Abstract
Human exposure to mercury is a leading public health problem. Artisanal and small-scale gold mining (ASGM) is a major source of global mercury emissions. Although occupational mercury exposure to miners (via mercury vapor inhalation) is known, chronic mercury exposure to nearby residents that are not miners (via mercury-contaminated fish consumption) is poorly characterized. We conducted a population-based mercury exposure assessment in 23 communities (19 rural, 4 urban) around the Amarakaeri Communal Reserve, which is bordered on the east by heavy ASGM activity. We measured total mercury in hair (N=2,083) and blood (N=476) from March-June 2015, performed follow-up measurements (N=723 hair and N=290 blood) from February-April 2016. Mercury exposure risk was highest in communities classified as indigenous, or native, regardless of proximity to mining activity. Residence in a native community (vs. non-native) was associated with mercury levels 1.9 times higher in hair (median native 3.5 ppm vs. median non-native 1.4 ppm total mercury) and 1.6 times higher in blood (median native 7.4 ng/mL vs median non-native 3.2 ng/mL total mercury). Unexpectedly, proximity to mining was not associated with exposure risk. These findings challenge common assumptions about mercury exposure patterns and emphasize the importance of population-representative studies to identify high risk sub-populations.
INTRODUCTION
Pollution is the largest environmental cause of death and disease worldwide(1). Environmental contamination disproportionately impacts the poor and vulnerable; nearly 92% of pollution-related deaths occur in low- and middle-income countries(2). Current impact estimates are limited by a lack of exposure data, and communities for which we have the least information are at highest risk(1). Informal resource extraction, including mining of valuable metals, is a lucrative but dangerous option in poor, resource-rich countries(2, 3). Artisanal and small-scale gold mining (ASGM) is an informal mining technique that commonly uses large inputs of mercury(2, 3). Miners mix liquid elemental mercury with soil or sediment to form mercury-gold amalgams, which they burn to yield a semi-pure gold alloy(2, 3). A fraction of this gaseous mercury is released to the global atmosphere(3). The remaining gaseous mercury is deposited on the terrestrial landscape and surface water, generally as oxidized inorganic divalent mercury(3); this aquatic contamination is compounded by miners disposing of unused liquid elemental mercury in local rivers(3). Anaerobic microorganisms in saturated soils, sediment and water convert inorganic mercury to methylmercury(3), which bioaccumulates and biomagnifies in the aquatic food web(3-5).
ASGM activity is rapidly expanding worldwide(2, 3), with significant but largely unmeasured health implications for mercury-exposed populations living near mining activity. Both inorganic mercury and methylmercury are highly neurotoxic metals that irreversibly damage the brain, most potently in the very young(4, 5). Miners are exposed to elemental mercury (commonly measured as oxidized inorganic divalent mercury in peripheral blood or urine) via inhalation of mercury vapor during amalgam burning(2, 4, 5). Residents of nearby communities may be exposed through consumption of fish contaminated with methylmercury (commonly measured in hair)(4, 5).
The scope and magnitude of ASGM-related mercury exposure to human populations is unknown. Although mercury exposure to miners has recently been quantified(1, 2, 6), miners represent only a fraction of those at risk. Fish contaminated with ASGM-mercury impact the food supply in wide geographic areas. Currently, an estimated 14-19 million miners are at risk for occupational exposure to mercury vapor, resulting in 2.9 million disability-adjusted life years (DALYs) lost(1, 2, 6, 7). Exposure risk to broader communities likely affects millions more, but reliable data are lacking. Available community-level mercury data near ASGM draw on small convenience samples, which are non-random, not generalizable to the population, and provide a poor measure of the exposure landscape(8-11).
Here, we conducted a population-level mercury exposure assessment at an ASGM site to fully characterize the scope and magnitude of mercury exposure to residents of communities not directly involved in mining. We conducted our study around the Amarakaeri Communal Reserve, a semi-protected, communally managed reserve in Madre de Dios, a southeastern region of Peru in the Amazon jungle(8-12). The Reserve is bordered on the east by heavy ASGM activity along the Madre de Dios River and its tributaries (Figure 1), but no mining activity is present near communities to the north and west of the Reserve.
Figure 1. Map of the Amarakaeri Communal Reserve study site.
The Amarakaeri Communal Reserve is located in the department of Madre de Dios in southeastern Peru, in the Amazon Basin. Study communities in progressively darker shades of purple with increasing median total hair mercury. Formal mining is shown in gold, informal mining is shown in yellow. The Madre de Dios River flows west to east, the Colorado River and Puquiri River flow southwest to northeast.
MATERIALS/SUBJECTS AND METHODS
Study design and sample collection
Baseline.
We conducted a population-level mercury exposure assessment of residents of 23 communities around the Amarakaeri Communal Reserve, a protected area within the department of Madre de Dios, in the southeastern portion of the Peruvian Amazon (Figure 1). In lieu of high quality, population-level exposure data to inform a random sample of individuals vulnerable to high mercury exposure, we focused on characterizing exposure in women of childbearing age (WCBA, ages 15-49), since developing fetuses and young children are most vulnerable to mercury toxicity(4, 5). To select our sample, we first included all known rural and indigenous communities around the reserve, and then selected the four largest urban towns (Salvacion, Boca Colorado, Huepetuhe, and Quincemil). We estimated a sample size of 1500 WCBA to obtain minimally sufficient confidence intervals around fertility and mortality estimates to detect changes over time. Based on our prior data in the region (13), we assumed an average of 4.6 people per household, an average of 1.2 WCBA per household with at least one WCBA in 65% of households, 85% of WCBA married or in a union, and an 8% non-response rate. We conducted sampling by creating a listing of all households in each community with a community leader and/or health director. All households with WCBA in rural or native communities (each with fewer than 75 households) were asked to enroll. In the four urban towns (each with more than 75 households), we randomly selected 50% of households to enroll. Households were visited by two study interviewers. We required nine out of every ten households to have at least one WCBA, for a final sample with 90% WCBA-containing households. We conducted baseline data collection from March-June 2015. After obtaining consent, we administered household surveys to the economic head of each enrolled household. We collected hair from every adult and child, if present, and peripheral blood from every consenting adult within a sentinel group, defined as one WCBA, plus her partner and child, if present. A “complete sentinel group” included one WCBA, her partner and her child, and an “incomplete sentinel group” included one WCBA and either her partner or her child or neither. In total, we collected survey data from N=4,083 participants in N=1,221 households in N= 23 communities. We collected hair samples from N=2,308 individuals in N=1,039 households in N=23 communities and blood samples from N=476 individuals from N=421 households in N=22 communities (Tables S7-S10). This study was approved by the Committee on Human Ethics from the Universidad Peruana Cayetano Heredia (OHRP registration IORG0000671, IRB00001014, study ID #63056). Dr. Pan’s research activities on the protocol are covered under an IRB Authorization Agreement (IAA) between Duke University and Cayetano, in which UPCH was designated as the IRB of record.
Follow-up.
We conducted a follow-up of households that included complete or incomplete sentinel groups. We calculated a target sample size of N=459 households for a follow-up study powered to detect a 10% difference in total hair mercury among complete sentinel groups with 80% power and a significance level of 5%, adjusted for a maximum of 40% intermittent non-response from adult males due to travel and ~20% loss of adult females or children. To select households balanced on economic and demographic factors, we ran Principal Components Analysis on 10 baseline survey variables from N=664 households with complete or incomplete sentinel groups with at least one blood sample from one household member. We summed the eight principal components that explained total variance in the 10 variables, stratified the sums into tertiles, and selected N=153 households per tertile. We prioritized selection of N=268 households with at least one complete sentinel group and randomly selected additional households using random number generation to reach our target sample size. We replaced households that moved away or declined participation with the household with the nearest factor sum value. We conducted follow-up data collection from February-April 2016. We administered household surveys to all consenting eligible households. We collected hair samples from consenting adults and children in complete sentinel groups and blood samples from consenting adults in sentinel groups. We collected hair samples from N=723 individuals in N=336 households in N=21 communities and blood samples from N=290 individuals from N=235 households in N=17 communities. (Tables S7-S10)
Biomarker testing
Hair.
We collected hair samples by cutting three tufts of hair from the occipital region of the scalp, stored samples individual in paper envelopes at room temperature, and transported to Duke University laboratories for analysis. We determined total mercury content in proximal 2 cm (unwashed) hair segments by thermal decomposition, amalgamation, atomic absorption spectrometry (Milestone DMA-80)(14). We calibrated the instrument with aqueous ionic mercury (Hg2+) acidified with 1 M nitric acid (Brooks Applied Labs, Bothell, WA). We performed instrument blank analyses throughout the batch run, at least once per 10 samples, to ensure no detectable analyte carryover. We verified our calibration and instrument performance with a hair certified reference material (DB001, European Reference Materials) analyzed at least once per 10 hair samples during a sample batch run. We accepted sample measurements if corresponding reference material measurements were within 10% of the certified mean value. One sample per every 100 samples, for a total of 25 samples, was selected randomly for testing in triplicate, to ensure technical reproducibility. The relative standard deviation of triplicates measurements was 10.7% (average among n=25). The lower limit of quantification, as determined by the standard deviation of at least triplicate blank analyses was <1 ng total mercury (approximately 0.05 ppm in hair, based typical analyzed sample mass of 0.02 g).
Blood.
We collected blood samples in BD Trace Element Vacutainer tubes and froze samples at −20°C within 6 hours of collection. All blood samples were analyzed at Research Triangle Institute, Research Triangle Park, North Carolina using a modification of a previous method (15). Control human blood samples (BioReclamationIVT, Westbury, NY), acid-matrix matched method blank digestions and method blank samples fortified with analyte elements were digested and analyzed as quality control samples alongside study samples. Acids were screened for background analyte concentrations prior to use. High-purity deionized water was used for all sample and standard preparations. We digested ~0.5 mL of each sample with 1 mL nitric acid and 0.05 mL hydrochloric acid at 60°C for 60 minutes. We cooled samples, added 0.5 mL hydrogen peroxide, and digested at 60°C for an additional 30 minutes. We spiked cooled samples with National Institute of Standards and Technology (NIST)-traceable of Y, Pr, and Bi internal standard stock solutions (High Purity Standards, Charleston, SC), each to a concentration of 10 ng/mL, to ensure that at least one of these elements would serve as a high-quality standard, in the event of unforeseen interference or background noise; in our final analysis, Pr was used as the internal standard for data processing. Au from a single element standard, (High Purity Standards) was also added to a concentration of 5 μg/mL to stabilize the Hg in solution and prevent carryover during the batch analyses. We brought the samples to volume with DI water, filtered samples through 0.45 μm Teflon filters, and analyzed samples on Thermo X-series 2 ICP-MS (Bremen, Germany), using low-volume Peltier-cooled impact bead spray chamber and Xt cones. We randomly selected one sample per every 20 samples, for a total of 26 samples for testing in duplicate, to ensure technical reproducibility. The relative % differences between duplicates were typically 20% or less. The lower limits of detection and quantification were evaluated on a per-batch basis; the limit of detection ranged from 0.251 ng/mL to 0.473 ng/mL, and the limit of quantification was fairly consistently at 0.50 ng/mL. The recovery of Hg-spiked samples was between 100-110% in all batch runs.
Statistical analysis
Variables.
We natural log (ln)-transformed right-skewed exposure variables total hair mercury (μ/g or parts per million, ppm) and total blood mercury (ng/mL). We defined indigenous, or native, communities as those listed on Peru’s Ministry of Culture database of indigenous populations at http://bdpi.cultura.gob.pe.
We measured socio-economic position (SEP) using both an asset index and occupation, to compensate for the limitations of each approach. Although asset indices are well-accepted measures of SEP, these indices may perform poorly in low-income countries. Occupation is an indicator of social class; however, it may be a confounder in these analyses, due to high exposure risk to miners and smelters. We applied a standard index based on survey items drawn from Peru’s Demographic and Health Survey (DHS). We used the polychoric principal components analysis (PCA) method to compute the index based on a subset of binary (yes/no) ownership of assets variables included in the study(16). Only variables where approximately less than 90% owned or at least 10% owned were included (Table S15). As recommended in the literature, we used the first principal component as a variable to represent asset-based socioeconomic status. The first principal component explained 39% of the variability in the assets. We defined occupation based on self-reported occupational categories: “Mining” included contract miners and daily hired miners, “Agriculture/Fishing” included those self-employed in agriculture or as fishermen, “Outdoor” included contract farmworkers, loggers, and other hired labor, “Professional/Urban” included professional workers, including those in stores and in transportation, and “Unemployed/Working at home” included homemakers and unemployed individuals. We assigned parental occupation to children.
We developed four variables to test for dependence of total hair mercury on proximity to mining: variable 1 - %formal mining within 15 km buffer around each household; variable 2 - %recent mining within 15 km buffer around each household; variable 3 - %formal mining within watershed upstream of each community; variable 4-%recent mining within watershed upstream of each (Table S4).
Household buffers were designed to capture mining activity within close proximity; watershed variables were designed to integrate all upstream mining activity to estimate cumulative exposure to mining. We defined “formal mining” as legal mining concessions leased by the Peruvian government, obtained as GIS layers from the Ministry of Energy and Mining of Peru (http://www.minem.gob.pe/), and limited to layers that specified gold as one of the metals extracted. We defined “recent mining” as regions of active gold mining as of 2016, estimated by the Monitoring of the Andean Amazon Project (MAAP)(17) using deforestation data detected on satellite images from 2013-2016(18-20) and gold mining footprint classification methods from Asner et al 2013(18). We determined 15 km household buffers using GPS coordinates taken by study field workers. Elevation-derived watersheds were developed throughout the study region using digital elevation models in GIS. “Upstream watershed” for each community was defined as the sum of the sub-basin for the given community and all upstream basins. Percent mining was defined as the percent area in a buffer of 15 km radius around each household or the percent area within a bounded upstream watershed area accounted for by land leased for mining or regions of active gold mining. Mining proximity variables were generated using ArcMap 10.5.
Temporal variability.
Our data include N=554 individuals from N=315 households in N=21 communities with both baseline and follow-up total hair mercury measurements and N=161 individuals from N=149 households in N=15 communities with both baseline and follow-up total blood mercury measurements. We computed the mean difference between baseline and follow-up mercury levels for each individual by community. We did not transform temporal exposure difference variables, because they were approximately normally distributed. We fit multivariate linear regression models with difference in total hair or blood mercury as the outcome, and age, sex, WCBA, socioeconomic position (using either asset index or occupation as proxy, in separate models), and residence in a native community as predictors, with random intercepts for household and community. We fit separate models for individual communities with at least 20 households, with a random intercept for household. To test the contributions of individual-level and community-level variance to temporal difference, we fit additional multivariate linear regression models with difference in total hair or blood mercury as the outcome, and individual-level and community-level variance in temporal exposure difference as predictors. In these models, the estimated intercepts are the estimated mean differences with corrected variance. We normalized the two difference measures to put them on the same scale and calculated the residual variance estimates at the individual and community levels.
Intra-cluster correlations.
We computed intra-cluster correlations in each community using mixed models with ln (baseline total hair mercury) as the outcome using complete baseline data. We computed three sets of correlations: 1. Unadjusted, 2. Adjusted for age, sex, and asset index, 3. Adjusted for age, sex, and occupation (Figure 4, Figure S5). We stratified the full dataset by age group: 1 - > 18 years old; 2 - 18 years old or under; 3 - > 12 years old (Figure S5).
Figure 4. Intra-cluster correlations for baseline total hair mercury levels for households by community.
Intra-cluster correlations (95% CI) in total hair mercury levels for households in each community at baseline in unadjusted models (red) and models adjusted for age, sex, and asset index (blue) or age, sex, and occupation (green). Data shown only for communities with at least 20 households. Communities are ordered by location around the reserve, beginning with Queros (bottom left on map in Figure 1) and ending with Quincemil (bottom right on map in Figure 1.)
Proximity to mining.
All four mining proximity variables were heavily skewed with many zeros (Table S4). We dichotomized all four variables based on the data distribution (Table S4): the formal watershed variable at 50%, formal household variable at 30%, and both informal mining variables at 0%. We fit separate multivariate linear regression models with ln (baseline total hair mercury) and ln (baseline total blood mercury) as outcomes, adjusted for age, sex, and residence in a native community, with random intercepts for household and community.
Hair-blood correlations.
We computed Spearman’s rank correlations between ln (total hair mercury) and ln (total blood mercury) for individuals with both sample types at baseline (N=433) and at follow-up (N=206).
RESULTS
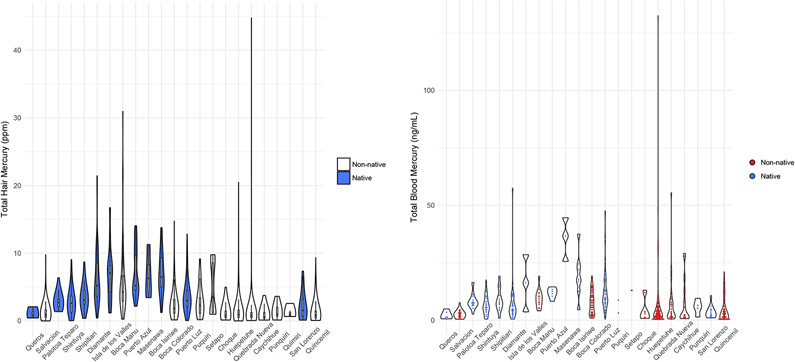
Mercury risk to non-miners living far from mining activity is likely through consumption of methylmercury-contaminated fish. Total mercury level in proximal 2-cm segments of head hair is an established exposure biomarker for methylmercury exposure over the prior 2-3 months(4, 5). In our sample, a significant fraction of individuals showed evidence of exposure that exceeds regulatory guidelines. Median total hair mercury in all baseline hair samples (N=2308) was 1.29 ppm (range 0.001-44.8 ppm). Approximately 60% (N=1376/2308) of individuals showed levels exceeding 1.0 ppm, which corresponds to the USEPA reference dose for methylmercury in blood(5), 53% (N=1214/2308) exceeded 1.2 ppm, which corresponds to the recommended update to the reference dose derived by the National Research Council(4), and 29% (N=665/2308) exceeded the World Health Organization exposure guideline of 2.5 ppm(21). Residence in a native community was associated with 1.9 times higher total hair mercury, as compared to non-native communities (β=0.92, 95% CI 0.45-1.4; median native 3.5 ppm vs. median non-native 1.4 ppm total hair mercury) (Figure 2, Table S1). Exposure to methylmercury in mining towns was comparatively lower, except for one outlier individual in Quebrada Nueva (44.8 ppm) who reported being active in mining (Figures 1-2, Tables S7-S10). Notably, the highest methylmercury exposure occurred in native communities located on the main stem of the Madre de Dios River, with the exception of three native communities with relatively low exposure: Queros, Palotoa Teparo, and San Lorenzo (Figures 1-2, Tables S7-S10).
Figure 2. Violin plots of total hair mercury and total blood mercury at baseline by study community.
Violin plots with inlaid boxplots of total hair mercury (left) and violin plots with inlaid dotplots of total blood mercury (right) distributions at baseline by community. Communities are ordered by location around the reserve, beginning with Queros (bottom left on map in Figure 1) and ending with Quincemil (bottom right on map in Figure 1.) Native communities are highlighted in royal blue. Data are shown on natural scale for ease of interpretation.
Miners are more commonly exposed to mercury vapor inhalation, from mercury-gold amalgam smelting(2, 6, 7). People living near mining, even those not actively engaged in mining, are assumed at high risk for vapor exposure, as well(7). Elemental mercury vapor is rapidly oxidized in the human body and is measured as inorganic mercury(4, 5). Hair is primarily a biomarker for methylmercury, not inorganic mercury; individuals with low total hair mercury may still have high inorganic mercury exposure(4, 5). Since both methylmercury and inorganic mercury exposures are reflected in blood(4, 5), we evaluated potential inorganic mercury exposure by measuring total mercury levels in peripheral blood samples. We anticipated that participants in mining towns would have high blood mercury. However, we observed similar patterns of hair and blood mercury in study participants. Residence in a native community was associated with 1.6 times higher total blood mercury (β=0.62, 95% CI 0.14-1.1; median native 7.4 ng/mL vs. median non-native 3.2 ng/mL total blood mercury) (Figure 2, Table S2). The same native communities with high total hair mercury exhibited high total blood mercury, and mining towns with low total hair mercury showed primarily low total blood mercury, with the exception of a few outliers with high exposures (Figures 1 and 2, Tables S7-S10). Six individuals had total blood mercury levels greater than 44 ng/mL, the blood concentration corresponding to 10 times the USEPA reference dose for methylmercury(4, 5); two of these individuals reported working in mining, two are farmers or fishermen in native communities, and the remaining two reported non-mining occupations but live in Huepetuhe, where ASGM activity is concentrated.
We evaluated the common assumption that proximity to mining activity is a risk factor for mercury exposure. Since most ASGM activity is both informal and illegal, human proximity to mining activity is difficult to characterize. We used two complementary approaches to estimate proximity. First, we defined legal mining concessions established by the Peruvian government as “formal mining.” Formal mining concessions may or may not contain active mining. Second, we used published data on recently deforested sites in the region, which represent regions of active mining as of 2016(17), to define “recent mining.” To test exposure risk in households living close to mining activity, we calculated the percent area of formal or recent mining activity in 15 km buffers around each household accounted for by formal or recent mining activity (Table S4). In addition, we calculated proximity to cumulative formal or recent mining activity within the watershed upstream of each community. No mining proximity variable predicted higher exposure risk when models were adjusted for residence in a native community (Tables S5 and S6). However, consistent with our observation that native communities have higher exposure than those in the mining region, residence in a native community still predicted 1.6 times higher blood mercury and more than 1.9 times higher hair mercury levels in models adjusted for proximity to mining (Tables S5 and S6).
Measuring chemical exposure at a single time point can be misleading. If people are chronically exposed to a relatively constant amount of chemical pollution, then one measurement provides an accurate assessment(4, 5). However, one test at one point in time cannot capture seasonal exposure to a dietary chemical, or well-spaced, short bursts of exposure(4, 5). To test whether exposure around the Reserve was stable and chronic, we evaluated temporal variability in mercury exposure at nine- to 12-month follow-up (Table S14) in a subset of households balanced on demographic and economic factors. We observed that total hair mercury was temporally stable, with no significant difference over time (mean difference was 0.43, P=0.12, corrected for correlation at the individual and community levels); however, total blood mercury was highly variable (corrected mean difference was −4.4, P=0.04), and residence in a native community predicted an approximate 9-10 ng/mL decrease in blood mercury from baseline to follow-up, as compared to non-native community residents (Figure 3, Figures S3 and S4, Tables S11a-d).
Figure 3. Temporal variability in total hair and total blood mercury levels by community.
Mean individual differences in total hair mercury (left) and total blood mercury (right). Error bars denote standard errors. Communities are ordered by location around the reserve, beginning with Queros (bottom left on map in Figure 1) and ending with Quincemil (bottom right on map in Figure 1.)
ASGM represents a unique exposure scenario. Unlike exposure settings in which people either breathe in mercury vapor (primarily occupational) or ingest methylmercury-contaminated food (primarily community), people living near ASGM may be at risk for both(11). Studies that measure only one biomarker can miss true exposure levels and types. To assess potential mixed exposures to multiple mercury species, we computed Spearman’s rank correlations between log-transformed values of total hair and total blood mercury measurements from the same individuals. We observed that hair-blood correlations were highly variable both within native and non-native communities and across time (Figures S1 and S2, Table S3).
Most exposure assessments near ASGM assume that individuals and households within communities experience similar exposures and that inter-individual or inter-household variability is low(11), likely due to a common food source. We tested this assumption by computing intra-cluster correlations (ICCs) for total hair mercury by community. We observed that ICCs were high in a subset of communities, indicating that, in these communities, the members of households are more similar to each other than they are to people in other households within the same community (Figure 4). This result was robust to adjustment with covariates, including age, sex, socioeconomic position represented by asset index or occupation (Figure 4, Figure S5, Tables S12a-d), community size, residence in native community, and proximity to mining (not shown).
DISCUSSION
Here, we show that native communities living around the Amarakaeri Communal Reserve are at highest risk for mercury exposure, not residents of mining towns. In fact, our results suggest that environmental mercury exposure risk, as measured by total mercury levels in hair or blood, remains high hundreds of kilometers away from mining activity. This result challenges the common assumptions that highest environmental methylmercury exposures occur in miners or residents of mining communities, and that residents of mining communities, even those not directly involved in mining activities, experience extreme mercury vapor inhalation(2, 6-11). ASGM is rapidly expanding in countries around the world, and is encroaching on previously unmined, resource-rich protected areas, including communally managed reserves(22-24). Our data suggest that broader communities living near ASGM, even if not involved in mining activity, are vulnerable to high mercury exposure.
Native communities in this study are likely exposed through consumption of contaminated fish from the Madre de Dios River, based on extensive prior evidence of fish as the primary source of human methylmercury exposure (4, 5). Methylmercury bioaccumulates in the aquatic food web, and is biomagnified as larger fish consume smaller fish(3, 25); therefore, fish at higher trophic levels have higher concentrations of methylmercury, relative to smaller fish in the same aquatic system(3, 25). We have previously shown that total mercury concentrations in river water, sediment and carnivorous fish in the Madre de Dios River are highest close to and downstream of mining activity(26), supporting ASGM activity as the primary source of mercury in contaminated river fish in this region. This result is not inconsistent with our observation of higher human mercury exposure far from mining. Most carnivorous fish in the Madre de Dios River are migratory (per personal communication with local ecologists); therefore, fish could be contaminated near mining and be caught by subsistence fishers far from mining. Alternatively, because commonly eaten carnivorous fish are large and relatively long-lived (per personal communication with local ecologists), these fish may have very high levels of methylmercury due to accumulation over a long period of time. Urban mining towns are heavily populated by immigrants from the Andean Highlands, whose dietary culture is more focused on non-fish food items(26, 27). In contrast, communities native to the Amazon rely heavily on subsistence fishing, and therefore likely consume more fish than do urban immigrants living closer to mining(27). Although fish from other regions of Peru are sold in local markets, we observed in an earlier pilot study along the Madre de Dios River that 71% of fish-consuming households reported obtaining their fish primarily from the river(27), indicating that likely fish consumption behavior can be linked to likely mercury exposure. However, we note several limitations to these conclusions, most notably that we did not directly measure fish intake in participants in this study. In addition, we did not assess mercury levels in local rice, which may be contaminated with methylmercury if grown in flooded paddies, nor did we assess rice intake in study participants.
Two native communities, Queros and Palotoa Teparo, did not fit this higher exposure pattern. Fishermen in these communities likely fish primarily in the nearest rivers, which are tributaries that feed into the Madre de Dios River, suggesting that highly contaminated fish were concentrated in the main stem of the river. These results constitute an important link between mining activity and mercury exposure to the surrounding population. Mining advocates claim that fish contamination is not a consequence of active gold mining in the region, but rather due to release of naturally occurring mercury or mobilization of residual mercury from prior mining that would result in fish contamination in the absence of active mining. However, our prior data show that active mining leads to contamination of riverine systems both through direct mercury inputs and through deforestation, which mobilizes existing mercury via increased soil erosion into the river(28). Our exposure data suggest that fish that are on the main stem of the river, closer to mining activity, are more highly contaminated than fish on tributaries with no active mining, strongly implicating active mining as a direct driver of fish contamination.
We observed three additional patterns in mercury exposure that both contradict common assumptions about exposure patterns near ASGM, and, critically, can be used to develop effective human biomonitoring programs. First, we showed that total hair mercury reliably represents chronic exposure over roughly one year. Although blood levels of other heavy metals (e.g., lead) are considered the biomonitoring “gold standard”(29), we observe significantly higher variability in total blood mercury levels, as compared to hair. Mercury is stably incorporated in growing hair strands; however, in blood, methylmercury has a half-life of 48-53 days and inorganic Hg2+ has a half-life of 35-67 days(4, 5). Therefore, high temporal variability in blood mercury within individuals indicates either that blood mercury levels do not represent steady-state exposure levels, which could be due to intermittent, acute exposures in a subset of study participants, or rapid clearance of mercury in a subset of study participants, or both. Since total hair mercury is a stable indicator of chronic methylmercury exposure(4, 5), which is the primary exposure concern to non-miners(11), these data strongly support hair as the biological matrix of choice for human biomonitoring in ASGM-impacted regions. Second, our data do not support mining towns as hotspots for non-occupational mixed species mercury exposures. Prior small studies in mining towns have suggested that mining town residents are at risk for mercury vapor inhalation, even if not actively working as miners or in gold shops that burn amalgams(8, 9, 11); if true, mining town residents could be exposed to mercury both via inhalation (reflected as inorganic mercury in blood) and via contaminated fish consumption (reflected as methylmercury in blood). We hypothesized that communities with primarily methylmercury exposure would show high hair-blood mercury correlations, because, if most mercury in blood is methylmercury, that exposure level would eventually be reflected in hair – conversely, communities with mixed exposures would show low correlations, since the inorganic fraction of blood mercury would not be reflected in hair. Instead, the high variability in hair-blood correlations in native and non-native communities, both at baseline and at follow-up, do not indicate clear patterns of mixed exposures in mining towns that are absent in non-mining towns. These data strongly support focusing human biomonitoring programs intended to capture risk to non-miners on methylmercury exposure in both mining and non-mining towns. However, we note that this conclusion assumes no non-fish, non-mining sources of mercury exposure, such as cigarette smoking, which can lead to low level increases in total hair or total blood mercury levels (30). Third, our observation of high ICCs intra-cluster correlations in some communities implies an unmeasured source of household-level variation in exposure risk. An important policy implication of this finding is that future human biomonitoring programs cannot select random households within a community for monitoring and safely assume that all households within a community are representative of community-level exposure.
Although the current scientific consensus is that the majority (80-90%) of total mercury in hair results from dietary ingestion of methylmercury, additional discussion of the use of total hair mercury as a biomarker for dietary methylmercury is warranted in ASGM communities, where multiple sources of hair mercury are possible. Although total hair mercury is a poor biomarker of inorganic mercury exposure, due to inconsistent and much lower rates of incorporation of inorganic mercury into hair, relative to methylmercury, total hair mercury has the potential to reflect both methylmercury exposure (via ingestion) and inorganic mercury exposure (via oxidation of inhaled gaseous mercury or ingestion of inorganic mercury), as well as ex situ routes of incorporation (e.g., adsorption of gaseous mercury onto hair.) The contributions of the first two sources are likely to be small; even in mixed exposures in controlled animal experiments and observational human studies, the majority of total hair mercury is still methylmercury, even accounting for fractions of inhaled mercury vapor, ingested inorganic mercury, or methylmercury that is demethylated in the body (31, 32). However, it is possible that the hair of individuals living very close to mining is externally contaminated with mercury, a concern which is partly mitigated by our measurement of both total hair and total blood mercury levels.
CONCLUSIONS
We anticipate that these data will directly inform similar assessments in comparable ASGM regions globally, as well as development of human biomonitoring programs in signatory countries of the Minamata Convention on Mercury(33). Comprehensive, population-level characterization of environmental pollutant exposures in high-risk, hard-to-measure parts of the world is the first critical step in reducing environmental injustice and protecting public health worldwide.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge local field workers and Jean-Pierre Muro of MEDLAB-Peru for assistance in data collection, as well as support from the Madre de Dios Regional Health Directorate (DIRESA), particularly Drs. Emperatriz Morales, Elvis Rojas and Fernando Mendieta. Support for this study was provided by the Inter-American Institute for Global Change Research (CRN 3034), Bass Connections at Duke University, and Hunt Oil Peru LLC (HOEP-QEHSS-140003).
FUNDING
This study was funded by the Inter-American Institute for Global Change Research (CRN 3034), Bass Connections at Duke University, and Hunt Oil Peru LLC (HOEP-QEHSS-140003). The study funders had no roles in study design, data collection, data analysis, data interpretation, or manuscript preparation. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Footnotes
Supplementary information is available at the Journal of Exposure Science and Epidemiology’s website.
CONFLICT OF INTERESTS
The authors declare that they have no competing financial interests in relation to this work.
AVAILABILITY OF DATA AND MATERIAL
Data on legal mining concessions leased by the Peruvian government are available from the Ministry of Energy and Mining of Peru (http://www.minem.gob.pe/). Data on recent mining activity are available on request from Matthew Finer of the Monitoring of the Andean Amazon Project (maaproject.org). Mercury exposure and epidemiological datasets generated during and/or analyzed during the current study are not publicly available, due to restrictions for protection of human subjects, but are available from the corresponding author on reasonable request.
REFERENCES
- 1.Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu NN, et al. The Lancet Commission on pollution and health. Lancet. 2017. [DOI] [PubMed] [Google Scholar]
- 2.Steckling N, Tobollik M, Plass D, Hornberg C, Ericson B, Fuller R, et al. Global Burden of Disease of Mercury Used in Artisanal Small-Scale Gold Mining. Ann Glob Health. 2017;83(2):234–47. [DOI] [PubMed] [Google Scholar]
- 3.Bank MS, Vignati DA, Vigon B. United Nations Environment Programme's Global Mercury Partnership: science for successful implementation of the Minamata Convention. Environ Toxicol Chem. 2014;33(6):1199–201. [DOI] [PubMed] [Google Scholar]
- 4.National Research Council. Toxicological effects of methylmercury. The National Academies Press: Washington, DC. 2000. [PubMed] [Google Scholar]
- 5.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for mercury. Atlanta, Georgia: U.S. Department of Health and Human Services. 1999. [Google Scholar]
- 6.Steckling N, Devleesschauwer B, Winkelnkemper J, Fischer F, Ericson B, Kramer A, et al. Disability Weights for Chronic Mercury Intoxication Resulting from Gold Mining Activities: Results from an Online Pairwise Comparisons Survey. Int J Environ Res Public Health. 2017;14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ha E, Basu N, Bose-O'Reilly S, Dorea JG, McSorley E, Sakamoto M, et al. Current progress on understanding the impact of mercury on human health. Environ Res. 2017;152:419–33. [DOI] [PubMed] [Google Scholar]
- 8.Ashe K. Elevated mercury concentrations in humans of Madre de Dios, Peru. PLoS One. 2012;7(3):e33305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yard EE, Horton J, Schier JG, Caldwell K, Sanchez C, Lewis L, et al. Mercury exposure among artisanal gold miners in Madre de Dios, Peru: a cross-sectional study. J Med Toxicol. 2012;8(4):441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langeland AL, Hardin RD, Neitzel RL. Mercury Levels in Human Hair and Farmed Fish near Artisanal and Small-Scale Gold Mining Communities in the Madre de Dios River Basin, Peru. Int J Environ Res Public Health. 2017;14(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibb H, O'Leary KG. Mercury exposure and health impacts among individuals in the artisanal and small-scale gold mining community: a comprehensive review. Environ Health Perspect. 2014;122(7):667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser B. Peruvian gold rush threatens health and the environment. Environ Sci Technol. 2009;43(19):7162–4. [DOI] [PubMed] [Google Scholar]
- 13.INEI. Peru: Encuesta Demografica de Salud Familiar (ENDES) 3014. . Instituto Nacional de Estadistica e Informatica Lima, Peru. 2015. [Google Scholar]
- 14.United States Environmental Protection Agency. Method 7473 (SW-846): Mercury is Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry, Revision 0. 1998.
- 15.Monastero RN, Karimi R, Nyland JF, Harrington J, Levine K, Meliker JR. Mercury exposure, serum antinuclear antibodies, and serum cytokine levels in the Long Island Study of Seafood Consumption: A cross-sectional study in NY, USA. Environ Res. 2017;156:334–40. [DOI] [PubMed] [Google Scholar]
- 16.Kolenikov S AG. Socioeconomic status measurement with discrete proxy variables: Is principal component analysis a reliable answer? Review of Income and Wealth. 2009;55(1):128–65. [Google Scholar]
- 17.Finer M OT, Novoa S. Gold Mining Deforests 32,000 Acres in southern Peruvian Amazon from 2013 to 2016. MAAP:50. Monitoring the Andean Amazon Project. 2016. [Google Scholar]
- 18.Asner GP, Llactayo W, Tupayachi R, Luna ER. Elevated rates of gold mining in the Amazon revealed through high-resolution monitoring. Proc Natl Acad Sci U S A. 2013;110(46):18454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen MC, Krylov A, Tyukavina A, Potapov PV, Turubanova S, Zutta B, Ifo S, Margono B, Stolle F, Moore R Humid tropical forest disturbance alerts using Landsat data. Environmental Research Letters. 2016;11(3):034008. [Google Scholar]
- 20.Hansen MC, Potapov PV, Moore R, Hancher M, Turubanova SA, Tyukavina A, et al. High-resolution global maps of 21st-century forest cover change. Science. 2013;342(6160):850–3. [DOI] [PubMed] [Google Scholar]
- 21.JECFA. Summary and conclusions of the 67th Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland: World Health Organization, International Programme on Chemical Safety.; 2004. [Google Scholar]
- 22.Asner GP, Tupayachi R. Accelerated losses of protected forests from gold mining in the Peruvian Amazon. Environmental Research Letters. 2016;12:094004. [Google Scholar]
- 23.Ingram V TJC, Schure J, Nkamgnia E, Tadjuidje MH Where artisanal mines and forest meet: Socio-economic and environmental impacts in the Congo Basin. Natural Resources Forum. 2011;35(4):304–20. [Google Scholar]
- 24.Hilson G Small-scale mining and its socio-economic impact in developing countries. Natural Resources Forum. 2002;26:3–13. [Google Scholar]
- 25.The United Nations Environment Programme. UNEP Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport. Geneva, Switzerland, Branch UC; 2013. [Google Scholar]
- 26.Diringer SE, Feingold BJ, Ortiz EJ, Gallis JA, Araujo-Flores JM, Berky A, et al. River transport of mercury from artisanal and small-scale gold mining and risks for dietary mercury exposure in Madre de Dios, Peru. Environ Sci Process Impacts. 2015;17(2):478–87. [DOI] [PubMed] [Google Scholar]
- 27.Wyatt L, Ortiz EJ, Feingold B, Berky A, Diringer S, Morales AM, et al. Spatial, Temporal, and Dietary Variables Associated with Elevated Mercury Exposure in Peruvian Riverine Communities Upstream and Downstream of Artisanal and Small-Scale Gold Mining. Int J Environ Res Public Health. 2017;14(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diringer SE, Berky AJ, Marani M, Ortiz EJ, Karatum O, Plata DL, Pan WK, Hsu-Kim H Deforestation due to artisanal and small-scale gold minng exacerbates soil and mercury mobilization in Madre de Dios, Peru. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klotz K, Goen T. Human Biomonitoring of Lead Exposure. Met Ions Life Sci. 2017;17. [DOI] [PubMed] [Google Scholar]
- 30.Afridi HI, Talpur FN, Kazi TG, Brabazon D. Assessment of toxic elements in the samples of different cigarettes and their effect on the essential elemental status in the biological samples of Irish hypertensive consumers. J Hum Hypertens. 2015;29(5):309–15. [DOI] [PubMed] [Google Scholar]
- 31.Yasutake A, Hachiya N. Accumulation of inorganic mercury in hair of rats exposed to methylmercury or mercuric chloride. Tohoku J Exp Med. 2006;210(4):301–6. [DOI] [PubMed] [Google Scholar]
- 32.Cernichiari E, Myers GJ, Ballatori N, Zareba G, Vyas J, Clarkson T. The biological monitoring of prenatal exposure to methylmercury. Neurotoxicology. 2007;28(5):1015–22. [DOI] [PubMed] [Google Scholar]
- 33.The United Nations Environment Programme (UNEP). The Minamata Convention on Mercury. 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.