spectrum multi-kinase inhibitor in relapsed/refractory NHL with correlative genomic analysis in a Phase I/II trial. The study included 33 patients with various sub-types of NHL who had received at least one prior therapy. The most common sub-types were diffuse large B-cell lymphoma (24%), follicular lymphoma, grade 1/2 (21%) and peripheral T-cell lymphoma not otherwise specified (PTCL-NOS; 21%). Most patients were heavily pre-treated, including 42% with more than four prior therapies, 67% with rituximab exposure and 24% with prior autologous transplant. In this cohort, dasatinib showed modest activity in evaluable patients with an objective response rate of 29% (7/24) and clinical benefit rate of 71% (17/24). In 32 patients with outcome data, median progression-free survival was 3 months and median overall survival was 22.4 months. There were two patients with sustained complete responses, both with PTCL-NOS histology. The side effect profile was consistent with prior studies, with pleural effusion being the most common non-haematological toxicity. Exploratory genomic analysis showed two cases of PTCL-NOS with sustained response had a common mutation in LRRK2 and high prevalence of FOXO1 mutation in relapsed/refractory follicular lymphoma.
Keywords: Dasatinib, relapsed/refractory lymphoma, NHL, Phase 2 study, mutation analysis
Introduction:
Non-Hodgkin lymphoma (NHL) is one of the most prevalent cancers in the Western world with an increasing incidence in US (Zelenetz, et al 2010) This group of lymphomas encompasses a heterogeneous group of diseases with a wide range of histology, pathogenesis and clinical course ranging from indolent to aggressive diseases. The most common indolent subtype of B-cell lineage is follicular lymphoma (FL), and that of the T-cell lineage is cutaneous T-cell lymphoma (CTCL), whereas aggressive subtypes include diffuse large B-cell lymphoma (DLBCL) and peripheral T-cell lymphoma (PTCL), representing approximately 80% of all NHL diagnoses in adults (Swerdlow et al 2016). There is often patient-to-patient variability in clinical course, with a significant proportion of patients relapsing or refractory to first line therapies. While many treatment difficulties exist in NHL, relapsed/refractory disease represents a major challenge and is currently under intense investigation, especially in the rituximab era for B-cell lineage lymphomas.
The use of the monoclonal antibody rituximab, as a single agent or in combination with cytotoxic chemotherapy, is currently the standard of care in the first line setting for B-cell NHLs and has significantly improved the prognosis of affected patients. However, rituximab resistance has been reported in multiple NHL subtypes, including relapsed FL or low-grade NHL, and is associated with poor prognosis (Davis, et al 2000, Hagberg and Gisselbrecht 2006, Martin, et al 2008). Patients with PTCL generally have a poor prognosis with current standard-of-care therapy, and no progress in their outcome has been achieved in the past two decades (Xu and Liu 2014). The prognosis of relapsed PTCL is very poor and novel effective treatments are urgently needed.
Autologous haematopoietic stem cell transplantation has been associated with extended survival in relapsed/refractory NHL, however the Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) demonstrated that, in the post-rituximab era, patients with DLBCL, the most common type of NHL in the US, derive less benefit from autologous transplant, with a dismal progression-free survival (PFS) of 20% at 3 years (Gisselbrecht, et al 2010). As patients relapse after autologous transplant, progress through multiple lines of treatment, the subsequent responses achieved are incrementally shorter, with eventual exhaustion of meaningful options. This clearly highlights the need for additional safe, effective and targeted agents for this high-risk population. Several novel classes of drugs, such as next generation monoclonal antibodies, antibody–drug or radioactive isotope conjugates and specific small-molecule inhibitors of oncogenic pathways, are emerging as therapeutic options in NHL. However, despite the recent array of drug approvals, long-term remission remains elusive for a significant proportion of NHL patients, particularly those with relapsed/refractory disease (Crump, et al 2017). In this context, we studied the safety and efficacy of single agent dasatinib in patients with relapsed/refractory NHL.
Dasatinib, originally developed as a pan-Src kinase inhibitor, is a potent and broad-spectrum multi-kinase inhibitor with proven safety and efficacy in chronic myeloid leukaemia (CML) and Philadelphia chromosome positive acute lymphoblastic leukaemia (Ph+ ALL) (Cortes, et al 2016, Lilly, et al 2010). Dasatinib has selective 100-fold higher affinity for ABL1 than imatinib mesylate (Shah, et al 2004), and targets several tyrosine kinase families that are implicated in cell survival in NHLs (Brave, et al 2008, Sprangers, et al 2006). The half-life of the drug is approximately 5 h, shown to be well tolerated in CML patients (Talpaz et al. 2004), and achieves sustained inhibition of Lyn kinase, which is critical for B-cell survival. Given that Lyn could be inhibited by dasatinib at tolerable doses, we initiated this phase 1/2 trial in patients with relapsed NHLs.
Materials and Methods:
Patients:
Eligible patients were 19 years or older with biopsy-proven relapsed/refractory NHL, either with primary refractory disease or relapsed after at least after one prior therapy. Histological diagnosis was made by two expert haematopathologists. Other eligibility criteria included Eastern Cooperative Oncology Group performance status score of 0–2, absolute neutrophil count ≥ 1 × 109/l, platelet count ≥50 × 109/l, total bilirubin < 2 × upper limit of normal, hepatic transaminases ≤ 2.5 × upper limit of normal and serum creatinine < 3 × upper limit of normal. Prior chemotherapy or radiation must have ended at least three weeks before study enrolment. Major exclusion criteria included history of other malignancies requiring systemic treatment within the previous 3 years, significant pleural or pericardial effusions, known bleeding and cardiac disorders. Pregnant or breast-feeding women and those unable to use an acceptable method of contraception were also excluded.
Study design:
This was an open label, Phase I/II trial (https://clinicaltrials.gov/ct2/show/NCT00550615). The primary end-point was maximum tolerable dose of dasatinib in the phase I stage and objective response rate (ORR) in the phase II stage. Dosing was continuous without interruptions, unless indicated. Patients underwent treatment until progression of disease or intolerable toxicity. Patients were evaluated for adverse events and dose-limiting toxicities at each visit using the National Cancer Institute Common Terminology Criteria for Adverse Events Criteria version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). The study protocol included pre-specified guidelines for dose reductions or discontinuation of therapy based on toxicity assessments. Patients underwent restaging every two cycles of therapy. Based on revised response criteria for malignant lymphoma (Cheson, et al 2007), response to therapy was classified as complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), early death or not evaluable (NE). All subjects were followed for survival analysis. All patients signed written informed consents. The study was conducted in accordance with the International Conference on Harmonization for Good Clinical Practice. Bristol-Myers Squibb (New York, NY, USA) supplied dasatinib.
Exploratory genomic analysis using next generation sequencing:
Archived formalin-fixed paraffin-embedded samples from 22 patients were successfully sequenced using the FoundationOne® Heme assay (Foundation Medicine, Cambridge, MA, USA), while 11 samples failed extraction. This next generation sequencing platform interrogated the entire coding sequence of 406 genes, selected introns of 31 genes involved in rearrangements, and utilizes RNA sequencing to interrogate 265 genes known to be somatically altered in human haematological malignancies (He, et al 2016). A minimum of 20% tumour content was required. Samples with median coverage <150x were excluded as sequencing failures. The mutant-allele frequency cut-off points were 1% for somatic base substitutions, 5% for putative driver somatic variants, 3% for previously described indels and 10% for putative driver indels for tumour suppressor genes. Gene amplifications/gains were defined at a copy number ≥6 and gene losses as copy number of 0. A minimum of 10 reads were needed for known fusions, and 50 for putative driver rearrangements. Any aberration not meeting stated criteria was defined as unknown significance. Details of methods have been published previously (Frampton, et al 2013, He, et al 2016). The data analysis was performed as previously described (Bouska, et al 2017a).
Statistical analysis:
Phase 1 employed a standard 3+3 dose-escalation. Maximum tolerable dose was defined as the next lowest dose level below which ≥ 2/3 or ≥ 3/6 patients experienced dose-limiting toxicities in cycle 1. The phase II stage used an optimal Simon two-stage design (Simon 1989). The following sample size calculation was performed prior to the start of the phase II portion of the study. With a power of 80%, at an alpha level of 0.05, and assuming that at least 30% of the population would respond to the treatment and that a response rate less than 10% was considered unacceptable, 10 evaluable patients were enrolled in the first stage. Evaluable patients were defined as those completing 2 cycles of therapy with documented response or documented progression of disease prior to completion of 2 cycles of therapy. Up to a maximum of 20 patients were to be enrolled in the first stage to obtain the 10 evaluable patients. When at least two of the 10 evaluable patients had a positive response, we continued to enrol patients to 29 evaluable patients in Stage 2 of the Phase II portion of this trial.
Response rates were calculated for phase I and phase II portions combined with exact 95% confidence intervals (CI). The Kaplan-Meier method was used to estimate the overall survival (OS) and PFS distributions. OS was calculated as the time from diagnosis to the date of death or last contact. Patients who were alive and failure-free at last contact were censored. PFS was calculated as the time from diagnosis to either: date of progression, death or last contact. Patients who were alive at last contact and who had not progressed were censored for the event-free survival analysis. The data analysis was conducted using SAS version 9.4 for personal computers (SAS Institute Inc., Cary, NC, USA).
Results:
Patient characteristics
The study enrolled 33 patients, including 14 patients in Phase 1 and 19 patients in phase II. Table I shows the demographics and baseline disease characteristics of the group. There were 20 men and 13 women with a median age of 59 years (range: 34–87 years). The most common NHL sub-type was DLBCL (24%), followed by FL, grade 1–2 (21%) and PTCL not otherwise specified (PTCL-NOS; 21%). Among prior treatment characteristics, 42% had received more than four lines of therapy, 67% had rituximab exposure and 24% had undergone autologous stem cell transplantation. The median duration of treatment with dasatinib was 7 cycles.
Table I.
Baseline characteristics of the study group
| Phase I N=14 | Phase II N=19 | Phase I/II N=33 | |
|---|---|---|---|
| Gender | |||
| Female | 6 (43%) | 7 (37%) | 13 (39%) |
| Male | 8 (57%) | 12 (63%) | 20 (61%) |
| Age (years) | |||
| Median (range) | 59 (49, 85) | 61 (34, 87) | 59 (34, 87) |
| Primary diagnosis | |||
| AITL | - | 1 (5%) | 1 (3%) |
| ALCL | - | 1 (5%) | 1 (3%) |
| BCLU | 1 (7%) | - | 1 (3%) |
| CLL | - | 3 (16%) | 3 (9%) |
| DLBCL | 2 (14%) | 6 (32%) | 8 (24%) |
| ENKL | - | 1 (5%) | 1 (3%) |
| FL 1–2 | 5 (36%) | 2 (11%) | 7 (21%) |
| MCL | 1 (7%) | 1 (5%) | 2 (6%) |
| MZL/LPL | 2 (14%) | - | 2 (6%) |
| PTCL-NOS | 3 (21%) | 4 (21%) | 7 (21%) |
| Histology | |||
| B cell | 11 (79%) | 12 (63%) | 23 (70%) |
| T cell | 3 (21%) | 7 (37%) | 10 (30%) |
| Number of prior therapies | |||
| 0–3 | 7 (50%) | 12 (63%) | 19 (58%) |
| 4+ | 7 (50%) | 7 (37%) | 14 (42%) |
| Stage at enrolment | |||
| 3 | 4 (29%) | 2 (11%) | 6 (18%) |
| 4 | 10 (71%) | 16 (84%) | 26 (79%) |
| NA | 1 (5%) | 1 (3%) | |
| Prior rituximab | 11 (79%) | 11 (58%) | 22 (67%) |
| Prior autologous transplant | 1 (7%) | 7 (37%) | 8 (24%) |
| Number of cycles of dasatinib | |||
| 1 | 3 (21%) | 8 (42%) | 11 (33%) |
| 2 | 4 (29%) | 3 (16%) | 7 (21%) |
| 4 | 1 (7%) | 5 (26%) | 6 (18%) |
| 5 | - | 1 (5%) | 1 (3%) |
| 6 | 1 (7%) | - | 1 (3%) |
| 8 | 1 (7%) | 1 (5%) | 2 (6%) |
| 10 | 1 (7%) | - | 1 (3%) |
| 17 | 1 (7%) | - | 1 (3%) |
| 38 | - | 1 (5%) | 1 (3%) |
| 99 | 2 (14%) | - | 2 (6%) |
AITL: Angio-immunoblastic T-cell lymphoma; ALCL: Anaplastic large cell lymphoma; BCLU: Unclassifiable B-cell lymphoma; CLL: Chronic lymphocytic leukaemia; DLBCL: Diffuse large B-cell lymphoma; ENKL: Extra nodal Natural Killer/T-cell lymphoma; FL 1–2: Follicular lymphoma, Grade 1/2; MCL: Mantle cell lymphoma; MZL/LPL: Marginal zone lymphoma/Lymphoplasmacytic lymphoma; NA: not available; PTCL: NOS-Peripheral T-cell lymphoma, not otherwise specified.
Dose-limiting toxicity and Safety profile
During the phase I study, three patients received 100 mg, three patients received 150 mg, and eight patients received 200 mg of dasatinib daily. No dose-limiting toxicities were encountered during the phase I portion of the study and, hence, the maximum tolerable dose (MTD) was determined to be 200 mg PO daily. The first stage of the phase II study enrolled 10 patients at the MTD. At the interim analysis, the dose was reduced to 150 mg PO daily when a higher incidence of grade 3 pleural effusions was noted (2 of 10 patients receiving 200 mg dose in the first stage of phase II). The remaining 9 patients were enrolled on the second stage of the phase II study at the 150 mg PO daily dose. All 33 patients enrolled were evaluable for safety. Tables II and III summarize the haematological toxicities and most frequently reported non-haematological toxicities (>10%), regardless of relationship to treatment. Grade 3 thrombocytopenia was the most common haematological toxicity, followed by anaemia and neutropenia. Among all grades, gastrointestinal side effects were the most common non-haematological toxicity followed by pleural effusion. There were no grade 4 toxicities.
Table: II.
Haematological Toxicities
| Adverse event | All grades* N=33 | Grade 1 N=33 | Grade 2 N=33 | Grade 3 N=33 |
|---|---|---|---|---|
| Anaemia | 17 (52%) | 7 (21%) | 7 (21%) | 4 (12%) |
| Thrombocytopenia | 17 (52%) | 4 (12%) | 8 (24%) | 7 (21%) |
| Leucopenia | 9 (27%) | 7 (21%) | 3 (9%) | 1 (3%) |
| Neutropenia | 8 (24%) | 4 (12%) | 3 (9%) | 4 (12%) |
If a patient had more than one event from Grade1–3, only one was counted in the percentage of all grades.
Table: III.
Most frequent Non-haematological toxicities (>10%)
| Adverse event | All grades* N=33 | Grade 1 N=33 | Grade 2 N=33 | Grade 3 N=33 |
|---|---|---|---|---|
| Gastrointestinal | 22 (67%) | 15 (45%) | 11 (33%) | 4 (12%) |
| Pleural effusion | 17 (52%) | 5 (15%) | 10 (30%) | 4 (12%) |
| Fatigue | 16 (48%) | 11 (33%) | 6 (18%) | - |
| Oedema | 9 (27%) | 6 (18%) | 4 (12%) | - |
| Myalgia | 9 (27%) | 3 (9%) | 7 (21%) | - |
| Rash | 8 (24%) | 6 (18%) | 3 (9%) | 1 (3%) |
| Fever | 7 (21%) | 5 (15%) | 2 (6%) | - |
| Shortness of breath | 7 (21%) | 3 (9%) | 3 (9%) | 3 (9%) |
| Anorexia | 6 (18%) | 1 (3%) | 5 (15%) | - |
| Headache | 6 (18%) | 5 (15%) | 1 (3%) | - |
| Infection | 6 (18%) | 2 (6%) | 6 (18%) | - |
| Cardiovascular | 5 (15%) | 4 (12%) | 1 (3%) | 2 (6%) |
| Neuropathy | 5 (15%) | 4 (12%) | 1 (3%) | - |
| Chills | 4 (12%) | 3 (9%) | 1 (3%) | - |
| Electrolyte abnormalities | 4 (12%) | 4 (12%) | - | - |
| Pericardial effusion | 4 (12%) | 4 (12%) | - | - |
If a patient had more than one event from Grade1–3, only one was counted in the percentage of all grades.
Among the entire cohort, 15 patients experienced 65 serious adverse events, of which 7 were related and 33 were possibly related to treatment with dasatinib. The most common serious adverse event was pleural effusion requiring hospitalization.
Efficacy:
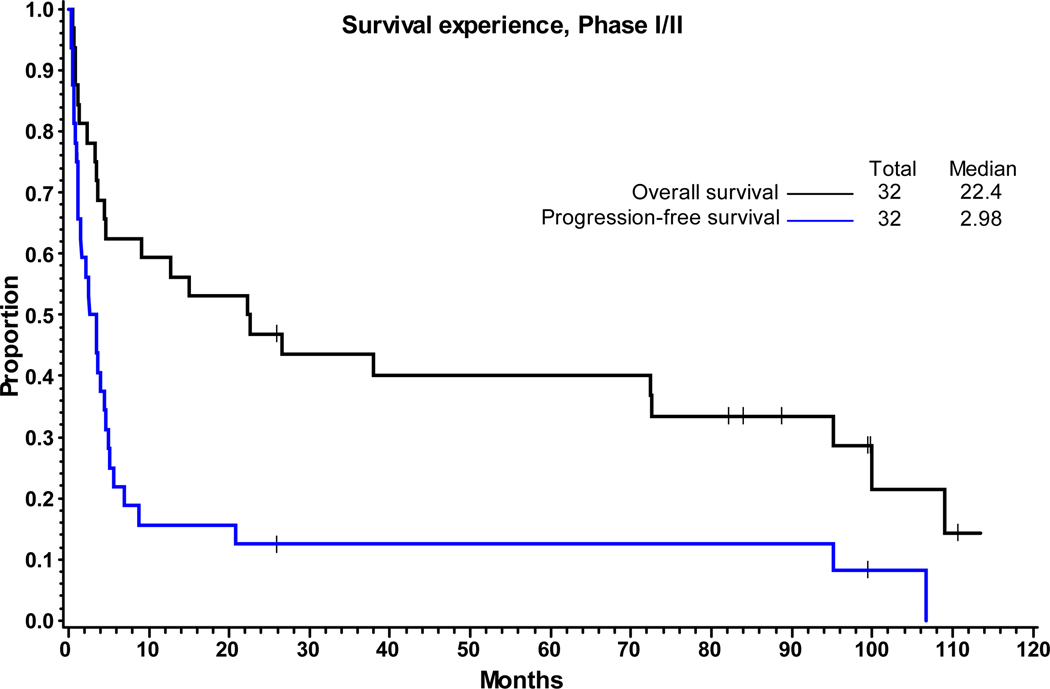
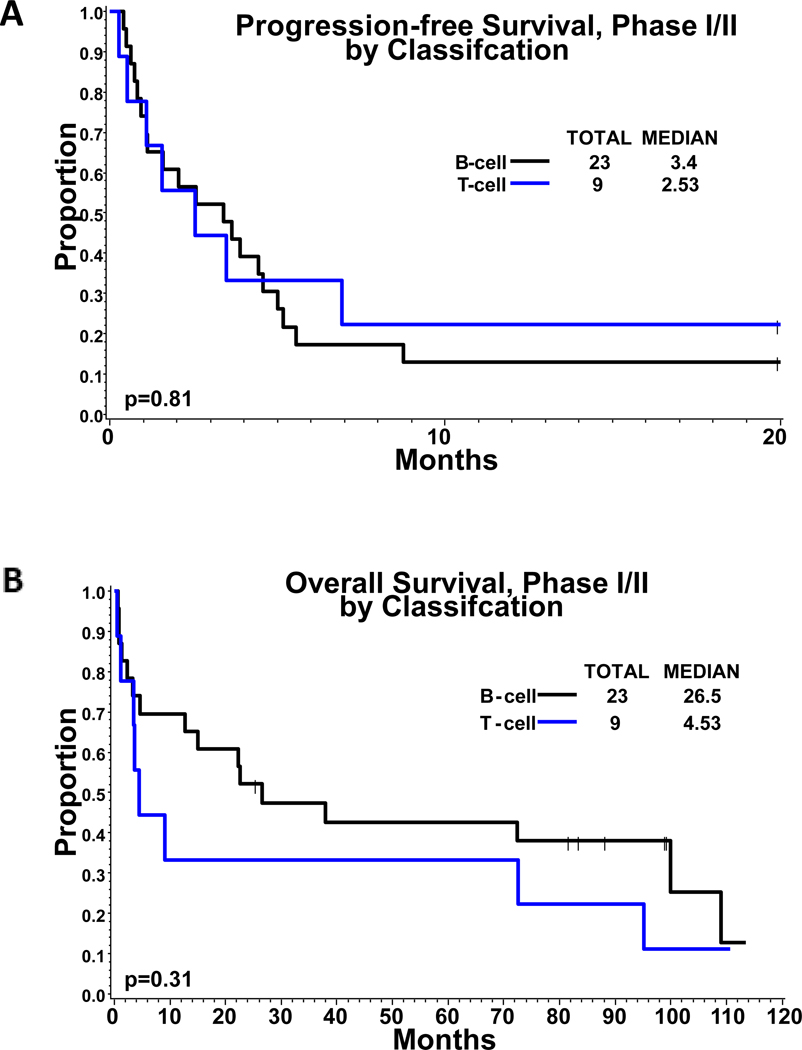
Response assessment was evaluable in 24 of the 33 patients enrolled in both phases of the trial combined. Nine patients discontinued treatment and were not evaluable (3 due to adverse events, 2 died and 4 withdrew consent). The ORR (CR+PR) was 29% (7/24); 95% exact CI of 13%−51%. The Clinical Benefit Rate (CR+PR+SD) was 71% (17/24); 95% exact CI of 49%−87%. The ORR in all subjects (Intent to treat population) was 21% (7/33) with 95% CI of 9%−39% and clinical benefit rate was 52% (17/33, 95% CI: 34%−69%). The median duration of response for the 7 patients who were responders (CR+PR) was 91.8 months by Kaplan-Meier estimate. The 7 responders included 5 PD and 2 that were alive and disease free at last follow-up. The median duration of response for the 17 patients who obtained clinical benefit (CR+PR+SD) is 3.5 months. Among these 17 patients, 2 were alive and disease-free at last follow-up. There was no significant difference in response rates between patients with B-cell lineage compared to T-cell lineage NHLs. Two patients (both PTCL-NOS) achieved and sustained a CR. At the time of analysis, 32 patients had survival data; 24 (75%) had died and 8 patients (25%) were alive at last contact. For PFS analysis, 32 subjects had available data; 27 failed (84%) and 5 were alive and failure-free at last contact (16%). The median follow-up of surviving patients was 94.1 months (range = 25.9 – 113.5 months). The median PFS and OS was 2.98 months and 22.4 months, respectively (Figure 1), with no significant difference in PFS or OS of either B- or T-cell lineage NHLs (PFS: 3.4 months vs 2.5 months, p=0.8; OS: 26.5 vs 4.5, p=0.3) (Figure 2). Additionally, we compared PFS and OS by relapse/refractory status of the patients and found that relapsed patients tended to have better survival outcomes compared to refractory patients (p<0.001, Figure S1). Among the B-cell sub-type, patients with indolent NHL showed marginally better PFS when compared to those with aggressive sub-types (p=0.06), but no significant difference in OS was observed (p=0.43) (Figure S2).
FIGURE 1: Survival Experience, Phase I/II.
Kaplan-Meier estimates of overall survival (OS) and progression-free survival (PFS) of 32 patients included in the survival analysis. The median PFS and OS was 2.98 months and 22.4 months, respectively.
FIGURE 2: Survival Comparison by B-cell Vs T-cell lineage.
Kaplan-Meier estimates of (A) progression-free survival and (B) overall survival compared by B cell vs. T cell lineage
Exploratory genomic analysis of the relapsed tumours:
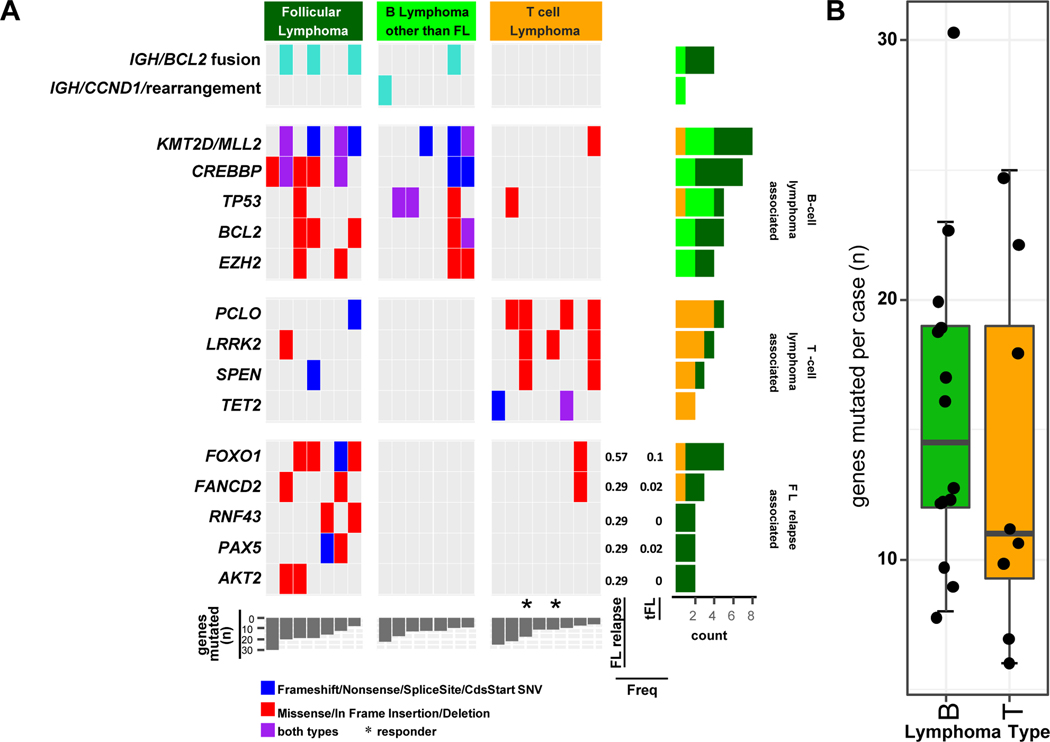
We observed a mutation spectrum consistent with diversity of the histological NHL cases in the study cohort, with mutations enriched in B-lineage (e.g. CREBBP, KMT2D [previously termed MLL2], BCL2, EZH2, and TP53) and T-lineage (e.g. TET2, LRRK2, PCLO, SPEN) lymphomas, as seen in de novo diseases(Iqbal, et al 2016, Iqbal, et al 2015). Consistent with this observation, mutation burden was higher in B-cell vs T-cell lymphomas. B-cell lineage lymphomas showed immunoglobulin translocation partners with BCL2 (3 of 6) in relapsed FL cases and with CCND1 in relapsed MCL case, whereas such events were not observed in T-cell lineage tumours. We also observed that clustering of the mutations in general showed distinct clusters characteristic of T or B-lineage lymphomas, indicating that the mutation spectrum is distinct in these tumour entities. Mutation in genes involved in nuclear factor (NF)-κB and PI3K-mTOR signalling pathways or DNA repair were enriched in T-cell lymphomas, whereas B-cell tumours were enriched with mutations in genes involved with epigenetic and BCR signalling. We examined the mutations in the tumours from the 2 PTCL-NOS cases that showed sustained CR and observed that both tumours had a LRRK2 mutation. Whether LRRK2 is a target of dasatinib is not known. CBL and EGFR, which are known targets of dasatinib, were mutated in two responders. As 7 of 22 patients had relapsed FL, we compared the genetic profiles with our published series of de novo and transformed FL cases (Bouska, et al 2017b) and observed that there was no major change in frequency of commonly mutated genes, e.g. KMT2D, CREBBP, EZH2 (40–~70&%). However, FOXO1 (p=0.009, 60% vs 5%), AKT2, FANCD2, PAX5, RNF43 (p<0.05, 30% vs < 5%) were significantly associated with relapsed FL cases (Figure 3).
FIGURE 3. Select gene rearrangements or genes found mutated in relapsed lymphoma cases.
A. The type of mutation is indicated according to the colour chart shown. The frequency of the follicular lymphoma (FL) relapse-associated genes in FL-relapse case in the current study compared to transformed FL cases (n=42) analysed in a previous study (Bouska et al, 2017) are denoted. The two cases that showed a complete response to Dasatinib are noted (*).
B. Boxplot of the number of genes mutated in B cell lymphoma and T cell lymphoma cases.
Discussion:
Several studies have demonstrated aberrant tyrosine kinase signalling in NHLs and these observations have led to the exploration of tyrosine kinase inhibitors in NHLs (Brave, et al 2008, Dickerson, et al 2013, Heldin 2013, Petersen, et al 2014). This is the first clinical trial of dasatinib in refractory NHL, where we observed an ORR of 32% and a clinical benefit rate of 52%, with a median PFS of 3 months and OS of 22.4 months. Two patients had a sustained CR and one patient showed PR, all three with the diagnosis of relapsed PTCL-NOS. Dasatinib is a potent, broad spectrum, ATP-competitive inhibitor of oncogenic tyrosine kinase families: BCR-ABL1, SRC, KIT, PDGFR and Ephrin receptor kinases. It has been shown that B-cell lines, with activated B-cell receptor (BCR) signalling regulated by Src kinases (LYN and SYK), are sensitive to dasatinib (Chen, et al 2008). Thus, SRC-kinase inhibition can completely abolish BCR-induced Ca2+ release in B cells (Sprangers, et al 2006, Zhu, et al 2002). This also results in inhibition of tyrosine-phosphorylation of MAPK1 and STAT5 molecules, which were sensitive to silencing of the SRC kinase LYN. Inhibition of SRC kinases resulted in growth arrest and cell death specifically in lymphoma cells (Chen, et al 2008, Yang, et al 2008, Zhao and Hsi 2010). Similarly, PDGFR-α is one of multiple targets of Src kinase and is expressed in multiple PTCL cell lines (Huang, et al 2010, Piccaluga, et al 2007, Piccaluga, et al 2005, Piva, et al 2010), which may explain the sensitivity of T-cell lines to dasatinib (Mahadevan, et al 2005, Thompson, et al 2005).
Our results show that dasatinib has modest activity as single agent in this cohort of heavily pre-treated patients with relapsed/refractory NHL. Despite the heterogeneity of the disease and prior treatment characteristics, response rates and survival outcomes are comparable to other salvage therapies in this setting (Chihara, et al 2017, Crump, et al 2017, Nagle, et al 2013). Among the 7 patients with PTCL-NOS histology, 2 patients achieved a durable CR, 2 patients had objective response and 1 patient had SD. Recently; it was shown that dasatinib efficiently inhibits aberrant VAV1 activation and subsequent signalling by RHOAG17V and VAV1 mutations in angioimmunoblastic T-cell lymphoma (AITL) (Fujisawa, et al 2017). Consistent with this observation, 1 AITL patient in our cohort achieved a short-lived PR. Among the B-cell lineage lymphomas, 1 FL and 1 MZL case each showed PR, whereas 4 FL (of 7) had SD. Single daily dosing of dasatinib has been studied, in both CML and Ph+ ALL, and found to be safe and effective (Lilly, et al 2010, Shah, et al 2008). Due to the potential convenience and equivalent efficacy, our study utilized the once daily dosing of dasatinib. The side effect profile was consistent with prior studies in CML and Ph+ ALL, with pleural effusion being the most common non-haematological toxicity (Cortes, et al 2016, Lilly, et al 2010).. We acknowledge several limitations of our study, including the open-label design, small number of patients and the heterogeneity of disease and treatment characteristics. However, the ORR of 29% and a clinical benefit rate of 71% are notable in this heavily pre-treated population, providing support for future studies to explore dasatinib as part of a combination therapy in patients with NHL or PTCL-NOS.
We performed exploratory mutation analysis in 22 of the 33 cases: The two cases of PTCL with sustained CR had a common mutation in LRRK2. The LRRK2 gene is known to be active in brain tissue as well as many other tissues throughout the body and has been implicated in the development of autosomal dominant Parkinson disease (Mata, et al 2006). The main known function of LRRK2 is the production of the protein dardarin and has multiple functions including GTP-ase and kinase function, and has been associated with T-cell activation (Cook, et al 2017, Guo, et al 2007). Whether LRRK2 is a target of dasatinib is not known and needs further investigation. In addition, 7 of the 22 relapsed NHL cases were FL that were refractory to prior rituximab-based therapy. We examined the mutation profile of these relapsed cases with our series (Bouska, et al 2017b) and observed that 4/7 carried recurrent mutations in FOXO1, RNF43 and AKT2, which are usually present in <5% of de novo FL cases. FOXO1 is a tumour suppressor gene and acts by transcriptionally activating genes involved in apoptosis and cell cycle arrest that was recently shown to predict an inferior 5-year failure-free survival after front-line chemo-immunotherapy in a cohort of patients with FL (Pastore, et al 2015). Thus, loss of these genes may contribute to chemo-resistant pathogenesis in FL.
In conclusion, dasatinib as single agent has shown encouraging activity in heavily pre-treated, recurrent or refractory indolent NHLs and modest activity in aggressive NHLs with an acceptable toxicity profile. In particular, PTCL cases (3 of 9) were highly sensitive to the drug and future studies may need to evaluate the expression of the B-cell kinase SYK, which is aberrantly expressed in majority of PTCLs (Feldman, et al 2008) and shown to be inhibited by dasatinib (Chen, et al 2008). This study also led us to ancillary genomic studies on relapsed cases, and in particular we observed recurrent FOXO1 mutation (4 of 7 patients) in relapsed FL, suggesting this mutant clone is persistent despite being heavily treated with immunochemotherapy or other salvage treatments. This gene was also included in a recently developed gene risk prognosticator for FL (Pastore, et al 2015), thus further investigation about its role in drug resistance in FL is required.
Supplementary Material
Acknowledgements
Preliminary data from this study was presented as a poster at the 52nd Annual American Society of Hematology Meeting, held 4-7 December 2010, at the Orange County Convention Center in Orlando, Florida.
Disclosures
BMW: Research support: Celgene. Advisory Board: Miragen. Consulting: Guidepoint Global and Navicor.
MAL: Research grant support: Amgen, Bristol-Myers Squibb Co, Celgene, Constellation Pharmaceuticals, Janssen Scientific Affairs, LLC, Jazz Pharmaceuticals, Juno Therapeutics
Memorial Sloan-Kettering Cancer Center, Pharmacyclics, TG Therapeutics. Consulting: AbbVie, ADC therapeutics, Astra-Zeneca/Acerta, Bayer, Celgene Corporation, Epizyme, Genentech, Gilead Sciences, Inc, Janssen/Pharmacyclics, Juno Therapeutics, Kite, Portola, Sanofi-Genzyme,Seattle Genetics, TG Therapeutics
AY: Honorarium for speaking engagements: Bayer, BMS, Celgene, Incyte, Janssen, Sanofi, Seattle Genetics, Takeda Millenium, Genentech and Merck; Research Support: Novartis, J&J, Curis, Roche, BMS
JOA: Consulting: Conatus – IDMC, Samus Therapeutics, Ascentage; Board of Directors: Tesaro Bio, Inc.
JMV: Research grant support: Acerta Pharma,Bristol – Myers Squibb, Celgene, Incyte Corp, Kite Pharma, Merck Sharp & Dohme Corp, Novartis, Seattle Genetics, Inc. Consulting/Honorarium: Novartis, Abbvie, Epizyme, Roche, Legend Pharmaceuticals, Kyopharm, Sandoz, Vaniam Group, Janssen/Pharmacyclic.
Footnotes
The remaining authors have nothing to disclose
References:
- Bouska A, Bi C, Lone W, Zhang W, Kedwaii A, Heavican T, Lachel CM, Yu J, Ferro R, Eldorghamy N, Greiner TC, Vose J, Weisenburger DD, Gascoyne RD, Rosenwald A, Ott G, Campo E, Rimsza LM, Jaffe ES, Braziel RM, Siebert R, Miles RR, Dave S, Reddy A, Delabie J, Staudt LM, Song JY, McKeithan TW, Fu K, Green M, Chan WC & Iqbal J. (2017a) Adult high-grade B-cell lymphoma with Burkitt lymphoma signature: genomic features and potential therapeutic targets. Blood, 130, 1819–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouska A, Zhang W, Gong Q, Iqbal J, Scuto A, Vose J, Ludvigsen M, Fu K, Weisenburger DD & Greiner TC (2017b) Combined copy number and mutation analysis identifies oncogenic pathways associated with transformation of follicular lymphoma. Leukemia, 31, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brave M, Goodman V, Kaminskas E, Farrell A, Timmer W, Pope S, Harapanhalli R, Saber H, Morse D, Bullock J, Men A, Noory C, Ramchandani R, Kenna L, Booth B, Gobburu J, Jiang X, Sridhara R, Justice R. & Pazdur R. (2008) Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clinical cancer research : an official journal of the American Association for Cancer Research, 14, 352–359. [DOI] [PubMed] [Google Scholar]
- Chen L, Monti S, Juszczynski P, Daley J, Chen W, Witzig TE, Habermann TM, Kutok JL & Shipp MA (2008) SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood, 111, 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A. & Zucca E. (2007) Revised response criteria for malignant lymphoma. Journal of Clinical Oncology, 25, 579. [DOI] [PubMed] [Google Scholar]
- Chihara D, Fanale MA, Miranda RN, Noorani M, Westin JR, Nastoupil LJ, Hagemeister FB, Fayad LE, Romaguera JE & Samaniego F. (2017) The survival outcome of patients with relapsed/refractory peripheral T‐cell lymphoma‐not otherwise specified and angioimmunoblastic T‐cell lymphoma. British journal of haematology, 176, 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DA, Kannarkat GT, Cintron AF, Butkovich LM, Fraser KB, Chang J, Grigoryan N, Factor SA, West AB & Boss JM (2017) LRRK2 levels in immune cells are increased in Parkinson’s disease. NPJ Parkinson’s disease, 3, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, Shah NP, Chuah C, Casanova L. & Bradley-Garelik B. (2016) Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. Journal of Clinical Oncology, 34, 2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, Link BK, Hay A, Cerhan JR & Zhu L. (2017) Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood, 130, 1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TA, Grillo-López AJ, White CA, McLaughlin P, Czuczman MS, Link BK, Maloney DG, Weaver RL, Rosenberg J. & Levy R. (2000) Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. Journal of Clinical Oncology, 18, 3135–3143. [DOI] [PubMed] [Google Scholar]
- Dickerson EB, Marley K, Edris W, Tyner JW, Schalk V, Macdonald V, Loriaux M, Druker BJ & Helfand SC (2013) Imatinib and Dasatinib Inhibit Hemangiosarcoma and Implicate PDGFR-beta and Src in Tumor Growth. Translational oncology, 6, 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman AL, Sun DX, Law ME, Novak AJ, Attygalle AD, Thorland EC, Fink SR, Vrana JA, Caron BL & Morice WG (2008) Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia, 22, 1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, Schnall-Levin M, White J, Sanford EM & An P. (2013) Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nature biotechnology, 31, 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa M, Sakata-Yanagimoto M, Nishizawa S, Komori D, Gershon P, Kiryu M, Tanzima S, Fukumoto K, Enami T. & Muratani M. (2017) Activation of RHOA–VAV1 signaling in angioimmunoblastic T-cell lymphoma. Leukemia. 32, 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, Bosly A, Ketterer N, Shpilberg O, Hagberg H, Ma D, Briere J, Moskowitz CH & Schmitz N. (2010) Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 28, 4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Gandhi PN, Wang W, Petersen RB, Wilson-Delfosse AL & Chen SG (2007) The Parkinson’s disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase thatstimulates kinase activity. Experimental cell research, 313, 3658–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H. & Gisselbrecht C. (2006) Randomised phase III study of R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL) followed by high-dose therapy and a second randomisation to maintenance treatment with rituximab or not: an update of the CORAL study. Annals of oncology, 17, iv31–iv32. [DOI] [PubMed] [Google Scholar]
- He J, Abdel-Wahab O, Nahas MK, Wang K, Rampal RK, Intlekofer AM, Patel J, Krivstov A, Frampton GM, Young LE, Zhong S, Bailey M, White JR, Roels S, Deffenbaugh J, Fichtenholtz A, Brennan T, Rosenzweig M, Pelak K, Knapp KM, Brennan KW, Donahue AL, Young G, Garcia L, Beckstrom ST, Zhao M, White E, Banning V, Buell J, Iwanik K, Ross JS, Morosini D, Younes A, Hanash AM, Paietta E, Roberts K, Mullighan C, Dogan A, Armstrong SA, Mughal T, Vergilio JA, Labrecque E, Erlich R, Vietz C, Yelensky R, Stephens PJ, Miller VA, van den Brink MR, Otto GA, Lipson D. & Levine RL (2016) Integrated genomic DNA/RNA profiling of hematologic malignancies in the clinical setting. Blood, 127, 3004–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C-H (2013) Targeting the PDGF signaling pathway in tumor treatment. Cell Communication and Signaling, 11, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, de Reynies A, de Leval L, Ghazi B, Martin-Garcia N, Travert M, Bosq J, Briere J, Petit B, Thomas E, Coppo P, Marafioti T, Emile JF, Delfau-Larue MH, Schmitt C. & Gaulard P. (2010) Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood, 115, 1226–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, Wilcox R, Naushad H, Rohr J, Heavican TB, Wang C, Bouska A, Fu K, Chan WC & Vose JM (2015) Genomic signatures in T-cell lymphoma: How can these improve precision in diagnosis and inform prognosis? Blood Rev. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Naushad H, Bi C, Yu J, Bouska A, Rohr J, Chao W, Fu K, Chan WC & Vose JM (2016) Genomic signatures in B-cell lymphoma: How can these improve precision in diagnosis and inform prognosis? Blood Rev, 30, 73–88. [DOI] [PubMed] [Google Scholar]
- Lilly MB, Ottmann OG, Shah NP, Larson RA, Reiffers JJ, Ehninger G, Müller MC, Charbonnier A, Bullorsky E. & Dombret H. (2010) Dasatinib 140 mg once daily versus 70 mg twice daily in patients with Ph‐positive acute lymphoblastic leukemia who failed imatinib: Results from a phase 3 study. American Journal of Hematology, 85, 164–170. [DOI] [PubMed] [Google Scholar]
- Mahadevan D, Spier C, Della Croce K, Miller S, George B, Riley C, Warner S, Grogan TM & Miller TP (2005) Transcript profiling in peripheral T-cell lymphoma, not otherwise specified, and diffuse large B-cell lymphoma identifies distinct tumor profile signatures. Molecular cancer therapeutics, 4, 1867–1879. [DOI] [PubMed] [Google Scholar]
- Martin A, Conde E, Arnan M, Canales MA, Deben G, Sancho JM, Andreu R, Salar A, Garcia-Sanchez P, Vazquez L, Nistal S, Requena MJ, Donato EM, Gonzalez JA, Leon A, Ruiz C, Grande C, Gonzalez-Barca E, Caballero MD (2008) R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: the influence of prior exposure to rituximab on outcome. A GEL/TAMO study. Haematologica, 93, 1829–1836. [DOI] [PubMed] [Google Scholar]
- Mata IF, Wedemeyer WJ, Farrer MJ, Taylor JP & Gallo KA (2006) LRRK2 in Parkinson’s disease: protein domains and functional insights. Trends in neurosciences, 29, 286–293. [DOI] [PubMed] [Google Scholar]
- Nagle SJ, Woo K, Schuster SJ, Nasta SD, Stadtmauer E, Mick R. & Svoboda J. (2013) Outcomes of patients with relapsed/refractory diffuse large B‐cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. American Journal of Hematology, 88, 890–894. [DOI] [PubMed] [Google Scholar]
- Pastore A, Jurinovic V, Kridel R, Hoster E, Staiger AM, Szczepanowski M, Pott C, Kopp N, Murakami M, Horn H, Leich E, Moccia AA, Mottok A, Sunkavalli A, Van Hummelen P, Ducar M, Ennishi D, Shulha HP, Hother C, Connors JM, Sehn LH, Dreyling M, Neuberg D, Moller P, Feller AC, Hansmann ML, Stein H, Rosenwald A, Ott G, Klapper W, Unterhalt M, Hiddemann W, Gascoyne RD, Weinstock DM & Weigert O. (2015) Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol, 16, 1111–1122. [DOI] [PubMed] [Google Scholar]
- Petersen DL, Krejsgaard T, Berthelsen J, Fredholm S, Willerslev-Olsen A, Sibbesen NA, Bonefeld CM, Andersen MH, Francavilla C. & Olsen JV (2014) B-lymphoid tyrosine kinase (Blk) is an oncogene and a potential target for therapy with dasatinib in cutaneous T-cell lymphoma (CTCL). Leukemia, 28, 2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccaluga PP, Agostinelli C, Zinzani PL, Baccarani M, Dalla Favera R. & Pileri SA (2005) Expression of platelet-derived growth factor receptor alpha in peripheral T-cell lymphoma not otherwise specified. Lancet Oncol, 6, 440. [DOI] [PubMed] [Google Scholar]
- Piccaluga PP, Agostinelli C, Califano A, Rossi M, Basso K, Zupo S, Went P, Klein U, Zinzani PL, Baccarani M, Dalla Favera R. & Pileri SA (2007) Gene expression analysis of peripheral T cell lymphoma, unspecified, reveals distinct profiles and new potential therapeutic targets. J Clin Invest, 117, 823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva R, Agnelli L, Pellegrino E, Todoerti K, Grosso V, Tamagno I, Fornari A, Martinoglio B, Medico E, Zamo A, Facchetti F, Ponzoni M, Geissinger E, Rosenwald A, Muller-Hermelink HK, De Wolf-Peeters C, Piccaluga PP, Pileri S, Neri A. & Inghirami G. (2010) Gene expression profiling uncovers molecular classifiers for the recognition of anaplastic large-cell lymphoma within peripheral T-cell neoplasms. J Clin Oncol, 28, 1583–1590. [DOI] [PubMed] [Google Scholar]
- Shah NP, Tran C, Lee FY, Chen P, Norris D. & Sawyers CL (2004) Overriding imatinib resistance with a novel ABL kinase inhibitor. Science (New York, N.Y.), 305, 399–401. [DOI] [PubMed] [Google Scholar]
- Shah NP, Kantarjian HM, Kim D-W, Réa D, Dorlhiac-Llacer PE, Milone JH, Vela-Ojeda J, Silver RT, Khoury HJ & Charbonnier A. (2008) Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and-intolerant chronic-phase chronic myeloid leukemia. Journal of Clinical Oncology, 26, 3204–3212. [DOI] [PubMed] [Google Scholar]
- Simon R. (1989) Optimal two-stage designs for phase II clinical trials. Controlled clinical trials, 10, 1–10. [DOI] [PubMed] [Google Scholar]
- Sprangers M, Feldhahn N, Herzog S, Hansmann M-L, Reppel M, Hescheler J, Jumaa H, Siebert R. & Müschen M. (2006) The SRC family kinase LYN redirects B cell receptor signaling in human SLP65-deficient B cell lymphoma cells. Oncogene, 25, 5056. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. (2016) The 2016 revision to the World Health Organization classification of lymphoid neoplasm. Blood, 127, 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpaz M, Kantarjian H, Shah NP, Donato N, Nicoll J, Bai SA, Huang F, Clark E, DeCillis AP, & Sawyers C. (2004) Hematologic and Cytogenetic Responses in Imatinib-Resistant Accelerated and Blast Phase Chronic Myeloid Leukemia (CML) Patients Treated with the Dual SRC/ABL Kinase Inhibitor BMS-354825: Results from a Phase I Dose Escalation Study..Blood, 104(11), 20. [Google Scholar]
- Thompson MA, Stumph J, Henrickson SE, Rosenwald A, Wang Q, Olson S, Brandt SJ, Roberts J, Zhang X, Shyr Y. & Kinney MC (2005) Differential gene expression in anaplastic lymphoma kinase-positive and anaplastic lymphoma kinase-negative anaplastic large cell lymphomas. Human pathology, 36, 494–504. [DOI] [PubMed] [Google Scholar]
- Xu B. & Liu P. (2014) No survival improvement for patients with angioimmunoblastic T-cell lymphoma over the past two decades: a population-based study of 1207 cases. PLoS One, 9, e92585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Lu P, Lee FY, Chadburn A, Barrientos JC, Leonard JP, Ye F, Zhang D, Knowles DM & Wang YL (2008) Tyrosine kinase inhibition in diffuse large B-cell lymphoma: molecular basis for antitumor activity and drug resistance of dasatinib. Leukemia, 22, 1755. [DOI] [PubMed] [Google Scholar]
- Zelenetz AD, Abramson JS, Advani RH, Andreadis CB, Byrd JC, Czuczman MS, Fayad L, Forero A, Glenn MJ, Gockerman JP, Gordon LI, Harris NL, Hoppe RT, Horwitz SM, Kaminski MS, Kim YH, Lacasce AS, Mughal TI, Nademanee A, Porcu P, Press O, Prosnitz L, Reddy N, Smith MR, Sokol L, Swinnen L, Vose JM, Wierda WG, Yahalom J. & Yunus F. (2010) NCCN Clinical Practice Guidelines in Oncology: non-Hodgkin’s lymphomas. Journal of the National Comprehensive Cancer Network : JNCCN, 8, 288–334. [DOI] [PubMed] [Google Scholar]
- Zhao X. & Hsi ED (2010) Dasatinib induces dephosphorylation of tyrosine proteins and apoptosis in both B-cell lymphoma lines and primary B-cell Non-Hodgkin Lymphoma cells in vitro for a subgroup of patients with phospho-Src (Y416) expression. [Google Scholar]
- Zhu D-M, Tibbles HE, Vassilev AO & Uckun FM (2002) SYK and LYN mediate B-cell receptor-independent calcium-induced apoptosis in DT-40 lymphoma B-cells. Leukemia & lymphoma, 43, 2165–2170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.