Abstract
Access to affordable, objective and scalable biomarkers of brain function is needed to transform the healthcare burden of neuropsychiatric and neurodegenerative disease. Electroencephalography (EEG) recordings, both resting and in combination with targeted cognitive tasks, have demonstrated utility in tracking disease state and therapy response in a range of conditions from schizophrenia to Alzheimer's disease. But conventional methods of recording this data involve burdensome clinic visits, and behavioural tasks that are not effective in frequent repeated use. This paper aims to evaluate the technical and human-factors feasibility of gathering large-scale EEG using novel technology in the home environment with healthy adult users. In a large field study, 89 healthy adults aged 40–79 years volunteered to use the system at home for 12 weeks, 5 times/week, for 30 min/session. A 16-channel, dry-sensor, portable wireless headset recorded EEG while users played gamified cognitive and passive tasks through a tablet application, including tests of decision making, executive function and memory. Data was uploaded to cloud servers and remotely monitored via web-based dashboards. Seventy-eight participants completed the study, and high levels of adherence were maintained throughout across all age groups, with mean compliance over the 12-week period of 82% (4.1 sessions per week). Reported ease of use was also high with mean System Usability Scale scores of 78.7. Behavioural response measures (reaction time and accuracy) and EEG components elicited by gamified stimuli (P300, ERN, Pe and changes in power spectral density) were extracted from the data collected in home, across a wide range of ages, including older adult participants. Findings replicated well-known patterns of age-related change and demonstrated the feasibility of using low-burden, large-scale, longitudinal EEG measurement in community-based cohorts. This technology enables clinically relevant data to be recorded outside the lab/clinic, from which metrics underlying cognitive ageing could be extracted, opening the door to potential new ways of developing digital cognitive biomarkers for disorders affecting the brain.
Keywords: EEG, EEG biomarker, cognition, gamification, mobile EEG
Introduction
Recent advances in digital technologies provide a wealth of opportunity in the management of health conditions. In neurological disease the heterogeneity and complexity of conditions, along with continuing reliance on traditional subjective measurement tools, have presented a challenge for the development of data-driven biomarkers for diagnosis, monitoring and prediction of therapeutic response (1–7). The suite of tools described in this paper was designed to enable longitudinal, in-home data collection of brain electrophysiology and cognitive performance. The platform comprises (1) a dry sensor, wireless electroencephalography (EEG) headset that records brain activity, (2) gamified versions of cognitive tasks, and (3) cloud-based storage and automatic processing—with the aim of identifying potential digital biomarkers with utility in neuropsychiatric and neurodegenerative disorders.
EEG directly reflects neural synaptic function, with similar patterns from animal to human (8–10) and thus has substantial potential as a brain-based, translatable biomarker for diseases such as schizophrenia (11–18), depression (19–21) and Alzheimer's disease (AD) (22–29). However, traditional research EEG setups are effortful and time-consuming, requiring expensive equipment and the support of personnel with technical training. Single or infrequent lab-based EEG recording sessions may be affected by a range of factors including fluctuations in levels of participants' mental alertness, fatigue and task-induced mental workload (30, 31). Similarly, cognition as traditionally measured in therapeutic research and practise tends to take the form of clinician administered batteries of neuropsychological tests [e.g., (32, 33)] which, whilst low burden and relatively inexpensive, are subject to variability in scores on repeated testing occasions (34). Infrequent, “snapshot” assessments are subject to measurement error arising from multiple factors, such as practise effects (35–37), the “white-coat effect” related to anxiety about suspected cognitive impairment (38), and day-to-day fluctuations in context (39), in mood and in perceived stress (40–44).
The adoption of modern technology into medicine allows for more innovative forms of data collection (e.g., wearable devices), increasing objectivity and taking advantage of powerful analytical tools to probe complex diseases. Further, digital tools may allow for more frequent sampling and detection of subtle daily fluctuations, at minimal disruption to the patient since data may be collected both inside and outside of the clinic. Progress in modern electronics and dry sensor technology means that EEG is emerging from amongst standard brain imaging methods as a mobile technology, suitable for deployment to very large cohorts for convenient at-home use (45). Likewise, neuropsychological testing can now be completed outside of the clinic through the use of automated, web-based assessments (46–49).
Mobile EEG systems are advancing quickly. Several studies have shown that it is possible in principle to collect EEG recordings using consumer-grade hardware, and from the data extract potentially useful neuronal signals, including spectral band-power measures (45, 50, 51) and task-evoked event-related potentials (ERPs) (52–55). However, studies using such devices have typically required some specialist equipment (e.g., a computer running bespoke software to present stimuli and record EEG), and a specialist experimenter to set up and supervise the recording. In addition, most consumer-grade EEG platforms operate using low numbers of electrodes, leaving some research questions and certain types of analysis out of reach for researchers. To the authors' knowledge, there exists no prior example of large-scale, unsupervised in-home, repeated sampling ERP research using a dry-sensor, portable, user-friendly EEG platform.
Innovative solutions can be deployed to enable us to carry out unsupervised data collection without placing undue burden on the user, such as ‘dry' sensors (i.e., eschewing the conductive gel used in the laboratory in favour of an easier electrode setup) and automated user-facing stepwise tutorials and notifications (to compensate for reduced environmental control outside the laboratory). Similarly, for use at home over repeated sessions, EEG/ERP tasks as used in research may not be particularly exciting or motivational for the user, but applying gamification can make these tasks more engaging and rewarding for participants (56) and gamified cognitive tasks can facilitate global data gathering on an unparalleled scale (57).
The study presented here was a first, proof-of-concept, field study to test the human-factors and technical feasibility of an early version of the Cumulus Neuroscience platform in a cohort of healthy adults spanning an age range up to 79 years old. In this paper we investigate the potential of this platform to capture in-home, frequent repeated measurement of EEG and behavioural metrics of cognitive ageing, metrics that also have broader appeal as potential cognitive biomarkers for the diagnosis and treatment of disorders affecting the brain. Use of the platform on a regular basis over 3 months assesses the acceptability of long-term use for future use cases where longitudinal progression tracking is required, avoids dependence on a single “snapshot” measurement, and allows for improved signal quality through aggregation of EEG data collected in the home (an unsupervised environment).
Analyses are presented that quantify reported ease-of-use, and resultant levels of weekly adherence over a period of 3 months of unsupervised at-home use. The gamified cognitive tasks are evaluated for face-validity, by comparing key known behavioural effects with data gathered in the home, and examining effects of age that have been reported in the literature. Similarly the EEG data is examined at grand-average level to confirm that it replicates the main features (waveform morphology and timing, frequency content, scalp topography) of the neural signatures that the gamified tasks are designed to elicit.
Method
Participants
89 healthy adult volunteers (67 female), aged between 40 and 79 years (mean 58.78, s.d. 8.86) with a Montreal Cognitive Assessment (MoCA) score ≥24 gave informed consent to take part in the procedures approved by Queen's University Belfast Ethics Committee. Recruitment channels included “Join Dementia Research,” local community groups and use of print media and social media.
The Platform
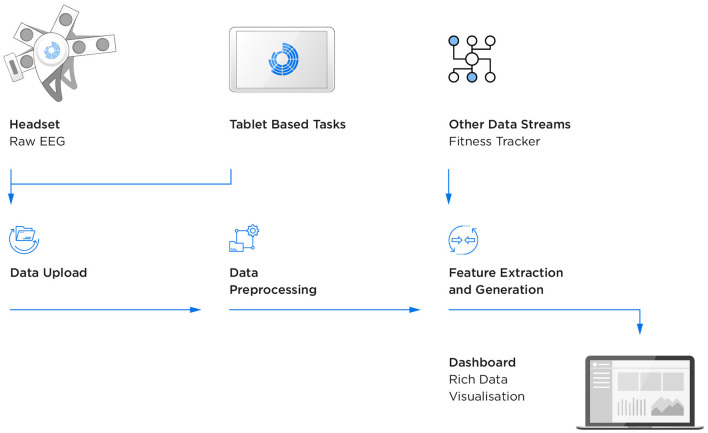
The platform was designed to enable frequent, objective sampling of brain-based markers of cognition inside and outside of the clinic/lab setting, using a dry sensor, wireless encephalography (EEG) headset that records brain activity, accompanied by gamified versions of cognitive tasks presented via a tablet-based app. Upon logging into the app, a stepwise tutorial guides the user through setup of the headset (covering placement on the head, positioning of the detachable mastoid sensors and feedback on sensor impedances) in preparation for recording data during the gamified tasks. Cloud-based secure methods are used for collection and automatic processing, as well as integration with other data streams (in this study participants wore a fitness tracker, the Withings Go) and web-based dashboards for monitoring and data visualisation on a daily, session-by-session basis (Figure 1).
Figure 1.
Flow of EEG and behavioural data.
EEG
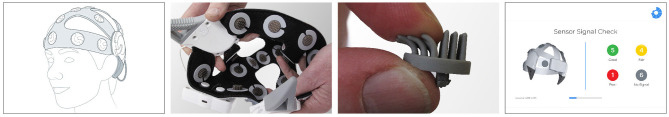
The wireless EEG headset (Figure 2) consists of dry flexible Ag/AgCl coated polymer sensors at 16 channels (O1, O2, P3, Pz, P4, Cz, FT7,FC3, FCz, FC4, FT8, Fz, AF7, AF8, FPz). The left and right mastoids are used for reference and driven-bias, with single-use, snap-on electrodes attached to wires extending from the headset. The electronics and sensors are mounted on flexible neoprene, and the stretchable structure incorporates anatomical landmarks in the form-fit of the headset to encourage consistent placement by users in line with the 10–20 sensor system. The analogue headset has high input impedance of 1 GΩ, a configurable driven bias function for common-mode rejection, built-in impedance checking, and configurable gain and sampling rates. An onboard processor and Bluetooth module transmit 250 Hz EEG data to the tablet, from where it is transferred to a cloud server for storage and processing. EEG recording and behavioural events are timestamp synchronised to ±2 ms.
Figure 2.
Sixteen-channel wireless headset designed with pliable sensors and the sensor signal quality check.
Cognitive Tasks
The gamified tasks (Figure 3) are based on well-known paradigms from experimental electrophysiology and cover a range of core cognitive functions. Cognitive/electrophysiological tasks were gamified with the aim of improving motivation, i.e., to enhance attentiveness during testing and to prevent boredom over repeated plays, while maintaining the core cognitive components of the original task. Feedback on gameplay performance was provided (e.g., points awarded for speed of responses where appropriate), along with personalised leaderboards to promote long-term adherence to the study schedule. Each daily session comprised a resting state plus two of the four other games (alternating between sessions). Participants also answered a daily health and lifestyle questionnaire to further contextualise the daily recordings. Daily sessions were designed to take <30 min total time from start to finish.
Figure 3.
Images of 2-stimulus visual oddball, flanker, n-back, and delayed match-to-sample gamified tasks.
Two-Stimulus Visual Oddball
This gamified version of the classic 2-stimulus visual oddball paradigm (58), presents target stimuli (aliens—requiring the participant to tap on the screen, n = 30) and non-target stimuli (visually different aliens—requiring no response from the participant, n = 70) across five levels of gameplay, as well as 15 “bonus” stars throughout the game (to enhance gameplay and not included for analysis). Behaviour (reaction time and response accuracy) and corresponding EEG correlates are indicative of neural dynamics of the decision-making process and the attention and working memory on which it relies (59–61). Using EEG, a positive voltage deflection can be observed over the parietal cortex starting ~300 ms following presentation of the stimulus, known as the P300 event-related potential (ERP). With advancing age, the amplitude of the P300 is known to decrease, and its latency known to increase (62–64).
Flanker
Inhibition and error awareness were probed using a gamified version of the Erikson Flanker task (65). Fish served as directional stimuli and were presented across five levels with a shoal of fish (flanking stimuli) appearing first, followed by the central (target) fish. The participant was asked to tap on the side of the screen corresponding to the direction of the central fish, ignoring the flanking fish (either congruent or incongruent stimuli, split evenly between the 150 trials), presenting a cognitive challenge reflected in behavioural responses (accuracy and reaction time) and EEG. The relevant EEG metric extracted from this task is the Error-related negativity (ERN)—a negative voltage deflection observed on error trials most prominently over the fronto-central scalp, followed by a subsequent positive rebound in the signal (the Error Positivity, or Pe) (66). Previous studies have consistently reported a decrease in the negative amplitude of the ERN with progressing age (67).
N-Back
The visual n-back paradigm (68) taxes working memory and executive function with age-related differences in behavioural performance, according to recent meta-analysis (69). In the current study, this game had a continuous short-sequence memorisation of 4 different playing cards where the participant was asked whether the current card is a “match” or “no-match” to the card seen 2 trials before. This 2-back paradigm consisted of 100 trials presented across two levels, with a 33% match rate.
Delayed Match-to-Sample
A visual delayed match-to-sample task, this task targets recognition memory, a key cognitive function known to be affected by age (70), across 50 trials, presented in blocks of 5, with 50% overall match rate (71). Each level is set in a specific location (beach, jungle, etc) where the user is presented with a variety of objects which must be encoded into memory to be retrieved after a brief (10-s) distractor game which involves connecting dots. Points are earned by identifying previously presented items at retrieval and rejecting unseen items.
Resting State
In this passive task (72) participants selected a relaxing scene (forest, park or beach) for 1 min of restful eyes open followed by 1 min eyes closed. This task elicits resting electrocortical activity and seeks to produce an increase in the neural oscillatory power of the alpha frequency band (7–13 Hz) when eyes are closed relative to eyes open, a physiological measure sensitive to a range of neurocognitive and psychiatric disorders, ageing, as well as sleep quality and caffeine intake (22, 73–75).
Procedure
Participants attended two in-lab sessions, at baseline and following 12 weeks of at-home use of the platform.
Lab Sessions
Lab sessions consisted of neuropsychological testing followed by in-lab use of the platform. Neuropsychological assessment was carried out for screening and to facilitate potential longitudinal follow-up and/or comparison with other datasets, and is not analysed in this paper. The MoCA (76) was selected as a screening tool using a cut-off of ≥24 to be representative of normal cognitive function, in line with findings reported in the Irish older adult general population (77, 78). Participants then completed tasks from the Cambridge Neuropsychological Test Automated Battery (CANTAB) covering multiple domains including psychomotor skills, executive function, memory and attention domains before completing a session with the platform. Participants provided ratings of the usability of the platform at baseline, 6 and 12 weeks into the study on the System Usability Scale (SUS), a 10-item industry standard questionnaire designed specifically to assess use of technology (79).
Home Sessions
Participants were asked to use the platform as described in section The Platform, at home, over the course of 12 weeks. These sessions were ~25–30 min and participants were asked to contribute 5 sessions per week (one session per day, days unspecified for participant convenience). Throughout the 12-week period, participants wore a fitness tracker to monitor their step count and sleep, and answered questions about daily well-being and lifestyle habits (not analysed in the current paper).
Analysis
To measure usability of the platform across age groups and feasibility of extracting features reflecting cognitive ageing, participants were assigned to three groups for analysis: those aged 40–54 (n = 26), 55–66 (n = 35), and 67–79 (n = 17) years. Usability measures used for analysis were adherence and participant-reported SUS scores, as well as technical measures of signal reliability. To investigate feasibility and explore effects of cognitive ageing, behavioural and EEG metrics were extracted across age groups. Additionally, event-related potential waveforms were plotted for comparison based on single game-play median epoch, single-participant averaged epochs and whole-sample grand averaged epochs. Validity of the approach can be established by confirming that behavioural and neural patterns observable in the literature (e.g., differences in timing between congruent and incongruent trials; the waveform and scalp topography of ERP components) are seen in the data recorded unsupervised in the home, and that age-related changes in these variables reflect the published consensus. Ninety five percentage confidence intervals are reported throughout using the upper and lower bounds.
Behavioural Analysis
Measures of accuracy and speed of response were extracted from the cognitive games played using the platform (2-stimulus oddball, flanker, n-back and delayed match-to-sample) to establish face validity against that which the literature leads us to expect. For this analysis, we averaged different behavioural measures across game-plays. To investigate reaction times, we chose the median reaction time per game-play, taking the median-average per participant to calculate age group mean comparisons and sample means. To compare rates of accuracy, we calculated percentage accuracy per game-play. We produced a mean accuracy rate per participant for age group and sample mean comparisons. Additionally, we calculated confidence intervals as an indication of variance. To visualise age group differences across game plays, the log-transformed game-play number was used as an explanatory variable of the different behavioural metrics per group in a linear model and a 95% confidence interval was calculated using 1,000 bootstrapping resamples.
EEG Analysis
The processing pipeline consists of filtering from 0.25 to 40 Hz, customised artefact removal, epoch extraction and baseline removal. Metric-based methods for removing invalid ERPs and PSDs were applied to outputs. Two event-related potential (ERP) components, the P300 (a positive-going waveform which peaks >300 ms after the presentation of an attended stimulus, associated with decision-making) and the Error-Related Negativity (a negative-going, response-locked waveform associated with error-awareness) were computed as the smoothed pointwise median of epochs within each session. Power spectral density (PSD) was computed using a 1,024 point Fast Fourier Transform (FFT) with Welch's method of averaging (using a 256 sample window) on the resting-state eyes-open and eyes-closed data. For this analysis, time-series data was converted to average reference to remove lateralised effects of the original single-mastoid reference. Mean and 95% confidence intervals were computed across all sessions from participants within each age group.
Results
Usability
Of the 89 healthy adult participants that enrolled in the study (67 female, mean age = 58.78, mean MoCA score = 27.12), 11 participants withdrew and 78 (61 female) completed the study (mean age = 58.99 mean MoCA score = 27.06), yielding an attrition rate of 12.40%. Data from those who withdrew was excluded from the following analyses reported.
Reasons for withdrawal cited were work/caring/other commitments (n = 3) and/or illness/health-related issues (n = 6). Two participants cited both health and caring commitments. One participant mentioned a faulty headset as an additional factor in the decision to withdraw; this participant's headset had required repair. Four participants did not give any reason. The mean number of sessions contributed by participants who withdrew was 16.82 [8.41–25.23], ranging from 1 (completed in-lab) to 45 sessions. The mean duration of at-home involvement by those who withdrew was 5.55 weeks [3.22–7.88].
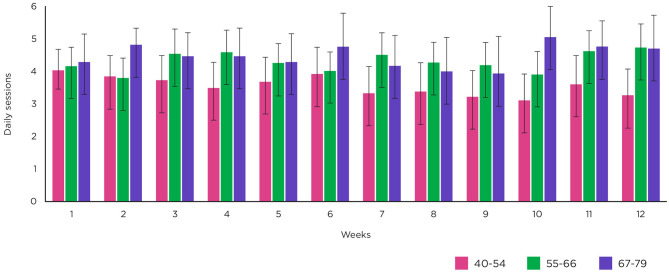
Figure 4 shows the rate of weekly adherence for those who completed the study (n = 78), including a breakdown of weekly adherence by age group [40–54 years (n = 26), 55–66 years (n = 35) and 67–79 years (n = 17)]. For those who completed, mean number of sessions contributed per week was 4.10 [3.97–4.23], out of a target of 5 per week and the mean total number of sessions contributed per participant was 49.14 [46.54–51.74]. By age group, mean number of sessions per week was 3.56 [3.33–3.78] for those aged 40–54, 4.31 [4.12–4.50] for those aged 55–66 and 4.48 [4.22–4.74] for those aged 67–79 years.
Figure 4.
Weekly adherence. Mean number of sessions per week across all participants and by age group. Error bars show upper and lower 95% confidence intervals.
Participants were asked to evaluate usability via the System Usability Scale (SUS) at 3 timepoints. Mean SUS scores were 76.59 [72.94–80.24] at baseline, 81.45 [78.33–84.57] at 6 weeks and 78.28 [74.74–81.81] at 12 weeks (see Table 1). It is worth noting that the mean SUS score at baseline from those who subsequently withdrew was 75.23 [64.42–86.04] and of those who were still enrolled at 6 weeks, mean SUS score was 74.64 [62.62–86.67].
Table 1.
System usability scale scores by age group at baseline, 6 and 12 weeks.
| 40–54 years | 55–66 years | 67–79 years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | n | Mean | 95% CI | n | Mean | 95% CI | n | |
| Baseline | 81.35 | ±5.99 | 26 | 77.94 | ±4.60 | 34 | 66.62 | ±9.01 | 17 |
| 6 weeks | 81.56 | ±5.81 | 24 | 85.44 | ±3.69 | 34 | 72.81 | ±7.08 | 16 |
| 12 weeks | 80.50 | ±6.04 | 25 | 81.50 | ±4.62 | 35 | 68.38 | ±8.05 | 17 |
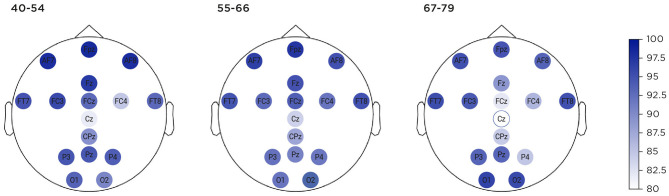
Ability to use the system to record usable EEG in the at-home setting, reported in Figure 5, was considered by measuring the percentage of time that individual sensors were connected to the scalp (i.e., recording non-saturated data) for the different age groups. Three thousand six hundred three sessions were successfully uploaded to the cloud server. Of these, 95.81% (3,452 sessions) contained portions of EEG data that could be used for analysis, even though certain sections of that session, or certain sensors, may be very noisy. One hundred and fifty-one sessions were rejected in their entirety, due to saturated data sections, high variance sections or gaps. By comparison, the behavioural data, where 99.03% (3,568 sessions) contained a complete set of response measures for at least one of the two games assigned per session. There were 116 sessions for which behavioural data, but not EEG data, was suitable for analysis.
Figure 5.
Percentage of time each of 16 channels recorded non-saturated data, shown across age groups.
Behaviour
To establish face validity of the gamified behavioural tasks, key metrics from each game were extracted to evaluate against what would be expected from traditional lab paradigms described in the literature. The temporal development of several illustrative metrics, stratified by age-group, is displayed in Figure 6.
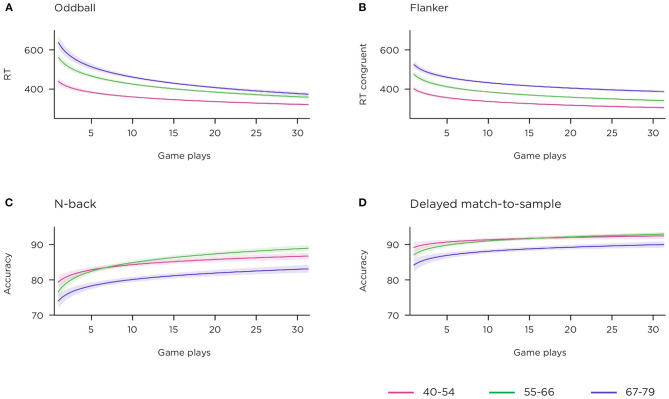
Figure 6.
Behavioural responses to gamified cognitive tasks over 12 weeks across age group. Shading indicates 95% confidence interval. (A) median correct RTs to targets in 2-stimulus oddball task; (B) median correct RTs to congruent trials in flanker task; (C) percentage accuracy, all trials, n-back task; (D) percentage accuracy, all trials, delayed match-to-sample task.
Two-Stimulus Oddball Game
Similar to lab versions of simple decision-making task which do not challenge the accuracy of responses, the gamified version demonstrated high accuracy, mean 97.71 [97.08–98.35]%. Age group means were 98.7 [98.32–99.08]% for 40–54 years, 98.05 [97.16–98.95]% for 55–66 years, and 95.5 [93.62–97.39]% for 67–79 years. As the game rewards speed of response (in its scoring procedure), and due to learning/strategizing effects, we expected reaction time (RT) to improve with repeated gameplays. Figure 6A shows the temporal trend of speed of response over consecutive sessions, and clear separation of the three age bands is visible. Reaction time per group, averaged over all sessions, was 366.76 [352.68–380.84] milliseconds (ms) for 40–54 years, 414.85 [393.17–436.54] ms for 55–66 years and 449.58 [423.45–475.70] ms for 67–79 years.
Flanker Game
This task is time-restricted, and encourages the player to make a trade-off between speed and accuracy of response (as erroneous response trials are those that yield the key EEG metric). Incongruent trials require inhibition and possible motor-replanning relative to congruent trials, and this is seen in an inhibition cost of 62.16 [56.62–67.7] ms. The inhibition cost per age group was 55.87 [48.28–63.45] ms for 40–54 years, 64.67 [56.0–73.34] ms for 55–66 years and 66.89 [52.99–80.8] ms for 67–79 years.
Incongruent trials induced many more errors [8.45 (7.06–9.84)] than congruent trials [2.02 (1.66–2.68)]. Looking at the temporal development of the congruent condition reaction times alone (Figure 6B), a pattern of learning from session to session, and separation of age bands, is visible.
N-Back Game
This game requires cycling of information in and out of short-term memory. RTs were slower for non-match [1,075.83 (990.77–1,160.9) ms] than match trials [919.56 (857.76–981.36) ms], however accuracy rates were higher for non-match [87.71 (86.41–89.01)%] vs. match trials [74.76 (71.91–77.60)%]. Accuracy rate was 76.49 [72.96–80.02]% for 40–54 years, 75.56 [70.57–80.55]% for 55–66 years and 70.46 [64.6–76.32]% for 67–79 years on match trials and 87.51 [85.73–89.29]% for 40–54 years, 89.29 [87.41–91.16]% for 55–66 years and 84.77 [81.47–88.08]% for 67–79 years on non-match trials. Figure 6C shows accuracy rates on all trials, by age group, across repeated game-plays.
Delayed Match-to-Sample Game
This is not a speed challenge task, however, RTs to match trials were faster than non-match trials 886.99 [847.27–926.71] ms vs. 1,060.31 [1,013.4–1,107.22] ms, a pattern reflected in RTs by age group: 796.27 [754.02–838.52] ms, 890.05 [840.75–939.35] ms and 1,027.89 [914.95–1,140.83] ms for match trials compared to 944.11 [898.82–989.41] ms, 1,079.58 [1,007.6–1,151.56] ms and 1,208.17 [1,102.96–1,313.39] ms for non-match trials, for 40–54, 55–66, and 67–79 years. Memory performance is known to decrease with age, and divergence can be seen in Figure 6D for the oldest age-band, though again not between the younger and middle bands. Separate examination of the accuracy for match and non-match trials showed that this difference in performance was primarily driven by the non-match trials. Over the sample, accuracy was 93.16 [92.04–94.28] for match vs. 81.82 [79.35–84.30] for non-match trials, while accuracy rates across the age groups showed more difference for non-match compared to match trials: 85.67 [82.61–88.72], 80.98 [76.72–85.24], and 77.38 [72.51–82.24], compared to 93.85 [92.41–95.28], 93.12[91.24–94.99], and 92.12 [89.44–94.81] for non-match and match trials, respectively, for the age groups 40–54, 55–66, and 67–79 years. These results suggest that the non-match trials acted as effective lures.
EEG
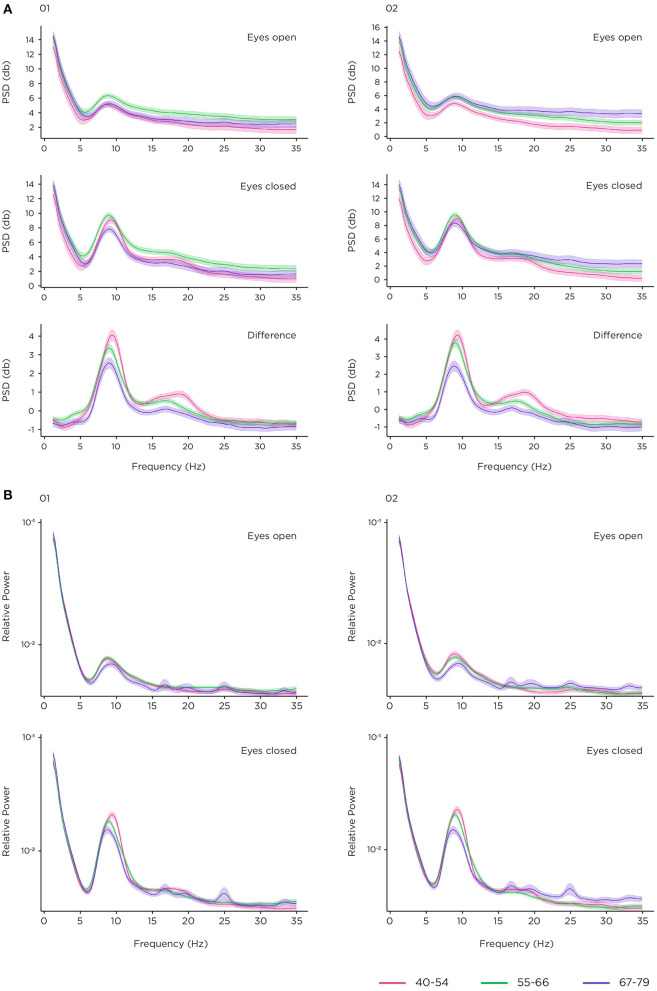
For the resting-state task, power spectral density (PSD) was plotted at occipital sites to explore the effectiveness of the platform to measure change in alpha power between the eyes-open and eyes-closed conditions of resting-state task, across the three different age bands (Figure 7). Data from all 78 participants was included in the analysis. The number of sessions per comparison at electrode site O1 for eyes-open/eyes-closed were 904 for 40–54 years, 1,511 for 55–66 years, and 791 for 67–79 years. For electrode site O2, number of sessions were 903 (40–54 years), 1,513 (55–66 years) and 792 (67–79 years). The eyes-open data clearly shows the expected 1/f pattern of signal power falling with increasing frequency, and an alpha band peak around 10 Hz. As expected, the alpha peak amplitude is increased in the eyes-closed condition, as well as in the lower beta band (15–20 Hz). Figure 7A displays the effect of age group on absolute band power. There is a clear monotonic decrease in power with age in the difference condition with the largest eyes-open/eyes-closed difference for those aged 40–54 and the smallest difference for those aged 67–79 in the alpha and lower beta range. Furthermore, it can be seen that the average peak alpha frequency is highest for younger participants, and lowest for older participants. No consistent pattern is apparent in alpha power for the eyes-open and eyes-closed conditions alone, although there are clear distinctions between groups in the gamma range (30–35 Hz). This may indicate a difference in noise floor between the age groups. We applied a suitable normalisation by taking the relative power on this analysis (80). Relative power is shown in Figure 7B, again demonstrating a stratified pattern of age group on alpha power and peak frequency, most evident in the graph of the eyes-closed condition. It is noticeable that there is more fluctuation in the higher frequencies for the oldest age group (67–79 year olds).
Figure 7.
Resting state task. (A) power spectral density (PSD) in decibels (dB) at O1 and O2 by age group in eyes-open and eyes-closed conditions, and the difference condition; (B) relative power at O1 and O2 by age group in eyes-open and eyes-closed conditions with logarithmic scaling for display only.
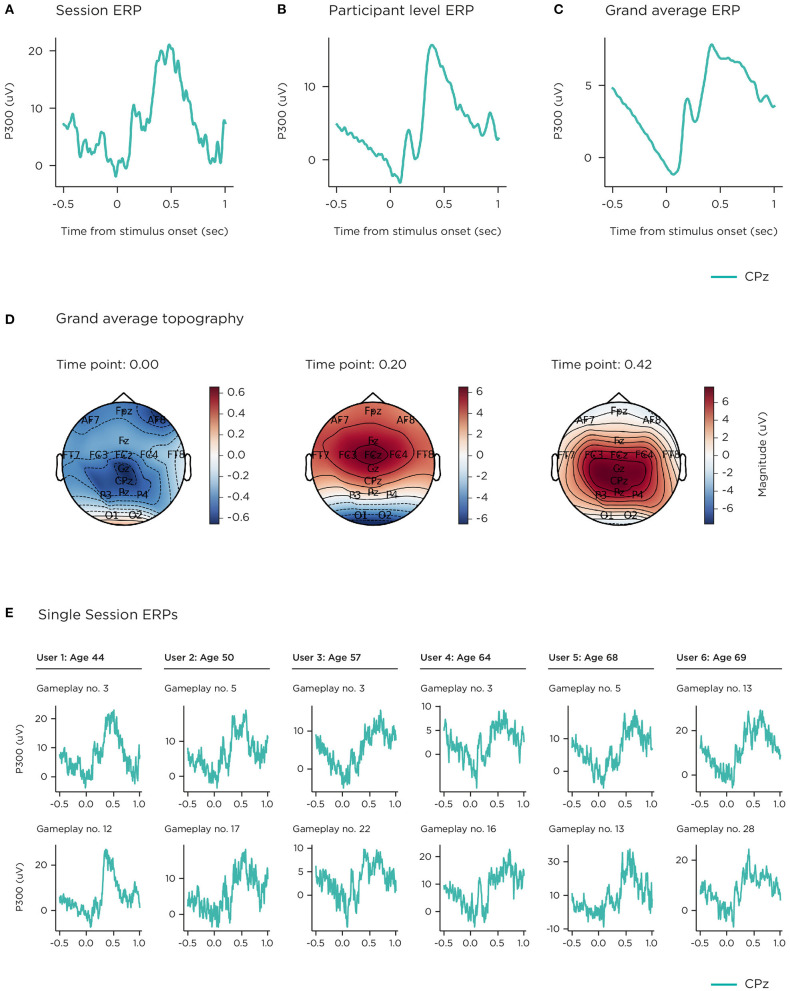
Evoked and event-related potentials elicited in the gamified 2-stimulus oddball and flanker tasks were also extracted at a single-session, single-participant and grand average level (shown in Figures 8, 9). Figure 8C illustrates the grand average ERP for target trials on the 2-stimulus oddball, at the centro-parietal location CPz, where the P300 is centred. This is a robust average over multiple sessions contributed by 77 participants, time-locked to the presentation of the stimulus (data from one participant, n = 26 sessions, did not meet quality thresholds for inclusion at this channel). Interpolated topographies (Figure 8D) across all 16 channels at ERP peaks are shown at 0, 200 and 420 ms post-stimulus onset. The principal waveform features of a P300 ERP are visible in the early sensory processing components (~0–250 ms with an occipital focus) and the P300 component (~300–500 ms, with a centro-parietal focus). A strong readiness potential can also be seen before stimulus presentation (−500–0 ms). The other two panels show the median stimulus-locked epoch from 29 correctly identified target trials from a single game-play session (Figure 8A), and the median-average across 18 out of a total of 21 game-play sessions (3 did not meet quality thresholds), contributed by a participant aged 44 years (Figure 8B). Figure 8E demonstrates examples of successful session-level ERPs evoked during a single game-play session, representing 6 users across the different age groups in the study (2 participants from each age group). Unsuccessful sessions yield waveforms that show a discernible ERP overlaid with noise, flat-line signals (e.g., due to an unconnected sensor), or noise of various heterogenous types.
Figure 8.
P300. (A) single-session median; (B) single-participant mean; (C) grand mean; (D) grand mean topographies selected at ERP peak timepoints; (E) examples of single-session median ERPs successfully recorded from game plays from 6 different participants (2 participants per age group).
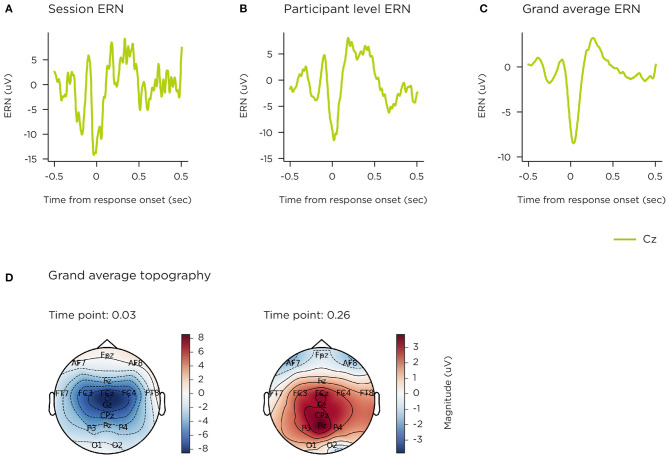
Figure 9.
ERN. (A) single-session median; (B) single-participant mean; (C) grand mean; (D) grand mean topographies selected at ERP peak timepoints.
Figure 9C illustrates the grand average difference ERP for error trials on the flanker task, at the central Cz location, where the ERN is observed (total 1,004 sessions). This is a robust average over multiple sessions contributed by 76 participants, time-locked to touch-response (single-channel data was excluded from 2 participants, who contributed 6 and 8 sessions). Grand average topography (Figure 9D) across all 16 channels at ERP peaks are shown at 30 and 260 ms post-stimulus onset. The event-related negativity (ERN) waveform is clearly represented (peaking at ~50 ms), with a central focus, as is the error positivity (Pe) with a centro-parietal focus (peak ~250 ms). Figure 9A shows the corresponding median response-locked epoch across 12 error trials following a single game-play session, contributed by a participant aged 52 years. Figure 9B displays that participant's median-average across 14 out of 16 game-play sessions.
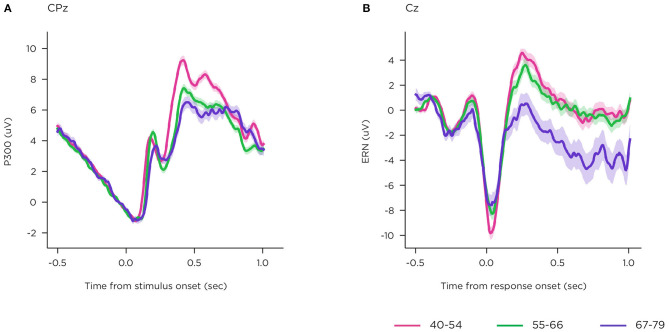
The P300 and ERN components were also compared by age group. Figure 10A shows grand-averaged epochs per age group on single channel CPz for the stimulus-locked ERP from the visual oddball, and Figure 10B shows these at Cz for the response-locked ERP of the flanker task. The number of participants and sessions per comparison at electrode site CPz were 26 and 397 for 40–54 years; 34 and 515 for 55–66 years; 17 and 240 for 67–79 years. For electrode site Cz, number of participants and sessions were 23 and 339 (40–54 years); 35 and 473 (55–66 years); 17 and 182 (67–79 years). The impact of signal variability from individual sessions (both noise, and genuine inter-individual differences) is quantified in the 95% confidence intervals illustrated.
Figure 10.
Showing age-related differences in event-related components recorded using the platform. (A) P300; (B) ERN.
The P300 shows early differences in latency in sensory processing, and separation in amplitude among the groups, where disruption increases with age. In the Flanker task ERP, the early ERN component is attenuated in both older groups, relative to the youngest group, and the later Pe component is reduced for the oldest group.
Discussion
Usability
This proof-of-concept paper reports findings from the first time that this novel EEG platform was deployed in-field. After a single training session, participants, including older adults up to the age of 79 years, were able to use the technology at home to successfully perform EEG and behavioural recordings without the supervision of trained technicians. This study yielded 3,603 uploaded sessions, >95% of which contained usable data (i.e., EEG and behavioural metrics could be extracted from the submitted data), providing encouraging evidence supporting the feasibility of this technological approach to cognitive neuroscience research.
Users were asked to trial and evaluate this new system for monitoring brain health at home and to contribute five 30-min recording sessions per week, which was a considerable effort given that no extrinsic incentives or disincentives were applied to promote adherence to this schedule. Adherence to this schedule was remarkably high, relative to other reports of in-home monitoring devices in older populations over similar time courses [~55% (81, 82)]. For the current study, attrition was low (12.4%) and the average contribution was >4 sessions per week (>80% adherence to schedule). Older adults had the highest rates of adherence, indicating that age was no impediment to using the system regularly. A high level of adherence was maintained throughout the 3-month period without substantial decline in the latter weeks of participation, testament to both the power of gamification and usability of the system motivating and facilitating repeated play, and the level of commitment from the study participants. Maintaining adherence in unsupervised environments is challenging and may be particularly so for psychiatric populations (83), however widespread evidence from other diseases, where there has been a broad uptake in new technologies, indicates that patient-centric digital monitoring provides more objective, frequent tracking with clear healthcare benefits (84–86) and have been shown to lead to better compliance relative to paper based assessments (86, 87).
Reported usability was somewhat lower for the oldest age-band, although it is worth noting that their lowest average score still falls within the range between “ok” and “good” (88). Contrary to our expectations, this did not result in reduced adherence, suggesting that many challenges of manual dexterity or familiarity with digital technologies had been successfully mitigated during the initial user-focused design process. However, signal reliability measures indicated that the oldest age band experienced the greatest difficulty in obtaining good sensor connectivity, particularly around the midline sensors (Cz) located at the top of the head. Younger participants achieved slightly better connectivity at Cz but also better connectivity on adjacent sensors. Head shape is variable at the crown, meaning that generic headset sizing options are not always optimal. That location may also require additional manual dexterity and adjustment, which is more difficult for older populations. In the light of these findings from the first deployment of this technology in-field, subsequent incremental improvements to the headset, app and enrolment training procedures have been deployed which have resulted in superior sensor connectivity and data quality (89, 90).
In addition to investigating the overall usability of the platform, empirical data collected in this study was used to assess the potential of collecting scientifically valid neurocognitive data from remote, fully autonomous participants.
Behaviour
All gamified cognitive tasks exhibited some degree of a learning effect. Reaction times generally decreased rapidly over the first five sessions of a given task (see Figure 6). This likely reflects the development of task-specific perceptual-motor skill, rather than a change in the underlying cognitive function probed by the task (i.e., “brain training”). Time spent developing task strategies, and learning the layout of the task environment, is likely to have enabled more effective allocation of visual attention and therefore faster responding (91). Age-related effects on speed of response were generally preserved throughout this learning phase and into asymptote, with the oldest participants consistently making their responses more slowly than other age groups, consistent with the literature. Regarding accuracy over time, participants on average improved very slowly and consistently on the n-back task throughout the study, as expected since n-back variants are typically included with brain training suites due to their inherent learnability (92). Participants, regardless of age band, demonstrated less of a learning effect in the visual delayed match-to-sample task, potentially reflecting the simplicity of this basic old/new matching task for healthy adults however again, the oldest participants scored lowest on this task throughout the study, consistent with age-related decline in memory performance. These learning curves themselves (Figure 6), enabled through the ability to collect multiple assessments over time, may be a rich source of data and potentially informative measures of underlying cognitive function (93), with recent research showing that rate of learning, in the context of a cognitive task conducted over multiple days, can differentiate groups by age (94) and neuropathology (95).
EEG
EEG signals described in this paper (resting-state spectral activity and the P300 and ERN ERPs) demonstrate morphology consistent with those elicited by non-gamified, laboratory paradigms described in the literature. Furthermore, grand average visualisations of P300 and ERN ERPs across age bands replicate classic electrophysiological patterns of age-related change.
The study design included repeated use to permit aggregation of EEG data collected in the home, as a means of improving reliability and signal to noise ratio. The focus in this paper is on group level grand-average analyses, common in cognitive neuroscience literature. Although, as can be seen in the data presented in this paper, it is feasible to obtain cognitive ERP components from users (across different age groups) based on single, home-based sessions. As might be expected, not all sessions were available for analysis with factors such as saturated signal or high variance rendering the data unusable. However, over 95% of sessions contained EEG from which at least a portion of the data was usable, even though certain sections of that session, or certain sensors, may have been noisy. In order to support participants to achieve good signal quality, the system included a sensor signal check step at the beginning of every session to give feedback on impedance levels to encourage self-adjustment for a good connection.
EEG devices that offer miniaturisation of the EEG amplifier, use of Bluetooth technology to transmit EEG data, precise stimulus event-marking, and a choice of wet or dry sensor set-ups, have been extensively evaluated in the literature [e.g., (54, 96–99)]. These demonstrate reduced set-up times and greater portability while generally maintaining good signal-to-noise ratio [e.g., (52, 53, 100, 101); but see Duvinage et al. (102), Maskeliunas et al. (103)]. However, the authors are not aware of reports of any other mobile EEG system for which repeated ERP data collection has been demonstrated in participants' home environment without a researcher present, as was the case in this study.
The P300 elicited from the 2-stimulus oddball task exhibited reduced amplitude and latency for the older age groups, consistent with previous studies (62–64). Whilst the underlying mechanisms are yet not fully elucidated, recent evidence points toward the P300 reflecting the accumulation of information leading to a decision (60), the ability to do this effectively being impaired by ageing and cognitive decline (104). ERP components evoked from the Flanker task also demonstrated sensitivity to ageing with a smaller ERN for the older age groups and a weak Pe for the oldest age band, reproducing known effects in the literature, reflecting a general weakening of the processes underpinning cognitive control in ageing populations (67, 105). Resting state EEG PSD demonstrated alpha band increase in the eyes closed condition relative to eyes open as expected. In the absolute power analyses differences in noise levels were observed. Grummet et al. (106) discusses variability in noise floor in dry EEG, which in this case may potentially be driven by factors such as systematic variation in the use of the headset, and age-related changes in skin condition (107). However, having controlled for differences in noise floor levels, we observed alpha band power and peak frequency stratification across that age groups that align with the effects of ageing on brain activity during resting state typically reported in the literature (74, 108, 109).
Conclusion
In this paper we have described the first large scale field trial of a new suite of tools to collect clinically relevant domain-specific markers of brain function and cognitive performance unsupervised in the home. Human-factors feasibility was demonstrated by high reported usability, low levels of withdrawal, and adherence of >80% over a 5-day-per-week, 3-month long, uncompensated participation. Newly gamified versions of established tasks were trialled and were successful in replicating key aspects of behaviour from their lab-based counterparts. Widespread learning effects were observed, as would be expected on repeated plays, but age-related differences were preserved over many weeks of repeated play. Grand average EEG data from the resting state, visual oddball and flanker tasks all illustrated core features of frequency content, waveform morphology and timing, and scalp topographies to confirm that they faithfully replicate the lab-based tasks on which they were modelled.
Challenges of data quality were encountered. On an average session, 14 or 15 sensors (of 16) provided EEG signals that could be analysed, the remainder lost due to issues with contact reliability with particular scalp locations and age cohorts. Certain sessions were evaluated as too noisy for inclusion in grand average analyses and early behavioural sessions proved more variable than later ones. Since this study was completed, incremental improvements to the headset, tablet-based app and participant familiarisation procedures have been made that have increased signal quality (89, 90).
While the focus of this paper is on ageing, and cognitive functions of relevance to Alzheimer's disease and other pathologies underlying dementia, this suite of tools can also include additional tasks (e.g., emotional face processing, passive auditory oddball) suitable for use in mood disorders, psychosis (110), and measurement of treatment response in psychiatry (89, 111).
Advances in wearable electronics, dry sensors and user-facing interactive technologies enable EEG as an easy-to-use affordable biomarker of cognition, grounded directly in brain function. Decades of scientific literature support EEG as an emerging translational biomarker for disease cases in neuropsychiatric (schizophrenia, depression) (20, 112, 113) and neurodegenerative (e.g., Alzheimer's) disease (114–116). Sampling a broad suite of cognitive functions (including memory, attention, and executive function) offers coverage of multiple cognitive domains, which has greater predictive accuracy for disease progression (117, 118). Cloud computing can securely collect data from distributed locations, automatically evaluate quality, and use machine learning techniques to derive composite markers based on neural activity and behavioural performance from single and multiple cognitive domains (119). These innovations in technology, supported by scientific literature, make it possible to use large-scale longitudinal sampling of real-world data, to support potential future use-cases in early detection, personalised medicine, progression tracking, and measurement of treatment response for neuropsychiatric disorders.
Data Availability Statement
The datasets presented in this article are not readily available because the data collected using the Cumulus Neuroscience platform is commercially sensitive and contains proprietary information. Requests to access supporting data will be considered from bona fide researchers upon reasonable request. Requests to access the datasets should be directed to alison@cumulusneuro.com.
Ethics Statement
The studies involving human participants were reviewed and approved by Queen's University Belfast Faculty Research Ethics Committee (EPS). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
EM, BMu, BMc, PP, and AB contributed to the conception and design of the study and paper. AB performed data collection. EM, LR-D, HN, MI, JD, FB, BMu, and AB performed analysis and interpretation. EM, JD, BMu, and AB wrote the manuscript. LR-D, HN, and MI wrote sections of the manuscript. All authors critically revised the manuscript and approve the submitted version.
Conflict of Interest
LR-D, AB, EM, JD, HN, MI, FB, and BMu are employees of the company Cumulus Neuroscience Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank Liam Watson and Aoife Sweeney for their part in data collection and Weronika Jaworowska-Bialko for her contributions to usability analysis. We also extend our gratitude to all our participants who gave up their time so generously to take part in this 12-week study. We would like to thank Xi Jiang and Albert Yang for their helpful and constructive feedback during review.
Footnotes
Funding. The study on which this paper is based was funded by the Innovate UK Biomedical Catalyst programme (Grant Number: IUK 102862). LR-D was supported by Science Foundation Ireland (18/IF/6272) and the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 893823.
References
- 1.Brietzke E, Hawken ER, Idzikowski M, Pong J, Kennedy SH, Soares CN. Integrating digital phenotyping in clinical characterization of individuals with mood disorders. Neurosci Biobehav Rev. (2019) 104:223–30. 10.1016/j.neubiorev.2019.07.009 [DOI] [PubMed] [Google Scholar]
- 2.Carpenter G, Harbin HT, Smith RL, Hornberger J, Nash DB. A promising new strategy to improve treatment outcomes for patients with depression. Popul Health Manage. (2019) 22:223–8. 10.1089/pop.2018.0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ermers NJ, Hagoort K, Scheepers FE. The predictive validity of machine learning models in the classification and treatment of major depressive disorder: state of the art and future directions. Front Psychiatry. (2020) 11:472. 10.3389/fpsyt.2020.00472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillan CM, Whelan R. What big data can do for treatment in psychiatry. Curr Opin Behav Sci. (2017) 18:34–42. 10.1016/j.cobeha.2017.07.003 [DOI] [Google Scholar]
- 5.Jollans L, Whelan R. Neuromarkers for mental disorders: harnessing population neuroscience. Front Psychiatry. (2018) 9:242. 10.3389/fpsyt.2018.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newson JJ, Thiagarajan TC. EEG frequency bands in psychiatric disorders : a review of resting state studies. Front Hum Neurosci. (2019) 12:521. 10.3389/fnhum.2018.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russ TC, Woelbert E, Davis KAS, Hafferty JD, Ibrahim Z, Inkster B, et al. How data science can advance mental health research. Nat Hum Behav. (2019) 3:24–32. 10.1038/s41562-018-0470-9 [DOI] [PubMed] [Google Scholar]
- 8.Clapp WC, Hamm JP, Kirk IJ, Teyler TJ. Translating long-term potentiation from animals to humans: a novel method for noninvasive assessment of cortical plasticity. Biol Psychiatry. (2012) 71:496–502. 10.1016/j.biopsych.2011.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drinkenburg WHIM, Ruigt GSF, Ahnaou A. Pharmaco-EEG studies in animals: an overview of contemporary translational applications. Neuropsychobiology. (2015) 72:151–64. 10.1159/000442210 [DOI] [PubMed] [Google Scholar]
- 10.Leiser SC, Dunlop J, Bowlby MR, Devilbiss DM. Aligning strategies for using EEG as a surrogate biomarker: a review of preclinical and clinical research. Biochem Pharmacol. (2011) 81:1408–21. 10.1016/j.bcp.2010.10.002 [DOI] [PubMed] [Google Scholar]
- 11.Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol Psychiatry. (2012) 71:98–104. 10.1016/j.biopsych.2011.08.023 [DOI] [PubMed] [Google Scholar]
- 12.Erickson MA, Ruffle A, Gold JM. A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biol Psychiatry. (2016) 79:980–7. 10.1016/j.biopsych.2015.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton HK, Boos AK, Mathalon DH. Electroencephalography and event-related potential biomarkers in individuals at clinical high risk for psychosis. Biol Psychiatry. (2020) 88:294–303. 10.1016/j.biopsych.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Light GA, Naatanen R. Mismatch negativity is a breakthrough biomarker for understanding and treating psychotic disorders. Proc Natl Acad Sci USA. (2013) 110:15175–6. 10.1073/pnas.1313287110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Näätänen R, Todd J, Schall U. Mismatch negativity (MMN) as biomarker predicting psychosis in clinically at-risk individuals. Biol Psychol. (2016) 116:36–40. 10.1016/j.biopsycho.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 16.Nagai T, Tada M, Kirihara K, Araki T, Jinde S, Kasai K. Mismatch negativity as a “translatable” brain marker toward early intervention for psychosis: a review. Front Psychiatry. (2013) 4:115. 10.3389/fpsyt.2013.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez VB, Roach BJ, Woods SW, Srihari VH, McGlashan TH, Ford JM, et al. Chapter 10 - Early auditory gamma-band responses in patients at clinical high risk for schizophrenia. In: Başar E, Başar-Eroĝlu C, Özerdem A, Rossini PM, Yener GG, editors. Application of Brain Oscillations in Neuropsychiatric Diseases: Selected Papers from “Brain Oscillations in Cognitive Impairment and Neurotransmitters”, Vol. 62. Istanbul: Elsevier; (2013). p. 1–375. [Google Scholar]
- 18.Perez VB, Woods SW, Roach BJ, Ford JM, McGlashan TH, Srihari VH, et al. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry. (2014) 75:459–69. 10.1016/j.biopsych.2013.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Normann C, Schmitz D, Fürmaier A, Döing C, Bach M. Long-Term plasticity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry. (2007) 62:373–80. 10.1016/j.biopsych.2006.10.006 [DOI] [PubMed] [Google Scholar]
- 20.Olbrich S, van Dinteren R, Arns M. Personalized medicine: review and perspectives of promising baseline EEG biomarkers in major depressive disorder and attention deficit hyperactivity disorder. Neuropsychobiology. (2015) 72:229–40. 10.1159/000437435 [DOI] [PubMed] [Google Scholar]
- 21.Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. J Abnorm Psychol. (2010) 119:502–12. 10.1037/a0019196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babiloni C, Lizio R, Del Percio C, Marzano N, Soricelli A, Salvatore E, et al. Cortical sources of resting state EEG rhythms are sensitive to the progression of early stage Alzheimer's disease. J Alzheimers Dis. (2013) 34:1015–35. 10.3233/JAD-121750 [DOI] [PubMed] [Google Scholar]
- 23.Choi J, Ku B, You YG, Jo M, Kwon M, Choi Y, et al. Resting-state prefrontal EEG biomarkers in correlation with MMSE scores in elderly individuals. Sci Rep. (2019) 9:10468. 10.1038/s41598-019-46789-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghorbanian P, Devilbiss DM, Verma A, Bernstein A, Hess T, Simon AJ, et al. Identification of resting and active state EEG features of alzheimer's disease using discrete wavelet transform. Ann Biomed Eng. (2013) 41:1243–57. 10.1007/s10439-013-0795-5 [DOI] [PubMed] [Google Scholar]
- 25.Lai CL, Lin RT, Liou LM, Liu CK. The role of event-related potentials in cognitive decline in Alzheimer's disease. Clin Neurophysiol. (2010) 121:194–9. 10.1016/j.clinph.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 26.Musaeus CS, Nielsen MS, Østerbye NN, Høgh P. Decreased parietal beta power as a sign of disease progression in patients with mild cognitive impairment. J Alzheimers Dis. (2018) 65:475–87. 10.3233/JAD-180384 [DOI] [PubMed] [Google Scholar]
- 27.Parra MA, Ascencio LL, Urquina HF, Manes F, Ibáñez AM. P300 and neuropsychological assessment in mild cognitive impairment and alzheimer dementia. Front Neurol. (2012) 3:172. 10.3389/fneur.2012.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smailovic U, Jelic V. Neurophysiological markers of alzheimer's disease: quantitative EEG approach. Neurol Ther. (2019) 8:37–55. 10.1007/s40120-019-00169-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tóth B, File B, Boha R, Kardos Z, Hidasi Z, Gaál ZA, et al. EEG network connectivity changes in mild cognitive impairment - Preliminary results. Int J Psychophysiol. (2014) 92:1–7. 10.1016/j.ijpsycho.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 30.Borghini G, Astolfi L, Vecchiato G, Mattia D, Babiloni F. Measuring neurophysiological signals in aircraft pilots and car drivers for the assessment of mental workload, fatigue and drowsiness. Neurosci Biobehav Rev. (2014) 44:58–75. 10.1016/j.neubiorev.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 31.Wilson GF, Russell CA, Monnin JW, Estepp JR, Christensen JC. How does day-to-day variability in psychophysiological data affect classifier accuracy? Proc Hum Fact Ergonom Soc. (2010) 1:264–8. 10.1177/154193121005400317 [DOI] [Google Scholar]
- 32.Duke Han S, Nguyen CP, Stricker NH, Nation DA. Detectable neuropsychological differences in early preclinical alzheimer's disease: a meta-analysis. Neuropsychol Rev. (2017) 27:305–25. 10.1007/s11065-017-9366-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassenstab J, Ruvolo D, Jasielec M, Xiong C, Grant E, Morris JC. Absence of practice effects in preclinical Alzheimer's disease. Neuropsychology. (2015) 29:940–8. 10.1037/neu0000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Binder LM, Iverson GL, Brooks BL. To err is human: “abnormal” neuropsychological scores and variability are common in healthy adults. Arch Clin Neuropsychol. (2009) 24:31–46. 10.1093/arclin/acn001 [DOI] [PubMed] [Google Scholar]
- 35.Cooley SA, Heaps JM, Bolzenius JD, Salminen LE, Baker LM, Scott SE, et al. Longitudinal change in performance on the montreal cognitive assessment in older adults. Clin Neuropsychol. (2015) 29:824–35. 10.1080/13854046.2015.1087596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falleti M, Maruff P, Collie A, Darby D. Practice effects associated with the repeated assessment of cognitive function using the CogState battery at 10-minute, one week and one month test-retest intervals. J Clin Exp Neuropsychol. (2006) 28:1095–112. 10.1080/13803390500205718 [DOI] [PubMed] [Google Scholar]
- 37.Goldberg TE, Harvey PD, Wesnes KA, Snyder PJ, Schneider LS. Practice effects due to serial cognitive assessment: implications for preclinical Alzheimer's disease randomized controlled trials. Alzheimers Dement Diag Assess Dis Monitor. (2015) 1:103–11. 10.1016/j.dadm.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mario B, Massimiliano M, Chiara M, Alessandro S, Antonella C, Gianfranco F. White-coat effect among older patients with suspected cognitive impairment: prevalence and clinical implications. Int J Geriatr Psychiatry. (2009) 24:509–17. 10.1002/gps.2145 [DOI] [PubMed] [Google Scholar]
- 39.Sliwinski MJ, Mogle JA, Hyun J, Munoz E, Smyth JM, Lipton RB. Reliability and validity of ambulatory cognitive assessments. Assessment. (2018) 25:14–30. 10.1177/1073191116643164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brose A, Schmiedek F, Lövdén M, Lindenberger U. Daily variability in working memory is coupled with negative affect: the role of attention and motivation. Emotion. (2012) 12:605–17. 10.1037/a0024436 [DOI] [PubMed] [Google Scholar]
- 41.Brose A, Lövdén M, Schmiedek F. Daily fluctuations in positive affect positively co-vary with working memory performance. Emotion. (2014) 14:1–6. 10.1037/a0035210 [DOI] [PubMed] [Google Scholar]
- 42.Hess TM, Ennis GE. Age differences in the effort and costs associated with cognitive activity. J Gerontol Ser B Psychol Sci Soc Sci. (2012) 67:447–55. 10.1093/geronb/gbr129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metternich B, Schmidtke K, Hüll M. How are memory complaints in functional memory disorder related to measures of affect, metamemory and cognition? J Psychosomat Res. (2009) 66:435–44. 10.1016/j.jpsychores.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 44.Stawski RS, Sliwinski MJ, Smyth JM. Stress-related cognitive interference predicts cognitive function in old age. Psychol Aging. (2006) 21:535–44. 10.1037/0882-7974.21.3.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashemi A, Pino LJ, Moffat G, Mathewson KJ, Aimone C, Bennett PJ, et al. Characterizing population EEG dynamics throughout adulthood. ENeuro. (2016) 3:1–13. 10.1523/ENEURO.0275-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cormack FK, Taptiklis N, Abbott RA, Anatürk M, Cartland I, Coppieters L, et al. Changes to validity of online cognitive assessment in young and older adults: a comparison to supervised testing using the cantab battery. Alzheimers Dement. (2016) 12:P286–7. 10.1016/j.jalz.2016.06.520 [DOI] [Google Scholar]
- 47.Lancaster C, Koychev I, Blane J, Chinner A, Chatham C, Taylor K, et al. Gallery game: smartphone-based assessment of long-term memory in adults at risk of Alzheimer's disease. J Clin Exp Neuropsychol. (2020) 42:329–43. 10.1080/13803395.2020.1714551 [DOI] [PubMed] [Google Scholar]
- 48.Moore RC, Swendsen J, Depp CA. Applications for self-administered mobile cognitive assessments in clinical research: a systematic review. Int J Methods Psychiatr Res. (2017) 26:e1562. 10.1002/mpr.1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Resnick HE, Lathan CE. From battlefield to home: a mobile platform for assessing brain health. MHealth. (2016) 2:30. 10.21037/mhealth.2016.07.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogers JM, Johnstone SJ, Aminov A, Donnelly J, Wilson PH. Test-retest reliability of a single-channel, wireless EEG system. Int J Psychophysiol. (2016) 106:87–96. 10.1016/j.ijpsycho.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 51.Wong SWH, Chan RHM, Mak JN. Spectral modulation of frontal EEG during motor skill acquisition: a mobile EEG study. Int J Psychophysiol. (2014) 91:16–21. 10.1016/j.ijpsycho.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 52.Badcock NA, Mousikou P, Mahajan Y, De Lissa P, Thie J, McArthur G. Validation of the emotiv EPOC® EEG gaming systemfor measuring research quality auditory ERPs. PeerJ. (2013) 2013:e38. 10.7717/peerj.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Badcock NA, Preece KA, de Wit B, Glenn K, Fieder N, Thie J, et al. Validation of the emotiv EPOC EEG system for research quality auditory event-related potentials in children. PeerJ. (2015) 3:e907. 10.7717/peerj.907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krigolson OE, Williams CC, Norton A, Hassall CD, Colino FL. Choosing MUSE: validation of a low-cost, portable EEG system for ERP research. Front Neurosci. (2017) 11:109. 10.3389/fnins.2017.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuziek JWP, Shienh A, Mathewson KE. Transitioning EEG experiments away from the laboratory using a Raspberry Pi 2. J Neurosci Methods. (2017) 277:75–82. 10.1016/j.jneumeth.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 56.Lumsden J, Edwards EA, Lawrence NS, Coyle D, Munafò MR. Gamification of cognitive assessment and cognitive training: a systematic review of applications and efficacy. JMIR Ser Games. (2016) 4:e11. 10.2196/games.5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coughlan G, Coutrot A, Khondoker M, Minihane AM, Spiers H, Hornberger M. Toward personalized cognitive diagnostics of at-genetic-risk Alzheimer's disease. Proc Natl Acad Sci USA. (2019) 116:9285–92. 10.1073/pnas.1901600116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herrmann CS, Knight RT. Mechanisms of human attention: event-related potentials and oscillations. Neurosci Biobehav Rev. (2001) 25:465–76. 10.1016/S0149-7634(01)00027-6 [DOI] [PubMed] [Google Scholar]
- 59.Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biol Psychol. (1995) 41:103–46. 10.1016/0301-0511(95)05130-9 [DOI] [PubMed] [Google Scholar]
- 60.Twomey DM, Murphy PR, Kelly SP, O'Connell RG. The classic P300 encodes a build-to-threshold decision variable. Euro J Neurosci. (2015) 42:1636–43. 10.1111/ejn.12936 [DOI] [PubMed] [Google Scholar]
- 61.Woods DL, Wyma JM, Yund EW, Herron TJ, Reed B. Age-related slowing of response selection and production in a visual choice reaction time task. Front Hum Neurosci. (2015) 9:193. 10.3389/fnhum.2015.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fjell AM, Walhovd KB. P300 and neuropsychological tests as measures of aging: scalp topography and cognitive changes. Brain Topogr. (2001) 14:25–40. 10.1023/A:1012563605837 [DOI] [PubMed] [Google Scholar]
- 63.Pavarini SCI, Brigola AG, Luchesi BM, Souza ÉN, Rossetti ES, Fraga FJ, et al. On the use of the P300 as a tool for cognitive processing assessment in healthy aging: a review. Dement Neuropsychol. (2018) 12:1–11. 10.1590/1980-57642018dn12-010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Dinteren R, Arns M, Jongsma MLA, Kessels RPC. P300 development across the lifespan: a systematic review and meta-analysis. PLoS ONE. (2014) 9:e0087347. 10.1371/journal.pone.0087347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. (2004) 111:931–59. 10.1037/0033-295X.111.4.931 [DOI] [PubMed] [Google Scholar]
- 66.Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GPH, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. (2001) 38:752–60. 10.1111/1469-8986.3850752 [DOI] [PubMed] [Google Scholar]
- 67.Hoffmann S, Falkenstein M. Aging and error processing: age related increase in the variability of the error-negativity is not accompanied by increase in response variability. PLoS ONE. (2011) 6:e0017482. 10.1371/journal.pone.0017482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong S, Reder LM, Yao Y, Liu Y, Chen F. Individual differences in working memory capacity are reflected in different ERP and EEG patterns to task difficulty. Brain Res. (2015) 1616:146–56. 10.1016/j.brainres.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 69.Bopp KL, Verhaeghen P. Aging and n-back performance: a meta-analysis. J Gerontol Ser B. (2018) 75:229–40. 10.1093/geronb/gby024 [DOI] [PubMed] [Google Scholar]
- 70.Grady C. The cognitive neuroscience of ageing. Nat Rev Neurosci. (2012) 13:491–505. 10.1038/nrn3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mecklinger A. On the modularity of recognition memory for object form and spatial location: a topographic ERP analysis. Neuropsychologia. (1998) 36:441–60. 10.1016/S0028-3932(97)00128-0 [DOI] [PubMed] [Google Scholar]
- 72.Alloway CED, Ogilvie RD, Shapiro CM. The alpha attenuation test: assessing excessive daytime sleepiness in narcolepsy-cataplexy. Sleep. (1997) 20:258–66. 10.1093/sleep/20.4.258 [DOI] [PubMed] [Google Scholar]
- 73.Barry RJ, Rushby J, Wallace M, Clarke A, Johnstone S, Zlojutro I. Caffeine effects on resting-state arousal. Clin Neurophysiol. (2005) 116:2693–700. 10.1016/j.clinph.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 74.Rossini PM, Rossi S, Babiloni C, Polich J. Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Progr Neurobiol. (2007) 83:375–400. 10.1016/j.pneurobio.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 75.Stampi C, Stone P, Michimori A. A new quantitative method for assessing sleepiness: the alpha attenuation test. Work Stress. (1995) 9:368–76. 10.1080/02678379508256574 [DOI] [Google Scholar]
- 76.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 77.Coen RF, Cahill R, Lawlor BA. Things to watch out for when using the montreal cognitive assessment (MoCA). Int J Geriatr Psychiatry. (2011) 26:107–8. 10.1002/gps.2471 [DOI] [PubMed] [Google Scholar]
- 78.Kenny RA, Coen RF, Frewen J, Donoghue OA, Cronin H, Savva GM. Normative values of cognitive and physical function in older adults: findings from the irish longitudinal study on ageing. J Am Geriatr Soc. (2013) 61:S279–90. 10.1111/jgs.12195 [DOI] [PubMed] [Google Scholar]
- 79.Brooke J. SUS: a “quick and dirty” usability scale. In: Jordan PW, Thomas B, Weerdmeester BA, McClelland IL, editors. Usability Evaluation in Industry. London: Taylor and Francis; (1996). p. 189–94. [Google Scholar]
- 80.McEvoy K, Hasenstab K, Senturk D, Sanders A, Jeste SS. Physiologic artifacts in resting state oscillations in young children: methodological considerations for noisy data. Brain Imag Behav. (2015) 9:104–14. 10.1007/s11682-014-9343-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, et al. Telemonitoring in patients with heart failure. N Engl J Med. (2010) 363:2301–9. 10.1056/NEJMoa1010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure. JAMA Intern Med. (2016) 176:310. 10.1001/jamainternmed.2015.7712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sajatovic M, Velligan DI, Weiden PJ, Valenstein MA, Ogedegbe G. Measurement of psychiatric treatment adherence. J Psychosomat Res. (2010) 69:591–9. 10.1016/j.jpsychores.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Argent R, Daly A, Caulfield B. Patient involvement with home-based exercise programs: can connected health interventions influence adherence? JMIR MHealth UHealth. (2018) 6:e47. 10.2196/mhealth.8518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maguire R, Fox PA, McCann L, Miaskowski C, Kotronoulas G, Miller M, et al. The eSMART study protocol: a randomised controlled trial to evaluate electronic symptom management using the advanced symptom management system (ASyMS) remote technology for patients with cancer. BMJ Open. (2017) 7:e015016. 10.1136/bmjopen-2016-015016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Masterson Creber RM, Hickey KT, Maurer MS. Gerontechnologies for older patients with heart failure: what is the role of smartphones, tablets, and remote monitoring devices in improving symptom monitoring and self-care management? Current Cardiovasc Risk Rep. (2016) 10:30. 10.1007/s12170-016-0511-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials. (2003) 24:182–99. 10.1016/S0197-2456(02)00320-3 [DOI] [PubMed] [Google Scholar]
- 88.Bangor A, Kortum PT, Miller JT. An empirical evaluation of the system usability scale. Int J Hum Comput Interact. (2008) 24:574–94. 10.1080/10447310802205776 [DOI] [Google Scholar]
- 89.Barbey F, Dyer JF, McWilliams EC, Nolan H, Murphy B. Conventional wet eeg vs dry-sensor wireless eeg: comparing signal reliability through measures of neuronal integrity. In: Advances in Alzheimer's and Parkinson's Therapies: An AAT-AD/PD Focus Meeting (2020). Available online at: https://aat-adpd.kenes.com/
- 90.Murphy B, Barbey F, Buick AR, Dyer JF, Farina F, McGuinness B, et al. Replicating lab electrophysiology with older users in the home, using gamified dry EEG. Alzheimers Dement. (2019) 15:P867. 10.1016/j.jalz.2019.06.4606 [DOI] [Google Scholar]
- 91.Reingold EM, Charness N, Pomplun M, Stampe DM. Visual span in expert chess players: evidence from eye movements. Psychol Sci. (2001) 12:48–55. 10.1111/1467-9280.00309 [DOI] [PubMed] [Google Scholar]
- 92.Colom R, Ramon FJ, Abad FJ, Shih PC, Privado J, Froufe M, et al. Adaptive n-back training does not improve fluid intelligence at the construct level: gains on individual tests suggest that training may enhance visuospatial processing. Intelligence. (2013) 41:712–27. 10.1016/j.intell.2013.09.002 [DOI] [Google Scholar]
- 93.Hassenstab J, Monsell SE, Mock C, Roe CM, Cairns NJ, Morris JC, et al. Neuropsychological markers of cognitive decline in persons with Alzheimer disease neuropathology. J Neuropathol Exp Neurol. (2015) 74:1086–92. 10.1097/NEN.0000000000000254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baker JE, Bruns L, Hassenstab J, Masters CL, Maruff P, Lim YY. Use of an experimental language acquisition paradigm for standardized neuropsychological assessment of learning: a pilot study in young and older adults. J Clin Exp Neuropsychol. (2019) 42:55–65. 10.1080/13803395.2019.1665626 [DOI] [PubMed] [Google Scholar]
- 95.Lim YY, Baker JE, Bruns L, Mills A, Fowler C, Fripp J, et al. Association of deficits in short-term learning and Aβ and hippocampal volume in cognitively normal adults. Neurology. (2020) 95:e2577–85. 10.1212/WNL.0000000000010728 [DOI] [PubMed] [Google Scholar]
- 96.De Vos M, Gandras K, Debener S. Towards a truly mobile auditory brain-computer interface: exploring the P300 to take away. Int J Psychophysiol. (2014) 91:46–53. 10.1016/j.ijpsycho.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 97.Hairston WD, Whitaker KW, Ries AJ, Vettel JM, Bradford JC, Kerick SE, et al. Usability of four commercially-oriented EEG systems. J Neural Eng. (2014) 11:046018. 10.1088/1741-2560/11/4/046018 [DOI] [PubMed] [Google Scholar]
- 98.Kam JWY, Griffin S, Shen A, Patel S, Hinrichs H, Heinze HJ, et al. Systematic comparison between a wireless EEG system with dry electrodes and a wired EEG system with wet electrodes. NeuroImage. (2019) 184:119–29. 10.1016/j.neuroimage.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Radüntz T, Meffert B. User experience of 7 mobile electroencephalography devices: comparative study. JMIR MHealth UHealth. (2019) 7:1–18. 10.2196/14474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Debener S, Minow F, Emkes R, Gandras K, de Vos M. How about taking a low-cost, small, and wireless EEG for a walk? Psychophysiology. (2012) 49:1617–21. 10.1111/j.1469-8986.2012.01471.x [DOI] [PubMed] [Google Scholar]
- 101.Stopczynski A, Stahlhut C, Larsen JE, Petersen MK, Hansen LK. The smartphone brain scanner: a portable real-time neuroimaging system. PLoS ONE. (2014) 9:e0086733. 10.1371/journal.pone.0086733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duvinage M, Castermans T, Petieau M, Hoellinger T, Cheron G, Dutoit T. Performance of the emotiv Epoc headset for P300-based applications. BioMed Eng Online. (2013) 12:1–15. 10.1186/1475-925X-12-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maskeliunas R, Damasevicius R, Martisius I, Vasiljevas M. Consumer grade EEG devices: are they usable for control tasks? PeerJ. (2016) 4:e1746. 10.7717/peerj.1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Porcaro C, Balsters JH, Mantini D, Robertson IH, Wenderoth N. P3b amplitude as a signature of cognitive decline in the older population: an EEG study enhanced by functional source separation. NeuroImage. (2019) 184:535–46. 10.1016/j.neuroimage.2018.09.057 [DOI] [PubMed] [Google Scholar]
- 105.Larson MJ, Clayson PE, Keith CM, Hunt IJ, Hedges DW, Nielsen BL, et al. Cognitive control adjustments in healthy older and younger adults: conflict adaptation, the error-related negativity (ERN), and evidence of generalized decline with age. Biol Psychol. (2016) 115:50–63. 10.1016/j.biopsycho.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 106.Grummett TS, Leibbrandt RE, Lewis TW, DeLosAngeles D, Powers DMW, Willoughby JO, et al. Measurement of neural signals from inexpensive, wireless and dry EEG systems. Physiol Measure. (2015) 36:1469. 10.1088/0967-3334/36/7/1469 [DOI] [PubMed] [Google Scholar]
- 107.Hurlow J, Bliss DZ. Dry skin in older adults. Geriatr Nurs. (2011) 32:257–62. 10.1016/j.gerinurse.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 108.Ishii R, Canuet L, Aoki Y, Hata M, Iwase M, Ikeda S, et al. Healthy and pathological brain aging: from the perspective of oscillations, functional, connectivity, signal complexity. Neuropsychobiology. (2017) 75:151–61. 10.1159/000486870 [DOI] [PubMed] [Google Scholar]
- 109.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. (1999) 29:169–95. 10.1016/S0165-0173(98)00056-3 [DOI] [PubMed] [Google Scholar]
- 110.Dyer JF, Barbey F, Barrett SL, Pickering EC, Buick AR, Mulholland C, et al. Gamified mobile EEG for early detection of psychotic disorders: identifying needs from clinicians and end-users. In: Nutt D, and Blier P, editors. British Association for Psychopharmacology Summer Meeting. Manchester (2019). p. A53. [Google Scholar]
- 111.Murphy B, Barbey F, Bianchi M, Buhl DL, Buick AR, Danyeli L, et al. Demonstration of a Novel Wireless EEG Platform to Detect the Acute and Long-Term Effects of Ketamine, in the Lab and in the Home. Glasgow: FENS; (2020). [Google Scholar]
- 112.Maran M, Grent-‘t-Jong T, Uhlhaas PJ. Electrophysiological insights into connectivity anomalies in schizophrenia: a systematic review. Neuropsychiatr Electrophysiol. (2016) 2:1–9. 10.1186/s40810-016-0020-5 [DOI] [Google Scholar]
- 113.Randeniya R, Oestreich LKL, Garrido MI. Sensory prediction errors in the continuum of psychosis. Schizophren Res. (2018) 191:109–22. 10.1016/j.schres.2017.04.019 [DOI] [PubMed] [Google Scholar]
- 114.Al-Nuaimi AHH, Jammeh E, Sun L, Ifeachor E. Complexity measures for quantifying changes in electroencephalogram in alzheimer's disease. Complexity. (2018) 2018:22–4. 10.1155/2018/891507918093675 [DOI] [Google Scholar]
- 115.Ferreira D, Jelic V, Cavallin L, Oeksengaard AR, Snaedal J, Høgh P, et al. Electroencephalography is a good complement to currently established dementia biomarkers. Dement Geriatr Cogn Disord. (2016) 42:80–92. 10.1159/000448394 [DOI] [PubMed] [Google Scholar]
- 116.Horvath A, Szucs A, Csukly G, Sakovics A, Stefanics G, Kamondi A. EEG and ERP biomarkers of Alzheimer's disease: a critical review. Front. Biosci. (2018) 23:4587. 10.2741/4587 [DOI] [PubMed] [Google Scholar]
- 117.Belleville S, Fouquet C, Hudon C, Zomahoun HTV, Croteau J. Neuropsychological measures that predict progression from mild cognitive impairment to alzheimer's type dementia in older adults: a systematic review and meta-analysis. Neuropsychol. Rev. (2017) 27:328–353. 10.1007/s11065-017-9361-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chehrehnegar N, Nejati V, Shati M, Rashedi V, Lotfi M, Adelirad F, et al. Early detection of cognitive disturbances in mild cognitive impairment: a systematic review of observational studies. Psychogeriatrics. (2020) 20:212–28. 10.1111/psyg.12484 [DOI] [PubMed] [Google Scholar]
- 119.Murphy B, Aleni A, Belaoucha B, Dyer JF, Nolan H. Quantifying cognitive aging and performance with at-home gamified mobile EEG. In: 2018 International Workshop on Pattern Recognition in Neuroimaging (PRNI). Singapore: (2018). p. 1–4. 10.1109/PRNI.2018.8423954 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this article are not readily available because the data collected using the Cumulus Neuroscience platform is commercially sensitive and contains proprietary information. Requests to access supporting data will be considered from bona fide researchers upon reasonable request. Requests to access the datasets should be directed to alison@cumulusneuro.com.