Abstract
Background:
Chronic pain is highly prevalent among people in methadone maintenance treatment (MMT) for opioid use disorder and is known to be an important contributor to treatment discontinuation and opioid relapse. Mindfulness-Oriented Recovery Enhancement (MORE) is one of the few interventions developed and tested as an integrated treatment to simultaneously address both pain and illicit opioid use; however, this study is the first to evaluate MORE as an adjunct to MMT.
Methods:
Randomized individuals in MMT (N=30) received MORE plus methadone TAU (n=15) or methadone TAU, only (n=15). Participants in the MORE arm received their MMT, as usual, and attended eight, weekly, two-hour MORE groups at their MMT clinics. Participants in the TAU arm received their MMT, as usual, and group or individual counseling, as required by the clinic. TAU counseling consisted of relapse prevention, cognitive-behavioral therapy, and supportive treatment. TAU participants did not receive any mindfulness-based intervention. Participants completed assessments at baseline, post-treatment (i.e., 8-weeks post-baseline), and follow-up (i.e., 16-weeks post-baseline).
Results:
Participants in MORE evidenced significantly fewer baseline adjusted days of illicit drug use and significantly lower levels of craving through 16-week follow-up compared to TAU. Also, participants in MORE reported significantly lower levels of pain, physical and emotional limitations, depression, and anxiety through 16-week follow-up compared to TAU. Conversely, participants in MORE reported significantly higher levels of well-being, vitality, and social functioning through 16-week follow-up compared to TAU.
Conclusion:
MORE could be an effective adjunct to MMT, and larger trials are warranted.
Keywords: chronic pain, MORE, mindfulness, methadone, opioid, savoring
1. Introduction
Opioid use is currently an epidemic in the United States. Over 800,000 Americans used heroin and over 10 million misused opioid medication in 2018 (Administration, 2019). Medication for opioid use disorder (MOUD) is the most effective intervention for OUD, and methadone maintenance treatment (MMT) is the most commonly used MOUD; however, 50% of people who begin MMT discontinue within twelve months (Bao et al., 2009), and 50% of people retained in MMT have an opioid relapse within six months (Naji et al., 2016). Chronic pain is an important contributor to treatment discontinuation and opioid relapse, and chronic pain is highly prevalent among people in MMT (55%−61%; Eyler, 2013). While several behavioral interventions, most notably cognitive-behavioral therapy (CBT) and mindfulness-based interventions (MBIs), are effective in addressing problems of pain and substance misuse separately (Garland et al., 2020; Goldberg et al., 2018; Hilton et al., 2017; Li et al., 2017) more focus on developing behavioral interventions that simultaneously address both problems is needed. Applying integrated treatments to these frequently co-occurring disorders could vastly improve outcomes for people with OUD and pain. Mindfulness-Oriented Recovery Enhancement (MORE) is one of the few interventions developed and tested as an integrated treatment to simultaneously address both pain and opioid use; however, this study is the first to evaluate MORE as an adjunct to MOUD (Garland, Hanley, Riquino, et al., 2019; Garland, Manusov, et al., 2014).
People who use substances often report that they do so to cope with pain or distress (Cicero & Ellis, 2017; Cooperman et al., 2015). Negative reinforcement models suggest that substances are used to escape or avoid physical or emotional discomfort (Baker et al., 2004; Carmody et al., 2007; Khantzian, 1997; Leventhal & Zvolensky, 2015; Wills & Shiffman, 1985). Pain and negative affect are associated with the initiation and maintenance of addictive behavior due to the perceived and actual benefits of drug use. Drug use temporarily alleviates physical and emotional pain, thereby negatively reinforcing continued use (Leventhal & Zvolensky, 2015). Yet, according to allostatic models of the downward spiral linking chronic pain to OUD (Elman & Borsook, 2016; Garland et al., 2013; Shurman et al., 2010), prolonged use of opioids to escape distress may shift hedonic set points in corticostriatal brain circuitry mediating reward and disruptimg emotion regulatory capacity. These allostatic changes are believed to compel opioid use as a means of preserving a dwindling sense of well-being. Consequently, OUD in the context of chronic pain involves a process of hedonic homeostatic dysregulation, in which the motivation to obtain natural rewards is re-organized around seeking drug-related reward and the desire to alleviate aversive states (e.g., affective distress and pain). As such, individuals in MMT need to learn how to replace drug use with naturally rewarding, healthy behaviors as a means of coping with painful or uncomfortable experiences and tolerating discomfort. Further, MMT patients would benefit from learning how to replace drug-induced rewards with natural rewards from healthful and socially-affiliative stimuli (Garland, 2016; Garland, Atchley, et al., 2019; Garland et al., 2015).
MORE integrates training in mindfulness, cognitive reappraisal skills, and savoring of natural rewards into an 8-week group therapy designed to remediate hedonic dysregulation in brain reward systems underpinning the downward spiral linking chronic pain to OUD and relapse (Garland, 2016). The intervention is based on dual-process theories of addiction tailored specifically to target the hedonic dysregulation that undergirds OUD (Koob & Volkow, 2009; Verdejo-Garcia & Bechara, 2009). Dual-process theories (Koob & Volkow, 2009; Verdejo-Garcia & Bechara, 2009) posit that addictive behaviors result from dysregulation of bottom-up neural circuitry that codes for the salience of reward-related stimuli as well as impaired top-down frontal-executive brain circuitry that subserves cognitive control processes (Robinson & Berridge, 2008), including metacognitive awareness and proactive regulation of attention and emotion (George et al., 2011). To the extent that OUD involves dysfunction in these automatic and controlled processes, it may be tractable to dual-process treatments. MORE aims to remediate hedonic dysregulation by strengthening top-down metacognitive control to restructure bottom-up reward learning from valuation of drug rewards back to valuation of natural rewards (Koob & Le Moal, 2008). In that regard, MORE modulates neurophysiological responses to drug and natural rewards among chronic opioid users (Garland, Atchley, et al., 2019).
Given the potential for MORE to improve MOUD outcomes among individuals with chronic pain, and to inform a larger trial, we conducted a Stage 1, pilot randomized controlled trial of MORE, as compared to treatment as usual (TAU) among 30 individuals in MMT for OUD. We previously reported that, in this study, based on ecological momentary assessment (EMA) data, MORE was associated with greater improvements in craving, positive affect, stress, and pain unpleasantness, as compared to TAU (Garland, Hanley, Kline, et al., 2019). In this paper, we present data on MORE feasibility in MMT and, to inform a larger clinical trial, secondary data analyses of the effects of MORE on illicit drug use, health, and well-being. We hypothesized that participants who receive MORE will have less illicit drug use, better mental and physical health, and increased well-being through 16-week follow-up, relative to those who receive TAU.
2. Material and Methods
2.1. Participants and Procedure
Participant (N=30) eligibility criteria included: 1) English-speaking, 2) age 18 or older, 3) receiving MMT for OUD, 4) experiencing at least mild, non-malignant, pain (score of at least 8 or higher on the Gracely Pain Scale) for at least three months (Gracely, McGrath, & Dubner, 1978), and 5) not actively psychotic or suicidal. Recruitment was conducted in two clinics in New Jersey, through referral by clinic staff, flyers posted in clinic waiting areas, and word of mouth. After informed consent, randomization to receive MORE plus methadone TAU (n=15) or methadone TAU, only (n=15) in two separate cohorts (one at each of the two clinics) occurred. Computer software created randomization tables with participant condition. Research staff kept the tables in sealed envelopes to maintain blinding until the study coordinator notified each participant of condition. Participants completed assessments at baseline, post-treatment (i.e., 8-weeks post-baseline), and follow-up (i.e., 16-weeks post-baseline). Participants received compensation for completing study assessments. Research staff registered the study with ClinicalTrials.gov (NCT03894501), and obtained study procedures approval from the Rutgers Health Sciences Institutional Review Board.
2.2. Study Interventions
2.2.1. MORE.
Participants in the MORE arm received their MMT, as usual (including methadone dosing and individual or group counseling, as required), and attended eight, weekly, two-hour MORE groups, a manual guided intervention, at their MMT clinics. In some cases, where group sessions are required for TAU (e.g., intensive outpatient treatment) one TAU group counseling session was replaced with a MORE group; however, other group sessions and individual sessions remained the same as TAU. An MMT clinician and a study clinician co-led study groups. MORE sessions involved mindfulness to prevent drug use and reduce pain, reappraisal to regulate negative emotions and craving, and savoring to augment natural reward processing and evoke positive emotion (Garland, 2013). Each session began with a mindful breathing or body scan meditation, followed by debriefing of that in-session meditation with a phenomenological and reinforcement-based processing approach (Garland, Hanley, Riquino, et al., 2019; Hanley & Garland, 2019). Next, therapists debriefed participants’ weekly homework practice of using mindfulness, reappraisal, and savoring to decrease pain and addictive behavior. Group therapists then introduced psychoeducational material, and sessions culminated with an experiential exercise. Therapists asked participants to practice 15 minutes of mindfulness/reappraisal/savoring skills each day. Study staff recorded all sessions and assessed for fidelity using the MORE Fidelity Measure (Hanley & Garland, 2019), and clinicians received weekly supervision.
2.2.2. TAU.
Participants in the TAU arm received their MMT, as usual, and group or individual counseling, as required by the clinic. TAU counseling consisted of relapse prevention, cognitive-behavioral therapy, and supportive treatment. TAU participants did not receive any mindfulness-based intervention.
2.3. Measures
2.3.1. Demographics.
At baseline, study staff asked participants about their age, race, education, employment, chronic pain conditions, length of time in methadone treatment, methadone dose, and drug use history.
2.3.2. Feasibility and engagement.
Research staff tracked number of sessions completed and homework practice (i.e., number of minutes per day). Completing at least four sessions qualified participants as “treatment completers.”
2.3.3. Drug use.
At baseline and each follow-up, research staff assessed drug use with questions based on the Addiction Severity Index (McLellan et al., 1992). Research staff asked participants if they used various drugs (i.e., heroin, cocaine, opioids, marijuana, amphetamines, inhalants, hallucinogens, benzodiazepines, zolpidem, methylphenidate, or other drugs) in the past 30 days and the number of days of use for each drug in the past 30 days. Drug use was also triangulated by ecological momentary assessment (EMA). The EMA survey approach involved 1) collecting event-contingent records of drug use when it occurred as well as 2) regular random assessments, prompted by random text messages, twice daily, via smartphones provided to study participants. For this analysis, “days of illicit drug use” was determined by counting the number of days each participant used drugs over the 16-week study period, based on past-30 day self-reports at the 8- and 16-week assessments. EMA data are presented elsewhere (Garland, Hanley, Kline, et al., 2019). Illicit drug use was defined as any use of the aforementioned illicit substances or prescription substances taken without a prescription or in ways or amounts not prescribed.
2.3.4. Craving.
Research staff assessed opioid craving with a version of the Penn Alcohol Craving Scale (PACS; (Flannery et al., 1999) that was adapted to assess craving to opioids. The PACS is a valid, reliable, and internally consistent measure that consists of five self-report items that have been found to predict relapse. The PACS items assess the intensity, frequency, ability to resist, and duration of opioid cravings over the past week. Originally developed to assess alcohol craving, the PACS was adapted and is used to measure craving for various substances, including opioids (Tsui et al., 2014). Previous trials of MORE for substance use disorders utilized adapted versions of the PACS (Garland et al., 2016).
2.3.5. Depression and anxiety.
Research staff evaluated symptoms of depression and anxiety at baseline and each follow-up, with the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977) and the Beck Anxiety Inventory (BAI; (Beck et al., 1988; Radloff, 1977). The CES-D scale is a widely used valid and reliable measure that consists of 20 items with potential scores ranging from 0 to 60. A score above 16 on the CES-D indicates clinically significant symptoms of depression. The BAI is also a widely used reliable and valid scale that consists of 21 items with potential scores ranging from 0 to 63. A score of 16 or higher indicates clinically significant symptoms of anxiety. Higher scores on the CES-D and BAI indicate greater depression and anxiety, respectively.
2.3.6. Pain, health, and well-being.
Research staff assessed pain, health, and well-being at baseline and each follow-up, with subscales of the RAND 36-Item Short Form Health Survey (Brazier et al., 1992). The scales include, pain (i.e., bodily pain severity and interference), physical functioning (i.e., ability to perform various physical activities such as walking), physical or emotional limitations (i.e., difficulty with daily activities or work due to physical or emotional problems), vitality (i.e., energy versus fatigue), well-being (i.e., anxiety and depression versus calmness and happiness), social functioning (i.e., impact of physical or emotional health on social interactions), and general health (i.e., perceived overall health). Scores for each factor range from 0 to 100, with higher scores indicating better functioning, health, and well-being and less pain, limitations, and symptom severity or interference as compared to lower scores.
2.4. Data Analytic Approach
We assessed outcomes with mixed model analysis of covariance for repeated measures (8 and 16-week follow-up), comprised of fixed effects including baseline measurement + treatment + week + treatment x week, with week treated as a categorical indicator. With this model, the “treatment” term estimates the average difference between MORE and TAU across follow-up weeks and the treatment x week term assesses whether this difference varies by 8- and 16- week follow-up. In sensitivity analyses, we added age, gender, and duration of MMT as covariates to mixed models. Paired samples t-tests examined change over time in each outcome following MORE and TAU.
3. Results
3.1. Sample characteristics
Based on recommendations for a Phase 1 pilot trial (Rounsaville, Carroll, & Onken, 2001), we aimed to enroll 15 participants per arm (N=30). During January and February, 2019, we screened 32 participants for eligibility; 2 declined to participate; and, research staff randomized 30 to the MORE or TAU arms (Figure 1). The average age of participants was, 50.4, with an equal distribution of males and females (50%, Table 1). The sample included Black/African American individuals (53%); White individuals (37%), and Hispanic individuals (20%). Most completed at least high school (70%); however, 83% did not have employment. Chronic pain was most commonly attributed to lower back issues (53%) and arthritis (40%). At baseline, the majority of participants had used heroin (67%) or opioid pain medications (13%) in the past 30 days. Participants had high levels of depression and anxiety, at baseline, with average CES-D and BAI scores of 25.4 (SD=14.2) and 40.7 (SD=13.2) respectively, and participants had significant pain (M[SD]=32.9 [25.6]) and physical limitations (M[SD]=32.5 [40.0]). Among participants, chronic pain was most commonly related to a lower back condition (53%), arthritis (40%), or migraine (17%). Twenty-seven percent reported taking non-prescription or prescription medication for pain (e.g. ibuprofen, acetaminophen, aspirin, naproxen, bupropion, or alprazolam). Only one participant reported having a prescription for an opioid pain medication (i.e., oxycodone). No statistically significant differences existed between-groups on any baseline variables.
Figure 1.
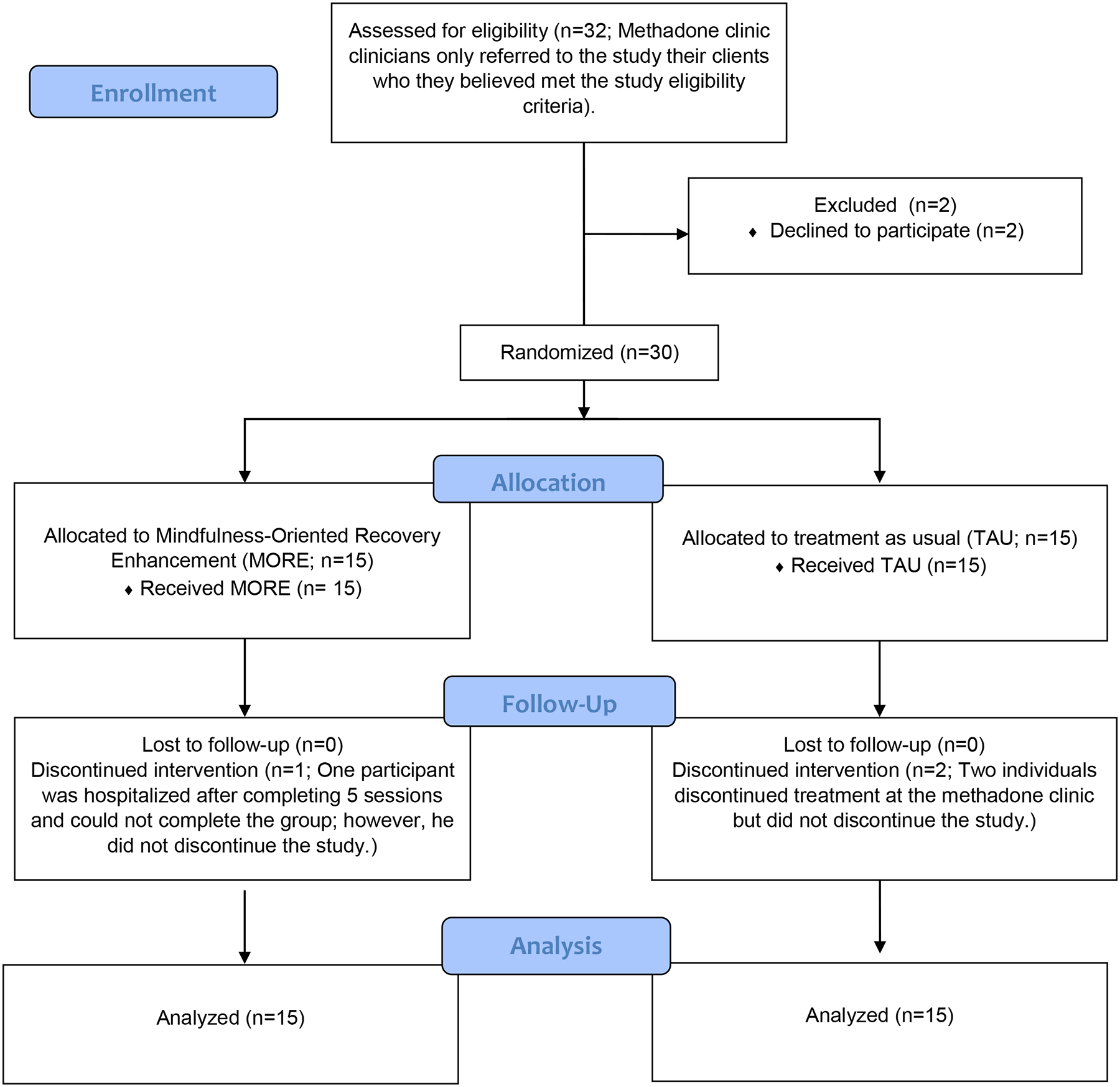
CONSORT Diagram
Table 1.
Baseline Demographic and Clinical Characteristics, Overall and by Treatment Groupa
| Measure | Entire sample (n=30) | MOREb (n = 15) | TAUc (n = 15) |
|---|---|---|---|
| Female, n (%) | 15 (50) | 8 (53) | 7 (47) |
| Age, m ± sd | 50.4 ± 8.8 | 47.9 ± 8.7 | 52.9 ± 8.4 |
| Racial/ethnic background, n (%)d | |||
| Black/African American | 16 (53) | 7 (47) | 9 (60) |
| White | 11 (37) | 7 (47) | 4 (27) |
| Hispanic | 6 (20) | 3 (20) | 3 (20) |
| Education, high school graduate or more, n (%) | 21 (70) | 10 (67) | 11 (73) |
| Unemployed, n (%) | 25 (83) | 12 (80) | 13 (87) |
| Primary pain condition, n (%)d | |||
| Low back pain | 16 (53) | 8 (53) | 8 (53) |
| Arthritis | 12 (40) | 6 (40) | 6 (40) |
| Migraine | 5 (17) | 2 (13) | 3 (20) |
| Other | 4 (13) | 2 (13) | 2 (13) |
| Taking over-the-counter or prescribed pain medication | 8 (27) | 3 (20) | 5 (33) |
| Illicitly used in the past 30 days, n (%) | |||
| Heroin | 20 (67) | 9 (60) | 11 (73) |
| Morphine | 2 (7) | 1 (7) | 1 (7) |
| Cocaine/crack | 7 (23) | 4 (27) | 3 (20) |
| Opioid pain medication (e.g., codeine, oxycodone) | 4 (13) | 1 (7) | 3 (20) |
| Benzodiazepines | 11 (37) | 7 (47) | 4 (27) |
| Stimulants (e.g., methylphenidate) | 4 (13) | 3 (20) | 1 (7) |
| Marijuana | 7 (24) | 4 (27) | 3 (20) |
| Sedatives/sleeping pills | 5 (17) | 3 (20) | 2 (13) |
| Other (e.g., hallucinogens, methamphetamine) | 0 (0) | 0 (0) | 0 (0) |
| Number of days of illicit use in past 30 days, m ± sd | |||
| Heroin | 9.1 ± 11.3 | 6.4 ± 9.2 | 11.7 ± 12.8 |
| Morphine | 0.6 ± 2.3 | 0.7 ± 2.6 | 0.5 ± 2.1 |
| Cocaine/crack | 3.6 ± 8.7 | 2.7 ± 8.0 | 4.5 ± 9.8 |
| Opioid pain medication (e.g., codeine, oxycodone) | 0.3 ± 0.7 | 0.1 ± 0.5 | 0.4 ± 0.8 |
| Benzodiazepines | 4.9 ± 9.2 | 6.3 ± 10.5 | 3.4 ± 7.9 |
| Stimulants (e.g., methylphenidate, amphetamine) | 2.3 ± 7.6 | 4.3 ± 10.5 | 0.2 ± 0.8 |
| Marijuana | 1.3 ± 3.7 | 1.1 ± 2.0 | 1.5 ± 4.9 |
| Sedatives/sleeping pills | 2.7 ± 7.7 | 2.3 ± 7.2 | 3.0 ± 8.4 |
| Total number of days of drug use in past 30 days, m ± sd | 24.6 ± 23.7 | 24.0 ± 17.4 | 25.20 ± 29.3 |
| Methadone dose, m ± sd | 89.5 ± 26.3 | 91.9 ± 25.6 | 87.0 ± 27.7 |
| Number of years on methadone, m ± sd | 2.8 ± 6.5 | 1.7 ± 2.2 | 4.0 ± 9.1 |
| Anxiety,e m ± sd | 40.7 ± 13.2 | 41.7 ± 14.5 | 39.7 ± 12.3 |
| Depression,f m ± sd | 25.4 ± 14.2 | 27.2 ± 15.3 | 23.5 ± 13.4 |
| Health and functioning,g m ± sd | |||
| Pain | 32.9 ± 25.6 | 28.3 ± 27.6 | 37.5 ± 23.5 |
| Physical functioning | 46.8 ± 28.6 | 47.9 ± 8.7 | 47.9 ± 8.7 |
| Physical limitations | 32.5 ± 40.0 | 23.3 ± 33.4 | 41.7 ± 45.0 |
| Emotional limitations | 43.3 ± 46.4 | 33.3 ± 43.6 | 53.3 ± 48.5 |
| Vitality | 43.8 ± 23.8 | 40.0 ± 26.7 | 47.7 ± 20.6 |
| Well-being | 56.7 ± 28.6 | 52.5 ± 29.5 | 60.8 ± 28.0 |
| Social functioning | 54.6 ± 34.7 | 45.8 ± 38.0 | 63.3 ± 20.4 |
| General Health | 41.4 ± 22.4 | 40.5 ± 25.0 | 42.3 ± 27.7 |
No statistically significant differences existed between-groups on any baseline variables.
MORE = Mindfulness-Oriented Recovery Enhancement
TAU = Treatment as Usual
Participants could report more than one category.
Measured with the Beck Anxiety Inventory. Higher scores indicate greater symptoms of anxiety.
Measured with the Center for Epidemiological Studies Depression Scale. Higher scores indicate greater symptoms of depression.
Measured with subscales of the RAND 36-Item Short Form Health Survey. Higher scores indicate better functioning, health, and well-being and less pain, limitations, and symptom severity or interference.
3.2. Feasibility and engagement
Participants in the MORE arm received a mean of 6.4 (SD=3.9) hours of counseling per week (including MORE and any other counseling services). Specifically, all participants (100%, n=15) completed at least four sessions, with 20% (n=3) completing four, 27% (n=5) completing five, 20% (n=3) completing six or seven, and the remaining 30% (n=5) completing all eight sessions. Given that clinics allowed participants in the MORE group to replace a required TAU group with the MORE group, those who received TAU, on average, received a similar number of counseling hours per week as the MORE group, 6.4 hours (SD=4.1). MORE and TAU participants did not differ significantly on any baseline demographic or clinical variables or amount of counseling time per week. One participant discontinued the MORE intervention after completing five sessions due to an unrelated hospitalization but was retained for follow-up; two participants in the TAU arm discontinued MMT but completed follow-up measures. Participants in the MORE group completed a mean of 23.8 (SD=25.3) homework practice minutes per day, and practiced mindfulness on 55.6% of days.
3.3. Outcomes
Results of baseline-adjusted mixed models for each outcome are reported in Table 2, with within-group tests of change over time reported in Table 3. In sensitivity analyses controlling for age, gender, and duration of MMT, the effects of MORE vs. TAU on all the outcomes reported below remained statistically significant.
Table 2.
Outcome Analyses over 16-weeks (N=30).
| Baseline Adjusted Outcome | MOREa (n=15) m(se) | TAUb (n=15) m(se) | Primary Analysis | Sensitivity Analysis |
|---|---|---|---|---|
| Days of Illicit Drug Use | 6.37 (2.76), [.67, 12.02] | 14.56 (2.77), [8.88, 20.25] | Fl,27.46=4.28, p=0.048 | Fl,24.09=5.86, p=0.023 |
| Days of Illicit Opiate Use | 2.47 (.97), [.47, 4.46] | 5.49 (.97), [3.49, 7.48] | F1,27.62=4.81, p=0.037 | Fl,27.45=5.08, p=0.032 |
| Opioid Cravingc | 15.52 (1.71) | 21.35 (1.72) | F1,26.97=5.76, p=0.024 | F1,24.35=6.95, p=0.014 |
| Paind | 50.76 (5.52) | 26.65 (5.54) | F1,27.05=9.34, p=0.005 | F1,24.26=8.29, p=0.008 |
| Emotional Limitationsd | 67.76 (9.65) | 31.07 (9.67) | F1,26.75=7.11, p=0.013 | F1,23.82=9.577, p=0.005 |
| Physical Limitationsd | 55.88 (9.65) | 26.97 (9.68) | F1,26.29=4.36, p=0.047 | F1,23.62=4.46, p=0.045 |
| Well-beingd | 64.65 (4.39) | 47.83 (4.40) | F1,26.43=7.23, p=0.012 | F1,24.02=11.51, p=0.002 |
| Vitalityd | 57.85 (4.14) | 38.45 (4.15) | F1,27.05=10.79, p=0.003 | F1,24.43=9.34, p=0.005 |
| Social Functioningd | 67.00 (6.27) | 44.69 (6.29) | F1,26.97=6.10, p=0.020 | F1,24.14=5.42, p=0.029 |
| General Healthd | 67.00 (6.27) | 44.69 (6.29) | F1,27.25=0.83, p=0.37 | F1,24.38=1.15, p=0.29 |
| Physical Functiond | 54.44 (5.69) | 45.09 (5.69) | F1,26..58=1.34, p=0.257 | F1,23.98=2.04, p=0.16 |
| Depressione | 34.11 (2.31) | 42.93 (2.34) | Fl,24.82=7.14, p=0.013 | F1,22.07=10.69, p=0.003 |
| Anxietyf | 41.05 (3.09) | 50.83 (3.09) | F1,26.13=4.96, p=0.035 | F1,23.16=6.97, p=0.015 |
MORE = Mindfulness-Oriented Recovery Enhancement
TAU = Treatment as Usual
Measured with the Penn Alcohol Craving Scale, adapted for opioid cravings. Higher scores indicate greater craving.
Measured with subscales of the RAND 36-Item Short Form Health Survey. Higher scores indicate better functioning, health, and well-being and less pain, limitations, and symptom severity or interference.
Measured with the Center for Epidemiological Studies Depression Scale. Higher scores indicate greater symptoms of depression.
Measured with the Beck Anxiety Inventory. Higher scores indicate greater symptoms of anxiety.
Note = The primary analysis reports the fixed effect of treatment. No significant treatment X time effects were observed, indicating that the effect of MORE vs TAU did not differ at 8- and 16-weeks. Sensitivity analyses included age, gender, and duration of methadone treatment as covariates.
Table 3.
Within-Group Change Over Time (N=30).
| MOREa (n=15) Baseline to Post MΔ (SEΔ), p-value | MOREa (n=15) Baseline to Follow-Up MΔ (SEΔ), p-value | TAUb (n=15) Baseline to Post MΔ (SEΔ), p-value | TAUb (n=15) Baseline to Follow-Up MΔ (SEΔ), p- value | |
|---|---|---|---|---|
| Days of Illicit Drug Use | −13.7 (5.9), p=.037 | −16.7 (5.4), p=.009 | −6.4 (4.4.), p=.17 | −6.3 (6.8), p=.37 |
| Days of Illicit Opiate Use | −4.8 (2.3), p=.057 | −4.6 (2.5), p=.08 | 10 (3.2), p=.008 | 10.1 (3.3), p=.008 |
| Opioid Cravingc | −4.9 (8.8), p =.048 | −7.4 (2.5), p=.01 | −0.3 (1.6), p=.87 | −0.6 (1.7), p=.74 |
| Paind | 15.5 (5.0), p=.008 | 250 (8.4), p=.01 | −11.0 (5.8), 2.7 p=.08 | −2.7 (7.9), p=.74 |
| Emotional Limitationsd | 28.9 (12.9), p=.043 | 30.9 (12.3), p=.026 | −13.3 (48.5), p=.31 | −21.4 (13.2), p=.16 |
| Physical Limitationsd | 26.6 (12.3), p=.048 | 30.4 (13.2), p=.038 | −11.6 (11.4), p=.32 | −8.9 (14.0), p=.54 |
| Well-beingd | 9.1 (5.9), p=.15 | 12.3 (6.9), p=.098 | −13.9 (4.3), p=.006 | −3.1 (4.07), p=.45 |
| Vitalityd | 12.6 (5.5), p=.04 | 19.3 (4.9), p=.002 | −7.3 (15.2), p=.08 | −3.9 (6.20), p=.54 |
| Social Functioningd | 20.0 (10.8), p=.09 | 16.1 (7.8), p=.06 | −17.5 (6.9), p=.025 | −8.9 (8.0), p=.29 |
| General Healthd | 1.5 (18.0), p=.78 | 5.8 (5.8), p=.34 | −1.2 (3.2), p=.71 | −0.3 (2.9), p=.92 |
| Physical Functiond | 1.1 (8.2), p=.89 | 17.6 (8.6), p=.06 | 7.0 (6.8), p=.32 | 3.3 (5.9), p=.59 |
| Depressione | 7.2 (2.6), p=.017 | 7.7 (2.6), p=.012 | 18.6 (3.1), p<.001 | 14.9 (2.7), p<.001 |
| Anxietyf | −0.5 (3.5), p=.89 | −0.6 (2.0), p=.78 | 10 (3.9), p=.02 | 10.5 (4.8), p=.046 |
Δ = Change over time (outcome measurement – baseline measurement).
MORE = Mindfulness-Oriented Recovery Enhancement
TAU = Treatment as Usual
Measured with the Penn Alcohol Craving Scale, adapted for opioid cravings. Higher scores indicate greater craving.
Measured with subscales of the RAND 36-Item Short Form Health Survey. Higher scores indicate better functioning, health, and well-being and less pain, limitations, and symptom severity or interference.
Measured with the Center for Epidemiological Studies Depression Scale. Higher scores indicate greater symptoms of depression.
Measured with the Beck Anxiety Inventory. Higher scores indicate greater symptoms of anxiety.
3.3.1. Days of illicit drug use and craving.
Participants in MORE evidenced significantly fewer baseline adjusted days of illicit drug use and significantly lower levels of craving through 16-week follow-up compared to TAU.
3.3.2. Pain, health, and well-being.
Participants in MORE reported significantly lower levels of pain through 16-week follow-up compared to TAU, as well as significantly lower levels of physical and emotional limitations. Conversely, participants in MORE reported significantly higher levels of well-being, vitality (i.e., energy as opposed to fatigue), and social functioning through 16-week follow-up compared to TAU. We observed no significant differences between MORE and TAU for physical functioning and general health through follow-up.
3.3.3. Depression and anxiety.
Participants in MORE reported significantly lower levels of depression and anxiety through 16-week follow-up compared to TAU. Participants in both groups experienced increased symptoms of depression over time, but TAU participants reported a significantly greater increase in depressive symptoms than those in MORE.
4. Discussion
To inform a larger trial, we conducted a Stage 1 RCT to obtain an initial indication of the effects of MORE on drug use, craving, health, and well-being among people receiving MMT in community clinics. Despite the study’s small sample size, participants in MORE reported significantly greater improvements in drug use, craving, mental and physical health, and well-being than those who received TAU only. These findings suggest that MORE could be an effective adjunct to MOUD treatment and larger clinical trials are warranted.
MORE was associated with significantly less illicit drug use, craving, physical pain, and emotional distress over the course of 16-weeks, as compared to TAU. By 16-week follow-up, participants in MORE evidenced an approximate 60% decrease in days of illicit drug use from baseline levels, whereas days of illicit drug use in TAU decreased by only 25%. Using this metric for comparison, in the present pilot RCT, adding MORE to usual care with MMT more than doubled its efficacy. Similarly, in a Stage 2 RCT of MORE (Garland, Manusov, et al., 2014) and a new full-scale clinical trial of MORE (Garland et al., under review) as a treatment for chronic pain among patients on long-term opioid therapy, MORE doubled the efficacy of a supportive psychotherapy control condition on reducing opioid misuse. With regard to illicit opiate use specifically, participants in TAU reported significantly more opiate use days over time, whereas those in MORE reported a mean reduction in opiate use days. As compared to TAU participants, participants in the MORE group also reported lower levels of craving throughout the 16-weeks of the study. Further, based on daily ecological momentary assessment data (EMA) collected in this study (and described elsewhere) we found that individuals who received MORE reported significantly weaker cravings and greater self-control over cravings than those in TAU (Garland, Hanley, Kline, et al., 2019). Further, participants in the MORE group reported less pain severity and interference, less difficulty with daily functioning due to physical or emotional limitations, and less depression and anxiety than those in TAU. It should be noted that participants in both groups experienced increased symptoms of depression over time, but TAU participants reported a significantly greater increase in depressive symptoms than those in MORE, suggesting that MORE may buffer against worsening depression symptom trajectories. Though MORE and TAU did not differ on the general health outcome, the effects of MORE on reduced physical and emotional suffering may have reduced the discomfort that drug use had previously served to alleviate, thereby decreasing participants’ need to use drugs to escape aversive experiences.
The observed effects on illicit drug use, physical pain, and emotional distress may be the result of the mindfulness and reappraisal techniques in MORE designed to enhance cognitive regulation of cue-reactivity and negative emotions. Mindfulness may decrease cue-reactivity by increasing metacognitive awareness of one’s attentional focus and habitual responses, and thereby enable individuals to shift attention to healthy stimuli, choose alternative healthy behaviors to drug use, and break the negative reinforcement cycles that perpetuate illicit drug use (Garland, Froeliger, & Howard, 2014). In that regard, MORE reduces drug cue-reactivity as indexed by changes in attentional bias (Garland, Baker, & Howard, 2017), heart rate responses (Garland, Froeliger, & Howard, 2014), cue-elicited salivation (Hanley & Garland, 2019), and neurophysiological indices (Garland et al., 2019). Further, MORE uses mindfulness to increase metacognitive awareness of the maladaptive thoughts that often feed the physical and emotional suffering leading to drug use; once recognized, mindfulness may then facilitate reappraisal of such maladaptive cognitions (Garland, Farb, Goldin, & Fredrickson, 2015). As opposed to other mindfulness-based interventions that do not focus on restructuring cognitive content, MORE teaches individuals to use mindfulness to first disrupt negative thoughts that promote pain and illicit drug use and then reframe them into adaptive appraisals to reduce suffering and foster adaptive behaviors.
In addition to the effects of MORE on physical pain, emotional distress, and illicit drug use, MORE was also associated with significant increases in positive outcomes, including better social functioning, greater vitality, and improved overall well-being as compared to TAU. These effects may stem from MORE’s emphasis on savoring natural rewards. MORE teaches mindfulness as a means of shifting attention away from drug-related cues and towards naturally rewarding stimuli (e.g., nature, social affiliation). Then, participants are instructed to savor positive emotions, sensations, and thoughts that flow from pleasant experience. This mindful savoring of positive objects and events can replace rumination on negative experience and facilitate positive affect regulation. Indeed, a prior RCT found that positive affect regulation was 2.75 times more likely among participants in MORE as compared to those in an active control condition (Garland et al., 2017). Previous studies of MORE show that mindfully savoring positive, healthy stimuli and events is associated with decreases in opioid craving (Garland, Froeliger, & Howard, 2014; Garland et al., 2015) and opioid misuse (Garland et al., 2017).
The statistically significant findings in this study are striking given the small sample size and limited power of the study. A larger, fully powered study is warranted to determine the mechanisms through which MORE influences outcomes among people on MMT. Additionally, a future study with a longer follow-up period would better demonstrate the long-term effects of MORE as an adjunct to MMT as follow-up was limited to 8-weeks post treatment in the present study. Further, drug use was not biochemically verified, and the present study included only a limited number of patients at two clinics in New Jersey. As such, findings from this study may underestimate drug use and may not generalize to other patients at the same clinics or to patients in other MMT clinics. Finally, these findings are only among individuals who are receiving MMT for an OUD and may not generalize to people receiving other medications for OUD (i.e., buprenorphine or naltrexone) or no MOUD.
Despite these limitations, the results of this study indicate that MORE could be an effective and powerful adjunct to MMT that can help to optimize treatment outcomes among people with OUD. Based on this pilot study, it seems that MORE can be easily integrated into existing MMT settings, and patients are willing and able to participate in the MORE intervention. MORE has the potential to not only decrease illicit drug use, but to better help people cope with craving and distress and increase rewarding and healthy experiences in their lives. Further, the effects of MORE seem to last for at least two months after the treatment period ends. Therefore, larger clinical trials with longer follow-up periods are warranted, and future implementation trials can help to optimize MORE integration into OUD treatment settings.
Highlights.
People who received MORE, as compared to TAU, had less drug use and craving.
People who received MORE, as compared to TAU, had less pain and limitations.
People who received MORE, as compared to TAU, had greater well-being and vitality.
People who received MORE, as compared to TAU, had greater social functioning.
Acknowledgements
This research was supported by grant number R21AT010109 from the National Center for Complimentary and Integrative Health and the National Institute on Drug Abuse. The authors thank Patricia Dooley-Budsock, Candra Savage, April Yeager, Katherine Kneisel, Joshua Antunes, Danisha Moodie, and the staff and clients at the New Brunswick Counseling Center and the Lennard Clinic for their help implementing this study.
Dr. Garland received honoraria and payment for delivering seminars, lectures, and teaching engagements (related to training clinicians in MORE and mindfulness) sponsored by institutions of higher education, government agencies, academic teaching hospitals, and medical centers. Dr. Garland also receives royalties from the sale of books related to MORE. Declarations of interest by other authors: none.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, & Fiore MC (2004). Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev, 111(1), 33–51. 10.1037/0033-295X.111.1.33 2004–10332-002 [pii] [DOI] [PubMed] [Google Scholar]
- Bao YP, Liu ZM, Epstein DH, Du C, Shi J, & Lu L (2009). A meta-analysis of retention in methadone maintenance by dose and dosing strategy. Am J Drug Alcohol Abuse, 35(1), 28–33. 10.1080/00952990802342899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, & Steer RA (1988). An inventory for measuring clinical anxiety: psychometric properties [Research Support, Non-U.S. Gov’tResearch Support, U.S. Gov’t, P.H.S.]. J Consult Clin Psychol, 56(6), 893–897. [DOI] [PubMed] [Google Scholar]
- Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, & Westlake L (1992). Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ, 305(6846), 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody TP, Vieten C, & Astin JA (2007). Negative affect, emotional acceptance, and smoking cessation. J Psychoactive Drugs, 39(4), 499–508. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, & Ellis MS (2017). The prescription opioid epidemic: a review of qualitative studies on the progression from initial use to abuse. Dialogues Clin Neurosci, 19(3), 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperman NA, Richter KP, Bernstein SL, Steinberg ML, & Williams JM (2015). Determining Smoking Cessation Related Information, Motivation, and Behavioral Skills among Opiate Dependent Smokers in Methadone Treatment. Subst Use Misuse, 50(5), 566–581. 10.3109/10826084.2014.991405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, & Borsook D (2016). Common Brain Mechanisms of Chronic Pain and Addiction. Neuron, 89, 11–36. 10.1016/j.neuron.2015.11.027 [DOI] [PubMed] [Google Scholar]
- Eyler ECH (2013). Chronic and acute pain and pain management for patients in methadone maintenance treatment. American Journal on Addictions, 22(1), 75–83. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Volpicelli JR, & Pettinati HM (1999). Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res, 23(8), 1289–1295. [PubMed] [Google Scholar]
- Garland E, Froeliger B, & Howard M (2014). Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Frontiers in psychiatry, 4, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL (2013). Mindfulness-Oriented Recovery Enhancement for Addiction, Stress, and Pain. NASW Press. [Google Scholar]
- Garland EL (2016). Restructuring reward processing with Mindfulness-Oriented Recovery Enhancement: novel therapeutic mechanisms to remediate hedonic dysregulation in addiction, stress, and pain. Annals of the New York Academy of Sciences, 1373, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Atchley RM, Hanley AW, Zubieta JK, & Froeliger B (2019). Mindfulness-Oriented Recovery Enhancement remediates hedonic dysregulation in opioid users: Neural and affective evidence of target engagement. Sci Adv, 5(10), eaax1569. 10.1126/sciadv.aax1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, & Howard MO (2014). Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology, 231(16), 3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, & Howard MO (2015). Neurophysiological evidence for remediation of reward processing deficits in chronic pain and opioid misuse following treatment with Mindfulness-Oriented Recovery Enhancement: exploratory ERP findings from a pilot RCT. Journal of behavioral medicine, 38(2), 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Zeidan F, Partin K, & Howard MO (2013). The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neuroscience & Biobehavioral Reviews. 10.1016/j.neubiorev.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Hanley AW, Kline A, & Cooperman NA (2019). Mindfulness-Oriented Recovery Enhancement reduces opioid craving among individuals with opioid use disorder and chronic pain in medication assisted treatment: Ecological momentary assessments from a stage 1 randomized controlled trial. Drug Alcohol Depend, 203, 61–65. 10.1016/j.drugalcdep.2019.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Hanley AW, Riquino MR, Reese SE, Baker AK, Salas K, Yack BP, Bedford CE, Bryan MA, Atchley R, Nakamura Y, Froeliger B, & Howard MO (2019). Mindfulness-oriented recovery enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial. J Consult Clin Psychol, 87(10), 927–940. 10.1037/ccp0000390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Howard MO, Zubieta J-K, & Froeliger B (2017). Restructuring hedonic dysregulation in chronic pain and prescription opioid misuse: effects of mindfulness-oriented recovery enhancement on responsiveness to drug cues and natural rewards. Psychotherapy and Psychosomatics, 86, 111–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, & Howard MO (2014). Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. Journal of Consulting and Clinical Psychology, 82(3), 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Roberts-Lewis A, Tronnier CD, Graves R, & Kelley K (2016). Mindfulness-Oriented Recovery Enhancement versus CBT for co-occurring substance dependence, traumatic stress, and psychiatric disorders: proximal outcomes from a pragmatic randomized trial. Behaviour Research and Therapy, 77, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Brintz CE, Hanley AW, Roseen EJ, Atchley RM, Gaylord SA, Faurot KR, Yaffe J, Fiander M, & Keefe FJ (2020). Mind-body therapies for opioid-treated pain: A systematic review and meta-analysis. JAMA Intern. Med, 180 (1), 91–105. 10.1001/jamainternmed.2019.4917. January 1 31682676. PMC6830441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Farb NA, Goldin P, & Fredrickson BL (2015). Mindfulness broadens awareness and builds eudaimonic meaning: A process model of mindful positive emotion regulation. Psychol. Inq, 26(4), 293–314. 10.1080/1047840X.2015.1064294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Lloyd A, Carroll FI, Damaj MI, & Koob GF (2011). Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology (Berl), 213(4), 715–722. 10.1007/s00213-010-2024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Tucker RP, Greene PA, Davidson RJ, Wampold BE, Kearney DJ, & Simpson TL (2018). Mindfulness-based interventions for psychiatric disorders: A systematic review and meta-analysis. Clin. Psychol. Rev, 59, 52–60. 10.1016/j.cpr.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH, McGrath F, & Dubner R (1978). Ratio scales of sensory and affective verbal pain descriptors. Pain., 5(1), 5–18. 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- Hanley AW, & Garland EL (2019). The Mindfulness-Oriented Recovery Enhancement Fidelity Measure (MORE-FM): Development and Validation of a New Tool to Assess Therapist Adherence and Competence. J Evid Based Soc Work, 1–15. 10.1080/26408066.2020.1833803 [DOI] [PubMed] [Google Scholar]
- Hilton L, Hempel S, Ewing BA, Apaydin E, Xenakis L, Newberry S, … Maglione MA (2017). Mindfulness meditation for chronic pain: Systematic review and meta-analysis. Ann. Behav. Med, 51(2), 199–213. 10.1007/s12160-016-9844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ (1997). The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry, 4(5), 231–244. 10.3109/10673229709030550 [DOI] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2008). Addiction and the brain antireward system. Annu Rev Psychol, 59, 29–53. 10.1146/annurev.psych.59.103006.093548 [DOI] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2009). Neurocircuitry of addiction Neurocircuitry of addiction, 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, & Zvolensky MJ (2015). Anxiety, depression, and cigarette smoking: a transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychol Bull, 141(1), 176–212. 10.1037/bul0000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Howard MO, Garland EL, McGovern P, & Lazar M (2017). Mindfulness treatment for substance misuse: A systematic review and meta-analysis. J. Subst. Abus. Treat, 75, 62–96. 10.1016/j.jsat.2017.01.008. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, & Argeriou M (1992). The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat, 9(3), 199–213. 10.1016/0740-5472(92)90062-s [DOI] [PubMed] [Google Scholar]
- Naji L, Dennis BB, Bawor M, Plater C, Pare G, Worster A, Varenbut M, Daiter J, Marsh DC, & Desai D (2016). A prospective study to investigate predictors of relapse among patients with opioid use disorder treated with methadone. Substance abuse: research and treatment, 10, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Robinson TE, & Berridge KC (2008). Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci, 363(1507), 3137–3146. 10.1098/rstb.2008.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville BJ, Carroll KM, & Onken LS (2001). A stage model of behavioral therapies research: Getting started and moving on from stage I. Clin. Psychol. Sci. Pract, 8(2), 133–142. 10.1093/clipsy.8.2.133. [DOI] [Google Scholar]
- Shurman J, Koob GF, & Gutstein HB (2010). Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain medicine (Malden, Mass.), 11, 1092–1098. 10.1111/j.1526-4637.2010.00881.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2019). Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Retrieved November 13, 2020 from https://www.samhsa.gov/data/
- Tsui JI, Anderson BJ, Strong DR, & Stein MD (2014). Craving predicts opioid use in opioid-dependent patients initiating buprenorphine treatment: a longitudinal study. Am J Drug Alcohol Abuse, 40(2), 163–169. 10.3109/00952990.2013.848875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, & Bechara A (2009). A somatic marker theory of addiction. Neuropharmacology, 56 Suppl 1, 48–62. 10.1016/j.neuropharm.2008.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, & Shiffman S (1985). Coping and substance use: A conceptual framework. In Shiffman S & Wills TA (Eds.), Coping and Substance Use. Academic Press. [Google Scholar]


