Abstract
Arnebia decumbens (Vent.) Coss. et Kralik, A. euchroma (Royle) Johnst and A. guttata Bunge, three commonly used traditional Chinese medicinal plants have been widely used for the clinical treatment of inflammatory diseases caused by fungal, bacterial, oxidation, and other related pathogens. However, precise identification at the similar species level is usually challenging due to the influence of the source of medicinal materials, traditional ethnic medicine and medicinal habits. Here we developed a comprehensive and efficient identification system for three source spices of Arnebiae Radix based on DNA barcoding and HPLC fingerprinting. A total of 599 samples from thirty-five wild populations were collected and identified by using DNA barcodes of ITS2 regions, and the chemotypes of seven naphthoquinoneswere revealed by HPLC quantitative analysis including principal component analysis and hierarchical clustering analysis. Our results showed that the ITS2 sequences can distinguish three source spices of Arnebiae Radix from adulterants. However, it was difficult to identify them by HPLC-specific chromatograms combined with chemometric analysis. These results indicated that DNA barcoding was a more powerful method than HPLC fingerprinting for the identification of related species that were genetically similar. DNA barcoding analysis could be a promising and reliable tool to accurately confirm the identities of medicinal materials, especially for those whose sources are multiple and difficult to be identified by conventional chromatography.
Keywords: Arnebiae Radix, DNA barcoding, ITS2, HPLC, identification
Introduction
Arnebiae Radix (Zicao in Chinese), a kind of traditional Chinese medicine, is the dried root bark of A. euchroma (Royle) Johnst. and A. guttata Bunge in the Chinese Pharmacopoeia (2020 version). Shikonin and its derivatives, red naphthoquinones, are widely found in the epidermis of the roots of Arnebiae Radix (Zhan et al., 2015) and have been widely demonstrated to possess various biological activities, such as anti-inflammatory (Fu et al., 2016; Sun et al., 2017; Guo et al., 2019), antibacterial (Zhao et al., 2017; Huang et al., 2020), and antiangiogenic (Liu C. et al., 2020) activities. Recently, it was reported that shikonin and its derivatives could induce apoptosis of many types of cancer cells and exhibit anticancer activities and antitumorigenic properties (Liao et al., 2020). Amebia Radix has been widely used in the medicine, printing and dyeing industry, cosmetics and food industries (Xu et al., 2014; Ma et al., 2021).
Furthermore, there are other plants of the genus Arnebia (Boraginaceae) that are also named Zicao in Xinjiang of China, such as A. decumbens (Vent.) Coss. et Kralik and A. tschimganica (Fedtsch.) G. L. Chu (Jia-Xin et al., 2018). The market for Arnebiae Radix is complicated due to the influence of the source of medicinal materials, traditional ethnic medicine and medicinal habits. Thus, it is difficult to identify the authenticity of medicinal Arnebiae Radix, and the identification problem needs to be solved urgently.
Currently, DNA barcoding has emerged as an effective tool for the identification of traditional Chinese medicine due to its species specificity. DNA barcoding has been performed to recognize animals, plants, and fungi (Chen et al., 2014; Gunnels et al., 2020; Behrens-Chapuis et al., 2021; Selva Pandiyan et al., 2020). As a valuable tool for biological identification, DNA barcoding can identify species efficiently and conveniently (Yu et al., 2021). Chen et al. found that the internal transcribed spacer 2 (ITS2) region can potentially be used as a standard DNA barcode to identify medicinal plants and their closely related species (Chen et al., 2010). ITS2 can serve as a novel universal barcoding for the identification of a broader range of plant taxa (Liu et al., 2012; Zhang et al., 2018; Khan et al., 2019). Moreover, high-performance liquid chromatography (HPLC) specific chromatograms, which can effectively determine the content of compounds, are widely used for authenticity confirmation and quality control of traditional Chinese medicines (Hu et al., 2020; Liu B. et al., 2020). Some researchers have indicated that the chemical components of different Arnebiae Radix vary, some of which may have good bioactivities (Feng et al., 2020; Liao et al., 2020; Mei et al., 2020).
Thus, authenticity assurance is crucial for their quality control. In this study, we intended to use the DNA barcoding technique and HPLC-specific chromatograms to identify three source spices of Arnebiae Radix. The results will facilitate exploring the genetic basis of chemical variations and developing strategies for the utilization and conservation of Arnebiae Radix.
Materials and Methods
Plant Materials
A total of 599 samples from thirty-five wild populations were collected and analyzed in this study (Table 1), including A. decumbens (Vent.) Coss. et Kralik (Ad:120 individuals from eight wild populations), A. euchroma (Royle) Johnst (Ae: 227 individuals from 13 wild populations), and A. guttata Bunge (Ag: 252 individuals from 13 wild populations). This study contained most of the Arnebiae radix species in Taiwan and China but did not include C. quinquesecta, because the species is a critically endangered medicinal plant and was not found in the field. Sampling from plantation populations or within short distances was avoided (>50 km). All samples were dried and stored immediately in silica gel after collection. Voucher specimens were deposited at Xinjiang Medical University. The geographic localities of each sampled population were determined using a Garmin GPS unit (Table 1).
TABLE 1.
Sample information of Arnebiae Radix and its adulterants in this study.
| Taxon | Sample | Locality | Longitude(E) | Latitude(N) | Altitude(m) | Sample Size |
|---|---|---|---|---|---|---|
| A. decumbens (vent.) coss. et kralik | Ad(FK) | Fukang city | 88°17′34″ | 44°24′39″ | 487 | 18 |
| Ad(TKS) | Tekesi county | 81°54′41.78″ | 43°12′10.64″ | 1375 | 16 | |
| Ad(MQ) | Miquan county | 87°26′47″ | 44°36′42″ | 707 | 15 | |
| Ad(KLMY) | Kelamayi city | 84°57′40″ | 45°11′48″ | 436 | 18 | |
| Ad(WS) | Wusu county | 84°57′40″ | 45°11′48″ | 379 | 15 | |
| Ad(SHZ) | Shihezi city | 86°14′28″ | 45°1′42″ | 472 | 15 | |
| Ad(BEJ) | Buerjin county | 86°92′34.51″ | 47°07′33.08″ | 497 | 11 | |
| Ad(SHW) | Shawan county | 85°55′17″ | 44°55′52″ | 564 | 12 | |
| Ae(WLMQ) | Wulumuqi county | 87°07′28.67″ | 43°17′19″ | 2,507 | 14 | |
| Ae(ML) | Mulei county | 90°31′09.79″ | 43°33′21.42″ | 2,568 | 5 | |
| Ae(BCH) | Baicheng county | 81°84′55.37″ | 41°82′11.06″ | 2,606 | 20 | |
| Ae(NLT) | Nalati town | 83°56′10.920″ | 43°10′11.24″ | 2,500 | 16 | |
| Ae(TSHKEG) | Tashikuergan county | 75º04′52.2″ | 37º49′58.7″ | 4,234 | 18 | |
| A. euchroma (royle) Johnst | Ae(ATSH) | Atushi county | 76°12′33.08″ | 39°27′43.50″ | 2,300 | 20 |
| Ae(WQ) | Wenquan county | 80°32′18″ | 45°2′18″ | 2,299 | 19 | |
| Ae(HJ) | Hejing county | 84º07′13.3″ | 42º42′00.5″ | 2,456 | 19 | |
| Ae(HCH) | Huocheng county | 81°09′53.83″ | 44°27′32.39″ | 2,502 | 21 | |
| Ae(JH) | Jinghe county | 83º15′44.7″ | 44º23′42.6″ | 2,144 | 19 | |
| Ae(GL) | Gongliu county | 82°23′19.10″ | 43°35′42.30″ | 2,530 | 19 | |
| Ae(AKS) | Akesu county | 80°29′31.22″ | 41°15′33.28″ | 2030 | 19 | |
| Ae(XY) | Xinyuan county | 83°27′66.19″ | 43°41′55.40″ | 2,201 | 18 | |
| Ag(HM) | Hami county | 93°50′53.05″ | 43°07′42.63″ | 861 | 18 | |
| Ag(FY) | Fuyun county | 89°1′2″ | 45°2′46″ | 1065 | 22 | |
| Ag(BLK) | Balikun county | 91°39′48.60″ | 43°47′25,6″ | 1632 | 17 | |
| Ag(NLK) | Nileke county | 82°10′3″ | 43°36′41″ | 765.4 | 16 | |
| Ag(TSHKEG) | Tashikuergan county | 75º28′58.3″ | 37º13′44.7″ | 3,780 | 14 | |
| Ag(QT) | Qitai county | 091°22′52.9″ | 44°58′0.5″ | 1167 | 19 | |
| Ag(HJ) | Hejing county | 86°0′23.56″ | 43°01′1.89″ | 2,253 | 17 | |
| A. guttata bunge | Ag(YW) | Yiwu county | 94°48′51.30″ | 43°19′25.3″ | 1414 | 19 |
| Ag(SHSH) | Shanshan county | 89°56′37.05″ | 43°06′2,0″ | 653 | 15 | |
| Ag(TL) | Tuoli county | 82°34′40″ | 45°35′0″ | 1532.3 | 17 | |
| Ag(ML) | Mulei county | 091°23′22.5″ | 45°03′20″ | 1312 | 23 | |
| Ag(QH) | Qinghe county | 90°22′25″ | 45°33′7″ | 1193 | 20 | |
| Ag(WQ) | Wenquan county | 81°8′18″ | 44°46′32″ | 1801.7 | 16 | |
| Ag(XY) | Xinyuan county | 82°29′45″ | 43°24′17″ | 894.1 | 19 |
Chemical Apparatus
Chemical standards including (β,β-dimethylacryl)shikonin (15102821), alkannin (15102721), deoxyalkannin (15062422) and acetylshikonin (15120431) were purchased from Tauto Biotech (Shanghai, China). β-Acetoxyisovalerylalkannin (P05M7F14235) was purchased from Yuanye Biotech (Shanghai, China). Isobutylshikonin (wkq16101302) was purchased from Weikeqi Biotech (Sichuan, China). (2-Methyl-n-nbutyl) shikonin (AV51-LDQR) was purchased from Tokyo Chemical Industry (Tokyo, Japan). The purity of the standards was above 98%. The petroleum ether (60–90°C) was analytically pure. All of the chemicals and reagents used in this study were of HPLC analytical grade.
DNA Extraction, PCR Amplification and DNA Sequencing
The material specimens were dried by natural methods, and 20 mg of dried plant material was used for DNA extraction. Genomic DNA was extracted with a DNA Secure Plant Kit from Tiangen Biotech (Beijing, China). The relative purity and concentration of extracted DNA were estimated by ethidium bromide staining on agarose gels and compared with known DNA concentration markers.
The extracted genomic DNA was amplified by polymerase chain reaction (PCR), using the ITS2 (ITS2F, 5′-ATGCGATACTTGGTGTGAAT-3′ and ITS2R, 5′-GACGCTTCTCCAGACTACAAT-3′). PCR amplifications were carried out in a volume of 20 μL using 1 μL of template DNA (50–100 ng), 2 μL of 10 × reaction buffer, 1.6 μL of dNTP mix (2.5 mM), 1.25 μL of 10 μM of each primer, 0.2 μL of Ex-Taq DNA polymerase (Takara Shuzo Co., Ltd., Otsu, Japan), and 12.7 μL of sterile distilled water. Reactions were run on a Veriti thermocycler (Applied Biosystems, United States). The PCR conditions for amplification consisted of one cycle of denaturation at 95°C for 5 min, 35 cycles of 1 min of denaturation at 94°C, 1 min of annealing at 55°C and 1 min 30 s of extension at 72°C, followed by an 8 min extension step at 72°C. PCR products were electrophoresed on 1.5% (w/v) agarose gels and purified through precipitation with 95% ethanol and 3 M sodium acetate (pH 5.2). All the purified PCR products were sequenced directly in both directions on an ABI 3730XL automated sequencer (Applied Biosystem, Foster City, CA, United States).
DNA Barcoding Analysis
Cutting and splicing of all ITS2 sequences, removal of the primer region and low-quality regions, manual correction, and stitching were performed by ContigExpress software. Then, the modified ITS2 sequences were submitted to DNAMAN software to compare the similarities of the samples. Finally, the modified ITS2 sequences were aligned, and the inter/intraspecific genetic distances were measured using MEGAX 10.2.4 software. A phylogenetic tree using GenBank sequences as outgroups was constructed based on standard parameters with bootstrap testing of 1,000 replicates.
HPLC Conditions
The medicinal powder precisely was weighed to approximately 1 g and placed in an Erlenmeyer flask; 50 ml of petroleum ether (60–90°C) was added. Then, the sample was accurately weighed and extracted with 30 min ultrasonication steps. After cooling and adding petroleum ether (60–90°C) to compensate for the decrease in weight, the sample was filtered. The resulting filtrate was measured (10 ml of A. euchroma, 30 ml of A. guttata Bunge, 30 ml of A. tschimganica, and 30 ml of A. decumbens (Vent.) Coss. et Kralik), evaporated to dryness, dissolved the residue in acetonitrile, transferred to a 10 ml volumetric flask, and dissolved in acetonitrile. The sample was then transferred to a 10 ml volumetric flask, diluted with acetonitrile to scale, shaken to mix well and prepared for analysis. HPLC chromatographic conditions were conducted as described by Ding et al. (Ding et al., 2019).
Chemometric Analysis
The reference chromatogram was generated using a Similarity Evaluation System for Chromatographic Fingerprint of TCM (Version 2012). Principal component analysis (PCA) and hierarchical clustering analysis (HCA) were performed by the professional software SIMCA 14.1 to demonstrate the variability of the relative peak areas of bioactive compounds. Outliers were identified and removed by DmodX.
Results
Genetic Divergence Determination
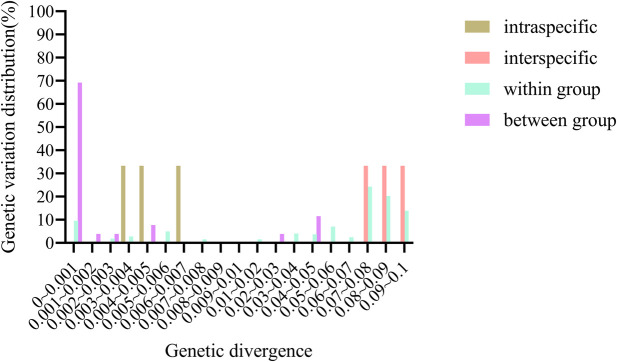
The length of the aligned ITS2 sequences was 399 bp, and the number of variable sites was 71 (Table 2). We used four parameters to characterize divergence. The intraspecific distance of ITS2 was 0.0025–0.006, and the interspecific distance was 0.0745–0.0915. Additionally, after grouping samples according to locality, the genetic distance within a group was 0–0.0494, and the genetic distance between groups was 0–0.0941. The analysis of the distribution of genetic distance (Figure 1) showed obvious barcoding gaps between samples, indicating that the ITS2 sequence has a strong ability to identify Arnebia genus samples at the species level.
TABLE 2.
Variable sites of the ITS2 region of the three Arnebiae Radix species.
| Site | Ad | Ae | Ag |
|---|---|---|---|
| 1 | −/A | −/A | −/A/G |
| 2 | −/A | −/G | −/A/G |
| 13 | C | C | C/T |
| 26 | C/T | C | C |
| 40 | C | C/T | C/T |
| 41 | G | A | A/G |
| 42 | C | C/T | C |
| 44 | T | T | A/T |
| 45 | C | C/T | A/C |
| 46 | G | C/G | G/T |
| 52 | C | C | C/T |
| 60 | C | A | A/C |
| 63 | A | A | A/T |
| 64 | G | A | A/G |
| 66 | A | A | A/G |
| 67 | C | A/C | A |
| 68 | T | T | C/T |
| 69 | T | G | G/T |
| 70 | G | T | A/T |
| 71 | C | T | T |
| 77 | T | G/T | C/T |
| 98 | G | G | A/G |
| 101 | C | T | T |
| 102 | C/T | T | T |
| 103 | G | G | G/T |
| 104 | G | A | A/T |
| 118 | A/G | G | A/G |
| 122 | A/T | T | T |
| 124 | C/T | C | C/T |
| 130 | C/T | C | C/T |
| 137 | G/T | T | G/T |
| 149 | A/G/T | A | A/T |
| 180 | A/C | A | A/C |
| 184 | C/T | C/T | C |
| 194 | A/G | A/G | A |
| 200 | A/G | G | A/G |
| 201 | C/T | T | T |
| 202 | G/T | G | G/T |
| 203 | C/G/T | T | C/T |
| 207 | C/T | C | C/T |
| 208 | C/G | G | G |
| 210 | A/G | A | A/G |
| 218 | A/T | A | A |
| 219 | G/T | G/T | G/T |
| 223 | C/T | C | C/T |
| 226 | G/T | G | G/T |
| 227 | C/T | C | C/T |
| 231 | C/T | C | C/T |
| 232 | A/C | C | A/C |
| 233 | A/G | A | A |
| 236 | C/T | T | C/T |
| 238 | G/T | G | G/T |
| 239 | C/T | T | T |
| 240 | G/T | T | G/T |
| 241 | C/G/T | C | C/T |
| 252 | C/T | C | C/T |
| 254 | G/T | G | G/T |
| 261 | C/G | C/G | G |
| 263 | C/T | C | C/T |
| 264 | C/T | C | C/T |
| 267 | C/G | C/G | G |
| 286 | C/T | T | C/T |
| 348 | A/G | A/G | G |
| 359 | C/T | C | C |
| 371 | A/T | A | A/T |
| 380 | −/G/T | G/T | −/T |
| 389 | −/A/G | G | −/G |
| 390 | −/A/G | G | −/A/G |
| 391 | −/C/T | C | −/C/T |
| 396 | −/C/T | C/T | −/C/T |
| 399 | −/G/T | −/T | −/G/T |
Ad, A. decumbens (Vent.) Coss. et Kralik; Ae, A. euchroma (Royle) Johnst; Ag, A. guttata Bunge; -missing variant site.
FIGURE 1.
Genetic divergence of the ITS2 region of the three Arnebiae Radix species.
Identification of Arnebiae Radix by DNA Barcoding
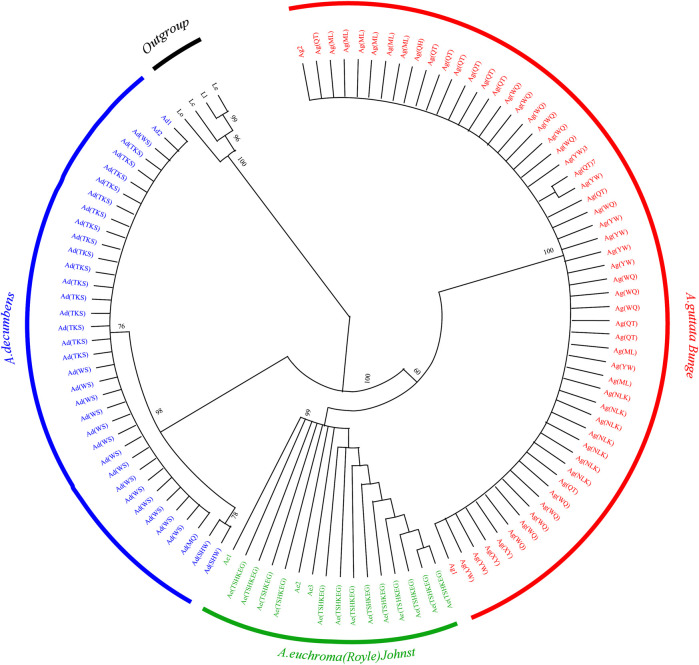
To identify the species of the 599 Arnebiae Radix samples more accurately and visually, we constructed a neighbor-joining tree based on the ITS2 sequences obtained from the samples and four ITS2 sequences of Boraginaceae downloaded from NCBI (Supplementary Table S1). The ITS2 sequences were divided into mutually exclusive monophyletic clades. A. guttata Bunge clustered into one subgroup, A. euchroma (Royle) Johnst could be clustered into one group, and A. decumbens (Vent.) Coss. et Kralik could be clustered into one group (Figure 2). The pairwise distance analysis supports this interpretation, revealing that ITS2, as a barcode, is able to distinguish between the three species of Arnebiae Radix.
FIGURE 2.
Results of the neighbor-joining tree based on ITS2 sequences of the 599 Arnebiae Radix samples. Supporting values (>50%) are indicated above or below the relevant branches. Some subtrees are compressed to show the samples completely in a picture. Ad, A. decumbens (Vent.) Coss. et Kralik; Ae, A. euchroma (Royle) Johnst; Ag, A. guttata Bunge. Ad1:17 samples of Ad(FK),18 samples of Ad(KLMY),nine samples of Ad(SHW) and 15 samples of Ad(SHZ); Ad2:11 samples of Ad(BEJ),14 samples of Ad(MQ),one samples of Ad(SHW), and one sample of Ad(FK);Ae1:19 samples of Ae(WQ), 14 samples of Ae(WLMQ), 19 samples of Ae(HJ), 16 samples of Ae(NLT),15 samples of Ae(XY), 20 samples of Ae(JH), 21 samples of Ae(HCH), 19 samples of Ae(GK) and 20 samples of Ae(ATSH);Ae2: six samples of Ae(TSHKEG);Ae3: five samples of Ae(ML) ,20 samples of Ae(BCH) and 19 samples of Ae(AKS);Ag1: nine samples of Ag(NLK), and 17 samples of Ag(XY);Ag2: four samples of Ag(TL), 14 samples of Ag(HJ), three samples of Ag(SHSH), and one samples of Ag(TSHKEG).
HPLC Fingerprint of Arnebiae Radix Samples
The results of the determination of seven naphthoquinones in the roots of three species of Arnebiae Radix from different habitats in Xinjiang are shown in Table 3. Naphthoquinones were found in the roots of all three Arnebiae Radix species, and there were differences among different species. The total amount of naphthoquinones in Ae was the highest (43.3505 mg/g), followed by that in Ag (11.4042 mg/g), and the lowest amount (6.0462 mg/g) was found for Ad. It was assumed that Ad was an annual herb, the rest were perennial herbs, and the accumulation of each component in plants was different. Ag and Ae both contain seven components, while β-acetoxyisovaleryl acarnin β and β'-dimethylacrylamine were not detected in Ad. According to the Chinese Pharmacopoeia (2020 edition), the content of β,β'-dimethylacrylamine in Arnebiae Radix should not be less than 0.30%. This study found that this component in Ae and Ag met the pharmacopoeia requirements. This study will provide a reference basis for exploring new drug sources.
TABLE 3.
Content of seven bioactive components of the 35 Arnebiae Radix samples. (mg/g).
| L-shikonin | Acetylshikonin | β-Acetoxyisovaleryl acarnin | Deoxyshikonin | Isobutyryl shikonin | β. β′-dimethylacrylamine | 2-methylbutyl Shikonin | Total | |
|---|---|---|---|---|---|---|---|---|
| Ag(HM) | − | 0.69 | 1.0 | − | − | 0.84 | 1.5 | 4.0 |
| Ag(FY) | − | 0.46 | 0.74 | − | − | 0.67 | 1.1 | 3.0 |
| Ag(BLK) | − | 1.1 | 0.87 | 0.26 | − | 0.65 | 1.2 | 4.1 |
| Ag(NLK) | 0.10 | 1.6 | 3.0 | 0.19 | 0.38 | 0.73 | 2.4 | 8.4 |
| Ag(TSHKEG) | 0.57 | 8.1 | 9.8 | 0.58 | 2.0 | 1.3 | 11 | 34 |
| Ag(QT) | 0.12 | 2.2 | 1.3 | 0.29 | − | 1.6 | 1.9 | 7.3 |
| Ag(HJ) | 0.11 | 1.6 | 3.2 | 0.21 | 0.55 | 0.95 | 4.0 | 11 |
| Ag(YW) | − | 0.96 | 1.0 | 0.23 | − | 0.82 | 1.4 | 4.5 |
| Ag(SHSH) | 0.94 | 2.9 | 14 | 0.21 | 1.8 | 1.33 | 15 | 36 |
| Ag(TL) | − | 1.3 | 1.2 | − | − | 0.44 | 1.0 | 4.0 |
| Ag(ML) | 0.15 | 3.3 | 0.81 | 0.23 | − | 0.55 | 1.1 | 6.1 |
| Ag(QH) | 0.14 | 1.5 | 1.6 | 0.26 | − | 1.31 | 2.1 | 6.9 |
| Ag(WQ) | − | 0.61 | 1.2 | − | − | 0.42 | 0.72 | 3.0 |
| Ag(XY) | 0.13 | 4.0 | 3.5 | 0.49 | 0.62 | 1.33 | 5.7 | 16 |
| Average | 0.28 | 2.2 | 3.1 | 0.29 | 1.1 | 0.92 | 3.6 | 11 |
| Ae(WLMQ) | 0.23 | 4.4 | 1.9 | − | 1.7 | 4.6 | 7.9 | 21 |
| Ae(ML) | 0.21 | 2.8 | 1.9 | − | 1.4 | 3.0 | 7.1 | 16 |
| Ae(BCH) | 0.25 | 2.7 | 1.3 | 0.23 | 1.2 | 3.0 | 6.6 | 15 |
| Ae(NLT) | 0.68 | 22 | 3.6 | 0.67 | 6.0 | 11 | 32 | 76 |
| Ae(TSHKEG) | 0.19 | 1.4 | 8.4 | − | 3.2 | 0.60 | 4.1 | 18 |
| Ae(ATSH) | 0.67 | 9.1 | 2.7 | 0.30 | 2.9 | 5.5 | 14 | 35 |
| Ae(WQ) | 1.07 | 9.4 | 3.7 | 0.37 | 8.6 | 8.0 | 17 | 48 |
| Ae(HJ) | 0.54 | 18.9 | 3.1 | 0.63 | 4.4 | 12 | 24 | 63 |
| Ae(HCH) | 0.68 | 12.6 | 5.4 | 0.36 | 7.1 | 4.1 | 17 | 47 |
| Ae(JH) | 0.75 | 12.2 | 4.7 | 0.69 | 6.8 | 5.1 | 15 | 46 |
| Ae(GL) | 0.80 | 20.0 | 4.2 | 0.64 | 4.2 | 14 | 26 | 70 |
| Ae(AKS) | 0.36 | 9.0 | 1.7 | − | 2.3 | 4.2 | 11 | 29 |
| Ae(XY) | 0.84 | 25.2 | 3.8 | 0.90 | 5.6 | 13 | 29 | 78 |
| Average | 0.56 | 11.5 | 3.6 | 0.53 | 4.2 | 6.8 | 16 | 43 |
| Ad(FK) | − | 4.4 | − | 0.25 | 0.43 | − | 3.3 | 8.4 |
| Ad(TKS) | − | 4.1 | − | 0.93 | 0.22 | − | 1.2 | 6.4 |
| Ad(MQ) | − | 1.7 | − | 0.29 | 0.14 | − | 0.81 | 3.0 |
| Ad(KLMY) | 0.13 | 8.2 | − | 0.66 | 0.32 | − | 2.4 | 12 |
| Ad(WS) | − | 2.5 | − | 0.21 | 0.25 | − | 1.8 | 4.7 |
| Ad(SHZ) | − | 3.1 | − | 0.42 | 0.25 | − | 1.3 | 5.0 |
| Ad(BEJ) | − | 2.4 | − | 0.20 | 0.32 | − | 2.4 | 5.3 |
| Ad(SHW) | − | 1.5 | − | 0.20 | 0.22 | − | 0.92 | 2.8 |
| Average | 0.13 | 3.5 | − | 0.39 | 0.27 | − | 1.8 | 6.0 |
Ad, A. decumbens; Ae, A. euchroma (Royle) Johnst; Ag, A. guttata Bunge.
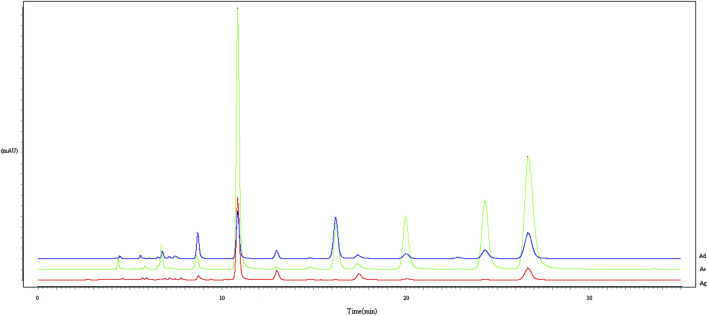
To establish the chromatographic fingerprint, 35 Arnebiae Radix samples from different species were analyzed under the optimized chromatographic analysis conditions. All chromatograms were matched through multipoint correction and free matching, and the bottom sample was the reference sample (Supplementay Figures S1, S2). The representative HPLC fingerprints were so similar that it was difficult to separate the three spaces visually (Figure 3).
FIGURE 3.
Representative HPLC fingerprints of three Arnebiae radix species. Ad, A. decumbens; Ae, A. euchroma (Royle) Johnst; and Ag, A. guttata Bunge.
Principal Component Analysis of HPLC Fingerprint of Arnebiae Radix Samples
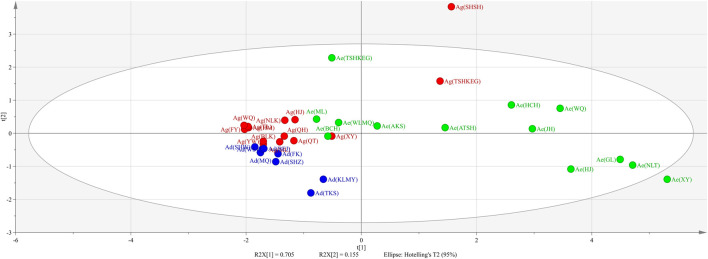
PCA, a multivariate method, is widely used in data analysis to summarize variation, and is implemented as a data-reduction technique to generate a visual scatter plot for the qualitative evaluation of similarities and differences within multivariate data. To differentiate all the Arnebiae Radix samples clearly, we carried out PCA according using the data for the seven common characteristic peaks. The score plot was structured based on the first three principal components, which accounted for more than 94.3% of the total variability. We discarded the other principal components because they had little effect on the model. The results showed that all samples were divided into six groups according to their different sources (Figure 4). Group 1 contained two samples belonging to Ag, Group 2 contained four samples belonging to Ae, and Group 3 contained four samples belonging to Ae. Group 4 included five samples belonging to Ae, Group 5 included two samples belonging to Ad, and Group 6 included 12 samples belonging to Ag and six samples belonging to Ad. The results were consistent with the HPLC fingerprint analysis. The results of the HPLC-specific chromatograms combined with PCA were not as accurate as those of DNA barcoding.
FIGURE 4.
Score plot of PCA of the 35 Arnebiae Radix samples. Ad, A. decumbens; Ae, A. euchroma (Royle) Johnst; Ag, A. guttata Bunge.
Hierachical Cluster Analysis of HPLC Fingerprint of Arnebiae Radix Samples
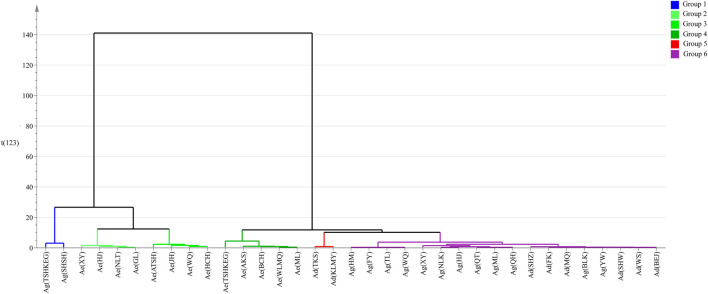
HCA is one of the most commonly used unsupervised pattern recognition methods, and is a useful multivariate statistical technique. It can create a cluster tree to assign a data set into groups according to similarity. To show the degree of similarity and difference among the 35 Arnebiae Radix samples more clearly, HCA was performed based on the results of PCA of all common characteristic peaks. As shown in Figure 5, all samples were divided into two main clusters according to their similarities and differences. Cluster one included three groups: Group 1 contained two samples belonging to Ag, Group 2 contained four samples belonging to Ae, and Group 3 contained four samples belonging to Ae. Cluster two was divided into three groups: Group 4 included five samples belonging to Ae, Group 5 included two samples belonging to Ad, and Group 6 included 12 samples belonging to Ag and six samples belonging to Ad. Group 4 merged with Group 5 to form a larger branch. All samples in the branch were gathered from Ad, Ae, and Ag. The results of the HPLC-specific chromatograms combined with HCA were not as accurate as those of DNA barcoding.
FIGURE 5.
The results of HCA of the 35 Arnebiae Radix samples. Calculated with Ward and stored by size. Ad, A. decumbens; Ae, A. euchroma (Royle) Johnst; and Ag, A. guttata Bunge.
Discussion
Arnebiae Radix, a commonly used herbal medicine in China, is also widely used in the food and cosmetics industries. As the ecological environment has been constantly destroyed, the wild resources of Arnebiae Radix have been sharply reduced, and cultivation is very difficult. There is a serious shortage of Arnebiae Radix supplies, leading to a complex and confusing market. The traditional classification method is based on the roots, leaves, flowers, fruits, and other organs of plants. Due to the lack of accurate identification characteristics, the processed commodities only retain the root, which creates great difficulties in the identification of Arnebiae Radix. However, the accuracy of the original medicinal materials is required to ensure the effectiveness and safety of clinical medication. DNA barcoding technology techniques are not influenced by organs, growth conditions, tissue differences or the external environment, among other factors (Bhargava and Sharma 2013; Mohammed Abubakar et al., 2017). In this study, the DNA barcoding technique showed the ability to scientifically and accurately identify the species. In the preliminary experimental stage, another three sequences, matK, rbcL, and ITS, were also considered, but it was found that there were many nested peaks within ITS, and the variable sites measured by matK and rbcL were not obvious. The established PCR-RFLP method based on the ITS2 sequence can identify A. euchroma (Royle) Johnst and A. guttata Bunge, as well as other plants also named Zicao in China (Qian et al., 2019). Thus, we examined the ITS2 sequence similarity, genetic distance and phylogenetic tree by using DNA barcoding technology based on its ability to differentiate Arnebiae Radix species. Our results suggested that ITS2 can discriminate A. euchroma (Royle) Johnst, A. guttata Bunge and A. decumbens (Vent.) Coss. et Kralik (Figure 2). As one of the most important markers in molecular phylogenetic research, the ITS2 sequence has obvious sequence variation at the species level or subspecies level, and it is an important candidate barcode for identification at the species level or subspecies level (Sickel et al., 2015; Cheng et al., 2016).
HPLC-specific chromatograms were established, and the contents of six hydroxyl naphthoquinones can be used to distinguish the different origins of Arnebiae Radix herbs (Ke et al., 2016). Therefore, we utilized HPLC methods to differentiate the three Arnebiae Radix species. Our results indicated that this method allows for the simultaneous discrimination of the seven main naphthoquinones in these samples. The lichen A. euchroma (Royle) Johnst shows high intraspecific chemical variations in Xinjiang, while A. guttata Bunge and A. decumbens (Vent.) Coss. et Kralikshow relatively less variation (Table 3). This result could partly be explained by the limited distribution of Ag and Ad, resulting in less variation. Ag and Ad have restricted geographic distributions in western and northern Xinjiang, whereas Ae has a rather wide distribution around Xinjiang. However, the samples could not be distinguished based on HPLC fingerprint (Figure 3), PCA (Figure 4) and HCA methods (Figure 5). Ecological factors, especially altitude, may be responsible for this result. Additionally, other environmental factors, such as light, temperature, air, soil, and moisture, also affect the content of the chemical components of plants. To further develop and utilize the plant resources of Arnebiae Radix, it is necessary to study more about the how genetic and environmental factors influence the metabolites of Arnebiae Radix.
In summary, this study has established a system for identifying the three Arnebiae Radix species based on DNA barcoding and chemical analysis methods. The results revealed that although the HPLC method cannot differentiate these samples, DNA barcoding can transcend the limitations of HPLC methods to ensure effective and universal proof of medicinal plants from different species. Similar results showed that DNA barcoding was a promising and reliable tool for the identification of three kinds of Plumeria flowers compared to HPLC-specific chromatograms, which are generally used (Zhao et al., 2018). Thus, DNA barcoding is more powerful than HPLC fingerprinting for species traceability in related species that are genetically similar. Our findings may be useful for the determination of naphthoquinones of Arnebiae Radix and provide a reference for the identification of traditional Chinese medicine based on DNA barcoding. Due to the narrow distribution of A. tschimganica (Fedtsch.) G. L. Chu, no sample was used in this experiment. It is necessary to expand the sample size and investigate the corresponding response of different growth periods and growing environments to provide a reference for the quality control and expansion of new drug sources of Arnebiae Radix.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
CL, DJ and HX designed researches, analyzed data and prepared the manuscript. HX and YW performed the experiment. PL and GR participated in the data analysis and prepared the manuscript. CL and DJ revised the manuscript. All authors read and approved the final article.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82060696) and Xinjiang Tianshan talent projects. We gratefully thank Wenhuan Ding, Dongdong Wang, Xuejia Zhang and Li Ma for the collection of wild Arnebiae Radix samples.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer (YS) declared a shared affiliation with several of the authors, DJ, PL, GR, CL, to the handling editor at time of review
References
- Behrens-Chapuis S., Herder F., Geiger M. F. (2021). Adding DNA Barcoding to Stream Monitoring Protocols - What's the Additional Value and Congruence between Morphological and Molecular Identification Approaches? PLoS One 16 (1), e0244598. 10.1371/journal.pone.0244598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava M., Sharma A. (2013). DNA Barcoding in Plants: Evolution and Applications of In Silico Approaches and Resources. Mol. Phylogenet. Evol. 67 (3), 631–641. 10.1016/j.ympev.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Chen S., Pang X., Song J., Shi L., Yao H., Han J., et al. (2014). A Renaissance in Herbal Medicine Identification: from Morphology to DNA. Biotechnol. Adv. 32 (7), 1237–1244. 10.1016/j.biotechadv.2014.07.004 [DOI] [PubMed] [Google Scholar]
- Chen S., Yao H., Han J., Liu C., Song J., Shi L., et al. (2010). Validation of the ITS2 Region as a Novel DNA Barcode for Identifying Medicinal Plant Species. PLoS One 5 (1), e8613. 10.1371/journal.pone.0008613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T., Xu C., Lei L., Li C., Zhang Y., Zhou S. (2016). Barcoding the Kingdom Plantae: New PCR Primers forITSregions of Plants with Improved Universality and Specificity. Mol. Ecol. Resour. 16 (1), 138–149. 10.1111/1755-0998.12438 [DOI] [PubMed] [Google Scholar]
- Ding W. H, Yan X. H, Ge L., Xu H. Y. (2019). Determination of Naphthoquinones in Roots of Four Species of Arnebia in Xinjiang by HPLC. Chin. Traditional Patent Med. 41 (04), 936–939. 10.3969/j.Issn.1001-1528.2019.04.047 [DOI] [Google Scholar]
- Feng J., Yu P., Zhou Q., Tian Z., Sun M., Li X., et al. (2020). An Integrated Data Filtering and Identification Strategy for Rapid Profiling of Chemical Constituents, with Arnebiae Radix as an Example. J. Chromatogr. A 1629, 461496. 10.1016/j.chroma.2020.461496 [DOI] [PubMed] [Google Scholar]
- Fu D., Shang X., Ni Z., Shi G. (2016). Shikonin Inhibits Inflammation and Chondrocyte Apoptosis by Regulation of the PI3K/Akt Signaling Pathway in a Rat Model of Osteoarthritis. Exp. Ther. Med. 12 (4), 2735–2740. 10.3892/etm.2016.3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnels T., Creswell M., McFerrin J., Whittall J. B. (2020). The ITS Region Provides a Reliable DNA Barcode for Identifying Reishi/lingzhi (Ganoderma) from Herbal Supplements. PLoS One 15 (11), e0236774. 10.1371/journal.pone.0236774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Sun J., Li D., Hu Y., Yu X., Hua H., et al. (2019). Shikonin Attenuates Acetaminophen-Induced Acute Liver Injury via Inhibition of Oxidative Stress and Inflammation. Biomed. Pharmacother. 112, 108704. 10.1016/j.biopha.2019.108704 [DOI] [PubMed] [Google Scholar]
- Hu L., Zhou C., Huang Y. C., Wang Y., Wei G., Liang Z., et al. (2020). HPLC Coupled with Electrospray Ionization Multistage MS/MS and TLC Analysis of flavones‐C‐glycosides and Bibenzyl of Dendrobium Hercoglossum. J. Sep. Sci. 43 (20), 3885–3901. 10.1002/jssc.202000647 [DOI] [PubMed] [Google Scholar]
- Huang X. Y., Fu H. L., Tang H. Q., Yin Z. Q., Zhang W., Shu G., et al. (2020). Optimization Extraction of Shikonin Using Ultrasound-Assisted Response Surface Methodology and Antibacterial Studies. Evid. Based Complement. Alternat Med. 2020, 1208617. 10.1155/2020/1208617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia-Xin T., F. G., Zhi-Lai Z., et al. (2018). Herbalogical Textual of Arnebiae Radix and Analysis of Medicinal Resources. Mod. Chin. Med. 20 (9), 1064–1067. 10.13313/j.issn.1673-4890.20180428002 [DOI] [Google Scholar]
- Ke Z., Rui S., Aijun T., Hong W., Jie L., Linong G., et al. (2016). HPLC Specific Chromatogram of Arnebia Euchroma and Determination of Six Naphthoquinones in Boraginaceous Herbs. Chin. J. Pharm. Anal. 36 (9), 1526–1535. 10.16155/j.0254-1793.2016.09.03 [DOI] [Google Scholar]
- Khan S. A., Baeshen M. N., Ramadan H. A., Baeshen N. A. (2019). ITS2: An Ideal DNA Barcode for the Arid Medicinal Plant Rhazya Stricta. Pharm. Med. 33 (1), 53–61. 10.1007/s40290-019-00266-3 [DOI] [PubMed] [Google Scholar]
- Liao M., Yan P., Liu X., Du Z., Jia S., Aybek R., et al. (2020). Spectrum-effect Relationship for Anti-tumor Activity of Shikonins and Shikonofurans in Medicinal Zicao by UHPLC-MS/MS and Chemometric Approaches. J. Chromatogr. B 1136, 121924. 10.1016/j.jchromb.2019.121924 [DOI] [PubMed] [Google Scholar]
- Liu B., Hu T., Yan W. (2020). Authentication of the Bilberry Extracts by an HPLC Fingerprint Method Combining Reference Standard Extracts. Molecules 25 (11), 2514. 10.3390/molecules25112514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., He L., Wang J., Wang Q., Sun C., Li Y., et al. (2020). Anti-angiogenic Effect of Shikonin in Rheumatoid Arthritis by Downregulating PI3K/AKT and MAPKs Signaling Pathways. J. Ethnopharmacology 260, 113039. 10.1016/j.jep.2020.113039 [DOI] [PubMed] [Google Scholar]
- Liu Z., Zeng X., Yang D., Ren G., Chu G., Yuan Z., et al. (2012). Identification of Medicinal Vines by ITS2 Using Complementary Discrimination Methods. J. Ethnopharmacology 141 (1), 242–249. 10.1016/j.jep.2012.01.057 [DOI] [PubMed] [Google Scholar]
- Ma S. J., Geng Y., Ma L., et al. (2021). Advances in Studies on Medicinal Radix Arnebia. Mod. Chin. Med. 23 (01), 177–184. 10.13313/j.issn.1673-4890.20190719004 [DOI] [Google Scholar]
- Mei L., Ling-Feng W., Hong-Liang J. (2020). Reviews on Natural Quinones and Their Bioactivities of Medicinal Zicao. Nat. Product. Res. Dev. 32 (04), 694–707. 10.16333/j.1001-6880.2020.4.021 [DOI] [Google Scholar]
- Mohammed Abubakar B., Mohd Salleh F., Shamsir Omar M. S., Wagiran A. (2017). Review: DNA Barcoding and Chromatography Fingerprints for the Authentication of Botanicals in Herbal Medicinal Products. Evid. Based Complement. Alternat Med. 2017, 1352948. 10.1155/2017/1352948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L., LIu J., Guo L., Zheng J. I., Ma S. (2019). Molecular Identification of Arnebiae Radix by PCR-RFLP Based on the ITS2 Sequence. Chin. J. Pharm. Anal. 36 (9), 1611–1617. 10.16155/j.0254-1793.2016.09.14 [DOI] [Google Scholar]
- Selva Pandiyan A., Siva Ganesa Karthikeyan R., Rameshkumar G., Sen S., Lalitha P. (2020). Identification of Bacterial and Fungal Pathogens by rDNA Gene Barcoding in Vitreous Fluids of Endophthalmitis Patients. Semin. Ophthalmol. 35, 358–364. 10.1080/08820538.2020.1864416 [DOI] [PubMed] [Google Scholar]
- Sickel W., Ankenbrand M. J., Grimmer G., Holzschuh A., Härtel S., Lanzen J., et al. (2015). Increased Efficiency in Identifying Mixed Pollen Samples by Meta-Barcoding with a Dual-Indexing Approach. BMC Ecol. 15, 26194794. 10.1186/s12898-015-0051-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W. X., Liu Y., Zhou W., Li H. W., Yang J., Chen Z. B. (2017). Shikonin Inhibits TNF-Alpha Production through Suppressing PKC-NF-kappaB-dependent Decrease of IL-10 in Rheumatoid Arthritis-like Cell Model. J. Nat. Med. 71 (2), 349–356. 10.1007/s11418-016-1064-3 [DOI] [PubMed] [Google Scholar]
- Xu C. M., Li D., Wang Y. Y., et al. (2014). Research Development of Food Pigment Biosynthesis. J. Chin. Inst. Food Sci. Technol. 14 (02), 225–233. 10.16429/j.1009-7848.2014.02.046 [DOI] [Google Scholar]
- Yu J., Wu X., Liu C., Newmaster S., Ragupathy S., Kress W. J. (2021). Progress in the Use of DNA Barcodes in the Identification and Classification of Medicinal Plants. Ecotoxicol. Environ. Saf. 208, 111691. 10.1016/j.ecoenv.2020.111691 [DOI] [PubMed] [Google Scholar]
- Zhan Z. L., Hu J., Liu T., Kang L. P., Nan T. G., Guo L. P. (2015). Advances in Studies on Chemical Compositions and Pharmacological Activities of Arnebiae Radix. Zhongguo Zhong Yao Za Zhi 40 (21), 4127–4135. [PubMed] [Google Scholar]
- Zhang J., Hu X., Wang P., Huang B., Sun W., Xiong C., et al. (2018). Investigation on Species Authenticity for Herbal Products of Celastrus Orbiculatus and Tripterygum Wilfordii from Markets Using ITS2 Barcoding. Molecules 23 (4). 10.3390/molecules23040967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Yu X., Shen J., Xu X. (2018). Identification of Three Kinds of Plumeria Flowers by DNA Barcoding and HPLC Specific Chromatogram. J. Pharm. Anal. 8 (3), 176–180. 10.1016/j.jpha.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Zhou Y., Wang L., Li M., Shi D., Li D., et al. (2017). Shikonin Alleviates the Biotoxicity Produced by Pneumococcal Pneumolysin. Life Sci. 177, 1–7. 10.1016/j.lfs.2017.04.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.