Summary
Background
Although many smokers use electronic cigarettes (e-cigarettes) to quit smoking, most continue to smoke while vaping. This dual use might delay cessation and increase toxicant exposure. We aimed to test the efficacy of a self-help intervention designed to help dual users to quit smoking.
Methods
In this three-arm randomised controlled trial we recruited individuals in the USA using Facebook and multimedia advertisements. Included participants were 18 years or older, smoked at least weekly in the preceding year, and vaped at least weekly in the preceding month. We used computer generated randomisation with balanced-permuted blocks (block size 10, with 2–4-4 ratio) to allocate participants to assessment only (ASSESS group), generic smoking cessation self-help booklets (GENERIC group), or booklets targeting dual users (eTARGET group). Individuals in the generic or targeted intervention groups received monthly cessation materials for 18 months, with assessments every 3 months for 24 months. The main outcome was self-reported 7-day point-prevalence smoking abstinence at each assessment point. All randomly allocated participants were included in primary analyses using generalised estimating equations for each of 20 datasets created by multiple imputation. Analysis of the χ2s produced an F test. The trial is registered with ClinicalTrials.gov, NCT02416011, and is now closed.
Findings
Between July 12, 2016, and June 30, 2017, we randomly assigned 2896 dual users (575 to assessment, 1154 to generic intervention, and 1167 to targeted self-help). 7-day point-prevalence smoking abstinence increased from 14% at 3 months to 42% at 24 months (F7,541·7=67·1, p<0·0001) in the overall sample. Targeted self-help resulted in higher smoking abstinence than did assessment alone throughout the treatment period (F1,973·8=10·20, p=0·0014 [α=0·017]). The generic intervention group had abstinence rates between those of the assessment and targeted groups, but did not significantly differ from either when adjusted for multiple comparisons (GENERIC vs eTARGET F1,1102·5=1·79, p=0·18 [α=0·05]; GENERIC vs ASSESS F1,676·7=4·29, p=0·039 [α=0·025]). Differences between study groups attenuated after the interventions ended.
Interpretation
A targeted self-help intervention with high potential for dissemination could be efficacious in promoting smoking cessation among dual users of combustible cigarettes and e-cigarettes.
Introduction
Electronic cigarette (e-cigarette) use (also known as vaping) has increased greatly over the past decade. In 2018, for example, 8 million adults in the USA alone reported vaping,1 primarily for the purpose of quitting or reducing smoking.2 The few randomised smoking cessation trials of e-cigarettes have indicated efficacy at least as high as nicotine replacement therapy (NRT).3 However, many individuals continue to smoke while vaping, representing a type of dual use, which refers to use of two nicotine or tobacco products concurrently. It is estimated that 40·8% of e-cigarette users are also cigarette smokers (ie, dual users).4 Unlike exclusive vaping, which can be viewed as a harm reduction strategy, dual use is associated with a high exposure to toxicants at levels that can be similar or higher than that of cigarette smoking alone.5 Dual use might also lead to greater nicotine dependence, prolonging smoking and impeding cessation.6,7 Almost half of dual users continue both smoking and vaping when followed up for a year, and 44% return to exclusive smoking.8,9 Therefore, although dual users are interested in quitting smoking and are more likely to make quit attempts than smokers who do not vape,10 they still have difficulty achieving smoking abstinence. Many dual users report a reduction in smoking after the onset of vaping; yet even low rates of smoking are associated with poor health outcomes and increased mortality.11 Additionally, smoking cessation interventions with better established efficacy (eg, behavioural counselling and pharmacotherapies such as NRT) are limited by poor population uptake.6 Together, these findings suggest that an intervention to help dual users to achieve and maintain smoking abstinence, particularly one that is easily disseminated, could have high public health impact.
In this three-arm randomised controlled trial, we tested the hypothesis that a self-help intervention designed specifically for dual users would improve smoking abstinence compared with a no-treatment control and an existing, efficacious, and generic self-help smoking cessation intervention.12 Secondary aims were to identify responsive prespecified subgroups, assess changes in vaping and its covariation with smoking, and compare the cost-effectiveness of interventions.
Methods
Study design and participants
This study was a randomised controlled trial done in the USA. Participants were recruited throughout the USA through Facebook and multimedia advertisements (newspapers, radio, TV, e-cigarette forums, and so on) for a study measuring attitudes and behaviours regarding cigarettes and e-cigarettes. Inclusion criteria comprised the following: age 18 years or older, smoked one or more combustible cigarettes per week over the preceding year, used e-cigarettes one or more times per week over the preceding month, not currently enrolled in a face-to-face smoking cessation programme, and able to speak and read English. The original inclusion criteria required daily smoking. However, early in the trial, it became apparent that many dual users were skipping smoking on some days. Therefore, to better reflect the dual-using population, we amended the use frequency criteria to equate them for smoking and vaping at one or more uses per week. The protocol was amended on Sept 25, 2016. We had recruited 652 participants up to that date. Participants were not necessarily seeking treatment or motivated to quit smoking or vaping. Participation was limited to one individual per street address. Participants gave oral informed consent. The study was approved by the Advarra Institutional Review Board. The study protocol is available upon request to the corresponding author.
Randomisation
We used a three-arm (1:2:2) design for this trial. Upon return of a baseline questionnaire, participants were randomly assigned to assessment only (ASSESS; the no-treatment control group), a generic self-help intervention (GENERIC),12 or a self-help intervention targeted to dual users (eTARGET). Randomisation was done with balanced-permuted block randomisation with a block size of 10 (2–4-4). Sequences were created a priori by the study statistician (SKS) and applied by a workflow and database software system. Given that interventions in this study were in the form of self-help booklets, research staff involved in the trial were not masked to group assignment. Participants were told during telephone screening that they might receive educational smoking-cessation materials, but they did not receive explicit information regarding the existence of study arms or group assignment.
Procedures
Individuals interested in the study could either call to inquire about the study or submit a brief survey with their contact information. Research staff explained the study over the telephone and screened those who expressed interest in participating. Eligible, consenting individuals were sent a baseline questionnaire (by postal mail or e-mail). When the baseline questionnaire was returned, individuals who still met inclusion criteria were randomly assigned to one of the three study groups. The ASSESS group controlled for the baseline and follow-up assessments and allowed for estimation of smoking abstinence among dual users in the absence of intervention. Participants assigned to the GENERIC group received smoking cessation materials found to be efficacious in our previous randomised controlled trial:12 an introductory Stop Smoking for Good brochure, ten Stop Smoking for Good didactic booklets, and nine How I Quit Smoking pamphlets, delivered over the course of 18 months. The content of these materials was based on cognitive-behavioural theory13,14 and empirical evidence regarding the nature of tobacco dependence, cessation, and relapse.15 These materials were originally designed as a means of translating the cognitive-behavioural counselling that occurs in a clinic into a written format that would be much more accessible to smokers. Participants in the eTARGET group received a guide designed specifically for dual users (If You Vape: a Guide to Quitting Smoking), which included an introductory If You Vape brochure, a series of ten If You Vape: Guide to Quitting Smoking booklets, and nine My Story pamphlets. We drew on materials from the GENERIC intervention, qualitative research and literature on dual users attempting to quit smoking, and existing empirical research and guidelines on the efficacious use of NRT, to address the special needs, circumstances, and risk factors of dual users.16 The materials in the If You Vape: Guide to Quitting Smoking resource emphasised the use of e-cigarettes for smoking cessation (eg, vaping when tempted to smoke, keeping e-cigarettes handy, or trying different flavours or devices until finding the most effective), encouraged users to taper and eventually terminate e-cigarette use towards the end of the intervention, and incorporated language, photographs, and graphics relevant and appealing for dual users. Links to all intervention materials are available in the appendix (p 3).
Participants assigned to the GENERIC or eTARGET groups were sent the intervention materials by postal mail, with the option of also receiving them electronically. In both groups, the initial brochure and the first booklet were mailed upon receipt of the completed baseline assessment. The remaining booklets were mailed 1, 2, 3, 5, 7, 9, 12, 15, and 18 months after the baseline assessment. The pamphlets were mailed 4, 6, 8, 10, 11, 13, 14, 16, and 17 months after baseline assessment.
The baseline questionnaire contained a demographic and tobacco history assessment, including the Fagerström Test for Nicotine Dependence (FTND)17 for the assessment of baseline cigarette dependence and the Heaviness of Smoking Index (HSI) to assess cigarette dependence before vaping initiation. Three motivation-related constructs were assessed: the Stages of Change algorithm,18 a situation-specific abstinence self-efficacy scale (SSE),19 and the Abstinence-Related Motivational Engagement (ARME) scale.20 The item “I am committed to being smoke-free” (a general measure of motivation to quit smoking, rated with a 5-point Likert scale) was also included. A questionnaire assessing e-cigarette use was developed mirroring the combustible cigarette scales and items. A full description of all study measures is available elsewhere.16
Full follow-up assessments with similar measures to the baseline questionnaire were done at 6, 12, 18, and 24 months after enrolment. Abbreviated assessments were administered at 3, 9, 15, and 21 months after baseline. Participants reporting smoking abstinence at 12 or 24 months and living within 100 miles of the research site were invited to complete a biochemical validation appointment (appendix p 6).
Participants were compensated US$10–20 for the first eight assessments and $40 for the final one, and they were eligible for $40–60 bonuses for completing at least seven assessments. Participants returning assessments within 1 week were sent inexpensive appreciation gifts.
Outcomes
The primary outcome was self-reported 7-day point-prevalence abstinence from smoking. Secondary outcomes were 7-day point-prevalence abstinence from vaping, and cost per incremental smoking cessation. We also report 30-day and 90-day point-prevalence abstinence rates, which reflect sustained abstinence.
Statistical analysis
Based on previous studies12,21 and considering that the study population was not limited to treatment-seeking smokers, we estimated abstinence rates increasing linearly from 0% at baseline to 15% at 18 months and beyond in the ASSESS group, 20% in the GENERIC group, and 25% in the eTARGET group. Sample size was estimated by use of GEESIZE version 3.1,22 with a first-order autocorrelation working correlation structure and coefficient of 0·7, and adjusted α of 0·017 (ASSESS vs eTARGET), 0·025 (ASSESS vs GENERIC), and 0·05 (GENERIC vs eTARGET) following Holm’s procedure.23 We assumed that abstinence rates would increase from 6 to 18 months and stabilise thereafter. To ensure 80% power or greater for all comparisons required the random assignment of 2065 participants in a 1:2:2 ratio (413 to ASSESS, 826 to GENERIC, and 826 to eTARGET). Assuming the 17% attrition we observed in our previous study,12 we initially planned to recruit 2500 individuals. However, the attrition rate for the first 575 participants was higher than expected at 27·5%, so on Feb 27, 2017, we amended the protocol to increase the target sample size to 2900 to account for this change.
We used SAS, version 9.4, for all analyses. Descriptive statistics were computed for demographic, smoking-related, and vaping-related variables, as well as bio-verification outcomes. Hypothesis testing variables were transformed as needed. The following prospective moderators and multiple imputation model variables were dichotomised before the analysis: married or living together, education beyond high school, committed to being smoke free, and annual household income ($20 000). An intention-to-treat approach was used for all analyses, with multiple imputation using the multivariate normal approach applied to manage missing data.24,25
Preliminary univariate and multivariate logistic regression analyses identified auxiliary variables for the imputation model (ie, baseline measures predicting smoking or unreturned surveys) to increase credibility of the missing-at-random assumption. After the imputation modelling, a post-hoc adjustment was applied to imputed smoking status values to reflect missing-not-at-random effects (ie, that individuals with missing smoking status data might be more likely to continue smoking than individuals who submit data) with a small to medium effect size (ie, Cohen’s d=0·35).24 The final smoking and vaping imputed values were dichotomised with use of adaptive rounding. 20 datasets were generated. Additional details on the multiple imputation procedure are presented in the appendix (p 7).
Analyses of intervention-based differences in smoking and vaping abstinence used generalised estimating equations (GEE), with each GEE targeting a treatment group-paired comparison (eg, eTARGET vs ASSESS). The covariates for the base model were treatment group, assessment (3–24 months), and their interaction. Within each of the 20 datasets, χ2 was the test statistic. Within each of the 20 datasets created by multiple imputation, χ2 was the test statistic for each effect. These 20 χ2 values were submitted to Allison’s COMBCHI.SAS macro implementing the method presented in Schafer25 to generate an FDF,DDF test of the effect that adjusts for the variability of the χ2 values across datasets. DF is from the χ2 and DDF is based on the original sample size with downward adjustment for the variability of the χ2 values across the imputed datasets. Given apparent differences between treatment (3–18 months) and post-treatment (21–24 months) smoking outcomes, we did separate analyses for these periods. To analyse whether the targeted intervention had a greater effect on a particular subgroup of dual users, seven prespecified moderators (sex, age, education, income, FTND at baseline, HSI pre-vaping, and planning to quit within 30 days) were evaluated by adding the moderator and its interaction with treatment group to the base model for eTARGET vs ASSESS. Significant interactions were explored through analyses of intervention effects within subgroups. Although not prespecified, three baseline e-cigarette variables were evaluated as moderators: e-cigarette type (refillable vs all others), daily vaping frequency, and whether vaping was used for quitting smoking. Finally, the association between smoking and vaping status was evaluated with use of GEE in a model predicting smoking status with treatment group (all three), assessment, time-varying vaping status, and the interaction of group with vaping status (more details in the appendix p 8).
We collected information on all resources needed for the intervention (eg, personnel, printing, and postage) and assigned appropriate unit prices for each resource type to do a deterministic cost-effectiveness calculation of cost per participant who quit smoking. Research-specific resources (eg, assessments) were excluded. Incremental cost-effective ratios of abstinence at 18 and 24 months were calculated for eTARGET and GENERIC interventions compared with the ASSESS group. Additionally, we did sensitivity analyses to explore the effect of varying intervention costs that might occur with different levels of automation for administering the intervention. This trial was registered on ClinicalTrials.gov, NCT02416011.
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between July 12, 2016, and June 30, 2017, 5827 individuals were recruited and assessed for eligibility, with 3611 individuals initially qualifying for inclusion. Of these, 3113 returned the baseline questionnaire. Subsequently, 2896 remained eligible and were enrolled, randomly assigned, and included in the analyses (figure 1). 575 participants were allocated to the ASSESS group, 1154 to the GENERIC group, and 1167 to the eTARGET group. Of the individuals enrolled, 2263 (78%) were recruited through Facebook and 401 (14%) through online advertisements. Of enrolled participants, 503 (17%) did not return any follow-up surveys, with percentages higher for eTARGET (odds ratio [OR] 1·63, 95% CI 1·21–2·18, p=0·0013) and GENERIC (1·89, 1·41–2·53, p<0·0001) than for ASSESS. Preliminary analyses to identify variables for the multiple imputation model found three additional predictors of not returning any follow-up surveys: being male (OR 2·25, 95% CI 1·80–2·82; p<0·0001), younger (0·95, 0·94–0·97; p<0·0001), and requesting paper surveys (1·45, 1·12–1·87, p=0·0047). The number of incomplete surveys increased from 814 (28%) at 3 months to 1531 (53%) at 21 months and decreased to 1274 (44%) at the final assessment (figure 1).
Figure 1:
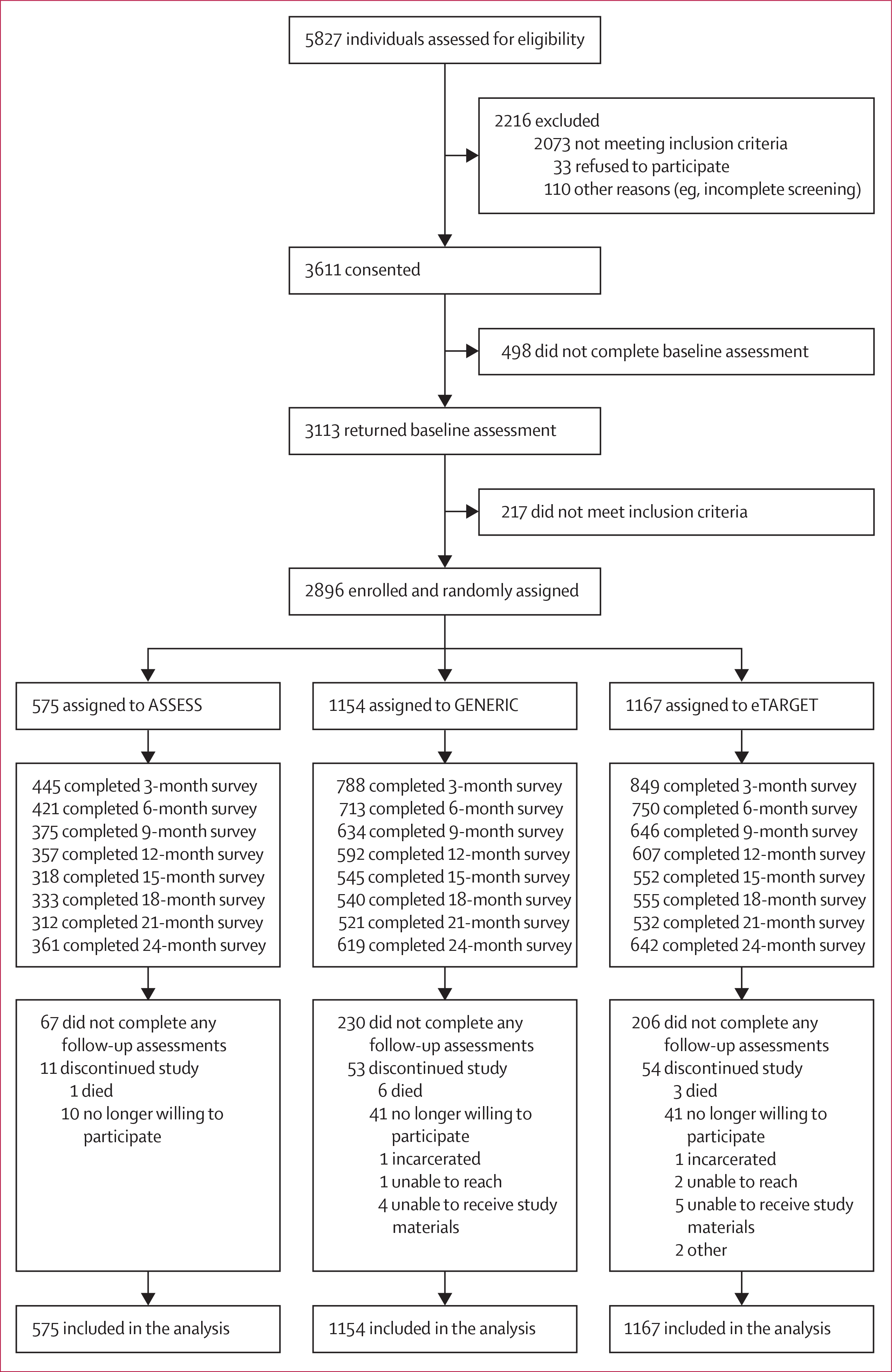
Trial profile
Most participants were non-Hispanic White, men, aged 18–31 years, educated beyond high school, and had an annual household income lower than $30 000 (table 1). Participants typically smoked one to ten cigarettes per day, had low to moderate cigarette dependence, and were considering quitting smoking within 6 months but not within the following 30 days; nearly half started vaping to quit smoking. Most participants had vaped for at least 1 year and reported 20 or more vaping episodes per day (table 1). Participants were asked which type of e-cigarettes they usually used. The most endorsed category was “refillable” at 2140 (74%), with only 86 (3%) indicating “cartridge” and 69 (2%) indicating “disposable”. The remaining 601 (21%) indicated “other” or did not answer.
Table 1:
Participant characteristics
| Overall (n=2896) | ASSESS (n=575) | eTARGET (n=1167) | GENERIC (n=1154) | |
|---|---|---|---|---|
| Number of missing surveys | 3·5 (3·2) | 2·9 (3·0) | 3·6 (3·2) | 3·7 (3·2) |
| Participants who returned all surveys | 954 (33%) | 222 (39%) | 381 (33%) | 351 (30%) |
| Participants who returned no surveys | 503 (17%) | 67 (12%) | 206 (18%) | 230 (20%) |
| Age, years | 29·9 (11·2) | 30·1 (11·2) | 30·0 (11·2) | 29·6 (11·4) |
| Sex | ||||
| Men | 1830 (63%) | 368 (64%) | 743 (64%) | 719 (62%) |
| Women | 1066 (37%) | 207 (36%) | 424 (36%) | 435 (38%) |
| Race or ethnicity | ||||
| White | 2550 (88%) | 513 (89%) | 1025 (88%) | 1012 (88%) |
| Non-White | 178 (6%) | 33 (6%) | 66 (6%) | 79 (7%) |
| More than one race | 150 (5%) | 27 (5%) | 66 (6%) | 57 (5%) |
| Hispanic or Latino ethnicity | 241 (8%) | 65 (11%) | 89 (8%) | 87 (8%) |
| Married or living together | 1001 (35%) | 207 (36%) | 424 (36%) | 370 (32%) |
| Education beyond high school | 1524 (53%) | 309 (54%) | 627 (54%) | 588 (51%) |
| Annual household income, US$* | ||||
| <10 000 | 533 (18%) | 119 (21%) | 187 (16%) | 227 (20%) |
| 10 000–19 000 | 568 (20%) | 113 (20%) | 227 (19%) | 228 (20%) |
| 20 000–29 000 | 535 (19%) | 96 (17%) | 220 (19%) | 219 (19%) |
| ≥30 000 | 1247 (43%) | 245 (43%) | 531 (46%) | 471 (41%) |
| Smoking frequency | ||||
| Daily | 1743 (60%) | 357 (62%) | 686 (59%) | 700 (61%) |
| 4–6 days per week | 727 (25%) | 140 (24%) | 300 (26%) | 287 (25%) |
| 1–3 days per week | 425 (15%) | 78 (14%) | 181 (16%) | 166 (14%) |
| Cigarettes per day | ||||
| 1–10 | 1663 (57%) | 328 (57%) | 670 (57%) | 665 (58%) |
| 11–20 | 972 (34%) | 189 (33%) | 391 (34%) | 392 (34%) |
| >20 | 259 (9%) | 58 (10%) | 104 (9%) | 97 (8%) |
| Years smoking before vaping | 12·9 (10·9) | 13·2 (10·6) | 13·0 (10·8) | 12·7 (11·1) |
| FTND at baseline (0–10) | 3·6 (2·4) | 3·6 (2·5) | 3·6 (2·4) | 3·7 (2·4) |
| Consider quitting in 6 months | 2103 (73%) | 414 (72%) | 843 (72%) | 846 (73%) |
| Plan to quit in 30 days | 755 (26%) | 147 (26%) | 304 (26%) | 304 (26%) |
| Committed to being smoke free: agree or strongly agree | 1258 (43%) | 237 (41%) | 501 (43%) | 520 (45%) |
| Vaping frequency† | ||||
| Daily | 2003 (69%) | 399 (70%) | 796 (68%) | 808 (70%) |
| 4–6 days per week | 448 (15%) | 91 (16%) | 188 (16%) | 169 (15%) |
| <4 days per week | 429 (15%) | 81 (14%) | 176 (15%) | 172 (15%) |
| Electronic cigarette events per day | ||||
| 1–9 | 626 (22%) | 118 (21%) | 251 (22%) | 257 (22%) |
| 10–19 | 464 (16%) | 96 (17%) | 197 (17%) | 171 (15%) |
| ≥20 | 383 (13%) | 74 (13%) | 160 (14%) | 149 (13%) |
| Continuously | 1418 (49%) | 286 (50%) | 557 (48%) | 575 (50%) |
| Time since starting to use electronic cigarettes | ||||
| <1 year | 867 (30%) | 180 (31%) | 354 (30%) | 333 (29%) |
| 1–2 years | 749 (26%) | 144 (25%) | 314 (27%) | 291 (25%) |
| >2 years | 1279 (44%) | 251 (44%) | 498 (43%) | 530 (46%) |
| Started vaping to help quit smoking | 1309 (45%) | 255 (44%) | 530 (45%) | 524 (45%) |
Data are n (%) or mean (SD). FTND=Fagerström Test for Nicotine Dependence.
13 respondents did not answer this item.
16 respondents did not answer this item.
1945 (84%) of 2321 participants in the eTARGET and GENERIC groups requested electronic copies of the booklets in addition to mailed hard copies. At the end of the intervention (18 months), 737 (68%) of 1091 respondents reported having read all or almost all the intervention materials, 287 (26%) read some, and 67 (6%) did not read any. Despite randomisation, ASSESS had a higher percentage of participants of Hispanic or Latino ethnicity than that in eTARGET and GENERIC, while eTARGET had a higher proportion of participants married or living together than GENERIC and of participants with annual income greater than $20 000 than ASSESS and GENERIC (table 1).
Less than 1% of data for any variable were missing for the baseline survey and for the returned follow-up surveys. The multiple imputation model included treatment group, the 16 variables representing smoking and vaping status at each follow-up (eg, 7-day abstinence at 3 months), prespecified moderators (sex, age, education, income, FTND, HSI pre-vaping, and planning to quit within 30 days), auxiliary variables (survey type, married or living together, non-Hispanic White versus minority, ARME, SSE, commitment, when started vaping, vaping days per week, and vaping events per day) identified by preliminary analyses, and variables representing the interaction of a moderator or auxiliary variable with condition. Relative efficiency for tests of variable mean differing from 0 was greater than 0·98 for all variables. These 20 datasets were used for all analyses of 7-day point prevalence. Parallel multiple imputation models were completed for 30-day and 90-day point-prevalence smoking abstinence.
Overall, 7-day point-prevalence smoking abstinence increased from 14% at 3 months to 42% at 24 months (F7,541·7=67·1, p<0·0001). 7-day smoking abstinence rates by group at 9, 18, and 24 months, including time-specific group comparisons, are presented in table 2. We plotted the 7-day point-prevalence abstinence by group at each assessment across the 20 imputed datasets (figure 2). Abstinence rates by treatment group and assessment are presented in the appendix (p 9). Across all post-baseline assessments, GEE analysis revealed a main effect of eTARGET over ASSESS (F1,955·8=7·27, p=0·0071, α=0·017), but not over GENERIC (F1,1271·4=1·05, p=0·31, α=0·05). The difference between GENERIC and ASSESS was not significant (F1,1071·3=3·53, p=0·06, α=0·025) with our adjustment for multiple comparisons. When the treatment period (up to 18 months from baseline) and post-treatment period (21–24 months from baseline) were analysed separately, eTARGET resulted in higher abstinence rates than ASSESS (F1,973·8=10·20, p=0·0014), but not higher than GENERIC (F1,1102·5=1·79, p=0·18) for the treatment period. The difference between GENERIC and ASSESS again did not reach significance (F1,676·7=4·29, p=0·039). For the post-treatment period, no significant differences were found between treatment groups (all paired comparisons by assessment period are presented in the appendix pp 10–11).
Table 2:
7-day abstinence rates for smoking and vaping at 9, 18, and 24 months
| 9 months |
18 months |
24 months |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Smoking abstinent—all | Smoking abstinent—HCD | Vaping abstinent—all | Smoking abstinent—all | Smoking abstinent—HCD | Vaping abstinent—all | Smoking abstinent—all | Smoking abstinent—HCD | Vaping abstinent—all | |
| ASSESS | 21·7% | 17·8% | 22·9% | 33·2% | 27·3% | 28·0% | 40·0% | 35·4% | 32·4% |
| eTARGET | 28·5% | 26·1% | 20·9% | 38·4% | 35·5% | 30·9% | 42·3% | 39·4% | 35·9% |
| GENERIC | 25·4% | 22·3% | 21·8% | 36·6% | 34·0% | 29·2% | 42·2% | 39·5% | 34·0% |
| eTARGET vs ASSESS | 1·44 (1·11–1·86)* | 1·64 (1·20–2·23)* | 0·89 (0·66–1·21) | 1·26 (0·98–1·61) | 1·47 (1·08–1·98)* | 1·15 (0·84–1·59) | 1·10 (0·86–1·41) | 1·18 (0·88–1·58) | 1·17 (0·88–1·54) |
| eTARGET vs GENERIC | 1·17 (0·96–1·43) | 1·23 (0·97–1·55) | 1·06 (0·74–1·21) | 1·08 (0·88–1·32) | 1·07 (0·84–1·35) | 1·08 (0·85–1·38) | 1·01 (0·81–1·24) | 1·01 (0·79–1·25) | 1·09 (0·88–1·35) |
| GENERIC vs ASSESS | 1·23 (0·95–1·59) | 1·33 (0·97–1·83) | 0·94 (0·71–1·25) | 1·16 (0·92–1·47) | 1·38 (1·04–1·82) | 1·07 (0·78–1·45) | 1·10 (0·87–1·39) | 1·19 (0·91–1·56) | 1·07 (0·82–1·41) |
Data are % or odds ratios (95% CI) based on logistic regression for the individual paired comparisons between treatment groups. All data are based on the 20 multiple imputation datasets. HCD=higher cigarette dependence at baseline (Fagerström test for nicotine dependence ≥2).
Odds ratios are statistically significant with α=0·0167 for eTARGET versus ASSESS, α=0·025 for GENERIC versus ASSESS, and α=0·05 for GENERIC versus eTARGET.
Figure 2: Percentage of smokers abstinent by study group for each assessment.
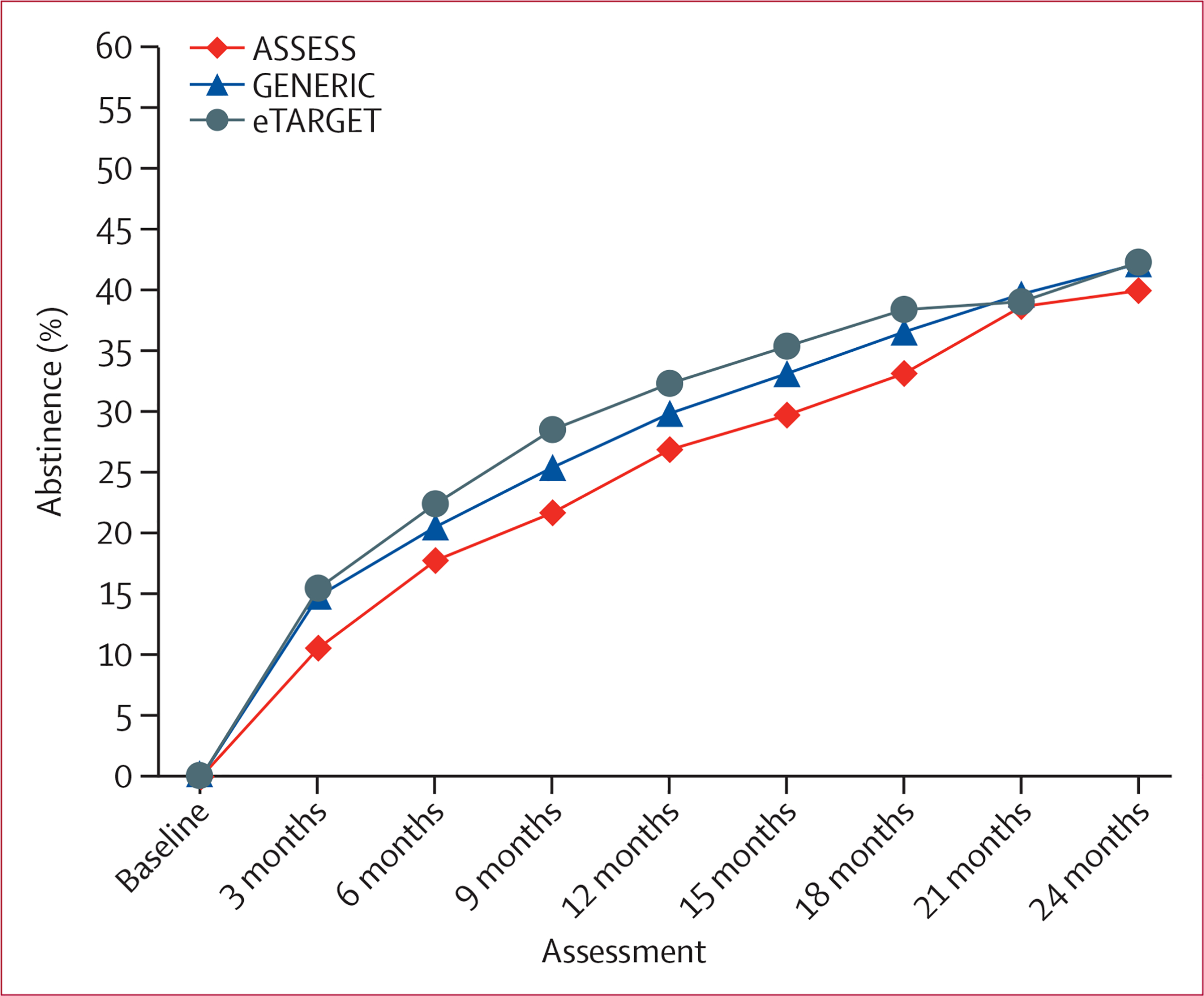
Percentage of abstinence averaged across 20 multiple imputed datasets. GENERIC and eTARGET interventions began just after baseline and ended at 18 months.
For 30-day point-prevalence by treatment group and assessment across all assessment points, the difference between eTARGET and ASSESS did not reach significance (F1,970·5=4·83, p=0·028). For the treatment period, this difference was significant (F1,682·6=5·82, p=0·016). No other paired comparisons were significant (appendix pp 9–11).
For 90-day point-prevalence across all assessment points, differences did not reach significance for eTARGET versus ASSESS (F1,1341·9=5·00, p=0·026) and for eTARGET versus GENERIC (F1,966·8=3·83, p=0·051). During the treatment period, the difference between eTARGET and GENERIC was significant (F1,926·9=4·49, p=0·034), whereas the difference did not reach significance for eTARGET versus ASSESS (F1,1186·2=4·58, p=0·033). No other paired comparisons were significant (appendix pp 9–11).
Abstinence rates and ORs at 18-month and 24-month assessments for all three abstinence indices for the full sample following multiple imputation, for responders only (ie, those who completed a given follow-up assessment), and for the full sample using the most conservative assumption of imputing missing smoking status as smoking are presented in the appendix (p 12).
We evaluated potential moderators of the eTARGET versus ASSESS effect for 7-day point-prevalence smoking abstinence over all assessments (appendix p 13). A significant moderator by treatment group interaction was observed for FTND, a measure of baseline cigarette dependence (F1,905·5=4·47, p=0·035). To illustrate the interaction, we plotted the 7-day point-prevalence abstinence rates for participants in the bottom quartile, who reported little to no cigarette dependence (FTND ≤1; 409 participants), versus those with higher dependence (FTND ≥2; 1333 participants; figure 3). Low-dependent smokers had higher abstinence rates but showed little benefit from the intervention during treatment (F1,3016·0=0·14, p=0·71) or post-treatment (F1,1072·9=0·33, p=0·57). In higher-dependence smokers, abstinence rates were higher for eTARGET than for ASSESS during treatment (6–8 percentage points, F1,1346·1=17·10, p<0·0001) but not during post-treatment (2–4 percentage points, F1,215·2=1·03, p=0·31).
Figure 3: Percentage of smokers abstinent for eTARGET and ASSESS for low and higher cigarette dependence.
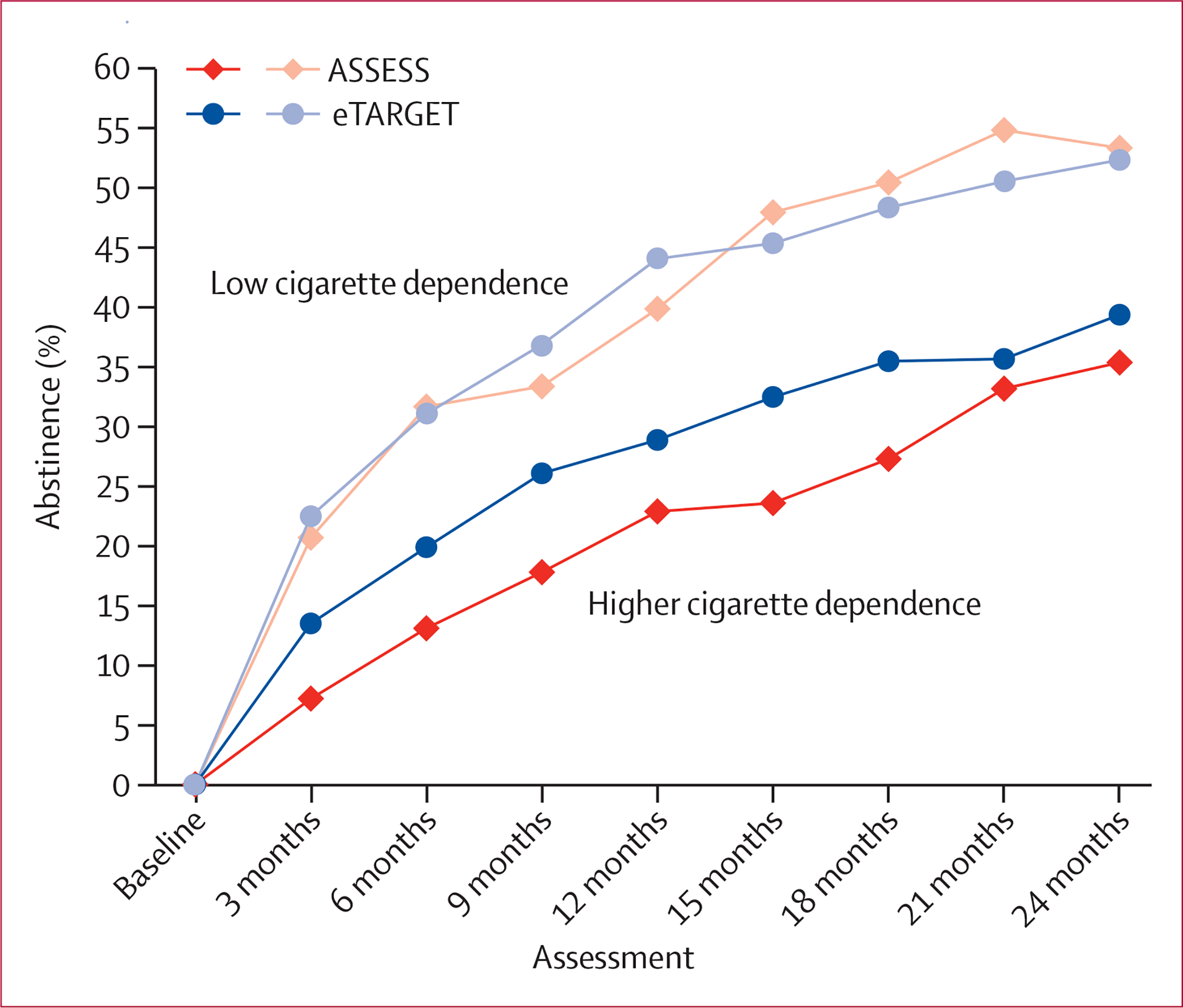
Percentage of abstinence averaged across 20 multiple imputed datasets. eTARGET intervention began just after baseline and ended at 18 months. The low cigarette dependence group (n=409) had Fagerström Test for Nicotine Dependence scores of 1 or lower at baseline, whereas the higher cigarette dependence group (n=1333) had scores of 2 or higher.
Vaping abstinence overall increased from 11% at 3 months to 34% at 24 months (F7,686·5=43·0, p<0·0001; table 2, appendix p 14). The difference between eTARGET and ASSESS did not reach significance over all assessments (F1,385·4=3·62, p=0·058) and over the treatment period (F1,525·7=3·94, p=0·048). No other treatment group comparisons reached significance (appendix p 15).
Finally, a separate GEE analysis across all assessments revealed that, in general, participants currently vaping were more likely to be abstinent from smoking (F1,157·9=4·85, p=0·029), with no significant differences across conditions. For example, at 18 months, 39% of current vapers were abstinent from smoking compared with 32% of non-vapers (appendix p 16).
The total intervention cost per participant was $0 for ASSESS, $52 for GENERIC, and $52 for eTARGET. Compared with ASSESS, the incremental cost per quitter at 18 months (end of treatment) was $1535 for GENERIC and $1000 for eTARGET. At 24 months, the incremental costs per quitter were $2369 for GENERIC and $2253 for eTARGET. Excluding individuals with very low cigarette dependence (FTND ≤1), the incremental cost per quitter was $781 for GENERIC and $640 for eTARGET at 18 months, and $1277 for GENERIC and $1312 for eTARGET at 24 months. Sensitivity analyses varying intervention costs at 10% increments are presented in the appendix (p 17).
Discussion
To our knowledge, the targeted intervention tested in this trial was the first specifically designed for dual users of combustible cigarettes and e-cigarettes. Our study is also one of the few trials that followed up vapers for over 12 months. Although the intervention did not endorse the initiation of vaping, it notably did not demonise or immediately discourage ongoing vaping. Instead, the intervention instructed current dual users to use e-cigarettes in ways thought to maximise their efficacy for smoking cessation, and later it also recommended cessation of vaping.
In the full study sample, the targeted intervention resulted in smoking abstinence rates approximately 5–10 percentage points higher than that of the assessment-only control over the 18 months of treatment. The generic intervention resulted in abstinence rates between those of the targeted and control groups. The level of baseline cigarette dependence was an important moderator. As would be expected, smokers who reported little or no baseline cigarette dependence had the greatest success in quitting smoking, upon which the intervention did not improve. However, the targeted intervention was efficacious compared with a no-treatment control among smokers with greater cigarette dependence (FTND ≥2), representing over 75% of our sample. Smokers who have higher dependency appear to have more difficulty in quitting smoking; thus the intervention might have been more valuable to them.26 Therefore, our intervention might help smokers who are more dependent make a complete switch to e-cigarettes, thereby reducing the harm from smoking.
Although the targeted intervention produced higher abstinence rates over the 18 months when the materials were distributed, differences declined after the treatment period. It is possible that the intervention accelerated smoking cessation that would have eventually occurred without treatment. Alternatively, the intervention might have been ended prematurely, and extending it might maintain its efficacy.
With vaping risks believed to be substantially lower than those of smoking,6 vaping cessation was not a primary target or outcome. However, the intervention communicated the advantages of e-cigarette cessation regarding health benefits, cost savings, and freedom from addiction. Although vaping decreased over time, no significant differences were observed across groups. In general, vaping was associated with a higher probability of smoking abstinence, as has been previously reported.6 A 2019 randomised controlled trial showing the efficacy of e-cigarettes also found that most individuals continued vaping after they quit smoking.27 Unlike pharmaceutical NRTs, e-cigarettes mimic the sensorimotor aspects of cigarette smoking; can, under certain circumstances, approximate more closely the pharmacokinetics of smoking;6 and are not accompanied by instructions on tapering off the product over time. Although long-term vaping appears to involve exposure to fewer toxicants than smoking, it is probably not benign. Interventions that facilitate vaping cessation without risking smoking relapse are needed.
Smoking abstinence rates across all three trial groups were higher than expected and higher than those reported in longitudinal studies of dual users, albeit with different inclusion and outcome criteria.8,9 Therefore, our findings support evidence from randomised controlled trials that e-cigarettes can be efficacious smoking cessation aids. We did not select for individuals seeking to quit smoking (and only 26% were planning to do so within the following 30 days), which might have suppressed intervention effects compared with other clinical trials. Additionally, because the study recruited only dual users, any individuals who had already switched completely from smoking to vaping were excluded, which would have attenuated the observed smoking cessation rates. Indeed, approximately 70% of our sample had been vaping for over 1 year without quitting smoking. Therefore, the sample could be conceptualised as comprising the residual subset of vapers who had so far been unsuccessful at quitting smoking. Conversely, the study probably attracted individuals committed to vaping and motivated to participate in research on the topic. These last characteristics might have increased the overall smoking cessation rates while also suppressing vaping cessation rates.
Self-help interventions are efficacious for smoking cessation, with potentially high reach at low cost.28 The economy of the targeted intervention yielded a cost per quitter lower than most smoking cessation interventions,29 particularly when excluding smokers with low dependence. Costs might be reduced substantially by using a mobile health format, which would also enable more frequent contact over a longer duration of treatment.
Limitations include a study sample recruited largely through social media, potentially limiting generalisability; low representation of racial and ethnic minorities, although this might reflect the demographics of e-cigarette use;30 minimal biochemical verification of smoking abstinence; and relatively high long-term attrition rates, possibly reflecting the young age and mobility of this sample, the study not being advertised as a cessation trial, and media reports regarding e-cigarette risks over the course of the study. However, 83% of participants responded to one or more of the follow-up assessments, which contributed to imputation of missing data. Importantly, follow-up assessment rates for research should not necessarily predict the acceptance or effect of the intervention when implemented in non-research settings. Finally, the study might have not captured the effect of newer and better performing devices, including pod systems that deliver protonated nicotine, which became popular during the course of the study.
In conclusion, our trial of an inexpensive self-help smoking cessation intervention for dual users of combustible and e-cigarettes showed that it improves smoking cessation, particularly during the 18-month intervention and among dual users with higher cigarette dependence. A 2020 Cochrane review of e-cigarette efficacy for smoking cessation calculated that e-cigarettes improve smoking cessation outcomes by about 6 percentage points at 6 or more months compared with smoking cessation without e-cigarettes.3 Effect sizes achieved in our study were of a similar size as those reported by Cochrane for nicotine e-cigarettes versus non-nicotine e-cigarettes or versus behavioural support or no support. This suggests that an additional effect of similar size could be achieved by adding self-help booklets. Moreover, because dual use is prevalent and self-help can be easily disseminated, when brought to scale, each percentage point can represent a substantial public health impact. Additional pragmatic trials of similar interventions in different populations and settings are needed to identify the most effective ways to support dual users in completely switching from smoking to vaping on their journey towards nicotine abstinence.
Supplementary Material
Research in context.
Evidence before this study
A Cochrane review published in 2020, reviewing research published up to January, 2020, reported the potential of electronic cigarettes (e-cigarettes) for smoking cessation. The review concluded, with moderate confidence, that e-cigarettes containing nicotine resulted in higher smoking abstinence rates compared with placebo e-cigarettes, and that e-cigarettes were more effective than nicotine replacement therapy for smoking cessation. The review also concluded, albeit with very low confidence on the basis of only four randomised controlled trials, that nicotine e-cigarettes were more effective than behavioural support alone or no support for smoking cessation. To extend these findings, we searched Cochrane, Ovid MEDLINE, and Embase databases on Oct 30, 2020, with no language restrictions, to identify additional randomised trials testing the efficacy of e-cigarettes for smoking cessation published since January, 2020. In line with the Cochrane search strategy, we searched for trials using the following terms: “e-cig$ OR electr$ cigar$ OR electronic nicotine OR (vape or vaper or vapers or vaping)”. We identified 60 articles with only one relevant randomised controlled trial that had not been included in the meta-analyses previously summarised. The study was done in the USA and randomly allocated 264 smokers uninterested in quitting to one of four conditions they were instructed to follow: use e-cigarettes ad libitum, completely substitute combustible cigarettes with e-cigarettes, completely substitute e-cigarettes with nicotine gum or lozenges, or continue smoking combustible cigarettes. 8 weeks after baseline, 7-day point-prevalence abstinence from smoking was significantly higher when instructing participants to completely substitute combustible cigarettes with e-cigarettes (32·9%) compared with instructions to substitute cigarettes with nicotine gum or lozenges (17·1%; p=0·039). We found no trials that tested interventions designed for smokers already using e-cigarettes (ie, dual users).
Added value of this study
Despite the growing evidence that e-cigarettes might aid smoking cessation, most smokers who initiate vaping continue to smoke, raising concern that e-cigarettes might also maintain smoking among many individuals otherwise motivated to quit. Such dual use maintains exposure to known smoking-related toxicants and possible vaping-related toxicants. To our knowledge, this study is the first to test an intervention specifically for dual users, with the goal of transforming their e-cigarettes from products that might maintain smoking into tools that could be used to promote smoking cessation. Our low-cost, easily disseminated intervention resulted in modest increases in smoking abstinence over 18 months, with stronger treatment effects predicted by higher baseline levels of cigarette dependence. Importantly, our findings expand the target of smoking cessation interventions involving e-cigarettes to encompass those who have already initiated vaping, including individuals who might have settled into a prolonged pattern of dual use.
Implications of all the available evidence
This study indicates that dual users could benefit from specific interventions that capitalise on their ongoing e-cigarette use. Future pragmatic research is needed to test alternative intervention methods, enhance long-term efficacy, keep pace with evolving e-cigarette products, assist with eventual e-cigarette cessation, and maximise implementation in clinical and other settings.
Acknowledgments
This work was supported by the National Institute on Drug Abuse of the NIH (R01DA037961). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work has also been supported in part by the Biostatistics and Bioinformatics Shared Resource and the Participant Research, Interventions, and Measures Resource at the H Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute designated Comprehensive Cancer Center (P30CA76292).
Declaration of interests
THB has received research support from the US National Institutes of Health (NIH), the American Cancer Society, the Florida Department of Health, and Pfizer; has collaborated on funded research with Voxiva, Optum, and the University of East Anglia (Norwich, UK); spent a sabbatical period at the Trimbos Institute and Utrecht University (Utrecht, Netherlands); is on the advisory board of, and holds restricted stock in, Hava Health, which is developing a pharmaceutical grade electronic nicotine delivery system for smoking cessation; participated in a Best Brains Exchange for Health Canada, providing advice on e-cigarette policy; and consulted for the Australian Government Solicitor regarding plain tobacco packaging. UM has received research support from the NIH and the Galician Plan of Research, Innovation, and Growth (Spain); and has received funding from the Barrie Foundation to receive predoctoral training at the University of Newcastle (Callaghan, NSW, Australia). VNS has received research support from the NIH and the Florida Department of Health. SKS has received research support from the NIH, the American Cancer Society, the Florida Department of Health, and Pfizer. DJD has received research support from the NIH, the American Cancer Society, and the Florida Department of Health; and has provided paid expert testimony in litigation against tobacco companies. MMB has received funding from the NIH, the Florida Department of Health, the US Department of Veterans Affairs, the US Centers for Disease Control and Prevention, the National Science Foundation, and the US Department of Housing & Urban Development; and has received research support from Gilead Sciences, Florida Blue Foundation, Bristol Myers Squibb Foundation, Merck Foundation, Maine Cancer Foundation, and Pfizer. PTH has received research support from the NIH, US Food and Drug Administration (FDA), and Virginia Foundation for Healthy Youth. TE conducts research supported by the National Institute on Drug Abuse of the NIH and the Center for Tobacco Products of the FDA; is a paid consultant in litigation against the tobacco industry and the electronic cigarette industry; is named on one patent for a device that measures the puffing behaviour of electronic cigarette users and on another patent for a smartphone app that determines electronic cigarette device and liquid characteristics; owns shares in a variety of mutual funds, the exact stock makeup of which he has no control, and owns shares in three publicly traded companies, none of which are in any way related to the tobacco industry, the electronic cigarette industry, or any other aspect of this work; and has served as a special government employee of the US Government in the context of his service on the FDA’s Tobacco Products Scientific Advisory Committee and the Department of Health and Human Services Secretary’s Advisory Committee on Human Research Protection. CRB has received research support from the New Zealand Ministry of Health, the Health Research Council of New Zealand, CureKids Foundation, Heart Foundation, Health Promotion Agency, and Auckland Council and Sanitarium; collaborates on funded research with Newcastle University (Australia) through a grant from the Australian National Health and Medical Research Council, with Zhejiang University (Hangzhou, China) and Kunming University (Yunnan, China) on an Education New Zealand Tripartite grant, and with the University of Malaya (Kuala Lumpur, Malaysia) on a University of Malaya Grand Challenges grant; received funding from Pfizer Australasia for a survey of the impact of COVID-19 on health workers in low-income and middle-income countries and from Johnson & Johnson Japan for consultancy on smoking cessation medication; and was a consultant to Moffit Cancer Center on this study through an NIH grant. The employees of Moffitt Cancer Center—UM, VNS, SKS, DJD, LRM, KOB, MMB, and THB—are eligible for sharing of any revenue that might be generated by products developed during their employment, including the intervention used in this study. LRM and KOB declare no additional competing interests.
Funding
National Institute on Drug Abuse, National Cancer Institute.
Footnotes
Data sharing
Deidentified data will be available with publication through the corresponding author after approval of a proposal with a signed data access agreement. Only deidentified data that underlie results reported in this Article can be shared with investigators who submit an approved proposal. The data can be used for only the aims stated in the approved proposal with investigator support.
See Online for appendix
Contributor Information
Ursula Martinez, Department of Health Outcomes and Behavior, H Lee Moffitt Cancer Center, Tampa, FL, USA; Department of Oncologic Sciences, University of South Florida, Tampa, FL, USA.
Vani N Simmons, Department of Health Outcomes and Behavior, H Lee Moffitt Cancer Center, Tampa, FL, USA; Department of Oncologic Sciences, University of South Florida, Tampa, FL, USA; Department of Psychology, University of South Florida, Tampa, FL, USA.
Steven K Sutton, Department of Biostatistics and Bioinformatics, H Lee Moffitt Cancer Center, Tampa, FL, USA; Department of Oncologic Sciences, University of South Florida, Tampa, FL, USA; Department of Psychology, University of South Florida, Tampa, FL, USA.
David J Drobes, Department of Health Outcomes and Behavior, H Lee Moffitt Cancer Center, Tampa, FL, USA; Department of Oncologic Sciences, University of South Florida, Tampa, FL, USA; Department of Psychology, University of South Florida, Tampa, FL, USA.
Lauren R Meltzer, Department of Health Outcomes and Behavior, H Lee Moffitt Cancer Center, Tampa, FL, USA.
Karen O Brandon, Department of Health Outcomes and Behavior, H Lee Moffitt Cancer Center, Tampa, FL, USA.
Margaret M Byrne, Department of Health Outcomes and Behavior, H Lee Moffitt Cancer Center, Tampa, FL, USA; Department of Oncologic Sciences, University of South Florida, Tampa, FL, USA; Department of Psychology, University of South Florida, Tampa, FL, USA.
Paul T Harrell, Department of Pediatrics, Eastern Virginia Medical School, Norfolk, VA, USA.
Thomas Eissenberg, Center for the Study of Tobacco Products, Department of Psychology, Virginia Commonwealth University, Richmond, VA, USA.
Christopher R Bullen, School of Population Health, Faculty of Medical and Health Science, University of Auckland, Auckland, New Zealand.
Thomas H Brandon, Department of Health Outcomes and Behavior, H Lee Moffitt Cancer Center, Tampa, FL, USA; Department of Oncologic Sciences, University of South Florida, Tampa, FL, USA.
References
- 1.Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults - United States, 2018. MMWR Morb Mortal Wkly Rep 2019; 68: 1013–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glasser AM, Collins L, Pearson JL, et al. Overview of electronic nicotine delivery systems: a systematic review. Am J Prev Med 2017; 52: e33–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann-Boyce J, McRobbie H, Lindson N, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 2020; 10: CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owusu D, Huang J, Weaver SR, et al. Patterns and trends of dual use of e-cigarettes and cigarettes among U.S. adults, 2015–2018. Prev Med Rep 2019; 16: 101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goniewicz ML, Smith DM, Edwards KC, et al. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw Open 2018; 1: e185937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services. Smoking cessation: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2020. [Google Scholar]
- 7.Martínez Ú, Martínez-Loredo V, Simmons VN, et al. How does smoking and nicotine dependence change after onset of vaping? A retrospective analysis of dual users. Nicotine Tob Res 2020; 22: 764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman B, Rostron B, Johnson SE, et al. Transitions in electronic cigarette use among adults in the Population Assessment of Tobacco and Health (PATH) Study, waves 1 and 2 (2013–2015). Tob Control 2019; 28: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piper ME, Baker TB, Benowitz NL, Jorenby DE. Changes in use patterns over 1 year among smokers and dual users of combustible and electronic cigarettes. Nicotine Tob Res 2020; 22: 672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen RL, Steinberg ML. Interest in quitting e-cigarettes among adults in the United States. Nicotine Tob Res 2020; 22: 857–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue-Choi M, Christensen CH, Rostron BL, et al. Dose-response association of low-intensity and nondaily smoking with mortality in the United States. JAMA Netw Open 2020; 3: e206436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandon TH, Simmons VN, Sutton SK, et al. Extended self-help for smoking cessation: a randomized controlled trial. Am J Prev Med 2016; 51: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandura A Social learning theory. Upper Saddle River, NJ: Prentice Hall, 1977. [Google Scholar]
- 14.Marlatt GA. Relapse prevention: theoretical rationale and overview of the model. In: Marlatt GA, Gordon JR, eds. Relapse prevention: maintenance strategies in the treatment of addictive behaviors. New York, NY: Guilford, 1985: 3–70. [Google Scholar]
- 15.Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annu Rev Psychol 2004; 55: 463–91. [DOI] [PubMed] [Google Scholar]
- 16.Meltzer LR, Simmons VN, Sutton SK, et al. A randomized controlled trial of a smoking cessation self-help intervention for dual users of tobacco cigarettes and e-cigarettes: intervention development and research design. Contemp Clin Trials 2017; 60: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991; 86: 1119–27. [DOI] [PubMed] [Google Scholar]
- 18.DiClemente CC, Prochaska JO, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS. The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. J Consult Clin Psychol 1991; 59: 295–304. [DOI] [PubMed] [Google Scholar]
- 19.Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addict Behav 1990; 15: 271–83. [DOI] [PubMed] [Google Scholar]
- 20.Simmons VN, Heckman BW, Ditre JW, Brandon TH. A measure of smoking abstinence-related motivational engagement: development and initial validation. Nicotine Tob Res 2010; 12: 432–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382: 1629–37. [DOI] [PubMed] [Google Scholar]
- 22.Rochon J Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med 1998; 17: 1643–58. [DOI] [PubMed] [Google Scholar]
- 23.Holm S A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6: 65–70. [Google Scholar]
- 24.Rubin D Multiple imputation for nonresponse in surveys. New York, NY: John Wiley & Sons, 1987. [Google Scholar]
- 25.Schafer JL. Analysis of incomplete multivariate data. London: Chapman and Hall, 1997. [Google Scholar]
- 26.Vangeli E, Stapleton J, Smit ES, Borland R, West R. Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addiction 2011; 106: 2110–21. [DOI] [PubMed] [Google Scholar]
- 27.Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med 2019; 380: 629–37. [DOI] [PubMed] [Google Scholar]
- 28.Livingstone-Banks J, Ordóñez-Mena JM, Hartmann-Boyce J. Print-based self-help interventions for smoking cessation. Cochrane Database Syst Rev 2019; 1: CD001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruger JP, Lazar CM. Economic evaluation of pharmaco- and behavioral therapies for smoking cessation: a critical and systematic review of empirical research. Annu Rev Public Health 2012; 33: 279–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villarroel MA, Cha AE, Vahratian A. Electronic cigarette use among U.S. adults, 2018. NCHS Data Brief 2020; 365: 1–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


