Abstract
Atrial fibrillation (AF) remains a growing problem in the United States and worldwide, imposing a high individual and health system burden, including increased resource consumption due to repeated hospitalizations, stroke, dementia, heart failure, and death. This comprehensive review summarizes the most recent data on sex-related differences in risks associated with AF. Women with AF have increased risk of stroke and death compared to men, and possible reasons for this disparity are explored. Women also continue to have worse symptoms and quality of life, and poorer outcomes with stroke prevention, as well as with rate and rhythm control management strategies. Many current rhythm control treatment strategies for AF, including cardioversion and ablation, are used less frequently in women as compared to men, whereas women are more likely to be treated with rate control strategies or antiarrhythmic drugs. Sex differences should be considered in treating women with AF to improve outcomes and women and men should be offered the same interventions for AF. We need to improve the evidence base to understand if variation in utilization of rate and rhythm control management between men and women represents health inequities or appropriate clinical judgement.
Keywords: catheter ablation, left atrial appendage closure, quality of life, stroke prevention, women
1 ∣. INTRODUCTION
Atrial fibrillation (AF) remains a growing problem in the United States and worldwide due to high healthcare burden, including increased resource consumption related to repeated hospitalizations, stroke, dementia, heart failure (HF), and death. In two community-based cohorts, even after accounting for age, duration of AF, and other comorbidities, AF conferred a pooled estimated incremental cost of US $18,601 (95% confidence interval [CI]: US $15,981–US $21,234) in 2009 dollars versus non-AF patients.1 Experts estimate that AF cost $28.4 (95% CI, 24.6–33.8) billion in 2016.2 Also troubling is the prediction that the incidence of AF is expected to continue to increase over the next decade.
The overall lifetime prevalence of AF is similar for both sexes. Yet, AF does not have similar consequences in women and men, an important consideration that has received limited attention. Accordingly, this review examines sex differences in AF with respect to presentation, clinical course, and outcomes.
2 ∣. EPIDEMIOLOGY
2.1 ∣. Sex differences in prevalence and incidence
The prevalence and incidence of AF varies by sex, age, race/ethnicity, intensity of screening, and over time. The prevalence in the United States was about 5.2 million in 20103 and 37.6 million in 2017 world-wide, with an estimated prevalence of 17.8 million women and 19.8 million men in 2017.4 Importantly, AF prevalence has been increasing in both sexes over time.5,6 Among individuals 45–94 years of age, in the Framingham Heart Study (FHS), between 1958–1967 and 1998–2007 the age-adjusted prevalence quadrupled in both women and men (from 13.7 to 49.4 and 20.4 to 96.2 cases per 1000 person-years, respectively).5 Since on average women live longer than men, the number of women and men with AF are similar despite the higher risk of AF in men. In a 5% sample from 2007 Medicare recipients with AF, 47,719 were women and 42,475 were men; the corresponding prevalence rate per 1000 beneficiaries was 63 and 88 in women and men, respectively.6 Regardless of sex, AF prevalence increases markedly with advancing age, and is higher in individuals of European ancestry versus other races/ethnicities.6
Similar to these prevalence data, the AF incidence rate has increased over time (Table 1).5,6 Tables 2 and 3 show incidence rates per 1000 person-years in various groups based on level of risk, and in different racial/ethnic groups.7,8
TABLE 1.
Age-adjusted incidence rate per 1000 person-years
| 1958–1967 | 1998–2007 | |
|---|---|---|
| Women | 2.5 | 8.6 |
| Men | 3.7 | 13.4 |
Source: Framingham Heart Study.5
TABLE 2.
Age-adjusted incidence rate per 1000 person-years by level of risk
| Age-adjusted incidence rate by risk factor status | |||
|---|---|---|---|
| Risk factor status |
|||
| Optimal | Borderline | Elevated | |
| Black women | 0 | 1.7 | 4.1 |
| White women | 2.0 | 2.7 | 6.0 |
| Black men | 0 | 2.6 | 6.0 |
| White men | 4.0 | 5.2 | 9.1 |
Source: Atherosclerosis Risk in Communities (ARIC) Study, 1987–2007.7
TABLE 3.
Age and sex adjusted incidence rate per 1000 person-years by race
| Age- and sex-adjusted incidence rates | |
|---|---|
| Chinese | 3.9 |
| Hispanic | 6.1 |
| Non-Hispanic Blacks | 5.8 |
| Non-Hispanic Whites | 11.2 |
Source: Multiethnic Study of Atherosclerosis (MESA), 2000–2010.8
Most studies report a higher incidence of AF in men versus women,5-7,9 for reasons that are unclear. However, in the CHARGE-AF model, based on three US cohort studies and replicated in two European cohorts, sex per se did not appear to be a risk factor for incident AF after accounting for participant height and other risk factors.9
2.2 ∣. Sex differences in lifetime risk
The FHS (European ancestry) suggested that at age 55, the lifetime risk of AF varied by sex, as well as the presence and burden of AF risk factors (Table 2).10 However, in the Atherosclerosis Risk in Communities (ARIC) cohort, the lifetime AF risk varied by sex in Whites, but not in Blacks (Table 4).11
TABLE 4.
Lifetime risk of AF according to race and sex in the ARIC at ages 45 and 55 years
| Lifetime risk of developing af by risk factor status, FHSa | |||
|---|---|---|---|
| Optimal | Borderline | Elevated | |
| Women | 20.5% | 28.0% | 34.6% |
| Men | 29.8% | 39.7% | 43.3% |
| Lifetime risk of AF according to race and sex, ARICb | ||
|---|---|---|
| Black | White | |
| Women | 22% | 30% |
| Men | 21% | 36% |
2.3 ∣. Sex differences in af burden: Role of screening
Detection of AF increases with the duration and intensity of screening. For instance, in a Swedish community-based screening study of individuals 74–75 years old, after 2 weeks of intermittent electrocardiogram (ECG) recordings, 3.0% were observed to have previously undiagnosed AF, and only 0.5% had AF detected on their initial ECG.12 A meta-analysis of 13 community-based screening studies confirmed a higher rate of newly detected AF in men versus women. However, the absolute numbers of AF cases detected were similar in men and women at age 80 years, presumably because of the greater number of older women in the sample.13
Cardiac implantable electronic devices (CIEDs) also have contributed to increased recognition of subclinical AF—that is, AF not previously diagnosed.14 A study of 3131 patients with CIEDs (30% women) reported that the presence of more than 6 h of AF per week was associated with increased odds of death and the association was stronger in women compared with men.15
2.4 ∣. Potential mechanisms for differences
Mechanisms underlying sex differences in AF are incompletely understood.16,17 Factors that contribute to increased incidence of AF in men are taller stature, higher body mass index (BMI), and larger atria and ventricles.9,18-21 The greater burden of atrial fibrosis in women with AF22,23 may predispose them to more complications with AF. In a consecutive case series, compared with men, women had more atrial fibrosis that was associated with higher prevalence of stroke or transient ischemic attack.23 A study of patients with persistent AF undergoing cryoballoon pulmonary vein isolation showed that the extent of left atrial low voltage areas (atrial fibrosis) predicted AF-free survival, and this type of ablation appeared highly effective in patients with persistent AF without atrial fibrosis.24
2.5 ∣. Mortality and AF
There is a significant AF-sex interaction with mortality, such that AF reduces the typical survival advantage enjoyed by women. In a meta-analysis of 30 studies with over 4.3 million participants, the ratio of relative risks (RRs) comparing women with men was 1.12 (95% CI, 1.07–1.17), based on a RR of mortality of 1.69 (95% CI, 1.50–1.90) in women, and 1.47 (95% CI, 1.32–1.65) in men with versus without AF.25 In absolute terms per 1000 patient-years, compared with men, women had a 1.8 (95% CI, 1.1–2.6) excess risk of death associated with AF. These investigators concluded that women compared to men with AF had a higher RR of stroke, all-cause and cardiovascular mortality, cardiac events, and HF.25
3 ∣. PRESENTATION, SYMPTOMS, AND DIAGNOSIS
Women with AF report more symptoms than men, but findings from different studies vary.26-30 A 2019 study reported that women were more likely to report AF symptoms, and poor AF-related quality of life than men.31 In a study of emergency department patients, women were more likely to have longer duration of symptoms and to present with atypical symptoms like weakness and/or fatigue.16,32
Women, compared with men, with AF also have more functional impairment, greater limitation in their daily activities, and lower quality of life scores. In some studies, such as the Outcomes Registry for Better Informed Treatment of AF (ORBIT-AF), women had less persistent forms of AF compared to men.27 However, in EORP-AF,33 there was no difference in the subtypes of AF duration between the two sexes. In ORBIT-AF, women had 24% lower quality of life score, even after adjustment for comorbidities.27
Diagnostically, stress testing, and transesophageal echocardiograms for left atrial appendage (LAA) investigation are less likely to be performed in women with AF.33,34 While some studies have not identified sex differences in the utilization of transthoracic echocardiograms or transesophageal echocardiograms, others have observed that these studies are ordered less often in women with AF.35,36
3.1 ∣. Prognosis of AF with HF
Women have a higher adjusted risk of incident HF (56.0 and 50.7 per 1000 person-years, respectively).25,37 Most likely due to less ischemic cardiomyopathy, women have less incidence of HF with reduced ejection fraction (12.4 vs. 27.2 events per 1000 person-years).37 On the other hand, because of the higher prevalence of valvular heart disease and hypertension in women,36,38 women have a higher incidence of HF with preserved ejection fraction (HFpEF; 35.1 vs. 21.2 events per 1000 person-years).
4 ∣. STROKE PREVENTION IN AF-SEX DIFFERENCES IN STROKE RISK
Many studies examining associations between sex and stroke risk in AF have concluded that women are at greater risk for stroke than men; a 20–30% excess risk versus men, even after adjusting for differences in stroke risk factors and anticoagulant treatment.25,39-44 A meta-analysis of 44 reports confirmed the higher risk of stroke in women (RR, 1.24; 95% CI, 1.14–1.36], and also confirmed that the risk increased after age 65 years.41 These findings led to the inclusion of female sex in the CHA2DS2-VASc stroke risk score.45-47
Although the weight of evidence points toward higher risk for stroke among women compared to men, the explanation for this disparity remains unclear. Hypotheses include biological differences between women and men, higher burden of atrial fibrosis, differences in distribution, manifestation, intensity of comorbid conditions related to stroke, and differences in treatment with anticoagulants. In addition, female sex has been associated with a twofold increased risk of severe disabling or fatal ischemic stroke in consecutive AF patients with stroke, according to the “Get With the Guidelines” stroke database, even after adjustment for possible confounders.48
A large retrospective Swedish cohort study of (231,077 people (48.1% women)) unselected patients with AF not receiving oral anticoagulation from 2006 to 2014 found that there was 1.5-fold higher risk of ischemic stroke in women compared to men. They found that this higher risk was the result of confounding by age. They also found that in the CHA2DS2-VASc low risk end, the score underestimated the ischemic stroke risk conferred by age 65–74 years but it overestimated the risk conferred by female sex.49
Whether variation in stroke severity is related to underlying treatment patterns remains uncertain. Data from the PINNACLE National Cardiovascular Data Registry suggested that women with AF were significantly less likely to receive oral anticoagulants at all levels of the CHA2DS2-VASc score, and men received oral anticoagulants more than women.50 Another American study of 2.3 million women and men with a new diagnosis of AF and CHA2DS2-VASc ≥2 from 2008 to 2015 also found women were not anticoagulated as frequently as men. The oral anticoagulation-eligible women were not as likely to receive anticoagulation; 50.0% women versus 43.9% men did not received anticoagulation. A mediation analysis revealed that not receiving oral anticoagulation partially mediated the observed increased risk of stroke but also decreased risk of intracranial bleeding in women.51 In the global FIELD (GARFIELD)-AF anticoagulant registry, there was no significant difference in the overall rate of anticoagulant use found between the sexes (61% for both).52
Direct oral anticoagulants were first introduced into clinical practice in 2011 and fewer women received oral anticoagulants (53% men vs. 48% women); women were also more likely to receive aspirin only (35% women vs. 30% men). However, these sex differences were no longer observed by 2015, except for women ≥80 years and those with complicated comorbidities.52,53
In the Atrial Fibrillation Follow up in Rhythm Management (AFFIRM) trial, women had a higher risk of stroke than men even when they had comparable time in the therapeutic anticoagulation range on warfarin. However, women were found to spend less time in therapeutic range compared to men.54
Two meta-analyses of major direct oral anticoagulant versus warfarin trials found that direct oral anticoagulants resulted in better outcomes for women. These outcomes included stroke, thromboembolism, and major bleeding.55,56 Four direct oral anticoagulants (apixaban, dabigatran, edoxaban, and rivaroxaban) were compared in a meta-analysis that found no significant difference with regard to safety and efficacy in women compared with warfarin, suggesting that they can be used interchangeably among women.57
Sex-specific differences in the distribution and effect of various CV risk factors are well documented, which may contribute to higher stroke risk in women versus men with AF.5,18,58,59 Compared with men, women with AF are, on average, older, and more likely to have thyroid dysfunction, hypertension, valvular heart disease, and HFpEF.16 Although studies have attempted to adjust for differences in risk factors between women and men, adjustment may be insufficient or incomplete. For example, a population-based study found that the 16% higher risk of stroke observed in women with AF disappeared once women and men were matched on time-varying risk factors instead of simply adjusting for baseline factors at cohort entry.60
The updated AF guidelines for anticoagulation from American and European organizations are compared in Table 5. Women with CHA2DS2-VASc scores of ≥3 and in men with CHA2DS2-VASc scores of ≥2 should be anticoagulated. Women with CHA2DS2-VASc scores of ≥2 have variable levels of recommendation strength. All the guidelines recommend no anticoagulation for patients with CHA2DS2-VASc scores of 0 among men or 1 among women.61-63 A study found that women with CHA2DS2-VASc of 2 or less had very low risk of stroke/systemic thromboembolism and therefore should not be anticoagulated. It was only when the CHA2DS2-VASc was ≥3 did women have a higher risk of stroke compared to men.64
TABLE 5.
Recommendations for stroke prevention for patients with nonvalvular atrial fibrillation
| 2019 | 2020 | |||
|---|---|---|---|---|
| ACC/AHA/HRS61 |
ESC/EACTS/EHRA62 |
|||
| CHA2DS2-VASc | Women | Men | Women | Men |
| 0 | - | - | - | - |
| 1 | None, ASA or OAC (IIb) | None, ASA or OAC (IIb) | - | OAC (IIa) |
| 2 | None, ASA or OAC (IIb) | OAC (I) | OAC (II) | OAC (I) |
| ≥3 | OAC (I) | OAC (I) | OAC (I) | OAC (I) |
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; ASA, aspirin; EACTS, European Association of Cardio-Thoracic Surgery; EHRA, European Heart Rhythm Association; ESC, European Society of Cardiology; HRS, Heart Rhythm Society; OSA, oral anticoagulation.
4.1 ∣. Percutaneous LAA closure devices
During AF, the LAA contractility is limited and blood flow is stagnant creating a nidus for clot formation, which increases with smaller LAA orifice and higher number of lobes.65 The LAA is considered the primary source of cardioembolic stroke in patients with nonvalvular AF.66 In a cohort with high AF stroke risk, women had more extensive LA remodeling and deterioration of LAA function than men, which may play a role in the higher stroke risk observed in women.67
The following LAA endocardial occlusion devices have been developed for stroke risk reduction in AF patients: the Watchman, the Amplatzer Cardiac Plug and Amulet, the WaveCrest, the Atri-Clip, and the Lariat. These devices are in different stages of “regulator approval” in the United States and Europe. The most extensively studied device is the Watchman and is approved by the US Food and Drug Administration.68 There was no significant interaction with sex and a composite efficacy endpoint of stroke, systemic embolism, and CV death was found in a meta-analysis of the PROTECT-AF (Percutaneous Left Atrial Appendage Closure vs. Warfarin for Atrial Fibrillation) and PREVAIL (Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients with Atrial Fibrillation vs. Long-Term Warfarin Therapy) trials.69 A small earlier study of the Watchman reported that there was a nonsignificant trend towards women having device-related thrombus (75 vs. 34%, p = .094) versus men.70 More recent data indicate no significant sex differences in device-related thrombus with the Watchman.71 In univariate and multivariate logistic regression model studies of the Amplatzer Cardiac Plug showed that female sex (OR, 4.22; p = .03) and cigarette smoking (OR, 5.79; p = .02) were predictors of device-related thrombus in adjusted models.72 Potential reasons for women having a higher incidence of device-related thrombus were not mentioned. Interestingly, a wider LAA mouth is associated with deeper implantation of the device, and subsequently, device-related thrombus, not smaller LAA.
Notably, mortality in candidates for LAA closure and/or undergo device implantation ranges between 5% and 10% a year, likely related to underlying comorbidities.73-75 A recent meta-analysis suggested improvement in survival with device implantation after analyzing the results of the PROTECT AF and PREVAIL trials. In a multicenter retrospective study of 101 patients (48% women) who underwent LAA device implantation due to a contraindication to anticoagulation, 85% of whom received an Amplatzer Cardiac Plug/Amulet device, male sex, and not female sex, was independently associated with late mortality.75
4.2 ∣. Surgical closure of LAA
LAA exclusion or excision may be considered in conjunction with surgical ablation for AF and at the time of cardiac operations in AF patients for thromboembolic prevention (Class IIA, level C expert opinion by the surgical guidelines, whereas the AHA/ACC/HRS guidelines consider it a Class IIB, Level of evidence B-nonrandomized data) and the European guidelines consider it a Class IIB, Level of Evidence C.61,62,76 Despite the high prevalence of postoperative AF, there is not sufficient evidence to recommend routine left atrial exclusion during heart surgery.77
Surgical occlusion of the LAA (LAAO) includes excision or amputation, and exclusion (clip/stapler, suture ligation). Success rates vary according to surgical procedure with highest success with excision/amputation techniques.78 A recent meta-analysis concluded that surgical LAAO was associated with a lower risk of thromboembolic events and stroke.79 In a retrospective cohort of 75,782 adults with LAAO during cardiac surgery, with 29% women, the hazard ratio for stroke was higher in women [0.80 (0.51–1.25)] than men [0.59 (95% CI, 0.39–0.91)] among propensity-matched patients with AF at baseline.80 However, there are currently no published data focused on differences in outcomes by sex in surgical closure of the LAA.
5 ∣. RATE CONTROL–SEX DIFFERENCES
5.1 ∣. Atrioventricular nodal blocking drugs
When treated for AF, women are more likely to receive rate control rather than rhythm control strategies compared to men,29 but reasons for this disparity are unclear. Rate control medication alone for AF was associated with comparatively poorer functional status and quality of life.31
Sex hormones affect the pharmacokinetics of drugs. Differences in physiology, pharmacokinetics, and pharmacodynamics are responsible for a greater risk for adverse drug reactions in women.81 Many factors cause sex-specific differences in pharmacotherapy in women, including a lower BMI and creatinine clearance, a higher proportion of body fat, and smaller organs.82 Drug absorption may also be slower in women. Sex differences are mainly modulated by the activity of drug-metabolizing enzymes, particularly the cytochrome (CYP) P450 system.81 CYP3A4 contributes to first-pass metabolism of most CV drugs, including diltiazem and verapamil. Higher expression of CYP3A4 messenger RNA and twofold higher CYP3A4 levels are found in women on liver biopsy.81 Whether there is a sex-specific difference for CYP2D6, which has a role in the metabolism of metoprolol and propranolol, is controversial.
Whereas it is clear that there are sex differences in β-blocker and calcium channel blocker metabolism, whether these differences affect the doses needed to achieve ventricular rate control in AF in men versus women is unknown. Differences in body size should be considered when selecting a specific drug dose, but other factors such as atrioventricular nodal conduction properties in an individual patient will also affect ventricular rate control.
Although β-blocker use has been reported to be similar in men and women (72.5% and 70.0%), women have been more likely to be prescribed digoxin (25.0% vs. 19.8%)29 which have resulted in higher serum levels of digoxin and a higher mortality rate compared to men, despite lower doses.83 On the other hand, a posthoc analysis of the Rivaroxaban versus Vitamin K Antagonist for Prevention of Stroke and Embolism Trial (ROCKET) AF trial, suggested a significant digoxin–sex interaction, with increased risk of all-cause mortality and vascular death among men with AF compared with women.84 In addition to mortality, two studies found an increased risk of breast cancer when cardiac glycosides were used for AF.85,86 The Women's Health Initiative, which enrolled 93,676 postmenopausal women from 1994 to 1998 and followed them for 15 years, reported that AF patients had a 5.7% incidence of invasive breast cancer. There was a 19% excess risk of invasive breast cancer with cardiac glycoside use. Adjusting for AF and other confounders, cardiac glycoside use was strongly associated with incident invasive breast cancer (hazard ratio [HR], 1.68; 95% CI, 1.33–2.12).85 A meta-analysis of 29 studies found that cardiac glycoside use was associated with increased risk of breast cancer by about a third (RR, 1.33; 95% CI, 1.25–1.42).86
5.1.1 ∣. Bradycardia and need for pacing
Despite higher resting sinus rates, women are more likely than men to have sinus node disease as the indication for permanent pacemaker implantation; however, in general, women are older at the time of need for pacemaker implantation.87 There are more men than women who receive permanent pacemakers under the age of 80 years, whereas the opposite is true after that age.87 Potential explanations for the older age at the time of development of bradycardia and the cause of bradycardia in women include the protective effect of sex hormones and later development of significant cardiovascular disease in women. Women, particularly those over 80 years old, may be more likely to receive single chamber ventricular pacing rather than dual chamber pacing system, although the reason may be because of advanced age than sex. Another potential explanation may be higher prevalence of persistent AF, especially if women are less likely to be treated with rhythm control.
Women also have higher rates of complications such as pocket hematoma and pneumothorax from permanent pacemaker implantation compared with men, adjusting for age, and type of device implanted.87 Smaller body habitus is one factor likely responsible for this observation.
6 ∣. RHYTHM CONTROL–SEX DIFFERENCES
In addition to the aforementioned ECG sex differences, on average women also have higher resting heart rates, shorter P wave duration, decreased QRS width, less frequent J waves, decreased ST segment abnormalities, and increased QT duration.88
Female sex is independently associated with adverse left atrial remodeling.89 Therefore, restoring and maintaining sinus rhythm was thought to present a strategy to improve quality of life and reduce mortality in women. However, in the Rate Control Efficacy (RACE) trial, women randomized to a rhythm control strategy using medications had a greater rate of cardiovascular events (i.e., cardiovascular death, HF, thromboembolic complications, bleeding, severe adverse effects of antiarrhythmic drugs (AADs), and need for pacemaker implantation), largely driven by adverse effects from AADs.90,91 Severe AAD-related side effects were experienced at a greater rate by women than men in the RACE trial, including sick sinus syndrome, torsade de pointes, and atrioventricular conduction during atrial flutter (4.7% vs. 1.5%), as well as pacemaker implantation (3.6% vs. 1.2%).91
Women have higher risk of proarrhythmia and adverse effects with AADs, including Class IA92 and class III AADs, including amiodarone, which has traditionally been thought to confer the lowest proarrhythmia risk.93 A study of patients over 65 years old with new-onset AF found that female sex was an independent risk factor for bradyarrhythmia and need for pacemaker placement (OR, 3.86 women vs. 1.52 men).94 With the exception of the AFFIRM trial,95 class III AADs have been shown in multiple studies to be associated with a greater risk of torsades de pointes (TdP) in women. Lack of sex-associated differences observed in the AFFIRM trial may have been driven by very close monitoring of medications and doses. The QT prolonging drugs, including sotalol, dofetilide, and ibutilide, are associated with a greater TdP risk in women.96-98 Interestingly, lower age (<61 years) for women, was associated with an increased adjusted probability of cardioversion with ibutilide98 that may be related to the longer repolarization time not only of the ventricles, but also of the atria, in premenopausal women, due to higher estrogen levels. In this study, the most pronounced QTc prolongation was observed in young women whose AF converted to sinus rhythm (86 ± 41 ms).98 Although dronedarone was shown to cause bradycardia and QT prolongation in the Effect of Dronedarone on Cardiovascular Events in AF (ATHENA) trial,93 sex differences in clinical outcomes were not evident, and dronedarone was believed linked to few sex-related side effects.99 However, a review of data between 2009 and 2011 from the Federal Drug Administration's adverse event reporting system, and found more adverse events, including TdP, reported with dronedarone than amiodarone (810 vs. 493). More women experienced cardiac arrhythmias due to TdP associated with dronedarone use than men (23 vs. 13 reports).100
Actual sex differences in AAD use for a rhythm control strategy in clinical practice are less clear. In the EORP-AF registry, rhythm control with AADs was less frequently used in women,29 while in the ORBIT-AF, no sex differences were found with regard to AAD use.27 Differences in these findings may represent physician preferences, and geographical and patient population differences. On the other hand, a multivariable adjusted analysis suggested that, among Medicare beneficiaries age ≥66 years at the time of AF diagnosis, women were slightly more likely than men to receive both rate (HR, 1.16; 95% CI, 1.15–1.17) and rhythm (HR, 1.04; 95% CI, 1.03–1.05) control.101
With regard to electrical cardioversion as treatment for AF, women are less likely to undergo electrical cardioversion, particularly if asymptomatic.29,36,102 In the EORP-AF registry, women were less likely to undergo electrical cardioversion (25.5% men vs. 18.9% women, p < .0001), and despite the potential for greater adverse effects of class III antiarrhythmic agents, women were more likely undergo pharmacologic cardioversion (28.2% vs. 22.4%, p = .0002).29 This was further confirmed in a substudy of the Prevention of Thromboembolic Events— European Registry in AF (PREFER in AF), which collected data on 7243 patients from seven European countries, and demonstrated less use of electrical cardioversion (14.9% vs. 20.6%, p < .0001) or ablation in women.26 In the United States, inpatient use of electrical cardioversion has been reported to be higher in men.103 Rates of AF recurrence after cardioversion are higher in women.104 Together with use of AAD therapy and renal failure, female sex predicted the risk of recurrence after successful cardioversion in a retrospective multicenter study of 1342 patients.105
6.1 ∣. AF ABLATION
Sex differences in clinical characteristics, referral patterns, procedural success, and complications of AF catheter ablation have been described. Women comprise the minority of patients in catheter ablation studies. Compared with men, women referred for AF are older,106-110 have a longer history of AF,106,110 are more likely to have valvular heart disease,106,108 hypertension,106 HFpEF,36 larger left atrial dimensions/volume index,106 and more comorbidities.111,112 Women, compared with men, are less likely to have “lone AF,”113 have a lower prevalence of paroxysmal AF,110 tend to be more symptomatic,114,115 and treated with more antiarrhythmic agents before ablation.109,110 Patients, especially women with HF and AF are not frequently treated with catheter ablation.116
Compared to men, it is established that women are less likely to undergo catheter ablation of AF.25,91,101,117,118 Based on the prevalence of AF in the population, this raises the possibility of potential referral bias against this invasive procedure in women. In a large observational cohort study in the United States that included 10,135 patients with AF (ORBIT-AF Registry), women were less likely to undergo AF ablation (4.9% vs. 5.9%, p = .04).91 Prospective observational registries in Western Europe and South Korea showed similar findings, with women significantly less likely to undergo AF ablation than men, despite having more severe symptoms.25,118 While temporal trends in referral for a “first” AF ablation procedure in Canada have shown an almost sevenfold increase in the rate of AF ablation over the past 10 years, there has been no increase in the relatively small proportion of women undergoing AF ablation.119 Women were three times less likely to be referred to a specialized outpatient arrhythmia clinic for management of AF.115 However, after referral to the arrhythmia clinic, there was no difference in the proportion of women and men undergoing AF ablation.115,120 Greater comorbidities and increased age at the time of diagnosis of AF may contribute to lower referral rates for women, suggesting sex differences as opposed to a referral bias. In addition, the possibility that women might be more likely to decline subspecialty referral for consideration of invasive procedures cannot be excluded. Further study is needed to determine whether sex differences in treatment decisions are due to patient preferences, eligibility for invasive therapy, other comorbidities/confounders, increased risk for complications, versus true treatment disparities.106-110,113,121,122
Sex differences in procedural outcomes with respect to maintenance of sinus rhythm have been examined. In contrast to one study that suggested no difference in early success rates with similar freedom from arrhythmia recurrences in men and women after about 2 years of follow-up,106 other studies have shown lower ablation success rates with higher recurrence rates in women.107,110,113,123,124 A pooled analysis of 20 studies revealed that women have a 20% greater risk of AF recurrence compared to men, with low heterogeneity across studies, and this difference in recurrence rates was partly attributed to the percent of nonparoxysmal AF patients.125 A more recent meta-analysis of 19 observational studies confirmed a lower rate of freedom from AF/AT recurrence in women than in men at 2.4-year follow-up (OR, 0.75; 95% CI, 0.69–0.81; p < .0001).126
Women appear to be referred later for ablation,106,110 with a longer duration of AF than men (mean, 60 vs. 47 months; p = .04). With a delay in referral for ablation in women, women may have larger left atrial sizes when adjusted for body size with more adverse electrical and structural remodeling that may be responsible for lower ablation success rates. Women may also be less likely to undergo re-do ablation procedures than men,110,112,127 and this may also help explain the potentially lower ablation success rates in women.
In addition, women are more likely to have non-pulmonary vein triggers than men,110,128 which may increase rates of arrhythmia recurrence, if these other triggers are not targeted during the procedure. Differences in outcomes between studies may be due to differences in clinical characteristics of various patient populations, duration and type of AF, procedural techniques, sample size, healthcare provider recommendations, or likelihood of patients to undergo re-do procedures. Alternatively, sex differences may be due to differences in LA remodeling or autonomic function, with lower LA endocardial voltage and higher parasympathetic activity described in women undergoing AF ablation in one study.123 In contrast, another study showed no systematic sex differences in underlying pulmonary vein or atrial substrate during electrophysiological testing with electroanatomic mapping in patients with or without a history of AF.129
Prior investigations have inconsistent findings related to sex differences in procedural complications following catheter ablation. While some studies have shown no differences in complication rates in men compared to women,106,108 others have demonstrated higher complication rates in women,107,110,112,113,121,122,130-132 largely driven from bleeding or vascular complications, such as hematomas and pseudoaneurysms, that have been reported to be higher in women than men,107,110,113,121,122 Women may also have a higher rate of cardiac tamponade112,133; in a worldwide survey of almost 35,000 AF ablation procedures, women had a twofold increased risk (1.24% in women vs. 0.67% in men, odds ratio 1.83, p < .001).
7 ∣. AF DURING PREGNANCY
Pregnancy is associated with significant hemodynamic changes, including increased blood volume, leading to four-chamber cardiac dilatation, and up to 50% increased cardiac output by 28–32 weeks' gestation.134 Heart rate increases in the setting of increased catecholamine concentrations and adrenergic receptor sensitivity.134 AF most often occurs during the third trimester or within 24 h postpartum. AF has been estimated to occur in 59.3 per 100,000 of all pregnancies, or in about 1.3% of pregnancies in women with pre-existing heart conditions.135,136 AF may occur in pregnant women with pre-existing heart disease such as hypertrophic cardiomyopathy, congenital heart disease, or valvular heart disease.137 AF may also occur in women with a structurally normal heart, either in isolation or in the setting of hyperthyroidism, pulmonary embolism, infection, drug toxicity, or pre-eclampsia.134 Hypertension, diabetes, and hyperlipidemia were associated with higher odds of AF in pregnancy, as well as older age and higher BMI.135
However, studies of AF in pregnancy are limited. Thus, there is little evidence to guide the optimal management of AF occurring in pregnancy. Recommended management is in general similar to that as in nonpregnant patients, except that treatment should be expedited and potential teratogenicity of medications needs to be considered.138 Transesophageal echocardiogram and synchronized electrical cardioversion are safe and usually successful in pregnant women, and should be performed in women with hemodynamic instability secondary to AF in women at all stages of pregnancy.62,138
Need for anticoagulation is currently guided by CHA2DS2-VASc scoring, though the added increased risk of thromboembolism due to pregnancy itself is unknown.138 Pregnancy is a prothrombotic state, for example associated with fivefold increased risk of venous thromboembolism.137,139 Additionally, CHA2DS2-VASc scoring has not been validated in pregnancy, and most pregnant women have a low CHA2DS2-VASc score (1 for female sex).138 Anticoagulation during pregnancy is further complicated by teratogenicity risk for vitamin K antagonists, continuously changing pharmacokinetics of low-molecular-weight heparin, and relatively limited data with direct oral anticoagulants during pregnancy.137,138,140 Low-molecular-weight heparin requires monitoring of peak and trough anti-Xa levels to ensure appropriate anticoagulation.137
Although AF is a relatively rare, potentially serious complication for both mother and fetus during pregnancy, women are now bearing children at older ages with more CV risk factors. As more women with congenital heart disease are surviving to adulthood, the incidence of AF in this population can be expected to increase.141 Table 6 is a summary of the 2020 ESC guidelines for management of AF in pregnant women. New recommendations include: (1) guidance for patients with hypertrophic cardiomyopathy, (2) specific AADs for rate and rhythm control, and (3) anticoagulants for different pregnancy stages.62
TABLE 6.
Recommendations for the management of AF during pregnancy
| Recommendations | Classa | Levelb |
|---|---|---|
| Acute management | ||
| Immediate electrical cardioversionc is recommended in case of haemodynamic instability or pre-excited AF | I | C |
| In pregnant women with HCM, cardioversionc should be considered for persistent AF | IIa | C |
| Ibutilide or flecainide IV may be considered for termination of AF in stable patients with structurally normal hearts | IIb | C |
| Long-term management (oral administration of drugs) | ||
| Therapeutic anticoagulation with heparin or VKA according to the stage of pregnancy is recommended for patients with AF | I | C |
| β-Selective blockers are recommended for rate control in AFd | I | C |
| Flecainide,e propafenone,e or sotalolf should be considered to prevent AF if atrioventricular nodal-blocking drugsf fail | IIa | C |
| Digoxing or verapamilg should be considered for rate control if β-blockers fail | IIa | C |
Note: Note that the former A to X categories of drugs—the classification system for counselling of pregnant women requiring drug therapy—was replaced by the Pregnancy and Lactation Labelling Rule, which provides a descriptive risk summary and detailed information on animal and clinical data, by the US FDA in June 15.
Abbreviations: AF, atrial fibrillation; ECG, electrocardiogram; HCM, hypertrophic cardiomyopathy; IV, intravenous; LV, left ventricular; QTc, corrected QT interval; US FDA, United States Food and Drug Administration; VKA, vitamin K antagonist.
Class of recommendation.
Level of evidence.
Cardioversion of AF should generally be preceded by anticoagulation.
Atenolol has been associated with higher rates of foetal growth retardation and is not recommended.
Flecainide and propafenone should be combined with atrioventricular nodal-blocking drugs, but structural heart disease, reduced LV function, and bundle branch block should be excluded.
Class III drugs should not be used in prolonged QTc.
Atrioventricular nodal-blocking drugs should not be used in patients with pre-excitation on resting ECG or pre-excited AF.
Source: 2020 Update of European AF guidelines62 Copyright obtained on December 9, 2020, from Oxford University Press.
7.1 ∣. Shared decision-making in AF management
Shared decision-making is a collaborative process whereby clinicians help patients reach a decision that is both informed by evidence and congruent with their personal values and preferences. The process involves exploring and comparing the benefits, harms, and risks of each option, relating these to what matters most to the patient, placing patients at the center of decisions about their treatment and care, with the decision reached jointly. Nowhere is this more important than for women with AF. Shared decision-making (per the Centers for Medicare and Medicaid Services) involves three components: (1) clear, accurate, and unbiased medical evidence about reasonable alternatives—including no intervention—and the risks and benefits of each, (2) clinician expertise in communicating and tailoring that evidence for an individual patient, and (3) patient values, goals, informed preferences and concerns that may include treatment burdens. Shared decision-making is a means to ensure better health decisions, that is, those that align with what matters most to the patient.142
It is important to discuss with patients their symptoms and, in particular, expectations of treatment—“feeling better, cure of the problem, living longer, staying out of the hospital, etc.” Management options should be presented in relationship to the patient's healthcare outcome preferences. This includes the risks and benefits, as well as the burdens of diagnostic testing, medical therapies, requisite follow-up, AF recurrence, etc. What is known about AF related to sex, age, and other variables specific to the patient should be addressed. In this regard, the fact that women on average have worse symptoms and quality of life should be reviewed, as well as worse outcomes with stroke prevention, rate and rhythm control strategies.
The “talk back” approach is valuable, having the patient repeat back what has been heard regarding the therapy or therapies eventually selected. Critical questions include:
How should the clinician assess the individual patient's healthcare preferences related to the management of atrial fibrillation?
How should the management options best be presented, in terms of their relationship to the patient's healthcare outcome preferences?
8 ∣. SUMMARY AND CONCLUSIONS
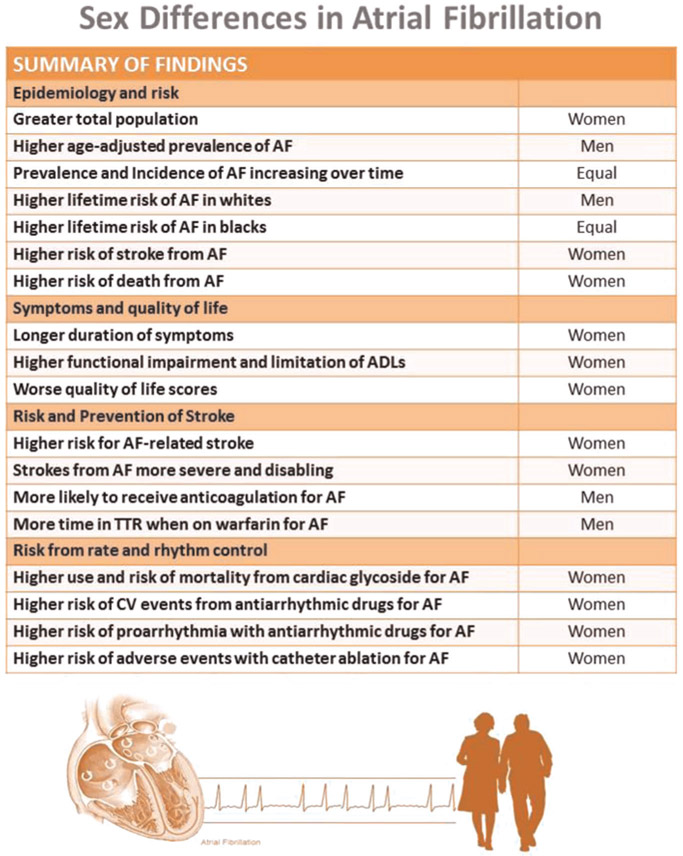
A summary of our findings appears in Figure 1. There is an epidemiologic difference in the prevalence of AF between women and men with a three to two male to female ratio; importantly, this does not consider age-related differences or the predominance of women in our elderly population. An overview of these data indicates that while women have a lower incidence and prevalence, they have a poorer prognosis with AF that includes increased risk of stroke, HF, and death. There are many knowledge gaps about pathophysiologic mechanisms accounting for these differences as summarized in Table 7 with recommendations for future research.
FIGURE 1.
Summary of sex differences in atrial fibrillation. ADLs, activity of daily living; AF, atrial fibrillation; CV, cardiovascular; TTR, time in therapeutic range
TABLE 7.
Critical knowledge gaps and recommendations for future research
| 1 | Increased prevalence and incidence over time for both sexes may be due to more people living longer. However, it may be possible to decrease incidence by decreasing the risk factors for AF such as obesity, hypertension, and heart failure. Studies are needed to ascertain if improved blood pressure control in older women decreases AF incidence |
| 2 | Unclear why there is a higher lifetime risk of AF in Whites versus individuals of other races/ethnicities. Studies to evaluate whether genetic versus environmental versus structural factors contribute to this risk |
| 3 | Studies to understand why women are not receiving anticoagulation when appropriate may decrease this disparity in treatment |
| 4 | Studies to understand the higher risk of death from AF in women are needed to decrease mortality from AF |
| 5 | Studies to better understand symptoms and what treatments are more effective in ameliorating them to improve quality of life of AF patients |
| 6 | Efforts needed to better educate healthcare professionals to avoid unnecessary or inappropriate use of cardiac glycosides may decrease risk of breast cancer and mortality from cardiac glycosides |
| 7 | Studies are needed to determine what measures can decrease risk from antiarrhythmic drugs |
| 8 | More efforts are needed to prevent vascular and bleeding risks in women may help decrease the risk of adverse events with catheter ablation |
Abbreviation: AF, atrial fibrillation.
It appears that women have different left atrial morphology, more atrial fibrosis associated with increased cerebral infarcts, and worse outcomes with cryoballoon pulmonary vein isolation, more nonpulmonary foci, and differences in membrane action potential duration. These differences are partially driven by estrogen. Although older, women benefit from treatment of AF including cardioversion and ablation and prevention of stroke including anticoagulation. These therapies are utilized less often in women than in men, the reasons for which are unclear and require further investigation.
The role of LAA occlusion is considered a class IIb for both men and women with AF. The role of LAA occlusion, and whether there are sex differences in the benefits and complications by sex requires further study.
There are widespread sex differences in CV structure and function, risk factors, epidemiology, disease manifestations, and clinical outcomes. AF is a complex disease, with sex differences in epidemiology, risk factors, treatment, and outcomes. Women have more severe symptoms, less effective treatment options, and worse outcomes. A better understanding of these differences is critical to improve outcomes and reduce disparities of care between women and men. These improvements include increased use of appropriate stroke prevention strategies, symptom control, better monitoring of medical therapy, and optimized procedural outcomes.
Abbreviations:
- AAD
antiarrhythmic drugs
- AF
atrial fibrillation
- BMI
body mass index
- CIED
cardiac implantable electronic device
- CYT
cytochrome
- EHRA
European Heart Rhythm Association
- FHS
Framingham Heart Study
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- LAA
left atrial appendage
- RR
relative risk
- TdP
torsades de pointes
Footnotes
Disclosures: Anne B. Curtis: advisory board: Abbott, Janssen Pharmaceuticals, Novartis, Sanofi Aventis, and Milestone Pharmaceuticals; honoraria for speaking: Medtronic, Inc., Biotronik, Novartis, and Boston Scientific; data monitoring board: Medtronic, Inc. Gerald V. Naccarelli: consultant: Acesion, Omeicos, Janssen, Sanofi, and Milestone; research grant: Janssen. Andrea M. Russo: research trials (funding to hospital): BMS, Boehringer Ingelheim, Boston Scientific, Kestra, and Medilynx; Steering Committee, research: Apple, Boston Scientific (no financial reimbursement), and Medtronic; consulting: Medtronic. Annabelle S. Volgman: Apple Inc. Stock. Albert L. Waldo: consultant: Biosense Webster, Milestoms Pharmaceuticals, and Cardiac Insight; speaker: Pfizer and Bristol-Myers Squib.
REFERENCES
- 1.Delaney JA, Yin X, Fontes JD, et al. Hospital and clinical care costs associated with atrial fibrillation for Medicare beneficiaries in the Cardiovascular Health Study and the Framingham Heart Study. SAGE Open Med. 2018;6:2050312118759444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dieleman JL, Cao J, Chapin A, et al. US health care spending by payer and health condition, 1996-2016. JAMA. 2020;323:863–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. [DOI] [PubMed] [Google Scholar]
- 4.Virani SS, Alonso A, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 5.Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez CJ, Soliman EZ, Alonso A, et al. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25(2):71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staerk L, Wang B, Preis SR, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ. 2018;361:k1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mou L, Norby FL, Chen LY, et al. Lifetime risk of atrial fibrillation by race and socioeconomic status: ARIC study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol. 2018;11:e006350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass Screening for untreated atrial fibrillation: the STROKESTOP Study. Circulation. 2015;131:2176–84. [DOI] [PubMed] [Google Scholar]
- 13.Lowres N, Chao TF, Chen SA, et al. Guideline-based screening for atrial fibrillation: meta-analysis of yield and stroke risk. Paper presented at: Circulation; 2017. [Google Scholar]
- 14.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 15.Piccini JP, Passman R, Turakhia M, Connolly AT, Nabutovsky Y, Varma N. Atrial fibrillation burden, progression, and the risk of death: a case-crossover analysis in patients with cardiac implantable electronic devices. Europace. 2019;21:404–413. [DOI] [PubMed] [Google Scholar]
- 16.Ko D, Rahman F, Schnabel RB, Yin X, Benjamin EJ, Christophersen IE. Atrial fibrillation in women: epidemiology, pathophysiology, presentation, and prognosis. Nat Rev Cardiol. 2016;13:321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrade JG, Deyell MW, Lee AYK, Macle L. Sex differences in atrial fibrillation. Can J Cardiol. 2018;34:429–436. [DOI] [PubMed] [Google Scholar]
- 18.Magnussen C, Niiranen TJ, Ojeda FM, et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation. 2017;136:1588–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McManus DD, Xanthakis V, Sullivan LM, et al. Longitudinal tracking of left atrial diameter over the adult life course: clinical correlates in the community. Circulation. 2010;121:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishi S, Reis JP, Venkatesh BA, et al. Race-ethnic and sex differences in left ventricular structure and function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Heart Assoc. 2015;4:e001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–730. [DOI] [PubMed] [Google Scholar]
- 22.Cochet H, Mouries A, Nivet H, et al. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J Cardiovasc Electrophysiol. 2015;26:484–492. [DOI] [PubMed] [Google Scholar]
- 23.Akoum N, Mahnkopf C, Kholmovski EG, Brachmann J, Marrouche NF. Age and sex differences in atrial fibrosis among patients with atrial fibrillation. Europace. 2018;20:1086–1092. [DOI] [PubMed] [Google Scholar]
- 24.Gramlich M, Maleck C, Marquardt J, et al. Cryoballoon ablation for persistent atrial fibrillation in patients without left atrial fibrosis. J Cardiovasc Electrophysiol. 2019;30:999–1004. [DOI] [PubMed] [Google Scholar]
- 25.Emdin CA, Wong CX, Hsiao AJ, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ. 2016;532:h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnabel RB, Pecen L, Ojeda FM, et al. Gender differences in clinical presentation and 1-year outcomes in atrial fibrillation. Heart. 2017;103:1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piccini JP, Simon DN, Steinberg BA, et al. Outcomes registry for better informed treatment of atrial fibrillation I and patients. Differences in clinical and functional outcomes of atrial fibrillation in women and men: two-year results from the ORBIT-AF Registry. JAMA Cardiol. 2016;1:282–291. [DOI] [PubMed] [Google Scholar]
- 28.Blum S, Muff C, Aeschbacher S, et al. Prospective assessment of sex-related differences in symptom status and health perception among patients with atrial fibrillation. J Am Heart Assoc. 2017;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lip GY, Laroche C, Boriani G, et al. Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Observational Research Programme Pilot survey on atrial fibrillation. Europace. 2015;17:24–31. [DOI] [PubMed] [Google Scholar]
- 30.Xiong Q, Proietti M, Senoo K, Lip GY. Asymptomatic versus symptomatic atrial fibrillation: a systematic review of age/gender differences and cardiovascular outcomes. Int J Cardiol. 2015;191:172–177. [DOI] [PubMed] [Google Scholar]
- 31.Gleason KT, Dennison Himmelfarb CR, Ford DE, et al. Association of sex, age and education level with patient reported outcomes in atrial fibrillation. BMC Cardiovasc Disord. 2019;19:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheuermeyer FX, Mackay M, Christenson J, et al. There are sex differences in the demographics and risk profiles of emergency department (ED) patients with atrial fibrillation and flutter, but no apparent differences in ED management or outcomes. Acad Emerg Med. 2015;22:1067–1075. [DOI] [PubMed] [Google Scholar]
- 33.Lip GY, Laroche C, Ioachim PM, et al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry). Eur Heart J. 2014;35:3365–3376. [DOI] [PubMed] [Google Scholar]
- 34.Magnussen C, Ojeda FM, Wild PS, et al. Atrial fibrillation manifestations risk factors and sex differences in a population-based cohort (from the Gutenberg Health Study). Am J Cardiol. 2018;122:76–82. [DOI] [PubMed] [Google Scholar]
- 35.Sinner MF, Greiner MA, Mi X, et al. Completion of guideline-recommended initial evaluation of atrial fibrillation. Clin Cardiol. 2012;35:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dagres N, Nieuwlaat R, Vardas PE, et al. Gender-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Heart Survey on Atrial Fibrillation. J Am Coll Cardiol. 2007;49:572–577. [DOI] [PubMed] [Google Scholar]
- 37.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ball J, Løchen ML, Wilsgaard T, et al. Sex differences in the impact of body mass index on the risk of future atrial fibrillation: insights from the Longitudinal Population-Based Tromsø Study. J Am Heart Assoc. 2018;7:e008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng EY, Kong MH. Gender differences of thromboembolic events in atrial fibrillation. Am J Cardiol. 2016;117:1021–1027. [DOI] [PubMed] [Google Scholar]
- 40.Wagstaff AJ, Overvad TF, Lip GY, Lane DA. Is female sex a risk factor for stroke and thromboembolism in patients with atrial fibrillation? A systematic review and meta-analysis. QJM. 2014;107:955–967. [DOI] [PubMed] [Google Scholar]
- 41.Marzona I, Proietti M, Farcomeni A, et al. Sex differences in stroke and major adverse clinical events in patients with atrial fibrillation: a systematic review and meta-analysis of 993,600 patients. Int J Cardiol. 2018;269:182–191. [DOI] [PubMed] [Google Scholar]
- 42.Raccah BH, Perlman A, Zwas DR, et al. Gender differences in efficacy and safety of direct oral anticoagulants in atrial fibrillation: systematic review and network meta-analysis. Ann Pharmacother. 2018;52(11):1135–1142. [DOI] [PubMed] [Google Scholar]
- 43.Bassand JP, Accetta G, GARFIELD-AF Investigators, et al. Risk factors for death, stroke, and bleeding in 28,628 patients from the GARFIELD-AF registry: rationale for comprehensive management of atrial fibrillation. PLOS One. 2018;13:e0191592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camm AJ, Accetta G, GARFIELD-AF Investigators, et al. Impact of gender on event rates at 1 year in patients with newly diagnosed non-valvular atrial fibrillation: contemporary perspective from the GARFIELD-AF registry. BMJ Open. 2017;7:e014579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 46.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 47.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Kardiol Pol. 2016;74:1359–1469. [DOI] [PubMed] [Google Scholar]
- 48.Martin RC, Burgin WS, Schabath MB, et al. Gender-specific differences for risk of disability and death in atrial fibrillation-related stroke. Am J Cardiol. 2017;119:256–261. [DOI] [PubMed] [Google Scholar]
- 49.Tomasdottir M, Friberg L, Hijazi Z, Lindböck J, Oldgren J. Risk of ischemic stroke and utility of CHA(2) DS(2)-VASc score in women and men with atrial fibrillation. Clin Cardiol. 2019;42:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson LE, Maddox TM, Lei L, et al. Sex differences in the use of oral anticoagulants for atrial fibrillation: a report from the National Cardiovascular Data Registry (NCDR(R)) PINNACLE Registry. J Am Heart Assoc. 2017;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yong CM, Tremmel JA, Lansberg MG, Fan J, Askari M, Turakhia MP. Sex differences in oral anticoagulation and outcomes of stroke and intracranial bleeding in newly diagnosed atrial fibrillation. J Am Heart Assoc. 2020;9:e015689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lip GY, Rushton-Smith SK, Goldhaber SZ, et al. Does sex affect anticoagulant use for stroke prevention in nonvalvular atrial fibrillation? The prospective global anticoagulant registry in the FIELD-Atrial Fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8:S12–S20. [DOI] [PubMed] [Google Scholar]
- 53.Loikas D, Forslund T, Wettermark B, Schenck-Gustafsson K, Hjemdahl P, von Euler M. Sex and gender differences in thromboprophylactic treatment of patients with atrial fibrillation after the introduction of non–vitamin K oral anticoagulants. Am J Cardiol. 2017;120:1302–1308. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan RM, Zhang J, Zamba G, Lip GY, Olshansky B. Relation of gender-specific risk of ischemic stroke in patients with atrial fibrillation to differences in warfarin anticoagulation control (from AFFIRM). Am J Cardiol. 2012;110:1799–1802. [DOI] [PubMed] [Google Scholar]
- 55.Pancholy SB, Sharma PS, Pancholy DS, Patel TM, Callans DJ, Marchlinski FE. Meta-analysis of gender differences in residual stroke risk and major bleeding in patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Am J Cardiol. 2014;113:485–490. [DOI] [PubMed] [Google Scholar]
- 56.Proietti M, Cheli P, Basili S, Mazurek M, Lip GY. Balancing thromboembolic and bleeding risk with non-vitamin K antagonist oral anticoagulants (NOACs): a systematic review and meta-analysis on gender differences. Pharmacol Res. 2017;117:274–282. [DOI] [PubMed] [Google Scholar]
- 57.Moseley A, Doukky R, Williams KA, Jaffer AK, Volgman AS. Indirect comparison of novel oral anticoagulants in women with nonvalvular atrial fibrillation. J Womens Health (Larchmt). 2017;26:214–221. [DOI] [PubMed] [Google Scholar]
- 58.Wei Y-C, George NI, Chang C-W, Hicks KA. Assessing sex differences in the risk of cardiovascular disease and mortality per increment in systolic blood pressure: a systematic review and meta-analysis of follow-up studies in the United States. PLOS One. 2017;12:e0170218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poorthuis MH, Algra AM, Algra A, Kappelle LJ, Klijn CJ. Female- and male-specific risk factors for stroke: a systematic review and meta-analysis. JAMA Neurol. 2017;74:75–81. [DOI] [PubMed] [Google Scholar]
- 60.Renoux C, Coulombe J, Suissa S. Revisiting sex differences in outcomes in non-valvular atrial fibrillation: a population-based cohort study. Eur Heart J. 2017;38:1473–1479. [DOI] [PubMed] [Google Scholar]
- 61.January CT, Wann LS, Calkins H, et al. AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;2019(140):e125–e151. [DOI] [PubMed] [Google Scholar]
- 62.Hindricks G, Potpara T, Dagres N, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) [published online ahead of print August 29, 2020]. Eur Heart J. 2020.ehaa612. [Google Scholar]
- 63.Linde C, Bongiorni MG, Birgersdotter-Green U, et al. Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace. 2018;20–1565. [DOI] [PubMed] [Google Scholar]
- 64.Nielsen PB, Skjoth F, Overvad TF, Larsen TB, Lip GYH. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: should we use a CHA2DS2-VA score rather than CHA2DS2-VASc? Circulation. 2018;137:832–840. [DOI] [PubMed] [Google Scholar]
- 65.Di Biase L, Santangeli P, Anselmino M, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012;60:531–538. [DOI] [PubMed] [Google Scholar]
- 66.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. [DOI] [PubMed] [Google Scholar]
- 67.Yu HT, Lee JS, Kim TH, et al. Advanced left atrial remodeling and appendage contractile dysfunction in women than in men among the patients with atrial fibrillation: potential mechanism for stroke. J Am Heart Assoc. 2016;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casu G, Gulizia MM, Molon G, et al. ANMCO/AIAC/SICI-GISE/SIC/SICCH consensus document: percutaneous occlusion of the left atrial appendage in non-valvular atrial fibrillation patients: indications, patient selection, staff skills, organisation, and training. Eur Heart J Suppl. 2017;19:D333–D353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol. 2015;65:2614–2623. [DOI] [PubMed] [Google Scholar]
- 70.Kaneko H, Neuss M, Weissenborn J, Butter C. Predictors of thrombus formation after percutaneous left atrial appendage closure using the WATCHMAN device. Heart Vessels. 2017;32:1137–1143. [DOI] [PubMed] [Google Scholar]
- 71.Dukkipati SR, Kar S, Holmes DR, et al. Device-related thrombus after left atrial appendage closure: incidence, predictors, and outcomes. Circulation. 2018;138:874–885. [DOI] [PubMed] [Google Scholar]
- 72.Saw J, Tzikas A, Shakir S, et al. Incidence and clinical impact of device-associated thrombus and peri-device leak following left atrial appendage closure with the Amplatzer cardiac plug. JACC Cardiovasc Interv. 2017;10:391–399. [DOI] [PubMed] [Google Scholar]
- 73.Reddy VY, Doshi SK, Kar S, et al. 5-year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–2975. [DOI] [PubMed] [Google Scholar]
- 74.Masoud A, Bartoletti S, Fairbairn T, et al. Outcome of left atrial appendage occlusion in high-risk patients. Heart. 2018;104:594–599. [DOI] [PubMed] [Google Scholar]
- 75.Regueiro A, Cruz-Gonzalez I, Bethencourt A, et al. Long-term outcomes following percutaneous left atrial appendage closure in patients with atrial fibrillation and contraindications to anticoagulation. J Interv Card Electrophysiol. 2018;52:53–59. [DOI] [PubMed] [Google Scholar]
- 76.Badhwar V, Rankin JS, Damiano RJ, Jr., et al. The Society of Thoracic Surgeons 2017 clinical practice guidelines for the surgical treatment of atrial fibrillation. Ann Thorac Surg. 2017;103:329–341. [DOI] [PubMed] [Google Scholar]
- 77.Dominguez H, Madsen CV, Westh ONH, et al. Does left atrial appendage amputation during routine cardiac surgery reduce future atrial fibrillation and stroke? Curr Cardiol Rep. 2018;20:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol. 2008;52:924–929. [DOI] [PubMed] [Google Scholar]
- 79.Atti V, Anantha-Narayanan M, Turagam MK, et al. Surgical left atrial appendage occlusion during cardiac surgery: a systematic review and meta-analysis. World J Cardiol. 2018;10:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yao X, Gersh BJ, Holmes DR Jr., et al. Association of surgical left atrial appendage occlusion with subsequent stroke and mortality among patients undergoing cardiac surgery. JAMA. 2018;319:2116–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nicolson TJ, Mellor HR, Roberts RR. Gender differences in drug toxicity. Trends Pharmacol Sci. 2010;31:108–114. [DOI] [PubMed] [Google Scholar]
- 82.Kaiser J Gender in the pharmacy: does it matter? Science. 2005;308(5728):1572. [DOI] [PubMed] [Google Scholar]
- 83.Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347:1403–1411. [DOI] [PubMed] [Google Scholar]
- 84.Washam JB, Stevens SR, Lokhnygina Y, et al. Digoxin use in patients with atrial fibrillation and adverse cardiovascular outcomes: a retrospective analysis of the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Lancet. 2015;385:2363–2370. [DOI] [PubMed] [Google Scholar]
- 85.Wassertheil-Smoller S, McGinn AP, Martin L, Rodriguez BL, Stefanick ML, Perez M. The associations of atrial fibrillation with the risks of incident invasive breast and colorectal cancer. Am J Epidemiol. 2017;185:372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Osman MH, Farrag E, Selim M, Osman MS, Hasanine A, Selim A. Cardiac glycosides use and the risk and mortality of cancer: systematic review and meta-analysis of observational studies. PLOS One. 2017;12:e0178611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nowak B, Misselwitz B, Erdogan A, et al. Do gender differences exist in pacemaker implantation? Results of an obligatory external quality control program. Europace. 2010;12:210–215. [DOI] [PubMed] [Google Scholar]
- 88.Boriani G, Lorenzetti S, Cerbai E, et al. The effects of gender on electrical therapies for the heart: physiology, epidemiology, and access to therapies. Europace. 2017;19:1418–1426. [DOI] [PubMed] [Google Scholar]
- 89.Smit MD, Crijns HJ, RACE II Investigators, et al. Effect of lenient versus strict rate control on cardiac remodeling in patients with atrial fibrillation data of the RACE II (RAte Control Efficacy in permanent atrial fibrillation II) study. J Am Coll Cardiol. 2011;58:942–949. [DOI] [PubMed] [Google Scholar]
- 90.van Gelder IC, Hagens VE, Kingma JH, et al. Rate control versus electrical cardioversion for atrial fibrillation: a randomised comparison of two treatment strategies concerning morbidity, mortality, quality of life and cost-benefit—the RACE study design. Neth Heart J. 2002;10:118–124. [PMC free article] [PubMed] [Google Scholar]
- 91.Rienstra M, Van Veldhuisen DJ, Hagens VE, et al. Gender-related differences in rhythm control treatment in persistent atrial fibrillation: data of the Rate Control Versus Electrical Cardioversion (RACE) study. J Am Coll Cardiol. 2005;46:1298–1306. [DOI] [PubMed] [Google Scholar]
- 92.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–2597. [DOI] [PubMed] [Google Scholar]
- 93.Hohnloser SH, Crijns HJ, ATHENA Investigators, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668–78. [DOI] [PubMed] [Google Scholar]
- 94.Essebag V, Hadjis T, Platt RW, Pilote L. Amiodarone and the risk of bradyarrhythmia requiring permanent pacemaker in elderly patients with atrial fibrillation and prior myocardial infarction. J Am Coll Cardiol. 2003;41:249–254. [DOI] [PubMed] [Google Scholar]
- 95.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 96.Torp-Pedersen C, Moller M, Bloch-Thomsen PE, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med. 1999;341:857–65. [DOI] [PubMed] [Google Scholar]
- 97.Gowda RM, Khan IA, Punukollu G, Vasavada BC, Sacchi TJ, Wilbur SL. Female preponderance in ibutilide-induced torsade de pointes. Int J Cardiol. 2004;95:219–222. [DOI] [PubMed] [Google Scholar]
- 98.Reisinger J, Gatterer E, Lang W, et al. Flecainide versus ibutilide for immediate cardioversion of atrial fibrillation of recent onset. Eur Heart J. 2004;25:1318–1324. [DOI] [PubMed] [Google Scholar]
- 99.Dorian P Clinical pharmacology of dronedarone: implications for the therapy of atrial fibrillation. J Cardiovasc Pharmacol Ther. 2010;15:15S–18SS. [DOI] [PubMed] [Google Scholar]
- 100.Kao DP, Hiatt WR, Krantz MJ. Proarrhythmic potential of dronedarone: emerging evidence from spontaneous adverse event reporting. Pharmacotherapy. 2012;32:767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhave PD, Lu X, Girotra S, Kamel H, Vaughan Sarrazin MS. Race- and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm. 2015;12:1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alegret JM, Vinolas X, Martinez-Rubio A, et al. Gender differences in patients with atrial fibrillation undergoing electrical cardioversion. J Womens Health (Larchmt). 2015;24:466–470. [DOI] [PubMed] [Google Scholar]
- 103.Rochlani YM, Shah NN, Pothineni NV, Paydak H. Utilization and predictors of electrical cardioversion in patients hospitalized for atrial fibrillation. Cardiol Res Pract. 2016;2016:8956020–8956025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gurevitz OT, Varadachari CJ, Ammash NM, et al. The effect of patient sex on recurrence of atrial fibrillation following successful direct current cardioversion. Am Heart J. 2006;152 (155):e9–e13. [DOI] [PubMed] [Google Scholar]
- 105.Hellman T, Kiviniemi T, Vasankari T, et al. Prediction of ineffective elective cardioversion of atrial fibrillation: a retrospective multi-center patient cohort study. BMC Cardiovasc Disord. 2017;17:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Forleo GB, Tondo C, De Luca L, et al. Gender-related differences in catheter ablation of atrial fibrillation. Europace. 2007;9:613–620. [DOI] [PubMed] [Google Scholar]
- 107.Zylla MM, Brachmann J, Lewalter T, et al. Sex-related outcome of atrial fibrillation ablation: insights from the German Ablation Registry. Heart Rhythm. 2016;13:1837–1844. [DOI] [PubMed] [Google Scholar]
- 108.Takigawa M, Kuwahara T, Takahashi A, et al. Differences in catheter ablation of paroxysmal atrial fibrillation between males and females. Int J Cardiol. 2013;168:1984–1991. [DOI] [PubMed] [Google Scholar]
- 109.Winkle RA, Mead RH, Engel G, Patrawala RA. Long-term results of atrial fibrillation ablation: the importance of all initial ablation failures undergoing a repeat ablation. Am Heart J. 2011;162:193–200. [DOI] [PubMed] [Google Scholar]
- 110.Patel D, Mohanty P, Di Biase L, et al. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm. 2010;7:167–172. [DOI] [PubMed] [Google Scholar]
- 111.Grecu M, Blomstrom-Lundqvist C, Kautzner J, et al. In-hospital and 12-month follow-up outcome from the ESC-EORP EHRA Atrial Fibrillation Ablation Long-Term registry: sex differences. Europace. 2020;22:66–73. [DOI] [PubMed] [Google Scholar]
- 112.Kaiser DW, Fan J, Schmitt S, et al. Gender differences in clinical outcomes after catheter ablation of atrial fibrillation. JACC Clin Electrophysiol. 2016;2:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang XD, Tan HW, Gu J, et al. Efficacy and safety of catheter ablation for long-standing persistent atrial fibrillation in women. Pacing Clin Electrophysiol. 2013;36:1236–1244. [DOI] [PubMed] [Google Scholar]
- 114.Reynolds MR, Lavelle T, Essebag V, Cohen DJ, Zimetbaum P. Influence of age, sex, and atrial fibrillation recurrence on quality of life outcomes in a population of patients with new-onset atrial fibrillation: the Fibrillation Registry Assessing Costs, Therapies, Adverse events and Lifestyle (FRACTAL) study. Am Heart J. 2006;152:1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roten L, Rimoldi SF, Schwick N, et al. Gender differences in patients referred for atrial fibrillation management to a tertiary center. Pacing Clin Electrophysiol. 2009;32:622–626. [DOI] [PubMed] [Google Scholar]
- 116.Samuel M, Abrahamowicz M, Joza J, Essebag V, Pilote L. Population-level sex differences and predictors for treatment with catheter ablation in patients with atrial fibrillation and heart failure. CJC Open. 2020;2:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kummer BR Bhave PD, Merkler AE, Gialdini G, Okin PM, Kamel H. Demographic differences in catheter ablation after hospital presentation with symptomatic atrial fibrillation. J Am Heart Assoc. 2015;4:e002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee JM, Kim TH, Cha MJ, et al. Gender-related differences in management of nonvalvular atrial fibrillation in an Asian population. Korean Circ J. 2018;48:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Avgil Tsadok M, Gagnon J, Joza J, et al. Temporal trends and sex differences in pulmonary vein isolation for patients with atrial fibrillation. Heart Rhythm. 2015;12:1979–1986. [DOI] [PubMed] [Google Scholar]
- 120.Gerstenfeld EP, Callans D, Dixit S, et al. Characteristics of patients undergoing atrial fibrillation ablation: trends over a seven-year period 1999-2005. J Cardiovasc Electrophysiol. 2007;18:23–28. [DOI] [PubMed] [Google Scholar]
- 121.Kuck KH, Brugada J, FIRE AND ICE Investigators, et al. Impact of female sex on clinical outcomes in the fire and ice trial of catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2018;11:e006204. [DOI] [PubMed] [Google Scholar]
- 122.De Greef Y, Stroker E, Schwagten B, et al. Complications of pulmonary vein isolation in atrial fibrillation: predictors and comparison between four different ablation techniques: results from the MId-delheim PVI-registry. Europace. 2017;20(8):1279–1286. [DOI] [PubMed] [Google Scholar]
- 123.Yu HT, Yang PS, Kim TH, et al. Poor rhythm outcome of catheter ablation for early-onset atrial fibrillation in women-mechanistic insight. Circ J. 2018;82:2259–2268. [DOI] [PubMed] [Google Scholar]
- 124.Heist EK, Chalhoub F, Barrett C, Danik S, Ruskin JN, Mansour M. Predictors of atrial fibrillation termination and clinical success of catheter ablation of persistent atrial fibrillation. Am J Cardiol. 2012;110:545–551. [DOI] [PubMed] [Google Scholar]
- 125.Vallakati A, Reddy M, Sharma A, et al. Impact of gender on outcomes after atrial fibrillation ablation. Int J Cardiol. 2015;187:12–16. [DOI] [PubMed] [Google Scholar]
- 126.Cheng X, Hu Q, Gao L, Liu J, Qin S, Zhang D. Sex-related differences in catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Europace. 2019;21:1509–1518. [DOI] [PubMed] [Google Scholar]
- 127.Russo AM, Zado E, Gerstenfeld EP, et al. Under-referral of women for atrial fibrillation ablation: can this be explained by gender differences in outcome? J Am Coll Cardiol. 2008;51.A6. [Google Scholar]
- 128.Lee SH, Tai CT, Hsieh MH, et al. Predictors of non-pulmonary vein ectopic beats initiating paroxysmal atrial fibrillation: implication for catheter ablation. J Am Coll Cardiol. 2005;46:1054–1059. [DOI] [PubMed] [Google Scholar]
- 129.Walters TE, Teh AW, Spence S, Morton JB, Kistler PM, Kalman JM. Absence of gender-based differences in the atrial and pulmonary vein substrate: a detailed electroanatomic mapping study. J Cardiovasc Electrophysiol. 2014;25:1065–1070. [DOI] [PubMed] [Google Scholar]
- 130.Spragg DD, Dalal D, Cheema A, et al. Complications of catheter ablation for atrial fibrillation: incidence and predictors. J Cardiovasc Electrophysiol. 2008;19:627–631. [DOI] [PubMed] [Google Scholar]
- 131.Shah RU, Freeman JV, Shilane D, Wang PJ, Go AS, Hlatky MA. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2012;59:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Elayi CS, Darrat Y, Suffredini JM, et al. Sex differences in complications of catheter ablation for atrial fibrillation: results on 85,977 patients. J Interv Card Electrophysiol. 2018;53:333–339. [DOI] [PubMed] [Google Scholar]
- 133.Michowitz Y, Rahkovich M, Oral H, et al. Effects of sex on the incidence of cardiac tamponade after catheter ablation of atrial fibrillation: results from a worldwide survey in 34 943 atrial fibrillation ablation procedures. Circ Arrhythm Electrophysiol. 2014;7:274–280. [DOI] [PubMed] [Google Scholar]
- 134.Cumyn A, Sauve N, Rey E. Atrial fibrillation with a structurally normal heart in pregnancy: an international survey on current practice. Obstet Med. 2017;10:74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee MS, Chen W, Zhang Z, et al. Atrial fibrillation and atrial flutter in pregnant women-a population-based study. J Am Heart Assoc. 2016;5:e003182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Salam AM, Ertekin E, van Hagen IM, et al. Atrial fibrillation or flutter during pregnancy in patients with structural heart disease: data from the ROPAC (Registry on Pregnancy and Cardiac Disease). JACC Clin Electrophysiol. 2015;1:284–292. [DOI] [PubMed] [Google Scholar]
- 137.Lip GYH, Collet JP, Caterina R, et al. Antithrombotic therapy in atrial fibrillation associated with valvular heart disease: a joint consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology Working Group on Thrombosis, endorsed by the ESC Working Group on Valvular Heart Disease, Cardiac Arrhythmia Society of Southern Africa (CASSA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), South African Heart (SA Heart) Association and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace. 2017;19:1757–1758. [DOI] [PubMed] [Google Scholar]
- 138.Katsi V, Georgiopoulos G, Marketou M, et al. Atrial fibrillation in pregnancy: a growing challenge. Curr Med Res Opin. 2017;33:1497–1504. [DOI] [PubMed] [Google Scholar]
- 139.Brenner B Haemostatic changes in pregnancy. Thromb Res. 2004;114:409–414. [DOI] [PubMed] [Google Scholar]
- 140.Hoeltzenbein M, Beck E, Meixner K, Schaefer C, Kreutz R. Pregnancy outcome after exposure to the novel oral anticoagulant rivaroxaban in women at suspected risk for thromboembolic events: a case series from the German Embryotox Pharmacovigilance Centre. Clin Res Cardiol. 2016;105:117–126. [DOI] [PubMed] [Google Scholar]
- 141.Simpson LL. Maternal cardiac disease: update for the clinician. Obstet Gynecol. 2012;119:345–359. [DOI] [PubMed] [Google Scholar]
- 142.Barry MJ, Edgman-Levitan S. Shared decision making—pinnacle of patient-centered care. N Engl J Med. 2012;366:780–781. [DOI] [PubMed] [Google Scholar]