Short abstract
Background
There are limited data on the effect of glenoid retroversion in clinical outcomes following reverse total shoulder arthroplasty (RTSA). The purpose of this study was to evaluate if surgical correction of retroversion affects outcomes following RTSA.
Methods
An institutional database was utilized to identify 177 patients (mean age: 68.2 ± 10.1 years) with minimum 2-year follow-up after primary RTSA. Glenoid version was measured on preoperative and postoperative radiographs. American Shoulder and Elbow Surgeons (ASES) scores and range of motion were collected before and after RTSA. Change in retroversion was determined by comparing preoperative and postoperative glenoid retroversion on radiographs using paired Wilcoxon signed-rank test. Spearman’s rank correlation was used to investigate relationships between ASES scores and glenoid retroversion.
Results
The mean postoperative ASES composite score (75.5 ± 22.7) was significantly higher than preoperative (36.8 ± 19.2; P < .0001). The mean preoperative glenoid retroversion was 9.1 ± 6.7° compared to 6.5 ± 5.1° postoperatively (P < .0001). There was no correlation between postoperative ASES scores and preoperative retroversion (r = .014, P = .85) or postoperative retroversion (r = −.043, P = .57). There was no statistical relationship between postoperative retroversion and range of motion, though there is a risk of inadequate power given the sample size.
Conclusions
Patient-reported outcomes and range of motion measurements following RTSA at short-term follow-up appear to be independent of either preoperative or postoperative glenoid retroversion.
Keywords: Reverse shoulder arthroplasty, glenoid retroversion, shoulder replacement outcomes
Introduction
Shoulder arthritis is a significant cause of pain, functional limitations, and decreased quality of life, all of which may be improved with shoulder arthroplasty.1,2 Shoulder replacement, with either anatomic or reverse total shoulder arthroplasty (RTSA), can provide excellent pain relief and improvements in shoulder function. Since the introduction of RTSA in Europe and the United States in here has been rising utilization of RTSA. In 2011, it was estimated that RTSA represented one-third of all shoulder arthroplasty in the United States, 3 and the proportion of RTSA compared to TSA has also dramatically increased. 4
In advanced cases of glenohumeral arthritis, the loss of articular cartilage on both the glenoid and humerus can lead to posterior subluxation of the humerus and posterior glenoid wear. Walch et al. has previously outlined the wear pattern for glenohumeral arthritis.5 According to this classification, glenoids with posterior wear include B2 (biconcave) and C type (greater than 25° of glenoid retroversion) glenoids, which can be a challenge for replacement procedures. 5 Biomechanical studies have shown that increased glenoid component retroversion leads to increased humeral contact forces in anatomic total shoulder arthroplasty (TSA) which can lead to increased wear and loosening; however, there are little data on the effect of glenoid retroversion in RTSA. 6
Various methods to manage posterior wear for reverse shoulder replacement have been described. Eccentric reaming and glenoid bone grafting are options to allow for correction of the retroverted glenoid to a more neutral surface.7–9 Lateralization with bone grafting has been reported to allow for excellent patient reported outcomes and range of motion after RTSA though these procedures are technically demanding. Recently, there has been much interest in novel augmented implants to address posterior glenoid erosion.10,11 Despite increasing interest and utilization of these implants, the effect of preoperative and postoperative retroversion on subjective and objective outcomes after RTSA remains undefined.
The primary purpose of this study was to evaluate the effect of preoperative and postoperative glenoid retroversion on clinical outcomes following RTSA. We hypothesized that increased preoperative and postoperative glenoid retroversion would negatively influence postoperative patient-reported outcomes and shoulder range of motion.
Materials and Methods
Patients were prospectively enrolled as part of an institutional shoulder arthroplasty database. Patients were treated by 1 of 3 fellowship-trained orthopedic surgeons between October 2007 and September 2016. Inclusion criteria included patients who underwent a primary RTSA for any indication and were at least 2 years postoperative from replacement surgery. A total of 426 patients were identified meeting inclusion criteria. Patients were excluded if they were lost to follow-up (N = 104), if there was no available preoperative (N = 101) or postoperative (N = 6) radiographs, if they were deceased (N = 15), or elected to be dropped from the database (N = 10). Patients with a failure or infection (N = 13), including prior to 2 years postoperative, were included for measurement of glenoid retroversion but excluded from analysis of final outcomes given interval revision surgery. All patients provided informed consent to participate.
Surgical Treatment
RTSA was performed in the beach chair position through a deltopectoral approach. The subscapularis was released with a peel and was not routinely repaired at the conclusion of the procedure. All patients were treated with the same implant system (Zimmer, Inc). The baseplate for this system has a trabecular metal surface, central peg, and 2 peripheral locking screws. A 25 mm baseplate was used with a 36 mm glenosphere. No augmented baseplates or bone grafting procedures were used on the glenoid side. The humeral stem was implanted in approximately 20° of retroversion and was routinely cemented in place. Postoperatively, patients were immobilized in a sling for 6 weeks. Formal physical therapy began at 6 weeks after surgery, with a goal for recovery of range of motion by 3 months postoperative.
Data Collection
Demographic variables, including age, sex, body mass index (BMI), and surgical laterality, were recorded. Patients completed preoperative and annual postoperative outcome evaluations with the American Shoulder and Elbow Surgeons (ASES) composite scores. The individual functional and pain subscores were also collected for analysis, and the most recent postoperative score available was used for analysis. Range of motion was measured by a trained clinical research coordinator who was blinded to the patients’ radiographic parameters. Range of motion measurements were made with a goniometer and included forward flexion, abduction, external rotation, and internal rotation (marked as the highest spinous process level).
Imaging Measurements
Preoperative and postoperative plain radiographs, including a true anteroposterior view of the glenohumeral joint in neutral rotation and an axillary lateral view, were collected. The most recent radiograph prior to surgery was selected for the preoperative measurements. Postoperatively, radiographs were selected within 1 year of surgery that most clearly demonstrated the baseplate position.
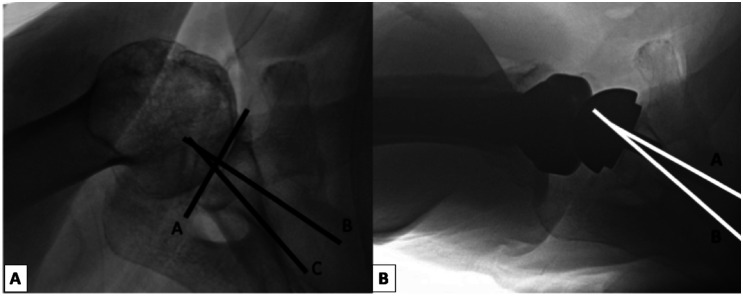
Glenoid retroversion was measured on the axillary lateral as previously described by Service et al. as the angle between the anterior–posterior glenoid face and the line perpendicular to the axis of the scapula (Figure 1).12 Glenoid retroversion was measured independently by 2 orthopedic surgeons (ECC and WX). The intraclass correlation coefficients demonstrated good reliability between reviewers for both preoperative retroversion (ICC = 0.86; 95% confidence interval = 0.81–0.89) and postoperative retroversion (ICC = 0.79; 95% CI = 0.73–0.83) measurements. The mean value between the 2 independent measurements was utilized for analysis.
Figure 1.
Retroversion measurements are shown on (A) preoperative axillary lateral radiograph and (B) postoperative axillary lateral radiograph following reverse total shoulder replacement. Preoperatively, a line along the face of the glenoid was drawn (A) and then a line perpendicular to this was drawn (B). A third line was drawn from the mid-point of the glenoid along the long axis of the scapula (C). The angle between B and C defined the glenoid retroversion. Postoperatively, a line along the central peg (A) was drawn and a line along the axis of the scapula was drawn (B). The angle between A and B defined the component retroversion.
Statistical Analysis
Values were reported as mean and standard deviation for continuous variables and number and percentage for categorical variables. Patients were classified into 3 groups ( < 10°, 10–20°, and >20°) for both preoperative retroversion measurement and postoperative retroversion measurement. The magnitude of change in retroversion was determined with by comparing preoperative glenoid retroversion to postoperative glenoid retroversion with a paired Wilcoxon signed rank test as well as a Fisher’s exact test to compare groups for preoperative and postoperative retroversion measurements. Univariate analysis was performed with Spearman’s rank correlation to investigate relationships between the ASES composite score and preoperative and postoperative glenoid retroversion measurements. We also performed univariate analysis to determine relationships between the ASES functional subscore, ASES pain subscore, forward flexion, abduction, external rotation, and internal rotation with preoperative and postoperative glenoid retroversion measurements. We compared the ASES scores for patients with < 10° of postoperative glenoid retroversion to those equal to or greater than 10° of postoperative glenoid retroversion with unpaired Student’s t tests. The change in retroversion after surgery was calculated, and the relationship between the change and version and ASES scores was tested with a Spearman’s rank correlation. Statistical analyses were performed using Stata (version 13.0, STATA Inc., College Station, Texas). All reported P values were 2-tailed with statistical significance established at P < .05.
Results
There were 177 patients available for follow-up, with a mean age of 68.2 ± 10.1 years and BMI of 28.1 ± 6.5 kg/m2 (Table 1). A total of 21 adverse events requiring revision surgery were reported by 13 patients (3.1%), including infection (N = 12, 7.4%), periprosthetic fracture (N = 7, 4.3%), and instability (N = 2, 1.2%). The preoperative retroversion for patients with eventual failure was 3.5° ± 8.0°. The postoperative retroversion for these patients was 2.4° ± 9.7°.
Table 1.
Patient Demographics.
| Mean (N) | Standard Deviation (%) | |
|---|---|---|
| Age (years) | 68.2 | 10.1 |
| Body mass index (kg/m2) | 28.1 | 6.5 |
| Sex | ||
| Male | 85 | 48.0 |
| Female | 92 | 52.0 |
| Final follow-up (years) | 3.8 | 1.8 |
| Preoperative glenoid retroversion (°) | 9.1 | 6.7 |
| Postoperative glenoid retroversion (°) | 6.5 | 5.1 |
The ASES composite score and range of motion measurements all significantly improved following RTSA (Table 2). The final mean ASES composite score was 75.5 ± 22.7, which was significantly higher than the preoperative ASES composite score (36.8 ± 19.2; P < .0001). The mean preoperative glenoid retroversion was 9.1 ± 6.7° and ranged from −4° to 36.5°. The postoperative glenoid retroversion was significantly lower (P < .0001) at 6.5 ± 5.1° and ranged from −1° to 32°.
Table 2.
Comparison Between Preoperative and Postoperative Variables for Patients Undergoing Reverse Total Shoulder Arthroplasty.
|
Preoperative |
Postoperative |
||||
|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | P | |
| ASES composite score | 36.8 | 19.2 | 75.1 | 23.2 | <.0001 |
| Forward flexion (°) | 82.3 | 40.7 | 133.7 | 29.8 | <.0001 |
| Abduction (°) | 70.6 | 41.4 | 127.3 | 36.1 | <.0001 |
| External rotation (°) | 33.2 | 24.3 | 35.1 | 20.1 | .47 |
| Internal rotation (median spinous level) | L3 | 5.0 | L1 | 5.6 | .007 |
| Glenoid retroversion (°) | 9.1 | 6.7 | 6.5 | 5.1 | <.0001 |
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
There was no significant correlation between the preoperative retroversion (Table 3) and the postoperative ASES Composite score (rho = .014, P = .85), ASES Function score (rho = −.044, P = .57), or ASES Pain score (rho = .11, P = .21). We observed no statistical relationship between preoperative retroversion and active postoperative forward flexion, abduction, or external rotation. There was a significant correlation between preoperative retroversion with active postoperative internal rotation (rho = .16, P = .047).
Table 3.
Relationship Between Preoperative Retroversion and Outcome Measurements.
| Spearman’s rho | P | |
|---|---|---|
| ASES composite score | .014 | .85 |
| ASES function subscore | −.044 | .57 |
| ASES pain subscore | .11 | .21 |
| Forward flexion | .045 | .58 |
| Abduction | .092 | .25 |
| External rotation | −.0062 | .94 |
| Internal rotation | .16 | .047 |
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
There were no significant correlations between the postoperative retroversion (Table 4) and ASES Composite score (rho = −.043, P = .57; Figure 2), ASES Function score (rho = −.026, P = .74), or ASES Pain score (rho = .029, P = .75). We observed no statistical relationship between postoperative retroversion and active postoperative forward flexion, abduction, external rotation, or internal rotation. There was no significant correlation between the change in version and the final ASES Composite score (rho = .078, P = .30) or between the change in version and the change in the ASES Composite score (rho = .17, P = .10).
Table 4.
Relationship Between Postoperative Retroversion and Outcome Measurements.
| Spearman’s rho | P | |
|---|---|---|
| ASES composite score | −.043 | .57 |
| ASES function subscore | −.026 | .74 |
| ASES pain subscore | .029 | .75 |
| Forward flexion | −.092 | .25 |
| Abduction | −.021 | .80 |
| External rotation | .079 | .33 |
| Internal rotation | .14 | .08 |
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
Figure 2.
A scatter plot is shown demonstrating the relationship between postoperative component retroversion and final ASES score. There was no significant relationship between these 2 variables (Spearman’s rho = −.043, P = .57). ASES, American Shoulder and Elbow Surgeons.
Discussion
We observed no relationship between either native glenoid retroversion or baseplate retroversion and postoperative patient-reported measures for patients treated with RTSA. For range of motion, the only significant association noted was between preoperative retroversion and final postoperative internal rotation. There were no other significant relationships between retroversion before or after surgery and final range of motion measurements. There was also no apparent influence of preoperative or postoperative retroversion on eventual failure. The findings of this study support consideration for positioning of the baseplate for RTSA in a retroverted position if there is sufficient bone for support and fixation (Figure 3).
Figure 3.
Retroversion measurements are shown on (A) preoperative axillary lateral radiograph and (B) postoperative axillary lateral radiograph following reverse total shoulder replacement for a patient with increased preoperative and postoperative retroversion. Retroversion measured 28° preoperatively and 17° postoperatively. The final ASES score was 100.
Component positioning influences the final impingement-free range of motion of the shoulder following RTSA. Keener et al. utilized CT-based modeling of glenohumeral joints with retroverted glenoids to determine the influence of various positions of the baseplate, including a more lateralized position and retroversion ranging from neutral to 20° of retroversion.13 Potential range of motion was optimized with a lateralized baseplate. Increasing retroversion led to more internal rotation at the expense of decreasing external rotation. A larger glenosphere and lateralized glenoid component also improve impingement-free range of motion in modeling studies.14,15 Humeral component alignment can also influence final functional outcomes after RTSA. Gulotta et al. demonstrated through a cadaveric study that humeral retroversion from 0 to 20° allows for maximizing internal rotation while maintaining flexion and external rotation.16 These simulation study designs, however, do not account for muscle quality or function and does not establish the active functional motion after shoulder replacement. In our study, there was no clear relationship between component retroversion and any range of motion parameter or functional outcome.
Surgical techniques and implants have been introduced to address glenoid wear and bone loss in RTSA, including bony increased offset reverse shoulder arthroplasty (BIO-RSA) and augmented baseplates, among other techniques.8,9 The BIO-RSA, where humeral head autograft is placed between the native glenoid and the baseplate to correct glenoid bone deficiencies, can produce good functional and subjective outcomes. Athwal et al., however, found no differences from patients with BIO-RSA or with standard RTSA with regard to range of motion or patient-reported outcome measures.17 In a randomized controlled trial of BIO-RSA versus standard RTSA, Greiner et al. also found no difference between groups in final range of motion or PROs.18 These observations are concordant with our findings that the final glenoid component alignment with regards to version may not have a meaningful influence on clinical results.
While good results may be expected with bone grafting at the glenoid, it is important to consider results without these additive procedures. McFarland et al. described 42 patients with primary glenohumeral osteoarthritis, an intact rotator cuff, and posterior wear pattern who were treated with RTSA.19 This group utilized a similar surgical technique with eccentric reaming as needed to allow for baseplate seating but no attempt for glenoid correction to near-neutral version. They observed a 2% baseplate failure rate and 19% rate of scapular notching with excellent patient reported outcome measures and range of motion recovery. Our results are in agreement with their findings that correction of retroversion or posterior bone loss may not be necessary to achieve successful outcomes after RTSA. One concern with an eccentric reaming strategy may be incomplete seating of the baseplate on native glenoid bone; however, a biomechanical study reported no difference in micromotion or fixation strength between 50% and complete seating of the baseplate. 20
Recently, augmented baseplates have been introduced to address glenoid retroversion with the intention to both preserve bone and improve postoperative shoulder function without need for structural bone grafting. The clinical evidence to support this utilization, however, is limited. There are limited reports on outcomes and longevity of augmented components. Given our observation that a persistently retroverted glenoid component may not negatively influence either range of motion or patient outcomes, we encourage further study on this topic before the widespread adoption of these implants that may add cost and surgical complexity without a clear added benefit in postoperative results.
Multiple factors contribute to outcomes after RTSA. Preoperative muscle quality can influence postoperative functional outcomes.21,22 The preoperative expectations, patient comorbidities, and patient mental health status can variably contribute to satisfaction and outcomes.23,24 We are not able to measure or control for all of these in this study, and there may be a subgroup of patients that benefits from correction of glenoid retroversion. We would encourage further study of the topic before the routine widespread utilization of augmented baseplates or other techniques beyond eccentric reaming to correct glenoid retroversion until the clear indication for these procedures is understood.
This study used radiographic measurements of retroversion and continuous values rather than the Walch classification system. Cross-sectional imaging provides for more accurate determination of glenoid version; however, obtaining CT or MRI scans is not part of our routine protocol preoperatively for patients with shoulder arthritis and is certainly not part of the postoperative evaluation process after RTSA.25–28 The intraclass coefficient correlations for glenoid retroversion measured on axillary lateral radiographs and CT scans has been reported as 0.67 to 0.69 for patients with shoulder arthritis.26,28 While the Walch classification offers a useful method for approaching glenoid deformity and wear patterns, the reproducibility of this system is moderate and further limits data analysis given its categorical nature. 29
This study does have multiple other limitations that deserve consideration. Our study is underpowered to definitively claim that there is no statistical association between retroversion and outcome scores. With a sample size of 165 patients, however, we could detect a correlation of 0.21 as statistically significant, and the strongest relationship observed in this cohort had a correlation coefficient of only 0.08. We determined retroversion measurements based on radiographs. While cross-sectional imaging may be more accurate, these studies are not standard-of-care for all surgeons, carry an increased risk of irradiation, and would be difficult to obtain routinely in the postoperative period for a large cohort of patients. We also do not have information regarding muscle quality or the status of the posterior rotator cuff, which may influence outcomes after RTSA. Our results do reflect early follow-up. We are unable to determine the effects of glenoid retroversion on potential baseplate loosening or failure over time, and this certainly warrants future evaluation. Our study includes all patients treated with RTSA and is not specific to advanced glenoid retroversion, which does represent a smaller subset within this group. Future comparative studies specifically on patients with severe retroversion may be warranted to better explore potential differences in this pathology. Humeral retroversion may contribute to motion and functional results after RTSA. All humeral components were implanted at approximately 20° of retroversion, though there may be variability as to the true alignment on the humeral side. There are multiple different implant systems with varying designs that may influence functional outcomes and only one was used in this study. Finally, all surgeries were performed by fellowship-trained surgeons at a tertiary academic center, so the results may not be generalizable to all practices.
Conclusion
In conclusion, we found no relationship with either preoperative glenoid retroversion or postoperative glenosphere retroversion with ASES scores or active range of motion following RTSA. Given the sample size, this study may be underpowered to detect smaller relationships as statistically significant. Patient-reported outcomes and range of motion measurements following RTSA appear to be independent of either preoperative or postoperative glenoid retroversion at short-term follow-up, and further studies are need to clarify these relationships at longer follow-up periods.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Drew Lansdown https://orcid.org/0000-0001-8086-6776
Edward C Cheung https://orcid.org/0000-0001-5125-4180
References
- 1.Bülhoff M, Sattler P, Bruckner T, et al. Do patients return to sports and work after total shoulder replacement surgery? The American journal of sports medicine 2015; 43: 423-427. DOI: 10.1177/0363546514557940. [DOI] [PubMed]
- 2.Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. JBJS 2014; 96: 198–205. DOI: doi: 10.2106/JBJS.M.00079. [DOI] [PubMed] [Google Scholar]
- 3.Westermann RW, Pugely AJ, Martin CT, et al. Reverse shoulder arthroplasty in the United States: a comparison of national volume, patient demographics, complications, and surgical indications. The Iowa orthopaedic journal 2015; 35: 1. [PMC free article] [PubMed] [Google Scholar]
- 4.Chalmers PN, Salazar DH, Romeo AA, et al. Comparative Utilization of Reverse and Anatomic Total Shoulder Arthroplasty: A Comprehensive Analysis of a High-volume Center. JAAOS-Journal of the American Academy of Orthopaedic Surgeons 2018; 26: e504–e510. DOI: doi: 10.5435/JAAOS-D-17-00075. [DOI] [PubMed] [Google Scholar]
- 5.Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. The Journal of arthroplasty 1999; 14: 756–760. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro TA, McGarry MH, Gupta R, Lee YS, Lee TQ. Biomechanical effects of glenoid retroversion in total shoulder arthroplasty. Journal of shoulder and elbow surgery 2007; 16: S90–S95. DOI: 10.1016/j.jse.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno N, Denard PJ, Raiss P, Walch G. Reverse Total Shoulder Arthroplasty for Primary Glenohumeral Osteoarthritis in Patients with a Biconcave Glenoid. JBJS 2013; 95: 1297–1304. DOI: 10.2106/jbjs.L.00820. [DOI] [PubMed] [Google Scholar]
- 8.Boileau P, Moineau G, Roussanne Y, O’shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clinical Orthopaedics and Related Research® 2011; 469: 2558–2567. DOI: 10.1007/s11999-011-1775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boileau P, Morin-Salvo N, Gauci M-O, et al. Angled BIO-RSA (bony-increased offset–reverse shoulder arthroplasty): a solution for the management of glenoid bone loss and erosion. Journal of shoulder and elbow surgery 2017; 26: 2133–2142. DOI: 10.1016/j.jse.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Wright TW, Roche CP, Wright L, et al. Reverse shoulder arthroplasty augments for glenoid wear: comparison of posterior augments to superior augments. Bulletin of the NYU Hospital for Joint Diseases 2015; 73: S124. [PubMed] [Google Scholar]
- 11.Jones RB, Wright TW, Roche CP. Bone Grafting the Glenoid Versus Use of Augmented Glenoid Baseplates with Reverse Shoulder Arthroplasty. Bulletin of the Hospital for Joint Diseases 2015; 73. [PubMed] [Google Scholar]
- 12.Service BC, Hsu JE, Somerson JS, Russ SM, Matsen FA. Does Postoperative Glenoid Retroversion Affect the 2-Year Clinical and Radiographic Outcomes for Total Shoulder Arthroplasty? Clinical Orthopaedics and Related Research® 2017; 475: 2726–2739. DOI: 10.1007/s11999-017-5433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keener JD, Patterson BM, Orvets N, Aleem AW, Chamberlain AM. Optimizing reverse shoulder arthroplasty component position in the setting of advanced arthritis with posterior glenoid erosion: a computer-enhanced range of motion analysis. Journal of shoulder and elbow surgery 2018; 27: 339–349. DOI: 10.1016/j.jse.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Berhouet J, Garaud P, Favard L. Evaluation of the role of glenosphere design and humeral component retroversion in avoiding scapular notching during reverse shoulder arthroplasty. Journal of shoulder and elbow surgery 2014; 23: 151–158. DOI: 10.1016/j.jse.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Werner BS, Chaoui J, Walch G. The influence of humeral neck shaft angle and glenoid lateralization on range of motion in reverse shoulder arthroplasty. Journal of Shoulder and Elbow Surgery 2017; 26: 1726–1731. DOI: 10.1016/j.jse.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Gulotta LV, Choi D, Marinello P, et al. Humeral component retroversion in reverse total shoulder arthroplasty: a biomechanical study. Journal of shoulder and elbow surgery 2012; 21: 1121–1127. DOI: 10.1016/j.jse.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Athwal GS, MacDermid JC, Reddy KM, et al. Does bony increased-offset reverse shoulder arthroplasty decrease scapular notching? Journal of Shoulder and Elbow Surgery 2015; 24: 468–473. DOI: 10.1016/j.jse.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg 2015; 24: 1397–1404. 2015/07/15. DOI: 10.1016/j.jse.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 19.McFarland EG, Huri G, Hyun YS, Petersen SA, Srikumaran U. Reverse Total Shoulder Arthroplasty without Bone-Grafting for Severe Glenoid Bone Loss in Patients with Osteoarthritis and Intact Rotator Cuff. J Bone Joint Surg Am 2016; 98: 1801–1807. 2016/11/04. DOI: 10.2106/JBJS.15.01181. [DOI] [PubMed] [Google Scholar]
- 20.Formaini NT, Everding NG, Levy JC, et al. The effect of glenoid bone loss on reverse shoulder arthroplasty baseplate fixation. Journal of shoulder and elbow surgery 2015; 24: e312–e319. DOI: 10.1016/j.jse.2015.05.045. [DOI] [PubMed] [Google Scholar]
- 21.Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of Fatty Infiltration of the Teres Minor Muscle on the Outcome of Reverse Total Shoulder Arthroplasty. The Journal of Bone & Joint Surgery 2007; 89: 934–939. DOI: 10.2106/JBJS.F.01075. [DOI] [PubMed] [Google Scholar]
- 22.Wiater BP, Koueiter DM, Maerz T, et al. Preoperative deltoid size and fatty infiltration of the deltoid and rotator cuff correlate to outcomes after reverse total shoulder arthroplasty. Clinical Orthopaedics and Related Research® 2015; 473: 663–673. DOI: 10.1007/s11999-014-4047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris BJ, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. Preoperative opioid use and outcomes after reverse shoulder arthroplasty. Journal of shoulder and elbow surgery 2015; 24: 11–16. DOI: 10.1016/j.jse.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Wong SE, Zhang AL, Berliner JL, Ma CB, Feeley BT. Preoperative patient-reported scores can predict postoperative outcomes after shoulder arthroplasty. Journal of shoulder and elbow surgery 2016; 25: 913–919. DOI: 10.1016/j.jse.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Hoenecke HR, Jr, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. Journal of shoulder and elbow surgery 2010; 19: 166–171. DOI: 10.1016/j.jse.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Nyffeler RW, Jost B, Pfirrmann CWA, Gerber C. Measurement of glenoid version: conventional radiographs versus computed tomography scans. Journal of Shoulder and Elbow Surgery 2003; 12: 493–496. DOI: 10.1016/s1058-2746(03)00181-2. [DOI] [PubMed] [Google Scholar]
- 27.Matsen FA, Gupta A. Axillary view: arthritic glenohumeral anatomy and changes after ream and run. Clinical Orthopaedics and Related Research® 2014; 472: 894–902. DOI: 10.1007/s11999-013-3327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho JC, Youderian A, Davidson IU, Bryan J, Iannotti JP. Accuracy and reliability of postoperative radiographic measurements of glenoid anatomy and relationships in patients with total shoulder arthroplasty. J Shoulder Elbow Surg 2013; 22: 1068–1077. 2013/02/05. DOI: 10.1016/j.jse.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Nowak DD, Gardner TR, Bigliani LU, Levine WN, Ahmad CS. Interobserver and intraobserver reliability of the Walch classification in primary glenohumeral arthritis. Journal of Shoulder and Elbow Surgery 2010; 19: 180–183. DOI: 10.1016/j.jse.2009.08.003. [DOI] [PubMed] [Google Scholar]