Short abstract
Introduction
Cutibacterium acnes (C. acnes) is a common pathogen in postoperative shoulder infections. The purpose of this study was to evaluate the time to positive cultures for C. acnes and compare our experience before and after implementation of a regulated anaerobic chamber system. We hypothesized that this would reduce the time to identify positive cultures.
Methods
This was a retrospective review of 34 patients with cultures obtained from the shoulder that were positive for C. acnes. The time until positive result was evaluated before and after implementation of a regulated anaerobic incubation chamber.
Results
Following implementation of the regulated anaerobic incubation chamber, the time until C. acnes culture growth significantly decreased from 6.5 days (range 3–10 days) to 4.9 days (range 2.75–10 days) (mean difference: 1.6 days, 95% confidence interval: 1.06–2.66 days; P = .002). True infections had a significantly shorter time to positive culture compared to contaminants (5.5 vs 6.8 days, respectively, P = .003). Increased number of positive culture specimens correlated with a shorter time to positivity (Spearman rank = −0.58, P = .007).
Conclusion
Improved anaerobic culture protocols and techniques may lead to greater accuracy and earlier diagnosis and initiation of treatment of postoperative shoulder infections.
Keywords: Cutibacterium acnes, anaerobic culture, shoulder, infection, anaerobic incubation chamber, anaerobic, Propionibacterium acnes
Introduction
Cutibacterium acnes (C. acnes), formerly Propionibacterium acnes, is a Gram-positive, anaerobic bacillus known to colonize lipid-laden hair follicles and sebaceous glands around the shoulder.1,2 C. acnes is implicated in nearly 50% of subacute and chronic infections following shoulder arthroplasty as well as other postoperative infections.3–9 Early reports of postoperative infections in shoulder surgery likely did not fully comprehend the role of C. acnes. More recent work recognizes that C. acnes is a common pathogen and that prolonged culture duration is required, with some investigators advocating culture incubation lengths as long as 21 days in order to increase the yield of positive cultures.10–13 However, extended culture duration may delay a diagnosis and treatment of infection, as well as lead to culture contamination and false-positive growth, thus creating a diagnostic challenge.5,6
Anaerobic microorganisms, such as C. acnes, require specific culture techniques to ensure growth and identification in the clinical setting. Standard anaerobic chamber systems rely on fixed concentrations of gas (commonly 5% carbon dioxide) to maintain a constant environment. Alternative techniques and protocols have been developed to improve the efficacy of anaerobic cultures.14–18 The anoxomat incubation system (AIS; Advanced Instruments, Norwood, MA) employs an automatic evacuation-replacement technique to create and maintain an anaerobic and microaerophilic environment for bacterial culture. 15
The purpose of this study was to evaluate the time to positive cultures for C. acnes and compare our experience before and after implementation of a regulated anaerobic chamber system. We hypothesized that this would reduce the time for growth.
Materials and Methods
Following Institutional Review Board approval, we retrospectively reviewed the records of 91 patients from October 1999 to September 2015 who had tissue cultures obtained during shoulder surgery at a large tertiary academic medical center. An automated anaerobic incubation chamber system (AIS; Advanced Instruments) was introduced in December 2011. There were 47 males and 44 females with an average age of 59.5 years old (range 32–83). Among the 91 surgeries during this period, 34 patients had positive C. acnes culture. There were no cases with negative cultures that were subsequently diagnosed with infection. Twenty-four of the patients had a history of prior shoulder arthroplasty, while 10 did not involve prior shoulder arthroplasty. Based upon the clinical evaluation, 19 of the patients had a suspected infection preoperatively, while 15 did not. Cultures were obtained on all patients for which an infectious etiology of pain or implant failure could not be excluded, even if clinical suspicion was low.
Patients had preoperative laboratory testing. The average number of cultures taken in each case was 4.6 (range 2–7). Tissue samples were taken at varying depths of dissection. For periprosthetic infection, superficial tissue as well as deep tissue including glenoid and humeral periprosthetic tissue and capsular tissue was sampled. Perioperative intravenous antibiotics were withheld until the culture specimens were collected.
Solitary positive cultures with no preoperative or intraoperative findings of infection were considered “contaminants” according to the definition proposed by Frangiamore et al. 6 Individuals were considered to have “true” infections if they had greater than one positive culture, or if they had one positive culture in addition to one of the following findings: elevated erythrocyte sedimentation rate, elevated C-reactive protein, purulent drainage, redness, swelling, or positive frozen section with acute inflammation consistent with infection. 6
Culture Technique
All surgical specimens were collected in a sterile manner and submitted to the laboratory by direct transport at the time of collection. Upon arrival, specimens were immediately minced and placed onto aerobic media (Sheep Blood agar [BAP], Colistin-Nalidixic-agar [CNA], Chocolate agar, MacConkey agar), anaerobic media (Kanamycin-Vancomycin agar, phenylethyl alcohol agar, anaerobic BAP), and thioglycolate broth (used for growth of small numbers of both aerobic and facultative anaerobes). Prereduced media (Brucella agar) aerobic plates and thioglycolate broth were incubated in an air incubator at 35°C. During the early period of the study, a standard (nonautomated) anaerobic chamber system was used to maintain a constant environment. In the latter part of the study period, an automated system was used, as this system was deemed an efficient and reliable method to culture anaerobic bacteria. No cultures were grown on both incubation systems. This regulated anaerobic chamber uses pressure measurements to control evacuation of air from the culture jars which is then followed by replacement of the gas mixture to create a specific anaerobic environment (80% nitrogen, 10% carbon dioxide, and 10% hydrogen). The cultures were stored at 35°C. This method of controlled evacuation has been previously validated as an efficient and reliable technique for culturing anaerobic bacteria. 15
The aerobic plates and the thioglycolate broth were first evaluated at 24 h following collection. Aerobic plates were checked daily and routinely held for 5 days before discarding. Anaerobic media and thioglycolate broth were held for 14 days. Anaerobic media were checked daily Monday through Saturday only. Four cases were reported positive on a Monday and it was not known if they were positive on the preceding Sunday. C. acnes organisms were ultimately identified by Matrix Assisted Laser Desorption/Ionization-Time-of-Flight and confirmed by standard biochemical and antibiotic disk tests.
Statistical Analysis
Descriptive statistics were reported as mean, standard deviation, and 95% confidence intervals (CIs). The average number of days until C. acnes cultures were positive was compared before and after implementation of the automated anaerobic incubation chamber using an independent samples t test. Data from pre- and postimplementation of the automated anaerobic incubation chamber was compared using an independent samples t test. Fisher’s exact test was used to analyze the relationship of revision arthroplasty to the culture results given the lower number of revision arthroplasty cases. A Mann–Whitney U test was used to compare the number of days until C. acnes culture positivity for contaminants versus true positives in order to account for the possibility of non-normal data. Finally, a Spearman rank correlation was used to assess the relationship between the proportion of positive samples for C. acnes and the time until the initial cultures became positive, again to account for the possibility of nonparametric data. A post hoc power analysis was utilized with a calculated power level of 95.7%, given the mean difference of 1.6 days. A P value of .05 was used to determine significance for all tests.
Results
The cohorts evaluated before and after the implementation of the automated anaerobic incubation chamber were similar (Table 1). There were 23 males and 11 females, and the mean age was 58.6 years old (range 18.3–83.6 years old). There was no significant difference between the distributions and types of cases (revision arthroplasty cases or nonarthroplasty cases) before and after initiation of automated anaerobic incubation chamber (Fisher’s exact = 5.5, P = .68). There were no other significant differences between pre- and postimplementation of the automated anaerobic incubation chamber with regard to patient demographics (Table 2).
Table 1.
List of Patient Demographics for Patients With Cultures Positive for Cutibacterium acnes.
| Gender | |
|---|---|
| Male | 23 |
| Female | 11 |
| Age (years ± SEM) | 58.6 ± 3.0 |
| Procedure performed | |
| Revision total shoulder arthroplasty | 16 |
| No infection suspected | 10 |
| Revision for infection | 6 |
| Revision hemiarthroplasty | 6 |
| No infection suspected | 2 |
| Revision for infection | 4 |
| Revision reverse total shoulder arthroplasty | 2 |
| No infection suspected | 1 |
| Revision for infection | 1 |
| Arthroscopic debridement | 5 |
| No infection suspected | 0 |
| Revision for infection | 5 |
| Clavicle incision and drainage | 3 |
| No infection suspected | 0 |
| Revision for infection | 3 |
| Rotator cuff repair | 1 |
| No infection suspected | 1 |
| Revision for infection | 0 |
| Coracoclavicular ligament reconstruction | 1 |
| No infection suspected | 1 |
| Revision for infection | 0 |
Abbreviation: SEM, standard error of the mean.
Table 2.
List of Patient Demographics and Time Until Cutibacterium acnes Culture Growth Pre- and Postautomated Anaerobic Chamber Implementation.
| Prechamber | Postchamber | P | |
|---|---|---|---|
| Gender | |||
| Male | 13 | 10 | .91 |
| Female | 6 | 5 | |
| Age (years ± SEM) | 57.9 ± 4.2 | 59.5 ± 4.4 | .8 |
| Procedure performed | |||
| Revision total shoulder arthroplasty | 8 | 5 | |
| Antibiotic spacer placement | 4 | 2 | |
| Arthroscopic debridement | 3 | 2 | |
| Revision hemiarthroplasty | 1 | 2 | .68 |
| Clavicle incision and drainage | 1 | 2 | |
| Revision reverse total shoulder arthroplasty | 0 | 2 | |
| Rotator cuff repair | 1 | 0 | |
| Coracoclavicular ligament reconstruction | 1 | 0 | |
| Days until C. acnes positive (± SEM) | 6.5 ± 0.4 | 4.9 ± 0.2 | .002 |
| Days until C. acnes positive without contaminants (± SEM) | 6.0 ± 0.4 | 4.6 ± 0.4 | .016 |
| Proportion of cultures positive after 7 days | 0.15 | 0.06 | .21 |
Abbreviation: SEM, standard error of the mean.
Thirty-four of the 91 cases (37%) had at least 1 culture positive for C. acnes with a mean number of positive samples of 2.8 (range 1–7). Nineteen samples were positive for C. acnes before implementation of the automated anaerobic incubation chamber, and 15 were positive after (Table 2). In addition to C. acnes, 8 patients grew coagulase-negative Staphylococci, 2 grew Staphylococcus aureus, 2 grew alpha-hemolytic Streptococci, and 1 grew Enterobacter.
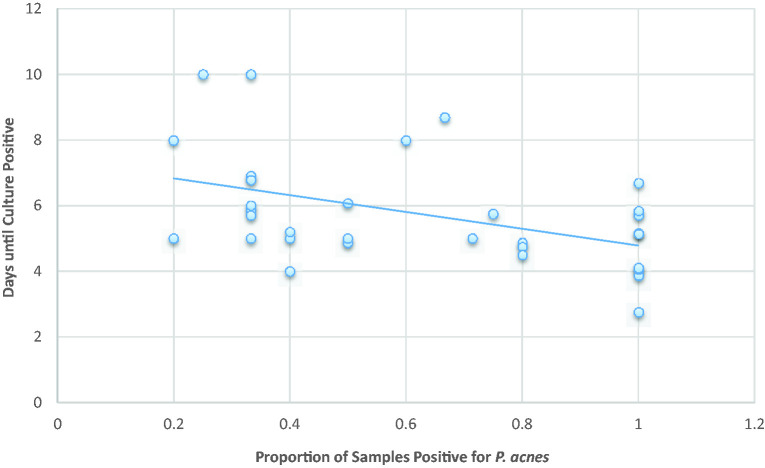
Figure 1.
Relationship between days until culture positivity and proportion of sample positive for C. acnes with linear correlation line superimposed.
Following implementation of the automated anaerobic incubation chamber, the average number of days until C. acnes positivity significantly decreased from 6.5 to 4.9 days (mean difference of 1.6 with 95% CI: 1.06–2.66 days, P = .002). When the cases that were thought to be contaminants were excluded from the mean culture times, there remained a significantly shorter time to positive cultures from 6.0 to 4.6 days (P = .016). There was no significant differences between time to culture positivity for revision arthroplasty cases with and without suspected infection (5.9 vs 5.7 days, P = .8).
Cases with true infections had a significantly shorter average time to C. acnes positivity compared to the contaminants (5.5 vs 6.8 days, respectively, mean difference of 1.3 days, 95% CI: 0.86–1.74 days, P = .003), regardless of the culture protocol. There was a significant negative correlation between the proportion of positive samples for C. acnes and average time until the culture positivity (Spearman rank = −0.58, P = .007) regardless of the culture protocol (Figure 1). Prior to initiation of the automated anaerobic incubation chamber, 3 cultures became positive after 7 days, whereas only 1 returned positive after 7 days following initiation of the system (at 10 days).
Discussion
The observed prolonged culture times reported to identify C. acnes pose problems for clinical management by both potentially delaying the onset of treatment as well as increasing the risk of obtaining false-positive cultures that lead to inappropriate treatment. Although most investigators recommend maintaining prolonged culture times, there has been little effort to standardize and optimize culturing practices in order to decrease culture times or eliminate false-positive cultures.19–21 In fact, many reports omit specific information regarding culturing protocol, making it difficult to generalize the findings. In this investigation, we found that implementing the automated anaerobic incubation chamber significantly decreased the time to positive C. acnes culture.
We found that the use of a combination of anaerobic prereduced media (Brucella agar) along with an automated regulated anaerobic incubation system resulted in an average time to positive C. acnes cultures lower than that typically reported in other studies. Butler-Wu et al. reported that nearly 70% of cultures became positive for C. acnes after more than 7 days. 13 Although other authors also report using prereduced Brucella media, none have reported the use of an automated anaerobic incubation chamber or other automated systems for jar evacuation-replacement for culture of anaerobes. Matsen et al. reported mean anaerobic culture times of 6.8 days for C. acnes growth, although they do not specifically comment on the method of anaerobic incubation. 14
Some authors favor anaerobic culture incubation periods of up to 14 to 21 days in order to increase anaerobic culture yield. 13 Although it is true that longer incubation periods will increase the number of positive samples, recent reports also suggest that later growing samples are more likely to be false positives. 6 Frangiamore et al. found that, among patients with only 1 positive C. acnes and no other clinical signs of infections, the mean time to positive culture growth was 4 days longer compared to cases with other clinical signs of infection. 6 When they excluded the cases with false-positive cultures, the time to culture positivity was only 5 days. 6 Our study also found that cases thought to have true infections had a significantly shorter time until culture positivity compared to the contaminant cases, regardless of culture technique (5.5 vs 6.8 days, respectively). In addition, the time to positivity was even less after implementation of the automated anaerobic incubation chamber for cases of true infection (4.6 days). Lastly, the proportion of positive samples also significantly correlated with time until C. acnes culture positivity, suggesting that the true infection group was associated with a decrease in time to positive sample result. Millet et al. recently reported that only 14% of their cultures were positive for C. acnes after 7 days, with those becoming positive after 7 days being more likely to have a lower proportion of positive samples. 5 In contrast to most other reports, using the regulated anaerobic chamber system, only 1 culture became positive for C. acnes after 7 days. Most previous investigators report that a majority of samples become positive after 7 days. 13
There are limitations to this study. As this is a retrospective study, data collection was not standardized. Nevertheless, the medical records that were reviewed demonstrated substantial homogeneity, as did the culture techniques at our local institution. Importantly, we did not directly compare the results of cultures of specimens using both culture techniques (ie, with and without the automated anaerobic incubation chamber system). As previously stated, anaerobic media were not checked for growth on Sundays, leading to a possible delay in identification of cultures positive for C. acnes. Four samples were reported as positive on a Monday: 2 prior to initiation of the chamber system and 2 samples after. This had no significant effect on the differences between the mean time to positive cultures. Finally, the definition of “contaminant” adopted from Frangiamore et al. may be imperfect in that the area of clinical infection may have only been sampled once. Therefore, patients with only 1 positive shoulder culture may not actually represent “contaminants.” To combat this bias, it is imperative that surgeons take multiple samples from varying locations and depths during the surgery, with multiple samples from surgical sites in which there is a suspicion for infection.
In conclusion, the findings of this study demonstrate that implementation of an automatically regulated anaerobic incubation system significantly reduced the time to positivity of C. acnes cultures obtained from the shoulder. Improved anaerobic culture protocols and techniques may lead to greater accuracy and earlier diagnosis and initiation of treatment of postoperative shoulder infection. Although additional studies must be performed, these benefits might ultimately result in overall cost savings to the health-care system if more reliable culture results ultimately result in a decreased latency to diagnosis and treatment of C. acnes infections.
Ethical Approval
This project was approved by the Lifespan/Rhode Island Hospital Intuitional Review Board (reference 410414; 45 CFR 46.110(5)).
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Leeming JP, Holland KT, Cunliffe WJ. The microbial ecology of pilosebaceous units isolated from human skin. Microbiology. 1984; 130:803–807. [DOI] [PubMed] [Google Scholar]
- 2.Patel A, Calfee RP, Plante M, Fischer SA, Green A. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elb Surg. 2009; 18:897–902. [DOI] [PubMed] [Google Scholar]
- 3.Hall GS, Pratt-Rippin K, Meisler DM, Washington JA, Roussel TJ, Miller D. Growth curve for Propionibacterium acnes. Curr Eye Res. 1994; 13:465–466. [DOI] [PubMed] [Google Scholar]
- 4.Dodson CC, Craig EV, Cordasco FA, et al. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elb Surg. 2010; 19:303–307. [DOI] [PubMed] [Google Scholar]
- 5.Millett PJ, Yen YM, Price CS, Horan MP, Van Der Meijden OA, Elser F. Propionobacter acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011; 469:2824–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frangiamore SJ, Saleh A, Grosso MJ, et al. Early versus late culture growth of Propionibacterium acnes in revision shoulder arthroplasty. J Bone Joint Surg Am. 2015; 97:1149–1158. [DOI] [PubMed] [Google Scholar]
- 7.Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;(382):206–216. [DOI] [PubMed] [Google Scholar]
- 8.Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007; 55:119–124. [DOI] [PubMed] [Google Scholar]
- 9.Pottinger P, Butler-Wu S, Neradilek MB, et al. Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Jt Surg Am. 2012; 94:2075–2083. [DOI] [PubMed] [Google Scholar]
- 10.Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008; 47:1403–1409. [DOI] [PubMed] [Google Scholar]
- 11.Kelly JD, Hobgood ER: Positive culture rate in revision shoulder arthroplasty. Clin Orthop Relat Res. 2009; 467:2343–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy PY, Fenollar F, Stein A, et al. Propionibacterium acnes postoperative shoulder arthritis: an emerging clinical entity. Clin Infect Dis. 2008; 46:1884–1886. [DOI] [PubMed] [Google Scholar]
- 13.Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011; 49:2490–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsen FA, Butler-Wu S, Carofino BC, Jette JL, Bertelsen A, Bumgarner R. Origin of propionibacterium in surgical wounds and evidence-based approach for culturing propionibacterium from surgical sites. J Bone Joint Surg Am. 2013; 95:e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Summanen P, McTeague M, Väisänen M-L, Strong C, Finegold S. Comparison of recovery of anaerobic bacteria using the Anoxomat®, Anaerobic Chamber, and GasPak®Jar Systems. Anaerobe. 1999; 5:5–9. [DOI] [PubMed] [Google Scholar]
- 16.Debelian GJ, Olsen I, Tronstad L. Electrophoresis of whole-cell soluble proteins of microorganisms isolated from bacteremias in endodontic therapy. Eur J Oral Sci. 1996; 104:540–546. [DOI] [PubMed] [Google Scholar]
- 17.Debelian GJ, Olsen I, Tronstad L. Bacteremia in conjunction with endodontic therapy. Dent Traumatol. 1995; 11:142–149. [DOI] [PubMed] [Google Scholar]
- 18.Saper D, Capiro N, Ma R, Li X. Management of Propionibacterium acnes infection after shoulder surgery. Curr Rev Musculoskelet Med. 2015; 8:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padegimas EM, Maltenfort M, Ramsey ML, Williams GR, Parvizi J, Namdari S. Periprosthetic shoulder infection in the United States: incidence and economic burden. J Shoulder Elb Surg. 2015; 24:741–746. [DOI] [PubMed] [Google Scholar]
- 20.Matsen FA, Russ SM, Bertelsen A, Butler-Wu S, Pottinger PS. Propionibacterium can be isolated from deep cultures obtained at primary arthroplasty despite intravenous antimicrobial prophylaxis. J Shoulder Elb Surg. 2015; 24:844–847. [DOI] [PubMed] [Google Scholar]
- 21.Ricchetti ET, Frangiamore SJ, Grosso MJ, et al. Diagnosis of periprosthetic infection after shoulder arthroplasty: a critical analysis review. JBJS Rev. 2013; 1:e3–e3. [DOI] [PubMed] [Google Scholar]