Abstract
Background:
As genomic science moves beyond government-academic collaborations into routine healthcare operations, nursing’s holistic philosophy and evidence-based practice approach positions nurses as leaders to advance genomics and precision health care in routine patient care.
Purpose:
To examine the status of and identify gaps for U.S. genomic nursing health care policy and precision health clinical practice implementation.
Methods:
We conducted a scoping review and policy priorities analysis to clarify key genomic policy concepts and definitions, and to examine trends and utilization of health care quality benchmarking used in precision health.
Findings:
Genomic nursing health care policy is an emerging area. Educating and training the nursing workforce to achieve full dissemination and integration of precision health into clinical practice remains an ongoing challenge. Use of health care quality measurement principles and federal benchmarking performance evaluation criteria for precision health implementation are not developed.
Discussion:
Nine recommendations were formed with calls to action across nursing practice workforce and education, nursing research, and health care policy arenas.
Conclusions:
To advance genomic nursing health care policy, it is imperative to develop genomic performance measurement tools for clinicians, purchasers, regulators and policymakers and to adequately prepare the nursing workforce.
Keywords: Genomics nursing, Health care quality, measurement, Performance measures, Precision health, Quality improvement, Genomic nursing health care, policy
Introduction
Precision health—tailored patient care based on an individual’s genomic or other omics, clinical, physiologic, lifestyle, behavioral and environmental characteristics—is moving closer to clinical utility validation and clinical care implementation (Collins & Varmus, 2015; National Institutes of Health, 2020a). Although geographical disparities exist, there are a number of examples of what can become part of routine care. These include incorporation of three generation family history into electronic health records (EHRs; Davis, 2019, Hickey et al. 2017) and screening for at-risk individuals for common hereditary cancer syndromes including BRCA1/2 (breast and ovarian cancer) and Lynch Syndrome (gastrointestinal, ovarian, endometrial, brain, urinary tract and sebaceous skin tumors; Buchanan et al., 2018; Williams et al., 2016). Other applications include noninvasive prenatal screening of cell-free DNA for genetic mutations during maternal health care visits and diagnosis of solid organ transplant rejection (Knight et al., 2019; Oellerich et al., 2020). Oncology uses targeted diagnosis and therapeutics for cancers based on cancer cell molecular signatures (Madhavan et al., 2018; Rehm, 2017). And there are a number of prescription drug selections and dosing that incorporates a patient’s genetic variation (Volpi et al., 2018). These advances are derived from foundational scientific investments in large-scale genomic research programs, computational science methodologies, and government-academic collaborations, leading to a paradigm shift in clinical practice (EHRs; Intermountain Healthcare, 2020; Williams, Buchanan et al., 2018; National Human Genome Research Institute [NHGRI], 2020a, 2020b, 2020c).
Large-scale population research initiatives are continuing to rapidly advance precision health interests. United States (U.S.) examples include the All of Us initiative at the National Institutes of Health (NIH) and the Veteran’s Administration (VA) Million Veteran Program (MVP; Bick et al, 2019; The All of Us Research Program Investigators, 2019; U.S. Department of Veteran’s Affairs, 2020). A recently published review (Fu et al., 2020) presents nursing perspectives on precision health and encourages nurse scientists and clinical leaders to contribute fully to the precision health effort by conducting novel genomic research on social determinants of health, environmental and lifestyle choices, and biological contributors for a wide variety of health conditions with All of Us data (The All of Us Research Program Investigators, 2019; NIH 2020b).
Nursing has contributed significant leadership to advance genomics and precision health patient care models. Examples include: development of academic frameworks to guide curriculums, nurse workforce education and training, and use of genomics in everyday clinical nursing practice across a range of patient care settings for pharmacogenetics, hereditary cancer screening, newborn screening and more (Calzone et al., 2014; Rogers et al., 2017). Nursing’s holistic philosophy and evidence-based practice approach positions nurses as leaders to implement precision health into routine patient care, including: prescribing and administering pharmacogenetic-based treatments and medications, conducting family history assessments to detect the presence of high-risk hereditary and multifactorial diseases, making referrals to genetic colleagues, interpreting common chromosomal, genetic and genomic laboratory tests, developing health plans for and providing counseling and education to patients, families, and communities (Fu et al., 2020; Grady, 2017; Grady & Gough, 2017; Starkweather et al., 2018a).
Health care quality improvement and performance measurement tools are instrumental to implementing new clinical advances and processes into routine health care delivery operations. These tools provide a way to objectively evaluate whether a health care team, organization or system is making positive changes that lead to improved health care delivery processes and outcomes and are linked to value-based financial reimbursement (Institute for Healthcare Improvement, 2020a). As genomic science moves beyond government-academic collaborations into routine health care operations, a health quality performance measurement and quality improvement infrastructure policy emphasis is imperative (Agency for Healthcare Research & Quality [AHRQ], 2013).
From January 2019 to July 2019, experts who served on the American Academy of Nursing’s (AAN) Genomic Nursing and Health care Expert Panel (EP) conducted a scoping review and policy priorities analysis, aiming to: (a) provide an overview of current status on U. S. genomic nursing health care policy; (b) identify gaps for implementing precision health in clinical practice; (c) identify opportunities for greater impact in genomic nursing using health care quality measurements and benchmarking standards for genomic outcomes, and; (d) promote innovative solutions for driving wider implementation of precision health by issuing calls to action for nursing practice workforce, education, research, and health care policy arenas.
Methods
Operationalization of “U.S. Nursing Genomic Health Care Policy” Definition
Genomic health policy is a growing area of interest as sequencing costs decline and genomic information is used by health care systems (Global Genomics Nursing Alliance, 2020; McCormick & Calzone, 2016; NHGRI, 2020f). The World Health Organization (WHO) defines health policy as: “decisions, plans, and actions that are undertaken to achieve specific health care goals within a society” (WHO, 2020). Since there is no single definition for nursing genomic health care policy, we applied the WHO (2020) definition of health policy to U.S. nursing genomic health care policy as “decisions, plans, and actions undertaken by nurses to achieve genomic or precision health care goals in an organization, health care system or country”. This broader policy definition can link nursing leadership activities across various precision health, genomic, and omic initiatives in a variety of nurisng environments (Table 1). We further operationalized nursing genomic health care policy as nursing efforts to make decisions and plans, as well as take actions to achieve genomic and precision health through tailored patient care based on an individual’s genomic or other omics, clinical, physiologic, lifestyle, behavioral and environmental characteristics.
Table 1 –
Selected Current and Emerging Genomic Concepts and Terminology
| Term | Definition |
|---|---|
| Artificial intelligence (AI) | An umbrella term lacking formal definition that encapsulates the abilities of computers to sense, reason, act and adapt without being programmed to do so (NIH, 2018). AI technologies are increasingly being used to combine omic data with clinical information in electronic health records. |
| Machine learning (ML) | Type of AI that uses algorithms whose performance improves as more data are used over time (NIH, 2018). |
| Deep learning (DL) | A subset of ML in which multilayered neural networks learn from vast amounts of data (NIH, 2018). |
| Big data | An emerging paradigm and health care ecosystem characterized by large-scale volume, variety, and velocity of biomedical data fields, types, structures, and processes (Luo et al., 2016). Examples include genetic/genomic sequence, cancer tumor expression data, electronic health record information, imaging/radiology data, clinical transcription patient notes, and many others. |
| Genomic medicine (health care) | Emerging health care discipline that involves use of genomic information about a patient as part of their clinical care and the health outcomes and policy implications of that clinical use (NHGRI, 2020d). |
| Omics | Scientific field associated with the measurement and analysis of large number of molecular measurements within a tissue or a cell (National Academies of Sciences Engineering, and Medicine, 2012). |
| Genomics | Study of all of a person’s genes (the genome), including interactions of those genes with each other and with the person’s environment (NHGRI, 2020e). |
| Metabolomics | The study of complete sets of small molecule metabolites and any relevant metabolic intermediates within a biological sample including carbohydrates, amino acids, lipids, nucleic acids, drug metabolite products, signaling molecules and others (National Academies of Sciences Engineering, and Medicine, 2012). |
| Microbiome | The interaction, function and structure of micro-organisms living in and on the human body (Regan et al., 2018). |
| Proteomics | The study of proteins expressed by an organism, tissue, or cell (National Academies of Sciences Engineering, and Medicine, 2012). |
| Transcriptomics | The study of complete sets of RNA transcripts from DNA in a cell or tissue, such as ribosomal RNA (rRNA), messenger RNA (mRNA), transfer RNA (tRNA), micro RNA (miRNA), and noncoding RNA (ncRNA), and others (National Academies of Sciences Engineering, and Medicine, 2012). |
| Precision medicine | An emerging approach for targeted disease prevention and treatment that takes into account people’s individual variations in genes, environment and lifestyle (Collins & Varmus, 2015). |
| Precision health | An approach that builds on precision medicine and includes targeted prevention and health promotion activities that work best for each unique person outside the setting of a doctor’s office or hospital, including evaluation of information from: family health history, personal smart devices and mobile health applications, social media and others (CDC, 2019). |
| Precision public health | An expansion of precision medicine that extends to beyond the individual to entire populations: delivering the “right intervention at the right time to the right population,” (CDC, 2018). |
Policy Task A (January to July 2019): Genomic Nursing and Health Care Expert Panel (EP) Scoping Review and Policy Brief
Figure 1 presents the cumulative process for Expert Panel (EP) policy tasks. In January 2019-July 2019, members of the American Academy (AAN) of Nursing Genomic Nursing and Health Care EP conducted a scoping review to examine current status of U.S. genomic nursing health care policy, to clarify key concepts and definitions, and to identify U.S. genomic nursing health care policy and implementation gaps (Policy Task A, Figure 1; Munn et al., 2018; Tricco et al., 2018). Specific research questions developed by the EP were: 1) what are the published U.S. nursing policies regarding genomic nursing and precision health; and 2) what U.S. federal-level health quality outcome measures are available that could be applied to precision health? PubMed was searched for systematic reviews, policy position statements, clinical research trials, mixed methods studies, and qualitative studies literature in the English language from January 1, 2014 to July 31, 2019. The following MeSH search terms were used: genomic nursing policy (n = 105), genetic nursing policy (n = 135), genetic/genomic nursing (n = 14), health care system genetic nursing implementation (n = 18), precision health nursing (n = 471), genetic/genomic health care quality measures (n = 1), genetic/genomic quality improvement (n = 0), genetic/genomic nursing workforce development (n = 0), nursing genetic and/or genomic education and training (n = 9). Additional grey literature resources were reviewed including non-profit health policy organizations and databases, think tank foundations, U.S. Congressional research reports, state and federal government initiative policy briefs and databases, and industry trade group reports. Sources of evidence are presented in Figure 2.
Figure 1 –
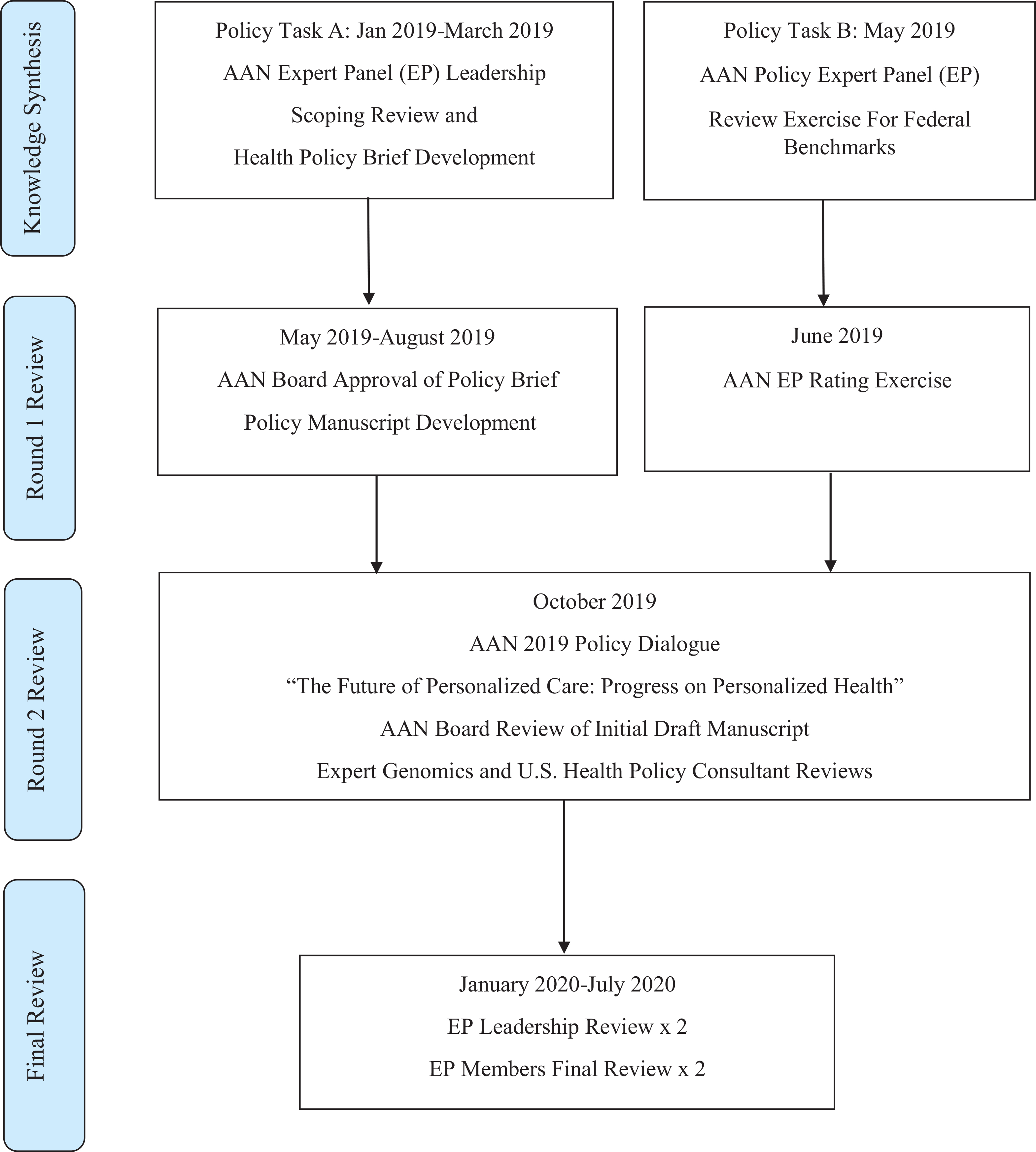
Methods for Policy development timeline flowchart by the American Academy of Nursing (AAN) Genomic Nursing and Health Care Expert Panel (EP).
Figure 2 –
Sources of evidence utilized for Policy Task A.
Policy Task B (May to June 2019): Genomic Nursing and Health Care Expert Panel (EP) Policy Priorities Analysis
Complementing this work from January to October 2019, a policy priorities analysis supporting the American Academy of Nursing (AAN) was conducted (Policy Task B; Figure 1; American Academy of Nursing, 2019a). All 24 AAN Expert Panels (EPs), including the Genomic Nursing and Health Care EP, were asked to review published AAN policy documents from 2015 to 2019 (Starkweather et al., 2017; Starkweather et al., 2018a, Starkweather et al., 2018b). EPs were asked to suggest federal level benchmark measures that could be used to evaluate the efficacy of organizational policy impact that could lead to change enactment. Benchmarking is a quantitative process by which an organization’s practice performance on a clinical process or health care outcome measure is compared with an external standard. This standard could be one developed by similar organizations in the region (local, state benchmarking), or by a federal government agency (Agency for Healthcare Research & Quality [AHRQ], 2013). The Genomic Nursing and Health Care EP generated 28 recommendations which were designated into one of three AAN policy priority areas: (a) advance health equity and champion wellness; (b) promote innovation and sustainability; and, (c) reduce patient, provider, and system burden (AAN, 2019a). EP members rated the strongest candidates which were forwarded to the AAN Board for formal review at the October 2019 Transforming Health, Driving Policy Conference meeting in Washington, D.C. (AAN, 2019b). Two external content experts and two internal program consultants reviewed the final combined Task A and B recommendations for scientific accuracy who had experience in: genomic medicine (non-federal health system perspective); senior DHHS administrative policy leadership, patient safety, health care quality and rural health care; intramural nursing science; and an AAN Board Member Liaison with nursing administration scholarship and nursing policy advocacy leadership expertise.
Findings
Policy Task A: Scoping Review and Policy Brief
Results of the scoping review confirmed that genomic nursing health care policy is an emerging area of scholarly inquiry. There is terminology inconsistency in the nursing profession due to a number of specialty movements (e.g., precision medicine, precision health, genetics, genomics, -omics and all its derivations, big data analytics, and others) in addition to technology lags between terms used in scientific versus clinical settings (Table 1). For this reason, when discussing policy development, formation, and enactment, a broader term based on the World Health Organization definition of health policy is recommended to define nursing genomic health care policy as “decisions, plans, and actions undertaken by nurses to achieve genomic or precision health care goals in an organization, health care system or country”. The adapted definition and operationalization of nursing genomic health care policy based on the WHO health policy term standard was able to capture all forms of nursing efforts in genomic and precision health.
Findings also show that nurse scholars are actively publishing in genomics and exerting influence in policy recommendations, policy formation and framework implementation across a wide variety of clinical disciplines. Specific examples are highlighted in Table 2 and include: ensuring adequate genomic education and training of nurses in the workforce to ensure precision health; implementing genomics in academic nursing; considering ethics and ethical legal social implications (ELSI); ensuring genomic nursing ethics for patient, family and community health care advocacy; implementing genomic policies at the level of health care institutions or regional health care settings (e.g., cancer screening, pharmacogenetic implementation); ensuring strong genomic inclusion in nursing science funding; ensuring minority and racial ethnic group inclusion for vulnerable population research; and representing the nursing profession on broader interdisciplinary panels such as the National Academies Roundtable on Genomics and Precision Health (National Academies of Sciences Engineering, and Medicine, 2020). Nursing scholarly writing has great philosophical variation in its use of key words and terms. For example, workforce development, labor demand and supply are commonly used terms in human resources and front-line workforce development planning initiatives, but are not always terms used by genomic nursing scientists (e.g., competency, knowledge, clinical practice, nursing practice). At the time of the literature search, we found limited publications in nursing on genetic or genomic health care quality measures (n = 1) and genetic/genomic quality improvement (n = 0). Ensuring uptake, presence and growth of precision health in the current health care system will require use of federal benchmarks and ratings used in standardized health quality performance assessments to demonstrate implementation in clinical environments. Currently, U.S. non-profit organization and federal database resources contain few health quality performance measures that could be applied to precision health.
Table 2 –
Selected Examples of Nursing Leadership in Genomic Nursing Health Care Policy and Translation of Precision Health into Clinical Settings
| Example | Setting and Intervention |
|---|---|
| Curriculum and Nursing Education Standards | |
| Doctoral nursing research | Creation of knowledge content areas needed for nursing scientists to perform omics research in health and disease states, and the fundamental core science areas required in undergraduate and graduate level nursing curricula (Regan et al., 2018). |
| Advance practice | Creation of essential genetic and genomics competencies for nurses with graduate degrees (Greco et al., 2011). |
| Baccalaureate | Establishment of the outcome indicators for essential nursing competencies and curricular guidelines for genetics and genomics (Calzone et al., 2011). |
| Nursing informatics | Developed recommendations for genetic and genomic competencies for nursing informatics internationally (McCormick & Calzone, 2017). |
| G2NA | Creation of the “Global Genomics Nursing Alliance” (G2NA) for the development of genomic competency education resources for the international community (Calzone et al., 2018c). |
| MINC | Development of “Method for Introducing a New Competency in Genomics,” a freely available patient care integration toolkit for hospital administrators and clinician educators at all levels of genomic competency (NHGRI, 2017). |
| Ethics, ELSI, and policy | Translation of the 2015 American Nurses Association Code of Ethics for Nurses with Interpretive Statements (Code) within the context of genetics and genomics by the Members of the Ethics and Public Policy Committee of the International Society of Nurses in Genetics (ISONG, Tluczek et al, 2018). |
| Application of Ethical Legal Social Implications (ELSI) framework for genomics for the improvement of cancer care outcomes (Hammer, 2019). | |
| Nurse led recommendation to strengthen federal regulation of laboratory-developed and direct-to-consumer (DTC) genetic testing (Starkweather et al, 2018b). | |
| Family history | Implementation of a pilot project in which nurses will obtain three-generation family health histories from 100 NIH Clinical Center patients in a sickle cell clinical trial followed by documentation and integration of the patient’s family pedigree into the electronic health record (Davis, 2019). |
| Hereditary cancer screening and oncology | Clinical overview for nurses of genetics and genetic tests in the clinical setting for identification of common single gene disorders such as hereditary cancer (breast/ovarian cancers, Lynch syndrome, others), nursing practice models, making appropriate referrals for genetic consultation and testing, providing patient education and family support to understand genetic test results and types of genetic testing technologies, provision of psychosocial support for family in the event of a positive or indeterminate genetic test, and to facilitate patient and family compliance with screening and treatment recommendations (Beamer, 2019; Eggert, 2017; Kelly, 2017; Montgomery et al., 2017). |
| Comprehensive review of genomics knowledge integration into clinical practice for oncology nursing (Aiello, 2017) and next generation sequencing, multi-gene panel testing for in oncology settings (Kelly, 2017). | |
| Overview of clinical implications for advance practice nurses in hereditary breast and ovarian cancers, Lynch syndrome detection (Williams et al., 2016). | |
| Multifactorial condition risks | Clinical overview for nurses of the genetic red flags in the detection of patient risks for higher risk of developing multifactorial conditions (those with combination of genetic and environmental factors) that have strong genetic components including: hypertension, hypercholesterolemia, coronary artery disease, stroke, autoimmune disease disorders, Alzheimer’s and Parkinson’s, and mental health disorders (Montgomery et al., 2017). |
| Clinical overview of familial hypercholesterolemia detection for advanced practice nurses (Williams et al., 2016). | |
| Neonatal screening and maternal health care | Nurse led policy recommendation for federal newborn screening requirements to be required in all 52 states, including 34 core heritable disorders and the reporting of another 26 secondary heritable disorders (Starkweather et al., 2017). |
| Developed policy translation framework for roles of nurses and nurse scientists in use of genome sequencing technologies for population screening in newborns (Taylor et al., 2017). | |
| Pharmacogenetics/pharmacogenomics (PGx) | Relevant summary of pharmacogenomic literature for Registered Nurses and Advanced Practice Nurses, websites including Clinical Pharmacogenetics Implementation Consortium (CPIC) and PharmGKB, and additional expert professional resources for pharmacogenomics in clinical nursing practice (Cheek et al., 2015). |
| Professional nurse certification in genomics | The Nurse Portfolio Credentialing Commission (NPCC) offers genomic health care practice certification and credentials for baccalaureate degree nurses (Clinical Genomics Nurse or CGN) and advanced practice degree nurses (Advanced Clinical Genomics Nurse or ACGN), (ISONG, 2018). |
| Racial minority population inclusion | Development of community-based participatory research framework for Black African immigrant participation in genomics research and DNA biobanking (Buseh et al., 2013). |
| Development of a clinical workforce survey instrument to assess multi-ethnic nurses’ knowledge and practice of genetics and genomics (Coleman et al, 2014). | |
| Application of the transdisciplinary ConNECT Framework principles (model for behavioral medicine science and practice) to precision health to achieve population health through emphasis of diversity inclusion and recruitment of underserved and vulnerable populations in nursing research and practice (Menon et al., 2019). | |
| Outlined contributions of nurse scientists in -omics based research to facilitate policy development for precision health in minority populations. Developed recommendations for improving diversity among nurse scientists, educators, study participants, and continuing education programs (Taylor & Mendoza, 2018). | |
| Symptom science | Defined use of symptom science and precision health in nursing research to further the understanding of patient symptoms and their self-management, including: omics requirements, data science infrastructure, research collaborations, analytical standards and controls, data workflows, data sharing and institutional policies, common data elements, and common data models for data integration (Cashion & Grady, 2015; Hickey et al., 2019). |
| Developed standardization of self-reported adverse event reporting into clinically meaningful grading metrics for pediatric patient-reported outcome symptoms across nine pediatric hospitals (McFatrich et al., 2020). | |
| Quality and patient safety | Nurse led policy recommendation to advance precision health implementation and nurses’ impact on health care quality and safety (Starkweather et al., 2018a). |
| Workforce development | Implemented a 12-month genomic competency pilot project for 8,150 hospital nurses in 23 Magnet facilities (21 intervention, 2 control). Intervention: hospital administrator-education dyads who received genomic training and resources followed by monthly supplementation and peer support to facilitate progress on institution-specific action plans. Outcome assessment: Genetic-Genomic Nursing Practice Survey data scores (Calzone et al., 2018a). |
| Development of required nursing education during new-hire employee onboarding for all nurses who provide direct patient care: Introduction to Genetics and Genomics in Health Care (Cusack & Feigenbaum, 2016; NIH Clinical Center Nursing Department, 2020a). | |
| Development of intermediate nursing education for advanced concepts for NIH Clinical Center clinical research nurses and research nurse coordinators who directly care for patients (Cusack & Feigenbaum, 2016; NIH Clinical Center Nursing Department, 2020b). | |
Policy Task B: Policy Priorities Analysis
Results of the AAN Expert Panel recommendation rating exercise are presented in Supplemental File 1. Ten items from 28 total published recommendations were selected for highest quality, priority, and relevance for federal benchmarking activity (Starkweather et al., 2017; Starkweather et al., 2018a, Starkweather et al., 2018b). Scope of these recommendations included: 1) newborn screening at the state and federal level; 2) health information technology standards for health system documentation of genomic information; 3) clinical decision support use and implementation for pharmacogenomic dosing; 4) nursing education and nursing workforce education and training; and, 5) strengthening of Health Insurance Portability and Accountability Act (HIPAA) rules to ensure patient confidentiality in precision health implementation. Federal benchmarks did not exist for most genomic nursing health care policy recommendations beyond numbers of funding metrics for content areas or numbers of newborn screening tests used at the state level as recommended by Department of Health and Human Services’ (DHHS).
As such, one of the leading conclusions from the policy priority analysis was to ensure nurse scholar participation in health policy technical panels that worked closely with the Department of Health and Human Services’ (DHHS) Office of the Secretary to work towards achieving a health care system infrastructure that could implement benchmark measurement standards. For example, there should be nurse scientist presence and input on the Health Level 7 (HL7) International ‘Clinical Genomics’ and ‘Clinical Decision Support’ work groups to ensure representation of nursing interests when recommendations are submitted to the DHHS Office of the National Coordinator (ONC) to develop EHR infrastructure interoperability standards (HL7 International, 2020a; HL7 International 2020b).
Discussion and Recommendations
Our findings should not be interpreted as a quantitative outcome since the Policy Task A included a scoping review, and the findings of the scoping review indicates that the size of literature does not allow for a systematic review. This work represents a starting point in characterizing a broader and more inclusive definition, concepts and terminology, such as genomic nursing health care policy. In addition, this work broadly outlined the current concepts and gaps in precision health implementation, such as nursing administration, human resources, nursing management and performance and quality improvement in clinical settings. A common challenge in health policy is facilitating evidenced-based policy making and implementation in emerging content areas (Collins, 2005). This work can inform future design of mature research projects to generate the outcome evidence required for sound U.S. policy formation (Ellenbecker & Edward, 2016; Wakefield, 2004). Because Policy Task B was an AAN assigned exercise reviewing published AAN policy statements, there is presence of AAN self-interest. However, the policy priorities analysis exercise confirmed the strategic value and importance of AAN’s vision to include nursing leadership involvement from a variety of sectors, and in addition to the scoping review, confirmed the need for expansion of precision health into this area.
Recommendations were made based on a synthesis of Policy Tasks A and B according to areas where nurses are actively publishing in genomic nursing health policy. For areas where there is limited literature (i.e., “genomic health care quality measures,” “genomic health care quality improvement”), salient health care quality improvement initiatives and trends are highlighted. Calls to action for nursing practice workforce and education, nursing research, and health policy arenas are emphasized.
Nursing Practice Workforce and Education
Recommendation 1. Ensure Adequate Genomic Nursing Skill Preparation and Labor Supply
Adequate genomic skill preparation and labor supply of the U.S. nursing workforce is a primary policy concern. Minimum essential genomic skills and competencies for preparation of all levels of nurses are well documented (Consensus Panel of Genetic/Genomic Nursing Competencies, 2009; Greco et al., 2011). According to the most recent U.S. nurse workforce survey, there are 3,957,661 Registered Nurses (RNs) in the U.S. (Health Resources and Services Administration, 2019). Census-based regional registered nurse (RN) labor demand and supply variations coupled with retiring RN baby-boomers (born between 1946 and 1964) and U.S. aging patient population trends must be accounted for when projecting health care systems’ capabilities to implement disruptive technologies such as next-generation genetic sequencing (Auerbach et al., 2017a; Auerbach, et al., 2017b). There are number of administrative leadership, financial, and organizational infrastructural capacity barriers that must be overcome when implementing RN competency for higher technology areas; strong nursing leadership and multidisciplinary collaborations with academic institutions must be a priority (Health Forum LLC, 2018; Vaughn et al, 2019). Fostering and maintaining adequate support of nurse educators and faculty to integrate genomic competencies into clinical practice is paramount (Aiello, 2017; Thompson & Brooks, 2011).
Recommendation 2. Ensure Implementation and Use of Genomic Nursing Education Competencies and Genomic Knowledge Matrices in Academic Nursing Programs Through Integration in Academic Nursing Accreditation Standards
Genomic nursing competencies and outcome indicators for all levels of nurses are established and endorsed by a number of nursing organizations, including the American Association of Colleges of Nursing (AACN; Consensus Panel of Genetic/Genomic Nursing Competencies, 2009; Greco et al., 2011). Doctoral knowledge elements for nurse scientist omics training are also established (Regan et al., 2018). Review of genomic nursing health policy trends, resources, and survey instruments consistently identify education and training, nurse educator and workforce capacity deficiencies as key challenges to genomic technology integration in clinical care settings (Anderson et al., 2015; Calzone et al., 2014; Calzone et al., 2016; Calzone et al., 2018a; Calzone et al., 2018b; Calzone et al., 2018c; Hickey et al., 2018; Maradiegue et al., 2013; Regan et al., 2018; Thompson & Vasquez Brooks, 2011; Rosenman et al., 2017). Nursing remains in need of additional support for training in genomics and clinical practice integration because significant portions of its workforce are associate degree prepared and educated prior to the uneven genomic competency adoption and implementation requirements in 2006 (Health Resources and Services Administration, 2019; National Academies of Science, Engineering, and Medicine, 2016). Currently, there is a draft 2020 nursing education accreditation “AACN Essentials Task Force” standards document for which nurse scientists, leaders, and educators can review and provide feedback to ensure genomic content is included for this revision cycle (AACN, 2020).
Recommendation 3. Ensure Adequate Inclusion of Genomics in Entry-Level Registered Nurse Licensure Exams
Entry into the nursing profession is regulated at the state level by National Council of State Boards of Nursing (NCSBN). The most important driver for integration of genomics into the nursing profession is through the NCSBN state board examination National Council Licensure Examination (NCLEX-RN), which impacts the education and service spaces of nursing practice and ensures safe patient care (Clarke, 2017; NCSBN, 2019). NCLX-RN content is developed with a strong emphasis on what is most likely to be encountered by practicing clinical nurses during their first-year post-graduation with an emphasis on the evaluation of safe and competent performance. Genomic information in the 2019 NCLEX-RN Text Plan is limited to collecting a family health history (NCSBN, 2019). Evaluation of recent NCLEX-RN test bank items reveals that these questions need to be current with clinical best practices utilizing genomics to ensure that all RNs demonstrate adequate understanding of biology to inform safe entry level practice, including pharmacogenetic drug dosing (NCSBN, 2018). Food and Drug Administration (FDA) presently includes 404 entries on their “Table of Pharmacogenomic Biomarkers in Drug Labeling” for drug labeling safety information, and there are 24 Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines that ensure safe and optimal drug therapy based on a patient’s pharmacogenetic test results (CPIC, 2020; FDA, 2020).
Recommendation 4. Ensure Inclusion of Genomics Content into Institutional Workforce Training That Occurs in Hospitals, Health Systems, and Health System Networks
The final mechanism for ensuring adequate RN workforce preparation in genomics is through the institutions that employ the largest numbers of RNs—hospitals, hospital systems, and health care networks. Please refer to Table 2 for seminal and emerging examples of current nursing genomic policy clinical translation initiatives. Because many RNs including ethnic minority nurses (Coleman et al, 2014) received their education before the genomic competencies were endorsed by AACN in 2006 for all levels of nursing practice, and NCSBN state licensure and certification renewal continuing education requirements vary widely, individual hospitals, systems, and networks bear significant responsibility in ensuring that their RN workforce is adequately trained to use genomics. Magnet® hospitals are a strategic nursing policy focal point for genomic implementation via nurse education leadership activities (Calzone et al., 2018a; Cusack & Feigenbaum, 2016; Jenkins et al., 2015). Since only 505 of the U.S.’s total 6,146 hospitals (8.2%) are Magnet certified, there is a significant genomic diffusion and implementation challenge at lesser-resourced community hospital settings including rural institutions (American Nurses Credentialing Center, 2019; American Hospital Association, 2020). Nurse leaders at non-Magnet facilities can coordinate genomic continuing education with other disciplines, such as engagement of staff RNs in an institutional-wide computer-based pharmacogenomics program (Rosenman et al., 2017). The NHGRI Method for Introducing a New Competency (MINC) website contains practical guidelines, implementation options, and evidence-based strategies for nurse administrators to customize genomic education initiatives that take into account budget, infrastructure, and staff expertise constraints (NHGRI, 2017).
Nursing Research: Health Care Performance Measurement and Implementation Science
Recommendation 5. Identify a Strategic Path Towards Achieving Precision Health Best Practice Implementation as Evidenced by Health Care Performance Measurement
Health care performance measurement is rooted in Donabedian’s triad framework of evaluating structure, process, and outcomes to define governance of health care system resources (Donabedian, 1966; Ayanian & Markel, 2015). In 2001, the Institute of Medicine (IOM) released its seminal report Crossing the Quality Chasm whereby pervasively flawed health care system practice variations resulted in suboptimal health care quality and patient outcomes (Institute of Medicine, 2001). In the years since, measuring and improving health care quality by using accurate, reliable, and valid health performance measures (e.g., EHR based, claims based, and patient experience surveys) has become a standard approach for ensuring that the U.S. health care delivery system delivers the “Quadruple Aim”—improved patient health and population outcomes, at lower costs, with an engaged health care workforce (Perla et al., 2018; Perla et al., 2015; Patient Protection and Affordable Care Act, 2010; Sikka et al., 2015). Standardized health care performance measures endorsed by the National Quality Forum (NQF), a non-profit organization committed to health care quality measurement and reporting, are used by federal programs [e.g., Centers for Medicare/Medicaid Services (CMS) and Agency for Healthcare Research Quality’s (AHRQ) Hospital Compare), employers, and purchasing groups to evaluate clinical performance and quality of care that drive consumer choice, organizational accountability, and value-based financial reimbursement (Damberg et al., 2012). Use of value-based health care quality improvement frameworks and evidenced based practice constructs to measure, monitor, and implement new technologies in clinical settings are instrumental tools in health care organizations and systems (Figure 3; Goldstein et al., 2018; Institute for Healthcare Improvement, 2020b; McNett et al., 2019; Priest et al, 2020; Yano, 2008).
Figure 3 –
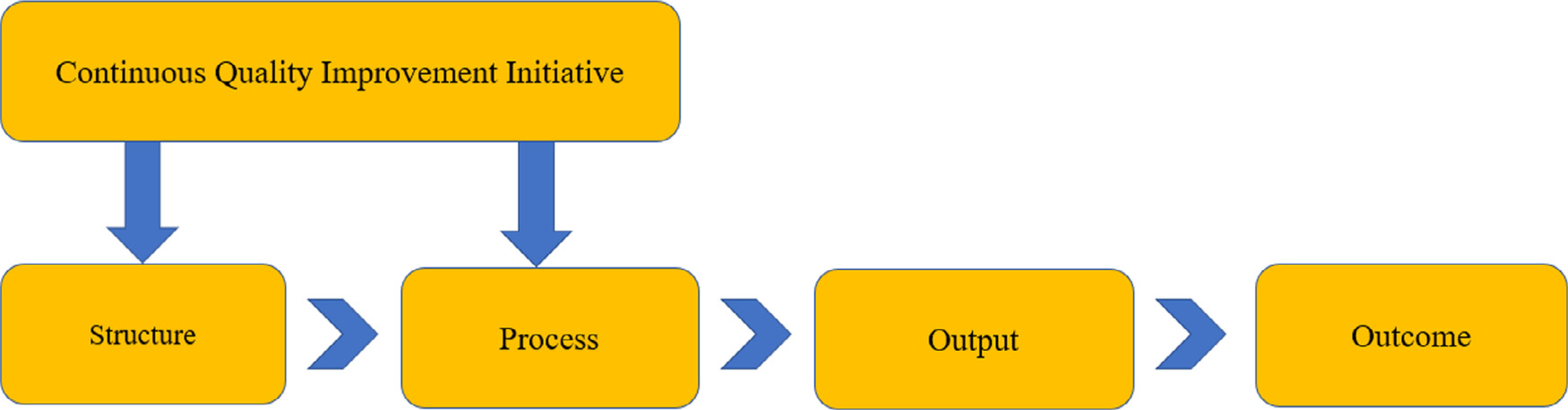
The quality improvement process using structure, process, output and outcome measures.
Figure 3 is the summary of a sample HHS HealthIT.gov continuous quality improvement initiative that outlines use of health care quality measures at each implementation step: structure, process, output, and outcome (AHRQ, 2020d; National Learning Consortium, 2013). Outcome measures can inform real-time adjustments in structures and processes to improve clinical health care performance.
Structure.
Measures that indicate a health care provider’s capacity, systems, and processes to provide high-quality health care. Examples: number or proportion of board-certified geneticists, genetics counselors, or certified advanced practice nurses in genetics that are practicing within an institution or health care system.
Process.
Measures what a health care provider or clinician does to maintain health for the patient (either healthy or with a disease). Example: the percentage of individuals with a high-risk family health history for colon cancer that receive education and counseling for recommended screening.
Output.
Measures that are a predecessor to the change in the patient’s status (clinical, administrative, efficiency, return on investment indicators). Examples: clinician documentation in the electronic health record (EHR) for family history screening at each visit; sequencing costs; utilization pattern.
Outcome.
Measures the end result of a health care service or intervention, or the health status of the patient (s). Example: rate of adverse events following use of genetic testing for coumadin dosing; patient reported satisfaction with a genomic health care service.
Despite performance measurement being an essential component to how the U.S. health care system implements evidence-based practice advances, genomics in health care quality measurement is largely undeveloped (Chou et al., 2018; Genomic Medicine Knowledge Base, 2020). Search queries of the NQF database and the Centers for Medicare & Medicaid Services (CMS) Core Quality Measures Collaborative for health performance measures with “genetics,” “genomics,” “family history,” “deoxyribonucleic acid” (DNA), “ribonucleic acid” (RNA) terms yields few results, primarily related to treatment of HIV and hepatitis C (Centers for Medicare & Medicaid, 2020a; National Quality Forum, 2020). Quality improvement is a key mechanism for health care systems to evaluate, implement, and reimburse for best evidence-based practices at the clinician, group, health care facility, or health plan levels. Lack of genomics-related performance measures indicates insufficient infrastructure for precision health implementation. Dissemination and implementation science is a well-established research priority that can facilitate clinical translation alignment with health care system, payer, and policy maker priorities such as health care quality performance measurement (National Institutes of Health, 2019; Santacroce et al., 2018; Williams et al., 2017).
Recommendation 6. Conduct Research to Contribute Towards and/or Build Institutional Level Health Care Performance Measures for Precision Health Implementation
There is an immediate need to quantify genomic health care processes and outcomes that define high quality precision health best practices (Williams, Buchanan et al., 2018; Williams, Chung et al., 2018; Williams, 2019). Geisinger is piloting a variety of institutional and system metrics to define precision health, including: process measures (e.g., if a lipid profile is performed after a high-risk genetic test result for familial hypercholesterolemia); intermediate biomarker measures (e.g., ensuring patient cholesterol levels are held at a specific threshold if they have a high-risk genetic test result); individual and group health measures (e.g., decrease in myocardial infarction rates in response to an intervention following a high-risk genetic test); patient and provider behavioral measures in response to genomic information (e.g., improved medication adherence, modification of care based on condition-specific evidence and clinical practice recommendations); and patient-reported outcome measures (e.g., patient satisfaction with service, patient engagement with self-care, knowledge about genetic disease, patient access to recommended care, patient self-assessed well-being, and family communication of genomic test information; Williams, 2019). Although development of genomic measures is observed in precision oncology, this area is challenged by rapidly changing science when aligning individual tumor scoring with population health applications (Discern Health, 2018). Development of standardized measures that are open-ended or technology agnostic allows for genomic science to change while providing performance improvement anchor tools for health care delivery teams.
Recommendation 7. Conduct Research to Contribute Towards and/or Build State/Regional Health Performance Measures for Precision Health Implementation
A number of state health departments are working with the Centers for Disease Control (CDC) to define hereditary cancer screening measures for public health (Doyle et al., 2018; Fussman et al., 2016; Michigan Department of Health and Human Services, 2020). The National Academy of Sciences, Engineering and Medicine formed the Genomics and Public Health Action Collaborative (GPHAC) to identify genomic performance objectives and outcome metrics for Hereditary Breast and Ovarian Cancer syndrome and Lynch Syndrome for use in public health programs and state health departments (Doyle et al., 2018). Chou et al. (2018) performed a first-pass pilot testing of standardized measures for comprehensive genetic service assessment to inform state-level quality improvement across eight midwestern states. Implementing quality measures at the state level allows health care providers and policymakers to evaluate if high quality and cost-effective precision health services are being provided; moreover, it sets the stage for expansion to a national benchmark standard.
Recommendation 8. Identify Realistic Development Targets for Precision Health Care Quality Performance Measurement at the National Level
Performance measures implemented at the national level are used in pay for performance and value-based public reporting programs. They are expensive to develop, maintain and harmonize; are subject to rapidly changing science, congressional policy amendments, U.S. health system infrastructure pressures; and must take into account multi-stakeholder burden and cost (Congressional Budget Office, 2017; Congressional Research Service, 2009). The NQF endorsement process is a standard path to demonstrate scientific transparency of performance measures’ validity, reliability, and utility at the national level. Measures are broadly categorized by endorsement status, developer steward, and the level of measurement (individual provider, group, institution, and system) and searchable in the NQF measure database (National Quality Forum, 2020). A descriptive survey of NQF performance measures used by 70 organizations for quality improvement, public reporting, payment, and accreditation purposes identified seven core measurement domains (structure, process, outcome, access, safety, costs, and patient experience) with process measures being the most dominant (Damberg et al., 2012).
Development of new or modification of existing standardized measures with genomic medicine components that align closely with those already in use, such as the CMS reporting programs, AHRQ Consumer Assessment of Health care Providers and Systems (CAHPS), AHRQ Hospital Consumer Assessment of Health care Providers and Systems (HCAHPS), and AHRQ Patient Safety Indicators, Inpatient Quality Indicators, may represent an easier pathway than developing new measures from scratch (AHRQ, 2020a, 2020b, 2020c; CMS, 2017; CMS, 2019; CMS, 2020a-b). Genetic conditions on CDC’s Tier 1 Classified Guidelines are especially favorable to assess for inclusion in the national quality, safety measurement strategy because there is the highest level of evidence to justify their use, such as the U.S. Preventive Services Task Force (USPSTF) recommendation on hereditary breast and ovarian cancer (BRCA1, BRCA2; USPSTF, 2019). Other examples include Lynch Syndrome and familial hypercholesterolemia. A recent example of modifying a national performance measure for genomic health care is Goehringer et al.’s (2019) genetic counseling Press Ganey Medical Practice patient-centeredness survey. Scores were benchmarked to medical practices nationally and used to inform real-time clinical practice improvements by supervisors in rural Pennsylvania to achieve improved patient experience, decreased health care costs, and higher compliance with recommended treatments. This pilot is being considered for the HCAHPS public reporting initiative on value of U.S. hospital care (Giammarco, 2020).
Health Care Policy Call to Action
Recommendation 9: Develop Nurse-Sensitive Quality Measures for Precision Health Care Implementation
An area of particular need in the U.S. health care system and nursing science, is evaluating, quantifying and defining nurse contributions to patients, providers, hospitals, health care teams and health systems to indicate high-performing nursing services (Beck et al., 2013; Montalvo, 2007; Naylor, 2007; Naylor et al., 2013). In 2004, a Nursing Steering Committee convened by NQF selected and endorsed 15 “nurse-sensitive” performance measures to define the first set of nationally standardized metrics of nurse contributions to inpatient care. These metrics were adopted for use in the National Database of Nursing Quality Indicators (NDNQI) to guide quality improvement efforts, insurers, and purchasers of care as to which hospitals have higher performing nursing services down to the unit level, and currently represents activities of ~2,000 U.S. hospitals and 98% of Magnet-accredited hospitals (Montalvo, 2007; National Quality Forum, 2004; NDNQI, 2020). Nurses are leaders and experts in performance measure development (Beck et al., 2013; Connor et al., 2016; Naylor et al., 2013). Development of genomic nurse-sensitive indicators could be piloted into the current national nursing performance measurement framework (Calzone et al., 2016; Garrard et al., 2016). For example, nursing leaders can promote modification of large nursing data sets, such as the NDNQI, to incorporate measures that can better advance precision health. When piloting genomic performance outcome measures, nurse scientists and health policy leaders should create conditions for genomic accountability without subjecting systems to excessive financial risk (Perla et al., 2018; Bleser et al., 2019).
Discussion
In 2001, authors of the IOM Crossing the Quality Chasm document stated if “the health care system cannot consistently deliver today’s science and technology, it is even less prepared to respond to even greater advances”, (Institute of Medicine, 2001). This quote still carries weight today. Without necessary genomic health care quality performance measurement tools for clinicians, purchasers, regulators and policymakers; an adequately prepared nursing workforce; frameworks, planning, and know-how for rapid cycle performance improvement/quality improvement; it will be difficult to move precision health beyond research trials at academic medical centers. Similar to other performance measurement health care policy ‘calls to action’, we opened the dialogue to defining a precision health care quality measurement strategy that is evidence based, harmonized, and multi-stakeholder (Lamb & Donaldson, 2011; National Quality Forum, 2019). Strategic integration of health care quality performance measurement principles and federal benchmarking infrastructure including development of genomic outcome measures and nurse contributions to genomic health care will facilitate broader expansion of precision health into routine health care operations.
Supplementary Material
Acknowledgments
Conflict of interest: Dr. Emma Kurnat-Thoma is supported by an NIH/NINR Clinical and Translational Intramural Research Training Award. This manuscript has been exclusively prepared for and submitted to Nursing Outlook for publication consideration and is not submitted elsewhere. The views expressed in this article are those of the authors and do not necessarily represent the official views of the National Institutes of Health or the U.S. Department of Health and Human Services.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.outlook.2020.12.006.
REFERENCES
- Agency for Healthcare Research and Quality (AHRQ). (2020a). All indicators resources. Retrieved from: https://www.qualityindicators.ahrq.gov/Modules/all_resources.aspx. Accessed June 11, 2020
- Agency for Healthcare Research and Quality (AHRQ). (2020b). The CAHPS program. Retrieved from: https://www.ahrq.gov/cahps/index.html. Accessed June 11, 2020.
- Agency for Healthcare Research and Quality (AHRQ). (2020c). The CAHPS hospital survey. Retrieved from: https://www.ahrq.gov/cahps/surveys-guidance/hospital/index.html. Accessed June 11, 2020.
- Agency for Healthcare Research and Quality (AHRQ). (2020d). Types of quality measures. Retrieved from: https://www.ahrq.gov/talkingquality/measures/types.html. Accessed June 11, 2020
- Agency for Healthcare Research and Quality (AHRQ). (2013). Module 7. Measuring and benchmarking clinical performance. Practice Facilitation Handbook. Rockville, MD: National Center for Excellence in Primary Care Research (NCEPCR). Retrieved from: https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod7.html Accessed June 11, 2020. [Google Scholar]
- Aiello L (2017). Genomics education: Knowledge of nurses across the profession and integration into practice. Clinical Journal of Oncology in Nursing, 21(6), 747–753, doi: 10.1188/17.CJON.747-753. [DOI] [PubMed] [Google Scholar]
- American Academy of Nursing. (2019a). Policy priorities 2019–2020. Retrieved from: https://higherlogicdownload.s3.amazonaws.com/AANNET/c8a8da9e-918c-4dae-b0c6-6d630c46007f/UploadedImages/Academy_Federal_Policy_Priorities_Approved_10_30_19.pdf. Accessed June 11, 2020
- American Academy of Nursing. (2019b). Transforming health, driving policy conference. Retrieved from: https://higherlogicdownload.s3.amazonaws.com/AANNET/c8a8da9e-918c-4dae-b0c6-6d630c46007f/UploadedImages/Final_2019_Onsite_Program.pdf. Accessed July 2, 2020. [Google Scholar]
- American Association of Colleges of Nursing (AACN). (2020). Essentials task force and AACN essentials DRAFT document for public comment. Retrieved from: https://www.aacnnursing.org/About-AACN/AACN-Governance/Committees-and-Task-Forces/Essentials
- American Hospital Association. (2020). Fast facts on U.S. hospitals, 2020. Retrieved from: https://www.aha.org/statistics/fast-facts-us-hospitals. Accessed June 11, 2020
- American Nurses Credentialing Center (ANCC). (2019). Number of hospitals in the United States with Magnet status. Campaign for Action. Retrieved from: https://campaignforaction.org/resource/number-hospitals-unitedstates-magnet-status/ Accessed June 11, 2020. [Google Scholar]
- Anderson G, Alt-White A, Schaa K, Boyd A, & Kasper C (2015). Genomics for nursing education and practice: Measuring competency. Worldviews on Evidence-Based Nursing, 12(3), 165–175, doi: 10.1111/wvn.12096. [DOI] [PubMed] [Google Scholar]
- Auerbach D, Beurhaus P, & Staiger D (2017a). How fast will the registered nurse workforce grow through 2030? Projections in nine regions of the country. Nursing Outlook, 65(1), 116–122, doi: 10.1016/j.outlook.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Auerbach D, Buerhaus P, & Staiger D (2017b). Millennials almost twice as likely to be registered nurses as baby boomers were. Health Affairs, 36(10), 1804–1807, doi: 10.1377/hlthaff.2017.0386. [DOI] [PubMed] [Google Scholar]
- Ayanian J, & Markel H (2015). Donabedian’s lasting framework for health care quality. New England Journal of Medicine, 375(3), 205–207, doi: 10.1056/NEJMp1605101. [DOI] [PubMed] [Google Scholar]
- Beamer L (2019). Hereditary breast and hereditary ovarian cancer: Implications for the oncology nurse. Seminars in Oncology Nursing, 35(1), 47–57, doi: 10.1016/j.soncn.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Beck S, Weiss M, Ryan-Wenger N, Donaldson N, Aydin C, Towsley G, & Gardner W (2013). Measuring nurses’ impact on health care quality. Medical Care, 51 (4), S15–S22, doi: 10.1097/MLR.0b013e3182802e8b. [DOI] [PubMed] [Google Scholar]
- Bick A, Akwo E, Robinson-Cohen C, Lee K, Lynch J, Assimes T, …, Miller DR, & VA Million Veteran Program. (2019). Association of APOL1 risk alleles with cardiovascular disease in african americans in the MVP. Circulation, 1–29, doi: 10.1161/CIRCULATIONAHA.118.036589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleser W, Saunders R, Winfield L, Japinga M, Smith N, Kaufman B, Crook HL, Muhlestein D, & McClellan M (2019). ACO serious illness care: Survey and case studies depict current challenges and future opportunities. Health Affairs, 6, 1011–1020, doi: 10.1377/hlthaff.2019.00013. [DOI] [PubMed] [Google Scholar]
- Buchanan A, Manickman K, Meyer M, Wagner J, Hallquist M, Williams J, Rahm AK, Williams MS, Chen Z-M, Shah CK, Garg TK, Lazzeri A, Schwartz MLB, Lindbuchler DM, Fan AL, Leeming R, Servano PO 3rd, Smith AL, Vogel VG, …, Murray M (2018). Early cancer diagnoses through BRCA 1/2 screening of unselected adult biobank participants. Genetics in Medicine, 20(5), 554–558, doi: 10.1038/gim.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buseh A, Underwood S, Stevens P, Townsend L, & Kelber S (2013). Black African immigrant community leaders’ views on participation in genomics research and DNA biobanking. Nursing Outlook, 61, 196–204, doi: 10.1016/j.outlook.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Calzone K, Culp S, Jenkins J, Caskey S, Edwards P, Fuchs M, Reints A, Stange B, Questad J, & Badzek L (2016). Test-retest reliability of the genetics and genomics in nursing practice survey instrument. Journal of Nursing Measurement, 24(1), 54–68, doi: 10.1891/1061-3749.24.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone K, Jenkins J, Culp S, & Badzek L (2018a). Hospital nursing leadership led interventions increased genomic awareness and educational intent in Magnet settings. Nursing Outlook, 66(3), 244–253, doi: 10.1016/j.outlook.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone K, Jenkins J, Culp S, Caskey S, & Badzek L (2014). Introducing a new competency into nursing practice. Journal of Nursing Regulation, 5(1), 40–47, doi: 10.1016/s2155-8256(15)30098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone K, Jenkins J, Prows C, & Masny A (2011). Establishing the outcome indicators for the essential nursing competencies and curricula guidelines for genetics and genomics. Journal of Professional Nursing, 27(3), 179–191, doi: 10.1016/j.profnurs.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone K, Kirk M, Tonkin E, Badzek L, Benjamin C, & Middleton A (2018b). The global landscape of nursing and genomics. Journal of Nursing Scholarship, 50(3), 249–256, doi: 10.1111/jnu.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone K, Kirk M, Tonkin E, Badzek L, Benjamin C, & Middleton A (2018c). Increasing nursing capacity in genomics: Overview of existing global genomics resources. Nurse Education Today, 69, 53–59, doi: 10.1016/j.nedt.2018.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashion A, & Grady P (2015). The NIH/NINR intramural research program and the development of the NIH symptom science model. Nursing Outlook, 63(4), 484–487, doi: 10.1016/j.outlook.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2019). Precision health: Improving health for each of us and all of us. Retrieved from: https://www.cdc.gov/genomics/about/precision_med.htm. Accessed June 11, 2020
- Centers for Disease Control and Prevention. (2018). Precision public health: What is it? Retrieved from: https://blogs.cdc.gov/genomics/2018/05/15/precision-public-health-2/. Accessed June 11, 2020
- Centers for Medicare & Medicaid Services. (2020a). Core quality measures overview. Retrieved from: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/Core-Measures.html. Accessed June 11, 2020
- Centers for Medicare & Medicaid Services. (2020b). Quality measures. Retrieved from: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/index.html. Accessed June 11, 2020
- Centers for Medicare & Medicaid Services. (2019). CMS patient safety indicators PSI 90. National quality strategy domain: Patient safety. Accessed 6/11/2020: Retrieved from: https://innovation.cms.gov/Files/fact-sheet/bpciadvanced-fs-psi90.pdf. Accessed June 11, 2020 [Google Scholar]
- Centers for Medicare & Medicaid Services. (2017). HCAHPS Fact Sheet. CAHPS hospital survey. Retrieved from: https://www.hcahpsonline.org/globalassets/hcahps/facts/hcahps_fact_sheet_november_2017.pdf. Accessed June 11, 2020
- Cheek D, Bashore L, & Brazeau D (2015). Pharmacogenomics and implications for nursing practice. Journal of Nursing Scholarship, 47(6), 496–504, doi: 10.1111/jnu.12168. [DOI] [PubMed] [Google Scholar]
- Chou A, Mulvihill J, Kaye C, Mann S, Williams M, & Williamson L (2018). Developing a genetic services assessment tool to inform quality improvement efforts in state genetic service delivery. Genetics in Medicine, 21 (4), 955–964, doi: 10.1038/s41436-018-0141-2. [DOI] [PubMed] [Google Scholar]
- Clarke S (2017). What you need to know about the NCLEX-RN. Nursing Management, 48(10), 21–23, doi: 10.1097/01.NUMA.0000524821.72029.0a. [DOI] [PubMed] [Google Scholar]
- Clinical Pharmacogenetics Implementation Consortium (CPIC). (2020). Guidelines. Retrieved from: https://cpicpgx.org/guidelines/. Accessed July 9, 2020 [Google Scholar]
- Coleman B, Calzone K, Jenkins J, Paniagua C, Rivera R, Hong O, …, Bonham V (2014). Multi-ethnic minority nurses’ knowledge and practice of genetics and genomics. Journal of Nursing Scholarship, 46(4), 235–244, doi: 10.1111/jnu.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F, & Varmus H (2015). A new initiative on precision medicine. New England Journal of Medicine, 372(9), 793–795, doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T (2005). Health policy analysis: A simple tool for policy makers. Public Health, 119, 192–196, doi: 10.1016/j.puhe.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Congressional Budget Office. (2017). Issues and challenges in measuring and improving the quality of health care. Washington DC: CBO. Working paper seriesRetrieved from: https://www.cbo.gov/system/files/115th-congress-2017-2018/workingpaper/53387-workingpaper.pdf Accessed June 11, 2020. [Google Scholar]
- Congressional Research Service. (2009). Measuring health care quality: measure development, endorsement, and implementation. Washington, DC: CRS. Retrieved from: https://www.everycrsreport.com/reports/R40749.html Accessed June 11, 2020. [Google Scholar]
- Connor J, Mott S, Green A, Larson C, & Hickey P (2016). Measurement of quality nursing practice in congenital cardiac care. American Journal of Critical Care, 25 (2), 128–135, doi: 10.4037/ajcc2016708. [DOI] [PubMed] [Google Scholar]
- Consensus Panel of Genetic/Genomic Nursing Competencies. (2009). Essentials of Genetic and Genomic Nursing: Competencies, Curricula, Guidelines, and Outcome Indicators (pp. 1–74) (2nd ed.). Silver Spring, MD: American Nurses Association. [Google Scholar]
- Cusack G & Feigenbaum K (2016). MINC implementation in the clinical center: Exemplar. Implementation of clinical competencies. Retrieved from: https://www.genome.gov/Pages/Careers/EducationalPrograms/ShortCourse/2016ShortCourse/GeneticsShortCoursePresentation2016.pdf. Accessed June 11, 2020
- Damberg C, Sorbero M, Lovejoy S, Lauderdale K, Werthjeimer S, Smith A, Waxman D, & Schnyer C (2012). An evaluation of the use of performance measures in health care. RAND Health Quarterly, 1(4), 3 PMID: 28083210. [PMC free article] [PubMed] [Google Scholar]
- Davis L (2019). Nursing department joins NIH genomics revolution. NIH Clinical Center News. Retrieved from: https://clinicalcenter.nih.gov/about/news/newsletter/2019/fall/story-02.html Accessed June 11, 2020. [Google Scholar]
- Discern Health. (2018). Quality measurement of personalized medicine. Tensions between personalization and standardization. Policy brief for Pharmaceutical Research and Manufacturers of America (PhRMA). Retrieved from: http://discernhealth.com/wp-content/uploads/2018/12/Quality-Measurement-of-Personalized-Medicine-Issue-Brief.pdf. Accessed June 11, 2020 [Google Scholar]
- Donabedian A (1966). Evaluating the quality of medical care. Milbank Quarterly, 44, 691–729, doi: 10.1111/j.1468-0009.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle D, Clyne M, Rodriguez J, Cragun D, Senier L, Hurst G, Chan K, & Chambers D (2018). Proposed outcome measures for state public health genomic programs. Genetic Medicine, 20(9), 995–1003, doi: 10.1038/gim.2017.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert J (2017). Genetics and genomics in oncology nursing. What does every nurse need to know? Nursing Clinics of North America, 52(1), 1–25, doi: 10.1016/j.cnur.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Ellenbecker C, & Edward J (2016). Conducting nursing research to advance and inform health policy. Policy, Politics & Nursing Practice, 17(4), 208–217, doi: 10.1177/1527154417700634. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA). (2020). Table of pharmacogenomic biomarkers in drug labeling. Retrieved from: https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling. Accessed June 11, 2020
- Fu M, Kurnat-Thoma E, Starkweather A, Henderson W, Cohen S, Cashion A, Williams J, Katapodi M, Reuter-Rice K, Hickey K, Barcelona de Mendoza V, Calzone K, Conley Y, Anderson C, Lyon D, Weaver M, Shiao P, Constantino R, Wung S, Hammer M, & Coleman B (2020). Precision health: A nursing perspective. International Journal of Nursing Science, 7(1), 5–12, doi: 10.1016/j.ijnss.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussman C, Schrager J, & Duquette D (2016). Breast and ovarian cancer personal/family history and genetic counseling utilization among Michigan women. Michigan BRFSS Surveillance Brief, 10(3), 1–2. Retrieved from: https://www.michigan.gov/documents/mdhhs/MIBRFSS_Surveillance_Brief_Dec_2016_Vol10No3_FINAL_547831_7.pdf Accessed June 11, 2020. [Google Scholar]
- Garrard L, Boyle D, Simon M, Dunton N, & Gajewski B (2016). Reliability and validity of the NDNQI injury falls measure. Western Journal of Nursing Research, 38(1), 111–128, doi: 10.1177/0193945914542851. [DOI] [PubMed] [Google Scholar]
- Genomic Medicine Knowledge Base. (2020). IGNITE genomic medicine knowledge base. The knowledge hub for genomic medicine. Retrieved from: https://gmkb.org/. Accessed June 11, 2020 [Google Scholar]
- Giammarco M (2020). Press Ganey’s genetic counselor survey component an opportunity for genetic counselors. Retrieved from: https://www.nsgc.org/p/bl/et/blogid=53&blogaid=1121. Accessed June 11, 2020
- Global Genomics Nursing Alliance (G2NA). (2020). Genomics policy research Retrieved from: https://genomics.research.southwales.ac.uk/global-leadership/. Accessed June 11, 2020
- Goehringer J, Bachman K, Sommer J, Faucett W, & Williams JL (2019). Developing a nationally benchmarked resource for practice outcome measurement. Poster abstract and presentation results. Poster presentation at the Annual Educational Conference of the National Society of Genetic Counselors. [Google Scholar]
- Goldstein K, Vogt D, Hamilton A, Frayne S, Gierisch J, Blakeney J, Sadler A, Bean-Mayberry B, Carney D, DiLeone B, Fox A, Klap R, Yee E, Romodan Y, Strehlow H, Yosef J, & Yano E (2018). Practice-based research networks add value to evidence-based quality improvement. Healthcare, 6, 128–134, doi: 10.1016/j.hjdsi.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady P (2017). Investigating the determinants of health: The role of nursing science. Nursing Outlook, 65(5), 489–491, doi: 10.1016/j.outlook.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Grady P, & Gough L (2017). Using nursing science to inform health policy: The role of the National Institute of Nursing Research. In Grady P, & Hinshaw A (Eds.), Using nursing research to shape health policy (pp. 131–149). New York, NY: Springer Publishing Company. [DOI] [PubMed] [Google Scholar]
- Greco K, Tinley S, & Seibert D (2011). Essential genetic and genomic competencies for nurses with graduate degrees. Silver Spring, MD: American Nurses Association and International Society of Nurses in Genetics. [DOI] [PubMed] [Google Scholar]
- Hammer M (2019). Beyond the helix: Ethical, legal, and social implications in genomics. Seminars in Oncology Nursing, 35(1), 93–106, doi: 10.1016/j.soncn.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Health Forum, LLC. (2018). Caring for communities: How hospitals are engaging in new payment models and addressing community needs. American Hospital Association Statistics Quick Report Retrieved from: https://www.ahadataviewer.com/quickreport/ Accessed June 11, 2020.
- Health Level 7 (HL7) International. (2020a). Clinical genomics. Retrieved from: http://www.hl7.org/Special/committees/clingenomics/overview.cfm. Accessed June 11, 2020
- Health Level 7 (HL7) International. (2020b). Clinical decision support. Retrieved from: http://www.hl7.org/Special/committees/dss/overview.cfm. Accessed June 11, 2020
- Health Resources and Services Administration (HRSA). (2019). 2018 national sample survey of registered nurses. Brief summary of results. Rockville, MD: National Center for Health Workforce Analysis. Bureau of Health Professions, Health Resources and Services Administration. Retrieved from: https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/nssrn-summary-report.pdf Accessed June 11, 2020. [Google Scholar]
- Hickey K, Bakken S, Byrne M, Bailey D, Demiris G, Docherty S, Dorsey S, Guthrie B, Heitkamper M, Jacelon C, Kelechi T, Moore S, Redeker N, Renn C, Resnick B, Starkweather A, Thompson H, Ward T, McCloskey D, Austin J, & Grady P (2019). Precision health: Advancing symptoms & self-management science. Nursing Outlook, 67(4), 462–475, doi: 10.1016/j.outlook.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey K, Katapodi M, Coleman B, Reuter-Rice K, & Starkweather A (2017). Improving utilization of the family history in the electronic health record. Journal of Nursing Scholarship, 49(1), 80–86, doi: 10.1111/jnu.12259. [DOI] [PubMed] [Google Scholar]
- Hickey K, Taylor J, Barr T, Hauser N, Jia H, Riga T, & Katapodi M (2018). Nursing genetics and genomics: The International Society of Nurses in Genetics survey. Nurse Educator Today, 63, 12–17, doi: 10.1016/j.nedt.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Healthcare Improvement (IHI). (2020a). Measures. Retrieved from: http://www.ihi.org/resources/Pages/Measures/default.aspx. Accessed June 29, 2020
- Institute for Healthcare Improvement (IHI). (2020b). Science of improvement: Establishing measures. Retrieved from: http://www.ihi.org/resources/Pages/HowtoImprove/ScienceofImprovementEstablishingMeasures.aspx. Accessed June 11, 2020
- Institute of Medicine. (2001). Crossing the quality chasm. Washington, D.C.: National Academies Press, doi: 10.17226/10027. [DOI] [Google Scholar]
- Intermountain Healthcare. (2020). Precision genomics. Retrieved from: https://intermountainhealthcare.org/services/cancer-care/precision-genomics/. Accessed June 11, 2020
- International Society of Nurses in Genetics (ISONG). (2018). Nurse portfolio credentialing commission (NPCC) for advanced clinical genomics nurse and the clinical genomics nurse (CGN). Retrieved from: https://www.isong.org/resources/Documents/NPCC%20BROCHURE%2011292018.pdf. Accessed June 11, 2020
- Jenkins J, Calzone K, Caskey S, Culp S, Weiner M, & Badzek L (2015). Methods of genomic competency integration into practice. Journal of Nursing Scholarship, 47(3), 200–210, doi: 10.1111/jnu.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P (2017). Next generation sequencing and multi-gene panel testing: Implications for the oncology nurse. Seminars in Oncology Nursing, 33(2), 208–218, doi: 10.1016/j.soncn.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Knight S, Thorne A, & Faro M (2019). Donor-specific cell-free DNA as a biomarker in solid organ transplantation: A systematic review. Transplantation, 103(2), 273–283, doi: 10.1097/TP.0000000000002482. [DOI] [PubMed] [Google Scholar]
- Lamb G, & Donaldson N (2011). Performance measurement—A strategic imperative and call to action. Nursing Outlook, 59, 336–338, doi: 10.1016/j.outlook.2011.09.002. [DOI] [Google Scholar]
- Luo J, Wu M, Gopukumar D, & Zhao Y (2016). Big data application in biomedical research and health care: A literature review. Biomedical Informatics Insights, 8, 1–10, doi: 10.4137/BII.S31559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan S, Subramaniam S, Brown T, & Chen J (2018). Art and challenges of precision medicine: Interpreting and integrating genomic data into clinical practice. American Society of Clinical Oncology Educational Book, 38, 546–553, doi: 10.1200/EDBK_200759. [DOI] [PubMed] [Google Scholar]
- Maradiegue A, Edwards Q, & Seibert D (2013). 5-years later—Have faculty integrated medical genetics into nurse practitioner curriculum? International Journal of Nursing Education Scholarship, 10(1), 245–254, doi: 10.1515/ijnes-2012-0007. [DOI] [PubMed] [Google Scholar]
- McCormick K, & Calzone K (2017). Genetic and genomic competencies for nursing informatics internationally. Stud Health Technol Inform, 232, 152–164, doi: 10.3233/978-1-61499-738-2-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick K, & Calzone K (2016). The impact of genomics on health outcomes, quality, and safety. Nurse Manager, 47(4), 23–26, doi: 10.1097/01.NUMA.0000481844.50047.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFatrich M, Brondon J, Lucas N, Hinds P, Maurer S, Mack J, Freyer D, Jacobs S, Baker J, Mowbray C, Wang M, Castellino S, Leahy A, & Reeve B (2020). Mapping child and adolescent self-reported symptom data to clinician-reported adverse event grading to improve pediatric oncology care and research. Cancer, 126(1), 140–147, doi: 10.1002/cncr.32525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNett M, Tucker S, & Melnyk M (2019). Implementation science: A critical strategy necessary to advance and sustain evidence-based practice. Worldviews on Evidence-Based Nursing, 16(3), 174–175, doi: 10.1111/wvn.12368. [DOI] [PubMed] [Google Scholar]
- Menon U, Ashing K, Chang M, & Christy S (2019). Application of the ConNECT framework to precision health and health disparities. Nursing Research, 68(2), 99–109, doi: 10.1097/NNR.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michigan Department of Health and Human Services. (2020). Michigan cancer genomics program. Retrieved from: https://www.michigan.gov/mdhhs/0,5885,7-339-73971_4911_4916_47257_68337_94208—,00.html. Accessed June 11, 2020
- Montalvo I (2007). The national database of nursing quality indicators (NDNQI). The Online Journal of Issues in Nursing, 12(3), doi: 10.3912/OJIN.Vol12No03Man02 Manuscript 2doi: 10.3912/OJIN.Vol12No03Man02. [DOI] [PubMed] [Google Scholar]
- Montgomery S, Brouwer W, Everett P, Hassen E, Lowe T, McGreal S, & Eggert J (2017). Genetics in the clinical setting. American Nurse Today, 12(10), 10–16. Retrieved from: https://www.americannursetoday.com/genetics-clinical-setting/ Accessed June 11, 2020. [Google Scholar]
- Munn Z, Peters M, Stern C, Tufanaru C, McArthur A, & Aromataris E (2018). Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Medical Research Methodology, 18(143), 1–7, doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences Engineering, and Medicine. (2020). Roundtable on genomics and precision health. Retrieved from: https://www.nationalacademies.org/our-work/roundtable-on-genomics-and-precision-health. Accessed June 11, 2020
- National Academies of Sciences, Engineering, and Medicine. (2016). Assessing progress on the institute of medicine report, the future of nursing. Washington, DC: The National Academies Press, doi: 10.17226/21838. [DOI] [Google Scholar]
- National Academies of Sciences Engineering, and Medicine. (2012). Evolution of translational omics: lessons learned and the path forward. Washington, D.C.: The National Academies Press, doi: 10.17226/13297. Retrieved from: https://www.ncbi.nlm.nih.gov/books/NBK202168/pdf/Bookshelf_NBK202168.pdf Accessed June 11, 2020. [DOI] [PubMed] [Google Scholar]
- National Council of State Boards of Nursing®. (2019). National council licensure examination (NCLEX)-RN examinations. Test plan for the NCLEX-RN. Retrieved from: https://www.ncsbn.org/2019_RN_TestPlan-English.pdf.Accessed July 9, 2020
- National Council of State Boards of Nursing®. (2018). NCLEX examinations RN. Exam 1. Retrieved from: https://drive.google.com/file/d/1HV8hWZ9aAvzvVykNCRZ3TpmcexFjapmV/view.Accessed July 9, 2020
- National Database of Nursing Quality Indicators (NDNQI). (2020). Press ganey NDNQI summary statistics. Retrieved from: http://www.health-links.me/web/ndnqi.html. Accessed June 11, 2020
- National Human Genome Research Institute (NHGRI). (2020a). Electronic medical records and genomics (eMERGE) network. Retrieved from: https://www.genome.gov/Funded-Programs-Projects/Electronic-Medical-Records-and-Genomics-Network-eMERGE. Accessed June 11, 2020
- National Human Genome Research Institute (NHGRI). (2020b). Innovation grants to nurture initial translational efforts (IGNITE) program network. Retrieved from: https://www.genome.gov/Funded-Programs-Projects/Implementing-Genomics-in-Practice-IGNITE. Accessed June 11, 2020
- National Human Genome Research Institute (NHGRI). (2020c). Clinical sequencing evidence-generating research (CSER) consortium. Retrieved from: https://cser-consortium.org/. Accessed June 11, 2020
- National Human Genome Research Institute (NHGRI). (2020d). Genomics and medicine. Retrieved from: https://www.genome.gov/health/Genomics-and-Medicine. Accessed June 11, 2020
- National Human Genome Research Institute (NHGRI). (2020e). A brief guide to genomics. Retrieved from: https://www.genome.gov/about-genomics/fact-sheets/A-Brief-Guide-to-Genomics. Accessed June 11, 2020
- National Human Genome Research Institute (NHGRI). (2020f). Policy and program analysis branch. Retrieved from: https://www.genome.gov/about-nhgri/Division-of-Policy-Communications-and-Education/Policy-and-Program-Analysis-Branch. Accessed June 11, 2020
- National Human Genome Research Institute (NHGRI). (2017). New toolkit helps nurses use genomics in patient care.: Retrieved from: https://www.genome.gov/news/news-release/New-toolkit-helps-nurses-use-genomics-in-patient-care. Accessed June 11, 2020
- National Institutes of Health. (2020a). All of Us research program. Retrieved from: https://allofus.nih.gov/news-events. Accessed June 11, 2020 [Google Scholar]
- National Institutes of Health. (2020b). All of Us research program: Research hub and data snapshots. Retrieved from: https://www.researchallofus.org/data-snapshots/. Accessed June 11, 2020
- National Institutes of Health. (2019). Funding opportunity announcement (FOA) PAR-19–274: Dissemination and implementation in health. Retrieved from: https://grants.nih.gov/grants/guide/pa-files/PAR-19-274.html. Accessed June 11, 2020
- National Institutes of Health. (2018). NIH workshop: Harnessing artificial intelligence and machine learning to advance biomedical research. Bethesda, MD: NIH. Workshop Report SummaryRetrieved from: https://datascience.nih.gov/sites/default/files/AI_workshop_report_summary_01-16-19_508.pdf Accessed June 11, 2020. [Google Scholar]
- National Institutes of Health Clinical Center Nursing Department. (2020a). NIH Clinical Center class information for introduction to genetics and genomics in healthcare. Retrieved from: https://ccnd.nihcc.recsolutions.com/ct/client/home/session_information/3572. Accessed June 11, 2020
- National Institutes of Health Clinical Center Nursing Department. (2020b). NIH Clinical Center class information for intermediate genetics and genomics in health care. Retrieved from: https://ccnd.nihcc.recsolutions.com/ct/client/home/session_information/3574. Accessed June 11, 2020
- National Learning Consortium. (2013). Continuous quality improvement (CQI) strategies to optimize your practice. A Primer. Washington DC: HHS HealthIT.gov. Retrieved from: https://www.healthit.gov/sites/default/files/tools/nlc_continuousqualityimprovementprimer.pdf Accessed June 11, 2020. [Google Scholar]
- National Quality Forum (NQF). (2020). Quality positioning system. Retrieved from: https://www.qualityforum.org/Home.aspx. Accessed June 11, 2020
- National Quality Forum (NQF). (2019). A national call to action: Quality and payment innovation in social determinants of health. Retrieved from: https://www.qualityforum.org/News_And_Resources/Press_Releases/2019/National_Quality_Forum_Leads_National_Call_to_Address_Social_Determinants_of_Health_through_Quality_and_Payment_Innovation.aspx. Accessed June 11, 2020
- National Quality Forum (NQF). (2004). National voluntary consensus standards for nursing-sensitive care: A consensus report. Washington, D.C.: NQF. Retrieved from: https://www.qualityforum.org/Publications/2004/10/National_Voluntary_Consensus_Standards_for_Nursing-Sensitive_Care_An_Initial_Performance_Measure_Set.aspx Accessed June 11, 2020. [Google Scholar]
- Naylor M (2007). Advancing the science in the measurement of health care quality influenced by nurses. Medical Care Research and Review, 614(2), 144S–169S, doi: 10.1177/1077558707299257. [DOI] [PubMed] [Google Scholar]
- Naylor M, Lustig A, Kelley H, Volpe E, Melichar L, & Pauly M (2013). Introduction, the interdisciplinary nursing quality research initiative (INQRI). Medical Care, 51(4), S1–S5, doi: 10.1097/MLR.0b013e31827dc3ab. [DOI] [PubMed] [Google Scholar]
- Oellerich M, Christenson R, Beck J, Schutz E, Sherwood K, Price C, Keown P, & Walson P (2020). Donor-derived cell free DNA testing in solid organ transplantation: A value proposition. The Journal of Applied Laboratory Medicine, doi: 10.1093/jalm/jfaa062. [DOI] [PubMed] [Google Scholar]
- Patient Protection and Affordable Care Act. (2010). 42 U.S. C. §18001.
- Perla R, Pham H, Gilfillan R, Berwick D, Baron R, Lee P, McCannon CJ, Progar K, & Shrank W (2015). Learning systems at scale. Where policy meets practice. JAMA, 314(20), 2131–2132, doi: 10.1001/jama.2015.15079. [DOI] [PubMed] [Google Scholar]
- Perla R, Pham H, Gilfillan R, Berwick D, Baron R, Lee P, …, Shrank W (2018). Government as innovation catalyst: Lessons from the early Center for Medicare and Medicaid innovation models. Health Affairs, 37 (2), 213–221, doi: 10.1377/hlthaff.2017.1109. [DOI] [PubMed] [Google Scholar]
- Priest J, Irwin D, Evans K, Oglesby A, & Brady B (2020). Benchmarking quality measures across U.S. payer types. Population Health Management, 23(2), 146–156, doi: 10.1089/pop.2019.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan M, Engler M, Coleman B, Daack-Hirsch, & Calzone K (2018). Establishing the genomic knowledge matrix for nursing science. Journal of Nursing Scholarship, 51(1), 50–57, doi: 10.1111/jnu.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm H (2017). Evolving health care through personal genomics. Nature Reviews Genetics, 18(4), 259–267, doi: 10.1038/nrg.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M, Lizer S, Doughty A, Hayden B, & Klein C (2017). Expanding RN scope of knowledge—Genetics/genomics the new frontier. Journal for Nurses in Professional Development, 33(2), 56–63, doi: 10.1097/NND.0000000000000340. [DOI] [PubMed] [Google Scholar]
- Rosenman M, Decker B, Levy K, Holmes A, Pratt V, & Eadon M (2017). Lessons learned when introducing pharmacogenomic panel testing into clinical practice. Value in Health, 20, 54–59, doi: 10.1016/j.jval.2016.08.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacroce S, Leeman J, & Song M (2018). A training program for nurse scientists to promote intervention translation. Nursing Outlook, 66(2), 149–156, doi: 10.1016/j.outlook.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikka R, Morath J, & Leape L (2015). The quadruple aim: Care, health cost and meaning in work. BMJ Quality and Safety, 24, 608–610, doi: 10.1136/bmjqs-2015-004160. [DOI] [PubMed] [Google Scholar]
- Starkweather A, Coleman B, Barcelona de Mendoza V, Fu M, Menzies V, O’Keefe M, & Williams J (2018b). Strengthen federal regulation of laboratory-developed and direct-to-consumer genetic testing. Nursing Outlook, 66, 101–104, doi: 10.1016/j.outlook.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Starkweather A, Coleman B, Barcelona de Mendoza V, Fu M, Taylor J, Henderson W, Kenner C, Walker D, Amankwaa L, & Anderson C (2017). Policy brief: Improve coverage of newborn genetic screening to include the Recommended Uniform Screening Panel (RUSP) and newborn screening registry. Nursing Outlook, 65(4), 480–484, doi: 10.1016/j.outlook.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkweather A, Coleman B, Barcelona de Mendoza V, Fu M, Menzies V, O’Keefe M, & Williams J (2018a). Strengthen federal and local policies to advance precision health implementation and nurses’ impact on healthcare quality and safety. Nursing Outlook, 66, 401–406, doi: 10.1016/j.outlook.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Taylor J, & Mendoza V (2018). Improving -omics based research and precision health in minority populations: Recommendations for nurse scientists. Journal of Nursing Scholarship, 50(1), 11–19, doi: 10.1111/jnu.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Wright M, Hickey K, & Housman D (2017). Genome sequencing technologies and nursing: What are the roles of nursing and nurse scientists? Nursing Research, 66(2), 198–205, doi: 10.1097/NNR.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The All of Us Research Program Investigators. (2019). The “All of Us” Research Program. New England Journal of Medicine, 381(7), 668–676, doi: 10.1056/NEJMsr1809937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H, & Vasquez Brooks M (2011). Genetics and genomics in nursing: Evaluating essentials in implementation. Nurse Education Today, 31(6), 623–627, doi: 10.1016/j.nedt.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tluczek A, Twal M, Beamer L, Burton C, Darmofal L, Kracun M, Zanni K, & Turner M (2018). How American Nurses Association code of ethics informs genetic/genomic nursing. Nursing Ethics, 26(5), 1505–1517, doi: 10.1177/0969733018767248. [DOI] [PubMed] [Google Scholar]
- Tricco A, Lillie E, Zarin W, O’Brien K, Colquhoun H, Levac D, Moher D, Peters M, Horsley T, Weeks L, Hempel S, Akl E, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson M, Garrity C, …, Straus S (2018). PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Annals of Internal Medicine, 169(7), 467–473, doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Veteran’s Affairs. (2020). Million veteran program. Office of Research & Development. Retrieved from: https://www.research.va.gov/mvp/ Accessed June 11, 2020. [Google Scholar]
- U.S. Preventive Services Task Force (USPSTF). (2019). Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer. USPSTF recommendation statement. JAMA, 322(7), 652–665, doi: 10.1001/jama.2019.10987. [DOI] [PubMed] [Google Scholar]
- Vaughn V, Saint S, Krein S, Forman J, Meddings J, Ameling J, Winter S, Townsend W, & Chopra V (2019). Characteristics of healthcare organizations struggling to improve quality: Results from a systematic review of qualitative studies. BMJ Quality and Safety, 28, 78–84, doi: 10.1136/bmjqs-2017-007573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi S, Bult C, Chisholm R, Deverka P, Ginsburg G, Jacob H, Kasapi M, McLeod H, Roden D, Williams M, Green E, Rodriguez L, Aronson S, Cavallari L, Denny J, Dressler L, Johnson J, Klein T, Leeder J, …, Relling M (2018). Research directions in the clinical implementation of pharmacogenomics—An overview of US programs and projects. Clinical Pharmacology and Therapeutics, 103(5), 778–786, doi: 10.1002/cpt.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield M (2004). Linking health policy to nursing and health care scholarship: Points to consider. Nursing Outlook, 52, 111–123, doi: 10.1016/S0029-6554(01)70039-5 doi: 10.1016/j.outlook.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Williams JK, Feero G, Leonard D, & Coleman B (2017). Implementation science, genomic precision medicine and improved health: A new path forward? Nursing Outlook, 65, 36–40, doi: 10.1016/j.outlook.2016.07.014. [DOI] [PubMed] [Google Scholar]
- Williams JK, Katapodi M, Starkweather A, Badzek L, Cashion A, Coleman B, Fu M, Lyon D, Weaver M, & Hickey K (2016). Advanced nursing practice and research contributions to precision medicine. Nursing Outlook, 64, 117–123, doi: 10.1016/j.outlook.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Williams JL, Chung W, Fedotov A, Kiryluk K, Weng C, Connolly J, Harr M, Hakonarson H, Leppig K, Larson E, Jarvik G, Vennstra D, Hoell C, Smith M, Holm I, Peterson J, & Williams M (2018). Harmonizing outcomes for genomic medicine: Comparison of eMERGE outcomes to ClinGen outcome/intervention pairs. Healthcare, 83(6), 1–16, doi: 10.3390/healthcare6030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M (2019). Early lessons from the implementation of genomic medicine programs. Annual Review of Genomics and Human Genetics, 20, 389–411, doi: 10.1146/annurev-genom-083118-014924. [DOI] [PubMed] [Google Scholar]
- Williams M, Buchanan A, Davis F, Faucett W, Hallquist M, Leader J, Martin C, McCormick C, Meyer M, Murray M, Rahm A, Schwartz M, Sturm A, Wagner J, Williams J, & Ledbetter D (2018). Patient-centered precision health in a learning health care system: Geisinger’s genomic medicine experience. Health Affairs, 37(5), 757–764, doi: 10.1377/hlthaff.2017.1557. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2020). Health policy. Retrieved from: https://www.who.int/topics/health_policy/en/. Accessed June 11, 2020
- Yano E (2008). The role of organizational research in implementing evidence-based practice: QUERI series. Implementation Science, 3(29), 1–15, doi: 10.1186/1748-5908-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


