Abstract
Diet is a significant factor in determining human well-being. Excessive eating and/or diets with higher than needed amounts of carbohydrates, salt, and fat are known to cause metabolic disorders and functional changes in the body. To compensate the ill effects, many designer diets including the Mediterranean diet, the Okinawa diet, vegetarian/vegan diets, keto diet, anti-inflammatory diet, and the anti-oxidant diet have been introduced in the past 2 decades. While these diets are either enriched or devoid of one or more specific components, a better way to control diet is to limit the amount of food consumed. Caloric restriction (CR), which involves limiting the amount of food consumed rather than eliminating any specific type of food, as well as intermittent fasting (IF), which entails limiting the time during which food can be consumed on a given day, have gained popularity because of their positive effects on human health. While the molecular mechanisms of these 2 dietary regimens have not been fully deciphered, they are known to prolong the life span, control blood pressure, and blood glucose levels. Furthermore, CR and IF were both shown to decrease the incidence of heart attack and stroke, as well as their ill effects. In particular, IF is thought to promote metabolic switching by altering gene expression profiles leading to reduced inflammation and oxidative stress, while increasing plasticity and regeneration.
Keywords: Dietary restriction, Brain, Stroke, Neuroprotection, Inflammation, Oxidative stress
Introduction
Over the last century, caloric intake has steadily increased, but physical activity/exercise in humans has not. This has led to faster progression of metabolism-related disorders such as obesity, diabetes, and hypertension, and the associated diseases like heart attack, kidney dysfunction, and stroke. Recent studies have shown that caloric restriction (CR) extends lifespan, decreases age-induced functional changes, and minimizes the incidence of age-related diseases in multiple species (Wan et al., 2010; Ran et al., 2015; Ciobanu et al., 2017; Wahl et al., 2017). When subjected to focal ischemia, animals subjected to CR had smaller infarcts and better neurologic outcome (Varendi et al., 2014; Ran et al., 2015; Ciobanu et al., 2017). More importantly, CR induces ischemic tolerance in aged rodents as well (Manzanero et al., 2011). CR can be achieved by consuming <70% of suggested calories/day, which is challenging in the longer term. Intermittent fasting (IF) is a variation of CR in which a person can consume 100% calories, but only during an 8 to 10 h period/day. For example, a person can eat normally between 9 am to 6 pm and then go on a fast from 6 pm to 9 am the next day. This is much easier as ~8 of those 15 fasting hours are sleeping time. In addition, IF can be followed in a flexible format. That means, IF can be practiced on all days of a week with time-restricted fasting for 12 to 16 h or can be followed as alternate day fasting. IF was shown to produce similar effects to CR including extension of life span, attenuation of neurodegenerative and cardiovascular diseases, and increased cerebral plasticity (Fann et al., 2017). IF also decreases blood pressure and promotes better glucose handling in humans (Tikoo et al., 2007; van Bilsen et al., 2014; Mattson et al., 2017; Erdem et al., 2018; Sutton et al., 2018; Badreh et al., 2019; Camelo et al., 2019). In addition, conditioning by IF was shown to minimize neuronal death and improve neurological recovery if rodents were subjected to focal ischemia (Manzanero S et al., 2014; Arumugam et al., 2010). While minimizing the post-stroke brain damage with pharmacologic therapies is important; modalities that can prevent stroke incidence and/or promote tolerance (so that the brain damage is lower in the unavoidable event of a stroke) are also extremely beneficial. The goal of this review article is to discuss the putative mechanisms that confer ischemic tolerance following IF.
Decreasing food intake (caloric restriction and IF) is different than maintaining a designer diet. For example, to follow a designer diet like the Mediterranean diet or the anti-inflammatory diet, the person will eat a specific kind of food rich in certain ingredients, whereas, in CR/IF there are no specific foods to eat or avoid. A person can eat any food, but the amount consumed (CR) or time during which a person can eat (IF) is restricted. As of today, food restriction studies are not combined with any designer diets.
IF leads to extensive transcriptional reprogramming
A recent study evaluated the detailed transcriptomic profiles in brain, heart, kidney, and muscle of mice subjected to various paradigms of IF (Kim et al., 2018). In this study, adult C57BL/6 male mice were subjected to either ad libitum (AL) feeding or IF for either 12h or 16h a day for 4 months. At the end of the IF period, the cerebral cortical tissue of the 3 cohorts of mice was analyzed by RNA-sequencing (RNA-seq). Interestingly, 16h IF, but not 12h IF, induced several changes in gene expression compared to AL control. Genes induced after IF include those that control signal transduction, cell communication, CNS development, positive regulation of metabolism, and cellular responses to stimuli. All these groups or genes are known to be beneficial. The authors also studied the effect of IF on stroke-induced gene expression changes. Focal cerebral ischemia was induced by transient middle cerebral artery occlusion (MCAO) in cohorts of mice subjected to either 12h or 16h IF or AL feeding. In these mice, the cerebral cortical transcriptome was assessed at 3h, 12h, 1 day, and 3 days of reperfusion. In the AL cohort of mice subjected to transient MCAO, genes that were involved in inflammation, cell stress response to cell death, apoptotic processes, and immune cell response/inflammation were observed to be significantly upregulated as expected. However, both 12h and 16h IF cohorts showed several post-MCAO changes compared to the AL cohort. In the IF groups subjected to focal ischemia, transcription, translation, oxidation-reduction process, response to reactive oxygen species, response to stress, ribosome biogenesis and assembly, cellular biosynthesis, oxidative phosphorylation, regulation of metabolism, and regulation of cell proliferation were the major biological processes upregulated compared to AL group. Furthermore, regulation of neuronal death, regulation of apoptosis, calcium signaling, glutamatergic signaling, cholinergic signaling, GABAergic signaling, dopaminergic signaling, MAPK signaling, mTOR signaling, and inflammatory mediator regulation were the major biological processes downregulated in IF/MCAO groups compared to AL/MCAO groups. In brief, IF led to molecular switching in the post-stroke brain that changed the balance towards a pro-cell survival and anti-inflammatory state (Fig. 1). Importantly, many genes that participate in pathways that mediate neuroplasticity, such as neurotrophic factors and neuronal energy metabolism such as peroxisome proliferator-activated receptors (PPARs) were upregulated in the IF groups compared to the AL groups. In addition, a metabolic shift from glucose to fatty acid oxidation and change in circadian rhythms was also seen in the IF groups (Kim et al., 2018).
Figure 1.
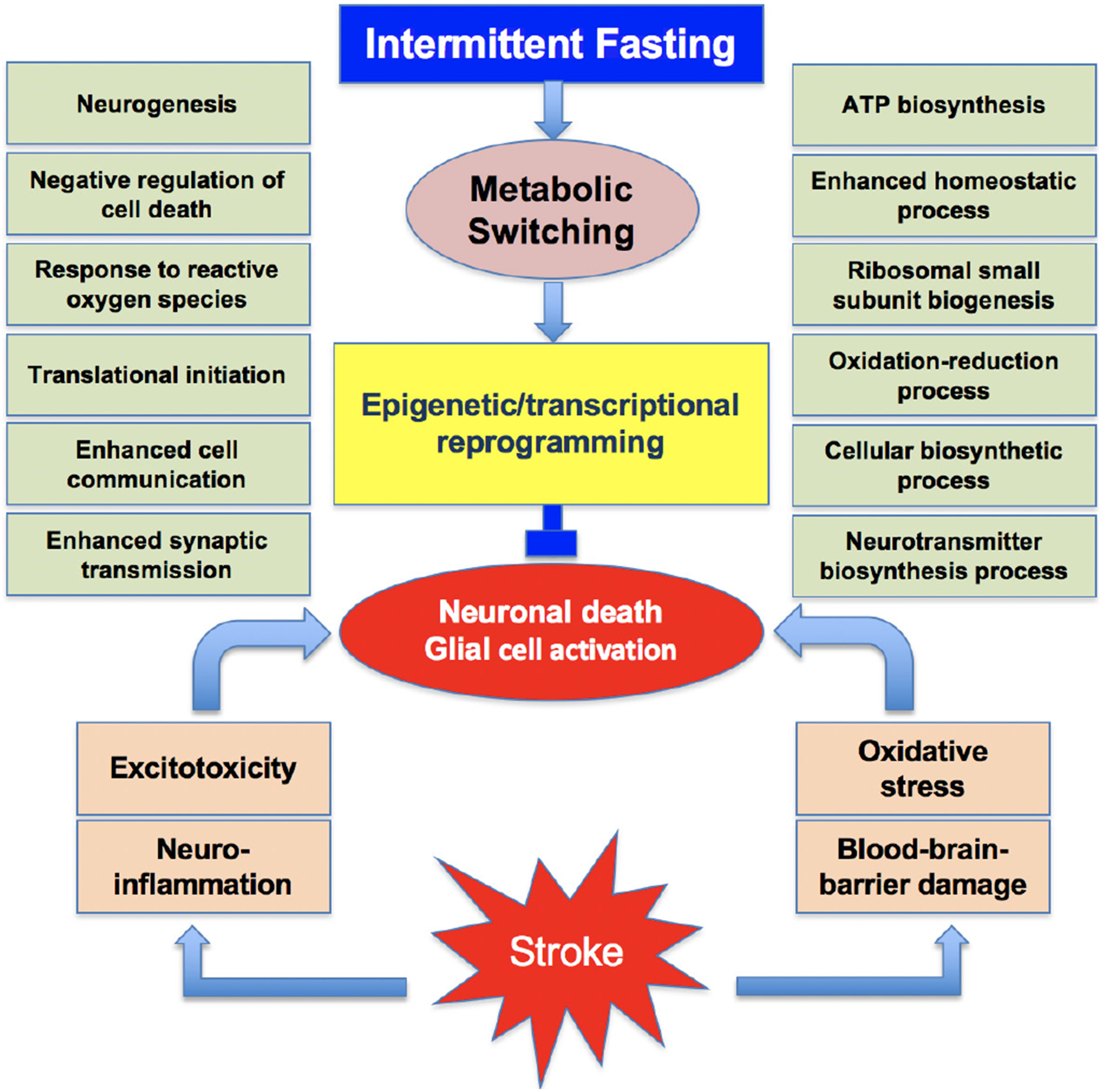
Many synergistic pathological events that include excitotoxicity, neuroinflammation, oxidative stress and blood-brain barrier damage promote the post-stroke brain damage. Intermittent fasting leads to metabolic switching that promotes epigenetic and transcriptional reprogramming. This induces the translation of many beneficial proteins concurrently inhibiting the translation of neurotoxic proteins. This promotes a neuroprotective phenotype.
Interestingly, other studies also found that IF improved glucose tolerance and altered the genes that control the circadian clock, aging, and autophagy in humans (Jamshed et al., 2019). When adult rats were subjected to IF for 1 week and then global cerebral ischemia was induced in them, they showed better recovery of cognitive function as assessed by a novel object recognition test and the Morris water maze test compared to the AL cohort 8 weeks after surgery (Hu et al., 2019). This study also found that the IF cohort of rats showed mitigated post-ischemic oxidative stress indicated by lower malondialdehyde levels and higher glutathione levels compared to the AL cohort. Furthermore, the IF group of rats showed higher expression levels of the anti-oxidant enzymes NADPH oxidase 1 and superoxide dismutase, and their upstream genes (Hu et al., 2019). This study further confirmed that IF leads to lower microglial activation and induced expression of sphingosine 1-phosphate receptor 1 leading to decreased post-ischemic inflammation compared with the AL group. In diet-induced obese mice, IF induced neuropeptide Y gene expression and increased norephinephrine levels in the hypothalamus leading to fat loss and retention of lean mass, which are beneficial to animals (Gotthardt et al., 2016). Serotonin controls feeding behavior in rodents and mammals, hence altering the serotonin transporter SERT can help to mitigate feeding related disorders. Severe IF (only 2 hours feeding per day) for 7 days in adult rats did not result in any changes in brain SERT expression, but did increase neuropeptide Y and proopiomelanocortin expression in the hypothalamus (Lauzurica et al., 2010). IF was also shown to induce anti-depressive effects by modulating the expression of certain genes. In mice subjected to 9 hours of fasting per day and administered imipramine, the p-CREB/CREB ratio in the hippocampus and frontal cortex was enhanced and the immobility time in the forced swim test was decreased (Li et al., 2014). The senescence-accelerated prone 8 (SAMP8) mice, which live shorter than normal mice, show many changes in the CNS, including decreased expression of sirtuin 1 and increased expression of forkhead box protein O1 (FoxO1) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) targets leading to altered transcriptional control of genes related to cell proliferation and survival (Tajes et al., 2010). In addition, these mice also showed reduced levels of brain derived neurotrophic factor (BDNF) and heat shock protein 70 (HSP70). Interestingly 8 weeks of IF in SMAP8 mice showed recovery of sirtuin 1, BDNF, and HSP70 expression concomitant with decreases in acetyled NF-kB targets, c-Jun N-terminal kinase 1 (JNK1), and FoxO1 (Tajes et al., 2010). Interestingly, even a single episode of a 36h fast was shown to alter the expression of genes that control fatty acid oxidation, cell cycle, apoptosis, and inflammation in the blood of humans (Elliott et al., 2014). These studies showed that IF can promote better cell survival via altered gene expression leading to increased quality of life.
IF induced transcriptional reprogramming is not restricted to higher level organisms. IF has also shown to alter transcriptional regulation in Drosophila that shows youthful metabolic and behavioral switching in the CNS (Zhang et al., 2018). In C. elegans, IF led to altered expression of the gene Rheb-1, which is a low molecular weight GTPase that is responsible for controlling the insulin/insulin-like growth factor signaling effector DAP-16. Furthermore, in worms that were subjected to IF, RHEB-1 and target of rapamycin (TOR) signaling worked together to down-regulate insulin-like peptide INS-7. All these changes are thought to promote the life span in C-elegans subjected to IF (Honjoh et al., 2009).
IF protects the brain by curtailing inflammation
Post-stroke brain damage is known to be significantly modulated by central and peripheral inflammation that starts within hours after the insult (Dirnagl et al., 1999; Yi et al., 2007; Kapadia et al., 2008; del Zoppo, 2009; Iadecola and Anrather, 2011; Kim et al., 2014). A hallmark of inflammation after stroke is the extravasation of leukocytes (macrophages and neutrophils) from blood to brain parenchyma (Jin et al., 2010; Yang et al., 2019a). These cells release free radicals and pro-inflammatory molecules that mediate neuronal death in the ischemic brain. Previous studies showed that IF significantly mitigates several markers of inflammation in both the CNS and the periphery (Fann et al., 2017; Ott et al., 2017; Hu et al., 2019). Mechanistically, this was thought to be implemented by suppressing both genetic and physiological modulation.
In adult men, levels of the pro-inflammatory cytokines interleukin (IL)-6, IL1β, and tumor necrosis factor alpha (TNF-α), and the number of circulating leukocytes were observed to be lower during the Ramadan fasting period than 1 week before the start of the fast or 1 month after the fast (Faris et al., 2012). In a model of myocardial infarction, prior IF decreased leukocyte infiltration in the at risk area of the heart and the levels of the pro-inflammatory cytokine IL-6 in plasma (Wan et al., 2010). Lipopolysaccharide (LPS) administration is known to induce neuroinflammation in rodents that promotes cognitive deficits. This was observed to be due to NF-kB activation and thus induction of expression of many of its downstream pro-inflammatory genes including TLR4, iNOS, IL-1α, IL-1β, IFN-γ, TNF-α, and IL-6 (Vasconcelos et al., 2014). However, in rats subjected to IF for 30 days, LPS induced NF-kB activation, and downstream gene expression and cognitive deficits were observed to be curtailed compared to AL fed rats (Vasconcelos et al., 2014). This study lends further support to the idea that IF can rectify inflammatory gene expression following an insult in both the periphery and brain. Mechanistically, increased due to fasting leads to induction of sirtuins like SIRT1, which are deacetylases. SIRTs in turn activate the expression of downstream transcription factors including nuclear factor erythroid 2-related factor 2 (NRF2), FOXO, and PPARγ coactivator 1α (PGC-1α) (de Cabo and Mattson, 2019; Liu et al., 2019b; Mattson, 2019). These factors are known to induce anti-inflammatory and anti-oxidant gene expression. SIRT1 also prevents NF-kB activation by deacetylating the RelA/P65 subunit, thereby mitigating NF-kB downstream pro-inflammatory gene expression (Yeung et al., 2004). Another putative transcriptional mechanism that decreases inflammation is NAD+-mediated inactivation of mammalian TOR, which in turn promotes autophagy and mitophagy, thus decreasing pro-inflammatory cytokine levels (Fann et al., 2017).
Importantly, IF alleviated LPS-induced neuroinflammation in both young and aged rats, which was shown to be mediated by sodium-potassium adenosine triphosphatase (Na,K-ATPase) (Vasconcelos et al., 2015). IF, when combined with resistance training, significantly decreased the levels of pro-inflammatory cytokines IL-6, IL-1β, and TNF-α in the blood of adult male humans (Moro T et al., 2016). Another study combined IF with supplementation of Ayurvedic herbs (leaf powder of Withania somnifera and dried stem powder of Tinospora cordifolia) in middle aged female rats. This combo treatment led to significantly reduced pro-inflammatory molecules IL-6, IL-1β, TNF-α, NF-kB, and Iba1 in the hippocampus (Singh et al., 2017). This study also observed that the rats subjected to IF + herbal supplements showed reduced anxiety-like behavior (Singh et al., 2017). These studies indicate that a combination of positive life style changes synergistically act to provide benefits to humans. In addition, IF was also protective in comorbid conditions. For example, when high-fat diet fed male mice were subjected to IF, there was a significant improvement in glucose tolerance and decreased fat mass associated with reduced levels of macrophage markers (Lgals3, Itgax, Ccl2, and Ccl3) indicating decreased inflammation (Liu et al., 2019a).
Inflammasomes are multimeric protein complexes, which are components of the innate immune system. When activated, they release several pro-inflammatory cytokines like IL-1β and IL-6 that promote cell death (Swanson et al., 2019). In particular, the NOD-, LRR- and pyrin domain-containing protein (NLRP) inflammasomes were shown to be activated by focal ischemia (Bauernfeind et al., 2009). Recent studies showed mice subjected to IF prior to stroke show reduced activation of NLRP inflammasomes, in addition to decreased levels of pro-inflammatory cytokines, which is beneficial for cells to survive after an ischemic attack (Fann et al., 2014). As inflammasomes are known to be activated by NF-kB, and as IF prevents NF-kB, it also leads to less inflammasome activation (Jung et al., 2009; Castello et al., 2010; Tajes et al., 2010; Fann et al., 2018). It was also shown that IF prevents post-ischemic activation of both NLRP1 and NLRP3 inflammasomes in mouse brain (Fann et al., 2014).
IF minimizes oxidative stress
Following stroke, white blood cells, including leukocytes and macrophages, that extravasate into brain parenchyma release several reactive oxygen species (ROS), free radicals (superoxide, hydroxyl radical, and peroxyl radical), and non-radicals (hydrogen peroxide and hyperchlorous acid). These cells also release reactive nitrogen species (RNS), radicals (nitric oxide and nitrogen dioxide), and non-radicals (peroxynitrite, dinitrogen trioxide, nitrous acid, nitroxyl anion, and nitrosyl cations), which are all neurotoxic (Sundararajan et al., 2005; Bowen et al., 2006; Kapadia et al., 2006; Satriotomo et al., 2006; Tureyen et al., 2007; Weston et al., 2007; Tang et al., 2008; Jin et al., 2010; Chu et al., 2014). Numerous studies have shown that IF before induction of stroke in rodent curtail release of ROS and RNS, as well as increase anti-oxidant defense by promoting the expression of enzymes like superoxide dismutase and catalase downstream to the transcription factor Nrf2 (Arumugam et al., 2010; Amigo and Kowaltowski, 2014; Ahn et al., 2018; Madkour et al., 2019). Fasting is known to cause metabolic switching resulting in lowered glucose and increased fatty acid use for energy production. IF promotes fatty acid production from triglycerides and the fatty acids are converted to ketone bodies that are used as a major source of fuel during IF (Mattson et al., 2017). Ketone bodies also modulate the expression of PGC-1α (Svensson et al., 2016). One of the mechanisms that is central to controlling oxidative stress after IF is thought to be initiated by the transcription factor PGC-1α, which promotes the levels of the antioxidant SIRT1 that in turn promotes the expression of mitochondrial uncoupling proteins (UCPs) UCP2 and UCP4 (Mattiasson et al., 2003; Bevilacqua et al., 2005; Liu et al., 2006; Canto and Auwerx, 2009; Chu et al., 2009; Haines et al., 2010; Canto and Auwerx, 2011). It was shown that when rodents were kept on IF prior to the induction of stroke, neuronal mitochondrial number and the levels of UCP2 and UCP4 were shown to be increased, which might lead to a higher rate of mitochondrial respiration (Fann et al., 2017). Overall, these effects decreased ROS levels, while concomitantly increasing their disposal leading to decreased oxidative stress after stroke.
IF is also thought to induce other transcriptional mechanisms that reduce oxidative stress. For example, IF activates the transcription factor NRF2, which induces many antioxidant enzymes including superoxide dismutase-2 and catalase. Furthermore, food restriction promotes mitochondrial biogenesis by activating transcription factors NRF2 and mitochondria transcription factor A (TFAM), and by modulating certain microRNAs (Hancock et al., 2011; Gouspillou and Hepple, 2013; Picca et al., 2013; Zhang et al., 2019). It was also suggested that IF might increase the availability of NAD+ due to mitochondrial uncoupling and also increase the respiratory rate that leads to reduced oxygen tension, subsequently lowering levels of superoxide production (Sanz et al., 2005; Haines et al., 2010; Fann et al., 2017; Klaus and Ost, 2019).
IF promotes brain plasticity
Rodents subjected to IF demonstrated improved cognitive function. Various studies with different regimens of IF showed improvement in verbal memory, spatial memory, working memory, associative memory, and executive function in adult as well as aged subjects (35 to 40 of Mattson). IF was shown to significantly increase the levels of BDNF, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) in young as well as middle-aged male mice (Arumugam et al., 2010). BDNF is known to activate TrkB-PI3K/Akt signaling that induces neurogenesis in the adult brain following focal ischemia (Liu et al., 2015; Kisoh et al., 2017; Yang et al., 2019b). Hypoxia-induced factor 1 alpha (HIF-1α) induced by IF promotes the expression of VEGF-A, which might also increase neurogenesis. A recent study showed conclusive evidence for increased activation of the Notch signaling pathway, as well as upregulation of BDNF and downstream p-CREB following IF, which are all known to mediate neural stem cell proliferation (Baik et al., 2019). These authors further showed increased levels of PSD95, which is a post-synaptic marker, and nestin, which is an immature neuronal marker in the hippocampus of mice subjected to IF for 3 months (Baik et al., 2019). Hence, the improved cognitive function after IF might be due to neurotrophic factors that stimulate neurogenesis and angiogenesis. A diet that mimics fasting for 4 days resulted in an increased number of progenitors and stem cells leading to higher regeneration in adult mice (Brandhorst S et al., 2015). These authors also showed that a fasting mimicking diet promoted increased IGF-1 levels, hippocampal neurogenesis, and improved cognitive performance in old mice as well (Brandhorst et al., 2015). It was also demonstrated that in amyloid precursor protein (APP) mutant mice IF enhanced hippocampal GABAergic tone leading to reduced anxiety-like behavior and improved hippocampus-dependent memory in a SIRT3 dependent manner (Liu et al., 2019b). Phosphatase and tensin homolog (PTEN) plays an essential role in modulating cell growth and survival. IF was shown to decrease impairment in contextual fear memory observed in PTEN haloinsufficient mice (Cabral-Costa JV 2018). A recent study showed that IF prevents TBI-induced hippocampal dependent learning and memory deficits in adult mice (Rubovitch et al., 2019).
Gap in our understanding of the clinical translation of IF for neuroprotection after stroke
Post-stroke brain damage is considered to be significantly dependent on age and sex. While at a younger age, males show higher brain damage and more severe loss of neurological function than females, this trend reverses at older age. Postmenopausal women show higher incidence of stroke and importantly more brain damage and significantly worsened neurological dysfunction than men at older ages (Li and McCullough, 2009; Liu et al., 2009b; Liu et al., 2009a; McCullough et al., 2009; Liu and McCullough, 2012). Hence, the efficacy of IF in protecting the brain after stroke as well as other conditions like TBI, spinal cord injury, and chronic neurodegenerative disorders need preclinical testing in both sexes and more importantly using older animals.
As the consumption of processed foods and calorically-rich foods with higher amounts of fats and sugars increased steadily in humans and the level of physical activity decreased, the incidence of metabolic syndromes like type-2 diabetes, obesity, and hypertension increased at an alarming rate. These changes are known to exacerbate the risk of CNS and peripheral organ diseases enormously. In particular, type-2 diabetes and hypertension are the major comorbid conditions that promote the incidence of stroke as well as exacerbated post-stroke brain damage. Hence, future studies need to test the efficacy of IF in comorbid animal models like diabetic and hypertensive rodents.
Although many diabetic and hypertensive individuals are on medications, diet plays a major role in deciding the health of these patients. Adopting either CR or IF was shown to promote better glycemic homeostasis in diabetic individuals (Mattson et al., 2017; Anton et al., 2018; Zubrzycki et al., 2018). In humans, IF was shown to decrease body weight, fasting glucose, and insulin sensitivity in overweight adults (Barnosky et al., 2014). IF was shown to result in better glycemic control in type-2 diabetic humans (Carter et al., 2016a, b). More importantly, in type-2 diabetes patients, IF was shown to be a safe and tolerable practice that promotes better fasting glucose levels (Arnason et al., 2017). In prediabetic humans, IF was shown to improve insulin sensitivity and β cell responsiveness, while decreasing oxidative stress and blood pressure (Sutton et al., 2018). A recent study showed that intermittent CR in db/db mice resulted in significantly reduced fasting blood glucose levels and improved insulin sensitivity (Wei et al., 2019). IF was also shown to prevent progression of nephropathy in type 1 diabetic rats (Tikoo et al., 2007) and prevented hyperglycemia in diabetes-susceptible New Zealand Obese mice (Baumeier et al., 2015). Mechanistically, IF was shown to enhance beta cell survival in diabetic mice by preserving organelle quality via the autophagy-lysosome pathway (Liu et al., 2017). IF was also shown to promote neogenesis of beta cells by autophagy-induced degradation of Notch1 leading to reversal of type-2 diabetes symptoms in rodents (DiNicolantonio and McCarty, 2019).
IF was also shown to decrease blood pressure in young and aged rodents (Badreh et al., 2019), as well as rodents on high-fat or high-fructose diets (Camelo et al., 2019). More importantly, IF was also shown to reduce blood pressure in humans with hypertension (Erdem et al., 2018) and or pre-diabetes (Sutton et al., 2018).
The mechanisms by which IF promotes ischemic tolerance is not yet completely deciphered. Recent studies showed that the gut microbiome influences the physiology of many organs including the brain. Stroke was shown to significantly alter the composition of the gut microbiome that significantly influences the post-stroke outcome. Furthermore, the gut microbiome depends on eating habits, and hence IF influences the composition of microbes in the gut. Hence, future studies are needed to understand the effect of IF on the gut microbiome in both sexes at different ages and also in comorbid subjects. The gut and the brain communicate with each other and the microbiota is a major mediator of the “gut-brain axis”. An imbalance in this axis is thought to mediate several disorders including brain dysfunction. Dysbiosis of the gut microbiome is currently thought to be a major proponent of inflammation and brain damage after stroke (Benakis et al., 2016; Singh et al., 2016; Spychala et al., 2018; Durgan et al., 2019; Park et al., 2019; Prame Kumar and Wong, 2019). The gut microbiota is known to release several short chain fatty acids (SCFAs) that play key roles in protecting the brain after stroke (Zeng and Cullen, 2004; Chen et al., 2019). A recent study showed that supplementation of SCFAs altered microglial activation and improved post-stroke motor function recovery in mice subjected to focal ischemia (Sadler et al., 2019). Several studies showed that CR impacts the gut microbial composition and impacts metabolism (Zhang et al., 2013; Duszka et al., 2018; Fabbiano et al., 2018; Zheng et al., 2018). CR was shown to promote reduction of the firmicutes/bacteriodetes ratio and expansion of lactobacilli leading to increased short-chain fatty acid synthesis, which is beneficial (Tanca et al., 2018). CR-mediated gut microbiome changes were shown to promote anti-inflammatory effects in rodents (Pan et al., 2018), as well as humans (Ott et al., 2017). These actions were thought to be due to expansion of lactobacillus in the gut (Fraumene et al., 2018). IF was also shown to decrease obesity and insulin resistance probably by shifting gut microbiota composition to a beneficial phenotype (Li et al., 2017). In type-2 diabetic db/db mice, IF increased lifespan, in addition to decreasing acellular capillaries and leukocyte infiltration (Beli et al., 2018). Furthermore, IF in db/db mice altered the gut microbiome with increased levels of firmicutes and decreased levels of bacteroidetes and verrucomicrobia, which is thought to be a beneficial phenotype (Beli et al., 2018). This beneficial phenotype of gut microbiota was also shown to be associated with increased levels of tauroursodeoxycholate (TUDCA), which is a neuroprotective bile acid formed by firmicutes (Beli et al., 2018). IF protects the brain from autoimmune conditions by altering the gut microbiota (Cignarella et al., 2018). Composition of gut microbiota also correlates with immune function (Li et al., 2019). Hence, future studies are needed to decipher if the beneficial effects of IF after stroke are due to rectifying the gut microbiota dysbiosis.
Conclusions
Overall, we conclude that IF promotes an adoptive (stress) response in the body that includes prevention of inflammation, better handling of oxidative stress, formation of more mitochondria, transcriptional switch to turn off neurotoxic and to turn on neuroprotective genes, as well as increase brain plasticity through neurogenesis/angiogenesis. IF was shown to protect mature neurons, and to promote regeneration and plasticity by inducing neurogenesis. Most of the studies to date indicated a beneficial effect of IF in various species. An attractive feature for adoption of IF in humans is the flexibility, as the regimen can be followed for 14 to 16 hours/day or fasting on alternate days or even normal feeding on 5 days and reduced calories on 2 days of the week. All of these adaptations are known to be beneficial by reducing the incidence and negative effects of major metabolic disorders like diabetes and hypertension, as well as subsequent diseases like heart attack, stroke, or neurodegenerative diseases.
References
- Ahn JH, Noh Y, Shin BN, Kim SS, Park JH, Lee TK, Song M, Kim H, Lee JC, Yong JH, Kang IJ, Lee YL, Won MH, Kim JD (2018) Intermittent fasting increases SOD2 and catalase immunoreactivities in the hippocampus but does not protect from neuronal death following transient ischemia in gerbils. Mol Med Rep 18:4802–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amigo I, Kowaltowski AJ (2014) Dietary restriction in cerebral bioenergetics and redox state. Redox Biol 2:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG 3rd, Leeuwenburgh C, Mattson MP (2018) Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity (Silver Spring) 26:254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnason TG, Bowen MW, Mansell KD (2017) Effects of intermittent fasting on health markers in those with type 2 diabetes: A pilot study. World J Diabetes 8:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R (2010) Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol 67:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badreh F, Joukar S, Badavi M, Rashno M, Dehesh T (2019) The Effects of Age and Fasting Models on Blood Pressure, Insulin/Glucose Profile, and Expression of Longevity Proteins in Male Rats. Rejuvenation Res. [DOI] [PubMed] [Google Scholar]
- Baik SH, Rajeev V, Fann DY, Jo DG, Arumugam TV (2019) Intermittent fasting increases adult hippocampal neurogenesis. Brain Behav:e01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnosky AR, Hoddy KK, Unterman TG, Varady KA (2014) Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res 164:302–311. [DOI] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E (2009) Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183:787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeier C, Kaiser D, Heeren J, Scheja L, John C, Weise C, Eravci M, Lagerpusch M, Schulze G, Joost HG, Schwenk RW, Schurmann A (2015) Caloric restriction and intermittent fasting alter hepatic lipid droplet proteome and diacylglycerol species and prevent diabetes in NZO mice. Biochim Biophys Acta 1851:566–576. [DOI] [PubMed] [Google Scholar]
- Beli E et al. (2018) Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes 67:1867–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J (2016) Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med 22:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME (2005) Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am J Physiol Endocrinol Metab 289:E429–438. [DOI] [PubMed] [Google Scholar]
- Bowen KK, Naylor M, Vemuganti R (2006) Prevention of inflammation is a mechanism of preconditioning-induced neuroprotection against focal cerebral ischemia. Neurochemistry international 49:127–135. [DOI] [PubMed] [Google Scholar]
- Brandhorst S et al. (2015) A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab 22:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camelo L, Marinho TS, Aguila MB, Souza-Mello V, Barbosa-da-Silva S (2019) Intermittent fasting exerts beneficial metabolic effects on blood pressure and cardiac structure by modulating local renin-angiotensin system in the heart of mice fed high-fat or high-fructose diets. Nutr Res 63:51–62. [DOI] [PubMed] [Google Scholar]
- Canto C, Auwerx J (2009) Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab 20:325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Auwerx J (2011) Interference between PARPs and SIRT1: a novel approach to healthy ageing? Aging (Albany NY) 3:543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S, Clifton PM, Keogh JB (2016a) Intermittent energy restriction in type 2 diabetes: A short discussion of medication management. World J Diabetes 7:627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S, Clifton PM, Keogh JB (2016b) The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res Clin Pract 122:106–112. [DOI] [PubMed] [Google Scholar]
- Castello L, Froio T, Maina M, Cavallini G, Biasi F, Leonarduzzi G, Donati A, Bergamini E, Poli G, Chiarpotto E (2010) Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kB activation. Free Radic Biol Med 48:47–54. [DOI] [PubMed] [Google Scholar]
- Chen R, Xu Y, Wu P, Zhou H, Lasanajak Y, Fang Y, Tang L, Ye L, Li X, Cai Z, Zhao J (2019) Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res 148:104403. [DOI] [PubMed] [Google Scholar]
- Chu AC, Ho PW, Kwok KH, Ho JW, Chan KH, Liu HF, Kung MH, Ramsden DB, Ho SL (2009) Mitochondrial UCP4 attenuates MPP+ - and dopamine-induced oxidative stress, mitochondrial depolarization, and ATP deficiency in neurons and is interlinked with UCP2 expression. Free Radic Biol Med 46:810–820. [DOI] [PubMed] [Google Scholar]
- Chu HX, Kim HA, Lee S, Moore JP, Chan CT, Vinh A, Gelderblom M, Arumugam TV, Broughton BR, Drummond GR, Sobey CG (2014) Immune cell infiltration in malignant middle cerebral artery infarction: comparison with transient cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 34:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, Phillips D, Weinstock GM, Fontana L, Cross AH, Zhou Y, Piccio L (2018) Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab 27:1222–1235 e1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciobanu O, Elena Sandu R, Tudor Balseanu A, Zavaleanu A, Gresita A, Petcu EB, Uzoni A, Popa-Wagner A (2017) Caloric restriction stabilizes body weight and accelerates behavioral recovery in aged rats after focal ischemia. Aging Cell 16:1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cabo R, Mattson MP (2019) Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med 381:2541–2551. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ (2009) Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience 158:972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNicolantonio JJ, McCarty M (2019) Autophagy-induced degradation of Notch1, achieved through intermittent fasting, may promote beta cell neogenesis: implications for reversal of type 2 diabetes. Open Heart 6:e001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends in neurosciences 22:391–397. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Lee J, McCullough LD, Bryan RM Jr. (2019) Examining the Role of the Microbiota-Gut-Brain Axis in Stroke. Stroke 50:2270–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duszka K, Ellero-Simatos S, Ow GS, Defernez M, Paramalingam E, Tett A, Ying S, Konig J, Narbad A, Kuznetsov VA, Guillou H, Wahli W (2018) Complementary intestinal mucosa and microbiota responses to caloric restriction. Sci Rep 8:11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott RM, de Roos B, Duthie SJ, Bouwman FG, Rubio-Aliaga I, Crosley LK, Mayer C, Polley AC, Heim C, Coort SL, Evelo CT, Mulholland F, Daniel H, Mariman EC, Johnson IT (2014) Transcriptome analysis of peripheral blood mononuclear cells in human subjects following a 36 h fast provides evidence of effects on genes regulating inflammation, apoptosis and energy metabolism. Genes Nutr 9:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem Y, Ozkan G, Ulusoy S, Arici M, Derici U, Sengul S, Sindel S, Erturk S, Turkish Society of H, Renal D (2018) The effect of intermittent fasting on blood pressure variability in patients with newly diagnosed hypertension or prehypertension. J Am Soc Hypertens 12:42–49. [DOI] [PubMed] [Google Scholar]
- Fabbiano S, Suarez-Zamorano N, Chevalier C, Lazarevic V, Kieser S, Rigo D, Leo S, Veyrat-Durebex C, Gaia N, Maresca M, Merkler D, Gomez de Aguero M, Macpherson A, Schrenzel J, Trajkovski M (2018) Functional Gut Microbiota Remodeling Contributes to the Caloric Restriction-Induced Metabolic Improvements. Cell Metab 28:907–921 e907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann DY, Ng GY, Poh L, Arumugam TV (2017) Positive effects of intermittent fasting in ischemic stroke. Exp Gerontol 89:93–102. [DOI] [PubMed] [Google Scholar]
- Fann DY, Santro T, Manzanero S, Widiapradja A, Cheng YL, Lee SY, Chunduri P, Jo DG, Stranahan AM, Mattson MP, Arumugam TV (2014) Intermittent fasting attenuates inflammasome activity in ischemic stroke. Exp Neurol 257:114–119. [DOI] [PubMed] [Google Scholar]
- Fann DY, Lim YA, Cheng YL, Lok KZ, Chunduri P, Baik SH, Drummond GR, Dheen ST, Sobey CG, Jo DG, Chen CL, Arumugam TV (2018) Evidence that NF-kappaB and MAPK Signaling Promotes NLRP Inflammasome Activation in Neurons Following Ischemic Stroke. Mol Neurobiol 55:1082–1096. [DOI] [PubMed] [Google Scholar]
- Faris MA, Kacimi S, Al-Kurd RA, Fararjeh MA, Bustanji YK, Mohammad MK, Salem ML (2012) Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr Res 32:947–955. [DOI] [PubMed] [Google Scholar]
- Fraumene C, Manghina V, Cadoni E, Marongiu F, Abbondio M, Serra M, Palomba A, Tanca A, Laconi E, Uzzau S (2018) Caloric restriction promotes rapid expansion and long-lasting increase of Lactobacillus in the rat fecal microbiota. Gut Microbes 9:104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotthardt JD, Verpeut JL, Yeomans BL, Yang JA, Yasrebi A, Roepke TA, Bello NT (2016) Intermittent Fasting Promotes Fat Loss With Lean Mass Retention, Increased Hypothalamic Norepinephrine Content, and Increased Neuropeptide Y Gene Expression in Diet-Induced Obese Male Mice. Endocrinology 157:679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouspillou G, Hepple RT (2013) Facts and controversies in our understanding of how caloric restriction impacts the mitochondrion. Exp Gerontol 48:1075–1084. [DOI] [PubMed] [Google Scholar]
- Haines BA, Mehta SL, Pratt SM, Warden CH, Li PA (2010) Deletion of mitochondrial uncoupling protein-2 increases ischemic brain damage after transient focal ischemia by altering gene expression patterns and enhancing inflammatory cytokines. J Cereb Blood Flow Metab 30:1825–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock CR, Han DH, Higashida K, Kim SH, Holloszy JO (2011) Does calorie restriction induce mitochondrial biogenesis? A reevaluation. FASEB J 25:785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjoh S, Yamamoto T, Uno M, Nishida E (2009) Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 457:726–730. [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhang M, Chen Y, Yang Y, Zhang JJ (2019) Postoperative intermittent fasting prevents hippocampal oxidative stress and memory deficits in a rat model of chronic cerebral hypoperfusion. Eur J Nutr 58:423–432. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J (2011) The immunology of stroke: from mechanisms to translation. Nat Med 17:796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM (2019) Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 87:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KJ, Lee EK, Kim JY, Zou Y, Sung B, Heo HS, Kim MK, Lee J, Kim ND, Yu BP, Chung HY (2009) Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm Res 58:143–150. [DOI] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R (2008) Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Frontiers in bioscience: a journal and virtual library 13:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia R, Tureyen K, Bowen KK, Kalluri H, Johnson PF, Vemuganti R (2006) Decreased brain damage and curtailed inflammation in transcription factor CCAAT/enhancer binding protein beta knockout mice following transient focal cerebral ischemia. J Neurochem 98:1718–1731. [DOI] [PubMed] [Google Scholar]
- Kim J et al. (2018) Transcriptome analysis reveals intermittent fasting-induced genetic changes in ischemic stroke. Hum Mol Genet 27:1497–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Kawabori M, Yenari MA (2014) Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Current medicinal chemistry 21:2076–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisoh K, Hayashi H, Itoh T, Asada M, Arai M, Yuan B, Tanonaka K, Takagi N (2017) Involvement of GSK-3beta Phosphorylation Through PI3-K/Akt in Cerebral Ischemia-Induced Neurogenesis in Rats. Mol Neurobiol 54:7917–7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus S, Ost M (2019) Mitochondrial uncoupling and longevity - A role for mitokines? Exp Gerontol 130:110796. [DOI] [PubMed] [Google Scholar]
- Lauzurica N, Garcia-Garcia L, Pinto S, Fuentes JA, Delgado M (2010) Changes in NPY and POMC, but not serotonin transporter, following a restricted feeding/repletion protocol in rats. Brain Res 1313:103–112. [DOI] [PubMed] [Google Scholar]
- Li B, Zhao J, Lv J, Tang F, Liu L, Sun Z, Wang L, Siwela SP, Wang Y, Song Y, Manchishi SM, Cui R (2014) Additive antidepressant-like effects of fasting with imipramine via modulation of 5-HT2 receptors in the mice. Prog Neuropsychopharmacol Biol Psychiatry 48:199–206. [DOI] [PubMed] [Google Scholar]
- Li J, McCullough LD (2009) Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab 29:670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Qi M, Gatesoupe FJ, Tian D, Jin W, Li J, Lin Q, Wu S, Li H (2019) Adaptation to Fasting in Crucian Carp (Carassius auratus): Gut Microbiota and Its Correlative Relationship with Immune Function. Microb Ecol 78:6–19. [DOI] [PubMed] [Google Scholar]
- Li X, Shimizu Y, Kimura I (2017) Gut microbial metabolite short-chain fatty acids and obesity. Biosci Microbiota Food Health 36:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hutchison AT, Thompson CH, Lange K, Heilbronn LK (2019a) Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obes Res Clin Pract 13:408–415. [DOI] [PubMed] [Google Scholar]
- Liu D, Chan SL, de Souza-Pinto NC, Slevin JR, Wersto RP, Zhan M, Mustafa K, de Cabo R, Mattson MP (2006) Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromolecular Med 8:389–414. [DOI] [PubMed] [Google Scholar]
- Liu F, McCullough LD (2012) Interactions between age, sex, and hormones in experimental ischemic stroke. Neurochemistry international 61:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Yuan R, Benashski SE, McCullough LD (2009a) Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab 29:792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD (2009b) Sex differences in caspase activation after stroke. Stroke 40:1842–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Javaheri A, Godar RJ, Murphy J, Ma X, Rohatgi N, Mahadevan J, Hyrc K, Saftig P, Marshall C, McDaniel ML, Remedi MS, Razani B, Urano F, Diwan A (2017) Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy 13:1952–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Zhou XY, Hou JC, Zhu H, Wang Z, Liu JX, Zheng YQ (2015) Ginsenoside Rd promotes neurogenesis in rat brain after transient focal cerebral ischemia via activation of PI3K/Akt pathway. Acta Pharmacol Sin 36:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cheng A, Li YJ, Yang Y, Kishimoto Y, Zhang S, Wang Y, Wan R, Raefsky SM, Lu D, Saito T, Saido T, Zhu J, Wu LJ, Mattson MP (2019b) SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat Commun 10:1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madkour MI, A TE-S, Jahrami HA, Sherif NM, Hassan RE, Awadallah S, Faris MAE (2019) Ramadan diurnal intermittent fasting modulates SOD2, TFAM, Nrf2, and sirtuins (SIRT1, SIRT3) gene expressions in subjects with overweight and obesity. Diabetes Res Clin Pract 155:107801. [DOI] [PubMed] [Google Scholar]
- Manzanero S, Gelderblom M, Magnus T, Arumugam TV (2011) Calorie restriction and stroke. Exp Transl Stroke Med 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T (2003) Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med 9:1062–1068. [DOI] [PubMed] [Google Scholar]
- Mattson MP (2019) An Evolutionary Perspective on Why Food Overconsumption Impairs Cognition. Trends Cogn Sci 23:200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Longo VD, Harvie M (2017) Impact of intermittent fasting on health and disease processes. Ageing Res Rev 39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Koerner IP, Hurn PD (2009) Effects of gender and sex steroids on ischemic injury. Handbook of clinical neurology 92:149–169. [DOI] [PubMed] [Google Scholar]
- Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, Paoli A (2016) Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med 14:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott B, Skurk T, Hastreiter L, Lagkouvardos I, Fischer S, Buttner J, Kellerer T, Clavel T, Rychlik M, Haller D, Hauner H (2017) Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci Rep 7:11955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Zhang L, Li M, Hu Y, Zeng B, Yuan H, Zhao L, Zhang C (2018) Predominant gut Lactobacillus murinus strain mediates anti-inflammaging effects in calorie-restricted mice. Microbiome 6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MJ, Pilla R, Panta A, Pandey S, Sarawichitr B, Suchodolski J, Sohrabji F (2019) Reproductive Senescence and Ischemic Stroke Remodel the Gut Microbiome and Modulate the Effects of Estrogen Treatment in Female Rats. Transl Stroke Res. [DOI] [PubMed] [Google Scholar]
- Picca A, Fracasso F, Pesce V, Cantatore P, Joseph AM, Leeuwenburgh C, Gadaleta MN, Lezza AM (2013) Age- and calorie restriction-related changes in rat brain mitochondrial DNA and TFAM binding. Age (Dordr) 35:1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prame Kumar K, Wong CH (2019) Imbalance in the force: the dark side of the microbiota on stroke risk and progression. Curr Opin Neurobiol 62:10–16. [DOI] [PubMed] [Google Scholar]
- Ran M, Li Z, Yang L, Tong L, Zhang L, Dong H (2015) Calorie restriction attenuates cerebral ischemic injury via increasing SIRT1 synthesis in the rat. Brain Res 1610:61–68. [DOI] [PubMed] [Google Scholar]
- Rubovitch V, Pharayra A, Har-Even M, Dvir O, Mattson MP, Pick CG (2019) Dietary Energy Restriction Ameliorates Cognitive Impairment in a Mouse Model of Traumatic Brain Injury. J Mol Neurosci 67:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler R, Cramer JV, Heindl S, Kostidis S, Betz D, Zuurbier KR, Northoff BH, Heijink M, Goldberg MP, Plautz EJ, Roth S, Malik R, Dichgans M, Holdt LM, Benakis C, Giera M, Stowe AM, Liesz A (2019) Short-chain fatty acids improve post-stroke recovery via immunological mechanisms. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A, Caro P, Ibanez J, Gomez J, Gredilla R, Barja G (2005) Dietary restriction at old age lowers mitochondrial oxygen radical production and leak at complex I and oxidative DNA damage in rat brain. J Bioenerg Biomembr 37:83–90. [DOI] [PubMed] [Google Scholar]
- Satriotomo I, Bowen KK, Vemuganti R (2006) JAK2 and STAT3 activation contributes to neuronal damage following transient focal cerebral ischemia. J Neurochem 98:1353–1368. [DOI] [PubMed] [Google Scholar]
- Singh H, Kaur T, Manchanda S, Kaur G (2017) Intermittent fasting combined with supplementation with Ayurvedic herbs reduces anxiety in middle aged female rats by anti-inflammatory pathways. Biogerontology 18:601–614. [DOI] [PubMed] [Google Scholar]
- Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, Dichgans M, Liesz A (2016) Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J Neurosci 36:7428–7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, Putluri N, Graf J, Bryan RM, McCullough LD (2018) Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol 84:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE (2005) Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience 130:685–696. [DOI] [PubMed] [Google Scholar]
- Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM (2018) Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab 27:1212–1221 e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson K, Albert V, Cardel B, Salatino S, Handschin C (2016) Skeletal muscle PGC-1alpha modulates systemic ketone body homeostasis and ameliorates diabetic hyperketonemia in mice. FASEB J 30:1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KV, Deng M, Ting JP (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19:477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajes M, Gutierrez-Cuesta J, Folch J, Ortuno-Sahagun D, Verdaguer E, Jimenez A, Junyent F, Lau A, Camins A, Pallas M (2010) Neuroprotective role of intermittent fasting in senescence-accelerated mice P8 (SAMP8). Exp Gerontol 45:702–710. [DOI] [PubMed] [Google Scholar]
- Tanca A, Abbondio M, Palomba A, Fraumene C, Marongiu F, Serra M, Pagnozzi D, Laconi E, Uzzau S (2018) Caloric restriction promotes functional changes involving short-chain fatty acid biosynthesis in the rat gut microbiota. Sci Rep 8:14778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XN, Cairns B, Cairns N, Yenari MA (2008) Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience 154:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikoo K, Tripathi DN, Kabra DG, Sharma V, Gaikwad AB (2007) Intermittent fasting prevents the progression of type I diabetic nephropathy in rats and changes the expression of Sir2 and p53. FEBS Lett 581:1071–1078. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, Vemuganti R (2007) Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem 101:41–56. [DOI] [PubMed] [Google Scholar]
- van Bilsen M, Daniels A, Brouwers O, Janssen BJ, Derks WJ, Brouns AE, Munts C, Schalkwijk CG, van der Vusse GJ, van Nieuwenhoven FA (2014) Hypertension is a conditional factor for the development of cardiac hypertrophy in type 2 diabetic mice. PLoS One 9:e85078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varendi K, Airavaara M, Anttila J, Vose S, Planken A, Saarma M, Mitchell JR, Andressoo JO (2014) Short-term preoperative dietary restriction is neuroprotective in a rat focal stroke model. PLoS One 9:e93911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos AR, Yshii LM, Viel TA, Buck HS, Mattson MP, Scavone C, Kawamoto EM (2014) Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J Neuroinflammation 11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos AR, Kinoshita PF, Yshii LM, Marques Orellana AM, Bohmer AE, de Sa Lima L, Alves R, Andreotti DZ, Marcourakis T, Scavone C, Kawamoto EM (2015) Effects of intermittent fasting on age-related changes on Na,K-ATPase activity and oxidative status induced by lipopolysaccharide in rat hippocampus. Neurobiol Aging 36:1914–1923. [DOI] [PubMed] [Google Scholar]
- Wahl D, Coogan SC, Solon-Biet SM, de Cabo R, Haran JB, Raubenheimer D, Cogger VC, Mattson MP, Simpson SJ, Le Couteur DG (2017) Cognitive and behavioral evaluation of nutritional interventions in rodent models of brain aging and dementia. Clin Interv Aging 12:1419–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, Mattson MP (2010) Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem 21:413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Zhao J, Bai M, Li C, Zhang L, Chen Y (2019) Comparison of glycemic improvement between intermittent calorie restriction and continuous calorie restriction in diabetic mice. Nutr Metab (Lond) 16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston RM, Jones NM, Jarrott B, Callaway JK (2007) Inflammatory cell infiltration after endothelin-1-induced cerebral ischemia: histochemical and myeloperoxidase correlation with temporal changes in brain injury. J Cereb Blood Flow Metab 27:100–114. [DOI] [PubMed] [Google Scholar]
- Yang C, Hawkins KE, Dore S, Candelario-Jalil E (2019a) Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol 316:C135–C153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang S, Liu J, Feng Y, Qi F, Zhao R (2019b) DNA Hypomethylation of GR Promoters is Associated with GR Activation and BDNF/AKT/ERK1/2-Induced Hippocampal Neurogenesis in Mice Derived From Folic-Acid-Supplemented Dams. Mol Nutr Food Res 63:e1801334. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23:2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Park SW, Kapadia R, Vemuganti R (2007) Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochem Int 50:1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Cullen BR (2004) Structural requirements for pre-microRNA binding and nuclear export by Exportin 5. Nucleic Acids Res 32:4776–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li S, Yang L, Huang P, Li W, Wang S, Zhao G, Zhang M, Pang X, Yan Z, Liu Y, Zhao L (2013) Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun 4:2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wang X, Qu JH, Liu B, Zhang P, Zhang T, Fan PC, Wang XM, Xiao GY, Su Y, Xie Y, Liu Y, Pei JF, Zhang ZQ, Hao DL, Xu P, Chen HZ, Liu DP (2019) Caloric Restriction Induces MicroRNAs to Improve Mitochondrial Proteostasis. iScience 17:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ratliff EP, Molina B, El-Mecharrafie N, Mastroianni J, Kotzebue RW, Achal M, Mauntz RE, Gonzalez A, Barekat A, Bray WA, Macias AM, Daugherty D, Harris GL, Edwards RA, Finley KD (2018) Aging and Intermittent Fasting Impact on Transcriptional Regulation and Physiological Responses of Adult Drosophila Neuronal and Muscle Tissues. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Wang S, Jia W (2018) Calorie restriction and its impact on gut microbial composition and global metabolism. Front Med 12:634–644. [DOI] [PubMed] [Google Scholar]
- Zubrzycki A, Cierpka-Kmiec K, Kmiec Z, Wronska A (2018) The role of low-calorie diets and intermittent fasting in the treatment of obesity and type-2 diabetes. J Physiol Pharmacol 69. [DOI] [PubMed] [Google Scholar]


